Abstract
Background
Oral rehydration solution (ORS) has reduced childhood deaths from diarrhoea in many countries. Recent studies suggest that the currently recommended formulation of ORS recommended by the World Health Organization (WHO) may not be optimal, and solutions that contain lower concentrations of sodium and glucose may be more effective.
Objectives
To compare reduced osmolarity ORS with WHO standard ORS in children with acute diarrhoea.
Search methods
CENTRAL (The Cochrane Library, Issue 3, 2004), MEDLINE (1966 to July 2004), EMBASE (1988 to July 2004), and Current Contents (July 2004) were searched. Additional trials were identified by hand searching. Content experts were contacted.
Selection criteria
Randomized controlled trials comparing reduced osmolarity ORS with the WHO standard ORS formulation. The primary outcome was unscheduled intravenous fluid infusion. Secondary outcomes were measures of clinical illness.
Data collection and analysis
Two reviewers extracted data. We tested for heterogeneity using the Chi‐square statistic, conducted sensitivity analysis by allocation concealment, and the regression approach to assess funnel plot asymmetry from selective trial publication.
Main results
The primary outcome, unscheduled intravenous fluid infusion, was reported in 11 trials. In a meta‐analysis of 8 trials, reduced osmolarity ORS was associated with fewer unscheduled intravenous fluid infusions compared with WHO standard ORS (Mantel Haenzel odds ratio 0.59, 95% confidence interval 0.45 to 0.79) with no evidence for heterogeneity between trials. No unscheduled intravenous fluid infusion therapy was required in any participant in three trials.
Eleven trials reported stool output, and data suggested less stool output in the reduced osmolarity ORS group. Vomiting was less frequent in the reduced osmolarity group in the six trials reporting this. Six trials sought hyponatraemia, with events in three studies, but no obvious difference between the two arms.
Authors' conclusions
In children admitted to hospital with diarrhoea, reduced osmolarity ORS when compared to WHO standard ORS is associated with fewer unscheduled intravenous fluid infusions, lower stool volume post randomization, and less vomiting. No additional risk of developing hyponatraemia when compared with WHO standard ORS was detected.
Keywords: Child, Preschool; Humans; Infant; Bicarbonates; Dehydration; Dehydration/etiology; Dehydration/therapy; Diarrhea; Diarrhea/complications; Fluid Therapy; Fluid Therapy/methods; Glucose; Osmolar Concentration; Potassium Chloride; Rehydration Solutions; Rehydration Solutions/therapeutic use; Sodium Chloride
Reduced osmolarity oral rehydration solution for treating dehydration caused by acute diarrhoea in children
Children with diarrhoea lose body water and sometimes become dehydrated. A solution of sugar and salt dissolved in water is widely used to treat dehydration caused by diarrhoea. This reviews shows that a solution of lower osmolarity than the current international standard means fewer children subsequently require an intravenous drip.
Background
Diarrhoea remains a leading cause of childhood death in middle and low income countries. The main complication is dehydration, which was treated with intravenous fluid infusion until the early 1960s. Oral rehydration solution (ORS) is now the mainstay of therapy and is particularly useful when intravenous fluids are in short supply, health services are basic, and there is a shortage of skilled personnel (Almroth 1995). The combination of salt and sugar enhances fluid absorption because sodium and glucose transport in the small intestine are coupled, and glucose promotes absorption of both sodium ions and water (Fordtran 1968). Diarrhoea is caused by derangement of fluid absorption and secretion from the gut, and coupling sodium and glucose allows absorption, even during active fluid secretion due to infection. Thus rehydration can take place even with large fluid losses, as seen in enterotoxic diarrhoea, such as that caused by cholera or infection with Escherichia coli (Guarino 2001).
ORS has proved both safe and effective worldwide in hospital settings, and is now widely used in the home to prevent dehydration (Mahalanabis 1973, Grant 1983).For more than two decades, the World Health Organization (WHO) has recommended the standard formulation of glucose‐based ORS with 90 mmol/L of sodium and 111 mmol/L of glucose and a total osmolarity of 311 mmol/L. It remains unclear however, whether this is the optimum level of sodium. Laboratory work suggests that lower concentrations of sodium and glucose enhance solute induced water absorption (Farthing 1988, Hunt 1992). Papers report patients experiencing blood sodium levels above the normal of 150 mmol/L with standard solution (Finberg 1973).
The objective of this review is to critically appraise and evaluate all relevant randomized controlled trials addressing comparative effects of reduced osmolarity ORS with WHO standard ORS. One potential adverse effect of reduced osmolarity ORS is a deficiency of sodium is the blood (hyponatraemia), which can give rise to convulsions. We are also exploring the risk of this adverse outcome through trial and observational data.
We confined the review to children, as they are most vulnerable to dehydration and electrolyte imbalance from diarrhoea, and are the targets for large primary care child investments that include ORS sachet distribution. Severity, duration, and volume of diarrhoea are often primary outcomes in clinical ORS studies, but we sought a pragmatic outcome relevant to health providers. ORS aims to rehydrate children and avoid the need for intravenous fluid infusion. We therefore identified unscheduled intravenous fluid infusion as a primary outcome as this represents failed oral therapy.
Objectives
To compare reduced osmolarity oral rehydration solution with the World Health Organization recommended strength for treating diarrhoea in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials, defined as a trial in which the subjects followed were assigned prospectively to one of two or more interventions by random allocation. This excludes quasi‐randomized designs.
Types of participants
Children with acute diarrhoea (history of less than 5 days).
Types of interventions
Experimental: Reduced osmolarity oral rehydration solution (total osmolarity 250 mmol/L or less with reduced sodium).
Control: World Health Organization standard oral rehydration solution (90 mmol/L sodium, 111mmol/L glucose, total osmolarity 311 mmol/L).
Types of outcome measures
Primary outcomes
Need for unscheduled intravenous fluid infusion during the course of treatment.
Secondary outcomes
Stool output.
Children vomiting during rehydration.
Asymptomatic hyponatraemia (defined as serum sodium less than 130mmol/L) during follow up.
Search methods for identification of studies
We used the following search terms to search all trial registers and electronic databases: child; diarrhoea; fluid therapy; oral rehydration; osmolar; and rehydration solutions.
We searched the following trial register: Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 3, 2004).
We searched the following electronic databases: MEDLINE (1966 to July 2004); EMBASE (1988 to July 2004); and Current Contents (July 2004).
We also checked the citations of existing reviews and trial reports.
For unpublished data and ongoing trials, we contacted current researchers and key agencies, including the World Health Organization, the Centers for Disease Control and Prevention, Atlanta (USA), and the International Centre for Diarrhoeal Disease Research, Bangladesh.
Data collection and analysis
Selection of studies
SH and SK independently applied the inclusion criteria to all identified trials, and differences were resolved by discussion with the PG.
Data extraction and management
SH and SK extracted data on relevant outcome measures using a standardized data abstraction form.
Assessment of risk of bias in included studies
Each included trial was assessed in terms of adequacy of concealment of allocation, generation of allocation sequence, blinding, and follow up of patients, using the guidelines of the Cochrane Infectious Diseases Group. Studies excluded were detailed in the 'Characteristics of excluded studies'.
