Abstract
Analysis of variation in whole-animal performance can shed light on causal connections between specific traits, integrated physiological capacities, and Darwinian fitness. Here, we review and synthesize information on naturally occurring variation in physiological performance capacities and how it relates to environmental adaptation in deer mice (Peromyscus maniculatus). We discuss how evolved changes in aerobic exercise capacity and thermogenic capacity have contributed to adaptation to high elevations. Comparative work on deer mice at high and low elevations has revealed evolved differences in aerobic performance capacities in hypoxia. Highland deer mice have consistently higher aerobic performance capacities under hypoxia relative to lowland natives, consistent with the idea that it is beneficial to have a higher maximal metabolic rate (as measured by the maximal rate of O2 consumption, VO2max) in an environment characterized by lower air temperatures and lower O2 availability. Observed differences in aerobic performance capacities between highland and lowland deer mice stem from changes in numerous subordinate traits that alter the flux capacity of the O2-transport system, the oxidative capacity of tissue mitochondria, and the relationship between O2 consumption and ATP synthesis. Many such changes in physiological phenotype are associated with hypoxia-induced changes in gene expression. Research on natural variation in whole-animal performance forms a nexus between physiological ecology and evolutionary biology that requires insight into the natural history of the study species.
Keywords: adaptation, aerobic capacity, aerobic metabolism, elevation, fitness, high, hypoxia, physiological performance, thermogenic capacity, VO2max
Abstract
El análisis de la variación en el desempeño de un animal puede dilucidar las conexiones causales entre rasgos específicos, capacidades fisiológicas integradas y aptitud darwiniana. En este trabajo se revisan y sintetizan información sobre la variación natural de las capacidades de desempeño fisiológico y cómo se relacionan con la adaptación ambiental en ratones ciervos (Peromyscus maniculatus). Se discuten cómo los cambios evolutivos en la capacidad de ejercicio aeróbico y la capacidad termogénica contribuyen a la adaptación a grandes altitudes. Estudios comparativos de ratones ciervos de alta y baja altitud revelan diferencias evolutivas en la capacidad de desempeño aeróbico durante la hipoxia. Los nativos de las tierras altas tienen capacidades de desempeño aeróbico consistentemente más altas en estado de hipoxia que los nativos de las tierras bajas, lo que coincide con la idea de que es beneficioso tener una tasa metabólica máxima más alta (medida como la tasa máxima de consumo de O2, VO2max) en un ambiente caracterizado por menores temperaturas y menor disponibilidad de O2. Las diferencias observadas en la capacidad de desempeño aeróbico entre ratones ciervos de tierras altas y bajas, provienen de cambios en numerosos rasgos subordinados que alteran la capacidad de flujo del sistema de transporte de O2, la capacidad oxidativa de las mitocondrias y la relación entre el consumo de O2 y la síntesis de ATP. Muchos de estos cambios en el fenotipo fisiológico están asociados con los cambios en la expresión génica inducidos por la hipoxia. La investigación sobre la variación natural en el completo desempeño de los animales crea un nexo entre la ecología fisiológica y la biología evolutiva que requiere del conocimiento de la historia natural de las especies estudiadas.
Whole-animal performance, defined as “…a quantitative measure of how well an individual performs a dynamic, ecologically relevant task” (Lailvaux and Husak 2014:286), is often positively associated with success in foraging, escaping predators, and competing for mates, and is therefore a strong predictor of lifetime reproductive success. For this reason, measures of whole-animal performance represent an obvious point of focus in studies of phenotypic evolution (Arnold 1983; Huey 1983; Djawdan and Garland 1988; Pough 1989; Bennett and Huey 1990; Bennett 1991; Djawdan 1993; Irschick and Garland 2001; Irschick et al. 2008; Careau and Garland 2012). In studies of terrestrial vertebrates, the most commonly examined performance capacities relate to aspects of locomotor activity such as burst speed (the greatest velocity an animal attains over a short distance), maximal exertion (the work output during activity to rapid exhaustion), and endurance (the length of time a given activity can be sustained—Bennett and Huey 1990; Bennett 1991; Irschick and Garland 2001). These various performance capacities may depend on somewhat different physiological and anatomical support systems but together they define a “locomotor performance space” that bounds most of the ecologically relevant behaviors and activities of an animal (Bennett and Huey 1990; Bennett 1991).
In endotherms, many ecologically relevant measures of physiological performance, such as the capacity for sustained exercise and the ability to be active in the cold, are directly related to aerobic metabolism. Given that O2 is required for ATP synthesis via oxidative phosphorylation involving the mitochondrial electron transport system, an animal’s rate of aerobic metabolism can be measured as the rate of O2 consumption (VO2). In homeothermic endotherms, the maximum metabolic rate, MMR (measured by the maximal rate of O2 consumption, VO2max) can be elicited by challenging an animal’s aerobic capacity via forced exercise (Weibel et al. 2004) or by challenging their thermoregulatory capacity via acute cold exposure (Rosenmann and Morrison 1974a; Rosenmann et al. 1975; Hinds et al. 1993). In response to cold exposure, most eutherian mammals maintain a constant body temperature by increasing metabolic heat production. If the cold stress is sufficiently severe, it will elicit the maximal rate of heat production, which, since it is fueled by aerobic metabolism, is reflected by the maximal rate of O2 consumption (also referred to as “summit metabolism”). Measures of VO2max elicited by aerobic exercise or cold exposure are often quite similar, particularly in small animals, although observed discrepancies may reflect training or acclimation effects, or differences in the physiological or anatomical support systems involved in locomotor exercise and thermostatic heat production (Chappell 1984; Conley et al. 1985; Hayes 1989a, 1989b; Hinds and Rice-Warner 1992; Hinds et al. 1993; Hammond et al. 2002; Chappell and Hammond 2004; Chappell et al. 2007; McClelland et al. 2017). Whereas exercise-induced VO2max is directly related to the oxidative capacity of skeletal muscle (Mitchell and Blomqvist 1971; Armstrong et al. 1992; Weibel and Hoppler 2005), cold-induced VO2max is related to capacities of the tissues involved in both shivering thermogenesis (the sustained, nonlocomotory contraction of skeletal muscle that produces metabolic heat as a by-product) and nonshivering thermogenesis (which, in eutherian mammals, involves metabolic heat production in skeletal muscle and brown adipose tissue [BAT]—Cannon and Nedergaard 2004).
Performance measures such as exercise- or cold-induced VO2max can be expected to provide strong, causal links between integrated physiological capacities and Darwinian fitness (Huey and Stevenson 1979; Bennett and Huey 1990; Bennett 1991). The adaptive significance of some physiological performance capacities is likely to be highly environment-specific. At high elevation, for example, the reduction in the ambient partial pressure of O2 (PO2) can impose severe constraints on aerobic metabolism, and can therefore impair capacities for sustained locomotion and thermogenesis (Segrem and Hart 1967; Rosenmann and Morrison 1974b, 1975; West 1984). In the case of small, winter-active rodents living in alpine or subalpine environments, a sustained capacity for aerobic thermogenesis may often be critical for survival during periods of extreme cold. When challenged with the combined stressors of cold and hypoxia, individuals that are capable of attaining a higher VO2max (i.e., those with a higher capacity for aerobic thermogenesis) can maintain a constant body temperature at lower ambient temperatures. Such individuals can therefore expand the active period of the torpor cycle, increasing opportunities for foraging, mating, etc., and should be less likely to die of hypothermia. Measurements of field metabolic rates of deer mice (Peromyscus maniculatus) living in the high alpine revealed that these animals are often operating close to their aerobic performance limits (Hayes 1989a, 1989b) and, even during summer months, thermoregulatory demands may exceed VO2max (Conley and Porter 1986; Hayes and O’Connor 1999). For rodents living at high elevation, the combination of increased thermoregulatory demand (due to low temperature) and reduced capacity for aerobic thermogenesis (due to low PO2) suggests that variation in cold-induced VO2max should have important fitness consequences.
Here, we review and synthesize information about how evolved changes in aerobic exercise capacity and thermogenic capacity in deer mice have contributed to their ability to survive and function in high-elevation environments. Deer mice are a model species for the study of environmental adaptation (MacMillen and Garland 1989; Storz and Nachman 2003; Storz and Hoekstra 2007; Bedford and Hoekstra 2015), and they are especially well-suited to the study of adaptive differentiation across elevational gradients. This is because deer mice have the broadest elevational distribution of any North American mammal, and can be surprisingly abundant in high alpine environments at elevations of ~4,300 m above sea level, the highest attainable elevation within the species’ geographic range. We begin by explaining the physiological challenges of life at high elevations, and how evolved changes in physiological performance capacities enable deer mice to meet these challenges. We also review and synthesize what is known about the physiological mechanisms underlying evolved changes in performance capacities in deer mice that are native to different elevations. We then address a number of questions about the evolution of such performance traits by natural selection: Is physiological performance capacity variable in natural populations? Are performance measures stable and consistent through time? Is performance capacity heritable? Is variation in performance capacity associated with variation in Darwinian fitness?
The Physiological Challenge of Life at High Elevations
High-elevation environments are characterized by low PO2, low temperature, high winds, high solar radiant energy, and low primary productivity, and therefore pose a number of physiological challenges to small endotherms like mice. To the extent that the elevational ranges of mammals are limited by physiological tolerances, O2 availability is likely to be an especially important factor. The decline in ambient PO2 with elevation results in a corresponding decline in the PO2 of inspired air in the lungs (alveolar PO2), so compensatory changes in the O2 transport pathway are required to minimize the corresponding reduction in O2 delivery to the cells of respiring tissues.
