Abstract
Background
Unit‐dose packaging of antimalarial drugs may improve the success of malaria treatments by making it easier for patients to take them correctly.
Objectives
To summarize the effects of unit‐dose packaged treatment on treatment failure and treatment adherence in people with uncomplicated malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (February 2009); CENTRAL (The Cochrane Library Issue 1, 2009); MEDLINE (1966 to February 2009); EMBASE (1980 to February 2009); LILACS (February 2009); conference proceedings, and reference lists of articles. We also contacted pharmaceutical companies, organizations, and researchers in the field.
Selection criteria
Randomized controlled trials (RCTs), cluster‐RCTs and quasi‐RCTs of unit‐dose packaged drugs for treating uncomplicated malaria.
Data collection and analysis
We independently assessed trial eligibility and risk of bias, and extracted data for an intention‐to‐treat analysis, where possible. We combined binary data using risk ratio (RR) and the fixed‐effect model, and presented them with 95% confidence intervals (CI). We attempted to contact trial authors for additional information.
Main results
One RCT (203 participants), three quasi‐RCTs (895 participants), and one cluster‐RCT (six health facilities) met the inclusion criteria. Trials were generally of poor methodological quality, and none adequately assessed treatment failure. Unit‐dose packaged drugs (in conjunction with prescriber training and patient information) appeared to be associated with higher participant‐reported treatment adherence in all trials.
A meta‐analysis of two trials (596 participants) showed that participant‐reported treatment adherence was slightly higher with blister‐packed tablets compared with tablets in paper envelopes (RR 1.18, 95% CI 1.12 to 1.25). Two trials using tablets in sectioned polythene bags as the intervention also noted an increase in participant‐reported treatment adherence: the cluster‐RCT (six clusters) compared it with tablets in paper envelopes, and the other trial compared it with syrup in bottles (RR 2.15, 95% CI 1.76 to 2.61; 299 participants).
Authors' conclusions
There is insufficient evidence to know if the effects of unit‐dose packaged antimalarial drugs reduce treatment failure. Unit‐dose packaging, supported by prescriber training and patient information, appears to improve participant‐reported treatment adherence, but these data come from trials with methodological limitations.
23 April 2019
No update planned
Research area no longer active
Co‐formulated antimalarial combination treatments are now widely available. This is an historical question and the review will no longer be updated.
Plain language summary
Unit‐dose packaging of drugs for treating malaria
Malaria is a parasitic disease spread by mosquitoes in areas such as sub‐Saharan Africa, South‐East Asia and South America. Millions of people are infected with malaria each year. It is thought that packaging a course of treatment in units of a single dose may better ensure the correct dosage is taken, thus increasing the success of treatment. The review found insufficient good quality evidence from randomized controlled trials to determine if unit‐dose packaging of drugs saves lives, but there is some indication that it might improve treatment adherence. More research is needed.
Background
Treating malaria
Malaria is caused by the Plasmodium parasite, which is spread by mosquitoes mainly in Africa, Asia, and South America. Millions of people are infected with malaria each year and many will die (WHO 2004). The World Health Organization (WHO) promotes rapid diagnosis and prompt treatment of malaria to reduce the burden of the disease (WHO 1993). There is a challenge in ensuring that people are receiving effective drugs to treat malaria. Once these drugs are available, interventions that aid people in taking the correct treatment regimen will help maximize their effectiveness. Suboptimal dosing results in low blood concentrations and inadequate exposure of the infecting parasite population to therapeutic concentrations of the drug (White 1998; Bloland 2003). This contributes to fewer cures and parasite recrudescence, and can contribute to the development of parasite resistance to antimalarial drugs.
Some antimalarial drugs, such as sulfadoxine‐pyrimethamine and mefloquine, require only a single dose, making the regimen straightforward. In fact, as many people attend health facilities for illness, health staff can directly supervise the dosing of these drugs. Regimens for other drugs, such as chloroquine and amodiaquine, span three days. If the drugs are acquired from a health facility the first dose can be ensured in a similar way, but subsequent doses may be missed as people usually take these at home. Children commonly receive bottles of chloroquine syrup for the treatment of malaria; adequate treatment would require caregivers to use appropriately sized dosing instruments, but this cannot always be ensured. Newly introduced courses of antimalarial treatment are increasingly complex. The WHO currently promotes artemisinin‐based combination therapy (ACT) and, unless the drugs are co‐formulated, this often requires people to follow the regimen for more than one antimalarial drug at a time. These regimens may be more difficult to follow correctly.
Understanding adherence
The ability of people to follow a given treatment regimen has been studied for some time. 'Compliance', 'concordance', and 'adherence' have all been used to describe the concept. Recently, some experts have discouraged the use of the term 'compliance' as it may imply that "the patient is docile and subservient to the provider" (Sumartojo 1993). Mullen and colleagues suggest 'concordance' may be more suitable as it reflects "the active exchange of information, negotiation, and spirit of cooperation" between patient and health provider (Mullen 1997). However, it is not widely used. Haynes and colleagues believe that completing treatment is usually an independent choice of the patient and is best described as 'adherence', a statement of fact rather than of blame of the patient, prescriber, or treatment (Haynes 2008). We use 'adherence' throughout this review.
Levels of adherence vary widely between treatments for all disease states (Sackett 1979). Recorded reasons for non‐adherence include adverse events, poor instructions, poor provider‐patient relationship, loss of drugs, forgetting to take treatment, patient's disagreement with the need for treatment, perceived ineffectiveness of the drug, and the inability to pay for it (Gomes 1998; Haynes 2008). People are less likely to take medicines once they feel they have recovered from an illness (White 1998). Most interventions to promote adherence are targeted at modifying the behaviour of the patient, caregiver, or health staff (Volmink 1997). Strategies include education and information campaigns, directly observed therapy, increased supervision of staff, home visits with follow up, and improved packaging of drugs.
Drug packaging
In a Cochrane Review, Haynes 2008 summarized the evidence around interventions used to help patients follow prescriptions for medications. Three trials included in the review looked at interventions with a drug‐packaging component. These trials generated conflicting results. Henry 1999 found that a package of "compliance enhancing measures" did not improve adherence or clinical outcome for Helicobacter pylori treatment; in fact, adherence and treatment success were high in both the control and treatment groups. Similarly, Becker 1986 did not find any improvement in adherence or blood pressure control for hypertensive patients receiving special medication packaging. However, Peterson 1984 found that adherence and clinical control improved significantly in participants given a combination of "compliance‐improving strategies" for the treatment of epilepsy. Another Cochrane Review Heneghan 2006 has examined reminder packaging as an intervention for improving adherence to self‐administered long‐term medications. Eight studies were included in this review. Results were inconclusive. Whilst the intervetion appeared to improve dosing and showed the possibility for an effect on adherence, no clinical benefits of the intervention was demonstarted. The current review looks at unit‐dose drug packaging as an adherence intervention for malaria treatment.
Historically, drugs were first packaged to preserve freshness, prevent contamination, and protect them from damage (Richardson 2003). It is also an effective way to make them more recognizable, help ensure expired drugs are not used, and prevent under or over weighing and counterfeiting. Over time, marketers realized the potential of packaging to aid brand advertising. More recently, the design of packaging has been considered a useful tool to enhance adherence to treatment. Packaging can be designed to enhance communication of the regimen to patients and providers. With the aid of improved packaging, providers should be better able to give the correct amount of the drug, inform patients of the treatment regimen, and highlight the need for full therapy; and patients should be better able to use the packaging to guide treatment, even when unsupervised.
Some drugs have a narrow therapeutic range and must be packaged individually for each age or weight category in order to avoid under or over dosing (Ondari 2003). Labelling of packs can be problematic as age estimations of mothers have frequently been found to be unreliable, and many rural villages have no weighing devices. The use of diagrams (for example, a crawling, walking or talking child used by Okonkwo 2001) may be an appropriate alternative in some settings. When drugs are packaged differently, the entire range must be made available. If not, there is the risk that people may try to alter the dose by doubling (using two packs) or halving the packs they can find. This adds a new potential for miscalculations and under dosing (Kilian 2003). Even when all presentations are available there is the possibility of over or under dosing at the extremes of the age range, especially among populations where malnutrition exists (Bloland 2003).
