Abstract
Research on emotional learning and memory is traditionally approached from one of two directions: episodic memory and classical conditioning. These approaches differ substantially in methodology and intellectual tradition. Here, we offer a new approach to the study of emotional memory in humans that involves integrating theoretical knowledge and experimental techniques from these seemingly distinct fields. Specifically, we describe how subtle modifications to traditional Pavlovian conditioning procedures have provided new insight into how emotional experiences are selectively prioritized in long-term episodic memory. We also speculate on future directions and undeveloped lines of research where some of the knowledge and principles of classical conditioning might advance our understanding of how emotion modifies episodic memory, and vice versa.
Introduction
The ability to remember emotional events can adaptively guide behavior in future situations. Consequently, memory systems are biased to remember experiences encoded around the time of heightened emotional arousal, referred to as the emotional enhancement of memory. This prioritization of emotional memory is adaptive insofar as it helps ensure that we remember people, places, stimuli, situations, and responses associated with important experiences. Research on emotional memory tends to fall into two largely isolated psychological disciplines with characteristically distinct academic traditions: episodic memory and classical (Pavlovian) conditioning. Emotional episodic memory research is generally concerned with explicitly stated knowledge of details surrounding an emotional experience. It is dominated by research on humans, but informed by a history of non-human animal research focused on hippocampal-dependent learning, such as spatial navigation and object recognition. Classical conditioning, on the other hand, describes both a learning process and an experimental procedure by which animals associate neutral stimuli in the environment with meaningful outcomes (e.g. aversive shock or appetitive reward). Neurobiological research on conditioning is predominately focused on the amygdala for its role in learning, storage, and retrieval of threat memories. The goal of this article is to bring into focus the correspondence between these traditionally separated areas of emotional memory research. We briefly describe how “emotional memory” is experimentally defined in episodic memory and conditioning research, describe challenges to studying emotional memory for each field, and discuss how integrating these areas of research can advance our understanding of emotional learning and memory.
Episodic memory and the trouble with isolating the role of emotion
Episodic memory refers to knowledge of the time, place, or other contextual details of an experience [1]. Research on human episodic memory dates back to at least the late nineteenth century [2-4], and has typically involved testing people’s ability to explicitly recall or recognize a variety of stimuli (words, images, etc.) or the associations between stimuli. Among the most widely replicated findings in episodic memory research is that emotional events are better remembered with more vividness and higher confidence than mundane or trivial everyday events (Figure 1) [5-7]. Functional neuroimaging research indicates that the amygdala responds to intrinsically emotional stimuli, and up-regulates processing in the hippocampus and neocortical regions to enhance long-term episodic memory for emotional versus neutral events [8,9]. Another hallmark of emotional episodic memory is that the memory advantage for emotional versus neutral details increases over time [10-12], a process that has been linked to the strength of connectivity between the amygdala and hippocampal complex at the time of encoding [13]. This would imply that emotional memory benefits are not determined entirely by biased encoding processes, which would predict superior memory immediately after encoding. It is widely recognized, however, that the memory advantage for emotional material is often confounded by a host of additional cognitive factors related to processing the emotional material, per se [14]. For instance, emotional items outcompete neutral items in the allocation of perceptual resources, making it difficult to disentangle heightened attention from strict emotional modulation of memory processes [15]. Another issue concerns the relatively restricted set of thematically related content (e.g., taboo words, violent scenes), as compared to neutral stimuli that can span a range of thematic content [16]. The interrelatedness of emotional items may simply make it easier to remember them.
