Abstract
BACKGROUND:
Restrictive transfusion strategies supported by large randomized trials are resulting in decreased blood utilization in cardiac surgery. What remains to be determined, however, is the impact of lower discharge hemoglobin (Hb) levels on readmission rates. We assessed patients with higher versus lower Hb levels on discharge to compare 30-day readmission rates after coronary artery bypass grafting (CABG).
METHODS:
We retrospectively evaluated 1552 patients undergoing isolated CABG at our institution from January 2013 to May 2016. We evaluated 2 Hb cohorts: “high” (above) and “low” (below) the mean discharge Hb level of 9.4 g/dL, comparing patient characteristics, blood utilization, and clinical outcomes including 30-day readmission rates. We further evaluated the effects of the lowest (<8 g/dL) discharge Hb levels on 30-day readmission rates by dividing the patients into 4 anemia cohorts based on discharge Hb levels: “no anemia” (>12 g/dL), “mild anemia” (10–11.9 g/dL), “moderate anemia” (8–9.9 g/dL), and “severe anemia” (<8 g/dL). Risk adjustment accounted for age, sex, Charlson comorbidity index, preoperative comorbidities, revision sternotomy, and patient blood management program implementation.
RESULTS:
The “high” and “low” groups had similar patient characteristics except for Hb levels (mean discharge Hb was 10.4 ± 0.9 vs 8.5 ± 0.6 g/dL, respectively). Notably, no evidence for a difference in 30-day readmission rates was noted between the “high” (76/746; 10.2%) and “low” (97/806; 12.0%) (P = .25) Hb cohorts. The 4 anemia cohorts had differences in age, revision sternotomy incidence, Hb levels, certain patient comorbidities, and time to readmission. On multivariable analysis, the risk-adjusted odds of readmission in the “low” Hb cohort (odds ratio, 1.16; 95% confidence interval, 0.84–1.61; P = .36) was not significant compared to the “high” Hb cohort. Compared to patients with discharge Hb ≥8 g/dL, patients with Hb <8 g/dL had a higher incidence of readmission (22/129; 17.1% vs 151/1423; 10.6%; P = .036). On multivariable analysis, Hb <8 g/dL on discharge was predictive of readmission (odds ratio, 1.77; 95% confidence interval, 1.05–2.88; P = .03). The most common reason for readmission was volume overload, followed by infection and arrhythmias.
CONCLUSIONS:
A discharge Hb level below the institution mean for CABG patients does not provide evidence for an association with an increased 30-day readmission rate. In the small number of patients discharged with Hb <8 g/dL, there is a suggestion of increased risk for readmission and larger more controlled studies are needed to verify or refute this finding.
According to the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality, blood transfusion is the most common procedure performed during inpatient hospitalizations.1 In 2012, The Joint Commission concluded their National Summit on Overuse and identified red blood cell (RBC) transfusions as 1 of the top 5 overused practices in US hospitals.2 Due in part to reports like these and in conjunction with numerous trials that associate restrictive transfusion practices with either the same or improved clinical outcomes, patient blood management (PBM) programs have been implemented in hospitals nationwide with the goal to reduce unnecessary RBC transfusions.3–7 The current AABB (formerly the American Association of Blood Banks) evidence-based recommendations for RBC transfusion include adherence to a hemoglobin (Hb) trigger of 7 g/dL in hemodynamically stable, adult hospitalized patients, and a Hb trigger of 8 g/dL in patients undergoing orthopedic or cardiac surgery or in patients with underlying cardiovascular disease. They also recommend the administration of a single-unit RBC transfusion followed by reassessment as standard of care for patients who are hemodynamically stable and not actively bleeding.8
There are now at least 11 randomized controlled trials (RCTs) that have compared restrictive versus liberal RBC transfusion strategies, including 5 that were done in the setting of cardiac surgery.9–19 While the results of these trials have been almost universally in favor of a restrictive approach, the practice could potentially lead to unintended consequences. Notably, the RCTs mentioned above did not include readmissions as a measured outcome. To date, the effect of patients being discharged with lower Hb levels as a result of restrictive transfusion protocols has not been fully evaluated, and of particular interest is the impact on hospital readmission rates after discharge. To our knowledge, only 1 study has reported the relationship between Hb levels and readmissions after cardiac surgery, which was a retrospective analysis by Shehata et al20 reporting that anemia on discharge did not increase readmissions. With the goal of confirming or refuting these results, we conducted a retrospective study assessing the relationship between discharge Hb levels and 30-day readmission rates after coronary artery bypass grafting (CABG). We tested the hypothesis that lower Hb levels, within the range of our normal practice, are not associated with increased readmissions. Furthermore, we evaluated if there is a lower limit at which there is an increased risk of unplanned, 30-day readmissions.
