Abstract
Introduction:
Eicosanoids are biological lipids that serve as both activators and suppressors of inflammation. Eicosanoid pathways are implicated in synovitis and joint destruction in inflammatory arthritis, yet they might also have a protective function, underscoring the need for a comprehensive understanding of how eicosanoid pathways might be imbalanced. Until recently, sensitive and scalable methods for detecting and quantifying a high number of eicosanoids have not been available.
Objective:
Here, we intend to describe a detailed eicosanoid profiling in patients with psoriatic arthritis (PsA) and evaluate correlations with parameters of disease activity.
Methods:
Forty-one patients with PsA, all of whom satisfied the CASPAR classification criteria for PsA, were studied. Outcomes reflecting the activity of peripheral arthritis as well as skin psoriasis, Disease Activity Score (DAS)28, Clinical Disease Index (CDAI) and Body Surface Area (BSA) were assessed. Serum eicosanoids were determined by LC-MS, and the correlation between metabolite levels and disease scores was evaluated.
Results:
Sixty-six eicosanoids were identified by reverse-phase LC/MS. Certain eicosanoids species including several pro-inflammatory eicosanoids such as PGE2, HXB3 or 6,15-dk,dh,PGF1a correlated with joint disease score. Several eicosapentaenoic acid (EPA)-derived eicosanoids, which associate with anti-inflammatory properties, such as 11-HEPE, 12-HEPE and 15-HEPE, correlated with DAS28 (Disease Activity Score) and CDAI (Clinical Disease Activity Index) as well. Of interest, resolvin D1, a DHA-derived anti-inflammatory eicosanoid, was down-regulated in patients with high disease activity.
Conclusion:
Both pro- and anti-inflammatory eicosanoids were associated with joint disease score, potentially representing pathways of harm as well as benefit. Further studies are needed to determine whether these eicosanoid species might also play a role in the pathogenesis of joint inflammation in PsA.
Keywords: eicosanoids, lipidomics, biomarkers, psoriatic arthritis
Background
Psoriatic arthritis (PsA) is a heterogeneous disorder with activity and severity ranging from mild synovitis to progressive debilitating erosive disease (Gravallese and Schett, 2018; Ritchlin et al., 2017). The pathophysiology of PsA involves chronic inflammation mediated by pro-inflammatory cytokines, including TNF, IL-17 and IL-23 (Gravallese and Schett, 2018; Ritchlin et al., 2017). It has become clear that many of the signaling pathways triggered by inflammatory cytokines that are activated during inflammation have a profound effect on core lipid metabolism of cells, and the study of the lipidome/metabolome has been successful in identifying new pathogenic targets (Johnson et al., 2016).
Although they represent only a small fraction of the total fatty acids in plasma, free fatty acids are highly metabolically active lipids and they also include the polyunsaturated fatty acids (PUFA): arachidonic acid (AA), linoleic acid (LA) and the nutritionally essential α-linolenic acid (α-LA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Quehenberger et al., 2010; Quehenberger and Dennis, 2011). Eicosanoids are a class of bioactive lipid mediators derived from the metabolism of AA and related PUFAs by cyclooxygenases (COX), lipoxygenases (LOX), cytochrome P450s, or non-enzymatic pathways (Buczynski et al., 2009). In general, AA gives rise to pro-inflammatory eicosanoids whereas α-LA, EPA and DHA give rise to anti-inflammatory eicosanoids (Buczynski et al., 2009). This class of lipids has been extensively studied over the years due to its implication in the inflammatory response. Some studies suggest that an alteration in AA metabolism is seen in the form of increased formation of pro-inflammatory eicosanoids and decreased production of PUFA-derived anti-inflammatory lipoxins, resolvins, protectins and maresins in several inflammatory diseases (Dennis and Norris, 2015; Serhan, 2014).
Until recently, sensitive and scalable methods for detecting and quantifying a large number of eicosanoids have not been available. Advances in mass spectrometry (MS) analytics now allow for high-throughput rapid and reliable quantification of more than 150 eicosanoids, both pro-and anti-inflammatory, in human plasma. This new technology addresses the question of how eicosanoid pathways may be active and involved in PsA pathogenesis. More important, it allows a more comprehensive, and detailed understanding of how eicosanoid pathways are active, imbalanced, and perturbed.
In this study, we describe the eicosanoid profiling in patients with PsA and its association with joint inflammation. We show that certain pro-inflammatory eicosanoids, such as PGE2, HXB3 or 6,15-dk,dh,PGF1a, but also some EPA-derived anti-inflammatory eicosanoids, such as 11-HEPE, 12-HEPE and 15 -HEPE, correlated with joint disease. Of interest, resolvin D1, a DHA-derived anti-inflammatory eicosanoid, was down-regulated in patients with high disease activity. These results suggest that an imbalance of pro- and anti-inflammatory eicosanoids might be associated with clinical inflammation in PsA patients and provide a foundation for more focused investigations into novel eicosanoid-based interventions to reduce PsA-related morbidity.
