Abstract
Social anxiety is a common disorder that has negative impacts across multiple domains of function. Several clinical groups are at elevated risk for social anxiety, including those with fragile X syndrome and those with autism spectrum disorder. Measuring social anxiety in these clinical subgroups is fraught with challenge, however, given the complexity of social anxiety and measurement limitations that are particularly acute in persons with neurodevelopmental disorders. The over-arching aim of this study was to contribute to our understanding of the nature of social anxiety in fragile X syndrome and its association with autism spectrum disorder. To address this aim, we created a multi-faceted composite representing behavioral and biological aspects of social anxiety and examined differences in two adolescent and young adult-aged groups: 59 males with fragile X syndrome and 18 males with autism spectrum disorder. Results indicated a lower score on the multivariate composite for the males with fragile X syndrome relative to autism spectrum disorder but with evidence that traits of autism and social anxiety overlap. We conclude that measuring anxiety and autism traits in fragile X syndrome and autism spectrum disorder is complex with features that overlap and interact in a dynamic manner.
Introduction
Anxiety is a multi-faceted construct associated with cognitive, affective, and biological factors that interact dynamically over the life course. Anxiety disorders constitute a common and debilitating mental health problem in adolescents and young adults, with 30% meeting criteria for an anxiety disorder and many more displaying symptoms of anxiety but not meeting full diagnostic criteria (Kessler & Wang, 2008). Given the high prevalence and significant negative impact of anxiety, research has increasingly focused on understanding the extent, nature, and sources of anxiety in clinical subgroups that are at elevated risk. Individuals with intellectual disability (ID) represent a clinical subgroup that presents with the full range of mental illnesses and are four times more likely to have anxiety than typical controls (Green, Berkovits, & Baker, 2014). Comorbidity of anxiety in persons with ID reduces function across multiple domains and inhibits their already impaired learning (Schneier et al., 1994), thereby highlighting the importance of identification, prevention, and treatment of anxiety in these individuals.
Characterizing anxiety in persons with ID is fraught with challenges, however, given both measurement limitations and ambiguous diagnostic demarcation. Measurement limitations reflect the lack of available standardized measures normed for persons with ID in addition to the inapplicability of many available measures, which typically rely on self-report, to ID populations given blunted self-reflection and communication impairments. Informant reports, although useful, are subject to many of the same limitations as in intellectually typical populations (e.g., limited knowledge of the informant about the full range of contexts in which the target individual participates). Likewise, differential diagnosis of anxiety co-morbid with ID is complicated given overlapping features between the disorders and minimal established guidelines upon which to make distinctions. Also, there has been an over-reliance in research on ID populations on conceptualizing and measuring anxiety as a unitary disorder, rather than recognizing discrete anxiety disorders. As a result, little is known about the nature and expression of anxiety in persons with ID, which makes the development of treatments and prevention strategies for this population difficult.
Social anxiety disorder, also known as social phobia, is a distinct anxiety disorder that is characterized by discomfort and/or fear around social interaction (American Psychological Association, 2013). Social anxiety is one of the most prevalent and impairing anxiety disorders, with nearly 10% of the general population (Nagata, Suzuki, & Teo, 2015) and a large subgroup of individuals with ID meeting criteria for the disorder. Diagnosing social anxiety in persons with ID is challenging, however, because social impairments are often associated with low cognitive skills and the differential diagnosis of social anxiety co-morbid with ID requires determining that the clinical presentation is not best accounted for by ID alone, which can be very difficult in practice. Moreover, like anxiety more generally, social anxiety is multidimensional and may be manifested in at least partly different ways across individuals with ID or in combination with other disorders.
In this study, we aimed to capture the features of social anxiety using a composite measure comprised of behavioral features derived from multiple sources (parent ratings and direct observation) and a biomarker (cortisol) across two distinct groups with ID, each of whom is at elevated risk for social anxiety disorder. Our goal was to determine if social anxiety is manifested to different degrees, as different symptom profiles, or with different correlates between fragile X syndrome (FXS) and nonsyndromic autism spectrum disorder (ASD). We use the term nonsyndromic to recognize ASD in individuals without a known genetic syndrome (e.g., FXS, tuberous sclerosis). Given that social anxiety is thought to characterize both of these neurodevelopmental disorders, studies that contrast features of social anxiety across these disorders have the potential to contribute to increased phenotypic specificity. Moreover, these data will advance our understanding of how common behavioral traits may reflect unique underlying problems across disorders and may provide clues to possible differential treatment options across the two disorders.
Anxiety in Nonsyndromic Autism Spectrum Disorder
A high prevalence of anxiety disorders has been reported in individuals with nonsyndromic ASD, with 40–60% of youth meeting diagnostic criteria for an anxiety disorder and up to 37% diagnosed with social anxiety disorder (Kerns et al., 2014; White, Oswald, Ollendick, & Scahill, 2009). Although nearly 40% of persons with nonsyndromic ASD have ID (Christensen et al., 2016), most studies of social anxiety in nonsyndromic ASD have examined the relationship of social anxiety to IQ among individuals in the typical IQ range, with little work focused on individuals with comorbid ID and ASD. This is of concern given that cognitive level has clearly been reported as an important factor in the prevalence and profile of anxiety disorders in other populations (Reardon, Gray, & Melvin, 2014). Specifically, generalized anxiety disorder and separation anxiety were reported as more prevalent in youth with nonsyndromic ASD not having an ID (IQ >70) than in those with nonsyndromic ASD and a comorbid ID, whereas the prevalence of specific phobia, panic disorder, and social phobia were not different in those with and without comorbid ID (Sukhodolsky et al., 2008). The finding of an effect of IQ on the prevalence of anxiety disorders in nonsyndromic ASD has also been found in studies of samples of ASD without ID (Kerns et al., 2014). To date, only one study has examined social anxiety in persons with comorbid ASD and ID contrasted to an etiologically distinct group with ID (Thurman, McDuffie, Hagerman, & Abbeduto, 2014). This work is critical to determine if the diagnosis or treatment of social anxiety in “low functioning” children with ASD who have comorbid ID should be unique or similar to that in those not having ID.
A number of mechanisms have been proposed to account for elevated social anxiety in ASD. In a large-scale twin study, results indicated that children with ASD, their siblings diagnosed with the broader autism phenotype, and their siblings unaffected by ASD all displayed heightened social anxiety compared to typical controls implying a strong heritable component (Hallett et al., 2013). Neural indicators reflecting increased lateral prefrontal activation during anticipation to reward (Mikita et al., 2016) and increased bilateral amygdala activation to social reward that parallels that of persons with social anxiety disorder have been reported in individuals with ASD (Richey et al., 2014). Finally, children with ASD and a co-morbid anxiety disorder displayed blunted cortisol and heart rate responses to stress that distinguished them from children with ASD who did not have anxiety and from a typically developing control group, suggesting that non-adaptive physiological modulation may be implied mechanistically (Hollocks, Howlin, Papadopoulos, Khondoker, & Simonoff, 2014).
