Abstract
The aim of this in vitro study was to synthesize three new methacrylate monomers based on the modification of saccharides structures (glucose-Gluc, sucrose-Sucr and chitosan-Chit) with glycidyl methacrylate, and to use them in the composition of dental adhesives. Three methacrylate saccharide monomers were synthesized and characterized by mid-IR, 1H and 13C NMR, antioxidant activity and cytotoxic effect. Monomers included: one monosaccharide – Gluc-MA; one disaccharide – Sucr-MA; and one polysaccharide – Chit-MA. Primers containing HEMA, methacrylate saccharide monomers at concentrations of 0 (control), 1, 2 or 4 wt%, 60 wt% ethanol aqueous solution (pH3.0) and initiator system were formulated. Primers were used in conjunction with a bond step and composite paste to restore caries-free third molars, and dentin bond strength (24 hours and 6 month of storage in water), and antimicrobial activity (Alamar Blue test) were tested. Degree of conversion (DC) and maximum rate of polymerization (Rpmax) of the primers themselves were also analyzed. The mid-IR, 1H and 13C spectrum confirmed the presence of vinyl group on the structure of saccharides. Chit-MA showed low antioxidant activity and did not present a cytotoxic effect. Gluc-MA and Sucr-MA possess antioxidant and cytotoxic activity, concentration dependent. In the presence of methacrylate saccharide monomers, the primers showed DC comparable to the control group, except Gluc-MA4%, Sucr-MA4% and Chit-MA1%, which showed a range of 64.6 from 58.5 %DC. Rpmax was not statistically different for all the groups (p = 0.01). The bond strength of Sucr-MA1% increased from 25.7 (±2.8) to 40.6 (±5.3) MPa after 6 months of storage. All the synthesized monomers showed some antimicrobial activity after polymerization. Gluc-MA and Chit-MA 4% and Sucr-MA 1, 2 and 4% led to decrease bacterial metabolism. Sucr-MA 1% showed better results regarding the decrease in bacterial metabolism and increasing the bond strength after 6 months of storage.
Keywords: Antibacterial adhesives, Dentine, Nuclear magnetic resonance spectroscopy, Mechanical properties of adhesives
1. INTRODUCTION
The development of adhesive systems revolutionized esthetic restorative procedures, by modifying the cavity preparation concepts and allowing for conservation of the remaining healthy tooth structure[1–3]. The total etching technique proposed by Fusayama et al. (1979) allowed for the hybridization of the demineralized dentin and has become the most common bonding mechanism in dental practice[4,5]. Dentin adhesion with three-step etch-and-rinse strategy was first used with clinical success around 1990’s[6]. However, adhesion failure between restorative materials and dental structures continues to be one of the biggest practical problems in clinical Dentistry, leading to marginal leakage, discoloration, marginal fractures, secondary caries, postoperative sensitivity and pulpal reactions[7–9]
The success of restorations with margins in enamel has been described since the introduction of the etching technique, which is credited to the high inorganic content (~90%) of enamel. On this substrate, the mechanical imbrication by tag formation within the demineralized tissue is very efficient, and leads to very stable bonding[10,11]. Dentin, however, still poses the major challenge for adhesive procedures, due to its tubular structure and organic and aqueous content[11–13]. The adhesive resin must infiltrate the wet network of exposed collagen fibrils, and polymerize in situ, forming what is known as the hybrid layer[5,14].
The monomer composition of dental adhesives is one of the determining factors in adhesion performance. Hydrophilic monomers are essential in adhering to a wet substrate such as dentin. 2-hydroxyethyl methacrylate (HEMA) is the hydrophilic monomer most commonly used in adhesive systems and is present in the composition of the primer (hydrophilic monomers with organic solvents with or without water in it formulation)[15]. This monomer has a hydrophilic moiety (hydroxyl) with affinity with the wet substrate and, a hydrophobic tail (methacrylate functionality) which promotes the polymerization with other monomers. HEMA has a low molar mass allowing the infiltration of the resin adhesive in the dental substrate. However, HEMA monomers do not form very strong polymer networks, due to the linear nature of its chains, and potential phase separation during polymerization in a highly solvated state, in the presence of tubular water[15,16].
Despite the advances achieved in dental adhesive technology, studies point to the degradation of the material over time in the presence of water[17]. This degradation may be a result of hydrolysis of the material and/or the collagen, thereby weakening the physical properties of resin-dentin bonding. The dentin-adhesive interface is porous and permeable, allowing the leaching of unreacted monomers, water sorption, swelling the polymer, and also be susceptible to the enzyme activity by metalloproteinases (MMPs), which degrade mainly type I collagen, hence exposing the fibrils at the bottom of the hybrid layer[18,19].
The biodegradation of the interface can also increase the bacterial infiltration through a gap between the restorative material and dentin (interproximal areas), which may lead to secondary caries formation[20,21]. Therefore, one additional feature of interest for adhesives is direct antimicrobial activity. Antimicrobial agents can potentially limit the infiltration, growth and the formation of a cariogenic biofilm, particularly by decreasing the viability of Streptococcus mutans (S. mutans), considered the main pathogen of tooth decay[22–25]. Using this concept, antibacterial agents with broad antimicrobial spectra have been added with no concern regarding the promotion of bacterial resistance and the production of undesirable outcomes on oral health[26,27]. Much attention has been given to antimicrobial compounds in natural products, as an alternative to synthetic compounds[28–30]. Thus, the search for new therapies to stabilize the resin-dentin interface is the key to improving the biomechanical and biochemical properties of hard dental tissues in restorative therapy.
A series of multi-functional monomers based on bile acids, colic acids and saccharides has shown high biocompatibility and low cytotoxicity compared to conventional polymers and monomers found in literature[30–38]. These bi- or multi-functional monomers are being used to provide resistance in the crosslinked monomer formed from the monomeric matrix[39]. Therefore, the aim of this in vitro study was to synthesize and characterize methacrylate monomers based on saccharides (mono-, di- and poly-saccharides) and analyze their behavior when incorporated into dental adhesive systems, including antimicrobial properties, hydrolytic stability and quality of the bonded interface with dentin. Our hypotheses were: (1) the synthesis route proposed here will successfully modify the saccharides structure to incorporate photopolymerizable groups; (2) the adhesives formulated with methacrylate saccharides monomers will demonstrate: a. greater antimicrobial activity against Streptococcus mutans; (3) greater/longer-lasting bonding to the dentin substrate when compared to control.
2. MATERIALS AND METHODS
2.1. Materials
All chemical reagents used in the synthesis were purchased from Sigma-Aldrich (St. Louis, MO, USA): D-(+)-Glucose ≥99,5%, Sucrose ≥99,5%, Chitosan low molecular, weight.; glycidyl methacrylate ≥97,0% (GMA), 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), Thiazolyl blue tetrazolium bromide (MTT), 4-methoxyphenol 99%, chloride acid 37% (HCI) and methylene chloride. The reagents used in primer formulation were: 2-hydroxyethyl methacrylate (HEMA) (ESSTECH, Essington, PA); Camphorquinone 99% (CQ) and Butylatedhydroxytoluene 99% (BHT) were purchased from Sigma-Aldrich; Ethyl 4-dimethylamino benzoate 99% (EDMAB) was purchased from Acros Organic, Pittsburgh, PA; and Phosphine oxide diphenyl (TPO) was purchased from Dispersions & Paper Chemicals, Mt. Olive, NJ; Ethanol 99% from Fischer Scientific (Fair Lawn, NJ, USA). For the antimicrobial test, the reagents used were purchased from BD diagnostics (Sparks, MD, USA): TSB- BBL™ Trypticase™ Soy Broth (TSB) and BD Bacto™ Brain Heart Infusion (BHI); and AlamarBlue™ (Thermo Fisher Scientific, Waltham, MA, USA).
2.2. Synthesis of saccharide methacrylate monomers
Acidic saccharide solutions (pH 3.5) were prepared based on Reis et al. (2009) and Chen &Park (2000)[40,41]: 1.0 g of saccharide (glucose, sucrose or chitosan) was dissolved into 100 mL of deionized water under a stirring speed of 750 rpm at room temperature. After the mixture had been completely homogenized, a 2.0 mol.L−1 HCI solution was dropped into the mixture to adjust the pH at 3.5 with the addition of 1.0% of 4-methoxyphenol (an inhibitor). After that, GMA was mixed into solution by a constant and vigorous stirring at 50 °C for 24 h. The proportions of GMA added for each saccharide solution corresponded to 50% substitution of the total hydroxyl groups. Thereafter, 50 mL of methylene chloride was added in the solution and then transferred to a separating funnel. The resulting organic solution containing unreacted GMA was discarded and the resulting aqueous solutions containing the final product were: glucose-methacrylate (Gluc-MA), sucrose-methacrylate (Sucr-MA) and chitosan-methacrylate (Chit-MA). If traces of water were present, the monomers were rotaevaporated and/or lyophilized. Figure 1 shows a chemical reaction scheme for the saccharide with GMA at pH 3.5 and the possible synthetized structures.
