Abstract
Soft tissue integration of medical implants is important to prevent bacterial infection and implant failure. A bioadhesive that forms firm binding between the implant and the surrounding tissue and facilitates the wound-healing process will be a great tool to establish the desired tissue-implant integration. In this project, we introduce a novel method that can be used to enhance integration between any implant material and any tissue using an enzyme-crosslinked gelatin hydrogel combined with polydopamine (PDA) coating. PDA coating was shown to enhance the binding between the gelatin hydrogel and three model implant materials – aluminum, poly(methyl methacrylate) (PMMA) and titanium. When combined with the gelatin hydrogel, pig cornea tissue adhered more strongly to the PDA coated surfaces than to the uncoated surfaces. The enzyme-crosslinked gelatin hydrogel was non-cytotoxic to human dermal fibroblasts and it also allowed the cells to adhere and proliferate. Altogether, the results indicate that the combination of PDA coating with gelatin hydrogel can be used to enhance the integration of various medical implants.
Keywords: gelatin, microbial transglutaminase, polydopamine coating, implant, biointegration
1. INTRODUCTION
Medical implants have been the only viable option for the patients who underwent irreversible loss of tissues or organs. One of the main causes of implant failure is poor biointegration. Biointegration refers to a seamless interconnection between a medical implant and recipient tissues, and is usually associated with strong adhesion at their interface1. Biointegration has been considered mostly for the implants in hard bone tissues such as in orthopedic and dental implants, for which it is more popularly termed, osseointegration2. However, biointegration with soft tissues is equally important and the lack of it can result in many detrimental clinical consequences. For instance, when dental implants do not integrate with the gum tissue (gingiva), it allows bacterial infection, which can cause serious dental health issues including peri-implantitis3. Another example that highlights the importance of soft tissue biointegration is found in artificial cornea. The current polymeric artificial corneas (keratoprosthesis, or KPro) do not integrate with the surrounding cornea tissue, creating a portal for bacteria into the vitreous humor, which leads to irreversible retinal blindness. Therefore, the patients who undergo KPro procedures have to take life-long prophylactic antibiotics4.
Various surface chemistries have been explored to improve the tissue-material interface for enhanced biointegration. Common surface chemistries that are found in the literature are modifying the implant surface with extracellular matrix (ECM)-derived molecules such as collagen and fibronectin 5–7, cell adhesive peptides8–10 or inorganic materials11–13. However, most surface chemistries developed thus far have had limited success in achieving the desired tissue-implant integration 14–17. Moreover, most of these immobilization methods are material-specific.
One alternative approach to improve biointegration is to apply a biodegradable adhesive at the tissue-implant interface, which can initially bind the tissue with the implant and serve as a temporary matrix to facilitate the wound-healing process at the tissue-material interface. However, the requirement of biocompatibility and biodegradability significantly reduces the choices of the bioadhesives and finding an adhesive that forms stable bonds both with tissues and with implant surfaces is very challenging.
In this research, we propose a novel method to improve biointegration of medical implants by gelatin hydrogel crosslinked with microbial transglutaminase (mTG), combined with polydopamine (PDA) coating of the implant surface. This method is not material- or tissue-specific, and can be applied to all types of medical implants and all types of human tissues.
We used gelatin hydrogels as an adhesive to attach a tissue to material surfaces. Gelatin is a protein derived from collagen and has been widely used as a biocompatible and biodegradable material to interface human tissues18–20. When mixed with mTG, gelatin not only crosslinks to form a hydrogel, but also can form covalent bonds with proteins of tissues because mTG catalyzes amide bond formation between glutamine and lysine residues, which gelatin and tissue proteins have in abundance21. However, gelatin hydrogel alone does not have good adhesion to the implant surfaces22.
To enhance adhesion between the gelatin hydrogel and the implant surface, PDA surface chemistry was employed. PDA forms a stable film virtually on all surfaces, including metals, metal oxides and polymers, as dopamine polymerizes at high pH23. Once PDA coating is formed, any amine- or thiol-containing molecules can be covalently immobilized on PDA-coated surfaces through Michael-type addition24. Therefore, when gelatin hydrogel is applied on PDA-coated implant surface, the primary amines of gelatin will form covalent bonds with PDA, establishing a firm adhesion between gelatin and implant surface. Since PDA can form on virtually any material surfaces and covalently bind to gelatin, and mTG can crosslink gelatin with the proteins from any tissues, this method in theory can be used to attach any implant materials to any tissues (Figure 1).
Figure 1. Schematic illustration of the use of gelatin adhesive and PDA-coating to enhance biointegration of medical implant.
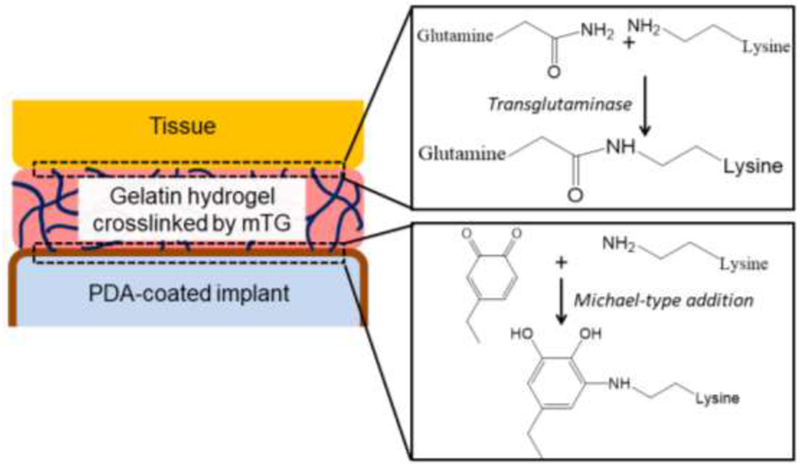
mTG ‘glues’ gelatin-tissue interface by transamidation between glutamine and lysine residues. The quinone groups from the PDA coating forms covalent bonds with the amine groups of gelatin through Michael-type addition.
