Abstract
Purpose:
To report the outcomes of Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) treatment in pediatric patients with chronic ocular surface disease associated with Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN).
Design:
Retrospective, interventional case series
Methods:
Patients ages 18 or younger seen in consultation for PROSE treatment at a single center between January 1992 and December 2016 with a history of SJS/TEN were reviewed. Demographics, etiology of SJS/TEN, age at treatment milestones, best corrected visual acuity (BCVA) at treatment milestones and treatment failures were recorded. BCVA at the initial presentation visit was compared to BCVA at the time of PROSE device dispense, and the last recorded visit.
Results:
27 females and 22 males were reviewed. Reported etiology was an antibiotic (n=19), anti-epileptic (n=9), anti-pyretic (n=9), other (n=3) and unknown (n=9). The mean age was 6.4 years at disease onset, and 9.3 years at time of initial presentation. The mean duration of follow up was 5.45 years. The median BCVA at the initial presentation was 0.6 logMAR (20/80 Snellen), and was significantly improved to 0.18 logMAR (20/30 Snellen) at the time a PROSE device was dispensed (p < 0.0001). The median BCVA at the last recorded visit was significantly improved to 0.18 logMAR (20/30 Snellen, p = 0.0004). There were 15 patients who failed PROSE treatment (30.6%).
Conclusions:
PROSE treatment is feasible in over two-thirds of pediatric patients with chronic ocular surface disease related to SJS/TEN and results in significant improvement in vision that is durable over a period of many years.
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are a spectrum of immune mediated mucocutaneous diseases that cause sloughing of the skin and mucous membranes. Ophthalmologists should be consulted as early as possible after the diagnosis is suspected to treat the acute disease and prevent long term complications. Chronic ocular surface disease occurs in 35%-79% of patients.1,2 In studies on TEN in pediatric patients, up to 70% were found to have chronic ocular sequelae.3 Ocular surface disease in SJS/TEN is multifactorial; disordered eyelid margin anatomy appears to be a key component in ocular surface disease with eyelid malposition, trichiasis, meibomian gland atrophy, and posterior eyelid margin keratinization leading to chronic corneal inflammation, neovascularization, scarring, and limbal stem cell dysfunction.4–6 Current treatment practices of both adult and pediatric disease are largely based on adult data.
Current approaches for treatment of the chronic phase of the disease include aggressive lubrication, punctal occlusion, topical corticosteroids, topical cyclosporine, eyelid surgery to correct malposition, and mucous membrane grafting to reconstruct the eyelid margin and fornix.2,4–6 Prosthetic replacement of the ocular surface ecosystem (PROSE) treatment has also been reported in the chronic phase of SJS/TEN7 with positive outcomes. Penetrating keratoplasty8 or keratoprosthesis9 surgeries are reserved for severe cases and visual outcomes are often poor.
Therapeutic contact lenses can be used as a non-surgical management for many different sequelae of chronic ocular surface disease. Contact lenses create a protective barrier between the corneal epithelium and an inhospitable surface environment. Options include soft (bandage) contact lenses, rigid gas permeable contact lenses, and scleral lenses. PROSE treatment uses a customized scleral lens prosthetic device that vaults over the cornea, creating a fluid filled reservoir on the ocular surface.10,11 It protects the corneal epithelium from eyelid trauma, acts as shield against evaporation, and allows for maintenance of a fluid reservoir over the cornea. The PROSE device has been used in adults to treat a number of ocular surface diseases including SJS/TEN, graft-versus-host disease, and severe dry eye syndrome.7,10–12 Previous studies have shown that PROSE treatment in the chronic phase of SJS/TEN can improve visual function and acuity.7,13 PROSE treatment can also prevent or mitigate progression of ocular disease in SJS/TEN. However, once a critical window of opportunity has passed, PROSE treatment may no longer be beneficial.6 Positive impact of other scleral lenses in SJS has been reported from France, Japan, and Brazil.14–16
Contact lenses can be used in children, despite obvious challenges related to cooperation, tolerance, and caregiver compliance.14 PROSE treatment in the pediatric population has been reported with SJS patients among the subjects described.17,18 However, there are no studies specifically evaluating PROSE treatment in children with SJS/TEN. In this study, we report outcomes of PROSE treatment in a pediatric population affected by SJS/TEN.