Data synthesis
We used the Mantel‐Haenszel odds ratio (OR) for binary outcomes. The odds ratios were not estimated when neither intervention group found any event, which are indicated in the MetaView figures. We used the Standardized Mean Difference (SMD) for continuous outcomes. We combined studies using a fixed effect method. For all estimates, we calculated 95% confidence intervals. We tested statistical heterogeneity using Chi‐square statistic with a P‐value less than 0.1 indicating statistical significance. We had prespecified potential sources of heterogeneity for analysis. We examined publication bias using a funnel plot, and a regression approach (Egger 1997) to assess funnel plot asymmetry. We conducted a sensitivity analysis in relation to adequate allocation concealment.
After presentation of the results, an expert consultation group from the World Health Organization recommended a stratified analysis by mmol sodium (less than 75 mmol and 75 to 85 mmol), and this analysis is now included (WHO 2001).
Results
Description of studies
We identified 41 studies for inclusion, and 16 studies met the inclusion criteria. The progress through the stages of meta‐analysis, using the process suggested in the QUOROM statement (QUOROM Group 1999), is shown below.
41 studies comparing oral rehydration solution (ORS) formulation for treating diarrhoea.
6 excluded as not randomized controlled trials (RCTs);
35 remaining RCTs of ORS comparing formulation for treating diarrhoea patients.
9 excluded as intervention was something other than reduced osmolarity ORS;
26 remaining RCTs reporting reduced osmolarity ORS in one treatment arm.
6 excluded if control group did not use World Health Organization (WHO) standard ORS;
20 remaining RCTs reporting comparison of reduced osmolarity ORS with WHO standard ORS.
2 excluded as not in children;
18 remaining RCTs reporting comparison of reduced osmolarity ORS with WHO standard ORS for treating children with diarrhoea.
2 excluded as no relevant outcomes reported;
16 remaining RCTs reporting comparison of reduced osmolarity ORS with WHO standard ORS in children with diarrhoea in relation to need of unscheduled intravenous fluid infusion therapy and some measures of clinical illness.
2 excluded (Mexico 1988, Mexico 1990b) as they appear to be duplicates of a third trial (Mexico 1990a). We have contacted the authors, but while awaiting clarification, we have included only the paper with the largest number of patients (Mexico 1990a).
13 remaining RCTs. As one paper reported on two trials, one in the USA and one in Panama, we present these as separate studies (Panama 1982, USA 1982).
This leaves a total of 14 included studies. These were from Egypt (2), Bangladesh (3), Mexico (1), Colombia (1), India (3), Panama (1), and the USA (1). Two other studies were multicentre trials; one was conducted in Brazil, India, Mexico, and Peru, and the other in Bangladesh, Brazil, India, Peru, and Vietnam.
Participants were children with acute non‐cholera diarrhoea in all trials except three, which included cholera patients (Bangladesh 1995b, CHOICE 2001, India 2000b). In all but one which included children up to 5 years old (India 2000a), the participants' ages ranged between 1 and 36 months. All children had some degree of clinical dehydration. One trial treated all children on day 1 with intravenous fluid infusion, and those still producing 80 ml/kg/24h were then randomized (Bangladesh 1995a). In five trials (CHOICE 2001, India 2000b, Panama 1982, WHO 1995, USA 1982) severely dehydrated children were included. Five trials included malnourished children (Bangladesh 1995b, Colombia 2000, Bangladesh 1995b, India 2000a, India 2000b, Mexico 1990a). The number of breastfed children was reported in eight trials (Bangladesh 1995a, Bangladesh 1995b, Bangladesh 1996a, CHOICE 2001, Colombia 2000, Egypt 1996b, India 2000b, WHO 1995). Fully weaned children were included in one trial (Egypt 1994).
We deviated slightly from the osmolarity definitions in our peer refereed protocol published in The Cochrane Library. For reduced osmolarity, we had defined this to be lower than 250 mmol/L, but some studies defined this as higher, and we therefore extended our limit to a total osmolarity of 270 mmol/L. For the WHO standard ORS, defined as a total osmolarity of 311 mmol/L, we also included two studies that used a slightly different WHO standard ORS with a total osmolarity of 331 mmol/L but with the same sodium and glucose combination (Panama 1982, USA 1982). All but two trials used a glucose based reduced osmolarity ORS; one used sucrose (Bangladesh 1996a), and one used L‐alanine with glucose (Bangladesh 1995a).
Risk of bias in included studies
Allocation
All of the studies were randomized controlled trials. Nine reported methods that assured adequate allocation concealment (WHO 1995, CHOICE 2001, Colombia 2000, Egypt 1996b, Bangladesh 1995a, Bangladesh 1995b, Bangladesh 1996a, India 2000a, India 2000b).
Blinding
Six studies (CHOICE 2001, Egypt 1996b, Bangladesh 1995b, Bangladesh 1996a, India 2000a, India 2000b) were double blinded. One of the Mexico studies was described as single blinded (Mexico 1990b), but this study is currently excluded from the analysis as it is thought to be a subset of patients reported in another paper which is included, where no details of blinding are given (Mexico 1990a). Eight studies did not mention blinding.
Inclusion of all randomized participants
Included trials had losses to follow up of less than 10% of randomized participants in all cases.
Effects of interventions
Meta‐analyses of the four outcomes are illustrated in the MetaView summary analysis.
Information for the primary outcome of the need for unscheduled intravenous fluid infusion was found in 11 trials (n = 1996). In the meta‐analysis of 8 trials, a statistically significant reduction for unscheduled intravenous infusion for participants receiving reduced osmolarity oral rehydration solution (ORS) when compared with World Health Organization (WHO) standard ORS was demonstrated (odds ratio 0.59, 95% confidence interval 0.45 to 0.79). 3 of the 11 trials reported that none of their patients needed intravenous fluid infusion in either group, and the odds ratios were not calculated for these trials.
11 trials reported stool output during rehydration. These trials measured stool output in various ways using different units. We therefore used the standardized mean difference to analyse these data. Since the stool output in diarrhoeal disease showed a positive skewed distribution with clinical improvement, we used a log‐normal approximation. The pooled standardized mean difference in the log scale is ‐0.23 (95% confidence interval ‐0.33 to ‐0.14), which suggests that the reduced osmolarity ORS resulted in significantly less stool output when compared with the WHO standard ORS. Data from one trial (India 2000a) were not combined with the others in the meta‐analysis because this trial measured stool output for a much longer period beyond rehydration phase. The individual results of all 12 trials are summarized in Appendix 1.
For children vomiting during rehydration, six trials reported these data. The tendency was for fewer patients to vomit in the reduced osmolarity ORS group (Odds ratio 0.71, 95% confidence interval 0.55 to 0.92).
For presence of hyponatraemia, six trials reported this outcome. Three of these six trials did not observe hyponatraemia in any participants, irrespective of their allocated group. The meta‐analysis of three trials, during which participants developed hyponatraemia, showed no significant difference between the groups (odds ratio 1.45, 95% confidence interval 0.93 to 2.26).
