Abstract
Background
Changes in clinical guidelines for primary prevention of cardiovascular disease (CVD) have widened eligibility for statin therapy.
Aim
To illustrate the potential impacts of changes in clinical guidelines.
Design and setting
Modelling the impacts of seven consecutive European guidelines based on a cohort of people aged ≥50 years from the Irish Longitudinal Study on Ageing.
Method
The eligibility for statin therapy of a sample of people without a history of CVD was established, according to changing guideline recommendations and modelled associated potential costs. The authors calculated the numbers needed to treat (NNT) to prevent one major vascular event in patients at the lowest baseline risk for which each of the seven guidelines recommended treatment, and for those at low, medium, high, and very-high risk according to 2016 guidelines. These were compared with the NNT that patients reported as required to justify taking a daily medicine.
Results
The proportion of patients eligible for statins increased from approximately 8% in 1987 to 61% in 2016; associated costs rose from €13.9 million to €107.1 million per annum. The NNT for those at the lowest risk for which each guideline recommended treatment rose from 40 to 400. By 2016, the NNT for low-risk patients was 400 compared to ≤25 very-high risk patients. The proportion of patients eligible for statins achieving NNT levels that patients regarded as justified to taking a daily medicine fell as guidelines changed over time.
Conclusion
Increased eligibility for statin therapy impacts large proportions of the present population and healthcare budgets. Decisions to take and reimburse statins should be considered on the basis of expected cost-effectiveness and acceptability to patients.
Keywords: drug costs; guideline; hydroxymethylglutaryl-CoA reductase inhibitors, patient preference; primary prevention
INTRODUCTION
Statins (HMG-CoA reductase inhibitors) are cholesterol-lowering drugs used in the prevention of cardiovascular disease (CVD). They are prescribed for those with known CVD (secondary prevention), as well as for those considered at risk of CVD but who have not yet had an event (primary prevention). The last 30 years have seen a large increase in the utilisation of statins,1–4 consistent with changes in recommendations by clinical guidelines.5 Debate has ensued about such changes,6–8 which have tended to expand the number of people eligible for treatment, particularly in primary prevention.9,10 The authors’ previous analysis found that almost two-thirds of people who were taking statins did so for primary prevention.11
While recent analyses have found statins to be cost-effective in primary prevention,12,13 issues such as the tolerability and safety of statins, or the views of patients on life-long drug therapy, were not considered.14 Accounting for even modest estimates of the disutility caused by daily medication use could negate the benefit of statins and result in net harm to low-risk people rather than net benefit.12 In short, while clinical guidelines and cost-effectiveness studies have broadly supported the widening use of statins in low-risk people, a major caveat exists in terms of acceptability to patients and benefit to society.
Decisions to take or prescribe a medicine involve a trade-off between the perceived benefits and harms and is particularly salient for those choosing to take a statin for the primary prevention of CVD. As the patient often feels healthy, they may perceive the medicine as unnecessary with uncertain benefits and potential side effects.15 Various methods to aid the decision-making of the individual patient and clinician now exist,16–19 such as the ‘number needed to treat’ (NNT); that is, the number of patients that must be treated to prevent one additional adverse outcome (for example, death or stroke) over a specified time period.20
The overarching aim of this study was to explore the impact of changing clinical guidelines on statins for prevention of cardiovascular events over time, incorporating patient preferences regarding preventive treatments. This involved four analyses. First, the authors estimated the increasing proportions of people who would be considered eligible for statin treatment according to each of seven European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines from 1987 to 2016. Second, the authors estimated the potential cost increases associated with each consecutive guideline recommendation. Third, the NNT to prevent one major vascular event in patients at the lowest baseline risk for which each guideline recommended treatment was calculated, as well as for those at low, medium, high, and very-high risk according to the most recent 2016 guideline.21 Finally, the authors compared these NNTs with those reported by patients as being the minimum benefit they would need to justify taking a daily medicine.15
How this fits in
Changes in clinical guidelines for primary prevention of cardiovascular disease (CVD) have widened eligibility for statin therapy. While previous studies have considered changes to clinical guidelines, the present study has analysed the impacts over a 30-year period, considering both societal perspectives and the perspective of the individual patient. The proportion of patients eligible for statins, associated costs, and numbers needed to treat to prevent cardiovascular events have risen significantly as the guidelines changed over time. However, fewer patients now achieve the numbers needed to treat levels that patients regard as justified to taking a daily medicine.