Although it is clear that social anxiety is highly prevalent in persons with ASD, there is a lack of consensus as to whether social anxiety in ASD is a common consequence of ASD (i.e., is part of the phenotype) or whether social anxiety is a unique comorbidity distinct from ASD (Kerns et al., 2014). Furthermore, controversy exists regarding whether the phenomenology of social anxiety in ASD is qualitatively similar to traditional Diagnostic and Statistical Manual (DSM) categorization or distinct and more reflective of ASD features (Kerns et al., 2014). In fact, a proposal has been made to designate anxiety disorders in ASD as either “traditional”, aligning with standard DSM criteria, or “atypical”, representing inconsistencies with DSM conventions and calling for the development of new instruments or the modifications of existing instruments to accurately reflect these categorizations of anxiety (Kerns et al., 2014).
In summary, social anxiety is highly prevalent in ASD. Cognitive ability likely has an effect on the prevalence and profile of social anxiety disorders in persons with ASD; however, the nature of this relationship is not consistent or clear. In fact, the majority of studies examining social anxiety in ASD have excluded persons with ID, have focused on the occurrence of symptoms of social anxiety rather than social anxiety disorder, and have seldom included cross-syndrome comparisons. The resulting gaps in our understanding of social anxiety in ASD will be addressed in the present study.
Anxiety in Fragile X Syndrome
FXS is a single-gene disorder that affects 1 in 3700 males and is the leading known inherited cause of ID (Schneider, Hagerman, & Hessl, 2009). Approximately 86% of males with FXS meet diagnostic criteria for an anxiety disorder (Cordeiro, Ballinger, Hagerman, & Hessl, 2011), and more individuals likely have subclinical symptoms. The co-occurrence of anxiety in FXS is associated with increased impairment at an individual level often leading to pharmacological treatment (Bailey, Raspa, Olmsted, & Holiday, 2008; Cordeiro et al., 2011), reduced employment, and higher financial burden at a family level (Ouyang, Grosse, Raspa, & Bailey, 2010). Although anxiety is frequently reported among males with FXS, only a single study has employed a diagnostic measure to evaluate anxiety, with most studies relying instead on broad-based rating scales of severity of anxiety-related symptoms. The majority of symptom-based research has reported elevations across broad indicators of anxiety and across multiple anxiety disorders, with social anxiety reported as particularly problematic (Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007; Thurman et al., 2014; Wheeler et al., 2014). These symptom-based findings are supported by diagnostic data indicating that social phobia was one of the most common anxiety disorders in males with FXS and ID, with 35% meeting DSM-IV criteria (Cordeiro et al., 2011). Of note, when the requirement of “worry” was removed in acknowledgment that many of the individuals could not express worry due to their ID, the prevalence of social phobia rose to 60% (Cordeiro et al., 2011). Compared to published work on idiopathic ID (n = 474; Dekker & Koot, 2003), males with FXS display rates of social phobia that are nearly 35 times higher than in idiopathic ID (60% versus 2%, respectively). The prevalence of social anxiety is significantly more common in adults than in children (Cordeiro et al., 2011), highlighting the importance of examining the predictors and consequences of social anxiety during the late adolescent and early adult years. This effort would help to direct treatment efforts aimed at improving adult outcomes in FXS, which are substantially lower than expected for their chronological age and IQ (Hartley et al., 2011).
Importantly, not only do males with FXS demonstrate elevated rates of social anxiety, but they also demonstrate increased risk for ASD (e.g., Clifford et al., 2007; Harris et al., 2008). Because the behavioral indicators of ASD overlap with those of social anxiety (e.g., gaze avoidance), it can be difficult to determine whether co-occurring symptoms contribute to diagnostic error with persons diagnosed with ASD rather than anxiety or if they represent a “true” comorbidity. In the only study to employ diagnostic measures of anxiety in FXS, the prevalence of social anxiety in males with FXS who met criteria for ASD was higher than those with FXS without ASD (Cordeiro et al., 2011). Likewise, in a series of studies examining dynamic aspects of social avoidance as one dimension of social anxiety (Roberts et al., 2007; Roberts et al., 2009), initial gaze avoidance during an interaction with an unfamiliar person appeared universal across boys with FXS with and without elevated symptoms of ASD, whereas sustained gaze avoidance was unique only to those with increased severity of ASD symptoms. Thus, initial within-group evidence suggests that DSM-based diagnostic categorization of social anxiety and observations of social withdrawal may differentiate males with FXS who do and do not have co-morbid ASD. However, it is again unclear if the social anxiety is comorbid with ASD or “biases” the clinical diagnosis toward ASD. In contrast, in one of the few cross-syndrome studies published, social anxiety did not differ across FXS (with or without ASD) and nonsyndromic ASD groups (Thurman et al., 2014). That said, the authors noted that social anxiety scores were correlated with general anxiety scores in FXS, but not in nonsyndromic ASD, suggesting between group differences in either the factors or mechanisms underlying performance on this measure (Thurman et al., 2014). Thus, this is a complex issue and it appears that findings depend greatly on measurement of social avoidance, suggesting the need to integrate multiple measurements of avoidance in assessing anxiety in FXS and ASD.
Multiple theories have been proposed to account for the elevated rate of anxiety in FXS with a number of studies focused on features of social anxiety in particular. Across studies, elevated physiological arousal is the primary putative candidate underlying features of social anxiety (Heilman, Harden, Zageris, Berry-Kravis, & Porges, 2011; Roberts et al., 2009). The role of physiological hyperarousal as a biological vulnerability to stress resulting in elevations of anxiety aligns with Barlow’s diathesis-stress model (Barlow, 2002). Hyperarousal in FXS is a well-established phenomenon with consistent reports of elevated baseline heart activity (Hall, Lightbody, Huffman, Lazzeroni, & Reiss, 2009; Klusek, Roberts, & Losh, 2015, for a review; Roberts, Tonnsen, Robinson, & Shinkareva, 2012) and heightened salivary cortisol (Hardiman & Bratt, 2016, for a review; Hessl et al., 2002).
Evidence supporting physiological arousal in direct association with anxiety symptoms, however, is mixed, with most work indicating systemic hyperarousal across baseline and stress-inducing conditions in FXS (Hall et al., 2009; Klusek, Martin, and Losh, 2013; Tonnsen, Malone, Hatton, & Roberts, 2013). Specific stress conditions, including conversation, the approach of a stranger, and cognitive challenges have not been associated with stimulus-bound increases in autonomic arousal as measured by heart activity (Roberts, Boccia, Bailey, Hatton, & Skinner, 2001; Klusek, et al., 2013; Tonnsen et al., 2013). Likewise, a number of behaviors reflective of elevated stress, including crying and task avoidance, have not been associated with elevated cortisol (see Hardiman & Bratt, 2016 for review). However, elevated salivary cortisol has been linked to features of social anxiety, as indexed by gaze avoidance (Hall et al., 2009), physical avoidance (Roberts et al., 2009), and elevated parent ratings of social avoidance (Matherly et al., in press). Thus, physiological arousal may contribute to the presence and expression of social anxiety in FXS, but the bulk of evidence supports the hypothesis that it is more likely a systemic rather than a stimulus-bound reaction (e.g., a pervasive condition of hyperarousal versus elevated arousal in response to discrete conditions).
In sum, existing evidence indicates that anxiety is common in males with FXS, with social anxiety being particularly prevalent and impairing. However, the associations of social anxiety with ID severity, ASD symptom severity, and chronological age are not well established in FXS, and differences in social anxiety presentation and source relative to nonsyndromic ASD are also unclear. These gaps in the literature can be attributed to the sparsity of studies, wide distribution of chronological ages in most work, a focus on social anxiety disorder versus symptoms of social anxiety, and measurement challenges. The present study was designed to address these gaps and limitations.