2. MATERIALS AND METHODS
2.1. Materials
Gelatin (Type 1, from bovine and porcine bones), dopamine hydrochloride, Trizma base were purchased from Sigma-Aldrich (St. Louis, MO). Microbial transglutaminase (mTG) was purchased from Ajinomoto (Fort Lee, NJ). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin and alamarBlue were purchased from Invitrogen (Frederick, MD). Phosphate Buffer Saline (PBS) was purchased from Fisher Scientific (Hampton, NH). Aluminum stubs (1/2’’ slotted head, 1/8’’ pin) were obtained from Ted Pella (Redding, CA). Poly(methyl methacrylate) (PMMA) and Titanium stubs (0.1cm thickness and 1.2cm in diameter) were purchased from JG Machine Company Inc (Wilmington, MA).
2.2. Gelatin hydrogel formation and rheological characterization
Gelatin was dissolved in neutral phosphate buffered saline (PBS) at 40°C to make 20% w/v solution. Microbial transglutaminase (mTG) at 20% w/v concentration was mixed with gelatin at 1:1 ratio to make 10% gelatin and 10% mTG solution. To make 10% gelatin and 4% mTG, gelatin and mTG was mixed at 1:0.4 ratio and PBS was added to adjust the concentration of gelatin to 10%. The crosslinking was done at 37°C in a humidified chamber. The gelatin hydrogel was then characterized with a rheometer (TA Instruments AR 550). A plane geometry of 2cm in diameter was used. A stress sweep was first performed to identify the linear viscoelasticity regime. For the time sweep, an oscillatory stress of 2 Pa at 10 rad/s was applied at 37°C.
2.3. Polydopamine coating and surface characterizations
Materials used in this project were aluminum, titanium and poly(methyl methacrylate) (PMMA). The polydopamine (PDA) coating was formed through a polymerization reaction of dopamine in 10 mM Tris buffer (pH 8.5) overnight under constant shaking. After PDA coating, the materials were thoroughly washed with DI water and air-dried.
The surface chemistry was characterized by Kratos Axis Ultra spectrometer (Kratos Analytical, Manchester, United Kingdom) equipped with a monochromatized Al Ka source.
2.4. Gelatin hydrogel-mediated adhesion test between the two identical material surfaces
A small volume of gelatin hydrogel (~30μL) was applied between the two surfaces. The surfaces were either coated with PDA or left uncoated. Once the gelatin hydrogel was cured at 37°C in a humidified chamber, the surfaces that were fixed at both ends were pulled in opposite direction using a mechanical tester (Instron) at the rate of 10 mm/min. The forces required to separate the surfaces were measured.
2.5. Tissue adhesion test
Fresh pig corneas were used to test the tissue-material adhesion. The pig corneas were extracted from the freshly obtained pig eyeballs using surgical scissors and a razor blade. The dissected pig cornea was placed on top of the material that was covered with gelatin and mTG mixture (volume = 50 µL). Both unmodified and PDA-coated surfaces were tested. The assembly was cured at 37°C in a hydrated environment overnight. For the force measurements, the free end of the cornea was fixed by tightening the grips of the mechanical tester against the cornea. Force was measured as a function of displacement at the rate of 10 mm/min.
2.6. Cytotoxicity test
Human dermal fibroblasts (hDFs), representing the resident cells of soft tissues, were cultured in a 24-well plate with Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cells of low passage number (< 5) were used for all studies. Once the cells reached 70% confluence, sterile gelatin and mTG solutions were mixed in a transwell insert to form a hydrogel (volume = 200 µL, 2 hour incubation at 37°C), which were then inserted in the wells of a 24-well plate in which hDFs were cultured. Timewise cell proliferation in the presence of mTG-crosslinked gelatin hydrogel was measured by almarBlue at day 1, day 4 and day 7. The fluorescence at 600 nm (excitation at 560nm) was measured and compared with the negative samples containing no cells. The proliferation was normalized to the group that was not exposed to the hydrogels at each time point.
After fixing the cells in 4% formaldehyde in PBS for 15 minutes, TUNEL assay was performed to visualize the occurrence of apoptotic cells, using Click-it® TUNEL assay from Life Technologies (Carlsbad, CA). This assay utilizes the binding of dUTP to fragmented DNA strands, followed by the addition of a azide-labeled fluorophore, allowing fluorescence microscope-based detection of apoptotic cells.