Figure 1.
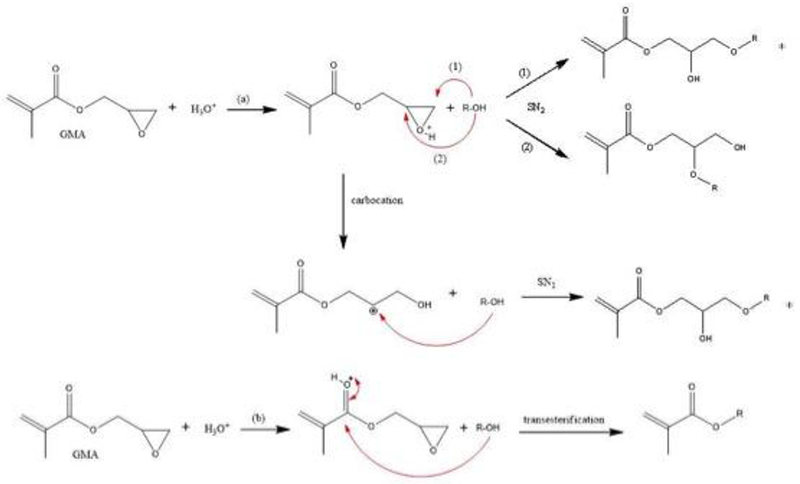
Scheme of chemical reaction of saccharides with GMA at pH 3.5 via (a) epoxy ring-opening mechanism and (b) transesterification. *racemic molecules. R-OH = glucose, sucrose and chitosan molecules.
2.2. Characterization of the synthesized saccharide methacrylates
The structures of synthesized saccharide methacrylates were verified by middle infrared spectroscopy, mid-IR, using Nicolet 6700 Thermo Scientific (Waltham, MA, USA) in the range of 4000 at 400 cm−1, with 32 scans at a resolution of 4 cm−1, using NaCl plates. The 1H Nuclear Magnetic Ressonance (1H NMR) spectra (Bruker AMX-400 MHz, Santa Barbara, CA, USA) and the 13C NMR spectra were recorded at 100 MHz, both in DMSO-d6 solvent.
2.3. Cytotoxicity and antioxidant assay of saccharides methacrylate monomers
For Cytotoxicity assay stock solutions were prepared: Gluc-MA and Sucr-MA both of the 500 g.L−1, and Chit-MA 20 g.L−1. The compounds were tested using African green monkey kidney cells, VERO (ATCC CCL-81)[42,43]. The cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM, Sigma St. Louis, Missouri, USA) supplemented with 10% fetal bovine serum, 10000 lU.mL−1 penicillin and 10 mg.mL−1 streptomycin, in a humidified environment with 5% CO2 at 37 °C. For the assays, 96-well plates were used, and in each well 1 χ 105 cells.mL−1 were incubated for 24 h at 37 °C in 5% CO2. Then, the medium was removed and the cells were incubated for 24 h with different concentrations of Gluc-MA, Sucr-MA and Chit-MA in PBS. Cell viability was assessed using the MTT assay. After 3 h incubation at 37 °C, 5% CO2, 95% air, MTT solution (1 mg.mL−1) was removed, and 50 μL were added to each well in order to solubilize the crystals formed of ethanol and then 150 μL of a solution containing PBS and isopropanol (1:1). The absorbance of each well was read on a microplate reader (Spectramax 190, Molecular Device) at 570 nm and 630 nm, being proportional to the number of living cells. Experiments were performed in quadruplicate for each concentration of each compound.
The antioxidant activity of the three compounds (Gluc-MA, Sucr-MA and Chit-MA) was evaluated on the radical cation 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+), according to previous studies, with some modifications [44,45]. The ABTS•+ was obtained after mixing of ABTS and potassium persulfate, this solution was allowed to stand for 12 h, in the dark, to obtain the radical. After, the ABTS•+ was diluted in 10 mM phosphate buffer (PB), pH 7.4, until the absorbance of ≅ 0.7 at 734.0 nm. The compounds were previously diluted in PB and incubated with the radical for 30 min in the dark at room temperature. The absorbance is measured at 734 nm, which corresponds to ABTS•+, which decays according to the antioxidant capacity of the compound tested. To obtain the percentage of inhibition of the radical, the following calculation was used:
| Eq. (1) |
where Ac is the absorbance of control and At is the absorbance of sample. The concentration of compounds leading to 50% reduction of ABTS•+ (IC50) was also determined by plotting the graph of percentage of scavenging.
2.4. Primer formulations
Acidic (pH 3.5) stock solutions, 10 % (w/v) of Gluc-MA, Sucr-MA and Chit-MA were prepared for later use in the composition of primers. These primers, with or without monomers, were prepared following the formulation: 60.0 wt% HEMA, 20.0 wt% Ethanol and 20.0 wt% stock solution; the photo-initiator system was 0.0012 mol% CQ, 0.0041 mol% EDMAB, 0.0019 mol% TPO and 0.0004 mol% BHT (inhibitor), according to the mass of HEMA. The final concentrations of each monomer in the primer system were 1.0%, 2.0% or 4.0%. The mixture was stored in glass bottles covered with aluminum foil and stirred for 24 h using a vortex mixer.
2.5. Degree of conversion and maximum rate of polymerization
Primers were placed in a silicone mold (6.8 mm in diameter, 0.8 mm in thickness), air dried for 30 s, sandwiched between two glass slides and clamped in a holder inside the IR chamber (Nicolet 6700). The peak area corresponding to the vinyl overtone absorption in the near-IR spectrum (6175 cm−1) was followed during continuous irradiation using 26 mW/cm2 (EXFO Acticure 4000, Mississauga, Ontario, Canada, λrange= 320-500 nm), for 15 min. The power output was measured using a power meter (Molectron, Portland, OR, USA), and the irradiance was calculated by dividing the power by the cross-sectional area of the power meter’s sensor. The degree of conversion (DC) was calculated as a function of the area of vinyl overtone peak (6125 cm−1) before and after 15 min of irradiation. The rate of polymerization was calculated as the first derivative of the conversion x time curve, and the maximum value (Rpmax) was recorded.
2.6. Dentin bond strength test
For this study, forty-two caries-free human third molars were extracted, selected and stored in 0.5% chloramine-T solution at 4 °C after debridement of soft tissues (Oregon Health and Science University, IRB protocol #00012056). In summary, the occlusal enamel was removed using a low-speed diamond saw (Struers, Acticurum 5, Cleveland, OH, USA), under constant water cooling. The exposed dentin surface was polished with 600-grit abrasive paper (Si-C) for 60 s under wet conditions to create a standardized smear layer. The samples were randomly assigned into seven groups (n=6), according to the primer used: Control (without synthetize monomers); Gluc-MA 1%; Gluc-MA 2%; Sucr-MA 1%; Sucr-MA 2%; Chit-MA 1%; and Chit-MA2%. The primer groups containing 4% of saccharides methacrylate monomers presented prefailures for all replicates.
The exposed dentin was etched with 32% phosphoric acid (ScotchbondTM Universal Etchant, 3M, St. Paul, MN, USA) for 15 s, rinsed with water for 30s and the water excess was removed with absorbent paper, in accordance with the wet-bonding technique. A thin layer of primer was applied, gently air-dried for 15 s, and photopolymerized with a LED light-curing unit (Valo Ultradent Products Inc., South Jordan, UT, USA) with mean irradiance of 1474.2 mW/cm2 for 20 s. A thin bond resin layer, composed by BisGMA:HEMA (60:40 %w/w) and photoinitiator system, was applied and then light cured for 60 s. Two increments of 1 mm of commercial resin composite (Ultra Universal Restorative Body Shade A2, 3M, St. Paul, MN, USA) was applied on the adhesive surface and each layer was light-cured for 20 s. The restored teeth were stored in water at 37 °C for 24 h.
After 24 h, restorations were sectioned perpendicular to the adhesive-tooth interface using a low speed diamond saw under constant water cooling, to obtain beam-shaped specimens of approximately 1.0 mm2 cross-sectional area. For each tooth, around 10 to 15 beams were obtained and these beams were randomly dived into two storage times (24 h and 6 months), stored in deionized water at 37 °C, changing the water every month. The beams, after the storage time, were fixed to a testing metallic apparatus with cyanoacrylate adhesive and tested in tension on a universal testing machine (Q-test, MTS, Eden Prairie, WI, USA) at a crosshead speed of 0.5 mm.min−1 until failure. Means and standard deviations were calculated and expressed in MPa.
