Abstract
This research was undertaken to investigate the global role of the plant inositol phosphorylceramide synthase (IPCS), a non-mammalian enzyme previously shown to be associated with the pathogen response. RNA-Seq analyses demonstrated that over-expression of inositol phosphorylceramide synthase isoforms AtIPCS1, 2 or 3 in Arabidopsis thaliana resulted in the down-regulation of genes involved in plant response to pathogens. In addition, genes associated with the abiotic stress response to salinity, cold and drought were found to be similarly down-regulated. Detailed analyses of transgenic lines over-expressing AtIPCS1-3 at various levels revealed that the degree of down-regulation is specifically correlated with the level of IPCS expression. Singular enrichment analysis of these down-regulated genes showed that AtIPCS1-3 expression affects biological signaling pathways involved in plant response to biotic and abiotic stress. The up-regulation of genes involved in photosynthesis and lipid localization was also observed in the over-expressing lines.
Introduction
According to UN estimates, growing at a rate of 1.1% per year, the world population is set to reach 9.8 billion by 2050 [1], which would require a 70% increase in food production [2]. A finite amount of arable land, coupled with the detrimental effects of climate change on crop yields, mean that strategies other than intensification will need to be employed to increase production. One that is being adopted, in combination with intensification, is the use of biotechnology to produce genetically modified crops with enhanced yields. The ability to make plants that are more tolerant to biotic and abiotic stress is predicated on identifying molecular targets which modulate plant stress responses.
One such target of interest is the non-mammalian plant enzyme inositol phosphorylceramide synthase (IPCS) which catalyses a key step in sphingolipid biosynthesis. Complex sphingolipids can be grouped into two main classes in plants: glycosylceramides and derivatives of inositol phosphorylceramide (IPC) [3]. IPCS is central to synthesis of the latter, catalyzing the transfer of phosphoinositol from phosphatidylinositol to ceramide to form IPC [4]. Ceramide is the base unit of complex sphingolipids and is composed of a long chain base (LCB) and a fatty acid (FA) component [5]. The structural diversity of complex sphingolipids is conferred by the FA and LCB, with variation in carbon chains (C16-26), hydroxylation and desaturation, and the addition of various saccharides/oligosaccharides attached, via a phosphoinositol group in some cases, to the primary hydroxyl of ceramide. These modifications account for the 168 sphingolipid species identified in Arabidopsis thaliana [6], and are involved in a plethora of biological pathways, including programmed cell death (PCD) [7], reproduction [8], senescence [9] and cold acclimation [10]. Disruption of the sphingolipid pathway has repeatedly been shown to be inextricably connected to plant defense signaling [11].
First identified in wax bean microsome [12] and later in A. thaliana [4, 13], IPCS has been shown to play a role as a negative regulator of PCD [13] and is required for reproduction and normal growth [14]. In planta three IPCS isoforms exist, and further characterization in Oryza sativa showed that the expression of all three IPCS isoforms was temporally altered to varying degrees in a tissue and stress specific manner [15]. For example, under cold stress OsIPCS1 (NP_001044812) and OsIPCS2 (NP_001055712) were up-regulated in roots and stems, but down-regulated in leaves; in contrast OsIPCS3 (NP_001055096) was up-regulated in all tissues. Together, these results suggested that OsIPCS1-3 have key roles in rice growth and abiotic stress responses [15]. With respect to the plant biotic stress response, T-DNA insertion mutants of AtIPCS2 (AT2G37940) in A. thaliana showed increased levels of ceramide and phytoceramide, both well-documented inducers of PCD, and displayed necrotic lesions associated with PCD [13]. When exposed to the biotropic pathogen Golovinomyces cichoracearum UCSC1, these plants showed a reduction in fungal mass compared to controls [13]. AtIPCS1 (AT3G54020) and AtIPCS3 (AT2G29525) have not been characterized so far.
The data from both monocot O. sativa (rice) and dicot A. thaliana [13, 15] indicate that manipulation of IPCS activity, chemically or genetically, could be used to modulate biotic and abiotic plant stress responses. To explore this further, in this study, A. thaliana lines over-expressing each IPCS isoform were created and RNA-Seq carried out to monitor conserved changes in the transcriptome.
Materials and methods
Over-expression of AtIPCS1-3 in Arabidopsis thaliana
PCR products of the full-length cDNA of AtIPCS1-3 [4] were cloned into pENTR/D-TOPO using T4-ligase (ThermoFisher) and into the destination vector pK7WG2 [16] via Gateway LR Clonase (ThermoFisher) to create pK7WG2_AtIPCS1-3.
Primers:
AtIPCS1-NotI-F:
GCGCGCGGCCGCCACAATGACGCTTTATATTCGCCGCG
AtIPCS1-AscI-R:
GCGCGGCGCGCCTCATGTGCCATTAGTAGCATTATCAGTGTG
AtIPCS2-NotI-F:
GCGCGCGGCCGCCACAATGACACTTTATATTCGTCGTGAATCTTCCAAG
AtIPCS2-AscI-R:
GCGCGGCGCGCCTCACGCGCCATTCATTGTGTTATC
AtIPCS3-NotI-F:
GCGCGCGGCCGCCACAATGCCGGTTTACGTTGATCGC
AtIPCS3-AscI-R:
GCGCGGCGCGCCTCAATGATCATCTGCTACATTGTTCTCGTTT
Agrobacterium tumefaciens strain C58C1 was transformed with pK7WG2_AtIPCS1-3, transformants plated on Luria broth (100 μg/μl rifampicin and 100 μg/μl spectinomycin) and incubated for 3 days at 28°C. Col-0 wild-type A. thaliana were subsequently transformed using the floral dipping method [17].
Arabidopsis thaliana growth conditions
Col-0 and AtIPCS1, 2 and 3 over-expressing plants were grown for 10 days on Murasige and Skoog (MS) agar before transfer to peat plugs. Growth conditions were 20 °with a 16-hour day / 8-hour night cycle.
RNA preparation
RNA extraction was carried out on samples flask frozen in nitrogen, using the ReliaPrep™ Tissue Miniprep System (Promega) according to the manufacturer’s protocol. Following DNase (ThermoFisher) treatment, the integrity of the RNA was determined by running the samples on a 2100 Bioanalyzer (Agilent) to obtain an RNA Integrity Number (RIN) score.
Quantification of AtIPCS1-3 in over-expressing Arabidopsis thaliana transgenic lines
cDNA samples prepared as above and the Applied Biosystems 7300 Real-Time PCR System and the SYBR Green Jump-Start Taq Ready Mix were used to quantify transcript as previously described [4, 18, 19]. Gene specific primers were designed using Primer3plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) for real-time PCR with PEX4 used as a reference gene. Primers: AtIPCS1_F: TGCGTCCCGTAAACATTACA, AtIPCS1_R: ACACCGTTCCCATTCAAGAG, AtIPCS2_F: TACCAGATCGGACTGCTGTG, AtIPCS2_F: GTGAACTCCGTTGCTGTCAA, AtIPCS3_F: CTGGGCCGAATTATCATTGT, AtIPCS3_R: CCTTCGTGTGCCGTATCTTT
RNA-Seq
Single end libraries for RNA-Seq were generated from DNase treated total RNA using TruSeq Stranded mRNA sample preparation kit according to manufacturer’s instructions (Illumina). Briefly, mRNAs were fragmented and purified for use as template for the synthesis of double stranded cDNA. End repair of the double stranded cDNA was carried out and the 3’ end adenylated. Sample specific indexing adapters were ligated to the ends of double stranded cDNA samples, amplified by PCR and then purified. Samples were normalized, pooled and then sequenced using a NextSeq 500 instrument (Illumina) to obtain 150 base pair single end reads.
RNA-Seq analyses
The RNA sequence data in Fastq format 11 were filtered and trimmed (sliding window 4:15 and 50 bp minimum) to remove low quality reads using Trimmomatic [20]. Reads were aligned to the Arabidopsis genome (Arabidopsis Araport 2017) using STAR [21]. The sequence alignment files were sorted by name14 for HTSeq-count and indexed using SAMtools [22]. Files were converted to BAM files and number of reads mapped onto a gene calculated using HTSeq package [23]. Gene counts were normalized and compared sample by sample using DESeq2 [24] (Bioconductor [25]) in R [26]. Differential expression was determined using with a log2 fold-change output. GO term enrichment was performed for analyses of genes up- and down-regulated in both biological replicates using the agriGO analysis tool (http://bioinfo.cau.edu.cn/agriGO/analysis.php) with the default settings [27]. Gene annotations was carried out against Arabidopsis gene model (TAIR9) background (https://www.arabidopsis.org/). These data are freely available in GEO (https://www.ncbi.nlm.nih.gov/geo/ GEO Accession GSE129016).
MapMan
Analyses were carried out using MapMan 3.5.1 R2 software [28]. RNA-Seq abundance data from the At2++ transgenic line were uploaded to MapMan and log2 fold change selected as the experimental data set for analyses. Mapping was carried out using Ath_AGI_TAIR9_Jan2010.
Osmotic stress assay
Seedlings were grown under standard conditions on MS agar for 8 days before floating on the various concentrations of mannitol (without MS) in a sterile culture dish under the same conditions as the plate (16h day photo-period, 20°C). Photographs were taken after 6 days.
Pathogen stress assay
Control and over-expressing transgenic lines were grown under short day conditions for 5 weeks and leaves excised then incubated on MS agar at 37°C for 72 hours following the addition of 5μl of Erwinia amylovora culture grown to an OD600 = 0.5.
Genevestigator
Genevestigator [29] was utilised to identify available data showing the upregulation of AtIPCS1, AtIPCS2 or AtIPCS3 in Arabidopsis thaliana seedlings exposed to different agents and conditions.
Results
Identification of genes down- or up-regulated on over-expression of AtIPCS
A. thaliana plants over-expressing the full-length cDNA of AtIPCS1, AtIPCS2 and AtIPCS3 respectively, were generated as described in Methods. For each isoform, two transgenic lines (biological replicates) over-expressing the respective AtIPCS were selected, one with high levels relative to wild-type Columbia-0 (Col-0) (At1-3++) and one with a lower level of over-expression (At1-3+) (Fig 1A–1C). Importantly, over-expression of one isoform did not affect the expression of the other two AtIPCS isoforms (S1 Fig). Genome wide transcriptomic data analyses indicated that the number of expressed genes detected was very similar in all transgenic lines and in the Col-0 control (Table 1).
Fig 1. Relative quantitation of mRNA levels of (A) AtIPCS1 (B) AtIPCS2 (C) AtIPCS3 in over-expressor transgenic lines compared to Col0 standardised to equate to a value of 1; qPCR was performed to measure mRNA levels in 10-day old seedlings.
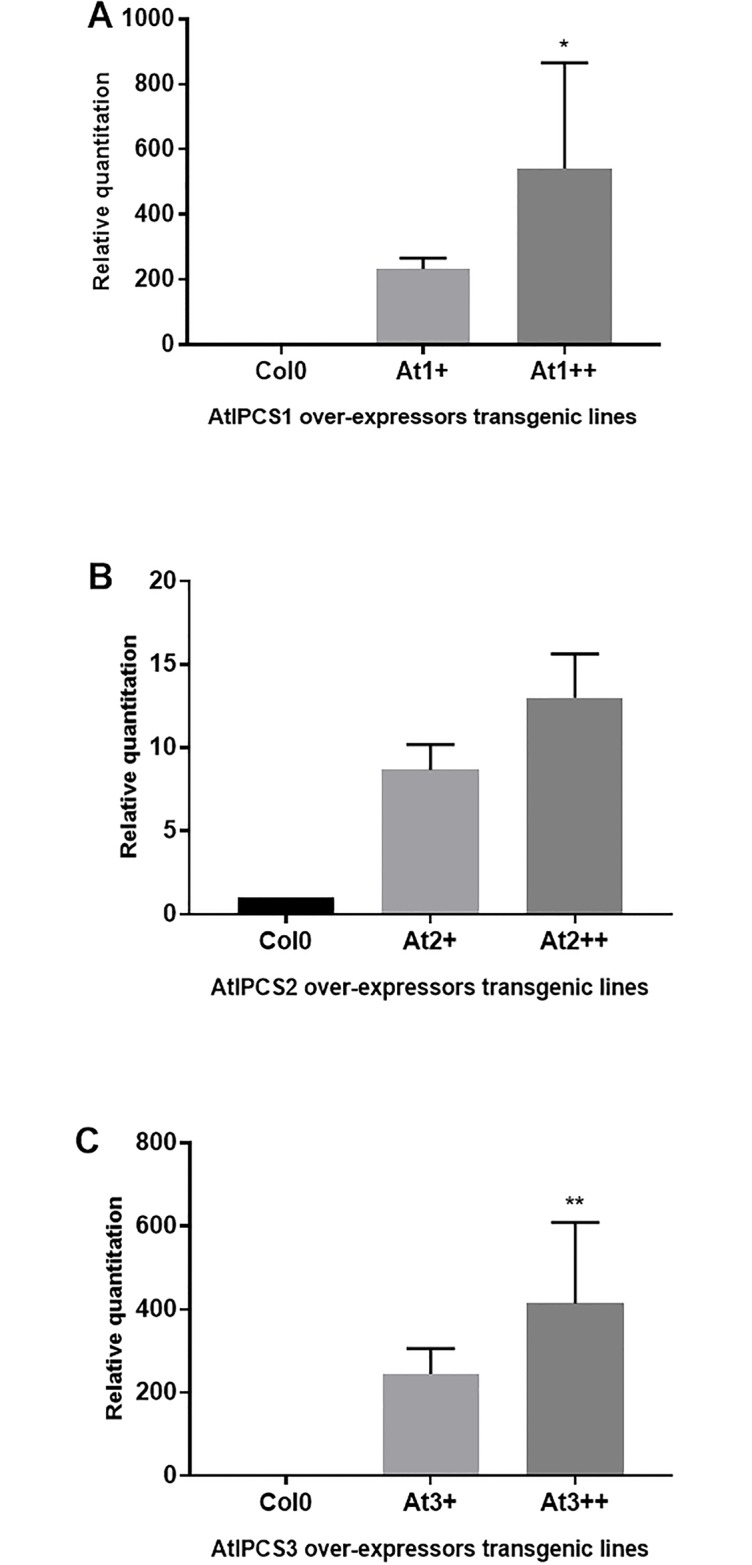
Relative quantitation was done after normalization using PEX4 levels; relative quantitation value is the mean of three biological replicates with standard deviation; significance of mRNA levels determined by one-way ANOVA with Turkey’s post hoc test (p < 0.05).