METHODS
Patient Identification
After institutional review board approval and determination that the requirement for written informed consent was waived by the institutional review board, data were obtained from our database designed to assess blood utilization and clinical outcomes at Johns Hopkins Hospital. All patients undergoing isolated CABG from January 2013 to May 2016 were identified from the database. Patients undergoing CABG in combination with other cardiac procedures such as valve repair or replacements, patients with missing admission or discharge Hb data, and patients with in-hospital deaths were excluded from the study. Given prior experiences with outcome studies, we chose the sample size based on data availability and previous outcome incidences. The mean Hb at discharge was calculated for the entire cohort and individual patients were divided into those with Hb levels above (“high”) or below (“low”) that value. Patients were also divided into 4 anemia cohorts based on Hb levels at discharge: “no anemia” (≥12 g/dL), “mild anemia” (10–11.9 g/dL), “moderate anemia” (8–9.9 g/dL), and “severe anemia” (<8 g/dL). Of note, a formal PBM program was initiated in January 2015, the details of which have been previously reported.21
Outcomes of Interest
The primary outcome for this study was all-cause, 30-day readmission. Secondary outcomes included length of stay (LOS) and a composite postoperative morbidity defined as having one or more hospital-acquired conditions. Hospital-acquired conditions as defined by International Classification of Diseases (ICD)-9 and ICD-10 codes included: (1) infection (Clostridium difficile, sepsis, surgical site infection, or drug-resistant infection); (2) thrombotic events (deep venous thrombosis, pulmonary embolism, or disseminated intravascular coagulation); (3) acute kidney injury; (4) respiratory event; and (5) major ischemic event (myocardial infarction, transient ischemic attack, or cerebrovascular accident). These diagnoses, if present on admission, did not contribute to the secondary outcomes.
Data Collection and Clinical Outcomes
Patient demographics, inpatient laboratory, blood product transfusion, and other relevant clinical data were identified and abstracted from an institutional blood management intelligence portal (Impact Online, Haemonetics Inc, Braintree, MA) that includes all blood components and Hb laboratory values for all inpatients (from admit to discharge). These data are extracted from 3 different computer systems within our medical center and made available through a proprietary, web-based application designed to provide data to assess and optimize PBM. Thirty-day readmission status was derived from the Maryland Health Services Cost Review Commission database. This database included readmission data from all hospitals in the state of Maryland and not just the primary institution. Readmission diagnoses were manually cross-referenced from the institutional electronic health record (Epic, Verona, WI). Relevant clinical outcomes and comorbidities were accessioned from DataMart (Hillsborough Township, NJ).
Assessment of Blood Utilization
For the purposes of this study, the admission Hb was defined as the first chronological measured Hb level for the hospital encounter while the discharge Hb was defined as the last measured Hb. As previously described, the transfusion trigger was defined as the nadir Hb during the hospitalization.22 Percent of patients transfused for all major blood products (RBC, plasma, and platelets) was assessed for the “high” and “low” discharge Hb groups. Each group was further evaluated to determine the total number of blood product units transfused and the setting of transfusion (ie, intraoperative or whole hospital stay).
Statistical Analysis
All data were processed and analyzed with the software programs Excel, v. 14.1.0 (Microsoft Inc, Redmond, WA) and JMP, v. 12.1.0 (SAS Institute, Cary, NC). Univariate analyses were performed to compare the 4 anemia cohorts and “high” and “low” discharge Hb groups for all patient characteristics. Normally distributed variables (ie, age, initial Hb, trigger Hb, and discharge Hb) were assessed using 1-way analysis of variance for the anemia cohorts. The same variables in the “high” and “low” groups were assessed using unpaired Student t tests. Nonnormally distributed continuous variables (ie, Charlson comorbidity index [CCI] and American Society of Anesthesiologists scores) were assessed using Kruskal-Wallis test for the anemia cohorts. The same variables in the “high” and “low” Hb groups were assessed using Mann-Whitney U test. Categorical variables (ie, age ≥65 years, sex, percentage revision sternotomy, and all comorbidities) were assessed using χ2 tests.
Multivariable logistic regression was performed to assess the adjusted relationship between “low” and “high” discharge Hb on our primary outcome (30-day readmissions). Variables included in the multivariable model were determined by clinically relevant patient characteristics and comorbidities. These include age, sex, CCI, PBM program implementation, percentage of patients undergoing revision sternotomy, renal disease, pulmonary disease, and diabetes mellitus. CCI was utilized to measure the burden of disease and case mix.23 Time period of precompared to post-PBM program implementation was included because a shift to a more restrictive transfusion practice could affect the primary and secondary outcomes. Discharge Hb was modeled 3 different ways: “low” Hb cohort compared to “high” Hb cohort, Hb as a continuous variable, and Hb as a dichotomous variable comparing patients discharged with Hb <8 g/dL to those discharged with Hb ≥8 g/dL.