Patients and methods
Patient selection and assessment.
41 adult patients with PsA fulfilling the classification for PsA (CASPAR) criteria were approached in University of California San Diego (UCSD) Arthritis Clinics for opportunistic sampling. The study was approved by the UCSD Institutional Review Board. Clinical assessment included the following: evaluation of the number of tender (TJC) and swollen joints (SJC) (out of 28); the number of tender entheseal sites; the percentage of the body surface affected by psoriasis; functional status as assessed by Health Assessment Questionnaire (HAQ); and assessments of pain; fatigue; global disease severity by patients; and a global assessment of disease by physicians, using a Visual Analogue Scale ranging from 0 to 10. Composite measures of peripheral arthritis were calculated using the above measures: Disease Assessment Score using a 28 joint count and C reactive protein (DAS28-CRP), Clinical Disease Activity Index (CDAI) and Simple Disease Activity Index (SDAI). Non-fasting blood samples were collected in the clinic by research personnel into 10 ml BD Vacutainer Blood Collection Tubes containing spray-coated silica and a polymer gel for serum separation. After 30 min incubation at room temperature, tubes were centrifuged for 10 min at 2000×g and sera were transferred into 1.7ml tubes and immediately frozen and stored at −80 °C until analysis.
Lipid extraction
All sera samples were stored at −80°C, thawed once, and immediately used for free fatty acid and eicosanoid isolation as described (Wang et al., 2014). Briefly, 50 µl sera was spiked with a cocktail of 26 deuterated internal standards that also included some selected PUFAs (individually purchased from Cayman Chemicals, Ann Arbor, MI) and brought to a volume of 1 ml with 10% methanol. The samples were then purified by solid phase extraction on Strata-X columns (Phenomenex, Torrance, CA), using an activation procedure consisting of consecutive washes with 3 ml of 100% methanol followed by 3 ml of water. The eicosanoids were then eluted with 1 ml of 100% methanol, and the eluent was dried under vacuum, dissolved in 50 µl of buffer A (consisting of water-acetonitril-acetic acid, 60:40:0.02 (v/v/v)), and immediately used for analysis).
LC-MS measure of eicosanoids
Eicosanoids in sera were analyzed and quantified by LC/MS/MS as previously described (Quehenberger et al., 2010; Wang et al., 2014). The methods used are in line with the proposed minimum reporting standards published in 2007 by Sumner et al (Sumner et al., 2007). Briefly, eicosanoids were separated by reverse-phase chromatography using a 1.7 μm 2.1 × 100 mm BEH Shield Column (Waters, Milford, MA) and an Acquity UPLC system (Waters). The column was equilibrated with buffer A, and 10 µl of sample was injected via the autosampler. Samples were eluted with a step gradient starting with 100% buffer A for 1 min, then to 50% buffer B (consisting of 50% acetonitril, 50% isopropanol, and 0.02% acetic acid) over a period of 3 min, and then to 100% buffer B over a period of 1 min. The LC was interfaced with an IonDrive Turbo V ion source, and mass spectral analysis was performed on a triple quadrupole AB SCIEX 6500 QTrap mass spectrometer (AB SCIEX, Framingham, MA). Eicosanoids were measured using electrospray ionization in negative ion mode and multiple reaction monitoring (MRM), using the most abundant and specific precursor ion/product ion transitions to build an acquisition method capable of detecting 158 analytes and 26 internal standards. The ionspray voltage was set at −4,500 V at a temperature of 550°C. Collisional activation of the eicosanoid precursor ions was achieved with nitrogen as the collision gas with the declustering potential, entrance potential, and collision energy optimized for each metabolite. Eicosanoids were identified by matching their MRM signal and chromatographic retention time with those of pure identical standards.
Eicosanoids and free fatty acids were quantitated by the stable isotope dilution method. Briefly, identical amounts of deuterated internal standards were added to each sample and to all the primary standards used to generate standard curves. To calculate the amount of eicosanoids and free fatty acids in a sample, ratios of peak areas between endogenous metabolite and matching deuterated internal standards were calculated. Ratios were converted to absolute amounts by linear regression analysis of standard curves generated under identical conditions (Loomba et al., 2015). Eicosanoid levels are shown as pmol/ml.
