Abstract
Minimally invasive point-of-care diagnostic devices are of great interest for rapid detection of biomarkers in diverse settings. Although blood is the most common source of biomarkers, interstitial fluid (ISF) is an alternate body fluid that does not clot or contain red blood cells that often complicate analysis. However, ISF is difficult to collect. In this study, we assessed the utility of a microneedle patch to sample microliter volumes of ISF in a simple and minimally invasive manner. We demonstrated the use of ISF collected in this way for therapeutic drug monitoring by showing similar vancomycin pharmacokinetic profiles in ISF and serum from rats. We also measured polio-specific neutralizing antibodies and anti-polio IgG in ISF similar to serum in rats immunized with polio vaccine. These studies demonstrate the potential utility of ISF collected by microneedle patch in therapeutic drug monitoring and immunodiagnostic applications.
Keywords: Interstitial fluid, Microneedle patch, Biomarker, Vancomycin, Polio specific antibodies, Therapeutic drug monitoring
1. Introduction
Rapid disease detection and treatment depend on identification and reliable measurement of biomarkers (Downing, 2010, Mabey, et al., 2004). Blood is the most common clinical sample for diagnostic assays (Anderson and Anderson, 2002). However, limitations of blood sampling include the need for expert training to collect blood by venipuncture, especially in neonates, infants and patients with fragile veins; difficulty of continuous monitoring due to blood clotting; and apprehension by patients associated with blood draws (Deacon and Abramowitz, 2006). Blood also requires downstream processing for most diagnostic assays to remove red blood cells (Yager, et al., 2008).
Other body fluids such as saliva and urine are more easily accessible (Pichini, et al., 1996) but have limited biomarkers of variable concentrations (Fischbach and Dunning, 2009). Interstitial fluid (ISF) is a body fluid that fills the space between cells in tissues and constitutes 75% of extracellular fluid and 15–25% of body weight (Fogh-Andersen, et al., 1995). Recent studies have shown that ISF contains systemic biomarkers, but also contains other biomarkers that are unique or at much higher concentration compared to serum or plasma (Fogh-Andersen, et al., 1995, Hadrévi, et al., 2013, Müller, et al., 2012, Niedzwiecki, et al., 2018, Samant and Prausnitz, 2018, Sloop, et al., 1987, Tran, et al., 2017). ISF is a clear fluid without red blood cells and does not clot, thus enabling simplified preparation for biochemical analysis.
ISF has been used for continuous glucose monitoring (Renard, 2008), analysis of tumor microenvironment (Celis, et al., 2004, Wiig, et al., 2010) and bioavailability of topical drugs (Herkenne, et al., 2008). ISF has been sampled by techniques such as reverse iontophoresis (Sieg, et al., 2004), which requires frequent calibration and is limited to small molecules; suction blister (Kiistala and Mustakallio, 1967), which requires ~1 hour to collect ISF and causes lasting skin damage; microdialysis (Schmidt, et al., 2008), which requires minor surgery and expert personnel; and laser microporation of skin followed by ISF collection under vacuum (Venugopal, et al., 2008) which requires specialized equipment and slow ISF collection rates.
More recently, microneedle (MN) technology has been developed for ISF sampling. MNs are micron-scale, sharp-tipped projections that can puncture micropores into the skin to access dermal ISF in a minimally invasive manner (Prausnitz, 2017). MN patches were developed initially for delivery of vaccines and drugs, where they have been shown to be painless, well-tolerated, easy-to-use and effective for delivering therapeutic molecules into skin (Vrdoljak, et al., 2016, Ye, et al., 2018). MNs have also been used for detection of glucose and other biomarkers in dermal ISF (Donnelly, et al., 2014, Tran, et al., 2017).
Approaches to ISF collection using MNs include extracting ISF from skin through micropores for extracorporeal analysis (Samant and Prausnitz, 2018), which typically takes 15 min or longer; swelling hydrogel-forming MNs with ISF in skin (Caffarel-Salvador, et al., 2015, Chang, et al., 2017), which generally collects nanoliter quantities of ISF, which is insufficient for many biomarker assays; selectively binding biomarkers to MN surfaces modified for in-situ capture of biomarkers (Coffey, et al., 2016, Muller, et al., 2012), which must be custom designed for each biomarker; and incorporation of sensors into MNs for in situ analysis of ISF (Miller, et al., 2011, Miller, et al., 2012, Miller, et al., 2014), which is technically challenging and requires a different sensor for each biomarker.
To overcome the various limitations of prior MN technology, we developed a MN patch that collects microliters of ISF within one minute that can be assayed for any biomarker of interest. We used this MN patch to sample ISF, determine ISF concentrations of a model drug (vancomycin) and a model immunomarker (anti-polio antibodies), and compare them to serum concentrations. We chose vancomycin, an antibiotic commonly used to treat gram-positive methicillin-resistant Staphylococcus aureus infections (MRSA), because its very narrow therapeutic index requires therapeutic drug monitoring to ensure efficacy and minimize toxicity during patient care (Ito, et al., 2016, Rybak, et al., 2009). We chose to monitor anti-polio antibodies because they can be measured using well-established immunoassays by our collaborators at the WHO Global Specialized Reference laboratory located at the Centers for Disease Control and Prevention (CDC).