The system of packaging adopted by each country and each pharmaceutical company varies widely. This is often due to the different and changing drug policies, various perceptions, the public and private sector involvement in drug and packaging production, and the level of technology available. Most packaging developments have been designed for use with tablets, despite children traditionally receiving syrup to treat malaria (it is considered easier to tolerate). Some investigators have developed special syrup bottles, but tablets are more readily packaged. In particular, blister packaging of tablets is becoming increasingly popular, especially with the spread of combination therapy (the two drugs can be packaged together in one blister (White 1999)). In fact, the WHO now suggests that all artemisinin‐based combination treatments should be blister packed to conform to good manufacturing practice standards (WHO 2003). Blister packaging requires a certain level of technology and, even within this packaging type, there is great variation in the products developed. For the purpose of this review, we have extracted as much descriptive information relating to each packaging intervention as possible and attempted to relate this to any heterogeneity in the effectiveness of interventions.
Designs that ensure suitability for illiterate or semi‐literate carers through the use of colour coding, pictures, and diagrams, as well as written instructions, should help optimize the effectiveness of interventions. One way to improve the suitability of the interventions is to consult with consumer groups at the design stage. Information from health workers often has to compete with other advice and perceptions before patients decide what to do about recommended treatments. When designing drug packaging, an understanding of the user group will help counteract any negative perspectives, reinforce positive ones, fill in missing information, answer questions, and overcome suspicion (Francis 1997). Many episodes of uncomplicated malaria are treated in the home after initiation at the health centre or entirely through self‐treatment (Biritwum 2000; Kilian 2003). People will often use drugs left over from a previous episode or will purchase them from private vendors (Biritwum 2000). In both scenarios, there will be no contact with medical facilities (Kilian 2003). In these cases, innovations that do not rely on the presence of health staff will be particularly useful.
This review has examined the use of one specific type of packaging, unit‐dose packaging. To make patients more aware of when to take each tablet, a full course of therapy is presented in a single pack, with the drugs to be taken together adjacent to each other, sometimes with markings or colours to indicate that they should be taken all at once; see Figure 1, Figure 2, Figure 3, and Figure 4 for examples.
1.
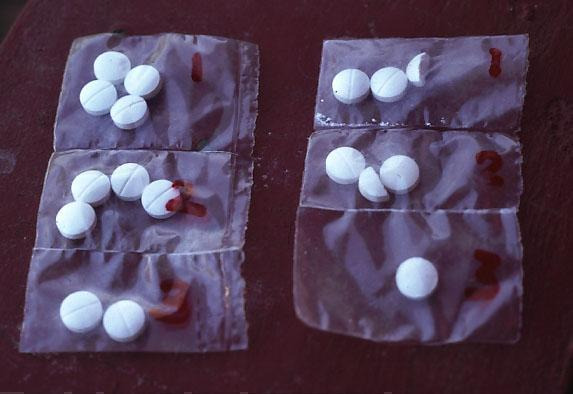
Sectioned polythene bags of chloroquine (from Ghana)
2.
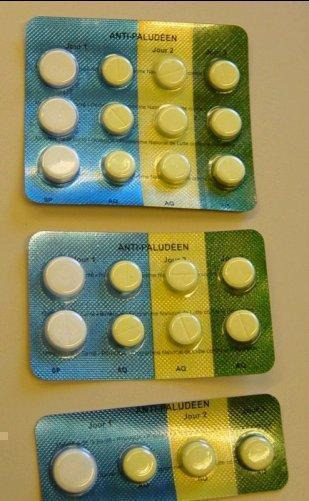
Blister‐packed sulfadoxine‐pyrimethamine and amodiaquine (from Rwanda)
3.
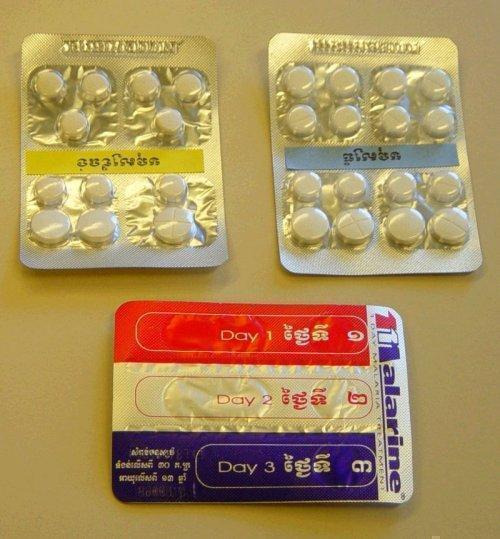
Blister‐packed artesunate and mefloquine (from Cambodia)
4.
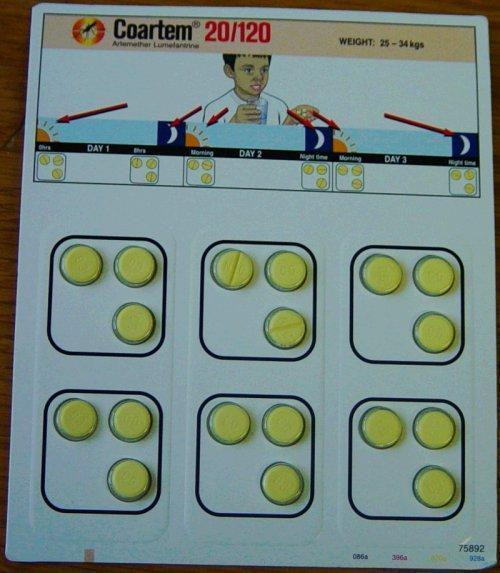
Blister‐packed artemether‐lumefantrine (trade name Coartem, Novartis)
Analysing the effectiveness of unit‐dose packaging
In reality, unit‐dose packaging is not likely to be provided as a single adherence intervention. Often, combining various interconnecting strategies helps ensure that the best patient outcomes can result (Haynes 1996). This must be taken into account when analysing which interventions are successful in which settings, and when defining, developing, documenting, and reproducing the interventions.
In addition, any difference in malaria cure rates or adherence levels between the intervention and control groups could be due to either the intervention (unit‐dose packaging) or to the non‐specific effects of increased attention paid to the intervention group. Depending on where and when the drugs are acquired, some form of health education may be given alongside the provision of antimalarial drugs. With the introduction of new unit‐dose packaged drugs, this health education may be more extensive and clear (structured and backed up by the pack, helping to reinforce messages). It could be that the packaging is a useful co‐intervention that increases provider instruction to the patient. It may also help private sellers with little medical knowledge to give correct information. This may be the mechanism for an effect on treatment adherence and cure rates. For the purpose of this review, we have assessed the extent to which co‐interventions (including information and education) have been reported and controlled for, and when "usual care" or "normal practice" was quoted as the control intervention we checked that this was adequately defined.
The aim of any adherence promotion in malaria treatment is to improve malaria cure rates (as measured by treatment failure), and we have used this as our primary outcome measure. Furthermore, while adherence to correct doses of malaria treatment has been found to correlate with improvement in the condition of patients (Okonkwo 2001), no clear connection has been found between malaria treatment adherence and cure (and some people may be cured at less than 100% adherence). Malaria cure rate may be improved with increased adherence, but we cannot guarantee that this is the mechanism for any observed change. Ensuring optimal treatment adherence may also help slow the development of parasite drug resistance by ensuring the correct drug concentration in the blood and the quickest cure, but we have not addressed this issue in the current review. Some researchers have hypothesized that as increased adherence to treatment will mean patients receive on average a higher dose of the drug, this may lead to an increase in the incidence of some adverse events. Hence, the current review also assesses the incidence of adverse events, where reported.
Malaria illness can take two forms, uncomplicated malaria and severe malaria. The most common symptom of uncomplicated malaria is fever, although patients may also complain of headache, aches and pains elsewhere in the body, and occasionally of abdominal pain and diarrhoea. Severe malaria is caused by infection with P. falciparum and usually occurs as a result of delay in treating an uncomplicated attack of falciparum malaria (WHO 2000). This review has examined the use of unit‐dose packaging for treating uncomplicated malaria only. Severe malaria is normally treated in the hospital or clinic setting with intravenous drugs; whether unit‐dose packaging interventions have an effect on treatment outcomes in this setting is a different question.
