Abstract
Increased estrogen is a strong epidemiologic risk factor for development of pulmonary arterial hypertension (PAH) in patients, associated with metabolic defects. Estrogens also drive penetrance in mice carrying mutations in BMPR2, the cause of most heritable PAH. The goal of the present study was to determine if inhibition of estrogens was effective in treatment of PAH in these mice.
The estrogen inhibitors fulvestrant and anastrozole were used in both a prevention and treatment paradigm in BMPR2 mutant mice, and tamoxifen used for treatment. BMPR2 mutant mice were also crossed onto estrogen receptor ESR1 and ESR2 knockout backgrounds to assess receptor specificity. Hemodynamic and metabolic outcomes were measured.
Estrogen inhibition both prevented and treated PAH in BMPR2 mutant mice. This was associated with reduction in metabolic defects including oxidized lipid formation, insulin resistance, and rescue of PPARg and CD36. The effect was mediated primarily through ESR2, but partially through ESR1.
Our data suggest that trials of estrogen inhibition in human PAH are warranted, and may improve pulmonary vascular disease through amelioration of metabolic defects. Although fulvestrant and anastrozole were more effective than tamoxifen, tamoxifen may be useful in pre-menopausal women, because of reduced risk of induction of menopause.
Introduction
Pulmonary Arterial Hypertension (PAH) is a disease which includes pulmonary vascular endothelial dysfunction, occlusion and dropout of the small and medium-sized pulmonary arteries, and hypertrophy and proliferation of smooth muscle and adventitial cells. These combine for a progressively worsening elevation of pulmonary vascular resistance[1, 2]. This eventually leads to right heart failure and death; no current therapy is curative.
The majority of cases of the heritable form of PAH (HPAH) are associated with mutations in BMPR2, the type 2 receptor for the BMP pathway[3]. In addition, BMPR2 is suppressed in most other forms of PAH, even in the absence of mutation [4]. Mice with BMPR2 mutation or deletion will spontaneously develop PAH[5–7]. However, penetrance in both mice and humans with BMPR2 mutation is incomplete: only 20% of humans with BMPR2 mutation overall will develop clinical PAH[8].
The strongest epidemiologic risk factor for many forms of PAH is female sex[9]. And, while only 20% of humans with a BMPR2 mutation develop PAH, there is a striking difference according to sex: 43% of females versus 14% of males with a BMPR2 mutation develop PAH in their lifetime[10]. Consistent with this finding, we demonstrated that estrogen metabolism was a strong predictor of penetrance in HPAH: women who preferentially metabolized estrogens into 16- estrogens such as 16αOHE1 developed PAH, whereas women who preferentially metabolized estrogen into 2- or 4- estrogens did not[11–13]. Estrogen metabolism also drives penetrance in men, although not to the same degree as in women[14].
The mechanism for female preponderance of human disease remains poorly explained, in part because in classical rodent models of PAH like hypoxia and monocrotaline, estrogen was protective[15]. This may be associated with a difference between endogenous and exogenous estrogens; potential differences include location of production (significant estrogens are made in the pulmonary vasculature) and the cyclic nature of natural estrogens[16]. Our data show that, as in human patients, BMPR2 mutant mice treated with 16αOHE1 developed PAH with higher penetrance and severity[14], more closely recapitulating human phenomenon than other models. Our prior work also suggested that 16αOHE1 promotes insulin resistance and other metabolic problems[14, 17]. Consistent with this finding, metabolic defects have been increasingly associated with PAH[18–20], and we have demonstrated that they exacerbate PAH in BMPR2 mutant mice[21, 22]. Although the mechanism linking metabolic defects to PAH is not clear, it may be associated with vascular dysfunction, proliferation, or production of damaging superoxides[23].
Because in classical PAH models estrogen inhibition is harmful, the current study sought to determine whether inhibition of endogenous estrogens was therapeutically effective at preventing or reversing established BMPR2-related PAH, and whether this was associated with improved metabolic metrics such as insulin resistance and oxidized lipids. Safety of anastrazole in postmenopausal PAH patients, with suggestion of efficacy, has already been established in a small trial[24]. Demonstration of efficacy and mechanism is thus a final step necessary in preclinical models to validate translation of this chain of research to patients.
Methods
Estrogen Inhibition Experiments
We used the Rosa26-rtTA2 x TetO7-Bmpr2R899X FVB/N mice as previously described[25, 26], called Rosa26-Bmpr2R899X or Bmpr2R899X for brevity. R899X is an arginine to termination mutation at amino acid 899 in the BMPRII tail domain found in family US33[6]. Expression of transgene occurs in all tissue types, but only after initiation of doxycycline.
Adult female Rosa26-only or Rosa26-Bmpr2delx4+ mice at a starting age of 8–10 weeks had transgene activated with doxycycline at 0.2 mg/g, and received either vehicle (see below) or treatment. No mice in these experiments received exogenous 16αOHE1. Mice were randomized to a treatment group, and the individual performing phenotyping was blinded as to group, as were the institutional specialty labs performing, for instance, insulin counts. Fulvestrant (Selleck Chemicals, Houston, TX ) was dissolved in ethanol to 100 mg/ml. Anastrozole (Sigma, St. Louis, MO) was dissolved in ethanol to 2 mg/ml. Inhibitor solution was diluted in peanut oil and injected subcutaneously into mouse daily. Fulvestrant dosage was 200 μg/mouse/day (~6 mg/kg) and anastrozole dosage was 10 μg/mouse/day (~0.3mg/kg). Medroxyprogesterone 17-acetate (Sigma, St. Louis, MO) was dissolved in ethanol to 50 mg/ml. Medroxyprogesterone 17-acetate (MPA) solution was diluted in peanut oil and injected intramuscularly into mouse at dosage of 1 mg/mouse every 3 weeks (30 mg/kg/3 weeks). Tamoxifen (Sigma, St. Louis, MO) was dissolved in ethanol to 100 mg/ml, it was diluted with peanut oil before injecting into mice. Injection dosage was 1 mg/mouse/day (~30 mg/kg/day).
Drug dosages used were converted from human dosages according to the method of Reagan-Shaw et al[27], using proportional body surface areas; this results in increasing dosages from human patient to mouse per unit weight by a factor of roughly 12. Doses used are well matched to those used in recent human and animal studies; for a 50kg human female, our dose would be equivalent to 1.2 mg/day, similar to the 1.0 mg/day used in the recent pilot study in human PAH patients[24].
For prevention experiments, animals received drug starting at time of doxycycline induction. For reversal experiments, animals received doxycycline only for four weeks, followed by an additional two weeks of both doxycycline and treatment (or vehicle).
After six weeks, animals underwent echocardiography, followed on the next day by hemodynamic phenotyping and tissue collection, as described below.
Echocardiography, and Hemodynamic Phenotyping
Two-dimensional echocardiography was performed using Vivo 770© High-Resolution Image System (VisualSonics© Toronto, Canada). Echocardiograms including B-mode, M-mode and spectral Doppler images were obtained the day prior to sacrifice under isoflurane anesthetic. Velocity time integral and heart rate were measured in the ascending aorta, diameter measured in the same location. Stroke volume [SV(calc)] was derived using the formula SV = {[π (Aortic diameter)2/4] * [Aortic velocity time integral]. Cardiac output was [CO (calc)] derived using formula CO = SV(calc) * heart rate (HR) [28, 29].
Hemodynamic phenotyping was performed as previously described[6, 25]. Briefly, mice are anesthetized with tribromoethanol, systemic pressure checked by tail cuff, and then undergo closed-chested intrajugular right cardiac catheterization.
Plasma estradiol measurements
Four female mice from each treatment were randomly chosen for estradiol measurements. Twenty-five μl plasma were dispensed into estradiol ELISA plate in triplicate (Cat# ES180S, Calbiotech Inc, El Cajon, CA). Estradiol ELISA was performed according to manufacturer’s instructions; the absorbance at 450nm was read with a micro plate reader (Bio-Rad Model 680). The concentration of plasma estradiol level was calculated and presented as pg/ml from the standard curve.