We tested for statistical heterogeneity of treatment effect across trials using the Chi‐square statistic for all meta‐analyses, and the statistic is presented in each meta‐analysis diagram. Results suggest no evidence of statistical heterogeneity (P‐value > 0.1) for any outcome considered.
A funnel plot was prepared with the primary outcome. The regression method used to assess funnel plot asymmetry yielded an intercept of ‐0.104 with a P‐value of 0.12, indicating no significant evidence of publication bias.
Sensitivity analysis carried out included studies where allocation concealment was clearly described as adequate and suggested little difference from the original meta‐analysis. For example, the pooled odds ratios of the seven trials for the primary outcome with adequate allocation concealment was 0.61 (95% confidence interval 0.46 to 0.82).
A stratified analysis by sodium content of the ORS is presented. Hyponatraemia was not detected in the three studies examining the very low sodium ORS.
Discussion
We intended to examine treatment effects in cholera subgroup compared with non‐cholera diarrhoea. A Cochrane Review of rice‐based rehydration compared with glucose oral rehydration solution (ORS) showed that rice water was associated with lower stool volumes in cholera patients but not in diarrhoea from other causes (Fontaine 2000). The available data were insufficient however. Three studies (CHOICE 2001, Bangladesh 1995b, India 2000b) involved cholera patients, but a subgroup analysis for cholera patients was not available for meta‐analysis. There were two studies (Farugue 1996, Alam 1999) in patients with cholera excluded from this review because they were in adults. Any recommendation for rehydration treatment for adults with cholera will need to take these and any other trials found through careful systematic searching into account.
This review examines trials of children admitted to hospital who were dehydrated because of diarrhoea. The trials do not provide any direct evidence for or against the use of ORS at home to prevent dehydration developing; nor do they provide any direct evidence that reduced osmolarity ORS is more effective in preventing dehydration in home‐based care in this group.
We stand by our selection of unscheduled intravenous fluid infusion rather than volume of diarrhoea as the primary outcome, as specified in the original protocol. Some specialists consider that volume of diarrhoea is more appropriate, probably because it reflects the animal and human perfusion experiments that provide part of the rationale for a reduced osmolarity ORS. Unscheduled intravenous fluid infusion is pragmatic; it provides a measure of failed oral rehydration and has implications for the healthcare resources. For these reasons, we preserved this as the primary outcome.
When we reviewed the studies for inclusion, most trials reported unscheduled intravenous fluid infusion in the details of trial implementation, where exclusions and dropouts were described. As this was identified as our primary outcome at the protocol stage, we sought out these data and presented them as the primary analysis, and it is our opinion this shows a clear effect. This highlights the value for careful attention to the protocol for a systematic review before examining the trials, and provides an illustration of how non‐specialist viewpoints can actually help in obtaining practical and useful answers from meta‐analysis.
We found that reduced osmolarity ORS has beneficial effects over the WHO standard ORS in reducing needs for unscheduled intravenous fluid infusion, stool output during rehydration, and the number of patients with vomiting during oral rehydration treatment. Reduced osmolarity ORS has no further risk of developing hyponatraemia as compared to the WHO standard ORS. We are currently exploring the feasibility of obtaining data on convulsions (as evidence of symptomatic hyponatraemia) for the authors of the largest trial (CHOICE 2001).
The research evidence presented here relates to the ORS used for treating children with dehydration. ORS is used much more widely for preventing dehydration developing in children with diarrhoea. While this seems appropriate, the applicability to prevention is a judgement, and highlights the need for a systematic review to examine the policies of ORS provision and ORS formula in preventing dehydration in children with diarrhoea.
Findings from this review indicate reduced osmolarity ORS is more effective than WHO standard ORS in the first line treatment of dehydration in children with diarrhoea. It is not easy to be sure however, that this finding applies to a subgroup of patients with severe diarrhoea caused by cholera, where electrolyte loss is profound. This could increase the risk of hyponatraemia, result in adverse clinical events, and attenuate the advantages of reduced osmolarity ORS.
There is the possibility that policymakers and clinicians will judge that cholera reverses the balance of benefits and harms (that is, hyponatraemia will be more common, and outweigh the advantages of reduced osmolarity solution). If this is the case, then one option is to provide WHO standard ORS for people with suspected cholera, or in areas where cholera is prevalent. This is likely to be a logistical problem in areas where diarrhoea is common and coexists with cholera. The single formula sachet aids implementation of this lifesaving intervention. Providing different formulations complicates distribution. It means health workers have a more complicated task in providing the appropriate ORS. These factors may actually impair the effective delivery of any ORS to children.
Policymakers need to be careful if they decide against change a shift to reduced osmolarity solution in areas where cholera is common because of a putative risk around hyponatraemia. If they do this, then they are obliged to prove or disprove their belief in the superiority of WHO standard ORS through a randomized controlled trial in children with clinical cholera. The WHO has convened a expert working group to consider this review and related evidence. The group recommended that ORS for treating diarrhoea in children with non‐cholera diarrhoea will be enhanced by shifting to a reduced osmolarity ORS, and propose a global shift to ORS with an osmolarity of 75 mEq/L of sodium (WHO 2001).
Authors' conclusions
Oral rehydration solution (ORS) has saved many children's lives in low and middle income countries, and the sachets are widely used in primary care, based on standards set by the World Health Organization (WHO). This review summarized data from existing studies, and provide some evidence that dehydrated children given a solution with a lower osmolarity were less likely to need an intravenous infusion than those given WHO standard ORS. These results have important implications for policy, and WHO and UNICEF, based on this review, related data, and expert discussions, are recommending reduced osmolarity ORS be accepted as standard (WHO 2001).
We found insufficient data on cholera in children to make recommendations for this condition. Since cholera is a secretory diarrhoea and electrolyte loss is profound, if reduced osmolarity ORS is to be used in cholera, more trials to investigate this should be undertaken.
There is a need for a good systematic review examining the influence of policies of ORS provision in preventing dehydration and hospital admissions in children with diarrhoea.
Acknowledgements
To: Christopher Duggan; Olivier Fontaine; Sheila Bird for comments. We note that the data presented and the views expressed are entirely the responsibility of the authors.
