Abstract
Background/Objectives:
To address the underrepresentation of older adults in clinical research, the National Institutes of Health will require investigators to include individuals across the lifespan. As investigators from other fields endeavor to recruit participants that are more representative of the patient population, geriatricians may have the opportunity to influence a broad range of research studies in older adults. Our aims were to elicit challenges to inclusion of older adults in clinical research and to develop a preliminary framework for communicating these challenges to non-geriatrics-trained researchers.
Design:
Communication framework development
Setting:
Academic hospital and Veterans Affairs Medical Center
Participants:
Non-geriatrician researchers and staff, aging research experts
Measurements:
Interviews were used to elicit challenges non-geriatrician investigators and research staff experience when conducting research that includes older adults and then solicit experienced aging researchers’ responses to these challenges.
Results:
Challenges described by non-geriatrician investigators included lack of knowledge, rigid study structures, and a disease-focused approach. Responses from our geriatrics experts included communicating practical advice for avoiding common pitfalls. Our resulting framework is the 5Ts: Target population, Team, Tools, Time, and Tips to Accommodate. This tool complements the 5Ms model for geriatric care and emphasizes representation of the Target population, building research Teams that include aging expertise, incorporating appropriate Tools for function and patient-reported outcomes, anticipating Time for longer study visits, and accommodating common needs with practical Tips. Limitations include convenience sampling and lack of formal qualitative thematic analysis.
Conclusion:
Communicating with non-geriatrician researchers using the 5Ts may offer a practical approach to avoiding barriers to inclusion of older adults in research and complements an existing framework for communicating the value of geriatric medicine. Next steps in developing the 5Ts will be to include additional stakeholders (e.g., national samples of non-geriatrician investigators, older adults and their families) and evaluating the impact of its implementation.
Keywords: 5Ts, communication framework, inclusion of older adults, geriatrics research
INTRODUCTION
In geriatric medicine, the patients geriatric healthcare providers spend the most time caring for—the oldest, most complex—are underrepresented in research.1,2 While the prevalence of most chronic diseases increases with age,3 older adults are often excluded from research studies explicitly through age cut-offs or implicitly by excluding those with co-occurring conditions.4,5 For example, more than 50% of those with chronic kidney disease (CKD) are 70 years and older;6,7 however, this age group comprises less than 5% of the clinical trial populations on which many CKD Clinical Practice Guideline recommendations were based.8,9 Older adults have been disproportionately excluded from studies of heart disease, cancer, and diabetes as well.10–12
Underrepresentation of older adults in clinical research results in evidence that may not be generalizable to those who experience the greatest burden of disease.13,14 To address this problem, the National Institutes of Health (NIH) “Inclusion Across the Lifespan” policy will require all grants on or after January 25, 2019 to submit a plan for including individuals of all ages.15 If age-based exclusions are proposed, a scientific justification must be provided. This policy will likely lead to opportunities for geriatricians and gerontologists to influence a broad range of research studies in older adults, as investigators from other fields endeavor to recruit and retain participants that are more representative of the patient population. Taking advantage of this opportunity will require aging research experts to effectively communicate with non-geriatrics-trained investigators. The purpose of this project was to elicit common challenges to inclusion of older adults in clinical research and develop a framework for communicating with non-geriatric-trained researchers to increase inclusion of older adults and as a first step towards improving the relevance of their work for older adult patients.
METHODS
Our approach included three main steps: 1) eliciting the challenges investigators and research staff face when conducting research that includes older adults, 2) asking aging research experts to respond to these challenges, and 3) developing a communication framework. Our approach assumed that underlying the current tendency to underrepresent medically complex older adults in research are a set of practical concerns or barriers regarding their inclusion. Thus, the first step was to create an inventory of the challenges and barriers perceived by investigators and use this list to work with researchers in the aging field to identify potential solutions.
First, our respondent group consisted of researchers and staff who, while not specifically trained in aging research methods, conduct research that is highly relevant to older populations. We selected five interviewees (GI Medicine, Cardiology, and Nephrology) at Duke University and the Durham Veterans Affairs Medical Center. We also interviewed one research coordinator in General Medicine/Endocrinology. Interviews followed a semi-structured interview guide, lasted approximately one hour, and were conducted by the same author (CBB). Interviews were not recorded; however, notes from the interviews were transcribed and used to identify key challenges to research in older adults from the perspective of our interviewees. Next, to check the face validity of responses and allow for elaboration of barriers described in our initial interviews, we conducted a round of interviews in which we asked interviewees to respond to the list of challenges. This round of interviews included seven investigators from General Medicine, Biostatistics, Epidemiology, Psychology, and Emergency Medicine. We compiled responses from both rounds of interviews and identified major themes.
