Abstract
The past two decades have witnessed soaring interest in the field of non-coding RNAs, largely attributed by its regulatory role in controlling two third of human transcriptional output. Though, there are several classes of non-coding RNAs found in nature, microRNAs takes the central stage because of their pleiotropic roles. In particular, extracellular microRNAs are gaining traction due to their relative stability and bio availability. Extracellular microRNAs has been shown to occur in all living organisms, including dietary plants. Some of the recent reports suggest that these dietary microRNAs pass through the gut, enter systemic circulation and exert biological effects on animal physiology. However, evidences against this hypothesis are also presented in literature and hence this area has been strongly debated. In this review, I will briefly summarise the evidences accumulated for and against this hypothesis and discuss potential implications of such findings in human health.
Keywords: Dietary non-coding RNAs, microRNAs and edible nanoparticles
1. Introduction
From the discovery of DNA double helical structure by Watson and Crick in 1947, the central dogma, in which RNA was considered as a mere messenger between the genetic code and proteins, has dominated the history of science. The non-coding function of RNA was only known for RNAs with housekeeping functions such as ribosomal RNAs (rRNAs) & transfer RNAs (tRNAs) and small nuclear/nucleolar RNAs (sn/sno RNAs), which ensure proper splicing and translation of mRNA. These non-coding RNAs were discovered about five to seven decades ago [1]. However, now it is apparent that there are other classes of non-coding RNA whose transcription is more pervasive and higher in abundance than earlier thought. This can, in fact, be attributed to the evolution of DNA sequencing technologies from earlier methods such as sanger sequencing and shot gun approach to massively parallel sequencing, adopted by sequencing platforms recently [2]. This led to the revelation that more than 70–90% of eukaryotic genome is transcribed at any given time and the abundance of ncRNAs exceeds the number of protein -coding mRNAs in eukaryotic cells [3]. What do these ncRNAs do? The answer appear to lie in their functions which is discrete from house keeping ncRNAs. There are several types of non-coding RNAs, distinctly classified based on characteristics such as size, function, localization, genomic location etc.
2. Non-coding RNA classification and function
A broad classification of ncRNAs based on size can divide them into long non-coding RNAs (>200 nts) and small non-coding RNAs (<200 nts). The long non-coding RNAs (lncRNAs) are further classified into several groups based on a) their genomic position, such as, enhancer lncRNAs, sense/anti-sense/overlapping lincRNAs with respect to neighbouring protein-coding gene, intergenic (lincRNAs) or intragenic lncRNAs and b) function: competing endogenous RNA (ceRNAs), natural anti-sense transcripts (NATs), circular RNAs (cRNAs) [4]. Emerging literature indicates that lncRNAs regulates the transcriptional output of human genome at multiple levels. About half of the long ncRNAs are commonly localized to the nucleus and regulate the transcription of target genes by altering the chromatin landscape. For this to occur, long ncRNAs either directly associate with the chromatin or recruit epigenetic complexes or act as a modular scaffold for recruitment of epigenetic factors on target gene promoter [4,5]. In other cases, NATs can suppress transcription, splicing and translation of target mRNAs by direct association. Even though, both ceRNAs and cRNAs perform a similar function by acting miRNAs sponges, cRNAs are derived from back splicing of intronic regions of protein-coding genes [6].
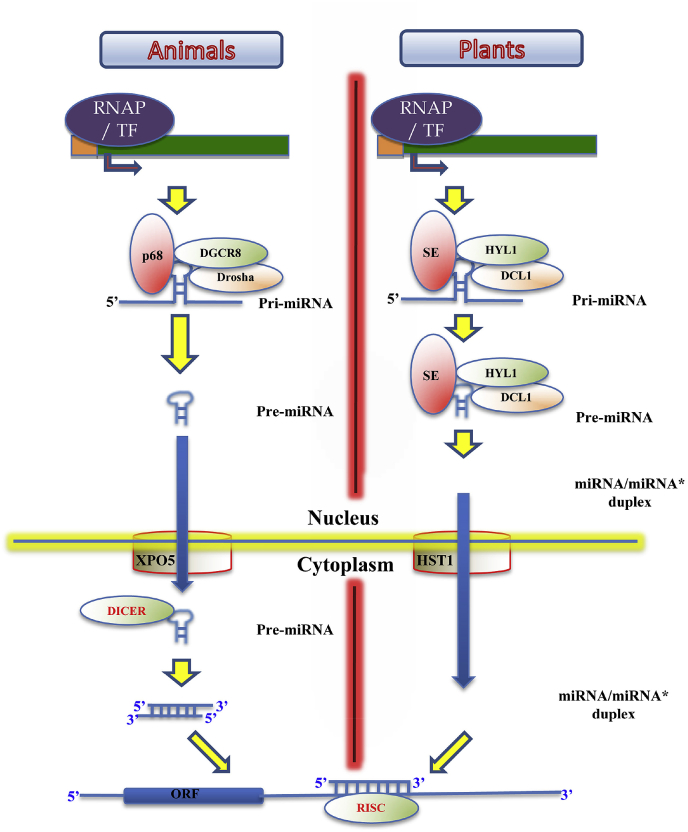
3. MicroRNAs: biogenesis and classification