Appendices
Appendix 1. Stool output
| Trials | Outcome | Value | ORS | Differences | |
| Low osmolarity | WHO standard | ||||
| WHO 1995 | Stool output at 24 h (g/kg) | Geometric mean | n = 221; mean = 65; 95% confidence interval 58 to 73 | n = 218; mean = 86; 95% confidence interval 77 to 96 | Ratio standard/reduced = 1.32 95% confidence interval 1.12 to 1.54 |
| India 2000a | Stool output during observation period (g/kg/d) | Arithmetic mean | n = 33; mean = 61.0; standard deviation = 24.5 | n = 37; mean = 75.0; standard deviation = 29.4 | — |
| Egypt 1996b | Stool output for rehydration phase (g/kg) | Geometric mean | n = 94; mean = 11; 95% confidence interval 8 to 14 | n = 96; mean = 15; 95% confidence interval 12 to 20 | — |
| Bangladesh 1995a | Stool output at 0 to 24 h (ml/kg) | Arithmetic mean | n = 19; mean = 156; standard deviation = 113.4 | n = 19; mean = 193; standard deviation = 71.2 | — |
| Colombia 2000 | Stool output for rehydration period (g/kg/h) | Arithmetic mean | n = 71; mean = 5.6; standard deviation = 5.1 | n = 69; mean = 6.3; standard deviation = 5.0 | — |
| Egypt 1994 | Stool output at 24 h (g/kg) | Arithmetic mean | n = 20; mean = 165; standard deviation = 52 | n = 21; mean = 260; standard deviation 114 | — |
| Bangladesh 1995b | Stool output at 0 to24 h (ml/kg) | Arithmetic mean | n = 30; mean = 109; standard deviation = 73.8 | n = 30; mean = 110; standard deviation = 42.7 | — |
| Bangladesh 1996a | Stool output at 0 to 24 h (g/kg) | Arithmetic mean | n = 18; mean = 80.9; standard deviation = 45.3 | n = 28; mean = 117.8; standard deviation = 81.0 | — |
| India 1984a; India 1984b | Stool output at 24 h (ml/kg) | Arithmetic mean | n = 22; mean = 82.3; standard deviation = 60 | n = 22; mean = 88.1; standard deviation = 58.2 | — |
| Panama 1982 | Stool output during first 8 h (ml/kg/h) | Arithmetic mean | n = 33; mean = 4.3; standard deviation = 4.6 | n = 30; mean = 4.3; standard deviation = 3.3 | — |
| USA 1982 | Stool output during the first 8 h (ml/kg/h) | Arithmetic mean | n = 15; mean = 4.2; standard deviation = 3.9 | n = 20; mean = 4.6; standard deviation = 4.0 | — |
| CHOICE 2001 | Stool output at 24 h (g/kg) | Arithmetic mean | n = 341; mean = 114; standard deviation = 73.9 | n = 334; mean = 125; standard deviation = 91.4 | — |
Data and analyses
Comparison 1.
Reduced osmolarity ORS compared to WHO standard ORS
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for unscheduled intravenous fluid infusion | 11 | 1996 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.79] |
| 2 Stool output | 11 | 1776 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.33, ‐0.14] |
| 3 Episode of vomiting during rehydration | 6 | 1305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 4 Presence of hyponatremia after rehydration | 6 | 1120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.93, 2.24] |
| 5 Need for unscheduled intravenous fluid infusion (sensitivity analysis) | 7 | 1688 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.82] |
| 6 Stool output (sensitivity analysis) | 6 | 1550 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.31, ‐0.11] |
Analysis 1.1.
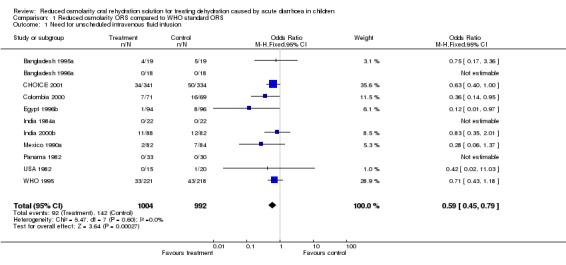
Comparison 1 Reduced osmolarity ORS compared to WHO standard ORS, Outcome 1 Need for unscheduled intravenous fluid infusion.
Analysis 1.2.

Comparison 1 Reduced osmolarity ORS compared to WHO standard ORS, Outcome 2 Stool output.
Analysis 1.3.
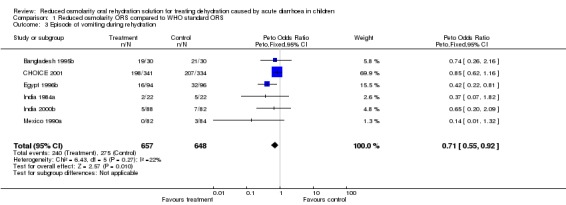
Comparison 1 Reduced osmolarity ORS compared to WHO standard ORS, Outcome 3 Episode of vomiting during rehydration.
Analysis 1.4.
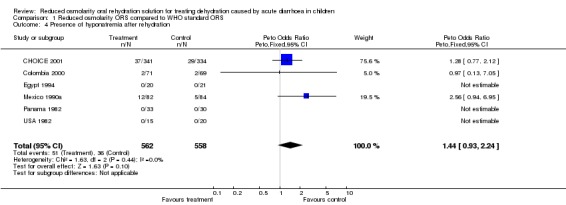
Comparison 1 Reduced osmolarity ORS compared to WHO standard ORS, Outcome 4 Presence of hyponatremia after rehydration.
Analysis 1.5.
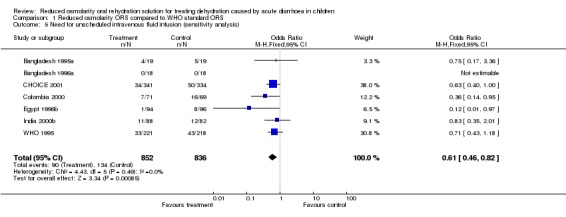
Comparison 1 Reduced osmolarity ORS compared to WHO standard ORS, Outcome 5 Need for unscheduled intravenous fluid infusion (sensitivity analysis).
Analysis 1.6.
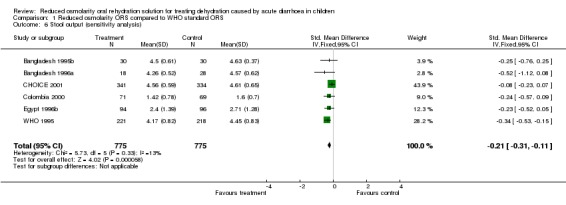
Comparison 1 Reduced osmolarity ORS compared to WHO standard ORS, Outcome 6 Stool output (sensitivity analysis).
Comparison 2.
Reduced osmolarity ORS (stratified by sodium concentration) compared to WHO standard ORS
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for unscheduled intravenous fluid infusion | 9 | 1925 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.44, 0.78] |
| 1.1 60 to 74 mmol | 4 | 584 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.15] |
| 1.2 75 mmol to 84 mmol | 5 | 1341 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.37, 0.76] |
| 2 Stool output | 7 | 1591 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.30, ‐0.10] |
| 2.1 60 to 74 mmol | 4 | 586 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.47, ‐0.15] |
| 2.2 75 to 84 mmol | 3 | 1005 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.26, ‐0.01] |
| 3 Episodes of vomiting | 6 | 1305 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.91] |
| 3.1 60 to 74 mmol | 2 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.47] |
| 3.2 75 to 84 mmol | 4 | 1201 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.93] |
| 4 Presence of hyponatraemia | 6 | 1171 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.93, 2.26] |
| 4.1 60 to 74 mmol | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 75 to 84 mmol | 3 | 981 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.93, 2.26] |
Analysis 2.1.

Comparison 2 Reduced osmolarity ORS (stratified by sodium concentration) compared to WHO standard ORS, Outcome 1 Need for unscheduled intravenous fluid infusion.
Analysis 2.2.

Comparison 2 Reduced osmolarity ORS (stratified by sodium concentration) compared to WHO standard ORS, Outcome 2 Stool output.
Analysis 2.3.