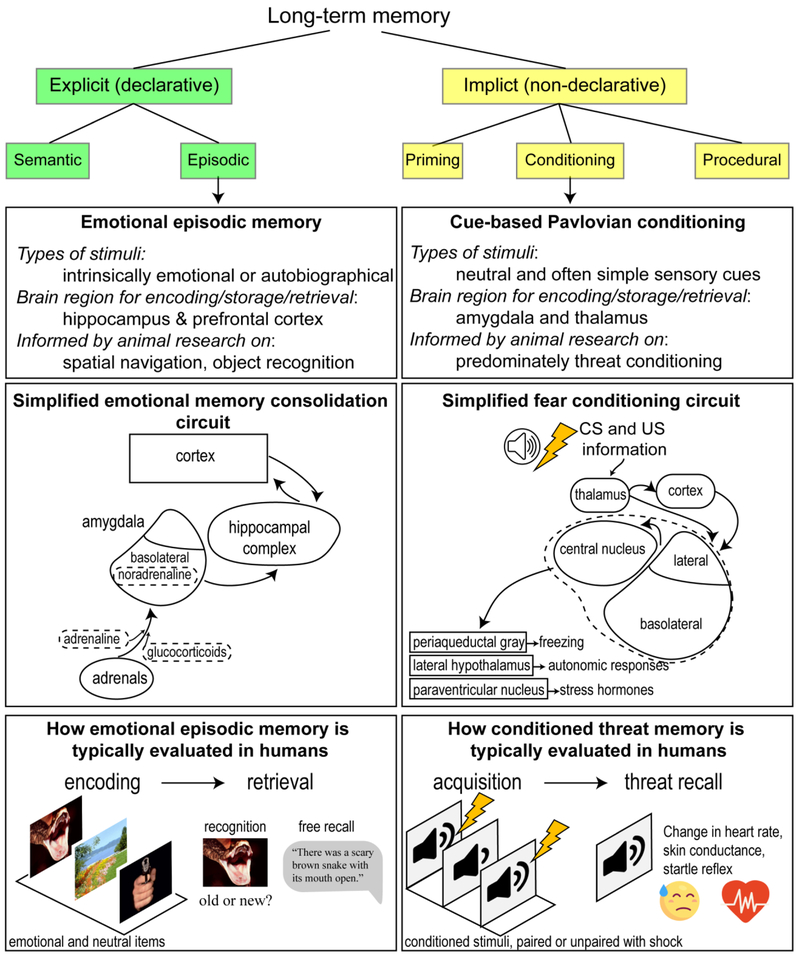
Figure 1.
A modified taxonomy of memory [68], showing how emotional memory is traditionally investigated along separate domains of inquiry that can broadly be divided between episodic memory and Classical (Pavlovian) conditioning.
One way to disentangle the effects of emotional arousal and heightened attention at the time of encoding is to present neutral stimuli in emotional and neutral contexts during encoding, and then test memory for neutral stimuli in isolation. These paradigms report that emotional contexts can enhance neutral item memory, a process associated with increases in amygdala-hippocampal activity and connectivity during encoding and retrieval [17-20]; however, the episodic enhancement effect in these studies tends to be minimal compared to typical performance for intrinsically emotional versus neutral stimuli. Another route to isolate the effect of emotion on episodic memory then is to modulate arousal after encoding. Pioneering research in rodents revealed that post-encoding arousal activates neurohormones (e.g. noradrenaline) in the amygdala that up-regulate hippocampal processes [21]. In humans, stress induction immediately after encoding via social stress (giving a public speech), physical stress (submerging the subjects arm in ice water), or a drug can also improve episodic memory for items encoded prior to the stressor [22]. While this provides compelling evidence of post-encoding arousal modulating long-term memory, many of the issues of dissociating different stages of memory processing remain pertinent. That is, early studies showed that immediate post-training treatments selectively affected memory for emotional material [23], therefore strengthening memory for items that already receive an encoding advantage. Interestingly, hippocampal encoding-related activity prior to post-encoding stress might already determine what neutral information gets prioritized by stress during a period of memory consolidation [24]. Recent evidence also shows that emotional experiences generate persistent emotional encoding states in amygdala-hippocampal networks that carry-forward in time, influencing encoding and enhancing memory formation for neutral information encountered several minutes afterward [25].
Given that characteristics of emotional stimuli can confound the interpretation of emotional memory enhancement, and the inconsistencies and complex selectivity of post-encoding manipulations, a methodology by which otherwise neutral information comes to acquire emotional significance would help disentangle intrinsic properties of the stimulus from emotion-mediated influences on memory. Pavlovian conditioning provides such a methodology.