Randomized controlled trials are rarely used to investigate complex interventions such as drug packaging. Most investigators use a community design, but it is often difficult to determine the treatment effect in this trial design. We have therefore not included community studies in this review, but we have discussed any pertinent observations made in such studies in the context of the main findings of the review.
Objectives
To summarize the effects of unit‐dose packaged treatment on treatment failure and treatment adherence in people with uncomplicated malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), and quasi‐RCTs; including trials randomized by cluster.
Types of participants
People diagnosed (clinically or confirmed with microscopy) with uncomplicated malaria infection (as defined by the trial authors).
Types of interventions
Intervention
Any programme that includes unit‐dose packaging of antimalarial drugs, that is, drugs packed in units of a single dose.
Control
Standard practice before the intervention, or alternative packaging intervention.
Intervention and control arm to receive the same antimalarial drug and any other interventions.
Types of outcome measures
Primary
Treatment failure on or by day 28: (1) including new infections and (2) adjusted to exclude new infections (detected by PCR).
Secondary
Treatment failure on or by day 14: (1) including new infections and (2) adjusted to exclude new infections (detected by polymerase chain reaction (PCR)).
Treatment adherence: participants completing the full treatment regimen, as approximated by the trial authors (for example, by pill counts or residual syrup measurement; patient interview; or drug concentration in urine or plasma).
Adverse events
Serious adverse events (fatal, life threatening, or require hospitalization).
Adverse events that result in the discontinuation of treatment.
Any other adverse events.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1. Cochrane Infectious Diseases Group Specialized Register (February 2009); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 1, 2009); MEDLINE (1966 to February 2009); EMBASE (1974 to February 2009); and LILACS (1982 to February 2009)
Conference proceedings
We searched the following conference proceedings for relevant abstracts: The Third Multilateral Initiative on Malaria Pan‐African Conference, Arusha, Tanzania, 18 to 22 November 2002; The Third European Congress on Tropical Medicine and International Health, Lisbon, Portugal, 8 to 12 September 2002; and The International Conference on Malaria: Current Status and Future Trends, Bangkok, Thailand, 16 to 19 February 2003; the Fourth Multilateral Initiative on Malaria Pan‐African Conference (Roll Back Malaria), Yarounde, Cameroon, 13 to 18 November 2005; the Fifth European Congress on Tropical Medicine and International Health, Amsterdam, the Netherlands, 24 to 28 May 2007; Stop Malaria Now! (an African‐European Initiative) International Malaria Conference, Bonn, Germany, 21 to 22 April 2008.
Researchers, organizations, and pharmaceutical companies
For unpublished and ongoing studies, we contacted individual researchers working in the field, the WHO, and the pharmaceutical companies GlaxoSmithKline, Merck, and Novartis (May 2009).
Reference lists
We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Lois Orton assessed the results of the literature search and obtained the full reports for all potentially relevant studies. Both authors independently assessed the potentially relevant studies for inclusion using an eligibility form based on the inclusion criteria. We scrutinized all potentially eligible studies for duplicate publication from the same data set, and resolved disagreements through discussion and, when necessary, by consulting a Cochrane Infectious Diseases Group Editor. We stated the reasons for excluding studies in the 'Characteristics of excluded studies'.
We described the usability of drug packaging (for literate and non‐literate people) using the following criteria (seeking further information from trial authors where necessary): regimen; packaging type; labelling and instructions on pack; and dosage presentations for age or weight categories.
We examined each trial to identify the presence of any possible co‐interventions, apart from the primary intervention. This included any health education (formal or informal), training, advice, poster, television or radio promotion, follow up, or support. We took this into consideration in the assessment of the efficacy of the primary intervention.
Data extraction and management
Both authors independently extracted data on the methods, types of participants, interventions, and outcomes. We compared the two sets of extracted data and discussed them to ensure accuracy and completeness. Where necessary, we sought the opinion of a Cochrane Infectious Diseases Group Editor to resolve any disagreements. Where required, we requested additional data from the trial authors.
As packaging interventions may be complex and unique, we also extracted additional information that helps the reader to understand the specific characteristics of the intervention.
Assessment of risk of bias in included studies
We independently assessed the methodological quality of each trial based on the guidance given in the Cochrane Handbook for Systematic Reviews of InterventionsHiggins 2008, and using the criteria outlined below, resolving disagreements through discussion. We displayed this information in a table and described it in the 'Risk of bias in included studies'.
We assessed the generation of the allocation sequence and allocation concealment as adequate, inadequate, or unclear according to Juni 2001. We considered the completeness of outcome data to be adequate if it was greater than 80%. We classified assessor blinding (all other types of blinding are redundant with pre‐packaging interventions) as present, absent, or unclear.
Data synthesis
We undertook a descriptive interpretation of results. We described all possible sources of heterogeneity between the interventions according to the criteria outlined above under description of intervention. Where possible, we also pooled the data in a meta‐analysis using the methods described below.
We used Review Manager 5.0 (RevMan 2008) to make comparisons between the treatment and control groups in an intention‐to‐treat analysis. We combined binary data using risk ratio (RR) and the fixed‐effect model and presented them with 95% confidence intervals (CIs). We assessed heterogeneity by visually examining the forest plots and by using the Chi2 test for heterogeneity with a 5% level of statistical significance, but detected none.
We will use the following planned analyses, which were not required in this version because of insufficient studies, when we update this review with new trials. (1) We will analyse continuous data using the mean difference with 95% confidence intervals. (2) If we detect statistically significant heterogeneity, and it is still appropriate to pool the data, we will use the random‐effects model. (3) We will explore potential sources of heterogeneity using subgroup analyses based on the criteria outlined under description of intervention, and the proportion of participants known to have completed the full treatment regimen. (4) We will conduct sensitivity analyses on the basis of trial methodological quality (using the quality criteria outlined above) and the adequacy of reporting of co‐interventions (in particular, whether or not it was explicitly verified that an equal level of concomitant health education was provided in the intervention and control arms of the trial). (5) We will consider publication bias using a funnel plot.
Results
Description of studies
Results of the search
We identified 57 articles from the search strategy and excluded 45 after scanning the titles. The remaining 12 papers reported 13 potentially relevant studies, of which eight did not meet the inclusion criteria (seeCharacteristics of excluded studies). Five trials met our inclusion criteria (Li 1998a; Li 1998b; Yeboah‐Antwi 2001; Ansah 2003; Lauwo 2006); they are detailed in the 'Characteristics of included studies' and summarized below.
Included studies
Trial design and location
Lauwo 2006 did not state the method of randomization used; Li 1998a, Li 1998b, and Ansah 2003 used alternate allocation techniques to allocate individuals, and Yeboah‐Antwi 2001 randomized health facilities (cluster‐randomization). Li 1998a and Li 1998b were conducted in Health and Epidemic Prevention Stations in Hunan Province, China, where malaria transmission is epidemic, with migration allowing imported cases. Lauwo 2006 was conducted in Papua New Guinea where malaria transmission is high and perennial. The other trials were conducted in Ghana: Ansah 2003 in Cape Coast, where malaria is highly endemic and perennial; and Yeboah‐Antwi 2001 in Wenchi District, where malaria transmission is intense and stable with slight seasonal variation.
Participants
RCTs
Lauwo 2006 randomized 203 adults with clinically and microscopically confirmed malaria into three treatment groups. We have excluded one treatment group because participants did not receive the same counselling co‐intervention as the other groups.
Quasi‐RCTs
Ansah 2003 randomized 299 children with clinically confirmed malaria to two treatment groups. Li 1998a and Li 1998b randomized 596 adults with slide‐confirmed P. vivax malaria to two treatment groups. Li 1998b was conducted two years later in the same region as Li 1998a, but when we contacted the trialists, they clarified that no participant was included in both trials.
Cluster‐RCT
Yeboah‐Antwi 2001 randomized six health facilities into two treatment groups. At these facilities, 616 children and adults with clinical malaria were recruited. Participants up to six years were given syrup, and those over six years received tablets. We have only included data for the 319 participants who received tablets, as the syrup was not unit‐dose packaged.
