Abstract
Background
Rotaviruses cause viral gastroenteritis and result in more deaths from diarrhoea in children under 5 years of age than any other single agent, particularly in low‐ and middle‐income countries.
Objectives
To assess rotavirus vaccines in relation to preventing rotavirus diarrhoea, death, and adverse events.
Search methods
We searched the Cochrane Infectious Diseases Group's trial register (October 2003), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 3, 2003), MEDLINE (1966 to October 2003), EMBASE (January 1980 to October 2003), LILACS (1982 to October 2003), Biological Abstracts (January 1982 to October 2003), reference lists of articles, and contacted researchers and rotavirus vaccine manufacturers.
Selection criteria
Randomized controlled trials comparing rotavirus vaccines to placebo, no intervention, or other rotavirus vaccines in children and adults.
Data collection and analysis
Two reviewers independently extracted data and assessed trial methodological quality, and contacted trial authors for additional information.
Main results
Sixty‐four trials provided information on efficacy and safety of three main types of rotavirus vaccine (bovine, human, and rhesus) for 21,070 children. Different levels of efficacy were demonstrated with different vaccines varying from 22 to 89% to prevent one episode of rotavirus diarrhoea, 11 to 44% to prevent one episode of all‐cause diarrhoea, and 43 to 90% to prevent one episode of severe rotavirus diarrhoea. Rhesus vaccine demonstrated a similar efficacy against one episode of rotavirus diarrhoea (37 and 44% respectively), and one episode of all‐cause diarrhoea (around 15%) for trials performed in high and middle‐income countries. Results on mortality and safety of the vaccines were scarce and incomplete. We noticed important heterogeneity among the pooled studies and were unable to discard a biased estimation of effect.
Authors' conclusions
Current evidence shows that rhesus rotavirus vaccines (particularly RRV‐TV) and the human rotavirus vaccine 89‐12 are efficacious in preventing diarrhoea caused by rotavirus and all‐cause diarrhoea. Evidence about safety, and about mortality or prevention of severe outcomes, is scarce and inconclusive. Bovine rotavirus vaccines were also efficacious, but safety data are not available. Trials of new rotavirus vaccines will hopefully improve the evidence base. Randomized controlled trials should be performed simultaneously in high‐, middle‐, and low‐income countries.
2010 Editor's Note: Several of the vaccines investigated in this review are no longer in routine clinical use (for example, live attenuated rhesus‐human reassortant tetravalent vaccine) and further new vaccines have been tested and approved for use since this review was written in 2004. For an up‐to‐date assessment of rotavirus vaccines currently approved for use, please see : Soares‐Weiser K, MacLehose H, Ben‐Aharon I, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No.: CD008521. DOI: 10.1002/14651858.CD008521.
23 April 2019
No update planned
Review superseded
This Cochrane Review has been superseded by Soares‐Weiser 2019 https://doi.org/10.1002/14651858.CD008521.pub4
Plain language summary
Rotavirus vaccines can prevent diarrhoea caused by rotavirus, but we are still not clear about safety and whether they prevent deaths
Rotavirus diarrhoea causes illness and death in young children. The benefits of the vaccine were different depending on the type of vaccine. The reviewers are unable to make conclusive recommendations regarding the use of rotavirus vaccines.
Background
Rotaviruses cause viral gastroenteritis, and result in more deaths from diarrhoea than any other single agent (Vesikari 1997). In middle‐ and low‐income countries, rotavirus kills an estimated 1600 to 2400 children every day (600,000 to 870,000 per year), and can cause up to 6% of all mortality in children under 5 years of age. It is estimated that rotavirus is responsible for 20 to 25% of all deaths due to diarrhoeal disease worldwide (Bresee 1999; Glass 1999).
Biology
People become infected with rotavirus by direct faecal‐oral spread, and symptoms develop 1 to 4 days after exposure. Babies in the first 3 months tend to have infections without symptoms because of the protection of antibodies transferred to the baby via the placenta and breastfeeding. In older infants, the disease can be without symptoms, or with mild or severe symptoms, which are characterized by vomiting, fever, watery diarrhoea, and dehydration (AAP 1998). Infants and children develop immunity to rotavirus infection during the first 2 or 3 years of life, which is thought to protect from subsequent episodes of severe disease (Vesikari 1997).
Rotaviruses have two proteins that induce neutralizing antibodies. These proteins have two different specific antibody‐inducing sections, called the P and G types (P is short for the VP4 protease‐cleaved protein, and G for the glycoprotein VP7) (Matson 1990). Children that are infected for the first time usually develop specific antibodies to the infecting rotavirus type, but with subsequent infections develop a broader immunity to other types.
Control
Efforts to prevent disease by improving sanitary conditions have not reduced exposure (Glass 1999; Peter 2002). More than 90% of children, in any region of the world, have been exposed and have measurable rotavirus antibody titres by the ages of 3 to 5 years. Hence, the high priority placed on the development of a safe and effective vaccine (Peter 2002).
Vaccines
Several rotavirus vaccines have been developed (Appendix 1) (Vesikari 1997; Bresee 1999). Early vaccine candidates were developed from an animal virus strain, but the efficacy of such vaccines was highly variable due to a predominant homotypic response of these so called monovalent vaccines. In an effort to induce a heterotypic response (tetravalent vaccines), combinations of animal or human‐animal reassortant strains or attenuated human strains of rotaviruses have been included in the second generation of vaccines (Henchal 1996).
The first vaccine tested in the early 1980s was a bovine, monovalent, live‐oral rotavirus vaccine for children (NCDV ‐ RIT 4237) (Appendix 1). The first trials were conducted in Finland (Vesikari 1997), and the vaccine seemed effective both against mild and severe rotavirus infection (Vesikari 1997). After this initial success, the monovalent, live‐oral bovine vaccines (RIT 4237 and WC3) were tested in low‐ and middle‐income countries, and much lower efficacy was reported. The hypothesis generated for such lower efficacy was that in these countries the rotavirus diarrhoea was caused by a different strain, not available in the vaccine. Both bovine rotavirus vaccines were therefore withdrawn and have not been used since then (Vesikari 1997).
Monovalent rhesus (RRV) and human attenuated (M37) vaccines were tested next. These vaccines appeared to be promising, but efficacy varied greatly between different countries. This variability was suggested to occur due to serotype‐specific protection produced by the monovalent vaccine. This finding was demonstrated in controlled trials conducted in Venezuela in the early 1990s (Perez‐Schael 1990b), and led to the development of a polyvalent reassortant vaccine protecting against the four major serotypes of rotavirus that were in common circulation worldwide.
To broaden the antigenic spectrum of the vaccine, rhesus‐human reassortant rotaviruses were developed. These polyvalent vaccines provide serotype‐specific immunity against all four predominant human rotavirus serotypes (G1, G2, G3, and G4), and were described as efficacious in reducing the rate of severe rotavirus diarrhoea by 80% ‐ a rate similar to the protection conferred by natural infection (Vesikari 1997).
In August 1998, the rhesus rotavirus tetravalent vaccine (RRV‐TV, RotaShield, Wyeth‐Lederle Vaccines and Pediatrics, Philadelphia, PA) was licensed by the US Food and Drug Administration for oral administration to infants at 2, 4, and 6 months of age. This vaccine is a live‐attenuated, orally administered product derived from four group A rotavirus. Three of the rotaviruses are single gene reassortants of the VP7 gene of human origin (types G1, G2, and G4), and the fourth strain is rhesus rotavirus (type G3), which is antigenically similar to human G3. No data are available to indicate whether this rotavirus vaccine protects against diarrhoea attributable to rotavirus strains not contained in the vaccine (Vesikari 1997).
Several studies in the USA, Finland, and Venezuela have found that RRV‐TV is very effective in rotavirus disease (Perez‐Schael 1997; Vesikari 1997; AAP 1998). However, other studies conducted in Peru and Brazil produced conflicting results that showed a much lower efficacy (Lanata 1996a; Linhares 1996).
Since the introduction of RRV‐TV, several cases of bowel obstruction and intussusception following administration of the vaccine have been reported, which have lead to the vaccine being withdrawn. In July 1999, the US Centers for Disease Control and Prevention advised doctors to temporarily stop giving children the vaccine against rotavirus after counting at least 20 infants who developed intussusception in the weeks after vaccination with RRV‐TV (CDC 1999; CDC 1999a; MMWR 1999a; MMWR 1999b). In October 1999, the vaccine's manufacturer decided to voluntarily withdraw the vaccine from the market because reports of intussusception possibly linked to the product have reached about 100 (MMWR 1999b).
RRV‐TV was also thought to cause febrile reactions 3 to 4 days after vaccination due to the viral antigens. These reactions seem to be more frequent and severe in older children who lack maternally acquired antibodies. Therefore the first dose of the vaccine was recommended to be given between age 2 and 6 months in the USA.
Using the same strategy as RRV‐TV, a quadrivalent, human‐bovine, reassortant vaccine has been tested. This vaccine incorporates human VP7 with bovine genes (G1 to G3) and VP4 reassortants. Results of efficacy trials with this vaccine are due to be published (Dr Penny Heaton, Vaccine Infectious Diseases ‐ Merck, personal communication).
Other vaccines have been developed (Bresee 1999; Merck 1999; Jones 2001). In Japan, an inactivated parenteral vaccine (BIRVI) with strains of human origin has been tested. In China, a live oral vaccine that uses a lamb strain appeared to be safe and immunogenic in phase II trials, and efficacy trials are now being planned. Vaccine‐like particles for use as an inactivated vaccine are being developed in Korea (Cunliffe 2002).
Sources of heterogeneity
The wide variation in protection observed in randomized controlled trials may be related to the response of the immune system to different strains of the rotavirus or of rotavirus vaccine, the study population, or the study design.
Rotavirus
Cross‐immunization is induced between some, but not all, human and animal strains. The differences in efficacy found in the various studies can be attributed in part to differences in serotypes of circulating strains and the failure of animal strains to elicit heterotypic protection.
Vaccines
Different vaccine formulations may have different efficacy. Premature conclusions about effectiveness were drawn from trials of bovine rotavirus vaccine in low‐ and middle‐income countries, and the same should not be repeated for other vaccines.
Study population
One possible explanation for low efficacy of rotavirus vaccine in low‐ and middle‐income countries may be the interference by other enteric viruses, which are a common cause of diarrhoea in children in such countries (Vesikari 1997). The epidemiology of rotavirus differs in low‐ and middle‐income compared to high‐income countries. In low‐ and middle‐income countries, the disease occurs all the year round (compared with seasonal peaks in high‐income countries), children are infected at an early age (6 to 9 months), immunization efforts require greater levels of coverage to be effective, and children are often subject to infections with strains that are not included in the current vaccines (Bresee 1999).
As a result, it has been speculated that rotavirus vaccine could be more efficacious if given at an earlier age, not associated with other vaccines (particularly 'diphtheria‐pertussis‐tetanus' (DPT) and oral polio vaccine (OPV)), or breastfeeding. Additionally, it has been suggested that higher titre and multiple doses of the vaccine are more efficacious (Vesikari 1997; Bresee 1999).
Study design
It is also plausible that some methodological issues, such as allocation concealment (adequate or unclear), exclusions after randomization (reported or not reported), sample size (< 1000, ≥ 1000), and length of follow up (one rotavirus season or more than one rotavirus season) could provide different levels of efficacy.
Significance of rotavirus vaccine
Finding that a rotavirus vaccine is efficacious might encourage immunization in the countries with the highest burden (Tucker 1998). This has to be weighed against the available resources (Griffiths 1995). Hence, to become a worthwhile intervention, vaccines against rotavirus will not only have to be proven to be efficacious and not related to major adverse events, but also affordable and cost‐effective in comparison with other potential interventions (Glass 1999).
Efficacy levels of as low as 50 to 60% against severe rotavirus disease could prevent a large number of deaths (Josefson 1997). Because rotavirus disease causes up to 6% of all mortality among children less than 5 years of age in low‐ and middle‐income countries, universal immunization with rotavirus vaccine may prevent a significant number of deaths, and the trade off against rare severe adverse effects may well be different from the situation in high‐income countries.
Objectives
To assess rotavirus vaccines in relation to preventing rotavirus diarrhoea, death, and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Children and adults.
Types of interventions
We included the following interventions and controls regardless of doses and/or schedules used in the different trials.
Intervention
Live‐attenuated bovine rotavirus vaccine (RIT‐4237, WC3, W179‐9, QHBRV).
Rhesus rotavirus vaccine (RRV, RRV‐TV).
Human‐attenuated rotavirus vaccine (M37, 89‐12).
Other rotavirus vaccines (BIRVI, Lamb RV).
Control
Placebo or no vaccination.
Any of the interventions mentioned above.
Types of outcome measures
Primary
Rotavirus diarrhoea
Episodes.
Severe episodes (defined as an episode that last for more than 24 hour of duration, with more than six stools in 24 hours, presence of vomiting, fever, and dehydration (WHO 1995)).
Episodes requiring hospitalization.
Episodes of more than four days duration.
Secondary
All‐cause diarrhoea
Episodes.
Episodes during first week after vaccine.
Severe episodes (defined as an episode that last for more than 24 hour of duration, with more than six stools in 24 hours, presence of vomiting, fever, and dehydration (WHO 1995)).
Episodes requiring re‐hydration.
Episodes requiring hospitalization.
Death: all‐cause.
Death: from rotavirus infection.
Malnutrition at follow up.
Drop outs before the end of the study.
Adverse events
Bowel obstruction and/or intussusception related to the use of the rotavirus vaccine.
Serious adverse events that are fatal, life threatening, or require hospitalization.
Systemic reaction, such as fever, related to the use of the rotavirus vaccine.
Adverse events that require discontinuation of vaccination schedule.
We excluded studies collecting only immunological data.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
We searched the Cochrane Infectious Diseases Group's trials register for relevant trials up to October 2003 using the search terms: rotavirus; diarrhoea; diarrhea; gastroenteritis; and vaccine. Full details of the Cochrane Infectious Diseases Group methods and the journals handsearched are published in The Cochrane Library in the section on Collaborative Review Groups.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 3, 2003), using the search terms: rotavirus; diarrhoea; diarrhea; gastroenteritis; and vaccine. We also searched the following electronic databases using the search terms in combination with the search strategy developed by The Cochrane Collaboration (Clarke 2003); MEDLINE (1966 to October 2003) using the search terms: rotavirus; rotavirus‐infections; diarrhoea; diarrhea; gastroenteritis; vaccines‐attenuated; and viral‐vaccines; EMBASE (January 1980 to October 2003) using the search terms: rotavirus; diarrhoea; diarrhea; gastroenteritis; vaccines‐attenuated; and viral‐vaccines; and LILACS (www.bireme.br; 1982 to October 2003) using the search terms: rotavirus; diarrhoea; diarrhea; gastroenteritis; and vaccine. Biological Abstracts (January 1982 to October 2003) using the search terms: rotavirus vaccine; diarrhoea; and diarrhea.
We checked the reference lists of all trials identified by the above methods. We also contacted the first or corresponding author of each included study as well as researchers active in the field, and a rotavirus vaccine manufacturer (Merck Sharp & Dohme) for information regarding unpublished trials or complementary information on their own trial.
Data collection and analysis
Selection of studies
Two reviewers (Karla Soares‐Weiser (KSW), Elad Goldberg (EG)) independently inspected the abstract of each reference identified by the search and determined the potential relevance of each article. For potentially relevant articles, or in cases of disagreement, we obtained the full article and independently inspected it and applied the inclusion criteria. Where we were unable to resolve the disagreement through discussion, we added the article to those 'awaiting assessment' and contacted the authors of the study for clarification. In an event of no reply from the authors within 6 months, a third reviewer (Leonard Leibovici (LL)) checked the article to solve disagreements. We also documented our justification for excluding studies from the review.
Data extraction and management
Two reviewers (KSW, EG) independently extracted data from the included trials. In case of any disagreement between the two reviewers, a third reviewer (LL) extracted the data. We discussed the data extraction, documented decisions, and where necessary, contacted the study authors for clarification.
We identified trials by the name of the first author and year in which the trial was first published. We extracted, checked, and recorded the following data.
1. Characteristics of trials.
Date location and setting of trial.
Publication status.
Case definitions used (clinical, serological, virological).
Sponsor of trial (specified, known or unknown).
2. Characteristics of participants.
Number of participants in each group.
Age, gender, nationality, ethnic group, and risk category.
Previous immunization status (if known).
Use of breast‐bottle feed during the vaccination.
Presence of any immunodeficiency.
3. Characteristics of interventions.
Type of vaccine, type of control, dose, and immunization schedule.
4. Characteristics of outcome measures.
Number of people who died in the vaccine and control group.
Number of people who developed rotavirus diarrhoea.
Number of people who developed any diarrhoea.
Number of hospitalizations for diarrhoea, dehydration, or malnutrition.
Systemic adverse events, specifically bowel obstruction.
Any adverse events.
Length of follow up (in months).
Loss to follow up (drop outs) before end of study.
We individually extracted adverse event data for each adverse event wherever possible. For trials reporting adverse events for more than one dose, we recorded the average number of people reporting each adverse event for each dose was recorded.
Assessment of risk of bias in included studies
Two reviewers (KWS, EG) assessed the included trials for methodological quality. We extracted information about the method of treatment allocation (random, quasi‐random, sequential, not stated), concealment of allocation (adequate (A), unclear (B), inadequate (C) according to Juni 2001), blinding (double, single, or not blind), sample size (< 1000, ≥ 1000), and exclusions after randomization (reported, not reported), and the follow‐up period (one rotavirus season after immunization, two or more rotavirus seasons).
Data synthesis
We analysed dichotomous data by calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed using 95% confidence intervals (CI). Additionally, we tested variability according to differences in type of vaccine (bovine, human, or rhesus).
Using the method of Newcombe‐Wilson hybrid score (not continuity corrected (Newcombe 1998)) and the correspondent 95% CI, we estimated the number of children needed to take rotavirus vaccine to avoid one case of rotavirus diarrhoea (NNT) for trials that tested each specific vaccine.
Heterogeneity and publication bias
We initially assessed heterogeneity on the results of the trials by inspecting of graphical presentations and by calculating a test of heterogeneity (chi‐squared test). However, we were aware of the fact that the chi‐squared test has a poor ability to detect statistically significant heterogeneity among studies. Therefore, we have also quantified the impact of heterogeneity in the meta‐analysis using a measure of the degree of inconsistency in the studies' results (Higgins 2003). This measure (I2) describes the percentage of total variation across studies that are due to heterogeneity rather than the play of chance (Higgins 2003). The values of I2 lies between 0 and 100%, and a simplified categorization of heterogeneity could be low, moderate, and high to I2 values of 25, 50, and 75% (Higgins 2003).
We anticipated between‐trial variation in estimation of vaccine efficacy for trials that used different rotavirus strains and were conducted in different geographic locations. We performed subgroup analyses in order to assess the impact of these possible sources of heterogeneity. In addition, we performed sensitivity analyses for the main outcome (rotavirus diarrhoea) in order to assess the robustness of the findings to different aspects of the trials methodology: concealment of allocation (adequate or unclear); exclusions after randomization (reported or not reported); sample size (< 1000, ≥1000); and length of follow up (one rotavirus season or more than one rotavirus season). We carried out further exploratory sensitivity analyses to test the impact of different types of vaccines, concomitant use of diphtheria‐tetanus‐pertussis (DPT) and/or oral polio vaccine (OPV), breastfeeding during vaccination, and number of doses of vaccine (single dose, two to three doses) in the pooled efficacy.
We used a fixed effect model throughout the review, except in the event of statistically significant heterogeneity between the trials (P < 0.10), when we chose the random effects model. And because we demonstrated heterogeneity in most of the outcomes when we performed the analyses using odds ratio or RR either on a fixed effect or random effects model (see Appendix 2 for an example), we chose to present the results using the RR with the correspondent 95% CI and a random effects model.
We observed moderate to high statistically heterogeneity among most of the outcomes evaluated on this review. Even when we analysed the vaccines separately, we observed heterogeneity in the combined trials for the following vaccines and outcomes: RRV‐TV (episodes of rotavirus diarrhoea, episodes of severe rotavirus diarrhoea), RRV (all‐cause diarrhoea), RIT‐4237 (episodes of rotavirus diarrhoea, episodes of severe rotavirus diarrhoea, all‐cause diarrhoea), and WC3 (episodes of rotavirus diarrhoea) vaccines.
We examined a funnel plot estimating the precision of trials (plots of logarithm of the RR for efficacy against the sample size) in order to estimate potential asymmetry.
Personal communication with authors and pharmaceutical industry
We contacted the corresponding authors of the 64 included trials, of which 25 replied and 20 provided additional data. We initially sought data on study design, death, bowel obstruction and/or intussusception, and dehydration. Because of the low response rate in the first round, we attempted to contact the authors for a second time using only e‐mail and requesting information only for deaths and adverse events. The results of the personal communication with the authors are summarized in Appendix 3. These data were previously published for only three of the trials (Joensuu 1997; Santosham 1997; Bresee 2001).
We also contacted Merck Sharp & Dohme − the pharmaceutical industry organizing the field trials of the new bovine quadrivalent vaccine − for further details on their trials. Dr Penny Heaton (Vaccine Infectious Diseases, Clinical Research) replied and informed us that most of the trials will be published, and they would prefer not to release any further information until after publication (e‐mail on 11 March 2002).
Results
Description of studies
Eligibility
We identified 138 potentially relevant references through our search strategy, and 1 trial through contact with authors, who also provided unpublished data (Perez‐Schael 2002). Of these, we included 64 trials (90 references), which are described below and in the 'Characteristics of included studies', and excluded 31 studies (33 references) and 16 reviews of the literature.
We have described the 31 excluded studies in the 'Characteristics of excluded studies'. Our main reasons for excluding these studies were because they were not randomized or had an inadequate allocation concealment (7), did not measure any clinical outcome (21), and because all participants received the vaccine and were randomized to different dietary components (3).
Two randomized controlled trials are still awaiting assessment because currently only an abstract without the required information has been published (Seth 2000; Vesikari 2002).
Participants
All but one study tested vaccines or placebo in children, age ranging from newborns to 12 years old; Barnes 1997 is a small safety study included a group of 10 adults (21 to 30 years old). Studies included only healthy children; in a single study, we found a description that children were included if they did not have any debilitating disease (Perez‐Schael 1990c).
Interventions
Details of the six different types of vaccines assessed in the included studies can be found in Appendix 4. Only one trial did not use placebo in the control group (Vesikari 1986a). In another trial (Santosham 1991a and Santosham 1991b), children were allocated to three groups: RIT 4237 (106 children); MMU18006 (108 children); placebo (107 children); the placebo group was arbitrarily divided into two arms, and we report each arm as a separate study.
Outcomes
Of the 64 included trials, 49 trials provided information on the safety of rotavirus vaccine in 16,602 children, and 40 trials provided information on the effectiveness of the vaccine to prevent rotavirus diarrhoea in 21,070 children. See the 'Characteristics of included studies for the complete list of outcomes provided by each study; no study reported on malnourishment.
Rotavirus was detected in all studies using serology and stool analysis by the ELISA method. An episode of diarrhoea was defined in the majority of the studies as at least two liquid or semi‐liquid stools in 24 hours. The definition of severity of diarrhoea varied according to the criteria used in the trial. Thirty‐seven studies used only a clinical definition of severe diarrhoeal diseases. The other studies used either the Clark 1988 scale (4 studies), Flores 1987 scale (7 studies), Rennels 1996 scale (3 studies), Ruuska 1990 scale (6 studies) or the World Health Organization (WHO) criteria (WHO 1995; 7 studies) for diarrhoea to measure the severity of diarrhoeal diseases.
Twenty‐three trials had an unbalanced randomization with more than one experimental group, which may affect the calculation of the NNT: 10 trials tested different strains of the same vaccine in two to four experimental groups; 10 trials tested different dosages of the same vaccine; and 3 trials tested different vaccines in two experimental groups. Of these three trials two provided data only on safety of the vaccine (children were followed up for up to 10 days after vaccination) (Flores 1990; Perez‐Schael 1994); we used data on the placebo and rhesus vaccines groups and discarded data on the M37 vaccine. The other trial provided data on the efficacy of two rotavirus vaccines (RIT 4237, MMU18006) against placebo (Santosham 1991a; Santosham 1991b).
Study location
We divided the trials according to the classification of high‐, middle‐, and low‐income countries of the World Bank (World Bank 2003). Trials were conducted in high‐income countries: Australia (2), Austria (1), Finland (12), Israel (1), Spain (1), Sweden (1), the United Kingdom (1), the United States of America (29); middle‐income countries: Brazil (1), Peru (3), and Venezuela (9); and low‐income countries: Bangladesh (1), Central African Republic (1), Gambia (1), Rwanda (1).
Risk of bias in included studies
Generation of allocation sequence
Seven trials reported information about generation of allocation sequence in the published version. They used random code assignment or computer‐generated randomization. The code was kept by the pharmaceutical industry or academic institutions (including World Health Organization and National Institute of Health, USA) and not broken until the end of the study. In eight trials, randomization was generated by a central computer, and pre‐coded vessels were administered sequentially to the children. It was not possible to determine the method used in the remaining 49 trials.
Concealment of allocation
Fifteen trials had adequate allocation concealment (information was available in the publication in seven trials and sought from authors in eight trials), and scored A. In the remained 49 trials it was not possible to determine the method used to conceal the allocation and these trials scored B (unclear).
Blinding
All trials used placebo in the control arm. Sixty‐one trials were reported to be double blind, and three studies provided no information. Only 29 out of the 61 studies reported a procedure to guarantee double blinding. The vaccine and placebo were of identical appearance in 28 trials. In another trial (Vesikari 1986a), parents and paediatricians were unaware of the code given to vaccine and placebo.
Exclusions after randomization
Exclusion of participants from the trials after randomization varied from 0 to 23%, except in Hanlon 1987, where 43% of the randomized children were excluded because they were absent during the epidemic period or too young to be vaccinated.
From the 40 included trials that provided data on efficacy: 6 did not provide any information about drop outs before end of study; in the other trials exclusion of participants from the trials after randomization varied from 0 to 23%, except in Hanlon 1987, where 43% of the randomized children were excluded because they were absent during the epidemic period or were too young to be vaccinated. Nineteen trials (n = 12,493) provided data for each group (vaccine or placebo) on the number of children dropped out before the end of the study. There was no statistically significant difference in the number of people who dropped out between the vaccine and placebo arms of the rhesus rotavirus vaccine trials (RR 0.95, 95% CI 0.85 to 1.06; Analysis 1.1).
1.1. Analysis.