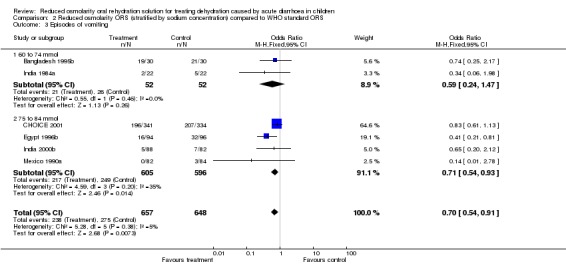
Comparison 2 Reduced osmolarity ORS (stratified by sodium concentration) compared to WHO standard ORS, Outcome 3 Episodes of vomiting.
Analysis 2.4.

Comparison 2 Reduced osmolarity ORS (stratified by sodium concentration) compared to WHO standard ORS, Outcome 4 Presence of hyponatraemia.
What's new
| Date | Event | Description |
|---|---|---|
| 22 October 2008 | Amended | Converted to new review format with minor editing. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 27 July 2004 | Amended | New studies found but not yet included or excluded. |
| 27 November 2001 | Amended | Changes made in response to feedback from specialists at the WHO/UNICEF oral rehydration salts formulation expert consultation. New York, 18 July 2001.
|
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT | |
| Participants | 55 children 2 to 15 months old Randomized after 1 day of rehydration Dehydration status not known |
|
| Interventions |
|
|
| Outcomes | Stool output (24 h, 96 h) Unscheduled IV Fluid intake Food intake Vomiting Body weight Stool frequency |
|
| Notes | — | |
| Methods | RCT (double blind) | |
| Participants | 50 children 5 to 24 months old with diarrhoea and mild to moderate dehydration Some with cholera |
|
| Interventions |
|
|
| Outcomes | Stool output (24 h, 48 h) Stool frequency Fluid intake Patients vomiting |
|
| Notes | — | |
| Methods | RCT (double blind) | |
| Participants | 46 children 6 to 30 months with diarrhoea and mild to moderate dehydration (WHO) | |
| Interventions |
|
|
| Outcomes | Stool output (24h, 48 h) ORS intake Unscheduled IV Urine output Stool frequency |
|
| Notes | — | |
| Methods | RCT (double blind) | |
| Participants | 671 children 1 to 24 months old with diarrhoea and some more severe dehydration. | |
| Interventions | (1) Low osmolarity glucose ORS (245 mosmol/L) (2) WHO standard ORS |
|
| Outcomes | Stool output (24 h and total) ORS intake (24 h, total) Vomiting in first 24 h Unscheduled IV in the first 24 h Frequency of hyponatraemia at 24 h Duration of diarrhoea |
|
| Notes | — | |
| Methods | RCT | |
| Participants | 140 boys 1 to 36 months old with diarrhoea and mild or moderate dehydration | |
| Interventions |
|
|
| Outcomes | Stool output rate at 24 h Fluid and food intake Weight gain Sodium and potassium levels Urine and vomit outputs Vomiting Unscheduled IV |
|
| Notes | — | |
| Methods | RCT, no details given | |
| Participants | 61 children 3 to 24 months old with diarrhoea and moderate dehydration (WHO definition) | |
| Interventions |
|
|
| Outcomes | Stool volume at 24 h Fluid intake Weight gain at 6 h Hyponatraemia; Duration of diarrhoea |
|
| Notes | — | |
| Methods | RCT (double blind) | |
| Participants | 190 boys 1 to 24 months with diarrhoea and dehydration (WHO criteria). | |
| Interventions |
|
|
| Outcomes | Stool output (24 h and total) Fluid intake Sodium Potassium Weight gain Children who vomited Mean weight gain Duration of diarrhoea Treatment failures |
|
| Notes | — | |
| Methods | RCT | |
| Participants | 65 infants 0 to 3 months old with acute non‐cholera diarrhoea and dehydration | |
| Interventions |
|
|
| Outcomes | Stool output (8 h, 24 h) Weight gain Fluid intake Unscheduled IV Haematologic and electrolyte measures Urine output Duration of diarrhoea after hospitalization |
|
| Notes | — | |
| Methods | RCT (double blind) | |
| Participants | 70 children 3 to 24 months with acute non‐cholera diarrhoea and some dehydration. | |
| Interventions | 1. Low osmolarity glucose ORS (224 mosmol/L) 2. WHO standard ORS |
|
| Outcomes | Number (%) of patients cured within 10 days Duration of diarrhoea Stool output (g/kg/d) Intake of ORS (ml/kg/d) Fluid intake (ORS + water + liquid food) % of weight gain Mean serum electrolytes |
|
| Notes | — | |
| Methods | RCT (double blind) | |
| Participants | 170 children 3 months to 5 years old with acute cholera and non‐cholera diarrhoea and some to severe dehydration |
|
| Interventions |
|
|
| Outcomes | Rehydration frequency (stool/4h) Rehydration ORS consumed (L) Rehydration duration (h) Maintenance frequency (stools/4h) Maintenance ORS consumed (L) Maintenance duration (h) Overall frequency (stool/4h) Overall ORS consumed (L) Overall duration (h) Weight gain (%) Caloric intake (kcal/kg/d) Serum sodium (mEq/L) Urine output (boys) (ml/k/h) Intravenous fluid (ml/kg) |
|
| Notes | — | |
| Methods | RCT | |
| Participants | 186 children 1 to 36 months old with diarrhoea and dehydration | |
| Interventions |
|
|
| Outcomes | Need of IV Sodium Potassium concentration |
|
| Notes | — | |
| Methods | RCT | |
| Participants | 94 well nourished children 3 months to 2 years old with diarrhoea and dehydration | |
| Interventions |
|
|
| Outcomes | Stool output (8 h and total illness) Unscheduled IV Fluid and electrolyte intake Weight gain Duration of diarrhoea after discharge Hyponatraemia Serum sodium Stool electrolyte |
|
| Notes | — | |
| Methods | RCT | |
| Participants | 52 well nourished children 3 months to 2 years old with diarrhoea and dehydration | |
| Interventions |
|
|
| Outcomes | Stool output (8 h and total illness) Unscheduled IV Fluid and electrolyte intake Weight gain Duration of diarrhoea after discharge Hyponatraemia Serum sodium |
|
| Notes | — | |
| Methods | Multicentred RCT (double blind) | |
| Participants | 447 children aged 1 to 24 months admitted to hospital with diarrhoea and mild to moderate dehydration (WHO classification) | |
| Interventions |
|
|
| Outcomes | Stool output at 24 h Fluid intake Mean daily consumption of formula milk and semi‐solid food. Weight gain Serum sodium on admission and at 24 h Need for unscheduled IV |
|
| Notes | Brazil, Peru, Mexico, India | |
Unscheduled IV: unscheduled intravenous fluid infusion; ORS: oral rehydration solution; RCT: randomized controlled trial; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Australia 1990 | They compared ORS‐26 (total 340 mosmol/L, sodium 26 mmol/L, glucose 2.7%) and ORS‐60 (total 240 mosmol/L, sodium 60 mmol/L, glucose 1.8%). The ORS‐26 was not WHO standard ORS. |