Current Study
Social anxiety is clearly prevalent and impairing in both FXS and nonsyndromic ASD, two etiologically distinct disorders that have a number of overlapping phenotypic features. Diagnostic determination of social anxiety in these neurodevelopmental disorders is made challenging by the multi-dimensional nature of social anxiety, features that overlap across disorders, and limitations in measurement that acutely impact studies of social anxiety in persons with ID. However, accumulating evidence indicates that it is critical to diagnose social anxiety in persons with ID so as to provide a foundation for the development of maximally efficacious treatments. As noted, such work is complex, and the inclusion of multiple measures of social anxiety, integration of biomarkers, and the use of cross-syndrome comparisons are essential to maximize the impact of this research (White et al., 2009).
The overarching goal of this study is to advance our understanding of the nature of social anxiety in males with FXS and its unique and shared associations with ASD. We accomplish this by creating a putative multivariate composite of social anxiety representing both behavioral and biological dimensions and examining mean differences on this composite across two groups – FXS and nonsyndromic ASD. We then conduct follow up analyses to determine which scales drive the group differences and how the groups differ on those scales. The objective is to understand similarities and differences in the extent, manifestation, and correlates of social anxiety between FXS and nonsyndromic ASD.
Methods
Participants
Participant data were collected as part of a larger longitudinal, multi-site study focused on language as one of several predictors of successful transition into adulthood (PI: Abbeduto) at the University of California, Davis MIND Institute and at the University of South Carolina. Data for the present cross-sectional analyses represent measures of social anxiety drawn from the first annual assessment. Subsequent analyses will extend this work by investigating the relationship of social anxiety and other aspects of the FXS phenotype to language and independent living in males with FXS during the critical transition into adulthood. The participants with FXS were recruited nationally for both sites through parent listservs, social media sites, advertisements by the National Fragile X Foundation and through the help of the Research Participant Registry Core of the Carolina Institute for Developmental Disabilities at the University of North Carolina at Chapel Hill for the USC site and the MIND Institute IDDRC Clinical Translational Core Registry at the UC Davis site. The nonsyndromic ASD group was largely recruited locally at each research site, through local postings, social media sites, parent support groups, and the South Carolina Department of Disabilities and Special Needs. The study received Institutional Review Board approval at both sites. The present analyses include a subset of measures from the project.
The participants were 77 adolescent and adult males aged 15–23 years (M = 18.18, SD = 2.3) with FXS (n= 59) or nonsyndromic ASD (n=18). The full mutation of the FMR1 gene (i.e., greater than 200 CGG repeats) was confirmed for the participants in the FXS group and ruled out in the nonsyndromic ASD group through review of records documenting molecular genetic testing prior to study participation. Of the FXS group, 62.7% were prescribed medication(s) at the time of the study, with 47.5% of the FXS group on medication(s) for anxiety. In the nonsyndromic ASD group, 55.6% were prescribed medication(s) with 27.8% on medication(s) for anxiety. In the FXS group, use of anxiety medication was significantly correlated with ADOS-2 severity score (r = .35, p < .01), but not with any of the social anxiety symptom variables. There were no significant correlations between anxiety medication use and any symptom variables in the nonsyndromic ASD group.
Measures
Social Avoidance Scale.
The Social Avoidance Scale, formerly referred to as the Social Approach Scale (SAS; Roberts et al., 2007; Roberts et al., 2009) is an experimental measure of multiple dimensions of social avoidance behavior. Capturing the complexity of this profile in FXS representing both social approach and social avoidance is not possible with most measurement tools as the majority of rating scales and diagnostic measures constrain responses about social avoidance to a binary “problem” or “not problem” designation. This fails to allow for nuances in behavior to be accurately captured as in when an individual is socially avoidant upon initial greeting only versus an individual who remains socially avoidant throughout the entire social exchange. Also, most measures provide only a single global composite score for social avoidance. We believe, however, that a multidimensional measure that captures the nuances of social avoidance may be needed characterize the social behaviors of persons with ID, especially given that their behavior may not conform to that of neurotypical individuals. Earlier social avoidance studies from our group (Roberts et al., 2007; Roberts et al., 2009) employed an experimental measure that included responses to both initial and prolonged social interaction across three dimensions of behavior: eye contact, facial expression, and bodily movement. Our results indicated that the majority of male children with FXS and less severe autism symptoms displayed initial social avoidance with a “warm-up” evident over time (Roberts et al., 2009). These data suggested that the initial social response of the children with FXS likely reflected social anxiety related to the onset of the research assessment.
In the present study, SAS ratings were made on three dimensions of behavior (Physical Movement, Facial Expression, and Eye Contact) upon initial interaction (i.e., the first minute of interaction when the participant arrives at the research site and first encounters the examiners) and after sustained interaction (i.e., the last hour of interaction to reflect social avoidance after given time to “warm up”). Physical approach represents bodily movement, with a high score indicating active avoidance such as walking away from the examiner. A high score on facial expression reflects elevated shyness or fear reflected by the position of the mouth, eyes, and brow regions. A high score on eye contact indicates minimal or poorly modulated eye contact. Data for this study came from the first minute of interaction on the first day of a two-day assessment averaged across all three dimensions as our focus was on basal levels of anxiety rather than in response to an experimental press. Raters were trained to ≥ 80% reliability on the SAS prior to data collection with an inter-rater reliability rate of 87% for the initial ratings for the first assessment day. Cronbach’s alpha for the mean initial interaction SAS score across the three dimensions for this study was α = .76. Raw scores for the measure range from 0–5 and were used for analysis. Ratings were made in real-time from direct observation.
Baseline Cortisol.
Baseline cortisol reflects activation of the hypothalamic-pituitary-adrenal axis, which contributes to emotional and behavioral regulation through its role as a biological stress response system. Elevated chronic or stimulus-bound responses represent a maladaptive response to stress and are associated with physical and mental health consequences including anxiety disorders (Zorn et al., 2017). Salivary cortisol was collected using either a salivette that was placed in the participant’s mouth for 1 minute (64% of sample) or using passive drool methods, depending on participant compliance. Saliva was collected via both methods on a subset of participants (n = 28) to test comparability across collection methods; the values obtained across methods were highly correlated at r = .73, p = .01. Samples were taken within 15 minutes of arrival at the testing site on the first day of the two-day assessment and prior to initiation of the research assessment as a “pre-assessment baseline” at approximately the same time of day (9:00 AM). To reduce potential contamination effects, participants were asked to refrain from drinking or eating dairy products or those with citric acid for approximately an hour prior to arrival (Schwartz, Granger, Susman, Gunnar, & Laird, 1998). The samples were then stored in a −20 °C freezer until shipped and analyzed by the Daacro Saliva Lab at Trier University, Trier, Germany. Cortisol levels were measured in micrograms/deciliters and determined employing a competitive solid phase time-resolved fluorescence immunoassay with flouromeric end point detection (DELFIA) using radioimmunoassay (Hessl et al., 2002; Roberts et al., 2009). The intra-assay coefficient of variation was between 4.0 to 6.7%, and the corresponding inter-assay coefficients of variation were between 7.1 to 9.0%. Each sample was assayed in duplicate with duplicate correlations of > .95.
