Abstract
Objective:
To test the hypothesis that Physical Activity (PA) improves cardiovascular disease (CVD)-related lipids beyond that associated with weight loss in adolescents with severe obesity, post-metabolic bariatric surgery (MBS).
Methods:
We used objective StepWatch™ PA data from 108 participants of the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study from baseline to 3 years post-MBS. Primary outcomes included absolute change in low density lipoprotein (LDL-C) and non-high density lipoprotein (non-HDL-C) from baseline. We adjusted for baseline measurement, visit, surgical procedure, and percent change in iliac waist circumference (IWC) or body mass index (BMI) from baseline in linear regression models using generalized estimating equations. PROC TRAJ in SAS generated optimal activity trajectories based on individual step count.
Results:
Despite low step counts and slow cadence, differences by activity trajectory were found. Greater absolute decreases in LDL-C and non-HDL-C (−15 mg/dL [95% CI: (−28, −2)], p =0.026 and −15 mg/dL [95% CI: (−28, −1)], p = 0.035), respectively, were associated with more activity (MA). MA was associated with greater resolution of triglycerides, LDL-C and non-HDL-C dyslipidemia and with greater weight loss 3 years post-MBS.
Conclusion:
More activity in adolescents was associated with improvements in CVD-related lipid measures and weight loss, post-MBS.
Keywords: physical activity, bariatric surgery, severe obesity, adolescent
INTRODUCTION
Severe obesity has long been recognized to increase cardiovascular disease (CVD) risk in adults and adolescents (1–3). Poor cardiovascular health is strongly associated with obesity (4) and cardiovascular health has been demonstrated to decrease with obesity severity (4, 5). The prevalence of severe obesity in childhood and adolescence, defined as ≥120% of the 95th percentile of BMI for sex and age, continues to rise and now impacts millions of youth (6). Obesity in adolescence tracks into adulthood, increasing the risk of cardio-metabolic morbidity 15-fold (7). Dyslipidemia in adolescence also predicts CVD risk in adulthood, particularly high LDL-C and non-HDL-C (3). Both obesity and dyslipidemia have been identified as important treatment targets in childhood and adolescence for the reduction of lifelong CVD risk, and metabolic bariatric surgery (MBS) has been demonstrated to effectively treat severe obesity and improve dyslipidemia in adolescents (3, 8).
Physical activity (PA) also improves lipid levels, reduces CVD risk and supports weight loss maintenance. However, PA participation wanes in adolescence (9, 10). At age 18, daily step counts of 8,000 – 9,000 steps/day at a moderate cadence of >87 steps per minute have been reported in adolescents of healthful weight (11, 12). Severe obesity is associated with approximately 50% fewer steps/day, slower cadence and more sedentary time (13, 14). Assessments of PA in adolescents with severe obesity, pre-and post-MBS, are sparse and largely based upon self-report, which has been repeatedly shown to poorly estimate actual PA (15, 16). PA participation and its potential to further improve lipid measures post-MBS have been examined in adults, but not in adolescents (17).
This evaluation examined the influence of PA on lipid changes in adolescents with severe obesity from baseline to 3 years post-MBS. We hypothesized that objectively measured PA would be positively associated with improvement in lipid measures. Study participants were enrolled in the Teen-LABS study, which collected longitudinal, prospective, observational clinical and laboratory data in adolescents undergoing MBS at five centers in the United States.
METHODS
Design
This analysis was designed to examine associations between objectively measured PA and changes in lipid measures before and after MBS (Roux-en-Y gastric bypass [RYGB, n=92] and vertical sleeve gastrectomy [VSG, n=16]), in adolescents. PA was defined by daily step counts and cadence (steps per minute). Moderate activity was defined as a cadence >80 steps per minute, as described by King et al. (18). The methods used for data collection have been described previously (19). The original protocol and data and safety monitoring plans were approved by the institutional review board (IRB) at each of the five participating institutions and by a data and safety monitoring board. All participants and/or their legally-authorized representatives provided written informed consent. The analysis plan was also reviewed and approved by the IRB at the University of Colorado, Anschutz Medical Campus.
Participants
Adolescents with severe obesity who participated in the Teen-LABS study received MBS (VSG or RYGB) and completed objective measurements of PA were included (19). More specifically, for inclusion in the analyses, participants had 1) StepWatch™ activity data for a minimum of 3 days (2 weekdays and 1 weekend day), 2) at least 6 hours with at least 10 steps per hour per day of recorded data, 3) data collection at baseline and at least one postoperative time point (6 months, 1 year, 2 years or 3 years), 4) time point-matched laboratory and anthropometric data and 5) no lipid-lowering medication(s). Original, objectively measured Teen-LABS data was used for all analyses. No significant differences were noted in demographics or baseline outcomes between the included and the excluded participant groups.
Outcomes of interest
The primary outcome of interest was change in lipid measures of cardiovascular risk, primarily non-HDL-C and LDL-C, calculated using the Friedewald equation and secondarily, HDL-C, plasma total cholesterol (TC), and triglycerides (TG). The clinical significance of the changes in lipid measures was also examined as percent resolution of dyslipidemia, defined as a change in individual lipid measures from outside of acceptable range at baseline to acceptable range at 1 and 2 years based upon 2011 guidelines from the National Heart Lung and Blood Institute (20). Additional secondary outcomes included weight change as iliac waist circumference (IWC) and BMI. Activity trajectory membership was the independent variable of interest and covariates chosen a priori included sex, race, visit and surgical procedure.