Small non-coding RNAs exist in several forms in nature. These are piwi-interacting RNAs, endogenous siRNAs and microRNAs (miRNAs), in which miRNAs play a major role in both development and diseases. Compared to long ncRNAs, miRNAs, on the other hand, are mechanistically simpler and exhibits less complexity. MiRNAs can also be classified based on its genomic location as intronic, intergenic and exonic, wherein, the latter constitutes less than 5% of total miRNAs [7]. In animals, synthesis of miRNAs begins with the transcription of a several kb long transcript known as primary microRNA (pri-miRNA) followed by its cleavage by nuclear localized microprocessor complex into 60-120 nt long precursor microRNA (pre-miRNA). The catalytic subunit of microprocessor complex consist of a class II ribonuclease enzyme, DROSHA and a double stranded RNA (dsRNA) binding protein, DiGeorge Syndrome critical protein 8 (DGCR8) [8]. Even though, the generation of pre-miRNA via the microprocessor complex appears to be the predominant pathway, pre-miRNAs can also be generated via DROSHA/DGCR8 independent ways. This is particularly exemplified in miRNAs derived from intronic regions of protein-coding genes (otherwise known as miRTRONs) wherein pre-miRNAs are derived through splicing mechanism [9]. In addition, generation of pre-miRNA from pri-miRNA can also be tightly regulated. This occurs via the modulation of microprocessor complex by post-translational modification or by the association of several other proteins to microprocessor complex such as, p68/72 RNA helicase, Mothers Against DPP Homologue 4 (SMAD) proteins, KH-type splice regulatory protein and elavl-like 1 (HuR) protein [8,[10], [11], [12], [13], [14]]. Notably, this regulation may affect either global miRNA biogenesis or affect the biogenesis of a subset of miRNAs. Once cleaved, pre-miRNA is then exported out of the nucleus via Exportin 5 (XPO5): Ran GTPase complex into the cytoplasm where it is further cleaved into miRNA:miRNA* duplex by DICER, an RNAse III class enzyme [15]. The duplex miRNA is then loaded on to the RNA induced silencing complex (RISC) wherein the guide strand is selectively retained and passenger strand (miRNA*) is degraded. The recruitment of RISC complex along with the guide strand (mature miRNA) on target mRNAs leads to translational repression followed by transcript degradation (Fig. 1) [[16], [17], [18]].
Fig. 1.
Comparison of miRNA biogenesis in animals and plants.
4. MicroRNAs in plants
In comparison to animals, miRNA biogenesis pathway differs significantly in plant kingdom (Fig. 1). Firstly, in plants, the derivation of miRNA duplex from pri-miRNA via pre-miRNA intermediate, is entirely carried out in the nucleus and the miRNA duplex is exported into the cytoplasm. Secondly, unlike animals, DICER LIKE-1 (DCL1), an RNAse III class enzyme, catalyses the conversion of pri-miRNA to pre-miRNA as well as the pre-miRNA to miRNA duplex [19]. In addition to DCL1, HYL1, a double stranded RNA binding protein and SE, a C2H2-type Zinc finger protein are essential for miRNA biogenesis in plants. The plant counterpart for XPO5 is known as HASTY (HST1) gene which exports the miRNA duplex from nucleus to cytoplasm [19,20]. In addition, the last nucleotide at the 3′ end of mature miRNA is 2′-O- methylated in plants, which is not observed in animal miRNAs (Fig. 1) [21]. In contrast to animals, plant target mRNAs possesses near perfect complimentary binding sites to miRNAs, leading to the hypothesis that transcript degradation being the major pathway for miRNA mediated suppression. However, contemporary research highlight the occurrence of translation inhibition as an additional pathway in plants and it may also take place on the endoplasmic reticulum [22]. About two and half decades ago, the first microRNA, lin14 was discovered by Ambrose and colleagues in C. elegans and now the number of miRNAs in both plant and animal kingdom is expanding at a constant pace [23]. The current version of miRbase released in march 2018 (v22) has 2654 mature and 1917 precursor human miRNAs. Arabidopsis thaliana, the model organism for plant biology, has 326 precursor and 428 mature miRNA. Commercially important crops such as soybean, rice and wheat possesses 684, 604, 122 pre-miRNAs and 756, 738 and 125 mature miRNAs, respectively. Emerging literature suggest the key role of specific miRNAs, especially in plant development, stress tolerance and disease resistance [19,24,25].
