Abstract
Multivitamin/mineral (MVM) supplements possess highly saline matrix which, unless eliminated, precludes accurate determination of trace amounts of toxic metal impurities by inductively coupled plasma mass spectrometry (ICP-MS). Multi-step separations (up to four-steps) are described in literature; often for single element determinations due to difficulties in removing the matrix components. In this study, we developed a three-step sequential coprecipitation procedure for simultaneous separation of As and Cd impurities from MVM supplements for determination by ICP-MS. The procedure provided effective elimination of salt matrix, including Ca, Mg and KCl along with the interfering molybdenum (Mo) and tin (Sn) from MVM solutions. KCl, Mo and Sn were removed by two-step Mg(OH)2 coprecipitation to about 34 µg mL−1 K (ca. 31 µg mL−1 Cl) and 0.4 µg mL−1 Mo. Levels of Sn and Na were not significant. A third coprecipitation of the resulting MVM solution with HF + NH4OH mixture precipitated virtually all Ca and Mg to as low as 1 and 10 µg mL−1, respectively. The recoveries for As and Cd in the spiked MVM solutions were about 96% and 95%, respectively. The accuracy of the method was validated with analysis of multivitamin/multielement tablets certified reference material (SRM 3280). Experimental values were 112 ± 37 ng g−1 for 75As, and 76 ± 5, 79 ± 5, and 78 ± 7 ng g−1 for 110Cd, 111Cd and 114Cd isotopes, respectively, that were not significantly different from the certified values of As (132 ± 44 ng g−1) and Cd (80.2 ± 0.9 ng g−1) at 95% confidence level. Several commercially available MVM supplements were analyzed with the procedure. Mean As levels measured in the tablets varied between 24 and 128 ng g−1 and that for Cd were between 28 and 125 ng g−1 indicating total amount of As or Cd ingested per serving size were below the safe daily exposure limits. In addition, the results obtained for As and Cd with the procedure were lower in comparison to the values reported in literature indicating that ICP-MS analysis of complex MVM supplements could be prone to higher risks of inaccuracy without removal of interfering matrix.
Keywords: Arsenic, cadmium, multivitamin/mineral supplement, coprecipitation, matrix removal, ICP-MS
1. Introduction
Dietary multivitamin/mineral (MVM) supplements contain essential nutrients, such as vitamins, minerals, amino acids, and macro/trace elements that are intended to augment daily diets. Over the last two decades, their consumption has increased significantly and today they have been increasingly used among children and adults, including pregnant women to remedy inadequate macro-and micro-nutrient intake [1,2,3,4], although there is no conclusive evidence to their potential benefits in treating nutritional deficiencies and medical conditions [5,6,7]. MVM supplements are regulated under the Dietary Supplement Health and Education Act of 1994 by the US Food and Drug Administration (FDA) [8], but there are no clear regulations for monitoring the manufacturing and public safety of these products. Consequently, consumers often rely on the contents labels and do not accurately know benefits and possible health risks of the products [3,9–12].
Various MVM supplements are commercially available to consumers under different brands in the form of hard tablets, liquid-gels (soft gels), chewable jellies and liquid concentrates, yet they all contain similar organic micronutrients, such as vitamins (A, B, C, D, E and K), flavonoids, folic acid and biotin. Among the elemental nutrients, calcium (Ca), copper (Cu), magnesium (Mg), iron (Fe), potassium (K), manganese (Mn), phosphorous (P), and zinc (Zn) make up the majority of elemental nutrients of MVM supplements. Other trace element nutrients, such as boron (B), chromium (Cr), iodine (I), molybdenum (Mo), nickel (Ni), selenium (Se), tin (Sn) and vanadium (V) are added at microgram per gram (ppm) levels [9,12,13]. Ideally, MVM supplements should not contain any potentially toxic elements, such as arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) that cause harmful effects on human health under chronic exposure. Nevertheless, they do contain toxic elements at varying levels depending on the raw minerals, their origin and chemical processes used in manufacturing [13–19].
In recent years, there has been an increasing interest in monitoring As and Cd impurities in dietary MVM supplements owing to their adverse effects on human health [4,9,12,13,15,16,20]. Arsenic is known to cause hepatic, renal, reproductive, and nervous systems disorders [21,22]. Cadmium is referred to as among the most toxic elements due to its long biological half-life (15–30 years). It largely accumulates in the kidneys in human body and hence chronic exposure to Cd could cause nephrotoxicity and kidney failure [23]. A number of studies have revealed that commercial MVM supplements contain both As and Cd impurities at varying levels [4,9,12,13,15,16,20]. Dolan et al. [15] reported as high as 4 µg g−1 As and 0.4 µg g−1 Cd in a number of dietary supplements. In another study, As levels from various MVM supplements were highly variable between 1 and as high as 16 µg g−1 while that for Cd were between 0.5 and 3.8 µg g−1 [20]. Evidently, As levels are in general an order of magnitude higher than Cd levels. Also, it should be noted that inorganic As species, As(III) and As(V), are more toxic than organic arsenic species, and hence speciation of As is important to elucidate the health risks associated with As contamination in MVM supplements.
Chronic exposure to high levels of As and Cd are concerning for humans, especially for children and pregnant women, and thus development of appropriate methods is imperative to accurately monitor their levels in MVM supplements. Currently, inductively coupled plasma mass spectrometry (ICP-MS) is the most popular technique for testing the toxic elements impurities in MVM supplements due mainly to its excellent sensitivity [15,16,20,24–26]. Nonetheless, determination of trace As and Cd impurities is a difficult task due to the highly saline nature of MVM supplements. High salt content hampers the detection capabilities of ICP-MS instrument. In addition, potassium chloride (KCl) matrix of MVM solutions induces spectral interferences through 40Ar35Cl on monoisotopic 75As while major Cd isotopes (e.g., 110Cd, 111Cd, 112Cd, 114Cd) suffer from the interferences of various molybdenum oxides, such as 94Mo16O, 95Mo16O, 96Mo16O, 98Mo16O. Tin (Sn) is also a common trace element nutrient of MVM supplements, which may confound the results of 112Cd and 114Cd isotopes due to overlaps of 112Sn and 114Sn respectively. To overcome such difficulties, high resolution (HR) ICP-MS and collision/reaction cell ICP-MS have been used in several studies [15,20], but it is hardly feasible to completely eliminate these spectral interferences even by using HR-ICP-MS instruments unless the interfering salt matrix (e.g., KCl, Mo and Sn) is removed prior to analysis.