The O2 and CO2 transport cascades.
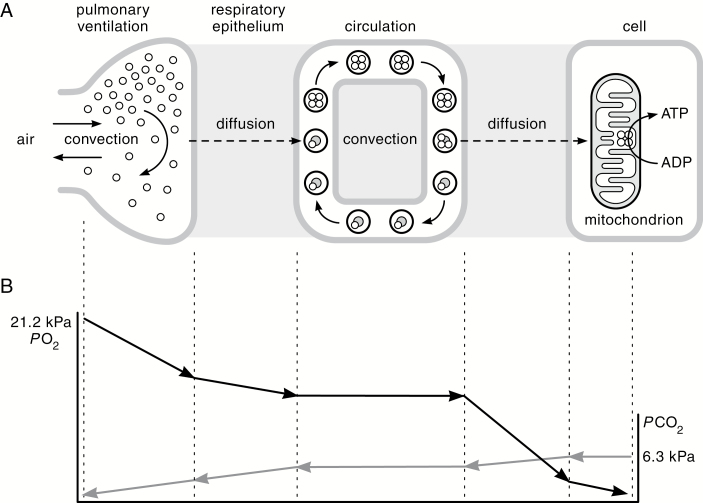
The cardiorespiratory system adjusts tissue O2 delivery in response to changes in O2 availability and metabolic demand via dynamic changes in several interacting transport steps. O2 from inspired air diffuses from terminal air spaces within the lungs (alveoli) into the arterial blood and is then transported convectively by the blood to the tissues. O2 then diffuses from the capillary blood into the cells of metabolizing tissues where it is used for respiration by mitochondria. The transport of O2 from inspired air to the tissue mitochondria involves four transfer steps that are arranged in series (Fig. 1A): 1) pulmonary ventilation, the convective movement of inspired tidal air into the residual lung volume; 2) diffusion of O2 across the alveolar membrane (the blood-gas interface); 3) convective movement of arterial blood to the capillaries of perfused tissues (with nearly all O2 chemically bound to hemoglobin [Hb]); and 4) diffusion of O2 from capillary blood to the mitochondria of tissue cells, where it is then used to generate ATP via oxidative phosphorylation. Differences in PO2 provide the driving force for O2 movement across each of the diffusive steps, and they account for much of the overall pressure gradient between the ambient PO2 of the outside air and the PO2 in the mitochondria (Fig. 1B). There is a reverse multistep pathway for the transport of CO2 from the cells, where it is produced as a by-product of oxidation, to the lungs, where it is expired (Fig. 1B). The pulmonary ventilation, alveolar diffusion, and tissue diffusion steps shown for O2 are essentially the reverse as those for CO2. However, the circulatory convection step is different because the majority of CO2 is transported in the blood as bicarbonate (HCO3−), formed from the reaction of CO2 and H2O in red blood cells, and a much smaller amount is transported as a carbamino compound via direct binding to Hb. In principle, acclimatization or adaptation to hypoxia could involve modifications of any number of the convective or diffusive steps in the O2 transport cascade (Storz et al. 2010b; Ivy and Scott 2015; McClelland and Scott, in press). It is instructive that genetically based changes in some of these same steps have contributed to an increased VO2max in rats that were selected for endurance running capacity (Henderson et al. 2002; Howlett et al. 2003, 2009; Gonzalez 2006a, 2006b; Kirkton et al. 2009).
Fig. 1.
The O2 and CO2 transport cascades. (A) Diagrammatic representation of compartments of respiratory gas transport in the mammalian cardiopulmonary system. O2 is transported from atmospheric air to the tissue mitochondria along a pathway with several diffusive and convective steps. The rate of O2 transport in the air compartment of the lungs is determined by the product of ventilation rate and the inspired-alveolar difference in PO2. The rate of O2 transport across the blood-gas interface is determined by the product of pulmonary O2 diffusion capacity and the alveolar-blood PO2 difference. The remaining steps include convective transport of O2 by hemoglobin (schematically depicted as a tetrameric [four-subunit] protein that reversibly binds up to four O2 molecules) and O2 diffusion across the blood-tissue interface to the mitochondria, culminating in the utilization of O2 to generate ATP via oxidative phosphorylation. (B) Diffusive O2 flux is driven by differences in PO2 between one compartment and the next. Blood PO2 declines from the afferent inlet of the tissue capillary bed to the efferent (venous) outlet as Hb unloads O2 to the perfused tissue. PO2 also declines with distance from the capillaries, so there should be a gradient of cellular PO2 reflecting variation in capillary PO2 and diffusion distance. There is an equivalent multistep pathway for the transport of CO2 in the reverse direction. Modified from Taylor and Weibel (1981) and Ivy and Scott (2015).
The Mechanistic Basis of Variation in Physiological Performance under Hypoxia
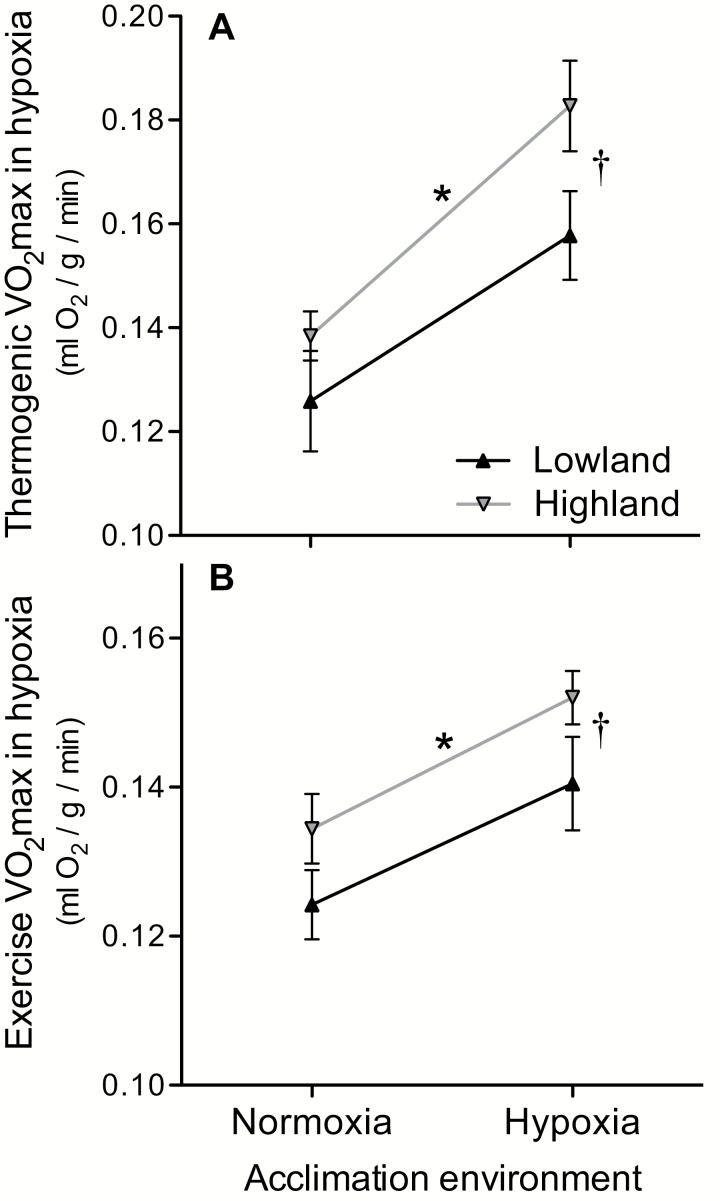
Comparative work on high-elevation deer mice from the Southern Rockies and low-elevation conspecifics from the Great Plains has revealed evolved differences in both aerobic exercise capacity and thermogenic capacity under hypoxia (Cheviron et al. 2012, 2013, 2014a; Lui et al. 2015; Lau et al. 2017; Tate et al. 2017; Fig. 2). Compared to lowland mice, highland natives have consistently higher aerobic performance capacities under hypoxia, consistent with the idea that it is beneficial to have a higher VO2max in an environment characterized by lower air temperatures and lower PO2. As explained below, available evidence indicates that the observed differences in hypoxic VO2max between deer mice from low and high elevations stem from changes in numerous subordinate traits that alter the flux capacity of the O2-transport system, the oxidative capacity of tissue mitochondria, and the relationship between O2 consumption and ATP synthesis. Many such changes in physiological phenotype are associated with hypoxia-induced changes in gene expression. By elucidating causal connections between changes in gene expression and changes in performance-related physiological traits, it is possible to identify and characterize components of the genomic transcriptional program that mediate the adaptive response to hypoxia (Storz and Cheviron 2016).
Fig. 2.
High-elevation deer mice (Peromyscus maniculatus) have higher aerobic capacities in hypoxia in comparison to lowland conspecifics. Performance capacities in hypoxia were measured as the maximal rate of O2 consumption (VO2max) during thermogenesis (A) or exercise (B). The absolute VO2max data (in units of ml/min) were corrected for body mass using a residual approach (see Lui et al. 2015 and Tate et al. 2017 for details) and are here divided by the body mass of an average-sized mouse (data represent means ± SE). Significant main effects of native elevation and hypoxia acclimation are denoted by * and †, respectively, in two-way ANOVA (P < 0.05).
Changes in the cardiorespiratory system.
Changes in the cardiorespiratory system of highland deer mice appear to involve a range of steps in the O2 transport cascade. Highland deer mice maintain higher O2 saturation in arterial blood than their lowland counterparts during hypoxia, both at rest and at VO2max (Ivy and Scott 2017; Tate et al. 2017). This could arise if highlanders have a greater capacity for O2 diffusion from alveolar air into the blood within the lungs. This has not yet been examined in highland deer mice, but studies in other highland mammals (including humans) and birds suggest that highlanders often have greater lung volumes or surface areas for gas exchange than lowlanders when compared in their native environment (Pearson and Pearson 1976; Scott et al. 2011; Brutsaert 2016; Maina et al. 2017; York et al. 2018).
