Abstract
Geographic atrophy (GA) is a progressive, advanced form of age-related macular degeneration leading to visual function impairment and irreversible vision loss. Standard clinical tests to evaluate visual function in patients with GA provide poor anatomic-functional correlation, while fundus imaging does not assess the visual function deficit. Microperimetry is a psychophysical visual function test that spatially maps retinal sensitivity and allows for correlation of anatomic features with visual function. In this review, we present an overview of mesopic microperimetry for GA, including: commercially available microperimetry devices, strategies to capture a mesopic microperimetry test, and strategies to assess and interpret microperimetry data in patients with GA. We demonstrate the importance of microperimetry data for assessing GA progression and for evaluating visual function loss through anatomic-functional correlations. Although valuable, current microperimetry tests require an extensive time commitment from patient and examiner, and the development of faster, more reproducible, and accessible methods is important to enable broader use of microperimetry in both clinical and research settings.
Keywords: microperimetry, geographic atrophy, age-related macular degeneration, anatomic-functional correlation, retinal sensitivity, visual function
1. Introduction
Geographic atrophy (GA) is a progressive and irreversible advanced form of age-related macular degeneration (AMD) that affects approximately 5 million people worldwide.43,60 The clinical diagnosis of GA is not common until over age 70 years, when the prevalence of GA increases exponentially with age.43,60 Patients with GA develop significant visual function impairment and irreversible vision loss. Standard visual acuity tests such as best-corrected visual acuity (BCVA) do not fully capture the functional impact of GA because lesions typically spare the foveal center until late in disease progression, causing vision measured on standard vision charts erroneously to seem unaffected.57
There are currently no approved treatments to reduce the progression of GA.21 To evaluate the efficacy of potential therapeutics in clinical trials, reliable methods are critical for monitoring both the structural and functional changes associated with GA progression. Fundus autofluorescence (FAF)32 and optical coherence tomography (OCT)45,71 provide invaluable new imaging data for defining and monitoring GA progression because the GA lesion can be identified and reproducibly measured even before the patient becomes aware of visual symptoms. Although relatively fast and easy to perform, FAF and OCT imaging do not assess the visual function deficit in GA. Meanwhile, standard methods for assessing visual function, such as visual acuity, generally provide poor anatomic-functional correlation.15 The decline in visual function due to GA can occur months to years before the patient perceives visual problems,65 and localized decreases in retinal sensitivity have been reported in intermediate AMD and in precursor lesions of GA.25 Therefore, there is a need for improved methods to simultaneously evaluate anatomic GA lesion progression and capture visual function changes over the course of AMD progression.
Microperimetry, or fundus-controlled perimetry, is a visual function test used to map point-wise retinal sensitivity and is particularly useful in dystrophic or degenerative conditions of the retina. Microperimetry has been shown to be useful in correlating retinal function with GA lesion features.5,6,15,59 Herein, we discuss mesopic microperimetry for the assessment of visual function loss in GA, including strategies to capture microperimetry tests and data analysis associated with these tests. In addition, we highlight important scientific questions related to visual function loss and disease progression that may be answered by further research using microperimetry on patients with GA.
2. Assessing geographic atrophy using imaging techniques
Although imaging GA provides key information about phenotypical characterization and disease progression, this may not be directly correlated with visual function measured by standard clinical tests. Common imaging modalities include FAF, OCT, color fundus photography (CFP), and near infrared (NIR) imaging.20,45 GA is characterized by sharply demarcated atrophic lesions of the outer retina, caused by loss of photoreceptors, retinal pigment epithelium (RPE), and choriocapillaris,4,21,46 which result in absolute scotomas in the corresponding lesion area.31,39 Imaging can also detect areas potentially predisposed to the future development of GA, termed nascent GA (also defined in more recent classification schemes as incomplete RPE and outer retinal atrophy, or iRORA).45 Nascent GA is characterized on OCT by the subsidence of the outer plexiform layer and inner nuclear layer and a hyporeflective wedge-shaped band, which result in relative scotomas in the corresponding lesion area.70 Over time, nascent GA can progress to GA. In previous studies reported in the literature, GA lesions were found to enlarge at rates between 0.53 and 2.6 mm2/year, depending on specific characteristics of each study cohort such as baseline GA lesion size in the affected eye and fellow eye.3,12,56
3. Assessing geographic atrophy through visual function
Monitoring the progressive decline of visual function is relevant because it provides insights into the impact of GA on patients’ lives. Metrics of visual function, including BCVA, low-luminance visual acuity (LLVA), reading speed, and patient-reported outcomes (PROs), provide valuable information about how GA affects patients on a daily basis.44 Often, however, BCVA does not correlate with visual dysfunction in patients with GA.57 Patients with foveal-sparing lesions and associated paracentral scotomas can experience apparent preservation of central vision based on a high-contrast single-letters test such as BCVA, even while parafoveal lesions enlarge over time.23,48 However, these patients may experience other visual function deficits during activities of everyday living, such as a blurred or distorted visual field or changes in reading speed.57
Microperimetry is a psychophysical visual function test used to spatially map retinal sensitivity, or the level of response of the retina to light stimuli (assuming that the posterior visual pathway is normal and subjects can perform the test).51 During a microperimetry test, light stimuli of various intensities is altered to determine the lowest level at which the participant can detect the light. Stimulus intensity is measured in decibels (dB), where a higher score indicates detection of a dimmer stimulus and thus higher retinal sensitivity; a score of 0 dB signifies an absolute scotoma (assuming there is no floor effect), reflecting a failure to detect the brightest stimulus available on the instrument. In contrast to traditional perimetry, microperimetry uses eye-tracking technology to lock onto specific fundus features.24 The stimulus display is adjusted many times per second (depending on eye-tracker frequency) to compensate for eye movements and to localize stimuli to specific retinal locations during an exam. This allows for follow-up examination of the same locations, which is essential for longitudinal analyses of change in sensitivity for correlation with structural changes.
The anatomic-functional correlation generated from associating functional data with retinal morphology (Fig. 1) makes microperimetry a scientifically valuable method for assessing changes in visual function due to intermediate AMD progression to GA or GA lesion progression.15 The decrease in visual function associated with GA progression is indicated by lower mean macular sensitivity and larger area of absolute scotoma.27,39 Besides its role as a clinical endpoint, microperimetry may potentially serve as a prognostic biomarker, as suggested by studies using flicker perimetry.25 The role of this visual function assessment as a predictor of GA progression is currently being investigated in ongoing clinical trials, such as the Development of Novel Clinical Endpoints in Intermediate AMD (MACUSTAR; NCT03349801) and the AMD Ryan Initiative Study (ARIS; NCT03092492).