Additionally, a Locally Weighted Scatterplot Smoothing curve comparing 30-day readmission rates and discharge Hb levels was made. Predetermined, explorative subgroup analyses were performed for each group based on age (<65 and ≥65 years). Data are given as mean ± standard deviation or median (interquartile range) as appropriate. P < .05 defined significance (2 sided). With the number of patients included in the analysis and an α level of .05, the available power is 85% to detect a clinically relevant difference in readmission rates (10% vs 15%) between the “high” and “low” Hb cohorts.
RESULTS
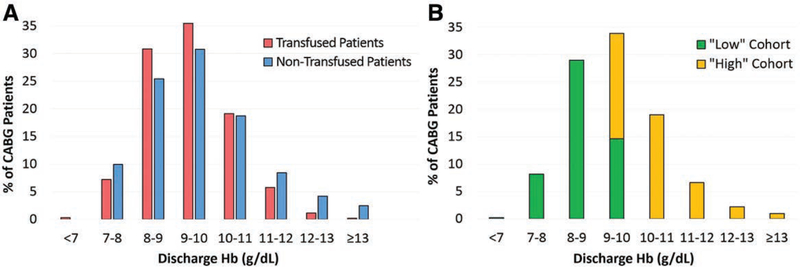
The mean discharge Hb for all patients (n = 1552) was 9.4 g/dL, and these were divided into the “high” (n = 746; ≥9.4 g/dL) and “low” (n = 806; <9.4 g/dL) discharge Hb cohorts. The patients were also divided into the 4 anemia cohorts described above: “no anemia” (n = 49, mean discharge Hb 12.7 ± 0.7 g/dL), “mild anemia” (n = 398, mean discharge Hb 10.6 ± 0.5 g/dL), “moderate anemia” (n = 976, mean discharge Hb 9.0 ± 0.6 g/dL), and “severe anemia” (n = 129, mean discharge Hb 7.6 ± 0.3 g/dL). Figure 1A shows the percentage of transfused and nontransfused patients in our patient population and the respective discharge Hb levels. Of note, the majority of our patients—both transfused and nontransfused—were discharged with Hb levels between 7 and 13 g/dL. Figure 1B demonstrates that the “low” and “high” groups had different discharge Hb levels.
Figure 1.
Discharge Hb levels and transfusion characteristics. A, The percentage of transfused and nontransfused coronary artery bypass grafting (CABG) patients by discharge hemoglobin (Hb) levels is shown with the vast majority of patients being discharged with Hb levels between 7 and 13 g/dL. There is only slight variability between transfused and nontransfused patients when stratified by discharge Hb levels. B, The percentage of “high” versus “low” cohort CABG patients and discharge Hb levels is shown in the figure. Patients in the “low” cohort are being discharged with lower Hb levels than their “high” cohort counterparts.
Table 1 compares demographic data between the “high” and “low” groups and among the 4 anemia cohorts. Supplemental Digital Content, Tables 1–2, http://links.lww.com/AA/C504, compares the 2 groups for patients ≥65 and <65 years of age, respectively. Of note, the older group of patients had a higher percentage of males in the “low” group. Furthermore, the younger group had a higher percentage of patients with CHF in the “low” group when compared to the “high” group. In both age groups, the “low” group had a significantly lower admission, nadir, and discharge Hb compared to the “high” group and is consistent with the results of the entire cohort. When comparing the older patients by anemia cohorts, there was a higher percentage of patients with revision sternotomies in the “severe anemia” cohort. The younger patients in the “no anemia” and “mild anemia” cohorts had a higher incidence of diabetes mellitus while those in the “severe anemia” and “moderate anemia” cohorts had a higher incidence of renal disease.
Table 1.