Anxiety Depression and Mood Scale: Avoidance Subscale.
The Anxiety Depression and Mood Scale (ADAMS; Esbensen, Rojahn, Aman, & Ruedrich, 2003) is a 28-item caregiver report with item responses ranging from 0 to 3. The measure includes five subscales – Mania/Hyperactivity, Depressed Mood, Social Avoidance, General Anxiety, and Obsessive/Compulsive. The ADAMS was normed for populations with ID. For this study, the Social Avoidance subscale was used as a measure of social anxiety, with acceptable internal consistency for our sample at α = .84. The Social Avoidance subscale includes, for example, the items “Withdraws from other people” and “Avoids peers”. The Social Avoidance scale was developed to reflect social anxiety (A. Esbensen, personal communication, 3/9/2017) and aligns with the revised DSM-5 classification of social anxiety disorder. Thus, we refer to the ADAMS Social Avoidance scale as social anxiety given the measure authors’ intent and to reflect theoretical advances and current DSM conventions. The participant’s parent, typically the biological mother, completed this measure. Raw scores from the ADAMS Social Avoidance subscale range from 0 to 21 and were used for analysis. Higher scores reflect greater problems.
Child Behavior Checklist: Anxiety Problems Subscale.
The Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001) is a 113-item caregiver rating scale of emotional and behavioral functioning for children adolescents between 6 and 18 years old. Caregivers rate their child’s behaviors over the past 6 months ranging from 0 to 3 on each item that factors into syndrome scores and DSM-oriented disorders. The Anxiety Problems Subscale is a DSM-oriented scale that measures features of Generalized Anxiety Disorder, Specific Phobia, Social Anxiety and Separation Anxiety Disorder. The Anxiety Problems subscale included the items “Fears going to school” and “Nervous, high-strung, or tense”. Although this measure does not focus on social anxiety, there are items within the subscale that have face validity as symptoms of social anxiety. The CBCL has adequate reliability and validity, including extensive normative data, and content and predictive validity in the differences of clinical samples of children compared with non-clinical samples (Achenbach & Rescorla, 2001). The participant’s parent, typically the biological mother, completed this measure. Approximately 31% of the current study sample scored above the clinical cutoff range for Anxiety Problems. Raw scores were used during analysis to avoid truncating scores and ranged from 0 to 12, with higher scores reflecting greater problem behavior (Nakamura, Ebesutani, Bernstein, & Chorpita, 2009). We observed marginal internal consistency for our sample (α = .68).
Autism Diagnostic Observation Schedule-2 and the Autism Diagnostic Interview-Revised.
ASD diagnosis was confirmed through the administration of the Autism Diagnostic Observation Schedule-Second Edition (ADOS-2; Lord et al., 2012) and Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994). The ADOS-2 is a semi-structured, standardized assessment of communication, social interaction, play, and restricted and repetitive behaviors. The ADOS-2 Comparison Score reflects the severity of symptoms across social affective domain and restricted and repetitive behavior domain relative to individuals of a similar chronological and language level. The Comparison Score is measured from 1 to 10 in the ADOS-2 and were used for analysis in the current study. The ADI-R is a standardized, diagnostic parent interview that assesses the presence of ASD behaviors relating to language and communication, reciprocal social interactions, and restricted, repetitive, or stereotyped behaviors. The ADI-R results in a categorical diagnosis of autism or no autism. In this study, the biological mother was interviewed in the ADI-R. Both the ADI-R and ADOS-2 were administered and scored by graduate-level professionals who had completed research reliability training. A second scorer measured inter-rater reliability in a randomly selected 15% of administrations. Across both sites, the inter-rater agreement was 81% for the ADOS-2 total score and 91% for the ADI-R total score. Using the Risi criteria (Risi et al., 2006) we subdivided the group with FXS into those with ASD (n = 44) and those without ASD (n = 15) to supplement our analyses using the ADOS-2 as a continuous measure. This was done to contribute to the ongoing debate regarding the nature of ASD in those with FXS. Thus, the primary analyses used the ADOS-2 calibrated severity score as a continuous measure with supplemental analyses examining ASD as a categorical factor.
The Leiter International Performance Scale-Revised.
The Leiter International Performance Scale-Revised (Leiter-R; Roid & Miller, 1997) is a measure of nonverbal intellectual ability. In this study, the Brief-IQ composite was administered, which consists of Figure Ground, Form Completion, Sequential Order, and Repeated Patterns subscales with scaled scores ranging from 36 to 169. The Brief-IQ has shown consistent internal reliability (α = 0.65 – 0.86), and previous studies have shown excellent psychometric properties for adolescents in both internal consistency (α = .89) and convergent validity with the Wechsler Intelligence Scale–Third Edition (WISC–III) Full Scale IQ (r = .85; Roid & Miller, 1997). To reduce potential floor effects, growth scores (GSV) were used in the analysis of group differences rather than standard scores.
Procedures
Although the assessment was conducted over two consecutive days, the SAS scoring utilize in this study study focuses on social avoidance responses at the outset of the assessment on the first day due to our interest in the initial social responses of males with FXS. The assessment was approximately five hours long on Day 1 for both sites. The first day of testing included assessments of language and cognition and the second day consisted of measures of ASD and parent interviews in a standardized order. All data reported here come from the first annual assessment in the longitudinal study.
Data Analysis
Analyses were conducted in SPSS (IBM Corp., 2015). First, descriptive statistics were examined for each of the anxiety symptom scales (see Tables 1a & ab) to examine means and ranges across groups and to identify potential violations of distributional assumptions. For our primary research question, group differences on a multivariate outcome variable (comprised of the SAS score, the ADAMS Social Anxiety scale score, baseline salivary cortisol, and the CBCL Anxiety Problems scale score) were tested with a one-way, between-subjects multivariate analysis of covariance (MANCOVA). This analytical approach was implemented given our focus on manifest variable comparisons consistent with the capacity of the data, which did not support the use of factor analysis (March, Hau, Balla, & Grayson, 1998). In addition, our sample size was inadequate for latent variable models (e.g., factor analyses, structural equation modeling), and we opted to use the MANCOVA as the most robust analytic model that allows for correlated dependent variables by design. Levene’s test indicated that the assumption of homogeneity of variance for the analysis was not violated (p = .409). Follow-up analyses corrected with Tukey’s HSD for multiple comparisons assessed specific group differences on the multivariate anxiety outcome. Finally, linear discriminant function coefficients were examined to determine how each of the dependent variables of the multivariate outcome contributed to differentiation between groups. These coefficients were subsequently multiplied by standardized z-scores for each outcome variable to create a weighted linear composite (Enders, 2003) for assessing the relationship between the multivariate anxiety outcome and ASD severity. To facilitate interpretation, the inverse was taken for the multivariate composite score so that a higher score reflected increased anxiety symptoms. To follow up on these primary findings, we tested whether mean differences were present across groups on those measures found to most strongly contribute to the multivariate composite using an ANOVA. We examined group differences first keeping the FXS group intact and comparing them to the nonsyndromic ASD group. We followed up these analyses by creating three groups, with the nonsyndromic ASD group contrasted to the group with FXS determined to meet diagnostic criteria for ASD (Risi et al., 2006) and the group with FXS not meeting criteria for ASD. These complementary approaches were used to contribute to the debate in the field regarding viewing ASD severity as a continuous symptom-based variable or as a categorical variable. ASD symptom severity and Leiter-R growth scores were included as covariates in the two-group continuous models with only Leiter-R growth scores co-varied in the three-group analyses given that ASD symptom severity is accounted for by the categorical designation and covariation of ASD symptom severity in the three-group analyses would violate assumptions given that a true pre-existing group difference that is dependent on the group membership exists (Miller & Chapman, 2001).