Comparison 1 Drop outs before end of study, Outcome 1 Drop outs before end of study.
Follow‐up period
The follow‐up period was less than a month in 21 trials, up to one year (one season) in 20 trials, and of one to four years (two or more seasons) in 23 trials.
We have provided additional information about the methodological quality of the trials in the Characteristics of included studies'.
Effects of interventions
Rotavirus diarrhoea
Episodes
Forty trials provided information for 21,070 children regarding diarrhoeal disease caused by rotaviruses. Rotavirus vaccine prevents rotavirus diarrhoea in all three groups of vaccines: rhesus (RR 0.59, 95% CI 0.50 to 0.70); bovine (RR 0.59, 95% CI 0.45 to 0.76); and human (RR 0.42, 95% CI 0.21 to 0.85) (Analysis 2.1).
2.1. Analysis.

Comparison 2 Rotavirus diarrhoea, Outcome 1 Episodes.
Converting these RR values to vaccine efficacy gives the following estimates for preventing on episode of rotavirus diarrhoea: 41% (95% CI 30 to 50%) for rhesus vaccines; 41% (95% CI 24 to 55%) for bovine vaccines; and 58% (95% CI 15 to 79%) for human vaccines.
Severe episodes
Rotavirus vaccines also showed a highly statistically significant benefit in preventing severe episodes of rotavirus infection (Analysis 2.2): rhesus (RR 0.42, 95% CI 0.31 to 0.57); bovine (RR 0.38, 95% CI 0.24 to 0.60); and human (RR 0.21, 95% CI 0.13 to 0.35).
2.2. Analysis.
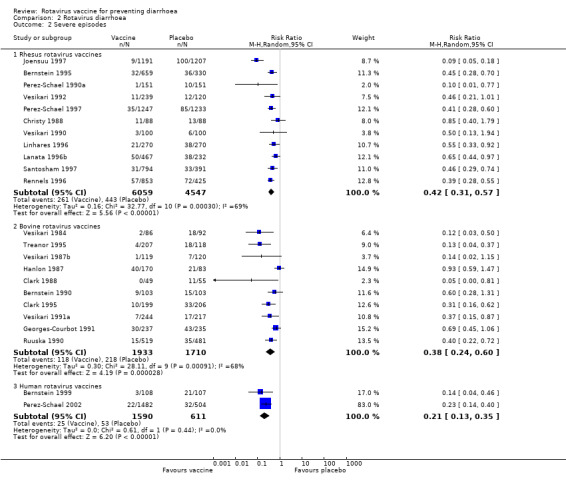
Comparison 2 Rotavirus diarrhoea, Outcome 2 Severe episodes.
Converting these RR values to vaccine efficacy gives the following estimates to prevent one episode of severe rotavirus diarrhoea: 58% (95% CI 43 to 69%) for rhesus vaccines; 62% (95% CI 40 to 76%) for bovine vaccines; and 79% (95% CI 65 to 87%) for human vaccines.
Episodes requiring hospitalization
Rotavirus vaccines also showed a highly statistically significant benefit in preventing severe rotavirus infection requiring hospitalization (Analysis 2.3): rhesus (RR 0.48, 95% CI 0.27 to 0.86); bovine (RR 0.37, 95% CI 0.18 to 0.74); and human (RR 0.21, 95% CI 0.09 to 0.48).
2.3. Analysis.

Comparison 2 Rotavirus diarrhoea, Outcome 3 Episodes requiring hospitalization.
Converting these RR values to vaccine efficacy gives the following estimates to prevent one episode of rotavirus diarrhoea requiring hospitalization: 52% (95% CI 14 to 73%) for rhesus vaccines; 63% (95% CI 26 to 82%) for bovine vaccines; and 79% (95% CI 52 to 91%) for human vaccines.
Episodes of more than four days duration
Only the rhesus vaccine significantly reduced the number of cases of rotavirus diarrhoea with more than four days of duration (rhesus: RR 0.61, 95% CI 0.42 to 0.87; bovine: RR 1.01, 95% CI 0.51 to 1.99; Analysis 2.4).
2.4. Analysis.

Comparison 2 Rotavirus diarrhoea, Outcome 4 Episodes of more than four days.
All‐cause diarrhoea
Episodes
Twenty‐three trials provided information about all‐cause diarrhoea for 11,296 children. A further nine trials provided information on episodes of diarrhoeal disease per person‐year in 3944 children. We could not combine the latter in a meta‐analysis and have summarized them in Analysis 3.1. Meta‐analysis was also not possible for human vaccines, as only one trial provided data on this outcome.
3.1. Analysis.
Comparison 3 All‐cause diarrhoea, Outcome 1 Episodes (number of episodes/number of person‐years).
| Episodes (number of episodes/number of person‐years) | ||||
|---|---|---|---|---|
| Study | Episodes (vaccine) | Person‐years vaccine | Episodes (placebo) | Person‐years placebo |
| Rhesus rotavirus vaccines | ||||
| Gothefors 1989 | 75 | 53 | 79 | 51 |
| Lanata 1996a | 9104 | 1032 | 3000 | 339 |
| Lanata 1996b | 6518 | 758 | 3200 | 386 |
| Linhares 1996 | 2579 | 361 | 2803 | 363 |
| Perez‐Schael 1990a | 294 | 141 | 290 | 130 |
| Santosham 1991a | 397 | 130 | 383 | 125 |
| Santosham 1997 | 2559 | 1391 | 1331 | 680 |
| Bovine rotavirus vaccines | ||||
| Bernstein 1990 | 153 | 102 (patients) | 179 | 103 (patients) |
| Lanata 1989 | 3432 | 358 | 1343 | 127 |
| Santosham 1991b | 404 | 125 | 383 | 125 |
| Human rotavirus vaccines | ||||
| Barnes 1997 | 62 | 40 (patients) | 23 | 20 (patients) |
| Georges‐Courbot 1991 | 503 | 232 (patients) | 455 | 230 (patients) |
We found a statistically significant beneficial effect of the rhesus vaccines (RR 0.86, 95% CI 0.80 to 0.92; Analysis 3.2) and bovine vaccines (RR 0.73, 95% CI 0.60 to 0.89; Analysis 3.2), and no statistically significant difference for human vaccines (RR 0.91, 95% CI 0.57 to 1.44; Analysis 3.2).
3.2. Analysis.

Comparison 3 All‐cause diarrhoea, Outcome 2 Episodes (number of cases/number of participants).
Converting these RR values to vaccine efficacy gives the following estimates to prevent one episode of diarrhoea: 14% (95% CI 8 to 20%) for rhesus vaccines; and 27% (95% CI 11 to 40%) for bovine vaccines.
Episodes during first week after vaccine (one or multiple doses)
The data indicated a non statistically significant difference between vaccine and placebo in the number of cases of diarrhoea in the first week after inoculation of the vaccine for both rhesus (RR 1.06, 95% CI 0.98 to 1.14) and bovine vaccines (RR 1.02, 95% CI 0.89 to 1.17), and a slightly statistically significant difference for the human vaccines (RR 1.94, 95% CI 1.03 to 3.65) (Analysis 3.3, fixed effect model).
3.3. Analysis.
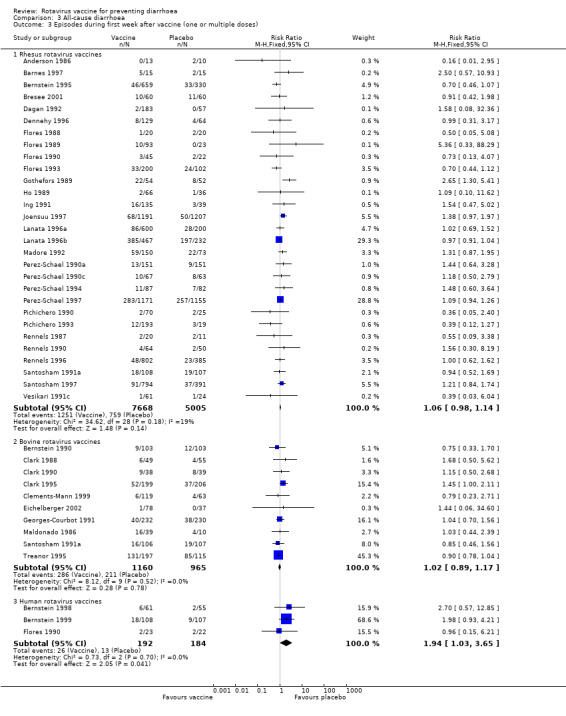
Comparison 3 All‐cause diarrhoea, Outcome 3 Episodes during first week after vaccine (one or multiple doses).
Severe episodes
Rotavirus vaccines showed a non‐statistically significant benefit in preventing episodes of severe diarrhoeal diseases: rhesus (RR 0.85, 95% CI 0.72 to 1.00); and bovine (RR 0.51, 95% CI 0.21 to 1.26) (Analysis 3.4).
3.4. Analysis.
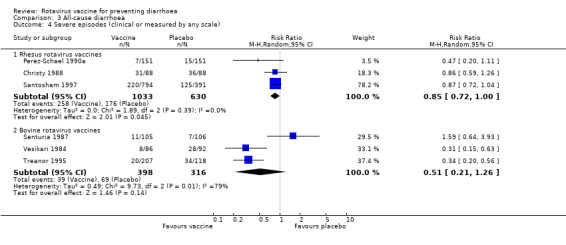
Comparison 3 All‐cause diarrhoea, Outcome 4 Severe episodes (clinical or measured by any scale).
Episodes requiring re‐hydration
Rotavirus vaccines also showed a statistically significant benefit in preventing diarrhoeal diseases requiring re‐hydration: rhesus (RR 0.51, 95% CI 0.30 to 0.88); and bovine (RR 0.42, 95% CI 0.26 to 0.67) (Analysis 3.5).
3.5. Analysis.

Comparison 3 All‐cause diarrhoea, Outcome 5 Episodes requiring re‐hydration.
Converting these RR values to vaccine efficacy gives the following estimates to prevent one episode of diarrhoea requiring re‐hydration: 49% (95% CI 12 to 70%) for rhesus vaccines; and 58% (95% CI 33 to 74%) for bovine vaccines.
Episodes requiring hospitalization
Rotavirus vaccines also showed a non statistically significant benefit in preventing cases of diarrhoea requiring hospitalization: rhesus (RR 0.81, 95% CI 0.66 to 0.99); and bovine (RR 0.55, 95% CI 0.16 to 1.91) (Analysis 3.6).
3.6. Analysis.

Comparison 3 All‐cause diarrhoea, Outcome 6 Episodes requiring hospitalization.
Death
All‐cause death
Published information about deaths was available in five studies. We received additional information for a further 18 studies after contacting the study authors. Overall, 57 cases of deaths occurred among the different trials. They occurred in trials of rhesus rotavirus vaccine (1 in a RRV‐MV trial, 36 in 6 RRV‐TV trials), live‐attenuated bovine rotavirus vaccines (8 in a RIT‐4237 trial, 11 in a WC3 trial), and human‐attenuated rotavirus vaccine (1 in a 89‐12 trial). Meta‐analysis was possible for 4 studies of rhesus rotavirus vaccines, accounting for 10 deaths in 6029 children. An additional 47 deaths occurred during the trials, but we have no information about the specific group to which these participants were allocated, and we could not pool these data together (seeAppendix 3 for details). The pooled data indicated a non‐statistically significant difference between children randomized to receive rhesus rotavirus vaccines or placebo in the number of deaths (RR 0.72, 95% CI 0.16 to 3.12; Analysis 4.1).
4.1. Analysis.

Comparison 4 Death, Outcome 1 All‐cause death.
Exploration of heterogeneity
We could not explore heterogeneity with the human rotavirus vaccines because of the small number of trials testing the vaccine. However, we were able to explore heterogeneity with trials from other classes, and the results are described below.
Risk of bias (Methodological quality)
Trials with adequate (A) or unclear (B) allocation concealment demonstrated a similar ratio of efficacy on preventing rotavirus diarrhoea for rhesus vaccines (A: RR 0.58, 95% CI 0.45 to 0.76; B: RR 0.57, 95% CI 0.48 to 0.67). As for bovine vaccines the comparison between adequate and unclear allocation concealment could not be undertaken because of small number of trials in at least one subgroup (Analysis 5.1 and Analysis 5.2).
5.1. Analysis.
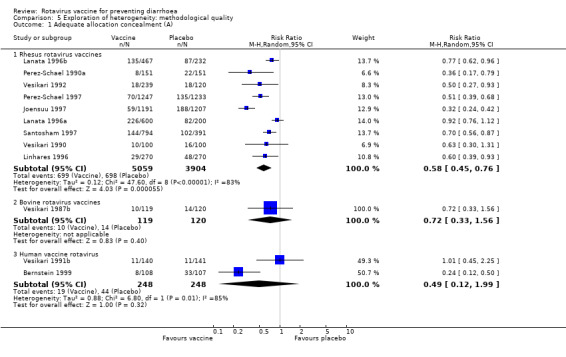
Comparison 5 Exploration of heterogeneity: methodological quality, Outcome 1 Adequate allocation concealment (A).
5.2. Analysis.

Comparison 5 Exploration of heterogeneity: methodological quality, Outcome 2 Unclear allocation concealment (B).
We also observed a similar RR in trials of rhesus vaccines that did report exclusions after randomization (RR 0.59, 95% CI 0.48 to 0.71) compared to those that did not (RR 0.58, 95% CI 0.44 to 0.76). Levels of efficacy were higher for bovine vaccine trials that reported exclusions (RR 0.50, 95% CI 0.26 to 0.96) than for trials that did not report exclusions after randomization (RR 0.61, 95% CI 0.46, 0.82) (Analysis 5.3 and Analysis 5.4).
5.3. Analysis.

Comparison 5 Exploration of heterogeneity: methodological quality, Outcome 3 Exclusions after randomization reported.
Regarding different sample size rhesus vaccine trials presented a slightly higher ratio of efficacy for larger trials when compared to trials with smaller sample size (< 1000 participants: RR 0.66, 95% CI 0.56 to 0.79; ≥ 1000 participants: RR 0.50, 95% CI 0.39 to 0.65). Once again we could not make the comparison for bovine vaccines because only one trial had a sample size of at least 1000 participants (Analysis 5.5 and Analysis 5.6).
Multiple versus single dose
Trials with multiple dose of rhesus rotavirus vaccine had a higher ratio of efficacy (RR 0.55, 95% CI 0.44 to 0.69) when compared to trials that used single dose of the vaccine (RR 0.64, 95% CI 0.50 to 0.82). Bovine vaccine trials in the other hand had similar ratio of efficacy for both multiple or single doses (multiple doses: RR 0.58, 95% CI 0.39 to 0.86; single dose: RR 0.57, 95% CI 0.40 to 0.83) (Analysis 6.1 and Analysis 6.2). All trials using multiple doses of vaccine were performed in the 1990s.
Administered with other vaccines
Rhesus vaccine trials that allowed the concomitant use of DPT and/or OPV and rotavirus vaccine demonstrated a higher ratio of efficacy on preventing rotavirus diarrhoea (RR 0.57, 95% CI 0.44 to 0.73) than trials that did not allow the concomitant use of DPT and/or OPV (RR 0.71, 95% CI 0.52 to 0.97). We could not make a comparison among bovine vaccine trials because many trials did not provide this information (Analysis 7.1, Analysis 7.2, and Analysis 7.3).
Breastfeeding
Rhesus vaccine trials that allowed breastfeeding had a smaller RR (0.52, 95% CI 0.32 to 0.86) than trials that did not allow breastfeeding at least one hour before or after vaccination (0.61, 95% CI 0.51 to 0.73). Conversely, bovine vaccine trials had a higher RR for trials that allowed breastfeeding at least one hour before or after vaccination (0.64, 95% CI 0.48 to 0.85) than the ones that did not (0.45, 95% CI 0.21 to 0.94) (Analysis 8.1 and Analysis 8.2). No information was provided about breastfeeding in four trials (Analysis 8.3).
8.1. Analysis.

Comparison 8 Exploration of heterogeneity: breastfeeding, Outcome 1 Breastfeeding allowed during vaccination.
8.2. Analysis.

Comparison 8 Exploration of heterogeneity: breastfeeding, Outcome 2 Breastfeeding not allowed for at least one hour before and after vaccination.
8.3. Analysis.

Comparison 8 Exploration of heterogeneity: breastfeeding, Outcome 3 No information.
Number of seasons of follow up
Rhesus vaccine trials had a similar ratio of efficacy for trials where children where followed up for one or at least two seasons (one season: RR 0.57, 95% CI 0.42 to 0.78; two or more seasons: RR 0.60, 95% CI 0.48 to 0.74). We found a slight difference among bovine vaccine trials that followed up for one season (RR 0.52, 95% CI 0.32 to 0.83) or at least two seasons (RR 0.60, 95% CI 0.43 to 0.84) (Analysis 9.1 and Analysis 9.2).
9.1. Analysis.
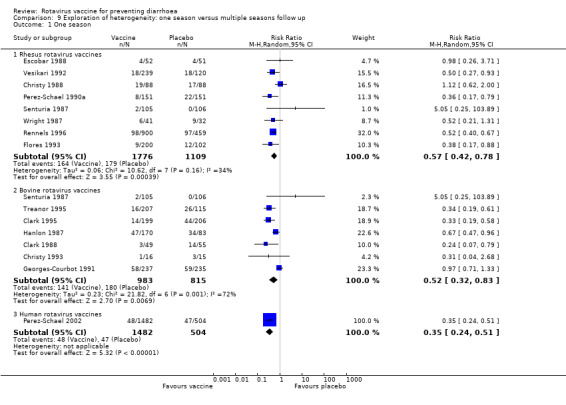
Comparison 9 Exploration of heterogeneity: one season versus multiple seasons follow up, Outcome 1 One season.
9.2. Analysis.

Comparison 9 Exploration of heterogeneity: one season versus multiple seasons follow up, Outcome 2 Two or more seasons.
Different rotavirus strains
We analysed the following vaccines separately for rotavirus diarrhoea, severe rotavirus diarrhoea, and all‐cause diarrhoea: rhesus tetravalent vaccine (RRV‐TV), rhesus monovalent vaccines (RRV, RRV‐D, RRV‐DS1, or RRV‐ST3), bovine vaccines (RIT‐4237 or WC3), bovine‐human vaccine (WC3‐QV), and human vaccines (89‐12 or M37). We observed a statistically significant beneficial effect for most vaccines, except for the bovine vaccine WC3 and the human vaccine M37 for rotavirus diarrhoea and severe episodes of rotavirus diarrhoea. The complete results are presented in Appendix 5 and Analysis 10.1, Analysis 10.2, and Analysis 10.3.
10.1. Analysis.

Comparison 10 Exploration of heterogeneity: different rotavirus vaccines, Outcome 1 Rotavirus diarrhoea: episodes.
10.2. Analysis.

Comparison 10 Exploration of heterogeneity: different rotavirus vaccines, Outcome 2 Rotavirus diarrhoea: severe episodes.
10.3. Analysis.

Comparison 10 Exploration of heterogeneity: different rotavirus vaccines, Outcome 3 All‐cause diarrhoea: episodes (number of cases/number of participants).
The NNT for each specific vaccine is available in Appendix 5. Eleven of the 40 trials that dealt with vaccine efficacy had more than one group of vaccine combined for the meta‐analysis (Lanata 1989; Mutz 1989; Vesikari 1992; Flores 1993; Bernstein 1995; Rennels 1995; Rennels 1996; Lanata 1996a; Lanata 1996b; Santosham 1997; Perez‐Schael 2002), which generated an imbalance in the number of participants and might imply possible bias on the calculation of the NNT (Altman 2002).
Country of origin: income status
We also analysed rotavirus vaccines according to the income status of country of origin. Trials of rhesus and human rotavirus vaccines were performed only in high‐ and middle‐income countries; only three small trials of bovine vaccine were performed in low‐income countries. We could only do subgroup analyses for rhesus rotavirus vaccine in high‐ and middle‐income countries. The results did not tend to be significantly different for any of the main outcomes. The complete results are presented in Analysis 11.1, Analysis 11.2, Analysis 11.3, Analysis 11.4, Analysis 11.5, Analysis 11.6, and Analysis 11.7.
11.1. Analysis.

Comparison 11 Exploration of heterogeneity: country of origin ‐ income status, Outcome 1 Rotavirus diarrhoea: episodes.
11.2. Analysis.

Comparison 11 Exploration of heterogeneity: country of origin ‐ income status, Outcome 2 Rotavirus diarrhoea: severe episodes.
11.3. Analysis.
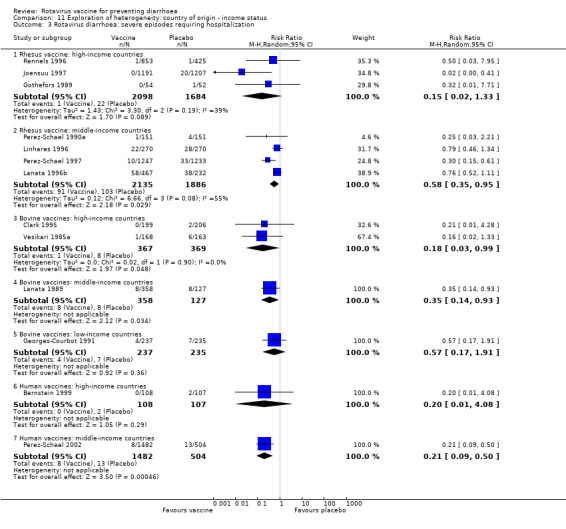
Comparison 11 Exploration of heterogeneity: country of origin ‐ income status, Outcome 3 Rotavirus diarrhoea: severe episodes requiring hospitalization.
11.4. Analysis.

Comparison 11 Exploration of heterogeneity: country of origin ‐ income status, Outcome 4 All‐cause diarrhoea: episodes (number of cases/number of participants).
11.5. Analysis.

Comparison 11 Exploration of heterogeneity: country of origin ‐ income status, Outcome 5 All‐cause diarrhoea: severe episodes (clinical or measured by any scale).
11.6. Analysis.

Comparison 11 Exploration of heterogeneity: country of origin ‐ income status, Outcome 6 All‐cause diarrhoea: episodes requiring hospitalization.
11.7. Analysis.

Comparison 11 Exploration of heterogeneity: country of origin ‐ income status, Outcome 7 All‐cause diarrhoea: episodes requiring re‐hydration.
Adverse events
Forty‐nine trials provided information on the safety of rotavirus vaccines for 16,602 children. We have summarized the results according to the outcomes collected in the published trials and personal communication with several authors.
Bowel obstruction and/or intussusception
Published information about cases of bowel obstruction and/or intussusception was available in two studies. We received additional information for a further 20 studies after contacting the study authors. Overall, four cases of intussusception were reported among trials of the rhesus rotavirus vaccine RRV‐TV. Additional cases of intussusception in trials conducted with RRV‐TV were described in the literature (Rennels 1998; Murphy 2001; Niu 2001; Zanardi 2001; Murphy 2002), but we failed to get this information after three attempts of contacting the authors.
The intussusception cases occurred between 6 and 44 days after vaccination: one was reported in the vaccine and three in the placebo group. A meta‐analysis was possible for three studies of rhesus rotavirus vaccines with a total of 4123 children. The pooled data indicated a non statistically significant difference in the number of intussusception between children randomized to receive rhesus rotavirus vaccines or placebo (RR 0.42, 95% CI 0.07 to 2.42; Analysis 12.1).
12.1. Analysis.
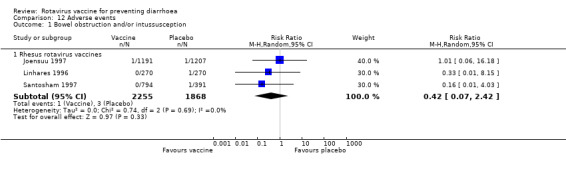
Comparison 12 Adverse events, Outcome 1 Bowel obstruction and/or intussusception.
Minor adverse events
Fever occurred more frequently among those taking rhesus vaccines (RR 2.00, 95% CI 1.51 to 2.64), and there was a non statistically significant difference between vaccine and placebo for bovine vaccines (RR 0.95, 95% CI 0.73 to 1.23) and human vaccines (RR 1.75, 95% CI 0.85 to 3.64) (Analysis 12.2).
12.2. Analysis.
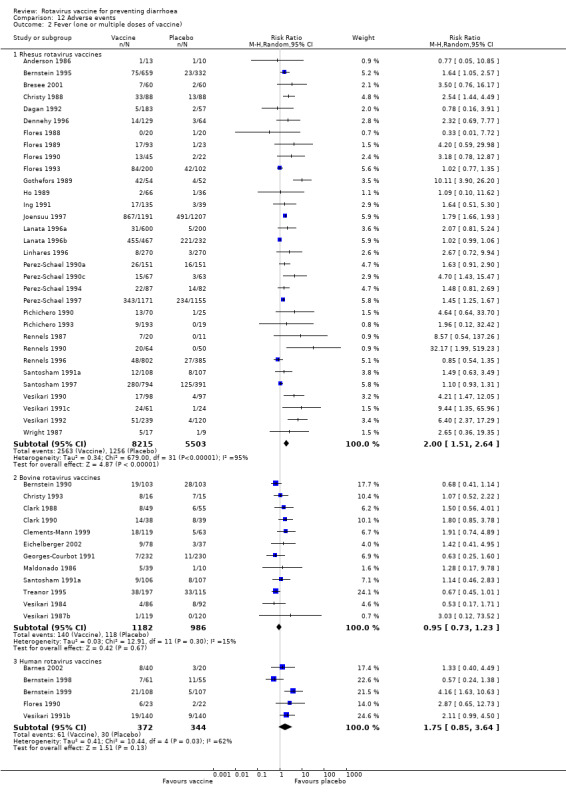
Comparison 12 Adverse events, Outcome 2 Fever (one or multiple doses of vaccine).
There was no statistically significant difference between vaccine and placebo in the numbers of episodes of vomiting (rhesus: RR 0.96, 95% CI 0.90 to 1.03; bovine: RR 1.05, 95% CI 0.90 to 1.22; human: RR 1.94, 95% CI 1.00 to 3.75; Analysis 12.3, fixed effect model), and irritability (rhesus: RR 1.01, 95% CI 0.85 to 1.20; bovine: RR 1.08, 95% CI 0.86 to 1.36; human: RR 0.70, 95% CI 0.51 to 0.98; Analysis 12.4, fixed effect model).
Funnel plot
Regarding the presence of possible publication bias, the funnel plot of the precision (inverse of the standard error) against the logarithm of the RR does not show an important asymmetry (Figure 1).
1.