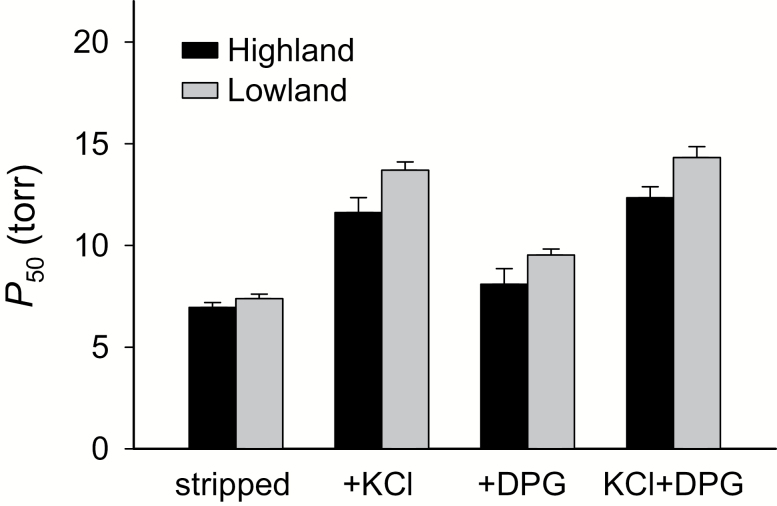
The higher arterial O2 saturation of highland mice also likely stems from an evolved increase in Hb-O2 affinity (Snyder et al. 1982; Snyder 1985; Storz 2007; Storz et al. 2009, 2010a; Natarajan et al. 2013, 2015a). In conjunction with potential variation in breathing or pulmonary O2 diffusion, an increased Hb-O2 affinity ensures that the arterial blood leaving the lungs is more highly saturated with O2 before it is delivered to the tissues. Deer mice native to the high alpine of the Rocky Mountains have evolved a significantly higher Hb-O2 affinity relative to lowland conspecifics from the prairie grasslands to the east (Storz et al. 2009, 2010a; Natarajan et al. 2015a; Jensen et al. 2016). This evolved change in Hb function involved an increase in intrinsic O2 affinity in combination with a suppressed sensitivity to the inhibitory effects of allosteric cofactors that are present in the red blood cell (Fig. 3). The allelic differences in Hb-O2 affinity are attributable to the additive and nonadditive effects of multiple amino acid mutations in the α- and β-chain subunits of the tetrameric (α2β2) Hb protein (Natarajan et al. 2013, 2015a).
Fig. 3.
Highland deer mice (Peromyscus maniculatus) from the Rocky Mountains have a higher Hb-O2 affinity than lowland mice from the Great Plains, as indicated by lower values of P50 (the PO2 at which Hb is 50% saturated). O2 equilibria were measured at pH 7.40, 37°C, in the presence and absence of allosteric cofactors that modulate Hb-O2 binding ([Cl−], 0.10 mol/l; [HEPES], 0.1 mol/l; DPG:tetrameric Hb ratio, 2.0: [heme], 0.2–0.3 mmol/l). P50 values (± SE) are reported for stripped Hbs in the absence of added anions, in the presence of Cl− alone (added as KCl), in the presence of DPG alone, and in the presence of both anions combined. This latter “KCl+DPG” treatment is most relevant to in vivo conditions in mammalian red blood cells, but measurements of O2 affinity under each of the four treatments provide insights into the functional mechanism responsible for observed differences in P50(KCl+DPG). Data from Natarajan et al. (2015a) (for additional details, see Storz et al. 2009, 2010a and Jensen et al. 2016).
Experimental studies of wild-derived strains of deer mice revealed that genetically based variation in Hb-O2 affinity is associated with whole-animal measures of both aerobic exercise capacity and thermogenic capacity under hypoxia (Chappell and Snyder 1984; Chappell et al. 1988). Complementing experimental studies of genetic variation in Hb function and its association with hypoxic VO2max, population genetic analyses of nucleotide variation at the underlying α- and β-globin genes have provided corroborative evidence for a history of spatially varying selection on Hb polymorphism between deer mouse populations that are native to different elevational zones (Storz and Kelly 2008; Storz et al. 2009, 2010a, 2012; Natarajan et al. 2015a). These results provide an additional line of evidence supporting the adaptive significance of the elevation-related variation in Hb function. Comparative studies suggest that evolved increases in Hb-O2 affinity have contributed to hypoxia adaptation in a diverse range of mammals and birds (Projecto-Garcia et al. 2013; Galen et al. 2015; Natarajan et al. 2015b, 2016, 2018; Tufts et al. 2015; Storz 2016, 2019; Jendroszek et al. 2018; Zhu et al. 2018), though there are exceptions (Revsbech et al. 2013; Cheviron et al. 2014b; Janecka et al. 2015).
The capacity for circulating blood to the tissues also appears to be enhanced in highland deer mice. Hypoxia acclimation increases the maximal heart rates achieved at VO2max in both highland and lowland deer mice, but highlanders appear to have evolved higher maximum stroke volumes, as indicated by a higher quotient of VO2max and heart rate, which reflects the O2 extracted from the blood per heartbeat (Tate et al. 2017). Highlanders also maintain higher heart rates than lowlanders during exposure to deep hypoxia at rest (Ivy and Scott 2017). These observations suggest that highlanders can sustain high cardiac outputs (the total rate of blood flow that is pumped by the heart) to help support intense aerobic activity or hypoxia resistance. Hematocrit (the ratio of the volume of red blood cells to the total volume of blood) also increases in response to chronic hypoxia (Tufts et al. 2013; Lui et al. 2015), which has long been considered a beneficial acclimatization response because it helps augment the O2 carrying capacity of blood (Siebenmann et al. 2017). However, many now consider an increased hematocrit to be nonadaptive at high elevation because it increases blood viscosity, which can decrease cardiac output and, hence, tissue O2 delivery (Winslow et al. 1985; Storz 2010; Storz et al. 2010b; Simonson et al. 2015; Moore 2017). The rise in hematocrit appears to be blunted in high-elevation deer mice, such that they have lower hematocrit than lowlanders after hypoxia acclimation (Lui et al. 2015). In principle, this reduction in hematocrit may be attributable to a reduction in erythropoiesis (red blood cell production) or an expansion of blood plasma volume. The observed patterns in deer mice are consistent with observations in other high-elevation species. Tibetan humans, for example, achieve higher maximal cardiac outputs and stroke volumes during exercise in hypoxia than Han Chinese lowlanders (Ge et al. 1995; Chen et al. 1997), and they have much lower blood Hb concentrations at high elevations (Beall and Reichsman 1984; Simonson 2015; Moore 2017). In deer mice and in numerous other highland taxa, the circulatory system is altered in several ways to optimize tissue O2 delivery in hypoxia.
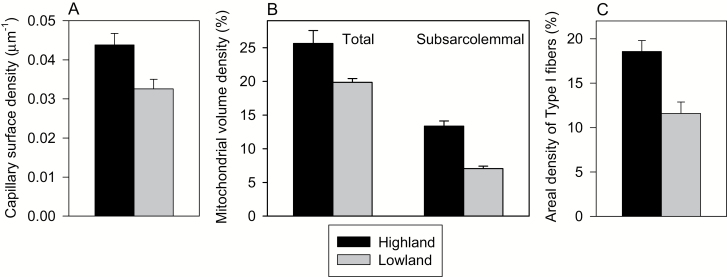
The ability to extract O2 from the blood appears to be augmented in highland deer mice due to evolved increases in the morphological capacity for O2 diffusion in skeletal muscles. Highlanders have greater capillary surface areas in both locomotory (gastrocnemius) and respiratory (diaphragm) muscles, as well as a higher volume density of mitochondria nearest to the capillaries (subsarcolemmal mitochondria) that reduces intracellular O2 diffusion distances (Figs. 4A and 4B; Lui et al. 2015; Scott et al. 2015; Lau et al. 2017; Mahalingam et al. 2017; Dawson et al. 2018). These evolved traits do not appear to exhibit significant plasticity, as they are unaffected by chronic hypoxia exposure during parental development (Nikel et al. 2017) and during adulthood (Lui et al. 2015; Dawson et al. 2018). These findings are consistent with studies in other mammals and birds, in which highlanders have been found to exhibit greater capillarity in skeletal muscle than lowlanders when compared in their native environment (Kayser et al. 1991; León-Velarde et al. 1993; Mathieu-Costello et al. 1998; Scott et al. 2015). The mechanisms underlying increases in muscle capillarity and O2 extraction in highland deer mice are not entirely clear, but we have shown that they are associated with differential expression of genes involved in energy metabolism, muscle development, and vascular development (Cheviron et al. 2014a; Scott et al. 2015), as described in more detail below.
Fig. 4.
Differences between highland and lowland deer mice (Peromyscus maniculatus) in the gastrocnemius muscle with respect to: (A) capillary surface density (µm−1), (B) total and subsarcolemmal mitochondrial volume densities (%), and (C) areal density of slow (Type I) oxidative fibers (%). Data are presented as trait means (± SE) measured in first-generation mice that were born and raised at sea level in laboratory conditions and acclimated for at least 6 weeks to hypobaric hypoxia equivalent to 4,300 m elevation (Lui et al. 2015; Mahalingam et al. 2017).