METHOD
Sample
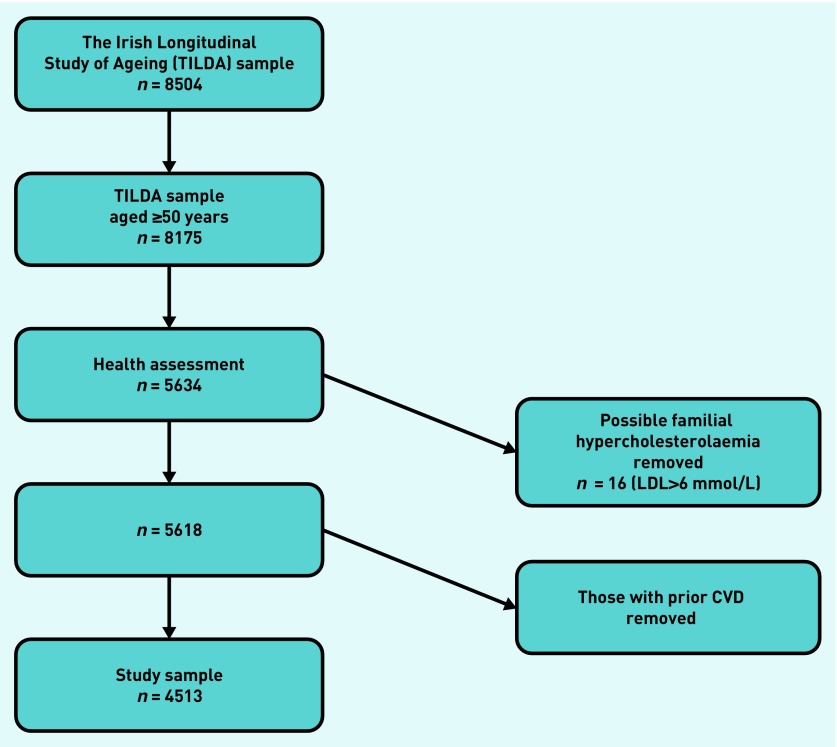
The sample comprised nationally representative participants from a community dwelling population aged ≥50 years from The Irish Longitudinal Study on Ageing (TILDA), who had no reported prior history of CVD (n = 4513) (Figure 1) TILDA collects health-related and sociodemographic data on a nationally representative sample of adults living in a community aged ≥50 years in Ireland and the sample in this study was based on the authors’ previous secondary analysis of these data.11
Figure 1.
Flow chart of the number of participants from the Irish Longitudinal Study on Ageing (TILDA) included in the analysis. CVD = cardiovascular disease. LDL = low-density lipoprotein.
Time trends in eligibility for statins for primary prevention
Each clinical guideline describes cholesterol thresholds that may warrant statin treatment, depending on a person’s baseline risk. Using these thresholds, the authors calculated the proportion of the TILDA sample that would be considered eligible for statin therapy. While these guidelines often recommend a trial of lifestyle changes to lower cholesterol levels before prescribing, for the purpose of this analysis, the authors assumed treatment thresholds above which statins would be prescribed based on each of the guidelines (further information is available from the authors on request).
Cost increases due to widening eligibility
The researchers used cost data from June 2016 to reflect the type, dosages, and frequency of statins reimbursed through the General Medical Service (GMS) in Ireland. A weighted average annual cost per patient taking statins of €149.33 was estimated. The population in Ireland ≥50 years is 1 446 460 individuals and based on a previous study of TILDA, the researchers estimated that 81% of these do not have prior CVD (n = 1 171 326).11 Total cost of statins was calculated by multiplying the estimated weighted average annual cost per patient by the estimated population of interest. Uncertainty was explored using a Monte Carlo simulation process, in which the proportions and units costs were assigned appropriate probability distributions, and 1000 replications of the total cost were generated to estimate 95% confidence intervals (CIs).
Changes in numbers needed to treat due to treatment threshold changes over time
Each clinical guideline defined baseline risk levels above which treatment with statins could be recommended, depending on the individual’s cholesterol levels. For example, in the 2016 guideline, a person at low risk whose baseline risk is <1%, could be recommended for statins if their low-density lipoprotein (LDL) was >4.9 mmol/L. Thus, all people at low risk would not be eligible for treatment, only those with LDL >4.9 mmol/L. However, as some people at low risk could be eligible, the lowest level of risk for the purposes of calculating the NNT was defined as 1%.
In 1994 and 1998 the guidelines recommended use of the Coronary Risk Chart to assess a person’s baseline risk of a fatal or non-fatal CVD event; the lowest risk at which statins could be recommended was 20%. From 2004, the Systematic Coronary Risk Evaluation (SCORE) risk assessment tool was recommended to assess a person’s baseline risk of a fatal CVD event. Thus, the risk bands used in this analysis may seem low to those more familiar with other risk assessment tools such as QRISK, which estimate a person’s risk of fatal and non-fatal CVD. Anyone whose baseline risk of a fatal CVD event is ≥5% is considered ‘high risk’ according to SCORE, whereas anyone whose baseline risk of a fatal or non-fatal CVD event is >20% is considered ‘high risk’ according to QRISK. The lowest risk at which statins could be recommended in 2004 and 2007 was 5%, and in 2012 and 2016, <1%. As two different types of risk assessment tool were used in the guidelines, it was necessary to equivalise each person’s baseline 10-year risk of fatal CVD events (SCORE) to the comparable risk of fatal and non-fatal CVD events (Coronary Risk Chart).