3. Materials and methods
3.1. Materials
Monovalent, bulk inactivated polio vaccine (IPV) (Mahoney strain of type 1, Middle East Forces (MEF) strain of type 2 and Saukett strain of type 3) was kindly provided by Bilthoven Biologicals (Bilthoven, Netherlands). The antigen concentrations were 1675, 963 and 950 D-antigen units (DU)/ml for IPV types 1, 2 and 3, respectively, determined by antigen-capture sandwich enzyme-linked immunosorbent (ELISA), as previously described (Edens, et al., 2015).
Vancomycin hydrochloride from Streptomyces Orientalis was purchased from Sigma Aldrich (St. Louis, MO). HPLC-MS grade acetonitrile, methanol and water were purchased from MedSupply Partners (Atlanta, GA) and formic acid (98%, ACS grade) was obtained from EMD Millipore Chemicals (Darmstadt, Germany).
3.2. Microneedle patch fabrication
MN patch dimensions were drafted using AutoCAD software (Autodesk, Cupertino, CA) to prepare patches by lithographically defined chemical etching (Tech Etch, Plymouth, MA). Patches were comprised of 9 MNs (650 μm long) each measuring 50 μm x 150 μm in cross section at the base and tapering to a tip of <1 μm radius of curvature.
Whatman grade 1 filter paper (Sigma Aldrich, St. Louis, MO) was cut into rectangular strips of desired dimensions (2 mm x 7 mm) using a CO2 laser (New Hermes Gravograph Model LS500XL, Gravotech, Duluth, GA). The patterns were made using a vector cut at 24 W power and a speed of 8 mm/s. To prevent burning due to excessive temperature rise during laser cutting, the heat capacity of the filter paper was increased by attaching it to an adhesive backing (3M, Maplewood, MN), followed by soaking in DI water until completely wet.
The final MN patch was prepared by adhering the paper strips to both sides of the base substrate of each MN patch to create a reservoir to collect ISF that flowed out of the skin during MN insertion.
3.3. ISF collection procedure
The MN patch was applied to rat skin up to 12 times while pinching the skin with a force of 20–40 N. The amount of ISF collected was estimated to be 4 μl once the 2 mm x 7 mm filter paper on both sides of the MN patch were saturated with ISF. This estimate was performed by determining sodium ion content in the MN patch paper backing using a sodium ion sensitive electrode (perfectION comb NA, Mettler Toledo, Columbus, OH). The sodium ion content measured in the samples was divided by the physiological sodium ion concentration in rat ISF of 143 mEq/L to determine ISF volume collected. This method relies on the expectation that sodium ion concentration is constant in the ISF of normal rats (Nachbaur, et al., 1977).
3.4. Vancomycin pharmacokinetic study
Procedures were performed on six Wistar rats (10–20 weeks old, female, Charles River Laboratories, Wilmington, MA) anesthetized by inhalation of isoflurane (AErrane, Baxter Healthcare, Deerfield, IL) in 100% oxygen during drug administration and sample collection. To administer vancomycin and collect blood, a silicone rubber tube was placed in the right jugular vein and kept locked with sodium heparin solution in physiological saline (100 U/ml). Care was taken to avoid administration of air bubbles, and blood samples were replaced with an approximately equal volume of heparinized saline to maintain blood volume. A 0.75 mg/ml solution of vancomycin hydrochloride in 0.9% NaCl injection solution (Hospira, Lake Forest, IL) was prepared and each rat was administered 1 mg/kg dose as a bolus via the jugular vein tube. Blood samples were obtained from the jugular tube before and 10, 20, 30, 45, 60 and 90 min after vancomycin administration. Companion ISF samples were also collected at the same time points. At the end of the study, the rats were euthanized by carbon dioxide gas asphyxiation. These experiments were approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee.
3.4.1. Extraction of vancomycin from ISF/serum samples
The extraction of vancomycin from ISF and serum samples was performed according to a previously reported method (Ito, et al., 2016). Serum samples were diluted by mixing 4 μl of serum in 100 μl of deionized water. ISF was extracted from MN patches by centrifuging 1 MN patch in 100 μl of deionized water at 6000 x g for 10 min. All samples were stored at 4 °C until testing. Then, 100 μL of formic acid/methanol (1:1, v/v) was added to each ISF or serum sample. The mixture was vortexed for 30 s and then centrifuged for 15 min at 12,000 x g. The supernatant was removed, diluted with 300 μL of high-performance liquid chromatography – mass spectrometry (HPLC-MS) grade water, and then transferred to an HPLC vial for HPLC-MS/MS analysis.
3.4.2. HPLC-MS/MS analysis
Calibrators were prepared by adding aliquots of vancomycin stock solution to a drug-free serum matrix to create the following concentrations: 1, 2, 6, 13, 32 and 75 μg/mL. The calibration curve was plotted using Mass Hunter QQQ Quantitative Analysis software (Agilent, Santa Clara, CA). The data were weighted 1/x and concentrations of vancomycin were calculated by the software.
Chromatography was performed by injecting 50 μl of extracted calibrator or sample onto an Agilent 1200 series HPLC fitted with an Agilent ZORBAX Eclipse C18 column (2.1 × 150 mm, 3.5 μm particle size). The column temperature was maintained at 35°C and the flow rate was maintained at 0.4 mL/min. Mobile phase A consisted of 0.25% formic acid in water and mobile phase B consisted of 0.25% formic acid in acetonitrile. Vancomycin was separated using a gradient method as follows: 5.0% mobile phase B from 0.00–3.00 min, increased to 30% mobile phase B from 3.01–10.00 min, increased to 90% mobile phase B from 10.01–12.00 min, decreased to 5.0% mobile phase B from 12.01–14.00 min, then maintained at 5.0% mobile phase B from 14.01–16.00 min.