Changes in metabolism.
At the cold temperatures that are characteristic of alpine and subalpine environments, sustained heat production requires the oxidation of lipids (mainly in the form of free fatty acids—Weber 2011; McClelland et al. 2017). The elevated cold-induced VO2max in highland deer mice is accompanied by elevated rates of fatty acid oxidation (Cheviron et al. 2012), and these rates are much higher than the maximal rates that can be achieved during exercise (Schippers et al. 2014; Lau et al. 2017). In contrast, highland native mice show a greater reliance on carbohydrate oxidation than lowland mice at equivalent intensities of submaximal exercise. Under hypoxia, carbohydrate oxidation is more efficient than lipid oxidation in terms of O2 economy, as it results in a greater yield of ATP per mole O2 consumed (Brand 2005). Since skeletal muscle is a primary consumer of O2 and metabolic substrates during exercise (Armstrong et al. 1992; Weibel and Hoppler 2005) and shivering thermogenesis (Nespolo et al. 1999; Van Sant and Hammond 2008), variation in muscle metabolism might provide insights into the physiological underpinnings of changes in whole-body fuel use.
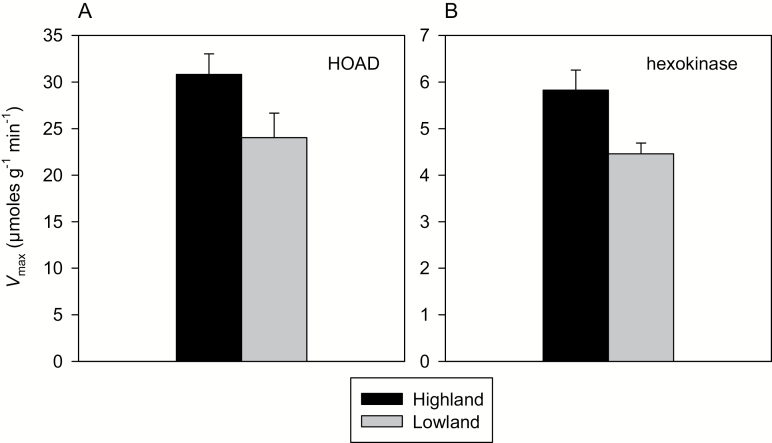
Many studies treat the gastrocnemius as a representative skeletal muscle that is used in both locomotion (Pearson et al. 2005) and shivering thermogenesis (Oufara et al. 1987). This muscle shows a greater capacity for fatty acid oxidation in highland deer mice, likely due to higher areal densities of oxidative Type I muscle fibers and greater mitochondrial volume densities (Fig. 4C), and is associated with enhanced thermogenic capacities (Cheviron et al. 2012; Lau et al. 2017). Interestingly, maximal activity of the fatty acid β-oxidation enzyme β-hydroxyacyl-CoA dehydrogenase (HOAD) is constitutively higher in muscle of highlanders (Fig. 5A), but remains unchanged in response to hypoxia and cold acclimation (Lau et al. 2017). In comparisons between highland and lowland mice, there is a good correspondence between the capacity for β-oxidation (HOAD activity) and whole-animal fatty acid oxidation, but such differences do not account for enhancements of performance due to acclimation. This suggests that other muscle traits involved in cellular uptake, cytosolic transport, and mitochondrial transport of fatty acids might be more labile in response to environmental stress.
Fig. 5.
Population differences in enzyme activities in the gastrocnemius muscle of highland and lowland deer mice (Peromyscus maniculatus). Apparent maximal activities (Vmax in µmoles of substrate per g tissue per minute) of (A) hexokinase (HK) and (B) β-hydroxyacyl-CoA dehydrogenase (HOAD). Data are presented as mean activities (± SE) measured in first-generation mice that were born and raised at sea level in laboratory conditions and acclimated for at least 6 weeks to hypobaric hypoxia equivalent to 4,300 m elevation (Lau et al. 2017).
Surprisingly, highland and lowland deer mice show little correspondence between exercise fuel use and the capacity for glycolytic flux in skeletal muscle. Highland deer mice and highland species of leaf-eared mice (genus Phyllotis) from the Andes do not show higher activities of several glycolytic enzymes despite exhibiting higher exercise carbohydrate use (Schippers et al. 2012; Lau et al. 2017). The exception is the enzyme hexokinase (HK), which showed increased maximal activity in highland deer mice after hypoxia acclimation (Fig. 5B), in conjunction with an increased reliance on carbohydrate use during exercise (Lau et al. 2017). In muscle, HK is responsible for the phosphorylation of blood-borne glucose and it is an important determinant of cellular uptake capacity that is linked to greater running endurance in laboratory mice (Fueger et al. 2005; Wasserman et al. 2010). Highland deer mice also tend to show an increased capacity for glucose production via gluconeogenesis in the liver compared to lowlanders (Lau et al. 2017), suggesting that an enhanced circulatory glucose supply is accompanied by an increased capacity for muscle uptake. These traits in highland deer mice are likely accompanied by other changes in the glucose oxidation pathway. For instance, the mitochondrial enzyme pyruvate dehydrogenase (PDH) directs pyruvate toward oxidative phosphorylation and is tightly regulated according to cellular energy supply and demand (LeMoine et al. 2011). However, whether PDH is differentially regulated during exercise in deer mice at high elevations is currently unknown.
With regard to population differences in vascular substrate supply, the higher surface densities of muscle capillaries of highland mice would increase surface area and conductance of metabolic substrates (Vock et al. 1996) in addition to increasing O2 diffusion capacities (Lui et al. 2015; Scott et al. 2015). The higher volume density of subsarcolemmal mitochondria also reduces the diffusion distances of substrates to the oxidative machinery of the cell (Mahalingam et al. 2017). These traits would increase the capacity for flux of both glucose and fatty acids. However, it is still unclear how the pathways for these two main substrates are differentially regulated in muscle fibers to ensure high rates of fatty acid oxidation during shivering and enhanced glucose oxidation during submaximal locomotion.
Changes in gene expression that underlie observed changes in physiological traits.
Many of the physiological differences between highland and lowland deer mice are associated with differences in the expression of genes that participate in functionally relevant pathways. As mentioned above, the elevated thermogenic capacity of highland deer mice is associated with an increased capacity to oxidize lipids as a primary fuel source during aerobic thermogenesis (Cheviron et al. 2012). These whole-animal differences in lipid catabolic capacities are in turn associated with differences in the activities of skeletal muscle enzymes that influence flux through fatty acid oxidation and oxidative phosphorylation pathways, and with concerted changes in the expression of genes in these same pathways. Highland deer mice exhibit a concerted upregulation of genes across lipid oxidation and oxidative phosphorylation pathways in comparison with their lowland counterparts (Cheviron et al. 2012, 2014a). Common-garden experiments have revealed evidence that many of the observed expression differences between populations are genetically based. Highland and lowland mice exhibit differences in the constitutive expression of dozens of genes that are strongly associated with differences in thermogenic capacity in hypoxia (Cheviron et al. 2014a; Lui et al. 2015; Scott et al. 2015; Lau et al. 2017). However, aerobic performance improves with hypoxia acclimation in both highland and lowland mice (Cheviron et al. 2013; Lui et al. 2015; Velotta et al. 2016; Lau et al. 2017; Tate et al. 2017). This improvement in performance is associated with environmentally induced changes in the expression of hundreds of genes, and these transcriptional responses are common to both highland and lowland natives (Cheviron et al. 2014a; Lui et al. 2015; Velotta et al. 2016).
The expression of a total of 1,127 genes in skeletal muscle was associated with variation in thermogenic performance in an experiment comparing wild-caught mice with the captive-bred progeny of wild-caught mice that were born and reared in a common-garden lab environment at low elevation (Cheviron et al. 2014a). Of these 1,127 genes, only 69 (6.1%) were differentially expressed between highland and lowland mice in both conditions. Within this subset, a number of genes participate in lipid metabolic processes, which were consistently upregulated in highlanders, mirroring whole-animal differences in lipid catabolic capacities and HOAD activity (Cheviron et al. 2012, 2014a; Lau et al., 2017). The remaining genes (93.9%) were differentially expressed between rearing conditions regardless of population of origin. Similarly, elevational differences in the capacity for nonshivering thermogenesis were largely environmentally induced, as were the associated changes in tissue-specific gene expression (Velotta et al. 2016). Together, these results suggest that while some of the observed population differences in thermogenic capacity are attributable to genetic differences in gene expression, transcriptomic changes also play an important role in the acclimatization responses that are common to both highland and lowland mice.
Different measures of thermogenic performance are associated with expression changes in overlapping sets of genes. Cheviron et al. (2014a) identified a total of five groups of co-expressed genes (co-expression modules) in skeletal muscle that were associated with different measures of thermogenic performance. Variation in total thermogenic capacity was associated with differential regulation of three of these modules, and thermogenic endurance (the amount of time that individuals can maintain 90% of VO2max—Cheviron et al. 2013, 2014a) was associated with four. Two modules were associated with both performance measures, and all of the performance-associated modules were enriched for genes that participate in similar biochemical/physiological processes, including tissue vascularization, skeletal muscle growth, metabolic fuel use, and mitochondrial oxidative capacity. These results suggest that the transcriptional underpinnings of thermogenic capacity and endurance are similar, a surprising result given that the two alternative measures of thermogenic performance exhibit different levels of plasticity (Cheviron et al. 2013).