Table 1. Genes expressed in Col-0 and AtIPCS1-3 over-expressing transgenic lines.
A. thaliana Col-0 parent; At1, 2 or 3 lines over-expressing AtIPCS1, 2 or 3 (2 lines of each); Rep—experimental replicates (3); FPKM—Fragments Per Kilobase Million.
| Genotype | Rep | Expressed genes (FPKM) | Average | Non-expressed genes (FPKM) | Average |
|---|---|---|---|---|---|
| Col-0 | 1 | 22740 | 22749 | 2524 | 2515 |
| Col-0 | 2 | 22700 | 2564 | ||
| Col-0 | 3 | 22807 | 2457 | ||
| At1+ | 1 | 22410 | 22518 | 2854 | 2746 |
| At1+ | 2 | 22503 | 2761 | ||
| At1+ | 3 | 22640 | 2624 | ||
| At1++ | 1 | 22914 | 22805 | 2350 | 2459 |
| At1++ | 2 | 22823 | 2441 | ||
| At1++ | 3 | 22677 | 2587 | ||
| At2+ | 1 | 22643 | 22631 | 2621 | 2633 |
| At2+ | 2 | 22546 | 2718 | ||
| At2+ | 3 | 22705 | 2559 | ||
| At2++ | 1 | 22520 | 22574 | 2744 | 2690 |
| At2++ | 2 | 22573 | 2691 | ||
| At2++ | 3 | 22628 | 2636 | ||
| At3+ | 1 | 22657 | 22595 | 2607 | 2669 |
| At3+ | 2 | 22593 | 2671 | ||
| At3+ | 3 | 22535 | 2729 | ||
| At3++ | 1 | 22708 | 22731 | 2556 | 2533 |
| At3++ | 2 | 22708 | 2556 | ||
| At3++ | 3 | 22777 | 2487 |
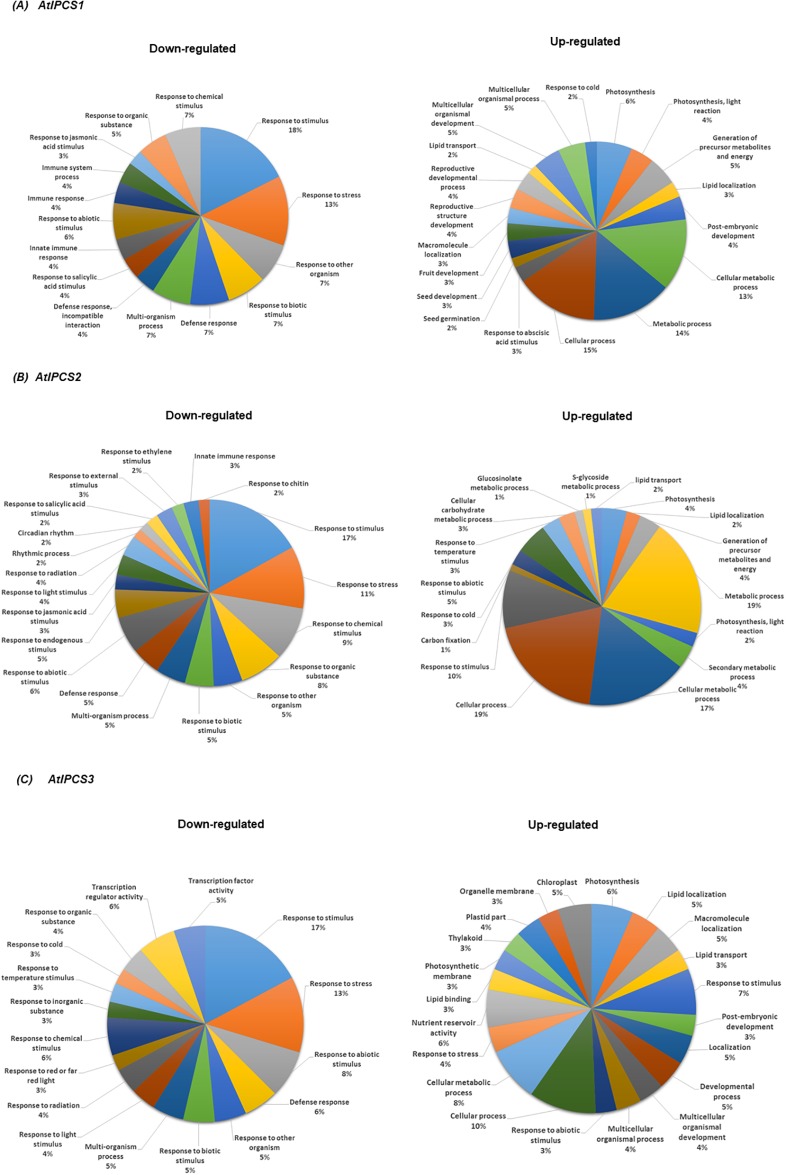
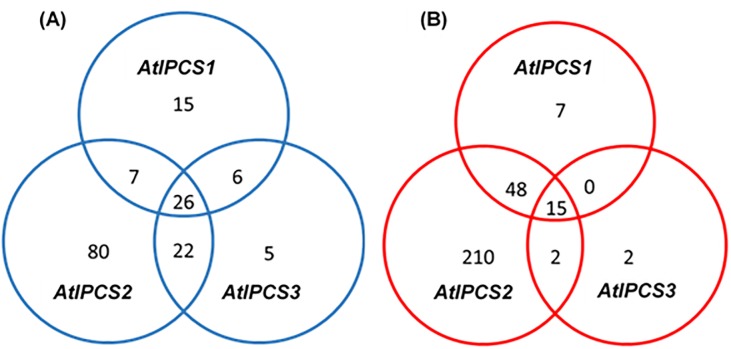
Genes that were identified as differentially expressed in both biological replicates, compared to Col0, were carried forward for further analyses. Function enrichment of the down-regulated genes using agriGO (http://bioinfo.cau.edu.cn/agriGO/index.php) revealed a significant enrichment of genes (Fisher exact test, two-tailed, p-value < 0.001) under the GO terms response to stimulus (p = 3.50E-18) and response to stress (p = 1.50E-14 ), whilst a significant amount of the up-regulated genes fell under the GO terms cellular process (p = 2.90E-07) and cellular metabolic process (p = 4.10E-08) (Fig 2). Those genes identified under GO term response to stimulus were the same genes as identified under response to stress and response to abiotic stress. Subsequently, to add specificity, focus was placed upon the latter two GO terms. AtIPCS2 over-expression led to the down-regulation of 135 genes, considerably more than AtIPCS1 (54) and AtIPCS3 (59) (Fig 3A). Of these, 26 genes were down-regulated in all lines. With respect to up-regulated genes, again most (275) were in response to AtIPCS2 over-expression, with 70 and 19 in AtIPCS1 and AtIPCS3 over-expressers respectively; 15 genes were found to be up-regulated in all lines (Fig 3B).
Fig 2. Pie chart of biological processes enriched for genes down- and up-regulated in response to the over-expression of (A) AtIPCS1, (B) AtIPCS2 and (C) AtIPCS3 isoforms when compared to wild type Col0.
Fig 3. Venn diagrams of number of genes that were down-regulated (A) or up-regulated (B) in response to AtIPCS1, AtIPCS2 and AtIPCS3 overexpression.
Log2 fold change relative to wild type Col-0.
Analyses of genes identified as responding negatively to AtIPCS over-expression
Global analyses of the down-regulated genes in response to AtIPCS1 over-expression revealed significant enrichment under the GO term GO:0006950, response to stress (p = 1.50E-14), 42.6% (23/54) when they represent only 6.14% of the Arabidopsis transcriptome (S1 Table). Similarly, on AtIPCS2 over-expression down-regulated genes were enriched under this term, 34.1% (46/135; p = 2.20E-22) (S2 Table); and on AtIPCS3 over-expression 40.7% (24/59; p = 2.40E-14). Other biological processes showing a significant enrichment of genes down-regulated in these transgenic lines, included those under GO terms: GO:0042221 (response to chemical stimulus); GO:0010033 (response to organic substances), GO:0051707 (response to other organisms); GO:0009607 (response to biotic stimulus); GO:0009628 (response to abiotic stimulus); and GO:0006952 (defense response) (S1–S3 Tables).
Of particular interest were genes that showed a dose-dependent decrease in expression associated with increased AtIPCS expression. Genes (GO:0006950, response to stress) whose decrease in expression was negatively correlated (log2 change ≥2) with higher AtIPCS1 levels (At1++ versus At1+], Fig 1A and Table 2) are: TYROSINE AMINOTRANSFERASE 3 (TAT3; AT2G24850); PLANT DEFENSIN 1.2B (PDF1.2B; AT2G26020); the salicylic acid inducible PATHOGENESIS-RELATED GENE, PR1 (AT2G14610) and PR2 (AT3G57260); LIPID TRANSFER PROTEIN (LTP; AT4G12490); and an ETHYLENE- AND JASMONATE-RESPONSIVE PLANT DEFENSIN (AT5G44420), a well characterized component of the defense network against pathogens [30] (Table 2).
Table 2. Genes showing a negative correlation with AtIPCS expression under GO term GO:0006950 (response to stress) compared to Col0.
At1-3+ over-expressing AtIPCS1-3; At1-3++ higher level expressers of AtIPCS1-3. Fold changes are the value of three technical triplicates of a transformed line. Transcripts listed had a p-value <0.001 (Wald test, cut off p-value < 0.05) and highlighted in bold are log2 fold change ≥ 2.