Methods
This retrospective cohort study was conducted in accordance with the Declaration of Helsinki and is compliant with the Health Insurance Portability and Accountability Act. It was approved by the New England Independent Review Board.
We conducted a database search of patients who were evaluated at BostonSight (BoS) in Needham, Massachusetts for PROSE treatment, from January 1, 1992 to December 31, 2016. The search criteria were for patients who had ocular surface disease caused by SJS or TEN and were age less than 18 years at the time of presentation. The medical records of these patients underwent retrospective review.
Patient demographics, reported etiology of SJS/TEN were recorded. Age was recorded from three milestones: disease onset, initial BoS presentation, and date of confirmed device wear. Symptoms at initial presentation were recorded. Notation of clinical findings at initial examination of these photophobic children was highly variable; for this reason, data on corneal findings at presentation was not extracted for this study. Eyes with a symblepharon to the cornea or within 2 mm of the limbus were categorized as “non-candidate” at initial presentation, were not subject to the trial and fitting process, and were not included in the analysis reported here. During the fitting process, a series of customized lenses with modifications to allow for physiologic function are designed, manufactured, and then subjected to on-eye trials of 1,3, and 6 hours at BoS. Patients undergoing PROSE treatment, and their caregivers, are trained to insert and remove the devices by specially trained technicians. Once the patient/caregiver can demonstrate that they are consistently able to apply and remove the device, and a device with optimal fit and physiologic tolerance is confirmed, they are dispensed with devices to begin wearing at home.
Best correct visual acuity (BCVA) was measured at each visit in standard conditions using a Snellen chart. The BCVA at initial presentation, at the visit during which devices were dispensed, and at the last recorded visit at the time of this study was extracted. For statistical analysis, this BCVA was converted to logMAR. Counting fingers acuity was assigned a logMAR equivalence of 2.0, and hand motion acuity assigned a logMAR of 3.0. Results are reported as median because logMAR is not a linear scale, and patients with CF, HM and LP vision skew the mean.
Daily wear time and device diameter was extracted from the last recorded visit. Patients who could not successfully wear devices were categorized as fitting, training, or wearing failures. Fitting failures were instances in which patients could not cooperate with serial lens trials. Training failures were instances in which the patient or caregiver could not cooperate for or manage application and removal of the device. Wearing failures were instances in which the patient had devices dispensed, but daily wear was abandoned. The age at consultation, BCVA at initial consultation, and BCVA when devices were dispensed were compared between patients who were successful and patients who failed treatment.
The statistical analysis was conducted using InStat (GraphPad Software, Inc, La Jolla, CA). Figure 1 is a flow chart showing the number of patients/eyes in whom BCVA was available and used for analysis. The Shapiro-Wilk test confirmed that the data had a Gaussian distribution. Two-tailed, unpaired t-tests were conducted to compare BCVA at the time of initial presentation versus initial PROSE device dispense date, and initial presentation versus last recorded visit. The age at presentation, initial BCVA, and BCVA at device dispense date were also compared between patients who failed treatment versus patients who succeeded using 2-tailed unpaired t-tests. Significance was defined as p < 0.05.
FIGURE 1.
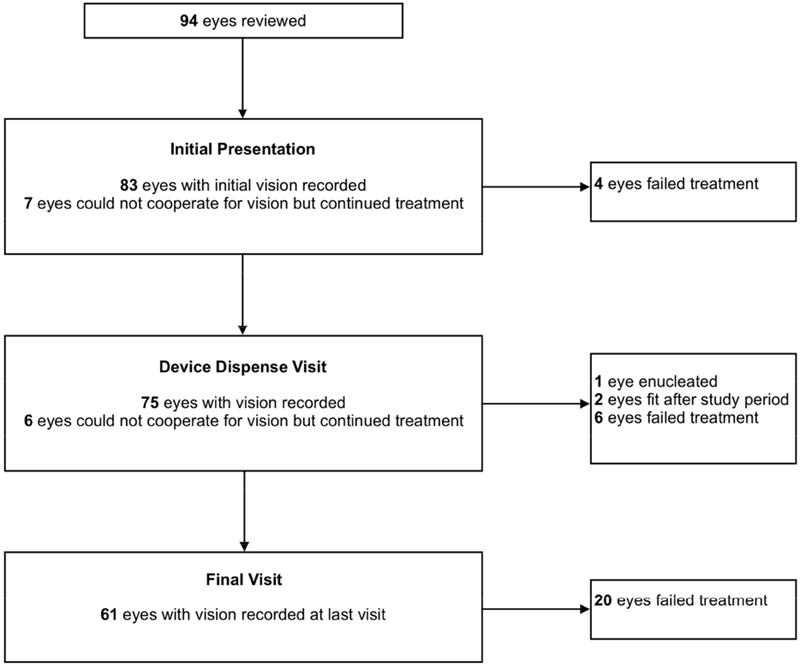
Flowchart of patients and eyes in the cohort.
