SUMMARY
Wind is a major navigational cue for insects, but how wind direction is decoded by central neurons in the insect brain is unknown. Here, we find that walking flies combine signals from both antennae to orient to wind during olfactory search behavior. Movements of single antennae are ambiguous with respect to wind direction, but the difference between left and right antennal displacements yields a linear code for wind direction in azimuth. Second-order mechanosensory neurons share the ambiguous responses of single antenna and receive input primarily from the ipsilateral antenna. Finally, we identify novel ‘wedge projection neurons’ that integrate signals across the two antennae and receive input from at least three classes of second-order neurons to produce a more linear representation of wind direction. This study establishes how a feature of the sensory environment – the wind direction – is decoded by neurons that compare information across two sensors.
Graphical Abstract
eTOC Blurb:
Suver et al. (2019) describe how walking flies use their two antennae to measure wind direction. They describe a mechanosensory pathway that encodes antennal movements, with higher-order neurons combining information from the two antennae to linearly encode wind direction.
INTRODUCTION
Animals have evolved a broad array of systems that enable them to extract relevant features of their sensory environment. These systems often achieve accurate encoding of the sensory world by comparing signals between sensors. For instance, in the auditory system of birds and mammals, coincidence detectors compare activity between neural ‘delay lines’ that receive inputs from opposite side of the head to compute sound direction (Konishi, 2003). Alternatively, perception can be enabled by comparing relative activities across sensors in the same region of space, but with different tuning properties. This occurs in touch perception in mammals, where input from mechanosensors with differing tuning properties in the same region of skin is integrated in the spinal cord (Abraira and Ginty, 2013). However, the central processing of sensory information is often challenging to study in vertebrates, particularly with single-cell precision.
One of the most iconic examples of paired sensors in the animal kingdom are arthropod antennae. Insects use their antennae for olfaction and thermosensation, and to perform a wide variety of mechanosensory behaviors. For instance, cockroaches use mechanosensory information from their antenna to guide high-speed locomotion (Camhi and Johnson, 1999), and to actively probe objects (Okada and Toh, 2006). Many insects also use their antennae to sense wind direction, an important cue for navigation. Examples include cockroaches, which can use wind to set a straight course in the absence of visual landmarks (Bell and Kramer, 1979), and desert ants that use wind to navigate towards a learned food source (Müller and Wehner, 2007). The ability to gauge wind direction is critical in many species for navigating towards an odor source (Wolf and Wehner 2000, Willis and Avondet, 2005, Bell and Wilson, 2016) because wind often provides a stronger direction cue than odor concentration in a turbulent environment (Murlis, Willis, Carde, 2000; Carde and Willis, 2008). Orientation to wind is abolished by stabilizing the antennae in cockroaches (Bell and Kramer, 1979), ants (Wolf and Wehner, 2000), and walking Drosophila (Álvarez-Salvado et al., 2018), indicating a critical role for antennal mechanoreceptors. Furthermore, experiments in flying flies suggest that information from both antennae must be combined to accurately gauge wind direction (Budick et al., 2007). This analysis is supported by video recordings of antennal movements in response to wind, which suggest that the two antennae are differentially displaced depending on the wind direction (Patella and Wilson, 2018; Yorozu et al., 2009). However, the neural circuits underlying this computation are not known.
In insects, movements of the antennae are detected by multiple sensors, including a large set of stretch receptors located in the second antennal segment, collectively known as the ‘Johnston’s Organ’ (JO; Johnston, 1855). Movements of the third antennal segment relative to the second are aided by a feather-like structure called the arista that acts as a sail and enables the fly to detect different types of mechanical stimuli including conspecific song (Göpfert and Robert, 2002). About 500 primary mechanosensory neurons, known as ‘Johnston’s Organ neurons’ (JONs) project from the antennae through the antennal nerve towards the central brain, to a region called the antennal mechanosensory and motor center (AMMC; Kamikouchi, Shimada and Ito, 2006). Traditionally, JONs have been broadly grouped into phasic (AB JONS) and tonic (CE JONs) classes that project to different regions of the AMMC and carry auditory (AB) or wind and gravity (CE) information (Kamikouchi et al., 2009; Yorozu et al., 2009). However, D JONs carry both phasic and tonic information (Matsuo et al. 2014), and it is likely that these broad classes are composed of many more specialized cell types (Kamikouchi et al., 2006; Patella and Wilson, 2018).
In the AMMC, mechanosensory information is processed by multiple second-order neurons, many of which project to the nearby ‘wedge’ (WED), a major third-order mechanosensory center (Kamikouchi et al., 2009; Lai et al., 2012; Matsuo et al., 2016a; Patella and Wilson, 2018; Vaughan et al., 2014). Among these second-order neurons, two classes known as APN2 and APN3 are thought to lie anatomically downstream of wind-sensitive JONs (Matsuo et al., 2016a; Vaughan et al., 2014). APN3 responds to tonic displacements of the antenna induced by a piezo probe (Chang et al., 2016), while APN2 has not been physiologically characterized. In contrast, the contralaterally projecting B1 neurons (also called APN1 in Vaughan et al., 2014) are thought to be downstream of the phasic AB JONs (Chang et al., 2016; Lai et al., 2012). B1 neurons were functionally implicated in the perception of courtship song in a previous study, while APN2 and 3 were found to be dispensable for this behavior (Vaughan et al., 2014). This suggests that the functional segregation of phasic auditory and tonic wind signals is maintained in second-order neurons. However, APN3 neurons respond to tonic displacements of the antenna as well as sound and sound-like stimuli (Chang et al., 2016). Furthermore, B1 neurons exhibit directionally tuned responses to piezo displacements (Azevedo and Wilson, 2017), complicating this simple picture. The responses of these neurons to wind, as opposed to piezo displacements of a single antenna, have not been measured.
Where might wind direction be computed from information about the displacements of the two antennae? A recent pan-neuronal imaging study in Drosophila suggested that information from the two antennae is first combined in the WED (Patella and Wilson, 2018). Because this study used pan-neuronal drivers to measure activity, however, it was not able to establish the identity of any specific neurons involved in this process or identify higher-order areas involved in wind encoding. In the central brain, neurons responding to wind stimuli and to tonic deflections of the antennae have been described in the central complex (in bees: Homberg, 1985, locusts: Homberg, 1994, and cockroaches: Ritzmann, Ridgel and Pollack, 2008). How information reaches these regions of the brain from peripheral processing centers in the AMMC and WED is unknown.
In this study, we use whole cell electrophysiology to identify WED projection neurons that compute wind direction and to elucidate presynaptic partners underlying this computation. We first show that walking flies combine information from both antennae to effectively orient to wind. Next, we perform a quantitative analysis of antennal movements in response to directional wind and show that each antenna provides an ambiguous signal about wind direction, but that this ambiguity can be resolved by a simple linear combination of displacement signals from the two antennae. Whole cell recordings demonstrate that second order neurons encode displacements of the ipsilateral antenna and encode wind direction with a similar ambiguity to a single antenna. Through a whole-cell electrophysiology screen, we identify novel wedge projection neurons (WPNs) that integrate information across the two antennae to generate a more linear encoding of wind direction in azimuth. Finally, we use pharmacological and genetic silencing experiments, along with trans-synaptic labeling, to show that multiple populations of second order neurons provide input to WPNs, and that these overlap with populations previously shown to play a role in auditory encoding and courtship behavior. In this study, we introduce novel neurons in the insect brain that integrate signals across the antennae, identify new regions of the dorsal brain as putative centers of mechanosensory processing, and provide insight into the circuit mechanisms by which mechanosensory neurons compute important features of the mechanosensory environment. Our results illustrate circuit mechanisms for decoding a salient feature of the environment – wind direction – from the activities of paired sensors.
RESULTS
Robust wind orientation behavior requires both antennae
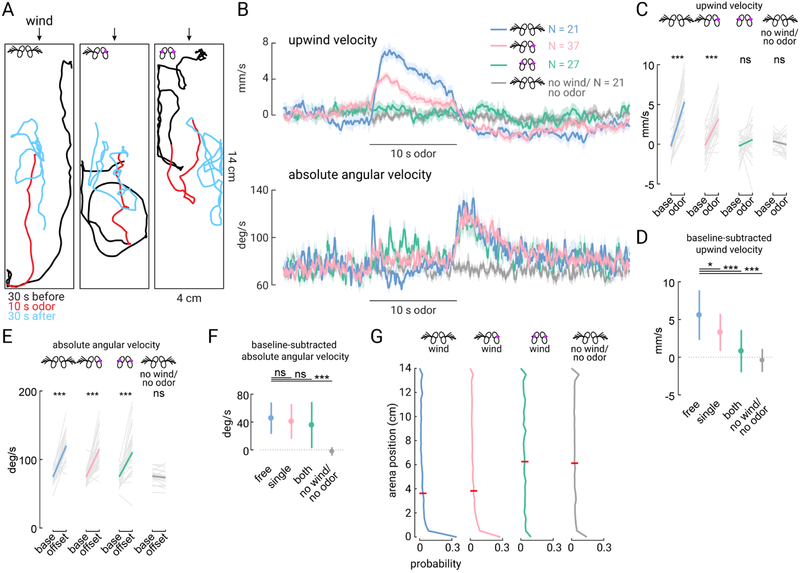
Walking Drosophila rely on antennal movements both to orient upwind in response to odor, and downwind in the absence of odor (Álvarez-Salvado et al., 2018). However, it is not known whether information from both antennae is required for these behaviors. To study how the two antennae work together to promote orientation to wind, we measured the behavioral responses of freely walking flies to wind and stabilized either one or both antennae (Figure 1A). As shown previously (Álvarez-Salvado et al., 2018), flies orient upwind in the presence of odor, and this orientation is blocked by stabilizing both antennae (Figures 1B–D). In contrast, local search behavior produced at odor offset is not impaired, indicating that flies with stabilized antennae can still detect the odor (Figures 1B, 1E, and 1F). In the absence of odor (blank trials), flies tend to aggregate at the downwind end of the tunnel; this downwind preference is lost when antennal inputs are blocked (Figure 1G).
Figure 1. Both antennae contribute to odor-driven upwind orientation.
(A) Example walking trajectories during single trials consisting of wind only (black, 30 s), wind with odor (1% apple cider vinegar, red, 10 s), and wind only again (cyan, 30 s). Wind direction indicated by arrows (top of this image). Examples show a fly with intact antennae (left), one antenna stabilized (center) or both antennae stabilized (right). (B) Top: Upwind velocity (average across flies +/− standard error) in response to a 10 s odor pulse for flies with intact antennae (blue, N=22 flies), one antenna stabilized (pink, N=37), both stabilized (green, N=27), or intact antenna but no wind and no odor (gray, N = 21). Bottom: absolute angular velocity (average across flies +/− standard error) for the same flies as above. (C) Odor-evoked upwind velocity for single flies (average of 5 s during odor versus 15 s pre-odor baseline). Flies with intact antennae or one antenna stabilized increase upwind velocity during the odor, but flies with both antennae stabilized or in the absense of wind and odor do not (Mann-Whitney U test, P = 0.000040, 0.00000067, 0.11, and 0.22, respectively). (D) Average baseline-subtracted upwind velocity +/− standard deviation for the single-fly averages plotted in (C). On average, flies with each treatment walk upwind less than flies with free antennae (Wilcoxon signed-rank test, P = 0.016, 0.0000012, and 0.000000082, respectively). In the absense of wind, upwind velocity is not significantly different from zero (Wilcoxon signed-rank test, P = 0.22). (E) Odor-offset evoked increase in absolute angular velocity for single flies (average of 2 s post-odor versus 15 s pre-odor baseline). Flies in all treatments exhibit an increase in angular speed after odor offset, except in the absense of wind (Mann-Whitney U test, P = 0.000053, 0.00000017, 0.000026, and 0.26). (F) Average baseline-subtracted absolute angular velocity +/− standard deviation for the single fly averages plotted in (E). Flies with stabilized antennae are not significantly different from intact flies (Wilcoxon signed-rank test, P = 0.33, 0.18). Velocity in the absense of wind velocity is not significantly different from zero (Wilcoxon signed-rank test, P = 0.0262). (G) Probability distribution of flies along the length of the walking arena during the wind-only stimulus (no odor) for flies with free antenna (N=22 flies), single antenna stabilized (N=36), both antenna stabilized (N=26), and free antenna but no wind and no odor (N = 21). Higher position values are upwind. Red bars indicate average position. Mean position of flies is significantly different between no wind and wind (grey vs. blue, Mann-Whitney U test, P = 0.000034). Mean position is not significantly different between flies with one antenna stabilized and flies with free antennae (pink vs. blue, P = 0.29), or between flies with both antennae stabilized and intact antennae but no wind (green vs. grey, P = 0.93). All comparisons were corrected with the Bonferroni method.