Pavlovian conditioning and the trouble of isolating individual learning experiences
Conditioning refers to both a learning process and an experimental procedure by which neutral conditional stimuli (CS) acquire the capacity to elicit learned behavioral conditional responses (CR) via association with a biologically salient unconditional stimulus (US). It is traditionally considered an implicit (non-declarative) form of memory mostly unconnected to declarative memory processes [26] (Figure 1). The dominant conditioning paradigm is threat conditioning. Threat conditioning has proved an indispensable model to answer questions on the nature of learning and memory representations in the brain; it is rapid, strong, stable, and has objective neural and behavioral correlates conserved across species [27,28]. The overwhelming advantage to threat conditioning protocols is the ease by which learning and memory strength can be inferred by the magnitude of the CR. For instance, if an animal learns that a CS predicts a painful shock (US), they will display an overt CR relevant to anticipation or delivery of the US (e.g., hypoanalgesia or freezing) indicating that they anticipate an impending threat. If during a retention test at some later time the CS elicits a CR of similar magnitude, then the experimenter can infer that the animal learned, stored, and retrieved a long-term memory of the CS-US association. While admittedly a simplified description of an animal learning experiment, the focus is ultimately on the expression (or lack thereof) of the CS-US memory as a measurable CR. In other words, the CR is the de facto memory, the magnitude of which reflecting the strength of the underlying memory trace.
It is well appreciated, however, that behavioral performance (the CR) at the time of test can be a poor indicator of the animal’s long-term memory, or ‘what it remembers,’ sometimes referred to as the learning versus performance distinction [29]. For instance, the animal might remember that the tone was paired with shock; but if it also remembers that the tone was sometimes presented without the shock—for instance during extinction—then it may not show a CR. The animal also likely formed a number of other associations with the CS (temporal, contextual), and the CS-US memory is also likely to engage a number of learning processes and response systems beyond the overt CR under investigation [30]. If the animal does not show a CR after a retention interval, it could be that the animal never formed a CS-US memory, the CR is temporarily inhibited, or the animal remembers a hybrid of competing excitatory and inhibitory CS associations. The CR thus provides an aggregate over the entire learning history and can thus be a poor indicator of what an animal remembers from individual learning events. How “memory” is defined in conditioning is especially critical when drawing inferences from protocols that putatively lead to persistent alteration of a threat memory [31], as in disrupting reconsolidation [32].
In summary, Pavlovian conditioning reveals how animals acquire adaptive emotional responses. But the CR is often measured as a unitary response that reflects one aspect of a CS-US memory built up over a series of trials [30,33]. A multitude of associations engaged by other behavioral systems might provide a more comprehensive reflection of what, precisely, an animal learns and remembers from the conditioning experience.
Charting the overlap between episodic memory and Pavlovian conditioning
Because human conditioning research is overwhelmingly informed by cue and context conditioning research in rodents, it is generally unconcerned with declarative memory processes. Likewise, because human episodic memory research is generally concerned with higher-order cognitive functions, it is generally unconcerned with putatively reflexive non-declarative memory systems. Indeed, even in the realm of human fear conditioning these forms of memory are typically viewed as operating independently; for instance, fear conditioned stimuli can evoke autonomic responses when presented subliminally [34], and ‘blind sight’ patients with lesions to the visual cortex show autonomic arousal and amygdala activity to emotional cues (e.g., pictures of a snake or a fearful face) in the absence of conscious awareness [35,36]. Put together, there is no obvious role for episodic memory processes in the basic conditioning preparation. Correspondingly, episodic memories are an example of one-shot learning in the absence of any obvious reinforcement; thus, there is no recognizable role for classical conditioning processes in typical episodic memory protocols. Notably, some forms of conditioning, such as context conditioning and trace conditioning, involve hippocampal processes. There is also some interesting speculation as to whether different forms of conditioning require conscious awareness, and may therefore constitute a form of explicit memory [37,38].
In conditioning, it is worth considering that memory retention overwhelming involves implicit measures of performance that are more or less independent of the hippocampus (e.g., freezing or sweating). But this emphasis on implicit behavioral response systems does not rule out learning in other memory systems. In fact, humans do form episodic memory during conditioning that can be expressed as propositional knowledge [39]. Because the cognitive demands tend to be low, this knowledge often takes the form of detailing the CS-US contingencies. To comprehensively examine the link between Pavlovian conditioning and episodic memory, we have augmented a Pavlovian conditioning task to increase the demands on episodic memory [40-44].
