Abstract
Metal tolerant bacterium Chryseobacterium sp. PMSZPI previously isolated and characterized from uranium ore deposit was studied for elucidating the role of metal transporter genes belonging to the Cation Diffusion Facilitator (CDF), Root-Nodulation-Division (RND) and PIB-type ATPase family in cadmium and uranium tolerance. The bacterium showed tolerance towards cadmium (MIC~6mM) and uranium (MIC~2mM) and was found to harbor metal transporter genes belonging to CDF, RND and PIB-type ATPase family of proteins. Expression studies by real-time PCR showed an upregulation of czcA(RND), czcD(CDF) and cadA(PIB-type ATPase) genes in presence of cadmium or uranium. Higher expression of czcA and czcD was found when the bacterium was treated with cadmium and uranium respectively. This study provides significant insight into the molecular mechanism that plays a role in cadmium and uranium tolerance in bacteria.
Introduction
Metal tolerance is a concerted effort of a number of factors that enables a particular microorganism to survive under stress conditions [1]. One of the known mechanisms is the role of metal transporters that are expressed by the host organism in response to the presence of metals [2]. The role of metal transporters through uptake, efflux or transportation to specialized compartments in a cell is one aspect of maintaining metal homeostasis [3, 4]. In bacteria, metal transporters are mainly found to comprise members of three broad families of proteins; Root Nodulation Diffusion family (RND), Cation diffusion family (CDF) and the PIB-type family [2, 5]. Genome-wide studies have revealed that these transporters are widely distributed in bacteria and Archaea [2, 5]. Members from these families have been described to play a role in conferring metal tolerance towards a wide range of divalent metal cations like Cu2+, Cd2+, Pb2+, Co2+ and Zn2+ [1, 5]. RND proteins are known to efflux metal cations across the membrane while CDF proteins are known to efflux excess divalent cations from the cytoplasm [5–9]. PIB-ATPase, on the other hand, has been reported to either export or import metal ion in bacteria [10–12]. Non-essential metals like cadmium (Cd) and uranium (U) are generally found in the environment, albeit at a very low concentration [13, 14]. Cadmium and U concentrations in the soil are approximately around 0.01–1.8 ppm and 1.8 ppm respectively [13, 14]. However, anthropogenic activities accompanying the growth of industries and mining has led to the increase in concentration and distribution of these metals in the environment [13, 14]. Cadmium is found in the environment as divalent cation whereas multivalent elements like U oxidation states depend on the redox conditions and pH of the existing environment.[14–16]. Furthermore, U speciation depends on the presence of competing organic (n-caboxylic acids) and inorganic cations (hydroxide, carbonates, phosphates, sulfates) that readily form complexes with U [16]. Both Cd and U have been reported to affect cellular function in bacteria and other higher life forms through oxidative stress by generation of free radical and reactive oxygen species[17, 18]. Furthermore, Cd have been found to compete with Zn and Mg for their active sites, inhibit heme synthesis and also cause epigenetic changes in DNA expression[19, 20]. Uranium has also been reported to cause DNA damage by causing single stand break and DNA lesions which induce mutation in the gene [21–23]. Chryseobacterium sp PMSZPI was isolated from U-deposit located at Domiasiat, India and showed multi-metal tolerant properties and possesses metal transporter belonging to PIB-type ATPase (cadA) family ([24]. In the present study, the presence of metal transporter genes belonging to RND (czcA) and CDF (czcD) in Chryseobacterium sp. PMSZPI (NCBI Accession No.JF768716) isolated from uranium deposit were investigated to elucidate the role of these transporters in U and Cd stress along with a previously described PIB-type ATPase (cadA)[24] gene found to occur in this isolate.
Material and methods
Screening of metal tolerant genes in PMSZPI
Screening for the presence of metal tolerant genes viz, czcA and czcD which are members of the RND and CDF family of metal transporting protein respectively in Chryseobacterium sp. was carried out using degenerate forward primer and reverse primer shown in S1 Table. These primers were designed using iCODEHOP software [25]. Metal transporter genes were amplified from genomic DNA extracted from the bacterial isolate using Bacterial genomic DNA extraction kit (HiMedia, India). PCR mixtures (25 μL) contained approximately 30ng of template DNA, 2μM forward primer, 2μM reverse primer, Taq DNA Polymerase buffer with 15 mM MgCl2, deoxynucleoside triphosphates (250μM each of dATP, dCTP, dGTP and dTTP) and 1.0U of Taq DNA polymerase. DNA amplification was carried out in a GeneAmpPCR system 9700 (Applied Biosystems, USA) with an initial denaturation step of 94°C for 5 min, followed by 30 cycles consisting of denaturation at 94°C for 1 min, annealing at 49°C for 1 min, and extension at 72°C for 1.5 min followed by a final extension step of 72°C for 5 min. The expected amplicons size for czcD and czcA genes are approximately 450–500 bps and 850–900 bps respectively.