Statistical Analysis
The SAS PROC TRAJ procedure was used for latent class modeling to separate participants by categorical activity trajectories using the average step count data for a visit. The optimal model was identified based on the process described by Andruff et al. to select the number of categorical activity trajectories and their respective shape (e.g., linear vs. quadratic) (21). Based upon this optimal model, two categories were identified that we label “less active” (LA) and “more active” (MA), that were linear and quadratic in shape, respectively. The probability of group trajectory membership for each individual was determined from the individual’s trajectory of average step counts across all available time points. The analyses examined lipid and anthropometric outcomes by time periods of clinical relevance including baseline to 1 year as a period of active weight loss and baseline to 2 years and 3 years, as periods of relative weight-loss maintenance.
Descriptive summaries included mean and standard deviation for continuous measures and count and percent of total for discrete measures. Baseline comparisons between activity categories were made using two-sample t-tests for continuous outcomes and two-sample tests of proportions for dichotomous outcomes. Triglyceride values were log-transformed.
Analysis of change in outcomes by trajectory categories used linear regression with generalized estimating equations, assuming an exchangeable working correlation structure to account for the longitudinal, correlated nature of our data to estimate the change from baseline to a measured period of time or visit. Change in lipid outcome from baseline were fit, adjusting for percent change from baseline to a given visit for both IWC and BMI as independent covariates of weight loss in order to examine the impact of general obesity as BMI and central obesity as IWC. The model also potentially included covariates for baseline lipid, sex, surgical procedure type, race/ethnicity (white, non-Hispanic vs. all others), PA trajectory group, study visit indicator, and the interaction between trajectory and visit indicator. To improve interpretability, reduced models were created by iteratively removing sex or race if the adjusted p>0.1, and the model refit before evaluating significance of the remaining covariate. Additionally, the interaction was removed if the adjusted p>0.05. Similar models were fit for change in central adiposity measure, but with percent change in the covariate excluded from the model. Graphs were created to illustrate individual and activity category trajectories, plotting average step count over time from baseline to specified visits and changes in the lipid and anthropometric measures of interest over time, as well. SAS v9.4 (SAS Institute Inc., Cary, NC, USA) was used for identifying trajectories and R v3.5.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for all other analyses and figures.(22)
RESULTS
Participants
Of the 242 adolescents enrolled in the Teen-LABS study, 125 provided PA data generated from wearing a StepWatch™ activity monitor. Of the 125 participants with objective PA data, 108 met criteria for inclusion in these analyses, with 14 classified as MA (mean probability of 97.8%) and 94 as LA (mean probability 99.2%). The baseline characteristics of the study participants are presented in Table 1. In this analysis of 108 participants, 77% were female, 75% were white, and 11% were Hispanic. The baseline mean (SD) age was 17.0 (1.6) years. The baseline mean (SD) BMI was 54.3 (9.87) kg/m2, and mean IWC by horizontal iliac crest measurement was 149.6 (17.3) cm. Baseline mean plasma TC was 161 (31.3) mg/dL, LDL-C 95 (28) mg/dL, non-HDL-C 122 (30.8) mg/dL, logTG was 5 (1), and HDL-C was 39 (9.5) mg/dL. When the cohort was examined by activity trajectory, MA vs. LA, there were no significant differences in baseline lipid values, race/ethnicity, or surgical procedure (Table 2).
Table 1.
Baseline summary table for all participants with baseline and at least one 3-day StepWatch™ record
As mean (SD) or N (%).
| Covariate | Overall (N=108) |
More Active (N=14) |
Less Active (N=94) |
|---|---|---|---|
| Female | 83 (76.9%) | 11 (78.6%) | 72 (76.6%) |
| Hispanic/Latino | 12 (11.1%) | 0 (0.0%) | 12 (12.8%) |
| Race: | |||
| White or Caucasian | 81 (75.0%) | 11 (78.6%) | 70 (74.5%) |
| Black or African American | 21 (19.4%) | 2 (14.3%) | 19 (20.2%) |
| Asian | 1 (0.9%) | 0 (0.0%) | 1 (1.1%) |
| American Indian or Alaskan Native | 1 (0.9%) | 0 (0.0%) | 1 (1.1%) |
| More Than One Race | 4 (3.7%) | 1 (7.1%) | 3 (3.2%) |
| Surgery Type RYB (vs. GS) | 92 (85.2%) | 13 (92.9%) | 79 (84.0%) |
| Number of Visits with Valid Step Data: | |||
| 1 | 38 (35.2%) | 8 (57.1%) | 30 (31.9%) |
| 2 | 31 (28.7%) | 1 (7.1%) | 30 (31.9%) |
| 3 | 23 (21.3%) | 4 (28.6%) | 19 (20.2%) |
| 4 | 13 (12.0%) | 1 (7.1%) | 12 (12.8%) |
| 5 | 3 (2.8%) | 0 (0.0%) | 3 (3.2%) |
Table 2.