Although highland and lowland deer mice exhibit persistent differences in thermogenic capacity, differences in thermogenic endurance are only evident in wild-caught mice (Cheviron et al. 2013). Moreover, thermogenic capacity and endurance are not correlated in the adult progeny of either highland or lowland mice that are born and reared at low elevation (Cheviron et al. 2013), suggesting that there may be a functional uncoupling of these two measures of performance. Similarity in the transcriptomic underpinnings of thermogenic capacity and endurance suggests that this functional uncoupling does not stem from gene expression differences in skeletal muscle. Instead, it may stem from regulatory variation that underlies plasticity in the nonshivering components of thermogenesis, or plasticity in upstream steps of the transport pathways for O2 or metabolic substrates.
In addition to the associations between transcriptomic variation and whole-organism performance, elevational differences in a number of subordinate traits have also been shown to be associated with a combination of plastic and genetically based differences in gene expression. For example, environmentally induced increases in the capacity for nonshivering thermogenesis under hypoxia is associated with upregulation of UCP1 in BAT as well as several other genes that positively regulate its expression (e.g., C/EBPb, CREB5, CREB3l1, CREB3l4—Velotta et al. 2016). Hypoxia-induced hypertrophy of the right ventricle is associated with the differential expression of genes that participate in interferon signaling pathways (Velotta et al. 2018), and different aspects of gastrocnemius muscle phenotype are also associated with gene expression differences (Lui et al. 2015; Scott et al. 2015). For example, the increased capillary surface area of highland deer mice is associated with constitutive differences in the expression of over 50 genes (Scott et al. 2015), including two (Cadherin-7 and Notch4) that play well-documented roles in angiogenesis (Hayward et al. 2011; Lv et al. 2013). Muscle fiber composition of the gastrocnemius, however, is associated with both constitutive expression differences and environmentally induced changes that are common to both populations. The numerical density of oxidative fibers, for example, is associated with two co-expression modules, one of which is comprised of 50 genes that exhibit constitutive expression differences, and the other is comprised of 274 environmentally sensitive genes that differ in expression among rearing conditions regardless of population of origin (Scott et al. 2015). Included within this group of genes are Ppargc1α and Tfam, two regulators of mitochondrial biogenesis whose expression levels are positively correlated with the density of oxidative fibers. However, neither of the two genes exhibit differences in constitutive expression between highland and lowland mice (Lui et al. 2015; Scott et al. 2015). In sum, these results suggest that aspects of both genotypic specialization and phenotypic plasticity in aerobic performance are associated with transcriptional variation at multiple levels biological organization.
Evolution of Physiological Performance Capacities
For a given trait to evolve by natural selection, it has to exhibit variation among individuals, the variation must be associated with fitness, and it must have a heritable basis. As described below, lab- and field-based studies of deer mice and other rodents have documented that capacities for aerobic exercise and thermogenesis meet each of these conditions.
Variability and heritability of physiological performance capacities in natural populations.
The skeletal and cranial traits that are routinely measured by mammalogists have high heritabilities (e.g., Lofsvold 1986, 1988), which is one reason why such characters have proven utility in systematics and taxonomy. By contrast, measures of whole-animal physiological performance tend to be far more labile, reflecting changes in transient physiological states and environmental conditions, and can be quite variable through the course of an individual’s lifetime. Thus, to assess the potential for performance traits to evolve by natural selection, it is necessary to confirm that measured values for individuals are stable and consistent through time and that some fraction of the observed interindividual variation has an additive genetic basis.
With regard to individual consistency through time, VO2max is highly repeatable with lab-reared deer mice (Hayes and Chappell 1986, 1990) and with wild-caught mice taken directly from the field (Hayes and O’Connor 1999). With regard to heritability, quantitative genetic experiments and artificial selection experiments involving house mice (Mus domesticus), rats (Rattus norvegicus), and bank voles (Myodes glareolus) have documented ample levels of additive genetic variance for exercise-induced VO2max and other measures of aerobic metabolism (Dohm et al. 1996, 2001; Swallow et al. 1998, 2009; Koch and Britton 2001; Sadowska et al. 2005, 2008; Gonzalez et al. 2006a; Meek et al. 2009; Wone et al. 2009, 2015; Stawski et al. 2015). Deer mice are unlikely to be very different in this regard. Moreover, as explained above, many of the subordinate traits that contribute to variation in VO2max have a well-documented genetic basis (e.g., Hb-O2 affinity), and must therefore contribute to the heritability of whole-animal performance capacities. Finally, in the case of between-population differences in performance capacities between deer mice from high and low elevations, common-garden experiments have demonstrated that a large fraction of observed population differences in mean VO2max persist after wild-caught animals are acclimated to the lab environment (which controls for effects of physiological plasticity during adulthood) and the differences also persist in the progeny of lab-born and lab-reared mice (which controls for effects of developmental plasticity—Cheviron et al. 2012, 2013, 2014a; Lui et al. 2015; Lau et al. 2017).
Physiology, performance, and fitness.
If a performance trait like VO2max is variable among individuals and if some fraction of that variation is heritable, then it is possible to measure the sign and magnitude of phenotypic selection in contemporary time (Arnold 1983; Bennett and Huey 1990; Husak 2015). This can be accomplished by measuring the statistical relationship between a given trait and estimates of organismal fitness or individual components of fitness (e.g., survival, mating success, or fecundity). The key parameter is the selection differential, defined as the difference in mean trait values between individuals who successfully reproduce in a given generation (actual parents) and the larger, more inclusive set of all potential parents. At the phenotypic level, selection describes the statistical change in trait means within the parental generation. Inheritance of the trait in question then determines the genetic response to selection, i.e., the change in the trait mean that is manifest in the next generation.
A multivariate approach can also be used to quantify the direct and indirect effects of selection that act simultaneously on multiple traits (Lande 1979; Lande and Arnold 1983). With estimates of additive genetic variances and covariances of the measured traits (estimated from genetic crosses) and observational data on selection (shifts in trait means within the parental generation), multivariate selection theory can be used to predict changes in trait means from one generation to the next. To predict the evolutionary response to selection on multiple traits, the single-trait selection differential is replaced by the “directional selection gradient,” which measures the direct effect of selection on a given trait while statistically controlling for correlated effects on other measured traits. The selection gradient can be measured as the partial regression of relative fitness on a given trait, holding all other traits constant. The value of the multivariate framework is that it can reveal how selection on one trait can produce correlated responses in unselected traits due to genetic correlations. Such correlated responses can provide insights into the causes of trade-offs and constraints that have always been of interest to physiologists (Arnold 1987; Garland and Carter 1994; Careau and Garland 2012; Storz et al. 2015).
Hayes and O’Connor (1999) performed an analysis of phenotypic selection on naturally occurring variation in thermogenic capacity in high-elevation deer mice in the White Mountains of eastern California. Deer mice are well-suited to studies of this kind because they often occur at relatively high population density, which facilitates repeated longitudinal sampling of individuals at specific field sites. These researchers measured cold-induced VO2max in deer mice sampled from an alpine fellfield habitat at ~3,800 m elevation. Estimates of survivorship based on a mark-release-recapture protocol revealed strong directional selection for increased thermogenic capacity in one out of three seasons. Hayes and O’Connor (1999) calculated the ambient air temperature at which an 18-g mouse in winter pelage would become hypothermic, and, based on their survivorship data, they estimated that the average survivor would have been able to avoid hypothermia at air temperatures a degree or two colder than the average nonsurvivor. They concluded that air temperatures during the course of their study would have been more than low enough to restrict aboveground activity, and that mice that went aboveground during the coldest times would have been at risk of death due to hypothermia.
A key question in such studies concerns the ecological relevance of the measured performance phenotype (Pough 1989; Irschick 2003). As pointed out by Bennett and Huey (1990:255), measurements of running speed and other aspects of locomotor performance “…define not only the limits of locomotor capacity per se, but also of behavioral ‘work’ of any type within a given metabolic mode. For example, maximal exertion may provide information on the ability to repel an intruder or perhaps to dig a burrow, not just on the ability to outrun a predator.” As an example of this reasoning, Hayes and O’Connor (1999:1285) suggested that the adaptive significance of thermogenic capacity in high-elevation deer mice may relate to the physiology of daily torpor, not just the ability to stave off hypothermia: “…if high VO2max was correlated with the ability to rewarm from torpor, mice that rewarmed more rapidly might be better able to exploit brief temporal windows when thermal conditions were warm enough for them to forage aboveground.” A high VO2max may also be associated with an increased capacity to recover from exercise-induced O2 debts.
Phenotypic evolution, organismal biology, and natural history.
Arnold (1987) discussed a number of reasons why the measurement of physiological performance capacities should be especially valuable in real-time studies of phenotypic selection and evolution. Bennett and Huey (1990:252) argued that physiologists can bring to such studies “…a thorough understanding of organismal function, an appreciation for the organism as an integrated unit, and the ability to analyze complex interactive effects of environmental factors on organismal capacities and performance. Most importantly, they can structure and execute experiments to test specific hypotheses about organismal function.” Research on natural variation in performance capacities forms a nexus between ecology, evolution, and diverse fields of functional biology (Bartholomew 1958). Such research plays to the strengths of mammalogists and other organismal biologists because it places a high premium on understanding the natural history of the study animal.
Acknowledgments
We thank N. Gutierréz-Pinto and two anonymous reviewers for helpful comments on the manuscript. Our research on the physiology of adaptation to high elevations in deer mice has been funded by the National Science Foundation (DEB-0614342 [JFS], IOS-1354390 [JFS], IOS-1354934 [ZAC], IOS-1634219 [ZAC], RII Track-2 FEC 1736249 [ZAC and JFS]), the National Institutes of Health/National Heart, Lung, and Blood Institute (HL087216 [JFS]), the Natural Sciences and Engineering Research Council of Canada (NSERC) (GRS and GBM), and the Canada Research Chairs Program (GRS).