Table 1a.
Descriptive Statistics for Anxiety Predictors and Composite Anxiety Indices Raw Scores Across Continuous Groups
| Groups | ||
|---|---|---|
| FXS n = 59 |
ASD n = 18 |
|
| Age | 18.34 (2.33) | 17.65 (2.39) |
| M (SD) | ||
| Leiter-R | 39.10 (5.31) | 58.65 (22.8) |
| M (SD) | ||
| ADOS-2 Severity | 5.79 (2.20) | 6.94 (1.59) |
| M (SD) | ||
| SAS Initial Average | 1.64 (1.25) | 2.14 (1.03) |
| M (SD) | ||
| Baseline Cortisol | .278 (.226) | .183 (.146) |
| M (SD) | ||
| ADAMS Avoidance | 6.58 (4.48) | 8.33 (4.89) |
| M (SD) | ||
| CBCL Anxiety Problems | 3.54 (2.29) | 4.11 (3.16) |
| M (SD) |
Table 1b.
Descriptive Statistics for Anxiety Predictors and Composite Anxiety Indices Raw Scores Across Categorical Groups
| Variables | Group | ||
|---|---|---|---|
| FXS-O n = 15 |
FXS-A n = 44 |
ASD n = 18 |
|
| Age | 18.27 (2.88) | 18.33 (2.17) | 17.39 (2.21) |
| M (SD) | |||
| Leiter-R | 42.43 (7.21) | 38.09 (4.12) | 58.65 (22.76) |
| M (SD) | |||
| ADOS-2 Severity | 3.27 (2.37) | 6.71 (1.33) | 6.69 (1.45) |
| M (SD) | |||
| SAS Initial Average | 1.49 (1.19) | 2.56 (1.18) | 2.15 (1.03) |
| M (SD) | |||
| Baseline Cortisol | .294 (.231) | .272 (.227) | .183 (.146) |
| M (SD) | |||
| ADAMS Avoidance | 4.73 (4.22) | 7.57 (4.38) | 8.33 (4.89) |
| M (SD) | |||
| CBCL Anxiety Problems | 2.58 (2.39) | 3.83 (2.21) | 4.11 (3.16) |
| M (SD) | |||
Note. Social Avoidance Scale Day 1, Scale 1, Rater 1 Average (SAS Initial Average); Anxiety, Depression, and Mood Scale Avoidance Subscale (ADAMS Avoidance); Child Behavior Checklist Anxiety Problems Subscale (CBCL Anxiety Problems)
Results
Omnibus Group Difference on Multivariate Anxiety Composite
Continuous Analysis.
The first MANCOVA model included all four anxiety symptom indicators across two primary diagnostic groups, FXS and nonsyndromic ASD, with Leiter-R growth scores and ADOS-2 severity scores co-varied. Two canonical variables emerged from the multivariate analyses with one nearing significance, λ = .77, F(2, 63) = 1.94, p = .06, R2c = .12. Baseline cortisol contributed least to the model and was not significantly correlated with the other dependent variables across both groups. Thus, we excluded cortisol from the final model.
In the revised model with cortisol excluded, two canonical variables emerged from the MANCOVA analyses with one reaching significance, λ = .79, F(2,63) = 2.61, p = .020, R2c = .114, indicating that the multivariate composite indexed a single underlying construct. Results indicated that the groups significantly differed on this multivariate composite, Wilks’ lambda, λ = .79, F(6, 122) = 2.615, p = .020, R2c = .191. The nonsyndromic ASD group scored higher on the composite (M=.1112) than the FXS group (M=.0708). Examination of the discriminant function coefficients for the composite indicated that group differences were primarily driven by the SAS (b = −.70305) and ADAMS Social Anxiety (b = −.50995) measures. The contributions of the CBCL anxiety scale (b = −.21772) discriminated groups to a lesser degree (see Table 2).
Table 2.
Standardized Discriminant Function Coefficients for Anxiety Composite
| Variable | Coefficient Weights |
|---|---|
| SAS Initial Average | −.70305 |
| ADAMS Avoidance | −.50995 |
| CBCL Anxiety Problems | −.21772 |
Note. Coefficients are regression weights that are multiplied by the z-scores used to create the multivariate anxiety composite with a higher coefficient reflecting higher symptomatology.
Categorical Analyses.
In this 3-group (FXS without ASD, FXS with ASD, and nonsyndromic ASD) analysis with the three anxiety symptom scales with IQ co-varied, the MANCOVA did not reach significance λ = .96, F(2, 67) = .8379, p = .478, R2c = .038.
Follow-up Analyses
Continuous Analysis.
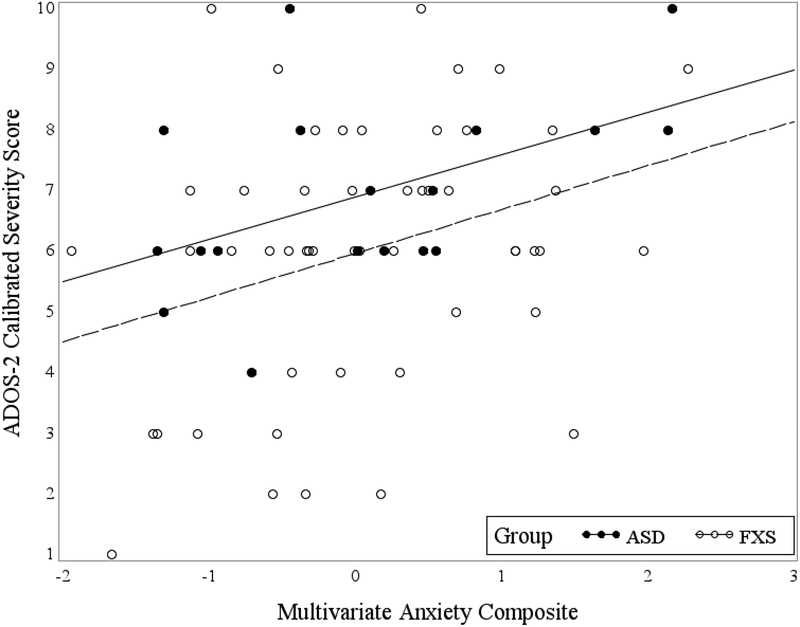
To follow-up on the primary analyses, we conducted two ANOVAs co-varying for IQ and ADOS-2 symptom severity to examine group differences (FXS vs. nonsyndromic ASD) on the measures that most strongly contributed to group discriminations on the composite. Although the FXS group had a higher mean score than the nonsyndromic ASD group on the SAS (2.288 versus 2.148 respectively), the groups were not significantly different; F(1, 74) = .149, p = .701, R2c = .142). Likewise, the nonsyndromic ASD group had a higher mean score than the FXS group on the ADAMS (8.33 versus 6.85 respectively), but the group difference was not significant; F(1, 74) = .193, p = .661, R2c = .085). To enhance interpretation of the multivariate composite with respect to its relationship to ASD severity, a Pearson correlation between the ADOS-2 and composite variable was computed. Results indicated a moderate relationship in both groups: r = .38, p = .007 in FXS and r = .49 and p = .037 in nonsyndromic ASD. Figure 1 illustrates a scatterplot of this relationship.