Interventions
Li 1998a and Li 1998b provided labelled and boxed blister packs of chloroquine and primaquine tablets and capsules; Yeboah‐Antwi 2001, Ansah 2003 and Lauwo 2006 provided simple labelled and sectioned polythene bags of chloroquine tablets (Yeboah‐Antwi 2001; Ansah 2003, see Appendix 2) or chloroquine and sulphadoxine‐pyrimethamine tablets (Lauwo 2006). In Li 1998a, Li 1998b, and Yeboah‐Antwi 2001 control participants received tablets or capsules in paper envelopes or loose, while in Ansah 2003 control participants received chloroquine syrup in bottles and in Lauwo 2006 control participants received chloroquine and sulphadoxine‐pyrimethamine tablets in separate polythene bags with pen‐written instructions.
Ansah 2003 trained prescribers in using a chart, showing the appropriate dosage by weight, to demonstrate to caregivers how to give the medicine. Yeboah‐Antwi 2001 trained intervention and control facility staff in prescribing and dispensing; all participants were given the same counselling in how to take the drug, but participants at the intervention facilities were given additional counselling. Li 1998a trained staff to give similar oral instructions at the intervention and control facilities. Lauwo 2006 provided staff with a written script containing information, questions, and special instruction including information on the risks associated with malaria if it was not quickly and appropriately treated, and on the need to adhere to medication directions, to complete treatment, to seek further medical attention if adverse effects were experienced, and to return to the hospital if there was no improvement after completion of the medication. Li 1998b did not provide any data about staff training or patient information. Participants were given the drugs to take at home in Li 1998a and Li 1998b; in Yeboah‐Antwi 2001 they were observed taking the first dose at the health centre; and in Ansah 2003 and Lauwo 2006 it is unclear where participants took the drugs.
Outcomes
None of the trials reported on the primary outcome measure, treatment failure, as defined in the inclusion criteria. Instead, Li 1998a tested for parasitaemia and clinical symptoms at day nine (eight‐day regimen), Lauwo 2006 reported cure according to medical notes (undefined) and participants' perception at day four of a three‐day regimen, and Ansah 2003 and Yeboah‐Antwi 2001 assessed wellness of the child at day four (three‐day regimen). Measurements taken at such early time points will not pick up relapses and recrudescence of infection. In an attempt to overcome this, Li 1998b followed a subset of 57 participants to day 100. All five trials measured treatment adherence (which they termed "compliance") through participant interview. Yeboah‐Antwi 2001 also measured the remaining drugs at the end of the treatment course, and Li 1998b marked the drugs with phenobarbital and measured its level in the blood as an indicator of treatment adherence.
Yeboah‐Antwi 2001 and Ansah 2003 recorded some adverse events, while Li 1998a, Li 1998b, and Lauwo 2006 did not measure the incidence of adverse events.
Risk of bias in included studies
See the 'Characteristics of included studies' for details about the methodology, and Appendix 3 for the risk of bias assessment.
Only the cluster‐RCT, Yeboah‐Antwi 2001, used an adequate method to generate the allocation sequence. The three quasi‐RCTs, Li 1998a, Li 1998b, and Ansah 2003, used alternate allocation, and Lauwo 2006 did not state the method used. None of the trials provide any details of the method of allocation concealment, although allocation was not likely to have been concealed in the quasi‐RCTs as the method of alternate allocation would lead to a predictable allocation sequence. The outcome assessor was not blinded in any of the trials (trialists provided additional data for Li 1998a and Li 1998b). It was not possible to blind participants or healthcare providers in Yeboah‐Antwi 2001 but, as the health facilities were far apart and communication was not good, it is unlikely that treatment allocation would influence participants in their choice of facility (intervention or control). It was only possible to calculate the completeness of outcome data for two of the trials, Ansah 2003 and Lauwo 2006. Ansah 2003 included 99% of the participants in the analysis, which we considered to be adequate, and Lauwo 2006 included 73%, which we considered to be inadequate.
The cluster‐RCT, Yeboah‐Antwi 2001, used three intervention and three control groups and treated the data as if it came from a trial that randomized individuals (not taking account of the clustering). As participants recruited at the same facility are likely to be more similar than participants recruited at different facilities, there is likely to be a high level of correlation between trial participants, which the trialists have not evaluated or considered.
Effects of interventions
Blister packs versus paper envelopes: 596 participants, two quasi‐RCTs
Although the trials did not assess treatment failure, Li 1998a found that all 324 participants (intervention and control) were aparasitaemic and asymptomatic at day nine of an eight‐day regimen, and Li 1998b found that one of the 57 participants still available for follow up at day 100 had recrudesced at day 88 (control group; reported not to have completed treatment).
A combined estimate from the two trials found that 18% more participants in the group using blister packs reported that they adhered to the full regimen (measured by participant interview), which is statistically significant (RR 1.18, 95% CI 1.12 to 1.25; 596 participants; Analysis 1.1). Li 1998b analysed the data separately for each drug and found a statistically significant difference, in favour of the intervention, in both drug groups (chloroquine 67/69 versus 56/67, P value < 0.05; primaquine 67/69 versus 49/67, P value 0.05; trialists' values). Li 1998b also measured drug marker levels and found no statistically significant difference in the median phenobarbital level‐to‐dose ratio between the intervention and control groups (trialists' data; seeAppendix 4).
1.1. Analysis.

Comparison 1 Blister‐packed tablets and capsules versus tablets and capsules in paper envelopes, Outcome 1 Treatment adherence (measured by interview).
Sectioned polythene bags versus bottled syrup: 299 participants, one quasi‐RCT
Ansah 2003 did not measure treatment failure, but by day four of the three‐day regimen most participants were considered fully recovered by their caregivers (intervention 144/155 versus control 137/144).
Participant‐reported treatment adherence (measured by participant interview) was 115% higher in those receiving tablets in sectioned polythene bags than in those receiving bottles of syrup (RR 2.15, 95% CI 1.76 to 2.61; 299 participants; Analysis 2.1). The volume of the instrument used to dispense the syrup to control participants was not considered in the assessment of adherence. This was found to vary from 1 ml to 9 ml, and most were less than the recommended 5 ml; when the trialists took spoon volume into account, only 12/140 of the control participants had taken the recommended 25 mg/kg total dose by day four, but not necessarily according to the prescribed dosage schedule.
2.1. Analysis.
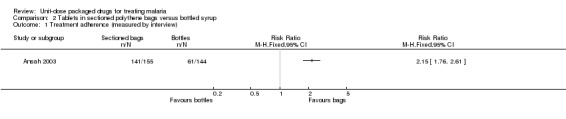
Comparison 2 Tablets in sectioned polythene bags versus bottled syrup, Outcome 1 Treatment adherence (measured by interview).
The trialists did not collect adverse event data systematically. Of the 155 participants receiving tablets, 28 vomited some medication and six vomited all tablets. The trialists reported that if those participants who vomited their tablets were considered non‐adherent, the difference in adherence between intervention and control is still statistically significant (76% versus 42%).
Sectioned polythene bags versus paper envelopes: six clusters, one cluster‐RCT
Yeboah‐Antwi 2001 did not assess treatment failure, but they reported that most participants' wellness had improved by day four of the three‐day regimen (intervention: 152 improved, 13 unchanged, two worsened; control: 143 improved, four unchanged, five worsened).
Treatment adherence (measured by participant interview and drug inspection) was higher in the group receiving tablets in sectioned polythene bags than in those receiving tablets in paper envelopes (137/167 intervention versus 92/152 control). Although reported as statistically significant, the trialists analysed the data without taking account of clustering, so the true result may not be.
The trialists reported a similar incidence of itching, dizziness, and other adverse events. They did not collect these data systematically, but reported them when given as a reason for not taking the drugs as recommended (seeAppendix 5).
Sectioned polythene bags versus polythene bags (unsectioned)
Lauwo 2006 did not assess treatment failure, but reported no significant difference in the cure rate at day four (intervention 77/91 versus control 96/112).