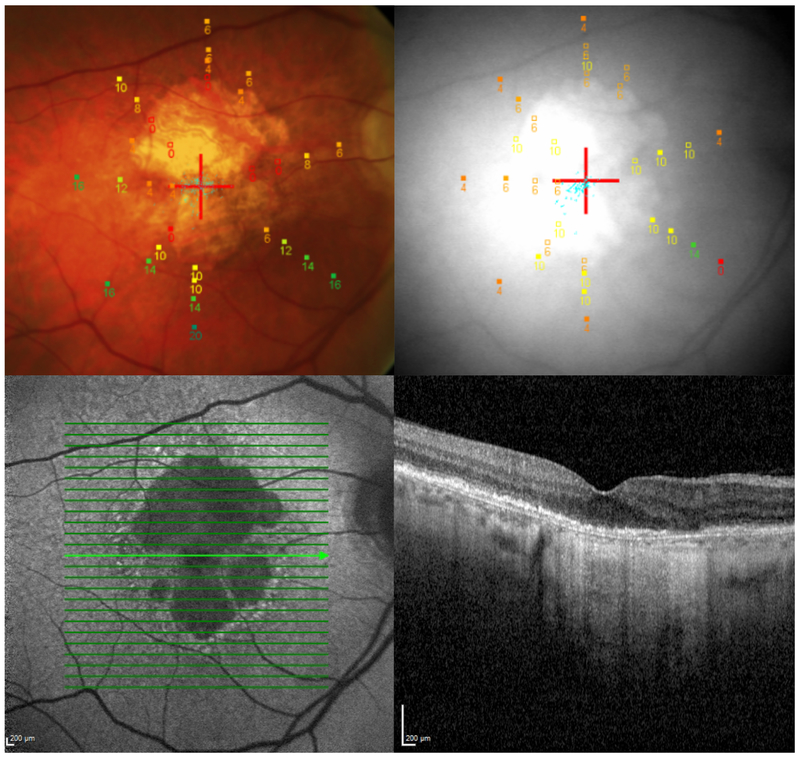
Fig. 1. Multimodal imaging and microperimetry in geographic atrophy.
Multimodal imaging of a patient with geographic atrophy: color fundus photograph (top left, with microperimetry grid overlaid and red cross indicating fixation target); near infrared image (top right, with microperimetry grid overlaid and red cross indicating fixation target); fundus autofluorescence image (bottom left); cross-sectional optical coherence tomography image (bottom right).
Microperimetry color guide: red = absolute scotoma (0 dB); orange/yellow = relative scotomatous point (intermediate retinal sensitivity); green = normal retinal sensitivity (20 dB).
Mesopic microperimetry assesses visual function under medium-light conditions in which both rod and cone receptors are functional.8,27,38 Mesopic microperimetry tests are typically administered in a dimly lit room without prior light/dark adaptation16 or using mydriatics.8,27,38 Alternatively, scotopic microperimetry is performed under dark-adapted conditions and predominantly measures the function of rod photoreceptor cells in healthy eyes.11,39 In diseased eyes in which rods are affected, dark-adapted sensitivity may also be mediated by cone photoreceptor cells. Recent research suggests that scotopic microperimetry may also provide valuable insights into GA pathology,33,35,53 but this type of microperimetry is not yet commonly employed outside the research setting and thus not discussed in this review. Herein, we highlight specifically mesopic microperimetry for the assessment of visual function loss in GA.
4. Strategies to capture a mesopic microperimetry test
There are several commercially available microperimetry devices, all with defining features that potentially drive one to be the instrument of choice over others. The key differences are range of stimulus intensity (0–20 dB to 0–50 dB), eye-tracker frequency (8–30 Hz),28 background illumination (1.27 cd/m2–10 cd/m2), and imaging features (CFP, scanning laser ophthalmoscope [SLO], OCT) [Table].15,26 Different features may be better suited to answering different questions; for example, imaging frequency, which is not necessarily the rate-defining step for technical stimulus placement or adjustment, may not be as important for consideration as dynamic range of the stimulus intensity.
Table.
Comparison among commercially available microperimetry devices
| Microperimeter | Release date |
Manufacturer | Imaging type | Anatomic- functional correlation |
Testing field |
Range of stimulus intensity |
Eye- tracker frequency |
Background illumination |
Maximum stimulus intensity |
|---|---|---|---|---|---|---|---|---|---|
| SLO 101a | 1982 | Rodenstock | SLO | ||||||
| Nidek MP-1 | 2002 | Nidek Technologies | IR and digital color fundus imaging | Measures sensitivity while imaging the retina | 45° | 0–20 dB | 25 Hz | 1.27 cd/m2 | 128 cd/m2 |
| Optos OCT/SLO | 2006 | Optos (previously OPKO Instrumentation) | SLO B&W fundus imaging with OCT | SLO fundus images align with topographic maps | 29.7° | 0–20 dB | 8 Hz | 10 cd/m2 | 125 cd/m2 |
| MAIA (Macular Integrity Assessment) | 2009 | CenterVue | SLO B&W fundus imaging | Greater dynamic range in stimulus sensitivity while imaging the retina | 36° | 0–36 dB | 25 Hz | 1.27 cd/m2 | 318 cd/m2 |
| Nidek MP-3 | 2015 | Nidek Technologies | Digital color fundus imaging | Greater dynamic range in stimulus sensitivity and isolation of cone function | 45° | 0–34 dB | 30 Hz | 1.27 cd/m2 or 10 cd/m2 | 3183 cd/m2 |
| Compass | 2015 | CenterVue | SLO fundus imaging | Sensitive simultaneous assessment of function and structure | 60° | 0–50 dB | 25 Hz | 10 cd/m2 | 3183 cd/m2 |
No longer commercially available.
B&W, black and white; cd, candela; dB, decibel; IR, infrared; OCT, optical coherence tomography; SLO, scanning laser ophthalmoscope.
As with any psychophysical exam, the quality of microperimetry results depends on patient performance. Because exam duration varies with acquisition parameters, limiting the length of the test can help avoid fatigue in an elderly population. Other important performance considerations include scheduling the test during a time when patients are more alert, alleviating patient discomfort with a comfortable chair and optimal positioning, and providing proper training for both patients and technicians administering the test.
The components of a microperimetry test may include the fixation test, the training test, and the perimetry test itself.
4.1. Fixation test
The fixation test quantifies fixation stability.26 All subjects have microsaccades under physiological conditions to prevent images from fading. Generally, these eye movements are small, but can become more pronounced with GA. During the fixation test, the microperimeter obtains real-time fundus images, generating multiple sets of x and y coordinates while the subject is fixating on a small target. These x and y coordinates are then used to calculate the degree of eccentric fixation and the stability of fixation with respect to anatomic landmarks.26
Patients with foveal GA typically experience difficulty with fixation. In some instances, patients can compensate by developing an eccentric site for fixation, a preferred retinal locus (PRL), outside the scotoma.55 The fixation test determines the PRL prior to the retinal sensitivity test, and can indicate if the PRL has changed or enlarged over time. When fixation is eccentric, one option is to decouple the test pattern from fixation and center on the fovea instead of at fixation. Foveal scotomas can produce unstable fixation during microperimetry and, with excessive fixation instability, the test may be unreliable depending on the device and exam protocol. Recent advances in microperimetry devices, however, may be able to overcome such potential limitations. For example, units such as the Nidek and CenterVue devices incorporate sensitive eye-tracking hardware that appears to compensate for fixation instability.9,35
4.2. Training test
The training test provides directions for the actual perimetry test along with an opportunity for patients to practice. This test is typically shorter than the study acquisition protocol and is performed before each new testing session to familiarize patients with the procedure. After the training test, the first microperimetry test is often discarded and the second test used as the patient’s “official” baseline microperimetry values.63 Patients may demonstrate better results during their second baseline microperimetry exam due to the learning effect: they are more comfortable with and knowledgeable about the testing requirements.