However, the focus of this review will be on some interesting discoveries made recently on the impact of dietary ncRNAs in human health. In particular, cross kingdom regulation of human transcriptome by dietary plant miRNAs is gaining some attention in the recent past. In this line, plant miRNAs were shown to cross the intestinal barrier, absorbed into the circulation and exhibit a regulatory effect on human mRNAs. However, these findings are refuted by several other research groups as erroneous and this remains a field of controversy till date. This review will evaluate and summarise the observations, counterarguments and the implications of such findings in human health and propose a mechanism on how this cross kingdom regulation may occur via the gastrointestinal barrier.
5. Dietary plant derived microRNAs: the treasure side
In 2008, seminal discovery by Zhang and colleagues demonstrated the presence of hundreds miRNAs in serum samples from healthy volunteers [26]. More importantly, this discovery revealed the stable nature of several miRNAs in extracellular environment, either as a naked duplex associated with proteins or encapsulated in vesicles such as exosomes. Later, several reports indicated the role of these circulating miRNAs as a novel mode of communication between different cell types/tissues, in which miRNAs secreted from one tissue exerts a regulatory effect on mRNA target/s in different tissue [27,28]. This raises an intriguing/interesting question, if miRNAs derived from dietary sources enters bloodstream, i) will it be stable and ii) will it exert a biological effect on human transcriptome? However, the harrowing passage of nucleic acids through the gastrointestinal tract is the limiting barrier for such transit to occur. The GI tract poses many obstacles for absorption of miRNAs from diet. This includes the extremely acidic pH of stomach, nucleases and bile salts secreted from pancreas and the intestinal flora, all of which has the capability to degrade the miRNA into individual nucleotides [29]. In spite of these odds, a later study by the same group, surprisingly detected the presence of plant miRNAs in human sera and claimed that these were derived from ingested food [30]. In particular, miR-156a and miR-168a, abundant in rice were shown to be present in serum samples of Chinese cohort and rice derived miR-168a was shown to target a human mRNA, low density lipoprotein receptor adaptor protein 1 (LDLRAP1) in human/mice liver. However, this study and other follow up reports from several labs were met with severe criticism from the research community, which are discussed further.
The phenomenon of dietary ncRNAs passing through GI tract with a systemic impact may not be uncommon (Table 1). Systemic delivery of siRNAs to model organism, C. elegans is often achieved by orally feeding these worms with E. coli overexpressing gene specific siRNAs indicating that orally delivered small RNA can cross the GI barrier [31]. This approach was also shown to knock down target genes in other insects and enhance pest mortality [32]. Hence, the detection of rice miRNAs in human plasma samples by Zhang and colleagues may not be surprising and was further corroborated by an independent study by Wang et al. (2012). In this case, using next generation small RNA (sRNA) sequencing datasets of human serum samples, the authors identified several exogenous sRNA species from gut microbiota and plant species. Notably, the most abundant sRNA was derived from staple food sources such as rice and corn [33]. Kendal Hirschi's lab from Bayer College of Medicine, made a similar discovery, in which plant miRNAs were detected in plasma and sera of mice fed with honeysuckle (HS) -rich chow diet. In particular, high levels of miR-2911, an atypical miRNA derived from 26S ribosomal RNA, was found in mice serum and urine, within 3.5 days post feeding. When miR-168a was combined with HS diet, higher plasma levels of miR-168a was noted compared to feeding miR-168a alone indicating that HS diet potentiates the absorption of miR-168a. This also raises an important proposition that specific dietary preferences may significantly influence the absorption of dietary miRNAs [34]. In addition, it was also shown that the plant derived miR-2911 exhibits better stability and intestinal absorption compared to synthetic miR-2911. This was attributed to the likely association of miR-2911 with proteins in plants but not exosomes [35]. Parallelly, Zhang's group assessed the functionality of HS derived miR-2911 on influenza viral infection. In their study, miR-2911 was found to be stable in decoction prepared from honeysuckle (HS) and feeding animals with HS decoction led to significant increase in circulatory miR-2911. It was further demonstrated that miR-2911 targets influenza A virus, both in vitro and in vivo and oral administration of either miR-2911 or HS decoction protected mice from H5N1 infection [36]. Supportive data for plant miRNAs targeting human transcriptome was also obtained by another study by Yang et al. (2016). In this report, the authors, sequenced and characterized the miRNA population from Moringa Oleifera seeds and found several homologues of human miRNAs in M. Oleifera. In particular, M. Oleifera miR-168a was predicted to be a functional homologue of human miR-579. Transfection of mol-miR-168a in hepatocarcinoma cell line led to a significant decreases in SIRT1 protein, a valid target of miR-579, further supporting this cross kingdom regulation [37]. Taken together, it is apparent that plant derived miRNAs are capable of passing through the GI tract and enter the circulation. The ability of plant miRNAs to pass through GI tract unperturbed could be due to the plant specific 2’ -O- methylation of plant miRNAs.- This may be true, since, human miRNAs, modified to mimic plant miRNAs, were able to pass through GI tract unaffected. In a manuscript by Vance and colleagues, the authors prepared a cocktail of tumour suppressor miRNAs, (miR-34a, miR-143 and miR-145) in which the 3′ terminal nucleotide was 2′-O- methylated to mimic plant miRNAs. Oral delivery of this cocktail led to a significant decrease in tumour burden in ApcMin/+ model of colon cancer indicating that 2′-O- methylation may have a role in stability of miRNA [38]. It would be interesting to see if transgenic plants engineered to express these miRNAs will have a similar effect compared to synthetic mimics. A recent study tested this possibility by engineering Arabidopsis thaliana to express an artificial miRNA sequence and murine miR-146a. Even though, the plants stably expressed the artificial miRNA and miR-146a equivalent to endogenous miR-2911, these miRNAs were not detected in circulation [39].