In this task, the CSs are trial-unique (i.e., non-repeating) images from a semantic category: one category is paired with the US (CS+) and a separate category is never paired with the US (CS−). Hence, we refer to the design as “category-conditioning.” Because trials are non-repeating, each trial effectively serves as an isolated event that either remains neutral or acquires emotional significance via a categorical association with the US. The paradigm still allows for typical measures of conditioning (e.g., sweating, startle, etc.). But the trial-unique nature of the design now affords a new opportunity to probe episodic memory for CS+ and CS− items afterwards (Figure 2). Although this modified conditioning design is superficially basic and straightforward, it represents a marked departure from traditional protocols that emphasize non-declarative measures of memory performance.
Figure 2.
A schematic of a category-conditioning paradigm. Basic-level exemplars from two different object categories (here, animals and tools) serve as trial-unique (i.e., non-repeating) conditioned stimuli in the framework of a conditioning paradigm. Exemplars can be presented before, during, or after conditioning with traditional measures to evaluate conditioning, such as skin conductance, fear-potentiated startle, and threat expectancy ratings. After learning, an episodic memory test could include CS+ and CS− exemplars encoded before, during, and after conditioning, as well as category-related foils (e.g., different animals or tools that were not presented during learning). An example of memory performance (corrected recognition) showing better memory for items from the CS+ than CS− category encoded before, during, and after conditioning.
So far our labs have mostly incorporated the broad superordinate categories of animate versus inanimate objects because their neural representations are separable in occipitotemporal regions [45]. This a priori distinction in how cortical regions represent category-level knowledge allows for inferences on how emotional learning modulates the representation of conceptual episodic knowledge using multivariate fMRI analysis tools [46]. Recently, deVoogd and colleagues incorporated animals and food (fruits and vegetables) [47,48] and we used animal subcategories (birds and fishes) [42] to similar effect. Overall, the neurobehavioral mechanisms underlying category-conditioning appear consistent with those supporting classical conditioning to a simple repeated CS, as well as systems supporting subsequent episodic memory for individual events.
Behaviorally, we have found that recognition memory is enhanced for CS+ versus CS− trials encoded prior to, during, and after Pavlovian conditioning [40-44]. Using partial CS-US pairings, we have verified that memory is enhanced for all CS+ items, regardless of whether a specific exemplars is paired the US. Thus, it is not the US itself that is “stamping” in item memory, but rather the association between the category and the US that seems relevant for the enhancement in episodic memory. The associative learning aspect of the design sets it apart from protocols in which unpredictable shocks are paired with unrelated neutral items [49]; a protocol that has inconsistent effects on enhancing episodic memory.
Critically, the category conditioning paradigm can address issues inherent to the use of intrinsically evocative stimuli in emotional memory research [14]. For instance, subjects show selective retroactive memory benefits for unique items from the CS+ object category encoded before conditioning [41,43], and after conditioning when the US is omitted [40,43]. The retroactive enhancement effect provides convincing evidence that conditioning-induced episodic memory enhancements are independent from biased processing at the time of encoding. Conditioning enhanced memory effects are also much weaker (or entirely absent) at immediate memory tests and emerge after a delay [40,41,43]. This suggest an important role in post-encoding consolidation processes, in keeping with models of arousal-mediated memory consolidation [50].