Sequencing and phylogenetic analysis of czcA and czcD genes
Amplicons of czcA and czcD from Chryseobacterium sp. were purified using Gel Extraction Kit (HiMedia, India) and sequenced using the Big Dye Terminator cycle sequencing kit v.3.1 (Applied Biosystems, USA) deploying the standard protocol and an automated Genetic Analyzer ABI 3130XL (Applied Biosystems, USA). The Basic Local Alignment Search Tool (BLAST, sub-program BLASTX) was used to determine the phylogenetic neighbors of their respective genes present in GenBank database (National Center for Biotechnology Information, Bethesda, USA)[26]. Phylogenetic analyses were carried out with nucleotide sequences of identified phylogenetic neighbors retrieved from Genbank and aligned using ClustalW of MEGA6 [27]. Phylogenetic tree construction was carried out using neighbor-joining method with 1000 bootstrap replications for nodal support. Phylogenetic analyses based on the maximum likelihood and maximum parsimony of czcA and czcD amino acid sequences were in agreement with the data generated by the above described neighbor joining method.
Analysis of minimum inhibitory concentration and viability test
One millilitre of mid-log phase cells (OD600 0.8) of the bacterium grown in LB medium was harvested and washed with sterile distilled water. The washed cells were then resuspended in one millilitre sterile Tris Buffered medium containing 20mM Tris-HCl pH 7.0, 80mM NaCl, 20mM KCl and 5% Glycerol. Varying concentrations of filter sterilized cadmium nitrate or uranyl nitrate solutions were added to each tube and the cell suspensions were incubated at 28°C for three hour in a shaker incubator. Five microlitre was then spotted in LB agar plate and incubated at 28°C for 48 hours. Viability tests of the isolate towards varying concentrations of metals were performed by serial dilutions and plating the inoculums in LB plates. The plates were then incubated at 28°C for 48 hours for observation.
RNA extraction and cDNA synthesis
Two millilitres of mid-log phase cells (OD600 0.8) grown in LB medium were harvested and washed with sterile distilled water. The washed cells were then resuspended in one millilitre sterile Tris Buffered medium containing 20mM Tris-HCl pH 7.0, 80mM NaCl, 20mM KCl and 5% Glycerol. For metal treatment, 0.5mM of cadmium or uranium was added to the individual cell suspension and incubated for 3 hours at 28°C. Metal treated/untreated cells were then harvested by centrifugation at 10500 X g (HERMLE,Germany) for 2 minutes. Total RNA was extracted using RNeasy Mini Kit (QIAGEN, USA) according to manufacturer’s instructions. On column DNase treatment was performed by adding 100μl of DNase treatment buffer contained 10units of RQ1 DNase (Promega,USA) and incubated for one hour at 37°C. Purified RNA was eluted after DNase treatment in nuclease-free water and quantified by NanoVue (GE Healthcare). For cDNA synthesis, 250ng of RNA was used and reverse transcription was performed using the iScript cDNA Synthesis Kit (BioRad, USA).
Transcription profiling using real-time PCR
Real-time PCR assays were performed in 10μl reaction volume containing 5μl of 2X GoTaq qPCR Master-mix (Promega, USA), 0.2μl each of forward and reverse primer and 1μl of 10X diluted cDNA. The temperature program for real-time PCR was 95°C for 2 minutes (Hot start activation) followed by 40 cycles of amplification (95°C for 15seconds and 60°C for 60 seconds) for 16S rRNA, czcA, cadA and czcD gene amplification (cadA occurrence was previously described in Kumar et al. 2013). The melting curve analysis was performed within the temperature range of 60–95°C. Primers used for real time PCR are listed in Table 1. The Ct values that were obtained from three independent experiments were used for calculating the fold change in expressions using 16S rRNA as an internal control. Statistical analysis was performed using Student’s t-test to determine whether the two dataset were significantly different (p>0.005).