ESR1/ESR2 X Bmpr2R899X Experiments
ERS1 or ESR2 knock-out mice were purchased from Jackson lab. They were firstly crossed with Rosa26 mice with C57BL/6 background. Then they were crossed with Rosa-R899X mice with FVB/N background. Controls (Rosa26-Bmpr2R899X only) were phenotyped on the same days as the ESR1 and ESR2 knockouts, and are a different group of animals than in any other experiment (in no experiment were control values ‘re-used’). Experiments were otherwise conducted exactly as for estrogen inhibition experiments, above.
Vessel Muscularization
Paraffin-embedded mouse lung sections were prepared as described previously. Slides were incubated with mouse monoclonal smooth muscle alpha actin (SMA) antibody (1:200; DAKO, Carpinteria, CA) overnight at 4°C. After 3 washes with PBS, the slides were treated with Alexa 488 fluorescent secondary antibody (1:1000; Invitrogen, Carlsbad, Calif., USA) for 1 hour at room temperature. After 3 washes with PBS, the slides were air dried and added with mounting medium (Vector Laboratories, Burlingame, CA) and covered by cover glass. SMA positive vessels were counted under 10x field with Nikon eclipse 90i upright fluorescent microscope for 10 randomly chosen fields per mouse.
Western Blotting
Tissues were homogenized in 500 μl of RIPA Buffer (PBS, 1% ipegal, 0.5% sodium deoxycholate, 0.1% SDS) with protease and phosphatase inhibitor cocktail (Sigma). Tissues were centrifuged at 4°C (15 min, 15,000 x g) and protein concentration was determined by Bradford microassay (Bio-Rad, Hercules, CA) on the supernatant. Equal amounts of protein extracts were denatured at 95°C in a denaturing sample buffer. Protein from each sample (20 μg) was separated by electrophoresis in 4–12% Bis-Tris gel and transferred onto a PVDF membrane; running buffers were either NuPage SDS Running Buffer (Life Technology) or Tris/Glycine/SDS buffer (Bio-Rad). The membrane was blocked for 1 hour at room temperature with phosphate-buffered saline containing 5% non-fat dry milk and 0.05% Tween-20 and probed overnight at 4°C with primary rabbit polyclonal antibody against PPAR (1:1000 Dilution, Abcam, Cambridge, MA), CD36 (1:1000 dilution, Novus Biological, Litteton, CO) and β-actin (1:1000, Abcam, Cambridge, MA). The membrane was then incubated at 37°C for 1 hour with horseradish peroxidase-labeled donkey anti-mouse immunoglobulin secondary antibody (1:5000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase was detected using the SuperSignal West Pico Chemiluminescent substrate (Thermo Scientific, Rockford, IL). Blots were imaged using either a Bio-Rad ChemiDoc Touch Imaging System or an Alpha Innotech Digital Imaging System. Densitometry was performed using ImageJ (public domain software published by the NIH).
Detection of mitochondrial superoxide
Mitochondrial superoxide was detected by MitoSox Red (Molecular Probes, Eugene, Oregon). Mouse PMVEC cells (WT orBMPR2R899X), and transgene induction were conducted as previously reported. The cells were grown till 50–80% confluence on chamber slides. MitoSOX was added to final concentration of 0.5 μM, Hoechst was added to a final concentration of 0.1 μg/ml. Cells were placed for 20 min at 37° incubator and then they were washed three times with Hank’s Buffered Salt Solution (HBSS) containing calcium and magnesium. The slides were added with mounting medium (Vector Laboratories, Burlingame, CA) and covered by coverslips. The digital images were taken by Nikon eclipse 90i upright fluorescent microscope with 100× oil immersion objective lens.
Measurement of mitochondrial oxygen uptake
Wild-type and BMPR2R899X murine pulmonary microvascular endothelial cells were harvested and cultured from the Immortomouse background as previously described[30]. 72 hours prior to experiments, cells were transitioned to 37°C, and doxycycline (300 ng/ml) was added to induce transgene expression. For the 24 hours prior to analysis, cells were treated with 1 μM 16αOHE1 or vehicle. Cells were washed in basal assay media from Seahorse Biosciences (North Billerica, MA). One hour prior to analysis, cells were equilibrated in a CO2-free incubator in Seahorse’s basal assay media supplemented with glucose (1g/L), L-glutamine (2mM), sodium pyruvate (1mM), ph 7.4. Cells were then analyzed in the Seahorse XFe96 Extracellular Flux Analyzer using the Mito Stress Test protocol with the following reagent concentrations (optimized for these analyses in separate experiments): oligomycin (2.5μM final well), FCCP (0.5μm final well), FCCP (0.5μm final well total 1μM) and rotenone and antimycin A (0.5μM final well) at manufacturer’s recommended concentrations. Data are reported as the average of 5–8 wells per individual condition in each experiment, with experiments performed in duplicate with 70K PMVECs per well.
Measurement of Glut4 mobilization in respons to insulin.
Glut4-GFP expression vector was purchased from OriGene (Rockville, MD). Cells were transfected with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to manufactory’s instructions. Transfection solutions were replaced by fresh medium 4 hrs after transfection, and cells were treated with 1 μM 16αOHE1 or vehicle for 18 hrs. Then the cells were serum-starved for 6 hrs, stimulated with 10 nM insulin for 1 hr, fixed, washed, and stained for DAPI and DY-554 phalloidin (red). Coverslips were mounted in Vectashield mounting medium (Vector Laboratories) and observed under microscope.
Immunohistochemical analysis of isoketal protein adducts and ceramides in murine lung
Immunolocalization of isoketal protein adducts and ceramides was performed on murine lung sections. Tissue sections were deparaffinized, rehydrated, and treated with 0.3% hydrogen peroxide as described previously. For isoketal staining, sections were incubated with in 0.1 M PBS (pH 7.4) containing 5% normal mouse serum and 5% bovine serum albumin for 30 min at RT to block nonspecific binding of the secondary antibody. Sections were incubated with 5 μg/ml D11 ScFv (Isoketal antibody) for 2 h at RT and then incubated with HRP-labeled anti-E Tag (1:500 dilution, GE Healthcare, Pittsburgh, PA) for 2 h at RT. For ceramide staining, antigen retrieval was performed in 10 mM citrate buffer. The sections were blocked with 5% BSA, followed by incubation with ceramide antibody [Enzo Life Sciences, Farmingdale, NY] overnight at 4°C. Next day, the sections were incubated with biotinylated secondary antibody followed by incubation with HRP-conjugated streptavidin. Diaminobenzidine (DAB) was used as a substrate for HRP (Vector Labs, Burlingame, CA). The sections were dehydrated and mounted in Cytoseal XYL (Richard-Allan Scientific, MI, USA) for light microscopic examination.
Statistical Methods
Statistical tests were performed using the JMP program (SAS, Cary, NC). One-way or multiple ANOVA were used to determine effects of interacting variables, with post-hoc Fischer’s LSD used to determine difference between individual groups. Significance of correlations were established using correlation z-test.
Animal Use Approval
The Institutional Animal Care and Use Committee at Vanderbilt University approved all animal studies.
Results:
Estrogen Inhibition Prevents Pulmonary Hypertension in BMPR2 mutant mice
Having previously shown that 16αOHE1 amplifies PAH, we first sought to determine whether estrogen inhibition, or androgen exposure, were protective, and if they worked synergistically. For estrogen inhibition, we used the combination of anastrozole and fulvestrant as commonly used for estrogen suppression in human breast cancer. We used them in combination only, rather than independently, because the goal of this initial study was to determine without the strongest possible pharmacologic signal if inhibition of estrogen signaling was protective in this model. Control (Rosa26 only) or Rosa26-Bmpr2R899X mice were fed doxycycline at 0.2g/kg for six weeks (a time in which Bmpr2R899X mice will typically develop elevated RVSP[25]), and then each genotype was split into four groups: plus or minus the androgen medroxyprogesterone 17-acetate (MPA, 1 mg/mouse every 3 weeks) and plus or minus a combination of the aromatase inhibitor anastrozole (10 μg/mouse/day) and the estrogen receptor inhibitor fulvestrant (200 μg/mouse/day) (referred to hence as anastrozole/fulvestrant). By multiple factor ANOVA, use of MPA had no significant effect on any variable measured, and so for the remainder of the studies they were grouped with the non-MPA animals. Furthermore, the results are not changed if MPA-exposed animals are excluded.