Baseline comparison of Less Active and More Active trajectories using two-sample t-tests or tests of proportions for p-values
| Covariate | More Active Mean (SD) | Less Active Mean (SD) | Difference [More-Less] (95% Cl) | p-value |
|---|---|---|---|---|
| HDL-C (mg/dL) | 44 (11) | 39 (9) | 5 (−1,12.0) | 0.104 |
| non-HDL-C(mg/dL) | 123 (31) | 121 (31) | 2 (−17,21) | 0.836 |
| LDL-C ( mg/dL) | 102(36) | 94 (27) | 8 (−14,29) | 0.448 |
| log(TG) | 5 (0) | 5 (1) | 0 (−0.5,0.1) | 0.117 |
| BMI (kg/m2) | 51 (8) | 55 (10) | −4 (−9,1) | 0.111 |
| IWC(cm) | 146 (15) | 150 (17) | −3(−13,6) | 0.459 |
| Body Fat (%) | 54 (6) | 54 (4) | 0.9 (−3,4) | 0.611 |
| Age (yrs.) | 17 (1) | 17 (2) | 0 (−1,1) | 0.458 |
| Total Cholesterol (mg/dL) | 167 (34) | 160 (31) | 7 (−13, 27) | 0.458 |
CI – confidence interval (95% confidence that the true population value will be located between these limits)
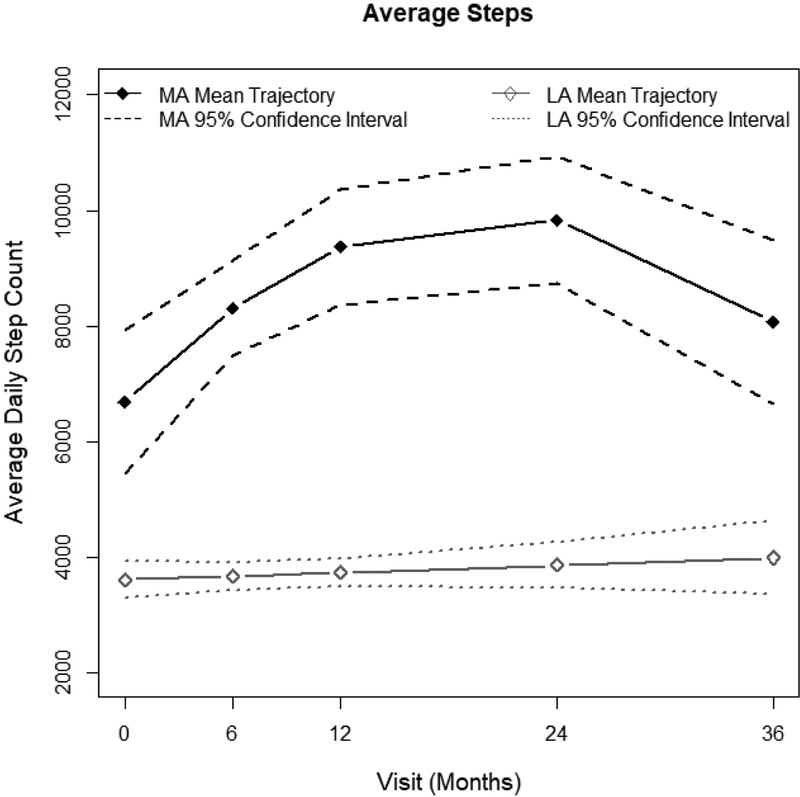
The MA trajectory average daily step count was >6,000 average steps at baseline and increased to >9,000 at 2 years. The LA trajectory was characterized by a stable daily step count of <4,000 steps per day from baseline to 3 years (Figure 1). Cadence was consistent at all time points, with no participant recording more than 4 minutes of moderate activity cadence per day (>80 steps/minute) (Table 3).
Figure 1.
Comparison of Less Active and More Active step counts over time
Table 3.
Summaries of average (SD) daily step count across time by sex
| All Participants | Boys | Girls | ||||
|---|---|---|---|---|---|---|
| Visit | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) |
| Baseline | 66 | 3,899 (1,818) | 10 | 4,035 (1,615) | 56 | 3,875 (1,865) |
| 6 Month | 68 | 4,186 (2,236) | 17 | 4,238 (1,953) | 51 | 4,168 (2,340) |
| 12 Month | 53 | 4,405 (1,820) | 14 | 4,745 (1,851) | 39 | 4,283 (1,818) |
| 24 Month | 35 | 4,560 (2,291) | 11 | 4,449 (1,689) | 24 | 4,611 (2,552) |
| 36 Month | 14 | 4,963 (3,473) | 5 | 6,657 (4,387) | 9 | 4,023 (2,681) |
Primary outcome
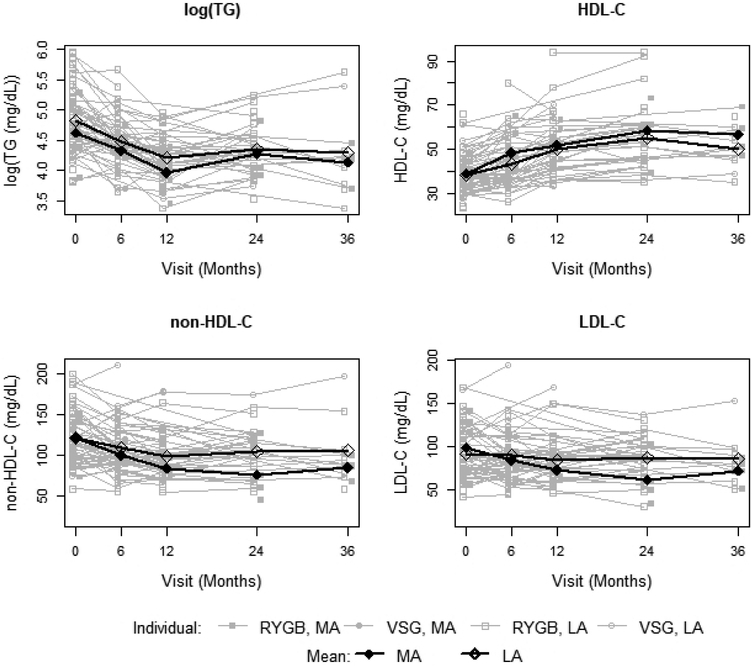
The MA trajectory was associated with significantly greater absolute decrease in non-HDL-C (−15 mg/dL, 95% CL: (−28, −1), p=0.035), adjusting for baseline non-HDL-C, time, percent change in IWC and procedure (Table 4). Similarly, a greater absolute decrease in LDL-C was associated with the MA trajectory, adjusting for baseline LDL-C, time, procedure, and for either percent change in IWC (−15 mg/dL, 95% CI: (−28, −2), p=0.026 or % change in BMI (−13 mg/dL, 95% CI: (−26, 0), p=0.044). In the same models, RYGB surgical procedure was associated with an additional absolute reduction of −19 mg/dL in measured non-HDL-C (95% CI: (−5, −33), p=0.007) and a reduction of −15 mg/dL in calculated LDL-C (95% CI: (−2, −27), p=0.019) over the VSG procedure (Table 4). Trajectories illustrating individual and activity trajectory mean longitudinal change in non-HDL-C and LDL-C by procedure are shown in Figure 2.
Table 4.
Absolute change in lipid outcome from baseline regression output with percent change in IWC or BMI as the adiposity measure (%Ad)
| Iliac Waist Circumference (IWC) | Body Mass Index (BMI) | |||
|---|---|---|---|---|