The category-conditioning protocol also allows memory strength to be evaluated as time-ordered function of when exactly each CS exemplar was encoded. This enables assessment for whether competing experiences of threat and extinction are represented as separate memory traces, as proposed by nearly all associative learning models [51-53]. Recently, we used category-conditioning to investigate whether an event boundary (i.e., a short break) separating conditioning from extinction segments emotional episodic memory for conceptually-related items [40]. Event segmentation has received considerable interest in the realm of human memory [54-60], as boundaries separating an otherwise continuous stream of experience have the power to organize episodic memory, but has received far less attention in the conditioning literature [but see 61]. Yet, event segmentation models [62] might provide an explanatory mechanism to address why extinction learning generates a secondary memory trace that competes with, but does not overwrite, threat memories [52]. We found that when a short break separated conditioning from extinction, and memory was tested after a 24-hour delay, there was a sharp drop in recognition memory for CS+ items encoded during extinction as compared to related CS+ exemplars encoded during conditioning [40]. There was no evidence of segmentation in memory strength for CS+ items encoded during conditioning versus extinction without a transition between phases, or when episodic memory was tested immediately. This perhaps suggests that event boundaries help shape the selective consolidation of emotional information, at the expense of related but conflicting information encoded shortly thereafter. Probing recognition memory as a time-ordered function of when each item was encoded has also afforded the opportunity to unite models of memory reconsolidation between the episodic and conditioning literatures. Specifically, we found that an isolated CS+ trial 10 minutes prior to extinction [cf. 63] appears to retroactively strengthen consolidated episodic memory for CS+ items encoded the previous day [42].
Work on event segmentation in episodic memory shares in common aspects of recent computational models on structured learning that clusters experience into distinct latent causes [64-67]. Specifically, latent cause theories of classical conditioning propose that the rapid change in associative value and accumulation of prediction errors provides allows animals to infer that conditioning and extinction arose from different latent states of the world, which separates the experience of acquisition and extinction into distinct memory traces. It is likely that the signals that generate a new latent cause in conditioning experiments share in common features of event boundaries that are shown to segment episodic memory, and may be one explanation for the segmentation in recognition memory between threat conditioning and extinction in the category conditioning paradigm [40].
Conclusions and future directions
Here we discussed how synthesizing elements of episodic memory and classical conditioning provides unique insight into the mechanisms of emotional memory. A “category conditioning” paradigm allows neutral stimuli to be “tagged” by emotional experiences before, during, or after learning to study the effects on the prioritization into long-term episodic memory that circumvents many of the confounds that have historically affected the field of emotional episodic memory [14]. At the same time, augmenting traditional conditioning protocols provides the possibility to move beyond a unitary CR as a measure of Pavlovian learning [29]. Episodic memory systems may be an underappreciated system engaged during Pavlovian conditioning that has, until recently, remained unmeasured in the field due to minimal demands on hippocampal encoding systems during standard cue conditioning preparations. Yet, real-world emotional experiences likely engage both Pavlovian and episodic memory mechanisms and, therefore, the integration of these seemingly disparate research fields is critical to understand human emotional memory and its malfunctioning in disease. Fortunately, by bringing together over a century of research on two mostly isolated fields, there arise a number of exciting avenues for future discovery on how emotional learning modulates episodic memory.
Highlights.
Episodic memory and classical conditioning offer distinct insights on emotional memory.
We discuss a protocol that synthesizes these historically separated lines of inquiry.
Integrating these approaches can advance our understanding of emotional memory.
Acknowledgments
The authors acknowledge funding from NIH R00 MH106719 to J.E.D., and an H2020 Marie Sklodowska-Curie fellowship and a Branco Weiss fellowship - Society in Science to M.C.W.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Nothing declared
References
- 1.Tulving E: Episodic memory: From mind to brain. Annual review of psychology 2002, 53:1–25. [DOI] [PubMed] [Google Scholar]
- 2.Müller GE, Pilzecker A: Experlmentelle beiträge zur lehre vom gedbeitragechtniss, vol 1: JA Barth; 1900. [Google Scholar]
- 3.Ebbinghaus H: Memory: A contribution to experimental psychology. Annals of neurosciences 2013, 20:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower GH: A brief history of memory research. The Oxford handbook of memory 2000:3–32. [Google Scholar]
- 5.LaBar KS, Cabeza R: Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience 2006, 7:54–64. [DOI] [PubMed] [Google Scholar]
- 6.Hamann S: Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences 2001, 5:394–400. [DOI] [PubMed] [Google Scholar]
- 7.Bennion KA, Ford JH, Murray BD, Kensinger EA: Oversimplification in the study of emotional memory. Journal of the International Neuropsychological Society 2013, 19:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·Reviews rules-of-thumb assumptions about the emotional memory enhancement effect and highlights potential confounds. Particularly, the authors highlight that emotion does not always enhance memory, that the effect of arousal on memory is complex, and how emotion can bias processing of information that may or may not lead to memory enhancement.