Table 1. List of primers for qRT-PCR studies.
| Primer | Oligo sequences 5’-3’ | Amplicon Size (bp) | Target Gene |
|---|---|---|---|
| ZP1Fc | GGTGGATAGAGATGGGCACG | 107 | 16S rRNA |
| ZP1Rc | AGTACCAGTGTGGGGGATCA | ||
| ZP1F1a | TGATGTTTTCGCAGCATTGG | 103 | czcA |
| ZP1R1a | TCCCAAAAGTCCCTCACTCC | ||
| PMZF2b | CCCGGTTGCTACTGCAATTC | 110 | cadA |
| PMZR2b | TTCCATTCACCGTTGCTTTCA | ||
| ZP1F1d | GGAGTCATGATTGCCGGAGTT | 120 | czcD |
| ZP1R1d | AGCCTCCATCAACAGTTTCCA |
Results
Cadmium and uranium tolerance
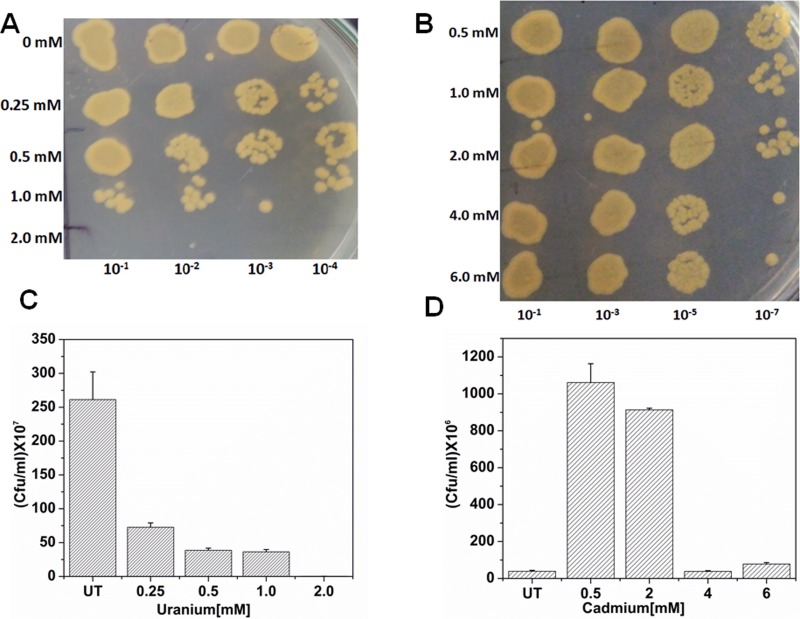
Cadmium and uranium tolerance capabilities of Chryseobacterium sp. was monitored by treating the bacterium with increasing concentrations of Cd or U. Chryseobacterium sp. showed notable tolerance towards Cd (MIC~6mM) as compared to uranium (MIC~2mM). Cell viability assay showed that Cd has an unusual growth stimulating effect on the bacterium up to a concentration of 2mM beyond which antagonistic effect of metal starts to show on the growth of bacterium (Fig 1D). Uranium, on the other hand, had an adverse effect on the growth of bacterium in the concentration range tested (Fig 1A and 1B).
Fig 1. Growth of Chryseobacterium sp. after treatment with various concentrations of metal.
A) Spot assay to determine the MIC towards Uranium; B) Plating method to determine the effect of U on Chryseobacteriumsp.; A) Spot assay to determine the MIC towards Cd; B) Plating method to determine the effect of Cd on Chryseobacterium sp.
Screening for metal tolerant genes
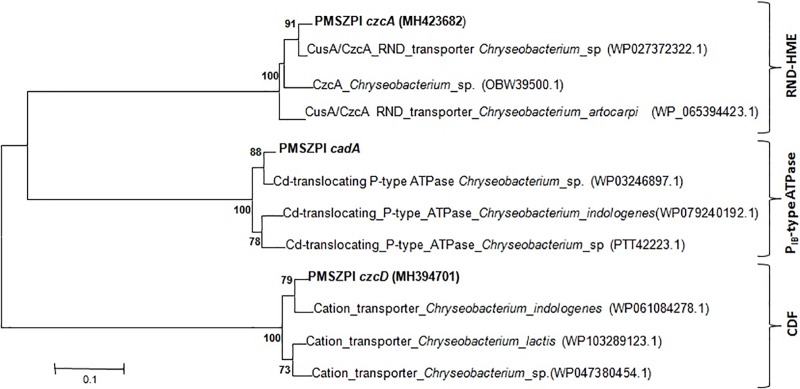
Metal transporter genes belonging to the member of RND-HME (czcA) and CDF (czcD) were amplified using primers listed on S1 Table. Chryseobacterium sp. PMSZPI was found to harbor all the genes known for the metal tolerant phenotype.BLASTX analyses of the sequenced nucleotide obtained for each amplicon showed that the putative czcA and czcD amplicons matched their respective genes from other Chryseobacterium sp. reported in the NCBI database (Table 2). The similarity percentage of each gene with respect to their close relative reported in the NCBI database is given in Table 2. Phylogenetic analyses of the sequenced amplicons using Neighbor-joining method showed that putative czcA and czcD genes from Chryseobacterium sp.PMSZPI are grouped in their respective gene families (Fig 2).