To examine whether input from both antennae is required for wind orientation, we stabilized either the left or right antenna and again measured flies’ responses to odor pulses. We were unable to distinguish between flies walking on the ceiling or the floor in our arena, so we combined data from flies with right or left antenna stabilized (which were not statistically different; data not shown). Stabilization of a single antenna reduced upwind velocity during odor by about half on average (Figure 1D). Local search after odor offset was unaffected, similar to flies that had both antennae glued (Figures 1E and 1F). Flies with one antenna glued also exhibited no change in average downwind aggregation (Figure 1G), perhaps because this measure reflects integrated downwind orientation over time. Together these findings suggest that flies require both antennae to perform normal upwind orientation in response to odor, but that a single antenna is sufficient to produce the baseline downwind orientation. These results led us to ask how wind direction signals are encoded by movements of the two antennae.
Single antenna motions are ambiguous with respect to wind direction
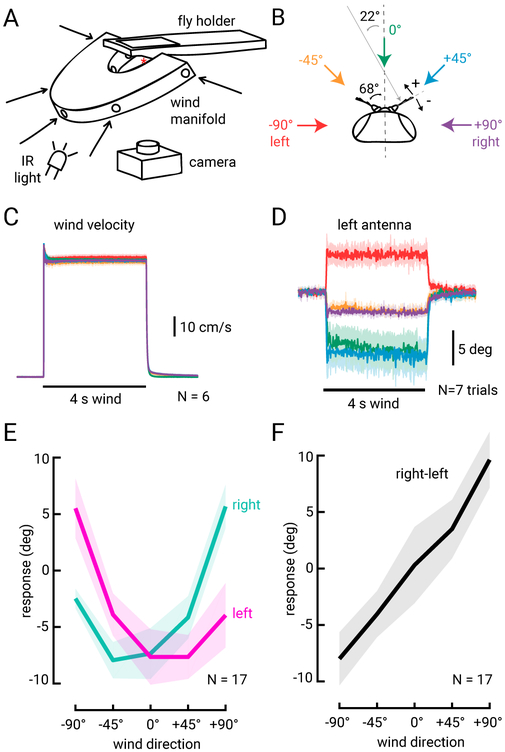
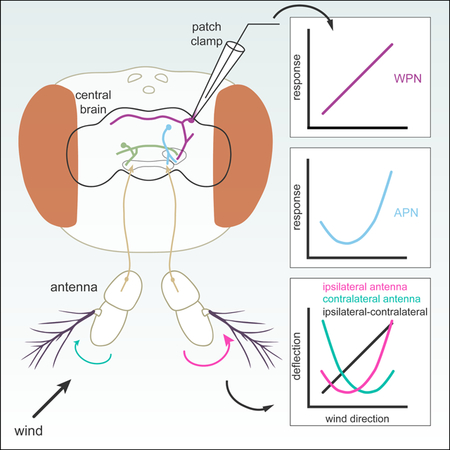
A previous study (Yorozu et al., 2009) showed that movements of the two antennae together can encode wind direction: wind from the front pushes both antennae towards the head, while wind from one side pulls the ipsilateral antenna away, and pushes the contralateral antenna towards the head. To examine how antennal displacements encode wind direction more quantitatively, we designed a stimulus apparatus to deliver wind to the fly from 5 different directions (Figures 2A and 2B). The fly was mounted in a holder with a fixed position relative to the stimulus ports, ensuring reliable positioning of the fly at the center of the five wind streams across experiments. We verified that the velocity of the wind was identical across directions, so that any difference in antennal movements would be due to directionality rather than velocity (Figure 2C).
Figure 2. Wind direction encoding by antennal displacement.
(A) Wind stimulus apparatus schematic. Wind is delivered to the fly from one of five directions through a manifold. The fly is mounted in a holder so that its head is centered at the intersection of the five channels. A camera is mounted below the fly, and the fly is lit by an infrared (IR) light aimed towards the fly from below. (B) Schematic of the fly head and antennae. We deliver wind to the fly from −90° (left), −45°, 0°, +45° and +90° (right) relative to the midline of the fly (dashed vertical line). We track antennal motion by measuring deflections of the aristae relative to the midline of the fly. Average resting inter-arista angle is 136° +/− 15°, and the wind angle normal to the arista’s axis is noted (22°) and indicated by the grey arrow. (C) Average wind velocity measured by a hot wire anemometer placed at the location of the fly head during experiments. Average steady state windspeed is 60 +/−1.4 cm/s. Standard deviation across N=6 measurements is plotted as a shaded region behind the averages (but is difficult to discern because they lie on top of one another). (D) Average left arista deflections across N=7 trials for each of the 5 wind directions for one fly. (E) Average steady state deflections (+/− standard deviation) relative to baseline position for the left (pink) and right (teal) antennae across N=17 flies. Averages are derived from 5 frames (83 ms total) directly before and at the end of the wind stimulus (see Methods). (F) Difference in antennal deflections between the two antennae (right-left, from the same flies as plotted in (E)). Standard deviation of the mean is plotted as a lighter shade around left, right, and right-left average deflections in (E) and (F).
We presented a 4 s tonic wind stimulus in random order with respect to direction, and recorded movements of the antenna using a camera mounted directly below the fly. By tracking position relative to the midline of the fly (Figure 2B), we found that the antennae are tonically displaced by the wind stimulus, remain deflected for the duration of the stimulus, then quickly relax back to their pre-wind position at stimulus offset (Figure 2D). We next examined displacements of the antennae as a function of wind orientation (Figures 2E and 2F). Each antenna exhibits a curved displacement function with the greatest displacement towards the head (minimum of the curve) at a point between 0° and 45° contralateral to that antenna. The right arista is positioned at approximately 68° relative to the midline at rest (Figure 2B); thus, displacement of the antenna towards the head should be maximal at ~−22° (normal to the arista), and displacement should decrease as the cosine of the angle as wind deviates from this direction. We observe exactly this relationship in the displacements of a single antenna (Figure 2E), each of which has a hook-shaped tuning curve for wind direction. However, because the two aristae are positioned at ~135° to one another, a nearly linear encoding of wind direction can be obtained by simply taking the difference between the two antennal displacements, as illustrated in Figure 2F. This analysis suggests that a single antenna provides an ambiguous representation of wind direction in azimuth, whereas a difference between the two antennae provides a more linear encoding of wind direction. The difference between antennal displacements also provides greater resolution for wind angles directly in front of the fly, where a single antenna provides a weak and ambiguous signal. These results raise the question of whether there are neurons in the brain that generate such representations.
Two classes of AMMC projection neurons encode tonic displacements of the ipsilateral antenna
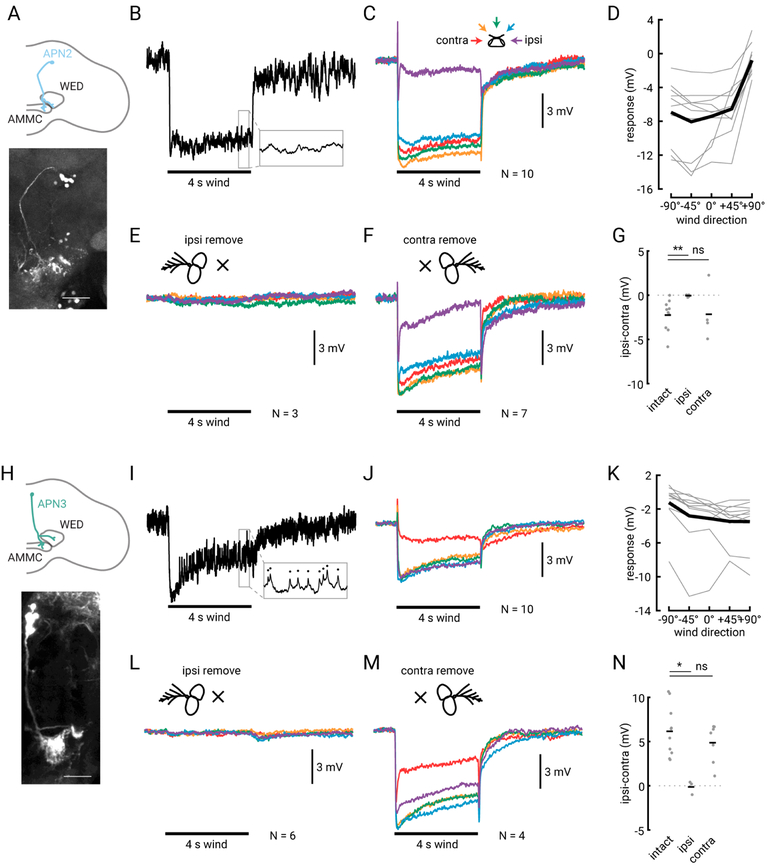
We next sought to characterize how antennal movements are represented by neurons in the central brain. First, we investigated the tuning of AMMC projection neurons APN2 and APN3, which are thought to be downstream of the wind-sensitive CE JONs (Chang et al., 2016; Vaughan et al., 2014). We performed whole cell patch clamp recordings from single, genetically identified APN2 or APN3 neurons from both the left or right side of the brain. Responses from neurons on the left side of the brain were flipped and averaged with neurons from the right side, and we describe neural responses to wind as ipsilateral or contralateral relative to cell body location. We presented 4 s tonic wind stimuli from five directions using the same apparatus described above (also see Figure 2). We found that APN2 neurons were tonically inhibited by wind from all directions except the ipsilateral side (Figures 3B–D). APN2 neurons were non-spiking and responded to tonic wind with graded changes in membrane potential and little adaptation (Figures 3B–D). All APN2 cells we recorded were least inhibited by ipsilateral wind, although we observed some variation in the amount of inhibition across cells (Figure 3D). The tuning curves for APN2 neurons had a hooked shape (Figure 3D) much like the tuning curve for ipsilateral antennal displacement.
Figure 3. Second-order APNs encode tonic deflections of the ipsilateral antenna.
(A) Schematic of APN2 anatomy (blue) on one half of the brain (gray outline), with processes in the AMMC and the WED. Below: GFP signal in 24C06-GAL4, the line we used to target APN2, in the right hemisphere of the brain. Scale bar is 30 μM. (B) Single raw trace of an intracellular recording from APN2 in response to a 4 s wind stimulus (black bar) delivered from 0°. Inset shows a 50 ms expanded trace. (C) Average membrane potential response across N=10 APN2 neurons (from 8 flies) to the five wind directions (color convention shown above the traces). (D) Average steady-state tuning (last 1 s of wind stimulus relative to last 1 s of baseline) for single cells (thin gray lines) and across flies (thick black line). (E) Average APN2 response to wind from five directions after ipsilateral antenna removal. (F) Average APN2 response to wind after contralateral antenna removal. (G) Response range (ipsilateral-contralateral wind response) for flies with intact antennae or with the ipsilateral or contralateral antenna removed. Single-fly differences plotted as grey dots; black bars indicate average across flies. Antennae-intact and ipsilateral removed are significantly different (two-sample student’s t-test, P = 0.0037). Antennae-intact and contralateral removed are not significantly different (two-sample t-test, P = 0.34). (H) Schematic of APN3 (green) depicted in the right hemisphere, with processes in the AMMC and WED. Below: GFP expression in 70G01-GAL4, the line we used to target APN3. Scale bar is 30 μM. (I) Single raw intracellular response from APN3 to wind from +45°. Inset shows small spikes, indicated by the black dots, that occur in a 50 ms segment during the wind stimulus. (J) Average membrane potential response of 10 APN3 neurons (from 10 flies) to wind from the five directions. (K) Steady state tuning of APN3 for single cells (thin lines) and across flies (thick line), computed in the same way as in (D). (L) Average APN3 response to wind from five directions after ipsilateral antenna removal. (M) Average APN3 response to wind after contralateral antenna removal. (N) APN3 response range (ipsilateral-contralateral wind response) for flies with intact antennae or with the ipsilateral or contralateral antenna removed. Symbols as in (G). Antennae-intact and ipsilateral removed are significantly different from one another (two-sample student’s t-test, P = 0.0093). Antennae-intact and contralateral removed are not significantly different (two-sample t-test, P = 0.94). All response traces are plotted on the same scale, and number of cells (each in a different fly) is listed to the right of the stimulus bar. See also Figure S1.