- 8.Murty VP, Ritchey M, Adcock RA, LaBar KS: fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia 2010, 48:3459–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolcos F, Katsumi Y, Weymar M, Moore M, Tsukiura T, Dolcos S: Emerging directions in emotional episodic memory. Frontiers in Psychology 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinsmith LJ, Kaplan S: Paired-associate learning as a function of arousal and interpolated interval. J Exp Psychol 1963, 65:190–193. [DOI] [PubMed] [Google Scholar]
- 11.Sharot T, Phelps EA: How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience 2004, 4:294–306. [DOI] [PubMed] [Google Scholar]
- 12.Yonelinas AP, Ritchey M: The slow forgetting of emotional episodic memories: an emotional binding account. Trends in Cognitive Sciences 2015, 19:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritchey M, Dolcos F, Cabeza R: Role of Amygdala Connectivity in the Persistence of Emotional Memories Over Time: An Event-Related fMRI Investigation. Cerebral Cortex 2008, 18:2494–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talmi D: Enhanced emotional memory: Cognitive and neural mechanisms. Current Directions in Psychological Science 2013, 22:430–436. [Google Scholar]; ··Puts forward an alternative to the “emotion enhanced memory consolidation” theory and suggests that emotion can mediate encoding and retrieval to enhance memory.
- 15.Cahill L, McGaugh JL: Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences 1998, 21:294–299. [DOI] [PubMed] [Google Scholar]
- 16.Talmi D, Schimmack U, Paterson T, Moscovitch M: The role of attention and relatedness in emotionally enhanced memory. Emotion 2007, 7:89. [DOI] [PubMed] [Google Scholar]
- 17.Maratos E, Dolan R, Morris J, Henson R, Rugg M: Neural activity associated with episodic memory for emotional context. Neuropsychologia 2001, 39:910–920. [DOI] [PubMed] [Google Scholar]
- 18.Smith AP, Stephan KE, Rugg MD, Dolan RJ: Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron 2006, 49:631–638. [DOI] [PubMed] [Google Scholar]
- 19.Smith AP, Dolan RJ, Rugg MD: Event-related potential correlates of the retrieval of emotional and nonemotional context. Journal of Cognitive Neuroscience 2004, 16:760–775. [DOI] [PubMed] [Google Scholar]
- 20.Takashima A, van der Ven F, Kroes MC, Fernández G: Retrieved emotional context influences hippocampal involvement during recognition of neutral memories. NeuroImage 2016, 143:280–292. [DOI] [PubMed] [Google Scholar]; ·Reports that if people at the time of retrieval think that a neutral image was encoded in an emotional context, irrespective of the veridical context, then activity in the amygdala and hippocampus is increased and retrieval of neutral items enhanced.
- 21.McGaugh JL: The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience 2004, 27:1–28. [DOI] [PubMed] [Google Scholar]
- 22.Shields GS, Sazma MA, McCullough AM, Yonelinas AP: The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychological bulletin 2017, 143:636. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·A comprehensive meta-analysis on how stress can have a variety of effects on episodic memory based on whether stress is administered prior, during, or after encoding or prior to retrieval.
- 23.Cahill L, Gorski L, Le K: Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory 2003, 10:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritchey M, McCullough AM, Ranganath C, Yonelinas AP: Stress as a mnemonic filter: Interactions between medial temporal lobe encoding processes and post-encoding stress. Hippocampus 2017, 27:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·Reports that items that evoke greater hippocampal activity during encoding are preferentially enhanced by post-encoding effects stress. These results indicate that post-encoding effects of stress on subsequent episodic memory are not independent initial encoding processes.
- 25.Tambini A, Rimmele U, Phelps EA, Davachi L: Emotional brain states carry over and enhance future memory formation. Nature Neuroscience 2017, 20:271–278. [DOI] [PubMed] [Google Scholar]; ··Reports that encoding of emotional stimuli creates a state-effect that carries over to enhance memory for subsequently encoded neutral stimuli. The emotional carry-over effect is reflected in emotion-related amygdala-hippocampal connectivity and evoked emotion-related amygdala activation at the time of subsequent encoding of neutral stimuli.