2.7. Cell adhesion test
Gelatin hydrogels (400μL) were formed in the wells of a 24-well plate by mixing sterile gelatin and mTG solutions. The gelatin hydrogels were cured for 2 hours under 37°C. hDFs were then seeded on each well at 3.5×103 cells/cm2. hDFs were allowed to grow for 7 days. Cell images were taken to observe adhesion and proliferation on the hydrogel surfaces. The cells were fixed in 4% formaldehyde in phosphate buffered saline (PBS) for 15 minutes, and stained with rhodamine-phalloidin (to stain actin cytoskeleton) and DAPI (to stain cell nuclei). Fluorescence microscope images were taken using EVOS FL Imaging System (Thermofisher, Waltham, MA).
2.8. Statistics
The data are presented as means ± standard deviation unless stated otherwise. The statistical significance of two sample groups was assessed by student-t test. For the statistical significance among multiple sample groups, ANOVA was performed followed by Tukey’s post hoc test using Origin 8.1.
3. RESULTS and DISCUSSIONS
3.1. Rheological Characterization of Gelatin Hydrogel
The gelation kinetics and viscoelastic properties of mTG-crosslinked gelatin hydrogel were characterized by rheometer. A time-sweep of the gelatin hydrogel (10% w/v) formation was recorded using two different mTG concentrations (4% w/v mTG, and 10% w/v mTG). In both cases, the elastic modulus (G’) was always higher than the viscous modulus (G’’) within minutes after mTG was added to the gelatin solution (Figure 2). When 10% (w/v) mTG was mixed with 10% gelatin, the gelation completed within 10 minutes, whereas it took more than 30 minutes when 10% gelatin was crosslinked with 4% mTG. The viscous moduli for both mTG concentrations were similar. Quick gelation time is desired during the implantation surgeries. 10% mTG also resulted in more rigid hydrogels. Therefore, 10% gelatin/10% mTG hydrogel was used in the later experiments.
Figure 2.
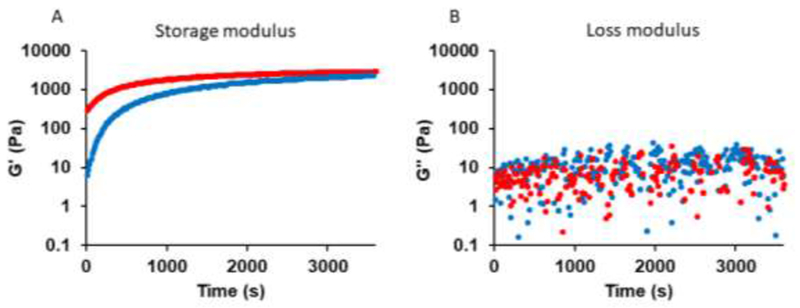
(A) G’ (storage modulus) and (B) G’’ (loss modulus) as a function of time for mTG-crosslinked gelatin hydrogels. Red and blue markers represent the mixture of 10% gelatin/10% mTG and 10% gelatin/4% mTG, respectively.
3.2. Characterization of PDA surface chemistry
PDA coating was achieved by immersing the test materials in a dopamine-containing alkaline buffer overnight. Aluminum (Al), poly(methyl methacrylate) (PMMA) and titanium (Ti) were chosen as model implant materials to demonstrate the universality of this method. The formation of uniform PDA coating on all surfaces was confirmed by two observations using X-ray photoelectron spectroscopy (XPS). First is the disappearance of the characteristic elemental peaks from XPS. From the survey and high-resolution spectra, aluminum and titanium peaks disappeared after the PDA coating (Figure 3A, Figure S1-S3). There were no characteristic elemental peaks for PMMA because dopamine and PDA all contained C, O and H (and additional N). Instead, we monitored the disappearance of the peaks at 288.5 eV in the high resolution spectra for C1s which exists only in PMMA. The successful formation of PDA film on all three material surfaces was also confirmed by the appearance of the identical C1s peaks (Figure 3B-D).
Figure 3. Confirmation of PDA coating by XPS.
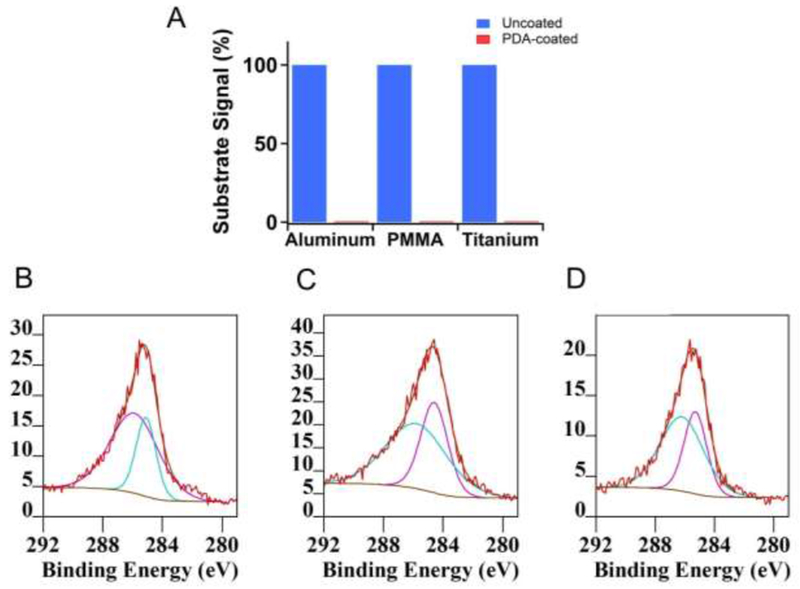
(A) Substrate characteristic XPS signals before and after PDA coating. Al 2p (73 eV), C1s (CO-O bond, 288.5 eV) and Ti2p (455 eV) signals were used for comparisons. (B)-(D) C 1s peaks of PDA-coated surfaces. (B) Aluminum, (C) PMMA, (D) titanium.