4.3. Microperimetry test
The test itself consists of stimuli presented at increasing intensities in a staircase pattern in order to calculate retinal sensitivity. The dynamic range of the stimulus varies; current commercially available devices provide stimulus intensity from 0 to 20 dB or 0 to 50 dB depending on the microperimeter. A limited dynamic range can lead to floor and ceiling effects observed when interpreting the results of the test; for example, if the lowest luminance stimulus gives a score of 20 dB, a ceiling effect occurs because the microperimeter is unable to produce any further reduction in stimulus intensity.8 In this instance, it is not possible to ascertain whether the retina could have been more sensitive. One approach to eliminate points that may reflect a floor or ceiling effect is to determine a priori the dynamic range of values that will be included in the analysis. Points including values 1 to 3 dB above the minimum or 1 to 3 dB below the maximum should be removed during analysis.
It is very important that the patient is not exposed to bright light (i.e., fundus imaging or clinical examinations) just prior to testing. Intense light will produce bleaching in the retina and could alter the perimetry test results. Different exam parameters, such as the stimulus size, duration, and pattern, are used to acquire data. Programmable parameters include: stimuli number, position, shape, color, and duration; background color; and fixation target size, shape, position, and color.26
Stimulus sizes are equivalent to the Goldmann stimuli for perimetry, with the area defined for round stimuli presented at a viewing distance of 300 mm: Goldmann I, 0.25 mm2; Goldmann II, 1.0 mm2; Goldmann III, 4.0 mm2; Goldmann IV, 16.0 mm2; Goldmann V, 64.0 mm2. The actual area of the retina that is stimulated by each of these beams will vary depending on several parameters, including the ocular axial length and angulation of the stimulus. Spot size 3 (0.43°; 125-μm diameter on the retina, assuming standard parameters) is equivalent to the Goldmann III test object and has been used in multiple studies;16,24,26,27,38 however, for instances when precise sensitivity measurements are required, spot size 3 may stimulate too large of an area of the retina.16 Spot size 2 (0.22°; 62.5-μm diameter on the retina, assuming standard parameters) may be used in these circumstances.30 The most common stimulus duration is 200 ms.16,24,27,38 The interval between stimuli is typically 1500 ms.24 A popular threshold strategy is the 4-2 staircase16,24,26,27,38 because it allows for sensitivity determinations at numerous locations within a reasonable-length test session.10 In the 4-2 strategy, an initial stimulus is presented; if the patient can detect it, the stimulus is made dimmer in 4 dB steps until the patient cannot detect the stimulus (because it is below the detection threshold). Then the stimulus is made brighter in 2 dB steps until the patient is able to detect the stimulus. Local sensitivity is taken as the value between the dimmest stimulus detected and the brightest stimulus not detected. For more precision in determining sensitivity, many manufacturers also provide a 4-2-1 staircase strategy to provide an additional reversal. The background illumination can be turned off (typically used for scotopic testing), 1.27 cd/m2 (mesopic testing),27,38 or 10 cd/m2 (photopic testing).16 The higher background illumination is more desirable because it effectively saturates rod photoreceptors and produces cone-mediated sensitivity values.34,52 The grid pattern is composed of testing points located at certain degrees from fixation, such as 10°, 16°, or 20°.16,27,38 The retinal sensitivity at these points is then compared with the sensitivity of normal eyes.16 Customized grids can also be used,38 for example, the Polar 3 Pattern for the Optos OCT/SLO.24 A grid pattern can have as many as 68 loci in a circular pattern covering the central macula.27 Use of different exam parameters can lead to differences in data acquisition, data analysis, and overall results.
The most common challenge associated with a microperimetry test is ensuring that the patient performs the test accurately. Patients experiencing ocular, cognitive, or physical problems may not able to complete the microperimetry test at all. For example, ocular conditions such as media opacities may confound the microperimetry report.49 Patients with cognitive problems (e.g., poor concentration) may not be able to focus for the duration of the test. In addition, many patients undergoing microperimetry tests are elderly and suffer from physical problems including fatigue and sore backs or necks. These patients may not be able to sit still long enough to complete the test.
5. Strategies to assess and interpret microperimetry data
5.1. Main test outputs
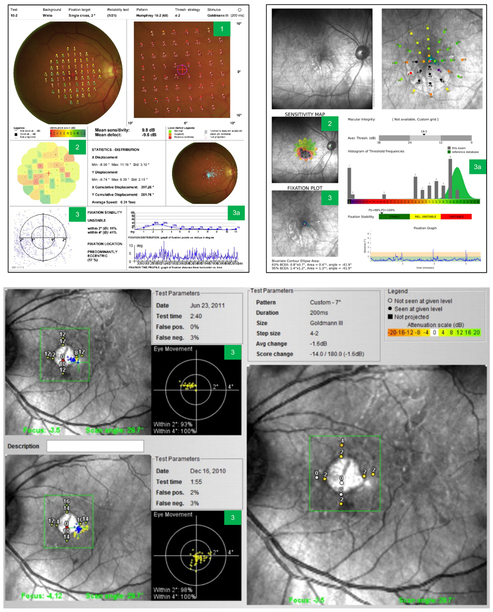
The main outputs from the microperimetry test are the local defect map, the interpolation map, and fixation stability (Fig. 2). Although only the Nidek MP-1 and Nidek MP-3 (Nidek Technologies, Padova, Italy) provide a local defect map, the Nidek and Macular Integrity Assessment (MAIA; CenterVue, Padova, Italy) devices provide an interpolation map, and all commercially available microperimeters including the Optos OCT/SLO (Optos, Dunfermline, Scotland, UK) assess fixation stability.
Fig. 2. Test outputs and how to read a microperimetry report.
Sample microperimetry reports from Nidek MP-1 (top left), MAIA (top right), and Optos OCT/SLO (bottom). Test outputs: 1) local defect map; 2) interpolation map; 3) fixation points; 3a) assessing fixation stability. The Optos microperimetry output shows progression data over time between 2 visits. MAIA, Macular Integrity Assessment; OCT, optical coherence tomography; SLO, scanning laser ophthalmoscope.