| GeneID | Gene annotation | At1+ | At1++ | At2+ | At2++ | At3+ | At3++ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | ||
| AT5G47220 | ERF2 (ETHYLENE RESPONSIVE ELEMENT BINDING FACTOR 2) | -2 | 1.46E-31 | -1.7 | 4.58E-25 | -1.1 | 7.33E-15 | ||||||
| AT2G24850 | TAT3 (TYROSINE AMINOTRANSFERASE 3) | -1.8 | 6.29E-17 | -4 | 1.33E-53 | -2.6 | 3.03E-28 | -2.3 | 4.60E-26 | -1.8 | 6.10E-18 | -2.3 | 1.65E-24 |
| AT1G16420 | MC8 (METACASPASE 8) | -2 | 1.68E-07 | -1.6 | 3.84E-06 | -1.2 | 0.00047 | -1 | 0.00242862 | -1.2 | 0.00058 | ||
| AT4G12480 | PEARLI 1; LIPID BINDING A PUTATIVE LIPID TRANSFER PROTEIN | -5 | 1.37E-159 | -1.9 | 1.94E-31 | -5.1 | 7.83E-172 | -1.2 | 8.99E-13 | -2 | 3.20E-32 | ||
| AT1G78290 | SERINE/THREONINE PROTEIN KINASE | -2 | 2.05E-47 | -1.8 | 8.25E-47 | -2 | 1.39E-54 | -1 | 2.73E-20 | -1.3 | 1.51E-29 | ||
| AT4G37990 | ELI3-2 (ELICITOR-ACTIVATED GENE 3–2) | -3 | 6.02E-17 | -2.3 | 7.52E-12 | -2.3 | 2.18E-12 | -1.8 | 3.22E-08 | -1.6 | 6.77E-07 | ||
| AT3G45290 | MLO3 (MILDEW RESISTANCE LOCUS O 3) | -1 | 7.16E-12 | -1.1 | 5.16E-09 | -1.3 | 7.16E-12 | 3.50E-08 | |||||
| AT2G26020 | PDF1.2B (PLANT DEFENSIN 1.2B) | -1.9 | 8.15E-18 | -5 | 1.44E-66 | -4.9 | 1.47E-60 | -4.9 | 3.31E-62 | -2.6 | 3.65E-30 | -3.4 | 3.34E-40 |
| AT2G32680 | RLP23 (RECEPTOR LIKE PROTEIN 23) | -2 | 2.69E-17 | -2.2 | 1.35E-13 | -2.3 | 3.16E-15 | 2.85E-235 | |||||
| AT2G26560 | PLA2A (PHOSPHOLIPASE A 2A) | -3 | 1.57E-78 | -1.4 | 1.57E-26 | -2.6 | 1.57E-78 | 2.27E-14 | |||||
| AT2G14610 | PR1 (PATHOGENESIS-RELATED GENE 1) | -3.4 | 1.83E-172 | -7.3 | 4.08E-238 | -7.3 | 1.05E-206 | -7.9 | 4.60E-217 | -4.6 | 1.09E-252 | -4.9 | 2.85E-235 |
| AT2G46830 | CCA1 (CIRCADIAN CLOCK ASSOCIATED 1) | -2 | 9.82E-14 | -2 | 3.12E-12 | -1.1 | 1.04E-05 | 5.91E-13 | |||||
| AT1G06160 | ORA59 (OCTADECANOID-RESPONSIVE ARABIDOPSIS AP2/ERF 59) | -1 | 1.14E-10 | -1.4 | 9.92E-17 | -2.5 | 9.89E-37 | -1.8 | 1.67E-24 | 2.31E-20 | |||
| AT3G49620 | DIN11 (DARK INDUCIBLE 11) | -4 | 1.16E-143 | -3.2 | 7.04E-106 | -4.1 | 5.37E-147 | 3.16E-94 | |||||
| AT1G06040 | STO (SALT TOLERANCE) | -1.1 | 2.13E-28 | -1.5 | 1.78E-49 | -1.3 | 2.23E-38 | -1.1 | 8.10E-27 | -1 | 6.04E-26 | -1.5 | 2.41E-48 |
| AT1G48000 | MYB112 (MYB DOMAIN PROTEIN 112) | -1 | 8.79E-05 | -2 | 1.97E-10 | -1.2 | 5.54E-05 | -1.2 | 6.45E-05 | -1.7 | 3.23E-08 | ||
| AT2G14560 | LURP1 (LATE UPREGULATED IN RESPONSE TO HYALOPERONOSPORA PARASITICA) | -2.7 | 5.62E-112 | -3.4 | 1.13E-162 | -4.1 | 5.67E-173 | -3.3 | 1.62E-149 | -3.2 | 9.88E-155 | -1.7 | 5.56E-82 |
| AT1G22770 | GI (GIGANTEA) | -2 | 2.40E-75 | -2.4 | 6.55E-94 | -2.3 | 8.70E-91 | -1.3 | 4.07E-33 | -1.7 | 1.27E-94 | ||
| AT5G10140 | FLC (FLOWERING LOCUS C) | -1.2 | 2.18E-09 | -1.5 | 4.16E-14 | -1.4 | 5.23E-11 | -1.3 | 4.55E-10 | -1.1 | 8.80E-12 | -1.7 | 3.25E-08 |
| AT3G1550 | ANAC055 (ARABIDOPSIS NAC DOMAIN CONTAINING PROTEIN 55) | -2 | 4.16E-28 | -1.3 | 2.83E-20 | -1.5 | 4.16E-28 | ||||||
| AT1G76930 | EXT4 (EXTENSIN 4) | -3 | 1.47E-142 | -1.2 | 1.21E-28 | -3.1 | 3.77E-175 | ||||||
| AT5G44420 | AN ETHYLENE- AND JASMONATE-RESPONSIVE PLANT DEFENSIN | -2.7 | 1.49E-54 | -6.1 | 3.17E-118 | -6 | 3.77E-111 | -6.1 | 3.17E-118 | -2.7 | 1.34E-55 | -3.6 | 7.78E-78 |
| AT2G23680 | STRESS-RESPONSIVE PROTEIN | -1 | 3.11E-14 | -1.1 | 3.58E-14 | -1.3 | 1.09E-18 | ||||||
| AT1G61120 | TPS04 (TERPENE SYNTHASE 04) | -3 | 1.57E-22 | -2.6 | 2.43E-15 | -1.7 | 5.55E-08 | -1.6 | 4.61E-07 | -1.8 | 6.72E-09 | ||
| AT1G75040 | PR5 (PATHOGENESIS-RELATED GENE 5) | -1.4 | 3.27E-22 | -1.7 | 2.13E-33 | -1.5 | 1.00E-25 | -1.8 | 5.50E-34 | ||||
| AT1G52890 | ANAC019 (ARABIDOPSIS NAC DOMAIN CONTAINING PROTEIN 19) | -2 | 4.84E-09 | -1.2 | 2.90E-05 | -1.5 | 5.04E-07 | ||||||
| AT3G57260 | PR2 (PATHOGENESIS-RELATED GENE 2) | -1.9 | 1.17E-30 | -5.3 | 4.64E-102 | -4.8 | 2.99E-86 | -4.4 | 4.08E-87 | -2 | 2.97E-36 | -2.2 | 8.40E-38 |
| AT1G18330 | EPR1 (EARLY-PHYTOCHROME-RESPONSIVE1) | -2 | 3.87E-53 | -1.6 | 3.85E-29 | -1.9 | 4.62E-40 | -1.3 | 1.98E-23 | -1.6 | 6.68E-30 | ||
| AT1G78410 | VQ MOTIF-CONTAINING PROTEIN | -1 | 2.59E-05 | -1.8 | 9.42E-08 | -1.6 | 1.24E-06 | -1.1 | 0.00072986 | -1.2 | 0.00016391 | ||
| AT5G19880 | PEROXIDASE | -1.4 | 0.00010488 | -1 | 0.00418 | ||||||||
| AT2G18050 | HIS1-3 (HISTONE H1-3) | -1 | 8.40E-31 | -2.5 | 2.90E-96 | -1.7 | 3.90E-55 | ||||||
| AT3G04720 | PR4 (PATHOGENESIS-RELATED 4) | -1 | 8.36E-46 | -1.6 | 7.55E-69 | -1.5 | 1.20E-55 | ||||||
| AT2G43510 | TI1; SERINE-TYPE ENDOPEPTIDASE INHIBITOR | -4 | 2.98E-227 | -2.5 | 6.34E-132 | -3.9 | 2.98E-227 | -1.1 | 2.90E-37 | -1.8 | 9.93E-77 | ||
| AT3G45860 | RECEPTOR-LIKE PROTEIN KINASE | -1.5 | 6.64E-09 | -2.9 | 8.20E-25 | -2.9 | 3.03E-22 | -3 | 8.20E-25 | -1.5 | 4.12E-09 | -1.7 | 4.81E-10 |
| AT3G51660 | MACROPHAGE MIGRATION INHIBITORY FACTOR FAMILY PROTEIN | -2 | 6.63E-15 | -1.2 | 1.40E-16 | -1.1 | 6.63E-15 | ||||||
| AT1G71030 | MYBL2 (ARABIDOPSIS MYB-LIKE 2) | -2 | 4.20E-88 | -1.2 | 1.04E-29 | -1.7 | 4.26E-55 | ||||||
| AT1G57630 | DISEASE RESISTANCE PROTEIN (TIR CLASS) | 3.1 | 1.80E-25 | -1.5 | 8.34E-09 | -2.5 | 4.73E-18 | ||||||
| AT5G59780 | MYB59 (MYB DOMAIN PROTEIN 59) | -2 | 1.15E-47 | -1.2 | 6.92E-29 | -1.7 | 9.77E-56 | ||||||
| AT4G12490 | LIPID TRANSFER PROTEIN (LTP) | -1.3 | 7.93E-05 | -4.6 | 1.76E-45 | -2.8 | 8.38E-18 | -4.7 | 1.76E-45 | -1.9 | 1.32E-08 | -2.6 | 1.46E-15 |
| AT4G23210 | PROTEIN KINASE FAMILY PROTEIN ENCODES A CYSTEINE-RICH RECEPTOR-LIKE KINASE (CRK13) | -3 | 1.31E-52 | -1.7 | 8.20E-24 | -3 | 1.31E-52 | ||||||
| AT1G09080 | ATP BINDING BIP3 | -1.1 | 7.65E-06 | -2.5 | 6.79E-21 | -1.6 | 4.53E-10 | -1.6 | 6.04E-11 | ||||
| AT4G02520 | GSTF2 (GLUTATHIONE S-TRANSFERASE PHI 2) | -2 | -1.2 | 2.82E-06 | -2.9 | 4.44E-28 | -1.4 | 3.33E-08 | -1.6 | 3.75E-10 | |||
| AT2G25000 | WRKY60 | -1.2 | 7.64E-11 | -1.4 | 9.79E-15 | ||||||||
| AT1G08320 | TGA9 | -1.1 | 1.21E-22 | ||||||||||
| AT3G52430 | PAD4 | -1.0 | 1.59E-15 | ||||||||||
| AT1G80840 | WRKY40 | -1.7 | 5.02E-23 | ||||||||||
| AT4G31800 | WRKY18 | -1.0 | 1.95E-20 | ||||||||||
| AT1G01560 | MPK11 | -2.0 | 5.24E-11 | -1.5 | 4.20E-07 | ||||||||
| AT1G73805 | SARD1 | -1.5 | 3.18E-29 | -1.3 | 5.55E-20 | -1.8 | 1.56E-35 | -1.3 | 6.19E-23 | ||||
| AT4G21534 | SPHINGOSINE KINASE 2 | -1.2 | 3.12E-21 | ||||||||||
| AT3G23250 | MYB15 | -1.9 | 5.76E-16 | -2.4 | 1.09E-20 | -1.4 | 4.96E-10 | ||||||
| AT4G25470 | DREB1C | -1.3 | 3.23E-04 | -1.1 | 2.06E-03 | -1.1 | 1.21E-03 | ||||||
| AT4G06746 | RAP2.9 | -1.1 | 3.27E-13 | ||||||||||
| AT4G25470 | DREB1B | -1.1 | 0.0014182 | -1.0 | 3.36E-03 | ||||||||
Interestingly, the dose response effects noted was largely specific for the AtIPCS1 isoform (Table 2). However, it is immediately clear that the plant defense system is more sensitive to increased AtIPCS2 expression (At2+ and At2++), despite the increase being relatively small (up to 10-fold) compared to AtIPCS1 and 3 (up to 390 and 440-fold respectively) (Fig 1A–1C and Table 2). The overall impact of AtIPCS2 over-expression may be due to an increases in what represents the most abundant AtIPCS transcript (approximately 100-fold AtIPCS1) in all tissues of wild type A. thaliana [4]. Interestingly, there is no major dose response apparent on an increase in the over-expression of AtIPCS3 (At3++ versus At3+, Table 2), despite an increase in AtIPCS3 transcript similar to that observed for AtIPCS1 (Fig 1C). However, transcripts that were down-regulated ≥2 log2 in AtIPCS3 (either line) and were also suppressed on AtIPCS1 or AtIPCS2 over-expression include: TAT3; lipid binding A putative lipid transfer protein (PEARLI 1; AT4G12480); PDF1.2B; PR1 and PR2; LATE UP-REGULATED IN RESPONSE TO HYALOPERONOSPORA PARASITICA (LURP1; AT2G14560); GIGANTEA (GI; AT1G22770); ETHYLENE- AND JASMONATE-RESPONSIVE ELEMENT PLANT DEFENSIN. Of the genes down-regulated with a log2 fold change ≥2 in response to AtIPCS2 over-expression a large number were specific: ELICITOR-ACTIVATED GENE 3–2 (ELI3-2; AT4G37990); RECEPTOR LIKE PROTEIN 23 (RLP23; AT2G32680); PHOSPHOLIPASE A 2A (PLA2A; AT2G26560); CIRCADIAN CLOCK ASSOCIATED 1 (CCA1; AT2G46830); OCTADECANOID-RESPONSIVE ARABIDOPSIS AP2/ERF 59 (ORA59; AT1G06160); DARK INDUCIBLE 11 (DIN11; AT3G49620); EXTENSIN 4 (EXT4; AT1G76930); TERPENE SYNTHASE 04 (TPS04; AT1G61120); HISTONE H1-3 (HIS1-3;AT2G18050); SERINE-TYPE ENDOPEPTIDASE INHIBITOR (TI1; AT2G43510); GLUTATHIONE S-TRANSFERASE PHI 2 (GSTF2; AT4G02520). From these analyses, it is clear that AtIPCS2 expression affects a wider network of genes (including some of those involved in plant defence responses) compared with AtIPCS1 and AtIPCS3 transgenic lines, perhaps due to its higher expression pattern in all tissues of Arabidopsis [4].
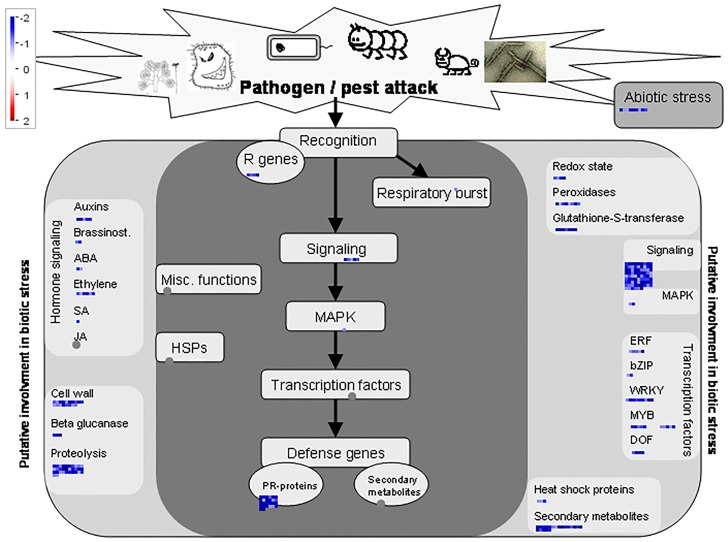
The effects of over-expression (At2++) were further analysed and visualised using MapMan (https://mapman.gabipd.org). These analyses illustrated multiple negative effects on metabolism (S2 Fig), however the clearest correlation of significantly down-regulated genes (log2-fold change) in At2++ was with the plant response to biotic stress: PR proteins, peroxidases, R genes, B-glucanases, heat shock proteins, transcriptional factors and signalling proteins involved in the response to pathogens (Fig 4). Furthermore, there is a remodelling of plant architecture as seen in the down-regulation of genes involved in cell wall modifications in response to pathogen attack (Fig 4).
Fig 4. Schematic, generated in MapMan, of genes identified as down-regulated in the At2++ over-expression line and enriched under plant response to biotic stress.
Log2 fold changes in gene expression are indicated by the colour scale. Abbreviations: Resistance (R) genes, salicylic acid (SA), jasmonic acid (JA), ethylene resposnse facotor (ERF), abscisic acid (ABA), DNA binding one zinc finger (DOF), heat-shock protein (HSP) and pathogenesis-related (PR) proteins.
The 26 genes down-regulated in response to the over-expression of AtIPCS1, 2 and 3 are primarily involved in the plant biotic stress response to pathogens (Table 3), including BDA1 a well characterised transmembrane protein found to be necessary in the regulation and augmentation of the plant response to pathogens [31]; and AED1 [32] an aspartyl protease implicated in Arabidopsis systemic acquired resistance. This conserved down-regulation of genes involved in the plant response to pathogens further stresses the functional importance of AtIPCS as a non-discriminate and far-reaching negative regulator of the response to biotic stress.