The researchers assumed that taking statins reduced a person’s risk of major vascular events by 25% (risk ratio [RR] 0.75, 95% CI = 0.70 to 0.80)22, and estimated the NNT to prevent one event for those at the lowest risk for which each guideline recommended treatment. Calculations of NNTs to prevent major vascular event in those at lowest risk according to all guidelines are available from the authors on request. The authors also calculated the NNTs for those considered low, medium, high, and very-high risk according to the most recent EAS/ESC guideline).21
Numbers needed to treat and the patient perspective
The researchers considered the NNTs estimated above in the context of a systematic review15 that reported on the minimum acceptable risk reduction that patients say is necessary to justify a daily intake of medication to prevent CVD events.15 Acceptable NNTs were reported in this review in terms of 5-year NNTs, and it was necessary to convert each person’s baseline 10-year risk to the equivalent 5-year risk. A wide variation in this preference was reported: between 46% and 87% (average 71%) of participants would consider taking a medication with an NNT ≤30. For illustrative purposes only, the authors used the NNT 30 as a proxy for acceptability of taking statins for life and assumed that an NNT >30 is not acceptable to any patient. Details of calculations to determine the proportions of patients eligible for statins, according to each guideline, who reach an NNT ≤30; and the proportion of those eligible for statins who would consider taking medication is available from the authors on request.
RESULTS
Time trends in eligibility for statins for primary prevention
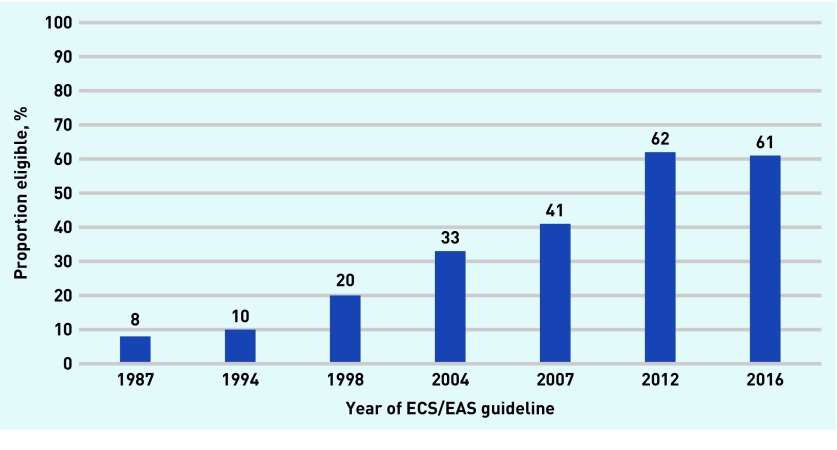
In 1987, approximately 8% of the sample group would have been eligible for statin therapy, and by 2016, 61%; an increase of 663% in eligibility. As the characteristics of the sample remained the same, changes in eligibility for statin therapy were due to changes in treatment thresholds of the guidelines and not changes in the sample (Figure 2).
Figure 2.
Proportion of participants eligible for statin therapy according to changes in ESC/EAS clinical guidelines 1987–2016. ESC/EAS = European Society of Cardiology/European Atherosclerosis Society.
Cost increases due to widening eligibility
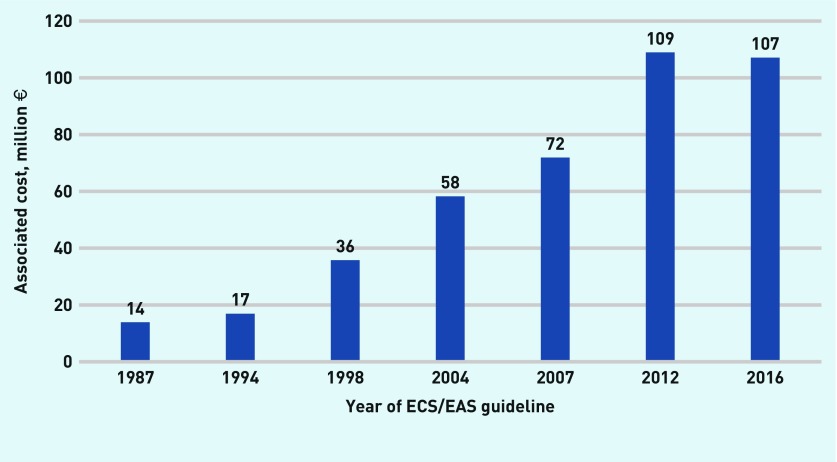
Assuming that all those eligible took statins according to each guideline, the overall national cost would increase from €13.9 million in 1987 to €107.1 million in real terms (2016 prices), and includes both public and private expenditure on statins (Table 1 and Figure 3).