Data Analysis
The data, consisting of 41 patient samples measured across clinical outcomes were processed using R, Version 3.4.1. (www.r-project.org). Continuous variables were expressed as mean ± standard deviation (SD) and the categorical variables as percentage. Pearson correlation was used to check correlation between disease activity scores. T-test was used to analyze statistically significant difference between the means of two groups, and the comparisons were then adjusted for BMI, therapy, and disease activity, by including the latter as covariates in a logistic regression model. Hierarchically clustered heatmaps were generated for correlations between each eicosanoid and clinical outcomes. Dendrograms were divided into flat clusters using a cophenetic distance metric. Linear regression was performed between each eicosanoid metabolite- clinical outcome pair, controlling for patient BMI, therapy, and disease activity using the Ordinary Least Square method. Normally distributed independent variables were standardized to a mean of 0 and a standard deviation of 1. Bejamini-Hochberg method was used to adjust for multiple comparisons.
Results
Patient demographics and disease characteristics
Patient characteristics are summarized in Table 1. Of the 41 PsA patients included in this study, 61% were males (N=25) and the mean age was 49.2±11.1 years. The mean number of tender joints and swollen joints were 1.7 ± 3 (range 0 to 10) and 2.1 ± 3.2 (range 0 to 10) respectively. The average DAS28-CRP score was 2.72 ± 1.3 (range 1–5.2). Twenty-seven patients (71%) had active skin disease, with an average BSA of 7.3 ± 15.7 (range 0–95%). Eight patients had enthesitis. The average body mass index (BMI) was 27.4±5.5. Sixty-five percent were receiving biological therapy, 56.5% of them in association with a synthetic disease modifying antirheumatic drug (sDMARDs), mostly methotrexate. 14.6% received sDMARDs as monotherapy, and 29.2% were on daily non-steroidal anti-inflammatory drugs (NSAIDs). Comorbidities are also summarized in Table 1.
Table 1.
Demographic and disease characteristics of patients included in our study
| Characteristic | Percentage/Mean (Standard Deviation) |
|---|---|
| Male | 61% |
| Age | 49.2 (11.1) |
| BMI | 27.4 (5.5) |
| Duration of PsA in years | 11.3 (10) |
| Duration of psoriasis in years | 18.4 (14.1) |
| Number of tender joints | 1.7 (range 0–10) |
| Number of swollen joints | 2.09 (range 0–10) |
| Pain | 2.8 (2.8) |
| Fatigue | 3.3 (3.3) |
| HAQ | 0.45 (0.55) |
| DAS28-CRP | 2.72 (1.28) |
| CDAI | 9.1 (9.6) |
| SDAI | 10.84 (10.25) |
| Enthesitis | 19.5% |
| Body surface affected by psoriasis (BSA) | 7.3 (range 0–95) |
| Treatment with TNF inhibitors | 56% |
| Diabetes Mellitus type 2 | 17% |
| High Blood Pressure | 44% |
| Dyslipidemia | 29.2% |
Eicosanoid profiling and clustering
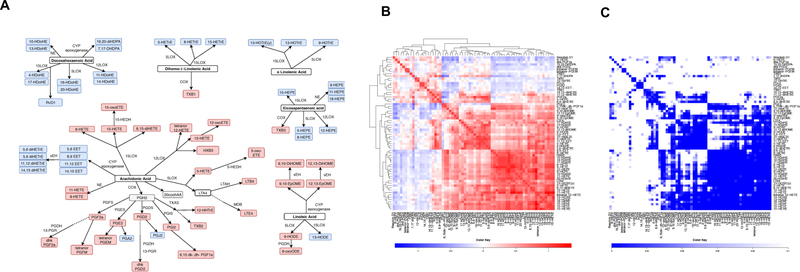
Sixty-six eicosanoids, which are derived from AA, EPA, LA, DGLA, αLA and DHA, were identified by Reverse-phase LC/MS. Among these, 18 (22.38%) belong to the cyclooxygenase (COX) pathway, 12 (11.94%) belong to the 5 lipoxygenase (LOX) pathway, 13 (23.88%) belong to the 15LOX pathway, 7 (13.43%) belong to the 12LOX pathway, 14 (20.89%) belong to the CYP pathway and the rest (2) are synthetized by non-enzymatic pathways. Twenty-seven of the detected eicosanoids are considered pro-inflammatory and 39 are anti-inflammatory species. Figure 1A shows the pro- (in red) and anti-inflammatory (in blue) eicosanoids and the different enzymatic eicosanoid pathways of the eicosanoids detected in our samples. We also analyzed eicosanoid clustering (Figure 1B, C). Concentrations of the circulating eicosanoids detected in our samples are shown in Table 2.
Fig. 1. Pro-inflammatory and anti-inflammatory eicosanoids.