Table 3. Genes down-regulated in all three AtIPCS over-expression lines compared to Col0.
At1-3+ over-expressing AtIPCS1-3; At1-3++ higher level expressors of AtIPCS1-3. Fold changes are the value of three technical triplicates of a transformed line. Transcripts listed had a p-value <0.001 (Wald test, cut off p-value < 0.05).
| Gene ID | Gene annotation | At1+ | At1 ++ | At2+ | At2++ | At3+ | At3++ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log2 fold change | p-value | log2 fold change | p-value | log2 fold change | p-value | log2 fold change | p-value | log2 fold change | p-value | log2 fold change | p-value | ||
| AT2G26020 | PDF1.2B (PLANT DEFENSIN 1.2B) | -1.9 | 8.15E-18 | -5 | 1.44E-66 | -4.9 | 1.47E-60 | -4.9 | 3.31E-62 | -2.6 | 3.65E-30 | -3.4 | 3.34E-40 |
| AT2G24850 | TAT3 (TYROSINE AMINOTRANSFERASE 3) | -1.8 | 6.29E-17 | -4 | 1.33E-53 | -2.6 | 3.03E-28 | -2.3 | 4.60E-26 | -1.8 | 6.10E-18 | -2.3 | 1.65E-24 |
| AT2G14610 | PR1 (PATHOGENESIS-RELATED GENE 1) | -3.4 | 1.83E-172 | -7.3 | 4.08E-238 | -7.3 | 1.05E-206 | -7.9 | 4.60E-217 | -4.6 | 1.09E-252 | -4.9 | 2.85E-235 |
| AT1G06040 | STO (SALT TOLERANCE) | -1.1 | 2.13E-28 | -1.5 | 1.78E-49 | -1.3 | 2.23E-38 | -1.1 | 8.10E-27 | -1 | 6.04E-26 | -1.5 | 2.41E-48 |
| AT2G14560 | LURP1 (LATE UPREGULATED IN RESPONSE TO HYALOPERONOSPORA PARASITICA) | -2.7 | 5.62E-112 | -3.4 | 1.13E-162 | -4.1 | 5.67E-173 | -3.3 | 1.62E-149 | -3.2 | 9.88E-155 | -2.2 | 5.56E-82 |
| AT5G10140 | FLOWERING LOCUS C | -1.2 | 2.18E-09 | -1.5 | 4.16E-14 | -1.4 | 5.23E-11 | -1.3 | 4.55E-10 | -1.1 | 8.80E-12 | -1.4 | 3.25E-08 |
| AT5G44420 | AN ETHYLENE- AND JASMONATE-RESPONSIVE PLANT DEFENSIN | -2.7 | 1.49E-54 | -6.1 | 2.23E-122 | -6 | 3.77E-111 | -6.1 | 3.17E-118 | -2.7 | 1.34E-55 | -3.6 | 7.78E-78 |
| AT3G57260 | PR2 (PATHOGENESIS-RELATED GENE 2) | -1.9 | 1.17E-30 | -5.3 | 4.64E-102 | -4.8 | 2.99E-86 | -4.4 | 4.08E-87 | -2 | 2.97E-36 | -2.2 | 8.40E-38 |
| AT3G45860 | RECEPTOR-LIKE PROTEIN KINASE | -1.5 | 6.64E-09 | -2.9 | 3.18E-24 | -2.9 | 3.03E-22 | -3 | 8.20E-25 | -1.5 | 4.12E-09 | -1.7 | 4.81E-10 |
| AT4G12490 | LIPID TRANSFER PROTEIN (LTP) | -1.3 | 7.93E-05 | -4.6 | 4.17E-43 | -2.8 | 8.38E-18 | -4.7 | 1.76E-45 | -1.9 | 1.32E-08 | -2.6 | 1.46E-15 |
| AT1G13470 | UNCHARACTERIZED | -2.1 | 2.12E-11 | -3.7 | 3.73E-29 | -3.3 | 2.67E-23 | -2.8 | 2.70E-18 | -2.1 | 6.88E-12 | -2.4 | 3.04E-14 |
| AT1G33960 | AVRRPT2-INDUCED GENE 1 | -1.5 | 6.39E-23 | -5.4 | 1.23E-127 | -5.7 | 9.38E-116 | -5.2 | 1.80E-119 | -2.2 | 7.45E-48 | -3.3 | 6.79E-81 |
| AT3G47480 | CALCIUM-BINDING EF-HAND FAMILY PROTEIN | -1.3 | 3.75E-11 | -3.0 | 3.27E-39 | -2.8 | 4.26E-33 | -3.3 | 6.57E-42 | -1.6 | 4.36E-16 | -2.2 | 5.55E-24 |
| AT4G03450 | ANKYRIN REPEAT FAMILY PROTEIN | -2.0 | 3.19E-10 | -3.4 | 9.96E-24 | -3.1 | 1.96E-20 | -3.2 | 1.03E-21 | -2.3 | 8.75E-13 | -2.1 | 1.90E-10 |
| AT4G23150 | CYSTEIN-RICH RLK | -1.7 | 5.61E-07 | -3.2 | 1.05E-20 | -2.9 | 5.61E-18 | -3.1 | 5.53E-20 | -2.1 | 1.08E-09 | -2.5 | 2.97E-13 |
| AT5G52760 | COPPER TRANSPORT PROTEIN FAMILY | -1.2 | 4.82E-05 | -2.6 | 2.28E-17 | -2.4 | 3.65E-14 | -2.7 | 1.53E-17 | -1.4 | 5.87E-07 | -2.2 | 2.08E-12 |
| AT5G54610 | BDA1 | -2.9 | 1.16E-25 | -4.2 | 8.48E-45 | -4.0 | 6.73E-39 | -3.7 | 2.34E-36 | -2.9 | 1.54E-26 | -2.2 | 4.99E-16 |
| AT5G10760 | AED1 | -1.7 | 2.88E-39 | -4.6 | 1.85E-126 | -4.2 | 6.95E-105 | -4.1 | 1.41E-112 | -2.1 | 1.09E-55 | -1.9 | 6.10E-45 |
| AT5G60900 | RLK1 | -1.1 | 9.02E-06 | -2.9 | 1.86E-24 | -2.3 | 1.51E-16 | -2.8 | 2.43E-22 | -1.3 | 1.25E-07 | -1.5 | 2.05E-09 |
| AT2G18660 | EGC2 | -1.6 | 2.23E-11 | -3.5 | 8.48E-35 | -3.9 | 7.62E-38 | -3.5 | 1.52E-33 | -2.3 | 8.03E-20 | -2.6 | 6.45E-22 |
| AT2G26400 | ARD1 | -2.0 | 2.90E-12 | -2.2 | 6.94E-15 | -2.9 | 2.32E-20 | -2.3 | 1.82E-15 | -2.4 | 2.33E-16 | -3.1 | 9.05E-23 |
| AT2G29120 | GLR2.7 | -1.2 | 6.10E-09 | -2.3 | 6.40E-25 | -1.8 | 1.01E-15 | -2.0 | 9.39E-20 | -1.0 | 1.54E-07 | -1.4 | 3.64E-11 |
| AT3G28510 | AAA-ATPase | -2.1 | 2.93E-23 | -1.4 | 2.97E-13 | -2.9 | 1.42E-32 | -1.8 | 1.70E-17 | -1.5 | 2.91E-14 | -2.2 | 6.87E-23 |
| AT1G52400 | BGLU18 | -1.4 | 1.35E-49 | -1.9 | 3.86E-91 | -1.8 | 1.92E-85 | -1.0 | 2.44E-28 | -1.1 | 2.69E-34 | -1.0 | 5.51E-28 |
| AT1G32960 | SBT3.3 | -1.4 | 5.56E-06 | -3.4 | 1.61E-25 | -3.5 | 3.54E-26 | -3.2 | 2.01E-22 | -2.0 | 6.04E-11 | -2.0 | 5.74E-10 |
| AT3G17609 | HYH | -1.7 | 1.92E-14 | -1.5 | 3.92E-12 | -1.6 | 8.78E-12 | -1.5 | 4.75E-11 | -1.5 | 1.75E-12 | -1.7 | 1.87E-13 |
Genes involved in the plant response to abiotic stress are also highlighted as down-regulated in Fig 4, although they did not map to specific pathways. Notably, the sphingolipid biosynthetic pathway itself has been linked to the abiotic stress response. Only one gene in this pathway, sphingosine kinase (AT4G21534), had significantly altered expression (down-regulated) in At2++ (see data deposited in GEO, Accession GSE129016). The protein product of this gene phosphorylates sphingosine (to S1P) and phytosphingosine (to phytoS1P) in plants [33], and increased levels of S1P and abscisic acid dependent stomatal closure have been reported in response to drought [34]. Knock-down of sphingosine kinase expression significantly decreased sensitivity to abscisic acid induced stomatal closure compared to Col0 [35], indicating that At2++ with reduced sphingosine kinase may be more sensitive to drought. One gene showed a negative correlation under GO term GO:009819 (drought recovery), a serine/theronine kinase (AT1G78290), a member of the SNF1-related protein kinase (SnRK2) family whose activity is activated by osmotic stress and dehydration [36]. Similarly, DREB genes have been implicated in the plant response to abiotic stress, including osmotic stresses such as high salinity and drought [37], and DREB1B and DREB1C were down-regulated in all the higher level over-expressors (At1-3++; Table 2). Together, these data place AtIPCS at the heart of the abiotic, as well as biotic, stress response.
Overall, these data show a complex picture of the modulation of the plant stress response on over-expression of AtIPCS isoforms. Some changes were relatively specific for an isoform, some showed dose response effects that correlated with AtIPCS expression levels, and all are genes modulated in response to biotic and abiotic stress.
Analyses of genes identified as responding positively to AtIPCS over-expression
Analyses of genes whose expression was positively correlated with AtIPCS1 over-expression (At1+ and At1++) revealed a significant enrichment (Fisher exact test, two tailed, p < 0.001) for those under GO term GO:0015979, photosynthesis (p = 9.10E-27) 25.7% (18/70) when they represent 0.43% of the Arabidopsis transcriptome (S4 Table). Similarly, for AtIPC2 over-expressor transgenic lines (At2+ and At2++) there was a significant enrichment (p = 1.70E-31) with 13.3% (30/226) of the genes associated under this GO term (S5 Table). AtIPC3 over-expressor lines (At3+ and At3++) also had a significant enrichment under photosynthesis (p = 6.00E-18)—34.5% (10/29). Other GO terms with significant enrichment for up-regulated genes in response to AtIPCS1, 2 or 3 over-expression include: GO:0010876 (lipid localization), GO:0006091 (generation of precursor metabolites and energy) and GO:0033036 (macromolecular localization) (S4–S6 Tables).
Utilising the differential over-expression in the analysed lines, the influence of AtIPCS1-3 expression levels on gene up-regulation was analysed. A dose dependent increase in the expression of up-regulated genes under GO term GO:0015979 (photosynthesis) was associated with the increase in AtIPCS1 and 2 transcript found in the transgenic lines (At1+ and At1++, and At2+ and At2++; Table 4). Those showing ≥2 log2 fold change dose response to both AtIPCS1 and AtIPCS2 are: YCF4, a PROTEIN REQUIRED FOR PHOTOSYSTEM I ASSEMBLY AND STABILITY (ATCG00520); a SUBUNIT OF THE CHLOROPLAST NAD(P)H DEHYDROGENASE COMPLEX, NDHI (ATCG01090); NADH DEHYDROGENASE ND1 NDHA (ATCG01100); PSAC SUBUNIT OF PHOTOSYSTEM I (ATCG01060); 49KDA PLASTID NAD(P)H DEHYDROGENASE SUBUNIT H PROTEIN (ATCG01110); NDHC, NADH DEHYDROGENASE D3 SUBUNIT OF THE CHLOROPLAST NAD(P)H DEHYDROGENASE COMPLEX (ATCG00440); YCF3, a PROTEIN REQUIRED FOR PHOTOSYSTEM I ASSEMBLY AND STABILITY (ATCG00360). All isoforms clearly influence the expression of the genes under this GO term and are therefore likely to influence photosynthesis itself. Over-expression of AtIPCS2 has the broadest and largest effect, correlating again with its status as the most abundant, and perhaps most important, AtIPCS isoform (approximately 100-fold AtIPCS1) in all tissues of wild type A. thaliana [4].
Table 4. Genes showing a positive correlation with AtIPCS expression under GO term GO:0015979 (photosynthesis).