Patient Characteristics for the 4 Anemia Cohorts and “High” Discharge Hb Versus “Low” Discharge Hb Cohorts
| Anemia Hb Cohorts | “Low” Versus “High” Hb Cohorts | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | “Severe” <8 g/dL (n = 129) | “Moderate” 8–9.9 g/dL (n = 976) | “Mild” 10–11.9 g/dL (n = 398) | “No” ≥12 g/dL (n = 49) | P Value | “Low” <9.4 g/dL (n = 806) | “High” ≥9.4 g/dL (n = 746) | P Value |
| Age (y) | 63 ± 11 | 65 ± 10 | 65 ± 11 | 61 ± 9 | .0016 | 65 ± 10 | 65 ± 10 | .40 |
| Age ≥65 y | 58 (45.0) | 523 (53.6) | 207 (52.0) | 12 (24.5) | .0003 | 410 (50.9) | 390 (52.3) | .58 |
| Sex (% male) | 99 (76.7) | 739 (75.7) | 294 (73.9) | 43 (87.8) | .15 | 623 (77.3) | 552 (74.0) | .13 |
| CCI | 2 (1–3) | 2 (1–4) | 2 (1–3) | 2 (1–3) | .80 | 2 (1–4) | 2 (1–3) | .83 |
| ASA score | 3 (3–4) | 3 (3–4) | 3 (3–4) | 3 (3–4) | .65 | 3(3–4) | 3(3–4) | .48 |
| % redo sternotomy | 9 (7.0) | 18 (1.8) | 12 (3.0) | 0 (0.0) | .009 | 21 (2.6) | 18 (2.4) | .81 |
| Initial Hb (g/dL) | 12.9 ± 1.9 | 13.0 ± 1.9 | 13.4 ± 2.0 | 14.8 ± 1.7 | <.0001 | 12.9 ± 1.9 | 13.5 ± 1.9 | <.0001 |
| Trigger Hb (g/dL)a | 7.2 ± 0.4 | 7.6 ± 0.7 | 7.9 ± 1.0 | 8.2 ± 1.3 | <.0001 | 7.5 ± 0.7 | 7.8 ± 1.0 | <.0001 |
| Discharge Hb (g/dL) | 7.6 ± 0.3 | 9.0 ± 0.6 | 10.6 ± 0.5 | 12.7 ± 0.7 | <.0001 | 8.5 ± 0.6 | 10.4 ± 0.9 | <.0001 |
| Comorbidities | ||||||||
| CHF | 28 (21.7) | 217 (22.2) | 93 (23.4) | 8 (16.3) | .71 | 186 (23.1) | 160 (21.5) | .44 |
| Hypertension | 104 (80.6) | 778 (79.7) | 322 (80.9) | 35 (71.4) | .51 | 642 (79.7) | 597 (80.0) | .85 |
| PVD | 24 (18.6) | 150 (15.4) | 64 (16.1) | 4 (8.2) | .39 | 125 (15.5) | 117 (15.7) | .92 |
| Pulmonary disease | 12 (9.3) | 168 (17.2) | 67 (16.8) | 12 (24.5) | .047 | 133 (16.5) | 126 (16.9) | .84 |
| Diabetes mellitus | 49 (38.0) | 262 (26.8) | 127 (31.9) | 13 (26.5) | .03 | 234 (29.0) | 217 (29.1) | .98 |
| Renal disease | 26 (20.2) | 195 (20.0) | 67 (16.8) | 1 (2.0) | .003 | 161 (20.0) | 128 (17.2) | .15 |
Values are reported as mean ± standard deviation, No. (%), or median (interquartile range) as appropriate.
Abbreviations: ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; CHF, congestive heart failure; Hb, hemoglobin; PVD, peripheral vascular disease.
Only patients who were transfused were included.
There was no evidence for a difference in 30-day readmission between the “high” and “low” groups (10.2% vs 12.0%, respectively; P = .25) (Table 2). However, compared to patients with discharge Hb ≥8 g/dL, patients with Hb <8 g/dL had a higher incidence of readmission (22/129; 17.1% vs 151/1423; 10.6%) (P = .036). The “low” group experienced a shorter median LOS (9 vs 8.5 days; P = .003) compared to the “high” group. In addition, the “high” group had a significantly higher rate of composite postoperative morbidity (52.7% vs 43.8%; P = .0005) compared to the “low” group. While a higher percentage of the “low” group received an RBC transfusion during their hospitalization (68.7% vs 63.7%; P = .04), they received approximately half a unit less per patient (3.0 ± 4.7 vs 3.5 ± 5.7 units; P = .04) compared to the “high” group. Of note, a higher percentage of the “high” group received plasma transfusions (56.0% vs 50.3%; P = .02) compared to the “low” group. There was no difference between groups for percentage of patients who were transfused platelets (50.1% vs 53.0%; P = .26).
Table 2.