5.1.1. Local defect map
The local defect map shows retinal sensitivity along with the infrared (IR) scanning laser image of the retina. It is used to quantify changes in retinal sensitivity over time and does not show overall retinal sensitivity, but rather changes in sensitivity compared with a normative database.1
5.1.2. Interpolation map
The interpolation map is a color-coded map of the retina showing the point-wise sensitivity in each region. It is used to visualize areas of poor retinal sensitivity at a glance. It is typically an en face image of the fundus with sensitivity values superimposed on the image.16 The sensitivity readout can also be superimposed onto OCT images to allow for accurate anatomic-functional correlation.38 The sensitivity at each test location appears as a color ranging from red (0 dB) to green (20 dB). It is possible to calculate mean retinal sensitivity and rate of scotoma enlargement from this superimposed map.38 The sensitivity map can also be used to compare the difference in sensitivity between 2 visits.24
5.1.3. Fixation stability
In contrast to the exam outputs indicating retinal sensitivity, the microperimetry grid contains points of fixation, or specific points at different degrees from fixation.16 One way of assessing fixation stability is to measure the fixation of the eye compared with anatomic landmarks. A tight pattern on the fixation scatter plot indicates good fixation, which correlates with good visual function. The semi-quantitative Fujii classification for fixation quality is the ratio of tracked fundus positions located within a certain radius from the fixation point (typically within 2° and 4° radii).14 Fixation is commonly classified in the literature as “stable” when 75% of fixations are within the 2° radius, “relatively unstable” when 75% of fixations are between the 2° and 4° radii, and “unstable” when <75% are within the 4° radius.26 As GA progresses, patients experience a decrease in fixation quality over time for both radii.27 Some microperimetry devices such as the Nidek MP-1 can show fixation stability over time.
Besides the semi-quantitative Fujii classification, fixation stability may also be quantified by the bivariate contour ellipse area (BCEA).54 BCEA is defined as a 2-dimensional ellipse on the retina where the fixation target is located at least 68%, 95%, or 99% of the time (BCEA 68, BCEA 95, or BCEA 99).40 BCEA is automatically calculated by the microperimeter and reported in square degrees. Another important fixation endpoint is the ability to accurately determine the fixation location. The distance from the PRL to the fovea can be calculated using the coordinates of the PRL centroid.26,50
5.2. Microperimetry study endpoints
The microperimetry test contains data for several potential study endpoints,59 some of which have been developed as global indices for following patients with glaucoma.36 These include: mean deviation and pattern standard deviation (PSD), which are weighted to account for local variation in sensitivity in normal eyes; mean defect and loss variance (LV), which are non-weighted; and the cumulative defect curve for visualizing global and local losses.
Additional summary endpoints have been developed primarily for patients with GA. These include number of scotomatous points, mean retinal sensitivity, perilesional sensitivity, point-wise or regional sensitivity, and fixation stability. These data are used to characterize functional impairment and assess disease severity. Herein, we describe the data obtained from the main endpoints in a microperimetry test.
5.2.1. Number of scotomatous points
The number of testing points that will be evaluated for response to a stimulus is selected at the beginning of the test. A scotoma is an area of reduced sensitivity in the visual field (Fig. 1).31 Absolute scotomatous points, also termed nonresponding points or “dense” scotomas, are testing points that do not provide a patient response even at a stimulus of maximum intensity (score of 0 dB).27,38 One study reported an average increase of +4.4 scotomatous points per year in eyes with GA, calculated from longitudinal data collected at 68 test points evenly distributed from the central 20° of the macula.27 These results indicate expansion of an absolute scotoma and correlation with enlargement of GA lesion area over time.
5.2.2. Mean retinal sensitivity (total retinal area)
Mean retinal sensitivity is the average of all point-wise sensitivities included in the test grid, which provides a measurement of overall retinal function within the tested area. Eyes with GA and intermediate AMD possess a macula-wide functional deficit, indicated by decreased mean retinal sensitivity.17,58,61,62,65,66 This functional decline may precede the development of GA.25,65
Several studies have used mesopic microperimetry to evaluate mean retinal sensitivity in the presence of reticular pseudodrusen (RPD). In eyes with foveal-sparing GA, the presence of pseudodrusen was highly predictive of mean retinal sensitivity (P = 0.004) and resulted in decreased retinal sensitivity across the entire macula.30 Similarly, the mean retinal sensitivity of eyes with RPD but without any macular abnormalities was lower than in normal eyes. The pattern of decreased sensitivity matched the distribution of RPD.29 Another study found a statistically significant difference in mean retinal sensitivity between eyes with RPD (and no GA) and eyes with early AMD (and no pseudodrusen): 5.9 ± 1.7 dB and 8.8 ± 2.4 dB, respectively (P < 0.001).41 However, in eyes with intermediate AMD, the presence of RPD was only marginally associated with mean retinal sensitivity as detected by microperimetry (P = 0.068).67
5.2.3. Perilesional sensitivity
Perilesional points are defined as loci immediately bordering a GA lesion, whereas extralesional points are separated from the GA border and corresponding absolute scotoma by more than 1 point.27 Eyes with GA may experience a decrease in sensitivity of perilesional points, reported in 1 cohort at a mean rate of −1.20 dB/year.27 When tracking the decrease in sensitivity over time for each point, perilesional points show lower sensitivity compared with extralesional points. Over a follow-up period of 2 years, microperimetry data showed that a decrease in perilesional sensitivity is related to enlargement of the GA lesion.27
In a study of eyes with GA, the retinal sensitivity near the border of the atrophic lesion, or junctional zone, was compared with the retinal sensitivity of eyes with early or intermediate AMD.16 The sensitivity was calculated at points located 10° from the foveal center and compared between the junctional zone of GA (defined as a ring 500 μm in width surrounding the lesion) and junctional subzones (defined as points on the margin of atrophy, points in the center of the junctional zone, or points on the outer border of the junctional zone). A drop in sensitivity was found at the GA junctional zone. This severe change in sensitivity – as opposed to the gradual lessening of sensitivity – was detected using the greater stimulus sensitivity of the Nidek MP-3 (0–34 dB stimulus). However, all macular regions outside of a lesion showed decreased sensitivity compared with eyes with mild or intermediate AMD, confirming that eyes with GA have a macula-wide functional deficit.16
Several studies of eyes with GA have found reduced retinal sensitivity at the border of GA lesions compared with adjacent points in the retina.17,37,42,58 In particular, retinal sensitivity was decreased in regions with photoreceptor damage (3.28 ± 2.70 dB) compared with undamaged regions (10.52 ± 4.12 dB).58 In eyes with foveal-sparing GA, the perilesional sensitivity of the foveal region was found to be higher than the perifoveal area of atrophy.13
In order to properly analyze junctional zone defects, the location and size of the GA lesion must be taken into account. At the border of a deep scotoma in one study, test-retest repeatability was found to be worse compared with other regions of the normal retina.69 Using the border of the optic nerve head as a model for the border of a deep scotoma, these findings suggest that measurements may be less reliable at the edge of a scotoma in GA. In addition, a larger GA region would have more junctional zone data points assessed and may lead to incorrect conclusions regarding the sensitivity of the junctional zone test spots relative to spots outside the junctional zone. Therefore, it remains to be confirmed whether decreased retinal sensitivity in the junctional zone of GA, as compared with better retinal sensitivity in areas away from the GA border, occurs due to underlying AMD.