At1-3+ over-expressing AtIPCS1-3; At1-3++ higher level expressers of AtIPCS1-3. Fold changes are the value of three technical triplicates of a transformed line. Transcripts listed had a p-value <0.001 (Wald test, cut off p-value < 0.05) and highlighted in bold log2 fold change ≥ 2.
| GeneID | Gene annotation | At1+ | At1++ | At2+ | At2++ | At3+ | At3++ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | ||
| ATCG00520 | ENCODES A PROTEIN REQUIRED FOR PHOTOSYSTEM I ASSEMBLY AND STABILITY | 1.3 | 0.000136 | 2 | 6.06E-09 | 2.4 | 3.56E-12 | 3.6 | 7.23E-26 | 2 | 1.23E-08 | ||
| ATCG01090 | ENCODES SUBUNIT OF THE CHLOROPLAST NAD(P)H DEHYDROGENASE COMPLEX NDHI | 1.5 | 1.14E-05 | 2.5 | 1.60E-13 | 2.7 | 1.01E-15 | 4 | 4.89E-33 | 2.2 | 6.40E-11 | ||
| ATCG00540 | ENCODES CYTOCHROME F APOPROTEIN | 1.1 | 0.00043 | 1.9 | 3.70E-09 | 2.2 | 1.72E-12 | 3.4 | 7.67E-27 | 1.7 | 3.65E-08 | ||
| ATCG01010 | CHLOROPLAST ENCODED NADH DEHYDROGENASE UNIT. NDHF | 2.2 | 2.06E-10 | 3.4 | 7.33E-22 | 1.8 | 2.59E-07 | ||||||
| ATCG00280 | CHLOROPLAST GENE ENCODING A CP43 SUBUNIT OF THE PHOTOSYSTEM II REACTION CENTER | 1.5 | 2.31E-06 | 1.6 | 1.08E-06 | 1.9 | 2.22E-09 | 3 | 1.05E-20 | 1.7 | 7.88E-08 | ||
| ATCG00680 | ENCODES FOR CP47, SUBUNIT OF THE PHOTOSYSTEM II REACTION CENTER | 1 | 0.000857 | 1.2 | 0.000146 | 1.5 | 8.93E-07 | 2.5 | 2.18E-15 | 1.3 | 2.90E-05 | ||
| AT4G28660 | PSB28 (PHOTOSYSTEM II REACTION CENTER PSB28 PROTEIN) | 1.6 | 6.17E-59 | 1.2 | 8.53E-37 | 0.5 | |||||||
| ATCG01100 | NADH DEHYDROGENASE ND1 NDHA | 1.6 | 2.92E-06 | 2.6 | 2.62E-15 | 2.9 | 1.82E-18 | 4 | 3.49E-33 | 2.4 | 9.53E-13 | ||
| AT3G04790 | RIBOSE 5-PHOSPHATE ISOMERASE-RELATED | 1.8 | 1.32E-101 | 1.3 | 6.95E-58 | 0.8 | 1.3 | 1.00E-52 | |||||
| ATCG00270 | PSII D2 PROTEIN PSBD | 1.8 | 5.79E-08 | 1.9 | 4.30E-09 | 2.4 | 7.28E-13 | 3.4 | 1.13E-25 | 2.1 | 3.43E-10 | ||
| ATCG01060 | ENCODES THE PSAC SUBUNIT OF PHOTOSYSTEM I | 1.3 | 5.19E-05 | 2.2 | 3.40E-12 | 2.6 | 8.05E-17 | 3.6 | 2.28E-30 | 2.1 | 1.50E-11 | ||
| AT3G55800 | SBPASE (SEDOHEPTULOSE-BISPHOSPHATASE); | 1.2 | 1.64E-100 | 1 | 4.49E-67 | 0.3 | |||||||
| AT4G09650 | ATPD (ATP SYNTHASE DELTA-SUBUNIT GENE); | 1.5 | 7.39E-119 | 1.2 | 3.91E-76 | 0.3 | 1.1 | 3.83E-57 | |||||
| ATCG00730 | A CHLOROPLAST GENE ENCODING SUBUNIT IV OF THE CYTOCHROME B6 | 1.3 | 2.93E-05 | 1.6 | 1.02E-06 | 1.9 | 4.38E-09 | 2.8 | 6.43E-19 | 1.6 | 3.63E-07 | ||
| ATCG01080 | NADH DEHYDROGENASE ND6 NDHG | 1.9 | 9.92E-08 | 3.1 | 5.52E-19 | 1.5 | 1.37E-05 | ||||||
| ATCG00710 | ENCODES A 8 KD PHOSPHOPROTEIN | 1 | 0.000866 | 1.2 | 1.80E-06 | 1.7 | 5.64E-08 | 2.8 | 1.16E-17 | 1.5 | 6.42E-06 | ||
| AT2G01590 | CRR3 (CHLORORESPIRATORY REDUCTION 3) | 1.2 | 1.06E-40 | 1 | 1.79E-31 | ||||||||
| ATCG00580 | PSII CYTOCHROME B559 | 1.1 | 0.0003654 | 1.3 | 8.33E-05 | 1.8 | 8.75E-09 | 2.4 | 2.18E-13 | 1.3 | 2.94E-05 | ||
| ATCG00720 | ENCODES THE CYTOCHROME B(6) SUBUNIT OF THE CYTOCHROME B6F COMPLEX | 1.1 | 0.0005436 | 1.4 | 5.73E-06 | 1.7 | 7.29E-08 | 2.9 | 4.62E-20 | 1.4 | 5.58E-06 | ||
| AT3G01440 | PSB-LIKE PROTEIN 2 (PQL2) | 1.8 | 7.06E-107 | 1.5 | 2.18E-13 | 0.5 | 4.69E-10 | 1.3 | 2.89E-50 | ||||
| AT1G14150 | PSB-LIKE PROTEIN 1 (PQL1) | 1.5 | 3.82E-75 | 1.2 | 1.54E-44 | 0.5 | 7.91E-09 | 1 | 4.06E-35 | ||||
| ATCG01110 | ENCODES THE 49KDA PLASTID NAD(P)H DEHYDROGENASE SUBUNIT H PROTEIN | 2.8 | 3.73E-19 | 4 | 4.72E-37 | 4.2 | 1.35E-40 | 4.7 | 5.23E-52 | 1 | 0.001626 | 3.6 | 5.95E-30 |
| AT1G19150 | LHCA6; CHLOROPHYLL BINDING PSI TYPE II CHLOROPHYLL A/B-BINDING PROTEIN | - | 1.2 | 2 | 2.50E-114 | 1.6 | 5.98E-73 | 0.7 | 1.71E-15 | 1.5 | 1.95E-66 | ||
| AT1G60950 | FED A; 2 IRON, 2 SULFUR CLUSTER BINDING | 1.6 | 1.03E-63 | 1.5 | 1.68E-54 | 1.2 | 1.99E-33 | ||||||
| ATCG00300 | ENCODES PSBZ, WHICH IS A SUBUNIT OF PHOTOSYSTEM II | 1.1 | 0.0022523 | 1.6 | 6.17E-06 | 1.7 | 8.98E-07 | 3.2 | 1.04E-19 | 1.6 | 2.72E-06 | ||
| ATCG00440 | ENCODES NADH DEHYDROGENASE D3 SUBUNIT OF THE CHLOROPLAST NAD(P)H DEHYDROGENASE COMPLEX NDHC | 1.3 | 0.0001679 | 2 | 3.59E-09 | 2.7 | 6.65E-16 | 3.4 | 4.14E-24 | 1.9 | 1.08E-08 | ||
| ATCG00340 | ENCODES THE D1 SUBUNIT OF PHOTOSYSTEM I AND II REACTION CENTERS. PSAB | 1.2 | 0.0001204 | 1.6 | 1.29E-06 | 1.8 | 1.35E-08 | 2.9 | 4.71E-19 | 1.6 | 1.30E-06 | ||
| ATCG00360 | ENCODES A PROTEIN REQUIRED FOR PHOTOSYSTEM I ASSEMBLY AND STABILITY | 1.5 | 6.99E-06 | 2.3 | 5.20E-12 | 2.7 | 1.25E-15 | 3.8 | 1.03E-29 | 2.1 | 4.94E-10 | ||
| AT3G16250 | NDF4 (NDH-DEPENDENT CYCLIC ELECTRON FLOW 1); | 1.4 | 1.02E-79 | 1.1 | 5.91E-52 | 0.4 | 1.70E-07 | ||||||
| AT3G62410 | PROTEIN BINDING CP12-2 ENCODES A SMALL PEPTIDE FOUND IN THE CHLOROPLAST STROMA | 1.8 | 5.62E-79 | 1.4 | 5.57E-49 | 0.3 | 0.006318 | 1.5 | 2.39E-51 | ||||
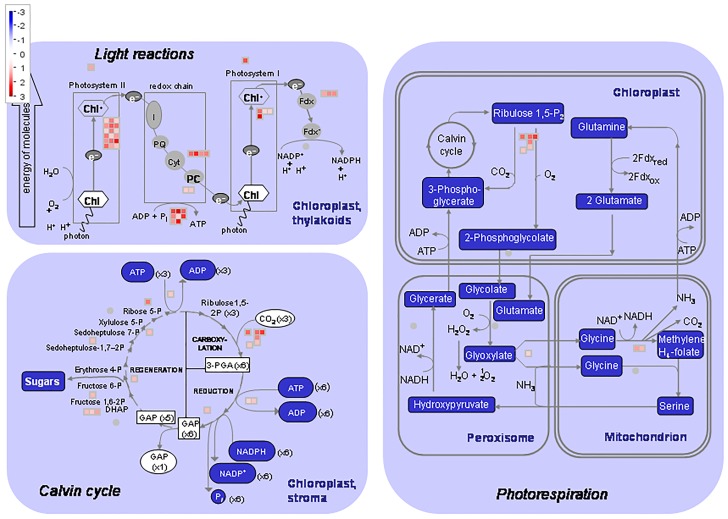
The effects of over-expression (At2++) were further analysed and visualised using MapMan (https://mapman.gabipd.org). This illustrated multiple positive transcriptional effects on genes associated with metabolism (S3 Fig), a greater effect than those negatively affected (S2 Fig). Amongst those particularly influenced were genes associated with the metabolism of light reactions and flavonoids. The effects clustered under light reactions correlated with the enrichment under GO term GO:0015979, photosynthesis discussed above. Further analyses using MapMan showed that genes up-regulated in At2++ had high enrichment under photorespiration, the Calvin cycle and light reactions (Fig 5). This indicated that there was an associated increase in energy production and, perhaps, the rate of growth.
Fig 5. MapMan schematic of showing genes identified as upregulated in the At2++ over-expression line enriched under the plant light reaction, Calvin cycle and photorespiration.
Log2 fold changes in gene expression are indicated by the colour scale.
The MapMan analyses (S3 Fig) also indicated an upregulation of flavonoid metabolism. Flavonoids are antioxidant molecules usually produced as a result of ROS accumulation in response to abiotic and biotic stress [38]. These data indicated that over-expression of AtIPCS2 may play a protective role in plant defense, not only as a negative regulator of plant pathogen defense genes, but also as a positive regulator of metabolites that have antioxidant properties.
After photosynthesis, the next best supported GO term for up-regulated genes across all AtIPCS isoform over-expressers was GO:0010876 (lipid localization; p = 8.20E-16, 1.80E-26 and 6.00E-18 for AtIPCS1, 2 and 3 respectively (S4–S6 Tables). The degree of enrichment across these genes (8/70, 17/275, and 7/19 for AtIPCS1, 2 and 3 respectively) was greater than that observed with GO:0015979 (photosynthesis); in addition, higher transcript log2 fold changes correlated with the rise in AtIPCS isoform expression levels (Table 5). Increased AtIPCS1 expression (At1+ to At1++; 200–390 fold wild type Col-0 (Fig 1A) saw ≥2 log2 increase in expression of the following genes under GO:0010876: OLEOSIN 1, 2 and 4 (OLEO1, 2 and 4; AT4G25140, AT5G40420 and AT3G27660); 2S SEED STORAGE PROTEIN 1–4 (AT4G27140, AT4G27150, AT4G27160 and AT4G27170); and LIPID TRANSFER PROTEIN (LPT; AT5G54740). All of these genes were also up-regulated in response to AtIPCS2 over-expression, including all 4 SEED STORAGE PROTEIN genes (AT4G27140, AT4G27150, AT4G27160 and AT4G27170). However, only isoforms 1, 3 and 4 increased ≥2 log2 in expression on further increase in AtIPCS2 expression (At2+ to At2++; 7–9 fold wild type Col-0; Fig 1B; Table 5). In addition, LIPID TRANSFER PROTEIN 4 (LTP4; AT5G59310); LIPID BINDING PROTEIN PREDICTED TO ENCODE A PR (PATHOGENESIS-RELATED) protein (LTP6; AT3G08770); and LIPID TRANSFER PROTEIN (LPT; AT5G55410 and AT2G37870) are all up-regulated ≥2 log2 on over-expression of this isoform. None of these are increased ≥2 log2 in the higher AtIPCS2 expressors, however LPT4 is decreased. As above, AtIPCS2 over-expression had the broadest effect on the selected genes (GO:0010876), again presumably due to its high levels in all tissues of wildtype A. thaliana [4]. All 4 SEED STORAGE PROTEIN genes, LTP (AT5G54740) and OLEO1 and 4, are up-regulated in response to AtIPCS3 over-expression. Furthermore, all are further up-regulated (≥2 log2) on increased expression (At3+ to At3++; 220–440 fold wild type Col-0; Fig 1C; Table 5). Although isoform specific effects, particularly with respect to AtIPCS2, were observed, over-expression of each lead to an up-regulation in expression of genes associated with GO term GO:0010876 (lipid localization). Analyses of the genes up-regulated in all over-expression lines demonstrated that they mainly encode seed storage proteins (Table 6), including CRUCIFERIN 2 and 3 genes (CRU2 and 3). CRU2 and 3 are expressed during the later stages of embryogenesis [39], with CRU3 having a role in protein and oil storage [40]. Together, these data indicated that the increased metabolic activity, perhaps induced by AtIPCS over-expression (S3 Fig), may result in an increase in protein and lipid storage during seed development.
Table 5. Genes showing a positive correlation with AtIPCS expression under GO term GO:0010876 (lipid localization).