Second, we presented the list of challenges to six geriatrics and gerontology experts during interviews. Experts were asked how they would respond to challenges described by non-aging research investigators and staff. Our goal in this step was to elicit data that could be organized into communication strategies and advice for responding to our non-geriatrician colleagues. Questions were phrased as “how would you respond to a colleague who experiences this challenge?” Notes from these interviews were used to develop a communication framework.
Lastly, we developed the communication framework based on these interviews. This included summarizing the challenges and responses from the first two steps. We used an iterative approach with a goal of developing a simple, easy-to-remember framework with wide applicability. We reviewed similar communication strategies used in clinical geriatrics. As the framework was developed, we presented drafts to colleagues in geriatrics as well our interviewees for feedback.
RESULTS
None of the investigators interviewed endorsed explicitly excluding older adults or the use of upper-age limits for study inclusion. However, they did report several barriers to engaging older adults in research and retaining them in their studies. These challenges were grouped into three main categories: knowledge, study structure, and disease-focused approach (Table 1). Interviewees reported that without training in geriatrics or aging research methods, they had little knowledge about addressing geriatric syndromes and common age-related impairments when these problems arose during enrollment or study participation. Lack of knowledge about existing measures or functional assessment tools and frustration with multiple ways to measure frailty or functional status were also reported as limitations.
Table 1.
Challenges faced by non-geriatrician subspecialists when conducting research that includes older adults
| Challenge | Examples | Need |
|---|---|---|
| Lack of knowledge | • No training in aging research, geriatric syndromes, or common age-related impairments • Unaware of knowledge gaps and high priority research questions in aging • Overwhelmed by range of existing measures (e.g., phenotypic frailty vs. deficit accumulation) • Not sure when to seek expertise in aging research |
Conceptual framework to anticipate problems and plan for solutions Team members with content expertise |
| Rigid study structure | • Emphasis on meeting recruitment targets • Inflexible and complex study protocols that often require multiple in-person visits • Concerns about high withdrawal rates and need to report unrelated adverse events • Regulatory requirements increase study complexity (e.g., consent forms) |
Operational approach to standardizing inclusion of older adults Practical strategies for accommodating those with age-related limitations |
| Focus on individual disease processes | • Underappreciate value of geriatrics/gerontology research expertise • Skeptical that mechanisms of disease differ in younger vs. older adults • Outcomes chosen based on relevance to disease of interest |
Concise description of value of a geriatrics approach |
Our interviewees also described challenges related to the inflexible study structure that they considered to be too burdensome for some older adults. They described these challenges often as being due to external forces beyond their control: penalties for low recruitment, protocols that other investigators designed, and onerous regulatory requirements. Although functional status and cognition may not be routinely assessed, indications of these problems (e.g., use of a wheelchair, having to repeat instructions) were used to avoid enrolling some older adults. Interviewees described the need for operationalized and standardized approaches to be included in their protocols. An additional need was for practical strategies to accommodate those with age-related impairments or multiple chronic conditions.
The third category of challenges identified by non-geriatrician interviewees related to the investigators’ desire to focus on individual diseases and the concern that aging effects or comorbid conditions would mask the ability to report on effects related to the single disease. Including older adults in their work was an unintended consequence of the higher prevalence of these conditions at older age, rather than an intentional desire to study aging. This disease focus lead some of our interviewees to question the value of geriatrics or gerontology research expertise.
Responses from our geriatrics and gerontology experts related to 1) communicating practical advice for avoiding pitfalls in research that includes older adults and 2) communicating geriatric principles and the value of a geriatric approach. The practical advice was related to understanding the population that the study findings will be generalized to, engaging interdisciplinary teams, and providing expertise in the use and interpretation of commonly used geriatric assessment tools. Communicating the value of a geriatric approach, which has been a goal of geriatrics medicine for many years,16 included knowledge of geriatric principles, common problems such as cognitive impairment, and the emphasis on patient-centered outcomes.