5.2.4. Point-wise sensitivity
Microperimetry provides a unique opportunity for anatomic-functional correlation because of its ability to measure point-wise sensitivity, or retinal sensitivity at precise, individual retinal locations.24 One study found that point-wise sensitivity in eyes with GA correlates with changes in retinal layer thickness identified by SD-OCT.47 These changes in retinal function may precede anatomical changes.25 Longitudinal functional changes also were detected in eyes with foveal-sparing GA: point-wise sensitivity decreased over 12 months, from 18.25 dB to 15.58 dB.65
Other studies have characterized the retinal point-wise sensitivity in nascent GA. In eyes with intermediate AMD and nascent GA, retinal sensitivity was found to be lower in regions of nascent GA (20.4 ± 0.8 dB) compared with non-atrophic regions (23.8–27.6 dB). If present, regions of drusen-associated atrophy detected on SD-OCT were consistently found to have lower sensitivity than other regions. However, unlike GA, these regions were not associated with an absolute scotoma.64
5.3. Strategies to assess the quality of microperimetry data
Different strategies to analyze microperimetry results provide different information about the status of retinal function in physiological and pathological conditions. Assessing the quality of microperimetry data is crucial to properly interpret the results of the exam in normal or pathological conditions. Typical measures for evaluating the data for reliability include test-retest variability, the coefficient of repeatability, and the reliability test.
5.3.1. Coefficient of repeatability/test-retest variability
The coefficient of repeatability (CoR) denotes the range of sensitivity values within which test-retest differences lie with 95% probability. CoR is a measure of intra-patient, intra-session, or inter-session reproducibility, depending on the study. Calculations may be performed for sensitivity measures in different regions of the retina to ensure that the same measures are repeatable for each patient. More reproducible results are indicated by smaller values, but CoR values may differ across microperimetry devices, study populations, and tested regions of the retina. Using the Optos OCT/SLO, the CoR of the inter-session test-retest repeatability for eyes with maculopathy (diagnosis of GA, macular edema, or other maculopathy) was found to be 2.15 dB for mean retinal sensitivity and 4.64 dB for point-wise sensitivity in 1 study.24 Using the MAIA, the CoR of the intra-session repeatability for eyes with AMD ranged from 1.08 to 2.32 dB for mean retinal sensitivity, and ranged from 4.12 to 4.37 dB for point-wise sensitivity.63 Using the Nidek MP-1, the CoR for intra-session repeatability for eyes with macular disease was 1.81 dB for mean retinal sensitivity.8
The test-retest variability using a single experienced examiner on the Nidek MP-1 was investigated in eyes with macular disease.8 In this study, test-retest variability was lowest for mean retinal sensitivity (1.81 dB) and highest for point-wise sensitivity (4.96 dB). However, floor and ceiling effects may have caused underestimation of variability for point-wise sensitivity, as the variability increased to 5.56 dB when test loci affected by floor or ceiling effects were removed. In eyes with AMD, test-retest variability on the MAIA was reported to be significantly different between the first and second exams, but not in subsequent exams.63 Therefore, it is recommended that the results from the first exam be discarded to minimize influence from this learning effect in the interpretation of results.
In addition to inconsistent patient responses, other parameters of the device may lead to increased variability. For example, if retinal tracking is not precise, then point-wise projection of the same stimuli on the retina may be different and may result in subsequent changes in retinal sensitivity.
5.3.2. Reliability test
A common reliability test used to evaluate potential false-positive results throughout the exam is the Heijl-Krakau method, also termed false-positive catch trials.18 Suprathreshold stimuli are projected over a physiological blind spot, such as the optic disc, which should have a score of 0 dB. Stimuli can be presented up to 16 times over the course of the exam.8 The Heijl-Krakau method is typically considered acceptable if the false-positive response rate is less than 20%25 or 25%.67 False-negative catch trials are currently not available for microperimetry devices.
6. Microperimetry data informing geographic atrophy
6.1. Anatomic-functional correlation
The anatomic-functional correlation provided by microperimetry is valuable for assessing GA. As discussed earlier, standard visual tests such as BCVA are not an ideal measure of visual function for patients with GA, especially those with foveal sparing. Although other tests such as LLVA, reading speed, or PRO assessments provide a more comprehensive overview of retinal function in patients with GA,44 they do not allow for a correlation between anatomy and functional outcomes. Microperimetry data provide a detailed assessment of localized anatomical and functional deficits in patients with GA.2
In eyes with GA, the number of scotomatous points increases over time, which is associated with an increase in GA lesion size.27 The correlation of structural images such as en face OCT with functional readings from microperimetry provides greater insight into the impact of GA on visual function, for example, by superimposing a microperimetry map over en face OCT images to calculate mean retinal sensitivity by region.38 The integrity of the inner segment–outer segment junctional layer appears to be inversely correlated with mean retinal sensitivity.22,42 Changes in retinal sensitivity also correlate with neurosensory retinal thickness,37 and occur with changes in the photoreceptor layer rather than the RPE layer.47
Measures of point-wise sensitivity can assist with accurately mapping where these changes are occurring, such as with nascent GA or its correlation with the border of GA, because mean retinal sensitivity does not include spatial information.24 However, point-wise sensitivity has more variability than mean retinal sensitivity, so the latter is often used to follow GA progression due to its better CoR.8
6.2. Progression of geographic atrophy
Microperimetry has become an important tool for the assessment of GA progression (Fig. 3). It has been used in both observational clinical studies and interventional clinical trials. Microperimetry data were collected as a secondary endpoint in a subset of patients in the lampalizumab clinical program, including the Proxima A (NCT02479386) and Proxima B (NCT02399072) natural history studies of patients with GA, as well as the phase 3 Chroma (NCT02247479) and Spectri (NCT02247531) interventional studies assessing the efficacy and safety of lampalizumab for reducing the rate of GA enlargement.19 In these clinical trials, predefined study endpoints assessing longitudinal GA progression were the number of absolute scotomatous points and the mean retinal sensitivity.