Results
Demographics and clinical characteristics
Forty-nine patients were identified for this study. The mean duration of follow-up was 5.45 years (range 1 visit – 23 years). The presumed cause of SJS/TEN was antibiotics in 39% of patients (n = 19), anti-epileptics in 18.3% (n = 9), anti-pyretic in 18.3% (n = 9), and other causes in 6% (1 case from mycoplasma, 1 case from quinine, 1 case from a viral infection). The etiology was unknown or not recorded in 18.3% of patients (n = 9). (Supplemental Table 1)
We report patient age at three important treatment milestones: age of disease onset, age at first presentation to BoS, and age at successful PROSE wear. The mean age of disease onset was 6.4 years (range 2-16). The mean age at presentation to BoS was 9.3 years (range 4-17). The mean age at the date of confirmed device wear was years (range 4-16). The distribution of age and genders of patients at the time of confirmed wear is presented in Figure 2.
FIGURE 2.
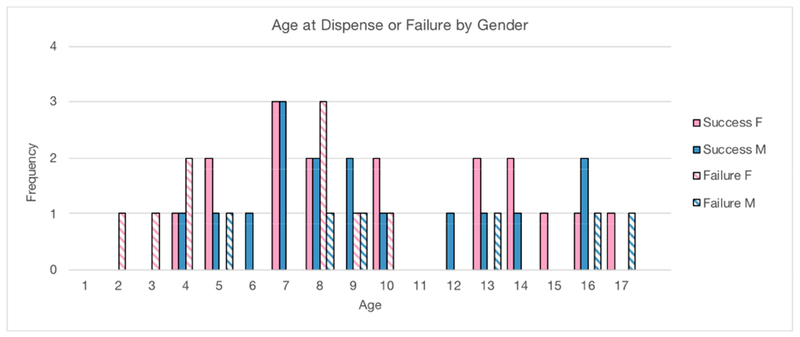
Solid bars: Distribution of patient age and gender at the date of confirmed PROSE device wear. Hatched bars: Distribution of patient age at presentation and gender who ultimately failed PROSE treatment.
Symptoms
Patient and/or caregiver-reported symptoms recorded at the initial presentation were tallied. Nearly half of the patients (n = 22) reported more than one symptom resulting in a total n greater than 49. The most common symptom was photophobia, which was reported by 34 patients and/or caregivers. Other symptoms included dryness/burning (n = 10), decreased vision (n = 12) and pain/discomfort (n = 10) and foreign body sensation/itching (n = 7).
Visual Outcomes
Best corrected visual acuity was extracted at three treatment milestones: initial presentation, device dispense date, and last recorded visit. Four patients were treated only in one eye and the untreated eye was excluded from the analysis. The median BCVA at initial presentation was 0.6 logMAR (Snellen 20/80, range 20/20 to hand motion; n = 83 eyes). The median BCVA at dispense date was 0.18 logMAR (Snellen 20/30, range 20/20 to hand motion; n = 75 eyes) which was significantly better than at initial presentation (unpaired t-test, p < 0.0001). The median visual acuity at the final visit recorded was 0.18 logMAR (Snellen 20/30, range 20/20 to hand motion; n = 61 eyes), which was a significant improvement compared to the initial BCVA (unpaired t-test, p = 0.0004). Impact on visual acuity is presented in Figure 3. Figure 4 is an illustrative example from this cohort demonstrating stability of the ocular surface and improvement in visual acuity over 9 years in a case of pediatric SJS/TEN.