The size of the circle is proportional to the size of the trial.
Discussion
Vaccine efficacy
Different rotavirus vaccines afforded different levels of efficacy. The pooled analysis showed an efficacy against one episode of rotavirus diarrhoea of 41% for rhesus and bovine vaccines, and of 58% for human vaccines. Efficacy against one episode of severe rotavirus diarrhoea varied from 79% for human vaccines to 58% for rhesus vaccines. Statistically significant, but lower efficacy was demonstrated against one episode of all‐cause diarrhoea, varying from 14% for rhesus vaccines to 27% for bovine vaccines. Heterogeneity among studies, however, compromise the results described here and should be taken into account. The pooled analysis for each specific vaccine showed that rhesus rotavirus vaccines (RRV‐TV, RRV‐MV), live‐attenuated bovine rotavirus vaccine (RIT‐4237), and human‐attenuated rotavirus vaccine (89‐12) were moderate to highly efficacious against one episode of rotavirus diarrhoea (42, 40, 35, and 68% respectively); and rhesus rotavirus vaccine (RRV‐TV), live‐attenuated bovine rotavirus vaccine (RIT‐4237), and human‐attenuated rotavirus vaccine (89‐12) were highly efficacious against one episode of severe rotavirus diarrhoea (60, 62, and 79% respectively).
Vaccine and mortality
We failed to demonstrate any impact of the tested vaccines on mortality because the included trials did not provide the relevant information. However, as a result of gathering together published and unpublished information, we became aware of 57 deaths that occurred in the rotavirus vaccine trials (rhesus rotavirus vaccines: 1 on RRV‐MV, 36 on RRV‐TV; bovine rotavirus vaccines: 8 on RIT‐4237, 11 on WC3; human rotavirus vaccine: 1 on 89‐12); all but six of these deaths occurred in low‐ or middle‐income countries. We could not combine these data because information about the allocation of children in the vaccine or placebo arms of the trial was provided only in three cases. However if one speculates that the non‐reported deaths might have happened in the vaccine arm of the trials one could say that one death occurred for every 200 children vaccinated with rhesus tetravalent vaccine (RRV‐TV), for every 150 children vaccinated with the bovine vaccine RIT‐4237, and for every 35 children vaccinated with the bovine vaccine WC3 (roughly estimations of the number of children needed to be vaccinated to cause one death, not based on accurate information). Yet, according to the investigators the cause of deaths on these trials was not related to the vaccine (see Appendix 3 for more details).
Furthermore, in our communication with investigators of the trials performed in South America (Brazil, Peru, and Venezuela), it became clear that these trials were conducted under ideal circumstances (ie rigorous surveillance and prompt treatment of children participating in the trials) that might not reflect the reality of treating rotavirus diarrhoea in poor communities worldwide. The investigators stated that if a child developed an episode of diarrhoea they would immediately start oral re‐hydration and daily visits to the house. This rigorous surveillance might have impacted on the relatively low mortality described in the trials. In addition, most of the trials reportedly tested the vaccine in low risk individuals, however, higher morbidity and mortality occurs in high‐risk children, particularly the malnourished ones.
Rare adverse events
We fail to provide any evidence about the possible link of the rhesus rotavirus vaccine RRV‐TV and intussusception (Murphy 2001; Cale 2002). In our communication with the principal investigators we also identified two cases of intussusception that occurred in a small trial conducted in China with the bovine vaccine WC3 (Dr Ho, personal communication).
Disease severity
Insufficient or no information was provided for the outcomes measuring the severity of the episodes of all‐cause diarrhoea and/or rotavirus diarrhoea (diarrhoea requiring hospitalization, re‐hydration, and malnutrition at follow up). In addition, the severity of the episode of diarrhoea was measured using different scales and might not represent the same spectrum of disease severity. Furthermore, once again the rigorous surveillance of children − particularly in the South American trials − might have influenced the low frequency of severe diarrhoea measured by hospitalization and dehydration.
High‐, middle‐, and low‐income countries
Rhesus rotavirus vaccines trials were performed in high‐ and middle‐income countries (South America). Contrary to what has been described in the literature (Josefson 1997; Bresee 1999; Cunliffe 2002; WHO/UNICEF 2002), when comparing the pooled results of studies performed in these two group of countries, rhesus vaccine demonstrated a similar efficacy against one episode of rotavirus diarrhoea (44% and 36%, respectively), or against one episode of diarrhoea (around 15%), and a slight difference favouring high‐income countries for the efficacy of the vaccine against one episode of severe rotavirus diarrhoea (61% and 50%, respectively). As for bovine vaccines, although they were tested in high‐, middle‐, and low‐income countries, we could only pool data for high‐income countries showing a 50% of efficacy against one episode of rotavirus diarrhoea, 69% against severe rotavirus diarrhoea, and 31% against all‐cause diarrhoea in such countries. We could not stratify or pool data for the three trials performed with the human rotavirus vaccines. Another important issue for the future implementation of rotavirus vaccines is whether rotavirus vaccines can become part of the World Health Organization's 'Expanded Program of Immunization'. From the sensitivity analyses performed on this review the concomitant use of DPT and/or OPV did not impact on the efficacy of the rotavirus vaccines. This is particularly relevant for the implementation of rotavirus vaccines in low‐ and middle‐income countries.
Limitations of this systematic review
To avoid publication bias, we have searched different databases and retrieved relevant publications regardless of language, and have contacted authors and checked the references of the included trials. We also performed a funnel plot for the main outcome, which showed no relevant asymmetry. We also made an additional effort in writing every contact author of the included trials in order to obtain further information about methodological issues and results of the published trials. Nevertheless, we were able to identify the procedure used to guarantee the concealment of the allocation in only 15 out of the 64 trials included in this review. Moreover, only 29 out of the 64 trials described the procedure used to guarantee the double blinding in their trials, and only in 19 out of the 40 trials reporting data on efficacy of rotavirus vaccines was it possible to perform the meta‐analysis on an intention‐to‐treat basis.
In addition, we could not fully determine the reasons for heterogeneity among studies, although we have tried to use different statistics to account for the given heterogeneity. Because of this we decided not to perform an overall meta‐analysis, which could provide an 'average' statement not easy to interpret quantitatively in relation to the benefits that might accumulate from any rotavirus vaccine. The percentage of total variation across studies due to heterogeneity (Higgins 2003) was around 70% for all group of vaccines in the main outcome (rotavirus diarrhoea) and for rhesus and bovine vaccines on severe rotavirus infection. This level of heterogeneity was maintained for the rhesus vaccine RRV‐TV and the bovine vaccine RIT‐4237, even when the meta‐analysis pooled together only trials that tested specifically these vaccines. Possible reasons that have been suggested in the literature are the concomitant use of other vaccines, the number of doses of the vaccines, whether mothers were allowed to breastfeed children during the vaccination, and whether trials were performed in low‐, middle‐, or high‐income countries. We performed subgroup analyses to evaluate the impact of these issues and observed that the concomitant use of rotavirus vaccine and DPT or OPV vaccines, and breastfeeding during vaccination did not appear to impact on the pooled data. Conversely, when trials were performed in high‐, middle‐, or low‐income countries, and used a single or multiple doses of vaccine, we observed a statistically significant difference between groups.
Authors' conclusions
Implications for practice.
Based on the available information gathered on this review, we cannot recommend rotavirus vaccines in routine clinical practice.
Rhesus, bovine, and human rotavirus vaccines were moderate to highly efficacious against rotavirus diarrhoea, and showed lower, but statistically significant levels of efficacy for all‐cause diarrhoea. However, results on mortality and adverse events during the follow up period of the different trials might have served to draw different conclusions about the efficacy of the vaccines. For example, we were unable to obtain detailed information about 47 of the 57 deaths occurred during follow up of different trials, and the lack of data on intussusception precludes the present use of the rhesus vaccine RRV‐TV. The high level of heterogeneity observed among different vaccines in the main outcomes could also make the results prone to possible overestimation of effects.
In addition, the current results are mainly based on trials performed in high‐ and middle‐income countries. Only three small trials were performed in low‐income countries. It is also important to state that the trials performed in South America countries were done under rigorous surveillance and might not reflect the reality of treating diarrhoea (with or without dehydration and malnutrition) in middle‐ and low‐income countries. Research has been performed on lamb and another human rotavirus vaccine, but efficacy trials are still not available.
Implications for research.
Based on the current evidence, we recommend the performance of additional placebo‐controlled randomized trials, particularly with the new bovine, human, lamb, and inactivated vaccines. The trials should be conducted in parallel in high‐, middle‐, and low‐income countries, have a simple design, and involve tens of thousands of children. Trials carried out this far had enough power to elucidate efficacy, but safety remains to be clarified. Outcomes of major importance, such as severe diarrhoea, intussusception, and death, should be collected (Verstraeten 2001). The lively debate that took place after the rhesus tetravalent rotavirus vaccine (RRV‐TV) was withdrawn from the market suggests that researchers and sponsors of rotavirus vaccine trials are concerned about the costs of trials if sample size is to aim at rare adverse events (Jacobson 2001). However, uncertainty regarding safety of rotavirus vaccines could end up being harmful and costly, particularly for those in middle‐ and low‐income countries where the vaccine is most needed. Even if use of intussusception as a primary outcome is precluded by the enormous sample size needed, data can be carefully collected for later use in systematic reviews and meta‐analyses.
The design and outcomes of trials are often tailored to the type of the disease occurring in high‐income countries, and to the outcomes of interest in these countries, but this might not be appropriate for use in low‐ and middle‐income countries (WHO/UNICEF 2002). This approach already led to early research being abandoned on bovine vaccines that appeared to be efficacious, and every effort should be make to avoid a repetition of the same mistake.
The proposed trials should account for the different agenda of rotavirus vaccines in different regions of the world (Josefson 1997; Weijer 2000). The design should take into account that rotavirus infections are caused by different strains, have a different severity and seasonability in high, middle and low‐income countries. In particular, trials should take into consideration that, although rotavirus diarrhoea accounts for a high number of hospitalization in high‐income countries, death is a rare event. Conversely, in middle‐ and low‐income countries diarrhoea accounts for approximately 13% of all deaths among children under 5 years of age and 20% of such deaths are related to rotavirus (Weijer 2000; Perez‐Schael 2001, WHO 2003).
Future trials should have a careful mechanism to promptly identify, treat, and record rare adverse events. Death should be considered a main outcome in such trials and a detailed report on mortality should be made available. Trials should be simple and aim to test the vaccine in real‐life situations, especially in middle‐ and low‐income countries.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2010 | Amended | Several of the vaccines investigated in this review are no longer in routine clinical use (for example, live attenuated rhesus‐human reassortant tetravalent vaccine) and further new vaccines have been tested and approved for use since this review was written in 2004. For an up‐to‐date assessment of rotavirus vaccines currently approved for use, please see : Soares‐Weiser K, MacLehose H, Ben‐Aharon I, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No.: CD008521. DOI: 10.1002/14651858.CD008521. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 11 August 2009 | Amended | Text added to abstract to highlight current actions: The authors are aware that further trials have been conducted for some of the vaccines included in this review. Authors are preparing new reviews that will update the evidence for vaccines approved for use and vaccines in development. |
| 22 October 2008 | Amended | Converted to new review format with minor editing. |
Acknowledgements
We thank the Nuffield Trust and The Child Health Field Bursary Scheme for providing funding for this review. We also thank the Infectious Diseases Group for supporting this work and Dr Harriet MacLehose for editing this review.
We express our gratitude to Iain Chalmers, for his support to this review and for helping securing funds for this work while director of the UK Cochrane Centre.
The following authors replied to our contact and kindly supplied additional information on their own trials: Dr David Bernstein, Dr Cynthia Christy, Dr Fred Clark, Dr Penelope Dennehy, Dr Marie Claude Georges‐Courbot, Dr Roger Glass, Dr Leif Gothefors, Dr Penny Heaton, Dr Mei‐Shang Ho, Dr Jaana Joensuu, Dr Claudio Lanata, Dr Alexandre Linhares, Dr Bonnie Maldonado, Prof Ingomar Mutz, Dr Catherine Peckham, Dr Irene Perez‐Schael, Dr Mathuram Santosham, Dr Lois Wagner, and Mr Robert Weatherholtz. Dr Roger Glass and Dr Irene Perez‐Schael also made some useful comments on previous version of this systematic review.
Appendices
Appendix 1. Types of oral rotavirus vaccines in research field trials
| Vaccine | Years on trial | Serotype |
| Live attenuated bovine rotavirus (NCDV ‐ RIT 4237) | 1982 to 1987 | G6, P6 |
| Live attenuated rhesus monovalent rotavirus (RRV ‐ MMU18006) | 1984 to 1988 | G3, P3 |
| Live attenuated bovine rotavirus (WC3) | 1986 to 1990 | G6, P5 |
| Live attenuated human rotavirus (M37) | 1990 to 1994 | G1 |
| Live attenuated rhesus‐human reassortant rotavirus tetravalent (RRV‐TV: RRV‐D + RRV‐DS1 + RRV + RRV‐ST3) | 1989 to present | G1‐4, P3 |
| Live attenuated bovine‐human ressortant multivalent (QHBRV) | 1994 to present | G1‐3, P5, P8 |
| Live attenuated human rotavirus (HRV 89‐12) | 1996 to present | G1 |
| Live attenuated human‐bovine rotavirus (W179‐9) | 1988 to present | G1, P8 |
Appendix 2. Rotavirus diarrhoea: overall effect by vaccine (random vs fixed effect model)
| Type of vaccine | Random‐effects model | Fixed‐effect model | Heterogeneity test | ||
| Risk ratio (95% CI) | Z statistics | Risk ratio (95% CI) | Z statistics | ||
| Rhesus tetravalent vaccine (RRV‐TV) | 0.58 (0.47 to 0.72) | 4.96, P < 0.00001 | 0.59 (0.54 to 0.64) | 11.96, P < 0.00001 | Chi squared 51.64, df 10, P < 0.00001, I squared 80.6% |
| Rhesus monovalent vaccine (RRV, RRV‐D, RRV‐DS1, or RRV‐ST3) | 0.60 (0.46 to 0.78) | 3.85, P 0.0001 | 0.59 (0.47 to 0.75) | 4.42, P < 0.00001 | Chi squared 9.80, df 8, P 0.28, I squared 18.4% |
| Bovine vaccine (RIT‐4237) | 0.65 (0.48 to 0.86) | 2.96, P 0.003 | 0.68 (0.58 to 0.79) | 4.84, P < 0.00001 | Chi squared 19.77, df 9, P 0.02, I squared 54.5% |
| Bovine vaccine (WC3) | 0.77 (0.46 to 1.28) | 1.00, P 0.32 | 0.85 (0.66 to 1.10) | 1.22, P 0.22 | Chi squared 5.09, df 2, P 0.08, I squared 60.7% |
| Bovine‐human vaccine (W179‐9) | 0.33 (0.19 to 0.56) | 4.00, P 0.00001 | 0.29 (0.17 to 0.51) | 4.36, P < 0.0001 | Chi squared 1.58, df 2, P 0.45, I squared 0% |
| Human vaccine (89‐12) | 0.32 (0.23 to 0.45) | 6.52, P < 0.00001 | 0.31 (0.22 to 0.44) | 6.60, P < 0.00001 | Chi squared 0.79, df 1, P 0.37, I squared 0% |
CI: confidence interval.
Appendix 3. Data on number of deaths and intussusception (published/unpublished)
| Trial | Type of vaccine | Intussusceptions | Deaths | Cause of deaths |
| Gothefors 1989 | RRV‐MV | No cases | No information | No information |
| Perez‐Schael 1990a | RRV‐MV | No cases | 1 case, group allocation unknown | Described by authors as "not related to vaccine" |
| Wright 1987 | RRV‐MV | No cases | No cases | No cases |
| Wright 1991 | RRV‐MV | No cases | No cases | No cases |
| Bresee 2001 | RRV‐TV | No cases | 2 cases, 1 case placebo group and 1 case vaccine group | Pneumonia and sudden death syndrome |
| Ing 1991 | RRV‐TV | No cases | No cases | No cases |
| Dennehy 1996 | RRV‐TV | No cases | No cases | No cases |
| Joensuu 1997 | RRV‐TV | Vaccine: 1 case, 6 days after thirrd dose; Placebo: 1 case, 44 days after second dose | 1 case, placebo group | Suffocation due to foreign body in the bronchia, 11 months old boy |
| Lanata 1996a | RRV‐TV | No cases | 13 cases, group allocation unknown | Described by authors as "not related to vaccine" |
| Lanata 1996b | RRV‐TV | No cases | 6 cases, group allocation unknown | Described by authors as "not related to vaccine" |
| Linhares 1996 | RRV‐TV | Placebo: 1 case, during follow up | 7 cases, group allocation unknown | Gastrointestinal reflux + asphyxia, measles + sepsis, poisoning, gastroenteritis + pneumonia, acute respiratory distress |
| Perez‐Schael 1997 | RRV‐TV | No cases | 3 cases, placebo group | Bronchial aspiration + diarrhoea + dehydration, pneumonia + sepsis, accidental intoxication |
| Santosham 1997 | RRV‐TV | Placebo: 1 case | 4 cases, vaccine group, all after one month of receiving the vaccine | HiB (hemophilus influenza B vaccine), sudden death syndrome, asphyxia, unknown |
| Lanata 1989 | RIT‐4237 | No cases | 8 cases | No information |
| Maldonado 1986 | RIT‐4237 | No cases | No cases | No cases |
| Senturia 1987 | RIT‐4237 | No cases | No cases | No cases |
| Mutz 1989 | RIT‐4237 | No cases | No cases | No cases |
| Christy 1993 | W179‐9 | No cases | No cases | No cases |
| Bernstein 1998 | HRV89‐12 | No cases | No cases | No cases |
| Bernstein 1999 | HRV89‐12 | No cases | 1 case | Pneumococcal sepsis |
| Georges‐Courbot 1991 | WC3 | No cases | 11 cases, group allocation unknown | Described by authors as "not related to vaccine" |
| Ho 1989 | WC3 | Vaccine: 2 cases (1 week and 1 month after) | No cases | No cases |
Appendix 4. Type of rotavirus vaccines assessed in the included trials
PFU: plaque‐forming units.
Appendix 5. Exploration of heterogeneity: different rotavirus vaccines
| Outcome | Vaccine | No. trials | No. participants | RR (95% CI) | NNT (95% CI) | Analysis |
| Rotavirus diarrhoea: episodes | Rhesus tetravalent (RRV‐TV) | 11 | 11,590 | 0.58 (0.47 to 0.72) | 19 (15 to 25) | Analysis 10.1 |
| Rhesus monovalent (RRV, RRV‐D, RRV‐DS1, or RRV‐ST3) | 9 | 1715 | 0.60 (0.46 to 0.78) | 13 (9 to 22) | Analysis 10.1 | |
| Bovine (RIT‐4237) | 10 | 3663 | 0.65 (0.48 to 0.86) | — | Analysis 10.1 | |
| Bovine (WC3) | 3 | 782 | 0.77 (0.46 to 1.28) | — | Analysis 10.1 | |
| Bovine‐human (W179‐9) | 3 | 433 | 0.33 (0.19 to 0.56) | 7 (5 to 12) | Analysis 10.1 | |
| Bovine‐human (WC3‐QV) | 1 | 405 | 0.33 (0.19 to 0.58) | 7 (5 to 13) | Analysis 10.1 | |
| Human (89‐12) | 2 | 2201 | 0.32 (0.23 to 0.45) | 11 (8 to 15) | Analysis 10.1 | |
| Human (M37) | 1 | 281 | 1.01 (0.45 to 2.25) | — | Analysis 10.1 | |
| Rotavirus diarrhoea: severe episodes | Rhesus tetravalent (RRV‐TV) | 7 | 9569 | 0.40 (0.28 to 0.57) | 18 (15 to 22) | Analysis 10.2 |
| Rhesus monovalent (RRV‐MMU 18006) | 4 | 1037 | 0.52 (0.27 to 0.97) | 23 (13 to 72) | Analysis 10.2 | |
| Bovine (RIT‐4237) | 5 | 2131 | 0.38 (0.19 to 0.79) | 24 (16 to 53) | Analysis 10.2 | |
| Bovine (WC3) | 3 | 782 | 0.57 (0.29 to 1.12) | 14 (8 to 37) | Analysis 10.2 | |
| Bovine‐human (W179‐9) | 1 | 325 | 0.14 (0.05 to 0.42) | — | Analysis 10.2 | |
| Bovine‐human (QHBRV) | 1 | 405 | 0.31 (0.16 to 0.62) | — | Analysis 10.2 | |
| Human (89‐12) | 2 | 2201 | 0.21 (0.13 to 0.35) | 14 (10 to 20) | Analysis 10.2 | |
| All‐cause diarrhoea: episodes | Rhesus tetravalent (RRV‐TV) | 5 | 6561 | 0.89 (0.84 to 0.93) | 12 (10 to 17) | Analysis 10.3 |
| Rhesus monovalent (RRV or RRV‐D or RRV‐DS1 or RRV‐ST3) | 6 | 1145 | 0.82 (0.69 to 0.98) | 8 (5 to 13) | Analysis 10.3 | |
| Bovine (RIT‐4237) | 7 | 2772 | 0.82 (0.67 to 1.01) | 16 (10 to 35) | Analysis 10.3 | |
| Bovine (WC3) | 1 | 104 | 0.48 (0.28 to 84) | — | Analysis 10.3 | |
| Bovine‐human (W179‐9) | 3 | 433 | 0.60 (0.50 to 0.72) | 4 (3 to 7) | Analysis 10.3 | |
| Human (M37) | 1 | 281 | 0.91 (0.57 to 1.44) | — | Analysis 10.3 |
Data and analyses
Comparison 1. Drop outs before end of study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Drop outs before end of study | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 14 | 11128 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.85, 1.06] |
| 1.2 Bovine rotavirus vaccines | 5 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.56, 1.22] |
Comparison 2. Rotavirus diarrhoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Episodes | 40 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 20 | 13305 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.50, 0.70] |
| 1.2 Bovine rotavirus vaccines | 17 | 5283 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.45, 0.76] |
| 1.3 Human rotavirus vaccines | 3 | 2482 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.85] |
| 2 Severe episodes | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 11 | 10606 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.31, 0.57] |
| 2.2 Bovine rotavirus vaccines | 10 | 3643 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.24, 0.60] |
| 2.3 Human rotavirus vaccines | 2 | 2201 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 3 Episodes requiring hospitalization | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Rhesus rotavirus vaccines | 7 | 7803 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.86] |
| 3.2 Bovine rotavirus vaccines | 4 | 1693 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.18, 0.74] |
| 3.3 Human rotavirus vaccines | 2 | 2201 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.48] |
| 4 Episodes of more than four days | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Rhesus rotavirus vaccines | 5 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.87] |
| 4.2 Bovine rotavirus vaccines | 3 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.51, 1.99] |
Comparison 3. All‐cause diarrhoea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Episodes (number of episodes/number of person‐years) | Other data | No numeric data | ||
| 1.1 Rhesus rotavirus vaccines | Other data | No numeric data | ||
| 1.2 Bovine rotavirus vaccines | Other data | No numeric data | ||
| 1.3 Human rotavirus vaccines | Other data | No numeric data | ||
| 2 Episodes (number of cases/number of participants) | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 11 | 7706 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.80, 0.92] |
| 2.2 Bovine rotavirus vaccines | 11 | 3309 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.60, 0.89] |
| 2.3 Human rotavirus vaccines | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.44] |
| 3 Episodes during first week after vaccine (one or multiple doses) | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Rhesus rotavirus vaccines | 29 | 12673 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.14] |
| 3.2 Bovine rotavirus vaccines | 10 | 2125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 3.3 Human rotavirus vaccines | 3 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.03, 3.65] |
| 4 Severe episodes (clinical or measured by any scale) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Rhesus rotavirus vaccines | 3 | 1663 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.00] |
| 4.2 Bovine rotavirus vaccines | 3 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.26] |
| 5 Episodes requiring re‐hydration | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Rhesus rotavirus vaccines | 5 | 5559 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.30, 0.88] |
| 5.2 Bovine rotavirus vaccines | 5 | 2148 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.26, 0.67] |
| 5.3 Human rotavirus vaccines | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.71] |
| 6 Episodes requiring hospitalization | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Rhesus rotavirus vaccines | 4 | 5023 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 0.99] |
| 6.2 Bovine rotavirus vaccines | 3 | 799 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.16, 1.91] |
Comparison 4. Death.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause death | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 4 | 6029 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.16, 3.12] |
Comparison 5. Exploration of heterogeneity: methodological quality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adequate allocation concealment (A) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 9 | 8963 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.45, 0.76] |
| 1.2 Bovine rotavirus vaccines | 1 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.56] |
| 1.3 Human vaccine rotavirus | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.99] |
| 2 Unclear allocation concealment (B) | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 11 | 4423 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.48, 0.67] |
| 2.2 Bovine rotavirus vaccines | 16 | 5040 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.43, 0.76] |
| 2.3 Human vaccine rotavirus | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.24, 0.51] |
| 3 Exclusions after randomization reported | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Rhesus rotavirus vaccines | 15 | 12009 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.48, 0.71] |
| 3.2 Bovine rotavirus vaccines | 5 | 1361 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.26, 0.96] |
| 4 Exclusions after randomization not reported | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Rhesus rotavirus vaccines | 5 | 1377 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.44, 0.76] |
| 4.2 Bovine rotavirus vaccines | 12 | 3918 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.46, 0.82] |
| 4.3 Human vaccine rotavirus | 3 | 2482 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.85] |
| 5 Sample size of less than 1000 participants | 33 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Rhesus rotavirus vaccines | 15 | 4975 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.56, 0.79] |
| 5.2 Bovine rotavirus vaccines | 16 | 4279 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.74] |
| 5.3 Human vaccine rotavirus | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.99] |
| 6 Sample size of at least 1000 participants | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Rhesus rotavirus vaccines | 5 | 8411 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.39, 0.65] |
| 6.2 Bovine rotavirus vaccines | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.61, 1.41] |
| 6.3 Human vaccine rotavirus | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.24, 0.51] |
5.4. Analysis.

Comparison 5 Exploration of heterogeneity: methodological quality, Outcome 4 Exclusions after randomization not reported.
5.5. Analysis.
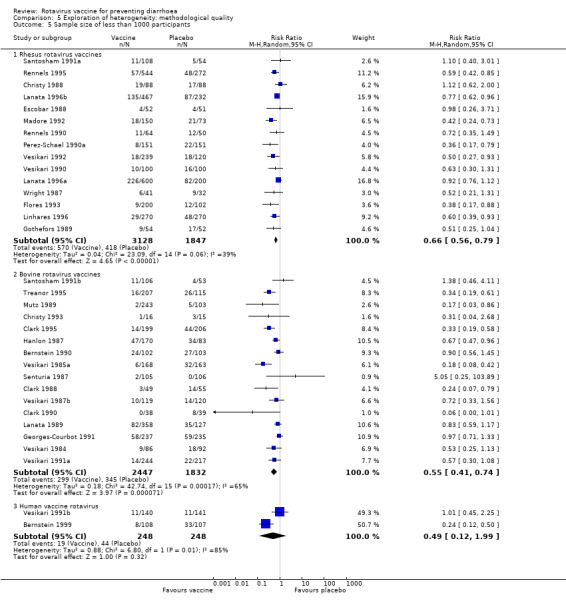
Comparison 5 Exploration of heterogeneity: methodological quality, Outcome 5 Sample size of less than 1000 participants.
5.6. Analysis.