3.3. Enhanced Adhesion between Implant Surfaces by PDA coating and gelatin hydrogel
One of the main hypotheses of this research is that PDA coating will enhance adhesion between gelatin hydrogel and the PDA-coated implant surface due to the covalent bond formation between the primary amines of gelatin and quinones of PDA coating. In order to test this hypothesis, we measured the adhesion strength between two material surfaces attached through a gelatin hydrogel (Figure 4A). When the gelatin hydrogel was fully cured with mTG between uncoated surfaces or PDA-coated surfaces, the tensile force to completely separate the surfaces was measured using a mechanical tester. Gelatin hydrogel had relatively weak adhesion strength to the uncoated material surfaces, with the rupture tensile stress ranging from 18 to 48 kPa, because the interactions between hydrogel and the surface were purely physical. However, when PDA coating was added to the surface, the adhesion between the gelatin hydrogel and the implant surfaces significantly increased for all three materials tested (Figure 4B). The increased adhesion is attributed to the covalent bond formation between the quinones of PDA coating and the lysine and arginine residues of gelatin hydrogel. The gelatin hydrogel was formed in a PBS at pH 7.4, which favors the formation of quinones and covalent attachment of amines.
Figure 4. The adhesion strength between two surfaces glued by gelatin hydrogel (10% w/v gelatin and 10% w/v mTG).
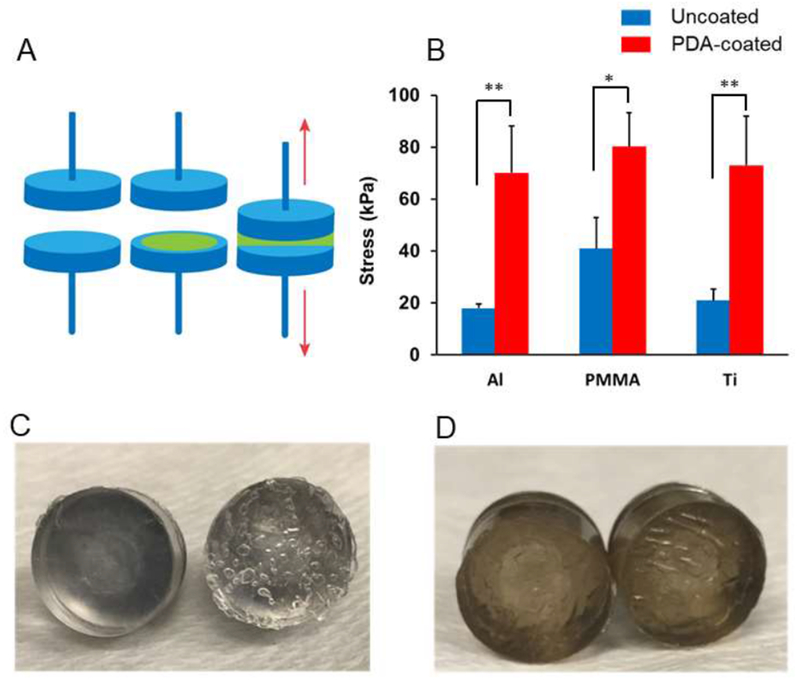
(A) The gelatin hydrogel was applied between two material surfaces and the force that separated the surface completely was measured. (B) PDA coating enhanced the adhesion between the two surfaces. * p < 0.05, ** p < 0.01 (n = 4). When separated, the gelatin hydrogel was found only on one side of the surface when the surfaces were not coated with PDA (C) but for PDA-coated surfaces, the gelatin adhesive was found on both sides (D).
After the adhesion test, it was observed that the gelatin hydrogel remained only on one of the two surfaces for uncoated materials (Figure 4C), whereas the hydrogel was found on both sides for the PDA-coated materials (Figure 4D). The area that was covered by hydrogel after the adhesion test quantitatively confirms this fact (Figure S4). These results indicate that the weakest link of the adhesion was the hydrogel-material interface for the uncoated materials whereas it was the cohesion of the hydrogel for the PDA-coated materials, which further supports our hypothesis that covalent bonds form between the PDA coating and the gelatin hydrogel. The formation of covalent bond formation between PDA coating and gelatin hydrogel is consistent with the previously published work25. The trend was identical for aluminum, PMMA and titanium, and there was no significant difference among the PDA-coated groups (p > 0.05). It was due to the universal nature of PDA coating. As long as the adhesion between hydrogel and surface is weaker than the cohesion within the hydrogel, PDA coating will enhance the binding between hydrogel and the surface. PDA coating has been utilized to covalently immobilize proteins, peptides and other biological macromolecules26–28. However, to the best of our knowledge, this is the first report that demonstrated the mechanical stability of the binding between PDA coating and hydrogels.