Literature Cited
- Armstrong R B, et al. . 1992. O2 delivery at VO2max and oxidative capacity in muscles of standardbred horses. Journal of Applied Physiology 73:2274–2282. [DOI] [PubMed] [Google Scholar]
- Arnold S J. 1983. Morphology, performance, and fitness. American Zoologist 23:347–361. [Google Scholar]
- Arnold S J. 1987. Genetic correlation and the evolution of physiology. Pp. 189–215 in New directions in ecological physiology (Feder M. E., Bennett A. F., Burggren W. W., and Huey R B, eds.). Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Bartholomew G A. 1958. The role of physiology in the distribution of terrestrial vertebrates. Pp. 81–95 in Zoogeography (Hubbs C L, ed.). American Association for the Advancement of Science, Washington, D.C. [Google Scholar]
- Beall C M, and Reichsman A. B.. 1984. Hemoglobin levels in a Himalayan high altitude population. American Journal of Physical Anthropology 63:301–306. [DOI] [PubMed] [Google Scholar]
- Bedford N L, and Hoekstra H. E.. 2015. Peromyscus mice as a model for studying natural variation. eLife 4. doi: 10.7554/eLife.06813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A F. 1991. The evolution of activity capacity. Journal of Experimental Biology 160:1–23. [DOI] [PubMed] [Google Scholar]
- Bennett A F, and Huey R. B.. 1990. Studying the evolution of physiological performance. Oxford Surveys in Evolutionary Biology 7:251–284. [Google Scholar]
- Brand M D. 2005. The efficiency and plasticity of mitochondrial energy transduction. Biochemical Society Transactions 33:897–904. [DOI] [PubMed] [Google Scholar]
- Brutsaert T. 2016. Why are high altitude natives so strong at high altitude? Nature vs. nurture: genetic factors vs. growth and development. Advances in Experimental Medicine and Biology 903:101–112. [DOI] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J. A. N.. 2004. Brown adipose tissue: function and physiological significance. Physiological Reviews 84:277–359. [DOI] [PubMed] [Google Scholar]
- Careau V, and Garland T. Jr.. 2012. Performance, personality, and energetics: correlation, causation, and mechanism. Physiological and Biochemical Zoology 85:543–571. [DOI] [PubMed] [Google Scholar]
- Chappell M A. 1984. Maximum oxygen consumption during exercise and cold-exposure in deer mice, Peromyscus maniculatus. Respiration Physiology 55:367–377. [DOI] [PubMed] [Google Scholar]
- Chappell M A, and Hammond K. A.. 2004. Maximal aerobic performance of deer mice in combined cold and exercise challenges. Journal of Comparative Physiology, B. Biochemical, Systemic, and Environmental Physiology 174:41–48. [DOI] [PubMed] [Google Scholar]
- Chappell M A, Hammond K. A., Cardullo R. A., Russell G. A., Rezende E. L., and Miller C.. 2007. Deer mouse aerobic performance across altitudes: effects of developmental history and temperature acclimation. Physiological and Biochemical Zoology 80:652–662. [DOI] [PubMed] [Google Scholar]
- Chappell M A, Hayes J. P., and Snyder L. R. G.. 1988. Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): physiology of β-globin variants and α-globin recombinants. Evolution 42:681–688. [DOI] [PubMed] [Google Scholar]
- Chappell M A, and Snyder L. R. G.. 1984. Biochemical and physiological correlates of deer mouse α-chain hemoglobin polymorphisms. Proceedings of the National Academy of Sciences of the United States of America 81:5484–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q H, et al. . 1997. Exercise performance of Tibetan and Han adolescents at altitudes of 3,417 and 4,300 m. Journal of Applied Physiology 83:661–667. [DOI] [PubMed] [Google Scholar]
- Cheviron Z A, Bachman G. C., Connaty A. D., McClelland G. B., and Storz J. F.. 2012. Regulatory changes contribute to the adaptive enhancement of thermogenic capacity in high-altitude deer mice. Proceedings of the National Academy of Sciences of the United States of America 109:8635–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron Z A, Bachman G. C., and Storz J. F.. 2013. Contributions of phenotypic plasticity to differences in thermogenic performance between highland and lowland deer mice. Journal of Experimental Biology 216:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron Z A, Connaty A. D., McClelland G. B., and Storz J. F.. 2014a. Functional genomics of adaptation to hypoxic cold-stress in high-altitude deer mice: transcriptomic plasticity and thermogenic performance. Evolution 68:48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron Z A, et al. . 2014b. Integrating evolutionary and functional tests of adaptive hypotheses: a case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Molecular Biology and Evolution 31:2948–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley K E, and Porter W. P.. 1986. Heat-loss from deer mice (Peromyscus) - evaluation of seasonal limits to thermoregulation. Journal of Experimental Biology 126:249–269. [DOI] [PubMed] [Google Scholar]
- Conley K E, Weibel E. R., Taylor C. R., and Hoppeler H.. 1985. Aerobic capacity estimated by exercise vs. cold-exposure - endurance training effects in rats. Respiration Physiology 62:273–280. [DOI] [PubMed] [Google Scholar]
- Dawson N J, Lyons S. A., Henry D. A., and Scott G. R.. 2018. Effects of chronic hypoxia on diaphragm function in deer mice native to high altitude. Acta Physiologica 223:e13030. [DOI] [PubMed] [Google Scholar]
- Dohm M R, Hayes J. P., and Garland T. Jr.. 1996. Quantitative genetics of sprint running speed and swimming endurance in laboratory house mice (Mus domesticus). Evolution 50:1688–1701. [DOI] [PubMed] [Google Scholar]
- Dohm M R, Hayes J. P., and Garland T. Jr.. 2001. The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics 159:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djawdan M. 1993. Locomotor performance of bipedal and quadrapedal heteromyid rodents. Functional Ecology 7:195–202. [Google Scholar]
- Djawdan M, and Garland T. Jr.. 1988. Maximal running speeds of bipedal and quadrapedal rodents. Journal of Mammalogy 69:765–772. [Google Scholar]
- Fueger P T, et al. . 2005. Hexokinase II protein content is a determinant of exercise endurance capacity in the mouse. The Journal of Physiology 566:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen S C, et al. . 2015. Contribution of a mutational hotspot to adaptive changes in hemoglobin function in high-altitude Andean house wrens. Proceedings of the National Academy of Sciences of the United States of America 112:13958–13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T. Jr., and Carter P. A.. 1994. Evolutionary physiology. Annual Review of Physiology 56:579–621. [DOI] [PubMed] [Google Scholar]
- Ge R.-L, et al. . 1995. Comparisons of oxygen transport between Tibetan and Han residents at moderate altitude. Wilderness and Environmental Medicine 6:391–400. [Google Scholar]
- Gonzalez N C, et al. . 2006a. Systemic oxygen transport in rats artificially selected for running endurance. Respiratory Physiology and Neurobiology 151:141–150. [DOI] [PubMed] [Google Scholar]
- Gonzalez N C, et al. . 2006b. Continued divergence in VO2max of rats artificially selected for running endurance is mediated by greater convective blood O2 delivery. Journal of Applied Physiology 101:1288–1296. [DOI] [PubMed] [Google Scholar]
- Hammond K A, Chappell M. A., and Kristan D. M.. 2002. Developmental plasticity in aerobic performance in deer mice (Peromyscus maniculatus). Comparative Biochemistry and Physiology, A. Molecular and Integrative Physiology 133:213–224. [DOI] [PubMed] [Google Scholar]
- Hayes J P. 1989a. Altitudinal and seasonal effects on aerobic metabolism of deer mice. Journal of Comparative Physiology, B. Biochemical Systemic and Environmental Physiology 159:453–459. [DOI] [PubMed] [Google Scholar]
- Hayes J P. 1989b. Field and maximal metabolic rates of deer mice (Peromyscus maniculatus) at low and high altitudes. Physiological Zoology 62:732–744. [Google Scholar]
- Hayes J P, and Chappell M. A.. 1986. Effects of cold-acclimation on maximum oxygen consumption during cold-exposure and treadmill exercise in deer mice, Peromyscus maniculatus. Physiological Zoology 59:473–481. [Google Scholar]
- Hayes J P, and Chappell M. A.. 1990. Individual consistency of maximal oxygen consumption in deer mice. Functional Ecology 4:495–503. [Google Scholar]
- Hayes J P, and O’Connor C S. 1999. Natural selection on thermogenic capacity of high-altitude deer mice. Evolution 53:1280–1287. [DOI] [PubMed] [Google Scholar]
- Hayward N M, et al. . 2011. Chronic hyperperfusion and angiogenesis follow subacute hypoperfusion in the thalamus of rats with focal cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism 31:1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson K K, et al. . 2002. Determinants of maximal O2 uptake in rats selectively bred for endurance running capacity. Journal of Applied Physiology 93:1265–1274. [DOI] [PubMed] [Google Scholar]
- Hinds D S, Baudinette R. V., MacMillen R. E., and Halpern E. A.. 1993. Maximum metabolism and the aerobic factorial scope of endotherms. Journal of Experimental Biology 182:41–56. [DOI] [PubMed] [Google Scholar]
- Hinds D S, and Rice-Warner C. N.. 1992. Maximum metabolism and aerobic capacity in heteromyid and other rodents. Physiological Zoology 65:188–214. [Google Scholar]
- Howlett R A, et al. . 2003. Selected contribution: skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. Journal of Applied Physiology 94:1682–1688. [DOI] [PubMed] [Google Scholar]
- Howlett R A, et al. . 2009. Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. Journal of Applied Physiology 106:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey R B. 1983. Natural variation in body temperature and physiological performance in a lizard (Anolis cristatellus). Pp. 484–490 in Advances in herpetology and evolutionary biology: essays in honor of Ernest E. Williams (Rhodin A. G. J. and Miyata K, eds.). Museum of Comparative Zoology, Cambridge, Massachusetts. [Google Scholar]