With these responses in mind, we are proposing the 5Ts (Figure 1). This framework describes maximizing generalizability by enrolling participants from the Target population, building research Teams that include geriatrics and gerontology expertise, incorporating appropriate Tools to measure function and patient-reported outcomes, anticipating Time for longer study visits, and accommodating older participants with comorbidities and age-related impairments by following practical Tips. This research communication framework is complementary to an existing framework for communicating the value of Geriatrics in clinical practice—The 5Ms (Mind, Mobility, Medications, Multi-complexity, and Matters Most to Me).17,18 While designed for clinical purposes, the 5Ms are clearly relevant to research that includes older adults as well. For example, Mind, referring to cognitive impairment, has important implications for assessing capacity to provide informed consent. There were some challenges described by our interviewees and responses from our experts that were specific to research and are not easily addressed in the 5Ms alone. Therefore, we believe that use of the 5Ts, to address practical research issues, along with the 5Ms, to describe geriatric principles, can serve as a comprehensive communication framework (Table 2).
Figure.
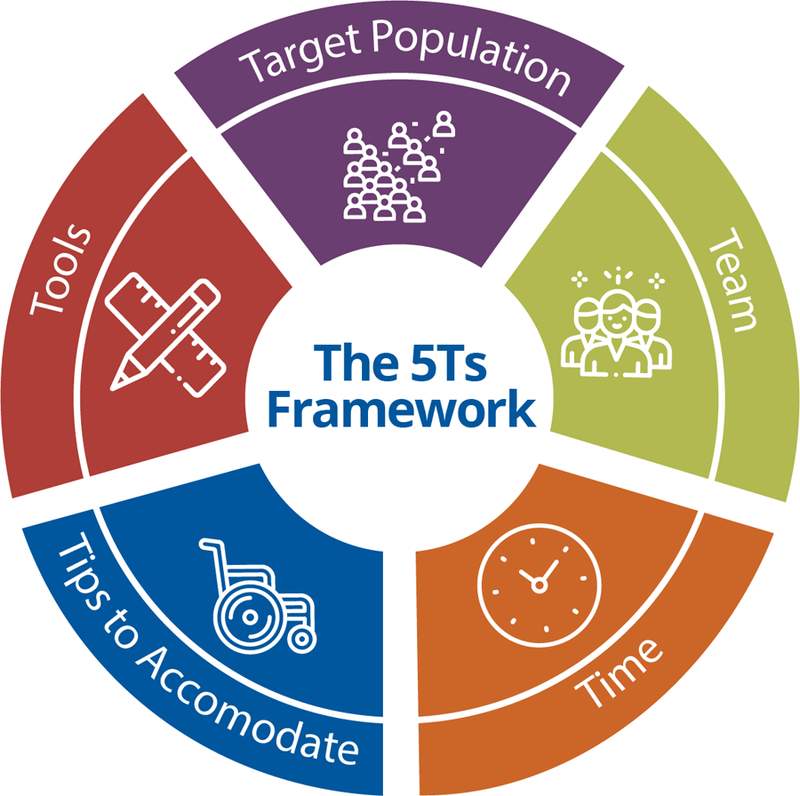
The 5Ts is a framework for communicating with non-geriatrics-trained researchers to increase inclusion of older adults in clinical research.
Table 2.
A comprehensive communication framework that includes the 5Ts to address practical research issues and the 5Ms to describe relevant geriatric principles
| Domain | Description | Example recommendations to address challenges |
|---|---|---|
| Target population | “At risk” or “real-world” population | • Avoid exclusions that limit study generalizability • Understand the prevalence of the studied condition in older adults |
| Team | Research team Family, informal caregivers |
• Engage geriatrician researchers and aging experts • Connect with caregivers and community resources |
| Tools | Measurement tools used in aging research | • Choose appropriate measures of function, physical performance, patient-reported outcomes, and the like • Balance data collection needs and participant burden |
| Time | Participant and study time | • Anticipate longer study visits for some participants • May need to accommodate comorbidities during long study visit days (e.g., snacks for diabetics, inform participants to bring afternoon medications) • May take longer to schedule follow-up visits if participants are dependent on others for transportation or scheduling |
| Tips to accommodate | Suggestions for improving recruitment and retention | • Budget for door-to-door transportation • Use pocket talkers, high-contrast print materials, large font size • Plan for higher attrition rate, which has implications for sample size/power calculations |
| Mind | Cognitive impairment Dementia Depression |
• Consider cognition when assessing for capacity to provide informed consent • Simplify study procedures • Include caregivers/proxy respondents |
| Mobility | Mobility limitations Restricted community mobility Functional limitations/Disability |
• Ensure access to study location • Schedule home visits or alternatives for those with mobility limitations • Include caregivers/proxy respondents |
| Medications | Polypharmacy Drug-drug interactions Adverse events |
• Review non-study medications • Discontinue potentially inappropriate medications • Anticipate common adverse events |
| Multi-complexity | Multiple chronic conditions Multiple age-related impairments Personal and environmental factors that contribute to complexity |
• Limit exclusions to maximize representativeness • Account for competing risks • Address trade-offs that occur in context of competing priorities |
| Matters most to me | Patient-centered outcomes | • Include outcomes that are important to patients such as function • Include other stakeholders (e.g., family/caregivers, health system) |
DISCUSSION
Conducting research in older populations is challenging, particularly for investigators who lack training in geriatrics or gerontology. During interviews with non-geriatrician researchers, the following needs were identified: knowledge about key concepts and available tools in aging research, more flexibility in study structures to accommodate older participants, and a better understanding of how to balance the need for generalizability of findings with the desire to focus on disease-specific effects. By interviewing aging research experts, we discussed practical strategies for anticipating common challenges and recognizing the value of a geriatric approach to research. Our resulting framework includes the 5Ts (Target population, Team, Tools, Time, and Tips to Accommodate), which could be used along with the 5Ms as a strategy for including special populations in research.