An Agilent 6410 triple quadrupole mass spectrometer was used to analyze samples in positive ion mode. The mass spectrometer parameters were as follows: capillary voltage of 3500 V, electron multiplier voltage at 300 V, gas temperature of 350 °C, nebulizer pressure of 240 kPa and gas flow rate of 10 L/min. Vancomycin was monitored using multiple reaction monitoring of the transition m/z 725.6→144.2. Chromatographic peaks were then manually integrated and analyzed using Agilent MassHunter software.
3.5. Polio immunization
Six Wistar rats (10–20 weeks old, female, Charles River Laboratories) were anesthetized by isoflurane (AErrane, Baxter Healthcare) inhalation during all procedures. IPV stock solutions were combined and diluted using medium 199 to a concentration of 44, 8 and 36 DUs of IPV types 1, 2 and 3, respectively in 100 μl, as determined by ELISA. We administered two 100 μl intramuscular injections per rat (one in each hind limb) for a total dose of 88, 16 and 72 DU of IPV types 1, 2 and 3, respectively.
Sera and ISF samples for polio-specific IgG testing were collected before and 1, 3 and 4 weeks after immunization. Twelve weeks after the initial vaccination, all animals were given a second dose of trivalent IPV in the same manner as the first dose. Sera and ISF samples for neutralizing antibody titer assay were collected before and 1, and 2 weeks after the second dose.
Blood samples (≤ 500 μl) were collected in microtainer collection tubes with clot activator (BD Diagnostics, Franklin Lakes, NJ) by tail bleeding after making a small incision in the tail using a surgical blade. ISF samples were collected from the lateral side of the rat after hair removal using electric shears followed by application of depilatory cream (Nair, Princeton, NJ). Hair removal was performed at least 1 day prior to ISF collection to avoid possible ISF contamination by the depilatory cream. At the end of the study, the rats were euthanized by carbon dioxide gas asphyxiation. These experiments have been approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee.
Serum was separated by centrifuging the blood samples at 6000 x g for 1.5 min in an Eppendorf Centrifuge 5415R (Eppendorf AG, Hamburg, Germany). For the anti-polio IgG testing, 6 μl of serum was diluted with 120 μl of dilution buffer (provided with the ELISA kit) and the MN patch containing 4 μl of ISF was placed in an Eppendorf tube containing 80 μl of dilution buffer and centrifuged at 6000 x g for 1.5 min.
For polio-specific neutralizing antibody titers, a 1:16 dilution of serum samples was performed by mixing 16 μl of serum with 240 μl of Dulbecco’s modified Eagle Medium (DMEM, Gibco, Grand Island, NY) and 2% fetal bovine serum (FBS, Gibco) and a 1:16 dilution of ISF samples was performed by extracting ISF from the MN patches by centrifuging in DMEM (2 MN patches per 120 μl) at 6000 x g for 1.5 min. The undiluted serum samples and the diluted ISF/serum samples were stored in Eppendorf tubes at −20 °C until used.
3.5.1. Anti-polio IgG measurements and polio-specific neutralizing antibody titers
A commercially available ELISA IgG assay (Anti-Polio viruses 1–3 IgG ELISA kit, Alpha Diagnostics Intl, San Antonio, TX) was used for the detection and quantitative determination of IgG antibodies to polio virus in serum and ISF specimens. Prior to testing, the sera and ISF samples were thawed and diluted to 1:100 by adding 480 μl and 320 μl, respectively, of low Nsb diluent (provided with the ELISA kit).
Neutralizing antibody titers to poliomyelitis were measured from collected sera and ISF samples at the WHO Global Specialized Reference laboratory at CDC (Atlanta, GA) using methods previously described (Greenwood, et al., 1996). Briefly, 80–100 CCID50 of Sabin strains 1, 2, and 3 poliovirus and two-fold serial dilutions of serum and ISF (starting at 1:4) were combined and incubated for 3 h at 35 °C prior to addition of HEp-2(C) cells. The plates were stained with crystal violet and cell viability was measured by optical density after 5 days of incubation at 35 °C and 5% CO2. Each sample was run in triplicate. The neutralization titers were estimated by the Spearman-Karber method (Finney, 1952) and reported as the reciprocal of the calculated endpoint. The limit of detection for this assay is a 2.5 log2 titer, and the precision of detection is ± 0.5 log2 titer.
3.6. Statistical analysis
Statistics were calculated using either Prism software version 7.0 (Graphpad, La Jolla, CA) or Excel (Microsoft, Redmond, WA). Arithmetic mean and median values of the samples are reported. Comparison between three or more samples was performed by one-way ANOVA or two-way ANOVA. Correlation between data was determined by Pearson’s correlation coefficient test. Probability (p) values of <0.05 were considered to be significant.