There was no statistically significant difference intreatment adherence between treatment groups (measured by participant interview; Analysis 3.1). However, the authors found both intervention and control group participants to have better adherence than a further control group which received neither packaging nor counselling.
3.1. Analysis.
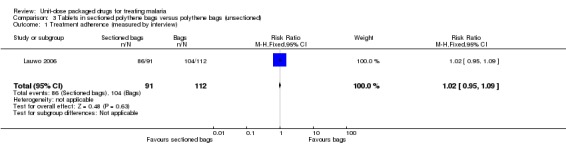
Comparison 3 Tablets in sectioned polythene bags versus polythene bags (unsectioned), Outcome 1 Treatment adherence (measured by interview).
Discussion
There is insufficient good quality evidence to determine the effect of unit‐dose packaged antimalarial drugs on treatment failure, but five small trials suggest that some designs may improve adherence to treatment for uncomplicated malaria when supported by prescriber training and patient information. However, the reliability of these conclusions is limited by the methodological quality of the trials that inform it.
Of the five RCTs that met our inclusion criteria, we were only able to calculate the number of randomized participants included in the analysis for two of them. Four used an inadequate or unclear method of allocation sequence generation, and the other randomized health facilities rather than patients. This cluster‐RCT included only a small number of health facilities, which reduces the statistical power of the trial, as it is likely that data for participants recruited at the same treatment facility will be more correlated than data for participants recruited at different facilities. In fact, the trialists ignored the possibility that data for participants were correlated in the analyses and, as such, this trial may overestimate the treatment effect. None of the trials blinded the outcome assessor, and it is likely that most trials did not conceal allocation of treatment, which allows the potential for bias. While there are limitations in the interpretation of the results of the included trials, they are supported by some observational studies that also suggest an improvement in adherence with the introduction of packaged antimalarial drugs (Browne 2001; Sirima 2003).
Patient interview was the predominant method used to approximate treatment adherence in the included trials (one trialist verified this measure by inspecting left‐over drugs). This is subject to bias, as participants will not always recall accurately how they took the drug. Another way to estimate what treatment was taken is to measure drug concentrations in the blood. However, this only provides a snap shot of medication taking. Reliable approaches to measuring treatment adherence are needed in order to assess the effects of adherence interventions accurately, such as unit‐dose packaging.
Most of the included trials assessed treatment adherence in terms of the participants drug‐taking behaviour in relation to the treatment guidelines. Although efforts were made by the trial authors to verify that prescribers were also adhering to the treatment guidelines this is difficult to ensure and, therefore, non‐adherence could be due to either prescriber or patient error. Yeboah‐Antwi 2001 looked at prescribers' ability to adhere to treatment guidelines and found that despite a background training session conducted for all prescribers (at intervention and control facilities), more participants at the intervention facilities were prescribed the correct doses. This suggests that unit‐dose packaging interventions help both the prescriber and the patient.
Some investigators have developed interventions for packaging paediatric syrup by adding dosing diagrams and spoons (Okonkwo 2001; Yeboah‐Antwi 2001), but at present no unit‐dose style packaging has been developed for syrup. Others have begun examining the use of unit‐dose packaged tablets for children instead. Ansah 2003 compared unit‐dose packaged tablets with bottled syrup, and found a large improvement in adherence. The increased ease of dosage due to a change from syrup to tablets will probably have played a large part in the observed effect in this trial. The caregivers involved in this trial accepted and even preferred tablets for their children (Ansah 2003). There was a slight problem with some children vomiting tablets. Investigators addressed this concern by suggesting caregivers crush and mix the tablets with sugar. However, as Standing 2004 asserts, it has not been shown that this method adequately delivers the drug, especially as the tablets have also been snapped in order to dose the children correctly. This is known to lead to wastage. One way to overcome this problem may be to manufacture special paediatric formulations, or to focus efforts on helping mothers to dose syrup correctly.
The design of packaging interventions, and the context in which they are used, is diverse and care should be taken when generalizing conclusions about their effectiveness from one design to another or from one region to another. Good drug‐packaging design is informed by users' needs. Qualitative investigation has shown that unit‐dose packaged drugs are generally acceptable or even preferred (Ansah 2003), but further studies should help determine the most effective design and elucidate which co‐interventions help maximize this effectiveness within and between treatment settings. Prescriber training and patient information is likely to be a critical factor in how well such interventions work in practice. In these trials, while it was usually intended for staff at the intervention and control facilities to receive comparable levels of training, it often transpired that staff at the intervention facilities received more comprehensive training and therefore gave the patients more comprehensive information. It is likely that the pack was used to guide the information given to patients. It was new and had to be explained. In fact, the Okonkwo 2001 trial found a significant difference in adherence between packaged drugs with verbal instructions and packaged drugs without verbal instructions (packaging not unit dose in this trial).
Normal practice circumstances are likely to differ dramatically from trial conditions. All trials included in this review were conducted intensively in a small locality, using a small number of government health facilities. Most prescribers were fairly highly qualified and were given training in how to dispense the unit‐dose packaged drugs with information to help patients take them correctly. If interventions such as these are going to be used in a wider setting, for example with multinationals producing packs on a large scale, the same level of one‐to‐one training is not likely to be provided to prescribers and dispensers, and the packs will have to work in a variety of settings in which they are unlikely to have been tested.
There is also the challenge of designing packs that can be used effectively in more rural settings. Here, drugs are more likely to be distributed by unregulated (normally unqualified) private drug vendors. In fact, some episodes of malaria will be treated in the home using leftover drugs without any contact with a health facility or drug vendor. In both of these situations, the packs will either have to be designed to stand alone (with no supportive co‐interventions) or shopkeepers will have to be trained in dispensing. As it is not always possible to ensure a good level of prescriber‐patient information, sector‐wide supportive interventions may be a useful tool to help increase the general awareness of unit‐dose packaged drugs (Marsh 2004). The WHO has recognized this and are preparing recommendations that packaged drugs be given as part of wider Information, Education, and Communication campaigns involving, for example, schools, printed posters, and television (Browne 2001).
We have found only five trials investigating the unit‐dose packaging of malaria treatment. These trials all used chloroquine‐based therapies, regimens which are no longer recommended for use in the treatment of uncomplicated falciparum malaria (WHO 2006). While all trials appear to show an increase in participant‐reported adherence with unit‐dose packaged drugs, they did not demonstrate an effect on treatment effectiveness and patient outcomes. Three of the four included trials used combination therapy (chloroquine‐based) (Li 1998a; Li 1998b; Lauwo 2006). However, in Li 1998a and Li 1998b the investigators did not optimize the potential of the packaging by presenting the drugs together in one pack. With the spread of multiple‐drug resistant malaria, it would be useful to know how effective unit‐dose packaging is when used with the currently recommended artemisinin‐based combination therapies (ACTs). As the majority of deaths due to malaria occur in children aged five years and under, efforts should be targeted at developing paediatric packs.
Authors' conclusions
Implications for practice.
Trials of unit‐dose packaged antimalarial drugs have not assessed treatment failure. When supported by prescriber training and patient information, some package designs appear to improve participant‐reported treatment adherence in the settings in which they have been tested. However, some methodological limitations of the included trials should be taken into consideration when interpreting these findings.
Implications for research.
Unit‐dose packaging interventions are complex interventions. Qualitative investigations could help determine the most reproducible and the most effective designs. Ideally, packs should be developed with input from the local community, with due consideration for the settings in which they will be employed and the need for any additional interventions (such as educational programmes) to help optimize their effectiveness.
Good quality RCTs of unit‐dose packaged combination therapies for malaria, particularly of formulations for children under five, are urgently needed. Investigators should assess treatment failure and adverse events, not just treatment adherence. There is a need to develop reliable methods of estimating treatment adherence in these trials.