A) Pro-inflammatory and anti-inflammatory eicosanoids detected in our patients, divided by pathways and origin PUFA. In grey, metabolites not identified by MS COX – cyclooxygenase; LOX – lypooxigenase; CYP – cytochrome P450; NE – non-enzymatic; PGFS – prostaglandin F synthase; PGES – prostaglandin E synthase; PGDS – prostaglandin D synthase; PGIS – prostaglandin I synthase; TXAS – thromboxane A2 synthase; LTAH – leukotriene A4 hydrolase; MDB – membrane dipeptidase; HEDH - hydroxyeicosanoid dehydrogenase; PGDH - hydroxyprostaglandin dehydrogenase; 13-PGR – 15-ketoprostaglandin∆13 reductase; sEH – soluble epoxide hydrolase. B) Heat map and hierarchical cluster analysis indicate positive relationships between circulating eicosanoids identified in blood from PsA patients (the heatmap was created using regression coefficients adjusted for BMI, BSA, NSAIDs and biological therapy. C) Eicosanoid regression p-values are displayed in C, where the row and column order are preserved from B
Table 2.
Mean (SD) of each circulating eicosanoid detected in 41 PsA patients
| Eicosanoid | Mean (SD) pmol/ml |
|---|---|
| TxB2 | 27.332 (32.049) |
| PGF2a | 1.269 (1.286) |
| PGE2 | 1.029 (1.232) |
| 6,15dk-,dh-PGF1a | 5.435 (6.886) |
| dhk PGF2a | 0.813 (1.018) |
| dhk PGD2 | 13.341 (5.663) |
| tetranor-PGFM | 1.609 (3.016) |
| tetranor-PGEM | 26.919 (63.712) |
| 9-HETE | 9.747 (13.861) |
| TXB1 | 0.278 (0.424) |
| TXB3 | 0.133 (0.244) |
| LTB4 | 0.31 (0.455) |
| LTE4 | 0.139 (0.182) |
| 5-HETE | 5.242 (6.475) |
| 5-oxoETE | 0.203 (0.505) |
| 9-HODE | 21.767 (20.02) |
| 9-oxoODE | 5.177 (4.807) |
| 12-HETE | 581.357 (988.653) |
| 12-oxoETE | 3.246 (7.153) |
| HXB3 | 7.054 (7.689) |
| 8-HETE | 7.764 (12.175) |
| 8,15-diHETE | 0.619 (1.171) |
| 15-oxoETE | 0.349 (0.852) |
| 9,10 EpOME | 4.258 (2.809) |
| 12,13 EpOME | 5.052 (4.786) |
| 9,10 diHOME | 8.081 (8.241) |
| 12,13 diHOME | 8.804 (7.916) |
| PGA2 | 0.263 (0.595) |
| PGJ2 | 0.127 (0.229) |
| 12-HHTrE | 71.954 (76.621) |
| 11-HETE | 5.961 (8.577) |
| 9-HEPE | 3.316 (5.152) |
| 11-HEPE | 0.67 (1.049) |
| 13 HDoHE | 0.43 (0.993) |
| 5-HEPE | 1.059 (2.126) |
| 8-HEPE | 2.999 (2.862) |
| 9-HOTrE | 1.656 (1.477) |
| 5-HETrE | 0.176 (0.463) |
| 16 HDoHE | 4.736 (6.834) |
| 20 HDoHE | 2.585 (4.27) |
| tetranor 12-HETE | 14.622 (38.727) |
| 12-HEPE | 32.511 (45.631) |
| 11 HDoHE | 6.285 (9.566) |
| 14 HDoHE | 109.937 (162.784) |
| 15-HETE | 14.395 (21.968) |
| 15-HEPE | 0.877 (1.484) |
| 13-HODE | 53.761 (64.177) |
| 13-HOTrE | 3.588 (5.313) |
| 13-HOTrE(y) | 0.795 (1.427) |
| 8-HETrE | 0.841 (1.184) |
| 15-HETrE | 1.903 (2.789) |
| 4 HDoHE | 0.529 (1.323) |
| 17 HDoHE | 4.201 (11.148) |
| Resolvin D1 | 0.273 (0.269) |
| 5,6-EET | 0.113 (0.276) |
| 8,9-EET | 2.294 (5.015) |
| 11,12-EET | 0.127 (0.335) |
| 14,15-EET | 0.215 (0.397) |
| 5,6-diHETrE | 1.894 (3.549) |
| 8,9-diHETrE | 0.983 (1.393) |
| 11,12-diHETrE | 0.822 (0.337) |
| 14,15-diHETrE | 1.181 (0.41) |
| 19,20 DiHDPA | 1.55 (0.735) |
| 7,17 dHDPA | 1.073 (0.782) |
| 18-HEPE | 0.546 (0.768) |
| 10 HDoHE | 2.03 (3.004) |
Linear regression analysis between grouped serum eicosanoids and clinical parameters of PsO and PsA.