Clinical Outcomes for the 4 Anemia Cohorts and “High” Discharge Hb Versus “Low” Discharge Hb Cohorts
| Anemia Hb Cohorts | “Low” Versus “High” Hb Cohorts | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | “Severe” <8 g/dL (n = 129) | “Moderate” 8–9.9 g/dL (n = 976) | “Mild” 10–11.9 g/dL (n = 398) | “No” ≥12 g/dL (n = 49) | P Value | “Low” <9.4 g/dL (n = 806) | “High” ≥9.4 g/dL (n = 746) | P Value |
| % Tx RBC (WH) | 77 (59.7) | 682 (69.9) | 256 (64.3) | 14 (28.6) | <.0001 | 554 (68.7) | 475 (63.7) | .04 |
| RBC unit/Pt (WH) | 3.0 ± 6.3 | 3.0 ± 4.1 | 4.2 ± 7.1 | 1.1 ± 2.4 | <.0001 | 3.0 ± 4.7 | 3.5 ± 5.7 | .04 |
| % Tx platelets | 67 (51.9) | 523 (53.6) | 199 (50.0) | 12 (24.5) | .0007 | 427 (53.0) | 374 (50.1) | .26 |
| % Tx plasma | 63 (48.8) | 504 (51.6) | 217 (54.5) | 39 (79.6) | .0007 | 405 (50.3) | 418 (56.0) | .02 |
| % Tx RBC (intraoperative) | 57 (44.2) | 522 (53.4) | 193 (48.5) | 8 (16.3) | <.0001 | 421 (52.2) | 359 (48.1) | .11 |
| RBC unit/Pt (intraoperative) | 0.94 ± 1.69 | 1.15 ± 1.72 | 1.25 ± 2.01 | 0.31 ± 0.94 | .003 | 1.09 ± 1.69 | 1.18 ± 1.88 | .35 |
| LOS (d) | 8 (6–12) | 9 (7–13) | 9 (7–14) | 8 (6–10) | .001 | 8.5 (6–13) | 9(7–14) | .003 |
| Morbidity | 49 (38.0) | 467 (47.9) | 210 (52.8) | 20 (40.8) | .02 | 353 (43.8) | 393 (52.7) | .0005 |
| 30-d readmission | 22 (17.1) | 109 (11.2) | 39 (9.8) | 3 (6.1) | .11 | 97 (12.0) | 76 (10.2) | .25 |
| Time to readmission (d): median | 7.5 (3–12.3) | 11 (6–17.5) | 14 (7–21) | 19 (7–30) | .02 | 11 (5–17) | 12 (6–20.5) | .20 |
| Time to readmission (d): mean | 9.3 ± 7.8 | 12.3 ± 7.7 | 14.6 ± 7.4 | 18.7 ± 11.5 | .04 | 11.9 ± 7.5 | 13.5 ± 8.1 | .18 |
| % of readmissions to same hospital | 14 (63.6) | 50 (45.9) | 16 (41.0) | 1 (33.3) | .35 | 51 (52.6) | 30 (39.5) | .09 |
Values are reported as mean ± standard deviation, No. (%), or median (interquartile range) as appropriate.
Abbreviations: Hb, hemoglobin; LOS, length of stay (median); Pt, patient; RBC, red blood cell; Tx, transfusion; WH, whole hospital stay.
When comparing the 4 anemia cohorts, there was a significant difference in the percentage of patients transfused with any type of product among the groups (Table 2). Less patients in the “no anemia” cohort were transfused (14/49; 28.6%) (P ≤ .0001). They were also transfused less (1.1 ± 2.4 units) (P ≤ .0001). Furthermore, the time to readmission increased with increasing discharge Hb levels. The “no anemia” cohort had a median time to readmission of 19 days while the “severe anemia” group had a median time to readmission of 7.5 days.
Multivariable analysis adjusted for age, sex, CCI, PBM program implementation, percentage revision sternotomy, renal disease, pulmonary disease, and diabetes mellitus was performed (Table 3). There was no significant increase in 30-day readmission rates between the “high” and “low” groups (odds ratio [OR], 1.16; 95% confidence interval [CI], 0.84–1.61; P = .36). Of note, CCI and history of renal disease were independent predictors of increased 30-day readmission rates. The same multivariable analysis was performed with discharge Hb modeled as a continuous and dichotomous variable. When discharge Hb was modeled as a continuous variable, the OR (95% CI) for each g/dL decrease in Hb was 1.15 (0.99–1.33) (P = .07). When discharge Hb was modeled as a dichotomous variable in the multivariable model, comparing the group with discharge Hb <8 g/dL to that with discharge Hb ≥8 g/dL, the OR (95% CI) was 1.77 (1.05–2.88) (P = .03).
Table 3.
Multivariable Logistic Regression: 30-d Readmission Rates
| Parameter | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age ≥65 y | 0.84 (0.61–1.16) | .30 |
| Male sex | 0.97 (0.67–1.43) | .88 |
| CCI (per unit increase) | 1.11 (1.01–1.21) | .02 |
| Pre (compared to post) PBM | 1.36 (0.97–1.90) | .07 |
| Redo sternotomy | 0.35 (0.06–1.17) | .09 |
| Renal disease | 2.35 (1.61–3.40) | <.0001 |
| Pulmonary disease | 1.17 (0.77–1.76) | .47 |
| Diabetes mellitus | 1.10 (0.77–1.55) | .60 |
| Low (compared to high) Hb cohorta,b | 1.16 (0.84–1.61) | .36 |
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; Hb, hemoglobin; PBM, patient blood management.
When discharge Hb was modeled as a continuous variable in the multivariable model, the odds ratio (95% CI) for each g/dL decrease in Hb was 1.15 (0.99–1.33) (P = .07).
When discharge Hb was modeled as a dichotomous variable in the multivariable model, comparing the group with discharge Hb <8 g/dL to that with discharge Hb ≥8 g/dL, the odds ratio (95% CI) was 1.77 (1.05–2.88) (P = .03).