Comparison 5 Exploration of heterogeneity: methodological quality, Outcome 6 Sample size of at least 1000 participants.
Comparison 6. Exploration of heterogeneity: multiple versus single dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Multiple doses (two or more) of rotavirus vaccine | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 8 | 9698 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.44, 0.69] |
| 1.2 Bovine rotavirus vaccines | 5 | 2148 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.86] |
| 1.3 Human rotavirus vaccines | 2 | 2201 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.23, 0.45] |
| 2 Single dose of rotavirus vaccine | 25 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 12 | 3607 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.50, 0.82] |
| 2.2 Bovine rotavirus vaccines | 12 | 3135 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.83] |
| 2.3 Human rotavirus vaccines | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.25] |
6.1. Analysis.

Comparison 6 Exploration of heterogeneity: multiple versus single dose, Outcome 1 Multiple doses (two or more) of rotavirus vaccine.
6.2. Analysis.

Comparison 6 Exploration of heterogeneity: multiple versus single dose, Outcome 2 Single dose of rotavirus vaccine.
Comparison 7. Exploration of heterogeneity: administered with other vaccines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Concomitant use of DPT or OPV allowed | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 8 | 10052 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.44, 0.73] |
| 1.2 Bovine rotavirus vaccines | 3 | 1071 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.18] |
| 1.3 Human rotavirus vaccines | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.24, 0.51] |
| 2 Concomitant use of DPT or OPV not allowed | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 6 | 1367 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.97] |
| 2.2 Bovine rotavirus vaccines | 7 | 2110 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 1.00] |
| 2.3 Human rotavirus vaccines | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.99] |
| 3 No information | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Rhesus rotavirus vaccines | 6 | 1886 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.43, 0.71] |
| 3.2 Bovine rotavirus vaccines | 7 | 2102 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.28, 0.79] |
7.1. Analysis.

Comparison 7 Exploration of heterogeneity: administered with other vaccines, Outcome 1 Concomitant use of DPT or OPV allowed.
7.2. Analysis.
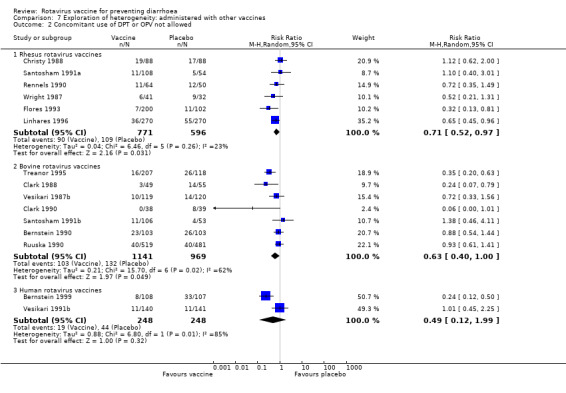
Comparison 7 Exploration of heterogeneity: administered with other vaccines, Outcome 2 Concomitant use of DPT or OPV not allowed.
7.3. Analysis.

Comparison 7 Exploration of heterogeneity: administered with other vaccines, Outcome 3 No information.
Comparison 8. Exploration of heterogeneity: breastfeeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breastfeeding allowed during vaccination | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 4 | 3652 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.32, 0.86] |
| 1.2 Bovine rotavirus vaccines | 9 | 3452 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.48, 0.85] |
| 2 Breastfeeding not allowed for at least one hour before and after vaccination | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 15 | 8468 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.51, 0.73] |
| 2.2 Bovine rotavirus vaccines | 6 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.94] |
| 2.3 Human rotavirus vaccines | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.99] |
| 3 No information | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Rhesus rotavirus vaccines | 1 | 1185 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.87] |
| 3.2 Bovine rotavirus vaccines | 2 | 877 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.20, 1.71] |
| 3.3 Human rotavirus vaccines | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.24, 0.51] |
Comparison 9. Exploration of heterogeneity: one season versus multiple seasons follow up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 One season | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 8 | 2885 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.78] |
| 1.2 Bovine rotavirus vaccines | 7 | 1798 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.32, 0.83] |
| 1.3 Human rotavirus vaccines | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.24, 0.51] |
| 2 Two or more seasons | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 12 | 10172 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.48, 0.74] |
| 2.2 Bovine rotavirus vaccines | 10 | 3726 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.43, 0.84] |
| 2.3 Human rotavirus vaccines | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.99] |
Comparison 10. Exploration of heterogeneity: different rotavirus vaccines.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rotavirus diarrhoea: episodes | 40 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus tetravalent vaccine (RRV‐TV) | 11 | 11590 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.47, 0.72] |
| 1.2 Rhesus monovalent vaccine (RRV, RRV‐D, RRV‐DS1, or RRV‐ST3) | 9 | 1715 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.78] |
| 1.3 Bovine vaccine (RIT‐4237) | 10 | 3663 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.48, 0.86] |
| 1.4 Bovine vaccine (WC3) | 3 | 782 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.46, 1.28] |
| 1.5 Bovine‐human vaccine (W179‐9) | 3 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.19, 0.56] |
| 1.6 Bovine‐human vaccine (WC3‐QV) | 1 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.19, 0.58] |
| 1.7 Human vaccine (89‐12) | 2 | 2201 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.23, 0.45] |
| 1.8 Human vaccine (M37) | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.25] |
| 2 Rotavirus diarrhoea: severe episodes | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus tetravalent vaccine (RRV‐TV) | 7 | 9569 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.28, 0.57] |
| 2.2 Rhesus monovalent vaccine (RRV‐MMU 18006) | 4 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 0.97] |
| 2.3 Bovine vaccine (RIT‐4237) | 5 | 2131 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.19, 0.79] |
| 2.4 Bovine vaccine (WC3) | 3 | 782 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.29, 1.12] |
| 2.5 Bovine‐human vaccine (W179‐9) | 1 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.05, 0.42] |
| 2.6 Bovine‐human vaccine (QHBRV) | 1 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.62] |
| 2.7 Human vaccine (89‐12) | 2 | 2201 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.13, 0.35] |
| 3 All‐cause diarrhoea: episodes (number of cases/number of participants) | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Rhesus tetravalent vaccine (RRV‐TV) | 5 | 6561 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.84, 0.93] |
| 3.2 Rhesus monovalent vaccine (RRV, RRV‐D, RRV‐DS1, or RRV‐ST3) | 6 | 1145 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.69, 0.98] |
| 3.3 Bovine vaccine (RIT‐4237) | 7 | 2772 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.67, 1.01] |
| 3.4 Bovine vaccine (WC3) | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.84] |
| 3.5 Bovine‐human vaccine (W179‐9) | 3 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.50, 0.72] |
| 3.6 Human vaccine (M37) | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.44] |
Comparison 11. Exploration of heterogeneity: country of origin ‐ income status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rotavirus diarrhoea: episodes | 40 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus vaccines: high‐income countries | 14 | 8182 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.46, 0.68] |
| 1.2 Rhesus vaccines: middle‐income countries | 6 | 5123 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.48, 0.83] |
| 1.3 Bovine vaccines: high‐income countries | 14 | 4073 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.35, 0.71] |
| 1.4 Bovine vaccines: middle‐income countries | 1 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.62, 0.97] |
| 1.5 Bovine vaccines: low‐income countries | 2 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.57, 1.18] |
| 1.6 Human vaccines: high‐income countries | 2 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 1.99] |
| 1.7 Human vaccines: middle‐income countries | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.24, 0.51] |
| 2 Rotavirus diarrhoea: severe episodes | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus vaccines: high‐income countries | 7 | 6585 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.25, 0.61] |
| 2.2 Rhesus vaccines: middle‐income countries | 4 | 4021 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.35, 0.72] |
| 2.3 Bovine vaccines: high‐income countries | 8 | 2918 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.46] |
| 2.4 Bovine vaccines: low‐income countries | 2 | 725 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.58, 1.09] |
| 2.5 Human vaccines: high‐income countries | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.46] |
| 2.6 Human vaccines: middle‐income countries | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.14, 0.40] |
| 3 Rotavirus diarrhoea: severe episodes requiring hospitalization | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Rhesus vaccine: high‐income countries | 3 | 3782 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.33] |
| 3.2 Rhesus vaccine: middle‐income countries | 4 | 4021 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.35, 0.95] |
| 3.3 Bovine vaccines: high‐income countries | 2 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 0.99] |
| 3.4 Bovine vaccines: middle‐income countries | 1 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.14, 0.93] |
| 3.5 Bovine vaccines: low‐income countries | 1 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.91] |
| 3.6 Human vaccines: high‐income countries | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.08] |
| 3.7 Human vaccines: middle‐income countries | 1 | 1986 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.09, 0.50] |
| 4 All‐cause diarrhoea: episodes (number of cases/number of participants) | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Rhesus vaccines: high‐income countries | 9 | 4924 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.94] |
| 4.2 Rhesus vaccines: middle‐income countries | 2 | 2782 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.83, 0.93] |
| 4.3 Bovine vaccines: high‐income countries | 10 | 3064 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.55, 0.86] |
| 4.4 Bovine vaccines: low‐income countries | 1 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.17] |
| 4.5 Human vaccines: high‐income countries | 1 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.57, 1.44] |
| 5 All‐cause diarrhoea: severe episodes (clinical or measured by any scale) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Rhesus vaccines: high‐income countries | 2 | 1361 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.73, 1.02] |
| 5.2 Rhesus vaccines: middle‐income countries | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.11] |
| 5.3 Bovine vaccines: high‐income countries | 3 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.21, 1.26] |
| 6 All‐cause diarrhoea: episodes requiring hospitalization | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Rhesus vaccine: high‐income countries | 4 | 5023 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 0.99] |
| 6.2 Bovine vaccines: high‐income countries | 2 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.04, 12.07] |
| 6.3 Bovine vaccines: low‐income countries | 1 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.91] |
| 7 All‐cause diarrhoea: episodes requiring re‐hydration | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Rhesus vaccine: high‐income countries | 1 | 1278 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.68] |
| 7.2 Rhesus vaccine: middle‐income countries | 4 | 4281 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.33, 1.05] |
| 7.3 Bovine vaccines: high‐income countries | 5 | 2148 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.26, 0.67] |
| 7.4 Human vaccines: high‐income countries | 1 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.71] |
Comparison 12. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bowel obstruction and/or intussusception | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Rhesus rotavirus vaccines | 3 | 4123 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.07, 2.42] |
| 2 Fever (one or multiple doses of vaccine) | 47 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Rhesus rotavirus vaccines | 32 | 13718 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.51, 2.64] |
| 2.2 Bovine rotavirus vaccines | 12 | 2168 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.73, 1.23] |
| 2.3 Human rotavirus vaccines | 5 | 716 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.85, 3.64] |
| 3 Vomiting (one or multiple doses of vaccine) | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Rhesus rotavirus vaccines | 16 | 10289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 3.2 Bovine rotavirus vaccines | 10 | 2016 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.90, 1.22] |
| 3.3 Human rotavirus vaccines | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.00, 3.75] |
| 4 Irritability (one or multiple doses of vaccine) | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Rhesus rotavirus vaccines | 5 | 807 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.85, 1.20] |
| 4.2 Bovine rotavirus vaccines | 3 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.36] |
| 4.3 Human rotavirus vaccines | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.51, 0.98] |
12.3. Analysis.

Comparison 12 Adverse events, Outcome 3 Vomiting (one or multiple doses of vaccine).
12.4. Analysis.