Table 1.
Proportion of participants eligible for statin therapy according to changes in ESC/EAS clinical guidelines and associated cost implications
| Year of clinical guideline | Proportion eligible for statins, % (SE) | Cost, € | 95% CI |
|---|---|---|---|
| 1987 | 7.95 (0.33) | 13 902 610 | 12 668 710 to 15 045 833 |
| 1994 | 9.68 (0.44) | 16 924 364 | 15 364 264 to 18 456 889 |
| 1998 | 20.46 (0.60) | 35 767 583 | 33 583 491 to 37 922 496 |
| 2004 | 33.33 (0.70) | 58 256 655 | 55 808 914 to 60 866 411 |
| 2007 | 41.14 (0.73) | 71 918 939 | 69 140 111 to 74 850 412 |
| 2012 | 62.33 (0.72) | 108 959 843 | 106 127 604 to 112 017 290 |
| 2016 | 61.27 (0.73) | 107 100 599 | 104 314 514 to 110 061 679 |
ESC/EAS = European Society of Cardiology/European Atherosclerosis Society. SE = standard error.
Figure 3.
Associated cost implications of changes in ESC/EAS clinical guidelines 1987–2016. ESC/EAS = European Society of Cardiology/European Atherosclerosis Society.
Changes in numbers needed to treat due to treatment threshold changes over time
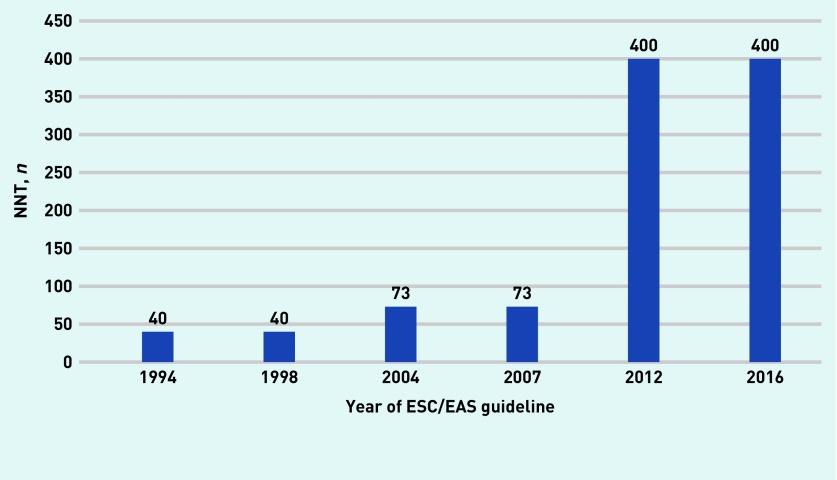
The NNT to prevent one major vascular event in those at the lowest levels of risk for which statins could be recommended was 40 according to the 1994 and 1998 guidelines; 73 according to the 2004 and 2007 guidelines; and 400 according to the 2012 and 2016 guidelines (Figure 4).
Figure 4.
NNT for those at the lowest baseline risk for which statins could be recommended according to ESC/EAS guidelines for the prevention of cardiovascular disease. The 1987 guidelines recommend treating anyone whose total cholesterol threshold exceeds 6.5 mmol/L, but does not describe the baseline level of risk required by a person to initiated treatment. Thus, the NNT cannot be calculated for 1987. Details of calculated risks are available from the authors on request. ESC/EAS = European Society of Cardiology/European Atherosclerosis Society. NNT = numbers needed to treat.
Of those at low risk, 400 people would have to be treated in the group to prevent one major vascular event; between 53 and 400 people at moderate risk; between 25 and 53 people at high risk; and ≤25 people at very-high risk according to the 2016 guideline (Table 2).
Table 2.
NNT to prevent one major vascular event in each sample profile
| Absolute 5 year risk of fatal and non-fatal CVD eventsa, % | RR reduction in major vascular events from taking statinsb (95% CI) | Absolute risk reduction of major vascular events from taking statins, % | NNT, n |
|---|---|---|---|
| Low risk (<1) | 0.75 (0.70 to 0.80) | 0.25 | ≥400 |
| Moderate risk (1–7.4) | 0.75 (0.70 to 0.80) | 0.25–1.9 | 400–53 |
| High risk (7.5–15.9) | 0.75 (0.70 to 0.80) | 1.9–4.0 | 53–25 |
| Very high risk (≥16) | 0.75 (0.70 to 0.80) | 4.0 | ≤25 |
Risk conversions are available from the authors on request.
RR reported for the outcome ‘Major vascular events’ as reported in Cholesterol Treatment Trialists Collaboration.26 NNT = numbers needed to treat. RR = risk ratio.