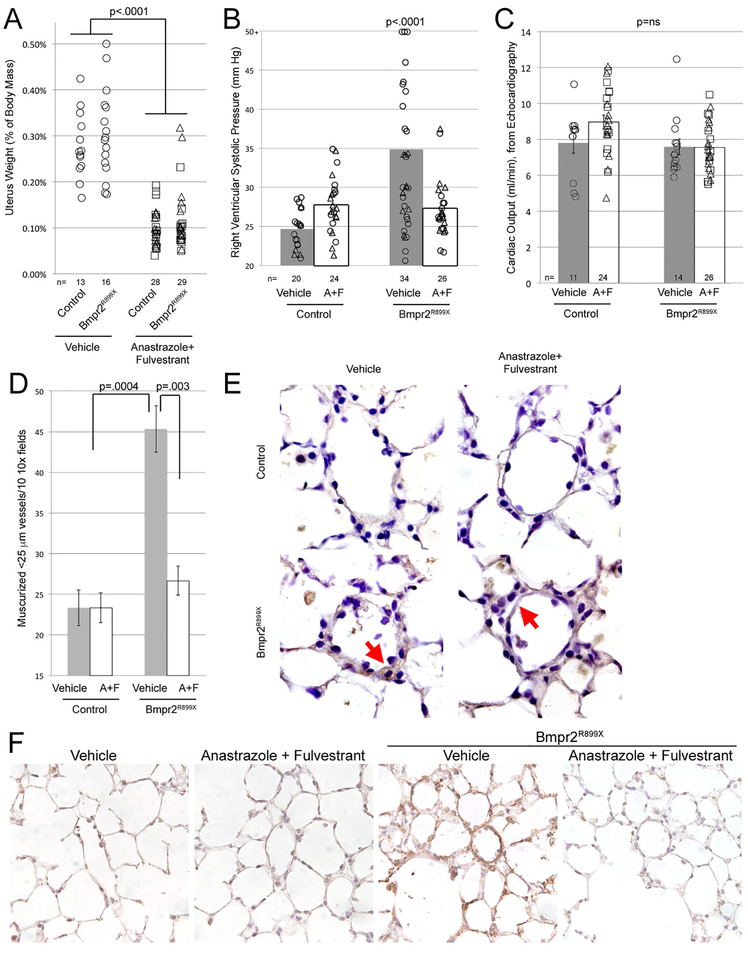
Use of anastrozole/fulvestrant was effective in suppressing estrogen signaling, as demonstrated by a strong reduction in uterus weight (Figure 1A). As expected, Bmpr2 mutant mice treated with vehicle had elevated RVSP, averaging 35 mm Hg, and about a 50% penetrance (Figure 1B). RVSP is variable in Bmpr2R899X mice because pulmonary vascular resistance is driven by loss and narrowing of vessels, rather than constriction[25]. Use of anastrozole/fulvestrant prevented most of the increase in RVSP in Bmpr2R899X mice, with RVSP averaging 27mmHg in the treatment group, and a penetrance of about 10%, p<0.0001 compared to vehicle. There was no effect on cardiac output in any group (Figure 1C). Fulton index (Supplemental Figure 1) is not informative in this model; we have previously published that the RV in Bmpr2 mutant mice (and patients) have an impaired hypertrophic response[19].
Figure 1 –
(A) Anastrozole & Fulvestrant (A+F) reduce uterine weights in mice (p<0.0001) as expected; by multiple ANOVA, R899X mutation did not have an effect on uterine weights. Each symbol is the measurement from one mouse. (B) Anastrozole & Fulvestrant delivered in osmotic pumps through the final four weeks of six weeks’ BMPR2R899X transgene induction prevents development of elevated RVSP (p<0.0001 by multiple ANOVA, using transgene and drug as variables). Each symbol is a value from one mouse; symbols that are triangles also received the androgen MPA, which did not impact RVSP and so have been not been separated. Numbers of mice in each group are listed at the bottom of the plot; mice were age-matched, and controls were done contemporaneously for each group. (C) Cardiac outputs were not significantly changed by either Anastrozole & Fulvestrant treatment or Bmpr2 mutation. (D) Anastrozole & Fulvestrant nearly normalized the increase in muscularized vessels normally seen in BMPR2R899X mice. By multiple ANOVA considering BMPR2 mutation and treatment as factors, BMPR2 mutation increases muscularized vessels (p=0.0004) while A+F decrease them, specifically in BMPR2 mutant mice (p=0.003). (E) Staining for oxidized lipids demonstrates increased oxidative stress in BMPR2R899X mice, normalized with Anastrozole & Fulvestrant. (F) Ceramide (toxic lipid) staining (brown) is increased in lungs from BMPR2R899X mice, and reduced by Anastrozole and Fulvestrant.
Estrogen inhibition with anastrozole/fulvestrant nearly normalized muscularized vessel counts (Figure 1D). Because we have previously shown increased oxidative stress as a marker of the metabolic defects in Bmpr2 mutant mice, we performed immunohistochemistry for oxidized lipids, and found increased oxidized lipid in the vessel wall in Bmpr2 mutant mice; however, this was normalized by estrogen inhibition (red arrows in Figure 1E). Estrogen inhibition does not appear to reduce oxidized lipid in infiltrating inflammatory cells, however (also visible in Figure 1E). We have previously shown an increase in accumulation of the toxic lipid ceramide with Bmpr2 mutation[31] as part of metabolic dysfunction. Ceramide was once again increased in the vascular walls of Bmpr2 mutant mice, and this was reduced by anastrozole/fulvestrant (Figure 1F).
Estrogen Inhibition Reverses Established PAH in BMPR2 mutant mice
Because reversal of established disease is particularly relevant to the PAH patient population, we allowed Bmpr2 mutant mice to develop PAH for six weeks, as in the above experiment, and then treated them with either anastrozole/fulvestrant or with the somewhat weaker estrogen receptor antagonist tamoxifen for an additional four weeks. Tamoxifen was added because it can be used in premenopausal women safely without inducing menopause.
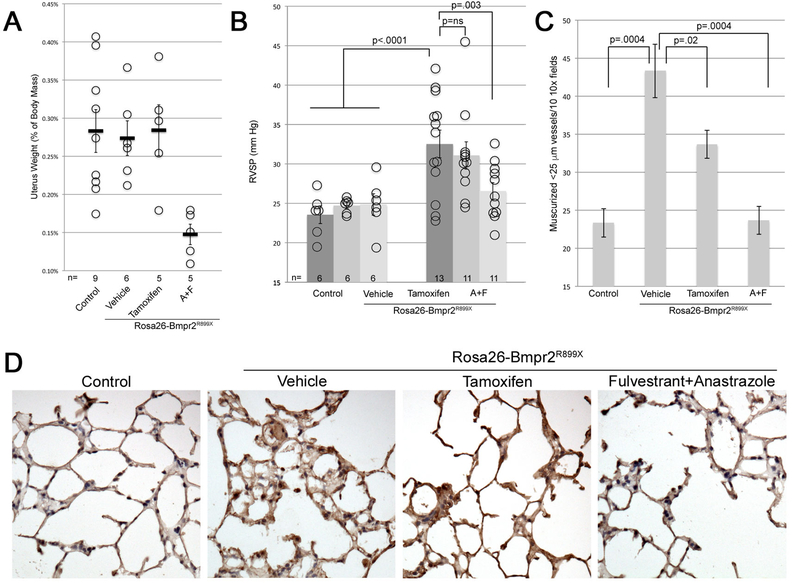
As expected, tamoxifen did not impact uterine weights, but anastrozole/fulvestrant reduced uterus weights (Figure 2A). Use of anastrozole/fulvestrant reduced plasma estrogen levels ~3.5x (Supplemental Figure 2A). Although as expected, tamoxifen did not impact circulating estrogen, it did reduce expression of known estrogen targets FHL1 and SNAT2[32] in lung by 50% and 35% respectively (Supplemental Figure 2B).