Table 1.
Dietary miRNAs, their bioavailability and putative targets.
| MicroRNA | Dietary Source | Target mRNA | Detection in human tissue | References |
|---|---|---|---|---|
| miR-156a | Rice | Human Serum | 32 | |
| miR-168a | Rice, Corn | human LDLRAP1 | Human Plasma | 33, 34 |
| 16S rRNA | Pseudomonas | Human Plasma | 33 | |
| 23S rRNA | Rhodococcus | Human Plasma | 33 | |
| miR-263a | Mosquito | Human Plasma | 33 | |
| Bantam | ||||
| miRNA | Housefly | Human Plasma | 33 | |
| miR-2911 | Honeysuckle | Influenza virus | Mice Serum, Urine | 34, 35, 36 |
| PB2 & NS1 protein | ||||
| miR-159a/e | A.thaliana, | Human TCF7 | Human Serum | 40 |
| Soybean | ||||
| miR-7267-3p | Ginger | LGG* ycnE | Gut microbiome | 56 |
| miR-167a-5p | Ginger | LGG* SpaC | Gut microbiome | 56 |
• LGG: Lactobacillus rhamnoses.
An independent study by Chin et al. (2016) attempted to establish dietary miRNA as a biomarker for breast cancer. In this report, authors specifically investigated the presence of plant miRNAs in NGS data set from 42 breast cancer patient serum samples. Interestingly, miR-159a/e, a plant miRNA conserved in A. thaliana and Soybean (Glycine max) was found in significant reads in patients sera and was further verified by quantitative RT-PCR. Moreover, the levels of miR-159 was higher in healthy control donors compared to patients, while also correlated negatively with metastatic status of patient cohort. Oral delivery of miR-159a reduced tumour incidences in xenograft tumour model, in vivo, via directly targeting TCF7, a key regulator of Wnt signalling pathway in breast cancer [40]. Though encouraging, it is also possible that the intestinal absorption of dietary miRNAs is likely due to a leaky gut in mice, caused by an underlying pathological condition, such as cancer. This appears to be true as it has recently been shown that dietary uptake of miRNAs are enhanced when mice are treated with aspirin or anti-CD3 antibodies, both of which increases the gut permeability indirectly via altering the immune system and inflammation [41].