Numbers needed to treat and the patient perspective
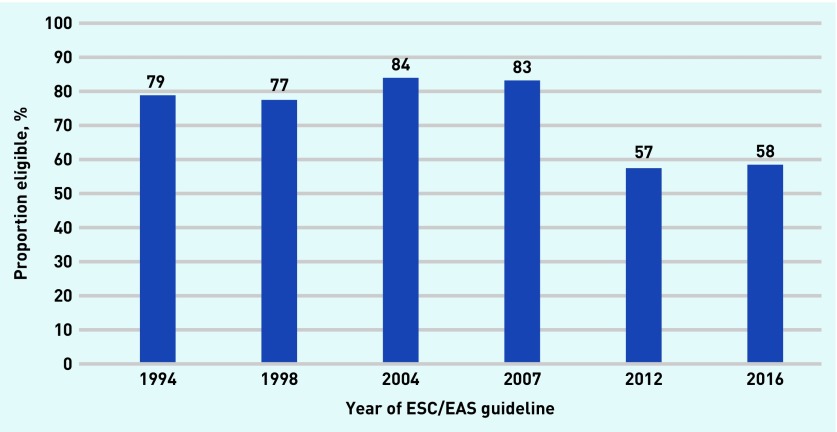
The proportion of people recommended for treatment who would reach an NNT ≤30 was 79% in 1994, rising to 84% in 2004, and falling to 58% in 2016 (Figure 5).
Figure 5.
Proportion of those eligible for statins who are above the acceptable NNT needed to justify taking a daily medication. The 1987 guidelines recommend treating anyone whose total cholesterol threshold exceeds 6.5 mmol/L, but does not describe the baseline level of risk required by a person to initiated treatment. Thus, the NNT cannot be calculated for 1987. Details of calculated risks are available from the authors on request. ESC/EAS = European Society of Cardiology/European Atherosclerosis Society. NNT = numbers needed to treat.
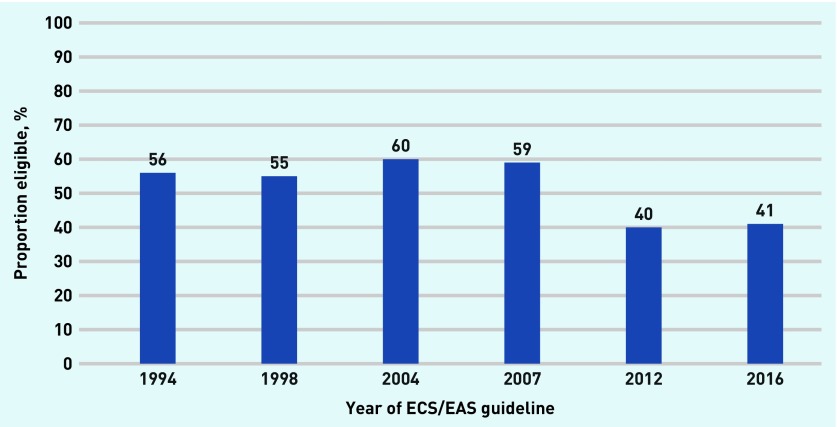
However, as Albarqouni et al reported in their systematic review, there is a wide range in the proportion of people who would find this NNT acceptable; between 46% and 87% (average 71%).15 Therefore, on average, the proportion of those who were eligible for statins, and who would find this NNT as an acceptable trade-off for taking a medicine for life fell, from 56% in 1994 to 41% in 2016 (Figure 6).
Figure 6.
Proportion of those eligible for statins who accept their NNT. The authors assumed that no participant whose NNT >30 accept taking statins. ESC/EAS = European Society of Cardiology/European Atherosclerosis Society. NNT = numbers needed to treat.
Only some of those classified as high and very-high risk, according to the 2016 EAS/ESC guidelines, would reach an acceptable NNT <30. Proportions of those eligible for statins according to the 2016 guideline with an NNT ≤30 who would accept the medication are available from authors on request.
DISCUSSION
Summary
Changes in clinical guideline recommendations have resulted in more than a 600% increase in statin eligibility for primary prevention, with 61% of the cohort becoming eligible for statin therapy by 2016 with significant cost implications. While 61% may be eligible based on guideline criteria, this does not necessarily represent the actual proportion of this patient population who are taking statins. Indeed, the authors’ previous analysis found that only 30% of the TILDA cohort took statins11 and adherence to statins in real life is reported to be, on average, 50%.23 However, the purpose of this article is to illustrate the potential impacts of widening statin eligibility based on changing guidelines over time.
Debate has ensued about clinical guideline recommendations,6–8 which have tended to expand the number of people eligible for treatment, without explicitly considering the adverse impacts of such measures.5,8 For example, a UK study found that if a new risk threshold were used, almost all of their sample would be indicated for statin treatment compared to less than three-quarters when the previously recommended risk threshold was used.24 A US study found that almost half of those currently not on statins would be recommended for statin treatment according to new guidelines; the largest potential change was in those without CVD or diabetes.25
However, for many of the individuals, the reduction in risk of cardiovascular events would not be large enough to justify taking a daily medication. Using the most conservative estimate, half of all those taking statins for primary prevention would not find it acceptable to take a statin for life. Using the least conservative measure, less than one-third of this patient population would find taking statins acceptable.