3.4. Tissue Adhesion Test
To test if mTG-crosslinked gelatin combined with PDA coating enhances adhesion between an implant surface and a tissue, adhesion between porcine cornea tissues and the model implant materials was measured. A freshly collected porcine cornea was placed on a test material through the mixture of gelatin and mTG (Figure 5A). Three things happened at the interface: (1) gelatin and cornea were crosslinked by mTG, (2) gelatin itself was crosslinked by mTG and formed a hydrogel, and (3) gelatin hydrogel adhered to the material surface either through physical binding (for uncoated materials) or a covalent bond (for PDA-coated materials). Once gelatin was fully cured overnight, the cornea and the material were mounted on a mechanical tester, and were pulled until the material and the tissue were completely separated as the force was measured. Unlike the previous material-material detachment, the tissue-material detachment process took place over a long displacement because of the stretching of the tissues during the measurement. As the tissue was pulled from the material surface, the force increased until the rupture began to take place (Figure 5B). The tensile adhesive strength was calculated by dividing the maximum tensile force by the contact area between the tissue and the material. Just as in the previous material-material adhesion test, addition of PDA coating significantly enhanced the adhesion between the tissue and the material surface for all three materials (Figure 5C), with the tensile strength ranging between 40 – 58 kPa. Although there were differences in the measured stresses among the PDA-coated groups, likely due to the slight sample-to-sample variations of the cornea tissue structures, there was no statistical significance of the differences (p > 0.05). Consistently, the weakest link during the measurement was the hydrogel-material interface for the uncoated surfaces and the cohesion of the hydrogel for the PDA-coated surfaces.
Figure 5. The adhesion strength between a material surface and a cornea tissue glued by a gelatin hydrogel.
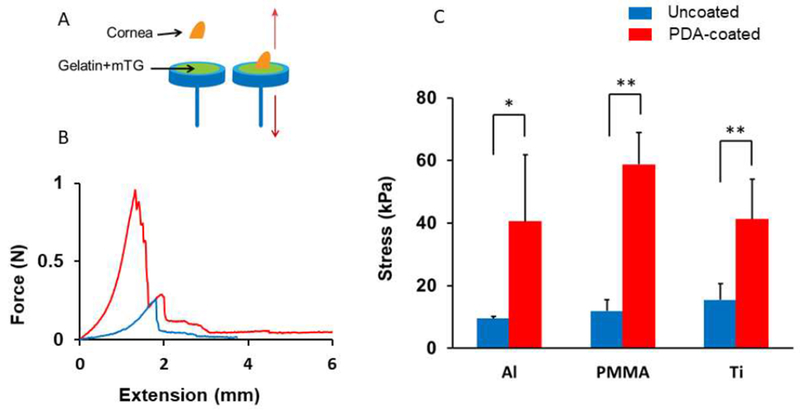
(A) The hydrogel was applied between a material and a porcine cornea, and the force was measured as the two were separated. (B) A representative force-extension curve for PDA-coated (red) and noncoated surface (blue). (C) PDA coating enhanced the adhesion between the tissue and the material. * p < 0.05, **p<0.01, (n = 4).
Although only cornea tissues were tested in this research, the same approach can be applied to any tissues such as skin and bone tissues, because all tissues contain proteins and can be crosslinked with gelatin through mTG as long as the tissue proteins contain either lysine or glutamine29.
In this study, the mechanical tests were performed one day after the material and tissue were assembled in a non-sterile environment. The viability of the tissue cells was not characterized. Therefore, long-term effects of the initial adhesion on the eventual biointegration should be tested in a more controlled environment. However, short-term studies have been widely used to test tissue adhesives29, and it is well known that the initial fixation between the implant and the surrounding tissue is a major determinant of the long-term biointegration30. Once the initial adhesion is established, the host cells can migrate through the hydrogel and deposit the native ECM at the implant-tissue interface further enhancing long-term biointegration. In case of implant-tissue detachment, the adhesion can be easily restored by the addition of gelatin and mTG, because the detachment will most likely be within the gelatin hydrogel itself.
3.5. Cytotoxicity and cell adhesion
Both gelatin and mTG are natural biodegradable materials and have been used in many applications for tissue engineering 31–33. We tested the cytotoxicity of gelatin and mTG mixture at two different mTG concentrations (4 and 10%) using human dermal fibroblasts (hDFs). hDFs were cultured in 24 well plates, and the gelatin was crosslinked by mTG in transwell inserts (Figure 6A). The cells were exposed to the crosslinked hydrogels through the transwell inserts and the cellular proliferation was measured by the alamarBlue assay on days 1, 4 and 7 (Figure 6B). Regardless of the concentration of mTG (4% and 10%) as crosslinking agent for gelatin hydrogels, the proliferation of hDFs was unaffected throughout the duration of the study, indicating that the mTG-crosslinked gelatin hydrogels or the unreacted mTG was not cytotoxic.
Figure 6.
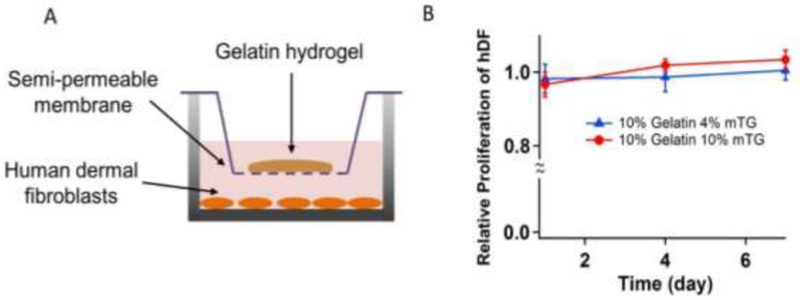
(A) hDFs were cultured while gelatin was crosslinked by mTG. (B) alamarblue proliferation assay. Gelation of gelatin by mTG did not affect hDF proliferation. The data were normalized to the proliferation of hDFs with no exposure to gelatin hydrogels (n = 4).