Comparison 12 Adverse events, Outcome 4 Irritability (one or multiple doses of vaccine).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1986.
| Methods | Randomization: no details Allocation concealment: no details Blinding: single (placebo and vaccine looked similar) Data collection: no details Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow‐up period: 8 days after vaccination |
|
| Participants | Age: 8 to 60 months Health status: healthy Breastfeeding: 50 to 75% Immunization status: no information Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology and stool analysis by ELISA |
|
| Interventions | 1. Rhesus vaccine (MMU 18006), 10^6.5 PFU single dose (n = 13) 2. Placebo (similac formula), 30 ml single dose (n = 10) 30 ml similac formula given with vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 8 days | |
| Notes | Study location: USA Two separate trials |
|
Barnes 1997.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (vaccine and placebo ampoules identified by participant number only) Data collection: no details Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow‐up period: 4 weeks after vaccination |
|
| Participants | Age: 3 to 360 months Health status: healthy Immunization status: no information Breastfeeding: no information Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology and stool analysis by ELISA |
|
| Interventions | 1. Human vaccine (RV3), 6.5 x 10^5 PFU, single dose (n = 15). 2. Placebo (uninfected monkey kidney culture fluids), single dose (n = 15) 1 h fasting and then 30 ml soy formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 4 weeks of vaccination | |
| Notes | Study location: Australia | |
Barnes 2002.
| Methods | Randomization: block randomization in groups of 6 independently done by the University of Melbourne Statistical Consulting Centre Allocation concealment: no details Blinding: double (vaccine and placebo ampoules identical) Data collection: no details Intention to treat: no Interim analysis: none Exclusion from analysis: 1/60 withdrawn from follow up by parents; 5/59 excluded from analysis due to wild‐type infection before or during trial Follow‐up period: 7 days after vaccination; 2 rotavirus seasons after completion of all vaccinations |
|
| Participants | Age: 3 months Health status: healthy Immunization status: vaccine administered not closer than 14 days to routine immunizations Breastfeeding: > 75% |
|
| Interventions | 1. Human vaccine (RV3), 6.5 x 10^5 PFU with soy‐based formula (n = 20) or without soy‐based formula (n = 20), 3 doses 2. Placebo (uninfected monkey kidney culture fluids) with soy‐based formula, 3 doses (n = 20) 1 h fasting and then 10 ml soy formula given before vaccine (only in 1 of 2 vaccine groups) or placebo 400 mg of sodium bicarbonate given to all groups before vaccination |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination. 2. Efficacy: diarrhoea within 2 rotavirus seasons of beginning of trial |
|
| Notes | Study location: Australia Clinical symptoms: clinical evaluation, severity score measured by Flores 1987 scale Laboratory studies: serology by ELISA, stool analysis by ELISA and PCR |
|
Bernstein 1990.
| Methods | Randomization: randomization code provided by Institut Merieux Allocation concealment: no details Blinding: double (no further details) Data collection: 7 September 1987 to 30 June 1989 Intention to treat: no Interim analysis: none Exclusion from analysis: 1\206 not evaluated; 42\205 not followed over the second year because of no consent Follow‐up period: 7 days after vaccination; 2 rotavirus seasons after beginning of trial |
|
| Participants | Age: 2 to 12 months Health status: healthy Breastfeeding: < 50% Immunization status: DTP and OPV vaccination not allowed within 2 weeks of WC3 vaccination |
|
| Interventions | 1. Bovine vaccine (WC3), 10^7 PFU, single dose (n = 103) 2. Placebo (diluent), 2 ml single dose (n = 103) 30 ml formula or 1 ml/kg antacid given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination 2. Efficacy: diarrhoea within 2 rotavirus seasons of beginning of trial |
|
| Notes | Study location: USA Feeding was withheld from infants for at least 1 h before vaccine. Breastfeeding also withheld for 1 h after vaccine Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 2 watery stools in 24 h); severity score measured by Clark 1988 scale Laboratory studies: serology by ELISA and fluorescent focus reduction assay; stool analysis by plaque assay and ELISA |
|
Bernstein 1995.
| Methods | Randomization: random code prepared by the pharmaceutical industry Allocation concealment: no details Blinding: double (identical appearing vials) Data collection: 10 October 1989 to 1 July 1991 Intention to treat: no Interim analysis: performed after first season; no changes made on the study protocol as a result of the interim analysis Exclusion from analysis: 17\1006 not vaccinated 91\989 not included in first year follow up; 34\898 not included in second year follow up Follow‐up period: 5 days after vaccination; 2 rotavirus seasons after beginning of trial |
|
| Participants | Age: 1 to 6.5 months Health status: healthy Breastfeeding: < 50% Immunization status: OPV vaccination given within 2 weeks of RRV vaccination in 120 participants |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 4 x 10^4 PFU, single dose (n = 332). 2. Monovalent vaccine (D x RRV), 4 x 10^4 PFU, single dose (n = 327) 3. Placebo (uninfected tissue culture fluids), single dose (n = 330) 400 mg sodium bicarbonate in 30 ml cow milk or soy formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination 2. Efficacy: gastrointestinal illness within 2 rotavirus seasons of beginning of trial |
|
| Notes | Country: USA Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 watery or loose stools in 24 h); severity score measured by Flores 1987 scale Laboratory studies: serology by ELISA and focus reduction assay; stool analysis by ELISA All other vaccines given as scheduled |
|
Bernstein 1998.
| Methods | Randomization: no details Allocation concealment: code was not available until end of study Blinding: double (no details) Data collection: enrolment between August 1995 and November 1995, end of vaccination December 1995 Intention to treat: no Interim analysis: none Exclusion from analysis: 1\116 not followed because of persistent otitis Follow‐up period: 1 week after each vaccination |
|
| Participants | Age: 1.5 to 60 months Health status: no information Breastfeeding: no information Immunization status: no vaccination allowed within 2 weeks of rotavirus vaccination Clinical symptoms: clinical evaluation, diarrhoea was defined as 3 or more unformed stools within 48 h. Fever was defined as a rectal temperature > 38.1 ºC in paediatric participants, confirmed within 20 minutes Laboratory studies: stool analysis by ELISA and PCR Serology by plaque reduction neutralization and ELISA |
|
| Interventions | 1. Human (89‐12), 10^5 PFU single dose for adults and children (n = 40), 2 doses for infants (n = 21) 2. Placebo (n = 55), no further details 50 ml sodium bicarbonate for adults and 0.5 ml/kg Mylanta‐DS for infants and children given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each vaccination | |
| Notes | Study location: USA Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 watery or loose stools in 24 h, fever defined as > 100.4 ºF) Laboratory studies: serology and stool analysis by ELISA Immunization with other vaccines separated by at least 2 weeks from the RV 3 separate trials: adults and children (2 to 12 years) for initial trials; infants (6 to 26 weeks) for last trial |
|
Bernstein 1999.
| Methods | Randomization: computer generated, blocks of 10, provided by sponsor Allocation concealment: code was not available until end of study Blinding: double (no further details) Data collection: August 1997 to May 1999 Intention to treat: no Interim analysis: none Exclusion from analysis: 1\215 not vaccinated because of persistent fever; 1\214 not re‐vaccinated because of congenital malformation; 1\213 died of pneumococcal sepsis; 28\212 were not available for second season follow up, no further details Follow‐up period: 1 week after each vaccination; 2 rotavirus season after beginning of trial |
|
| Participants | Age: 10 to 16 weeks Health status: healthy Breastfeeding: 50 to 75% Immunization status: no other vaccine allowed within 2 weeks of vaccination |
|
| Interventions | 1. Human (89‐12), 10^5 PFU, two doses (n = 108) 2. Placebo (tissue culture medium), 1 ml, 2 doses (n = 107) 2 ml antacid given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each vaccination. 2. Efficacy: gastroenteritis (rotavirus or other) within the study period (18 months) |
|
| Notes | Study location: USA Feeding withheld for at least 1 h before and after vaccine Clinical symptoms: clinical evaluation (gastroenteritis defined as vomiting, ≥ 3 loose stools), severity score measured by Flores 1987 scale Laboratory studies: serology by ELISA and antigen reduction assay; stool analysis by ELISA All other immunizations separated from RV by at least 2 weeks |
|
Bresee 2001.
| Methods | Randomization: block randomization in groups of 6 Allocation concealment: no details Blinding: double (vaccine and placebo that tasted and appeared identical) Data collection: October 1998 to February 1998 enrolment, no further details Intention to treat: no Interim analysis: none Exclusion from analysis: 9\120 not vaccinated because of parental refusal (8) or death (1 case due to severe pneumonia); 2\111 did not complete trial because of death (1 case due to suspected sudden infant death syndrome) or relocation (1) Follow‐up period: 1 week after each vaccination |
|
| Participants | Age: 2 to 6 weeks Health status: healthy, full term Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 4 x 10^5 PFU, 3 doses (n = 60) 2. Placebo (lyophilized growth medium with 10% SPG), 3 ml, 3 doses (n = 60) |
|
| Outcomes | Safety: clinical symptoms within 7 days of each vaccination | |
| Notes | Study location: Bangladesh Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology by ELISA and by neutralizing antibody titers; stool analysis by ELISA, polyacrylamide gel electrophoresis (PAGE) and by reverse transcriptase (RT)‐PCR |
|
Christy 1988.
| Methods | Randomization: sequential enrolment and vaccination according to table of random numbers Allocation concealment: no details Blinding: double (similar appearance to vaccine and placebo vials) Data collection: October 1986 to July 1986 Intention to treat: no Interim analysis: none Exclusion from analysis: 1\176 for "reasons unrelated to trial" Follow‐up period: 10 days after each vaccination; 10 months after beginning of trial |
|
| Participants | Age: 2 to 4 months Health status: healthy Breastfeeding: < 50% Immunization status: DPT and polio vaccinations not allowed within 4 weeks before and 3 weeks after vaccination |
|
| Interventions | 1. Rhesus (MMU 18006), 10^4 PFU, single dose (n = 88) 2. Placebo (soy formula), 1 ml single dose (n = 88) 400 mg sodium bicarbonate in 30 ml soy formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 10 days of each vaccination. 2. Efficacy: gastroenteritis (rotavirus or other) within 10 months of beginning of trial |
|
| Notes | Study location: USA All feedings withheld for 1 h before and after vaccine Clinical symptoms: clinical evaluation, severity measured by WHO criteria (fever (≥ 38) vomiting, dehydration, > 6 stools in 24 h, and illness duration ≥ 24 h) Laboratory studies: serology by micro neutralization assay and ELISA; stool analysis by ELISA |
|
Christy 1993.
| Methods | Randomization: block randomization scheme Allocation concealment: no details Blinding: double (no further details) Data collection: 21 November 1991 to 1 June 1992 Intention to treat: no Interim analysis: none Exclusion from analysis: 4/31 excluded for having diarrhoea from wild‐type rotavirus; 1/27 did not receive second dose "for reasons unrelated to vaccination" Follow‐up period: 10 days after each vaccination; 6 months after beginning of trial |
|
| Participants | Age: 2 to 6 months Health status: no information Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Bovine + human reassortant (WI79‐9),10^7.5 PFU, 2 doses (n = 16) 2. Placebo (cell culture medium with cherry syrup), 2.5 ml, 2 doses (n = 15) 400 mg sodium bicarbonate in 30 ml soy formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 10 days of each vaccination 2. Efficacy: gastroenteritis within 6 months of beginning of trial |
|
| Notes | Study location: USA Breastfeeding withheld for 1 h before vaccine Clinical symptoms: clinical evaluation (significant diarrhoea defined as ≥ 2 watery stools in 24 h) Laboratory studies: stool analysis by ELISA; serology by micro‐neutralization |
|
Clark 1988.
| Methods | Randomization: serial coding according to table of random numbers Allocation concealment: code unbroken until end of trial, no further details Blinding: double (numbered identical vials) Data collection: September 1985 to June 1986 Intention to treat: unknown (only number of infants completing the trial mentioned) Interim analysis: none Exclusion from analysis: unknown Follow‐up period: 1 week after each vaccination; 10 months after beginning of trial |
|
| Participants | Age: 3 to 12 months Health status: healthy Breastfeeding: < 50% Immunization status: DPT vaccination not allowed within 1 week before vaccination |
|
| Interventions | 1. Bovine (WC3), 10^7.5 PFU, single dose (n = 49) 2. Placebo (cell culture medium with 0.5 ml cherry syrup), 2.5 ml single dose (n = 55) 30 ml formula or 85 mg antacid given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each vaccination 2. Efficacy: gastroenteritis (rotavirus or other) within 10 months of beginning of trial |
|
| Notes | Study location: USA No feeding 1 h before vaccine, and no breastfeeding 1 h after vaccine Clinical symptoms: clinical evaluation (significant diarrhoea defined as ≥ 2 watery stools in 24 h); severity score measured by Clark 1988 (modified Duffy 1986) scale Laboratory studies: serology by plaque reduction neutralization; stool analysis by PAGE‐SS analysis and ELISA At least 1 week between DTP and RV |
|
Clark 1990.
| Methods | Randomization: table of random numbers Allocation concealment: no details Blinding: double (numbered vials) Data collection: September 1987 to July 1988 Intention to treat: no details, only number of infants completing the trial mentioned Interim analysis: none Exclusion from analysis: no details Follow‐up period: 1 week after each vaccination; 17 weeks after vaccination |
|
| Participants | Age: 2 to 11 months Health status: healthy Breastfeeding: < 50% Immunization status: DPT and polio vaccinations not allowed within 7 days of vaccination |
|
| Interventions | 1. Bovine + human reassortant (WI79‐9), 2 x 10^7 PFU, 2 doses (n = 38) 2. Placebo (cell culture medium with 20% cherry syrup), 2 doses (n = 39) 30 ml formula or 85 mg antacid given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each vaccination 2. Efficacy: gastroenteritis within 17 weeks of vaccination |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation; severity score measured by modified Duffy 1986 scale Laboratory studies: serology by plaque reduction neutralization test; stool analysis by latex agglutination test At least 7 days between DTP and RV |
|
Clark 1995.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (no further details) Data collection: no information Intention to treat: no Interim analysis: none Exclusion from analysis: 12/417 were not evaluated, no further details Follow‐up period: 1 week after each vaccination; 1 rotavirus season after vaccination |
|
| Participants | Age: 2 to 6 months Health status: no information Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Human‐bovine QHBRV, 4 x 10^7 PFU, 3 doses (n = 199) 2. Placebo (no details), 3 doses (n = 206) |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each vaccination 2. Efficacy: gastroenteritis within 1 rotavirus season of vaccination |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation; severity score measured by modified Duffy 1986 scale Laboratory studies: stool analysis by solid phase immunoassay and electropherotype analysis |
|
Clements‐Mann 1999.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (no further details) Data collection: no information Intention to treat: no Interim analysis: none Exclusion from analysis: 5/182 were excluded for exceeding the age limit Follow‐up period: 1 week after each vaccination |
|
| Participants | Age: 1.5 to 60 months Health status: no information Breastfeeding: no information Immunization status: no vaccination allowed within 2 weeks of rotavirus vaccination |
|
| Interventions | 1. Human‐bovine D x UK, 10^4.8 PFU, single dose (n = 17) 2. Human‐bovine D x UK, 10^5.8 PFU, single dose (n = 20) 3. Human‐bovine DS‐1 x UK, 10^4.3 PFU, single dose (n = 18) 4. Human‐bovine DS‐1 x UK, 10^5.3 PFU, single dose (n = 11) 5. Human‐bovine P x UK, 10^4.3 PFU, single dose (n = 10) 6. Human‐bovine P x UK, 10^5.3 PFU, single dose (n = 21) 7. Human‐bovine ST3 x UK, 10^4.8 PFU, single dose (n = 8) 8. Human‐bovine ST3 x UK, 10^5.8 PFU, single dose (n = 14). 9. Placebo (buffered solution), single dose (n = 63) 30 ml of formula mixed with 0.4 g of sodium bicarbonate given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each vaccination | |
| Notes | Study location: USA 1 h fasting before vaccination Clinical symptoms: clinical evaluation; diarrhoea was defined as 3 or more unformed stools within 48 h; fever was defined as a rectal temperature > 38.1 ºC in paediatric participants, confirmed within 20 minutes Laboratory studies: stool analysis by ELISA and PCR; serology by plaque reduction neutralization and ELISA |
|
Dagan 1992.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (vaccine and placebo vials looked alike and were coded) Data collection: study started July 1988, no further details. Intention to treat: no Interim analysis: none Exclusion from analysis: 19\240 did not receive second vaccine Follow‐up period: 10 days after each vaccine, 1 year after beginning of trial |
|
| Participants | Age: 2 days old infants Health status: healthy Breastfeeding: > 75% Immunization status: no OPV vaccines allowed within 2 weeks of vaccination |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (D x RRV + DS1 x RRV + RRV + ST3 x RRV), 4 x 10^4 PFU, 2 doses (n = 183) 2. Placebo (uninfected cell culture fluid), 2 doses (n = 57) 200 mg citrate‐bicarbonate in 20 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 10 days of each vaccination | |
| Notes | Study location: Israel Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology by ELISA At least 14 days were allowed between OPV and RV |
|
De Mol 1986.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (no further details) Data collection: April 1985 to August 1985 Intention to treat: no information Interim analysis: none Exclusion from analysis: no information Follow‐up period: 4 months after beginning of trial |
|
| Participants | Age: 3 to 8 months Health status: without fever Breastfeeding: > 75% Immunization status: no OPV vaccines allowed within 2 weeks of vaccination |
|
| Interventions | 1. Bovine RIT 4237, single dose (n = 122), no further details 2. Placebo (no further details), single dose (n = 123) |
|
| Outcomes | 1. Efficacy: gastroenteritis within 4 months of vaccination | |
| Notes | Study location: Rwanda Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology and stool analysis by ELISA |
|
Dennehy 1996.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (vaccine and placebo vials looked identical) Data collection: 1 July 1991 to 1 December 1991 Intention to treat: no Interim analysis: none Exclusion from analysis: 2\195 were not followed Follow‐up period: 5 days after each vaccine |
|
| Participants | Age: 6 to 24 weeks Health status: healthy Breastfeeding: no information Immunization status: no DTP, OPV, or Hib vaccines allowed within 2 weeks of vaccination |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 4 x 10^6 PFU, single dose (n = 69) 2. Rhesus + human tetravalent vaccine (RRV‐TV), 4 x 10^5 PFU, single dose (n = 60) 3. Placebo (tissue culture medium), single dose (n = 64) 3 ml sodium citrate‐bicarbonate given with vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination | |
| Notes | Study location: USA At least 2 weeks were allowed between DTP, OPV, Hib, and RV Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 looser than normal stools in 24 h and 1 or more vomiting; fever defined as > 38 ºC) Laboratory studies: serology by ELISA |
|
Eichelberger 2002.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (no further details) Data collection: no information Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow‐up period: 1 week after each vaccination |
|
| Participants | Age: 1.5 to 60 months Health status: no information Breastfeeding: no information Immunization status: no vaccination allowed within 2 weeks of rotavirus vaccination |
|
| Interventions | 1. Human‐bovine Wa x UK, 10^6.3 PFU, single dose (n = 21) 2. Human‐bovine Wa x UK, 10^7.3 PFU, single dose (n = 21) 3. Human‐bovine Wa x (DS‐1xUK), 10^6.4 PFU, single dose (n = 14) 4. Human‐bovine Wa x (DS‐1xUK), 10^7.4 PFU, single dose (n = 22) 5. Placebo (buffered soy‐based formula), single dose (n = 37) 30 ml of formula mixed with 0.4 g of sodium bicarbonate given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each vaccination | |
| Notes | Study location: USA 1 h fasting before vaccination Clinical symptoms: clinical evaluation; diarrhoea was defined as 3 or more unformed stools within 48 h; fever was defined as a rectal temperature > 38.1 ºC in paediatric participants, confirmed within 20 minutes Laboratory studies: stool analysis by ELISA and PCR; serology by plaque reduction neutralization and ELISA |
|
Escobar 1988.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (vaccine and placebo were identical) Data collection: no details Intention to treat: no information Interim analysis: no information Exclusion from analysis: no information Follow‐up period: 3 weeks after vaccination |
|
| Participants | Age: 6 to 18 months Health status: without diarrhoea, no further details Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Bovine RIT 4237, 0.5 ml, 10^8 DICT 50, single dose (n = 52) 2. Placebo (same preparation without the virus), single dose (n = 51) 110 ml of formula given before vaccine or placebo |
|
| Outcomes | 1. Efficacy: rotavirus diarrhoea within 3 weeks of vaccination | |
| Notes | Study location: Spain Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology and stool analysis by ELISA |
|
Flores 1988.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (no details) Data collection: no details Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow up‐period: 10 days after vaccination |
|
| Participants | Age: newborns Health status: healthy newborns and mothers, normal pregnancy Breastfeeding: > 75% Immunization status: no information |
|
| Interventions | 1. Rhesus (MMU18006) vaccine, 10^4 PFU, single dose (n = 20) 2. Placebo (buffered formula), single dose (n = 20) 200 mg citrate‐bicarbonate in 15 ml formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 10 days of vaccination | |
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 liquid or semi‐liquid stools in 24 h Laboratory studies: serology by ELISA, complement‐fixation assay, plaque reduction neutralization assay, tube neutralization assay, and VP‐7 competition assay; stool analysis by ELISA |
|
Flores 1989.
| Methods | Randomization: random code assignment to 5 groups Allocation concealment: code prepared in advance, not disclosed to field team, broken at NIH laboratory after follow up ended Blinding: double (same size of ampoules given to each group). Data collection: no details Intention to treat: no Interim analysis: none Exclusion from analysis: 20\116 excluded from study for having diarrhoea, nausea, or vomiting in the week prior to vaccination. Follow‐up period: 1 week after vaccination |
|
| Participants | Age: 1 to 5 months Health status: healthy Breastfeeding: no information Immunization status: polio vaccine not given within 2 weeks of vaccination |
|
| Interventions | 1. Rhesus + human (D x RRV), 10^4 PFU, single dose (n = 24) 2. Rhesus + human (DS1 x RRV), 10^4 PFU, single dose (n = 25) 3. Rhesus (RRV 3), 10^4 PFU, single dose (n = 21) 4. Rhesus + human (D x RRV + RRV), 5 x 10^3 PFU, single dose (n = 23) 5. Placebo (formula alone or with red soda), 1 ml single dose (n = 23) 400 mg citrate‐bicarbonate in 30 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination | |
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 watery or loose stools in 24 h; severity score measured by Flores 1987 scale Laboratory studies: serology by ELISA and plaque reduction neutralization assay; stool analysis by ELISA and cultures |
|
Flores 1990.
| Methods | Randomization: random order assignment to 4 groups Allocation concealment: code not disclosed to field team until end of analysis, broken at NIH Blinding: double (no further details) Data collection: no details Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow‐up period: 1 week after vaccination |
|
| Participants | Age: 10 to 20 weeks Health status: no information Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Rhesus + human quadrivalent vaccine (3 RRV + D x RRV + DS1 x RRV + ST3 x RRV), 10^4 PFU, single dose (n = 23) 2. Rhesus + human quadrivalent vaccine (3 RRV + D x RRV + DS1 x RRV + ST3 x RRV), 5 x 10^4 PFU, single dose (n = 22) 3. Human vaccine (M37), 10^4 PFU, single dose (n = 23) 4. Placebo (formula), 1 ml, single dose (n= 22) 400 mg citrate‐bicarbonate in 30 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination | |
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 watery or loose stools in 24 h Laboratory studies: serology by ELISA and plaque reduction neutralization assay; stool analysis by ELISA and cultures |
|
Flores 1993.
| Methods | Randomization: no details Allocation concealment: code not revealed at any time to field team, no further details Data collection: no details Blinding: double (no further details) Intention to treat: no Interim analysis: yes Exclusion from analysis: 22\302 dropped from study, no further details Follow‐up period: 1 week after each vaccination, passive follow up for 8 months |
|
| Participants | Age: newborns Health status: healthy Breastfeeding: no information Immunization status: OPV and DPT vaccinations were given 2 weeks after each RRV dose |
|
| Interventions | 1. Rhesus + human quadrivalent vaccine (3 RRV + D x RRV + DS1 x RRV + ST3 x RRV), 10^5 PFU, 3 doses (n = 101) 2. Rhesus + human quadrivalent vaccine (3 RRV + D x RRV + DS1 x RRV + ST3 x RRV), 10^6 PFU, 3 doses (n = 99) 3. Placebo (uninfected tissue culture fluids), 3 doses (n = 102) 400 mg citrate‐bicarbonate in 30 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination 2. Efficacy: diarrhoea (rotavirus or other) within 8 months |
|
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 watery or loose stools in 24 h Laboratory studies: serology by ELISA and plaque reduction neutralization assays; stool analysis by ELISA Vaccine given between OPV and DPT vaccines. |
|
Georges‐Courbot 1991.
| Methods | Randomization: table of random numbers, administered in numeric order in the first dose Allocation concealment: no details Blinding: double (identical precoded vials) Data collection: no information Intention to treat: no Interim analysis: yes Exclusion from analysis: 69\539 did not receive vaccine (7 deaths), 56/470 did not finish follow up (4 deaths) Follow‐up period: 9 months after first vaccination |
|
| Participants | Age: 3 months Health status: healthy Breastfeeding: > 75% Immunization status: DTCP vaccine given with rotavirus vaccine |
|
| Interventions | 1. Bovine (WC3), 10^7 PFU, 2 doses (n = 237) 2. Placebo (sacharose and lactose substrates), 2 doses (n = 235) Milk given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination 2. Efficacy: diarrhoea within 9 months of first vaccination |
|
| Notes | Country: Central African Republic Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 watery or loose stools in 24 h; severity score measured by Clark 1988 (modified Duffy 1986) scale Laboratory studies: stool analysis by latex agglutination and ELISA; serology by plaque reduction neutralization assay |
|
Gothefors 1989.
| Methods | Randomization: no details Allocation concealment: no details Blinding: no details Data collection: started December 1984, lasted 2 winter seasons Intention to treat: no Interim analysis: none Exclusion from analysis: 2\106 excluded for infection with serotype distinct from vaccine strain Follow‐up period: 2 winter seasons. |
|
| Participants | Age: 4 to 12 months Health status: no information Breastfeeding: < 50% Immunization status: no information |
|
| Interventions | 1. Rhesus (MMU18006), 1 ml vaccine diluted 1\10 orally, single dose (n = 54) 2. Placebo (distilled water with added color), single dose (n = 52) |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination 2. Efficacy: diarrhoea within 2 winter seasons (rotavirus or other) |
|
| Notes | Country: Sweden Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 watery or loose stools in 24 h, clinically significant diarrhoea defined as > 3 loose stools\24 h and symptoms > 48 h, WHO criteria Laboratory studies: stool analysis and serology by ELISA |
|
Hanlon 1987.
| Methods | Randomization: no details Allocation concealment: no details Blinding: none (one group not blinded because of IM vaccination) Data collection: January 1985 to March 1986 Intention to treat: no Interim analysis: none Exclusion from analysis: 180\433 excluded (absent during epidemic period or too young to be vaccinated) Follow‐up period: 7 days after vaccination; 1 rotavirus season |
|
| Participants | Age: > 2.5 months Health status: no information Breastfeeding: no information Immunization status: OPV and IPV given with rotavirus vaccine, no further details |
|
| Interventions | 1. Bovine (RIT 4237) 10^7.8 per monodose vial and OPV, 3 doses (n = 78) 2. Bovine (RIT 4237) 10^7.8 per monodose vial and IPV, 3 doses (n = 92) 3. Placebo (uninfected primary monkey kidney cells) 0.5 ml and OPV, 3 doses (n = 83) Vaccine given with OPV or IM polio vaccine |
|
| Outcomes | 1. Efficacy: diarrhoea (rotavirus) within 1 rotavirus season | |
| Notes | Study location: Gambia Mothers encouraged to breastfeed before and after vaccination Clinical symptoms: clinical evaluation; diarrhoea defined as ≥ 3 loose to watery stools in 24 h); severity measured by WHO criteria (fever (≥ 38 ºC) vomiting, dehydration, > 6 stools in 24 h, and illness duration ≥ 24 h) Laboratory studies: stool analysis by ELISA and silver staining polyacrylamide gel technique; serology by standard viral neutralization methods |