Supplemental Digital Content, Tables 3–4, http://links.lww.com/AA/C504, show patient outcomes for patients ≥65 and <65 years of age, respectively. Of note, there was no difference in rates of 30-day readmission between the “high” and “low” groups in the older (9.7% vs 11.5%, respectively; P = .43) and in the younger (10.7% vs 12.6%, respectively; P = .40). When comparing the older patients by anemia cohorts, those patients in the “no anemia” cohort were transfused less and received fewer RBC units per patient. They also had a slightly lower LOS when compared to the other anemia cohorts. When comparing the younger patients by anemia cohorts, we find similar results in regards to transfusions and RBC units received per patient. Furthermore, the percentage of patients receiving platelet transfusions were less in the “no anemia” cohort, but these patients had a higher percentage of plasma transfusions.
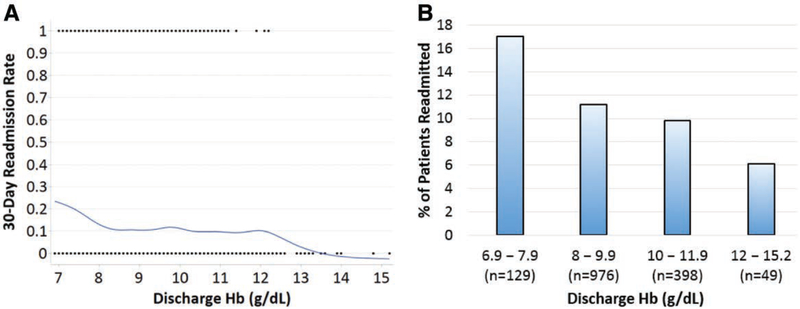
The Locally Weighted Scatterplot Smoothing curve demonstrates the difference in 30-day readmission rates as a function of discharge Hb (Figure 2A). The figure suggests that patients discharged with a Hb value <8 g/dL had a higher unadjusted rate of 30-day readmissions while those discharged above 12 g/dL may have a lower rate of 30-day readmissions. Of note, there was a plateau in 30-day readmission rates in patients discharged with a Hb level between 8 and 12 g/dL. Figure 2B also demonstrates a higher 30-day readmission rate seen in patients with Hb levels <8 g/dL at discharge when compared to the other Hb cohorts.
Figure 2.
Discharge Hb levels and 30-day readmissions. A, A Locally Weighted Scatterplot Smoothing (LOWESS) curve comparing 30-d readmission rates and discharge hemoglobin (Hb) levels is shown. There is an increase in 30-d readmission rates when discharge Hb levels are <8 g/dL and a plateau in the 30-d readmission rate between 8 and 12.5 g/dL. B, The percentage of patients readmitted and discharge Hb levels are shown in the figure. Again, there is a higher 30-d readmission rate seen in patients with Hb levels <8 g/dL at discharge when compared to the higher Hb cohorts.
The reasons for 30-day readmissions are shown in Table 4. Pleural effusions and signs of fluid overload accounted for 15% of readmissions, followed closely by surgical site infections (saphenous vein graft site or sternal incision site) at 14.5%. Ischemic heart disease accounted for 3.5% of readmissions. Of note, 16 patients (9.2%) were reportedly readmitted within 30 days, but no official documentation was found in our institutional electronic health records.
Table 4.
Reasons for 30-d Readmissions
| Readmission Reason | No. Readmissions (n = 173) (%) |
|---|---|
| Pleural effusion/volume overload | 26 (15.0) |
| Infection (SVG or sternal) | 25 (14.5) |
| Arrhythmia | 13 (7.5) |
| Hemorrhagic disorder | 12 (6.9) |
| Gastrointestinal disorder | 10 (5.8) |
| Heart failure | 10 (5.8) |
| Infection: other | 9 (5.2) |
| Pulmonary disease | 8 (4.6) |
| Thromboembolic disorder | 7 (4.0) |
| Ischemic heart disease | 6 (3.5) |
| Cardiac: other | 5 (2.9) |
| Othera | 26 (15.0) |
| Missing data | 16 (9.2) |
Abbreviation: SVG, saphenous vein graft.
Other reasons included anemia, syncope, anxiety, epididymitis, heat exhaustion, altered mental status, leukocytosis of unknown origin, hypocalcemia, musculoskeletal pain, transient ischemic attack, vision loss, seizure-like activity, peripheral neuropathy, right tibial nonunion, hyponatremia, hyperkalemia, hypoglycemia, and planned surgery.
DISCUSSION
Based on the results of our study, there is no evidence for an association between patients discharged with lower Hb levels after CABG and readmissions within 30 days of discharge, unless their discharge Hb level was <8 g/dL. In this small group of patients (8.3% of our study population) with the lowest discharge Hb, there was a slight but statistically significant increase in readmissions. Given the retrospective nature of our study, we believe this finding needs to be examined further, potentially from secondary analyses from previously published RCTs.