FIGURE 3.
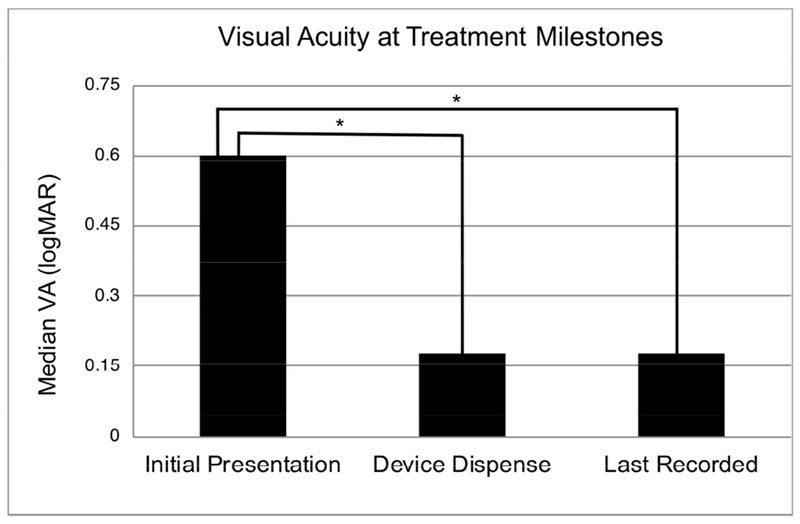
Best Corrected Visual Acuity (BCVA) at Treatment Milestones. The BCVA at the date of device dispense was significantly better than the BCVA at initial consultation. The BCVA at the final visit recorded in patients who achieved consistent daily wear was also significantly better than BCVA at initial consultation. * indicates statistical significance (p < 0.05).
FIGURE 4.
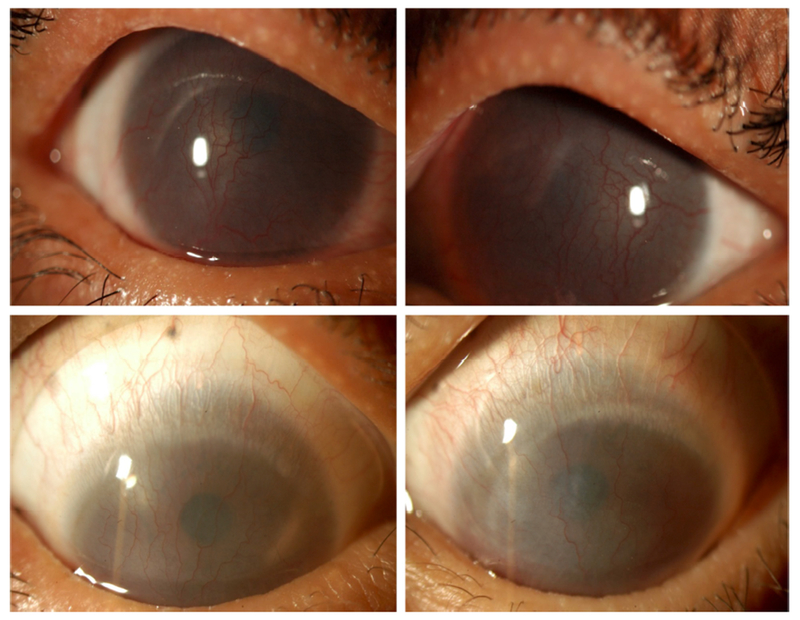
A 14-year-old patient referred for PROSE treatment 8 years after Stevens-Johnson Syndrome as a reaction to an antibiotic at age 6. She was photophobic with best corrected visual acuity (BCVA) of counting fingers at 2 feet in the right eye, and counting at 1 foot in the left eye at initial consultation. Twelve fitting and training visits over the next 6 months were necessary in order to dispense devices. The patient returned after 2 months of daily wear and reported less photophobia. Her BCVA in PROSE devices was 20/50 in the right eye (top left) and 20/400 in the left (top right). Nine years later, at age 23, her BCVA in PROSE devices was 20/30 in the right eye (bottom left) and 20/60 in the left eye (bottom right).
Wearing characteristics
Patients in whom daily wear was confirmed reported a mean daily wear time of hours per day. The mean diameter of the PROSE device in these patients was mm (range 14mm – 21mm).