Table 2. BLASTX analysis of metal transporters from Chryseobacterium sp. (PMSZPI).
| Gene Name (Accession. No) |
The closest match of metal tolerant genes from PMSZPI with those in NCBI Database after BLASTX | Similarity Percentage |
|---|---|---|
| czcA(MH423682) | CusA/CzcA family heavy metal efflux RND transporter [Chryseobacterium sp. UNC8MFCol] (WP_027372322.1) | 98 |
| czcD(MH394701) | Cation diffusion facilitator family transporter [Chryseobacterium sp. ISE14] (WP109713444.1) | 97 |
| cadA(JN034431) | Cadmium-translocating P-type ATPase [Chryseobacterium sp. ISE14] (WP_103246897.1) | 98 |
Fig 2. Phylogenetic analysis of CDF, RND-HME and PIB-type ATPases.
Neighbor-joining method of translated amino acid sequences from czcD, czcA and cadA genes were carried out using MEGA.6.0 software with 1000 bootstrap support.
Expression of czcA, czcD and cadAin the presence of Cd and U
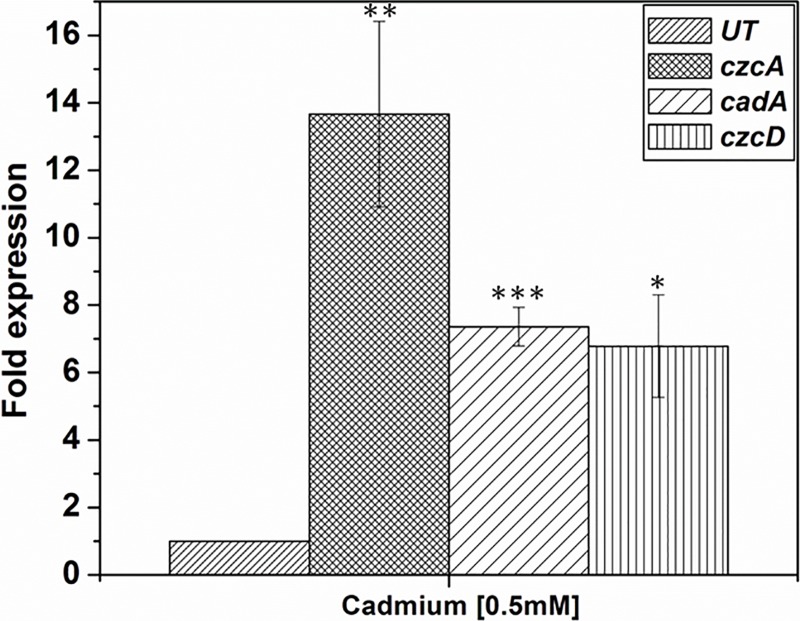
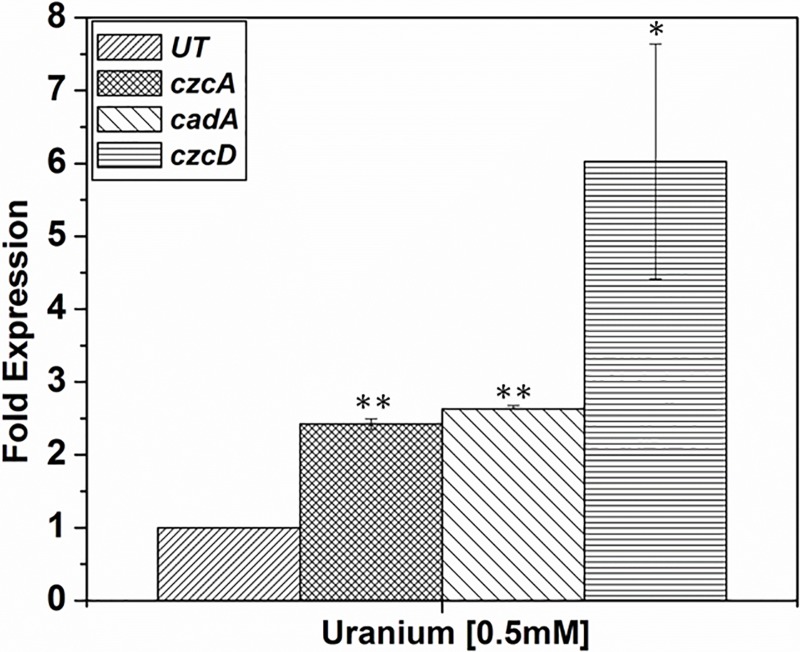
Previously described cadA gene belonging to the family of PIB-type ATPase from Chryseobacterium sp. PMSZPI [28] along with the newly identified czcA and czcD genes belonging to the RND family and CDF family of metal transporters were studied for their role in U or Cd stress. Expression of metal tolerant genes was studied by monitoring the level of mRNA transcripts of czcA, czcD and cadA genes in the presence of Cd or U. The level of expression was normalized to the expression of 16S rRNA both in treated/untreated cell. In this study, all the three genes under study were found to be upregulated in the presence of Cd or U. In this study, czcA cadA and czcD gene expressions were found to be 13, 7 and 6-fold upregulated respectively in the presence of 0.5mM Cd (Fig 3). Similarly, czcA and cadA genes were found to be 2-fold upregulated and czcD was 6-fold upregulated in the presence of 0.5mM U (Fig 4).
Fig 3. Expression of metal transporter genes in the presence of cadmium.
Expression of czcA, czcD and cadA genes in response to treatment with 0.5mM Cd. Expression of these genes was normalized to the expression of the 16SrRNA gene. Statistical significance was performed using Student’s test (p ≤0.05*; p ≤0.01≥0.001 **; p ≤0.001***.).
Fig 4. Expression of metal transporter genes in the presence of uranium.
Expression of czcA, czcD and cadA genes in response to treatment with 0.5mM U. Expression of these genes was normalized to the expression of the 16SrRNA gene. Statistical significance was performed using Student’s test (p ≤0.05 *; p ≤0.01≥0.001 **; p ≤0.001 ***.).
Nucleotide sequence accession number
The GenBank accession numbers obtained for czcA and czcD genes reported in this study are MH423682 and MH394701 respectively.
Discussion
Metal tolerant bacteria have been isolated from naturally occurring or anthropogenic metal-rich environment for their use in bioremediation, agriculture or understanding the mechanism for their survival in such extreme habitat [24, 28–31]. These isolates tend to possess additional features that allow them to exist in such extreme habitat [29]. Chryseobacterium sp. PMSZPI was previously isolated from uranium deposit and identified using biochemical and molecular methods [24]. PMSZPI showed significant tolerance towards U and Cd as evident from MIC and viability studies (Fig 1). This isolate was also found to tolerate high concentration of copper and zinc as reported in earlier studies [24]. Interestingly, the growth of PMSZPI seems to increase with the treatment of low