In contrast, APN3 neurons were inhibited by wind from all directions except the contralateral side (Figures 3I–K, Figure S1), the opposite of the ipsilateral antenna’s wind tuning. APN3 neurons produced small-amplitude spikes (28.6 Hz baseline, Figure 3I) as reported previously. APN3 responses were generally smaller and more variable than those of APN2 neurons (Figures 3I–K); the driver line we used to target APN3 also labels more neurons (approximately 11 per hemisphere in 70G01-GAL4) than the driver we used to target APN2 (approximately 6 per hemisphere in 24C06-GAL4).
Given these results, we wondered whether APNs receive any input from the contralateral antenna, or whether they are solely ipsilateral. A recent study (Chang et al., 2016) found that APN3 neurons did not respond to piezo displacements of the contralateral antenna. We removed either the ipsilateral or contralateral antenna and measured the responses of APN2 and APN3 to the five wind directions. Removing the ipsilateral antenna entirely abolished the wind response in both APN2 and APN3 (Figure 3E, 3G, 3L and 3N; two-sample t-test, P = 0.0037 and 0.011, relatively). However, removing the contralateral antenna had no significant effect on the tuning or magnitude of the response in either neuron (Figure 3F, 3G, 3M, and 3N, P = 0.34 and 0.94, relatively). We did notice a slight increase in adaptation in APN2 neurons when the contralateral antenna was removed (Figure 3F), and a slight increase in response magnitude in APN3, however not all flies exhibited these changes (data not shown), so we think they may simply represent variability across cells. Given these results, we conclude that both APN populations encode displacements of the ipsilateral antenna, suggesting that integration across the two antennae occurs downstream.
A class of wedge projection neurons integrates information from the two antennae to generate a linear encoding of wind direction.
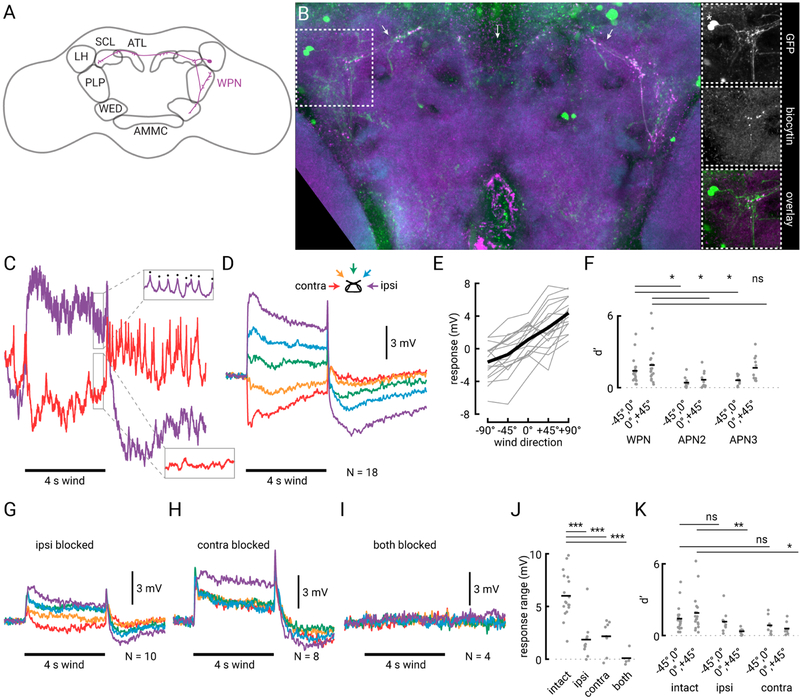
To identify wind-sensitive neurons downstream of the APNs that might integrate information from the two antennae, we performed an electrophysiological screen of projection neurons with putative input processes in or near the wedge (Jenett et al., 2012). We measured the wind responses of many candidate neurons (26 distinct classes), and found few that were both tonically activated by wind and exhibited strong directional tuning (Figure S2). However, we discovered one class of neurons, which we call ‘wedge projection neurons’ (WPNs), that consistently responded to wind stimuli in a tonic, directional manner (Figure 4). The line we found (70B12-GAL4) strongly labels only two of these neurons on each side of the brain, and the two cells are anatomically and physiologically similar. By intracellularly filling two ipsilateral WPNs (Figures 4A and 4B), as well as staining for pre-synaptic markers (Figure S3), we found that these cells receive putative input in the wedge, and project dorsally to the posterior lateral protocerebrum (PLP), where one arm of the neuron branches off. The main branch then projects dorsomedially towards the superior clamp (SCL) and antler (ATL), before crossing the midline to innervate the contralateral ATL and SCL (Figures 4A and 4B). WPNs appear to have large output puncta in the ATL and SCL (Figures 4B and S3). We note that these neurons appear to be members of a large anatomical class that projects along the same characteristic tract from the WED dorsally, crossing the midline at the level of the ATL and SCL (Figure S2E). By recording from other neurons in this class, we found some that were tuned (like the WPNs) for ipsilateral wind, though with a smaller dynamic range, while others responded only transiently to wind with no direction tuning (Figure S2F). Thus, the two neurons labeled in the driver line 70B12-GAL4 are likely members of a larger mechanosensory projection to the dorsal brain.
Figure 4. Wedge projection neurons combine input from the two antennae to linearly encode wind direction.
(A) Schematic of a single wedge projection neuron (WPN) with processes in the WED, PLP, SCL and ATL. (B) Maximum z-projection showing two WPNs filled on the right hemisphere of the brain (magenta) overlaid with GFP (green) from the driver line used to target these cells (70B12-GAL4). Neuropil is shown in blue (left image only). Dotted white box indicates 60×60 μM region of interest expanded on the right. Arrows indicate axon tract that runs across the brain (see also Figure S2E). Top right: GFP signal only. Asterisk indicates two cell bodies on the left side of the brain. Middle right: contralateral intracellular fill(s) in the expanded region. Note proximity of biocytin-filled puncta from ipsilateral (right hemisphere) cells and processes of contralateral cells labeled with GFP. Bottom right: overlay of GFP and biocytin in the expanded region. (C) Raw intracellular responses from one WPN to a 4 s ipsilateral (purple) and contralateral (red) wind stimulus. The neuron produces small-amplitude spikes at rest and during ipsilateral wind (upper box, spikes indicated by the black dots) and is inhibited by contralateral wind (lower box). (D) Average membrane potential of N=18 WPNs (from 17 flies) during wind from the five directions (schematic depicted above the traces). Same scale as (C). (E) Average steady state response (difference between 1 s at the end of the wind stimulus and 1 s baseline immediately before wind presentation). Individual WPN averages in grey; average across 18 cells indicated by the thick black line. (F) Discriminability index (d’) between responses to wind delivered from frontal directions (−45° vs. 0° and 0° vs. +45°) for WPN, APN2, and APN3. Single fly average d’ values plotted as grey dots, and horizontal bars indicate average across flies. Asterisks indicate if the WPN’s d’ values are different from the corresponding values in APN2 or APN3 (two-sample t-test, P = 0.012, 0.026, 0.040, and 0.68). (G) Average WPN response from N=10 flies with mechanosensory input from the ipsilateral antenna blocked. (H) Average wind response from N=8 WPNs in flies with contralateral antenna input blocked. (I) Average responses from N=4 WPNs in flies with input from both antennae blocked. (J) Response range (difference between steady state ipsilateral and contralateral WPN wind response) for flies with free, ipsilaterally-blocked, contralaterally-blocked, or dual-blocked antennae. Symbols as in (F). Mean responses from flies with ipsilateral, contralateral, or both antennae blocked are significantly different than flies with free antennae (two-sample t-test, P = 0.00022, 0.00012, and 0.000029, respectively). (K) Discriminability index (d’) between frontal directions (−45° vs. 0° and 0° vs. +45°) for WPNs with free antennae, ipsilateral blocked, or contralateral blocked. Symbols as in (F). Asterisks indicate d’ values for intact flies that are different from the corresponding d’ values in flies with blocked antenna (two-sample t-test, P = 0.55, 0.0046, 0.21, and 0.030). See also Figure S2–5.
To characterize the wind responses of WPNs, we presented wind to the fly from five directions and measured their responses using whole cell electrophysiology. WPNs were excited by wind delivered from the ipsilateral side and inhibited by wind from the contralateral side (Figures 4C–E). Distinct from the APNs, they exhibited an even gradient of tuning to intermediate wind angles (Figures 4D and 4E). WPNs produced small-amplitude spikes at a low baseline firing rate (8.4 Hz) and responded to tonic wind stimuli with both graded changes in membrane potential and changes in firing rate (Figures 4C and D, Figure S4). WPNs also exhibited a greater ability to differentiate between wind angles in front of the fly (0° vs 45° ipsilateral or contralateral) than APNs for most angles, as measured by the d-prime statistic (Figure 4F). Single neurons had somewhat differing levels of inhibition; however, the dynamic range of individual cells was similar (Figure 4E).
The striking linearity of the WPN’s wind response across azimuthal angles (Figure 4E), and the greater discriminability in front of the fly (Figure 4F), were both reminiscent of the difference between displacements of the two antennae (Figure 2F). Thus, we wondered whether WPNs integrate information from both antennae. To address this question, we blocked input from either the ipsilateral (Figure 4G) or contralateral antenna (Figure 4H), and recorded wind responses in WPNs. Both manipulations greatly diminished directional wind responses in WPNs but did not abolish them, indicating that WPNs receive directional wind information from both antennae. Stabilizing or removing either antenna had statistically indistinguishable effects (Figure S5) and led to a significant reduction in dynamic range (Figures 4G, 4H, and 4J), as well as significant reduction in discriminability of some neighboring frontal angles (Figure 4K). Further, when we blocked input from both antennae, WPNs no longer responded to wind (Figure 4I), indicating that WPNs receive all their input from the antennae. These results indicate that WPNs perform integration across the two antennae to generate a more linear representation of wind direction in azimuth and to increase the discriminability of frontal wind angles.