After the proliferation assay was completed, the TUNEL assay was performed to visualize the presence of apoptotic cells (Figure 7). None of the groups that were tested (no hydrogel, 10% gelatin/4% mTG, 10% gelatin/10% mTG) showed any indication of apoptosis. Since the gelation is completed within the first hour, this seven-day study results indicate that gelatin crosslinking by mTG will have minimal toxicity to the host when implanted.
Figure 7.
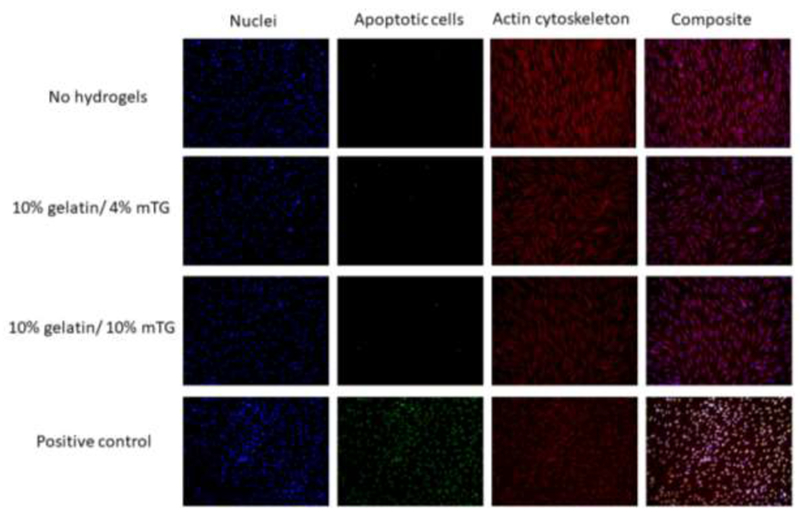
Apoptosis assay. The hDFs cultured in 24 well plates were exposed to no hydrogels, 10% gelatin/4% mTG hydrogels and 10% gelatin/10% mTG hydrogels through the transwell inserts over 7 days. Cell nuclei were stained blue with DAPI, apoptotic cells were stained green with TUNEL assay and actin cytoskeleton was stained red with rhodamine-labeled phalloidin. The positive control was generated by treating the no-hydrogel samples with DNase I. The composite images were generated by merging all three channels (blue, green, red) using imageJ.
Another component of the system is PDA coating. PDA coating has been found to make various material surfaces more biocompatible34. For example, PDA-coating has been found to enhance cell adhesion and proliferation for human corneal epithelial cells and human corneal fibroblasts on PMMA surfaces35.
For the method described in this article to be clinically relevant, the gelatin hydrogel should not only be non-cytotoxic but also facilitate cell adhesion and proliferation for the optimal tissue-material integration. hDFs were seeded directly on mTG-crosslinked gelatin hydrogels (Figure 8A). After 7 days of culture, hDFs were fixed and stained with rhodamine-phallodin and DAPI, which stains actin cytoskeleton and cell nuclei, respectively. The fluorescent images showed that the cells cultured on gelatin hydrogels adhered and proliferated as well as on the tissue culture polystyrene (TCPS) surface (Figure 8B, C), which is the first step of biointegration. Since gelatin hydrogel is biodegradable, the host cells such as fibroblasts can migrate through the action of various gelatinases (e.g. MMP-2, MMP-9)36 and secrete the native ECM, accomplishing biointegration.
Figure 8.
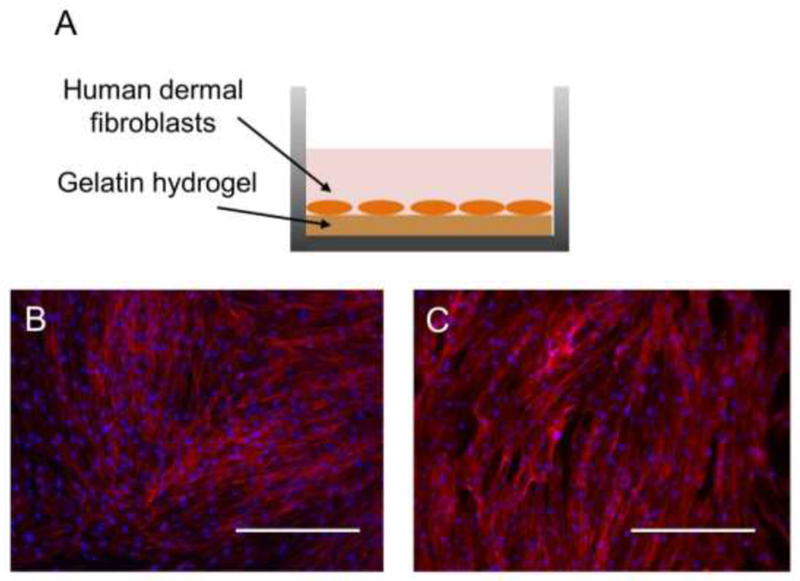
(A) hDFs were cultured directly on the surface of the gelatin hydrogel or on the tissue culture polystyrene (TCPS). (B) hDFs adhered and proliferated on the gelatin hydrogel (B) as well as on TCPS (C). Blue = cell nuclei, red = actin cytoskeleton. Scale bar = 400 µm.