concentration of Cd, even though it is known that Cd have no essential biological role (Fig 1D). Metal tolerance is a concerted effort of various molecular actors that maintain the metal homeostasis of both essential and non-essential metals that enter the cytoplasm of the microorganism [7, 32, 33]. Cadmium toxicity in living organisms is a result of its incursion into various molecular processes. One of the molecular mechanism to decrease Cd-toxicity is the involvement of metal efflux proteins[2, 34]). PMSZPI was found to harbor all the three member of metal efflux proteins that are known to play a major role in maintaining the cellular metal homeostasis in bacteria [2]. The three metal transporters were phylogenetically distinct from each other as evident from BLASTX and Phylogenetic analyses (Table 2 and Fig 2). Metals tolerant genes belonging to the family of CDF (czcD), RND-HME(czcA) and PIB-type ATPase (cadA) have been studied for their role in Cd2+ Cu+/2+ Zn2+ Pb2+ tolerance in bacteria[2]. Similarly, czcD, czcA and cadA genes from PMSZPI were found to be upregulated in response to Cd treatment indicating that these transporters have a role in Cd tolerance. Previous studies showed that there is the interplay between the czcCBA efflux system, PIB-type ATPases and CDF protein in Ralstonia metallidurans where the former was found to play a major role in metal detoxification [7, 8]. Similarly, in this study, we found that czcA expression was 2-fold higher as compared to cadA and czcD expression in the presence of Cd indicating that czcCBA efflux system might play a major role in Cd tolerance in this isolate. The interaction of U with various life forms is a widely studied area due to the recent increase in U exploration for its use in energy generation and weaponry. Understanding uranium toxicity is one the aspect that most researchers are trying to elucidate by studying its molecular interactions with biomolecules and their metabolic processes [16]. The mechanisms of Utolerance in bacteria are carried out through various biological processes like bioreduction, biomineralization, biosorption and bioaccumulation where they have been reported to play a major role in decreasing the toxicity of U [35]. Recent studies have been carried out to understand the molecular response towards uranium’s interaction with bacterial cells. These studies included proteomic analysis [36, 37], transposon-mediated mutagenesis [38] and whole genome transcriptional and functional analysis of genes in a microorganism that showed U tolerance capabilities[39–41]. These studies revealed the expression of genes involved in bioprecipitation, metal efflux, and oxidative stress besides other genes involved in cell metabolism. In this study, increase in the expression of metal efflux proteins belonging to the member of RND-HME, CDF and PIB-type ATPase was seen during U treatment indicating that these transporters play a part in U tolerance in Chryseobacterium sp. Furthermore, there is a 6-fold upregulation of czcD gene as compared to cadA and czcA during U treatment indicating that CDF protein may have a primary role in U tolerance. As expected, CDF, RND-HME and PIB-type ATPases which are known to play a major role in Cd tolerance in other microorganisms showed higher expressions when treated with Cd as compared to U. The findings of the present study revealed that these efflux proteins in Chryseobacterium sp. are also involved in U tolerances. However, toxicity of U depends on its speciation which is determined by the physicochemical components of that ecosystem. So, one of the major challenges in studying U interaction with biomolecules in situ is its speciation and complex formation in natural conditions. Nonetheless, this study showed that metal efflux proteins were found to respond toward U stress indicating that they may play a role either directly or indirectly in U tolerance.