Wedge projection neurons are not synaptically connected
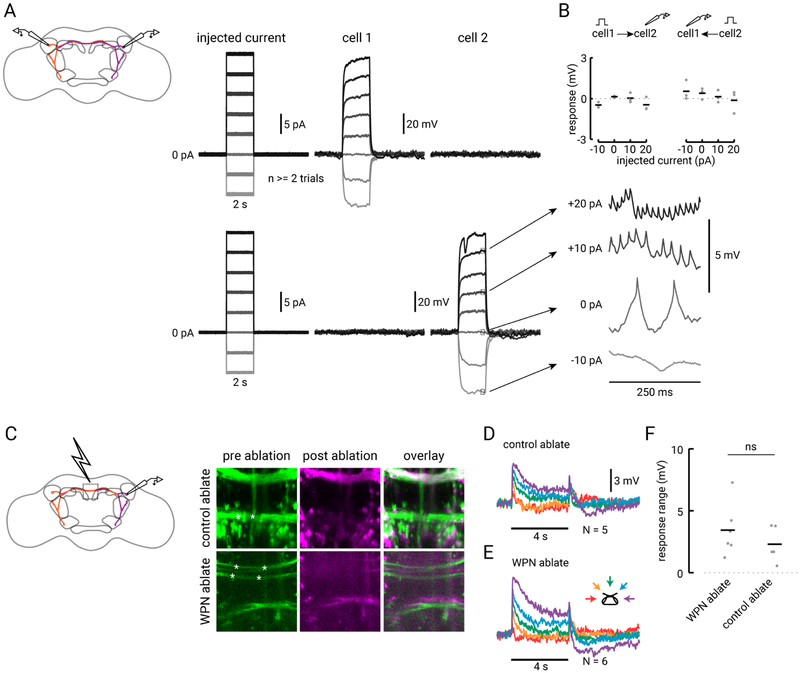
We next aimed to identify where information from the two antennae is exchanged. By visualizing the processes of single WPNs filled during recordings (see Methods), we noticed that the outputs of these neurons overlap closely with the processes of WPNs on the contralateral side of the brain (Figure 4B). Therefore, we hypothesized that WPNs on opposite sides of the brain might synapse onto each other to exchange wind direction information. To test this hypothesis, we performed dual whole cell patch clamp recordings from pairs of WPNs on opposite sides of the brain (Figure 5). When we injected current into one neuron, we observed an increase in the rate of spiking in that neuron, but saw no response in the contralateral WPN, and vice versa (Figure 5A). We observed a complete absence of response in three pairs of contralateral WPNs in different flies (Figure 5B), supporting the notion that WPNs are not synaptically connected.
Figure 5. The contralateral arm of the WPN does not contribute to its wind tuning.
(A) Dual whole cell recording from two WPNs on opposite sides of the brain (depicted in purple and orange in the schematic to the left). On the right, top traces show current injected into cell 1 (cell body located in the right hemisphere) and resulting response in the contralateral cell 2 (left hemisphere). Below traces show current injected into cell 2 and resulting response in cell 1. Expanded traces on the right show typical changes in spike rate due to current steps in that neuron. (B) Average response of contralateral partner to current pulses for three pairs of simultaneously recorded WPNs. None of the average responses across cells are significantly different from zero (one-sample t-test, from left to right, P = 0.056, 0.077, 0.89, 0.26, 0.33, 0.24, 0.65, 0.83). (C) Ablation of WPN or control axons using a 2-photon laser light pulse. Left: central region where axons were targeted for lesioning. Right: Example z-projections of a 41.18 μM square central region of the brain where either a control set of axons (top row; 30 μM deep) or the WPN axons (bottom row; 20 μM deep) are lesioned. Left, pre-ablation GFP signal from 70B12-GAL4, with focal point of laser indicated by the white asterisks. Center: post-ablation stack of the GFP signal in the same region (magenta). Right: overlay of pre- and post-ablation images. (D) Average wind response of N=5 WPNs for flies in which we lesioned control axons. (E) Average wind response for N=6 WPNs with severed axons. (F) Mean steady state difference between ipsilateral and contralateral wind response in flies with either WPN or control axons lesioned. Average for the two conditions are not significantly different (two-sample t-test, P = 0.34).
One concern we had with this experiment was that WPNs have very fine and extended processes, so current injection at the soma might not significantly affect activity at distant synapses. In a complementary experiment, we used a high-energy 2-photon light pulse to lesion the contralateral projections of the WPNs and measured their resulting wind tuning (Figure 5C). As a control, in separate experiments, we lesioned a set of axons labeled in the same line that cross the midline more ventrally and do not appear to have overlapping processes with the WPNs. Figure 5 shows images from two examples in which we ablated either control axons or WPN axons in the center of the brain. We found that wind responses in WPN-lesioned and control-lesioned animals were similar (Figures 5D–F). Both groups showed reduced inhibition, but this reduction was not different between the two groups (Figure 5F; two-sample student’s t-test, P = 0.34), suggesting that it arises from factors related to the lesioning protocol (see Methods). Together, the negative results from the dual patch and laser ablation experiments support the idea that information exchange between WPNs does not contribute to their wind tuning. Thus, we predict that WPNs receive information from the contralateral antenna from some other neuron.
Multiple AMMC projection neurons contribute to WPN wind tuning
Because the WPN’s wind tuning does not appear to depend on its contralateral projections, we next asked whether integration across the antennae occurs at WPN inputs in the wedge. To address this question, we first generated a 70B12-lexA driver line, and verified that the same two neurons are labeled by this line (Figure S3). Next, since other second-order mechanosensory neurons have been shown to receive large inputs through electrical synapses (Azevedo and Wilson, 2017), we asked whether wind responses in WPNs depend on chemical synaptic transmission. We blocked excitatory chemical transmission using the acetylcholine receptor antagonist methyllycaconitine (MLA, 1:1000) and found that this completely abolished the steady state wind response in WPNs (Figures 6A and 6B), indicating that tonic WPN responses require excitatory chemical synaptic transmission.
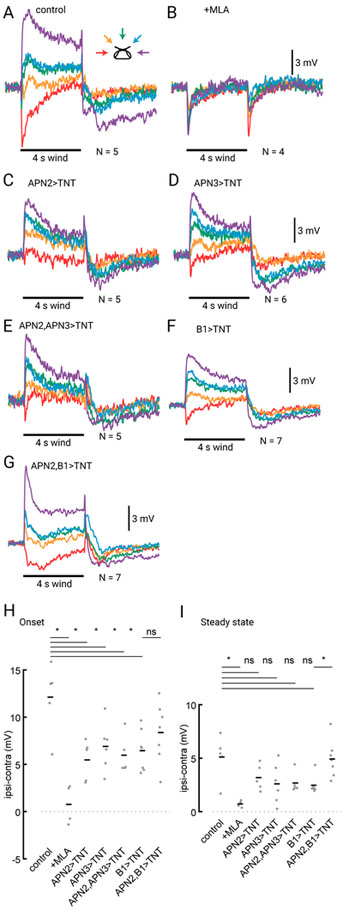
Figure 6. WPNs receive input from multiple AMMC projection neuron populations.
(A) Average responses of WPNs to a 4 s wind stimulus. (B) Average response of WPNs after bath-application of methyllycaconitine (MLA; 1:1000). (C) Average WPN response in flies expressing tetanus toxin (TNT) in APN2. (D) Average WPN response in flies expressing TNT in APN3. (E) Average WPN response in flies expressing TNT in both APN2 and APN3. (F) Average WPN responses in flies expressing TNT in B1. (G) Average WPN wind responses in flies expressing TNT in both APN2 and B1. Number of flies contributing to each average trace in (A)-(G) is listed to the right of the wind stimulus bar. See also Figure S6. (H) Mean response range at wind onset (ipsilateral minus contralateral WPN response average over 800 ms near wind onset) in individual flies (gray dots) and across flies (horizontal black lines). Expression of TNT in any of the AMMC projection neurons (APN2, APN3, and B1) and in both APN2 and APN3 results in a significant change in response range (two-sample t-test; from left to right, P = 0.0010, 0.0085, 0.022, 0.012, and 0.0075). Silencing APN2 and B1 does not result in a significantly different response range than silencing B1 alone (P=0.22). (I) Mean response range at steady state for conditions in (A)-(G). (from left to right, P = 0.0046, 0.11, 0.066, 0.047, 0.012, and 0.0057).
Next, we proceeded to inactivate putative presynaptic partners of the WPNs by driving expression of tetanus toxin, which blocks chemical synaptic transmission. We silenced either APN2 or APN3 (Figures 6C and 6D) and found that in each case the wind response in WPNs was significantly diminished. This effect was most pronounced in the onset response to wind (Figure 6H), however, we also observed a decrease in the tonic wind response in some cases (Figure 6I). Silencing APN2 and APN3 simultaneously also produced a decrease in wind tuning but did not abolish wind responses (Figures 6E, 6G and 6H). This result indicates that at least one other class of neuron must provide input to the WPNs.
What other neurons might provide input to WPNs? One compelling candidate are the B1 neurons. These AMMC projection neurons receive input from the antenna ipsilateral to their cell bodies (Azevedo and Wilson, 2017), and project both ipsilaterally and contralaterally. They have been thought to be mostly auditory, based on their anatomical position downstream of the sound-sensitive A/B JONs (Kamikouchi et al., 2009; Vaughan et al., 2014), and the finding that silencing them significantly impairs courtship song responses in females (Vaughan et al., 2014). However, a recent study (Azevedo and Wilson, 2017) showed that these neurons respond directionally to displacements of the antenna induced by a piezoelectric probe, and that a subset of these are tuned for low frequencies, suggesting a possible role in wind direction encoding. To test the hypothesis that B1 neurons contribute to WPN directional responses, we silenced B1 neurons, and recorded wind responses in WPNs. We found that this resulted in a diminished WPN wind response (Figures 6F–H).
In addition, we asked whether optogenetic activation of APNs and B1 neurons could drive responses in WPNs (Figure S6). We found that light alone could depolarize WPNs (Figure S6C). Optogenetic activation of APN2 with CsChrimson however produced a depolarization with a distinctly different time course: a transient depolarization, followed by a hyperpolarization, (Figure S6A–B) compared to genetic controls. Optogenetic activation of B1 neurons resulted in a transient hyperpolarization of WPN membrane potential (Figure S6G–H)— the opposite of what we observed with APN2 activation. APN3 activation produced no significant effect (Figure S6D–E). Although the effects of activation were small, they are consistent with the idea that APN2 and B1 neurons may provide input, directly or indirectly, onto WPNs with opposite sign.
Curiously, silencing of B1 and APN2 neurons together resulted in a milder deficit than silencing either population alone (Figure 6G). This result argues that additional populations of neurons must provide directional input to WPNs. We therefore used trans-synaptic tracing to investigate what additional neural populations might play a role in wind processing.
Trans-synaptic tracing suggests a larger circuit participating in wind processing
To broadly investigate circuits that might play a role in wind processing, we used trans-Tango (Talay et al., 2017) to identify neurons downstream of primary wind-sensitive mechanoreceptor neurons (JO-CE neurons labeled by JO31-GAL4) as well as the APN2, APN3, and B1 neurons labeled by each of our drivers. Consistent with previous reports, we found that JO-CE trans-Tango labeled both APN2 and APN3 as postsynaptic partners (Figure 7A, Video S1). In addition, this technique labeled at least two prominent commissural populations, including what we believe to be the B1 neurons and a second, more posterior tract with posterior cell bodies (Figure 7A) that may include AMMC-A1 and/or AMMC-Bb (Figure 7A; Matsuo et al., 2016b) and aLN (GCI; Vaughan et al., 2014). These findings support the idea that B1 neurons receive information from “wind” JONs and can participate in wind direction encoding. We also observed some labeling of WPN-like neurons, suggesting that WPNs may receive input directly from JONs or from another neuron labeled in this line with expression in the WED (Figure 7A, inset).
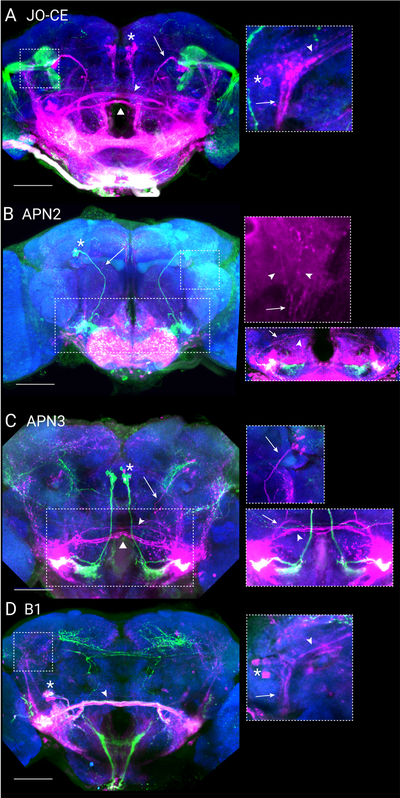
Figure 7. Trans-synaptic tracing labels multiple channels for wind encoding.