After failure, a Scanning Electron Microscope (Quanta 250, FEI Company, Hillsboro, OR, EUA) was used to examine the surface morphology and identify the different failure patterns analyzing the dentin surface of each beam tested. Fractured surfaces were allowed to air-dry overnight and were sputter coated with gold (MED 010, Balzers, Balzer, Liechtenstein). The failure patterns were classified as (1) adhesive failure between dentin and the adhesive, (2) cohesive failure in the adhesive system, (3) adhesive failure between adhesive system and resin composite and, (4) mixed failure (characterized for having more than one type of failure).
2.7. Antimicrobial test of primers
Streptococcus mutans (UA159 the American Type Culture Collection (ATCC), Manassas, VA) were grown aerobically from frozen stock cultures using BHI broth for 24 h, at 37 °C in atmosphere of 5% CO2. The cultures were monitored in visible light at 600 nm to ensure that bacteria was growing in logarithmic phase and the starter culture was standardized at an optical density (OD) of 0.30, which represents a bacterial concentration of 9 χ 107 CFU.mL−1, based on previous calibration studies. The final dilution of 1:100 subculture of S. mutans in TSB with 3 wt% sucrose (culture media) was placed in each well using a sterile pipette (5.0 mL).
Six specimens were prepared for each primer group. One commercial resin composite(Ultra universal Restorative A2 Body Shade, 3M, St. Paul, MN, USA) was placed in a mold and polymerized between two glass slides for 20s each side, to obtain disc-shaped specimens (9 × 2 mm).The specimens were sterilized under UV light for 10 min each side[46]. After that, 35 uL of primer was spread across the surface of the composite disc and photopolimerized with continuing visible light irradiation (λ = 320-500 nm) (EXFO Acticure 4000, Mississauga, Ontario, Canada) 80 mW.cm−2, at a distance of 7 cm for 15 min and then, sterilized again under UV light for 10 min.
The specimens, after the sterilization process, were placed at the bottom of a 6 well culture plates containing the culture media with bacteria (1:100 subculture). The specimens were incubated in 5% CO2 at 37 °C for 5 days, with daily media replacement. Alamar Blue assay was performed at 24 h and 5 days growing. The discs were washed with PBS 1×, dipped once in a well, and 1 mL of 10% AlamarBlue™ in TSB with 3% sucrose was added to each sample in a new 24 well plate and incubated for 1 h. After that, 200 μL of each sample was placed in 96 well in triplicate, and the fluorescence intensity was measured using a plate reader (Synergy HT, Bioteklntruments, Winnoski, VT, USA) with excitation at 530 nm and emission at 580 nm.
2.8. Statistical analysis
The data from degree of conversion and maximum rate of polymerization were evaluated using one-way analysis of variance (ANOVA). Dentin bond strength and antimicrobial test were analyzed by two-way ANOVA (2 variables: primer group and storage time) followed by Turkey’s test. A value of p < 0.05 was considered significant. Failure pattern was analyzed using descriptive statistics.
3. RESULTS
3.1. Characterization of the saccharide methacrylate synthesis
Figure 2 shows the mid-IR spectra of the GMA, Gluc-MA, Sucr-MA and Chit-MA. The wavenumber 3376 cm−1 refers to the presence of the hydroxyl group of the saccharides structure and due the ring-opening reaction of epoxy group. The absorption bands at approximately 1710 and 1637 cm−1 correspond to C=O and C=C vibrational stretching of the ester group (methacrylate), respectively[47,48]. Another feature of vibrational deformation C-H can be seen in the region in 1450 cm−1, wherein the first adsorption band may be due to the CH2 group adjacent to the carbonyl group[47,49]. Signals related to the epoxy group of GMA were observed in the region of 910 and 840 cm−1, but both signals were not found in other spectra[50].
Figure 2.
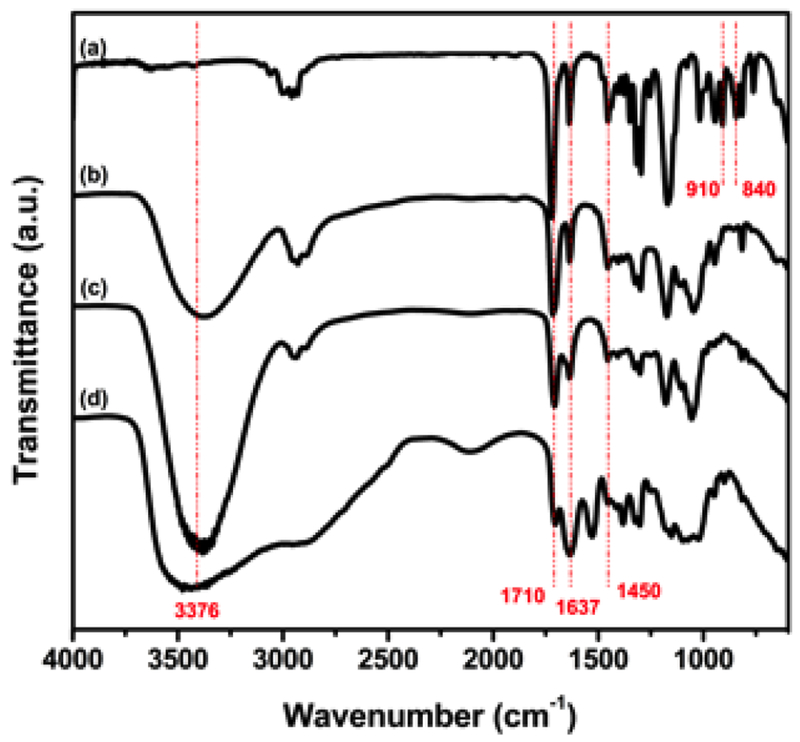
Mid-IR spectra of monomers of (a) GMA, (b) Gluc-MA, (c) Sucr-MA, (d) Chit-MA.
Figure 3 shows the 1H NMR spectrum of GMA and the reaction products of saccharides with GMA. Hydrogens bonded to carbon of vinyl group have been identified in two distinct chemical shifts, at δ 6.06 ppm and 5.68 ppm, to the product of the isomers of the epoxide ring-opening reaction, and δ 5.98 ppm and 5.61 ppm to the product of transesterification reactions. The signals from protons referring to the methyl bonded to carbon of vinyl group are identified at δ 1.88 ppm (epoxide ring-opening reaction) and δ 1.83 ppm (transesterification reaction). The glyceryl spacers showed signs that confirmed the reaction by epoxide ring-opening and their isomers, as follows: protons of the ethyl group attached to the oxygen of the methyl methacrylate group at range δ 4.14 - 4.10 ppm and δ 4.01 – 3.97 ppm, respectively; signals region at δ 3.72 - 3.65 ppm and δ 3.54 – 3.50 ppm correspond to the hydrogen remaining epoxide group, respectively, being the first region that corresponds to the product where the attack occurs to the carbon of the epoxy group with impediment steric lower; the hydrogens of the ethyl group, showed signs in the region δ 3.39 – 3.34 ppm. The remaining signals are related to protons of the saccharide structures.
Figure 3.
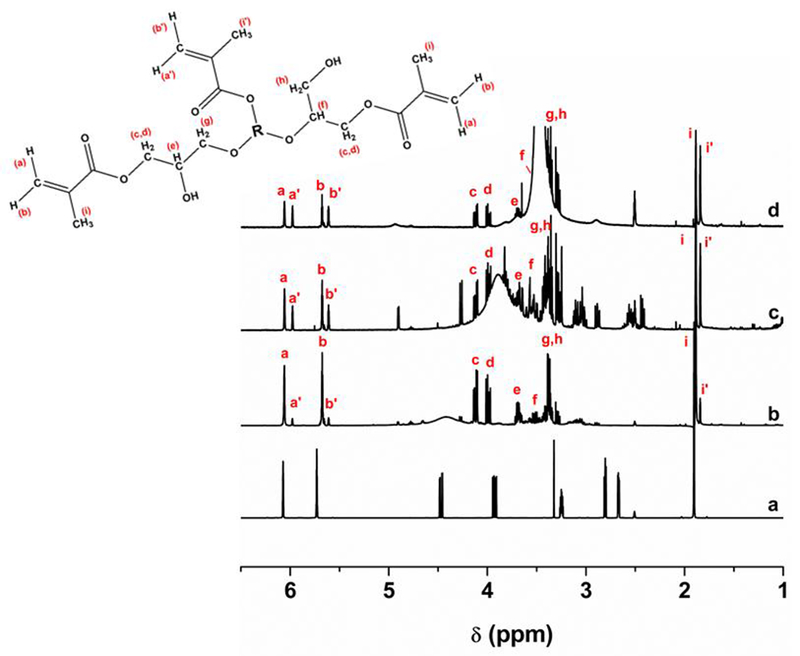
1H NMR spectra of (a) GMA, (b) Gluc-MA, (c) Sucr-MA and (d) Chit-MA (d). R = Glucose, Sucrose or Chitosan structure.