Treatment failure
Fifteen of 49 patients (30.6%) failed PROSE treatment. There were 9 patients who were categorized as fit or training failures and 6 patients who were categorized as wearing failures. Two patients in the wearing failure category were fitted and trained but were lost to follow up after devices were dispensed. The age at presentation and gender of these failures is also represented in Figure 2. Specific details of these patients are provided in Table 1. The mean age at the time of initial presentation was 8.3 years (range 2-17) in this sub-group. This was not different from the mean age of 9.2 years for those who were successful PROSE users (unpaired t-test, p = 0.24). In patients who failed treatment, the median BCVA at initial presentation was 1.0 logMar (Snellen 20/200, range 20/30 to LP; n = 24 eyes). This was worse, but not statistically different compared to the median BCVA of 0.6 logMAR in patients who were successful in PROSE treatment (Snellen 20/80, range 20/20 to LP; n = 60 eyes; unpaired t-test, p = 32). In patients who failed treatment, the median BCVA at the time of device dispense was 0.18 logMar (Snellen 20/30, range 20/20 to LP; n = 20 eyes) which was not significantly different compared to the median BCVA of 0.18 logMAR (Snellen 20/30, range 20/20 to CF; n = 55) in patients with successful treatment (unpaired t-test, p = 0.82). (Figure 5)
Table 1.
Age, Gender, and Cause of Failure in Patients who Failed Treatment.
| AGE, SEX | REASON FOR FAILURE |
|---|---|
| 2, F | Training failure; could not cooperate for trial lenses |
| 3, F | Training failure; could not cooperate for fitting |
| 4, F | Training failure; could not cooperate for fitting |
| 4, F | Training failure; difficult for patient and parents to insert and remove |
| 5. M | Wearing failure; did not notice any improvement in symptoms |
| 8, F | Wearing failure; lost to follow up |
| 8, F | Training failure; could not insert device |
| 8, F | Wearing failure |
| 8, M | Training failure |
| 9, F | Training failure |
| 9, M | Wearing failure; abandoned wear possibly due to no perceived difference in symptoms or vision |
| 10, F | Training failure |
| 13, M | Wearing failure; lost to follow up |
| 16, M | Training failure |
| 17, M | Wearing failure; could not tolerate daily wear |
FIGURE 5.
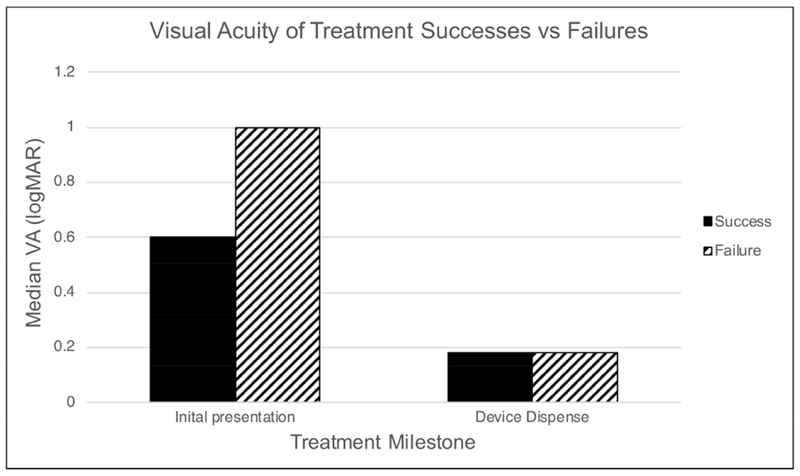
Best Corrected Visual Acuity (BCVA) of Treatment Successes compared to Failures. The median BCVA at the initial consultation was better in patients who were treatment successes compared to treatment failures, but this was not statistically significant (p = 0.32). The median BCVA at the device dispense visit was also not different between patients who were treatment successes vs treatment failures (p = 0.82).
Discussion
Ocular surface disease in the sub-acute and chronic phases of SJS/TEN is generally thought of as progressive, causing pain, photophobia, and loss of vision which is at best only partially responsive to medical intervention. Pediatric patients present additional challenges because of limitations in cooperation and communication. Local therapies such as lubricating eye drops, ointments, and topical medications create logistical demands on caregivers and require cooperation of the child. Even with optimal adherence and cooperation, symptoms may not be adequately controlled. In this cohort, all patients were managed initially by a primary ophthalmologist, and referred to BoS because of ongoing symptoms despite topical therapy and/or surgical interventions.