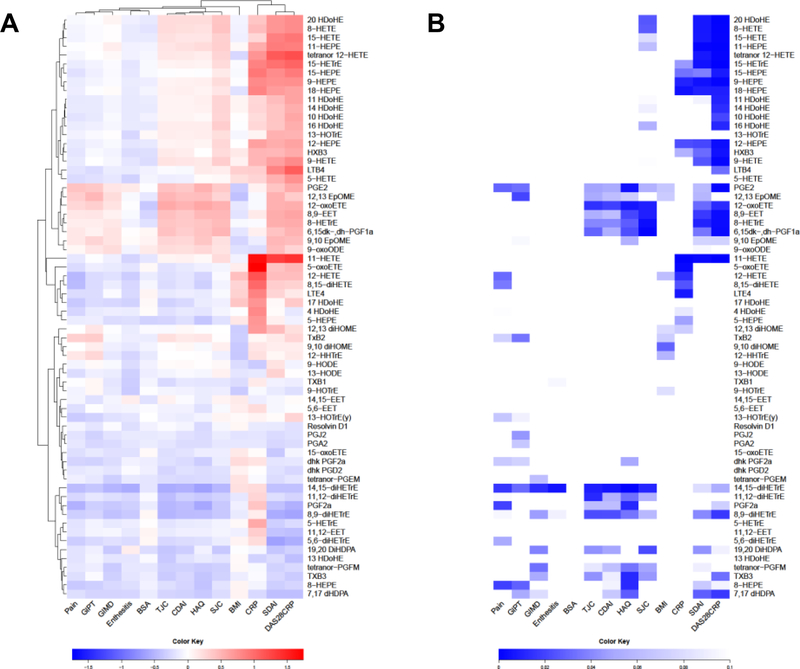
Linear regression was performed between each eicosanoid-clinical parameter pair, controlling for BMI, NSAIDs and biological therapy, by including these factors as covariates in the model. We also adjusted joint disease scores (TJC, SJC, DAS28, CDAI and SDAI) for BSA, and BSA score was also adjusted for CDAI. The regression coefficients for each eicosanoid-clinical parameter pair were used to form a clustered heatmap to lend insight into which groups of eicosanoids were correlated with which clinical parameters (Figure 2A). p-values and q-values of the regression analysis of metabolite levels are displayed in Figure 2B and Supplementary Figure 1A, where the row and column order are preserved from Figure 2A. Several eicosanoids positively correlated with parameters of joint disease activity (tender joint count -TJC-, swollen joint count -SJC-, CDAI, SDAI and DAS28-CRP) including several pro-inflammatory eicosanoid such as PGE2, 12-oxo-HETE, HXB3 or 6,15-dk,dh,PGF1a, but interestingly also several anti-inflammatory eicosanoids such as 11-HEPE, 12-HEPE and 15-HEPE among others. Interestingly, we also detected some eicosanoids that negatively correlated with joint disease activity including some anti-inflammatory eicosanoids such as 8,9-diHETrE, 11,12-diHETrE,14,15-diHETrE, 19,20-diHDPA and 7,17 DHDPA. Other clinical scores that cluster together were patients’ clinical scores (pain, HAQ, fatigue and global patient score), with a similar eicosanoid profiling as CDAI, TJC and TJC.
Fig. 2. Clinical score correlations with eicosanoids.
A) Linear regression was performed between each clinical score – eicosanoid pair, controlling for BMI, NSAIDs and biological therapy. We also adjusted joint disease scores (TJC, SJC, DAS28, CDAI and SDAI) for BSA, and BSA score was also adjusted for CDAI. Other factors, including comorbidities, gender and age were not found to influence eicosanoid levels and were not included in the model. The regression coefficients for each pair were used to form a clustered heatmap, to lend insight into which clinical scores were correlated with which groups of eicosanoids. Row clusters have been identified by cophenetic cutting of the row dendrogram. B) Metabolite regression p-values are displayed in Figure 2B, where the row and column order are preserved from Figure 2A TJC: tender joint count; SJC: swollen joint count; CDAI: clinical Disease Activity Index; SDAI: simple Disease Activity Index; HAQ: Health Assessment Questionnaire; GIMD: physician global assessment; GIPT: patient global assessment; BMI: body mass index; BSA: body surface area; DAS28CRP: disease assessment score 28; NSAIDS: non-steroidal anti-inflammatory drugs
Logistic regression analysis between grouped serum eicosanoids and DAS28CRP
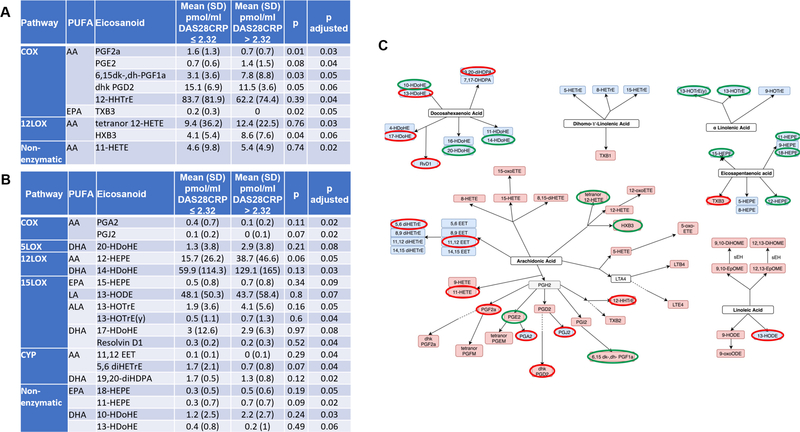
We further analyzed whether some eicosanoids could differentiate between high and low disease activity scores. Figure 3A, B and Supplemental Table S1 and Figure S1B show logistic regression analysis adjusted by BMI, BSA, NSAIDs and biological therapy of each eicosanoid in patients in remission or with low disease activity (DAS28CRP ≤ 2.32), compared to patients with high disease activity (DAS28CRP > 2.32). Figure 3C shows the significant eicosanoids within the different PUFA and enzymatic pathways. Of interest, while most of the EPA-derived anti-inflammatory eicosanoids were elevated in the high disease activity, some of the DHA- anti-inflammatory eicosanoid, including resolvin D1 and 17-HDoHE, were down-regulated in these group of patients.