In the present analysis it was assumed that the relative risk reduction from taking statins was similar across subgroups. However, when stratified by sex, smaller non-significant reductions in major vascular events were reported for females,26 which would further reduce acceptability. The evidence supporting statin use in older people is mixed,27–29 and should also be considered in terms of acceptability for this group. Albarqouni et al reported that in one study only 3% of older people (mean age 76 years [SD 7 years]) who live in a community would agree to a medication with adverse effects that could affect their activities of daily living, and half would not agree to take the medication if it was associated with even mild fatigue or nausea.15 In addition, younger people in the intermediate risk group would be much less healthy in relative terms to their peers than those in the older age groups, who make up the majority of this shift in usage. Studies have found that statin use can be associated with an increased risk of myopathy, rhabdomyolysis, diabetes, and haemorrhagic stroke,30 although debate has ensued as to the prevalence and significance of such adverse effects.31
The implications of the present findings are that pharmacoeconomic evaluations and clinical practice guidelines, as currently conducted, may not be sufficient to evaluate the value of statin therapy and recommend appropriate usage. There have been calls to incorporate evidence from sources other than conventional clinical trials into the development of clinical guidelines, including the views and experience of those using the intervention.32 Cost-effectiveness analyses (CEAs) have sometimes, albeit rarely, included NNT ratios, and this may increase the understanding and relevance of such publications for prescribers and patients.33 It can be seen that a patient’s preference for taking a daily medication is an important factor in assessing whether statin use represents net benefit. The consideration of such preferences should be incorporated into future CEAs and clinical guidelines.
Strengths and limitations
To the authors’ knowledge, this study is the first to simulate the effect of evolving guidelines over a 30 year period and can inform the debate on appropriate prescribing. The study has some limitations: the authors assumed that statin therapy was initiated at certain thresholds, even though some of these patients may be recommended lifestyle changes rather than statin therapy. The estimation of baseline risk within the TILDA sample was complex. Some of the sample may have already been taking statins and therefore their original baseline risk would have been higher, and the risk estimated for the current study may underestimate this original baseline risk as their cholesterol was now well controlled. The authors did not assess the changes in types of statins, dosage, or generic substitution over the period considered and, in the absence of patient-level data relating to type and dosage of statin, the authors employed an estimated average unit cost. All estimates in the cost analysis were presented in real terms based on 2016 prices which, while medical inflation has not been significant, may not reflect current prices. Finally, the estimates relating to patient preferences were based on a published systematic review.15 This review found that the assessment methods used to examine patient preferences were heterogeneous with a wide variety of estimates reported. Therefore, an accurate quantitative summary estimate of the minimum acceptable risk reduction could not be calculated, and the authors used a NNT of 30 as a proxy for acceptability of taking statins for life for illustrative purposes only. It is important to point out that each of the four aspects of the results of this article would require much more detailed analysis to ascertain the real-world impacts of widening eligibility for statins use. In addition, the generalisability of these findings may be limited, since they are based on a study of one country. The sample used was nationally representative of the Irish population aged ≥50 years and the costs based on reimbursement data from Ireland. The patient preferences were taken from a systematic review of patient preferences, which included studies from many countries.15 It is possible that patient preferences differed in individual countries according to social and cultural norms. In addition, prescribing, insurance and reimbursement practices, as well as drug costs, will differ from country to country. However, the authors believe that their analysis offers an important and original insight to inform decision-making in clinical practice and policy.
Comparison with existing literature
As noted above, to the authors’ knowledge, this study is the first that has examined the influence of changing clinical guidelines over a 30 year period. However, other studies have considered the impacts of single changes to guidelines, both in Europe and the US. For example, McFadden et al 24 estimated numbers affected by changes in 2014 to UK guidelines for statin use in primary prevention of CVD. The guidelines had previously recommended that statin treatment was indicated for those whose baseline risk exceeded 20% (QRISK), but the new guidelines recommended that those whose risk exceeded 10% now be considered for treatment. The authors estimated that 58% of males and 55% of females would be indicated for treatment by 5 years, and 71% of males and 73% of females by 10 years using the 20% threshold. Using the proposed threshold of 10%, 84% of males and 90% of females would be indicated for treatment by 5 years and 92% of males and 98% of females by 10 years. Ueda et al 34 determined risk factor levels required to exceed the risk threshold for statin therapy, and to estimate the number of adults in England who would require statin therapy under these new guidelines. They found that:
‘Even with optimal risk factor levels, males of different ethnicities would exceed the 10% risk threshold between the ages of 60 and 70 years, and females would exceed the threshold between 65 and 75 years. Under the NICE guidelines, 11.8 million males and females (37% of the adults aged 30–84 years) would require statin therapy, most of them (9.8 million) for primary prevention. When analysed by age, 95% of males and 66% of females without CVD in ages 60–74 years, including all males and females in ages 75–84 years, would require statin therapy.’