6. Dietary plant miRNAs; the fairy tale side
Despite the numerous reports emerging in support of dietary miRNAs impacting human health, the bioavailability of these miRNAs still remains controversial due to several reasons. This includes the lack of reproducibility of the data in different laboratories, technical errors in sequence analysis and in some cases, study design per se. The concept of dietary miRNA's uptake and cross kingdom regulation was first refuted by Dickinson et al. (2013) who fed mice with a control chow diet or diet complemented with 41% rice or rice based chow (75% rice) alone. However, when total RNA from plasma and liver tissues were subjected to small RNA sequencing, rice derived miR-168a showed less than 10 reads out of more than 10 million reads. Moreover, this was observed only in a subset of mice fed with rice diet, raising concern over previous studies [42]. Similarly, serum from healthy athletes who ingested fruits containing high levels of miR-156a, miR-159a and miR-169a did not display the presence of these miRNAs. In addition, these miRNAs were also not detected in gut tissues of honeybees when these insects were fed with pollen, rich in these miRNAs, further raising doubt about the bioavailability of plant derived miRNAs [43]. Furthermore, in a separate study, mice were either fed with a control diet or diet supplemented with miRNA population isolated from corn or autoclaved corn powder RNA for two weeks. No significant enrichment of corn miRNAs were detected in any of the groups and it was postulated that the majority of miRNAs are extensively degraded during the digestive process [44]. As reproducibility being a concern on one side, analytical and primer designing errors appear to have majorly influenced several published reports on dietary miRNA's bioavailability in human circulation. In a manuscript published by Pasterllo et al. (2017), the authors initially showed the detection of plant miRNAs in serum samples of healthy cohorts who consumed broccoli rich diet but later retracted this manuscript citing faulty primer design strategies leading to spurious amplification [45]. Moreover, several publicly available NGS data sets appear to be commonly contaminated with plant derived miRNAs [46]. A detailed analysis of 824 sequencing datasets from human tissues and sera, revealed the presence of non-human miRNA sequences, including plant derived miRNAs, though at a low abundance. In addition, there was no significant correlation between the presence and abundance of dietary miRNAs with organs exposed to dietary intake such as liver versus organs that are not directly exposed to dietary intake such as cerebral-spinal fluid, further supporting the contamination viewpoint [47].
But where does this contaminating miRNAs comes from? One possible source, as Wilmes and colleagues describe in their latest report, can be the extraction columns in commercial miRNA purification kits, indicating the introduction of exogenous miRNAs from unexpected sources. This was proved by an RNA isolation procedure performed with nuclease free water alone as input sample. As expected, subjecting nuclease free water for RNA isolation through these columns yielded several small RNAs. Notably, these contaminating small RNA sequences were shown to be present in public datasets, though at a lower level. Treating the columns with sodium hypochlorite followed by washing with nuclease free water, prior to RNA isolation led to a significant reduction of these contaminating small RNA population [48]. In addition to this, there are several other challenging technical issues with the detection of dietary miRNAs per se in human extracellular fluids. Purposefully, the literature pertaining to the uptake of milk derived dietary miRNAs and their detection in human serum was avoided in this review, especially due the sequence similarity between several cow and human miRNAs. For instance, miR-21-5p and miR-30a-5p are identical between these two species which led to the proposition that these miRNAs cannot be used for dietary uptake of miRNAs from cow's milk [49]. Other technical issues that can confound the interpretation of results and lead to spurious detection of xenomiRNAs in human extracellular fluid are, i) normalization controls used for plant miRNAs ii) the RNA isolation method (commercial columns versus Trizol isolation methods, as discussed above), iii) the stability of the chosen miRNA candidate, a factor that is also affected by the sequence and nucleotide composition, iv) or the choice of choosing the candidate miRNA itself, such as miR-168a, which is conserved across several kingdoms, including single cell organism, raising the possibility that the diet derived miRNA could actually be from gut microbiome or bacterial contamination and v) the parameters used in NGS data analysis, filtering and stringency cut off factors that can lead to spurious detection of fragmented small RNAs from human genome sequences as dietary miRNAs [50,51].
7. Edible exosomes or nanoparticles; an alternate solution for the transit of miRNA via GI tract?
The first hint of RNA as a mediator of extracellular communication between different parts or systemic spread of message in plants was proposed about 2 decades ago [52]. In this form of communication, plants appear to execute a mechanism akin to exosomes seen in mammalian cells. In recent past, evidence is mounting for the presence of exosome like nanoparticles (also known as edible nanoparticles, ENPs) in plants and they are secreted in response to infection and stress [53]. The presence of these nano-sized vesicles from plants was observed almost five decades ago and were thought to be technical artefacts and hence were not given much attention [54,55]. It is evident from recent literature that these ENPs from plant sources are rich in bioactive compounds including small RNA population. It is proposed that miRNAs encapsulated in plant ENPs may exhibit better bioavailability compared to miRNAs that are naked or associated with proteins. This is vindicated by a study by Zhang et al. (2016) in which the authors purified ∼230 nm ENPs from edible ginger plant and tested its activity against inflammatory bowel disease (IBD) in mice models. This study demonstrated successful uptake of Ginger ENPs by intestinal epithelial cells and macrophages in vivo. Ginger ENPs also exhibited anti-inflammatory properties in vivo, thereby preventing chronic colitis and inflammation associated cancer. Further characterization of these ENPs revealed the presence of lipids, proteins, phytochemicals and ∼125 miRNAs, probably attributing to the observed effect [56]. In addition, siRNAs against CD98, packaged into ginger ENPs showed better bioavailability compared to naked delivery. These siRNA packed ENPs, when orally administered, were able to pass through the digestive system and reduce the expression of CD98 in colon tissues [57]. The increased bioavailability of multiple cargo molecules in ENPs, has raised significant excitement in the field for exploring ENPs from a wide variant of plants. In this line, profiling the small RNA composition of ENPs isolated from 11 different edible fruits and vegetables identified abundant plant miRNAs within these ENPs, and many of them were predicted to target human mRNAs [58]. However, some of these ENP derived miRNAs may also apparently target key enzymatic and structural proteins of specific bacterial species in gut microbiota and thereby influence the barrier function and physiology of human gut. Tent et al. (2018) elegantly demonstrated this concept wherein, ENPs derived from Ginger is taken up by the human gut commensal bacteria, Lactobacillus rhamnosus (LGG). Furthermore, Ginger derived miR-7267-3p, that was delivered to the LGG via ENPs, was shown to target LGG monooxygenase ycnE, an enzyme involved in tryptophan metabolism. Inhibition of ycnE leads to increased accumulation of indole-3-carboxaldehyde (I3A), a ligand for the activation of aryl hydrocarbon receptor pathway which induces IL-22 synthesis and thereby increasing gut barrier function. This was further evidenced when orally delivered small RNA population from ginger ENP was able to protect mice from dextran sulphate induced colitis [59]. Thus, it is tempting to speculate that ENPs, in all likelihood, may act as a carrier for the dietary miRNAs to pass through the GI tract to human circulation.