We envision using the 5Ts in several ways. Geriatricians who already serve as collaborators or consultants to non-geriatrician investigators could use this tool to better articulate anticipated challenges to inclusion of older adults and proactively offer solutions. For example, we have used the tool to guide discussions during grant planning and to develop human subjects protection sections that justify our study population and procedures. If made available directly to non-geriatrician investigators, the 5Ts could also be used as a checklist when operationalizing study protocols and, when appropriate, to identify the need for additional expertise (e.g., add geriatrician to the Team). The 5Ts could also be used by research infrastructure programs, such as the Clinical and Translational Science Awards (CTSA) which are tasked with promoting the inclusion of special populations and underserved in translational research across the lifespan, to help organize and deliver needed resources to support investigators at their institutions. The 5Ts framework may have implications for special populations, beyond older adults: by encouraging investigators 1) to consider members of the Target population who have been traditionally underserved, 2) to broaden research Teams to include community research partners, and 3) to identify Tips to Accommodate participants from diverse backgrounds and resource needs.
This is the ideal time to develop and disseminate a communication framework for anticipating and addressing challenges in research that includes older adults. The NIH “Inclusion Across the Lifespan” policy begins in 2019 and will require more rigorous approaches to including older adults or reporting a scientific rationale for not doing so. The NIH and Food and Drug Administration have both conducted workshops in preparation for this policy change;19,20 however, awareness beyond aging research communities may be limited. This framework is also aligned with ongoing efforts that are part of the National Institute on Aging (NIA) Grants for Early Medical and Surgical Subspecialists, the Dennis W. Jahnigen Career Development Award, and the American Geriatrics Society (AGS) Geriatrics-for-Specialists Initiative which are building an aging research workforce of non-geriatrician subspecialists.
We acknowledge there are limitations to our approach. Interviewees were from a single academic medical center in medical subspecialties. Although we used semi-structured interviews to identify common barriers, interviews were not recorded, and formal thematic analysis was not conducted. While our focus was on eliciting challenges non-geriatrics-trained investigators experience, there is also an opportunity to learn from older adults who have and have not participated in research. Further, this framework has not yet been implemented or evaluated, therefore the effectiveness of the 5Ts for supporting inclusion of older adults in research remains to be proven. Despite these limitations, the simple design of this framework, alignment with the clinical 5Ms of geriatrics, practical applicability, and engagement of several stakeholders during development should be considered strengths. To better refine this framework, next steps include expanding our data collection to include a larger and more representative group of stakeholders (e.g., national samples of geriatrics and non-geriatrics trained investigators, older adults and their families) and evaluating the impact of implementation of the 5Ts within and outside of the field of geriatrics.
Just as most older patients will not be cared for exclusively by geriatricians, most research protocols will not be written, reviewed, or coordinated by investigators and research staff with formal training in geriatrics or gerontology. Communicating with non-geriatrician researchers using the 5Ts is a practical approach to avoiding barriers to inclusion of older adults in research and complements the 5Ms framework designed to communicate the value of geriatric medicine in clinical practice.
Impact Statement:
We certify that this work is novel. We have developed a new framework for communicating with non-geriatrician researchers to help avoid barriers to inclusion of older adults in research.
ACKNOWLEDGEMENTS
This work was conducted as part of the Tideswell Emerging Leaders in Aging Program. We thank Tideswell at UCSF, AGS, and ADGAP for supporting this leadership program and this project. Special thanks to members of the Research and Evaluation small group for their feedback and encouragement.