Fig. 3. Pro- and anti-inflammatory eicosanoids associated with DAS28-CRP.
Logistic regression was performed between each eicosanoid (pmol/ml) in patients with DAS ≤ 2.32 compared with patients with DAS > 2.32. A) Pro-inflammatory eicosanoids with p value < 0.1 after adjusting for BMI, BSA, NSAIDs and biological therapy. B) Anti-inflammatory eicosanoids with p value < 0.1 after adjusting for BMI, BSA, NSAIDs and biological therapy. Other factors, including comorbidities, gender and age were not found to influence eicosanoid levels and were not included in the model. C) Significant eicosanoids (p < 0.1) are circled in green if upregulated in patients with DAS > 2.32, and in red if downregulated in these patients. DAS28: disease activity score; NSAIDs: non-steroidal anti-inflammatory drugs
Discussion
Data regarding the role of eicosanoids in PsA is very scarce, as most of the research is associated with rheumatoid arthritis (RA) pathogenesis. Evidence that the COX pathway might be involved in the pathogenesis of RA dates back to the 1970s, when elevated prostaglandin levels were reported in synovial fluid from patients with RA (Korotkova and Jakobsson, 2014). Since then, studies in animals and patients have established a pivotal and complex role of prostaglandins and other eicosanoids in RA. For instance, expression of 5LOX and 15LOX was also increased in rheumatoid synovium (Gheorghe et al., 2009). LTB4, a metabolite of AA via 5LOX, has been related to inflammation in arthritis for a long time. Two independent studies demonstrated that LTB4 and BLT1 receptors are indispensable for the development of arthritis in the K/BxN model of RA (Chen et al., 2006; Kim et al., 2006). Another study demonstrated that inhibition of 5LOX in fibroblast-like synovioctyes (FLS) and knocking out 5LOX gene in a mouse model of RA decreased inflammatory cytokines expression and paw inflammation (Lin et al., 2014). More importantly, in RA, despite the wide range of sDMARDs and biological therapies that potently suppress specific inflammatory mechanisms, the COX and LOX pathways remain overexpressed in inflamed tissue in some patients with RA, which might contribute to subclinical inflammation and disease relapse (Korotkova and Jakobsson, 2014).
In PsA, pharmacological therapies begin with NSAIDs, suggesting that at least COX pathway is critical in PsA pathogenesis. However, the role of LOX and cytochrome P450 (CYP) enzymes, which catalyze several AA-derived pro-inflammatory eicosanoids and most of the PUFA-derived anti-inflammatory eicosanoids, is unknown in this disease. In our study, several eicosanoids were positively correlated with parameters of joint disease activity (tender joint count -TJC-, swollen joint count -SJC-, CDAI, SDAI and DAS28CRP) including several AA-derived pro-inflammatory eicosanoids such as PGE2, HXB3 or 6,15-dk,dh,PGF1a, being HXB3 and tetranor 12-HETE, 12LOX derived eicosanoids. More interestingly several EPA-derived anti-inflammatory eicosanoids, such as 11-HEPE, 12-HEPE and 15-HEPE, were also upregulated in active patients, suggesting that EPA-derived anti-inflammatory eicosanoids amount might be increased to try to balance the inflammation induced by AA-derived pro-inflammatory eicosanoids. Yet, we also detected some DHA-derived anti-inflammatory eicosanoids that did negatively correlate with joint disease activity, including anti-inflammatory eicosanoids such as resolvin (Rv)D1 and 17-HDoHE. RvD1 is one of the pro-resolving lipid mediators shown to actively contribute to the resolution of several inflammatory processes (Serhan, 2014). The injection of RvD3 reduced joint leukocytes as well as paw joint eicosanoids, clinical scores and edema in the K/BxN serum transfer inflammatory arthritis model (Arnardottir et al., 2016). Resolvins are also potential analgesics: systemic treatment with 17-HDoHE, the precursor of RvD, reduced inflammatory pain in an adjuvant-induced arthritis model (Xu and Ji, 2011). The fact that we detected a negative correlation between these pro-resolving lipids mediators and joint activity could suggest that a disbalance between pro- and anti- inflammatory eicosanoid species might play a role in the pathogenesis of joint inflammation in PsA and that these could be used for the treatment of inflammatory arthritis.