(A) trans-Tango driven by primary wind mechanoreceptors (JO31-GAL4, green) labels putative postsynaptic neurons (magenta). Neuropil is shown in blue. Multiple second-order wind neurons classes are labeled, including APN2 (arrow indicates axon tract), APN3 (asterisk indicates location of cell body cluster), B1 (arrowhead points to axon tract passing the midline), and an additional commissural axon tract (triangle). Scale bar is 50 μM. Inset is a maximum projection of putative postsynaptic WPN-like processes (50×50 μM in size and 10 μM deep). Asterisk indicates cell body leading to WPN-like processes, arrow indicates axons leading towards WED, and arrowhead indicates axons leading towards characteristic contralateral branch. (B) trans-Tango driven by APN2 (24C06-GAL4). Same colors and scale as (A). Upper right: Partial maximum projection (expanded 50×50 μM region, 14 μM deep, postsynaptic label shown alone for clarity) reveals WPN-like axon tract that leads dorsally out of the wedge (arrow) and branches into two distinct tracts in the PLP (arrowheads) Lower right: 10 μM deep maximum projection reveals B1 neurons (inset is 70×200 μM). Arrow indicates branching point of axons leading to the AMMC and WED, arrowhead indicates contralaterally-projecting central axon. (C) trans-Tango driven by APN3 (70G01-GAL4) labels APN2 (arrow indicates axon tract; also see inset to the right), B1 (arrowhead), and an additional commissural axon tract (triangle). Asterisk shows location of APN3 cell bodies, some of which are self-labeled. Upper right inset: z-projection (100 μM square, 26 μM deep) showing post-synaptic APN2 cell bodies and axon (marked by arrow) leading down towards the WED. Same colors and scale as (A). Lower right inset: narrower z-projection (10 μM deep) highlights B1 neurons (arrowhead) and an additional cell type leading to the WED (arrow; same scale as left image). (D) trans-Tango driven by B1 (45D07-GAL4). Same colors and scale as (A). Inset shows maximum projection of putative postsynaptic WPN-like processes (50×50 μM in size and 6 μM deep). Asterisk indicates cell body leading to WPN-like processes, arrow indicates axons leading towards the WED, arrowhead indicates axons leading towards characteristic contralateral branch.
Next, we used trans-Tango to trace putative postsynaptic targets of APN2 (24C06-GAL4; Figure 7B). This labeled WPN-like processes, which can be identified most clearly where the dorsal axon exiting the wedge sends a major branch into the posterior lateral protocerebrum (Figure 7B, inset). APN2>trans-Tango also labeled the same two commissures as in the JO-CE trans-Tango, however we believe this labeling arises from a cluster of small cell bodies near the B1 cell bodies that are also labeled in 24C06-GAL4 (Video S2). In contrast, APN3>trans-Tango did not appear to label WPNs. Some diffuse labeling was present along the region that runs between the WED and the posterior lateral protocerebrum (PLP), but these processes were much more anterior than the WPN axon tract between these structures (Figure 7C, Video S3). These results support our conclusions from silencing and optogenetic activation (Figure 6 and Figure S6), that APN3 neurons may connect only indirectly to WPNs. APN3>trans-Tango did label many putative second-order mechanosensory neurons, including APN2, B1, AMMC-Db1, and at least one other commissure (Figure 7, Video S3). This diversity of putative downstream partners suggests that APN3 broadly interconnects mechanosensory circuitry. Finally, we analyzed the trans-Tango signal driven by B1 neurons. B1>trans-Tango labeled distinct WPN-like processes at the posterior face of the brain and the corresponding cell body cluster (Figure 7D), consistent with the hypothesis that B1 neurons provide input to WPNs. We observed no labeling of APN2 or APN3 in B1>trans-Tango.
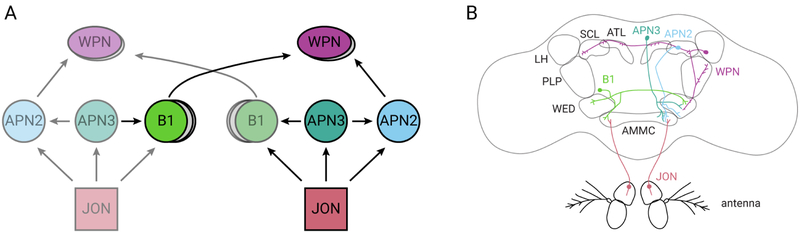
Together, our physiological and anatomical results suggest that wind direction is computed in the dendrites of WPNs at the level of the WED. At a minimum, this computation likely involves ipsilateral antenna deflection information, carried by APN2 neurons, and contralateral antenna deflection information, carried by B1 neurons (Figures 8A and 8B). However, our double silencing results (APN2 and B1 neurons) argue that WPNs must receive additional sources of directional information. One source could be the posterior contralateral projection observed in JO-CE> trans-Tango (AMMC-A1, AMMC-Bb, or aLN). WPNs may also receive direct input from CE-JONs (Figure 7A). Finally, it is unclear whether these inputs synapse directly onto our two WPNs, or pass through an intermediary, such as other WPN-like neurons. Future studies using connectomic approaches will be necessary to reconstruct the full complement of inputs onto WPNs.
Figure 8. Summary diagram of the wind processing circuit.
(A) Hypothesized circuit diagram of wind neurons in this study, using previously established nomenclature for brain regions (Ito et al., 2014). Arrows indicate hypothesized direction of information flow. Gray circles indicate the presence of additional contralaterally-projecting or WPN-like neurons. (B) Schematic depicting anatomical projection patterns for single neurons from the classes included in (A) (same colors). Neuropil regions are outlined in gray.
DISCUSSION
Wind direction as a mechanosensory computation
Animals use a wide array of sensory cues to navigate through the world. Visual cues are often used for navigation, because they can provide fast, reliable information about an animal’s location in space. However, mechanosensory cues can also provide spatial information. Animals that hunt in the dark, such as owls and bats, rely on highly specialized mechanosensory systems to compute the location of prey from sound stimuli. Studies of these systems have revealed many basic principles of neural computation (Konishi, 2003).
Wind is one such mechanosensory cue used by animals to navigate through space. When wind direction is stable, it can provide an orientation cue that permits dispersal over long distances (Bell and Kramer, 1979), or it can serve as a compass to help locate a feeding site or nest (Müller and Wehner, 2007). Odor molecules are typically dispersed by wind; consequently, many animals have evolved strategies of using wind direction cues, in combination with olfactory cues, to locate food or mates (David et al., 1983; Flugge, 1934; Kennedy and Marsh, 2004; Lacey et al., 2014; Wolf and Wehner, 2000). In flying animals, orientation to wind is thought to require measurements of optic flow (Kennedy 1941; Kennedy and Marsh 1974; Murlis et al. 2000), because wind displaces the entire animal as well as its mechanoreceptors. In contrast, walking animals can derive wind direction directly from mechanosensation (Álvarez-Salvado et al., 2018; Bell and Kramer, 1979; Wolf and Wehner, 2000). Although this strategy, known as “odor-gated anemotaxis,” has been studied most extensively in invertebrates, there is some evidence that vertebrates use this strategy as well. For example, rats can use their vibrissae to determine wind direction (Yu et al., 2016), and wolves will orient upwind after scenting their prey (Mech, 1966).
Like sound localization, computing wind direction also requires the nervous system to combine information from multiple sensors. Wind-sensing has been best-studied in the cercal system, in which a set of paired appendages on the abdomen of crickets and other orthopteran insects provide wind direction cues. The cercus is covered with an array of bristles, each of which has a preferred axis of deflection, that generate an array of direction-tuned axonal fields in the abdominal ganglion. These fields are sampled by interneurons to generate a new array of cosine-tuned wind sensors, each aligned with a few cardinal axes (Boyan and Ball, 1990; Jacobs, Miller, Aldworth 2008).
In contrast, the neural basis of Wind-sensing by the antennae has been less studied, despite their critical role during odor-gated wind orientation in many walking insects (Álvarez-Salvado et al., 2018; Wolf and Wehner, 2000). In this study, we showed that flies require signals from both antennae to perform full upwind orientation during walking olfactory navigation. We saw no effect of single antenna stabilization on downwind preference in the absence of odor. This may be because our measurement of downwind preference reflects the animals’ integrated behavior over time, or because flies are able to use active movements of the body, or of the antenna, to compensate for the loss of one sensor. Flies can actively position their antennae using muscles in the base of the antennae (Miller, 1950) and could use such movements to gain additional information about wind direction. We measured a slight increase in angular velocity during the odor stimulus, suggesting that flies might use body scanning to compensate for the loss of one antenna, and observed qualitative examples of looping. However, future studies will be necessary to understand how flies combine active movement with antennal mechanosensation during wind orientation.
Here we found that the geometry of the antennae is crucial for the ability of the fly to compute wind direction. We found that a single antenna is maximally deflected by wind perpendicular to the arista, with displacement falling off symmetrically with deviations from this angle. Thus, a single antenna encodes an ambiguous signal about wind direction. Our findings are consistent with previous work (Morley et al., 2012) showing that the mechanical sensitivity of the antennae are greatest in the frontal field at 45° relative to the midline. The central neurons we characterized in this study encode either the displacement of one antenna (with the associated ambiguity), or a signal closely resembling the difference between the two antennal displacements. The geometry of the antennae varies greatly across insect evolution (Krishnan and Sane, 2015); for instance different fly species from the same superfamily have distinct antennal morphologies (Sinclair and Cumming, 2006). How these different geometries might impact wind perception and sensitivity is an open question.
Wind circuits in the fly brain
Previous studies hypothesized that antennal displacement signals must be integrated to form a central representation of wind direction (Patella and Wilson, 2018; Yorozu et al., 2009). Wind direction is first represented by tonically responding JONs (Kamikouchi et al., 2009; Yorozu et al., 2009), and functional imaging from JON axon terminals has shown that the E region of the AMMC responds to deflections of the ipsilateral antenna towards the head (“push”), while the C region responds to deflections away from the head (“pull”; Kamikouchi et al., 2009; Yorozu et al., 2009). Unlike in the mosquito (Andres et al. 2016), Drosophila JONs do not receive synaptic input (Kamikouchi et al. 2010). Thus integration is unlikely to occur at the level of JONs. Recently, pan-neuronal imaging identified a region of the WED that responds selectively to ipsilateral pull and contralateral push, the displacement pattern produced by ipsilateral wind (Figure 2, Patella & Wilson, 2018). However, this study did not identify specific neurons that perform these computations.
Here we have identified a novel class of neurons, which we call WPNs, that integrate displacement information from the two antennae. These neurons are excited by ipsilateral wind and inhibited by contralateral wind, resulting in a rather linear encoding of wind direction in azimuth. In addition, responses to nearby frontal wind angles are well-differentiated from one another. Both these coding features closely resemble the difference in displacement signals we measured across the two antennae during wind stimulation and are distinct from the tuning exhibited by a single antennae. To directly test whether WPNs perform integration, we recorded their responses in flies with stabilized antennae, and found that stabilization of either antenna reduced wind tuning and discriminability of frontal angles, consistent with integration.