Certified reference materials serve an indispensable role for quality assurance and development of suitable methods for elemental analysis of MVM supplements [16,27]. Recent reports, however, indicate that accurate measurements may not even be achieved with suitable reference materials owing to the fact that interferences of salt matrix could substantially confound the results from ICP-MS analysis. In their study, for instance, Avula et al. [20] used SRM 3280 (Multivitamin/Multielement Tablets standard reference material) in elemental analysis of various commercial MVM supplements by collision cell ICP-MS, but no values were reported for As and Cd, presumably chloride and molybdenum matrices of SRM 3280 precluded accurate measurements indicating the need for more sophisticated sample preparation tactics for analysis of complex MVM supplements.
In this study, we developed a sequential coprecipitation procedure for simultaneous separation of As and Cd from MVM matrix along with effective removal of salt matrix and interfering elements, including Na, K, Ca, Mg, Cl, Mo and Sn. Both As and Cd were quantitatively separated from the matrix of MVM solutions by sequential coprecipitations with triethylamine (TEA) and a mixture of hydrofluoric acid (HF) and ammonium hydroxide (NH4OH). The procedure was validated with analysis of SRM 3280 (Multivitamin/Multielement Tablets) and applied to the determination of As and Cd impurities in various commercial MVM supplements by ICP-MS.
2. Experimental
2.1. Instrumentation
Measurements were made with a Varian 820MS inductively coupled plasma mass spectrometer (Varian, Australia). The ICP-MS instrument was equipped with collision reaction interface (CRI) technology utilizing H2 and He gases, a peltier-cooled double-pass glass spray chamber, an all-teflon Ari-mist nebulizer (SCP Science, Champlain NY), quartz torch, CRI-type Pt sampler and skimmer cones and all-digital detector (DDEM, Model AF250, ETP Australia). The instrument was optimized daily with 5 μg L−1 solution of 138Ba, 25Mg, 115In, 140Ce, 208Pb solution for optimal sensitivity, oxides (156CeO+/ 140Ce+ < 3%) and doubly charged ions (138Ba2+/ 138Ba+ < 2%). An internal standard (IS) solution was used for correcting the matrix effects and instrumental drift. The IS solutions contained 5 μg L−1 of germanium (Ge), rhodium (Rh), rhenium (Re), and bismuth (Bi). 72Ge and 185Re were used for As and Cd, respectively. All standard and sample solutions were introduced to ICP-MS instrument manually. Data were acquired by ICP-MS Expert software package (Version 2.2 b126). Data were collected in mode peak hop mode using 75As, 110Cd, 111Cd and 114Cd isotopes. The concentrations of matrix elements, including Na, K, Ca, Mg, and P, and interfering Mo and Sn were measured simultaneously along with As and Cd. Measurements for 23Na, 25Mg, 26Mg, 31P, 39K, 43Ca and 44Ca were made with CRI mode. Hydrogen (H2) was used as CRI gas (65 mL min-1) that was introduced through the skimmer cone. Mo and Sn determinations were made with 95Mo and 98Mo, 118Sn and 120Sn isotopes using the same instrumental conditions for As and Cd. The operating conditions of the ICP-MS instrument are shown in Table 1.
Table 1.
Operating parameters for Varian 820-MS ICP-MS instrument
| RF Power | 1.4 kW |
| Plasma argon flow | 17 L min-1 |
| Auxiliary argon flow | 1.7 L min-1 |
| Nebulizer argon flow | 1.1 L min-1 |
| Sheath argon flow | 0.2 L min-1 |
| Sampling depth | 6.5 mm |
| Pump rate | 6 rpm; 0.2 L min-1 |
| Stabilization time | 20 s |
| Spray chamber temperature | 4 oC |
| Scan mode | Peak hopping |
| Dwell time | 20 ms |
| Points/peak | 1 |
| Scans/peak | 5 |
| Scans/replicate | 3 |
| Normal mode (non-CRI) | |
| Isotopes monitored |
55Mn, 63Cu, 65Cu, 66Zn, 68Zn, 75As, 95Mo, 98Mo, 110Cd, 111Cd, 114Cd, 118Sn, 120Sn |
| CRI mode settings | |
| CRI gas/port | H2 / Skimmer cone |
| CRI gas flow rate | 65 mL min-1 |
| Isotopes monitored |
23Na, 25Mg, 26Mg, 31P, 39K, 43Ca, 44Ca, 56Fe, 57Fe |
2.2. Materials and solutions
All solutions were prepared with double deionized water (18.0 MΩ cm resistivity). Trace metal grade nitric acid (HNO3, BDH Aristar Plus), hydrochloric acid (HCl, Fisher Scientific)) and hydrofluoric acid (HF, Fisher Scientific) were used in sample dissolution and/or preparation of standard solutions. They were obtained from Fisher Scientific. Hydrogen peroxide (H2O2, Sigma Aldrich, 99.99%) was used for digesting multivitamin/mineral samples. Trace metal grade triethylamine (TEA, 99.8%, Acros Organics) and ammonium hydroxide (NH4OH, BDH Chemicals, Aristar Plus) were used for coprecipitations. A 1.0 μg mL−1 multielement standard solution was prepared in 5% (v/v) HNO3 from 1000 μg mL−1 single element stock solutions (High Purity Standards, Fisher Scientific). The standard solution contained Al, As, Ba, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Sb, Se, Sr, Tl, V and Zn and used for spike studies and preparing calibration solutions. A tin (Sn) standard solution (10 μg mL−1) was prepared separately in 2% (v/v) HCl and 2% (v/v) HNO3 from 1000 μg mL−1 single element stock solution.
For method development, commercially available Rite Aid complete multivitamin/multimineral supplements was used. A stock solution was prepared by acid dissolution and analyzed for elemental content. Details of preparation and analyses are provided in the next section. Method validation was performed with SRM 3280 (multivitamin/multielement tablets) standard reference material purchased from National Institutes of Standards and Technology (NIST). Several multivitamin/mineral (MVM) samples were purchased from local pharmacy stores and analyzed for As and Cd impurities. All MVM samples were in the form hard tablets with the following codes: Century Adults (MVM-1), Rite Aid MVM (MVM-2), Kroger Complete MVM Supplement (MVM-3), Centrum Adults MVM (MVM-4), Equate MVM (MVM-5), Walgreens MV (Adults 50+) (MVM-6), SpectraVite CVS (MVM-7) and One-a-Day Men (MVM-8).