In the current method, adhesion between the hydrogel and the implant surface mainly depends on the strength of the hydrogel. We utilized unmodified gelatin due to its low price and the ease of chemistry. Alternative methods can be easily applied to further enhance the mechanical property of the hydrogel. For example, silk hydrogels, which are known to have much higher tensile strength, can be used instead of, or in combination with gelatin hydrogels37,38. Utilizing an interpenetrating network (IPN) with a different hydrogel is another option39.
Another important factor is the stability of the PDA coating. In a short-term, we showed here that PDA coating is mechanically stable and withstands high external forces. The long-term stability of PDA coating is well-documented in the literature40–42, although it remains to be seen if PDA coating can withstand the same level of external forces in vivo.
4. CONCLUSION
The biointegration of medical implants is important to prevent bacterial infection and implant failure. This project proposes a method of combining an mTG-crosslinked gelatin hydrogel and PDA coating to increase the adhesion between any type of implants and any type of tissues. PDA coating can form on virtually all materials, and mTG-crosslinked gelatin can form covalent bonds with both PDA and any tissues. When applied to aluminum, titanium, and PMMA, three widely used materials as medical implants, PDA coating combined with gelatin hydrogel significantly increased the adhesion between the implant surface and pig cornea tissues. The mTG-crosslinked gelatin hydrogels were not cytotoxic to hDFs and enabled their adhesion and proliferation. This novel method can be applied to the interface of various medical implants and human tissues for enhanced biointegration.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by an NIH COBRE Center of Integrated Biomedical and Bioengineering Research (CIBBR, P20 GM113131) through an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences.
Footnotes
SUPPORTING INFORMATION: XPS survey spectra, C1s high resolution spectra, area covered by the hydrogel after the mechanical testing.
REFERENCES
- 1.Jeong KJ & Kohane DS Surface modification and drug delivery for biointegration. Therapeutic delivery 2, 737–752 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Raphel J, Holodniy M, Goodman SB & Heilshorn SC Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 84, 301–314, doi: 10.1016/j.biomaterials.2016.01.016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rompen E, Domken O, Degidi M, Pontes AE & Piattelli A The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res 17 Suppl 2, 55–67, doi: 10.1111/j.1600-0501.2006.01367.x (2006). [DOI] [PubMed] [Google Scholar]
- 4.Behlau I et al. Infectious endophthalmitis in Boston keratoprosthesis: incidence and prevention. Acta Ophthalmol 92, e546–555, doi: 10.1111/aos.12309 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Bellis SL Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 32, 4205–4210, doi: 10.1016/j.biomaterials.2011.02.029 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sverzut AT et al. Effects of type I collagen coating on titanium osseointegration: histomorphometric, cellular and molecular analyses. Biomed Mater 7, 035007, doi: 10.1088/1748-6041/7/3/035007 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R et al. Simple coating with fibronectin fragment enhances stainless steel screw osseointegration in healthy and osteoporotic rats. Biomaterials 63, 137–145, doi: 10.1016/j.biomaterials.2015.06.025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyes CD, Petrie TA, Burns KL, Schwartz Z & Garcia AJ Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 28, 3228–3235, doi: 10.1016/j.biomaterials.2007.04.003 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagel M et al. Multifunctional coating improves cell adhesion on titanium by using cooperatively acting peptides. Angewandte Chemie International Edition 55, 4826–4830 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Alas GR, Agarwal R, Collard DM & Garcia AJ Peptide-functionalized poly[oligo(ethylene glycol) methacrylate] brushes on dopamine-coated stainless steel for controlled cell adhesion. Acta Biomater 59, 108–116, doi: 10.1016/j.actbio.2017.06.033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L et al. Hydroxyapatite for keratoprosthesis biointegration. Investigative ophthalmology & visual science 52, 7392–7399, doi: 10.1167/iovs.11-7601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riau AK et al. Functionalization of the Polymeric Surface with Bioceramic Nanoparticles via a Novel, Nonthermal Dip Coating Method. ACS applied materials & interfaces 8, 35565–35577, doi: 10.1021/acsami.6b12371 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Riau AK, Venkatraman SS, Dohlman CH & Mehta JS Surface Modifications of the PMMA Optic of a Keratoprosthesis to Improve Biointegration. Cornea 36 Suppl 1, S15–s25, doi: 10.1097/ico.0000000000001352 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Schliephake H et al. Effect of RGD peptide coating of titanium implants on periimplant bone formation in the alveolar crest. An experimental pilot study in dogs. Clin Oral Implants Res 13, 312–319 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Petrie TA, Capadona JR, Reyes CD & Garcia AJ Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials 27, 5459–5470, doi: 10.1016/j.biomaterials.2006.06.027 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Broggini N et al. Evaluation of chemically modified SLA implants (modSLA) biofunctionalized with integrin (RGD)- and heparin (KRSR)-binding peptides. Journal of biomedical materials research. Part A 100, 703–711, doi: 10.1002/jbm.a.34004 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Korn P et