In this cohort we found that the most common presenting symptom by far was photophobia, which was present in 69% of patients. This finding is likely a reflection of how children express their visual discomfort. Younger children may not have the vocabulary to express symptoms of pain or burning but react with withdrawal to light. For the same reason, reduced vision may be under-represented as a complaint in this population. It should be noted that symptoms recorded were based on self or caregiver report; symptoms were not specifically or systematically queried. Symptoms are difficult to quantify in children; discussion on the change in symptoms with PROSE treatment is beyond the scope of this study. Several surveys have been validated to evaluate visual symptoms and function in ocular surface disease, but none have been designed for pediatric patients.19 Our clinical impression is that PROSE treatment in children results in marked reduction of photophobia and improvement in overall visual function, but neither were measured in the pediatric patients at this single center.
There was an interval of 3 years between the mean age of disease onset and the mean age of presentation to BoS for PROSE treatment. Lack of awareness by physicians and patients as well as limited access to PROSE treatment are all likely contributory. During the interval covered in this study, availability of PROSE treatment increased with the establishment of a network of 12 centers in the continental United States and several abroad. Fitting PROSE devices and training in their application and removal typically requires multiple visits over weeks and sometimes even years depending on the child’s capacity for cooperation, all which may serve as a deterrent for families.
PROSE treatment failure rates are not widely reported. An earlier study from this same center suggested a failure rate of 25.8% in a cohort of pediatric patients with a range of diagnoses.16 We report a 30.6% (15/49) failure rate in pediatric patients with SJS/TEN. The age of patients that failed treatment in our study ranged from 2-17 years, with a mean of 8.3 years, which is younger, but not significantly different when compared to patients who were able to achieve daily wear. This suggests that age is not a limiting factor in training success. Lower baseline BCVA was associated with PROSE failure but not to the point of statistical significance in this cohort of modest size. The capacity for fixation and handling of PROSE is hindered by poor visual acuity. Whether this signifies more advanced disease with features that are not amenable to PROSE wear, such as severe corneal opacification, vs logistical difficulties with application and removal secondary to a poor fellow eye remains to be ascertained.
Ocular surface disease from SJS/TEN is a chronic disease and requires lifelong ophthalmologic care. We found that some patients who failed to achieve consistent daily wear were able to return for fitting and training at a later time and achieve success. The average duration of the fitting and training process in this cohort was 3.88 months, but the range varied from a single session to 3 years. This highlights the importance of perseverance when training and fitting children. Our experience is that if the first few attempts are not successful, it is still worthwhile to have the patient return after an interval of 6 to 12 months. Repeated trials and failures during a shorter interval tend to be stressful for the child, caretakers, and clinicians. The adage “if at first you don’t succeed, try, try again,” applies here, but only if enough time elapses to allow for maturation and control over reflex squeezing and blinking. This is different from our experience with adults in whom reinforcement of strategies to reduce reflex squeezing are most successful if undertaken on a consecutive daily basis, with nearly all patients fitted and trained within a two-week period of successive daily visits.
We also note a very broad range of final device diameters, with a mean of 17.4 mm which is smaller than typically used in our practice. Smaller trial lenses may be selected for these patients to avoid interaction with symblephara and for ease of application in a patient with less than ideal cooperation. Smaller lenses may be associated with compression, impingement, and suction, all of which can lead to hypoxia and neovascularization. It is our practice, once successful PROSE wear is achieved and photophobia is improved, to modify fit including increasing diameter to the 18-19.0 mm range, to reduce likelihood of complications related to smaller scleral lenses. In SJS/TEN, the presence of symblephara sometimes necessitates continuing in a smaller diameter, which requires attentive monitoring of the cornea and adjustment of haptic contour as required.