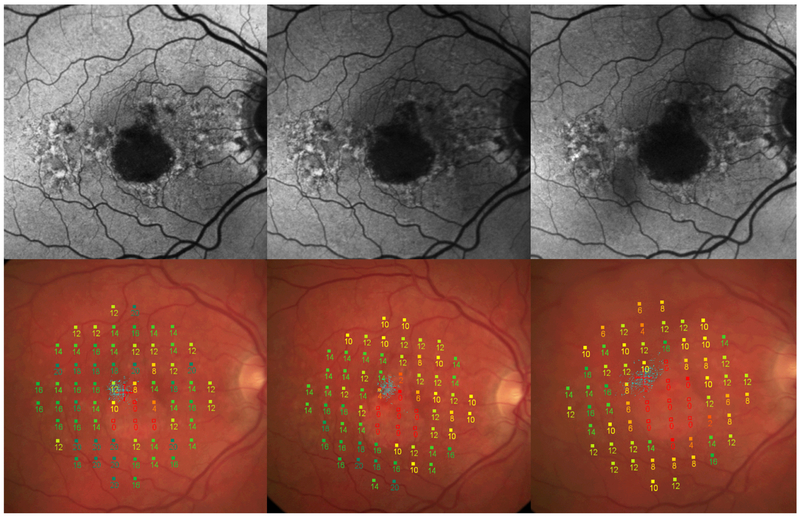
Fig. 3. Microperimetry assessments of geographic atrophy progression over 1 year.
Fundus autofluorescence images (top) and color fundus photography images (bottom, with microperimetry grid overlaid) from the same patient showing geographic atrophy progression at baseline (left), 6 months (middle), and 12 months (right).
Eyes with GA may experience an average increase of absolute scotomatous points per year, which correlates with increased size of the GA lesion over time.27 Microperimetry can also detect a severe drop in sensitivity over time at the junctional zone. Eyes with GA were found to have decreased sensitivity in all retinal regions outside GA lesions, compared with eyes with mild/intermediate AMD, suggesting that these patients with GA have a more global functional deficit.16 Another study found that there is a faster decline in sensitivity for perilesional points as compared with extralesional points, which correlates with loss of sensitivity in the junctional zone of atrophy.27 Fixation stability also decreases over time.
In a different study, microperimetry data from patients with GA were divided by topography or function and analyzed retrospectively.7 Topographical divisions were the central macula or paracentral macula; functional divisions were the region of dense scotoma, the edge of a dense scotoma, or remaining retina. There were no changes between the topographical regions. However, a significant decrease in retinal sensitivity was found for functional regions at the edge of a dense scotoma. These changes in regional sensitivity could be detected within 1 year of follow-up.
7. Conclusions
Different strategies to analyze microperimetry results provide different information about retinal pathology. Microperimetry assesses visual function at specific points of the retina, providing a unique opportunity for anatomic-functional correlation that has been demonstrated in several macular diseases.24,27,37,38,47 Despite the compelling scientific value of using microperimetry to evaluate GA, there is a paucity of GA-specific studies in the literature. The majority of microperimetry literature discusses patients with various macular diseases or AMD in different stages or reports on small sample sizes. More GA-specific data are needed to standardize microperimetry protocols for the disease and support robust interpretation.
Given the extensive time commitment for current microperimetry tests, the development of faster, more accessible methods is important for the future of perimetry as a ubiquitous clinical test. Using devices with more precise retinal tracking may also be helpful in decreasing unreliable testing. More rapid devices are being investigated as user-friendly alternatives to traditional microperimetry testing. For example, measures of mean central retinal sensitivity obtained using the tablet-based PsyPad examination were not significantly different from those obtained using the MAIA microperimeter. The PsyPad was able to generate a dynamic range of 31 dB, despite the fact that the test was administered on a tablet.68 Development of a test delivered on a portable, generally available device increases the possibility that patients may be able to self-monitor at home.
At the current time, anatomic assessment of GA via multimodal fundus imaging is commonly used to diagnose and monitor the disease; however, microperimetry offers an attractive addition to imaging methods for detecting conversion from intermediate AMD to GA and for quantifying GA progression through the detection and quantification of local functional deficits in the retina. By correlating these functional deficits with changes in retinal morphology, microperimetry has become a scientifically valuable method for assessing visual function loss in GA secondary to AMD.
Method of Literature Search.
Studies reporting on mesopic microperimetry and its use to evaluate GA were identified via a PubMed literature search using the terms “microperimetry,” “geographic atrophy,” “macular degeneration,” “retinal sensitivity,” “perilesional sensitivity,” and “fixation patterns,” and sorted for relevance. The search covered through March 2018. Additional articles were identified from the references within the publications identified by the primary search.
Studies reporting microperimetry data from eyes with GA, nascent GA, or advanced AMD were included; studies only reporting data from eyes with early or intermediate AMD were excluded. Conference abstracts and case reports were excluded. Studies where the whole article was not written in English were also excluded.
Acknowledgements
Funding was provided by F. Hoffmann-La Roche Ltd. for third-party writing assistance, which was provided by Charlotte A. Osborne, PhD, of Envision Pharma Group.
Funding
This work was supported by F. Hoffmann-La Roche Ltd.
Abbreviations:
- AMD
age-related macular degeneration
- BCEA
bivariate contour ellipse area
- BCVA
best-corrected visual acuity
- CFP
color fundus photograph
- CoR
coefficient of repeatability
- dB
decibel
- FAF
fundus autofluorescence
- GA
geographic atrophy
- iRORA
incomplete RPE and outer retinal atrophy
- LLVA
low-luminance visual acuity
- LV
loss variance
- MAIA
Macular Integrity Assessment
- NIR
near infrared
- OCT
optical coherence tomography
- PRL
preferred retinal locus
- PRO
patient-reported outcome
- PSD
pattern standard deviation
- RPD
reticular pseudodrusen
- RPE
retinal pigment epithelium
- SD-OCT
spectral-domain optical coherence tomography
- SLO
scanning laser ophthalmoscope
Footnotes
Disclosures
KGC: Consultant: Acucela, Inc., Astellas Pharma, Genentech, Inc., Heidelberg Engineering, Inc., Novartis, Ophthotech, Roche
PJP: Consultant: Bayer, Genentech, Inc., Novartis, Roche
YJS: Consultant: Astellas Pharma, Cell Cure/BioTime, Inc., Genentech, Inc., Optos, Paraxel, Regeneron, RegenxBio, Inc.