4. Results
4.1. ISF collection by microneedle patch
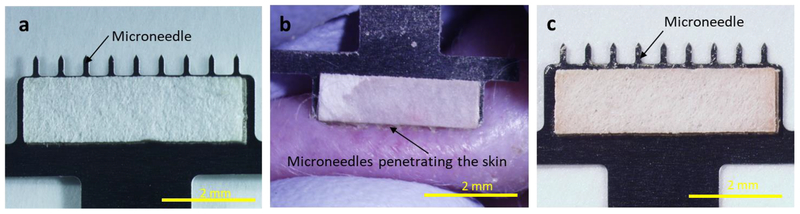
We developed a MN patch designed to collect ISF from skin. The MN patch comprised an array of nine, 650 μm long MNs (to create micropores in the skin surface) extending from a backing layer (to facilitate handling), all made of stainless steel. Two strips of filter paper (to create a reservoir calibrated to collect ~2 μl of ISF in each strip) were each adhered to one side of the patch backing (Figure 1A). ISF collection was performed by applying the MN patch to the skin, thereby creating micropores and inducing flow of ISF out of the skin and into the paper reservoirs (Figure 1B). MN insertion was repeated at a rate of ~1 insertion per second until 4 μl of ISF was collected, as indicated by fully wetted paper reservoirs (Figure 1C). This process usually required 10–12 MN patch insertions, which was completed in less than 1 min.
Fig. 1.
Collection of ISF using a microneedle (MN) patch. a) Representative photographic image of a MN patch showing a row of nine MNs measuring 650 μm in length extending from a patch backing with rectangular strips of filter paper adhered to both sides. b) Representative photographic image of ISF collection from hairless rat skin in vivo. Upon application of the MN patch to the skin, clear interstitial fluid (ISF) flowed out of the skin through micropores created by the MNs and was collected in the paper reservoirs. The paper reservoir shown in this image is mostly wetted by ISF, with only the upper left corner still dry, as indicated by different visual appearance. c) Representative photographic image of a MN patch after ISF collection by repeated application to skin. The amount of ISF collected on each strip of paper was ~2 μl, for a total of ~4 μl per MN patch
4.2. Vancomycin pharmacokinetics
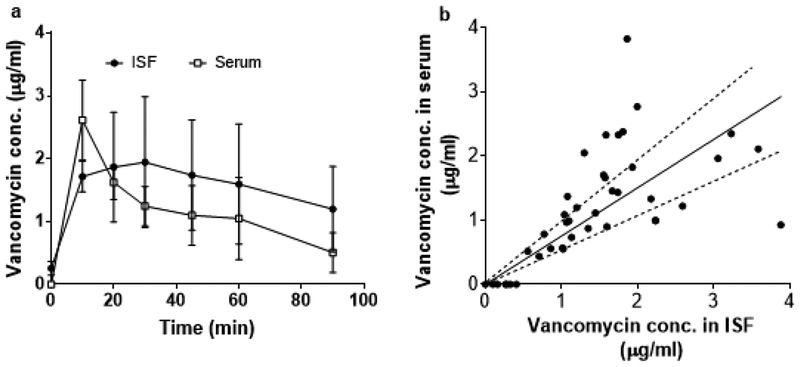
To assess the utility of ISF collection by MN patch, we studied the pharmacokinetics of vancomycin in ISF compared to serum of rats. For both ISF and serum, vancomycin concentration was rapidly increased by the first measurement made 10 min after bolus injection of vancomycin (Figure 2A). After reaching a peak, the vancomycin concentration decayed, which followed the expected pharmacokinetic curve for this drug (Rybak, 2006). Plotting paired individual ISF and serum drug levels yielded a roughly linear correlation with a slope of 0.75 ± 0.06 (R2 = 0.40), indicating a generally higher vancomycin concentration in ISF compared to serum.
Fig. 2.
Pharmacokinetics of vancomycin in rat ISF and serum. a) Pharmacokinetic profile of vancomycin concentration in ISF and serum samples collected from rats administered a 1 mg/kg bolus intravenous injection of vancomycin. Data points show mean ± standard deviation (SD) (n = 6 rats). b) Correlation between vancomycin concentration in paired serum and ISF samples (r= 0.61, p<0.005, Pearson’s correlation coefficient test). Dashed lines represent ± 30 % of the slope of the linear regression line (shown as solid black line), which are included as a visual guide. Data are the same as shown in (a)
A one-way ANOVA analysis of the pharmacokinetic profiles of each rat showed no statistically significant difference between ISF and serum concentrations (p ≥ 0.15) (Figure 2A). Pharmacokinetic analysis showed that the average peak vancomycin concentration (Cmax) and area under the curve (AUC) were not significantly different in ISF and serum (Student’s t-test, p > 0.8). A ~17 min delay in time to peak vancomycin concentration (Tmax) in ISF compared to serum was statistically significant (Student’s t-test, p < 0.05). This is consistent with prior literature, which reported correlation of vancomycin levels in ISF and blood and a delayed time for equilibration of vancomycin levels between ISF and blood (Ito, et al., 2016, Kiang, et al., 2014).
In this study, each rat had ISF collected seven times over the course of 90 min (i.e., a total of 42 ISF collections among the six rats in the study). Each ISF collection was done at a different skin site. These ISF collection procedures involving insertion of MNs into the skin up to 12 times each were well tolerated, with only very mild erythema and edema seen at the site of ISF collection. There were no other notable effects of the ISF collection procedure on the animals.