|
Ho 1989.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double for 2 groups (no further details) Data collection: September 1987 to January 1988 Intention to treat: no information Interim analysis: no information Exclusion from analysis: no information Follow‐up period: 5 days after vaccination |
|
| Participants | Age: 2 to 3 months Health status: healthy Breastfeeding: < 50% Immunization status: DPT/OPV given with RRV in one group, and 2 weeks apart in a second group |
|
| Interventions | 1. Rhesus (MMU18006) 10^4 PFU alone, single dose (n = 30). 2. Rhesus (MMU18006) 10^4 PFU with OPV/DTP, single dose (n = 36) 3. Placebo (soybean formula) with OPV/DTP single dose (n = 36) 400 mg sodium bicarbonate in 30 ml soybean formula given before vaccine or placebo 1 group received the vaccine together with OPV\DTP (this group received acetaminophen prophylactically for 24 h) The second group received the vaccine after 2 weeks of OPV\DTP |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination | |
| Notes | Study location: USA Breast or bottle feeding delayed for 1 h before and after RV Clinical symptoms: clinical evaluation (irritability defined as > 20 minutes unexplained crying, gastroenteritis defined as diarrhoea and vomiting) Laboratory studies: serology by ELISA |
|
Ing 1991.
| Methods | Randomization: prepared randomization schedule Allocation concealment: no details Blinding: none Data collection: January 1989 to November 1989 Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow‐up period: 5 days after vaccination; 2 months after vaccination |
|
| Participants | Age: 1.5 to 4 months Health status: healthy |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 4 x 10^5 PFU, with OPV/DTP vaccination, single dose (n = 135). 2. OPV/DTP alone (n = 39) 400 mg sodium bicarbonate in 25 ml soybean formula given before vaccine |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination 2. Illness or diarrhoea within 2 months of vaccination |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 loose to watery stools in 24 h) Laboratory studies: serology by ELISA |
|
Joensuu 1997.
| Methods | Randomization: computer‐generated randomization schedule, blocks of four, provided by the pharmaceutical industry Allocation concealment: code sent to investigator in sealed envelopes and returned to pharmaceutical industry unbroken at the end of the study Blinding: double (except 4th year of follow up), identically appearing vaccine and placebo Data collection: September 1993 to June 1997 Intention to treat: yes Interim analysis: none Exclusion from analysis: 38\2398 excluded, no further details Follow‐up period: 5 days after vaccination; 4 rotavirus seasons after vaccination |
|
| Participants | Age: 1.5 to 4.5 months Health status: healthy Breastfeeding: no information Immunization status: all other vaccinations given according to schedule without delays |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 4 x 10^5 PFU, 3 doses (n = 1191) 2. Placebo (tissue culture fluid), 3 ml, 3 doses (n = 1207) 3 ml sodium bicarbonate and citric acid buffer given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination. 2. Efficacy: gastroenteritis (rotavirus or other) within 4 rotavirus seasons |
|
| Notes | Study location: Finland Clinical symptoms: clinical evaluation (gastroenteritis defined as ≥ 3 looser than normal stools or ≥ 3 loose stools and one vomiting episode in 24 h); severity score measured by Ruuska 1990 scale Laboratory studies: stool analysis by ELISA and PCR; serology by ELISA |
|
Lanata 1989.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (identical pre‐coded vials) Data collection: November 1984 to July 1986 Intention to treat: no Interim analysis: none Exclusion from analysis: 32\43 excluded for not receiving all 3 doses; 50/391 did not finish surveillance (8 deaths, no details) Follow‐up period: 7 days after vaccination; 18 months after vaccination |
|
| Participants | Age: 2 to 18 months Health status: no information Breastfeeding: 50 to 75% (vaccinees only) Immunization status: no information Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 liquid or semi‐liquid stools in 24 h), WHO dehydration criteria used Laboratory studies: stool analysis by ELISA; serology by CF. |
|
| Interventions | 1. Bovine (RIT 4237), 10^8.3 TCID50, 3 doses (n = 358 child years). 2. Placebo (uninfected primary monkey kidney cells), 0.5 ml, 3 doses (n = 127 child years) 100 ml evaporated milk given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination 2. Efficacy: diarrhoea within 18 months of vaccination |
|
| Notes | Study location: Peru | |
Lanata 1996a.
| Methods | Randomization: 12 letter codes allocated by block randomization Allocation concealment: code kept at NIH and WHO, and not revealed until study ended Blinding: double (identical appearing vaccine and placebo vials) Data collection: August 1987 to October 1990 Intention to treat: no Interim analysis: none Exclusion from analysis: 115\800 not followed 24 months (13 deaths unrelated to vaccine) Follow‐up period: 6 days after vaccination; 24 months after vaccination |
|
| Participants | Age: 2 months Health status: healthy Breastfeeding: no information Immunization status: DPT and OPV vaccination began 1 month after rotavirus vaccination |
|
| Interventions | 1. Rhesus (MMU18006) 10^4 PFU, single dose (n = 200) 2. Rhesus + human (D x RRV) 10^4 PFU, single dose (n = 200) 3. Rhesus + human (DS1 x RRV), 10^4 PFU, single dose (n = 200) 4. Placebo (milk or minimum essential media), single dose (n = 200) 400 mg sodium bicarbonate in 30 ml milk given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 6 days of vaccination. 2. Efficacy: diarrhoea (rotavirus or other) within 24 months of vaccination |
|
| Notes | Study location: Peru Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 liquid or semi‐liquid stools in 24 h), WHO dehydration criteria used Laboratory studies: stool analysis by ELISA; serology by ELISA and plaque reduction neutralization assay |
|
Lanata 1996b.
| Methods | Randomization: computerized randomization, blocks of 12 Allocation concealment: pre‐coded vessels administered sequentially to children; code kept at pharmaceutical industry and not broken until end of study Blinding: double (identical appearing vaccine and placebo vials) Data collection: October 1988 to October 1990 Intention to treat: no Interim analysis: none Exclusion from analysis: 60\700 excluded for not completing 3 doses of vaccine; 60\640 not followed for 24 months (6 deaths unrelated to vaccine) Follow‐up period: 6 days after vaccination; 24 months after vaccination |
|
| Participants | Age: 2 months Health status: healthy Breastfeeding: no information Immunization status: DPT and polio vaccinations given with RRV |
|
| Interventions | Rhesus + human tetravalent vaccine (RRV + D x RRV + DS1 x RRV + ST3 x RRV), 10^4 PFU, 3 doses (n = 467) Placebo (uninfected cell cultures), 1 ml, 3 doses (n = 232) 400 mg sodium bicarbonate in 30 ml milk given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 6 days of vaccination 2. Efficacy: diarrhoea (rotavirus or other) within 24 months of vaccination |
|
| Notes | Study location: Peru Breastfeeding withheld for 1 h before and after RV Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 liquid or semi‐liquid stools in 24 h), WHO dehydration criteria used; severity score measured by Kapikian's scale (modified Flores 1987) Laboratory studies: stool analysis by ELISA; serology by ELISA and plaque reduction neutralization assay Children were concurrently immunized with IPV, DTP, BCG (at birth), and MMR |
|
Linhares 1996.
| Methods | Randomization: block randomization, numbered serially Allocation concealment: code kept by pharmaceutical industry and WHO and not broken until end of study Blinding: double (identical appearing vaccine and placebo vials) Data collection: February 1990 to January 1991 Intention to treat: no Interim analysis: none Exclusion from analysis: 74\540 not followed 24 months (8 deaths, no further details) Follow‐up period: 7 days after each vaccination; 24 months after vaccination |
|
| Participants | Age: 1 to 2 months Health status: healthy Breastfeeding: no information Immunization status: OPV vaccine given at least 15 days before or after RRV |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 10^4 PFU, 3 doses (n = 270) 2. Placebo (uninfected cell cultures), 1.2 ml, 3 doses (n = 270) 400 mg sodium bicarbonate in 30 ml milk given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination. 2. Efficacy: diarrhoea (rotavirus or other) within 24 months of vaccination |
|
| Notes | Study location: Brazil Breastfeeding or bottle feeding withheld for 1 h before or after vaccine OPV given at an interval of at least 2 weeks before or after RV Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 liquid or semi‐liquid stools in 24 h), WHO dehydration criteria used; severity score measured by Flores scale Laboratory studies: stool analysis by ELISA; serology by ELISA and plaque reduction neutralization assay |
|
Madore 1992.
| Methods | Randomization: table of random numbers Allocation concealment: no details Blinding: double (identical placebo and vaccine vials) Data collection: 15 September 1987 to 8 August 1990 Intention to treat: yes Interim analysis: none Excluded subjects: none Follow‐up period: 10 days after vaccination; 3 years after enrolment |
|
| Participants | Age: 2 to 4 months Health status: healthy Breastfeeding: < 50% Immunization status: DPT and OPV vaccinations not given 4 weeks before and 3 weeks after RRV |
|
| Interventions | 1. Rhesus (MMU 18006), 10^4 PFU, single dose (n = 76) 2. Rhesus + human (RRV x D), 10^4 PFU, single dose (n = 74) 3. Placebo (soy formula), 1 ml single dose (n = 73) 400 mg sodium bicarbonate in 30 ml soy formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 10 days of vaccination 2. Efficacy: gastroenteritis (rotavirus or other) within 3 years of enrolment |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 loose stools in 24 h), severity measured by WHO criteria (fever (≥ 38) vomiting, dehydration, > 6 stools in 24 h and illness duration ≥ 24 h) Laboratory studies: serology by micro‐neutralization assay and ELISA; stool analysis by ELISA All other vaccines withheld for 4 weeks before and 3 weeks after RV |
|
Maldonado 1986.
| Methods | Randomization: block randomization 2:2:1 Allocation concealment: no details Blinding: double (coded preparations) Data collection: enrolment between June 1984 and January 1985, no further details Intention to treat: no Interim analysis: none Exclusion from analysis: 11\54 excluded for non‐compliance Follow‐up period: 7 days before and 7 days after vaccination |
|
| Participants | Age: < 1 month Health status: no information Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Bovine (RIT 4237), 10^8 TCID50, 2 doses (n = 19) 2. Bovine (RIT 4237), 10^7 TCID50, 2 doses (n = 20) 3. Placebo (uninfected tissue culture fluid), 0.5 ml, 2 doses (n = 10) Formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination | |
| Notes | Study location: USA Clinical symptoms: clinical evaluation (diarrhoea defined as any noticeable change in frequency of stools or development of of watery/loose stools) Laboratory studies: serology by plaque reduction neutralization; stool analysis by ELISA |
|
Mutz 1989.
| Methods | Randomization: confirmed by investigator, no further details Allocation concealment: no details Blinding: double (confirmed by investigator), no further details Data collection: no details Intention to treat: no Interim analysis: none Exclusion from analysis: no details Follow‐up period: 3 to 4 weeks after vaccination; 6 months after end of safety period |
|
| Participants | Age: > 1 month Health status: no information Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Bovine (RIT 4237), 10^6.5 TCID50, 1 dose (n = 243) 2. Placebo (n = 103), no further details |
|
| Outcomes | 1. Safety: clinical symptoms within 3 to 4 weeks of vaccination 2. Efficacy: diarrhoea within 6 months of safety period ending |
|
| Notes | Study location: Austria Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology by ELISA; stool analysis (no details) |
|
Perez‐Schael 1990a.
| Methods | Randomization: code assigned to vials Allocation concealment: vials administered serially to participants; codes partially disclosed at NIH before end of trial to analyse reactions to vaccine, and kept at NIH until end of study Blinding: double (vaccine and placebo looked alike) Data collection: vaccination between February 1985 and February 1986, end of study February 1987 Intention to treat: no Interim analysis: yes, after phase 1 Exclusion from analysis: 63\320 dropped from follow up Follow‐up period: 1 week after vaccination, 1 year after safety period |
|
| Participants | Age: 1 to 10 months Health status: no information Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Rhesus (MMU‐18006), 10^4 PFU, single dose (n = 151) 2. Placebo (buffered formula), single dose (n = 151) 400 mg citrate‐bicarbonate in 30 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination 2. Efficacy: diarrhoea within 1 year of vaccination |
|
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 watery or loose stools in 24 h) Laboratory studies: serology by ELISA and plaque reduction neutralization assay; stool analysis by ELISA |
|
Perez‐Schael 1990c.
| Methods | Randomization: code, no further details Allocation concealment: code broken at NIH after completion of safety analysis but not disclosed to field team Blinding: double, no further details Data collection: no information Intention to treat: yes Interim analysis: yes, doses were evaluated sequentially Exclusion from analysis: none Follow‐up period: 1 week after vaccination |
|
| Participants | Age: 2.5 to 5 months Health status: no debilitating disease Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Rhesus + human quadrivalent vaccine (3 RRV + D x RRV + DS1 x RRV + ST3 x RRV), 0.25 x 10^4 PFU or 0.5 x 10^4 PFU or 10^4 PFU, single dose (n = 67) 2. Placebo (buffered formula), single dose (n = 63) 400 mg citrate‐bicarbonate in 30 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination | |
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology by ELISA and plaque reduction neutralization assay; stool analysis by ELISA |
|
Perez‐Schael 1994.
| Methods | Randomization: confirmed by letter to author, no further details. Allocation concealment: no details Blinding: double (confirmed by letter to author), no further details Data collection: no details Intention to treat: no details Interim analysis: none Exclusion from analysis: no details Follow‐up period: 1 week after vaccination |
|
| Participants | Age: 2.5 to 5 months Health status: no information Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Rhesus + human quadrivalent vaccine (3 RRV + D x RRV + DS1 x RRV + ST3 x RRV), 10^4 PFU or 5 x 10^4 PFU, 2 doses (n = 87) 2. Placebo, 2 doses (n = 82), no further details 400 mg bicarbonate in 30 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 1 week of vaccination | |
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology by ELISA and plaque reduction neutralization assay; stool analysis by ELISA and cultures |
|
Perez‐Schael 1997.
| Methods | Randomization: computerized randomization algorithm that balanced the number of children in groups of 500 numbers Allocation concealment: sequential code numbers Blinding: double (identical looking vaccine and placebo) Data collection: March 1992 to October 1995 Intention to treat: yes for safety, no for efficacy Interim analysis: none Exclusion from analysis: 443\2480 dropped from study, similar distribution in vaccine and placebo groups according to author, no further details Follow‐up period: 6 days after each vaccination; 19 to 20 months after vaccination |
|
| Participants | Age: newborns (< 1 month) Health status: healthy Breastfeeding: > 75% Immunization status: DPT vaccination given with RRV, OPV not given within 2 to 4 weeks of RRV |
|
| Interventions | 1. Rhesus + human quadrivalent vaccine (RRV‐TV), 4 x 10^5 PFU, 3 doses (n = 1247) 2. Placebo (uninfected tissue culture fluids), 2.5 ml, 3 doses (n = 1233) 400 mg citrate‐bicarbonate in 30 ml similac formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 6 days of vaccination 2. Efficacy: gastroenteritis within 19 to 20 months of vaccination |
|
| Notes | Study location: Venezuela Clinical symptoms: clinical evaluation (gastroenteritis defined as ≥ 3 watery or loose stools or 1 bloody stool with or without vomiting in 24 h); WHO dehydration criteria used; severity score measured by Ruuska 1990 scale Laboratory studies: stool analysis by ELISA and PCR; serology by ELISA Children were concurrently immunized with DTP; IPV was separated from RV by at least 2 weeks 85 to 95% of children were breastfeeding while receiving at least 1 of the doses of RV |
|
Perez‐Schael 2002.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double, no further details Data collection: no details Intention to treat: no details Interim analysis: yes Exclusion from analysis: no details Follow‐up period: 6 months after vaccination |
|
| Participants | Age: 1.5 to 3 months Health status: healthy Breastfeeding: no information Immunization status: DPT, HBV, and HiB vaccination given with RRV; OPV not given within 2 weeks of RRV |
|
| Interventions | 1. Human 89‐12 (RIX4414) 105.0 PFU/dose, 2 doses (n = 493) 2. Human 89‐12 (RIX4414) 105.5 PFU/dose, 2 doses (n = 496) 3. Human 89‐12 (RIX4414) 106.0 PFU/dose, 2 doses (n = 493) 4. Placebo, 2 doses (n = 504), no further details |
|
| Outcomes | 1. Efficacy: rotavirus gastroenteritis within 6 months of vaccination | |
| Notes | Study location: Venezuela, Brazil, Mexico Clinical symptoms: clinical evaluation; severity score measured by Ruuska 1990 scale Laboratory studies: stool analysis by ELISA Data taken from poster presented at ICAAC 2002 This is an interim analysis of an ongoing trial |
|
Pichichero 1990.
| Methods | Randomization: code supplied by pharmaceutical industry Allocation concealment: no details Blinding: double (coded vials) Data collection: before September 1987, no further details Intention to treat: no Interim analysis: none Exclusion from analysis: 1\96 gave only safety information because of no consent Follow‐up period: 1 week after each vaccination |
|
| Participants | Age: 2 to 5 months Health status: healthy Breastfeeding: < 50% Immunization status: no medication given within 2 weeks before vaccination |
|
| Interventions | 1. Rhesus (MMU 18006), 10^3 PFU, single dose (n = 22) 2. Rhesus (MMU 18006), 10^4 PFU, single dose (n = 25) 3. Rhesus (MMU 18006), 10^5 PFU, single dose (n = 23) 4. Placebo (buffered formula), 1ml, single dose (n = 25) Half the infants received 30 ml formula before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination | |
| Notes | Study location: USA All feedings withheld for 1 h before and after RV Clinical symptoms: clinical evaluation (diarrhea defined as ≥ 3 looser than usual stools in 24 h) Laboratory studies: serology by ELISA and plaque reduction neutralization assay; stool analysis by ELISA and cultures |
|
Pichichero 1993.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (identical appearing vials) Data collection: 31 May 1988 to 2 March 1988 Intention to treat: no Interim analysis: none Exclusion from analysis: 1\213 did not supply safety information Follow‐up period: 1 week after each vaccination |
|
| Participants | Age: 1.5 to 4 months Health status: healthy Breast feeding: > 75% Immunization status: no medication given within 2 weeks before vaccination |
|
| Interventions | 1. RRV S1, 10^4 or 10^5 PFU, single dose (n = 40) 2. RRV S2, 10^4 or 10^5 PFU, single dose (n = 37) 3. RRV S3, 10^4 or 10^5 PFU, single dose (n = 39) 4. RRV S4, 10^4 or 10^5 PFU, single dose (n = 39) 5. RRV‐TV, 10^4 or 10^5 PFU, single dose (n = 38) 6. Placebo, single dose (n = 19), no details |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination | |
| Notes | Study location: USA No buffer or formula given with vaccine; feeding allowed after vaccination Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 looser than usual stools in 24 h) Laboratory studies: serology by ELISA and plaque reduction neutralization |
|
Rennels 1987.
| Methods | Randomization: code, no further details Allocation concealment: no details Blinding: double (coded preparations) Data collection: May 1985 to October 1985 Intention to treat: yes Interim analysis: none Excluded subjects: none Follow‐up period: 7 days after vaccination |
|
| Participants | Age: 3 to 12 months Health status: healthy Breastfeeding: < 50% Immunization status: no other vaccines allowed within 2 weeks of vaccination |
|
| Interventions | 1. Rhesus (MMU 18006), 10^4 PFU, single dose (n = 12) 2. Rhesus (MMU 18006), 10^3 PFU, single dose (n = 8) 3. Placebo (buffered formula), 1 ml, single dose (n = 11) 400 mg sodium bicarbonate in 30 ml formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination | |
| Notes | Study location: USA Breastfeeding or bottle feeding withheld for 1 h before or after vaccine Clinical symptoms: clinical evaluation (diarrhea defined as ≥ 3 loose stools in 24 h) Laboratory studies: serology by plaque reduction neutralization assay; stool analysis by ELISA and cultures |
|
Rennels 1990.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (coded preparations) Data collection: no information Interim analysis: none Excluded subjects: 2\114 did not complete surveillance Follow‐up period: 7 days after vaccination, 2 years after vaccination |
|
| Participants | Age: 2 to 5 months Health status: healthy Breastfeeding: < 50% Immunization status: all other vaccinations not given within 2 weeks of RRV |
|
| Interventions | 1. Rhesus (MMU 18006), 10^4 PFU, single dose (n = 64) 2. Placebo (formula), single dose (n = 50) 30 ml formula containing 400 mg sodium bicarbonate given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination. 2. Efficacy: diarrhoea (rotavirus or other) within 2 years of vaccination |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 looser than normal stools in 24 h); severity score measured by Flores 1987 scale Laboratory studies: serology by plaque reduction neutralization assay; stool analysis by ELISA |
|
Rennels 1995.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double, no further details Data collection: vaccination between 10 August 1989 and 16 July 1990 Intention to treat: no Interim analysis: none Excluded participants: 173\989 did not complete surveillance Follow‐up period: 2 years after vaccination |
|
| Participants | Age: 1 to 6.5 months Health status: healthy Breastfeeding: < 50% Immunization status: no information |
|
| Interventions | 1. Rhesus + human monovalent vaccine (RRV‐S1), 4 x 10^4 PFU, 3 doses (n = 280) 2. Rhesus + human quadrivalent vaccine (RRV‐TV), 4 x 10^4 PFU, 3 doses (n = 264) 3. Placebo (tissue culture medium), 3 doses (n = 272) 30 ml formula containing 400 mg sodium bicarbonate given before vaccine or placebo |
|
| Outcomes | 1. Efficacy: gastroenteritis (rotavirus related) within 2 years of vaccination | |
| Notes | Study location: USA Clinical symptoms: clinical evaluation (gastroenteritis defined as ≥ 3 loose stools in 24 h or emesis) Laboratory studies: serology by ELISA and fluorescent focus reduction assay |
|
Rennels 1996.
| Methods | Randomization: code provided by pharmaceutical industry, no further details Allocation concealment: no details Blinding: double (identical appearing vials) Data collection: vaccination between 5 July 1991 and 24 January 1992 Intention to treat: no Interim analysis: none Excluded participants: 91\1278 did not complete efficacy surveillance Follow‐up period: 5 days after vaccination, 1 rotavirus season after vaccination |
|
| Participants | Age: 1.25 to 6.25 months Health status: healthy Breastfeeding: < 50% Immunization status: all other vaccines allowed with RRV |
|
| Interventions | 1. Rhesus + human monovalent vaccine (RRV‐S1), 4 x 10^5 PFU, 3 doses (n = 425) 2. Rhesus + human quadrivalent vaccine (RRV‐TV), 4 x 10^5 PFU, 3 doses (n = 428) 3. Placebo (tissue culture medium), 3 doses (n = 425) 3 ml sodium bicarbonate given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination 2. Efficacy: gastroenteritis (rotavirus or other) within 1 rotavirus season |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 loose stools in 24 h; gastroenteritis defined as diarrhea, vomiting, or both); severity score measured by Rennels 1996 (modified Flores 1987) scale Laboratory studies: serology and stool analysis by ELISA Other vaccines allowed with RV but not required |
|
Ruuska 1990.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double (identical vaccine and placebo appearance) Data collection: October 1984 to June 1987 Intention to treat: no Interim analysis: none Exclusion from analysis: 22% of first group and 13% of second group did not finish follow up, no further details Follow‐up period: 7 days after vaccination, 24 to 32 months after safety period |
|
| Participants | Age: newborns Health status: healthy Immunization status: no vaccine except BCG given before rotavirus vaccine |
|
| Interventions | 1. Bovine (RIT 4237), 10^8.3 TCID, single dose (n = 519 person years) 2. Placebo (uninfected tissue culture), single dose (n = 481 person years) |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination 2. Efficacy: diarrhoea within 2 seasons of vaccination |
|
| Notes | Study location: Finland Clinical symptoms: definitions and laboratory methods mentioned in different paper No buffer or formula given with vaccine |
|
Santosham 1991a.
| Methods | Randomization: table of random numbers Blinding: no details Data collection: 1 January 1992 to 31 January 1994 Intention to treat: no Interim analysis: none Exclusion from analysis: 11/332 were not followed because the left the area Follow‐up period: 5 days after vaccination, 17 months after vaccination |
|
| Participants | Age: 2 to 5 months Health status: no illness or immunodeficiency Breastfeeding: < 50% Immunization status: OPV vaccination not given within 3 weeks of rotavirus vaccination |
|
| Interventions | 1. Rhesus (MMU18006), 10^4 PFU, single dose (n = 108) 2. Placebo (soy formula), 1 ml, single dose (n = 107) 400 mg sodium bicarbonate in 30 ml soy formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination. 2. Efficacy: diarrhoeal illness (rotavirus or other) within 17 months of vaccination |
|
| Notes | Study location: USA Clinical symptoms: diarrhoea ≥ 3 watery stools in 24 h Laboratory studies: serology and stool analysis by ELISA OPV given at an interval of at least 3 weeks before or after RV Breastfeeding allowed during vaccination |
|
Santosham 1991b.
| Methods |
Santosham 1991a and 1991b are two arms of a single trial See Santosham 1991a for details |
|
| Participants | See Santosham 1991a for details | |
| Interventions | 1. Bovine (RIT 4237), 10^8 PFU, single dose (n = 106) 2. Placebo (soy formula), 1 ml, single dose (n = 107) 400 mg sodium bicarbonate in 30 ml soy formula given before vaccine or placebo |
|
| Outcomes | See Santosham 1991a for details | |
| Notes | See Santosham 1991a for details | |
Santosham 1997.
| Methods | Randomization: blocks of 6 to receive either RRV‐TV, RRV‐S1, or placebo. Packages containing 3 doses of study drug, code‐labelled, were provided to the study sites Allocation concealment: schedule generated by the biostatistics division at Wyeth Ayerst Blinding: no details Data collection: enrolment between September 1985 and February 1987, no further details Intention to treat: yes Interim analysis: none Exclusion from analysis: 101/1185 did not complete 2 years follow up, 171\1084 did not complete 2 years follow up, no further details Follow‐up period: 5 days after vaccination, 2 years after beginning of trial |
|
| Participants | Age: 1.5 to 6 months Health status: healthy Breastfeeding: no information Immunization status: all other vaccines allowed with RRV |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 10^5 PFU, 3 doses (n = 396) 2. Rhesus + human monovalent vaccine (RRV‐S1), 4 x 10^5 PFU, 3 doses (n = 398) 3. Placebo (tissue culture medium), 3 doses (n = 391) 3 ml sodium citrate bicarbonate given with vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of vaccination 2. Efficacy: gastroenteritis within 2 years of beginning of trial |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation (gastroenteritis defined as ≥ 3 watery or loose stools in 24 h); severity score measured by Rennels 1996 (modified from Flores 1987) scale Laboratory studies: serology and stool analysis by ELISA Simultaneous administration of routine childhood vaccines was permitted but not required |
|
Senturia 1987.
| Methods | Randomization: no details Allocation concealment: no details Blinding: double, no further details Data collection: winter 1985 to 1986 Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow‐up period: 1 winter season after vaccination |
|
| Participants | Age: 8 to 48 months Health status: no information Breastfeeding: < 50% Immunization status: no information |
|
| Interventions | 1. Bovine (RIT 4237), single dose (n = 105), no further details 2. Placebo (no details), single dose (n = 106) Milk drink given before vaccine or placebo |
|
| Outcomes | 1. Efficacy: diarrhoea (rotavirus or other) within 1 winter season of vaccination | |
| Notes | Study location: UK Clinical symptoms: clinical evaluation, no further details Laboratory studies: stool analysis by ELISA; serology by serum neutralization |
|
Treanor 1995.
| Methods | Randomization: block 2:1 (Rochester), 1:1 (Philadelphia), no further details Allocation concealment: no details Blinding: double, no further details Data collection: 18 June 1992 to 15 June 1993 Intention to treat: no information Interim analysis: no information Exclusion from analysis: 13/325 did not complete the study because of relocation out of the area or negative compliance Follow‐up period: 7 days after vaccination, 1 winter season after vaccination |
|
| Participants | Age: 2 to 8 months Health status: healthy Breastfeeding: < 50% Immunization status: no other vaccine given within 7 days of rotavirus vaccination |
|
| Interventions | 1. Bovine + human (WI79‐9), 10^7.3 PFU, 3 doses (n = 207). 2. Placebo (cell culture media and cherry syrup), 2.5 ml, 3 doses (n = 118) 30 ml soy or milk formula or 400 mg sodium bicarbonate in 30 ml soy formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination 2. Efficacy: gastroenteritis (rotavirus or other) within 1 winter season |
|
| Notes | Study location: USA Breastfeeding and bottle feeding withheld for 1 h before and after RV Clinical symptoms: clinical evaluation (gastroenteritis defined as ≥ 1 watery stool or ≥ 3 liquid stools and\or ≥ 1 vomiting episode in 24 h); severity score measured by Clark 1988 (modified Duffy 1986) scale Laboratory studies: stool analysis by ELISA, gel electrophoresis, and PCR Other vaccines given at least 7 days after RV |