Although these findings are certainly promising, this study is not without limitations, the heterogeneity of our PsA patients being the main limitation. Patients had long-standing disease and were exposed to various therapies prior to the study which could represent activated pathways resistant to current DMARDs and biological therapies. Confirmation of our results with a larger sample size from prospective cohorts of patients with new onset inflammatory arthritis before treatment initiation is necessary to strengthen our conclusions. Comparison with other arthritides would help to determine if the described eicosanoid changes are specific to PsA or secondary to systemic inflammation. Yet, we believe that this work can improve our limited understanding of role of eicosanoids beyond arachidonic acid metabolites and leukotrienes in PsA and may lay the groundwork for a more targeted investigation of novel eicosanoid- and lipidomics-based studies in PsA.
Conclusions
Sensitive and scalable methods for detecting and quantifying a high number of eicosanoids are now available. In this study 66 eicosanoids were identified, and both pro- and anti-inflammatory eicosanoids were associated with joint disease score, potentially representing pathways of harm as well as benefit. Further studies are needed to determine whether these eicosanoid species might also play a role in the pathogenesis of joint inflammation in PsA.
Supplementary Material
Supplementary Table 1: Eicosanoids associated with DAS28. T-test and then logistic regression was performed between each eicosanoid in patients with DAS ≤ 2.32 compared with patients with DAS > 2.32. Pro-inflammatory (A) and anti-inflammatory (B) eicosanoids (pmol/ml) after adjusting for BMI, BSA, NSAIDs and biological therapy are shown in this table.
Supplementary Figure 1: A) Linear regression was performed between each clinical score –eicosanoid pair, controlling for BMI, NSAIDs and biological therapy. We also adjusted joint disease scores (TJC, SJC, DAS28, CDAI and SDAI) for BSA, and BSA score was also adjusted for CDAI. This figure displays metabolite regression adjusted q-values after applying Benjamini-Hochberg false discovery rate to correct for multiple testing. B) Logistic regression was performed for each eicosanoid (pmol/ml) in patients with DAS ≤ 2.32 compared with patients with DAS > 2.32 adjusted for BMI, BSA, NSAIDs and biological therapy. This figure displays metabolite regression adjusted p-values after applying Benjamini-Hochberg false discovery rate to correct for multiple testing. TJC: tender joint count; SJC: swollen joint count; CDAI: clinical Disease Activity Index; SDAI: simple Disease Activity Index; HAQ: Health Assessment Questionnaire; GIMD: physician global assessment; GIPT: patient global assessment; BMI: body mass index; BSA: body surface area; DAS28CRP: disease assessment score 28; NSAIDS: non-steroidal anti-inflammatory drugs.
Acknowledgments
FUNDING SOURCES
Research reported in this publication was supported by the National Institutes of Health under Award Numbers K08AR064834, R03AR068094 and UL1TR000100 to MG, R01ES027595 and S10OD02002 to MJ, and P30DK063491 to OQ. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research was also supported by grant from Spanish Society of Rheumatology to RC.
ABBREVIATIONS
- (PsA)
Psoriatic arthritis
- (RA)
Rheumatoid arthritis
- (DAS)28
Disease Activity Score
- (CDAI)
Clinical Disease Index
- (BSA)
Body Surface Area
- (EPA)
Eicosapentaenoic acid
- (PUFA)
Polyunsaturated fatty acids
- (AA)
Arachidonic acid
- (LA)
Linolenic acid
- (α-LA)
α-linolenic acid
- (DHA)
docosahexaenoic acid
- (COX)
cyclooxygenases
- (LOX)
lipoxygenases
- (SD)
standard deviation
- (sDMARDs)
Synthetic disease modifying antirheumatic drug
- (NSAIDs)
Non-steroidal anti-inflammatory drugs
- (TJC)
Tender joints
- (SJC)
Swollen joints
- (HAQ)
Health Assessment Questionnaire
- (FLS)
Fibroblast-like synovioctyes
- (CRP)
C-reactive protein
Footnotes
DECLARATIONS
ETHICAL APPROVAL
Patients were enrolled following written informed consent. Ethical approval was granted by the Institutional Review Board (IRB) at UCSD.