- Huey R B, and Stevenson R. D.. 1979. Integrating thermal physiology and ecology of ectotherms - discussion of approaches. American Zoologist 19:357–366. [Google Scholar]
- Husak J F. 2015. Measuring selection on physiology in the wild and manipulating phenotypes (in terrestrial nonhuman vertebrates). Comprehensive Physiology 6:63–85. [DOI] [PubMed] [Google Scholar]
- Irschick D J. 2003. Measuring performance in nature: implications for studies of fitness within populations. Integrative and Comparative Biology 43:396–407. [DOI] [PubMed] [Google Scholar]
- Irschick D J, and Garland T. Jr.. 2001. Integrating function and ecology in studies of adaptation: investigations of locomotor capacity as a model system. Annual Review of Ecology and Systematics 32:367–396. [Google Scholar]
- Irschick D J, Meyers J. J., Husak J. F., and Le Galliard J. F.. 2008. How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evolutionary Ecology Research 10:177–196. [Google Scholar]
- Ivy C M, and Scott G. R.. 2015. Control of breathing and the circulation in high-altitude mammals and birds. Comparative Biochemistry and Physiology, A. Molecular and Integrative Physiology 186:66–74. [DOI] [PubMed] [Google Scholar]
- Ivy C M, and Scott G. R.. 2017. Control of breathing and ventilatory acclimatization to hypoxia in deer mice native to high altitudes. Acta Physiologica 221:266–282. [DOI] [PubMed] [Google Scholar]
- Janecka J E, et al. . 2015. Genetically based low oxygen affinities of felid hemoglobins: lack of biochemical adaptation to high-altitude hypoxia in the snow leopard. Journal of Experimental Biology 218:2402–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendroszek A, et al. . 2018. Allosteric mechanisms underlying the adaptive increase in hemoglobin-oxygen affinity of the bar-headed goose. Journal of Experimental Biology 221:jeb.185470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Storz J. F., and Fago A.. 2016. Bohr effect and temperature sensitivity of hemoglobins form highland and lowland deer mice. Comparative Biochemistry and Physiology, A. Molecular and Integrative Physiology 195:10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser B, Hoppeler H., Claassen H., and Cerretelli P.. 1991. Muscle structure and performance capacity of Himalayan Sherpas. Journal of Applied Physiology 70:1938–1942. [DOI] [PubMed] [Google Scholar]
- Kirkton S D, et al. . 2009. Continued artificial selection for running endurance in rats is associated with improved lung function. Journal of Applied Physiology 106:1810–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L G, and Britton S. L.. 2001. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiological Genomics 5:45–52. [DOI] [PubMed] [Google Scholar]
- Lailvaux S P, and Husak J. F.. 2014. The life history of whole-organism performance. Quarterly Review of Biology 89:285–318. [DOI] [PubMed] [Google Scholar]
- Lande R. 1979. Quantitative genetic analysis of multivariate evolution, applied to brain-body size allometry. Evolution 33:402–416. [DOI] [PubMed] [Google Scholar]
- Lande R, and Arnold S. J.. 1983. The measurement of selection on correlated characters. Evolution 37:1210–1226. [DOI] [PubMed] [Google Scholar]
- Lau D, et al. . 2017. Acclimation to hypoxia increases carbohydrate use during exercise in high-altitude deer mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 312:R400–R411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMoine C M R, Morash A. J., and McClelland G. B.. 2011. Changes in HIF-1α protein, pyruvate dehydrogenase phosphorylation, and activity with exercise in acute and chronic hypoxia. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 301:R1098–R1104. [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Sanchez J., Bigard A. X., Brunet A., Lesty C., and Monge C.. 1993. High altitude tissue adaptation in Andean coots: capillarity, fiber area, fiber type and enzymatic activities of skeletal muscle. Journal of Comparative Physiology, B. Biochemical Systemic and Environmental Physiology 163:52–58. [DOI] [PubMed] [Google Scholar]
- Lofsvold D. 1986. Quantitative genetics of morphological differentiation in Peromyscus. I. Tests of the homogeneity of genetic covariance structure among species and subspecies. Evolution 40:559–573. [DOI] [PubMed] [Google Scholar]
- Lofsvold D. 1988. Quantitative genetics of morphological differentiation in Peromyscus. II. Analysis of selection and drift. Evolution 42:54–67. [DOI] [PubMed] [Google Scholar]
- Lui M A, et al. . 2015. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 308:R779–R791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S, Cheng G., Zhou Y., and Xu G.. 2013. Thymosin beta4 induces angiogenesis through Notch signaling in endothelial cells. Molecular and Cellular Biochemistry 381:283–290. [DOI] [PubMed] [Google Scholar]
- MacMillen R E, and Garland T. Jr.. 1989. Adaptive physiology. Pp. 143–168 in Advances in the study of Peromyscus (Rodentia) (Layne J. N. and Kirkland G L, eds.). Texas Tech University Press, Lubbock, Texas. [Google Scholar]
- Mahalingam S, McClelland G. B., and Scott G. R.. 2017. Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. Journal of Physiology 595:4785–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina J N, McCracken K. G., Chua B., York J. M., and Milsom W. K.. 2017. Morphological and morphometric specializations of the lung of the Andean goose, Chloephaga melanoptera: a lifelong high-altitude resident. PLoS One 12:e0174395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Costello O, Agey P. J., Wu L., Szewczak J. M., and MacMillen R. E.. 1998. Increased fiber capillarization in flight muscle of finch at altitude. Respiration Physiology 111:189–199. [DOI] [PubMed] [Google Scholar]
- McClelland G B, Lyons S. A., and Robertson C. E.. 2017. Fuel use in mammals: conserved patterns and evolved strategies for aerobic locomotion and thermogenesis. Integrative and Comparative Biology 57:231–239. [DOI] [PubMed] [Google Scholar]
- McClelland G B, and Scott G. R.. In press. Evolved mechanisms of aerobic performance and hypoxia resistance in high-altitude natives. Annual Review of Physiology 81. doi:10.1146/annurev-physiol-021317-121527. [DOI] [PubMed] [Google Scholar]
- Meek T H, Lonquich B. P., Hannon R. M., and Garland T. Jr.. 2009. Endurance capacity of mice selectively bred for high voluntary wheel running. Journal of Experimental Biology 212:2908–2917. [DOI] [PubMed] [Google Scholar]
- Mitchell J H, and Blomqvist G.. 1971. Maximal oxygen uptake. New England Journal of Medicine 284:1018–1022. [DOI] [PubMed] [Google Scholar]
- Moore L G. 2017. Measuring high-altitude adaptation. Journal of Applied Physiology 123:1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, et al. . 2015a. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Molecular Biology and Evolution 32:978–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Hoffmann F. G., Weber R. E., Fago A., Witt C. C., and Storz J. F.. 2016. Predictable convergence in hemoglobin function has unpredictable molecular underpinnings. Science 354:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Inoguchi N., Weber R. E., Fago A., Moriyama H., and Storz J. F.. 2013. Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340:1324–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, et al. . 2018. Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLoS Genetics 14:e1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, et al. . 2015b. Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genetics 11:e1005681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespolo R F, Opazo J. C., Rosenmann M., and Bozinovic F.. 1999. Thermal acclimation, maximum metabolic rate, and nonshivering thermogenesis of Phyllotis xanthopygus (Rodentia) in the Andes Mountains. Journal of Mammalogy 80:742–748. [Google Scholar]
- Nikel K, Shanischara N. K., Ivy C., Dawson N. J., and Scott G. R.. 2017. Effects of hypoxia at different life stages on locomotory muscle phenotype in deer mice native to high altitude. Comparative Biochemistry and Physiology, B. Biochemistry and Molecular Biology 224:98–104. [DOI] [PubMed] [Google Scholar]
- Oufara S, Barre H., Rouanet J.-L., and Charonnet J.. 1987. Adaptation to extreme ambient temperatures in cold-acclimated gerbils and mice. American Journal of Physiology 253:R39–R45. [DOI] [PubMed] [Google Scholar]
- Pearson K G, Acharya K. G., and Fouad K.. 2005. A new electrode configuration for recording electromyographic activity in behaving mice. Journal of Neuroscience Methods 148:36–42. [DOI] [PubMed] [Google Scholar]
- Pearson O P, and Pearson A.. 1976. A stereological analysis of the ultrastructure of the lungs of wild mice living at low and high altitude. Journal of Morphology 150:359–368. [DOI] [PubMed] [Google Scholar]
- Pough F H. 1989. Organismal performance and Darwinian fitness - approaches and interpretations. Physiological Zoology 62:199–236. [Google Scholar]
- Projecto-Garcia J, et al. . 2013. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proceedings of the National Academy of Sciences of the United States of America 110:20669–20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech I G, et al. . 2013. Hemoglobin function and allosteric regulation in semi-fossorial rodents (family Sciuridae) with different altitudinal ranges. Journal of Experimental Biology 216:4264–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmann M, and Morrison P.. 1974a. Maximum oxygen consumption and heat loss facilitation in small homeotherms by He-O2. American Journal of Physiology 226:490–495. [DOI] [PubMed] [Google Scholar]
- Rosenmann M, and Morrison P.. 1974b. Physiological responses to hypoxia in the tundra vole. American Journal of Physiology 227:734–739. [DOI] [PubMed] [Google Scholar]
- Rosenmann M, and Morrison P.. 1975. Metabolic response of highland and lowland rodents to simulated high altitudes and cold. Comparative Biochemistry and Physiology A 51:523–530. [DOI] [PubMed] [Google Scholar]