|
Vesikari 1984.
| Methods | Randomization: no details Allocation concealment: code not available until end of study Blinding: double (code‐labelled vials) Data collection: January 1983 to June 1983 Intention to treat: no Interim analysis: none Exclusion from analysis: 12/190 did not complete vaccination or follow up Follow‐up period: 10 days after vaccination, 5 months after vaccination |
|
| Participants | Age: 8 to 11 months Health status: healthy Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Bovine (RIT 4237), 10^8.1 TCID50, single dose (n = 86) 2. Placebo (uninfected primary monkey kidney cells), 0.5 ml, single dose (n = 92) |
|
| Outcomes | 1. Clinical symptoms within 10 days of vaccination 2. Diarrhoea and other gastrointestinal upsets within 5 months of vaccination |
|
| Notes | Study location: Finland No restriction on breastfeeding or bottle feeding were imposed Clinical symptoms: clinical evaluation (clinically significant diarrhoea defined as watery stools > 24 h) Laboratory studies: serology and stool analysis ELISA |
|
Vesikari 1985a.
| Methods | Randomization: predetermined scheme Allocation concealment: code unavailable until end of study Blinding: double (indistinguishable vaccine and placebo) Data collection: September 1983 to April 1985 Intention to treat: no Interim analysis: yes Exclusion from analysis: 19/347 were not followed until end of study Follow‐up period: 10 days after vaccination, 2 rotavirus season after vaccination |
|
| Participants | Age: 6 to 12 months Health status: healthy Breastfeeding: 50 to 75% Immunization status: no information |
|
| Interventions | 1. Bovine (RIT 4237), 10^8.1 TCID50, single dose (n = 168) 2. Placebo (uninfected primary monkey kidney cells), 0.5 ml, single dose (n = 163) |
|
| Outcomes | 1. Clinical symptoms within 10 days of vaccination 2. Diarrhoea and other gastrointestinal upsets within 2 rotavirus seasons of vaccination |
|
| Notes | Study location: Finland Clinical symptoms: clinical evaluation (clinically significant diarrhoea defined as diarrhoea requiring rehydration) Laboratory studies: serology and stool analysis by ELISA No specific orders concerning feeding |
|
Vesikari 1986a.
| Methods | Randomization: coded list of the 2 vaccines Allocation concealment: code not broken until end of trial Blinding: double (parents and paediatricians unaware of the code) Data collection: vaccination between 10 December 1984 and 17 December 1984, no further details Intention to treat: no Interim analysis: none Exclusion from analysis: 2/51 excluded for lost incomplete data Follow‐up period: 7 days after vaccination |
|
| Participants | Age: 8 months Health status: healthy Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Bovine (RIT 4237), 10^8.3 TCID50, single dose (n = 24) 2. Rhesus (MMU18006), 10^5 PFU, single dose (n = 25) Ordinary meal or breast milk given before vaccines |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination | |
| Notes | Study location: Finland Clinical symptoms: clinical evaluation, no definitions given Laboratory studies: serology by ELISA CF and plaque reduction neutralization assay; stool analysis by ELISA |
|
Vesikari 1987b.
| Methods | Randomization: done at pharmaceutical industry Allocation concealment: code kept at pharmaceutical industry until end of study Blinding: double (identical vaccine and placebo appearance). Data collection: enrolment at October 1984 Intention to treat: no information Interim analysis: yes Exclusion from analysis: no information Follow‐up period: 10 days after vaccination, 16 months after vaccination |
|
| Participants | Age: newborns Health status: healthy Breastfeeding: > 75% Immunization status: no other vaccine given before rotavirus vaccination |
|
| Interventions | 1. Bovine (RIT 4237), 10^8.3 TCID50, single dose (n = 119) 2. Placebo (uninfected tissue culture fluid), 0.6 ml, single dose (n = 120) Ordinary meal or breast milk given before vaccine or placebo |
|
| Outcomes | 1. Clinical symptoms within 10 days of vaccination 2. Diarrhoea (rotavirus or other) within 16 months |
|
| Notes | Study location: Finland Clinical symptoms: clinical evaluation, no definitions given Laboratory studies: stool analysis and serology by ELISA |
|
Vesikari 1990.
| Methods | Randomization: no details Allocation concealment: code kept at WHO until end of trial Blinding: double (identical vaccine and placebo appearance) Data collection: July 1985 to June 1987 Intention to treat: yes to efficacy, no to safety Interim analysis: none Exclusion from analysis: 5/200 did not finish safety follow up Follow‐up period: 7 days after vaccination, 2 rotavirus seasons after vaccination |
|
| Participants | Age: 2 to 5 months Health status: healthy Breastfeeding: > 75% Immunization status: no information |
|
| Interventions | 1. Rhesus (MMU18006), 10^4 PFU, single dose (n = 100). 2. Placebo (minimal essential medium), 1 ml, single dose (n = 100) 30 ml soy milk formula given before vaccine or placebo |
|
| Outcomes | 1. Clinical symptoms within 7 days of vaccination 2. Diarrhoea (rotavirus or other) within 2 rotavirus seasons |
|
| Notes | Study location: Finland Most of the infants were breastfed. Feeding withheld for 90 minutes before vaccination and 2 h after vaccination Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 unformed stools); severity score by Vesikary 1990 scale Laboratory studies: stool analysis by ELISA; serology by plaque reduction neutralization assay and tube neutralization assay |
|
Vesikari 1991a.
| Methods | Randomization: sequentially numbered code from 1 to 252 Allocation concealment: no details Blinding: double (identical vaccine and placebo appearance) Data collection: February 1985 to June 1987 Intention to treat: no Interim analysis: none Exclusion from analysis: 41/252 did not finish follow up Follow‐up period: 2 rotavirus seasons |
|
| Participants | Age: newborns Health status: healthy Breastfeeding: > 75% Immunization status: no information |
|
| Interventions | 1. Bovine (RIT 4237), 10^8.3 TCID50, 2 doses (n = 244 person years) 2. Placebo (uninfected tissue culture fluid), 2 doses (n = 217 person years) 0.5% sodium bicarbonate in 50 ml 5% glucose solution given before vaccine or placebo |
|
| Outcomes | 1. Diarrhoea (rotavirus or other) within 2 rotavirus seasons | |
| Notes | Study location: Finland All infants breastfed at first vaccination, 64% breastfed at second vaccination Clinical symptoms: clinical evaluation, severity score by Ruuska 1990 scale Laboratory studies: stool analysis by latex test and ELISA; serology by ELISA |
|
Vesikari 1991b.
| Methods | Randomization: computer‐generated randomization list Allocation concealment: code kept at WHO, not available until end of trial Blinding: double, no further details Data collection: October 1989 to June 1990 Intention to treat: yes for efficacy, no for safety Interim analysis: none Exclusion from analysis: 2/282 excluded from safety analysis. Follow‐up period: 7 days after vaccination, 6 to 8 months after vaccination |
|
| Participants | Age: 2 to 7 months Health status: healthy Breastfeeding: no information Immunization status: all other vaccinations not given within 2 weeks of rotavirus vaccination |
|
| Interventions | 1. Human (M37), 10^4 PFU, (n = 102) single dose 2. Human (M37), 10^5 PFU, (n = 39) single dose 3. Placebo (dilution medium), single dose (n = 141) 400 mg sodium bicarbonate in 30 ml soy milk formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination. 2. Efficacy: diarrhoea (rotavirus or other) within 6 to 8 months of vaccination |
|
| Notes | Study location: Finland Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 watery or loose stools in 24 h); severity score by Ruuska 1990 scale Laboratory studies: stool analysis by ELISA; serology by ELISA and plaque reduction neutralization assay |
|
Vesikari 1991c.
| Methods | Randomization: randomization list Allocation concealment: no details Blinding: no details Data collection: no information Intention to treat: no Interim analysis: none Exclusion from analysis: 6/89 excluded from analysis Follow‐up period: 7 days after vaccination |
|
| Participants | Age: 2 to 4 months Health status: healthy Breastfeeding: no information Immunization status: no information. |
|
| Interventions | 1. Rhesus + human monovalent vaccine (D x RRV or DS1 x RRV), 10^4 PFU, single dose (n = 61) 2. Placebo (dilution medium), single dose (n = 24) 400 mg sodium bicarbonate in 40 ml soy milk formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of vaccination | |
| Notes | Study location: Finland Clinical symptoms: clinical evaluation, no further details Laboratory studies: stool analysis by ELISA; serology by ELISA and plaque reduction neutralization assay |
|
Vesikari 1992.
| Methods | Randomization: computer‐generated randomization list Allocation concealment: code kept at WHO, not available until end of trial Blinding: double, no details Data collection: August 1987 to June 1989 Intention to treat: yes for efficacy, no for safety Interim analysis: none Exclusion from analysis: None Follow‐up period: 7 days after vaccination, 7 to 10 months after vaccination |
|
| Participants | Age: 2 to 5 months Health status: healthy Breastfeeding: no information Immunization status: no information |
|
| Interventions | 1. Rhesus + human monovalent vaccine (D x RRV or DS1 x RRV), 10^4 or 10^5 PFU, single dose (n = 239) 2. Placebo (essential medium), single dose (n = 120) 400 mg sodium bicarbonate in 30 ml soy milk formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 7 days of each dose 2. Efficacy: diarrhoea (rotavirus or other) within 7 to 10 months of vaccination |
|
| Notes | Study location: Finland Clinical symptoms: clinical evaluation (diarrhoea defined as ≥ 3 watery or loose stools in 24 h); severity score by Ruuska 1990 scale Laboratory studies: serology and stool analysis by ELISA |
|
Vesikari 1999.
| Methods | Randomization: schedule generated by pharmaceutical industry Allocation concealment: no details Blinding: double (code‐labelled package) Data collection: no data Intention to treat: no Interim analysis: none Exclusion from analysis: 6\249 did not receive 3 doses because of medical problems Follow‐up period: 5 days after each dose |
|
| Participants | Age: 2.5 to 3.5 months Health status: healthy Breastfeeding: no information Immunization status: pentavalent DTP + Hib + polio vaccine and HBV vaccine given with RRV‐TV |
|
| Interventions | 1. Rhesus + human tetravalent vaccine (RRV‐TV), 4 x 10^5 PFU, 3 doses (n = 126). 2. Placebo (identical preparation without vaccine), 3 doses (n = 123) 2.5 ml citric acid and bicarbonate given with vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 5 days of each dose | |
| Notes | Study location: Finland Clinical symptoms: clinical evaluation, no further details Laboratory studies: serology by ELISA Vaccine given with pentavalent (DTP + HiB + IPV) vaccine and hepatitis B vaccine |
|
Wright 1987.
| Methods | Randomization: no details Allocation concealment: code broken at the end of the trial, no further details Blinding: yes, no details Data collection: winter 1984 to spring 1986 Intention to treat: yes Interim analysis: none Exclusion from analysis: none Follow‐up period: 3 days before vaccination and 9 days after vaccination |
|
| Participants | Age: 4 to 70 months Health status: no information Breastfeeding: no information Immunization status: no information. |
|
| Interventions | 1. Rhesus (MMU18006), 3.6 x 10^4 to 7.5 x 10^6 TCID, single dose (n = 41) 2. Placebo (no details), single dose (n = 39) Sodium bicarbonate tablet or in formula given before vaccine or placebo |
|
| Outcomes | 1. Safety: clinical symptoms within 9 days of vaccination 2. Efficacy: fever, gastrointestinal illness, and upper respiratory illness during 2 winter seasons |
|
| Notes | Study location: USA Clinical symptoms: clinical evaluation (fever defined as ≥ 38.3 ºC; gastrointestinal illness defined as > 3 stools in 24 h or 1 vomiting in 24 h; upper respiratory tract illness defined as any respiratory sign leading to clinical impression of illness) Laboratory studies: stool analysis by cell culture and ELISA; serology by ELISA, complement fixation, immune adherence haemaglutination, and tube neutralization assays |
|
Vaccines: BCG: Bacillus Calmette‐Guerrin vaccine; DTP: diphtheria‐tetanus‐pertussis vaccine; HiB: haemophilus influenza B vaccine; IPV: intramuscular polio vaccine; MMR: measles‐mumps‐rubella vaccine; OPV: oral polio vaccine; RRV: rhesus rotavirus vaccine; RV: rotavirus vaccine; DTCP: diphtheria‐tetanus‐coqueluche‐polio; RRV‐TV: rhesus‐human reassortant rotavirus vaccine.
Institutions: NIH: National Institute of Health; WHO: World Health Organization.
Serology: PFU: plaque‐forming units; ELISA: enzyme‐linked immunosorbent assay; PCR: polymerase chain reaction; SPG: Shirasu porous glass; CF: complement fixation; TCID: tissue culture infectious doses; PAGE‐SS: polyacrylamide gel electrophoresis and silver stain.
IM vaccination: intra‐muscular vaccination; ICAAC: Interscience Conference on Antimicrobial Agents and Chemotherapy HBV: hepatitis B virus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ceyhan 1993 | Randomization of children to breastfeed or bottle feed, not to vaccine. Only collected immunological data. Study design: RCT. Study location: Turkey. |
| Christy 1986 | Not randomized. Study design: clinical trial. Study location: USA. |
| Clements‐Mann 2001 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Friedman 1993 | Not randomized, only collected immunological data. Study design: clinical trial. Study location: Israel. |
| Giammanco 1988 | Only collected immunological data. Study design: RCT. Study location: Italy. |
| Halsey 1988 | Not randomized. Study design: clinical trial. Study location: USA. |
| Isolauri 1995 | Randomization of children to receive Lactobacillus casei GG or not with the rotavirus vaccine. All children received the rotavirus vaccine. Study design: RCT. Study location: Finland. |
| Jalil 1991 | Not randomized. All children received the rotavirus vaccine. Study design: CCT. Study location: Pakistan. |
| Jin 1996 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Kobayashi 1993 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Kobayashi 1994 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Losonsky 1988 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Markwick 1998 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Midthun 1989 | Only collected immunological data. Study design: RCT. Study location: Venezuela. |
| Padilla 1992 | Only collected immunological data. Study design: clinical trial. Study location: USA. |
| Pang 1999a | Only collected immunological data. Study design: RCT. Study location: Finland. |
| Pang 1999b | Only collected immunological data. Study design: RCT. Study location: Finland. |
| Pang 1999c | Only collected immunological data. Study design: RCT. Study location: Finland. |
| Pang 1999d | Only collected immunological data. Study design: RCT. Study location: Finland. |
| Pickering 1995 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Simasathien 1994 | Only collected immunological data. Study design: RCT. Study location: Thailand. |
| Tajima 1990 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Ukae 1994 | Not randomized. Study design: CCT. Study location: Japan. |
| Vesikari 1983 | Not randomized. Study design: clinical trial. Study location: Finland. |
| Vesikari 1985b | Only collected immunological data. Study design: RCT. Study location: Finland. |
| Vesikari 1986b | Only collected immunological data. Study design: RCT. Study location: Finland. |
| Vesikari 1987a | Only collected immunological data. Study design: RCT. Study location: Yugoslavia. |
| Vodopija 1986 | Only collected immunological data. Study design: RCT. Study location: Yugoslavia. |
| Wright 1991 | Only collected immunological data. Study design: RCT. Study location: USA. |
| Zoppi 1986 | Randomization of children to receive milk or soy formula, not to vaccine. Only collected immunological data. Study design: RCT. Study location: Italy. |
| Zoppi 1989 | Randomization of children to receive milk or soy formula, not to vaccine. Only collected immunological data. Study design: RCT. Study location: Italy. |
RCT: randomized controlled trial CCT: controlled clinical trial (not randomized)
Characteristics of studies awaiting assessment [ordered by study ID]
Seth 2000.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Vesikari 2002.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Differences between protocol and review
1. Objectives: we re‐worded the objectives to make them clearer.
2. Change in interventions. We added: W179‐9 (live‐attenuated bovine rotavirus vaccine); QHBRV (live‐attenuated bovine rotavirus vaccine); 89‐12 (human attenuated rotavirus vaccine); and other rotavirus vaccines (BIRVI, Lamb RV). We removed NCDV (live‐attenuated bovine rotavirus vaccine); WC‐QV (live‐attenuated bovine rotavirus vaccine); and M37 (human attenuated rotavirus vaccine).
3. Types of outcome measures. We re‐worded the following outcomes to make them clearer:
"Number of people who developed diarrhoea from rotavirus infection" becomes "Rotavirus diarrhoea: episodes".
"Number of deaths from rotavirus infection" becomes "Death: from rotavirus infection".
"Hospitalization with rotavirus infection" becomes "Rotavirus diarrhoea: episodes requiring hospitalization"
"Diarrhoea from any cause" becomes "All‐cause diarrhoea: episodes".
"Diarrhoea requiring hospitalization" becomes "All‐cause diarrhoea: episodes requiring hospitalization".
"Diarrhoea requiring intravenous infusion" becomes "Rotavirus diarrhoea: episodes requiring re‐hydration" and "All‐cause diarrhoea: episodes requiring re‐hydration"
The outcome "Death from diarrhoea" is no longer used (incorporated in the other 'death' outcomes).
4. The following outcomes are used in the review but were not pre‐specified in the protocol, because severity of rotavirus vaccine was measured in different ways and we thought that it would be useful to provide all information: rotavirus diarrhoea: severe episodes; rotavirus diarrhoea: episodes of more than four days duration; all‐cause diarrhoea: episodes during first week after vaccine; and all‐cause diarrhoea: severe episodes.
5. Elad Goldberg and Leonard Leibovici joined the review team. Prof Shai Ashkenazi left the review team.
Contributions of authors
Karla Soares‐Weiser: protocol writing, searching, trial selection, data extraction and assimilation, statistical analyses, and review writing. Elad Goldberg: trial selection, data extraction and assimilation, and review writing. Ghandi Tamimi: protocol writing; location of information on rare adverse events. Femi Pitan: protocol writing. Leonard Leibovici: data extraction and assimilation (as third author to resolve disagreements), statistical analyses, and review writing.
Sources of support
Internal sources
Al‐Ahli Hospital, Palestine Authority, Not specified.
Lagos University Teaching Hospital, Nigeria.
Rabin Medical Center, Beilinson Campus, Israel.
External sources
Nuffield Trust, UK.
Cochrane Child Health Field Bursary Scheme, Canada.
Declarations of interest
None declared.
Unchanged
References
References to studies included in this review
Anderson 1986 {published data only}
- Anderson E, Belshe R, Bartram J, Crookshanks‐Newman F, Chanock R, Kapikian A. Evaluation of rhesus rotavirus vaccine (MMU 18006) in infants and young children. Journal of Infectious Diseases 1986;153(5):823‐31. [DOI] [PubMed] [Google Scholar]
Barnes 1997 {published data only}
- Barnes G, Lund J, Adams L, Mora A, Mitchell S, Caples A, et al. Phase 1 trial of a candidate rotavirus vaccine (RV3) derived from a human neonate. Journal of Paediatrics and Child Health 1997;33(4):300‐4. [DOI] [PubMed] [Google Scholar]
Barnes 2002 {published data only}
- Barnes GL, Lund JS, Mitchell SV, Bruyn L, Piggford L, Smith AL, et al. Early phase II trial of human rotavirus vaccine candidate RV3. Vaccine 2002;20(23‐24):2950‐6. [DOI] [PubMed] [Google Scholar]
Bernstein 1990 {published data only}
- Bernstein D, Sander D, Smith V, Schiff G, Ward R. Protection from rotavirus reinfection: 2‐year prospective study. Journal of Infectious Diseases 1991;164(2):277‐83. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Smith V, Sander D, Pax K, Schiff G, Ward R. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. Journal of Infectious Diseases 1990;162(5):1055‐62. [DOI] [PubMed] [Google Scholar]
- Ward R, Bernstein D. Protection against rotavirus disease after natural rotavirus infection. Journal of Infectious Diseases 1994;169(4):900‐4. [DOI] [PubMed] [Google Scholar]
Bernstein 1995 {published data only}
- Bernstein D, Glass R, Rodgers G, Davidson B, Sack D. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. US Rotavirus Vaccine Efficacy Group. Journal of the American Medical Association 1995;273(15):1191‐6. [PubMed] [Google Scholar]
- Ward R, Bernstein D. Lack of correlation between serum rotavirus antibody titers and protection following vaccination with reassortant RRV vaccines. US Rotavirus Vaccine Efficacy Group. Vaccine 1995;13(13):1226‐32. [DOI] [PubMed] [Google Scholar]
Bernstein 1998 {published and unpublished data}
- Bernstein D, Smith V, Sherwood J, Schiff G, Sander D, DeFeudis D, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89‐12. Vaccine 1998;16(4):381‐7. [DOI] [PubMed] [Google Scholar]
Bernstein 1999 {published and unpublished data}
- Bernstein D, Sack D, Rothstein E, Reisinger K, Smith V, O'Sullivan D, et al. Efficacy of live, attenuated, human rotavirus vaccine 89‐12 in infants: a randomised placebo‐controlled trial. Lancet 1999;354(9175):287‐90. [DOI] [PubMed] [Google Scholar]
- Bernstein DI, Sack DA, Reisinger K, Rothstein E, Ward RL. Second‐year follow‐up evaluation of live, attenuated human rotavirus vaccine 89‐12 in healthy infants. Journal of Infectious Diseases 2002;186(10):1487‐9. [DOI] [PubMed] [Google Scholar]
Bresee 2001 {published data only}
- Bresee JS, Arifeen S, Azim T, Chakraborty J, Mounts AW, Podder G, et al. Safety and immunogenicity of tetravalent rhesus‐based rotavirus vaccine in Bangladesh. Pediatric Infectious Disease Journal 2001;20(12):1136‐43. [DOI] [PubMed] [Google Scholar]
Christy 1988 {published and unpublished data}
- Christy C, Madore H, Pichichero M, Gala C, Pincus P, Vosefski D, et al. Field trial of rhesus rotavirus vaccine in infants. Pediatric Infectious Disease Journal 1988;7(9):645‐50. [DOI] [PubMed] [Google Scholar]
Christy 1993 {published and unpublished data}
- Christy C, Offit P, Clark H, Treanor J. Evaluation of a bovine‐human rotavirus reassortant vaccine in infants. Journal of Infectious Diseases 1993;168(6):1598‐9. [DOI] [PubMed] [Google Scholar]
Clark 1988 {published data only}
- Clark H, Borian F, Bell L, Modesto K, Gouvea V, Plotkin S. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. Journal of Infectious Diseases 1988;158(3):570‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 1990 {published data only}
- Clark H, Borian F, Plotkin S. Immune protection of infants against rotavirus gastroenteritis by a serotype 1 reassortant of bovine rotavirus WC3. Journal of Infectious Diseases 1990;161(6):1099‐104. [DOI] [PubMed] [Google Scholar]
Clark 1995 {published data only}
- Clark H, Offit P, Ellis R, Eiden J, Krah D, Shaw A, et al. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. Journal of Infectious Diseases 1996;174 Suppl 1:73‐80. [DOI] [PubMed] [Google Scholar]
- Clark H, White C, Offit P, Stinson D, Eiden J, Weaver S, et al. Preliminary evaluation of safety and efficacy of quadrivalent human‐bovine reassortant rotavirus vaccine [abstract]. Pediatric Research 1995;37:172A. [Google Scholar]
Clements‐Mann 1999 {published data only}
- Clements‐Mann M, Makhene M, Mrukowicz J, Wright P, Hoshino Y, Midthun K, et al. Safety and immunogenicity of live attenuated human‐bovine (UK) reassortant rotavirus vaccines with VP7‐specifiity for serotypes 1, 2, 3 or 4 in adults, children and infants. Vaccine 1999;17(20‐21):2715‐25. [DOI] [PubMed] [Google Scholar]
Dagan 1992 {published data only}
- Dagan R, Kassis I, Sarov B, Midthun K, Davidson B, Vesikari T, Sarovi I. Safety and immunogenicity of oral tetravalent human‐rhesus reassortant rotavirus vaccine in neonates. Pediatric Infectious Diseases Journal 1992;11(12):991‐6. [DOI] [PubMed] [Google Scholar]
- Dagan R, Kassis I, Sarov B, Midthun K, Sarov I. Safety and immunogenicity of oral rhesus rotavirus tetravalent vaccine (ORRTV) in neonates. Pediatric Research 1991;29:117. [DOI] [PubMed] [Google Scholar]
De Mol 1986 {published data only}
- Mol P, Zissis G, Butzler J, Mutwewingabo A, Andre FE. Failure of live, attenuated oral rotavirus vaccine. Lancet 1986;2(8498):108. [DOI] [PubMed] [Google Scholar]
Dennehy 1996 {published and unpublished data}
- Dennehy PH, Rodgers GC Jr, Ward RL, Markwick AJ, Zito ET. Comparative evaluation of reactogenicity and immunogenicity of two dosages of oral tetravalent rhesus rotavirus vaccine. US Rhesus Rotavirus Vaccine Study Group. Pediatric Infectious Disease Journal 1996;15(11):1012‐8. [DOI] [PubMed] [Google Scholar]
Eichelberger 2002 {published data only}
- Eichelberger M, Sperber E, Wagner M, Hoshino Y, Dudas R, Hodgins V, et al. Clinical evaluation of a single oral dose of human‐bovine (UK) reassortant rotavirus vaccines Wa x UK (P1A[8],G6) and Wa x (DS‐1 x UK) (P1A[8],G2). Journal of Medical Virology 2002;66(3):407‐16. [DOI] [PubMed] [Google Scholar]
Escobar 1988 {published data only}
- Escobar Castro H, Perdomo Giraldi M, Tamariz‐Martel Moreno A, Suarez Cortina L. Oral vaccination with live attenuated rotavirus [Vacunacion oral con rotavirus vivo atenuado]. Anales Espanoles de Pediatria 1988;28(6):527‐9. [PubMed] [Google Scholar]
Flores 1988 {published data only}
- Flores J, Daoud G, Daoud N, Puig M, Martinez M, Perez‐Schael I, et al. Reactogenicity and antigenicity of rhesus rotavirus vaccine (MMU‐18006) in newborn infants in Venezuela. Pediatric Infectious Disease Journal 1988;7(11):776‐80. [DOI] [PubMed] [Google Scholar]
- Kapikian AZ, Flores J, Midthun K, Hoshino Y, Green KY, Gorziglia M, et al. Strategies for the development of a rotavirus vaccine against infantile diarrhea with an update on clinical trials of rotavirus vaccines. Advances in Experimental Medicine and Biology 1989;257:67‐89. [DOI] [PubMed] [Google Scholar]
Flores 1989 {published data only}
- Flores J, Perez‐Schael I, Blanco M, Vilar M, Garcia D, Perez M, et al. Reactions to and antigenicity of two human‐rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. Journal of Clinical Microbiology 1989;27(3):512‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flores 1990 {published data only}
- Flores J, Perez‐Schael I, Blanco M, White L, Garcia D, Vilar M, et al. Comparison of reactogenicity and antigenicity of M37 rotavirus vaccine and rhesus‐rotavirus‐based quadrivalent vaccine. Lancet 1990;336(8711):330‐4. [DOI] [PubMed] [Google Scholar]
Flores 1993 {published data only}
- Flores J, Perez‐Schael I, Blanco M, Rojas AM, Alfonzo E, Crespo I, et al. Reactogenicity and immunogenicity of a high‐titer rhesus rotavirus‐based quadrivalent rotavirus vaccine. Journal of Clinical Microbiology 1993;31(9):2439‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Georges‐Courbot 1991 {published and unpublished data}
- Georges‐Courbot MC, Monges J, Siopathis MR, Roungou JB, Gresenguet G, Bellec L, et al. Evaluation of the efficacy of a low‐passage bovine rotavirus (strain WC3) vaccine in children in Central Africa. Research in Virology 1991;142(5):405‐11. [DOI] [PubMed] [Google Scholar]
Gothefors 1989 {published and unpublished data}
- Gothefors L, Wadell G, Juto P, Taniguchi K, Kapikian A, Glass RI. Prolonged efficacy of rhesus rotavirus vaccine in Swedish children. Journal of Infectious Diseases 1989;159(4):753‐7. [DOI] [PubMed] [Google Scholar]
Hanlon 1987 {published data only}
- Hanlon P, Hanlon L, Marsh V, Byass P, Shenton F, Hassan‐King M, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet 1987;1(8546):1342‐5. [DOI] [PubMed] [Google Scholar]
Ho 1989 {published and unpublished data}
- Ho MS, Floyd RL, Glass RI, Pallansch MA, Jones B, Hamby B, et al. Simultaneous administration of rhesus rotavirus vaccine and oral poliovirus vaccine: immunogenicity and reactogenicity. Pediatric Infectios Disease Journal 1989;8(10):692‐6. [DOI] [PubMed] [Google Scholar]
Ing 1991 {published and unpublished data}
- Ing DJ, Glass RI, Woods PA, Simonetti M, Pallansch MA, Wilcox WD, et al. Immunogenicity of tetravalent rhesus rotavirus vaccine administered with buffer and oral polio vaccine. American Journal of Diseases of Children 1991;145(8):892‐7. [DOI] [PubMed] [Google Scholar]
Joensuu 1997 {published data only}
- Joensuu J, Koskenniemi E, Pang X, Vesikari T. Randomised placebo‐controlled trial of rhesus‐human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet 1997;350(9086):1205‐9. [DOI] [PubMed] [Google Scholar]
- Joensuu J, Koskenniemi E, Vesikari T. Prolonged efficacy of rhesus‐human reassortant rotavirus vaccine. Pediatric Infectious Disease Journal 1998;17(5):427‐9. [DOI] [PubMed] [Google Scholar]
- Joensuu J, Koskenniemi E, Vesikari T. Symptoms associated with rhesus‐human reassortant rotavirus vaccine in infants. Pediatric Infectious Disease Journal 1998;17(4):334‐40. [DOI] [PubMed] [Google Scholar]
- Takala A, Koskenniemi E, Joensuu J, Makela M, Vesikari T. Economic evaluation of rotavirus vaccinations in Finland: randomized, double‐blind, placebo‐controlled trial of tetravalent rhesus rotavirus vaccine. Clinical Infectious Diseases 1998;27(2):272‐82. [DOI] [PubMed] [Google Scholar]
- Vesikari T. New vaccine is effective against rotavirus‐induced diarrhea. American Family Physician 1997;55(4):1337. [Google Scholar]
Lanata 1989 {published and unpublished data}
- Lanata C, Black R, del‐Aguila R, Gil A, Verastegui H, Gerna G, et al. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. Journal of Infectious Diseases 1989;159(3):452‐9. [DOI] [PubMed] [Google Scholar]
Lanata 1996a {published and unpublished data}
- Lanata C, Black R, Flores J, Lazo F, Butron B, Linhares A, et al. Immunogenicity, safety and protective efficacy of one dose of the rhesus rotavirus vaccine and serotype 1 and 2 human‐rhesus rotavirus reassortants in children from Lima, Peru. Vaccine 1996;14(3):237‐43. [DOI] [PubMed] [Google Scholar]
Lanata 1996b {published and unpublished data}
- Lanata CF, Black RE, Flores J, Lazo F, Butron B, Linares A, et al. Safety, immunogenicity, and protective efficacy of one and three doses of the tetravalent rhesus rotavirus vaccine in infants in Lima, Peru. Journal of Infectious Diseases 1996;174(2):268‐75. [DOI] [PubMed] [Google Scholar]