The 13C NMR spectra of the GMA and of the monomers are shown in Figure 4, which also shows signals that confirmed the formation of the final products from synthesis. The signals corresponding to the methyl methacrylate group were identified, such as carbonyl carbons at δ 168.23 ppm for the isomeric products of the epoxide ring-opening reaction, and δ 166.58 ppm for the transesterification products; the signals corresponding to the vinyl carbon were clearly observed at δ 136.70 ppm and δ 125.66 ppm (epoxide ring-opening) and also at δ 135.94 ppm and δ 124.97 ppm (transesterification); and methyl carbon at δ 18.07 ppm and δ 18.00 ppm. The signals at spectrum region of δ 80-60 ppm revealed the presence of the glyceryl spacer from GMA[40]. Furthermore, the signals that correspond to the carbons at d and e, were identified at δ 76.08 ppm and δ 72.47 ppm, where in the first chemical shift refers to the product of the reaction SN2 of carbon with high steric impediment. The other 13C signals are relative to the saccharides structure.
Figure 4.
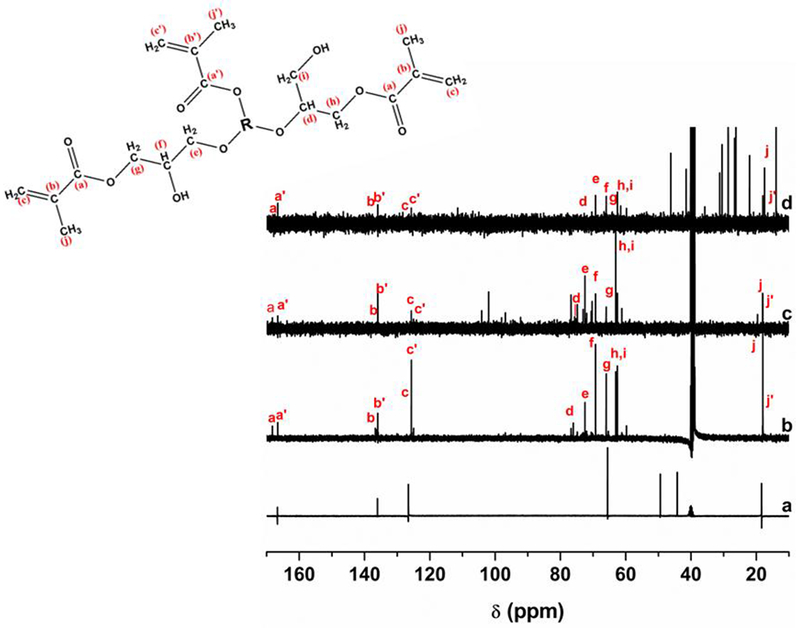
13C NMR spectra of (a) GMA, (b) Gluc-MA, (c) Sucr-MA and (d) Chit-MA (d). R = Glucose, Sucrose or Chitosan structure.
3.2. Cytotoxicity and antioxidant assay of saccharides methacrylate monomers
In this assays cytotoxicity effect of three compounds against Vero cells were evaluated. Chit-MA did not present cytotoxicity over Vero cells in the test concentration (at the maximum concentration of 0.5 %), after 24h of incubation, (data not shown). Gluc-MA and Sucr-MA compounds, were cytotoxic against Vero cell line after 24 h of treatment and they exhibit dose-dependent decrease in cell viability in response to increasing concentrations (Figure 5). Gluc-MA displayed greater cytotoxicity compared with Sucr-MA, the cell viability decrease 70% (0.313 %) to 39% (0.625 %), while that Sucr-MA 79% (0.625 %).
Figure 5.
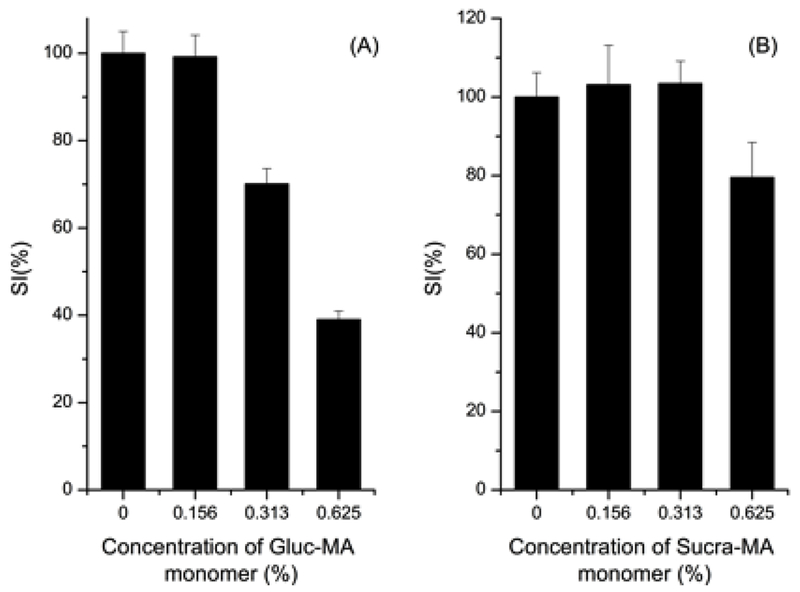
Cytotoxicity effect of compounds against Vero cells of Gluc-MA (A) and Sucr-MA (B). Data represent the mean ±standard error.
The ABTS•+ assay is one of the most widely used tests for analyzing the antioxidant property of various compounds groups. The scavenging activity of the compounds can be seen in Figure 6, Gluc-MA has the highest antioxidant effect on ABTS•+ (IC50 of ≅ 0.2 mg.mL−1, equivalent to 0.02%), followed with compound Sucr-MA, which showed approximately 2-fold higher IC50 (IC50 of ≅ 0.5 mg.mL−1, equivalent to 0.05%). The Chit-MA presented low activity on the ABTS•+, none of the concentrations tested inhibited the radical by 50%.
Figure 6.
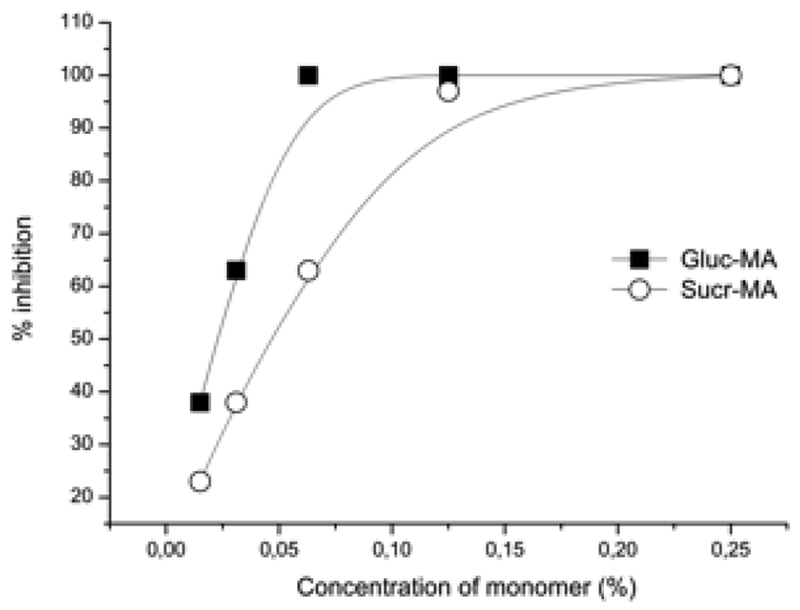
Antioxidant effect of the Gluc-MA and Sucr-MA monomers on ABTS•+.
3.3. Degree of conversion and polymerization kinetics
Table 1 shows the degree of conversion and the rate of polymerization values for the control group (without saccharides methacrylate monomer), Gluc-MA, Sucr-MA and Chit-MA monomers, at a concentration of 1%, 2% and 4%. Most of the primers groups had not statistical difference when compared to the control group (p> 0.001). The DC values decreased with increasing concentration of Gluc-MA and Sucr-MA and presented statistical difference from the control group. For the maximum rate of polymerization, the interaction between the factors (p = 0.001) were not statistically significant.