8. Concluding remarks
Even though, the cross kingdom regulation of human transcriptome by dietary plant derived miRNAs sounds exiting at first, this field may require more thorough investigation and in depth analysis before making valid conclusions [60]. The bioavailability of dietary miRNAs is still controversial and if it is proven to be true, the impact of this discovery may have two far reaching outcomes; 1) Oral delivery of therapeutically important miRNAs, either as a part of food or as edible transgenic plants expressing target miRNA, for various target diseases will likely be possible, 2) on the flip side, it also raises safety issues concerning the ingestion of genetically modified crops. Several confounding factors may affect the bioavailability of the plant derived miRNAs. A major portion of human diet is often subjected to harsh conditions such as high temperature and pressure in the form of cooking. How does this process affect the bioavailability of the miRNAs? At least, this has been investigated with Artichoke derived miRNAs, wherein, it was shown that 81% of the total miRNA population may be degraded after cooking artichoke plants in water at 100 °C for 15 min [61]. The stability of dietary miRNA may also depend on the mature miRNA sequence since mutating miR-2911 with various sequence combinations showed distinct dietary absorption of this miRNA [41]. As noted earlier, other confounding factors such as the dietary preference, the composition of diet in terms of the relative proportion of proximate principles (water, fibre, carbohydrate, protein and mineral salts), relative ENP content may as well play a critical role in determining the passage of miRNA/s from food through the digestive system to systemic circulation. On the other hand, the patho-physiological state of GI tract is another confounding factor which may significantly impact the dietary uptake of miRNAs. Taking these factors into account may aid in better mechanistic understanding of the fate of dietary miRNAs.
Acknowledgements
The author wishes to acknowledge support from Central Food Technological Research Institute (CSIR-CFTRI) and Council of Scientific and Industrial Research (CSIR), Government of India, India.
References
- 1.Yang J.X., Rastetter R.H., Wilhelm D. In: Non-coding RNAs: an Introduction BT - Non-coding RNA and the Reproductive System. Wilhelm D., Bernard P., editors. Springer Netherlands; Dordrecht: 2016. pp. 13–32. [Google Scholar]
- 2.Zhang J., Chiodini R., Badr A., Zhang G. The impact of next-generation sequencing on genomics. J Genet Genomics. 2011;38:95–109. doi: 10.1016/j.jgg.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kung J.T.Y., Colognori D., Lee J.T. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon R.A., Jaé N., Holdt L., Dimmeler S. Long noncoding RNAs: from clinical genetics to therapeutic targets? J. Am. Coll. Cardiol. 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 5.Salehi S., Taheri M.N., Azarpira N. State of the art technologies to explore long non‐coding RNAs in cancer. J. Cell Mol. Med. 2017;21:3120–3140. doi: 10.1111/jcmm.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene J., Baird A.-M., Brady L. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38. doi: 10.3389/fmolb.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slezak-Prochazka I., Kluiver J., de Jong D. Cellular Localization and Processing of Primary Transcripts of Exonic MicroRNAs. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 9.Westholm J.O., Lai E.C. Mirtrons: microRNA biogenesis via splicing. Biochimie. 2011;93:1897–1904. doi: 10.1016/j.biochi.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blahna M.T., Hata A. Smad-mediatedregulation of microRNA biosynthesis. FEBS Lett. 2012;586:1906–1912. doi: 10.1016/j.febslet.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller-Pace F.V., Moore H.C. RNA helicases p68 and p72: multifunctional proteins with important implications for cancer development. Future Oncol. 2011;7:239–251. doi: 10.2217/fon.11.1. [DOI] [PubMed] [Google Scholar]
- 12.Trabucchi M., Briata P., Garcia-Mayoral M. The RNA-binding protein KSRP promotes the biogenesis of a subset of miRNAs. Nature. 2009;459:1010–1014. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundaram G.M., Common J.E.A., Gopal F.E. ‘See-saw’ expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature. 2013;495:103–106. doi: 10.1038/nature11890. [DOI] [PubMed] [Google Scholar]