What's new
| Date | Event | Description |
|---|---|---|
| 26 May 2009 | New search has been performed | One new trial included in the review. Inclusion criteria updated to exclude controlled before and after studies. Treatment failure at day 14 removed from primary outcome measures and made secondary. No change to conclusions. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 3 November 2008 | Amended | Converted to new review format with minor editing. |
| 11 January 2005 | Amended | Issue 2, 2005 (Deviations from protocol) (1) Title: changed from 'Pre‐packaged drugs for treating malaria' to 'Unit‐dose packaged drugs for treating malaria' for a more specific definition of the intervention. (2) Types of intervention: different drug formulations may now be used in the intervention and control arms, as these may form useful comparisons, but we will not combine the data with data from studies using the same formulation in intervention and control arms. (3) Types of outcomes: treatment failure outcome measures altered to conform to new standards; this involved removing "parasite failure rate" and replacing "early" and "late" treatment failure with treatment failure on or by day 14/28. (4) Methods of the review: inclusion of 80% of all randomized participants considered adequate, rather than 90%, to take into account the difficulty in carrying out studies of this type of intervention. |
Acknowledgements
None stated.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | packaging | drug packaging | drug packaging | drug packaging | drug packaging |
| 2 | malaria | tablet | DRUG PACKAGING | DRUG PACKAGING | malaria |
| 3 | — | capsule | tablet | DRUG DOSAGE FORM | 1 and 2 |
| 4 | — | syrup | capsule | tablet | — |
| 5 | — | blister pack | syrup | TABLET | — |
| 6 | — | unit dose pack | blister pack | capsule | — |
| 7 | — | 1 or 2 or 3 or 4 or 5 or 6 | unit dose pack | syrup | — |
| 8 | — | malaria | 1 or 2 or 3 or 4 or 5 or 6 or 7 | blister pack | — |
| 9 | — | 7 and 8 | malaria | unit dose pack | — |
| 10 | — | — | MALARIA | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 | — |
| 11 | — | — | 9 or 10 | malaria | — |
| 12 | — | — | 8 and 11 | MALARIA | — |
| 13 | — | — | — | 11 or 12 | — |
| 14 | — | — | — | 10 and 13 | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Alderson 2004); upper case MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Interventions
| Trial | Regimen | Packaging type | Labels/instructions | Training/counselling | Dosage presentation |
| Ansah 2003 | 3‐day regimen of chloroquine Intervention: tablets Control: syrup |
Intervention: hermetically sealed, sectioned polythene bags with daily dose of tablets in each section Control: bottles of syrup |
Intervention: each section labelled 1, 2, 3 (day to be taken) Control: unclear |
Intervention and control: prescribers given chart showing appropriate dosage by weight; trained in using chart to tell caregivers how to give the medicine | Intervention: 8 dosage presentations available (by weight) Control: no dosage presentations; all receive 60 ml syrup and instructed to give weight‐tailored dose |
| Lauwo 2006 | 3‐day regimen of chloroquine, plus sulphadoxine‐pyrimethamine on the first day Intervention: tablets Control: tablets |
Intervention: sealed clear sectioned polythene bags stapled to card base with daily dose of tablets in each colour‐coded section Control: separate polythene bags for chloroquine and sulphadoxine‐pyrimethamine |
Intervention: name of drugs and instructions written under each section Control: pen‐written instructions |
Intervention and control: counselling given by intern pharmacists from written script covering risks of malaria if treated quickly and appropriately; need to adhere to medication directions, to complete treatment, to seek further medical attention if experience adverse events; and to return to outpatients if no improvement | Intervention: no dosage presentations available Control: no dosage presentations available |
| Li 1998a | 1. 3‐day regimen of chloroquine 2. 8‐day regimen of primaquine Intervention and control: tablets |
Intervention: 1 boxed blister pack for each drug course; tablets to be taken each day together in 1 blister Control: paper envelopes |
Intervention: drug name on blister pack (back) and box (front); dosage instructions and precautions on back of box; instruction leaflet inside box Control: none |
Intervention: health staff trained to give oral instructions to patients; patients asked to read instruction leaflet and indicate understanding; instructions repeated if poor understanding indicated Control: health staff trained to give oral instructions to patients; instructions repeated if poor understanding indicated |
Intervention: 1 dosage presentation, for adults Control: none |
| Li 1998b | 1. 3‐day regimen of chloroquine 2. 8‐day regimen of primaquine Intervention and control: tablets/capsules |
Intervention: 1 boxed blister pack for each drug; tablets/capsules to be taken each day together in 1 blister Control: paper envelopes |
Intervention: drug name on blister pack (back) and box (front); dosage instructions and precautions on back of box; instruction leaflet inside box Control: none |
Intervention and control: no information | Intervention: 1 dosage presentation, for adults Control: none |
| Yeboah‐Antwi 2001 | 3‐day regimen of chloroquine Intervention and control: tablets |
Intervention: hermetically sealed, sectioned polythene bags with daily dose of tablets in each section Control: paper envelopes |
Intervention: bag labelled with name of drug; each section labelled 1, 2, 3 (day to be taken) Control: none |
Intervention: prescribers and dispensers trained in prescribing and dispensing drugs; told to use labelling when instructing participants on how to take medication Control: same counselling as intervention group in terms of how much drug to take, but there were significant differences in all other aspects of counselling; more participants in the intervention group were told the names of drugs, purpose of drugs, how long to take them, and some possible side effects Note: staff at the control facilities were more qualified as dispensers than those at the intervention facilities; all participants in the intervention group were served by auxiliary staff with no baseline training; about 2/3 participants in the control group were served by a dispensing assistant/attendant with training in dispensing |
Intervention: 3 dosage presentations available, 1 for each age group: 7 to 11 year; 12 to 15 years; and 16 and above years Control: no dosage presentations available |
Appendix 3. Risk of bias assessmenta
| Trial name | Allocation sequence generation | Allocation concealment | Blinding of outcome assessor | Inclusionb |
| Ansah 2003 | Inadequate | Unclear | Absent | Adequate |
| Lauwo 2006 | Unclear | Unclear | Absent | Inadequate |
| Li 1998a | Inadequate | Unclear | Absent | Unclear |
| Li 1998b | Inadequate | Unclear | Absent | Unclear |
| Yeboah‐Antwi 2001c | Adequate | Unclear | Absent | Unclear |
aSee the 'Assessment of risk of bias in included studies' for methods and the 'Characteristics of included studies' for the methods used in each trial. bInclusion of all randomized participants in the final analysis. cTrialists did not take clustering into account in the analysis of this cluster‐randomized controlled trial.
Appendix 4. Treatment adherence (level to dose ratio of drug marker; from Li 1998b)
| Antimalarial drug | Tablets and capsules | |
| Blister packsa | Paper envelopesa | |
| Chloroquine | Number of participants: 36 Median: 3.86 Range: 1.2 to 18.85 |
Number of participants: 23 Median: 3.46 Range: 1.99 to 30.9 |
| Primaquine | Number of participants: 31 Median: 7.25 Range: 2.29 to 19.99 |
Number of participants: 34 Median: 7.12 Range: 3.74 to 30.24 |
Appendix 5. Adverse events (from Yeboah‐Antwi 2001a)
| Adverse eventb | Sectioned bagsc | Paper envelopesd |
| Itching | 12/167 | 16/152 |
| Dizziness | 6/167 | 5/152 |
| "Other" | 5/167 | 3/152 |
aCluster‐randomized controlled trial. bNumber of participants experiencing the event. cSectioned polythene bags (tablets). dTablets.
Data and analyses
Comparison 1. Blister‐packed tablets and capsules versus tablets and capsules in paper envelopes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment adherence (measured by interview) | 2 | 596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.12, 1.25] |
Comparison 2. Tablets in sectioned polythene bags versus bottled syrup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment adherence (measured by interview) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Tablets in sectioned polythene bags versus polythene bags (unsectioned).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment adherence (measured by interview) | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ansah 2003.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation Allocation concealment: no information provided Blinding of outcome assessor: none Completeness of outcome data: 99% |
|
| Participants | Participants: 299 analysed (155 intervention; 144 control); 301 initially recruited Inclusion criteria: aged 0 to 5 years; brought to 1 of 2 clinics with malaria Exclusion criteria: none given, but 2 participants excluded on developing severe malaria Mean age: not stated Age range: not stated Male to female ratio: not stated Education: not stated |
|
| Interventions | See Appendix 2 for details | |
| Outcomes |
Not included in review:
|
|
| Notes | Location: Cape Coast, Ghana Health facilities: 2 public health centres Endemicity: highly endemic malaria Antimalarial drug resistance: not stated Malaria diagnosis: clinical (Plasmodium falciparum causes most morbidity and mortality in this area) |
|
Lauwo 2006.