Table 1.
Means and standard deviations of degree of conversion (DC), maximum rate of polymerization (Rpmax) and dentin bond strength (MPa) at 24 hours and 6 month.
| Primer Group | DC (%) | RPmax (% S−1) | Dentin Bond strength (MPa) |
|
|---|---|---|---|---|
| 24 hours | 6 month | |||
| Control | 61.3 (0.8)abc | 0.713 (0.006)ab | 28.0 (3.8)a,A | 22.5 (3.4)b,B |
| Gluc-MA1% | 58.5 (1.5)cd | 0.650 (0.013)ab | 27.9 (3.9)a,A | 23.1 (5.7)b,B |
| Gluc-MA2% | 62.9 (0.7)ab | 0.706 (0.019)ab | 25.3 (5.1)a,A | 23.5 (3.5)b,A |
| Gluc-MA4% | 52.3 (2.7)e | 0.707 (0.042)ab | -- | -- |
| Sucr-MA1% | 64.6 (0.8)a | 0.618 (0.026)b | 25.7 (2.8)a,B | 40.6 (5.3)a,A |
| Sucr-MA2% | 61.1 (0.6)bc | 0.637 (0,059)ab | 16.6 (2.3)b,B | 27.4 (2.3)b,A |
| Sucr-MA4% | 53.5 (0.2)e | 0.690 (0,011)ab | -- | -- |
| Chit-MA1% | 57.4 (0.5)d | 0.737 (0.039)a | 27.7 (4.6)a,A | 21.5 (4.2)b,B |
| Chit-MA2% | 62.1 (0.9)ab | 0.632 (0,067)ab | 27.0 (4.8)a,A | 22.0 (2.3)b,B |
| Chit-MA4% | 61.9 (0.2)ab | 0.711 (0,041)ab | -- | -- |
Distinct lowercase letters indicate differences in column.
Distinct capital letters indicate differences between storage times for bond strength.
3.4. Dentin bond strength
Dentin bond strength values are described in mean (standard deviation) after 24 hours and 6 month of water storage (Table 1). Sucr-MA2% presented the lowest bond strength compared to the other groups after 24 h of storage (p < 0.0001). After 6 months, Sucr-MA1% increased the bond strength value and was higher compared to the other groups (p = 0.0002). Sucr-MA2% also increased the bond strength value after 6 months of storage compared to the same group at 24 h. Gluc-MA2% presented stable bond strength after 6 months of storage. The others primer groups showed a decrease after 6 months. The interaction between the primer and storage time was not statistically significant (p < 0.0001). All groups presented specimens with pre-test failures (5 to 10 beans) and no values were added to the statistical analysis for these specimens.
From the tested specimens, the predominant type of failure was type I, which corresponded to adhesive failure between dentin and the adhesive for all groups tested, followed by type II (cohesive in adhesive system). Adhesive failure between adhesive and the resin composite (type III) was not frequent. Mixed failure (type IV) was also predominant in Gluc-MA1% and Gluc-MA2% groups after 24 hours of storage (35.0 % and 37.7 %). The Sucr-MA1% group had a significant increase in failure pattern type II, after 6 month of storage. The same behavior was observed for Chit-MA groups. Figure 7 illustrates the failure pattern percentage across the groups and storage time, and Figure 8 shows representatives images of each failure type.
Figure 7.
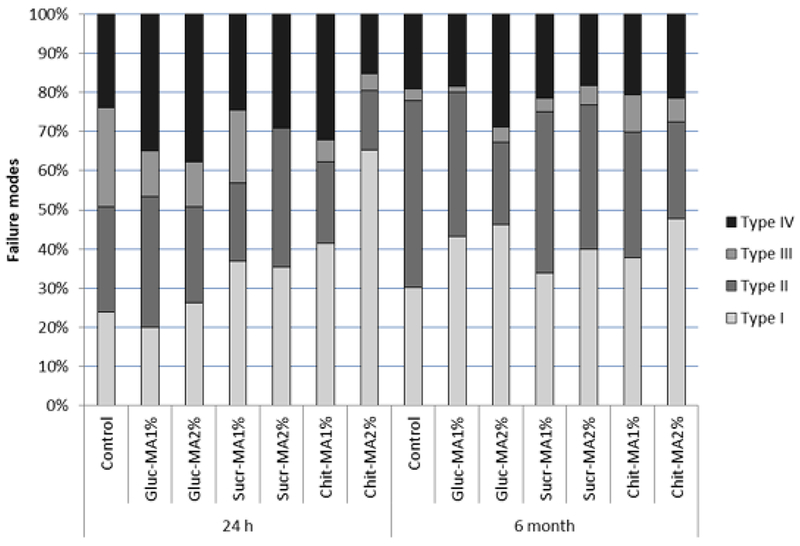
Failure pattern (percentage), of tested groups, after 24 hours and 6 month of storage. Type I: Adhesive failure between dentin and adhesive; Type II: Cohesive failure in the adhesive system; Type III: Adhesive failure between adhesive and resin composite; and Type IV: Mixed.
Figure 8.
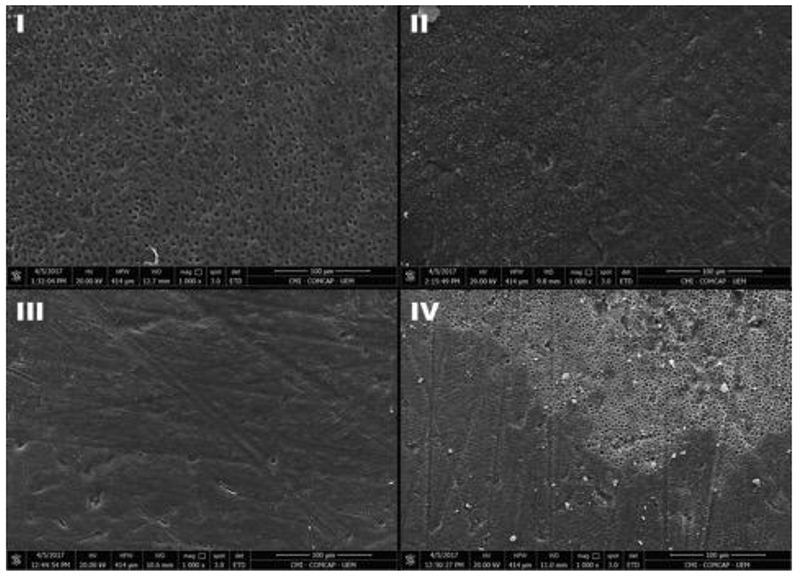
Representative images of each failure type (1000× magnification). (I) Type I; (II) Type II; (III) Type III; and (IV) Type IV.
3.5. Antimicrobial test
After 24 h and 5 days of incubation, the biofilm formed on the adhesive-coated disc was analyzed by Alamar Blue assay based on the reduction of resazurin, a blue dye that can be reduced by bacterial metabolic activity to pink resorufin, which has fluorescence (to quantify the metabolism of the bacteria)[51]. Figure 9 (a) and (c) demonstrate that, at 24 hours, groups containing 4 % of Gluc-MA and Chit-MA in the composition of primers showed antimicrobial activity, but only Chit-MA maintained this effect after 5 days of incubation. However, Sucr-MA (Figure 9 (b)) led to antimicrobial inhibition with 1 % of monomer to 4% of monomer in primer composition for 24 h of growth. After 5 days of growing, the antimicrobial capacity followed the same trend, although no statistical difference was observed for the concentrations of 2 and 4%. In most groups, there was a decrease in the biofilm formation as the specimens were stored for longer times.
Figure 9.