In reviewing our data and considering clinical experience retrospectively, the authors present these observations. (1) Patients who could not cooperate for therapeutic soft contact lens (bandage lens) insertion, either by an eye health professional or caregiver, typically could not cooperate for PROSE fitting and training. On the other hand, if patients or caregivers had a history of successful contact lens wear, they had less difficulty in taking on application and removal of the PROSE device and the demands of a daily lens disinfection regimen. (2) While age in general is not a limiting factor, children ages 4 and younger are less likely to be successful with PROSE treatment due to limited capacity to cooperate for fitting and daily wear regimens. The youngest patient included in the study was 2 years old at the time of initial presentation. This patient was not able to cooperate with fitting and was determined to be a treatment failure. An additional three patients under the age of 5 were also treatment failures. There were two 4 years old patients who were treatment successes. Both were cooperative enough to complete fitting within 3 months but did not achieve daily wear at home until they presented again 3-4 years later. The youngest patients to achieve daily consistent wear without any significant delay were 5 years old.
Although pediatric patients may be included in reports on therapeutic contact lens, scleral lens, or PROSE treatment, there are few reports specifically on the pediatric experience. In 2008, Gungor et al. reviewed this center’s use of PROSE devices in 31 pediatric patients that were under age 13, with a range of ages 7 months - 12.92 years, and a mean of 7.75 years.16 Rathi et al. subsequently reported on the use of PROSE devices in patients under age 16, with a range of 8-16 years, and mean of 12.85 years.15 Both studies reported that daily wear of PROSE devices in children was feasible.
Of note, SJS/TEN was the most common indication for PROSE treatment in both of these studies. Overall, ocular surface disease is a more common indication for PROSE treatment in the pediatric population (70-87%)17,18 compared to the adult population (43-53%)10,12 wherein corneal ectasia and refractive correction account for a larger proportion. Our study is the first to specifically address PROSE treatment in pediatric patients with ocular surface disease from SJS/TEN. The median initial vision of patients at presentation was 20/80 which improved to 20/30 with PROSE treatment. This is consistent with the visual improvement found in PROSE use in adults with SJS.7 Furthermore, we show that PROSE treatment is durable in these children over a mean follow up time of 5.45 years.
A major limitation of this study is that it is a retrospective review with no control group. There may be a selection bias in that patients seen at this single center may be different from pediatric SJS/TEN patients in general. Furthermore, follow up data was only available for those who continued with PROSE treatment, introducing another element of selection bias. It is possible that those who failed PROSE treatment also had similar improvement and stability of vision. A recent retrospective comparative case series, however, does suggest worsening disease over time in a control group of pediatric SJS/TEN patients who did not receive PROSE treatment compared to those who did.20 We also acknowledge that vision was tested according to clinical rather than scientific standards, with poor quantification of low vision or pre-literate vision. Patients who did not cooperate with visual acuity testing were excluded from the statistical analysis. This bias could potentially result in artifactually better baseline BCVA. If capacity for cooperation correlates with reduced visual acuity rather than pain or photophobia, our methodology would underestimate the degree of improvement in BCVA with PROSE treatment.
In conclusion, despite the obvious challenges of daily application and removal of a scleral lens prosthetic device in the pediatric age group, PROSE treatment is feasible and can result in improved vision in children as young as age 4 with chronic ocular surface disease from SJS/TEN. Improvement in BCVA remains stable in children wearing PROSE devices over a period of more than 5 years.
Supplementary Material
Ocular surface disease is a significant complication of Stevens Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). Prosthetic replacement of the ocular surface ecosystem (PROSE) treatment has been reported in the chronic phase of SJS/TEN. This study reports outcomes of PROSE treatment in pediatric SJS/TEN. Treatment is feasible and can result in improved vision in children as young as age 4. Improvement in BCVA remains stable over a period of more than 5 years.
Acknowledgements
a. Funding/Support: Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health under Award Number K23EY028230. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
b. Financial Disclosures: No financial disclosures
c. Other acknowledgements: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available at AJO.com.