| Outcome: log(TG) | Coefficient (95% Cl) | p-value | Coefficient (95% Cl) | p-value |
| Intercept | 1.91 (1.22, 2.59) | <0.001 | 2.09 (1.41, 2.78) | <0.001 |
| Baseline log(TG) | −0.48 (−0.62, −0.34) | <0.001 | −0.50 (−0.64, −0.35) | <0.001 |
| White, non-Hispanic (vs. “Other”) | 0.16 (0.04,0.27) | 0.007 | 0.20 (0.08, 0.32) | <0.001 |
| %Ad Change from Baseline | 0.01 (0.00, 0.01) | 0.255 | 0.01 (0.00, 0.02) | 0.041 |
| MA (vs. LA) | −0.10 (−0.27, 0.06) | 0.224 | −0.09 (−0.25, 0.07) | 0.282 |
| Baseline to 1 Year | −0.25 (−0.34, −0.16) | <0.001 | −0.22 (−0.31, −0.14) | <0.001 |
| Baseline to 2 Years | −0.07 (−0.21, 0.07) | 0.327 | −0.09 (−0.22, 0.05) | 0.222 |
| Baseline to 3 Years | −0.12 (−0.37, 0.13) | 0.343 | −0.15 (−0.39, 0.10) | 0.237 |
| VSG Procedure (vs. RYGB) | 0.11 (−0.08, 0.30) | 0.266 | 0.09 (−0.10, 0.29) | 0.337 |
| Outcome: HDL-C | Coefficient (95% Cl) | p-value | Coefficient (95% Cl) | p-value |
| Intercept | 16.51 (5.88, 27.14) | 0.002 | 12.55 (3.01, 22.09) | 0.010 |
| Baseline HDL | −0.28 (−0.54, −0.03) | 0.026 | −0.23 (−0.47, 0.01) | 0.056 |
| White, non-Hispanic(vs. “Other”) | −3.93 (−7.54, −0.31) | 0.033 | −4.37 (−8.10, −0.65) | 0.021 |
| Male | −3.14 (−6.45, 0.16) | 0.062 | −3.21 (−6.43, 0.01) | 0.050 |
| %Ad Change from Baseline | −0.17 (−0.39, 0.06) | 0.152 | −0.22 (−0.38, −0.06) | 0.009 |
| MA (vs. LA) | 1.51 (−3.63, 6.64) | 0.565 | 0.39 (−4.71, 5.49) | 0.881 |
| Baseline to 1 Year | 6.17 (4.24, 8.11) | <0.001 | 5.10 (2.97,7.22) | <0.001 |
| Baseline to 2 Years | 9.83 (7.06, 12.60) | <0.001 | 9.45 (6.64, 12.25) | <0.001 |
| Baseline to 3 Years | 7.31 (3.53, 11.08) | <0.001 | 7.34 (3.67, 11.01) | <0.001 |
| VSG Procedure (vs. RYGB) | −0.64 (−4.84, 3.56) | 0.765 | −1.00 (−5.26, 3.26) | 0.645 |
| Outcome: non-HDL-C | Coefficient (95% Cl) | p-value | Coefficient (95% Cl) | p-value |
| Intercept | 44.39 (22.52, 66.26) | <0.001 | 51.78 (29.42, 74.13) | <0.001 |
| Baseline non-HDL | −0.43 (−0.57, −0.29) | <0.001 | −0.42 (−0.56, −0.28) | <0.001 |
| %Ad Change from Baseline | 0.52 (−0.08, 1.12) | 0.092 | 0.69 (0.21, 1.18) | 0.005 |
| MA (vs. LA) | −14.62 (−28.18, −1.06) | 0.035 | −12.78 (−26.07, 0.51) | 0.059 |
| Baseline to 1 Year | −7.76 (−12.78, −2.75) | 0.002 | −6.19 (−11.19, −1.18) | 0.015 |
| Baseline to 2 Years | −4.02 (−12.01, 3.96) | 0.323 | −3.44 (−10.77, 3.89) | 0.358 |
| Baseline to 3 Years | −3.45 (−17.05, 10.15) | 0.619 | −3.98 (−17.90, 9.95) | 0.576 |
| VSG Procedure (vs. RYGB) | 19.36 (5.30, 33.42) | 0.007 | 19.33 (5.13, 33.54) | 0.008 |
| Outcome: LDL-C | Coefficient (95% Cl) | p-value | Coefficient (95% Cl) | p-value |
| Intercept | 41.37 (23.48, 59.26) | <0.001 | 47.24 (29.21, 65.26) | <0.001 |
| Baseline LDL | −0.43 (−0.59, −0.27) | <0.001 | −0.42 (−0.58, −0.26) | <0.001 |
| %Ad Change from Baseline | 0.47 (−0.01, 0.94) | 0.053 | 0.59 (0.21, 0.97) | 0.002 |
| MA (vs. LA) | −14.83 (−27.88, −1.78) | 0.026 | −13.26 (−26.14, −0.38) | 0.044 |
| Baseline to 1 Year | −3.32 (−8.18, 1.53) | 0.180 | −2.04 (−6.80, 2.72) | 0.401 |
| Baseline to 2 Years | −2.46 (−9.30, 4.38) | 0.481 | −1.34 (−7.69, 5.00) | 0.679 |
| Baseline to 3 Years | −3.76 (−14.64, 7.12) | 0.498 | −4.10 (−15.40, 7.19) | 0.476 |
| VSG Procedure (vs. RYGB) | 15.12 (2.39, 27.86) | 0.020 | 15.27 (2.48, 28.05) | 0.019 |