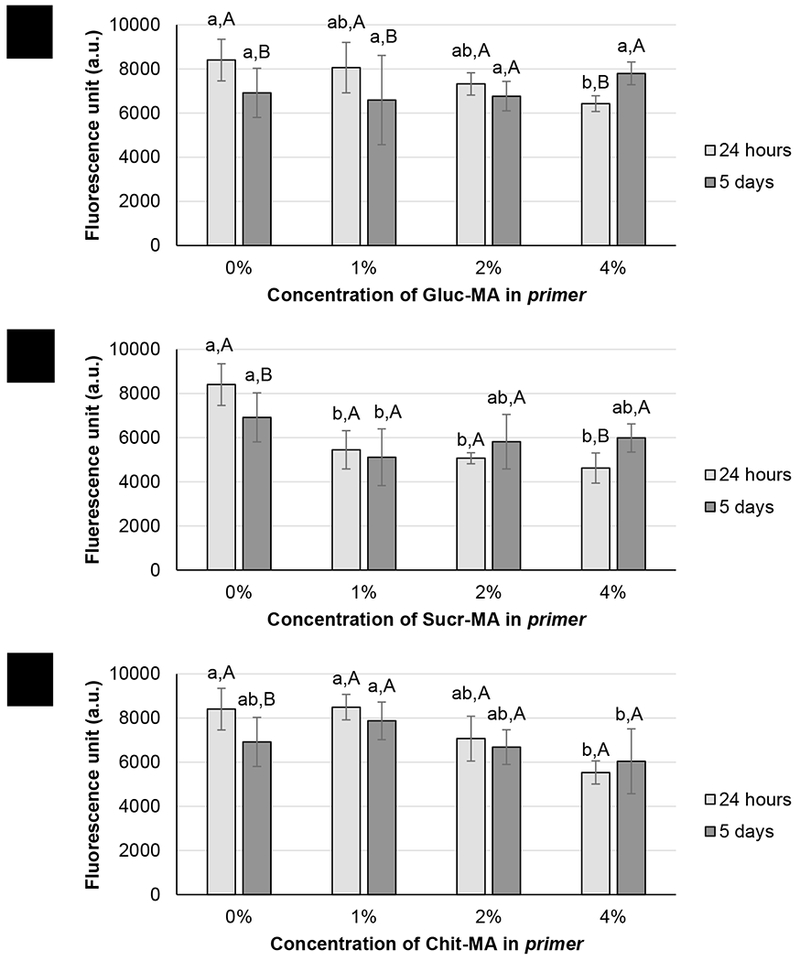
Metabolic activity of Streptococcus mutans for primer groups containing (a) Gluc-MA, (b) Sucr-MA and (c) Chit-MA, after 24 hours and 5 days of incubation. The vertical lines indicate standard deviations. Different lower case letters indicate statistical differences between the groups for the same period of growth; different capital letters indicate statistical differences between 24 h and 5 days of growth within the same group.
4. DISCUSSION
The use of GMA as a modifier has been described as a suitable method for the production of vinyl macromolecules. This method is based on the incorporation of carbon-carbon bonds derived from GMA structure in the macromolecule structures (saccharides), which allows them to undergo a gelation process through a radical polymerization reaction which promotes crosslinking[40,52–54].
The mid-IR results showed absorption signals that identified the incorporation of the methacrylate group by ring-opening reaction of the epoxy group and the transesterification reaction with hydroxyl groups (saccharides). Bands concerning the vibrational stretching of carbonyl group and carbon-carbon bond, from methacrylate group, were observed in the spectra[37]. Absorption signals of epoxy group, from GMA, were not detected in the synthesized structures spectrums, indicating that the addition of the methacrylate group in the saccharide reaction was successful[37,50].
The data 1H and 13C NMR showed that the reaction in acidic media in which the molecule of GMA was attacked by saccharide structure, occurred through the transesterification reaction and epoxy ring-opening mechanism. Signals referring to the methyl methacrylate group, for both mechanisms, were identified due to chemical shifts[40]. This difference is related to coupling of hydrogen atoms with other atoms in distinct neighborhood. Other chemical shifts were found in the spectral region for ethyl group derived epoxy ring, which is attached to oxygen of saccharide structure confirming the epoxy ring-opening reaction. Thus, the first hypothesis is accepted.
Dental adhesive systems must be biocompatible, so that they can be applied on living tissues. The interaction of these materials and their components with the cells is responsible for many of immune alterations[55]. Besides that, they may cause inflammation and necrosis[56]. In this way, cytotoxicity assays can be used to evaluate the biocompatibility of materials[57]. Vero cells can be used to evaluate the cytotoxicity of different materials for biological use; a recent study showed that three different dental adhesives did not show cytotoxicity on the Vero cells evaluated by the MTT assay[43]. Our results demonstrate that the tested materials have low cytotoxicity, in the concentrations tested, on Vero cells.
Some inflammatory processes, such as Periodontal Diseases (PD), which occur, especially due to the presence of pathogenic microorganisms, can cause a number of damaging effects on the oral cavity[58,59]. These effects are due to the formation of reactive oxygen species (ROS), which aim to neutralize these microorganisms, but also attack the tissue. Materials with antioxidant properties, such as dental adhesive systems, may help in reducing the effects of ROS. According to the results obtained, Gluc-MA and Sucr-MA monomers demonstrated a concentration-dependent effect on the ABTS•+, which can act on the ROS that have pro-oxidant activity, and determine a protection in the events generated by inflammatory processes of the buccal cavity.
All groups presented similar conversion when compared to the control group, except for the Gluc-MA4%, Sucr-MA4% and Chit-MA1%, which showed statistically lower DC values. The higher concentration of Gluc-MA4% and Sucr-MA4% contributed to decrease in the DC values due the increased diffusion limitations for these high molecular weight and higher viscosity monomers[60]. Another factor related to this decrease in conversion for those specific groups is the increased concentration of radicals, which can rapidly react with each other, inducing premature termination of the polymerization[61]. The maximum rate of polymerization did not significantly differ in the different systems, with or without saccharide methacrylate monomers. These results demonstrate that, up to a certain threshold, the addition of the saccharide methacrylates does not affect polymerization of the main primer monomer, HEMA.
The predominant organic component of dentin is Type I collagen, which is essential to establishing mechanical retention on the hybrid layer, which in turn increases bond strengths of adhesives to dentin. To maintain the stability of collagen fibrils, besides covalent intermolecular crosslinking, other agents have been used to induce the formation of intramolecular, intermolecular and intermicrofibrillar crosslinking in biological tissues[62,63]. Collagen crosslinking agents include compounds such as aldehydes and other natural compounds, including molecules of the proantocyanidin family, and even saccharides such as chitosan [64]. According to the data obtained in the present study, the addition of saccharide methacrylate monomers on primers up to 2 wt% did not affect the bond strength at 24 h, except for Sucr-MA2%, which presented the lowest value of bond strength. After 6 months of storage, however, the bond strength of adhesive restorations was shown to greatly improve with the addition of the saccharide monomers, at least for the sucrose compositions. While the control group and the groups containing Gluc-MA and Chit-MA presented a decrease in bond strength of about 20%, the use of Sucr-MA at 1 and 2 wt% led to a 57 and 65 % increase in bond strength, respectively, after 6 months storage. It can be speculated that the disaccharide structure of the sucrose favorably interacted with the demineralized dentin to improve its hydrolytic stability by establishing crosslinks with exposed collagen[6,65]. These crosslinks represent physical bonds between the organic portion of the dentin through electrostatic interactions of pendant hydroxyl groups (OH) from Sucr-MA. The amount of hydroxyls available from Sucr-MA is greater than Gluc-MA, and lower than in Chit-MA structure. However, the hydroxyl groups are more susceptible and exposed in Sucr-MA than in Chit-MA. In the latter, the polysaccharide nature of the molecule (a natural polymer) produces a steric impediment to the formation of intermolecular crosslinks. In addition, another factor that can improve the mechanical properties would be intermicrofibrilar crosslinks from chemical bond between collagen fibrils via polymerization with Sucr-MA molecules before crosslinking. Besides that, covalent bonds of intermolecular crosslinks with the adhesive system may form, promoting the increase of the bond strength[31,59]. These hypotheses will be confirmed in future studies including enzymatic challenge and zymography of the bonded interface using Sucr-MA monomers, especially at lower concentrations such as 1 wt%.
The failure pattern classification from bond strength specimens is an important tool to identify the weakest area of dentin–composite interface created by adhesives[20]. The predominant occurrence of Type I and II failure modes suggest that the weakest area of the adhesion is still the dentin-adhesive interface and the cohesive adhesion inside the adhesive layer. However the same pattern was found for the control group, indicating that the monomers additions were not able to interfere with the adhesion failure mechanism.
While the crosslinking potential of saccharide molecules has been somewhat explored in the literature, their antimicrobial activity, specifically related to oral biofilm, has not been reported so far. New ester formation from transesterification reaction generating sugar ester compounds, has been extensively investigated due the antifugal and antibacterial properties[66–68]. In addition, the presence of the vinyl ester group (methyl methacrylate), from epoxy-opening ring, has a bacterial inhibition [69,70]. This study showed antimicrobial activity with primers containing saccharides-methacrylate monomers, hence, the second hypothesis must be accepted. It is suggested that this phenomenon involves the presence of acyl group, donor of saccharides ester that can modify the physiological function of the bacteria[71]. Studies revealed that the action of sucrose-ester on bacteria do not appear to occur by solubilizing the cell membrane, but due to a autolytic enzymatic stimulus, called bacterial autolysis[72,73]. The results of the present study demonstrate, especially for the sucrose-based monomers, that the metabolic activity of S. mutans decreases in the presence of saccharides, and the effect is sustainable for at least the short period of incubation used in this study (5 days).