Schoen et al 25 reported that changes to treatment guidelines from 2001 Adult Treatment Panel III (ATPIII) to 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines would expand the number of patients recommended to receive statins, particularly among patients who were previously thought to be at moderate risk, and would increase the intensity of treatment for many patients at high risk. Abramson and Wright35 quantified this increase, noting that the changes:
‘… increased the number of Americans for whom statins are recommended from 13 million to 36 million, most of whom do not yet have but are estimated to be at moderately elevated risk of developing coronary heart disease.’
Implications for research and practice
Changes in recommendations for the use of statins would result in almost two-thirds of those aged ≥50 years in Ireland and similar countries being considered eligible for statin therapy. This has implications for the medicalisation of large proportions of the population, as well as for already resource constrained healthcare budgets. The value for money of the widening use of statins should be considered from both a societal and individual perspective. The decision to take and reimburse statins could be informed by NNTs, which are large in some risk categories. As can be seen from the present analysis, a proportion of the sample would require significantly greater reductions in absolute risk to justify taking a daily medication. The patient’s decision to take statins should be considered in the context of shared decision-making, and the relevant NNT, so that informed choices can be made relevant to their individual baseline risk.
Acknowledgments
The authors would like to thank TILDA for access to data, as well as Ronán Conroy for the Stata code for the SCORE and Coronary Risk Assessment. They also thank Michael Barry and Kathleen Bennett for access to Primary Care Reimbursement Service data, which were used to calculate statin costs over time. Researchers interested in using TILDA data may access the data for free from the following sites: Irish Social Science Data Archive (ISSDA) at University College Dublin http://www.ucd.ie/issda/data/tilda/; Interuniversity Consortium for Political and Social Research (ICPSR) at the University of Michigan http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/34315
Funding
The present study is part of Paula Byrne’s PhD which is funded by the SPHeRE Health Research Board structured PhD programme (grant reference: SPHeRE/2013/1). Rafael Perera is supported by the NIHR Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Ethical approval
Ethical approval for the TILDA study was received from the Trinity College Research Ethics Committee and all participants provided written informed consent. TILDA has both publicly available data, which can be accessed as described in the Acknowledgements section, as well as additional data that is available only for research purposes.
Provenance
Freely submitted; externally peer reviewed
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Walley T, Folino-Gallo P, Stephens P, Van Ganse E. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br J Clin Pharmacol. 2005;60(5):543–551. doi: 10.1111/j.1365-2125.2005.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feely J, McGettigan P, Kelly A. Growth in use of statins after trials is not targeted to most appropriate patients. Clin Pharmacol Ther. 2000;67(4):438–441. doi: 10.1067/mcp.2000.105152. [DOI] [PubMed] [Google Scholar]
- 3.Kildemoes HW, Støvring H, Andersen M. Driving forces behind increasing cardiovascular drug utilization: a dynamic pharmacoepidemiological model. Br J Clin Pharmacol. 2008;66(6):885–895. doi: 10.1111/j.1365-2125.2008.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeWilde S, Carey IM, Bremner SA, et al. Evolution of statin prescribing 1994–2001: a case of agism but not of sexism? Heart. 2003;89(4):417–421. doi: 10.1136/heart.89.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moynihan RN, Cooke GP, Doust JA, et al. Expanding disease definitions in guidelines and expert panel ties to industry: a cross–sectional study of common conditions in the United States. PLoS Med. 2013;10(8):e1001500. doi: 10.1371/journal.pmed.1001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenland P, Bonow RO. Interpretation and use of another statin guideline. JAMA. 2016;316(19):1977–1979. doi: 10.1001/jama.2016.15087. [DOI] [PubMed] [Google Scholar]
- 7.Redberg RF, Katz MH. Statins for primary prevention: the debate is intense, but the data are weak. JAMA. 2016;316(19):1979–1981. doi: 10.1001/jama.2016.15085. [DOI] [PubMed] [Google Scholar]
- 8.Unruh L, Rice T, Rosenau PV, Barnes AJ. The 2013 cholesterol guideline controversy: Would better evidence prevent pharmaceuticalization? Health Policy. 2016;120(7):797–808. doi: 10.1016/j.healthpol.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Abramson JD, Rosenberg HG, Jewell N, Wright JM. Should people at low risk of cardiovascular disease take a statin? BMJ. 2013;347:f6123. doi: 10.1136/bmj.f6123. [DOI] [PubMed] [Google Scholar]