Funding source: Work reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553. Dr. Bowling’s work was supported by that National Heart, Lung, and Blood Institute (R01HL133618). Dr. Whitson’s contributions were further supported by the Duke Pepper Older Americans Independence Center (P30AG028716). This work was also supported by the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT), (CIN 13-410) at the Durham VA Health Care System. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Department of Veterans Affairs.
Footnotes
Conflict of Interest: The authors have no financial or other conflicts with this manuscript.
Sponsor’s Role: The sponsors were not involved in the design, methods, data collection, or analysis of the study and had no role in the preparation of the manuscript.
References
- 1.Thake M, Lowry A. A systematic review of trends in the selective exclusion of older participant from randomised clinical trials. Arch Gerontol Geriatr 2017;72:99–102. [DOI] [PubMed] [Google Scholar]
- 2.Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med 2011;26(7):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380(9836):37–43. [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz JH. The exclusion of older people from participation in cardiovascular trials. Virtual Mentor 2014;16(5):365–368. [DOI] [PubMed] [Google Scholar]
- 5.Cherubini A, Del Signore S, Ouslander J, Semla T, Michel JP. Fighting against age discrimination in clinical trials. J Am Geriatr Soc 2010;58(9):1791–1796. [DOI] [PubMed] [Google Scholar]
- 6.Bowling CB, Muntner P. Epidemiology of chronic kidney disease among older adults: a focus on the oldest old. J Gerontol A Biol Sci Med Sci 2012;67(12):1379–1386. [DOI] [PubMed] [Google Scholar]
- 7.Bowling CB, Sharma P, Fox CS, O’Hare AM, Muntner P. Prevalence of reduced estimated glomerular filtration rate among the oldest old from 1988–1994 through 2005–2010. JAMA 2013;310(12):1284–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hare AM, Hotchkiss JR, Kurella Tamura M, et al. Interpreting treatment effects from clinical trials in the context of real-world risk information: end-stage renal disease prevention in older adults. JAMA Intern Med 2014;174(3):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Hare AM, Kaufman JS, Covinsky KE, Landefeld CS, McFarland LV, Larson EB. Current guidelines for using angiotensin-converting enzyme inhibitors and angiotensin II-receptor antagonists in chronic kidney disease: is the evidence base relevant to older adults? Ann Intern Med 2009;150(10):717–724. [DOI] [PubMed] [Google Scholar]
- 10.Bourgeois FT, Orenstein L, Ballakur S, Mandl KD, Ioannidis JPA. Exclusion of Elderly People from Randomized Clinical Trials of Drugs for Ischemic Heart Disease. J Am Geriatr Soc 2017;65(11):2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Carpena-Ruiz M, Montero-Errasquin B, Sanchez-Castellano C, Sanchez-Garcia E. Exclusion of older adults from ongoing clinical trials about type 2 diabetes mellitus. J Am Geriatr Soc 2013;61(5):734–738. [DOI] [PubMed] [Google Scholar]
- 12.Hamaker ME, Stauder R, van Munster BC. Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National Institutes of Health Clinical Trial Registry. Oncologist 2014;19(10):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guiding principles for the care of older adults with multimorbidity: an approach for c. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc 2012;60(10):E1–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott IA, Guyatt GH. Cautionary tales in the interpretation of clinical studies involving older persons. Arch Intern Med 2010;170(7):587–595. [DOI] [PubMed] [Google Scholar]
- 15.Bernard MA, Clayton JA, Lauer MS. Inclusion Across the Lifespan: NIH Policy for Clinical Research. JAMA 2018;320(15):1535–1536. [DOI] [PubMed] [Google Scholar]
- 16.Tinetti M Mainstream or Extinction: Can Defining Who We Are Save Geriatrics? J Am Geriatr Soc 2016;64(7):1400–1404. [DOI] [PubMed] [Google Scholar]
- 17.Tinetti M, Huang A, Molnar F. The Geriatrics 5M’s: A New Way of Communicating What We Do. J Am Geriatr Soc 2017;65(9):2115. [DOI] [PubMed] [Google Scholar]
- 18.Allen K, Ouslander JG. Age-Friendly Health Systems: Their Time Has Come. J Am Geriatr Soc 2018;66(1):19–21. [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. Public Workshop: Evaluating Inclusion and Exclusion Criteria in Clinical Trials Available at: https://www.fda.gov/downloads/RegulatoryInformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/FDARA/UCM613054.pdf 2018.
- 20.National Institutes of Health. Inclusion Across the Lifespan: June 1–2, 2017 Workshop Summary Available at: https://report.nih.gov/FileLink.aspx?rid=953 2018.