To localize the site of integration we performed paired recordings from WPNs, 2-photon lesions of WPN processes, and recordings, activations and silencing experiments of putative upstream partners of WPNs. Taken together these experiments support a model in which information from the two antennae is integrated in the WED, by combining ipsilateral information carried by APN2 neurons with contralateral information carried by B1 neurons. However, our experiments also suggest that the circuit underlying the computation of wind direction is more complex than this simple model. First, we were unable to completely abolish directional wind responses by silencing any pair of inputs. This suggests that at least three and likely more populations carry wind direction information directly or indirectly to WPNs. At least two prominent contralateral tracts were labeled by trans-synaptic tracing downstream of wind-sensitive JONs. Some CE JONs are known to project contralaterally (Kamikouchi et al., 2009), and these might contribute to integrative responses in WPNs as well. Second, the effects of activating putative upstream partners (APN2 and B1 neurons) were generally weak and biphasic. This could indicate that connections between AMMC projection neurons and WPNs are indirect, going either through WED local neurons, or through other WPN-like neurons. Finally, we observed several paradoxical responses in the wind circuit. For example, APN3 neurons responded differently to stimuli that displaced the ipsilateral antenna by the same amount (−90° and +45°). In addition, we found that silencing both B1 and APN2 neurons resulted in milder deficits than silencing either population alone. Both of these findings suggest a role for recurrent connections that might amplify or modulate responses to antennal displacement. A comprehensive understanding of computations in the wind direction circuit will likely require EM reconstruction, which has very recently been accomplished for the well-studied olfactory projection neurons of the fruit fly (Frechter et al., 2018), as well as genetic tools for silencing larger numbers of GAL4 lines simultaneously.
Potential downstream targets and functions of wind neurons
WPNs project to multiple targets in the brain, suggesting a broad dispersal of wind information throughout the dorsal brain. These regions include the posterior lateral protocerebrum (PLP), superior clamp (SCL), and antler (ATL). Although morphologically similar regions of the brain have been described in other insects (von Hadeln et al., 2018; Immonen et al., 2017), to our knowledge, little is known about the function of these regions of the brain. Neurons in the central complex of other insects have been shown to respond to mechanosensory stimuli (Homberg, 1985, 1994; Ritzmann et al., 2008), but it is not yet clear how information from the APNs or WPNs might be routed to the central complex. Each brain region innervated by the wind neurons in our study (AMMC, WED, PLP, SCL, ATL; see Figure 8) is also the site of input for descending neurons (Hsu and Bhandawat, 2016; Namiki et al., 2017), raising the possibility that wind information is relayed to motor systems multiple times. The identification of WPNs as mechanosensory neurons provides a starting point for investigating the function of these areas of the insect brain.
How might the wind direction information encoded by the WPNs be used during behavior? Because WPNs are activated by ipsilateral wind, they could produce upwind orientation by activating neurons that drive ipsilateral turns. Conversely, they could produce downwind orientation by activating neurons that drive contralateral turns. The contralateral projections of the WPNs, which we found did not contribute to their wind-tuning, might carry information to such motor systems. WPNs may also participate in other forms of orientation such as detecting body orientation relative to gravity (Kamikouchi et al., 2009) or measuring the direction from which a song is produced during courtship behavior (Morley et al., 2012).
Here we have shown how the fly measures wind direction, enabled by the geometry of the antennae, and by computations in a neural circuit that integrates information across sensors to generate a representation of a feature of the external world. We identify new regions of the central brain as mechanosensory processing centers and locate two sites of mechanosensory information exchange across the two sides of the brain. Together, these results reveal novel pathways and encoding strategies for wind information and highlight the importance and prevalence of wind information in the Drosophila brain.
STAR METHODS:
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and fly lines should be directed to and will be fulfilled by the lead contact, Dr. Katherine Nagel (katherine.nagel@nyumc.org).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
For all experiments, we used adult female Drosophila melanogaster, between 2 and 7 days old.
Fly strains
For behavioral experiments, we used flies bearing norpA36 backcrossed into an isogenic w1118 strain (Bloomington #5905) that exhibits robust walking and olfactory search behavior (Álvarez-Salvado et al., 2018). To target cell bodies for electrophysiological recordings, we expressed cytoplasmic GFP (typically 10XUAS-IVS-Syn21-GFP-p10 but also 10xUAS-CD8:GFP) under control of the following driver lines: R24C06- GAL4 (APN2), R70G01- GAL4 (APN3), and R70B12- GAL4 (WPN). We made flies carrying R70B12-lexA using standard techniques (Pfeiffer et al., 2008; Pfeiffer et al., 2010). This was recombined with lexAOP-GFP (from Bloomington #52265) to visualize WPNs while using the GAL4-UAS system to silence putative upstream neurons. For Chrimson activation experiments, we used flies with the following genotypes: 70B12-lexAop, lexAop-GFP / UAS-CsChrimson; R24C06- GAL4/UAS-10xGFP and R70B12-lexAop, lexAop-GFP/UAS-CsChrimson; R70G01- GAL4/UAS-10xGFP. For inactivation experiments, we used the three following genotypes: R70B12-LexAop, LexAop-GFP/UAS-TNTe; R24C06- GAL4and R70B12-lexAop, lexAop-GFP/UAS-TNTe; R70G01- GAL4, and R70B12-lexAop, lexAop-GFP/UAS-TNTe; R45D07- GAL4. Control flies were R70B12-lexAop, lexAop-GFP/+. For trans-synaptic tracing using trans-Tango, we crossed UAS-myrGFP, QUAS-mtdTomato (3x HA); trans-Tango flies to R24C06- GAL4 (APN2), R70G01- GAL4 (APN3), R45D06- GAL4 (B1) or JO31- GAL4 (JO-CE neurons). In the text, we refer to all GAL4-driver lines from the Janelia Flylight collection without the ‘R’ prefix for simplicity. All the genotypes we in this study are listed in the following table.
| Figure 1 | w1118norpA36; ; (5905) |
| Figure 3B-E,J(APN2) | R24C06- GAL4/ 10XUAS-IVS-Syn21-GFP-p10 |
| Figure 3F-IJ (APN3) | R70G01- GAL4/ 10XUAS-IVS-Syn21 –GFP-p10 and R70G01- GAL4, 10xUAS-CD8:GFP |
| Figure S1 (APN3) | R70G01- GAL4/10XUAS-lVS-Syn21-GFP-p10 and R70G01- GAL4, 10xUAS-CD8:GFP |
| Figure 4B-E, G-K (WPN) |
w+;; R70B12- GAL4/ 10XUAS-lVS-Syn21 -GFP-p10 and R70B12-lexA, lexAOP-GFP/ + |
| Figure 4F (WPN, APN2, and APN3) |
w+; R70B12-GAL4/ 10XUAS-IVS-Syn21 -GFP-p10, R70B12-lexA, lexAOP-GFP/+,; R24C06-GAL4/10XUAS-IVS-Syn21- GFP-p10;,; R70G01-GAL4/10XUAS-lVS-Syn21-GFP-p10; and; R70G01-GAL4, 10xUAS-CD8:GFP; |
| Figure S2 |
XX-GAL4/10XUAS-IVS-Syn21-GFP-p10 where driver ‘XX’ is noted in the figure. |
| Figure S3A | R70B12-lexA,lexA0P-GFP/UAS-nsyb.eGFP;UAS-tdTOM/+ |
| Figure S3B | R70B12-lexA,lexAOP-GFP/+;R70B12-GAL4,UAS-tdTOM |
| Figure S4 |
w+;; R70B12-GAL4/10XUAS-IVS-Syn21-GFP-p10 and R70B12- lexA, lexAOP-GFP/+ |
| Figure S5 |
w+;; R70B12-GAL4/10XUAS-IVS-Syn21-GFP-p10 and R70B12- lexA, IexAOP-GFP/ + |
| Figure 5A-B (WPN) | R70B12-lexA,lexAOP-GFP/ + |
| Figure 5C-D (WPN) | R70B12-GAL4/ 10XUAS-IVS-Syn21 -GFP-p10 |
| Figure 6A (WPN) | R70B12-lexA,lexAOP-GFP/+ |
| Figure 6B (WPN) | R70B12-lexA,lexA0P-GFP/ + |
| Figure 6C (WPN) | R70B12-lexA,lexA0P-GFP/ UAS-TNT; R24C06-GAL4/ + |
| Figure 6D (WPN) | R70B 12-lexA,lexA0P-GFP/ UAS-TNT; R70G01-GAL4/ + |
| Figure 6E (WPN) | R70B12-/lexA,lexAOP-GFP/UAS-TNT; R24C06-GAL4/70G01- GAL4/ + |
| Figure 6F(WPN) | R70B12-lexA,lexA0P-GFP/UAS-TNT; R45B07-GAL4 |
| Figure 6G | R70B12-lexA,lexAOP-GFP/UAS-TNT; R45B07-GAL4/R24C06- GAL4 |
| Figure 6H,I (WPN) | R70B12-lexA,lexAOP-GFP/+ R70B12-lexA,lexA0P-GFP/ UAS-TNT; R24C06-GAL4/ + R70B12-lexA,lexA0P-GFP/ UAS-TNT; R70G01-GAL4/ + R70B12-lexA,lexA0P-GFP/UAS-TNT; R24C06-GAL4/R70G01 -GAL4 R70B12-lexA,lexA0P-GFP/UAS-TNT; R45B07-GAL4/+ R70B12-lexA,lexA0P-GFP/UAS-TNT; R45B07-GAL4/R24C06- GAL4 |
| Figure S6A-B (WPN) | R70B12-LexAop, LexAop-GFP / UAS-CsChrimson; R24C06- GAL4/UAS-10xGFP |
| Figure S6C (WPN) | R70B12-LexAop, LexAop-GFP |
| Figure S6D-E (WPN) | R70B12-LexAop, LexAop-GFP/UAS-CsChrimson; R70G01- GAL4/UAS-10xGFP and R70B12-LexAop, LexAop-GFP |
| Figure S6F (APN2) | R70B12-LexAop, LexAop-GFP / UAS-CsChrimson; R24C06- GAL4/UAS-10xGFP |
| Figure S6G-H (WPN) | R70B12-LexAop, LexAop-GFP / UAS-CsChrimson; R45B07- GAL4/UAS-10xGFP |
| Figure S6I (WPN) | R70B12-LexAop, LexAop-GFP, R70B12-LexAop, LexAop- GFP / UAS-CsChrimson; R24CO6-GAL4/UAS-1OxGFP, R70B12-LexAop, LexAop-GFP / UAS-CsChrimson; R70G01- GAL4 / UAS-10xGFP and R70B12-LexAop, LexAop-GFP / UAS- CsChrimson; R45B07-GAL4/UAS-10xGFP |
| Figure 7A (JO-CE) |
UAS-myrGFP, QUAS-mtdTomato (3x HA)/+; trans-Tango/ J031- GAL4; |
| Figure 7B (APN2) |
UAS-myrGFP, QUAS-mtdTomato (3x HA); trans-Tango/+; R24C06-GAL4/ + |
| Figure 7C (APN3) |
UAS-myrGFP, QUAS-mtdTomato (3x HA); trans-Tango/+, R70G01-GAL4/+ |
| Figure 7D (Bl) | UAS-myrGFP, QUAS-mtdTomato (3xHA); trans-Tango/+, R45D06-GAJL4/+ |
METHOD DETAILS
Behavioral Experiments
For behavioral experiments, we used the same miniature wind tunnel apparatus described in Álvarez-Salvado et al. (2018). As previously described, flies were constrained to walk within a shallow arena. A constant airflow travelled through the arena at 11.9 cm/s as measured by a calibrated hot wire anemometer. A 10 s pulse of odor (1% apple cider vinegar, Pastorelli) was introduced by a set of solenoid valves located immediately below the arena. Advection of the odor through the arena was measured by photo-ionization detector (miniPID, Aurora Scientific, Aurora Canada). Flies randomly experienced one of 3 conditions on a given trial: wind only, a 10 s odor pulse in constant wind (30 s wind, 10 s odor, 30 s wind) or no wind. We collected mated females 3–7 days before the experiment and housed them in custom time shift boxes on light cycles of 12 h. We deprived flies of food for 20–24 h before experiments in an empty polystyrene vial with a small amount of shredded damp Kimwipe on the bottom to humidify the air. Experiments lasted approximately two hours were started at either ZT1 or ZT3 (1 or 3 hours after lights on).