- 10.Goldacre B, Smeeth L. Mass treatment with statins. BMJ. 2014;349:g4745. doi: 10.1136/bmj.g4745. [DOI] [PubMed] [Google Scholar]
- 11.Byrne P, Cullinan J, Murphy C, Smith SM. Cross-sectional analysis of the prevalence and predictors of statin utilisation in Ireland with a focus on primary prevention of cardiovascular disease. BMJ Open. 2018;8(2):e018524. doi: 10.1136/bmjopen-2017-018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heller DJ, Coxson PG, Penko J, et al. Evaluating the impact and cost-effectiveness of statin use guidelines for primary prevention of coronary heart disease and stroke. Circulation. 2017;136(12):1087–1098. doi: 10.1161/CIRCULATIONAHA.117.027067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McConnachie A, Walker A, Robertson M, et al. Long-term impact on healthcare resource utilization of statin treatment, and its cost effectiveness in the primary prevention of cardiovascular disease: a record linkage study. Eur Heart J. 2014;35(5):290–298. doi: 10.1093/eurheartj/eht232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor FC, Huffman M, Ebrahim S. Statin therapy for primary prevention of cardiovascular disease. JAMA. 2013;310(22):2451–2452. doi: 10.1001/jama.2013.281348. [DOI] [PubMed] [Google Scholar]
- 15.Albarqouni L, Doust J, Glasziou P. Patient preferences for cardiovascular preventive medication: a systematic review. Heart. 2017;103(20):1578–1586. doi: 10.1136/heartjnl-2017-311244. [DOI] [PubMed] [Google Scholar]
- 16.Polak L, Green J. Using quantitative risk information in decisions about statins: a qualitative study in a community setting. Br J Gen Pract. 2015. [DOI] [PMC free article] [PubMed]
- 17.Trevena LJ, Davey HM, Barratt A, et al. A systematic review on communicating with patients about evidence. J Eval Clin Pract. 2006;12(1):13–23. doi: 10.1111/j.1365-2753.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonner C, Jansen J, McKinn S, et al. Communicating cardiovascular disease risk: an interview study of General Practitioners’ use of absolute risk within tailored communication strategies. BMC Fam Pract. 2014;15:106. doi: 10.1186/1471-2296-15-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trevena L. Assessing, communicating, and managing risk in general practice. Br J Gen Pract. 2014. [DOI] [PMC free article] [PubMed]
- 20.Chatellier G, Zapletal E, Lemaitre D, et al. The number needed to treat: a clinically useful nomogram in its proper context. BMJ. 1996;312(7028):426–429. doi: 10.1136/bmj.312.7028.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 22.Cholesterol Treatment Trialists’ (CCT) Collaborators. Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28(5):574–580. doi: 10.1016/j.cjca.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.McFadden E, Stevens R, Glasziou P, Perera R. Implications of lower risk thresholds for statin treatment in primary prevention: analysis of CPRD and simulation modelling of annual cholesterol monitoring. Prev Med. 2015;70:14–16. doi: 10.1016/j.ypmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoen MW, Salas J, Scherrer JF, Buckhold FR. Cholesterol treatment and changes in guidelines in an academic medical practice. Am J Med. 2015;128(4):403–409. doi: 10.1016/j.amjmed.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Cholesterol Treatment Trialists’ (CCT) Collaboration. Fulcher J, O’Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–1405. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 27.Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+- year olds. Age Ageing. 2010;39(6):674–680. doi: 10.1093/ageing/afq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts CG, Guallar E, Rodriguez A. Efficacy and safety of statin monotherapy in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2007;62(8):879–887. doi: 10.1093/gerona/62.8.879. [DOI] [PubMed] [Google Scholar]
- 29.Savarese G, Gotto AM, Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 62(22):2090–2099. doi: 10.1016/j.jacc.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 30.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 31.Krumholz HM. Statins evidence: when answers also raise questions. BMJ. 2016;354:i4963. doi: 10.1136/bmj.i4963. [DOI] [PubMed] [Google Scholar]
- 32.Wieringa S, Dreesens D, Forland F, et al. Different knowledge, different styles of reasoning: a challenge for guideline development. BMJ Evid Based Med. 2018;23(3):87–91. doi: 10.1136/bmjebm-2017-110844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg V, Shen X, Cheng Y, et al. Use of number needed to treat in cost-effectiveness analyses. Ann Pharmacother. 2013;47(3):380–387. doi: 10.1345/aph.1R417. [DOI] [PubMed] [Google Scholar]
- 34.Ueda P, Lung TW, Clarke P, Danaei G. Application of the 2014 NICE cholesterol guidelines in the English population: a cross-sectional analysis. Br J Gen Pract. 2017. [DOI] [PMC free article] [PubMed]
- 35.Abramson J, Wright JM. Are lipid-lowering guidelines evidence-based? Lancet. 2007;369(9557):168–169. doi: 10.1016/S0140-6736(07)60084-1. [DOI] [PubMed] [Google Scholar]