2.3. Preparation and characterization of MVM stock solution for method optimization
For method development and optimization of experimental conditions, samples of Rite Aid MVM tablets were dissolved/digested in HNO3 and H2O2. This brand was chosen as the contents label declared the closest essential and trace element nutrient composition to that of SRM 3280. In addition, Rite Aid MVM contained very high level of molybdenum (160 µg/tablet as sodium molybdate) along with typical chloride (72 mg/tablet as KCl) which were key elements to optimize the procedures for As and Cd determinations. A total of 24 g MVM sample (16 tablets) were digested. A sample of 3 g (2 tablets, total 8 sets) were digested in 5 mL HNO3 and 4 mL H2O2 in teflon vessels (Savillex) on a digestion block (DigiPrep, SCP Science, Champlain, NY). The tablets were first digested in 5 mL HNO3 at 120 oC for 3 h, and an additional 2 h at 140 oC. Then, vessels were briefly cooled, opened and 4 mL H2O2 was added slowly. The contents were digested at 140 oC for additional hour to destroy the organics. These temperatures were opted to avoid over-boiling and excessive pressure build-up in teflon vessels from large amount sample.
After digestion, the MVM solutions were heated to near dryness at 120 oC to evaporate HNO3 The resulting residue/pellet was suspended in 10 mL of 1% (v/v) HNO3 and centrifuged to remove undissolved TiO2 and SiO2. The supernatant solution was evaporated in the teflon vessels again. The residue was then dissolved and heated in 2 mL HNO3 and 1 mL H2O2 for complete digestion until dryness. Finally, all digests were dissolved in 5 mL of 5% (v/v) HNO3 and combined in a 50-mL test tube. This solution was centrifuged for 30 min at 10,000 rpm on a centrifuge (Thermo Scientific Sorvall ST 16) to remove the suspending particles. The supernatant solution was transferred to an acid-cleaned polypropylene bottle and completed to 500 mL with deionized water. The acid concentration of the MVM stock solution was around 0.4% (v/v) HNO3.
For elemental analysis, 0.1 mL aliquots (n = 5) of the MVM stock were diluted to 2 mL with 5% (v/v) HNO3. They were then analyzed by ICP-MS. Determinations of 23Na, 25Mg, 26Mg 31P, 39K, 43Ca, 44Ca, 56Fe and 57Fe were made with CRI mode (see Table 1), while 55Mn, 63Cu, 65Cu, 66Zn, 68Zn, 95Mo, 98Mo, 118Sn and 120Sn were measured using normal operating conditions (non-CRI, Table 1). For Zn determinations, 66Zn results were not utilized due to interference of 26Mg40Ar. The concentrations of the major matrix and interfering elements in 500 mL stock solution were as follows: Ca (4.6 ± 0.3 mg mL−1), Mg (3.6 ± 0.2 mg mL−1), P (2.9 ± 0.3 mg mL−1), K (4.2 ± 0.3 mg mL−1), Fe (91 ± 3 µg mL−1), Cu (65 ± 5 µg mL−1), Mn (96 ± 8 µg mL−1) and Zn (532 ± 30 µg mL−1). Mo and Sn concentrations were 4.0 ± 0.3 µg mL−1 and 0.10 ± 0.004 µg mL−1, respectively. The concentration of Sn was lower than the declared value (ca. 0.5 µg mL−1 equivalent to 10 µg Sn/tablet). To compensate for this deficiency, Sn concentrations in experimental MVM solutions were adjusted to 0.5 µg mL−1 from 10 µg mL−1 Sn stock solution throughout the method development studies.
2.4. Dissolution of SRM 3280 and commercial MVM samples
A sample of 10 tablets of SRM 3280 (1.5 g per tablet) were ground and homogenized. About 0.125 g sub-samples (n = 5) were digested in 4 mL HNO3 in teflon vessels for 3 h at 160 ˚C on a digestion block. Then, the vessels were carefully opened and 2 mL H2O2 were added to samples. The samples were heated for another 3 h at 160 ˚C. The lids were opened and contents were heated to dryness at 120 oC. The residue was dissolved with 2 mL of 0.5% (v/v) HNO3 and transferred to 2-mL microcentrifuge tubes, and then centrifuged at 10,000 rpm for 15 min to remove undissolved material. The supernatant solution was transferred to new micro-centrifuge tubes and the volume was completed to 2 mL. For commercial MVM tablets, 5 tablets (ca. 6 to 7.5 g sample) for each brand were ground, homogenized and stored in plastic bottles. About 0.15 g samples (n = 5) were digested in HNO3 and H2O2 digested and prepared similarly as for the SRM 3280. The solutions were then processed concurrently with the coprecipitation procedure for separation of As and Cd from the MVM salt matrix for ICP-MS analysis.
2.5. Optimization of coprecipitation procedure
Coprecipitations in stock MVM solution was carried out in a sequential manner starting with TEA-assisted Mg(OH)2 of As and Cd and other trace element nutrients. The schematic of the coprecipitation procedure is outlined in Fig. 1. Initially, a one-step or two-step coprecipitation with TEA was examined for removal of matrix salts and interfering elements (e.g., Mo and Sn). A volume of 1.5 mL of MVM stock solution (n = 4) was placed into 2-mL micro-centrifuge tubes, and spiked with 30 µL of 1.0 µg mL−1 multielement solution and 100 µL of 10 µg mL−1 Sn solution to introduce a spike concentration for 15 µg L−1 for As and Cd, and 0.5 µg mL−1 for Sn in 2 mL volume. First precipitation was performed by adding 100 µL TEA to the solutions. An intense Mg(OH)2 coprecipitation occurred due to high level of Mg in highly saline medium. The suspensions were allowed to wait for about 5 min and then centrifuged at 12,000 rpm for 15 min. After discarding the supernatant solutions, the pellets were dissolved in 0.75 mL of 10% (v/v) HNO3 and completed to 1 mL with water. A volume of 0.5 mL was pipetted out and completed to 1 mL with 5% (v/v) HNO3 to determine recoveries of As and Cd, and elimination profiles of the matrix elements.
Fig. 1.