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: no information provided Blinding of outcome assessor: none Completeness of outcome data: 73% |
|
| Participants | Participants: 103 analysed (91 intervention; 112 control); 180 initially recruited Inclusion criteria: adult; clinical and microscopic diagnosis of malaria; prescribed standard antimalarial drugs; spoken and understood English, Tok Pisin or Motu Exclusion criteria: inability to return for follow‐up interview on day 4 Mean age: not stated Age range: not stated Male to female ratio: 242:194 Education: not stated |
|
| Interventions | see Appendix 2 for details | |
| Outcomes | 1. Treatment failure at day 4
2. Treatment adherence at day 4: interview ‐ good adherence if remember instructions and complete medication as prescribed or directed Not included in review: 3. Health professionals' reactions to the packaging |
|
| Notes | Location: Port Moresby, Papua New Guinea Health facilities: outpatient department of Port Moresby General Hospital Endemicity: not stated Antimalarial drug resistance: chloroquine and amodiaquine Malaria diagnosis: clinical and microscopic |
|
Li 1998a.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation Allocation concealment: no information provided Blinding of outcome assessor: none Completeness of outcome data: number analysed provided but number recruited not provided |
|
| Participants | Participants: 324 analysed (161 intervention; 163 control); no data provided on how many participants were initially recruited Inclusion criteria: slide positive for Plasmodium vivax malaria; aged > 15 years; ambulatory Exclusion criteria: major clinical symptoms requiring hospitalization; malaria treatment in the previous 6 months Mean age: 31 years Age range: 16 to 63 years Male to female ratio: 300:24 Education: not stated |
|
| Interventions | See Appendix 2 for details | |
| Outcomes |
|
|
| Notes | Location: Hunan province, China Health facilities: staff highly qualified, each station had good working relationship with provincial authorities Endemicity: epidemic (imported) malaria Antimalarial drug resistance: not stated Malaria diagnosis: microscopically confirmed Plasmodium vivax malaria |
|
Li 1998b.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation Allocation concealment: no information provided Blinding of outcome assessor: none Completeness of outcome data: number analysed provided but number recruited not provided |
|
| Participants | Participants: 272 analysed (138 intervention; 134 control); no information provided on the number of participants initially recruited Inclusion criteria: slide positive for Plasmodium vivax malaria; aged > 15 years; ambulatory Exclusion criteria: major clinical symptoms requiring hospitalization; malaria treatment in the previous 6 months Mean age: not stated Age range: 11 to 67 years Male to female ratio: mostly male Education: not stated |
|
| Interventions | See Appendix 2 for details | |
| Outcomes |
Not included in review:
|
|
| Notes | Location: Hunan province, China; conducted in same region as Li 1998a, but different participants (clarified following correspondence with trialists) Health facilities: staff highly qualified, each station had good working relationship with provincial authorities Endemicity: epidemic (imported) malaria Antimalarial drug resistance: not stated Malaria diagnosis: microscopically confirmed Plasmodium vivax malaria |
|
Yeboah‐Antwi 2001.
| Methods | Cluster‐randomized controlled trial Generation of allocation sequence: adequate Allocation concealment: no information provided Blinding of outcome assessor: none Completeness of outcome data: number analysed provided but number recruited unclear Trialists did not take clustering into account in the analysis of this cluster‐randomized controlled trial |
|
| Participants | Participants: facilities randomized rather than participants; 3 intervention facilities (262 participants) and 3 control facilities (247 participants); 190 participants excluded from this review as they did not receive unit‐dose packaged drugs; unclear how many were recruited initially Inclusion criteria: diagnosed with malaria; treated with chloroquine; living in the town of the facility Exclusion criteria: diagnosed as having malaria but not treated with chloroquine; living outside the town where the facility is located Mean age: not stated Age range: adults and children (7+ years) Male to female ratio: 197:312 Education: 309 none; 170 primary; 30 other Note: these data are for the entire trial; we have excluded participants receiving syrup from the review |
|
| Interventions | See Appendix 2 for details | |
| Outcomes |
Not included in review:
|
|
| Notes | Location: Wenchi District, Brong Ahafo Region, Ghana Health facilities: no data available Endemicity: intense stable malaria transmission with slight seasonal variations (45.9% to 46.8%) Antimalarial drug resistance: chloroquine resistance said to be low (only drug used at health centres, health posts and rural clinics) Malaria diagnosis: confirmed clinically (Plasmodium falciparum is the main cause of morbidity and mortality in this region) |
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agyepong 2002 | This trial was not controlled |
| Browne 2001 | This trial was not controlled |
| Cullinan 1997 | Participants included in this trial had severe malaria |
| Krudsood 2002 | Both treatment groups received identical packaging intervention |
| Okonkwo 2001 | The packaging used in this trial was not unit‐dose packaging |
| Pagnoni 1997 | This trial was not controlled |
| Shwe 1998 | Both treatment groups received identical packaging interventions |
| Sirima 2003 | This trial was not controlled |
Differences between protocol and review
2005, Issue 2 (first review version): (1) Title: changed from 'Pre‐packaged drugs for treating malaria' to 'Unit‐dose packaged drugs for treating malaria' for a more specific definition of the intervention. (2) Types of intervention: different drug formulations may now be used in the intervention and control arms, as these may form useful comparisons, but we will not combine the data with data from studies using the same formulation in intervention and control arms. (3) Types of outcomes: treatment failure outcome measures altered to conform to new standards; this involved removing "parasite failure rate" and replacing "early" and "late" treatment failure with treatment failure on or by day 14/28. (4) Methods of the review: inclusion of 80% of all randomized participants considered adequate, rather than 90%, to take into account the difficulty in carrying out studies of this type of intervention.
Contributions of authors
Lois Orton extracted and analysed data and drafted the review. Guy Barnish extracted data and commented on the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
Declarations of interest
Guy Barnish was involved in one of the included trials, however he knows of no conflicts of interest. Lois Orton has no known conflicts of interest.
Unchanged
References
References to studies included in this review
Ansah 2003 {published data only}
- Ansah EK, Gyapong JO, Agyepong IA, Evans DB. Improving adherence to malaria treatment for children: the use of pre‐packed chloroquine tablets vs. chloroquine syrup. Tropical Medicine and International Health 2003;6(7):496‐504. [DOI] [PubMed] [Google Scholar]
Lauwo 2006 {published and unpublished data}
- Lauwo JAK, Hombhanje FW, Tulo SP, Maibani G, Bjorge S. Impact of pre‐packaging antimalarial drugs and counselling on compliance with malaria treatment at Port Moresby General Hospital Adult Outpatient Department. Papua New Guinea Medical Journal 2006;49:14‐21. [PubMed] [Google Scholar]
Li 1998a {published and unpublished data}
- Li Q, Duan J, Tang L, Zhang X, Liang J, Hay A, et al. The effect of drug packaging on patients' compliance with treatment for Plasmodium vivax malaria in China. Bulletin of the World Health Organization 1998;76 Suppl 1:21‐7. [PMC free article] [PubMed] [Google Scholar]
Li 1998b {published and unpublished data}
- Li Q, Duan J, Tang L, Zhang X, Liang J, Hay A, et al. The effect of drug packaging on patients' compliance with treatment for Plasmodium vivax malaria in China. Bulletin of the World Health Organization 1998;76 Suppl 1:21‐7. [PMC free article] [PubMed] [Google Scholar]
Yeboah‐Antwi 2001 {published and unpublished data}
- Yeboah‐Antwi K, Gyapong JO, Asare IK, Barnish G, Evans DB, Adjei S. Impact of prepackaging antimalarial drugs on cost to patients and compliance with treatment. Bulletin of the World Health Organization 2001;79(5):394‐9. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Agyepong 2002 {published data only}
- Agyepong IA, Ansah E, Gyapong M, Adjei S, Barnish G, Evans D. Strategies to improve adherence to recommended chloroquine treatment regimes: a quasi‐experiment in the context of integrated primary health care delivery in Ghana. Social Science & Medicine 2002;55:2215‐26. [DOI] [PubMed] [Google Scholar]
Browne 2001 {unpublished data only}
- Browne ENL, Hall‐Baidu D, Sangber‐Dery F, Owusu‐Antwi F. Early appropriate management of fevers in children aged 6 months to 6 years in Ghana [unpublished]. World Health Organization TDR PROJECT No. 990614 2001.