At1-3 over-expressing AtIPCS1-3; At1-3+ higher level expressers of AtIPCS1-3. Fold changes are the value of three technical triplicates of a transformed line. Transcripts listed had a p-value <0.001 (Wald test, cut off p-value < 0.05) and highlighted in bold log2 fold change ≥ 2.
| GeneID | Gene annotation | At1+ | At1++ | At2+ | At2++ | At3+ | At3++ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | ||
| AT5G59310 | LTP4 (LIPID TRANSFER PROTEIN 4) | 3.7 | 2.07E-28 | 1 | 0.00358 | ||||||||
| AT5G48490 | DIR1 LIPID TRANSFER PROTEIN | 1.2 | 4.21E-16 | 1.3 | 4.78E-18 | ||||||||
| AT3G08770 | LTP6; LIPID BINDING PREDICTED TO ENCODE A PR (PATHOGENESIS-RELATED) PROTEIN | 2 | 3.96E-65 | 1.3 | 4.44E-27 | ||||||||
| AT5G40420 | OLEO2 (OLEOSIN 2) | 1.4 | 1.45E-07 | 4.6 | 3.63E-70 | 2.5 | 9.95E-21 | 2.8 | 5.74E-25 | ||||
| AT4G25140 | OLEO1 (OLEOSIN 1) | 3.5 | 4.38E-45 | 7.1 | 2.82E-192 | 4.6 | 2.85E-80 | 5 | 1.20E-91 | 2.5 | 1.01E-21 | 6.1 | 3.11E-141 |
| AT4G27140 | 2S SEED STORAGE PROTEIN 1 | 4.2 | 5.35E-65 | 8 | 1.03E-238 | 4.3 | 1.28E-68 | 6.3 | 1.95E-148 | 3 | 4.86E-32 | 7.2 | 6.10E-193 |
| AT4G27150 | 2S SEED STORAGE PROTEIN 2 | 3.8 | 2.16E-40 | 7.3 | 2.16E-147 | 4.7 | 1.09E-61 | 5.4 | 1.89E-81 | 2.3 | 8.24E-16 | 6.4 | 7.03E-115 |
| AT4G27160 | 2S SEED STORAGE PROTEIN 3 | 5 | 1.84E-81 | 8.5 | 2.51E-239 | 4.2 | 4.18E-58 | 6.3 | 1.28E-129 | 3.6 | 4.03E-43 | 7.3 | 2.59E-176 |
| AT5G64080 | LPT (LIPID TRANSFER PROTEIN) | 1.6 | 1.28E-30 | 1.2 | 3.85E-18 | ||||||||
| AT5G05960 | LPT (LIPID TRANSFER PROTEIN) | 1.7 | 4.62E-51 | 1.2 | 6.31E-26 | ||||||||
| AT3G18280 | LPT (LIPID TRANSFER PROTEIN) | 1.3 | 7.33E-28 | 1.1 | 3.46E-19 | ||||||||
| AT5G54740 | LPT (LIPID TRANSFER PROTEIN) | 5.1 | 3.42E-97 | 8.2 | 3.86E-259 | 5.8 | 1.57E-127 | 6.3 | 5.98E-153 | 3.9 | 4.20E-57 | 7.7 | 9.99E-230 |
| AT5G55410 | LPT (LIPID TRANSFER PROTEIN) | 2.2 | 4.37E-12 | 2 | 1.50E-09 | ||||||||
| AT4G27170 | 2S SEED STORAGE PROTEIN 4 | 2.7 | 1.03E-19 | 5.7 | 5.84E-88 | 1.8 | 6.48E-09 | 3.9 | 2.71E-39 | 1.8 | 6.80E-09 | 4.9 | 9.93E-64 |
| AT3G27660 | OLEO4 (OLEOSIN 4) | 2.1 | 1.35E-15 | 5.2 | 7.26E-100 | 2.8 | 6.81E-27 | 2.6 | 5.28E-23 | 1.1 | 8.65E-05 | 4 | 2.28E-58 |
| AT2G37870 | LPT (LIPID TRANSFER PROTEIN) | 2.9 | 2.88E-27 | 1.5 | 4.73E-08 | ||||||||
| AT3G01570 | OLE05 (OLEOSIN 5) | 1.5 | 8.26E-13 | 1.3 | 1.92E-08 | ||||||||
Table 6. Genes up-regulated in all three AtIPCS over-expression lines compared to Col0.
At1-3+ over-expressing AtIPCS1-3; At1-3++ higher level expressers of AtIPCS1-3. Fold changes are the value of three technical triplicates of a transformed line. Transcripts listed had a p-value <0.001 (Wald test, cut off p-value < 0.05).
| GeneID | Gene annotation | At1+ | At1++ | At2+ | At2++ | At3+ | At3++ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | log2fold | p-value | ||
| AT1G03880 | CRUCIFERIN 2 | 2.4 | 1.77E-17 | 6.9 | 2.47E-149 | 2.8 | 1.65E-22 | 4.4 | 2.05E-59 | 1.9 | 1.19E-10 | 5.5 | 6.22E-96 |
| AT1G14940 | POLYKETIDE CYCLASE | 1.3 | 0.00013457 | 3.9 | 3.80E-37 | 2.9 | 1.09E-19 | 1.2 | 0.000415868 | 1.2 | 0.000363067 | 3.9 | 5.89E-37 |
| AT1G68250 | UNCHARACTERIZED | 3.3 | 1.29E-35 | 5.5 | 4.67E-100 | 3.3 | 8.42E-35 | 4.7 | 2.00E-72 | 1.6 | 1.42E-08 | 4.7 | 1.07E-72 |
| AT1G75830 | PDF1.1 | 2.7 | 3.86E-42 | 6.1 | 4.61E-234 | 4.3 | 6.31E-116 | 4.1 | 6.34E-102 | 1.6 | 6.48E-15 | 5.3 | 1.15E-174 |
| AT4G27150 | 2S SEED STORAGE PROTEIN 2 | 3.8 | 2.16E-40 | 7.3 | 2.16E-147 | 4.7 | 1.09E-61 | 5.4 | 1.89E-81 | 2.3 | 8.24E-16 | 6.4 | 7.03E-115 |
| AT2G27380 | EPR1 | 2.9 | 3.08E-31 | 6.0 | 6.18E-135 | 5.1 | 6.03E-100 | 2.9 | 4.27E-33 | 1.5 | 4.85E-09 | 5.6 | 8.36E-120 |
| AT3G27660 | OLEO4 (OLEOSIN 4) | 2.1 | 1.35E-15 | 5.2 | 7.26E-100 | 2.8 | 6.81E-27 | 2.6 | 5.28E-23 | 1.1 | 8.65E-05 | 4.0 | 2.28E-58 |
| AT4G25140 | OLEO1 (OLEOSIN 1) | 3.5 | 4.38E-45 | 7.1 | 2.82E-192 | 4.6 | 2.85E-80 | 5.0 | 1.20E-91 | 2.5 | 1.01E-21 | 6.1 | 3.11E-141 |
| AT4G28520 | CRU3 | 5.8 | 9.91E-131 | 9.6 | 0 | 6.0 | 5.56E-142 | 7.3 | 9.09E-207 | 4.2 | 6.51E-67 | 8.3 | 2.55E-271 |
| AT5G44120 | CRA1 | 5.6 | 2.15E-133 | 9.0 | 0 | 6.8 | 2.63E-201 | 6.1 | 5.74E-158 | 3.6 | 1.13E-55 | 7.9 | 9.89E-267 |
| AT3G22640 | PAP85 | 3.2 | 2.74E-52 | 5.5 | 5.80E-166 | 3.2 | 9.47E-51 | 2.6 | 1.55E-32 | 1.5 | 6.57E-11 | 4.1 | 4.59E-89 |
| AT4G27160 | 2S SEED STORAGE PROTEIN 3 | 5.0 | 1.84E-81 | 8.5 | 2.51E-239 | 4.2 | 4.18E-58 | 6.3 | 1.28E-129 | 3.6 | 4.03E-43 | 7.3 | 2.59E-176 |
| AT4G27140 | 2S SEED STORAGE PROTEIN 1 | 4.2 | 5.35E-65 | 8.0 | 1.03E-238 | 4.3 | 1.28E-68 | 6.3 | 1.95E-148 | 3.0 | 4.86E-32 | 7.2 | 6.10E-193 |
| AT4G27170 | 2S SEED STORAGE PROTEIN 4 | 2.7 | 1.03E-19 | 5.7 | 5.84E-88 | 1.8 | 6.48E-09 | 3.9 | 2.71E-39 | 1.8 | 6.80E-09 | 4.9 | 9.93E-64 |
| AT5G54740 | LPT (LIPID TRANSFER PROTEIN) | 5.1 | 3.42E-97 | 8.2 | 3.86E-259 | 5.8 | 1.57E-127 | 6.3 | 5.98E-153 | 3.9 | 4.20E-57 | 7.7 | 9.99E-230 |
Phenotype of Arabidopsis over-expressing AtIPCS isoforms
To examine the effects of these global alterations in gene expression on plant development, the phenotypes of A. thaliana over-expressing each of the AtIPCS isoforms (both levels) were analysed. All lines showed early flowering (4 days earlier than wild type Col-0) associated with the formation of bolts (Fig 6). This correlated with a slight (<2-fold log2) up-regulation of the florigen FT (see data deposited in GEO, Accession GSE129016), a well characterized systematic signal for plant transition from the vegetative to the reproductive (flowering) phase [41]. However, the mechanism underlying this phenotype remains unclear, and perhaps reflects the metabolic changes indicated in the analyses above.
Fig 6.
At 44 days wild type Col-0 had flowered (A and B). The AtIPCS over-expressing lines had also flowered at this time point, however all had bolted: (C) At1+; (D) At1++; (E) At2+; (F) At2++; (G) At3+; (H) At3++.
Reflecting the broad negative regulatory effect AtIPCS over-expression has on biotic and abiotic stress responses in Arabidposis (transcriptomic data—Table 2 and Fig 4), the phenotypes of Arabidopsis At1++, At2++ and At3++ were observed under osmotic (abiotic) and pathogen (biotic) stress. Firstly, the over-expressing lines were analysed for tolerance to osmotic stress using the non-ionic osmolyte, mannitol (S4 Fig). In agreement with the down-regulation of genes involved in the abiotic stress response (Table 2; Fig 4), At1++, At2++ and At3++ over-expressing lines were all more susceptible to osmotic stress at high concentrations of mannitol (500mM).
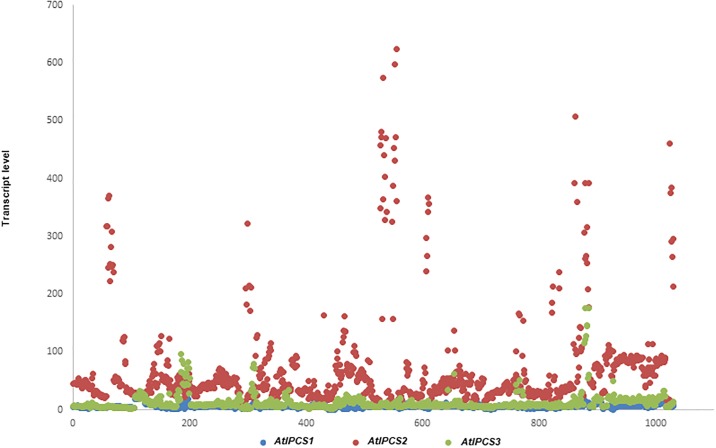
Subsequently, the phenotype of the pathogen response was assessed. Previously, a specific role for AtIPSC2 in plant resistance to biotrophic pathogens (powdery mildew, G. cichoracearum) was proposed. A homozygote AtIPSC2 T-insert mutant showed a reduction in fungal mass compared with a G. cichoracearum infected control, whereas resistance to the hemibiotrophic pathogen Pseudomonas syringae was unaffected [13]. The AtIPSC2 T-insert mutant resistance to G. cichoracearum was associated with increased PR1 [13], whereas as we demonstrated that AtIPCS over-expression reduced PR1 (and PR2) expression. This places AtIPCS at the centre of the biotic stress response. Therefore, to examine the potential role of AtIPCS in the necrotophic pathogen response, Arabidopsis At1++, At2++ and At3++ were challenged with Erwinia amylovora. Interestingly, based on the spread of the pathogen across the surface of the leaves, At2++ and At3++, but not At1++, were less susceptible than the Col0 controls (S5 Fig). At first glance these results appear counter intuitive, the over-expression lines showing down-regulation of the biotic response transcriptome but phenotypically showing a rise in pathogen resistance. Therefore, these data were considered in light of the expression data available in Genevestigator for AtIPCS1-3 (Fig 7). Response to various stimuli is observed for AtIPCS2 with a fold increase of up to 600 compared to the highest 100 fold increase observed for AtIPCS1 during developmental leaf senescence [42]. The highest increase in AtIPCS2 transcript levels was found to be in response to plants treated with ozone to activate apoplastic reactive oxygen species (ROS) signalling, correlating with the increase in transcript associated with antioxidant flavonoid metabolism seen in At2++ (S3 Fig). AtIPCS1 also showed an increase in the transcript, although 40-fold lower than AtIPCS2 [43]. Notably, AtIPCS1 and 2 transcript increases were also observed in ozone tolerant plants [44]. Other elicitors of AtIPCS2 transcript increase include the fungi Botryris cinereal [45] and Blumeria graminis [46], the bacterium P. syringae [47], and bacterial flagellin protein [48]. Therefore, increased expression of AtIPCS2 (and perhaps AtIPCS1 and 3) is part of the response to necrotropic, biotrophic and hemibiotropic pathogens. All this in a background of the suppression of the biotic stress response at the transcriptomic level.
Fig 7. Predicted AtIPCS1-3 expression in response to pathogens, pathogen effectors and chemical stimuli.
x-axis: experiment number; y-axis: transcript level. Produced from RNA-Seq data using Genevestigator.
Discussion
Each of the 3 IPCS isoforms are differentially expressed in the tissues of A. thaliana [4], and in Oryza sativa (rice) IPCS expression in response to specific abiotic stimulus is tissue specific [15]. To further probe the downstream effects of IPCS, in this study we analysed the transcriptomic response to the over-expression of AtIPCS1, 2 and 3.