| Australia 1993 | They compared Glucolyte (total 343 mosmol/L, sodium 26 mmol/L, glucose 145 mmol/L) and Gastrolyte (total 240 mosmol/L, sodium 60 mmol/L, glucose 90 mmol/L). The Glucolyte was not WHO standard ORS. This was not an RCT but a open‐label study. |
| Bangladesh 1978 | They compared two isotonic sucrose (111 mmol/L) based and glucose (111 mmol/L) based ORS solutions. They did not use reduced osmolarity ORS. |
| Bangladesh 1991 | Maltodextrin containing ORS and WHO standard ORS were compared. They did not clearly report the composition of fluid or exact osmolarities but only mentioned 50 g of maltodextrin was added in the place of glucose which suggests no reduced osmolarity ORS was used. |
| Bangladesh 1996b | In this RCT, they compared WHO standard ORS (311 mosmol/L) and low osmolar ORS (249 mosmol/L). This was excluded because the study was performed in adult patients. |
| Bangladesh 1999 | This is a RCT comparing WHO standard ORS and low osmolarity ORS. This was excluded because this study was performed in adult patients. |
| Costa Rica 1987 | They compared solution A (WHO standard ORS, 311 mosmol/L) and solution B (Pedialyte total 309 osmol/L). They did not use reduced osmolarity ORS. |
| Ecuador 1995 | This community study was not a RCT but a crossover design in 4 communities. They compared glucose based ORS (310 to 330 mosmol/L) and rice based ORS (220 to 240 mosmol/L). None of their outcomes were relevant for this review. |
| Egypt 1996a | The intervention group was maltodextrin ORS, and therefore does not meet the inclusion criteria. |
| Finland 1985 | ORS‐60 (total 304 mosmol/L, sodium 60 mmol/L, glucose 144 mmol/L) and WHO standard ORS (total 331 mosmol/L) were used. ORS‐60 was not a reduced osmolarity ORS. |
| Finland 1986 | They compared two glycin supplemented ORS (total osmolarity 360 mmol/L and 280 mmol/L) and an ORS with sodium 60 mmol/L (total osmolarity 304 mmol/L). They did not use reduced osmolarity ORS or WHO standard ORS. |
| Finland 1993 | Two ORS‐60 solutions (sodium 60 mmol/L, each) were compared. One is isotonic (304 mosmol/L and has higher glucose concentration (144 mmol/L), the other hypo‐osmolar solution (224 mosmol/L) has 84 mmol/L of glucose. They did not use WHO standard ORS. |
| Finland 1997 | They compared one standard ORS (sodium 60 mmol/L, total 304 mosmol/L) and the low osmolarity ORS (sodium 60 mmol/L, total 224 mosmol/L). They did not use WHO standard ORS. |
| Finland 1998 | Two hypotonic ORS with osmolarities of 224 osmol/L (sodium 60 mmol/L, glucose 84 mmol/L) and 204 mosmol/L (sodium 60 mmol/L, glucose 64 mmol/L) were compared. They did not use WHO standard ORS and this was not a RCT but an alternate allocation trial. |
| France 1990 | They compared solution A (total 326 osmol/L, sodium 49 mmol/L glucose 110 mmol/L) and solution D (total 240 osmol/L, sodium 60 mmol/L, glucose 90 mmol/L). They did not use WHO standard ORS. |
| Guinea‐Bissau 1999 | This is a community‐based RCT where they used WHO standard ORS of 311 osmol/L and reduced osmolarity ORS of 224 osmol/L. None of their outcomes were relevant for this review. |
| India 1978 | In this RCT, they used solution A (sodium 90 mmol/L, potassium 15 mmol/L, chlorine 75 mmol/L, bicarbonate 30 mmol/L, glucose 90 mmol/L) and B (sodium 50 mmol/L, potassium 15 mmol/L, chlorine 50 mmol/L, bicarbonate 15 mmol/L, glucose 170 mmol/L. Both solutions have total osmolarity of 300 mosmol/L. |
| India 1984b | In this randomized study, they compared WHO standard ORS (sodium 90 mmol/L, potassium 20 mol/L, bicarbonate 30 mmol/L, chlorine 80 mmol/L, and glucose 111 mmol/L, total 331 mmol/L) and glycin fortified ORS of which osmolarity is not lower because 111 mmol/L of glycine was added. |
| Iran 1983 | They compared sucrose high sodium (sodium 90 mmol/L, sucrose 111 mol/L, total 331 osmol/L) and sucrose low sodium (sodium 58 mmol/L, total 278 osmol/L) ORS. They did not use WHO standard ORS. |
| Mexico 1988 | Appears to contain same patients as Mexico 1990a. |
| Mexico 1990b | Appears to contain same patients as Mexico 1990a. |
| Myanmar 1991 | They compared WHO standard ORS (311 mmol/L) and maltodextrin/glycine/clycyl‐clycine ORS (326 mmol/L). They did not use reduced osmolarity ORS. |
| Russia 1997 | They compared WHO standard ORS (331 mmol/L), low ORS (224 mmol/L), and IV fluid infusion, and secondarily lactobacillus GG or placebo. None of their outcomes were relevant for this review. |
| Turkey 1985 | This is not an RCT but a comparison of data between two separate studies using ORS‐60 and ORS‐90. |
| Turkey 1986 | This is not an RCT but a comparison between treatment effects of ORS‐60 (sodium 60 mmol/L) in malnourished infants with infectious diarrhoea and in a previous study of well‐nourished patients. This paper is not an RCT but a comparison of data between two separate studies. |
| USA 1972 | They used two hypotonic solutions. This is not an RCT. They did not use WHO standard ORS. |
| USA 1986 | They used solution A (sodium 50 mmol/L, glucose 111 mEq/L, 389 mosmol/L) and B (sodium 50 mmol/L, glucose 111 mEq/L, 278 mosmol/L). Solution A had 111 mEq/L of glycine additionally. They did not use WHO standard ORS. |
IV: intravenous; ORS: oral rehydration solution; RCT: randomized controlled trial; WHO: World Health Organization.
Contributions of authors
Seokyung Hahn and Yaejean Kim wrote the protocol, conducted the data extraction, data analysis and interpretation, and drafted the paper. Paul Garner advised on inclusion criteria and outcomes for the protocol, quality assessment and analysis, and helped write the review. Paul Garner is the guarantor.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
Medical and Pharmaceutical Statistics Research Unit, University of Reading, UK.
Seoul National University Hospital, Korea, South.
External sources
World Health Organization, Switzerland.
Department for International Development, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (eg employment, consultancy, stock ownership, honoraria, expert testimony).
The World Health Organization provided funds for us to conduct this review.