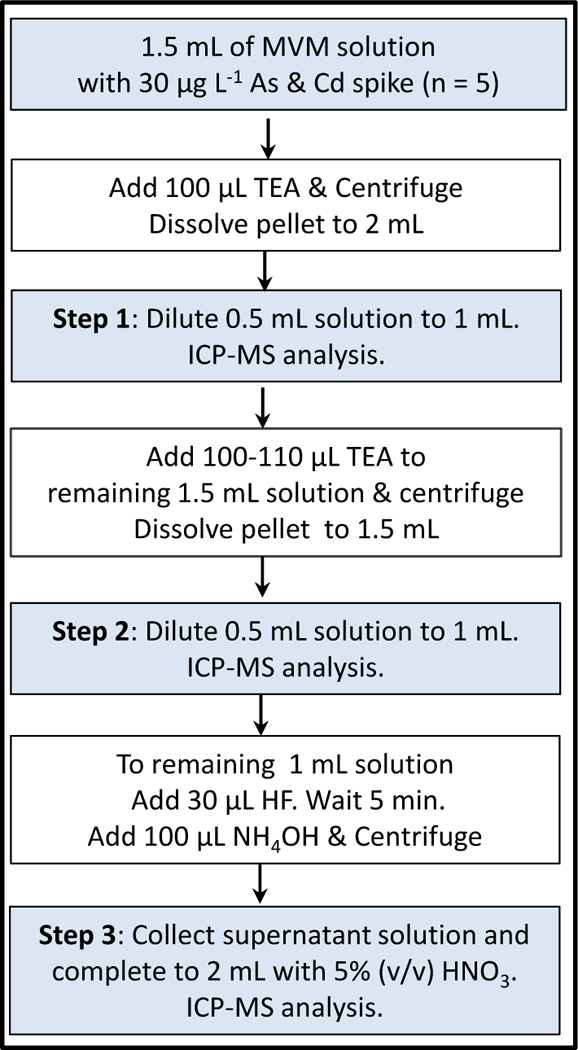
Coprecipitation scheme utilized for separation of As and Cd from MVM matrix. Steps 1, 2, and 3 refer to sampling after each treatment.
The remaining 1.5 mL solution was further treated with 100–110 µL TEA for second Mg(OH)2 coprecipitation, which occurred fast but not as intense as the first indicating partial removal of the salt matrix in first precipitation. Similarly, suspensions were allowed for completion of precipitation and then centrifuged. The pellets were dissolved in 0.6 mL of 10% (v/v) HNO3 and completed to 1.5 mL with deionized water. A volume of 0.5 mL was taken and completed to 1 mL with 5% (v/v) HNO3 to determine As and Cd recoveries and matrix removal efficiency as in the first precipitation.
The third step involved the removal of persistent Ca and Mg via simultaneous CaF2 – Mg(OH)2 coprecipitation. A volume of 30 µL HF was first added to the remaining 1 mL MVM solution to induce CaF2 precipitation. This step was relatively slow due to partial removal of Ca in previous two steps; solutions showed faint turbidity (e.g., weak precipitation) in about 10 min. Then, the same solutions were treated with 50, 100 and 150 µL volumes of NH4OH that accelerated CaF2 precipitation by inducing Mg(OH)2 coprecipitation. After completion of precipitation, suspensions were centrifuged similarly. At this stage, the supernatant solutions were transferred to new 2-mL micro-centrifuge tubes and completed to 2 mL with 5% (v/v) HNO3. Solutions were analyzed for As and Cd and the remaining matrix components by ICP-MS.
3. Results and discussion
3.1. Elemental recoveries and matrix elimination via sequential TEA coprecipitations
Major nutrient elements including, Ca, Mg, K, P, Cl, and Na make up about 35% of salt matrix of most MVM supplements. This amount increases to about 37% when trace metal nutrients, Fe, Mn, Cu and Zn are added. Triethylamine (TEA) is an alkylamine (weak base, pKb = 3.25) which readily generates a pH between 10 and 11 in its aqueous solutions. Unlike NH4OH, TEA does not coordinate with divalent or trivalent metal ions; consequently it does not form any water-soluble complexes as NH4OH does (e.g., Cd(NH3)42+). With this feature, TEA has been shown to be a very valuable reagent providing robust and quantitative scavenging of various of trace transition elements and heavy metals, including As and Cd, in the presence of Mg matrix [28]. Nevertheless, Mg and Ca, the two major matrix elements of MVM solutions, are carried over substantially in TEA-assisted Mg(OH)2 coprecipitations; it was not feasible to fully eliminate these elements despite three-step sequential coprecipitation with TEA [16].
Similar pattern was observed in this study for Mg, Ca, P, K and Na (Fig. 2) via one-step or 2-step TEA coprecipitation. As shown in Fig. 2A, single precipitation with TEA was not effective for removal of Mg, Ca and P although K matrix decreased to as low as 380 µg mL−1 from 4,200 µg mL−1 (ca. 91% K removal). With two-step coprecipitation with TEA (e.g., TEA-TEA), Mg levels dropped from 3,600 µg mL−1 to 1910 µg mL−1 (ca. 47% removal) while Ca was reduced from 4,600 µg mL−1 to about 2350 µg mL−1 (ca. 49% removal). In contrast, most Mo and Sn were eliminated from MVM solutions (Fig. 2B) via two-step coprecipitation; Mo concentration dropped from 4 µg mL−1 to about 0.6 µg mL−1, and Sn concentration was about 0.2 µg mL−1.
Fig. 2.
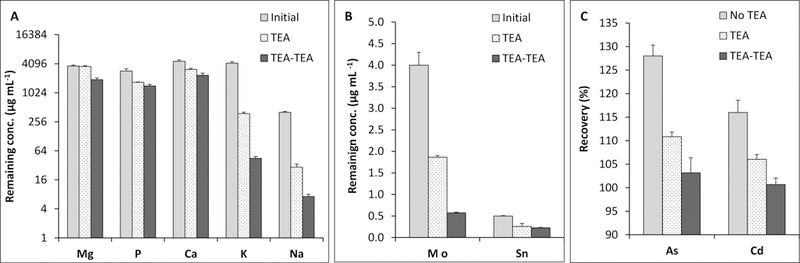
Recoveries for As and Cd, and the elimination profiles of interfering matrix elements with sequential coprecipitation with TEA in MVM solutions (n = 5). Initial values for the matrix elements refer to the concentrations in MVM stock solution. As and Cd recoveries without TEA are obtained with direct measurements of the MVM stock solutions spiked with 15 µg L−1 As and Cd.
The recoveries for As (128%) and Cd (116%) were significantly high from direct determinations (e.g., no TEA, Fig. 2C) indicating significant interferences of ArCl+ and various MoO+ on As and Cd. It should be noted these values are for the artificially spiked MVM solutions (15 µg mL−1 As or Cd) and thus the interferences are expected to worsen for actual samples with lower As and Cd impurities. It is also evident from Fig. 2C that both As and Cd coprecipitated onto Mg(OH)2 with one-and two-step coprecipitation. For one-step TEA coprecipitation, recoveries were 111% and 106% for As and Cd, respectively. In comparison to direct determination, interferences were alleviated but could not be fully eliminated since interring matrix levels were still significantly high; 380 µg mL−1 K (equivalent to 725 µg mL−1 KCl) and 1.9 µg mL−1 Mo (Fig. 2A and 2B). After two-step (TEA-TEA) coprecipitation, K and Mo concentrations decreased to about 45 µg mL−1 and 0.6 µg mL−1 affording more accurate determinations; the recoveries for As and Cd were about 103% and 101%, respectively.