Figure 2 –
(A) Anastrozole & Fulvestrant but not Tamoxifen used as treatment reduces uterine weights in mice (p=0.002 for comparison to control, or 0.007 for comparison to vehicle). Each symbol is the measurement from one mouse; median and SEM are indicated. (B) Anastrozole & Fulvestrant delivered in osmotic pumps through the final four weeks of ten weeks’ BMPR2R899X transgene induction reverses development of elevated RVSP (p=0.003 by multiple ANOVA, using transgene and drug as variables). Tamoxifen used the same way did not have a significant effect on RVSP but RVSP trended down. Each symbol is a value from one mouse. (C) Anastrozole & Fulvestrant used as treatment nearly normalized the increase in muscularized vessels normally seen in BMPR2R899X mice; Tamoxifen had intermediate effect. By ANOVA, BMPR2 mutation increases muscularized vessels (p=0.0004) while A+F (p=0.0004) and Tamoxifen (p=0.02) both decrease them. (D) Ceramide (toxic lipid) staining (brown) is increased in lungs from BMPR2R899X mice, and reduced by Anastrozole and Fulvestrant, but not by Tamoxifen.
Vehicle treated Bmpr2R899X mice had penetrance and pressures comparable to previous experiments, which were largely reversed by treatment with anastrozole/fulvestrant, but with less effect by tamoxifen (Figure 2B). Counts of muscularized small vessels showed a strong increase in Bmpr2R899X mice, with modest reduction with tamoxifen, and normalization with anastrozole/fulvestrant (Figure 2C). Ceramide was increased in the vascular walls of Bmpr2 mutant mice, and this was reduced by anastrozole/fulvestrant, but not apparently in tamoxifen (Figure 2D).
Knockout of Either ESR1 or ESR2 partially protects BMPR2 Mutant Mice
Because pharmacologic methods to inhibit estrogen receptors may lack specificity and vary by cell type, we used genetic knockouts to attempt to determine whether the estrogen effect was mediated through ESR1 or ESR2. These mice thus each had two transgenes and two knockout alleles (Rosa26-rtTA2M2, TetO7-Bmpr2R899X, and two alleles knocked out of either ESR1 or ESR2). Adult animals were fed doxycycline for 6 weeks to induce Bmpr2R899X transgene expression.
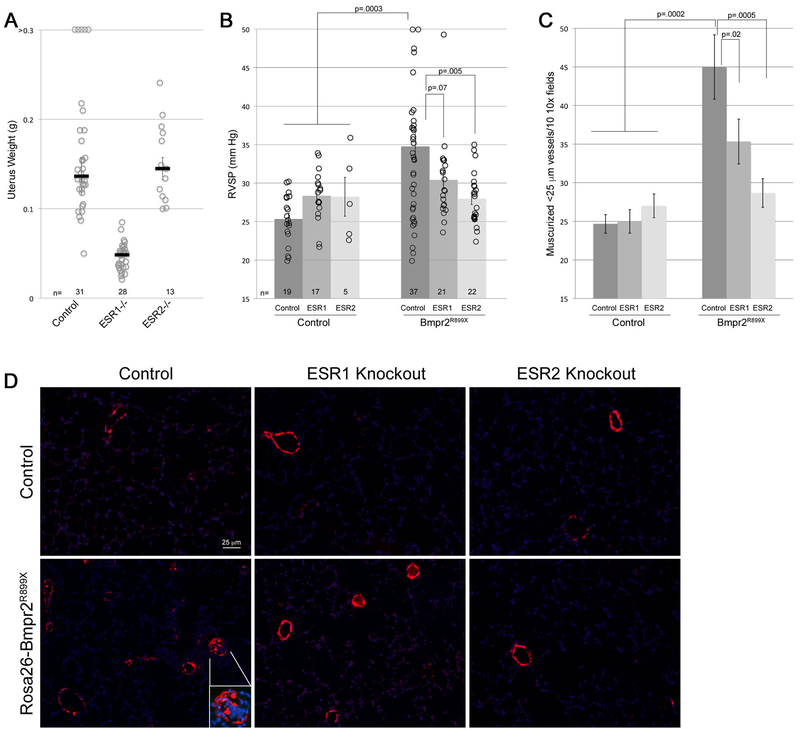
As expected ESR1 but not ESR2 knockout resulted in reduction in uterine weight (Figure 3A) [33]. Western blots for ESR1, ESR2, and the non-canonical receptor GPER in ESR1 and ESR2 knockout and heterozygous mice show the expected and specific loss of protein (Supplemental Figure 3). There appears to be compensatory increase in GPER, but not the reciprocal ESR, with either ESR1 or ESR2 knockout.
Figure 3 –
(A) ESR1 but not ESR2 knockout reduces uterine weights in mice (p<0.0001) as expected[33]; by multiple ANOVA, R899X mutation did not have an effect on uterine weights. Each symbol is the measurement from one mouse; median and SEM are indicated. (B) ESR1 knockout partially and ESR2 knockout completely reduces RVSP in both male and female BMPR2R899X mice. Each symbol is a value from one mouse. By multiple ANOVA, considering sex, BMPR2 mutation, and ESR status as factors, BMPR2 mutation increases RVSP (p=0.0003 by MANOVA), ESR1 trends towards restoration (p=0.07), and ESR2 completely restores (p=0.005) RVSP. Sex did not have significant interaction (all mice received 16αOHE1 in pumps). Numbers of mice in each group are listed at the bottom of the plot; mice were age-matched, and controls were done contemporaneously for each group. (C) ESR1 knockout mice partially and ESR2 knockout mice completely reduce muscularized small vessels in BMPR2R899X mice to normal. By multiple ANOVA, considering sex, BMPR2 mutation, and ESR status as factors, BMPR2 mutation increases muscularized vessels (p=0.0002 by MANOVA), and both ESR1 (p=0.02), and ESR2 (p=0.0005) normalize muscularized vessels. (D) Immunohistochemistry for smooth muscle actin (red) counterstained with DAPI (blue). Apparent intensity of DAPI staining has been reduced to clarify actin. Muscularized and partially muscularized small vessels are roughly doubled in BMPR2R899X mice, and there is the occasional occluded vessel (see inset). ESR1 knockout reduces muscularization, but still has some occluded vessels (top two in field); ESR2 knockout normalizes muscularization, and occluded vessels are no longer apparent.
Closed-chested measurement of RVSP in spontaneously breathing mice found 20/37 Bmpr2R899X mice with pressures over 32 mm Hg (54%) (Figure 3B), while this number was lower in Bmpr2R899X mice with ESR1 knockout (7/21 (33%)), and 3/22 (14%) in Bmpr2R899X mice with ESR2 knockout. The trend was similar for muscularization, with numbers of muscularized small vessels in Bmpr2R899X mice nearly double that of controls (Figure 3C, 3D), and these numbers reduced somewhat in ESR1 and more in ESR2 knockouts. The occluded vessels that can occasionally be seen in Bmpr2R899X mouse lungs were still present in ESR1 knockouts, but not apparent in ESR2 knockouts (Figure 3D). Taken together, these data suggest that the effects of estrogen in PAH are mediated partially through both ESR1 and ESR2, with ESR2 predominating.
Estrogen Inhibition Reverses BMPR2 Mutation-Induced Insulin Resistance
We and others have previously shown that PPARγ and CD36 expression are suppressed in BMPR2 mutant mice and human PAH patients and insulin resistance is increased[22, 34].