Figure 1.
Multivariate anxiety composite
Categorical Analysis.
A one-way ANOVA across the three diagnostic groups (FXS without ASD, FXS with ASD, and nonsyndromic ASD) and the anxiety indices controlling for IQ indicated significant differences on the SAS (F(2, 75) = 3.408, p = .039) and a trend toward a significant difference on the ADAMS Social Anxiety scale (F(2, 75) = 3.025, p = .055). Post hoc LSD analysis showed that the significant difference on the SAS scale reflected lower scores for the FXS without ASD group than for the group with FXS with comorbid ASD (p = .043). On the ADAMS Social Anxiety Scale, post hoc LSD analysis indicated a trend for the FXS without ASD to have lower scores than the nonsyndromic ASD group (p = .068).
Discussion
FXS is a single-gene neurodevelopmental disorder associated with a high rate (60%) of social anxiety (Cordeiro et al., 2011). Despite the high prevalence and significant negative impact of social anxiety on males with FXS and their families, little is known about its mechanistic underpinnings and clinical profile. This is due, in part, to the complex nature of social anxiety along with complications and limitations of measurement, leaving the field with important gaps in what is known about the nature and manifestation of social anxiety in FXS and related clinical groups. Furthermore, a more thorough understanding of the pathogenesis of anxiety in FXS can elucidate the impact and potential contribution to other co-morbid conditions, notably ASD. In the present study, our aim was to advance understanding of the nature, correlates, and mechanisms of social anxiety in males with FXS with a focus on its association with ASD.
Group Differences on Multivariate Composite
In recognition of the multifaceted nature of social anxiety, we created a composite comprised of multiple indices selected to measure various facets of social anxiety gleaned from different measurement sources and examined differences across males with FXS and a nonsyndromic ASD group. Two parent-rating scales were included in our composite with one standardized on neurotypical individuals (CBCL Anxiety) and the second on persons with intellectual and developmental disabilities (ADAMS Social Anxiety). In addition, we included a direct observation of initial social avoidance defined by eye contact, physical movement and facial expression (SAS), as well as a physiological biomarker (salivary cortisol).
Initial composite analyses utilizing all four anxiety measures indicated that no significant latent factors emerged and that cortisol contributed least to the composite. To refine the model, we excluded cortisol from the composite. New analyses with the three anxiety indices (cortisol dropped) suggested a single, multidimensional construct that was significant. We confirmed this by computing correlations that indicated no relationship between cortisol and the other three anxiety indices or the anxiety composite. These findings suggest that baseline cortisol represents a variable independent from the other three anxiety markers. This is consistent with our previous work indicating no relationship of baseline cortisol to initial SAS ratings in boys with FXS (Roberts et al., 2009). It is also consistent with evidence from an independent group of investigators showing that cortisol was not related to the anxiety subscale of the CBCL in males with FXS (Hessl et al., 2002).
In studies examining the relationship of ASD symptoms to cortisol in FXS, elevated baseline cortisol has been associated with increased ASD symptom severity (Roberts et al., 2009) and reduced eye contact (Hall et al., 2009) with a tendency to associate positively with the withdrawal subscale of the CBCL (Hessl et al., 2001). In contrast, elevated baseline cortisol has been associated with reduced ASD symptom severity in one study (Hall et al., 2009). Thus, salivary cortisol has been associated with ASD and anxiety symptoms, but not consistently. Given that both elevated and blunted salivary responses reflect stimulus-bound stress reactivity depending on the nature and duration of the stressor (Laurent, Gilliam, Wright, & Fisher, 2015; Slopen, McLaughlin, & Shonkoff, 2014), these findings may indicate both short and long-term effects of stress in children with FXS. Collectively, the current study and existing data suggest that the relationship between cortisol and the FXS phenotype is unclear with associations between cortisol and both ASD and anxiety features in some work but not in others.
Results using the revised three-index multivariate composite indicated that the groups differed on a single underlying function, with the FXS group having a lower score than the nonsyndromic ASD group. The SAS most strongly contributed to the group differences with the ADAMS also contributing to a moderate degree and the CBCL contributing the least. Given our follow-up analyses showing no group differences on any of the individual measures, the multivariate composite appears to represent a stronger discriminating factor than the SAS and ADAMS individually when the FXS participants are not dichotomized into those with and without ASD. In contrast, when the FXS participants are categorized as having comorbid ASD or not, the multivariate composite does not discriminate across these three groups while the individual measures do. The group with FXS without ASD displayed lower SAS scores than the group with FXS with co-morbid ASD and lower scores on the ADAMS Social Anxiety subscale than the nonsyndromic ASD group. Thus, the two groups with ASD (FXS and nonsyndromic ASD) are not distinguishable on either the composite or the individual measures, whereas the group with FXS without ASD is also not distinguishable from the two groups with ASD on the composite but is at the individual measure level for traits of social avoidance and social anxiety. These findings align with both a theoretical model and empirical data supporting the notion that representing complex traits, such as social anxiety, is best accomplished by employing multiple indicators.
It is unclear whether the composite reflects social anxiety or is simply an alternative indicator of ASD symptom severity as evidence supports both interpretations. In support of the composite reflecting ASD symptom severity, there is a moderate to strong relationship between the ADOS-2 severity score and the composite score for both the nonsyndromic ASD and FXS groups. Reflecting on components of the composite measure may provide further insights into this issue. The SAS measures initial social avoidance in an experimental setting and the ADAMS is a parental rating of social withdrawal across multiple settings. Thus, both of these measures contain features that overlap with the social impairments associated with ASD diagnostic criteria. In contrast, the CBCL measures aspects of social anxiety along with generalized anxiety. Collectively, these points speak to the possibility that the initial SAS rating and Social Anxiety on the ADAMS may index aloofness and avoidance in males with FXS comorbid with ASD, which are core features associated with ASD; yet, social avoidance is also a key feature of social anxiety, again highlighting the challenge when features overlap across disorders.
Alternatively, evidence from our study suggests that the composite is not simply a reflection of ASD features as the three-group analyses with the FXS group dichotomized into those with and without ASD indicated that the composite did not discriminate across these three groups. Thus, despite the group with FXS without ASD having lower SAS scores than the group with FXS with ASD and lower mean social anxiety scores on the ADAMS than the group with nonsyndromic ASD, the groups were not discriminated when the composite was used. Collectively, our results suggest that anxiety and ASD symptoms are closely related in complex ways in these groups and that the measures employed in this study may lack sufficient discrimination or may differ across groups. This conclusion is supported by work showing a bidirectional relationship between internalizing symptoms and ASD features across childhood in a longitudinal community twin sample (Hallett, Ronald, Rijsdijk, & Happé, 2010). Specifically, ASD-related social affective impairments and restricted and repetitive behaviors were associated with social anxiety with genetic contributions implied (Hallett, Ronald, Rijsdijk, & Happé, 2012). Also, social anxiety on the ADAMS has been shown to be more strongly related to the general anxiety scale on the ADAMS for those with FXS than it was for those with nonsyndromic ASD (Thurman et al., 2014) indicating that the expression of dimensions of anxiety may differ across clinical groups.