References
- 1.Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. 2013;69(2):187.e1–16. [DOI] [PubMed] [Google Scholar]
- 2.Jain R, Sharma N, Basu S, et al. Stevens-Johnson syndrome: The role of an ophthalmologist. Surv Ophthalmol. 2016;61(4):369–99. [DOI] [PubMed] [Google Scholar]
- 3.Quirke KP, Beck A, Gamelli RL, Mosier MJ. A 15-year review of pediatric toxic epidermal necrolysis. J Burn Care Res. 2015;36(1):130–6. [DOI] [PubMed] [Google Scholar]
- 4.Ciralsky JB, Sippel KC, Gregory DG. Current ophthalmologic treatment strategies for acute and chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Curr Opin Ophthalmol. 2013;24(4):321–8. [DOI] [PubMed] [Google Scholar]
- 5.Iyer G, Srinivasan B, Agarwal S, Kamala Muralidharan S, Arumugam S. Comprehensive approach to ocular consequences of Stevens Johnson Syndrome - the aftermath of a systemic condition. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):457–67. [DOI] [PubMed] [Google Scholar]
- 6.Kohanim S, Palioura S, Saeed HN, et al. Acute and Chronic Ophthalmic Involvement in Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis - A Comprehensive Review and Guide to Therapy. II. Ophthalmic Disease. Ocul Surf. 2016;14(2):168–88. [DOI] [PubMed] [Google Scholar]
- 7.Papakostas TD, Le HG, Chodosh J, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology. 2015;122(2):248–53. [DOI] [PubMed] [Google Scholar]
- 8.Tugal-Tutkun I, Akova YA, Foster CS. Penetrating keratoplasty in cicatrizing conjunctival diseases. Ophthalmology. 1995;102(4):576–85. [DOI] [PubMed] [Google Scholar]
- 9.Sayegh RR, Ang LP, Foster CS, Dohlman CH. The Boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol. 2008;145(3):438–44. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005;31(3):130–4. [DOI] [PubMed] [Google Scholar]
- 11.Stason WB, Razavi M, Jacobs DS, et al. Clinical benefits of the Boston Ocular Surface Prosthesis. Am J Ophthalmol. 2010;149(1):54–61. [DOI] [PubMed] [Google Scholar]
- 12.Agranat JS, Kitos NR, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem: impact at 5 years. Br J Ophthalmol. 2016;100(9):1171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heur M, Bach D, Theophanous C, Chiu GB. Prosthetic replacement of the ocular surface ecosystem scleral lens therapy for patients with ocular symptoms of chronic Stevens-Johnson syndrome. Am J Ophthalmol. 2014;158(1):49–54. [DOI] [PubMed] [Google Scholar]
- 14.Tougeron-Brousseau B, Delcampe A, Gueudry J, Vera L, Doan S, Hoang-Xuan T, Vision-related function after scleral lens fitting in ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2009. December;148(6):852–9 [DOI] [PubMed] [Google Scholar]
- 15.Sotozono C, Yamauchi N, Maeda S, Kinoshita S. Tear exchangeable limbal rigid contact lens for ocular sequelae resulting from Stevens-Johnson syndrome or toxic epidermal necrolysis. Am J Ophthalmol. 2014. November;158(5):983–93 [DOI] [PubMed] [Google Scholar]
- 16.La Porta Weber S, Becco de Souza R, Gomes JÁP, Hofling-Lima AL. The Use of the Esclera Scleral Contact Lens in the Treatment of Moderate to Severe Dry Eye Disease. Am J Ophthalmol. 2016. March;163:167–173 [DOI] [PubMed] [Google Scholar]
- 17.Rathi VM, Mandathara PS, Vaddavalli PK, Srikanth D, Sangwan VS. Fluid filled scleral contact lens in pediatric patients: challenges and outcome. Cont Lens Anterior Eye. 2012;35(4):189–92. [DOI] [PubMed] [Google Scholar]
- 18.Gungor I, Schor K, Rosenthal P, Jacobs DS. The Boston Scleral Lens in the treatment of pediatric patients. J AAPOS. 2008;12(3):263–7. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs DS, Barrett A. Corneal Disease in Children: Contact Lenses In: Colby K. editors. Corneal Diseases in Children, Essentials in Ophthalmology. Cham, Switzerland: Springer International Publishing AG; 2017. [Google Scholar]
- 20.Basu S, Shanbhag SS, Gokani A, Kedar R, Bahuguna C, Sangwan VS. Chronic Ocular Sequelae of Stevens-Johnson Syndrome in Children: Long-term Impact of Appropriate Therapy on Natural History of Disease. Am J Ophthalmol. 2018;189:17–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