To block mechanosensory input from the Johnston’s organ in one or both antennae, we gently anesthetized a fly over ice, and removed the fly’s arista with a sharp pair of forceps by pinching it off precisely near its base. We then applied a small amount of UV glue (Aquaseal UV Field Repair Adhesive) to the antennal joint between the 2nd and 3rd segments near the ablated arista and cured for 10 s. We used a small drop of glue so that the majority of sensilla on the third segment were unobscured. We allowed flies to recover for 20–24 h before the experiment, during which time they were deprived of food, but not water (similar to all other behavioral experiments). In a pilot set of experiments, we observed that the glue was no longer present on the antennae for some flies (we hypothesize that some were able to groom it off during the long starvation period). Thus, after each experiment, we gently removed each fly from the behavioral arena and inspected its antenna(e) to verify that the manipulation persisted throughout the starvation period and experiment. For control flies, we performed a “sham” manipulation in which we cold0anesthetized and exposed the flies to UV light for the same duration as experimental flies.
We analyzed behavioral data using similar protocols to those described in Álvarez-Salvado et al. (2018). Briefly, we tracked the position and orientation of flies in real time using custom Labview software (National Instruments) and further analyzed the data in Matlab (Mathworks). We discarded any trials with tracking errors, and low-pass filtered coordinates and orientations at 2.5Hz using a 2-pole Butterworth filter. Trials in which flies moved less than 25 mm, and flies that had fewer than 5 trials that met our criteria were discarded. In addition, we excluded trials in which flies spend more than 25% of the period used for analysis within 3 mm of the edge of the arena. Because odor reaches flies at different times depending on their position in the arena, we aligned trajectories for upwind velocity to the actual time the odor reached the fly based on their position and the delay as recorded by the PID (miniPID, Aurora Systems). When measuring angular velocity, we aligned trials to the end of the odor presentation. All flies that were excluded from the odor condition were subsequently excluded from the wind preference assay. One additional fly was excluded from the arena position analysis (Figure 1G) in both glue and single glue conditions compared to Figure 1B because of lack of movement during wind-only trials. Behavioral parameters (upwind velocity and absolute angular velocity) were computed only using segments in which flies moved at greater than 1 mm/s.
Upwind velocity and angular speed were computed for the 5 s following odor onset and the 2 s following the offset of the odor, respectively, on a fly-by-fly basis and averaged. We averaged behavior from 10 s after the trial began to 5 s before odor onset for a total of 15s to compute the baseline level of activity.
Wind stimulus apparatus
We custom-designed the wind stimulus delivery apparatus (Autodesk Inventor Professional) to precisely align a mounted fly at the center of 5 wind streams of equal velocity. The apparatus was 3D printed (Makerbot Replicator 2) and consisted of 3 parts: the wind manifold, the physiology holder, and the base. The wind manifold had five symmetric wind channels with a diameter of 0.1352 in (a bit wider than a fly). The physiology holder was created based on previous designs (Maimon et al., 2010; Weir et al., 2016) (see also http://ptweir.github.io/flyHolder/), and we designed the base to hold the manifold and the physiology holder at a constant angle and position. The manifold was attached to a base by a mounting screw which also allowed us to fix the apparatus to a post. The physiology holder attached to the base in a fixed position using magnets. When the fly was mounted, the openings of the channels were 0.55 inches away from the fly’s head.
To generate the wind stimuli, we passed house air over a charcoal filter and a water reservoir (to humidify the air). Wind was controlled by a set of 5 solenoid valves (Lee Company LHDA1233115H) that directed air flow through one of the five channels, or to a vacuum. Wind and vacuum flow rates were set using flow meters (Cole-Parmer PMR1–010608). Valves were controlled from Matlab via a digitizer (National Instruments BNC-2090A) that interfaced with a microcontroller board (Arduino Mega 2560). The Arduino decoded control signals and controlled a set of relays to direct the wind flow through one of the five channels, or to the vacuum. We placed flyback diodes across the leads of the solenoid valves to eliminate a large voltage spike that we otherwise picked up with the recording electrode.
We measured wind velocity at the location of the fly using an anemometer (Dantec Dynamics MiniCTA 54T42) with a probe (Dantec Dynamics 55P16) placed in the position normally occupied by the fly. Windspeed at the position of a mounted fly was 59.57 (+/− 1.4) cm/s. By mounting the probe in parallel with the wind channels, such that the filament was perpendicular to this flow, we ensured that it was circularly symmetric with respect to the five channels. We mounted the probe 6 times in this way and averaged the results to obtain a realistic measurement of stimulus variability (e.g. resulting from small deviations in the fly’s size and/or position in the physiology holder). To calibrate the probe and calculate absolute wind velocity, we created a pseudo-laminar wind flow with a C-mount tube filled with coffee straws and a mass flow meter placed 20 mm from the anemometer probe. A mass flow meter (Aalborg Mass Flow Controller GFC17 0–2L) was then used to relate air velocity (computed by dividing the volume flow rate by the cross-sectional area of the tube) to the voltage readout of the anemometer. The linear range of this function (20 to 70 cm/s at the anemometer) was used to determine absolute wind velocity.
Antenna tracking
To monitor movements of the antennae in response to wind, we acquired videos of the fly from below using an Allied Vision Guppy Pro F-031B camera with an Infinistix 68mm/2.00x lens with 90-deg mirror running at 60 Hz. We triggered the camera and acquired videos using Matlab. For all flies, we first defined the midline axis of the head using points at the top and bottom of each eye (at the edge of the retina) consistently across animals. To quantify antenna displacements over time (Figure 2D), we then used simple feature extraction techniques to detect the angle of the arista, relative to the midline of the head. Briefly, to detect the line of the main branch of the arista in each image, we performed the Hough transform of the image, found the peaks of the resulting Hough transform matrix, and detected line segments corresponding to these peaks. Errors were detected by excluding angles that were far out of the range of realistic antennal deflections (outside of the 30 deg range in which the antenna was displaced in these experiments). In the single fly tracking example shown in Figure 2D, we observed three instances in which there were large active deflections of the antenna; we omitted the trial block in which these occurred, to quantify the passive deflections of the antenna only. We averaged over 7 trials (7 videos) per direction for this example fly to obtain the average antenna deflection over time.
To quantify displacement as a function of wind direction (Figure 2E and 2F), we found that hand-tracking the antenna was the most accurate method for quantification. For these data, two people (M.D. and S.S.) were blinded to the wind direction in each trial. They hand-digitized the position of the arista in individual video frames using two points to define the line of the main branch. For each trial/video, we computed the average baseline arista position across 5 frames just before the wind stimulus turned on, and the steady state deflection across 5 points at the end of the wind stimulus. We did this for every fly, averaged across trials within a fly (over a minimum of 1 and maximum of 10 trials per direction, typically 5), then averaged across flies.
Electrophysiology
We performed whole cell patch clamp recordings in tethered female flies using previously described methods and solutions (Maimon et al., 2010; Wilson and Laurent, 2005). Briefly, we cold-anesthetized flies and glued them to a plastic holder using UV-curing glue (Kemxert Corp York KOA 300). The holders were made by 3D printing a plastic base (MakerBot Replicator 2), and attaching metal cutouts (Etchit) to the holder using UV-curing clue following guidelines outlined in (Weir et al., 2016). We removed the front two legs at the base of the femur, and the rest of the legs at the base of the coxa, to prevent interference with the wind stimulus. We then applied UV glue to the proboscis, securing it to the head and to the front two coxa, to prevent excessive brain movement. To access the brain, under saline, we removed the cuticle at the back of the head using a small hypodermic needle (30G), and then using fine forceps, we gently removed only the trachea on the surface of the brain over the region where our cell bodies resided. For most flies, we also removed muscle 1 which would otherwise obscure cell bodies of interest. We broke through the sheath of the brain and cleaned the area only around the cell bodies of interest by using positive pressure to apply a small amount of collagenase (5% in extracellular saline; Worthington Biochemical Corporation Collagenase Type 4) with a fine0tip electrode (approximately 5–10 μM diameter at the tip). Extracellular saline, composed of 103 mM NaCl, 3 mM KCl, 5 mM TES, 8 mM trehalose dihydrate, 10 mM glucose, 26 mM NaHCO3, 1 mM NaH2PO4H20, 1.5 mM CaCl22H2O, and 4 mM MgCl26H2O, was continuously perfused over the brain during recordings.
For whole cell patch clamp recordings, we pulled 7–13 MΩ glass pipettes using a Sutter Instruments P-1000 puller (smaller resistance electrodes were used with the smaller WPN and APN3 cell bodies, and larger with the larger APN2 cell bodies). Our intracellular solutions contained 140 mM KOH, 140 mM aspartic acid, 10 mM HEPES, 1 mM EGTA, 1 mM KCl, 4 mM MgATP, 0.5 mM Na3GFP, and 13 mM biocytin hydrazide (for visualization of neural processes). The average membrane potential of the wind neurons were −22.3 mV, −22.0 mV, and −26.4 mV for WPNs, APN2, and APN3, respectively. These values are not adjusted for an estimated −13 mV junction potential measured in a previous preparation with identical solutions, preparations, and electrodes (Suver et al., 2016).
We used the following three patch clamp amplifiers in our electrophysiology experiments: A-M Systems Model 2400 (for part of the wind neuron screen), Multiclamp 700B (Molecular Devices; for dual-patch and B1 recordings), and an Axopatch 200B (Molecular Devices) paired with additional preamplification by a Brownlee Precision Model 410 preamplifier for the remaining (majority) of experiments. We analyzed data and controlled stimuli with custom Matlab software. Control signals were relayed to stimulus devices via a digitizer (National Instruments BNC-2090A). We acquired all data at 10 kHz and then downsampled to 1kHz for analysis. Before downsampling, we detected spikes by smoothing (over a window of 20 samples), differentiating, and thresholding the membrane potential. False-positives were eliminated by excluding any spikes that occurred within 4 ms of each other, and the accuracy of this spike detection was confirmed by eye for each cell type. We filtered out spikes from the membrane potential averages for neurons that produce small spikes (APN3 and WPN) by lowpass filtering at 20 Hz. All average APN and WPN recordings are presented as averages across flies in which we obtained a stable recording for a minimum of 2 of each of the 5 wind directions, and a maximum of 15 trials/direction (7 trials on average). For all neural classes, we targeted a mixture of left and right-hemisphere cell bodies for recordings, and responses from neurons in the left hemisphere were flipped and averaged with those on the right. We targeted all APN2 neurons for recordings using 24C06-GAL4, and APN3 neurons using 70G01-GAL4. However, we targeted eight WPNs using cytoplasmic GFP under the control of 70B12-GAL4, and ten using cytoplasmic GFP under control of 70B12-LexA; we observed no difference between these groups.
Pharmacology
To block cholinergic synaptic transmission, we added 1:1000 methyllycaconitine (1mM solution in dH20 stored at 4°C) to our extracellular saline.
Optogenetic activation
For Chrimson activation experiments, we raised flies on typical agar food supplemented with 50 μL all-trans retinal (35mM stock dissolved in ethanol, stored at 4°C) mixed into 1 tablespoon of hydrated potato flakes. We targeted WPN neurons using 70B12-lexA and expressed UAS-CsChrimson under the control of either 24C06-GAL4 (for APN2 activation) or 70G01-GAL4 (for APN3 activation). We performed light control recordings in flies with the following genotype: 70B12-lexA/lexAop-10xGFP; 24C06-GAL4. Responses elicited in APN neurons were relatively small (a little over 5 mV, see Figure S6), so we used the lowest light intensity that produced a maximum response from APN2 neurons (2.23 mW/cm2; Figure S6).