3.2. Removal excess Mg and Ca matrix with combined HF-NH4OH coprecipitation
Sequential coprecipitations with TEA were not effective for eliminating Mg, P and Ca matrices that consequently carried over into the analysis solutions. As a result, the salt matrix content was still high to cause significant suppression of sensitivity (ca. 35–40%) and salt deposition on the sample and skimmer cones of the ICP-MS instrument. To overcome this hurdle, we attempted to retain As and Cd as fluoride complexes in the solution while precipitating Ca and Mg as CaF2 and Mg(OH)2. It was previously reported that both As and Cd form fluoride complexes in acidic solutions [29]. Fluoride complexes of As (e.g., arsenic hexafluoride AsF6-) appear to be highly stable. We verified this phenomenon with preliminary trials that once a small volume of HF (e.g., 20–30 µL) was added to stock MVM solutions, it was not feasible to fully scavenge As from solution, even with an excess volume of TEA (e.g., 200 µL). In contrast, CdF+ is a weak complex (Kq = 5.8 – 6.4) [30], and thus coprecipitated with Mg(OH)2 upon addition of TEA [16]. Here we utilized NH4OH instead of TEA to avoid precipitation of Cd from weak CdF+ complex, since Cd2+ forms cadmium ammonia complex, Cd(NH3)42+, in ammonical solutions.
The results for combined HF + NH4OH coprecipitation are illustrated in Fig. 3A-C comparatively for HF and HF + NH4OH treatments. The MVM solutions coprecipitated twice with TEA (e.g., TEA-TEA) were treated with either 30 µL HF only or a combination of 30 µL HF and 100 µL NH4OH (see section 2.5). It was evident that addition of HF resulted in significant removal of Mg (482 µg mL−1) and Ca (963 µg mL−1) via CaF2 coprecipitation (Fig. 3A). However, a more drastic decrease occurred for Mg and Ca levels with HF + NH4OH treatment when the pH of colloidal solutions containing HF were raised to alkaline pH (ca. pH 10) with NH4OH resulting in coprecipitation of virtually all Mg and Ca (Fig. 3A). The analysis solutions contained about 7 and 1.5 µg mL−1 Mg and Ca. The HF + NH4OH treatment also favored the removal of P to some extent in comparison to the levels with TEA-TEA coprecipitation; P concentration was reduced to about 615 µg mL−1 from 1415 µg mL−1 measured in TEA-TEA treatment (see Fig. 2A). As can be seen in Fig. 3C that As and Cd were in the solution after HF + NH4OH treatment; recoveries were 96 ± 7% for As and 95 ± 6% for Cd that were similar to those with HF treatments; 100 ± 6% for As and 95 ± 5% for Cd. Mo levels did decrease slightly (ca. to 0.4 µg mL−1) with HF + HN4OH coprecipitation, but Sn was almost completely removed from MVM solutions; residual Sn concentration was as low as 0.019 µg mL−1 (ca. 96.2% removal) (Fig. 3B).
Fig. 3.
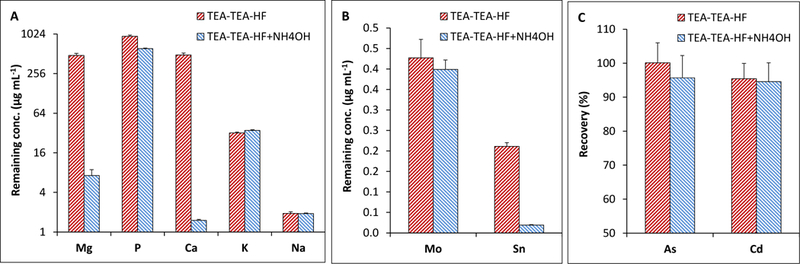
Recoveries for As and Cd, and the elimination profiles of interfering matrix elements with HF and HF + NH4OH coprecipitation from MVM solutions after TEA coprecipitation.
In a separate trial, the volume of NH4OH was examined for HF + NH4OH coprecipitation step. Namely, the NH4OH volume added to the MVM solutions was increased from 50 to 150 µL. The results are summarized in Table 2 for As and Cd along with interfering elements. The recoveries for As and Cd were quantitative with up 100 µL NH4OH that was also adopted as optimum volume. With 50 µL NH4OH, weak precipitation occurred which was not sufficient for eliminating Mg and Ca effectively. Adding 150 µL NH4OH, on the other hand, caused intense precipitation that resulted in partial precipitation of As and Cd that decreased the recoveries to about 86% and 62%, respectively. Additional experiments performed also showed that a volume of NH4OH between 75 to 110 µL could be used without impacting As and Cd retention in solution. In case HF, the volume was kept at 30 µL since no significant improvement was observed in preliminary trials. Contrary, adding more HF (ca. 50 µL) to solutions required more NH4OH to neutralize the acid and to initiate the precipitation without any improvement in the matrix removal.
Table 2.
Effect of NH4OH volume on the recoveries of As and Cd as well as the removal of interfering matrix elements from MVM stock solution with HF + NH4OH coprecipitation. Values are mean ± standard deviation of five separate preparations (n = 5)
| Analyte (recovery) |
NH4OH volume (µL) |
||
|---|---|---|---|
| 50 | 100 | 150 | |
| As (%) | 97 ± 3 | 99 ± 2 | 86 ± 2 |
| Cd (%) | 98 ± 7 | 95 ± 4 | 62 ± 4 |
| Matrix element (concentration) |
|||
| Mo (µg mL−1) | 0.68 ± 0.06 | 0.43 ± 0.04 | 0.35 ± 0.04 |
| Sn (µg mL−1) | 0.2 ± 0.04 | 0.05 ± 0.08 | 0.02 ± 0.02 |
| Mg (µg mL−1) | 1283 ± 33 | 10 ± 2 | 4 ± 0.6 |
| P (µg mL−1) | 1263 ± 136 | 812 ± 46 | 783 ± 34 |
| Ca (µg mL−1) | 418 ± 41 | 1 ± 0.5 | 0.5 ± 0.2 |
| K (µg mL−1) | 35 ± 3 | 34 ± 2 | 36 ± 3 |
| Na (µg mL−1) | 4 ± 0.5 | 3 ± 0.7 | 4 ± 0.4 |
3.3. Method validation with analysis of SRM 3280
Samples of SRM 3280 (multivitamin/multielement tablets) were analyzed using the optimized coprecipitation procedure. A sample of 10 tablets (15 g) of SRM 3280 was ground with agate mortar and mill to make a homogenized batch. About 0.125 g samples (n = 5) were weighed and digested in teflon vessels in 4 mL HNO3 and 2 mL H2O2 and prepared as described in section 2.4. Blank solutions (n = 10) were prepared with 30 µL HF and 210 µL TEA in 2-mL centrifuge tubes. These solutions were first evaporated on a hot-block digester at 100 oC and then completed to 2 mL with 1% (v/v) HNO3. Determinations were made using aqueous external standards from 0 to 20 µg L−1 As and Cd. Limits of detection (LOD) were estimated as 0.013 µg L−1 for As and 0.006 µg L−1 Cd from the blank solutions using the 3σ method.