Figure 2.
Lipid outcomes by activity trajectory and individual procedure
Secondary outcomes
HDL-C increased in association with both activity trajectories throughout active weight loss, baseline to 1 year, and throughout weight loss maintenance to 3 years, (p<0.001 for both models) in association with time and percent change in BMI. Female sex was associated with an additional mean absolute HDL-C increase of 3 mg/dL (95% CI: (0, 6), p = 0.05) in the model adjusting for % change in BMI. Non-white race was associated with a mean absolute HDL-C increase of 3.9 mg/dL (95% CI: (0.3, 7.5), p=0.029) or 4.4 mg/dL (95% CI: (0.7, 9.10), p=0.021) after adjusting for % change in IWC or % change in BMI, respectively. Surgical procedure (RGYB vs. VSG) did not appear to influence improvement in HDL-C (p>0.6 for both models).
Trajectories illustrating individual and activity trajectory mean longitudinal change in HDL-C and TG by procedure are also shown in Figure 2. Improvement in TG was not associated with activity trajectory (p>0.2 for all models) but was strongly associated with the active weight loss period from baseline to 1 year (p<0.001 for both models), indicating the predicted additional decrease of 0.22 to 0.25 in log TG value in year 1 was largely influenced by weight loss. The influence of change in BMI on log TG was significant (p=0.041) as indicated by a decrease of 0.01 in log triglyceride value for every 1% decrease in BMI.
Of clinical significance, a greater percentage of elevated measures of non-HDL-C, LDL-C and TG converted to acceptable values in association with the MA trajectory over time compared with the LA trajectory. This was based upon the 2011 Integrated Guidelines (20) (Table 5). Overall, the conversion of elevated lipid measures to acceptable lipid values occurred more often with MA trajectory membership, compared to the LA trajectory membership.
Table 5.
Counts (%) of those with acceptable lipid status at baseline, year 1, and year 2 by activity trajectory
| TG | Less Active Trajectory | More Active Trajectory |
| Baseline | 27/94 (28.7%) | 5/14 (35.7%) |
| Year 1 | 49/61 (80.3%) | 9/9 (100%) |
| Year 2 | 27/37 (70.3%) | 5/6 (83.3%) |
| HDL-C | Less Active Trajectory | More Active Trajectory |
| Baseline | 20/94 (21.3%) | 4/14 (28.6%) |
| Year 1 | 37/61 (60.7%) | 6/9 (66.7%) |
| Year 2 | 25/37 (67.6%) | 4/6 (66.7%) |
| non-HDL-C | Less Active Trajectory | More Active Trajectory |
| Baseline | 52/94 (55.3%) | 6/14 (42.9%) |
| Year 1 | 50/61 (82%) | 8/9 (88.9%) |
| Year 2 | 27/37 (73%) | 5/6 (83.3%) |
| LDL-C | Less Active Trajectory | More Active Trajectory |
| Baseline | 70/94 (74.5%) | 7/14 (50%) |
| Year 1 | 53/61 (86.9%) | 8/9 (88.9%) |
| Year 2 | 31/37 (83.8%) | 6/6 (100%) |
Acceptable values defined as: HDL-C>45mg/dL, LDL-C<110 mg/dL, non-HDL-C<120 mg/dL, TG<90 mg/dL.(20)
Importantly, the MA trajectory was also associated with an 11% greater reduction in BMI at 3 years compared to the LA trajectory, 95% CI: (−22, −0.2), p=0.046. White, non-Hispanic race was also more likely to experience weight loss with an average 7% greater reduction in BMI, 95% CI: (−11, −4), p<0.001. (BMI and central adiposity regression tables not shown).