There has been a nationwide effort to decrease transfusion overuse in the perioperative setting. Goodnough et al3 demonstrated that the implementation of a blood management program resulted in decreased blood utilization, decreased mortality, and unchanged readmission rates. Restrictive transfusion Hb triggers have been shown to be noninferior to liberal triggers even in the highest risk patients undergoing surgery. The Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS) trial evaluated the impact of liberal (Hb threshold <10 g/dL) versus restrictive (Hb threshold <8 g/dL) transfusion strategies in patients with high cardiovascular risks undergoing surgery for hip fractures.16 They concluded that using a liberal transfusion strategy did not reduce in-hospital morbidity or mortality rates. These findings are consistent with findings in high-risk patients undergoing cardiac surgery as well. The Transfusion Requirements in Cardiac Surgery (TRICS) III trial evaluated over 5000 patients comparing a restrictive versus liberal transfusion strategy for patients undergoing cardiac surgery. The study concluded that a restrictive strategy was noninferior to a liberal strategy, which is consistent with several other large RCTs in cardiac surgery.9,10,14,18,19 Unfortunately, none of the aforementioned studies evaluate the effect of a lower discharge Hb—a direct result of a restrictive transfusion strategy—on the rate of 30-day readmissions.
Shehata et al20 evaluated just over 2000 patients undergoing CABG and/or cardiac valve procedures and found that discharge Hb concentration was not associated with 30-day readmissions. Our findings were similar for both our younger and older patients. There are, however, some notable differences and similarities between our studies. First, we evaluated 2 groups of patients (discharge Hb ≥9.4 and <9.4 g/dL). Both studies further stratified patients into 4 anemia cohorts and found similar patterns of increased readmissions in the lowest Hb group—ours being borderline statistically significant and Shehata et al’s20 being just shy of statistical significance. Additionally, Shehata et al20 included patients undergoing CABG/valve procedures while our study focused on patients undergoing only CABG to improve the homogeneity of our patient population. Our study also subdivided patients into age-specific cohorts. The longstanding belief that older patients do better when transfused to higher Hb levels was not supported by the TRICS III trial, where patients with ≥75 years of age had increased adverse outcomes with liberal transfusions.9 Our findings were similar in that the older subgroup did not benefit from a higher discharge Hb.
Interestingly, Shehata et al20 noted that the primary reason for readmission in their patient population was infection, while our primary reason was pleural effusions or signs and symptoms of volume overload, followed closely by infection. Despite these notable differences, our conclusions support the finding that a relatively lower discharge Hb level was not associated with a difference in the rate of 30-day readmission except for in the small number of patients discharged with Hb levels <8 g/dL. In addition, our study demonstrates a potential association with shorter LOS and lower rates of composite morbidity with lower discharge Hb levels. One possible explanation for this finding is that patients in the “high” cohort received more RBC units per patient compared to the “low” cohort, and RBC transfusions have been associated with increased hospitalacquired infections, thrombotic events, and LOS.24–26
Our study also evaluates the importance of not only the Hb transfusion threshold (Hb “trigger” before the transfusion) but also the Hb at discharge (Hb “target” after the transfusion). Mazer et al9 utilized intraoperative Hb triggers of <7.5 and <9.5 g/dL, but the target Hb levels were approximately 9 and 10 g/dL, respectively. Similarly, Hajjar et al10 utilized postoperative Hb triggers for transfusion of <8 and <10 g/dL in the intensive care unit but the mean target Hb levels were 9.1 and 10.5 g/dL. In comparison, our study suggests an increased risk for readmissions when the target Hb level is too low (<8 g/dL). Therefore, a Hb trigger level of 8 g/dL for transfusions may be noninferior, whereas a Hb target level of <8 g/dL may actually be too restrictive and predispose to readmissions. Additionally, those discharged with a Hb level <8 g/dL were also readmitted earlier than those with higher discharge Hb levels.
Our study is limited in that it represents findings associated with a retrospective, observational design, which despite concerted attempts we recognize can never fully prevent some degree of confounding. There could be recognized or unrecognized differences in patient cohorts with higher or lower Hb levels during their hospitalizations. The “high” and “low” groups in our study, however, were very similar in terms of patient characteristics. Our evaluation of patients with discharge Hb levels <8 g/dL was limited by the fact that there are only a few patients who are discharged with Hb levels <8 g/dL. Our results are further limited by the exclusion of patients who died in the hospital and those with missing data. In addition, we do not account specifically for variation in surgical technique or surgical provider. Fortunately, there was minimal surgeon turnover during the time of the study. Importantly, we were unable to obtain the diagnosis associated with the 30-day readmission for 16 patients.
In conclusion, our study suggests that among patients who undergo CABG, a discharge Hb below the institutional mean is not associated with an increased 30-day readmission rate. However, a discharge Hb level <8 g/dL may be a risk factor for increased 30-day readmissions. Our study adds to the growing body of literature suggesting the noninferiority of a restrictive approach to RBC transfusion but also demonstrates the need for further studies to assess lower Hb levels as a risk factor for readmissions.