For SRM 3280, after removal of the undissolved material (e.g., TiO2 matrix), the supernatant solutions were treated according to the coprecipitation procedure described in Fig. 1. Namely, a volume of 0.5 mL solution was taken and completed to 1 mL with 1% (v/v) HNO3 through Steps 1 and 2 to verify the effects of interfering salt matrix on As and Cd determination. The results for As, Cd and interfering matrix elements measured in SRM 3280 solutions are summarized in Table 3. The certified values of As and Cd in SRM 3280 are 132 ± 44 ng g−1 and 80.15 ± 0.86 ng g−1, respectively. As can be seen that both As and Cd values were inaccurately high at Step 1, about 38% for As and 45% to 56% for Cd, due to the presence of significant Cl-(e.g., KCl or K) and Mo matrices. Residual K concentration was around 461 µg mL−1 that reflects about 419 µg mL−1 Cl-or 880 µg mL−1 KCl in SRM 3280 solutions. High As levels were indicative of significant contribution from 40Ar35Cl on monoisotopic 75As. For Cd, interfering Sn was removed mostly, but Mo concentration in analysis solutions was about 1.7 µg mL−1 that caused significant interferences on 110Cd, 111Cd and 114Cd isotopes from 94Mo16O, 95Mo16O and 98Mo16O, respectively. These spectral interferences on As and Cd were mostly alleviated with second coprecipitation (Step 2) that substantially reduced the matrices of interfering Cl-(80 µg mL−1 K = 150 µg mL−1 KCl) and Mo (0.42 µg mL−1). Consequently the measured As and Cd values were in agreement with the certified values at 95% confidence level (Step 2). These results were also consistent with those obtained with the spiked stock MVM solutions for which quantitative recoveries for As and Cd were achieved after second Mg(OH)2 coprecipitation (see Fig. 2C). Similarly, the SRM 3280 solutions possessed high levels of Mg, P and Ca as occurred for the stock MVM solutions (Fig. 2A). After the coprecipitation with HF – NH4OH (Step 3), the remaining Mg and Ca were effectively eliminated along with further reduction of P levels producing more conducive solutions for ICP-MS analysis. Concerning As and Cd, As concentration was slightly lower than that for at Step 2, however, both As and Cd concentrations were consistent with the certified values at 95% confidence level. It should be noted that SRM 3280 solutions contained substantial amount of Ca (2358 µg mL−1) and P (2133 µg mL−1) at Step 2. The decrease in measured As value at Step 3 could indicate a contribution from 44Ca31P on 75As at Step 2, which was also eliminated when Ca matrix was reduced from 2358 µg mL−1 to as low as 9 µg mL−1.
Table 3.
Concentrations of As and Cd determined in SRM 3280 by ICP-MS along with residual solution concentrations of interfering matrix elements across each coprecipitation step. Values are mean ± standard deviation of five separate preparations (n = 5). Values in parenthesis indicate per cent deviation (inaccuracy) from the certified value of As and Cd.
| Analyte | Certified value (ng g-1) |
Step 1 | Step 2 | Step 3 | |
|---|---|---|---|---|---|
| 75As (ng g−1) | 132 ± 44 | 182 ± 22 | 141 ± 18 | 112 ± 37 | |
| (+38%) | (+7%) | (−15%) | |||
| 110Cd (ng g−1) | 116 ± 14 | 77 ± 9 | 76 ± 5 | ||
| 80.2 ± 0.9 | (+45%) | (−4%) | (−5%) | ||
| 111Cd (ng g−1) | 118 ± 15 | 78 ± 6 | 79 ± 5 | ||
| (+48%) | (−2%) | (−2%) | |||
| 114Cd (ng g−1) | 125 ± 14 | 88 ± 7 | 78 ± 7 | ||
| (+56%) | (+10%) | (−2%) | |||
| Residual concentration of the matrix and interfering elements | |||||
| Mo (µg mL−1) | 1.7 ± 0.1 | 0.42 ± 0.05 | 0.37 ± 0.05 | ||
| Sn (µg mL−1) | 0.10 ± 0.01 | 0.02 ± 0.005 | 0.02 ± 0.003 | ||
| Mg (µg mL−1) | 2331 ± 145 | 1224 ± 159 | 21 ± 5 | ||
| P (µg mL−1) | 2921 ± 280 | 2133 ± 190 | 1451 ± 110 | ||
| Ca (µg mL−1) | 3688 ± 262 | 2358 ± 200 | 9 ± 3 | ||
| K (µg mL−1) | 461± 28 | 80 ± 8 | 55 ± 7 | ||
| Na (µg mL−1) | 4 ± 1 | nd | nd | ||
nd = not determined
3.4. Application to commercial MVM tablets
A sample of 5 tablets of commercial MVM were ground and homogenized. About 0.150 g samples (n = 5) were weighed and digested in teflon vessels in 4 mL HNO3 and 2 mL H2O2 as described for the SRM 3280 samples. Digests were diluted to 2 mL and treated entirely with the coprecipitation procedure. The results obtained after HF + NH4OH treatment (e.g., Step 3) are provided in Table 4. Arsenic concentration varied between 24 and 128 ng g−1, and that for Cd were between 28 and 125 ng g−1 in the tablets. These concentrations were equivalent to 0.031 – 0.180 µg As day−1 (e.g. serving size) and 0.035 – 0.162 µg Cd day−1. Both As and Cd are classified as toxic elements and thus their daily intake from MVM supplements is regulated. The minimum risk levels (MRLs) for adults under chronic exposure are estimated to be 21 µg day−1 for As (e.g., 0.3 µg kg−1 day−1) [22] and 7 µg day−1 for Cd (0.1 µg kg−1 day−1) [31]. The concentrations of As and Cd measured from the commercial MVM tablets were below the MRLs indicating that total amount of As or Cd ingested daily were within the safe exposure limits. In their survey of MVM supplements, Avula et al. [20] also reported As and Cd impurities below the MRLs, however, the measured concentrations were higher; As levels varied 0.6 and 15.9 µg day−1 and that for Cd were between 0.3 to 3.8 µg day−1. In contrast, both As and Cd levels determined in this study with the coprecipitation procedure were much lower indicating that ICP-MS analysis of complex MVM supplements for As and Cd impurities without implementing suitable analytical approaches could possess higher risks of inaccuracy.