Multiple genes responded both positively and negatively, and specifically, in response to elevated AtIPCS1, 2 and 3. Analyses of genes up-regulated in response to AtIPCS over-expression showed most enrichment under GO term GO:0010876 (lipid localization), with levels showing strong correlation with increased expression of AtIPCS1 and 3, indicating a regulatory (rheostat) function (Table 5). Specifically, 2S SEED STORAGE PROTEIN 1, 2, 3 and 4 genes, LIPID TRANSFER PROTEIN gene (LPT; AT5G54740) and OLEOSIN 1, 2 and 4 genes (OLEO1, 2 and 4) are up-regulated in response in AtIPCS1, and further up-regulated with increased expression. In addition, all apart from OLEO2 responded similarly to AtIPCS3 (Table 5), and all were up-regulated in response to AtIPCS2 over-expression. Several other LPT genes also positively responded to AtIPCS2 over-expression. OLE genes encode oleosins that prevent the abnormal fusion of oil bodies in seeds during imbibition and thereby protect the seeds from undergoing mechanical stress that would result in mortality [49]. Also upregulated were the 2S SEED STORAGE PROTEIN genes which act as nitrogen and sulphur reserves for seeds during germination [50]. Both were particularly influenced by the expression levels of AtIPCS1 and 3 (Table 5) suggesting these isoforms have a role in seed development. A small number of genes were enriched under GO term GO:000979 (post-embryonic development) and up-regulated in response to AtIPCS1 and 3 (S4 and S6 Tables). An shown in S7 Table, the effects seen with AtIPCS1 and 3 were again magnified on increased expression. Notably, in addition to those genes already discussed, up-regulated CRUCIFERIN 2 and 3 genes (CRU2 and 3) are seed storage proteins expressed during the later stages of embryogenesis [39] with CRU3 having a role in protein and oil storage [40]. From these data, it appears that AtIPCS is involved in regulating protein and lipid storage in seeds. This potential role is further supported by the observation that 1-CYSTEIN PEROXIREDOXIN 1 (AtPER1) and EXTENSIN PROLINE-RICH 1 (AtEPR1) are also up-regulated in response to AtIPCS. Both are expressed in the embryo and in developing seeds, providing protection from ROS during seed desiccation [51, 52]. EMBRYOGENIC CELL PROTEIN 31 gene (AtECP31) and CALEOSIN PROTEIN gene 1 (AtCOL1), both expressed in the later stages of seed development, are important for seed viability and desiccation tolerance [53, 54]. Therefore, AtIPCS may be important in the protection, viability and therefore the germination of seeds. The mechanisms behind this are not known, however the function of these enzymes in regulating ceramide and phytoceramide levels point towards multiple roles in the signal transduction networks underlying development [5].
The next most enriched genes up-regulated in response to AtIPCS over-expression were under GO term GO:0015979 (photosynthesis). The importance of this in relation to IPCS functionality is unclear. However, the most compelling change in transcript levels was seen under the influence of AtIPCS2 over-expression; whilst AtIPCS3 appeared to function as a rheostat (Table 4) with increased expression inducing the up-regulation of all 6 genes influenced (≥2 log2). Notably this isoform is least expressed in rosette and cauline leaves of A. thaliana [4] perhaps indicating a role of up-regulation in photosynthetic regulation. However, the mechanism behind this possible function is unclear.
To further visualise the possible effects of AtIPCS over-expression, and given the scale and scope of its influence on the transcriptome, the AtIPCS2 higher level over-expressor (At2++) data was analysed using MapMan (S3 Fig). The expression of many genes associated with metabolism were positively influenced, particularly those associated light reactions and antioxidative flavonoids. Further analyses showed up-regulated genes to be enriched under photorespiration, the Calvin cycle and light reactions (Fig 5), perhaps positively influencing energy production and the rate of growth. Interestingly, all AtIPCS over-expressing lines showed bolts (Fig 6) perhaps indicating accelerated growth, however this requires further analysis.
Flavonoid metabolism was also up-regulated in At2++ Arabidopsis at the transcriptome level, and these antioxidant molecules are synthesized to protect plant tissues from ROS produced in response to abiotic and biotic stress [38]. Therefore, over-expression of AtIPCS2 may also have a protective role in plant defense, a stress response. Interestingly, and perhaps correlating with this, on over-expression of each of the isoforms down-regulated genes were significantly enriched under GO term GO:0006950 (response to stress) (S1–S3 Tables). These included Pathogenesis-Related (PR) genes, PR1 and 2 were particularly effected and transcript levels were reduced ≥2 log2 (Table 2), this effect was magnified on increased expression of AtIPCS1 suggesting that this isoform may have a regulatory role in the stress response. (Table 2). Systematic Acquired Resistance (SAR) is characterised by the increased expression of the PR genes which are induced in response to elevated endogenous growth hormones such as salicylic acid (SA) and ethylene (ET), the levels of which increase in response to infection [55]. A. thaliana manipulated to express elevated levels of PR1, 2 and 5 are resistant to the oomycete obligate biotroph Hyaloperonospora parasitica and the bacterium biotroph Pseudomonas syringae pv. Maculicola [56]. Furthermore, PR genes are also induced by environmental stress such as cold and light [57]; PR1, PR2 and PR5 were induced in cold treated and drought stressed A. thaliana [58, 59].
Plant Defensin genes, PDF1.2B and an ET- AND JA-RESPONSIVE PLANT DEFENSIN, are similarly repressed in response to over-expression of AtIPCS1, 2 and 3, and again the effect was magnified on increased expression of (rheostatic) AtIPCS1 (Table 2). Like the PR genes, PDF genes are markers of SAR induced by endogenous growth hormones in response to biotic and abiotic stress [55]. Wound associated TYROSINE AMINO TRANSFERASE 3 (TAT3) was also induced in A. thaliana in response to an endogenous growth hormone (JA) [60], and was down-regulated in response to all isoforms but with a magnified effect on increased expression of AtIPCS1 (Table 2). LATE UP-REGULATED IN RESPONSE TO HYALOPERONOSPORA PARASITICA (LURP1) was down-regulated in response to over-expression of all isoforms and, as the name suggests, has been shown to be needed for basal resistance to the oomycete Hyaloperonospora parasitic [61]. More specifically PHOSPHOLIPASE 2A (PLA2A) expression is negatively regulated only by over-expression of AtIPCS2. An orthologue of this enzyme is induced in mosaic virus infected tobacco leaves independently of the growth hormone JA [62] (Table 2).
Similarly, in relation to abiotic stress response, MYB112, which is induced in salt stressed plants [63], responded specifically and negatively to AtIPC2 over-expression. CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and GIGANTEA (GI), which are part of the photoperiodic control of flowering [64, 65], are specifically negatively regulated by over-expression of AtIPCS2 and 3, and AtIPCS2 respectively (Table 2). Notably, in support of these effects, the AtIPCS over-expressing lines displayed an early flowering phenotype.
These data indicating the role of AtIPCS over-expression in the suppression of biotic and, to a lesser extent, abiotic stress responses, are supported by the MapMan analyses (Fig 4). At a phenotypic level, the transcriptomic findings correlate with a decreased tolerance for osmotic (abiotic) stress, albeit only at high concentrations of the non-ionic osmolyte, mannitol (S5 Fig). However, the relative resistance of the AtIPCS2 and 3 over-expressor lines (At2++ and At3++) to challenge with the necrotroph Erwinia amylovora (S4 Fig) is difficult to reconcile with the transcriptomic data showing down-regulation of the pathogen response. However, Genevestigator analyses (Fig 7) indicated that increased expression of AtIPCS2 (and perhaps AtIPCS1 and 3) is part of the response to necrotropic, biotrophic and hemibiotropic pathogens. The mode of this in biotic stress response is unclear, however Genevestigator showed that the AtIPCS2 transcript level positively correlated with ROS signalling, as could the indicated increase in antioxidant flavonoid metabolism noted in this work (S3 Fig). Clearly this warrants further investigation, however it notable that the response to E. amylovora, and other pathogens, in Arabidopsis includes ROS [66].
Together these data suggest some specificity in the influence of each AtIPCS isoform and that AtIPCS1 expression may act as a rheostat of SAR and the response to biotic and abiotic stress. Furthermore, the observation that AtIPCS expression negatively influences both growth hormone dependent (e.g. PR) and independent responses (PL2A) indicated its role in a wide variety of defence networks perhaps reflecting the role of phytoceramide as an indiscriminate pro-apoptotic signal [13].
Conclusion
Transcriptomic analyses of A. thaliana indicated that AtIPCS1-3 over-expression positively correlated with the expression of genes encoding storage proteins essential for normal seed development (S7 Table). As such, the enzyme may be crucial for seed survival, maturation and germination. Furthermore, these data also indicated that AtIPCS acts as a negative regulator of the plant defense response to pathogens and abiotic stress (Tables 2–4), a process associated with PCD. Importantly, these findings were also corroborated by data available from Genevestigator (Fig 7) and phenotypic observations (S4 and S5 Figs).
The negative association of biotic and abiotic stress responses to AtIPCS expression indicates the potential to engineer tolerance in crop plants.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
At1-3+ over-expressing AtIPCS1-3; At1-3++ higher level expressers of AtIPCS1-3. Log2 change ≥1 shown, ≥2 in bold.
(TIF)
Relative quantitation was done after normalization using PEX4 levels; relative quantitation value is the mean of three biological replicates with standard error.
(TIF)
Log2 fold changes in gene expression are indicated by the colour scale, with the distribution of genes in different pathways and expression levels shown. Abbreviations: carbohydrates (CHO), tricarboxylic acid (TCA) cycle, oxidative pentose phosphate (OPP) pathway, sulphur containing glucosinates synthesis (S-misc), nitrogen containing glucosinate synthesis (N-misc).
(TIF)
Log2 fold changes in gene expression are indicated by the colour scale, with the distribution of genes in different pathways and expression levels shown. Abbreviations: carbohydrates (CHO), tricarboxylic acid (TCA) cycle, oxidative pentose phosphat pathway, sulphur containing glucosinates synthesis (S-misc), nitrogen containing glucosinate synthesis (N-misc).
(TIF)
Col0 and overexpressing lines At1++, At2++ and At3++. Mannitol concentrations in mM. At the highest concentration (500mM) chlorosis in the over-expressing lines, but not Col0, was apparent.
(TIF)
(A) Col0 (B) At1++ (C) At2++ (D) At3++ (E) Plot of ratio of area infected by Erwinia amylovora to uninfected area for Col0 and over-expression lines. AtIPCS2 and 3 over-expressors are less susceptible to the pathogen compared to Col0 and AtIPCS1 over-expressor, based on the spread of the pathogen across the surface of the leaf. The pathogen has spread to occupy a large area of Col0 and AtIPCS1 over-expressor leaves, compared to a less aggressive spread seen on the leaves of AtIPCS2 and 3 over-expressors. In addition, a distinctive yellow colour is observed at the outer boundaries of the area occupied by the pathogen, indicating a measured response that favours plant survival. These observations may be linked to the role of AtIPCS as a negative regulator of the plant response to biotic stress.
(TIF)
Acknowledgments
We gratefully acknowledge the funding provided by the British Biotechnology Research Council (BB/K012703/1) and the collaboration with Bayer AG Crop Sciences. Special thanks goes to Dr Mag Leighton for critical input.
Data Availability
All transcriptomic data used in the study are publicly available in GEO (GEO accession number GSE129016).
Funding Statement
Funding was provided by the British Biotechnology Research Council (BB/K012703/1). The funder provided support in the form of salaries for authors [ECP], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Bayer Crop Sciences provided support in the form of salaries for authors [RS] and was involved in the study design and data collection, but did not have any additional role in the decision to publish or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Agricultural Development Economics Division FaAo, United Nations. How to feed the world 2050—High level expert forum. 2009.
- 2.United Nations DoEaSA, Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Tables ESA/P/WP/248. 2017.