4.3. Immune responses to inactivated polio vaccination
4.3.1. Anti-polio IgG responses
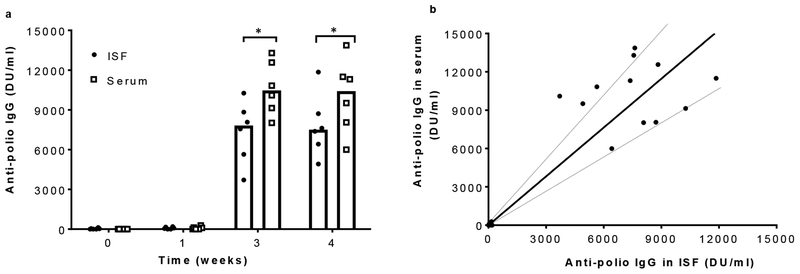
We also assessed the utility of ISF collection by MN patch in detection of anti-polio IgG responses in rats immunized with trivalent inactivated polio vaccine. Anti-polio IgG titers were negligible before and one week after vaccination. Three and four weeks after vaccination, IgG titer in both ISF and serum increased dramatically, but titers in ISF were ~23% lower than in serum (Student’s t-test, p < 0.03). Plotting paired individual ISF and serum IgG titers yielded a linear correlation with a slope of 1.27 ± 0.09 (R2 = 0.81), indicating a generally lower IgG titer in ISF compared to serum.
4.3.2. Polio-specific neutralizing antibody responses
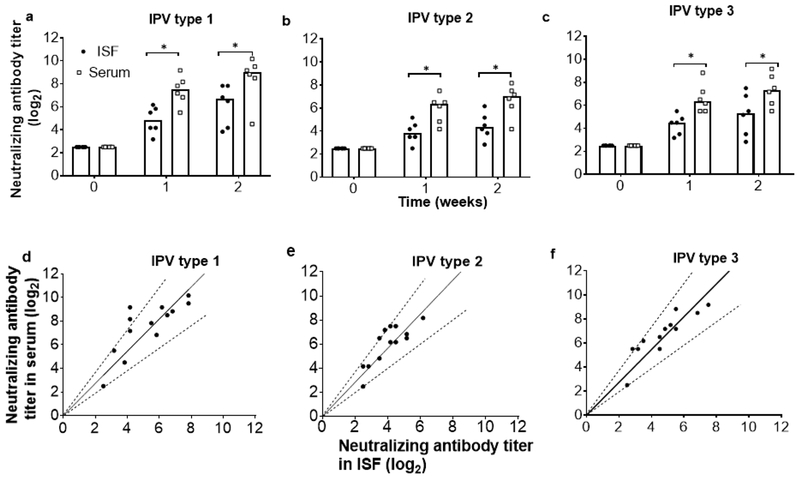
After observing the anti-polio IgG response in ISF, we performed a follow-up study to detect polio-specific neutralizing antibody responses by collecting ISF and companion blood samples after vaccinating the rats with a booster dose of trivalent inactivated polio vaccine. Before vaccination, neutralizing antibody titers in ISF and serum samples for all three serotypes were below the limit of detection (Figure 4a). One and two weeks after vaccination, neutralizing antibody titers increased in both ISF and serum, but titers in ISF were ~30% lower than in serum (Student’s t-test, p<0.01). This rapid increase of titer within one week is expected because responses to booster vaccination are typically seen on this time scale (Plotkin, et al., 2004). Paired individual ISF and serum titers were linearly correlated with a slope of 1.36 ± 0.07 (R2 = 0.79), 1.41 ± 0.06 (R2 = 0.78) and 1.36 ± 0.06 (R2 = 0.82) for IPV types 1, 2 and 3, respectively, indicating a generally lower neutralizing antibody titer in ISF compared to serum.
Fig. 4.
Neutralizing antibody responses to inactivated polio vaccination. Neutralizing antibody titers to a) IPV type 1, b) IPV type 2 and c) IPV type 3 in ISF and serum samples collected before and 1 and 2 weeks after administration of a booster dose of trivalent IPV by intramuscular injection in rats (the first dose was administered 12 weeks earlier). The limit of detection for the assay was 2.5 log2. Each data point represents a single animal while the bars represents the median values of each group (n = 6 rats). Asterisk (*) represents a significant difference (Student’s t-test, p < 0.01). Correlation between neutralizing antibody titers in paired serum and ISF samples for d) IPV type 1, (r= 0.89, p<0.005, Pearson’s correlation coefficient test), e) IPV type 2 (r= 0.87, p<0.005) and f) IPV type 3 (r= 0.91, p<0.005). Dotted lines represent ± 30% of the slope of the linear regression line (shown as solid black line), which are included as a visual guide. Data are the same as shown in (a)
These were the same rats that were used in the study of polio-specific IgG, meaning that each rat had ISF collected seven times over the course of 14 weeks. The procedures were well tolerated, with only very mild, transient erythema and edema seen at the site of ISF collection.
5. Discussion
5.1. MN patch for simplified ISF sampling
ISF is a relatively unexplored body fluid in major part due to lack of simple, reliable techniques to sample this fluid. In this study, we used a simple MN patch-based method to collect microliters of ISF in a minimally invasive manner within one minute. The MN patch consists of a steel MN array with strips of filter paper adhered on both sides of the patch backing. The MN patch should be inexpensive and the manufacturing process should be straightforward. ISF collection can be performed without expert training and the amount of ISF sampled can be controlled by changing the number of insertions into skin and the size/type of paper used for the ISF reservoir. We have performed repeated ISF collection on the same rats during these studies (i.e., ISF was collected a total of 84 times among 12 rats) and found that the procedure was well tolerated with a mild, localized erythema at the MN insertion site which resolved within one day (data not shown).