Compliance with ethical standards
The article does not contain any studies with human participants or animals performed by any of the authors.
Supporting information
(PDF)
Acknowledgments
The Authors gratefully acknowledge the UGC-SAP (No.4-7/2016/DRS-I) and DST-FIST(SR/FST/LSI-666/2016) Programmes for providing equipment support to Biotechnology & Bioinformatics, North Eastern Hill University. Macmillan Nongkhlaw gratefully acknowledges the financial support received from Department of Science and Technology, GOI under the DST-INSPIRE faculty program (Sanction No. DST/INSPIRE Faculty Award /2012 Dated 1st February, 2013)
Data Availability
CzcA and czcD genes reported in this study are available freely in NCBI database at Accession Numbers MH423682 and MH394701, respectively.
Funding Statement
This work was funded by Department of Science and Technology, INDIA, under the DST-INSPIRE faculty program that was awarded to Dr Macmillan Nongkhlaw.
References
- 1.Monsieurs P, Moors H, Van Houdt R, Janssen PJ, Janssen A, Coninx I, et al. Heavy metal resistance in Cupriavidus metallidurans CH34 is governed by an intricate transcriptional network. Biometals. 2011;24(6):1133–51. 10.1007/s10534-011-9473-y . [DOI] [PubMed] [Google Scholar]
- 2.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev. 2003;27(2–3):313–39 10.1016/S0168-6445(03)00048-2 . [DOI] [PubMed] [Google Scholar]
- 3.Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109(10):4644–81. 10.1021/cr900077w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nat Rev Microbiol. 2009;7(1):25–35. 10.1038/nrmicro2057 . [DOI] [PubMed] [Google Scholar]
- 5.Arguello JM, Raimunda D, Gonzalez-Guerrero M. Metal transport across biomembranes: emerging models for a distinct chemistry. J Biol Chem. 2012;287(17):13510–7. 10.1074/jbc.R111.319343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nies DH. The cobalt, zinc, and cadmium efflux system CzcABC from Alcaligenes eutrophus functions as a cation-proton antiporter in Escherichia coli. J Bacteriol. 1995;177(10):2707–12 10.1128/jb.177.10.2707-2712.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legatzki A, Grass G, Anton A, Rensing C, Nies DH. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J Bacteriol. 2003;185(15):4354–61 10.1128/JB.185.15.4354-4361.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anton A, Grosse C, Reissmann J, Pribyl T, Nies DH. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J Bacteriol. 1999;181(22):6876–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anton A, Weltrowski A, Haney CJ, Franke S, Grass G, Rensing C, et al. Characteristics of zinc transport by two bacterial cation diffusion facilitators from Ralstonia metallidurans CH34 and Escherichia coli. J Bacteriol. 2004;186(22):7499–507. 10.1128/JB.186.22.7499-7507.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axelsen KB, Palmgren MG. Evolution of substrate specificities in the P-type ATPase superfamily. J Mol Evol. 1998;46(1):84–101 . [DOI] [PubMed] [Google Scholar]