- 3.Lynch DV, Dunn TM. An introduction to plant sphingolipids and a review of recent advances in understanding their metabolism and function. New phytologist. 2004;161(3):677–702. [DOI] [PubMed] [Google Scholar]
- 4.Mina J, Okada Y, Wansadhipathi-Kannangara N, Pratt S, Shams-Eldin H, Schwarz R, et al. Functional analyses of differentially expressed isoforms of the Arabidopsis inositol phosphorylceramide synthase. Plant molecular biology. 2010;73(4–5):399–407. 10.1007/s11103-010-9626-3 [DOI] [PubMed] [Google Scholar]
- 5.Young SA, Mina JG, Denny PW, Smith TK. Sphingolipid and ceramide homeostasis: potential therapeutic targets. Biochem Res Int. 2012;2012:248135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markham JE, Jaworski JG. Rapid measurement of sphingolipids from Arabidopsis thaliana by reversed‐phase high‐performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21(7):1304–14. 10.1002/rcm.2962 [DOI] [PubMed] [Google Scholar]
- 7.Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT. Ceramides modulate programmed cell death in plants. Genes & development. 2003;17(21):2636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich CR, Han G, Chen M, Berg RH, Dunn TM, Cahoon EB. Loss-of-function mutations and inducible RNAi suppression of Arabidopsis LCB2 genes reveal the critical role of sphingolipids in gametophytic and sporophytic cell viability. The Plant Journal. 2008;54(2):284–98. 10.1111/j.1365-313X.2008.03420.x [DOI] [PubMed] [Google Scholar]
- 9.Coupe SA, Watson LM, Ryan DJ, Pinkney TT, Eason JR. Molecular analysis of programmed cell death during senescence in Arabidopsis thaliana and Brassica oleracea: cloning broccoli LSD1, Bax inhibitor and serine palmitoyltransferase homologues. Journal of Experimental Botany. 2004;55(394):59–68. 10.1093/jxb/erh018 [DOI] [PubMed] [Google Scholar]
- 10.Dutilleul C, Benhassaine-Kesri G, Demandre C, Rézé N, Launay A, Pelletier S, et al. Phytosphingosine-phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. New Phytologist. 2012;194(1):181–91. 10.1111/j.1469-8137.2011.04017.x [DOI] [PubMed] [Google Scholar]
- 11.Berkey R, Bendigeri D, Xiao S. Sphingolipids and plant defense/disease: the "death" connection and beyond. Front Plant Sci. 2012;3(68):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bromley PE, Li YO, Murphy SM, Sumner CM, Lynch DV. Complex sphingolipid synthesis in plants: characterization of inositolphosphorylceramide synthase activity in bean microsomes. Archives of Biochemistry and Biophysics. 2003;417(2):219–26. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Yang X, Tangchaiburana S, Ndeh R, Markham JE, Tsegaye Y, et al. An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. The Plant Cell. 2008;20(11):3163–79. 10.1105/tpc.108.060053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tartaglio V, Rennie EA, Cahoon R, Wang G, Baidoo E, Mortimer JC, et al. Glycosylation of inositol phosphorylceramide sphingolipids is required for normal growth and reproduction in Arabidopsis. The Plant Journal. 2017;89(2):278–90. 10.1111/tpj.13382 [DOI] [PubMed] [Google Scholar]
- 15.Liao P, Huang J, Tong P, Nie W, Yan X, Feng Y, et al. Characterization and expression analysis of inositolphosphorylceramide synthase family genes in rice (Oryza sativa L.). Genes & Genomics. 2017;39(5):485–92. [Google Scholar]
- 16.Karimi M, Inzé D, Depicker A. GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends in plant science. 2002;7(5):193–5. [DOI] [PubMed] [Google Scholar]
- 17.Clough SJ, Bent AF. Floral dip: a simplified method forAgrobacterium-mediated transformation ofArabidopsis thaliana. The plant journal. 1998;16(6):735–43. [DOI] [PubMed] [Google Scholar]
- 18.Ali HZ, Harding CR, Denny PW. Endocytosis and Sphingolipid Scavenging in Leishmania mexicana Amastigotes. Biochem Res Int. 2012;2012:691363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alqaisi AQI, Mbekeani AJ, Llorens MB, Elhammer AP, Denny PW. The antifungal Aureobasidin A and an analogue are active against the protozoan parasite Toxoplasma gondii but do not inhibit sphingolipid biosynthesis. Parasitology. 2018;145(2):148–55. 10.1017/S0031182017000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobin A, Gingeras TR. Mapping RNA-seq Reads with STAR. Current protocols in bioinformatics. 2015:11.4 1-.4. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–9. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biology. 2014;15(12):550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nature methods. 2015;12(2):115–21. 10.1038/nmeth.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ihaka R, Gentleman R. R: a language for data analysis and graphics. Journal of computational and graphical statistics. 1996;5(3):299–314. [Google Scholar]
- 27.Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic acids research. 2010;38(suppl_2):W64–W70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, et al. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal. 2004;37(6):914–39. [DOI] [PubMed] [Google Scholar]
- 29.Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, et al. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Advances in bioinformatics. 2008;2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penninckx I, Eggermont K, Terras F, Thomma B, De Samblanx GW, Buchala A, et al. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. The Plant Cell. 1996;8(12):2309–23. 10.1105/tpc.8.12.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Zhang Y, Ding P, Johnson K, Li X, Zhang Y. The ankyrin-repeat transmembrane protein BDA1 functions downstream of the receptor-like protein SNC2 to regulate plant immunity. Plant physiology. 2012;159(4):1857–65. 10.1104/pp.112.197152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breitenbach HH, Wenig M, Wittek F, Jorda L, Maldonado-Alconada AM, Sarioglu H, et al. Contrasting roles of apoplastic aspartyl protease AED1 and legume lectin-like protein LLP1 in Arabidopsis systemic acquired resistance. Plant Physiology. 2014:pp. 114.239665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coursol S, Fan L-M, Le Stunff H, Spiegel S, Gilroy S, Assmann SM. Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature. 2003;423(6940):651–4. 10.1038/nature01643 [DOI] [PubMed] [Google Scholar]
- 34.Egan JF, Maxwell BD, Mortensen DA, Ryan MR, Smith RG. 2, 4-dichlorophenoxyacetic acid (2, 4-D)–resistant crops and the potential for evolution of 2, 4-D–resistant weeds. Proceedings of the National Academy of Sciences. 2011;108(11):E37–E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worrall D, Liang YK, Alvarez S, Holroyd GH, Spiegel S, Panagopulos M, et al. Involvement of sphingosine kinase in plant cell signalling. The Plant Journal. 2008;56(1):64–72. 10.1111/j.1365-313X.2008.03579.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizoguchi M, Umezawa T, Nakashima K, Kidokoro S, Takasaki H, Fujita Y, et al. Two closely related subclass II SnRK2 protein kinases cooperatively regulate drought-inducible gene expression. Plant Cell Physiol. 2010;51(5):842–7. 10.1093/pcp/pcq041 [DOI] [PubMed] [Google Scholar]
- 37.Lata C, Prasad M. Role of DREBs in regulation of abiotic stress responses in plants. Journal of experimental botany. 2011;62(14):4731–48. 10.1093/jxb/err210 [DOI] [PubMed] [Google Scholar]
- 38.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Current opinion in plant biology. 2002;5(3):218–23. [DOI] [PubMed] [Google Scholar]
- 39.Pang PP, Pruitt RE, Meyerowitz EM. Molecular cloning, genomic organization, expression and evolution of 12S seed storage protein genes of Arabidopsis thaliana. Plant Molecular Biology. 1988;11(6):805–20. 10.1007/BF00019521 [DOI] [PubMed] [Google Scholar]
- 40.Lin Y, Pajak A, Marsolais F, McCourt P, Riggs CD. Characterization of a cruciferin deficient mutant of Arabidopsis and its utility for overexpression of foreign proteins in plants. PLoS One. 2013;8(5):e64980 10.1371/journal.pone.0064980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araki T. Transition from vegetative to reproductive phase. Curr Opin Plant Biol. 2001;4(1):63–8. [DOI] [PubMed] [Google Scholar]
- 42.Brusslan JA, Bonora G, Rus-Canterbury AM, Tariq F, Jaroszewicz A, Pellegrini M. A genome-wide chronological study of gene expression and two histone modifications, H3K4me3 and H3K9ac, during developmental leaf senescence. Plant physiology. 2015;168(4):1246–61. 10.1104/pp.114.252999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu E, Vaahtera L, Brosché M. Roles of defense hormones in the regulation of ozone-induced changes in gene expression and cell death. Molecular plant. 2015;8(12):1776–94. 10.1016/j.molp.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 44.Xu E, Vaahtera L, Horak H, Hincha DK, Heyer AG, Brosche M. Quantitative trait loci mapping and transcriptome analysis reveal candidate genes regulating the response to ozone in Arabidopsis thaliana. Plant, cell & environment. 2015;38(7):1418–33. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Kracher B, Ziegler J, Birkenbihl RP, Somssich IE. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100. Elife. 2015;4:e07295 10.7554/eLife.07295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maekawa T, Kracher B, Vernaldi S, van Themaat EVL, Schulze-Lefert P. Conservation of NLR-triggered immunity across plant lineages. Proceedings of the National Academy of Sciences. 2012;109(49):20119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howard BE, Hu Q, Babaoglu AC, Chandra M, Borghi M, Tan X, et al. High-throughput RNA sequencing of pseudomonas-infected Arabidopsis reveals hidden transcriptome complexity and novel splice variants. PLoS One. 2013;8(10):e74183 10.1371/journal.pone.0074183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, et al. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Molecular plant. 2008;1(3):423–45. 10.1093/mp/ssn019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimada TL, Shimada T, Takahashi H, Fukao Y, Hara-Nishimura I. A novel role for oleosins in freezing tolerance of oilseeds in Arabidopsis thaliana. The Plant Journal. 2008;55(5):798–809. 10.1111/j.1365-313X.2008.03553.x [DOI] [PubMed] [Google Scholar]
- 50.Krebbers E, Herdies L, De Clercq A, Seurinck J, Leemans J, Van Damme J, et al. Determination of the processing sites of an Arabidopsis 2S albumin and characterization of the complete gene family. Plant Physiology. 1988;87(4):859–66. 10.1104/pp.87.4.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dietz K-J. Plant peroxiredoxins. Annual review of plant biology. 2003;54(1):93–107. [DOI] [PubMed] [Google Scholar]
- 52.Stacy RA, Nordeng TW, Culiáñez-Macià FA, Aalen RB. The dormancy-related peroxiredoxin anti-oxidant, PER1, is localized to the nucleus of barley embryo and aleurone cells. The Plant Journal. 1999;19(1):1–8. [DOI] [PubMed] [Google Scholar]
- 53.Poxleitner M, Rogers SW, Lacey Samuels A, Browse J, Rogers JC. A role for caleosin in degradation of oil-body storage lipid during seed germination. The Plant Journal. 2006;47(6):917–33. 10.1111/j.1365-313X.2006.02845.x [DOI] [PubMed] [Google Scholar]
- 54.Yang H, Saitou T, Komeda Y, Harada H, Kamada H. Late embryogenesis abundant protein in Arabidopsis thaliana homologous to carrot ECP31. Physiologia Plantarum. 1996;98(3):661–6. [Google Scholar]
- 55.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. 10.1146/annurev.phyto.42.040803.140421 [DOI] [PubMed] [Google Scholar]
- 56.Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. The Plant Cell Online. 1994;6(12):1845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seo PJ, Lee AK, Xiang F, Park CM. Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol. 2008;49(3):334–44. 10.1093/pcp/pcn011 [DOI] [PubMed] [Google Scholar]
- 58.Liu W-X, Zhang F-C, Zhang W-Z, Song L-F, Wu W-H, Chen Y-F. Arabidopsis Di19 functions as a transcription factor and modulates PR1, PR2, and PR5 expression in response to drought stress. Molecular plant. 2013;6(5):1487–502. 10.1093/mp/sst031 [DOI] [PubMed] [Google Scholar]
- 59.Seo PJ, Kim MJ, Park JY, Kim SY, Jeon J, Lee YH, et al. Cold activation of a plasma membrane-tethered NAC transcription factor induces a pathogen resistance response in Arabidopsis. The Plant Journal. 2010;61(4):661–71. 10.1111/j.1365-313X.2009.04091.x [DOI] [PubMed] [Google Scholar]
- 60.Titarenko E, Rojo E, Leon J, Sanchez-Serrano JJ. Jasmonic acid-dependent and -independent signaling pathways control wound-induced gene activation in Arabidopsis thaliana. Plant Physiol. 1997;115(2):817–26. 10.1104/pp.115.2.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knoth C, Eulgem T. The oomycete response gene LURP1 is required for defense against Hyaloperonospora parasitica in Arabidopsis thaliana. The Plant Journal. 2008;55(1):53–64. 10.1111/j.1365-313X.2008.03486.x [DOI] [PubMed] [Google Scholar]
- 62.Dhondt S, Geoffroy P, Stelmach BA, Legrand M, Heitz T. Soluble phospholipase A2 activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes. The Plant Journal. 2000;23(4):431–40. [DOI] [PubMed] [Google Scholar]
- 63.Lotkowska ME, Tohge T, Fernie AR, Xue G-P, Balazadeh S, Mueller-Roeber B. The Arabidopsis transcription factor MYB112 promotes anthocyanin formation during salinity and under high light stress. Plant physiology. 2015:pp. 00605.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy YY, Dean C. Control of flowering time. Current opinion in plant biology. 1998;1(1):49–54. [DOI] [PubMed] [Google Scholar]
- 65.Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, et al. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. The Plant Cell. 2005;17(8):2255–70. 10.1105/tpc.105.033464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vrancken K, Holtappels M, Schoofs H, Deckers T, Valcke R. Pathogenicity and infection strategies of the fire blight pathogen Erwinia amylovora in Rosaceae: state of the art. Microbiology. 2013;159(Pt 5):823–32. 10.1099/mic.0.064881-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
At1-3+ over-expressing AtIPCS1-3; At1-3++ higher level expressers of AtIPCS1-3. Log2 change ≥1 shown, ≥2 in bold.
(TIF)
Relative quantitation was done after normalization using PEX4 levels; relative quantitation value is the mean of three biological replicates with standard error.
(TIF)
Log2 fold changes in gene expression are indicated by the colour scale, with the distribution of genes in different pathways and expression levels shown. Abbreviations: carbohydrates (CHO), tricarboxylic acid (TCA) cycle, oxidative pentose phosphate (OPP) pathway, sulphur containing glucosinates synthesis (S-misc), nitrogen containing glucosinate synthesis (N-misc).
(TIF)
Log2 fold changes in gene expression are indicated by the colour scale, with the distribution of genes in different pathways and expression levels shown. Abbreviations: carbohydrates (CHO), tricarboxylic acid (TCA) cycle, oxidative pentose phosphat pathway, sulphur containing glucosinates synthesis (S-misc), nitrogen containing glucosinate synthesis (N-misc).
(TIF)
Col0 and overexpressing lines At1++, At2++ and At3++. Mannitol concentrations in mM. At the highest concentration (500mM) chlorosis in the over-expressing lines, but not Col0, was apparent.
(TIF)
(A) Col0 (B) At1++ (C) At2++ (D) At3++ (E) Plot of ratio of area infected by Erwinia amylovora to uninfected area for Col0 and over-expression lines. AtIPCS2 and 3 over-expressors are less susceptible to the pathogen compared to Col0 and AtIPCS1 over-expressor, based on the spread of the pathogen across the surface of the leaf. The pathogen has spread to occupy a large area of Col0 and AtIPCS1 over-expressor leaves, compared to a less aggressive spread seen on the leaves of AtIPCS2 and 3 over-expressors. In addition, a distinctive yellow colour is observed at the outer boundaries of the area occupied by the pathogen, indicating a measured response that favours plant survival. These observations may be linked to the role of AtIPCS as a negative regulator of the plant response to biotic stress.
(TIF)
Data Availability Statement
All transcriptomic data used in the study are publicly available in GEO (GEO accession number GSE129016).