The MN patch used in this study is a lab prototype used to assess the feasibility of ISF collection for future medical applications. Next steps in this research include developing methods to recruit ISF that are simpler and more patient-friendly than repeated microneedle insertions, further study and optimize analytical methods best suited for use with ISF collected by MN patch, and studies in human subjects and further device development to optimize for usability, efficacy and safety.
5.2. Drug monitoring in ISF
While other body fluids such as saliva and urine have been investigated for therapeutic drug monitoring (Pichini, et al., 1996), ISF is a body fluid of increasing interest as a source of biomarkers providing dermal and systemic physiological information, as well as drug pharmacokinetics. Most research has been focused on detection of glucose in ISF, and indwelling subcutaneous sensors are now commonly used to continuously measure glucose concentration in subcutaneous ISF as a surrogate for blood glucose levels in diabetic patients (El-Laboudi, et al., 2013).
Other biomarkers have also been investigated in ISF, largely for research purposes. A comprehensive review of 87 individual pharmacokinetics comparisons of various classes of antibiotics demonstrated different ratios of drug concentration in ISF versus plasma, which was largely explained by different degrees of protein binding (Kiang, et al., 2014). Other drugs such as scopolamine, theophylline, methotrexate, vancomycin, carbamazepine and phenobarbital have also been measured in ISF and had similar AUC in serum/plasma and ISF samples (Kiang, et al., 2012, Lindberger, et al., 2002, Stetina, et al., 2005). On the other hand, some drugs such as phenytoin were not detectable in ISF (Lindberger, et al., 2002). These studies involved ISF collection using microdialysis and ultrafiltration probes, which cause significant skin damage, are time consuming to perform, require expert personnel and specialized equipment, and are not suitable for routine clinical use.
We demonstrated the ability of a minimally invasive MN patch to quickly collect ISF and detect biomarkers of interest, which provides an alternative approach to therapeutic drug monitoring in ISF. We collected microliters of ISF within 1 min, which should be sufficient to run many assays to detect biomarkers of clinical significance (Garg, et al., 1999, Loewenstein, et al., 2013). This contrasts with other MN-based methods that typically sample nanoliters of ISF and/or require as long as an hour of collecting (Caffarel-Salvador, et al., 2015, Samant and Prausnitz, 2018).
We used vancomycin as a model drug to explore the potential use of this MN device for therapeutic drug monitoring. In this study, vancomycin was administered via bolus intravenous injection and the PK sampling schedule was selected mainly to correlate drug concentrations in ISF and blood and not to simulate clinical use scenarios. Pharmacokinetics of vancomycin showed similar AUC and Cmax values in ISF and serum samples in rats, although vancomycin concentrations overall were slightly higher on average compared to serum levels. A delay in Tmax was observed in ISF, which is consistent with literature (Ito, et al., 2016, Kiang, et al., 2014). More studies are needed to understand the factors that influence drug distribution between ISF and blood. For example, vancomycin was shown to distribute into ISF at levels similar to serum in healthy patients with limb infections (Housman, et al., 2015), but had wide variability in vancomycin penetration into ISF in diabetic patients (Hamada, et al., 2015).
5.3. Immunodiagnostic markers in ISF
We also used ISF collected by MN patch to detect polio-specific IgG and neutralizing antibodies, which were at similar concentrations in ISF and plasma, although ISF levels were slightly lower. It has been shown that skin vaccination, especially by MN patch, can provide stronger, broader, longer-lasting and otherwise improved humoral and cellular immune responses compared to intramuscular injection (Suh, et al., 2014). Likewise, skin-associated B cells are believed to play an important role in skin immunity and inflammation (Geherin, et al., 2012). Therefore, the ability to measure local antibody titers in tissue ISF could play an important role in understanding tissue-specific immune responses.
6. Conclusion
We studied ISF collected from the skin using a minimally invasive, simple-to-use, low-cost MN patch that can collect microliters of ISF within 1 min. Of interest to therapeutic drug monitoring, we performed a pharmacokinetic study of vancomycin in rats and found that AUC and Cmax were similar in ISF, but Tmax was delayed in ISF by ~17 min compared to serum. Another study examined immunologic biomarkers in ISF by administering inactivated polio vaccine to rats and determined that polio-specific IgG and neutralizing antibody titers were similar in ISF and companion serum samples. We conclude that ISF collection using a novel MN patch can provide simple and rapid collection of microliters of ISF for future medical and research applications.
Fig. 3.
Anti-polio IgG responses to inactivated polio vaccination in rats. a) Anti-polio IgG titers determined by ELISA in ISF and serum samples collected before and 1, 3 and 4 weeks after vaccination with trivalent IPV by intramuscular injection. Each data point represents a single animal while the bars represents the median values of each group (n = 6 rats). Asterisk (*) represents a significant difference (Student’s t-test, p < 0.03). b) Correlation between anti-polio IgG titers in paired serum and ISF samples (r = 0.90, p<0.005, Pearson’s correlation coefficient test). Dotted lines represent ± 30% of the slope of the linear regression line (shown as solid black line), which are included as a visual guide. Data are the same as shown in (a)
Table 1.