- 14.Sundaram G.M., Ismail H.M., Bashir M. EGF hijacks miR-198/FSTL1 wound-healing switch and steers a two-pronged pathway toward metastasis. J. Exp. Med. 2017;214:2889–2900. doi: 10.1084/jem.20170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muqbil I., Bao B., Abou-Samra A.B. Nuclear export mediated regulation of MicroRNAs: potential target for drug intervention. Curr. Drug Targets. 2013;14:1094–1100. doi: 10.2174/1389450111314100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilczynska A., Bushell M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015;22:22–33. doi: 10.1038/cdd.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez A., Griffiths-Jones S., Ashurst J.L., Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacFarlane L.-A., Murphy P.R. MicroRNA: biogenesis, function and role in cancer. Curr. Genom. 2010;11:537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H., Yu H., Tang G., Huang T. Small but powerful: function of microRNAs in plant development. Plant Cell Rep. 2018;37:515–528. doi: 10.1007/s00299-017-2246-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J.-K. Reconstituting plant miRNA biogenesis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9851–9852. doi: 10.1073/pnas.0805207105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu B., Yang Z., Li J. Methylation as a crucial step in plant microRNA biogenesis. Science. 2005;307:932–935. doi: 10.1126/science.1107130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S., Liu L., Zhuang X. microRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell. 2013;153:562–574. doi: 10.1016/j.cell.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 24.Wang H.-L.V., Chekanova J.A. In: Long Noncoding RNAs in Plants BT - Long Non Coding RNA Biology. Rao M.R.S., editor. Springer Singapore; Singapore: 2017. pp. 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Meng J., Cui J., Luan Y. Characterization and function of MicroRNA(∗)s in plants. Front. Plant Sci. 2017;8:2200. doi: 10.3389/fpls.2017.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X., Ba Y., Ma L. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi T., Komatsu S., Ichikawa D. Circulating MicroRNAs: a next-generation clinical biomarker for digestive system cancers. Int. J. Mol. Sci. 2016;17:1459. doi: 10.3390/ijms17091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Liang H., Zen K., Zhang C.-Y. Secreted microRNAs from tumor cells can suppress immune function. OncoImmunology. 2016;5 doi: 10.4161/2162402X.2014.982407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y., Zhang Y., Dong P. Digestion of nucleic acids starts in the stomach. Sci. Rep. 2015;5:11936. doi: 10.1038/srep11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Hou D., Chen X. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res. 2012;22:107–126. doi: 10.1038/cr.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammell C.M., Hannon G.J. Inducing RNAi in C. elegans by feeding with dsRNA-expressing E. coli. Cold Spring Harb. Protoc. 2012 doi: 10.1101/pdb.prot072348. 2012:pdb.prot072348. [DOI] [PubMed] [Google Scholar]
- 32.Wuriyanghan H., Rosa C., Falk B.W. Oral Delivery of Double-Stranded RNAs and siRNAs Induces RNAi Effects in the Potato/Tomato Psyllid, Bactericerca cockerelli. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K., Li H., Yuan Y. The complex exogenous RNA spectra in human plasma: an interface with human gut biota? PLoS One. 2012;7 doi: 10.1371/journal.pone.0051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J., Farmer L.M., Agyekum A.A.A., Hirschi K.D. Detection of dietary plant-based small RNAs in animals. Cell Res. 2015;25:517–520. doi: 10.1038/cr.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J., Hotz T., Broadnax L. Anomalous uptake and circulatory characteristics of the plant-based small RNA MIR2911. Sci. Rep. 2016;6:26834. doi: 10.1038/srep26834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z., Li X., Liu J. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015;25:39–49. doi: 10.1038/cr.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirrò S., Zanella L., Kenzo M. MicroRNA from Moringa oleifera: identification by high throughput sequencing and their potential contribution to plant medicinal value. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mlotshwa S., Pruss G.J., MacArthur J.L. A novel chemopreventive strategy based on therapeutic microRNAs produced in plants. Cell Res. 2015;25:521–524. doi: 10.1038/cr.