CONSENT FOR PUBLICATION:
N/A
AVAILABILITY OF DATA AND MATERIALS
All data generated or analyzed during this study are included in this published article
COMPETING INTERESTS
Roxana Coras declares that she has no conflict of interest.
Arthur Kavanaugh declares that he has no conflict of interest.
Tristan Boyd declares that he has no conflict of interest.
Quyen Huynh declares that she has no conflict of interest.
Brian Pedersen declares that he has no conflict of interest.
Aaron M Armando declares that he has no conflict of interest.
Signe Dahlberg-Wright declares that she has no conflict of interest.
Sara Marsal declares that she has no conflict of interest.
Mohit Jain declares that he has no conflict of interest.
Taraneh Paravar declares that she has no conflict of interest.
Oswald Quehenberger declares that he has no conflict of interest.
Monica Guma declares that she has no conflict of interest.
The authors report no conflict of interest.
REFERENCES
- Arnardottir HH, Dalli J, Norling LV, Colas RA, Perretti M and Serhan CN (2016) Resolvin D3 Is Dysregulated in Arthritis and Reduces Arthritic Inflammation. J Immunol 197, 2362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Dumlao DS and Dennis EA (2009) Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J Lipid Res 50, 1015–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF and Lee DM (2006) Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med 203, 837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA and Norris PC (2015) Eicosanoid storm in infection and inflammation. Nat Rev Immunol 15, 511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheorghe KR, Korotkova M, Catrina AI, Backman L, af Klint E, Claesson HE, Radmark O and Jakobsson PJ (2009) Expression of 5-lipoxygenase and 15-lipoxygenase in rheumatoid arthritis synovium and effects of intraarticular glucocorticoids. Arthritis Res Ther 11, R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese EM and Schett G (2018) Effects of the IL-23-IL-17 pathway on bone in spondyloarthritis. Nat Rev Rheumatol [DOI] [PubMed]
- Johnson CH, Ivanisevic J and Siuzdak G (2016) Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 17, 451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ND, Chou RC, Seung E, Tager AM and Luster AD (2006) A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med 203, 829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova M and Jakobsson PJ (2014) Persisting eicosanoid pathways in rheumatic diseases. Nat Rev Rheumatol 10, 229–41. [DOI] [PubMed] [Google Scholar]
- Lin HC, Lin TH, Wu MY, Chiu YC, Tang CH, Hour MJ, Liou HC, Tu HJ, Yang RS and Fu WM (2014) 5-Lipoxygenase inhibitors attenuate TNF-alpha-induced inflammation in human synovial fibroblasts. PLoS One 9, e107890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Quehenberger O, Armando A and Dennis EA (2015) Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res 56, 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E and Dennis EA (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51, 3299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quehenberger O and Dennis EA (2011) The human plasma lipidome. N Engl J Med 365, 1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchlin CT, Colbert RA and Gladman DD (2017) Psoriatic Arthritis. N Engl J Med 376, 957–970. [DOI] [PubMed] [Google Scholar]
- Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ and Viant MR (2007) Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Armando AM, Quehenberger O, Yan C and Dennis EA (2014) Comprehensive ultra-performance liquid chromatographic separation and mass spectrometric analysis of eicosanoid metabolites in human samples. J Chromatogr A 1359, 60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ and Ji RR (2011) Resolvins are potent analgesics for arthritic pain. Br J Pharmacol 164, 274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Eicosanoids associated with DAS28. T-test and then logistic regression was performed between each eicosanoid in patients with DAS ≤ 2.32 compared with patients with DAS > 2.32. Pro-inflammatory (A) and anti-inflammatory (B) eicosanoids (pmol/ml) after adjusting for BMI, BSA, NSAIDs and biological therapy are shown in this table.
Supplementary Figure 1: A) Linear regression was performed between each clinical score –eicosanoid pair, controlling for BMI, NSAIDs and biological therapy. We also adjusted joint disease scores (TJC, SJC, DAS28, CDAI and SDAI) for BSA, and BSA score was also adjusted for CDAI. This figure displays metabolite regression adjusted q-values after applying Benjamini-Hochberg false discovery rate to correct for multiple testing. B) Logistic regression was performed for each eicosanoid (pmol/ml) in patients with DAS ≤ 2.32 compared with patients with DAS > 2.32 adjusted for BMI, BSA, NSAIDs and biological therapy. This figure displays metabolite regression adjusted p-values after applying Benjamini-Hochberg false discovery rate to correct for multiple testing. TJC: tender joint count; SJC: swollen joint count; CDAI: clinical Disease Activity Index; SDAI: simple Disease Activity Index; HAQ: Health Assessment Questionnaire; GIMD: physician global assessment; GIPT: patient global assessment; BMI: body mass index; BSA: body surface area; DAS28CRP: disease assessment score 28; NSAIDS: non-steroidal anti-inflammatory drugs.