- 26.Squire LR, Zola SM: Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences of the United States of America 1996, 93:13515–13522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, Heitland I, Hermann A, Kuhn M, Kruse O: Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience & Biobehavioral Reviews 2017, 77:247–285. [DOI] [PubMed] [Google Scholar]; ··An exceptionally comprehensive and practical overview of on the human fear conditioning research and practices.
- 28.Pape HC, Pare D: Plastic Synaptic Networks of the Amygdala for the Acquisition, Expression, and Extinction of Conditioned Fear. Physiological Reviews 2010, 90:419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouton ME, Moody EW: Memory processes in classical conditioning. Neuroscience & Biobehavioral Reviews 2004, 28:663–674. [DOI] [PubMed] [Google Scholar]
- 30.Delamater AR: On the nature of CS and US representations in Pavlovian learning. Learning & behavior 2012, 40:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lattal KM, Wood MA: Epigenetics and persistent memory: implications for reconsolidation and silent extinction beyond the zero. Nat Neurosci 2013, 16:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nader K, Schafe GE, Le Doux JE: Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 2000, 406:722–726. [DOI] [PubMed] [Google Scholar]
- 33.Delamater AR: Issues in the extinction of specific stimulus-outcome associations in Pavlovian conditioning. Behavioural processes 2012, 90:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight DC, Waters NS, Bandettini PA: Neural substrates of explicit and implicit fear memory. NeuroImage 2009, 45:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamm AO, Weike AI, Schupp HT, Treig T, Dressel A, Kessler C: Affective blindsight: intact fear conditioning to a visual cue in a cortically blind patient. Brain 2003, 126:267–275. [DOI] [PubMed] [Google Scholar]
- 36.Morris JS, DeGelder B, Weiskrantz L, Dolan RJ: Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 2001, 124:1241–1252. [DOI] [PubMed] [Google Scholar]
- 37.Bekinschtein TA, Shalom DE, Forcato C, Herrera M, Coleman MR, Manes FF, Sigman M: Classical conditioning in the vegetative and minimally conscious state. Nature Neuroscience 2009, 12:1343–U1176. [DOI] [PubMed] [Google Scholar]
- 38.Lovibond PF, Shanks DR: The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology-Animal Behavior Processes 2002, 28:3–26. [PubMed] [Google Scholar]
- 39.Mitchell CJ, De Houwer J, Lovibond PF: The propositional nature of human associative learning. Behavioral and Brain Sciences 2009, 32:183–246. [DOI] [PubMed] [Google Scholar]
- 40.Dunsmoor JE, Kroes MCW, Moscatelli CM, Evans MD, Davachi L, Phelps EA: Event segmentation protects emotional memories from competing experiences encoded close in time. Nature Human Behaviour 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patil A, Murty VP, Dunsmoor JE, Phelps EA, Davachi L: Reward retroactively enhances memory consolidation for related items. Learning & Memory 2017, 24:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroes MC, Dunsmoor JE, Lin Q, Evans M, Phelps EA: A reminder before extinction strengthens episodic memory via reconsolidation but fails to disrupt generalized threat responses. Scientific Reports 2017, 7:10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunsmoor JE, Murty VP, Davachi L, Phelps EA: Emotional learning selectively and retroactively strengthens memories for related events. Nature 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·Shows that category conditioning can enhance memory specifically for neutral items related to the conditioned category that were encoded before conditioning, dependent on a period of consolidation.
- 44.Dunsmoor JE, Martin A, LaBar KS: Role of conceptual knowledge in learning and retention of conditioned fear. Biological Psychology 2012, 89:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin A: The representation of object concepts in the brain. Annual Review of Psychology 2007, 58:25–45. [DOI] [PubMed] [Google Scholar]
- 46.Dunsmoor JE, Kragel PA, Martin A, LaBar KS: Aversive Learning Modulates Cortical Representations of Object Categories. Cerebral Cortex 2014, 24:2859–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Voogd LD, Fernández G, Hermans EJ: Disentangling the roles of arousal and amygdala activation in emotional declarative memory. Social Cognitive and Affective Neuroscience 2016, 11:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]; ·Uses the category-conditioning paradigm to show that amygdala activity, but not measures of arousal, during encoding predicts subsequent emotion enhanced episodic memory. Hence, arousal may not be the driving factor that stamps neutral items into long-term episodic memory.