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J., Primo C., Elbaz-Younes I., Hirschi K.D. Bioavailability of transgenic microRNAs in genetically modified plants. Genes Nutr. 2017;12:17. doi: 10.1186/s12263-017-0563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chin A.R., Fong M.Y., Somlo G. Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Res. 2016;26:217–228. doi: 10.1038/cr.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J., Elbaz-Younes I., Primo C. Intestinal permeability, digestive stability and oral bioavailability of dietary small RNAs. Sci. Rep. 2018;8:10253. doi: 10.1038/s41598-018-28207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickinson B., Zhang Y., Petrick J.S. Lack of detectable oral bioavailability of plant microRNAs after feeding in mice. Nat. Biotechnol. 2013;31:965. doi: 10.1038/nbt.2737. [DOI] [PubMed] [Google Scholar]
- 43.Snow J.W., Hale A.E., Isaacs S.K. Ineffective delivery of diet-derived microRNAs to recipient animal organisms. RNA Biol. 2013;10:1107–1116. doi: 10.4161/rna.24909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H., Davis C.D., Wang T.T.Y. Extensive degradation and low bioavailability of orally consumed corn miRNAs in mice. Nutrients. 2018;10:215. doi: 10.3390/nu10020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastrello C., Tsay M., McQuaid R. Retraction: circulating plant miRNAs can regulate human gene expression in vitro. Sci. Rep. 2017;7:46826. doi: 10.1038/srep46826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tosar J.P., Rovira C., Naya H., Cayota A. Mining of public sequencing databases supports a non-dietary origin for putative foreign miRNAs: underestimated effects of contamination in NGS. RNA. 2014;20:754–757. doi: 10.1261/rna.044263.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang W., Bang-Berthelsen C.H., Holm A. Survey of 800+ data sets from human tissue and body fluid reveals xenomiRs are likely artifacts. RNA. 2017;23:433–445. doi: 10.1261/rna.059725.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heintz-Buschart A., Yusuf D., Kaysen A. Small RNA profiling of low biomass samples: identification and removal of contaminants. BMC Biol. 2018;16:52. doi: 10.1186/s12915-018-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fromm B., Tosar J.P., Lu Y. Human and cow have identical miR-21-5p and miR-30a-5p sequences, which are likely unsuited to study dietary uptake from cow milk. J. Nutr. 2018;148:1506–1507. doi: 10.1093/jn/nxy144. [DOI] [PubMed] [Google Scholar]
- 50.Witwer K.W. Alternative miRNAs? Human sequences misidentified as plant miRNAs in plant studies and in human plasma. F1000Research. 2018;7:244. doi: 10.12688/f1000research.14060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Witwer K.W., Zhang C.-.Y. Diet-derived microRNAs: unicorn or silver bullet? Genes Nutr. 2017;12:15. doi: 10.1186/s12263-017-0564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voinnet O., Baulcombe D.C. Systemic signalling in gene silencing. Nature. 1997;389:553. doi: 10.1038/39215. [DOI] [PubMed] [Google Scholar]
- 53.Hansen L.L., Nielsen M.E. Plant exosomes: using an unconventional exit to prevent pathogen entry? J. Exp. Bot. 2018;69:59–68. doi: 10.1093/jxb/erx319. [DOI] [PubMed] [Google Scholar]
- 54.Marchant R., Robards A.W. Membrane systems associated with the plasmalemma of plant cells. Ann. Bot. 1968;32:457–471. [Google Scholar]
- 55.Halperin W., Jensen W.A. Ultrastructural changes during growth and embryogenesis in carrot cell cultures. J Ultrasructure Res. 1967;18:428–443. doi: 10.1016/s0022-5320(67)80128-x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M., Viennois E., Prasad M. Edible ginger-derived nanoparticles: a novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang M., Wang X., Han M.K. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12:1927–1943. doi: 10.2217/nnm-2017-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao J., Feng S., Wang X. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6 doi: 10.7717/peerj.5186. e5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teng Y., Ren Y., Sayed M. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–652. doi: 10.1016/j.chom.2018.10.001. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fromm B., Kang W., Rovira C. Plant microRNAs in human sera are likely contaminants. J Nutr Biochem. 2018 doi: 10.1016/j.jnutbio.2018.07.019. Sep 5:S0955. [DOI] [PubMed] [Google Scholar]
- 61.Cavallini A., Minervini F., Garbetta A. High degradation and no bioavailability of artichoke miRNAs assessed using an in vitro digestion/Caco-2 cell model. Nutr. Res. 2018;60:68–76. doi: 10.1016/j.nutres.2018.08.007. [DOI] [PubMed] [Google Scholar]