Cullinan 1997 {published data only}
- Cullinan TR, Pieterick C. Packaged treatment for first‐line care in cerebral malaria and meningitis. Bulletin of the World Health Organization 1998;76(3):257‐64. [PMC free article] [PubMed] [Google Scholar]
Krudsood 2002 {published data only}
- Krudsood S, Looareesuwan S, Silachamroon U, Chalermrut K, Pittrow D, Canbon N, et al. Artesunate and mefloquine given simultaneously for three days via a prepacked blister is equally effective and tolerated as a standard sequential treatment of uncomplicated acute Plasmodium falciparum malaria: randomized, double‐blind study in Thailand. American Journal of Tropical Medicine and Hygiene 2002;67(5):465‐72. [DOI] [PubMed] [Google Scholar]
Okonkwo 2001 {published data only}
- Okonkwo PO, Akpala CO, Okafor HU, Mbah AU, Nwaiwu O. Compliance to correct dose of chloroquine in uncomplicated malaria correlates with improvement in the condition of rural Nigerian children. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(3):320‐4. [DOI] [PubMed] [Google Scholar]
Pagnoni 1997 {published data only}
- Pagnoni F, Convelbo N, Tiendrebeogo J, Cousens S, Esposito F. A community‐based programme to provide prompt and adequate treatment of presumptive malaria in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(5):512‐7. [DOI] [PubMed] [Google Scholar]
Shwe 1998 {published data only}
- Shwe T, Lwin M, Aung S. Influence of blister packaging on the efficacy of artesunate + mefloquine over artesunate alone in community‐based treatment of non‐severe falciparum malaria in Myanmar. Bulletin of the World Health Organization 1998;76 Suppl 1:35‐41. [PMC free article] [PubMed] [Google Scholar]
Sirima 2003 {published data only}
- Sirima SB, Konate A, Tiono AB, Convelbo N, Cousens S, Pagnoni F. Early treatment of childhood fevers with pre‐packaged antimalarial drugs in the home reduces severe malaria morbidity in Burkina Faso. Tropical Medicine and International Health 2003;8(2):133‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins J, editors. Highly sensitive search strategy for identifying reports of randomized controlled trials in MEDLINE. Cochrane Reviewers' Handbook 4.2.2 [Updated March 2004]; Appendix 5b. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 25 January 2005).
Becker 1986
- Becker LA, Glanz K, Sobel E, Mossey J, Zinn SL, Knott KA. A randomized trial of special packaging of antihypertensive medications. Journal of Family Practice 1986;22(4):357‐61. [PubMed] [Google Scholar]
Biritwum 2000
- Biritwum RB, Welbeck J, Barnish G. Incidence and management of malaria in two communities of different socio‐economic level, in Accra, Ghana. Annals of Tropical Medicine and Parasitology 2000;94(8):771‐8. [DOI] [PubMed] [Google Scholar]
Bloland 2003
- Bloland PB, Kachur SP, Williams HA. Trends in antimalarial drug deployment in sub‐Saharan Africa. The Journal of Experimental Biology 2003;206(21):3761‐9. [DOI] [PubMed] [Google Scholar]
Francis 1997
- Francis F. The packaging of anti‐malarials in Africa: communication issues [oral presentation]. The Tropical Diseases and Health Sector Reform Task Force Meeting, 10‐12 September 1997, Liverpool.
Gomes 1998
- Gomes M, Wayling S, Pang L. Interventions to improve the use of antimalarials in south‐east Asia: an overview. Bulletin of the World Health Organization 1998;76 Suppl 1:9‐19. [PMC free article] [PubMed] [Google Scholar]
Haynes 1996
- Haynes RB, McKibbon KA, Kanani R. Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet 1996;348(9024):383‐6. [DOI] [PubMed] [Google Scholar]
Haynes 2008
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008, Issue 2. [Art. No.: CD000011. DOI: 10.1002/14651858.CD000011.pub3] [DOI] [PubMed] [Google Scholar]
Heneghan 2006
- Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self‐administered long‐term medications. Cochrane Database of Systematic Reviews 2006, Issue Issue 1. [DOI: 10.1002/14651858.CD005025.pub2.] [DOI] [PubMed] [Google Scholar]
Henry 1999
- Henry A, Batey RG. Enhancing compliance not a prerequisite for effective eradication of Helicobacter pylori: the HelP Study. The American Journal of Gastroenterology 1999;94(3):811‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. [Updated September 2008]. The Cochrane Collaboration, 2008. [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilian 2003
- Kilian AHD, Tindyebwa D, Gulck T, Byamukama W, Rubaale T, Kabagambe G, et al. Attitude of women in western Uganda towards pre‐packed, unit‐dosed malaria treatment for children. Tropical Medicine and International Health 2003;8(5):431‐8. [DOI] [PubMed] [Google Scholar]
Marsh 2004
- Marsh VM, Mutemi WM, Willetts A, Bayah K, Were S, Ross A, et al. Improving malaria home treatment by training drug retailers in rural Kenya. Tropical Medicine and International Health 2004;9(4):451‐60. [DOI] [PubMed] [Google Scholar]
Mullen 1997
- Mullen PD. Compliance becomes concordance. BMJ 1997;314(7082):691‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ondari 2003
- Ondari C. Personal communication 20 March 2003.
Peterson 1984
- Peterson GM, McLean S, Millingen KS. A randomised trial of strategies to improve patient compliance with anticonvulsant therapy. Epilepsia 1984;25(4):412‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Richardson 2003
- Richardson, Edwards. Inc. A brief history of packaging. www.richanded.com/html/packaging_history.htm (accessed 18 September 2003).
Sackett 1979
- Sackett DL, Snow JC. The magnitude of adherence and nonadherence. In: Haynes RB, Taylor DW, Sackett DL editor(s). Compliance in health care. Baltimore: John Hopkins University Press, 1979:11‐22. [Google Scholar]
Standing 2004
- Standing JF, Wong ICK. Chlorproguanil‐dapsone for malaria. Lancet 2004;364(9447):1752‐3. [DOI] [PubMed] [Google Scholar]
Sumartojo 1993
- Sumartojo E. When tuberculosis treatment fails. A social behavioural account of patient adherence. American Review of Respiratory Diseases 1993;147(5):1311‐20. [DOI] [PubMed] [Google Scholar]
Volmink 1997
- Volmink J, Garner P. Systematic review of randomised controlled trials of strategies to promote adherence to tuberculosis treatment. BMJ 1997;315(7120):1403‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1998
- White NJ. Why is it that antimalarial drug treatments do not always work?. Annals of Tropical Medicine and Parasitology 1998;92(4):449‐58. [DOI] [PubMed] [Google Scholar]
White 1999
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. Lancet 1999;353(9168):1965‐7. [DOI] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. A global strategy for malaria for malaria control. Geneva: World Health Organization, 1993. [Google Scholar]
WHO 2000
- World Health Organization, Gilles HM. Management of severe malaria: a practical handbook. 2nd Edition. World Health Organization: Geneva, 2000. [Google Scholar]
WHO 2003
- Malaria Control Department, World Health Organization. An update on quality assurance and procurement through WHO for improving access to artemisinin‐based combination treatments (ACTs) for malaria. http://www.paho.org/English/AD/DPC/CD/mal‐acts‐update‐7‐03.pdf 2003 (accessed 11 November 2003).
WHO 2004
- World Health Organization. The world health report 2004: changing history. World Health Organization: Geneva, 2004. [Google Scholar]
WHO 2006
- World Health Organization. Guidelines for the treatment of malaria 2006.
References to other published versions of this review
Orton 2005
- Orton L, Barnish G. Unit‐dose packaged drugs for treating malaria. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD004614. DOI: 10.1002/14651858.CD004614.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