Summary
Findings from this study confirm that characterizing anxiety as part of the FXS phenotype is complex with a number of cautions raised regarding measurement. First, an attempt to create a multivariate social anxiety composite comprised of parental rating scales, direct observations and a physiological biomarker revealed that the biomarker, salivary cortisol, was unrelated to the other scales and its inclusion prevented the identification of a clear unifying latent trait across the multiple measures. This suggests that salivary cortisol may be a biomarker indicative of a more generalized disrupted arousal system rather than one that is mechanistically specific to social avoidance. Second, we found that the initial rating of the SAS and the social avoidance scale of the ADAMS were strongly associated with the ADOS-2 severity score across both the FXS and nonsyndromic ASD groups, with these two measures contributing most strongly to the composite. The current results are generally consistent with our earlier findings that initial social avoidance characterized all males with FXS, whereas prolonged social avoidance was associated only with boys with FXS who had elevated ASD features (Roberts et al., 2007). As such, we interpret the composite to reflect ASD symptom severity along with elements of social anxiety, recognizing that features of ASD and social anxiety clearly overlap and that measures of these complex traits likely represent elements from both disorders (e.g., social anxiety could drive scores on the ADOS-2 and vice versa).
Limitations and Future Directions
Although there are a number of strengths of this study, including our focus on males who are low functioning, implementation of multiple measures including a biomarker in a cross syndrome design, limitations exist. Our sample sizes are small, particularly for the nonsyndromic ASD group. We also were not able to recruit a sample with nonsyndromic ASD that was matched on both nonverbal IQ and expressive language to our sample with FXS despite that being our goal. The primary barrier we encountered was that males with nonsyndromic ASD who met the minimum language requirements nearly universally had a higher nonverbal IQ than those with FXS. Participants with nonsyndromic ASD who had a similar nonverbal IQ were often non-verbal or did not meet the minimum language inclusion criteria highlighting a potential phenotypic distinction across these two groups (Thurman et al., 2017). In addition, the instruments were constrained by those available through the larger parent study that this study was drawn from. Future studies should have larger samples, include females, include more targeted instruments (e.g., the Aberrant Behavior Checklist – FXS adaptation; Sansone et al., 2012) 15and employ a longitudinal design to examine potential developmental effects that are beyond the scope of this study. Also, future work should examine these relationships in younger children to determine if these same relationships are present earlier in development and if precursors for later emerging anxiety symptoms might be present in young children which could direct treatment efforts. Questions regarding the inter-relationship between anxiety and ASD features in FXS clearly need additional study including longitudinal studies that investigate the emergence, trajectory, and co-occurrence of both disorders. Future work that investigates the potential role of the GABA system are needed particularly given its potential as a targeted treatment (Lozano, Hare, & Hagerman, 2014). This is particularly salient given recent evidence that social communication difficulties in a population-based sample predicted elevated features of social anxiety while social anxiety did not predict later social communication symptoms (Pickard, Rijsdijk, Happé, & Mandy, 2017).
References
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms and Profiles. Journal of Child Neurology, 40(4), 791–799. 10.1016/S1474-4422(03)00585-4 [DOI] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, & Holiday DB (2008). Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics, Part A, 146(16), 2060–2069. 10.1002/ajmg.a.32439 [DOI] [PubMed] [Google Scholar]
- Barlow DH (2002). Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic, 2nd ed. American Journal of Psychiatry (Vol. 159). 10.1176/appi.ajp.159.8.1453 [DOI] [Google Scholar]
- Casey MB, & Sleigh MJ (2001). Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology, 39(2), 107–123. 10.1002/dev.1035 [DOI] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN, … Yeargin-Allsopp M (2016). Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morbidity and Mortality Weekly Report. Surveillance Summaries, 65(3), 1–23. 10.15585/mmwr.ss6503a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, & Loesch DZ (2007). Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders, 37(4), 738–747. 10.1007/s10803-006-0205-z [DOI] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, & Hessl D (2011). Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders, 3(1), 57–67. 10.1007/s11689-010-9067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker MC, & Koot HM (2003). DSM-IV Disorders in Children With Borderline to Moderate Intellectual Disability. II: Child and Family Predictors. Journal of the American Academy of Child & Adolescent Psychiatry, 42(8), 923–931. 10.1097/01.CHI.0000046891.27264.C1 [DOI] [PubMed] [Google Scholar]
- Enders CK (2003). Performing multivariate group comparisons following a statistically significant MANOVA. Measurement and Evaluation in Counseling and Development, 36, 40–56. [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, & Ruedrich S (2003). Reliability and Validity of an Assessment Instrument for Anxiety, Depression, and Mood among Individuals with Mental Retardation. Journal of Autism and Developmental Disorders, 33(6), 617–629. [DOI] [PubMed] [Google Scholar]
- Green S. a, Berkovits LD, & Baker BL (2014). Symptoms and Development of Anxiety in Children with or Without Intellectual Disability. Journal of Clinical Child and Adolescent Psychology : The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53, 4416(December), 37–41. 10.1080/15374416.2013.873979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, & Reiss AL (2009). Physiological Correlates of Social Avoidance Behavior in Children and Adolescents With Fragile X Syndrome. Journal of the American Academy of Child & Adolescent Psychiatry, 48(3), 320–329. 10.1097/CHI.0b013e318195bd15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett V, Lecavalier L, Sukhodolsky DG, Cipriano N, Aman MG, McCracken JT, … & Sikich L (2013). Exploring the manifestations of anxiety in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(10), 2341–2352. 10.1007/s10803-013-1775-1.Exploring [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, & Happé F (2010). Association of autistic-like and internalizing traits during childhood: A longitudinal twin study. American Journal of Psychiatry, 167(7), 809–817. 10.1176/appi.ajp.2009.09070990 [DOI] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, & Happé F (2012). Disentangling the associations between autistic-like and internalizing traits: A community based twin study. Journal of Abnormal Child Psychology, 40(5), 815–827. 10.1007/s10802-011-9596-1 [DOI] [PubMed] [Google Scholar]
- Hardiman RL, & Bratt A (2016). Hypothalamic-pituitary-adrenal axis function in Fragile X Syndrome and its relationship to behaviour: A systematic review. Physiology & Behavior, 167, 341–353. 10.1016/j.physbeh.2016.09.030 [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, … Hagerman RJ (2008). Autism Profiles of Males With Fragile X Syndrome. American Journal on Mental Retardation, 113(6), 427–438. 10.1352/2008.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Harden ER, Zageris DM, Berry-Kravis E, & Porges SW (2011). Autonomic regulation in fragile X syndrome. Developmental Psychobiology, 53(8), 785–795. 10.1002/dev.20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, & Reiss AL (2001). The Influence of Environmental and Genetic Factors on Behavior Problems and Autistic Symptoms in Boys and Girls With Fragile X Syndrome. Pediatrics, 108(5), e88–e88. 10.1542/peds.108.5.e88 [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, & Reiss AL (2002). Cortisol and behavior in fragile X syndrome. Psychoneuroendocrinology, 27(7), 855–872. 10.1016/S0306-4530(01)00087-7 [DOI] [PubMed] [Google Scholar]