DISCUSSION
These results demonstrate that using objectively-measured PA, a relationship between PA and improvement in lipid status beyond weight loss post-MBS in severely obese adolescents was demonstrated. As has previously been reported in adults post-MBS, PA was low (24), well below the equivalent of recommended 60 minutes of moderate-to-vigorous PA daily in adolescence (23), or 30 minutes of moderate-to-vigorous PA on at least 5 days of the week for adults, or the typical 8,000–9,000 steps/day at a moderate cadence (11, 23). Additionally, cadence was too low to be considered moderate activity with fewer than 4 high-step-count minutes recorded by any participant. Yet, despite overall low step counts and slow cadence, objectively-measured PA post-MBS was associated with a greater improvement in absolute change in LDL-C, adjusting for baseline lipid value, percent BMI change, and procedure despite overall low step counts. PA was also associated with greater improvement in non-HDL-C, accounting for baseline lipid value, surgical procedure, and either percent BMI change or percent IWC change. Also, membership in the MA trajectory effectively doubled the percentage of participants with resolution of dyslipidemia at 2 years, as indicated by the return to acceptable values of LDL-C, non-HDL-C and TG (20). In contrast, <9% of LDL-C, non-HDL-C and TG dyslipidemia resolved from baseline to 2 years in association with the LA trajectory. Importantly, by 36 months post-MBS, these results demonstrate a normalization of dyslipidemia in participants falling within the MA trajectory category. Of particular interest, our study did not find that activity was associated with longitudinal increases in HDL-C or clinical remission of HDL-C dyslipidemia, as has been previously reported in adults (25). We found that HDL-C increased from baseline to 2 years post-MBS in association with time, suggesting the improvement in HDL-C at each time-point was related to the impact of MBS, rather than PA. Additionally, the association of increased PA with greater weight loss and improved weight loss maintenance post-MBS suggests the possibility of an expected causal relationship. Although supported by previously published reports in adults (24) this cannot be confirmed without future studies designed to measure these effects in adolescents. The MA trajectory was associated with an 11% greater decrease in BMI at 3 years, extending well beyond the period of active weight loss, from baseline to 12 months. The unexpected finding that RYGB may influence a larger reduction in non-HDL-C and LDL-C over VSG is worthy of further examination and confirmation as the VSG participants were few. PA-associated improvements in lipid outcomes persisted even when adjusted for percent change in BMI.
An augmented activity-associated decrease in CVD-related lipid values and greater long-term weight loss post-MBS in adolescents likely predict better long-term outcomes and improved cardio-metabolic status. This is important because CVD is strongly associated with dyslipidemia and BMI (7, 26). Improved lipid measures and clinical remission of dyslipidemia in association with weight loss post-MBS has previously been reported in adults and adolescents (8, 25, 27–29). Although percent BMI change was the primary contributor to improvements in LDL-C, non-HDL-C, HDL-C and TG post-MBS, physical activity was associated with further decrease in CVD-related lipids, LDL-C and non-HDL-C. Likewise, clinical dyslipidemia has previously been reported to resolve in 66% of adolescents post-MBS (30). In this analysis, more activity was associated with almost complete resolution of dyslipidemia. While both physical activity and MBS have been reported to increase HDL-C (31), our study found that physical activity was not associated with an increase in HDL-C. It may be that the low step counts and slow cadence of the population were insufficient to increase HDL-C. This is worthy of future exploration in adolescents with severe obesity.
PA has also been previously shown to support weight loss and weight loss maintenance resulting from both lifestyle modification and MBS in adults, however self-reported PA did not distinguish maintainers from those who failed to maintain weight loss in adolescents, post-MBS (15, 32). By examining objectively-measured activity data, our study demonstrated that more objectively-measured physical activity was associated with greater weight loss in the weight maintenance period from 1 year to 3 years, well beyond the period of active weight loss at year 1.
These results are unique and highlight the value of PA to modify cardio-metabolic risk and improve individual long-term outcomes as an adjunct therapy to MBS in adolescents with severe obesity. These study results are strengthened by the longitudinal study design, the use of an objective PA measure and the analysis of individual longitudinal activity trajectories rather than median or group means, accounting for sex, race, procedure, baseline data, and percent change in BMI or IWC. There are limitations to be considered as well, including limited PA participation among the adolescent-with-severe-obesity population, fewer VSG participants, limited StepWatch™ data available from the Teen-LABS participants, and limitation of recoding steps and cadence with StepWatch™. Participant self-selection may have influenced the participation with step data collection and continued participation over time. Also, individual dietary intake was not available in the Teen-LABS data set, although pre- and post-MBS dietary instructions were consistent for all of the participants.