Linhares 1996 {published and unpublished data}
- Linhares AC, Gabbay YB, Mascarenhas JD, Freitas RB, Oliveira CS, Bellesi N, et al. Immunogenicity, safety and efficacy of tetravalent rhesus‐human, reassortant rotavirus vaccine in Belem, Brazil. Bulletin of the World Health Organization 1996;74(5):491‐500. [PMC free article] [PubMed] [Google Scholar]
- Linhares AC, Lanata CF, Hausdorff WP, Gabbay Y, Black RE. Reappraisal of the Peruvian and Brazilian lower titer tetravalent rhesus‐human reassortant rotavirus vaccine efficacy trials: analysis by severity of diarrhea. Pediatric Infectious Disease Journal 1999;18(11):1001‐6. [DOI] [PubMed] [Google Scholar]
Madore 1992 {published data only}
- Madore H, Christy C, Pichichero M, Long C, Pincus P, Vosefsky D, et al. Field trial of rhesus rotavirus or human‐rhesus rotavirus reassortant vaccine of VP7 serotype 3 or 1 specificity in infants. The Elmwood, Panorama, and Westfall Pediatric Groups. Journal of Infectious Diseases 1992;166(2):235‐43. [DOI] [PubMed] [Google Scholar]
Maldonado 1986 {published and unpublished data}
- Maldonado Y, Hestvik L, Wilson M, Townsend T, O'Hare J, Wee S, et al. Safety and immunogenicity of bovine rotavirus vaccine RIT 4237 in 3‐month‐old infants. Journal of Pediatrics 1986;109(6):931‐5. [DOI] [PubMed] [Google Scholar]
Mutz 1989 {published and unpublished data}
- Mutz I, Krainer F, Deutsch J, Kunz C, Teuwen D. A trial of RIT‐4237 rotavirus vaccine in 1‐month‐old infants. European Journal of Pediatrics 1989;148(7):634‐5. [DOI] [PubMed] [Google Scholar]
Perez‐Schael 1990a {published and unpublished data}
- Flores J, Perez‐Schael I, Gonzalez M, Garcia D, Perez M, Daoud N, et al. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet 1987; Vol. 1, issue 8538:882‐4. [DOI] [PubMed]
- Perez‐Schael I, Garcia D, Gonzalez M, Gonzalez R, Daoud N, Perez M, et al. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. Journal of Medical Virology 1990;30(3):219‐29. [DOI] [PubMed] [Google Scholar]
- Perez‐Schael I, Garcia D, White L, Blanco M, Gonzalez R, Urbina G, et al. Field tests for anti‐rotavirus vaccine carried out in Venezuela [Pruebas de campo de una vacuna anti‐rotavirus realizadas en Venezuela (1985‐1989)]. Archivos Venezolanos de Puericultura y Pediatria 1990;53 Suppl(2):29‐36. [Google Scholar]
- Perez‐Schael I, Gonzalez M, Daoud N, Perez M, Soto I, Garcia D, et al. Reactogenicity and antigenicity of the rhesus rotavirus vaccine in Venezuelan children. Journal of Infectious Diseases 1987;155(2):334‐8. [DOI] [PubMed] [Google Scholar]
Perez‐Schael 1990c {published data only}
- Perez‐Schael I, Blanco M, Vilar M, Garcia D, White L, Gonzalez R, et al. Clinical studies of a quadrivalent rotavirus vaccine in Venezuelan infants. Journal of Clinical Microbiology 1990;28(3):553‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Schael 1994 {published and unpublished data}
- Perez‐Schael I, Blanco M, Garcia D, White L, Alfonzo E, Crespo I, et al. Evaluation of the antigenicity and reactogenicity of varying formulations of the rhesus rotavirus‐based quadrivalent and the M37 rotavirus vaccine candidates. Journal of Medical Virology 1994;42(4):330‐7. [DOI] [PubMed] [Google Scholar]
Perez‐Schael 1997 {published and unpublished data}
- Perez‐Schael I, Guntinas MJ, Perez M, Pagone V, Rojas AM, Gonzalez R, et al. Efficacy of the rhesus rotavirus‐based quadrivalent vaccine in infants and young children in Venezuela. New England Journal of Medicine 1997;337(17):1181‐7. [DOI] [PubMed] [Google Scholar]
Perez‐Schael 2002 {unpublished data only}
- Perez‐Schael I, Salinas B, Linhares A, Guerrero M, Ruiz‐Palacios G, Clemens S, Jacquet J, Vos B. Protective Efficacy Of An Oral Human Rotavirus (HRV) Vaccine In Latin American Infants. 42nd Annual ICAAC. San Diego, California, 2002:18, LB‐23.
Pichichero 1990 {published data only}
- Pichichero ME, Losonsky GA, Rennels MB, Disney FA, Green JL, Francis AB, et al. Effect of dose and a comparison of measures of vaccine take for oral rhesus rotavirus vaccine. The Maryland Clinical Studies Group. Pediatric Infectious Disease Journal 1990;9(5):339‐44. [DOI] [PubMed] [Google Scholar]
Pichichero 1993 {published data only}
- Pichichero ME, Losonsky GA. Asymptomatic infections due to wild‐type rotavirus may prime for a heterotypic response to vaccination with rhesus rotavirus. Clinical Infectious Diseases 1993;16(1):86‐92. [DOI] [PubMed] [Google Scholar]
- Pichichero ME, Marsocci SM, Francis AB, Green JL, Disney FA, Rennels MB, et al. A comparative evaluation of the safety and immunogenicity of a single dose of unbuffered oral rhesus rotavirus serotype 3, rhesus/human reassortant serotypes 1, 2 and 4 and combined (tetravalent) vaccines in healthy infants. Vaccine 1993;11(7):747‐53. [DOI] [PubMed] [Google Scholar]
Rennels 1987 {published data only}
- Losonsky GA, Rennels MB, Kapikian AZ, Midthun K, Ferra PJ, Fortier DN, et al. Safety, infectivity, transmissibility and immunogenicity of rhesus rotavirus vaccine (MMU 18006) in infants. Pediatric Infectious Disease 1986;5(1):25‐9. [DOI] [PubMed] [Google Scholar]
- Rennels MB, Losonsky GA, Levine MM, Kapikian AZ. Preliminary evaluation of the efficacy of rhesus rotavirus vaccine strain MMU 18006 in young children. Pediatric Infectious Disease 1986;5(5):587‐8. [DOI] [PubMed] [Google Scholar]
- Rennels MB, Losonsky GA, Shindledecker CL, Hughes TP, Kapikian AZ, Levine MM. Immunogenicity and reactogenicity of lowered doses of rhesus rotavirus vaccine strain MMU 18006 in young children. Pediatric Infectious Disease Journal 1987;6(3):260‐4. [DOI] [PubMed] [Google Scholar]
Rennels 1990 {published data only}
- Rennels MB, Losonsky GA, Young AE, Shindledecker CL, Kapikian AZ, Levine MM. An efficacy trial of the rhesus rotavirus vaccine in Maryland. The Clinical Study Group. American Journal of Diseases of Children 1990;144(5):601‐4. [DOI] [PubMed] [Google Scholar]
Rennels 1995 {published data only}
- Rennels MB, Wasserman SS, Glass RI, Keane VA. Comparison of immunogenicity and efficacy of rhesus rotavirus reassortant vaccines in breastfed and nonbreastfed children. US Rotavirus Vaccine Efficacy Group. Pediatrics 1995;96(6):1132‐6. [PubMed] [Google Scholar]
Rennels 1996 {published data only}
- Dennehy PH, Rodgers GC Jr, Ward RL, Markwick AJ, Mack M, Zito ET. Comparative evaluation of reactogenicity and immunogenicity of two dosages of oral tetravalent rhesus rotavirus vaccine. US Rhesus Rotavirus Vaccine Study Group. Pediatric Infectious Disease Journal 1996;15(11):1012‐8. [DOI] [PubMed] [Google Scholar]
- Griffiths RI, Anderson GF, Powe NR, Oliveras E, Herbert RJ, Grant CC, et al. Economic impact of immunization against rotavirus gastroenteritis. Evidence from a clinical trial. Archives of Pediatrics & Adolescent Medicine 1995;149(4):407‐14. [DOI] [PubMed] [Google Scholar]
- Rennels MB, Glass RI, Dennehy PH, Bernstein DI, Pichichero ME, Zito ET, et al. Safety and efficacy of high‐dose rhesus‐human reassortant rotavirus vaccines‐‐report of the National Multicenter Trial. United States Rotavirus Vaccine Efficacy Group. Pediatrics 1996;97(1):7‐13. [PubMed] [Google Scholar]
- Rennels MB, Ward RL, Mack ME, Zito ET. Concurrent oral poliovirus and rhesus‐human reassortant rotavirus vaccination: effects on immune responses to both vaccines and on efficacy of rotavirus vaccines. The US Rotavirus Vaccine Efficacy Group. Journal of Infectious Diseases 1996;173(2):306‐13. [DOI] [PubMed] [Google Scholar]
Ruuska 1990 {published data only}
- Ruuska T, Vesikari T, Delem A, Andre F, Beards G, Flewett T. Evaluation of RIT 4237 bovine rotavirus vaccine in newborn infants: correlation of vaccine efficacy to season of birth in relation to rotavirus epidemic period. Scandinavian Journal of Infectious Diseases 1990;22(3):269‐78. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Ruuska T, Delem A, Andre F. Neonatal rotavirus vaccination with RIT 4237 bovine rotavirus vaccine: a preliminary report. Pediatric Infectious Disease Journal 1987;6(2):164‐9. [DOI] [PubMed] [Google Scholar]
Santosham 1991a {published data only}
- Santosham M, Letson GW, Wolff M, Reid R, Gahagan S, Adams R, et al. A field study of the safety and efficacy of two candidate rotavirus vaccines in a Native American population. Journal of Infectious Diseases 1991;163(3):483‐7. [DOI] [PubMed] [Google Scholar]
Santosham 1991b {published data only}
- Santosham M, Letson GW, Wolff M, Reid R, Gahagan S, Adams R, et al. A field study of the safety and efficacy of two candidate rotavirus vaccines in a Native American population. Journal of Infectious Diseases 1991;163(3):483‐7. [DOI] [PubMed] [Google Scholar]
Santosham 1997 {published and unpublished data}
- Santosham M, Moulton LH, Reid R, Croll J, Weatherholt R, Ward R, et al. Efficacy and safety of high‐dose rhesus‐human reassortant rotavirus vaccine in Native American populations. Journal of Pediatrics 1997;131(4):632‐8. [DOI] [PubMed] [Google Scholar]
Senturia 1987 {published and unpublished data}
- Senturia YD, Peckham CS, Cordery M, Chrystie IA, Banatvala JE, Andre FE. Live attenuated oral rotavirus vaccine. Lancet 1987;2(8567):1091‐2. [DOI] [PubMed] [Google Scholar]
Treanor 1995 {published data only}
- Clark HF, Offit PA, Ellis RW, Eiden JJ, Krah D, Shaw AR, et al. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. Journal of Infectious Diseases 1996;174 Suppl 1:73‐80. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Clark HF, Pichichero M, Christy C, Gouvea V, Shrager D, et al. Evaluation of the protective efficacy of a serotype 1 bovine‐human rotavirus reassortant vaccine in infants. Pediatric Infectious Disease Journal 1995;14(4):301‐7. [DOI] [PubMed] [Google Scholar]
Vesikari 1984 {published data only}
- Vesikari T, Isolauri E, D'Hondt E, Delem A, Andre FE. Increased "take" rate of oral rotavirus vaccine in infants after milk feeding. Lancet 1984;2(8404):700. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Isolauri E, D'Hondt E, Delem A, Andre FE, Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet 1984;1(8384):977‐81. [DOI] [PubMed] [Google Scholar]
Vesikari 1985a {published data only}
- Vesikari T. Rotavirus infections and their prevention by vaccination. Antiviral Research 1985;Suppl 1:293‐300. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Isolauri E, Delem A, d'Hondt E, Andre FE, Beards GM, et al. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. Journal of Pediatrics 1985;107(2):189‐94. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Isoulari E, Andre F. Protection of children for two years against rotavirus diarrhea by RIT 4237 (NCDV) rotavirus vaccine. In: Holmgren J, Linderg A, Mollby R editor(s). Development of vaccines and drugs against diarrhea. 11th Nobel Conference, Stockolm 1985. Lund, Sweden: Studentlitteratur, 1986:189‐94. [Google Scholar]
Vesikari 1986a {published data only}
- Vesikari T, Kapikian AZ, Delem A, Zissis G. A comparative trial of rhesus monkey (RRV‐1) and bovine (RIT 4237) oral rotavirus vaccines in young children. Journal of Infectious Diseases 1986;153(5):832‐9. [DOI] [PubMed] [Google Scholar]
Vesikari 1987b {published data only}
- Vesikari T, Ruuska T, Delem A, Andre FE. Neonatal rotavirus vaccination with RIT 4237 bovine rotavirus vaccine: a preliminary report. Pediatric Infectious Disease Journal 1987;6(2):164‐9. [DOI] [PubMed] [Google Scholar]
Vesikari 1990 {published data only}
- Vesikari T, Rautanen T, Varis T, Beards GM, Kapikian AZ. Rhesus Rotavirus candidate vaccine. Clinical trial in children vaccinated between 2 and 5 months of age. American Journal of Diseases of Children 1990;144(3):285‐9. [PubMed] [Google Scholar]
Vesikari 1991a {published data only}
- Vesikari T, Ruuska T, Delem A, Andre FE, Beards GM, Flewett TH. Efficacy of two doses of RIT 4237 bovine rotavirus vaccine for prevention of rotavirus diarrhoea. Acta Paediatrica Scandinavica 1991;80(2):173‐80. [DOI] [PubMed] [Google Scholar]
Vesikari 1991b {published data only}
- Vesikari T, Ruuska T, Koivu HP, Green KY, Flores J, Kapikian AZ. Evaluation of the M37 human rotavirus vaccine in 2‐ to 6‐month‐old infants. Pediatric Infectious Disease Journal 1991;10(12):912‐7. [DOI] [PubMed] [Google Scholar]
Vesikari 1991c {published data only}
- Vesikari T, Varis T, Green K, Flores J, Kapikian AZ. Immunogenicity and safety of rhesus‐human rotavirus reassortant vaccines with serotype 1 or 2 VP7 specificity. Vaccine 1991;9(5):334‐9. [DOI] [PubMed] [Google Scholar]
Vesikari 1992 {published data only}
- Vesikari T, Ruuska T, Green KY, Flores J, Kapikian AZ. Protective efficacy against serotype 1 rotavirus diarrhea by live oral rhesus‐human reassortant rotavirus vaccines with human rotavirus VP7 serotype 1 or 2 specificity. Pediatric Infectious Disease Journal 1992;11(7):535‐42. [DOI] [PubMed] [Google Scholar]
Vesikari 1999 {published data only}
- Vesikari T, Joensuu J, Baer M, Kayhty H, Olander RM, Sormunen H, et al. Concurrent administration of rhesus rotavirus tetravalent (RRV‐TV) vaccine with pentavalent diphtheria‐pertussis‐tetanus‐Haemophilus influenzae beta‐inactivated polio and hepatitis B vaccines. Acta Paediatrica 1999;88(5):513‐20. [PubMed] [Google Scholar]
Wright 1987 {published data only}
- Wright PF, Tajima T, Thompson J, Kokubun K, Kapikian A, Karzon DT. Candidate rotavirus vaccine (rhesus rotavirus strain) in children: an evaluation. Pediatrics 1987;80(4):473‐80. [PubMed] [Google Scholar]
References to studies excluded from this review
Ceyhan 1993 {published data only}
- Ceyhan M, Kanra G, Secmeer G, Midthun K, Davidson BL, Zito ET, et al. Take of rhesus‐human reassortant tetravalent rotavirus vaccine in breast‐fed infants. Acta Paediatrica 1993;82(3):223‐7. [DOI] [PubMed] [Google Scholar]
Christy 1986 {published data only}
- Christy C, Madore HP, Treaner JJ, Pray K, Kapikian AZ, Chanock RM, et al. Safety and immunogenicity of live attenuated rhesus monkey rotavirus vaccine. Journal of Infectious Diseases 1986;154(6):1045‐7. [DOI] [PubMed] [Google Scholar]
Clements‐Mann 2001 {published data only}
- Clements‐Mann ML, Dudas R, Hoshino Y, Nehring P, Sperber E, Wagner M, et al. Safety and immunogenicity of live attenuated quadrivalent human‐bovine (UK) reassortant rotavirus vaccine administered with childhood vaccines to infants. Vaccine 2001;19(32):4676‐84. [DOI] [PubMed] [Google Scholar]
Friedman 1993 {published data only}
- Friedman MG, Segal B, Zedaka R, Sarov B, Margalith M, Bishop R, et al. Serum and salivary responses to oral tetravalent reassortant rotavirus vaccine in newborns. Clinical and Experimental Immunology 1993;92(2):194‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giammanco 1988 {published data only}
- Giammanco G, Grandi V, Lupo L, Mistretta A, Pignato S, Teuween D, et al. Interference of oral poliovirus vaccine on RIT 4237 oral rotavirus vaccine. European Journal of Epidemiology 1988;4(1):121‐3. [DOI] [PubMed] [Google Scholar]
Halsey 1988 {published data only}
- Halsey NA, Anderson EL, Sears SD, Steinhoff M, Wilson M, Belshe RB, et al. Human‐rhesus reassortant rotavirus vaccines: safety and immunogenicity in adults, infants, and children. Journal of Infectious Diseases 1988;158(6):1261‐7. [DOI] [PubMed] [Google Scholar]
- Midthun K, Halsey NA, Jett‐Goheen M, Clements ML, Steinhoff M, King JC, et al. Safety and immunogenicity of human rotavirus vaccine strain M37 in adults, children, and infants. Journal of Infectious Diseases 1991;164(4):792‐6. [DOI] [PubMed] [Google Scholar]
Isolauri 1995 {published data only}
- Isolauri, E, Joensuu, J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 1995;13(3):310‐2. [DOI] [PubMed] [Google Scholar]
Jalil 1991 {published data only}
- Jalil F, Zaman S, Carlsson B, Glass RI, Kapikian AZ, Mellander L, et al. Immunogenicity and reactogenicity of rhesus rotavirus vaccine given in combination with oral or inactivated poliovirus vaccines and diphteria‐tetanus‐pertussis vaccine. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991;85(2):292‐6. [DOI] [PubMed] [Google Scholar]
Jin 1996 {published data only}
- Jin Q, Ward RL, Knowlton DR, Gabbay YB, Linhares AC, Rappaport R, et al. Divergence of VP7 genes of G1 rotaviruses isolated from infants vaccinated with reassortant rhesus rotaviruses. Archives of Virology 1996;141(11):2057‐76. [DOI] [PubMed] [Google Scholar]
Kobayashi 1993 {published data only}
- Kobayashi M, Araki K, Thompson J, Tollefson SJ, Wright PF. Sequential administration of two human‐rhesus rotavirus reassortant strains of VP7 serotype 1 and 2 specificity to infants and young children. Journal of Infectious Diseases 1993;167(3):748‐52. [DOI] [PubMed] [Google Scholar]
Kobayashi 1994 {published data only}
- Kobayashi M, Thompson J, Tollefson SJ, Reed GW, Wright PF. Tetravalent rhesus rotavirus vaccine in young infants. Journal of Infectious Diseases 1994;170(5):1260‐3. [DOI] [PubMed] [Google Scholar]
Losonsky 1988 {published data only}
- Losonsky GA, Rennels MB, Lim Y, Krall G, Kapikian AZ, Levine MM. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatric Infectious Disease 1988;7(6):388‐93. [DOI] [PubMed] [Google Scholar]
Markwick 1998 {published data only}
- Markwick AJ, Rennels MB, Zito ET, Wade MS, Mack ME. Oral tetravalent rotavirus vaccine can be successfully coadministered with oral poliovirus vaccine and a combined diphtheria, tetanus, pertussis and Haemophilus influenzae type b vaccine. US Rhesus Rotavirus Vaccine Study Group. Pediatric Infectious Disease Journal 1998;17(10):913‐8. [DOI] [PubMed] [Google Scholar]
Midthun 1989 {published data only}
- Midthun K, Pang LZ, Flores J, Kapikian AZ. Comparison of immunoglobulin A (IgA), IgG, and IgM enzyme‐linked immunosorbent assays, plaque reduction neutralization assay, and complement fixation in detecting seroresponses to rotavirus vaccine candidates. Journal of Clinical Microbiology 1989;27(12):2799‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Padilla 1992 {published data only}
- Padilla‐Noriega L, Fiore L, Rennels M, Losonsky G, Mackow E, Greenberg H. Humoral immune responses to VP4 and its cleavage products VP5* and VP8* in infants vaccinated with rhesus rotavirus. Journal of Clinical Microbiology 1992;30(6):1392‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pang 1999a {published data only}
- Pang XL, Joensuu J, Hoshino Y, Kapikian AZ, Vesikari T. Rotaviruses detected by reverse transcription polymerase chain reaction in acute gastroenteritis during a trial of rhesus‐human reassortant rotavirus tetravalent vaccine: implications for vaccine efficacy analysis. Journal of Clinical Virology 1999;13(1‐2):9‐16. [DOI] [PubMed] [Google Scholar]
Pang 1999b {published data only}
- Pang XL, Vesikari T. Human astrovirus‐associated gastroenteritis in children under 2 years of age followed prospectively during a rotavirus vaccine trial. Acta Paediatrica 1999;88(5):532‐6. [DOI] [PubMed] [Google Scholar]
Pang 1999c {published data only}
- Pang XL, Koskenniemi E, Joensuu J, Vesikari T. Effect of rhesus rotavirus vaccine on enteric adenovirus‐‐associated diarrhea in children. Journal of Pediatric Gastroenterology and Nutrition 1999;29(3):366‐9. [DOI] [PubMed] [Google Scholar]
Pang 1999d {published data only}
- Pang XL, Joensuu J, Vesikari T. Human calicivirus‐associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trial. Pediatric Infectious Disease Journal 1999;18(5):420‐6. [DOI] [PubMed] [Google Scholar]
Pickering 1995 {published data only}
- Pickering LK, Morrow AL, Herrera I, O'Ryan M, Estes MK, Guilliams SE, et al. Effect of maternal rotavirus immunization on milk and serum antibody titers. Journal of Infectious Diseases 1995;172(3):723‐8. [DOI] [PubMed] [Google Scholar]
Simasathien 1994 {published data only}
- Migasena S, Simasathien S, Samakoses R, Pitisuttitham P, Sangaroon P, Steenis G, et al. Simultaneous administration of oral rhesus‐human reassortant tetravalent (RRV‐TV) rotavirus vaccine and oral poliovirus vaccine (OPV) in Thai infants. Vaccine 1995;13(2):168‐74. [DOI] [PubMed] [Google Scholar]
- Simasathien S, Migasena S, Samakoses R, Pitisuttitham P, Sangaroon P, Aree C, et al. Vaccination of Thai infants with rhesus‐human reassortant tetravalent oral rotavirus vaccine. Pediatric Infectious Disease Journal 1994;13(7):590‐6. [DOI] [PubMed] [Google Scholar]
Tajima 1990 {published data only}
- Tajima T, Thompson J, Wright PF, Kondo Y, Tollefson SJ, King J, et al. Evaluation of a reassortant rhesus rotavirus vaccine in young children. Vaccine 1990;8(1):70‐4. [DOI] [PubMed] [Google Scholar]
Ukae 1994 {published data only}
- Ukae S, Nakata S, Adachi N, Kogawa K, Chiba S. Efficacy of rhesus rotavirus vaccine MMU‐18006 against gastroenteritis due to serotype 1 rotavirus. Vaccine 1994;12(10):933‐9. [DOI] [PubMed] [Google Scholar]
Vesikari 1983 {published data only}
- Vesikari T, Isolauri E, Delem A, D'Hondt E, Andre FE, Zissis G. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet 1983;2(8354):807‐11. [DOI] [PubMed] [Google Scholar]
Vesikari 1985b {published data only}
- Vesikari T, Ruuska T, Bogaerts H, Delem A, Andre F. Dose‐response study of RIT 4237 oral rotavirus vaccine in breast‐fed and formula‐fed infants. Pediatric Infectious Disease 1985;4(6):622‐5. [DOI] [PubMed] [Google Scholar]
Vesikari 1986b {published data only}
- Vesikari T, Ruuska T, Delem A, Andre FE. Oral rotavirus vaccination in breast‐ and bottle‐fed infants aged 6 to 12 months. Acta Paediatrica Scandinavica 1986;75(4):573‐8. [DOI] [PubMed] [Google Scholar]
Vesikari 1987a {published data only}
- Vesikari T, Rautanen T, Isolauri E, Delem A, Andre FE. Immunogenicity and safety of a low passage level bovine rotavirus candidate vaccine RIT 4256 in human adults and young infants. Vaccine 1987;5(2):105‐8. [DOI] [PubMed] [Google Scholar]
Vodopija 1986 {published data only}
- Vodopija I, Baklaic Z, Vlatkovic R, Bogaerts H, Delem A, Andre FE. Combined vaccination with live oral polio vaccine and the bovine rotavirus RIT 4237 strain. Vaccine 1986;4(4):233‐6. [DOI] [PubMed] [Google Scholar]
Wright 1991 {published and unpublished data}
- Wright PF, King J, Araki K, Kondo Y, Thompson J, Tollefson SJ, et al. Simultaneous administration of two human‐rhesus rotavirus reassortant strains of VP7 serotype 1 and 2 specificity to infants and young children. Journal of Infectious Diseases 1991;164(2):271‐6. [DOI] [PubMed] [Google Scholar]
Zoppi 1986 {published data only}
- Zoppi G, Ferrarini G, Rigolin F, Bogaerts H, Andre FE. Response to RIT 4237 oral rotavirus vaccine in breast‐fed and formula‐fed infants. Helvetica Paediatrica Acta 1986;41(3):203‐8. [PubMed] [Google Scholar]
Zoppi 1989 {published data only}
- Zoppi G, Mantovanelli F, Pittschieler K, Delem A, Teuwen DE. Response to RIT 4237 oral rotavirus vaccine in human milk, adapted‐and soy‐formula fed infants. Acta Paediatrica Scandinavica 1989;78(5):759‐62. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Seth 2000 {published data only}
- Seth R, Mohan A. Efficacy of monovalent human rotavirus vaccine 89‐12 in infants. National Medical Journal of India 2000;13(5):253‐4. [PubMed] [Google Scholar]
Vesikari 2002 {published data only}
- Vesikari T, Karvonen A, Espo M, Korhonen T, Delem A, Vos B. Efficacy evaluation of an oral human rotavirus (HRV) vaccine in previously uninfected Finnish infants. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy. San Diego, CA, 2002.
Additional references
AAP 1998
- American Academy of Pediatrics. Prevention of rotavirus disease: guidelines for use of rotavirus vaccine. Pediatrics 1998;102(6):1483‐91. [DOI] [PubMed] [Google Scholar]
Altman 2002
- Altman DG, Deeks JJ. Meta‐analysis, Simpson's paradox, and the number needed to treat. BMC Medical Research Methodology 2002;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bresee 1999
- Bresee JS, Glass RI, Ivanoff B, Gentsch JR. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine 1999;17(18):2207‐22. [DOI] [PubMed] [Google Scholar]
Cale 2002
- Cale CM, Klein NJ. The link between rotavirus vaccination and intussusception: implications for vaccine strategies. Gut 2002;50(1):11‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 1999
- Center for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine‐‐United States, 1998‐1999. Journal of American Medical Association 1999;282(6):520‐1. [PubMed] [Google Scholar]
CDC 1999a
- Center for Disease Control and Prevention. Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports 1999;48(RR‐2):1‐20. [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman A, editors. Optimal search strategy for RCTs. Cochrane Reviewers' Handbook 4.2.0 [updated March 2003]; Appendix C. In: The Cochrane Library [database on CDROM]. The Cochrane Collaboration. Chichester: Wiley; 2003, issue 3.
Cunliffe 2002
- Cunliffe NA, Bresee JS, Hart CA. Rotavirus vaccines: development, current issues and future prospects. Journal of Infection 2002;45(1):1‐9. [DOI] [PubMed] [Google Scholar]
Flores 1987
- Flores J, Perez‐Schael I, Gonzalez M, Garcia D, Perez M, Daoud N, et al. Protection against severe rotavirus diarrhoea by rhesus rotavirus vaccine in Venezuelan infants. Lancet 1987;1(8538):882‐4. [DOI] [PubMed] [Google Scholar]
Glass 1999
- Glass RI, Bresee JS, Parashar UD, Holman RC, Gentsch JR. First rotavirus vaccine licensed: is there really a need?. Acta Paediatrica. Supplement 1999;88(426):2‐8. [DOI] [PubMed] [Google Scholar]
Griffiths 1995
- Griffiths RI, Anderson GF, Powe NR, Oliveras E, Herbert RJ, Grant CC, et al. Economic impact of immunization against rotavirus gastroenteritis. Evidence from a clinical trial. Archives of Pediatrics & Adolescent Medicine 1995;149(4):407‐14. [DOI] [PubMed] [Google Scholar]
Henchal 1996
- Henchal LS, Midthun K, Goldenthal KL. Selected regulatory and scientific topics for candidate rotavirus vaccine development. Journal of Infectious Diseases 1996;174 Suppl 1:112‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency is preferable to testing for heterogeneity in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobson 2001
- Jacobson RM, Adegbenro A, Pankratz VS, Poland GA. Adverse events and vaccination‐the lack of power and predictability of infrequent events in pre‐licensure study. Vaccine 2001;19(17‐19):2428‐33. [DOI] [PubMed] [Google Scholar]
Jones 2001
- Jones T. Rotavirus vaccine AVANT/GlaxoSmithKline. Current Opinion in Investigational Drugs 2001;2(7):893‐5. [PubMed] [Google Scholar]
Josefson 1997
- Josefson D. Rotavirus vaccine is effective in developing countries. BMJ 1997;315:1111‐6. [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in healthcare: Assessing the quality of controlled trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matson 1990
- Matson DO, Estes MK. Impact of rotavirus infection at a large at a large pediatric hospital. Journal of Infectious Diseases 1990;162(3):598‐604. [DOI] [PubMed] [Google Scholar]
Merck 1999
- Merck, Co. Rotavirus W179‐9 vaccine. Drugs in R&D 1999;2(3):220‐1. [DOI] [PubMed] [Google Scholar]
MMWR 1999a
- MMWR. Intussusception among recipients of rotavirus vaccine‐‐United States, 1998‐1999. Morbidity and mortality weekly report 1999;48(27):577‐81. [PubMed] [Google Scholar]
MMWR 1999b
- MMWR. Withdrawal of rotavirus vaccine recommendation. Morbidity and mortality weekly report 1999;48(43):1007. [PubMed] [Google Scholar]
Murphy 2001
- Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, et al. Intussusception among infants given an oral rotavirus vaccine. New England Journal of Medicine 2001;344(8):564‐72. [DOI] [PubMed] [Google Scholar]
Murphy 2002
- Murphy TV, Gargiullo PM, Wharton M. More on rotavirus vaccination and intussusception. New England Journal of Medicine 2002;346(3):211‐2. [DOI] [PubMed] [Google Scholar]
Newcombe 1998
- Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Statistics in Medicine 1998;17(8):873‐90. [DOI] [PubMed] [Google Scholar]
Niu 2001
- Niu MT, Erwin DE, Braun MM. Data mining in the US Vaccine Adverse Event Reporting System (VAERS): early detection of intussusception and other events after rotavirus vaccination. Vaccine 2001;19(32):4627‐34. [DOI] [PubMed] [Google Scholar]
Perez‐Schael 1990b
- Perez‐Schael I, Blanco M, Vilar M, Garcia D, White L, Gonzalez R, et al. Clinical studies of a quadrivalent rotavirus vaccine in Venezuelan infants. Journal of Clinical Microbiology 1990;28(3):553‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Schael 2001
- Perez‐Schael I. Future of research into rotavirus vaccine. Developing countries must apply mathematics to take their own decisions. BMJ 2001;322(7278):106‐7. [PubMed] [Google Scholar]
Peter 2002
- Peter G, Myers MG, National Vaccine Advisory Committee, National Vaccine Program Office. Intussusception, rotavirus, and oral vaccines: summary of a workshop. Pediatrics 2002;110(6):1‐6. [DOI] [PubMed] [Google Scholar]
Rennels 1998
- Rennels MB, Parashar UD, Holman RC, Le CT, Chang HG, Glass RI. Lack of an apparent association between intussusception and wild or vaccine rotavirus infection. Pediatric Infectious Disease Journal 1998;17(10):924‐5. [DOI] [PubMed] [Google Scholar]
Tucker 1998
- Tucker AW, Haddix AC, Bresee JS, Holman RC, Parashar UD, Glass RI. Cost‐effectiveness analysis of a rotavirus immunization program for the United States. Journal of American Medical Association 1998;279(17):1371‐6. [DOI] [PubMed] [Google Scholar]
Verstraeten 2001
- Verstraeten T, Baughman AL, Cadwell B, Zanardi L, Haber P, Chen RT, Vaccine Adverse Event Reporting System Team. Enhancing vaccine safety surveillance: a capture‐recapture analysis of intussusception after rotavirus vaccination. American Journal of Epidemiology 2001;154(11):1006‐12. [DOI] [PubMed] [Google Scholar]
Vesikari 1997
- Vesikari T. Rotavirus vaccines against diarrhoeal disease. Lancet 1997;350(9090):1538‐41. [DOI] [PubMed] [Google Scholar]
Weijer 2000
- Weijer C. The future of research into rotavirus vaccine. BMJ 2000;321(7260):525‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1995
- World Health Organization. Diarrhoeal Disease Control Programme. The treatment of diarrhoea: a manual for physicians and other senior health workers. 3rd Edition. Geneva: World Health Organization, 1995. [Google Scholar]
WHO 2003
- World Health Organization. Child and Adolescent Health and Development. Proportional mortality among children under fives, regions [2001]. http://www.who.int/child‐adolescent‐health/OVERVIEW/CHILD_HEALTH/map_01_regions.jpg (accessed 23 October 2003).
WHO/UNICEF 2002
- World Health Organization. Dept. of Vaccines and Biologicals. State of the world's vaccines and immunization. Geneva: World Health Organization, 2002. [Google Scholar]
World Bank 2003
- The World Bank Group. Data & statistics: country group. http://www.worldbank.org/data/countryclass/classgroups.htm (accessed 23 October 2003).
Zanardi 2001
- Zanardi LR, Haber P, Mootrey GT, Niu MT, Wharton M. Intussusception among recipients of rotavirus vaccine: reports to the vaccine adverse event reporting system. Pediatrics 2001;107(6):E97. [DOI] [PubMed] [Google Scholar]