- Hollocks MJ, Howlin P, Papadopoulos AS, Khondoker M, & Simonoff E (2014). Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology, 46, 32–45. 10.1016/j.psyneuen.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Kerns CM, Kendall PC, Berry L, Souders MC, Franklin ME, Schultz RT, … Herrington J (2014). Traditional and atypical presentations of anxiety in youth with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(11), 2851–2861. 10.1007/s10803-014-2141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, & Wang PS (2008). The Descriptive Epidemiology of Commonly Occurring Mental Disorders in the United States *. Annu. Rev. Public Health, 29, 115–29. 10.1146/annurev.publhealth.29.020907.090847 [DOI] [PubMed] [Google Scholar]
- Klusek Jessica, Martin Gary, and Losh M (2013). Physiological Arousal in Autism and Fragile X Syndrome: Group Comparisons and Links With Pragmatic Language. American Journal on Intellectual and Developmental Disabilities, 118(6), 475–495. 10.1016/j.humov.2008.02.015.Changes [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Roberts JE, & Losh M (2015). Cardiac autonomic regulation in autism and Fragile X syndrome: A review. Psychological Bulletin, 141(1), 141–175. 10.1037/a0038237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Gilliam KS, Wright DB, & Fisher PA (2015). Child anxiety symptoms related to longitudinal cortisol trajectories and acute stress responses: Evidence of developmental stress sensitization. Journal of Abnormal Psychology, 124(1), 68–79. 10.1037/abn0000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism diagnostic observation schedule: ADOS-2. Western Psychological Services; Los Angeles, CA. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Lozano R, Hare EB, Hagerman RJ. (2014). Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr Dis Treat. 16;10:1769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherly S, Klusek J, Thurman AJ, McDuffie A, Abbeduto L, & Roberts JE (in press). Cortisol profiles differentiated in adolescents and young adults males with fragile X syndrome versus autism spectrum disorder. Developmental Psychobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, & Chapman JP (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40–48. [DOI] [PubMed] [Google Scholar]
- Nagata T, Suzuki F, & Teo AR (2015). Generalized social anxiety disorder: A still-neglected anxiety disorder 3 decades since Liebowitz’s review. Psychiatry and Clinical Neurosciences. 10.1111/pcn.12327 [DOI] [PubMed] [Google Scholar]
- Nakamura BJ, Ebesutani C, Bernstein A, & Chorpita BF (2009). A Psychometric Analysis of the Child Behavior Checklist DSM-Oriented Scales. Journal of Psychopathology and Behavioral Assessment, 31(3), 178–189. 10.1007/s10862-008-9119-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang L, Grosse S, Raspa M, & Bailey D (2010). Employment impact and financial burden for families of children with fragile X syndrome: Findings from the National Fragile X Survey. Journal of Intellectual Disability Research, 54(10), 918–928. 10.1111/j.1365-2788.2010.01320.x [DOI] [PubMed] [Google Scholar]
- Pickard H, Rijsdijk F, Happé F, & Mandy W (2017). Are Social and Communication Difficulties a Risk Factor for the Development of Social Anxiety? Journal of the American Academy of Child & Adolescent Psychiatry, 56(4), 344–351.e3. 10.1016/j.jaac.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon TC, Gray KM, Melvin GA. Anxiety disorders in children and adolescents with intellectual disability: prevalence and assessment. Res Dev Disabil. 2014;36C:175–190. [DOI] [PubMed] [Google Scholar]
- Richey J. a., Rittenberg a., Hughes L, Damiano CR, Sabatino a., Miller S, … Dichter GS (2014). Common and distinct neural features of social and non-social reward processing in autism and social anxiety disorder. Social Cognitive and Affective Neuroscience, 9(3), 367–377. 10.1093/scan/nss146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, … Pickles A (2006). Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 45(9), 1094–103. 10.1097/01.chi.0000227880.42780.0e [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long ACJ, & Kaufmann WE (2009). Autistic behavior in boys with fragile X syndrome: Social approach and HPA-axis dysfunction. Journal of Neurodevelopmental Disorders, 1(4), 283–291. 10.1007/s11689-009-9028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen B, Robinson A, & Shinkareva SV (2012). Heart activity and autistic behavior in infants and toddlers with fragile X syndrome. American Journal on Intellectual and Developmental Disabilities, 117(2), 90–102. 10.1352/1944-7558-117.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LAH, Hatton DD, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37(9), 1748–1760. 10.1007/s10803-006-0305-9 [DOI] [PubMed] [Google Scholar]
- Sansone SM, Widaman KF, Hall SS, Reis AL, Lightbody A, Kaufmann WE, Berry-Kravis E, Lachiewicz A, Brown EC, & Hessl D (2012). Psychometric study of the abberrant behavior checklist in fragile X syndrome and implications for targeted treatment. Journal of Autism and Developmental Disabilities, 42(7), 1377–1392. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3290710/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Hagerman RJ, & Hessl D (2009). Fragile X syndrome - From genes to cognition. Developmental Disabilities Research Reviews, 15(4), 333–342. 10.1002/ddrr.80 [DOI] [PubMed] [Google Scholar]
- Schneier FR, Heckelman LR, Garfinkel R, Campeas R, Fallon BA, Gitow A, … Liebowitz MR (1994). Functional impairment in social phobia. The Journal of Clinical Psychiatry, 55(8), 322–31. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8071299 [PubMed] [Google Scholar]
- Schwartz EB, Granger D. a, Susman EJ, Gunnar MR, & Laird B (1998). Assessing salivary cortisol in studies of child development. Child Development. 10.1111/j.1467-8624.1998.tb06173.x [DOI] [PubMed] [Google Scholar]
- Slopen N, McLaughlin KA, & Shonkoff JP (2014). Interventions to Improve Cortisol Regulation in Children: A Systematic Review. PEDIATRICS, 133(2), 312–326. 10.1542/peds.2013-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, … Vitiello B (2008). Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. Journal of Abnormal Child Psychology, 36(1), 117–128. 10.1007/s10802-007-9165-9 [DOI] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman R, & Abbeduto L (2014). Psychiatric symptoms in boys with fragile X syndrome: A comparison with nonsyndromic autism spectrum disorder. Research in Developmental Disabilities, 35(5), 1072–1086. 10.1016/j.ridd.2014.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman AJ, McDuffie A, Hagerman RJ, Josol CK, & Abbeduto L (2017). Language skills of males with fragile X syndrome or nonsyndromic autism spectrum disorder. Journal of autism and developmental disorders, 47(3), 728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Malone PS, Hatton DD, & Roberts JE (2013). Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. Journal of Abnormal Child Psychology, 41(2), 267–280. 10.1007/s10802-012-9671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler A, Raspa M, Bann C, Bishop E, Hessl D, Sacco P, & Bailey DB (2014). Anxiety, attention problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. American Journal of Medical Genetics, Part A, 164(1), 141–155. 10.1002/ajmg.a.36232 [DOI] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, & Scahill L (2009). Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review, 29(3), 216–229. 10.1016/j.cpr.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn JV, Schur RR, Boks MP, Kahn RS, Joels M, & Vinkers CH (2017). Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology, 77, 25–36. 10.1016/j.psyneuen.2016.11.036 [DOI] [PubMed] [Google Scholar]