DGB: Consultant: Acucela, Inc., Applied Genetic Technologies Corp., Editas Medicine, Genentech, Inc., Ionis Pharmaceuticals, Nacuity Pharmaceuticals, NightstaRx
DVD: Research funding: Genentech, Inc., Regeneron; Consultant: Bayer, Genentech, Inc., Santen
MSI: Consultant: Alimera Sciences, Allergan, Boehringer Ingelheim, Genentech, Inc., Omeros, Quark, ThromboGenics
RHG: Consultant: Bayer, Genentech, Inc., Novartis, Roche
CDL: None
SG, HL, and DF are employees of Genentech, Inc. DF reports stock/stock options from F. Hoffmann-La Roche Ltd.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acton JH, Greenstein VC. Fundus-driven perimetry (microperimetry) compared to conventional static automated perimetry: similarities, differences and clinical applications. Can J Ophthalmol. 2013;48:358–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acton JH, Smith RT, Hood DC, Greenstein VC. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:7618–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batioglu F, Gedik Oguz Y, Demirel S, Ozmert E. Geographic atrophy progression in eyes with age-related macular degeneration: role of fundus autofluorescence patterns, fellow eye and baseline atrophy area. Ophthalmic Res. 2014;52:53–9 [DOI] [PubMed] [Google Scholar]
- 4.Biarnes M, Mones J, Alonso J, Arias L. Update on geographic atrophy in age-related macular degeneration. Optom Vis Sci. 2011;88:881–9 [DOI] [PubMed] [Google Scholar]
- 5.Cassels NK, Wild JM, Margrain TH, et al. The use of microperimetry in assessing visual function in age-related macular degeneration. Surv Ophthalmol. 2018;63:40–55 [DOI] [PubMed] [Google Scholar]
- 6.Chaikitmongkol V, Tadarati M, Bressler NM. Recent approaches to evaluating and monitoring geographic atrophy. Curr Opin Ophthalmol. 2016;27:217–23 [DOI] [PubMed] [Google Scholar]
- 7.Chen FK, Patel PJ, Webster AR, et al. Nidek MP1 is able to detect subtle decline in function in inherited and age-related atrophic macular disease with stable visual acuity. Retina. 2011;31:371–9 [DOI] [PubMed] [Google Scholar]
- 8.Chen FK, Patel PJ, Xing W, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50:3464–72 [DOI] [PubMed] [Google Scholar]
- 9.Cideciyan AV, Swider M, Aleman TS, et al. Macular function in macular degenerations: repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012;53:841–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Convento E, Barbaro G. Technical insights on the interpretation of automatic microperimetry, in Midena E (ed). Perimetry and the Fundus: An Introduction to Microperimetry, Thorofare (N.J.): Slack, 2007, pp. 229–37 [Google Scholar]
- 11.Crossland MD, Luong VA, Rubin GS, Fitzke FW. Retinal specific measurement of dark-adapted visual function: validation of a modified microperimeter. BMC Ophthalmol. 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125:369–90 [DOI] [PubMed] [Google Scholar]
- 13.Forte R, Querques G, Querques L, et al. Multimodal evaluation of foveal sparing in patients with geographicatrophy due to age-related macular degeneration. Retina. 2013;33:482–9 [DOI] [PubMed] [Google Scholar]
- 14.Fujii GY, de Juan E Jr., Sunness J, et al. Patient selection for macular translocation surgery using the scanning laser ophthalmoscope. Ophthalmology. 2002;109:1737–44 [DOI] [PubMed] [Google Scholar]
- 15.Hanout M, Horan N, Do DV. Introduction to microperimetry and its use in analysis of geographic atrophy in age-related macular degeneration. Curr Opin Ophthalmol. 2015;26:149–56 [DOI] [PubMed] [Google Scholar]
- 16.Hariri AH, Tepelus TC, Akil H, et al. Retinal sensitivity at the junctional zone of eyes with geographic atrophy due to age-related macular degeneration. Am J Ophthalmol. 2016;168:122–8 [DOI] [PubMed] [Google Scholar]
- 17.Hartmann KI, Bartsch DU, Cheng L, et al. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011;31:1323–31 [DOI] [PubMed] [Google Scholar]
- 18.Heijl A, Krakau CE. An automatic static perimeter, design and pilot study. Acta Ophthalmol (Copenh). 1975;53:293–310 [DOI] [PubMed] [Google Scholar]
- 19.Holz FG, Sadda SR, Busbee B, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136:666–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holz FG, Sadda SR, Staurenghi G, et al. Imaging protocols in clinical studies in advanced age-related macular degeneration: recommendations from Classification of Atrophy Consensus Meetings. Ophthalmology. 2017;124:464–78 [DOI] [PubMed] [Google Scholar]
- 21.Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079–91 [DOI] [PubMed] [Google Scholar]
- 22.Landa G, Su E, Garcia PM, et al. Inner segment-outer segment junctional layer integrity and corresponding retinal sensitivity in dry and wet forms of age-related macular degeneration. Retina. 2011;31:364–70 [DOI] [PubMed] [Google Scholar]
- 23.Lindner M, Nadal J, Mauschitz MM, et al. Combined fundus autofluorescence and near infrared reflectance as prognostic biomarkers for visual acuity in foveal-sparing geographic atrophy. Invest Ophthalmol Vis Sci. 2017;58:BIO61–7 [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Bittencourt MG, Wang J, et al. Retinal sensitivity is a valuable complementary measurement to visual acuity — a microperimetry study in patients with maculopathies. Graefes Arch Clin Exp Ophthalmol. 2015;253:2137–42 [DOI] [PubMed] [Google Scholar]
- 25.Luu CD, Dimitrov PN, Robman L, et al. Role of flicker perimetry in predicting onset of late-stage age-related macular degeneration. Arch Ophthalmol. 2012;130:690–9 [DOI] [PubMed] [Google Scholar]
- 26.Markowitz SN, Reyes SV. Microperimetry and clinical practice: an evidence-based review. Can J Ophthalmol. 2013;48:350–7 [DOI] [PubMed] [Google Scholar]
- 27.Meleth AD, Mettu P, Agron E, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci. 2011;52:1119–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morales MU, Saker S, Wilde C, et al. Reference clinical database for fixation stability metrics in normal subjects measured with the MAIA microperimeter. Transl Vis Sci Technol. 2016;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ooto S, Ellabban AA, Ueda-Arakawa N, et al. Reduction of retinal sensitivity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2013;156:1184–91.e2 [DOI] [PubMed] [Google Scholar]
- 30.Ooto S, Suzuki M, Vongkulsiri S, et al. Multimodal visual function testing in eyes with nonexudative age-related macular degeneration. Retina. 2015;35:1726–34 [DOI] [PubMed] [Google Scholar]
- 31.Panorgias A, Zawadzki RJ, Capps AG, et al. Multimodal assessment of microscopic morphology and retinal function in patients with geographic atrophy. Invest Ophthalmol Vis Sci. 2013;54:4372–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfau M, Goerdt L, Schmitz-Valckenberg S, et al. Green-light autofluorescence versus combined blue-light autofluorescence and near-infrared reflectance imaging in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:BIO121–30 [DOI] [PubMed] [Google Scholar]
- 33.Pfau M, Lindner M, Fleckenstein M, et al. Test-retest reliability of scotopic and mesopic fundus-controlled perimetry using a modified MAIA (macular integrity assessment) in normal eyes.Ophthalmologica. 2017;237:42–54 [DOI] [PubMed] [Google Scholar]