Table 4.
Arsenic and cadmium concentration determined in various multivitamin/mineral dietary supplements by ICP-MS using the developed coprecipitation. Values are mean ± standard deviation of five separate preparations (n = 5). Daily serving size is calculated as the average mass (µg) of As and Cd values per tablet.
| Sample | As (ng g−1) | Average As (µg/serving size) |
Cd (ng g−1) | Average Cd (µg/serving size) |
|---|---|---|---|---|
| MVM-1 | 71 ± 8 | 0.089 | 28 ± 2 | 0.035 |
| MVM-2 | 100 ± 6 | 0.075 | 54 ± 6 | 0.081 |
| MVM-3 | 56 ± 5 | 0.068 | 67 ± 3 | 0.087 |
| MVM-4 | 63 ± 8 | 0.078 | 58 ± 7 | 0.076 |
| MVM-5 | 128 ± 11 | 0.180 | 97 ± 4 | 0.135 |
| MVM-6 | 44 ± 5 | 0.063 | 60 ± 7 | 0.087 |
| MVM-7 | 29 ± 4 | 0.038 | 125 ± 12 | 0.162 |
| MVM-8 | 24 ± 5 | 0.031 | 106 ± 16 | 0.138 |
4. Conclusion
Despite the complex nature of MVM supplements, most studies concerning the safety of these products still rely on direct ICP-MS analysis to determine both essential elements and heavy metals. However, analysis of MVM supplements is a far more challenging task than it appears for accurate determination of toxic heavy metal impurities, even with highly sensitive ICP-MS technology. The results with MVM stock solutions and SRM 3280 samples showed that single step matrix elimination may not be sufficient to achieve accurate determinations. Here, a coprecipitation procedure is developed affording simultaneous determination of As and Cd impurities from MVM supplements with ICP-MS. Interfering salt matrix of MVM solutions comprising Mo, Sn, K, Cl, Ca and Mg were effectively eliminated through sequential coprecipitations. The mixture of HF-NH4OH was used judiciously to retain As and Cd in solution to eliminate predominant Ca and Mg. Analysis of commercial MVM supplements indicated lower As and Cd impurities in comparison to those reported in literature previously. It is therefore concluded that appropriate analytical methods should be developed to provide more accurate information on potential toxic element contamination in MVM supplements.
Highlights.
Three-step sequential coprecipitation was developed to determine toxic metal impurities from multivitamin/mineral (MVM) supplements.
The procedure is faster and allows simultaneous separation of As and Cd from MVM matrix.
KCl matrix, and interfering Mo and Sn were effectively removed.
Persistent Ca and Mg were completely eliminated from MVM solutions.
The method is validated with analysis of SRM 3280 for As and Cd.
Commercial MVM supplements contain lower As and Cd impurities than minimum risk levels (MRLs).
Acknowledgements
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (Grant No: G12RR013459) and from the Department of Energy Office of Environmental Management (DOE-EM) Minority Serving Institution Partnership Program (MSIPP) managed by the Savannah River National Laboratory (SRNL) under Savannah River Nuclear Solutions (SRNS) contract 291435. The views expressed herein are those of the authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT, Why US adults use dietary supplements, JAMA Intern. Med 173 (2013) 355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- [2].Dickinson A, Blatman J, El-Dash N, Franco JC, Consumer usage and reasons for using dietary supplements: Report of a series of surveys, J. Am. Coll. Nutr 33 (2014) 176–182. doi: 10.1080/07315724.2013.875423. [DOI] [PubMed] [Google Scholar]
- [3].Bailey RL, Gahche JJ, V Lentino C, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF, Dietary supplement use in the United States, 2003–2006, J Nutr 141 (2011) 261–266. doi: 10.3945/jn.110.133025.participant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Krawczyk M, Determination of macro and trace elements in multivitamin dietary supplements by high-resolution continuum source graphite furnace atomic absorption spectrometry with slurry sampling, J. Pharm. Biomed. Anal 88 (2014) 377–384. doi: 10.1016/j.jpba.2013.09.016. [DOI] [PubMed] [Google Scholar]
- [5].Kmietowicz Z, Multivitamin and mineral supplements in pregnancy are unnecessary expense, review finds, BMJ 354 (2016) i3821. doi: 10.1136/bmj.i3821. [DOI] [PubMed] [Google Scholar]
- [6].Jenkins DJA, Spence JD, Giovannucci EL, in Kim Y, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S, Paquette M, Patel D, Mitchell S, Kavanagh M, Tsirakis T, Bachiri L, Maran A, Umatheva N, McKay T, Trinidad G, Bernstein D, Chowdhury A, Correa-Betanzo J, Del Principe G, Hajizadeh A, Jayaraman R, Jenkins A, Jenkins W, Kalaichandran R, Kirupaharan G, Manisekaran P, Qutta T, Shahid R, Silver A, Villegas C, White J, Kendall CWC, Pichika SC, Sievenpiper JL, Supplemental Vitamins and Minerals for CVD Prevention and Treatment, J. Am. Coll. Cardiol 71 (2018) 2570–2584. doi: 10.1016/j.jacc.2018.04.020. [DOI] [PubMed] [Google Scholar]
- [7].Theal R, Tay VXP, Hickman IJ, Conflicting relationship between dietary intake and metabolic health in PTSD: A systematic review, Nutr. Res 54 (2018) 12–22. doi: 10.1016/j.nutres.2018.03.002. [DOI] [PubMed] [Google Scholar]
- [8].FDA, Dietary supplement Health and education act of 1994, U.S Food Drug Adm; NA; (1995) 20. doi: 10.2165/00128415-201013150-00060. [DOI] [Google Scholar]
- [9].van der Voet GB, Sarafanov A, Todorov TI, Centeno JA, Jonas WB, Ives JA, Mullick FG, Clinical and analytical toxicology of dietary supplements: a case study and a review of the literature., Biol. Trace Elem. Res 125 (2008) 1–12. doi: 10.1007/s12011-008-8157-0. [DOI] [PubMed] [Google Scholar]