- 11.Lewinson O, Lee AT, Rees DC. A P-type ATPase importer that discriminates between essential and toxic transition metals. Proc Natl Acad Sci U S A. 2009;106(12):4677–82. 10.1073/pnas.0900666106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maynaud G, Brunel B, Yashiro E, Mergeay M, Cleyet-Marel JC, Le Quere A. CadA of Mesorhizobium metallidurans isolated from a zinc-rich mining soil is a P(IB-2)-type ATPase involved in cadmium and zinc resistance. Res Microbiol. 2014;165(3):175–89. 10.1016/j.resmic.2014.02.001 . [DOI] [PubMed] [Google Scholar]
- 13.Kim ND, Fergusson JE. The concentrations, distribution and sources of cadmium, copper, lead and zinc in the atmosphere of an urban environment. Sci Total Environ. 1994;144(1–3):179–89 . [DOI] [PubMed] [Google Scholar]
- 14.Ribera D, Labrot F, Tisnerat G, Narbonne JF. Uranium in the environment: occurrence, transfer, and biological effects. Rev Environ Contam Toxicol. 1996;146:53–89 . [DOI] [PubMed] [Google Scholar]
- 15.Wedepohl KH. The composition of the continental crust. Geochim Cosmochim Acta. 1995;59(7):1217–32. [Google Scholar]
- 16.Maher K, Bargar JR, Brown GE Jr. Environmental speciation of actinides. Inorg Chem. 2013;52(7):3510–32. 10.1021/ic301686d . [DOI] [PubMed] [Google Scholar]
- 17.Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, et al. Cadmium stress: an oxidative challenge. Biometals. 2010;23(5):927–40. 10.1007/s10534-010-9329-x . [DOI] [PubMed] [Google Scholar]
- 18.Periyakaruppan A, Kumar F, Sarkar S, Sharma CS, Ramesh GT. Uranium induces oxidative stress in lung epithelial cells. Arch Toxicol. 2007;81(6):389–95. 10.1007/s00204-006-0167-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulis JM. Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals. 2010;23(5):877–96. 10.1007/s10534-010-9336-y . [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Li Y, Shao C, Tan Y, Cai L. Cadmium and its epigenetic effects. Curr Med Chem. 2012;19(16):2611–20 . [DOI] [PubMed] [Google Scholar]
- 21.Faa A, Gerosa C, Fanni D, Floris G, Eyken PV, Lachowicz JI, et al. Depleted Uranium and Human Health. Curr Med Chem. 2018;25(1):49–64. 10.2174/0929867324666170426102343 . [DOI] [PubMed] [Google Scholar]
- 22.Miller AC, Stewart M, Brooks K, Shi L, Page N. Depleted uranium-catalyzed oxidative DNA damage: absence of significant alpha particle decay. J Inorg Biochem. 2002;91(1):246–52 . [DOI] [PubMed] [Google Scholar]
- 23.Yazzie M, Gamble SL, Civitello ER, Stearns DM. Uranyl acetate causes DNA single strand breaks in vitro in the presence of ascorbate (vitamin C). Chem Res Toxicol. 2003;16(4):524–30. 10.1021/tx025685q . [DOI] [PubMed] [Google Scholar]
- 24.Kumar R, Nongkhlaw M, Acharya C, Joshi SR. Uranium (U)-tolerant bacterial diversity from U ore deposit of Domiasiat in North-East India and its prospective utilisation in bioremediation. Microbes Environ. 2013;28(1):33–41 10.1264/jsme2.ME12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyce R, Chilana P, Rose TM. iCODEHOP: a new interactive program for designing COnsensus-DEgenerate Hybrid Oligonucleotide Primers from multiply aligned protein sequences. Nucleic Acids Res. 2009;37(Web Server issue):W222–8. 10.1093/nar/gkp379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–9. 10.1093/molbev/msm092 . [DOI] [PubMed] [Google Scholar]
- 28.Nongkhlaw M, Kumar R, Acharya C, Joshi SR. Occurrence of horizontal gene transfer of P(IB)-type ATPase genes among bacteria isolated from the uranium rich deposit of Domiasiat in North East India. PLoS One. 2012;7(10):e48199 10.1371/journal.pone.0048199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mergeay M, Monchy S, Vallaeys T, Auquier V, Benotmane A, Bertin P, et al. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: towards a catalogue of metal-responsive genes. FEMS Microbiol Rev. 2003;27(2–3):385–410 10.1016/S0168-6445(03)00045-7 . [DOI] [PubMed] [Google Scholar]