In summary, in adolescents with severe obesity, PA appears to further reduce lipid-related-CVD risk and support long-term weight loss maintenance, post-MBS. As such, PA support should be included as an integral part of the chronic care plan for all adolescents undergoing MBS. Further research is needed to determine best practices and strategies to modify PA behavior among adolescents with severe obesity. Additionally, a better understanding of the frequency, intensity and duration of PA needed pre- and post-MBS is needed to modify specific lipid and weight maintenance outcomes longitudinally in adolescents and adults. Additional study is needed to confirm the potential influence of surgical procedure type in the reduction of CVD-related LDL-C and non-HDL-C and weight maintenance.
STUDY IMPORTANCE:
What is already known about this subject?
Dyslipidemia and obesity in adolescence track into adulthood and greatly increase cardiovascular disease (CVD) risk.
Dyslipidemia remits in the majority of adolescents with severe obesity, post-metabolic bariatric surgery.
Metabolic bariatric surgery effectively treats severe obesity in adolescents, yet weight loss and weight loss maintenance remain variable.
Physical activity improves lipid levels, reduces CVD risk, improves weight loss and supports weight loss maintenance.
What does this study add?
Greater decreases in CVD-related lipids, specifically LDL-C and non-HDL-C, are associated with more physical activity post-metabolic bariatric surgery in adolescents with severe obesity.
LDL-C and non-LDL-C dyslipidemia remission rates increase in association with more physical activity in adolescents with severe obesity, post-metabolic bariatric surgery.
Weight loss maintenance, indicated by an 11% greater decrease in BMI at 3 years, was associated with more physical activity in adolescents with severe obesity, post-metabolic bariatric surgery.
Acknowledgments
DISCLOSURES: Robert H. Eckel reports grants from NINDS, grants from NIA, grants from NHLBI, grants from Ionis Pharmaceutical, grants from Uniqure Biopharma, grants from CTSA Pilot Project, grants from Endece, LLC, personal fees from Cardiometabolic Health Congress, personal fees from Prime, personal fees from Medtelligence, personal fees from Medscape, personal fees from Medical Education Resources, personal fees from Vox Media, personal fees from Sanofi/Regeneron, personal fees from Merck, personal fees from Novo Nordisk, personal fees from Kowa, outside the submitted work.
Thomas H. Inge has served as a consultant for Zafgen Corporation, Biomedical Insights, and L&E Research, and received honoraria from Standard Bariatrics, UpToDate, and Independent Medical Expert Consulting Services, all unrelated to this project.
FUNDING: The Teen-LABS consortium is funded by cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), through grants: UM1DK072493 (PI, Dr. Thomas Inge, University of Colorado, Denver), and UM1DK095710 (PI, Dr. Changchun Xie, University of Cincinnati). Dr. Paula Holland Price is supported by the National Heart, Lung and Blood Institute (NHLBI): 5T32HL116276–05.
Footnotes
Paula Holland Price, Alexander M. Kaizer, Stephen M. Daniels, and Todd M. Jenkins declare no conflicts of interest.
References
- 1.Shah AS, Dolan LM, Khoury PR, Gao Z, Kimball TR, Urbina EM. Severe Obesity in Adolescents and Young Adults Is Associated With Subclinical Cardiac and Vascular Changes. The Journal of Clinical Endocrinology & Metabolism. 2015;100(7):2751–7. doi: 10.1210/jc.2014-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med. 2015;373(14):1307–17. doi: 10.1056/NEJMoa1502821. [DOI] [PubMed] [Google Scholar]
- 3.Koskinen J, Juonala M, Dwyer T, Venn A, Thomson R, Bazzano L, et al. Impact of Lipid Measurements in Youth in Addition to Conventional Clinic-Based Risk Factors on Predicting Preclinical Atherosclerosis in Adulthood: International Childhood Cardiovascular Cohort Consortium. Circulation. 2018;137(12):1246–55. doi: 10.1161/CIRCULATIONAHA.117.029726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fyfe-Johnson AL, Ryder JR, Alonso A, MacLehose RF, Rudser KD, Fox CK, et al. Ideal Cardiovascular Health and Adiposity: Implications in Youth. J Am Heart Assoc. 2018;7(8). doi: 10.1161/JAHA.117.007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung ST, Onuzuruike AU, Magge SN. Cardiometabolic risk in obese children. Ann N Y Acad Sci. 2018;1411(1):166–83. doi: 10.1111/nyas.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016. Pediatrics. 2018;141(3). doi: 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kivimäki M, Kuosma E, Ferrie JE, Luukkonen R, Nyberg ST, Alfredsson L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. The Lancet Public Health. 2017;2(6):e277–e85. doi: 10.1016/s2468-2667(17)30074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. The Lancet Diabetes & Endocrinology. 2017;5(3):165–73. doi: 10.1016/s2213-8587(16)30315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallis FJ, Prochaska JJ, Taylor CW. A review of correlates of physical activity of children and adolescents. Medicine & Science in Sports & Exercise. 2000;32(5):963–75. [DOI] [PubMed] [Google Scholar]
- 10.Evans JMM, Shelia CM, Kirk A, Crombie IK. Tracking of physical activity behaviours during childhood, adolescence and young adulthood: a systematic review. J Epidemiol Community Health. 2009;63(Suppl 2):9. doi: 10.1136/jech.2009.096701i. [DOI] [Google Scholar]