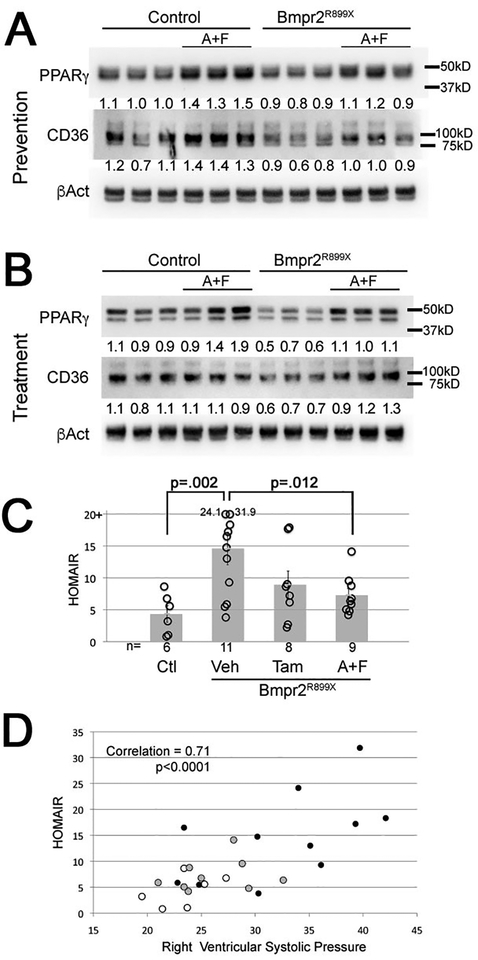
Here we found that these changes were prevented (Figure 4A) and reversed (Figure 4B) by estrogen inhibition. Suppression of PPARγ and CD36 in the vehicle BMPR2 mutant groups is more impressive in the reversal study, presumably because the animals have had longer to develop disease (10 weeks rather than 6 weeks). Measurement of insulin resistance by measurement of blood glucose and insulin showed, as before, a strong increase in HOMA-IR in BMPR2 mutants, substantially reduced by anastrozole/fulvestrant, and trending down with tamoxifen (Figure 4C). Plotting HOMA-IR against RVSP shows that the two are strongly correlated, r=0.71 (Figure 4D). These data suggest that the effect of estrogen inhibition on RVSP is closely correlated with its metabolic effects.
Figure 4 –
(A) Anastrozole & Fulvestrant used preventatively result in increased PPARγ (p=0.001) and CD36 (p=0.02) in whole lung, which are otherwise suppressed by R899X mutation (p=0.003 for PPARγ and p=0.007 for CD36) by two way ANOVA. Numbers are densitometry for the bands above; each lane is whole lung from a different animal. (B) Anastrozole & Fulvestrant used as treatment result in increased PPARγ (p=0.019) and CD36 (p=0.027) in whole lung, which are otherwise suppressed by R899X mutation (p=0.047 for PPARγ and p=0.043 for CD36) by two way ANOVA. Numbers are densitometry for the bands above; each lane is whole lung from a different animal. (C) Anastrozole & Fulvestrant (A+F) used as a treatment significantly reduce insulin resistance in R899X mice (p=0.012), while Tamoxifen (Tam) trends towards improvement (p=0.06). Tests are Fisher’s LSD after ANOVA (p=0.01 for rejection of null). Note that insulin resistance in even control mice is quite high, as they are FVB/N mice on western diet. (D) Insulin resistance is highly correlated with right ventricular systolic pressure (correlation=0.71, p<0.0001). Open circles are Rosa26-only controls; grey circles are R899X mice treated with Anastrozole & Fulvestrant; filled circles are R899X mice treated with vehicle.
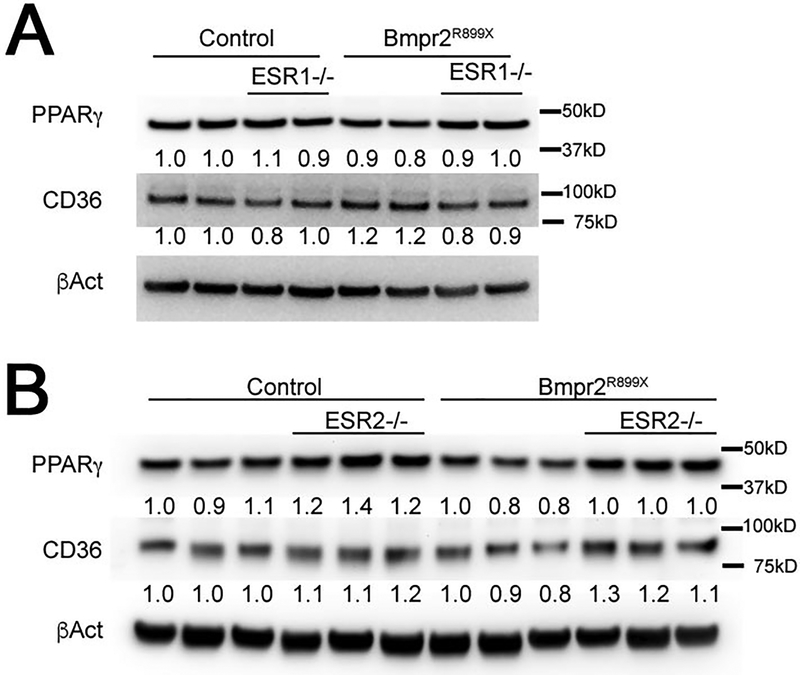
We also measured PPARγ and CD36 in ESR1 and ESR2 knockout mice with and without BMPR2 mutation. ESR1 knockout did not have a consistent effect (Figure 5A), while ESR2 knockout led to increased PPARγ and CD36 in both control and BMPR2 mutant mice (Figure 5B). This supports the hemodynamic measurements in Figure 3 which suggested that ESR2 is more responsible for the negative effects of estrogen in PAH than is ESR1.
Figure 5 –
(A) By two way ANOVA, ESR1 knockout does not affect PPARG or CD36 in whole lung, although PPARG is suppressed by R899X mutation (p=.03) (B) By two-way ANOVA, PPARG is suppressed by R899X mutation (p=.02) in whole lung and induced by ESR2 knockout (p=0.001); CD36 is also induced by ESR2 knockout (p=−.001).
Estrogen Drives Insulin Resistance on a Cellular Level
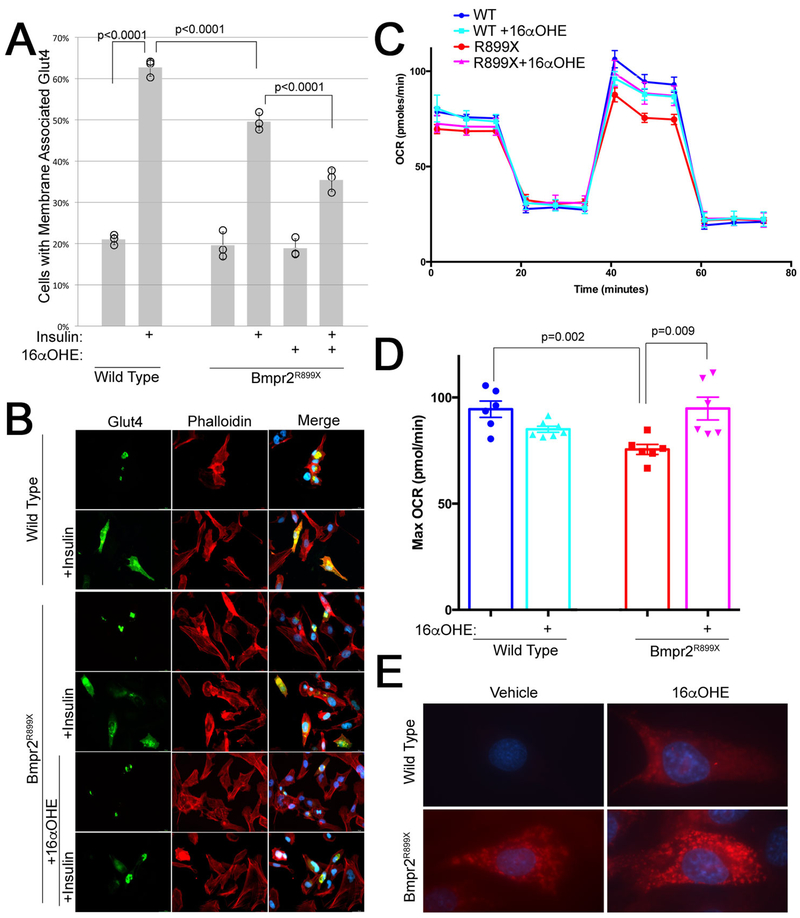
Although glucose metabolism is regulated on a whole-organism level by the interaction of multiple organs, on a cellular level, insulin resistance is characterized by reduced mobilization of the glucose transporter Glut4 in response to insulin. We have previously shown a basic defect at this level in BMPR2 mutant human endothelial cells; specifically, insulin signaling is intact but Glut4 fails to mobilize[35]. Here we replicate that result, and show that estrogenic 16αOHE1 significantly worsens the insulin response in pulmonary microvascular endothelial cells (PMVEC) cultured from Bmpr2R899X mice (Figure 6A, 6B). 16αOHE1 was used instead of E2, because it is more estrogenic than E2, and E2 in patients is preferentially metabolized to 16αOHE1[11].