- 30.Kartik VP, Jinal HN, Amaresan N. Characterization of cadmium-resistant bacteria for its potential in promoting plant growth and cadmium accumulation in Sesbania bispinosa root. Int J Phytoremediation. 2016;18(11):1061–6. 10.1080/15226514.2016.1183576 . [DOI] [PubMed] [Google Scholar]
- 31.Lu M, Jiao S, Gao E, Song X, Li Z, Hao X, et al. Transcriptome Response to Heavy Metals in Sinorhizobium meliloti CCNWSX0020 Reveals New Metal Resistance Determinants That Also Promote Bioremediation by Medicago lupulina in Metal-Contaminated Soil. Appl Environ Microbiol. 2017;83(20). 10.1128/AEM.01244-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hynninen A, Touze T, Pitkanen L, Mengin-Lecreulx D, Virta M. An efflux transporter PbrA and a phosphatase PbrB cooperate in a lead-resistance mechanism in bacteria. Mol Microbiol. 2009;74(2):384–94. 10.1111/j.1365-2958.2009.06868.x . [DOI] [PubMed] [Google Scholar]
- 33.Kirsten A, Herzberg M, Voigt A, Seravalli J, Grass G, Scherer J, et al. Contributions of five secondary metal uptake systems to metal homeostasis of Cupriavidus metallidurans CH34. J Bacteriol. 2011;193(18):4652–63. 10.1128/JB.05293-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–89. 10.1146/annurev.micro.50.1.753 . [DOI] [PubMed] [Google Scholar]
- 35.Newsome L MK, Lloyd JR The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem Geol. 2014;363:164–84. [Google Scholar]
- 36.Yung MC, Ma J, Salemi MR, Phinney BS, Bowman GR, Jiao Y. Shotgun proteomic analysis unveils survival and detoxification strategies by Caulobacter crescentus during exposure to uranium, chromium, and cadmium. J Proteome Res. 2014;13(4):1833–47. 10.1021/pr400880s . [DOI] [PubMed] [Google Scholar]
- 37.Gallois N, Alpha-Bazin B, Ortet P, Barakat M, Piette L, Long J, et al. Proteogenomic insights into uranium tolerance of a Chernobyl's Microbacterium bacterial isolate. J Proteomics. 2018;177:148–57. 10.1016/j.jprot.2017.11.021 . [DOI] [PubMed] [Google Scholar]
- 38.Yung MC, Park DM, Overton KW, Blow MJ, Hoover CA, Smit J, et al. Transposon Mutagenesis Paired with Deep Sequencing of Caulobacter crescentus under Uranium Stress Reveals Genes Essential for Detoxification and Stress Tolerance. J Bacteriol. 2015;197(19):3160–72. 10.1128/JB.00382-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu P, Brodie EL, Suzuki Y, McAdams HH, Andersen GL. Whole-genome transcriptional analysis of heavy metal stresses in Caulobacter crescentus. J Bacteriol. 2005;187(24):8437–49. 10.1128/JB.187.24.8437-8449.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutcliffe B, Chariton AA, Harford AJ, Hose GC, Greenfield P, Elbourne LDH, et al. Effects of uranium concentration on microbial community structure and functional potential. Environ Microbiol. 2017;19(8):3323–41. 10.1111/1462-2920.13839 . [DOI] [PubMed] [Google Scholar]
- 41.Junier P VE, Bernier-Latmani R. The Response of Desulfotomaculum reducens MI-1 to U(VI) Exposure: A Transcriptomic Study. Geomicrobiology Journal. 2011;28 (5–6):483–96. 10.1080/01490451.2010.512031 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
CzcA and czcD genes reported in this study are available freely in NCBI database at Accession Numbers MH423682 and MH394701, respectively.