Unchanged
References
References to studies included in this review
- Saker SA, Majid N, Mahalanabis D. Alanine‐ and glucose‐based hypo‐osmolar oral rehydration solution in infants with persistent diarrhoea: a controlled trial. Acta Paediatrica 1995;84:775‐80. [DOI] [PubMed] [Google Scholar]
- Mahalanabis D, Faruque ASG, Hoque SS, Faruque SM. Hypotonic oral rehydration solution in acute diarrhoea: a controlled clinical trial. Acta Paediatrica 1995;84:289‐93. [DOI] [PubMed] [Google Scholar]
- Faruque ASG, Mahalanabis D, Hamadani J, Hoque SS. Hypo‐osmolar sucrose oral rehydration solution in acute diarrhoea: a pilot study. Acta Paediatrica 1996;85:1247‐8. [DOI] [PubMed] [Google Scholar]
- CHOICE study group. Multicenter randomized double blind clinical trial to evaluate the efficacy and safety of a reduced osmolarity oral rehydration solution in children with acute watery diarrhoea. Pediatrics 2001;107:613‐8. [DOI] [PubMed] [Google Scholar]
- Bernal C, Velasquez C, Garcia G, Uribe G, Palacio C. Oral rehydration with a low‐osmolarity solution in children dehydrated by diarrheric diseases. A controlled clinical study [Hidratacion oral con una solucion de baja osmolaridad en ninos deshidratados por enfermedades diarreicas: un estudio clinico controlado]. Saludarte 2000;1:6‐23. [PubMed] [Google Scholar]
- El‐Mougi M, El‐Akkad N, Hendawi A, Hassan M, Amer A, Fontaine O, Pierce N F. Is a low‐osmolarity ORS solution more efficacious than standard WHO ORS solution?. Journal of Pediatric Gastroenterology and Nutrition 1994;19(1):83‐6. [DOI] [PubMed] [Google Scholar]
- Santosham M, Fayad I, Zikiri MA, Hussein A, Amponsah A, Duggan C, et al. A double‐blind clinical trial comparing World Health Organization oral rehydration solution with a reduced osmolarity solution containing equal amounts of sodium and glucose. Journal of Pediatrics 1996;128(1):45‐51. [DOI] [PubMed] [Google Scholar]
- Bhargava SK, Sachdev HP, Das Gupta B, Daral TS, Singh HP, Mohan M. Oral rehydration of neonates and young infants with dehydration diarrhea: compararison of low and standard sodium content in oral rehydration solutions. Journal of Pediatric Gastroenterology and Nutrition 1984;3(4):500‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dutta P, Dutta S, Manna B, Chatterjee MK, De A, Bhattacharya SK. Hypo‐osmolar oral rehydration salts solution in dehydrating persistent diarrhoea in children: double‐blind, randomized, controlled clinical trial. Acta Paediatrica 2000;89:411‐6. [DOI] [PubMed] [Google Scholar]
- Alam S, Afzal K, Maheshwari M, Shukia I. Controlled trial of hypo‐osmolar versus World Health Organization oral rehydration solution. Indian Pediatrics 2000;37:952‐9. [PubMed] [Google Scholar]
- Moreno‐Sanchez H, Velasques‐Jones L, Becerra FC, Faure A, Maulen I, Leon M de, et al. A comparative study on two oral rehydration solutions(ORS) containing 60 or 90 mmol/L of sodium and of different osmolalities [Estudio comparativo de dos soluciones de hidratacion oral conteniendo 60 o 90 mmol/L de sodio y con diferente osmolalidad]. Boletin Medico del Hospital Infantil de Mexico 1990;47(9):630‐5. [PubMed] [Google Scholar]
- Santosham M, Daum L Dillman RS, Rodriguez JL, Luque S, Russel R, et al. Oral rehydration therapy of infantile diarrhea: a controlled study of well‐nourished children hospitalized in the United States and Panama. New England Journal of Medicine 1982;306(18):1070‐6. [DOI] [PubMed] [Google Scholar]
- Santosham M, Daum L, Dillman RS, Rodriguez JL, Luque S, Russel R, et alA, Benenson AS, Sack RB. Oral rehydration therapy of infantile diarrhea: a controlled study of well‐nourished children hospitalized in the United States and Panama. New England Journal of Medicine 1982;306(18):1070‐6. [DOI] [PubMed] [Google Scholar]
- International Study Group on Reduced‐osmolarity ORS solutions. Multicentre evaluation of reduced‐osmolarity oral rehydration salts solution. Lancet 1995;345:282‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
- Cleghorn GJ, Shepherd RW, Patrick MK. Comparison of two oral rehydration solutions in children with gastroenteritis in Australia. Clinical Therapeutics 1990;12 Suppl A:81‐5. [PubMed] [Google Scholar]
- Wall CR, Shepherd RW, Patric M, Chin S, Cleghorn G. Osmolality electrolyte and carbohydrate type and oral rehydration solutions: A controlled study to compare the efficacy of two commercially available solutions(osmolalities 240 mmol/L and 34 mmol/L). Journal of Diarrhoeal Diseases Research 1993;11(4):222‐6. [PubMed] [Google Scholar]
- Sack DA, Chowdhury AMAK, Eusof A, Ali MA, Merson MH, Islam S, et al. Oral hydration in rotavirus diarrhoea: a double blind comparison of sucrose with glucose electrolyte solution. Lancet 1978;2:280‐3. [DOI] [PubMed] [Google Scholar]
- Akbar MS, Baker KM, Aziz MA, Khan WA, Salim AFM. A randomised, double‐blind clinical trial of a maltodextrin containing oral rehydration solution in acute infantile diarrhoea. Journal of Diarrhoeal Diseases Research 1991;9(1):33‐7. [PubMed] [Google Scholar]
- Faruque D, Mahalanabis ASG, Hamadani v, Zetterstrom JD. Reduced ormolarity oral rehydration salt in cholera. Scand Journal of Infectious Diseases 1996;28:87‐90. [DOI] [PubMed] [Google Scholar]
- Alam NH, Majumder RN, Fuchs GJ, CHOICE group. Efficacy and safety of oral rehydration solution with reduced osmolarity in adults with cholera: a randomised double‐blind clinical trial. Lancet 1999;354:296‐9. [DOI] [PubMed] [Google Scholar]
- Pizarro D, Castillo B, Posada G, Lizano C, Mata L. Efficacy comparison of oral rehydration solutions containing either 90 or 75 millimoles of sodium per liter. Pediatrics 1987;79(2):190‐5. [PubMed] [Google Scholar]
- Barclay DV, Gil‐Ramos J, Mora JO, Dirren H. A packaged rice‐based oral rehydration solution for acute diarrhea. Journal of Pediatric Gastroenterology and Nutrition 1995;20(4):408‐16. [DOI] [PubMed] [Google Scholar]
- El‐Mougi M, Hendawi A, Koura H, Hegazi E, Fontaine O, Pierce NF. Efficy of standard glucose‐based and reduced osmolarity maltodextrin‐based oral rehydration solution: effect of sugar malabsorption. Bulletin of the World Health Organization 1996;74(5):471‐7. [PMC free article] [PubMed] [Google Scholar]
- Isolauri E. Evaluation of an oral rehydration solution with Na 60 mmol/l in infants hospitalized for acute diarrhoea or treated as outpatients. Acta Paediatrica Scandinavica 1985;74:643‐9. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Isolauri E. Glycine supplemented oral rehydration solutions for diarrhoea. Archives of Disease in Childhood 1986;61:372‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautanen T, El‐RAdhi S, Vesikari T. Clinical experience with a hypotonic oral rehydration solution in acute diarrhoea. Acta Paediatrica 1993;82:52‐4. [DOI] [PubMed] [Google Scholar]