Comparison of AUC, Cmax and Tmax values of vancomycin pharmacokinetics between ISF and serum
| Pharmacokinetic parameter1 | ISF | Serum | Ratio (ISF:serum) |
|---|---|---|---|
| AUC (μg.min/ml) | 138 ± 43 | 106 ± 25 | 1.3 ± 0.2 |
| Cmax (μg/ml) | 2.5 ± 1 | 2.6 ± 0.6 | 0.9 ± 0.2 |
| Tmax (min) | 26.6 ± 16* | 10 ± 0 | 2.7 ± 1.6 |
Data are presented as mean ± SD (n= 6 rats). AUC, area under the concentration-time curve. Cmax, maximum concentration. Tmax, time required to reach maximum concentration. Asterisk (*) denotes parameter with statistically significant difference (p < 0.05) between ISF and the companion serum value.
7. Acknowledgments
The authors would like to thank William Weldon of the CDC for conducting serum and ISF neutralizing antibody assays and Donna Bondy for administrative support. Mark Prausnitz is an inventor of patents that have been or may be licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products, including Micron Biomedical. These potential conflicts of interest have been disclosed and are being managed by Georgia Tech and/or Emory University.
References
- [1].Anderson NL, Anderson NG, Mol. Cell. Proteomics 1(11), 845 (2002). [DOI] [PubMed] [Google Scholar]
- [2].Caffarel-Salvador E, Brady AJ, Eltayib E, Meng T, Alonso-Vicente A, Gonzalez-Vazquez P, Torrisi BM, Vicente-Perez EM, Mooney K, Jones DS, PloS one 10(12), e0145644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Celis JE, Gromov P, Cabezón T, Moreira JM, Ambartsumian N, Sandelin K, Rank F, Gromova I, Mol. Cell. Proteomics 3(4), 327 (2004). [DOI] [PubMed] [Google Scholar]
- [4].Chang H, Zheng M, Yu X, Than A, Seeni RZ, Kang R, Tian J, Khanh DP, Liu L, Chen P, Adv. Mater 29(37), (2017). [DOI] [PubMed] [Google Scholar]
- [5].Coffey JW, Meliga SC, Corrie SR, Kendall MA, Biomaterials 84, 130 (2016). [DOI] [PubMed] [Google Scholar]
- [6].Deacon B, Abramowitz J, J. Anxiety Disord. 20(7), 946 (2006). [DOI] [PubMed] [Google Scholar]
- [7].Donnelly RF, Mooney K, Caffarel-Salvador E, Torrisi BM, Eltayib E, McElnay JC, Ther. Drug Monit 36(1), 10 (2014). [DOI] [PubMed] [Google Scholar]
- [8].Downing GJ, Biomarkers: Facing the Challenges at the Crossroads of Research and Health Care, Pharmaceutical Science Encyclopedia, John Wiley & Sons, Inc., 2010, pp. 1. [Google Scholar]
- [9].Edens C, Dybdahl-Sissoko NC, Weldon WC, Oberste MS, Prausnitz MR, Vaccine 33(37), 4683 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].El-Laboudi A, Oliver NS, Cass A, Johnston D, Diabetes Technol. Ther 15(1), 101 (2013). [DOI] [PubMed] [Google Scholar]
- [11].Finney DJ, Statistical method in biological assay, Charles Griffin: London: 1952. [Google Scholar]
- [12].Fischbach FT, Dunning MB, A manual of laboratory and diagnostic tests, Lippincott Williams & Wilkins; 2009. [Google Scholar]
- [13].Fogh-Andersen N, Altura BM, Altura BT, Siggaard-Andersen O, Clin. Chem 41(10), 1522 (1995). [PubMed] [Google Scholar]
- [14].Garg SK, Potts RO, Ackerman NR, Fermi SJ, Tamada JA, Chase HP, Diabetes Care 22(10), 1708 (1999). [DOI] [PubMed] [Google Scholar]
- [15].Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, Young AJ, Hay JB, Debes GF, J. Immunol, 1102639 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Greenwood B, Hall A, Rowe M, Whittle H, George M, Al-Ghassani A, Elbualy M, Malankar P, Suleiman A, Clements G, Bulletin of the World Health Organization 74, 253 (1996).8789924 [Google Scholar]
- [17].Hadrévi J, Ghafouri B, Sjörs A, Antti H, Larsson B, Crenshaw A, Gerdle B, Hellström F, Eur. J. Appl. Physiol 113(12), 2977 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hamada Y, Kuti JL, Nicolau DP, J. Antimicrob. Chemother 70(7), 2064 (2015). [DOI] [PubMed] [Google Scholar]
- [19].Herkenne C, Alberti I, Naik A, Kalia YN, Mathy F-X, Préat V, Guy RH, Pharm. Res 25(1), 87 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Housman ST, Bhalodi AA, Shepard A, Nugent J, Nicolau DP, Journal of the American Podiatric Medical Association 105(5), 381 (2015). [DOI] [PubMed] [Google Scholar]
- [21].Ito Y, Inagaki Y, Kobuchi S, Takada K, Sakaeda T, Int. J. Med. Sci 13(4), 271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kiang TK, Häfeli UO, Ensom MH, Clin. Pharmacokinet 53(8), 695 (2014). [DOI] [PubMed] [Google Scholar]
- [23].Kiang TK, Schmitt V, Ensom MH, Chua B, Häfeli UO, J. Pharm. Sci 101(12), 4642 (2012). [DOI] [PubMed] [Google Scholar]
- [24].Kiistala U, Mustakallio K, J. Invest. Dermatol 48(5), 466 (1967). [PubMed] [Google Scholar]
- [25].Laboratories CR, https://www.criver.com/sites/default/files/resources/BaselineHematologyandClinicalChemistryValuesforCharlesRiverWistarRats%5BCrlWIBR%5DasaFunctionofSexandAgeSpring1998.pdf. (Accessed 18 April 2018).