5. CONCLUSION
Saccharide methacrylate monomers, Gluc-MA, Sucr-MA and Chit-MA, showed promising results when added to dental adhesives. Sucr-MA 1% showed the best results regarding the decrease in bacterial metabolism, low cytotoxicity and increasing the bond strength after 6 months of storage.
6. ACKNOWLEDGEMENTS
The authors would like to thank CNPq Brazil (#200116/2014-2), Inomat – Nacional Institute (INCT) and NIH/NIDCR (#1U01 DE02756) for funding. Dr. Kristen Lampi and Satin Salehi (in memorian) for the relief with the bacterial experiments and Dr. Ana Paula Piovezan Fugolin for the support with collecting microtensile bond strength data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- [1].Gordan V V, Vargas MA, Cobb DS, Denehy GE. Evaluation of acidic primers in microleakage of Class 5 composite resin restorations. Oper Dent 2017;23:244–9. [PubMed] [Google Scholar]
- [2].Retief DH. Effect of conditioning the enamel surface with phosphoric acid. J Dent Res 1973;52:333–41. [DOI] [PubMed] [Google Scholar]
- [3].Moura SK, Murad CG, Reis A, Klein-Júnior CA, Grande RHM, Loguercio AD. The influence of air temperature for solvent evaporation on bonding of self-etch adhesives to dentin. Eur J Dent 2014;8:205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fusayama T, Nakamura M, Kurosaki N, Iwaku M. Non-pressure adhesion of a new adhesive restorative resin. J Dent Res 1979;58:1364–70. [DOI] [PubMed] [Google Scholar]
- [5].Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res 1982;16:265–73. [DOI] [PubMed] [Google Scholar]
- [6].Farias DCS, Caldeira de Andrada MA, Boushell LW, Walter R. Assessment of the initial and aged dentin bond strength of universal adhesives. Int J Adhes Adhes 2016;70:53–61. [Google Scholar]
- [7].Baratieri LN. Adesivos Odontológicos In: Santos, editor. Estética Restaurações Adesivas Diretas em dentes anteriores Frat. 2a ed., São Paulo: 1995, p. 135–205. [Google Scholar]
- [8].Kidd EA. Microleakage: a review. J Dent 1976;4:199–206. [DOI] [PubMed] [Google Scholar]
- [9].Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007;28:3757–85. [DOI] [PubMed] [Google Scholar]
- [10].Oliveira NA De, Diniz LSM, Svizero N da R, D’Alpino PHP, Pegoraro CACC. Sistemas adesivos: Conceitos atuais e aplicações clínicas. Rev Dentística Line 2010;9:1–9. [Google Scholar]
- [11].Silva EO de S, Beltrani FC, Shibayama R, Contreras AFR, Hoeppner MG. Sistemas adesivos: conceito, aplicação e efetividade. Arq Cinência Da Saúde Da UNIPAR 2010;14:81–7. [Google Scholar]
- [12].Silverstone LM, Saxton CA, Dogon IL, Fejerskov O. Variation in the pattern of acid etching of human dental enamel examined by scanning electron microscopy. Caries Res 1975;9:373–87. [DOI] [PubMed] [Google Scholar]
- [13].Swift EJ, Perdigão J, Heymann HO. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int 1995;26:95–110. [PubMed] [Google Scholar]
- [14].Manfroi FB, Marcondes ML, Somacal DC, Borges GA, Júnior LHB, Spohr AM. Bond Strength of a Novel One Bottle Multi-mode Adhesive to Human Dentin After Six Months of Storage. Open Dent J 2016;10:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc 2003;69:726–31. [PubMed] [Google Scholar]
- [16].Reis AF, Pereira PNR, Giannini M. Sistemas Adesivos - Atualidades E Perspectivas. In: CIOSP, editor. 25 Congr. Int. Odontol. São Paulo, São Paulo: 2007, p. 85–117. [Google Scholar]
- [17].Giannini M, Seixas CA, Reis AF, Pimenta LA. Six-month storage-time evaluation of one-bottle adhesive systems to dentin. J Esthet Restor Dent 2003;15:43–8. [DOI] [PubMed] [Google Scholar]
- [18].Tay FR, Pashley DH, Suh BI, Carvalho RM, Itthagarun A. Single-step adhesives are permeable membranes. J Dent 2002;30:371–82. [DOI] [PubMed] [Google Scholar]
- [19].Hashimoto M, Ohno H, Sano H, Kaga M, Oguchi H. In vitro degradation of resindentin bonds analyzed by microtensile bond test, scanning and transmission electron microscopy. Biomaterials 2003;24:3795–803. [DOI] [PubMed] [Google Scholar]
- [20].André CB, Gomes BPFA, Duque TM, Stipp RN, Chan DCN, Ambrosano GMB, et al. Dentine bond strength and antimicrobial activity evaluation of adhesive systems. J Dent 2015;43:466–75. [DOI] [PubMed] [Google Scholar]
- [21].Nakamura M, Oyane A, Shimizu Y, Miyata S, Saeki A, Miyaji H. Physicochemical fabrication of antibacterial calcium phosphate submicrospheres with dispersed silver nanoparticles via coprecipitation and photoreduction under laser irradiation. Acta Biomater 2016;46:299–307. [DOI] [PubMed] [Google Scholar]
- [22].Beighton D The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol 2005;33:248–55. [DOI] [PubMed] [Google Scholar]
- [23].Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev 1986;50:353–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van Houte J Role of micro-organisms in caries etiology. J Dent Res 1994;73:672–81. [DOI] [PubMed] [Google Scholar]
- [25].Marsh PD. Antimicrobial strategies in the prevention of dental caries. Caries Res 1993;27:72–6. [DOI] [PubMed] [Google Scholar]
- [26].Falsetta ML, Klein MI, Lemos JA, Silva BB, Agidi S, Scott-Anne KK, et al. Novel Antibiofilm Chemotherapy Targets Exopolysaccharide Synthesis and Stress Tolerance in Streptococcus mutans To Modulate Virulence Expression In Vivo. Antimicrob Agents Chemother 2012;56:6201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mcdonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev 1999;12:147–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aguiar TR, Vidal CMP, Phansalkar RS, Todorova I, Napolitano JG, McAlpine JB, et al. Dentin Biomodification Potential Depends on Polyphenol Source. J Dent Res 2014;93:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhao W, Xie Q, Bedran-Russo AK, Pan S, Ling J, Wu CD. The preventive effect of grape seed extract on artificial enamel caries progression in a microbial biofilm-induced caries model. J Dent 2014;42:1010–8. [DOI] [PubMed] [Google Scholar]
- [30].Diolosà M, Donati I, Turco G, Cadenaro M, Di Lenarda R, Breschi L, et al. Use of Methacrylate-Modified Chitosan to Increase the Durability of Dentine Bonding Systems. Biomacromolecules 2014;15:4606–13. [DOI] [PubMed] [Google Scholar]
- [31].Chen WQ, Wei H, Li SL, Feng J, Nie J, Zhang XZ, et al. Fabrication of starshaped, thermo-sensitive poly(N-isopropylacrylamide)-cholic acid-poly(ε-caprolactone) copolymers and their self-assembled micelles as drug carriers. Polymer (Guildf) 2008;49:3965–72. [Google Scholar]
- [32].Strandman S, Le Dévédec F, Zhu XX. Thermosensitivity of Bile Acid-Based Oligo(ethylene glycol) Stars in Aqueous Solutions. Macromol Rapid Commun 2011;32:1185–9. [DOI] [PubMed] [Google Scholar]
- [33].Wan T, Liu Y, Yu J, Chen S, Li F, Zhang X, et al. Synthesis and characterization of star oligo/poly(2,2-dimethyltrimethylene carbonate)s containing cholic acid moieties. J Polym Sci Part A Polym Chem 2006;44:6688–96. [Google Scholar]
- [34].Giguère G, Zhu XX. Functional Star Polymers with a Cholic Acid Core and their Thermosensitive Properties. Biomacromolecules 2010;11:201–6. [DOI] [PubMed] [Google Scholar]
- [35].de Oliveira HFN, Felisberti MI. Amphiphilic copolymers of sucrose methacrylate and acrylic monomers: Bio-based materials from renewable resource. Carbohydr Polym 2013;94:317–22. [DOI] [PubMed] [Google Scholar]
- [36].Ates B, Koytepe S, Karaaslan MG, Balcioglu S, Gulgen S. Biodegradable nonaromatic adhesive polyurethanes based on disaccharides for medical applications. Int J Adhes Adhes 2014;49:90–6. [Google Scholar]