Figure 6 –
(A) Insulin mobilization of GLUT4 in mPMVEC is decreased in BMPR2R899X mice, and decreased further by pretreatment with 16αOHE1. Insulin mobilization was determined by counting perinuclear vs membrane associated GFP-tagged GLUT4 in each of 100 cells in each of three technical replicates (% in each replicate indicated by circles). Every group has three technical replicates: some are overlapping. Error bars are standard deviation; differences are significant at p<0.0001 by ANOVA, with comparisons indicated by Fisher’s LSD. Differences are also significant at p=0.01 by Wilcoxon non-parametric test. (B) Live cell images of mPMVEC transiently transfected with GFP-tagged Glut4, untreated and pretreated with 16αOHE1, either with vehicle or 15 minutes after treatment with insulin (which ought to mobilize Glut4 to the cell surface). (C) Seahorse Extracellular Flux analysis using the Mito Stress Protocol shows an increase in maximum oxygen consumption rate with FCCP treatment (@~40–60 minutes). (D) Quantitation of maximum oxygen consumption rate from data in (C). (E) A Mitosox Red assay suggests that the increased oxygen consumption in Bmpr2 mutant cells with 16αOHE1 is driven by increased mitochondrial superoxide production.
Comparing maximum oxygen consumption rate (OCR) in Bmpr2R899X PMVEC compared to wild-type PMVEC by Seahorse extracellular flux analysis, we find that PMVECs from Bmpr2R899X mice have a significantly lower maximum OCR (Figure 6C, 6D) in standard media. However, the addition of 16αOHE1 increases maximum OCR in Bmpr2R899X mutants; in contrast, 16αOHE1 lowers maximum OCR in WT cells (Figure 6C, 6D). These data suggest that estrogens modify the already perturbed cellular metabolic activity in the Bmpr2 mutant setting.
Although we do not know in detail how estrogen impacts metabolism in these cells, staining live cells with the superoxide detector Mitosox Red suggests that increased oxygen consumption with 16αOHE1 is caused by increased superoxide production (Figure 6E).
Discussion
Over the past decade, the PAH research community has made tremendous strides towards developing molecularly targeted therapies. There is broad consensus in the field that in addition to, or perhaps because of, suppressed BMP signaling, progression of PAH requires dedifferentiation and proliferation, inflammation, and metabolic changes. Trials targeting each of these have either recently been completed[36–38], are in progress[39], or are in development[40]; at this point debate is on how to effectively intervene, more than where we need to intervene. The field is thus in a state in which our goal is effectively translating our molecular findings into treatments.
Female sex is the strongest epidemiologic risk factor for PAH, with a 3:1 female: male ratio. However, until recently, the reasons for this were unclear: estrogen and female sex were protective in classical PAH models like hypoxia and monocrotaline[15]. Recently, however, a chain of research has contributed new information regarding sex and PAH. PAH patients have increased level of estradiol[41], while increased risk of PAH in patients is strongly associated with preferential metabolism of estrogens into 16- estrogens such as 16αOHE1[11, 12, 42]. There are underlying genetic variations in both aromatases which produce parent estrogens[41] and in CYP1B1 which modulates estrogen metabolism[11, 43] associated with outcomes in both portopulmonary and idiopathic pulmonary hypertension. Increase in 16αOHE1 is important because it covalently binds the receptor and is thus much more estrogenic than 2- and 4- estrogens.
Treating BMPR2 mutant mice with 16αOHE1 increases penetrance and severity of disease[14], associated with markers of increased metabolic problems[17]. Markers of metabolic derangements, including the metabolic syndrome, are strongly associated with disease in patients[18–20] and are causal in BMPR2 mutant mice[21, 22]. Metabolic problems may be important on their own – for instance, by facilitating proliferation – but they may also be important because they result in increased production of reactive oxygen and nitrogen species. These phenomenon are heavily interlinked, and both have been extensively studied in PAH (reviewed in [44]). Estrogens, and in particular 16αOHE1, have recently been shown to induce ROS production in an NADPH-dependent fashion[45], supporting the link between estrogens, metabolism, and ROS.
In the present study, we finalize preparations for translation by addressing key remaining issues. We demonstrate that estrogen inhibition both prevents and reverses PAH in the context of BMPR2 mutation, likely through canonical estrogen receptor signaling. We show that estrogen inhibition reverses markers of metabolic defects including HOMA-IR, and that estrogen impacts insulin resistance on a cellular level. The combination of these data provides a possible mechanism of action, and demonstrates efficacy in mice, paving the way for clinical trials in patients using estrogen inhibition to impact the metabolic axis.
A key question is whether the estrogen is modulating metabolism directly, or indirectly through modulation of the BMP pathway. Estrogen suppresses BMPR2 signaling in human pulmonary artery smooth muscle cells[46], possibly by direct binding of the estrogen receptor to the BMPR2 promoter[47], which can lead to increased proliferation in cell culture. Since the BMP pathway regulates metabolism, at least some of the effect may be through ESR1 (ERα) mediated suppression of BMPR2. ESR1 SNPs have previously been associated with PH risk in portopulmonary hypertension[41]. However, some of the effect may be direct; ESR2 (ERβ) can localize to the mitochondria, where it directly forces a metabolic shift supportive of proliferation[48]. Since we have previously a shown a strong impact of estrogen on mitochondrial morphology[17], at least part of the estrogen effect on metabolism likely bypasses BMPR2. Because both ESR1 and ESR2 have plausible and distinct modes of action, which effects dominate is likely exquisitely dependent on the precise conditions and organ under investigation. For instance, in the present study, since BMPR2 is genetically suppressed, the role of ESR1 in suppressing BMPR2 may be less important. The increase in GPER with estrogen receptor knockout may be relevant to outcomes as well; GPER has previously been shown to increase with pharmacologic estrogen blockade as well[49]. Increased signaling through GPER has potential benefits to PAH[50].
Another interesting point is the question of whether the source of the estrogens is important. Most previous studies showing a protective effect of estrogen used male rodents with exogenous estrogen administered; by contrast, anastrozole is protective in female but not male rodents using either hypoxia or sugen/hypoxia models[16]. Moreover, only female mice develop PH in the dexfenfluramine model[51] – and ovariectomy prevents PH in serotonin transporter overexpressing mice[52]. The theory is that the site of estrogen production is important, and that extragonadal estrogen production may be of particular importance. In support of this, we have previously shown that mode of estrogen metabolism correlates with disease penetrance in male PAH patients as well[17]. This suggests that in male and postmenopausal patients, peripheral estrogen production, for instance in adipose tissue[53], may still be a pathologic source of estrogen driving disease.