- 34.Pfau M, Lindner M, Gliem M, et al. Mesopic and dark-adapted two-color fundus-controlled perimetry in patients with cuticular, reticular, and soft drusen. Eye (Lond). doi: 10.1038/s41433-018-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfau M, Lindner M, Müller PL, et al. Effective dynamic range and retest reliability of dark-adapted two-color fundus-controlled perimetry in patients with macular diseases. Invest Ophthalmol Vis Sci. 2017;58:BIO158–67 [DOI] [PubMed] [Google Scholar]
- 36.Pfau M, Lindner M, Steinberg JS, et al. Visual field indices and patterns of visual field deficits in mesopic and dark-adapted two-colour fundus-controlled perimetry in macular diseases. Br J Ophthalmol. 2018;102:1054–9 [DOI] [PubMed] [Google Scholar]
- 37.Pilotto E, Benetti E, Convento E, et al. Microperimetry, fundus autofluorescence, and retinal layer changes in progressing geographic atrophy. Can J Ophthalmol. 2013;48:386–93 [DOI] [PubMed] [Google Scholar]
- 38.Pilotto E, Convento E, Guidolin F, et al. Microperimetry features of geographic atrophy identified with en face optical coherence tomography. JAMA Ophthalmol. 2016;134:873–9 [DOI] [PubMed] [Google Scholar]
- 39.Pilotto E, Guidolin F. Geographic atrophy, in Midena E (ed). Microperimetry and Multimodal Retinal Imaging, Berlin, Heidelberg, Springer Berlin Heidelberg, 2014, pp. 77–88 [Google Scholar]
- 40.Pilotto E, Guidolin F, Convento E, et al. Fundus autofluorescence and microperimetry in progressing geographic atrophy secondary to age-related macular degeneration. Br J Ophthalmol. 2013;97:622–6 [DOI] [PubMed] [Google Scholar]
- 41.Querques G, Massamba N, Srour M, et al. Impact of reticular pseudodrusen on macular function. Retina. 2014;34:321–9 [DOI] [PubMed] [Google Scholar]
- 42.Querques L, Querques G, Forte R, Souied EH. Microperimetric correlations of autofluorescence and optical coherence tomography imaging in dry age-related macular degeneration. Am J Ophthalmol. 2012;153:1110–5 [DOI] [PubMed] [Google Scholar]
- 43.Rudnicka AR, Kapetanakis VV, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160:85–93.e3 [DOI] [PubMed] [Google Scholar]
- 44.Sadda SR, Chakravarthy U, Birch DG, et al. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina. 2016;36:1806–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology. 2018;125:537–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye. 1988;2:552. [DOI] [PubMed] [Google Scholar]
- 47.Sayegh RG, Kiss CG, Simader C, et al. A systematic correlation of morphology and function using spectral domain optical coherence tomography and microperimetry in patients with geographic atrophy. Br J Ophthalmol. 2014;98:1050–5 [DOI] [PubMed] [Google Scholar]
- 48.Sayegh RG, Sacu S, Dunavölgyi R, et al. Geographic atrophy and foveal-sparing changes related to visual acuity in patients with dry age-related macular degeneration over time. Am J Ophthalmol. 2017;179:118–28 [DOI] [PubMed] [Google Scholar]
- 49.Schönbach EM, Ibrahim MA, Kong X, et al. Metrics and acquisition modes for fixation stability as a visual function biomarker. Invest Ophthalmol Vis Sci. 2017;58:BIO268–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schönbach EM, Ibrahim MA, Strauss RW, et al. Fixation location and stability using the MP-1 microperimeter in Stargardt disease. Ophthalmol Retina. 2017;1:68–76 [DOI] [PubMed] [Google Scholar]
- 51.Seiple W, Rosen RB, Castro-Lima V, Garcia PM. The physics and psychophysics of microperimetry. Optom Vis Sci. 2012;89:1182–91 [DOI] [PubMed] [Google Scholar]
- 52.Simunovic MP, Moore AT, MacLaren RE. Selective automated perimetry under photopic, mesopic, and scotopic conditions: detection mechanisms and testing strategies. Transl Vis Sci Technol. 2016;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinberg JS, Sassmannshausen M, Pfau M, et al. Evaluation of two systems for fundus-controlled scotopic and mesopic perimetry in eye with age-related macular degeneration. Transl Vis Sci Technol. 2017;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinman RM. Effect of target size, luminance, and color on monocular fixation. J Opt Soc Am. 1965;55:1158–64 [Google Scholar]
- 55.Sunness JS, Applegate CA. Long-term follow-up of fixation patterns in eyes with central scotomas from geographic atrophy that is associated with age-related macular degeneration. Am J Ophthalmol. 2005;140:1085–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114:271–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sunness JS, Rubin GS, Zuckerbrod A, Applegate CA. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind. 2008;102:600–10 [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi A, Ooto S, Yamashiro K, et al. Photoreceptor damage and reduction of retinal sensitivity surrounding geographic atrophy in age-related macular degeneration. Am J Ophthalmol. 2016;168:260–8 [DOI] [PubMed] [Google Scholar]
- 59.Wong EN, Chew AL, Morgan WH, et al. The use of microperimetry to detect functional progression in non-neovascular age-related macular degeneration: a systematic review. Asia Pac J Ophthalmol (Phila). 2017;6:70–9 [DOI] [PubMed] [Google Scholar]
- 60.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–6 [DOI] [PubMed] [Google Scholar]
- 61.Wu Z, Ayton LN, Guymer RH, Luu CD. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. 2014;121:1612–9 [DOI] [PubMed] [Google Scholar]
- 62.Wu Z, Ayton LN, Guymer RH, Luu CD. Comparison between multifocal electroretinography and microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:6431–9 [DOI] [PubMed] [Google Scholar]
- 63.Wu Z, Ayton LN, Guymer RH, Luu CD. Intrasession test–retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7378–85 [DOI] [PubMed] [Google Scholar]
- 64.Wu Z, Ayton LN, Luu CD, Guymer RH. Microperimetry of nascent geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;56:115–21 [DOI] [PubMed] [Google Scholar]
- 65.Wu Z, Ayton LN, Luu CD, Guymer RH. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015;133:442–8 [DOI] [PubMed] [Google Scholar]
- 66.Wu Z, Ayton LN, Luu CD, Guymer RH. Relationship between retinal microstructures on optical coherence tomography and microperimetry in age-related macular degeneration. Ophthalmology. 2014;121:1445–52 [DOI] [PubMed] [Google Scholar]
- 67.Wu Z, Ayton LN, Makeyeva G, et al. Impact of reticular pseudodrusen on microperimetry and multifocal electroretinography in intermediate age-related macular degeneration: the influence of RPD on retinal and visual function. Invest Ophthalmol Vis Sci. 2015;56:2100–6 [DOI] [PubMed] [Google Scholar]
- 68.Wu Z, Guymer RH, Jung CJ, et al. Measurement of retinal sensitivity on tablet devices in age-related macular degeneration. Transl Vis Sci Technol. 2015;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Z, Jung CJ, Ayton LN, et al. Test–retest repeatability of microperimetry at the border of deep scotomas. Invest Ophthalmol Vis Sci. 2015;56:2606–11 [DOI] [PubMed] [Google Scholar]
- 70.Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121:2415–22 [DOI] [PubMed] [Google Scholar]
- 71.Yehoshua Z, Rosenfeld PJ, Gregori G, et al. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography. Ophthalmology. 2011;118:679–86 [DOI] [PMC free article] [PubMed] [Google Scholar]