al. Chondroitin sulfate and sulfated hyaluronan-containing collagen coatings of titanium implants influence peri-implant bone formation in a minipig model. Journal of biomedical materials research. Part A 102, 2334–2344, doi: 10.1002/jbm.a.34913 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Rose JB et al. Gelatin-Based Materials in Ocular Tissue Engineering. Materials (Basel, Switzerland) 7, 3106–3135, doi: 10.3390/ma7043106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue K et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73, 254–271, doi: 10.1016/j.biomaterials.2015.08.045 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattazzo F et al. Gelatin-genipin-based biomaterials for skeletal muscle tissue engineering. Journal of biomedical materials research. Part B, Applied biomaterials, doi: 10.1002/jbm.b.34057 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Kieliszek M & Misiewicz A Microbial transglutaminase and its application in the food industry. A review. Folia Microbiol (Praha) 59, 241–250, doi: 10.1007/s12223-013-0287-x (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HW, Kim HE & Salih V Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials 26, 5221–5230, doi: 10.1016/j.biomaterials.2005.01.047 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Lee H, Dellatore SM, Miller WM & Messersmith PB Mussel-inspired surface chemistry for multifunctional coatings. Science (New York, N.Y.) 318, 426–430, doi: 10.1126/science.1147241 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Rho J & Messersmith PB Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv Mater 21, 431–434, doi: 10.1002/adma.200801222 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van De Walle E et al. Polydopamine-Gelatin as Universal Cell-Interactive Coating for Methacrylate-Based Medical Device Packaging Materials: When Surface Chemistry Overrules Substrate Bulk Properties. Biomacromolecules 17, 56–68, doi: 10.1021/acs.biomac.5b01094 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Ko E, Yang K, Shin J & Cho SW Polydopamine-assisted osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromolecules 14, 3202–3213, doi: 10.1021/bm4008343 (2013). [DOI] [PubMed] [Google Scholar]
- 27.Lim K et al. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater 15, 127–138, doi: 10.1016/j.actbio.2014.12.015 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Toma M & Tawa K Polydopamine Thin Films as Protein Linker Layer for Sensitive Detection of Interleukin-6 by Surface Plasmon Enhanced Fluorescence Spectroscopy. ACS applied materials & interfaces 8, 22032–22038, doi: 10.1021/acsami.6b06917 (2016). [DOI] [PubMed] [Google Scholar]
- 29.McDermott MK, Chen T, Williams CM, Markley KM & Payne GF Mechanical properties of biomimetic tissue adhesive based on the microbial transglutaminase-catalyzed crosslinking of gelatin. Biomacromolecules 5, 1270–1279, doi: 10.1021/bm034529a (2004). [DOI] [PubMed] [Google Scholar]
- 30.Javed F, Ahmed HB, Crespi R & Romanos GE Role of primary stability for successful osseointegration of dental implants: factors of influence and evaluation. Interventional Medicine and Applied Science 5, 162–167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuwahara K, Yang Z, Slack GC, Nimni ME & Han B Cell delivery using an injectable and adhesive transglutaminase-gelatin gel. Tissue engineering. Part C, Methods 16, 609–618, doi: 10.1089/ten.TEC.2009.0406 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Bode F et al. Hybrid processes in enzymatically gelled gelatin: impact on , macroscopic properties and cellular response. Soft matter 9, 6986–6999, doi: 10.1039/c3sm00125c (2013). [DOI] [PubMed] [Google Scholar]
- 33.Yang G et al. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 4, e2497, doi: 10.7717/peerj.2497 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding YH, Floren M & Tan W Mussel-inspired polydopamine for bio-surface functionalization. Biosurface and biotribology 2, 121–136, doi: 10.1016/j.bsbt.2016.11.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong KJ et al. Polydopamine coatings enhance biointegration of a model polymeric implant. Soft Matter 7, 8305–8312 (2011). [Google Scholar]
- 36.Lindner D et al. Differential expression of matrix metalloproteases in human fibroblasts with different origins. Biochemistry research international 2012, 875742, doi: 10.1155/2012/875742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elliott WH et al. Silk Hydrogels of Tunable Structure and Viscoelastic Properties Using Different Chronological Orders of Genipin and Physical Cross-Linking. ACS applied materials & interfaces 7, 12099–12108, doi: 10.1021/acsami.5b02308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim UJ et al. Structure and properties of silk hydrogels. Biomacromolecules 5, 786–792, doi: 10.1021/bm0345460 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Xiao W et al. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater 7, 2384–2393, doi: 10.1016/j.actbio.2011.01.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X et al. Mussel-inspired polydopamine: a biocompatible and ultrastable coating for nanoparticles in vivo. ACS nano 7, 9384–9395, doi: 10.1021/nn404117j (2013). [DOI] [PubMed] [Google Scholar]
- 41.Zeng R, Luo Z, Zhou D, Cao F & Wang Y A novel PEG coating immobilized onto capillary through polydopamine coating for separation of proteins in CE. Electrophoresis 31, 3334–3341, doi: 10.1002/elps.201000228 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Wei H et al. Stability of polydopamine and poly(DOPA) melanin-like films on the surface of polymer membranes under strongly acidic and alkaline conditions. Colloids and surfaces. B, Biointerfaces 110, 22–28, doi: 10.1016/j.colsurfb.2013.04.008 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