These and other scientific questions remain to be addressed. In the long term, estrogens may have a protective impact upon the function of the right ventricle in humans with PAH, which remains an opportunity for further study[43, 54–56]. Although clearly neither estrogen nor insulin resistance is enough on their own to cause PAH, as the vast majority of women and diabetics do not develop PAH, in the context of BMPR2 mutation these are strong drivers of progression to clinical disease. However, we still don’t understand mechanisms in detail. Exposure to estrogens and exposure to a BMPR2 mutation are associated with fragmented mitochondria[17], increased oxidative stress[57], alterations in protein, sugar, and lipid handling[18], and increased insulin resistance[14, 22]. Although all of these phenomena are related, we don’t as yet have a top-to-bottom mechanism linking these to either estrogen or BMPR2, and we don’t know which of these are pathogenic, and which are bystanders. In addition, we don’t know the precise contribution, if at all, of noncanonical estrogen signaling.
While scientifically interesting, it is not mandatory to answer all of these questions before proceeding to translation. Particularly in men and in post-menopausal women, strong estrogen inhibition is safe, addresses many molecular pathologies causally linked to development of PAH, and reverses established disease in mice carrying the same BMPR2 mutation found in many patients. In fact, a short phase II human PAH trial using a form of estrogen modification was recently completed (Kawut et al, NCT01545336); it was safe, well tolerated, and showed hints of efficacy[24]. One of the central questions in clinical translation is which drug to use. Our data suggest that while stronger inhibition, as through anastrozole and fulvestrant, is more effective, and thus likely preferable for post-menopausal patients and men, tamoxifen has some efficacy, and as it is easier to use in pre-menopausal women[58], it is worth determining whether it can be substituted in these younger patients so as to avoid induction of menopause.
The present study contributes critical background work to further demonstrate the efficacy of estrogen inhibition and to elucidate mechanisms including improvement in some metabolic irregularities present in PAH. By adding to the groundwork needed to proceed to human trials, we look forward to proceeding with additional testing and early translational studies.
Supplementary Material
Supplemental Figure 1 – Fulton Index (right ventricle weight divided by weight of the left ventricle and septum) is not changed in any group in prevention studies (A), treatment studies (B), and estrogen receptor knockout studies (C). Fulton index is not informative in this model because the RV in Bmpr2 mutant mice (and patients) has an impaired hypertrophic response[19].
Supplemental Figure 2 – (A) Plasma estrogens are inhibited by the combination of anastrozole and fulvestrant, but not by tamoxifen, as expected. (B) Expression of known estrogen targets FHL1 and SNAT2 in whole lung are suppressed by tamoxifen treatment. This was assessed by quantitative RT-PCR, normalized to expression of the housekeeping gene HPRT and to vehicle values. Error bars in both cases are standard error of the mean; round symbols are the values from individual mice, and bars mark averages.
Supplemental Figure 3 – Estrogen receptor levels from whole lung as assessed by western blot, with nearest protein ladder marker indicated. Note that although there does not appear to be an increase in ESR1 in ESR2 knockout mice, or an increase in ESR2 in ESR1 knockout mice, there is a clear increase in GPER, the G-protein coupled estrogen receptor, in heterozygous and homozygous ESR1 and ESR2 knockouts. Numbers indicate densitometry, normalized to wild-type.
Funding Sources
These studies were supported by NIH P01 HL108800, NIH R01 HL 095797 and HL 122417, NIH K08 HL121174, a Parker B. Francis fellowship (JPF), American Heart Association Fellow to Faculty Award #13FTF16070002 (ELB) and the Gilead Sciences Research Scholars Program in Pulmonary Arterial Hypertension (ELB). The Seahorse Biosciences Extracellular Flux Analyzer used in the present study is housed and managed within the Vanderbilt High-Throughput Screening Core Facility, an institutionally supported core, and was funded by NIH Shared Instrumentation Grant S10 OD018015.
Footnotes
Disclosures:
No conflicts of interest exist for any of the authors related to this manuscript.
References:
- 1.de Jesus Perez VA. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail Rev 2015: 19. [DOI] [PubMed] [Google Scholar]
- 2.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012: 186(3): 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cogan JD, Vnencak-Jones CL, Phillips JA, 3rd, Lane KB, Wheeler LA, Robbins IM, Garrison G, Hedges LK, Loyd JE. Gross BMPR2 gene rearrangements constitute a new cause for primary pulmonary hypertension. Genet Med 2005: 7(3): 169–174. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol 2010: 298(4): H1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 2004: 94(8): 1109–1114. [DOI] [PubMed] [Google Scholar]
- 6.West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D, Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol 2008: 295(5): L744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 2008: 118(7): 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, Appleby S, Shanahan CM, Bloch KD, Pepke-Zaba J, Upton P, Morrell NW. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med 2015: 192(7): 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahm T, Tuder RM, Petrache I. Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2014: 307(1): L7–26. [DOI] [PubMed] [Google Scholar]
- 10.Larkin EK, Newman JH, Austin ED, Hemnes AR, Wheeler L, Robbins IM, West JD, Phillips JA, 3rd, Hamid R, Loyd JE. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2012: 186(9): 892–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JA, 3rd. Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J 2009: 34(5): 1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, Lane K, Meyrick B, Loyd J. Gene expression in BMPR2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC Med Genomics 2008: 1: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, Thomas M, Mair KM, MacLean MR. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation 2012: 126(9): 1087–1098. [DOI] [PubMed] [Google Scholar]
- 14.Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, Johnson J, Loyd JE, Hemnes A, Austin E, West J. Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ 2013: 3(3): 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17beta-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 2012: 185(9): 965–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, MacLean MR. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med 2014: 190(4): 456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, Shay S, Trammell A, Phillips JA, Hamid R, Cogan JD, Dawson EP, Womble KE, Hedges LK, Martinez EG, Wheeler LA, Loyd JE, Majka SJ, West J, Austin ED. Estrogen Metabolite 16alpha-Hydroxyestrone Exacerbates Bone Morphogenetic Protein Receptor Type II-Associated Pulmonary Arterial Hypertension Through MicroRNA-29-Mediated Modulation of Cellular Metabolism. Circulation 2016: 133(1): 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR, West JD. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ 2012: 2(2): 201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, Disalvo T, West J. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2014: 189(3): 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 2011: 30(8): 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fessel JP, Flynn CR, Robinson LJ, Penner NL, Gladson S, Kang CJ, Wasserman DH, Hemnes AR, West JD. Hyperoxia synergizes with mutant bone morphogenic protein receptor 2 to cause metabolic stress, oxidant injury, and pulmonary hypertension. Am J Respir Cell Mol Biol 2013: 49(5): 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, Hemnes AR. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J 2013: 41(4): 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 2015: 131(19): 1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, Palevsky HI, Palmisciano AJ, Patel M, Pinder D, Propert KJ, Smith KA, Stanczyk F, Tracy R, Vaidya A, Whittenhall ME, Ventetuolo CE. Anastrozole in Pulmonary Arterial Hypertension. A Randomized, Double-Blind, Placebo-controlled Trial. Am J Respir Crit Care Med 2017: 195(3): 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, Harral JW, Talati M, Lane KB, Fagan KA, West J. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2012: 302(5): L474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasuda T, Tada Y, Tanabe N, Tatsumi K, West J. Rho-kinase inhibition alleviates pulmonary hypertension in transgenic mice expressing a dominant-negative type II bone morphogenetic protein receptor gene. Am J Physiol Lung Cell Mol Physiol 2011: 301(5): L667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008: 22(3): 659–661. [DOI] [PubMed] [Google Scholar]