- 48.de Voogd LD, Fernández G, Hermans EJ: Awake reactivation of emotional memory traces through hippocampal–neocortical interactions. NeuroImage 2016, 134:563–572. [DOI] [PubMed] [Google Scholar]; ·Uses the category-conditioning paradigm to show that conditioning-related neural activity reactivates during post-encoding consolidation periods specifically in neocortical representational regions for the CS+ category. Thus the paradigm allowed the researchers to demonstrate that the neural reactivations were learning and memory specific and not due to some none-specific state effects.
- 49.Schwarze U, Bingel U, Sommer T: Event-related nociceptive arousal enhances memory consolidation for neutral scenes. J Neurosci 2012, 32:1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGaugh JL: Consolidating memories. Annual review of psychology 2015, 66:1–24. [DOI] [PubMed] [Google Scholar]
- 51.Pearce JM, Hall G: A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review 1980, 87:532–552. [PubMed] [Google Scholar]
- 52.Bouton ME: Context and behavioral processes in extinction. Learning & Memory 2004, 11:485–494. [DOI] [PubMed] [Google Scholar]
- 53.Larrauri JA, Schmajuk NA: Attentional, associative, and configural mechanisms in extinction. Psychological review 2008, 115:640–676. [DOI] [PubMed] [Google Scholar]
- 54.Brunec IK, Moscovitch M, Barense MD: Boundaries Shape Cognitive Representations of Spaces and Events. Trends in cognitive sciences 2018. [DOI] [PubMed] [Google Scholar]
- 55.DuBrow S, Davachi L: Temporal binding within and across events. Neurobiology of learning and memory 2016, 134:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben-Yakov A, Eshel N, Dudai Y: Hippocampal immediate poststimulus activity in the encoding of consecutive naturalistic episodes. Journal of Experimental Psychology: General 2013, 142:1255–1263. [DOI] [PubMed] [Google Scholar]
- 57.Ezzyat Y, Davachi L: What constitutes an episode in episodic memory? Psychological Science 2011, 22:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudai Y, Karni A, Born J: The consolidation and transformation of memory. Neuron 2015, 88:20–32. [DOI] [PubMed] [Google Scholar]
- 59.DuBrow S, Rouhani N, Niv Y, Norman KA: Does mental context drift or shift? Current opinion in behavioral sciences 2017, 17:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baldassano C, Chen J, Zadbood A, Pillow JW, Hasson U, Norman KA: Discovering event structure in continuous narrative perception and memory. Neuron 2017, 95:709–721. e705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gershman SJ, Monfils M-H, Norman KA, Niv Y: The computational nature of memory modification. eLife 2017, 6:e23763. [DOI] [PMC free article] [PubMed] [Google Scholar]; ··Provides a computational modeling framework to interpret the conditions that promote formation of a new memory trace or updating (reconsolidation) of an old memory trace.
- 62.Zacks JM, Swallow KM: Event segmentation. Current Directions in Psychological Science 2007, 16:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA: Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 2010, 463:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gershman SJ, Blei DM, Niv Y: Context, learning, and extinction. Psychol Rev 2010, 117:197–209. [DOI] [PubMed] [Google Scholar]
- 65.Redish AD, Jensen S, Johnson A, Kurth-Nelson Z: Reconciling reinforcement learning models with behavioral extinction and renewal: implications for addiction, relapse, and problem gambling. Psychological review 2007, 114:784. [DOI] [PubMed] [Google Scholar]
- 66.Courville AC, Daw ND, Touretzky DS: Bayesian theories of conditioning in a changing world. Trends in cognitive sciences 2006, 10:294–300. [DOI] [PubMed] [Google Scholar]
- 67.Gershman SJ, Niv Y: Exploring a latent cause theory of classical conditioning. Learn Behav 2012, 40:255–268. [DOI] [PubMed] [Google Scholar]
- 68.Squire LR: Memory systems of the brain: a brief history and current perspective. Neurobiology of learning and memory 2004, 82:171–177. [DOI] [PubMed] [Google Scholar]