- [10].Dwyer JT, Frances Picciano M, Betz JM, Fisher KD, Saldanha LG, Yetley EA, Coates PM, Milner JA, Whitted J, Burt V, Radimer K, Wilger J, Sharpless KE, Holden JM, Andrews K, Roseland J, Zhao C, Schweitzer A, Harnly J, Wolf WR, Perry CR, Progress in developing analytical and label-based dietary supplement databases at the NIH Office of Dietary Supplements, J. Food Compos. Anal 21 (2008) 83–93. doi: 10.1016/j.jfca.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dwyer JT, Coates PM, Smith MJ, Dietary supplements: Regulatory challenges and research resources, Nutrients 10 (2018) 1–24. doi: 10.3390/nu10010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marrero J, Jiménez R, Leiva E, Londonio A, Smichowski P, Inductively coupled plasma optical emission spectrometric determination of fifteen elements in dietary supplements : Are the concentrations declared in the labels accurate ?, Microchem. J 108 (2013) 81–86. doi: 10.1016/j.microc.2012.12.013. [DOI] [Google Scholar]
- [13].Ludvíková I, Tomáš C, Pouzar M, Krejc A, Elemental analysis of nutritional preparations by inductively coupled plasma mass and optical emission spectrometry ˇ ernohorsky, 132 (2012) 588–596. doi: 10.1016/j.foodchem.2011.10.076. [DOI] [PubMed] [Google Scholar]
- [14].Van Der Voet GB, Sarafanov A, Todorov TI, Centeno JA, Jonas WB, Ives JA, Mullick FG, Clinical and analytical toxicology of dietary supplements: A case study and a review of the literature, Biol. Trace Elem. Res 125 (2008) 1–12. doi: 10.1007/s12011-008-8157-0. [DOI] [PubMed] [Google Scholar]
- [15].Dolan SP, Nortrup DA, Bolger PM, Capar SG, Analysis of dietary supplements for arsenic, cadmium, mercury, and lead using inductively coupled plasma mass spectrometry, J. Agric. Food Chem (2003). doi: 10.1021/jf026055x. [DOI] [PubMed] [Google Scholar]
- [16].White J, Çelik A, Washington R, Yılmaz V, Mitchum T, Arslan Z, Sequential coprecipitation and matrix removal for determination of cadmium impurities from multivitamin supplements by inductively coupled plasma mass spectrometry and method validation by isotope dilution analysis of SRM 3280 multivitamin/multielement tablets, Microchem. J 139 (2018) 242–249. doi: 10.1016/j.microc.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Thuppil V, Kales SN, Lead, mercury, and arsenic in US-and Indian-manufactured Ayurvedic medicines sold via the Internet, JAMA 300 (2008) 915–923. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].García-Rico L, Leyva-Perez J, Jara-Marini ME, Content and daily intake of copper, zinc, lead, cadmium, and mercury from dietary supplements in Mexico, Food Chem. Toxicol 45 (2007) 1599–1605. doi: 10.1016/j.fct.2007.02.027. [DOI] [PubMed] [Google Scholar]
- [19].Korfali SI, Hawi T, Mroueh M, Evaluation of heavy metals content in dietary supplements in Lebanon., Chem. Cent. J 7 (2013) 10. doi: 10.1186/1752-153X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Avula B, Wang Y, Duzgoren-aydin NS, Khan IA, Inorganic elemental compositions of commercial multivitamin / mineral dietary supplements : Application of collision / reaction cell inductively coupled-mass spectroscopy, Food Chem 127 (2011) 54–62. doi: 10.1016/j.foodchem.2010.12.083. [DOI] [Google Scholar]
- [21].Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC, A Review of arsenic poisoning and its effects on human health, Crit. Rev. Environ. Sci. Technol 293 (1999) 37–41. doi: 10.1080/10643389991259227. [DOI] [Google Scholar]
- [22].Agency for Toxic Substances, Disease Registry (ATSDR). Toxicological profile for arsenic Atlanta, GA: U.S. Department of Health and Human Services; (2007) 24 [Google Scholar]
- [23].Jarup L, Low level exposure to cadmium and early kidney damage: the OSCAR study, Occup. Environ. Med 57 (2000) 668–672. doi: 10.1136/oem.57.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bu K, V Cizdziel J, Reidy L, Analysis of herbal supplements for selected dietary minerals and trace elements by laser ablation-and solution-based ICPMS, Microchem. J 106 (2013) 244–249. doi: 10.1016/j.microc.2012.07.011. [DOI] [Google Scholar]
- [25].Filipiak-Szok A, Kurzawa M, Szłyk E, Determination of toxic metals by ICP-MS in Asiatic and European medicinal plants and dietary supplements, J. Trace Elem. Med. Biol 30 (2015) 54–58. doi: 10.1016/j.jtemb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- [26].Smichowski P, Londonio A, The role of analytical techniques in the determination of metals and metalloids in dietary supplements : A review, Microchem. J 136 (2018) 113– 120. doi: 10.1016/j.microc.2016.11.007. [DOI] [Google Scholar]
- [27].Thompson RQ, Christopher SJ, Novel separation for the determination of cadmium by isotope dilution ICP-MS in samples containing high concentrations of molybdenum and tin, Anal. Methods 5 (2013) 1346–1351. doi: 10.1039/c2ay26212f. [DOI] [Google Scholar]
- [28].Arslan Z, Oymak T, White J, Triethylamine-assisted Mg(OH)2 coprecipitation/preconcentration for determination of trace metals and rare earth elements in seawater by inductively coupled plasma mass spectrometry (ICP-MS), Anal. Chim. Acta 1008 (2018) 18–28. doi: 10.1016/j.aca.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Arslan Z, Analysis of fish otoliths by electrothermal vaporization inductively coupled plasma mass spectrometry : aspects of precipitating otolith calcium with hydrofluoric acid for trace element determination, Talanta 65 (2005) 1326–1334. doi: 10.1016/j.talanta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [30].Mesaric SS, Hume DN, The formation constants of copper, cadmium, and zinc fluoride complexes, Inorg. Chem 2 (1963) 1063–1064. doi: 10.1021/ic50009a043. [DOI] [Google Scholar]
- [31].Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, Rudisill C, Toxicological profile for cadmium, U.S. Dep. Heal. Hum. Serv (2012) 487 https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf. [PubMed] [Google Scholar]