- 28.Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, Hoffman EP. Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord 2008: 18(5): 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka N, Dalton N, Mao L, Rockman HA, Peterson KL, Gottshall KR, Hunter JJ, Chien KR, Ross J, Jr. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 1996: 94(5): 1109–1117. [DOI] [PubMed] [Google Scholar]
- 30.Majka S, Hagen M, Blackwell T, Harral J, Johnson JA, Gendron R, Paradis H, Crona D, Loyd JE, Nozik-Grayck E, Stenmark KR, West J. Physiologic and Molecular Consequences of Endothelial Bmpr2 Mutation. Respir Res 2011: 12(1): 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talati M, Hemnes A. Fatty acid metabolism in pulmonary arterial hypertension: role in right ventricular dysfunction and hypertrophy. Pulm Circ 2015: 5(2): 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itoh T, Karlsberg K, Kijima I, Yuan YC, Smith D, Ye J, Chen S. Letrozole-, anastrozole-, and tamoxifen-responsive genes in MCF-7aro cells: a microarray approach. Mol Cancer Res 2005: 3(4): 203–218. [DOI] [PubMed] [Google Scholar]
- 33.Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. The Journal of steroid biochemistry and molecular biology 2000: 74(5): 287–296. [DOI] [PubMed] [Google Scholar]
- 34.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, Rabinovitch M, Doyle RL. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009: 33(2): 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aaron T, Megha T, Evan B, Niki P, Santhi G, Tom B, Anna H. BMPR2 Mutation Results In Impaired GLUT4 Trafficking. A52 MECHANISMS OF PULMONARY VASCULAR DISEASE. American Thoracic Society, 2013; pp. A1735–A1735. [Google Scholar]
- 36.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 2007: 115(10): 1275–1284. [DOI] [PubMed] [Google Scholar]
- 37.Frost AE, Barst RJ, Hoeper MM, Chang HJ, Frantz RP, Fukumoto Y, Galie N, Hassoun PM, Klose H, Matsubara H, Morrell NW, Peacock AJ, Pfeifer M, Simonneau G, Tapson VF, Torres F, Dario Vizza C, Lawrence D, Yang W, Felser JM, Quinn DA, Ghofrani HA. Long-term safety and efficacy of imatinib in pulmonary arterial hypertension. J Heart Lung Transplant 2015: 34(11): 1366–1375. [DOI] [PubMed] [Google Scholar]
- 38.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007: 11(1): 37–51. [DOI] [PubMed] [Google Scholar]
- 39.Spiekerkoetter E, Sung YK, Sudheendra D, Bill M, Aldred MA, van de Veerdonk MC, Vonk Noordegraaf A, Long-Boyle J, Dash R, Yang PC, Lawrie A, Swift AJ, Rabinovitch M, Zamanian RT. Low-Dose FK506 (Tacrolimus) in End-Stage Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2015: 192(2): 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long L, Ormiston ML, Yang X, Southwood M, Graf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD, Morrell NW. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 2015: 21(7): 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts KE, Fallon MB, Krowka MJ, Brown RS, Trotter JF, Peter I, Tighiouart H, Knowles JA, Rabinowitz D, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, Kawut SM, Pulmonary Vascular Complications of Liver Disease Study G. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med 2009: 179(9): 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dempsie Y, MacRitchie NA, White K, Morecroft I, Wright AF, Nilsen M, Loughlin L, Mair KM, MacLean MR. Dexfenfluramine and the oestrogen-metabolizing enzyme CYP1B1 in the development of pulmonary arterial hypertension. Cardiovascular research 2013: 99(1): 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ventetuolo CE, Mitra N, Wan F, Manichaikul A, Barr RG, Johnson C, Bluemke DA, Lima JA, Tandri H, Ouyang P, Kawut SM. Oestradiol metabolism and androgen receptor genotypes are associated with right ventricular function. Eur Respir J 2016: 47(2): 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fessel JP, West JD. Redox biology in pulmonary arterial hypertension (2013 Grover Conference Series). Pulm Circ 2015: 5(4): 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hood KY, Montezano AC, Harvey AP, Nilsen M, MacLean MR, Touyz RM. Nicotinamide Adenine Dinucleotide Phosphate Oxidase-Mediated Redox Signaling and Vascular Remodeling by 16alpha-Hydroxyestrone in Human Pulmonary Artery Cells: Implications in Pulmonary Arterial Hypertension. Hypertension 2016: 68(3): 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mair KM, Yang XD, Long L, White K, Wallace E, Ewart MA, Docherty CK, Morrell NW, MacLean MR. Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 2015: 191(6): 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, Phillips Iii JA, Gaddipati R, Gladson S, Gu E, West J, Lane KB. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ 2012: 3(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao TL, Tzeng CR, Yu CL, Wang YP, Kao SH. Estrogen receptor-beta in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis. Ann N Y Acad Sci 2015: 1350: 52–60. [DOI] [PubMed] [Google Scholar]
- 49.Barton M Not lost in translation: Emerging clinical importance of the G protein-coupled estrogen receptor GPER. Steroids 2016: 111: 37–45. [DOI] [PubMed] [Google Scholar]
- 50.Alencar AK, Montes GC, Montagnoli T, Silva AM, Martinez ST, Fraga AG, Wang H, Groban L, Sudo RT, Zapata-Sudo G. Activation of GPER ameliorates experimental pulmonary hypertension in male rats. Eur J Pharm Sci 2017: 97: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dempsie Y, Morecroft I, Welsh DJ, MacRitchie NA, Herold N, Loughlin L, Nilsen M, Peacock AJ, Harmar A, Bader M, MacLean MR. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation 2008: 117(22): 2928–2937. [DOI] [PubMed] [Google Scholar]
- 52.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, MacLean MR. The serotonin transporter, gender, and 17beta oestradiol in the development of pulmonary arterial hypertension. Cardiovascular research 2011: 90(2): 373–382. [DOI] [PubMed] [Google Scholar]
- 53.Goss PE, Tye LM. Anastrozole: a new selective nonsteroidal aromatase inhibitor. Oncology (Williston Park) 1997: 11(11): 1697–1703; discussion 1707–1698. [PubMed] [Google Scholar]
- 54.Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am J Respir Crit Care Med 2011: 183(5): 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, Fierst J, Whitson J, Cucci AR, Brown MB, Lahm T. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol 2015: 308(9): L873–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu A, Hacker T, Eickhoff JC, Chesler NC. Estrogen Preserves Pulsatile Pulmonary Arterial Hemodynamics in Pulmonary Arterial Hypertension. Ann Biomed Eng 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lane K, Talati M, Austin E, Hemnes A, Johnson J, Fessel J, Blackwell T, Mernaugh R, Robinson L, Fike C, Roberts Ii L, West J. Oxidative injury is a common consequence of BMPR2 mutations. Pulmonary Circulation 2011: 1(1): 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colleoni M, Munzone E. Picking the optimal endocrine adjuvant treatment for pre-menopausal women. Breast 2015: 24 Suppl 2: S11–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Fulton Index (right ventricle weight divided by weight of the left ventricle and septum) is not changed in any group in prevention studies (A), treatment studies (B), and estrogen receptor knockout studies (C). Fulton index is not informative in this model because the RV in Bmpr2 mutant mice (and patients) has an impaired hypertrophic response[19].
Supplemental Figure 2 – (A) Plasma estrogens are inhibited by the combination of anastrozole and fulvestrant, but not by tamoxifen, as expected. (B) Expression of known estrogen targets FHL1 and SNAT2 in whole lung are suppressed by tamoxifen treatment. This was assessed by quantitative RT-PCR, normalized to expression of the housekeeping gene HPRT and to vehicle values. Error bars in both cases are standard error of the mean; round symbols are the values from individual mice, and bars mark averages.
Supplemental Figure 3 – Estrogen receptor levels from whole lung as assessed by western blot, with nearest protein ladder marker indicated. Note that although there does not appear to be an increase in ESR1 in ESR2 knockout mice, or an increase in ESR2 in ESR1 knockout mice, there is a clear increase in GPER, the G-protein coupled estrogen receptor, in heterozygous and homozygous ESR1 and ESR2 knockouts. Numbers indicate densitometry, normalized to wild-type.