- Rautane T, Kurki S, Vesikari T. Randomised double blind study of hypotonic oral rehydration solution in diarrhoea. Archives of Disease in Childhood 1997;76:272‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautanen T, Isolauri E, Salo E, Vesikari T. Management of acute diarrhoea with low osmolarity oral rehydration solutions and Lactobacillus strain GG. Archives of Disease in Childhood 1998;79:157‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet E, Guillot M, Luyer B, Morin C, Pollet F, Meynard C. Comparison of two oral rehydration solutions in eutrophic infants with moderate acute diarrhea: results of an interim analysis. Clinical Therapeutics 1990;12 Suppl A:104‐12. [PubMed] [Google Scholar]
- Valentiner‐Branth P, Steinsland H, Gjessing HK, Santos G, Bhan MK, Dias F, et al. Community‐based randomized controlled trial of reduced osmolarity oral rehydration solution in acute childhood diarrhea. Pediatric Infectious Diseases Journal 1999;18:789‐95. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Mahalanabis D, Jalan KN, Maitra TK, Agarwal SK, Dutta B, et al. Oral rehydration in infantile diarrhoea. Archives of Disease in Childhood 1978;53:284‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partra FC, Mahalanabis D, Jalan KN, Sen A, Banerjee P. In search of a super solution: controlled trial of glycin‐glucose oral rehydration solution in infantile diarrhoea. Acta Paediatrica Scandanavica 1984;73:18‐21. [DOI] [PubMed] [Google Scholar]
- Saberi MS, Assaee M. Oral hydration of diarrhoeal dehydration : Comparison of high and low sodium concentration in rehydration solutions. Acta Paediatrica Scandanavica 1983;72:167‐70. [DOI] [PubMed] [Google Scholar]
- Martinez‐Pantaleon 0, Faure‐Vilchis A, Gomez‐Najera RI, Hernandez‐Lopez M, Velasquez‐Jones L. Comparative study of oral rehydration solutions containing either 90 or 60 millimoles of sodium per liter. [Estudio comparativo de dos soluciones de rehidratacion oral conteniendo 90 o 60 milimoles de sodio por litro]. Boletin Medico del Hospital Infantil de Mexico 1998;45(12):817‐22. [MEDLINE: ] [PubMed] [Google Scholar]
- Velasquez‐Jones L, Becerra F, Faure A, Leon M, Moreno H, Maulen I, et al. Clinical experience in Mexico with a new oral rehydration solution with lower osmolality. Clinical Therapeutics 1990;12 Suppl A:95‐103. [PubMed] [Google Scholar]
- Khin‐Maunh‐U, Myo‐Khin, Nyunt‐Nyunt‐Wai, Mu‐Mu‐Khin, Mya‐Thi, Thein‐Thein‐Myint. Comparison of glucose/elctrolyte and maltodextrin/glycine/glycyl‐glycine/electrolye oral rehydration solution in acute diarrhea in children. Journal of Pediatric Gastroenterology and Nutrition 1992;13:397‐401. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Isolauri E, Burkanova L, Lukovnikova S, Vesikari R. A trial in the Karelian Republic of oral rehydration and Lactobacillus GG for treatment of acute diarrhoea. Acta Paediatrica 1997;86:460‐5. [DOI] [PubMed] [Google Scholar]
- Sokucu S, Marin L, Gunoz H, Aperia A, Neyzi O, Zetterstrom R. Oral rehydration therapy in infectious diarrhoea. Comparison of rehydration solutions with 60 and 90 mmol sodium per litre. Acta Paediatrica Scandinavica 1985;74:489‐84. [DOI] [PubMed] [Google Scholar]
- Marin L, Gunoz H, Sokucu S, Saner G, Aperia A, Neyzi O, et al. Oral rehydration therapy in malnourished infants with infectious diarrhoea. Acta Paediatrica Scandinavica 1986;75:477‐82. [DOI] [PubMed] [Google Scholar]
- Hirschhorn N, Cash RA, Woodward WE, Spivey GH. Oral fluid therapy of Apache children with acute infectious diarrhoea. Lancet 1972;7766:15‐8. [DOI] [PubMed] [Google Scholar]
- Santosham M, Burns BA, Reid R, Letson W, Duncan B, Powlesland JA, et al. Glycine‐based oral rehydration solutioin: reassessment of safety and efficacy. Jounal of Pediatrics 1986;109(5):795‐801. [DOI] [PubMed] [Google Scholar]
Additional references
- Alam NH, Majumder RN, Fuchs GJ, CHOICE study group. Efficacy and safety of oral rehydration solution with reduced osmolarity in adults with cholera: a randomised double‐blinded clinical trial. Lancet 1999;354:296‐9. [DOI] [PubMed] [Google Scholar]
- Almroth S, Latham MC. Rational home management of diarrhoea. Lancet 1995;345:709‐11. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analyses detected by a simple graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farthing MJ. History and rationale of oral rehydration and recent develpments in formulating an optimal solution. Drugs 1988;36 Suppl 4:80‐90. [DOI] [PubMed] [Google Scholar]
- Faruque ASG, Mahalanabis D, Hamadani JD, Zetterstrom R. Reduced osmoloarity oral rehydration salt in cholera. Scandanavian Journal of Infectious Diseases 1996;28:87‐90. [DOI] [PubMed] [Google Scholar]
- Finberg L. Hypernatremic (hypertonic) dehydration in infants. New England Journal of Medicine 1973;289:196‐8. [DOI] [PubMed] [Google Scholar]
- Fontaine O, Gore SM, Pierce NF. Rice‐based oral rehydration solution for treating diarrhoea (Cochrane Review). Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
- Fordtran JS, Rector FC, Carter NW. The mechanisms of sodium absorption in the human small intestine. Journal of Clinical Investigation 1968;47:884‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JP. The state of the world's children 1982‐1983. UNICEF. New York: UNICEF, 1983. [Google Scholar]
- Guarino A, Albano F, Guandalini S. Oral rehydration: Toward a real solution. Journal of Pediatric Gastroenterology and Nutrition 2001;33 Suppl 4:2‐12. [DOI] [PubMed] [Google Scholar]
- Hunt JB, Elliotte EJ, Fairclough PD, Clark ML, Farthing MJ. Water and solute absorption from hypotonic glucose‐electrolyte solutions in human jejunum. Gut 1992;33(4):479‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalanabis M, Choudfuri AB, Bagchi NG. Oral fluid therapy of cholera among Bangladesh refugees. Johns Hopkins Med J 1973;132:197‐205. [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, Okin I, Rennie D, Stroup DF, for the Quorum Group. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUORUM statement. Lancet 1999;354:1896‐1900. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Expert consultation on oral rehydration salts (ORS) formulation. Child and Adolescent Health and Development2001; Vol. WHO/FCH/CAH/01.22:[18 July 2001].
References to other published versions of this review
- Hahn S, Kim Y, Garner P. Reduced osmolarity oral rehydration salts solution for treating diarrhoea‐associated dehydration in children: systematic review. British Medical Journal 2001;323:81‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