- [37].Torabi S, Mahdavian AR, Sanei M, Abdollahi A. Chitosan and functionalized acrylic nanoparticles as the precursor of new generation of bio-based antibacterial films. Mater Sci Eng C 2016;59:1–9. [DOI] [PubMed] [Google Scholar]
- [38].Bowen RL. Polymerizable Cyclodextrin derivates for use dental applications. US5981740, 1999. [Google Scholar]
- [39].Giannini M, Makishi P, Ayres APA, Vermelho PM, Fronza BM, Nikaido T, et al. Self-Etch Adhesive Systems: A Literature Review. Braz Dent J 2015;26:3–10. [DOI] [PubMed] [Google Scholar]
- [40].Reis A V, Fajardo AR, Schuquel ITA, Guilherme MR, Vidotti GJ, Rubira AF, et al. Reaction of Glycidyl Methacrylate at the Hydroxyl and Carboxylic Groups of Poly(vinyl alcohol) and Poly(acrylic acid): Is This Reaction Mechanism Still Unclear? J Org Chem 2009;74:3750–7. [DOI] [PubMed] [Google Scholar]
- [41].Chen J, Park K. Synthesis of fast-swelling, superporous sucrose hydrogels. Carbohydr Polym 2000;41:259–68. [Google Scholar]
- [42].Mattos AC de, Altmeyer C, Tominaga TT, Khalil NM, Mainardes RM. Polymeric nanoparticles for oral delivery of 5-fluorouracil: Formulation optimization, cytotoxicity assay and pre-clinical pharmacokinetics study. Eur J Pharm Sci 2016;84:83–91. [DOI] [PubMed] [Google Scholar]
- [43].Catunda RQ, Vieira JRC, de Oliveira EB, da Silva EC, Brasil VLM, Perez DE da C. Citotoxicity evaluation of three dental adhesives on vero cells in vitro. J Clin Exp Dent 2017;9:e61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dalmolin LF, Khalil NM, Mainardes RM. Delivery of vanillin by poly(lactic-acid) nanoparticles: Development, characterization and in vitro evaluation of antioxidant activity. Mater Sci Eng C 2016;62:1–8. [DOI] [PubMed] [Google Scholar]
- [45].Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231–7. [DOI] [PubMed] [Google Scholar]
- [46].André CB, dos Santos A, Pfeifer CS, Giannini M, Girotto EM, Ferracane JL. Evaluation of three different decontamination techniques on biofilm formation, and on physical and chemical properties of resin composites. J Biomed Mater Res Part B Appl Biomater 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ibrahim M, Alaam M, El-Haes H, Jalbout AF, Leon A De. Analysis of the structure and vibrational spectra of glucose and fructose. Eclética Química 2006;31:15–21. [Google Scholar]
- [48].Almeida JF, Ferreira P, Lopes A, Gil MH. Photocrosslinkable biodegradable responsive hydrogels as drug delivery systems. Int J Biol Macromol 2011;49:948–54. [DOI] [PubMed] [Google Scholar]
- [49].Kumirska J, Czerwicka M, Kaczyński Z, Bychowska A, Brzozowski K, Thöming J, et al. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar Drugs 2010;8:1567–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hu G, Yu D, Liang H, Min C. Synthesis and characterization of novel aliphatic amine-containing dimethacrylate cross-linkers and their use in UV-curable resin systems. Polym Sci Ser B 2011;53:181–7. [Google Scholar]
- [51].Welch K, Cai Y, Strømme M. A Method for Quantitative Determination of Biofilm Viability. J Funct Biomater 2012;3:418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].van Dijk-Wolthuis WNE, Kettenes-van den Bosch JJ, van der Kerk-van Hoof A, Hennink WE. Reaction of Dextran with Glycidyl Methacrylate: An Unexpected Transesterification. Macromolecules 1997;30:3411–3. [Google Scholar]
- [53].Sutter M, Siepmann J, Hennink WE, Jiskoot W. Recombinant gelatin hydrogels for the sustained release of proteins. J Control Release 2007;119:301–12. [DOI] [PubMed] [Google Scholar]
- [54].Guilherme MR, Reis A V., Takahashi SH, Rubira AF, Feitosa JPA, Muniz EC. Synthesis of a novel superabsorbent hydrogel by copolymerization of acrylamide and cashew gum modified with glycidyl methacrylate. Carbohydr Polym 2005;61:464–71. [Google Scholar]
- [55].Kleinsasser NH, Wallner BC, Harréus UA, Kleinjung T, Folwaczny M, Hickel R, et al. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J Dent 2004;32:229–34. [DOI] [PubMed] [Google Scholar]
- [56].De Lourdes Rodrigues Accorinte M, Loguercio AD, Reis A, Muench A, De Araújo VC. Adverse effects of human pulps after direct pulp capping with the different components from a total-etch, three-step adhesive system. Dent Mater 2005;21:599–607. [DOI] [PubMed] [Google Scholar]
- [57].Demirci M, Hiller KA, Bosl C, Galler K, Schmalz G, Schweikl H. The induction of oxidative stress, cytotoxicity, and genotoxicity by dental adhesives. Dent Mater 2008;24:362–71. [DOI] [PubMed] [Google Scholar]
- [58].Gonçalves D, Correa FOB, Khalil NM, De Faria Oliveira OMM, Orrico SRP. The effect of non-surgical periodontal therapy on peroxidase activity in diabetic patients: A case-control pilot study. J Clin Periodontol 2008;35:799–806. [DOI] [PubMed] [Google Scholar]
- [59].Battino M, Ferreiro MS, Fattorini D, Bullon P. In vitro antioxidant activities of mouthrinses and their components. J Clin Periodontol 2002;29:462–7. [DOI] [PubMed] [Google Scholar]
- [60].Pfeifer CS, Shelton ZR, Braga RR, Windmoller D, Machado JC, Stansbury JW. Characterization of dimethacrylate polymeric networks : A study of the crosslinked structure formed by monomers used in dental composites. Eur Polym J 2011;47:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dewaele M, Asmussen E, Peutzfeldt A, Munksgaard EC, Benetti AR, Finné G, et al. Influence of curing protocol on selected properties of light-curing polymers: Degree of conversion, volume contraction, elastic modulus, and glass transition temperature. Dent Mater 2009;25:1576–84. [DOI] [PubMed] [Google Scholar]
- [62].Sung H-W, Chang W-H, Ma C-Y, Lee M-H. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res 2003;64A:427–38. [DOI] [PubMed] [Google Scholar]
- [63].Bedran-Russo AKB, Pereira PNR, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B Appl Biomater 2007;80:268–72. [DOI] [PubMed] [Google Scholar]
- [64].Bedran-Russo AKB, Pereira PNR, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res Part B Appl Biomater 2007;80B:268–72. [DOI] [PubMed] [Google Scholar]
- [65].Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater 2006;22:211–22. [DOI] [PubMed] [Google Scholar]
- [66].Xin L Antimicrobial structure-efficacy relationship of sugar fatty acid esters. J Chem Pharm Res 2014;6:944–6. [Google Scholar]
- [67].Lee KP, Kim HK. Antibacterial Effect of Fructose Laurate Synthesized by Candida antarctica B Lipase-Mediated Transesterification. J Microbiol Biotechnol 2016;26:1579–85. [DOI] [PubMed] [Google Scholar]
- [68].Wagh A, Shen S, Shen FA, Miller CD, Walsh MK. Effect of Lactose Monolaurate on Pathogenic and Nonpathogenic Bacteria. Appl Environ Microbiol 2012;78:3465–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shi Y, Li J, Chu Y-H. Enzyme-catalyzed regioselective synthesis of sucrosebased esters. J Chem Technol Biotechnol 2011;86:1457–68. [Google Scholar]
- [70].Kummerow FA. Carbohydrate Polyesters as Fat Substitutes. J Am Coll Nutr 1995;14:668–668. [Google Scholar]
- [71].Zhang X, Song F, Taxipalati M, Wei W, Feng F. Comparative Study of Surface-Active Properties and Antimicrobial Activities of Disaccharide Monoesters. PLoS One 2014;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Boscolo M Sucroquímica: Síntese e potencialidades de aplicações de alguns 30 derivados químicos de sacarose. Quim Nova 2003;26:906–12. [Google Scholar]
- [73].Sikes A Feasibility of using food-grade additives to control the growth of Clostridium perfringens. Int J Food Microbiol 1999;46:179–85. [DOI] [PubMed] [Google Scholar]