Supplementary Material
KEY POINTS.
Question: Does a lower discharge hemoglobin level affect 30-day readmission rates in patients undergoing coronary artery bypass grafting?
Finding: A discharge hemoglobin below the institutional mean for patients undergoing coronary artery bypass grafting did not result in increased 30-day readmission rates except in patients discharged with hemoglobin levels <8 g/dL.
Meaning: Further studies are needed to determine if lower discharge hemoglobin levels increase 30-day readmission rates, especially in patients discharged with hemoglobin levels <8 g/dL.
ACKNOWLEDGMENTS
The authors thank the Armstrong Institute of Patient Safety and Quality for providing readmissions data for the manuscript.
Funding: S.M.F. has received consulting fees from Haemonetics and Medtronic.
C.H.B. has consulted for and received grant support from Medtronic in areas unrelated to this study. Funding: 1 K76 AG057020.
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
REFERENCES
- 1.Healthcare Cost and Utilization Project. Most frequent procedures performed in US hospitals, 2010. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb149.pdf. Published February 2013. Accessed March 4, 2018.
- 2.The Joint Commission. Proceedings from the National Summit on Overuse. Available at: https://www.jointcommission.org/overuse_summit/. Published July 8, 2013. Accessed March 4, 2018.
- 3.Goodnough LT, Maggio P, Hadhazy E, et al. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54:2753–2759. [DOI] [PubMed] [Google Scholar]
- 4.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–3417. [DOI] [PubMed] [Google Scholar]
- 5.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–1186. [DOI] [PubMed] [Google Scholar]
- 6.Ferraris VA, Davenport DL, Saha SP, Bernard A, Austin PC, Zwischenberger JB. Intraoperative transfusion of small amounts of blood heralds worse postoperative outcome in patients having noncardiac thoracic operations. Ann Thorac Surg. 2011;91:1674–1680. [DOI] [PubMed] [Google Scholar]
- 7.Shishehbor MH, Madhwal S, Rajagopal V, et al. Impact of blood transfusion on shortand long-term mortality in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2009;2:46–53. [DOI] [PubMed] [Google Scholar]
- 8.Sadana D, Pratzer A, Scher LJ, et al. Promoting high-value practice by reducing unnecessary transfusions with a patient blood management program. JAMA Intern Med. 2018;178:116–122. [DOI] [PubMed] [Google Scholar]
- 9.Mazer CD, Whitlock RP, Fergusson DA, et al. ; TRICS Investigators and Perioperative Anesthesia Clinical Trials Group. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl J Med. 2017;377:2133–2144. [DOI] [PubMed] [Google Scholar]
- 10.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–1567. [DOI] [PubMed] [Google Scholar]
- 11.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. [DOI] [PubMed] [Google Scholar]
- 12.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. [DOI] [PubMed] [Google Scholar]
- 13.Holst LB, Haase N, Wetterslev J, et al. ; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–1391. [DOI] [PubMed] [Google Scholar]
- 14.Murphy GJ, Pike K, Rogers CA, et al. ; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997–1008. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix J, Hébert PC, Hutchison JS, et al. ; TRIPICU Investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators Network. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. [DOI] [PubMed] [Google Scholar]
- 16.Carson JL, Terrin ML, Noveck H, et al. ; FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson CS, Hannay HJ, Yamal JM, et al. ; Epo Severe TBI Trial Investigators. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA. 2014;312:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch CG, Sessler DI, Mascha EJ, et al. A randomized clinical trial of red blood cell transfusion triggers in cardiac surgery. Ann Thorac Surg. 2017;104:1243–1250. [DOI] [PubMed] [Google Scholar]
- 19.Bracey AW, Radovancevic R, Riggs SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070–1077. [DOI] [PubMed] [Google Scholar]
- 20.Shehata N, Forster A, Li L, et al. Does anemia impact hospital readmissions after coronary artery bypass surgery? Transfusion. 2013;53:1688–1697. [DOI] [PubMed] [Google Scholar]
- 21.Frank SM, Thakkar RN, Podlasek SJ, et al. Implementing a health system-wide patient blood management program with a clinical community approach. Anesthesiology. 2017;127:754–764. [DOI] [PubMed] [Google Scholar]
- 22.Frank SM, Resar LM, Rothschild JA, Dackiw EA, Savage WJ, Ness PM. A novel method of data analysis for utilization of red blood cell transfusion. Transfusion. 2013;53:3052–3059. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682. [DOI] [PubMed] [Google Scholar]
- 24.Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA. 2014;311:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattakos G, Koch CG, Brizzio ME, et al. Outcome of patients who refuse transfusion after cardiac surgery: a natural experiment with severe blood conservation. Arch Intern Med. 2012;172:1154–1160. [DOI] [PubMed] [Google Scholar]
- 26.Koch CG, Li L, Duncan AI, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–1616. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.