- 11.Tudor-Locke C, Craig Cora L, Beets Michael W, Belton S, Cardon Greet M, Duncan S, et al. How many steps/day are enough? for children and adolescents. International Journal of Behavioral Nutrition and Physical Activity. 2011;8(1):78. doi: 10.1186/1479-5868-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tudor-Locke C, Schuna JM Jr., Han H, Aguiar EJ, Larrivee S, Hsia DS, et al. Cadence (steps/min) and intensity during ambulation in 6–20 year olds: the CADENCE-kids study. Int J Behav Nutr Phys Act. 2018;15(1):20. doi: 10.1186/s12966-018-0651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berglind D, Willmer M, Tynelius P, Ghaderi A, Naslund E, Rasmussen F. Women undergoing Roux-en-Y Gastric Bypass surgery: Family resemblance in pre- to postsurgery physical activity and sedentary behavior in children and spouses. Surg Obes Relat Dis. 2015;11(3):690–6. doi: 10.1016/j.soard.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Lent MR, Bailey-Davis L, Irving BA, Wood GC, Cook AM, Hirsch AG, et al. Bariatric Surgery Patients and Their Families: Health, Physical Activity, and Social Support. Obes Surg. 2016;26(12):2981–8. doi: 10.1007/s11695-016-2228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryder JR, Gross AC, Fox CK, Kaizer AM, Rudser KD, Jenkins TM, et al. Factors associated with long-term weight-loss maintenance following bariatric surgery in adolescents with severe obesity. Int J Obes (Lond). 2018;42(1):102–7. doi: 10.1038/ijo.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corder K, Ekelund U, Steele RM, Wareham NJ, Brage S. Assessment of physical activity in youth. Journal of applied physiology (Bethesda, Md : 1985). 2008;105(3):977–87. doi: 10.1152/japplphysiol.00094.2008. [DOI] [PubMed] [Google Scholar]
- 17.Shah M, Snell PG, Rao S, Adams-Huet B, Quittner C, Livingston EH, et al. High-volume exercise program in obese bariatric surgery patients: a randomized, controlled trial. Obesity (Silver Spring). 2011;19(9):1826–34. doi: 10.1038/oby.2011.172. [DOI] [PubMed] [Google Scholar]
- 18.King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR, et al. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2). Surg Obes Relat Dis. 2012;8(5):522–32. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inge TH, Zeller M, Harmon C, Helmrath M, Bean J, Modi A, et al. Teen-Longitudinal Assessment of Bariatric Surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg. 2007;42(11):1969–71. doi: 10.1016/j.jpedsurg.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent Class Growth Modelling: A tutorial. Tutorials in Quantitative Methods for Psychology. 2013;9(1). doi: 10.20982/tqmp.09.1.p042. [DOI] [Google Scholar]
- 22.R: A language and environment for statistical computingR Foundation for Statistical Computing Vienna, Austria: 2018. Available from: https://www.R-project.org/. [Google Scholar]
- 23.Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. 2nd ed. Washington, DC: US Department of Health and Human Services; 2018. [Google Scholar]
- 24.Herman KM, Carver TE, Christou NV, Andersen RE. Keeping the weight off: physical activity, sitting time, and weight loss maintenance in bariatric surgery patients 2 to 16 years postsurgery. Obes Surg. 2014;24(7):1064–72. doi: 10.1007/s11695-014-1212-3. [DOI] [PubMed] [Google Scholar]
- 25.Bays HE, Jones PH, Jacobson TA, Cohen DE, Orringer CE, Kothari S, et al. Lipids and bariatric procedures part 1 of 2: Scientific statement from the National Lipid Association, American Society for Metabolic and Bariatric Surgery, and Obesity Medicine Association: FULL REPORT. J Clin Lipidol. 2016;10(1):33–57. doi: 10.1016/j.jacl.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Freedman DS, Ogden CL, Kit BK. Interrelationships between BMI, skinfold thicknesses, percent body fat, and cardiovascular disease risk factors among US children and adolescents. BMC Pediatr. 2015;15. doi: ARTN18810.1186/s12887-015-0493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bays H, Kothari SN, Azagury DE, Morton JM, Nguyen NT, Jones PH, et al. Lipids and bariatric procedures Part 2 of 2: scientific statement from the American Society for Metabolic and Bariatric Surgery (ASMBS), the National Lipid Association (NLA), and Obesity Medicine Association (OMA). Surg Obes Relat Dis. 2016;12(3):468–95. doi: 10.1016/j.soard.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Michalsky MP, Inge TH, Jenkins TM, Xie C, Courcoulas A, Helmrath M, et al. Cardiovascular Risk Factors After Adolescent Bariatric Surgery. Pediatrics. 2018;141(2). doi: 10.1542/peds.2017-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah AS, Jenkins T, Gao Z, Daniels SR, Urbina EM, Kirk S, et al. Lipid changes 8 years post gastric bypass in adolescents with severe obesity (FABS-5+ study). Int J Obes (Lond). 2017;41(10):1579. doi: 10.1038/ijo.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, et al. Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents. N Engl J Med. 2016;374(2):113–23. doi: 10.1056/NEJMoa1506699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kjellmo CA, Karlsson H, Nestvold TK, Ljunggren S, Cederbrant K, Marcusson-Stahl M, et al. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J Clin Lipidol. 2018;12(1):193–202. doi: 10.1016/j.jacl.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, et al. How many steps/day are enough? for adults. International Journal of Behavioral Nutrition and Physical Activity. 2011;8(1):79. doi: 10.1186/1479-5868-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]