- [26].Lindberger M, Tomson T, Ståhle L, Basic Clin. Pharmacol. Toxicol 91(4), 158 (2002). [DOI] [PubMed] [Google Scholar]
- [27].Loewenstein D, Stake C, Cichon M, Am. J. Emerg. Med 31(8), 1236 (2013). [DOI] [PubMed] [Google Scholar]
- [28].Mabey D, Peeling RW, Ustianowski A, Perkins MD, Nat. Rev. Microbiol 2(3), 231 (2004). [DOI] [PubMed] [Google Scholar]
- [29].Miller PR, Gittard SD, Edwards TL, Lopez DM, Xiao X, Wheeler DR, Monteiro-Riviere NA, Brozik SM, Polsky R, Narayan RJ, Biomicrofluidics 5(1), 013415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Miller PR, Skoog SA, Edwards TL, Lopez DM, Wheeler DR, Arango DC, Xiao X, Brozik SM, Wang J, Polsky R, Talanta 88, 739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Miller PR, Xiao X, Brener I, Burckel DB, Narayan R, Polsky R, Adv. Healthcare Mater 3(6), 876 (2014). [DOI] [PubMed] [Google Scholar]
- [32].Mü AC, Breitwieser FP, Fischer H, Schuster C, Brandt O, Colinge J, Superti-Furga G, Stingl G, Elbe-Bürger A, Bennett KL, J. Proteome Res 11(7), 3715 (2012). [DOI] [PubMed] [Google Scholar]
- [33].Muller DA, Corrie SR, Coffey J, Young PR, Kendall MA, Anal. Chem 84(7), 3262 (2012). [DOI] [PubMed] [Google Scholar]
- [34].Nachbaur J, Clarke M, Provost J, Dancla J, Lab. Anim. Sci 27(6), 972 (1977). [PubMed] [Google Scholar]
- [35].Niedzwiecki MM, Samant P, Walker DI, Tran V, Jones DP, Prausnitz MR, Miller GW, Anal. Chem 90(6), 3786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pichini S, Altieri I, Zuccaro P, Pacifici R, Clin. Pharmacokinet 30(3), 211 (1996). [DOI] [PubMed] [Google Scholar]
- [37].Plotkin SA, Orenstein WA, Offit PA, Vaccines, Saunders 2004. [Google Scholar]
- [38].Prausnitz MR, Annu. Rev. Chem. Biomol. Eng 8, 177 (2017). [DOI] [PubMed] [Google Scholar]
- [39].Renard E, Curr. Diabetes Rev 4(3), 169 (2008). [DOI] [PubMed] [Google Scholar]
- [40].Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, Dalovisio JR, Levine DP, Am. J. Health-Syst. Pharm 66(1), 82 (2009). [DOI] [PubMed] [Google Scholar]
- [41].Rybak MJ, Clin. Infect. Dis 42(Supplement_1), S35 (2006).16323118 [Google Scholar]
- [42].Samant PP, Prausnitz MR, Proc. Natl. Acad. Sci 115, 4583 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schmidt S, Banks R, Kumar V, Rand KH, Derendorf H, J. Clin. Pharmacol 48(3), 351 (2008). [DOI] [PubMed] [Google Scholar]
- [44].Sieg A, Guy RH, Delgado-Charro MB, Clin. Chem 50(8), 1383 (2004). [DOI] [PubMed] [Google Scholar]
- [45].Sloop CH, Dory L, Roheim PS, Lipid Res J. 28(3), 225 (1987). [PubMed] [Google Scholar]
- [46].Stetina P, Madai B, Kulemann V, Kirch W, Joukhadar C, International Journal of Clinical Pharmacology & Therapeutics 43(3), 134 (2005). [DOI] [PubMed] [Google Scholar]
- [47].Suh H, Shin J, Kim Y-C, Clin. Exp. Vaccine Res 3(1), 42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tran BQ, Miller PR, Taylor RM, Boyd G, Mach PM, Rosenzweig CN, Baca JT, Polsky R, Glaros T, J. Proteome Res 17(1), 479 (2017). [DOI] [PubMed] [Google Scholar]
- [49].Venugopal M, Feuvrel KE, Mongin D, Bambot S, Faupel M, Panangadan A, Talukder A, Pidva R, IEEE Sens. J 8(1), 71 (2008). [Google Scholar]
- [50].Vrdoljak A, Allen EA, Ferrara F, Temperton NJ, Crean AM, Moore AC, J. Controlled Release 225, 192 (2016). [DOI] [PubMed] [Google Scholar]
- [51].Wiig H, Tenstad O, Iversen PO, Kalluri R, Bjerkvig R, Fibrog. Tissue Repair 3(1), 12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yager P, Domingo GJ, Gerdes J, Annu. Rev. Biomed. Eng 10, 107 (2008). [DOI] [PubMed] [Google Scholar]
- [53].Ye Y, Yu J, Wen D, Kahkoska AR, Gu Z, Adv. Drug Delivery Rev 127, 106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]