- Rosenmann M, Morrison P., and Feist D.. 1975. Seasonal changes in metabolic capacity of red-backed voles. Physiological Zoology 48:303–310. [Google Scholar]
- Sadowska E T, Baliga-Klimczyk K., Chrzaścik K. M., and Koteja P.. 2008. Laboratory model of adaptive radiation: a selection experiment in the bank vole. Physiological and Biochemical Zoology 81:627–640. [DOI] [PubMed] [Google Scholar]
- Sadowska E T, et al. . 2005. Genetic correlations between basal and maximum metabolic rates in a wild rodent: consequences for evolution of endothermy. Evolution 59:672–681. [PubMed] [Google Scholar]
- Schippers M.-P, LeMoine C. M. R., and McClelland G. B.. 2014. Patterns of fuel use during locomotion in mammals revisited: the importance of aerobic scope. Journal of Experimental Biology 217:3193–3196. [DOI] [PubMed] [Google Scholar]
- Schippers M.-P, Ramirez O., Arana M., Pinedo-Bernal P., and McClelland G. B.. 2012. Increase in carbohydrate utilization in high-altitude Andean mice. Current Biology 22:2350–2354. [DOI] [PubMed] [Google Scholar]
- Scott G R, Elogio T. S., Lui M. A., Storz J. F., and Cheviron Z. A.. 2015. Adaptive modifications of muscle phenotype in high-altitude deer mice are associated with evolved changes in gene regulation. Molecular Biology and Evolution 32:1962–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G R, Schulte P. M., Egginton S., Scott A. L. M., Richards J. G., and Milsom W. K.. 2011. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Molecular Biology and Evolution 28:351–363. [DOI] [PubMed] [Google Scholar]
- Segrem N B, and Hart J. S.. 1967. Oxygen supply and performance in Peromyscus. Comparison of exercise with cold exposure. Canadian Journal of Physiology and Pharmacology 45:543–549. [DOI] [PubMed] [Google Scholar]
- Siebenmann C, Robach P., and Lundby C.. 2017. Regulation of blood volume in lowlanders exposed to high altitude. Journal of Applied Physiology 123:957–966. [DOI] [PubMed] [Google Scholar]
- Simonson T S. 2015. Altitude adaptation: a glimpse through various lenses. High Altitude Medicine and Biology 16:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson T S, et al. . 2015. Low haemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. Journal of Physiology 593:3207–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L R G. 1985. Low P50 in deer mice native to high altitude. Journal of Applied Physiology 58:193–199. [DOI] [PubMed] [Google Scholar]
- Snyder L R G, Born S., and Lechner A. J.. 1982. Blood oxygen affinity in high- and low-altitude populations of the deer mouse. Respiration Physiology 48:89–105. [DOI] [PubMed] [Google Scholar]
- Stawski C, Koteja P., Sadowska E. T., Jefimow M., and Wojciechowski M.. 2015. Selection for high activity-related aerobic metabolism does not alter the capacity of non-shivering thermogenesis in bank voles. Comparative Biochemistry and Physiology, A. Molecular and Integrative Physiology 180:51–56. [DOI] [PubMed] [Google Scholar]
- Storz J F. 2007. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. Journal of Mammalogy 88:24–31. [Google Scholar]
- Storz J F. 2010. Genes for high altitudes. Science 329:40–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F. 2016. Hemoglobin-oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? Journal of Experimental Biology 219:3190–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F. 2019. Hemoglobin: insights into protein structure, function, and evolution. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Storz J F, Bridgham J. T., Kelly S. A., and Garland T. Jr.. 2015. Genetic approaches in comparative and evolutionary physiology. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 309:R197–R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F, and Cheviron Z. A.. 2016. Functional genomic insights into regulatory mechanisms of high-altitude adaptation. Advances in Experimental Medicine and Biology 903:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F, and Hoekstra H. E.. 2007. The study of adaptation and speciation in the genomic era. Journal of Mammalogy 88:1–4. [Google Scholar]
- Storz J F, and Kelly J. K.. 2008. Effects of spatially varying selection on nucleotide diversity and linkage disequilibrium: insights from deer mouse globin genes. Genetics 180:367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F, and Nachman M. W.. 2003. Natural selection on protein polymorphism in the rodent genus Peromyscus: evidence from interlocus contrasts. Evolution 57:2628–2635. [DOI] [PubMed] [Google Scholar]
- Storz J F, Natarajan C., Cheviron Z. A., Hoffmann F. G., and Kelly J. K.. 2012. Altitudinal variation at duplicated β-globin genes in deer mice: effects of selection, recombination, and gene conversion. Genetics 190:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F, Runck A. M., Moriyama H., Weber R. E., and Fago A.. 2010a. Genetic differences in hemoglobin function between highland and lowland deer mice. Journal of Experimental Biology 213:2565–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F, et al. . 2009. Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proceedings of the National Academy of Sciences of the United States of America 106:14450–14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J F, Scott G. R., and Cheviron Z. A.. 2010b. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. Journal of Experimental Biology 213:4125–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow J G, Garland T. Jr., Carter P. A., Zhan W. Z., and Sieck G. C.. 1998. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). Journal of Applied Physiology 84:69–76. [DOI] [PubMed] [Google Scholar]
- Swallow J, Hayes J. P., Koteja P., and Garland T. Jr.. 2009. Selection experiments and experimental evolution of performance and physiology. Pp. 301–351 in Experimental evolution: concepts, methods, and applications of selection experiments (Garland T. Jr. and Rose M R, eds.). University of California Press, Berkeley, California. [Google Scholar]
- Tate K B, et al. . 2017. Circulatory mechanisms underlying adaptive increases in thermogenic capacity in high-altitude deer mice. Journal of Experimental Biology 220:3616–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C R, and Weibel E. R.. 1981. Design of the mammalian respiratory system. 1. Problem and strategy. Respiration Physiology 44:1–10. [PubMed] [Google Scholar]
- Tufts D M, Revsbech I. G., Cheviron Z. A., Weber R. E., Fago A., and Storz J. F.. 2013. Phenotypic plasticity in blood–oxygen transport in highland and lowland deer mice. Journal of Experimental Biology 216:1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts D M, et al. . 2015. Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Molecular Biology and Evolution 32:287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velotta J P, Ivy C. M., Wolf C. J., Scott G. R., and Cheviron Z. A.. 2018. Maladaptive phenotypic plasticity in cardiac muscle is attenuated in high-altitude deer mice. Evolution 72:2712–2727. [DOI] [PubMed] [Google Scholar]
- Van Sant M J, and Hammond K. A.. 2008. Contribution of shivering and nonshivering thermogenesis to thermogenic capacity for the deer mouse (Peromyscus maniculatus). Physiological and Biochemical Zoology 81:605–611. [DOI] [PubMed] [Google Scholar]
- Velotta J P, Jones J., Wolf C. J., and Cheviron Z. A.. 2016. Transcriptomic plasticity in brown adipose tissue contributes to an enhanced capacity for non-shivering thermogenesis in deer mice. Molecular Ecology 25:2870–2886. [DOI] [PubMed] [Google Scholar]
- Vock R, Weibel E. R., Hoppler H., Ordway G., Weber J.-M., and Taylor C. R.. 1996. Design of the oxygen and substrate pathways. V. Structural basis of vascular substrate supply to muscle cells. Journal of Experimental Biology 199:1675–1688. [DOI] [PubMed] [Google Scholar]
- Wasserman D H, Kang L., Ayala J. E., Fueger P. T., and Lee-Young R. S.. 2010. The physiological regulation of glucose flux into muscle in vivo. Journal of Experimental Biology 214:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J.-M. 2011. Metabolic fuels: regulating fluxes to select mix. Journal of Experimental Biology 214:286–294. [DOI] [PubMed] [Google Scholar]
- Weibel E R, Bacigalupe L. D., Schmitt B., and Hoppler H.. 2004. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respiratory Physiology and Neurobiology 140:115–132. [DOI] [PubMed] [Google Scholar]
- Weibel E R, and Hoppler H.. 2005. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. Journal of Experimental Biology 208:1635–1644. [DOI] [PubMed] [Google Scholar]
- West J B. 1984. Human physiology at extreme altitude on Mount Everest. Science 223:784–788. [DOI] [PubMed] [Google Scholar]
- Winslow R M, et al. . 1985. Effects of hemodilution on O2 transport in high-altitude polycythemia. Journal of Applied Physiology 59:1495–1502. [DOI] [PubMed] [Google Scholar]
- Wone B W, et al. . 2015. A strong response to selection on mass-independent maximal metabolic rate without a correlated response in basal metabolic rate. Heredity 114:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wone B W, Sears M. W., Labocha M. K., Donovan E. R., and Hayes J. P.. 2009. Genetic variances and covariances of aerobic metabolic rates in laboratory mice. Proceedings of the Royal Society of London, B. Biological Sciences 276:3695–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J M, Scadeng M., McCracken K. G., and Milsom W. K.. 2018. Respiratory mechanics and morphology of Tibetan and Andean high-altitude geese with divergent life histories. Journal of Experimental Biology 221:jeb170738. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. . 2018. Divergent and parallel routes of biochemical adaptation in high-altitude passerine birds from the Qinghai-Tibet Plateau. Proceedings of the National Academy of Sciences of the United States of America 115:1865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]