2-photon laser ablation
For lesioning, we mounted flies in the same way as for electrophysiology experiments (see Electrophysiology above). Immediately before lesioning, we removed the cuticle and trachea (again identically to electrophysiology experiments). The brain was submerged in fresh saline, but was not perfused, for approximately 20 min. Recording was delayed by about 50 minutes (on average) relative to recordings without laser ablation. We performed axotomies using an apparatus pioneered by Tsai et al. (2009) and optimized by Koyama et al. (2016). We used a high-power (8W) fixed wavelength (1040 nm) low-repetition rate (200 kHz) pulsed infrared laser (305 fs) with integrated pulse picker (Spirit One, Spectra-Physics). The high-power laser beam was concentrically aligned with a standard Ti-Sapphire two-photon imaging laser (Mai Tai DeepSea), steered using a ThorLabs microscope controlled with ThorImage 3.0, and focused on to the sample using a 40x objective (Olympus LUMPLFLN 40x/0.8 W). For each axon, we delivered 1–2 repeats of a 10-pulse long train at 10 kHz. Pulse power was 39 mW measured at the sample with a power meter (ThorLabs, PM 100D and S130C). We acquired pre-lesion z-stacks immediately before axotomy, and post-lesion z-stacks approximately 10 s after exposure to the high-power pulse train.
Immunohistochemistry and anatomy
To visualize processes of recorded neurons, we gently removed the fly from the physiology holder and dissected the fly’s brain in PBS immediately after recording. We then fixed the brain for 15 min in 4% paraformaldehyde solution (in PBS). Next, we washed the brain in PBS three times and stored at 4°C until antibody staining (within three days). We incubated brains in blocking solution (5% normal goat serum in PBST) for 20–60 min and incubated them for 24 hours at room temperature in a primary antibody solution containing 1:10 mouse anti0bruchpilot (nc82, Developmental Studies Hybridoma Bank) and 1:1000 rabbit anti-GFP (Thermo Fisher Scientific #A6455) in block. After three washes in PBS, we incubated the brains in a secondary antibody solution containing 1:250 anti-mouse Alexa Fluor 633 Thermo Fisher Scientific A-21052), 1:250 anti-rabbit Alexa Fluor 488 (Thermo Fisher Scientific A-11034), and 1:1000 streptavidin Alexa Fluor 568 (Thermo Fisher Scientific S-11226) in block, again at room temperature for 24 hours. We washed brains three times in PBS and mounted them in vectashield (Vector Labs H-1000). Slides were imaged immediately or stored at 4°C until imaging. We imaged brains at 20x magnification on a Zeiss LSM 800 confocal microscope with 20x objective (Zeiss W Plan0Apochromat 20x/1.0 DIC CG=0.17 M27 75mm). All brains were imaged at 1 μM depth resolution. The final images presented in our figures are maximum z-projections.
To define neuropil regions of interest in Figure 8 and Figure S2E, we traced these regions on small overlaid z-projections each at different depths corresponding to the structure. We further consulted standard brain maps to guide these boundaries relative to other structures in the brain (VirtualFlyBrain.org and Ito et al., 2014).
For trans-Tango images and for comparing expression of 70B12-GAL4 and 70B12-lexA drivers, we used the above immunohistochemistry and imaging protocol, except that we used a primary antibody solution containing 1:50 chicken anti-GFP (Thermo Fisher Scientific PA1–9533), 1:500 rabbit anti-RFP (abcam ab62341) and secondary solution containing 1:250 anti-chicken Alexa Fluor 488 (Thermo Fisher Scientific A-11039) and 1:250 anti-rabbit Alexa Fluor 568 (Thermo Fisher Scientific A-11011), and anti-mouse Alexa Fluor 633 (Thermo Fisher Scientific A-21052) in block. We raised trans-Tango flies at 18°C and aged them until they were 10–20 days old.
QUANTIFICATION AND STATISTICAL ANALYSIS
For both the behavioral and electrophysiology data, we used the Jarque-Bera test determine if the data sets were normally distributed. The majority of data groups from the electrophysiology recordings were normal, thus we computed statistics for these experiments using standard parametric tests (two-sample student’s t-test) and corrected for multiple comparisons with the Bonferroni method. For the behavioral experiments, the majority of data sets were not normally distributed, so we used non-parametric statistics to compare these results. We performed a Wilcoxon signed rank test to compare the mean upwind velocity and absolute angular speed values across flies with the mean baseline value (Figure 1C and 1E). In Figure 1D and 1F, we subtracted the baseline from the response for each individual fly and compared between conditions by performing a Mann-Whitney U test on the baseline subtracted parameters. The positions of the flies used to generate the probability distributions in 1G were computed using the last 30 s of trials to account for the fact that the preceding trial can influence the arena position of a fly at the beginning of the trial. We computed probability distributions for each fly in a given condition and averaged these to generate the plots shown. We performed a Mann-Whitney U test on the average arena positions across flies to determine if there was a significant difference in position across the conditions in Figure 1G. In Figure 1C–G, we corrected the threshold for significance with the Bonferroni method.
For all of the behavioral and electrophysiology data, we present standard deviation around mean values, with one exception. For Figure 1B, we depict standard error around the mean time traces, because standard deviation was large enough that it obscured the underlying average trend. For this data, however, we plot standard deviation and single-fly averages in subsequent subfigures (Fig. 1D–F) to fully depict the variability present in the data set. We computed the d-prime (d’, also called the discriminability index in Figure S2D) statistic in Figures 4K and S2 for pairs of directions for each neuron using the following equation:
where μ1 and μ2 are the average steady state responses of the neuron for two adjacent wind directions, and σ1 and σ2 are their standard deviations, respectively, across trials. For Figure S2, we then averaged the d’ statistic across all neurons to compute the average d’ value.
In the behavioral experiments, ‘N’ refers to the number of flies. In all other experiments, ‘N’ may refer either to the number of flies or to the number of cells (this is noted explicitly in the text, in the figure legends or figures themselves).
DATA AND SOFTWARE AVAILABILITY
Data is available at Dryad and analysis software is available at Github: https://github.com/nagellab/Suveretal2019.
Supplementary Material
Video S1. Related to Figure 7. Confocal image stack of postsynaptic targets of JO-CE neurons using trans-Tango. Green signal shows GFP signal from started line (JO-CE neurons), magenta labels postsynaptic targets, and neuropil is labeled in blue.
Video S2. Related to Figure 7. Confocal image stack of APN2s and their postsynaptic targets using trans-Tango. Green signal shows GFP signal from started line (APN2s), magenta labels postsynaptic targets, and neuropil is labeled in blue.
Video S3. Related to Figure 7. Confocal image stack of APN3s and their postsynaptic targets using trans-Tango. Green signal shows GFP signal from started line (APN3s), magenta labels postsynaptic targets, and neuropil is labeled in blue.
Video S4. Related to Figure 7. Confocal image stack of B1 neurons and their postsynaptic targets using trans-Tango. Green signal shows GFP signal from starter line (B1 neurons), magenta labels postsynaptic targets, and neuropil is labeled in blue.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| mouse anti-nc82 | Developmental Studies Hybridoma Bank |
nc82-s RRID: AB_2314866 |
| rabbit anti-GFP | Thermo Fisher Scientific | A6455 |
| chicken anti-GFP | Thermo Fisher Scientific | PA1–9533 |
| streptavidin Alexa Fluor 568 | Thermo Fisher Scientific | S-11226 |
| rabbit anti-dsred | abcam | 632496 |
| anti-mouse Alexa Fluor 633 | Thermo Fisher Scientific | A-21052 |
| anti-rabbit Alexa Fluor 488 | Thermo Fisher Scientific | A-11034 |
| anti-chicken Alexa Fluor 488 | Thermo Fisher Scientific | A-11039 |
| anti-rabbit Alexa Fluor 568 | Thermo Fisher Scientific | A-11011 |
| anti-mouse Alexa Fluor 633 | Thermo Fisher Scientific | A-21052 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Methyllycaconitine citrate (MLA) | Sigma | M168 |
| all trans retinal | Sigma | R2500 |
| Experimental Models: Organisms/Strains | ||
| R70B12-GAL4 | Bloomington Drosophila Stock Center (BDSC) | RRID:BDSC_39519 |
| R70B12-lexA | This manuscript | N/A |
| R70B12-lexA, lexAOP-GFP | This manuscript (recombined with Bloomington # 52265) |
N/A |
| R24C06- GAL4 | BDSC | RRID:BDSC_49073 |
| R70G01- GAL4 | BDSC | RRID:BDSC_39546 |
| R70G01-lexA | BDSC | RRID:BDSC_52855 |
| R70G01- GAL4, 10xUAS-CD8:GFP |
Chang et al. 2016 (Rachel Wilson) |
N/A |
| R45D07- GAL4 | BDSC | RRID:BDSC_49562 |
| JO31- GAL4 (NP6250) | Flybase: FBal0240898 | |
| +(HCS);+;P{10xUAS-IVS-Syn21-GFP-p10}attP2 | Michael Dickinson (wild-type backcrossed onto chromosome I using GFP construct from Pfeiffer, Truman, & Rubin, 2012) |
N/A |
|
w[1118]; P{y[+t7.7] w[+mC]=20XUAS-IVS- CsChrimson.mVenus}attP40 (UAS-Chrimson) |
BDSC | RRID:BDSC_55135 |
| w[*]; P{w[+mC]=UAS-TeTxLC.tnt}E2 (UAS-TNTe) | Matthieu Louis | |
| UAS-myrGFP, QUAS-mtdTomato (3x HA); trans-Tango | Gilad Barnea | N/A |
| w1118norpA36 (5905) | Alvarez-Salvado et al. (2018), Nicholas Stavropolous |
N/A |
| UAS-tdTOM (III) | BDSC | RRID:BDSC_36328 |
| w[*]; P{w[+mC]=UAS-nSyb.eGFP} (II) | BDSC | RRID:BDSC_6921 |
| R13B10-GAL4 | BDSC | RRID:BDSC_48548 |
| R20D06-GAL4 | BDSC | RRID:BDSC_48892 |
| R21G01-GAL4 | BDSC | RRID:BDSC_48951 |
| R25A01-GAL4 | BDSC | RRID:BDSC_49102 |
| R25B07-GAL4 | BDSC | RRID:BDSC_49112 |
| R28B05-GAL4 | BDSC | RRID:BDSC_49447 |
| R29CW-GAL4 | BDSC | RRID:BDSC_49481 |
| R30E10-GAL4 | BDSC | RRID:BDSC_49638 |
| R37F05-GAL4 | BDSC | RRID:BDSC_49961 |
| R38H10- GAL4 | BDSC | RRID:BDSC_49961 |
| R46D02- GAL4 | BDSC | RRID:BDSC_50263 |
| R49C12- GAL4 | BDSC | RRID:BDSC_38676 |
| R52F04- GAL4 | BDSC | RRID:BDSC_38839 |
| R54H01- GAL4 | BDSC | RRID:BDSC_47650 |
| R58H12- GAL4 | BDSC | RRID:BDSC_46415 |
| R72E03- GAL4 | BDSC | RRID:BDSC_47445 |
| R72G12- GAL4 | BDSC | RRID:BDSC_39796 |
| R75D06- GAL4 | BDSC | RRID:BDSC_39892 |
| R76E07- GAL4 | BDSC | RRID:BDSC_39929 |
| R79C11-GAL4 | BDSC | RRID:BDSC_40033 |
| R92B01- GAL4 | BDSC | RRID:BDSC_40601 |
Highlights:
Walking flies require both antennae for robust olfactory navigation behavior
The difference between antennal displacements generates a linear code for wind direction
Second-order APN neurons encode ipsilateral antenna deflections
Higher-order WPNs encode wind direction by integrating information from the two antennae
ACKNOWLEDGEMENTS
The authors would like to thank Nicholas Stavropoulos for helping us generate the 70B12-lexA flies, Matthieu Louis for UAS-TNTe flies, Mustafa Talay and Gilad Barnea for the trans-Tango flies, and Allison Baker-Chang and Rachel Wilson for the R70G01-GAL4, 10xUAS-CD8:GFP fly stock. We also thank Gerry Rubin for sharing FlyLight expression data and Heather Dionne and Gerry Rubin for the R70B12 plasmid. We thank Dimitry Rinberg for use of his 3D printer and for critical feedback on this work. Greg Suh also provided critical feedback on the manuscript. Henry Haeberle help set up the Thorlabs software for 2-photo lesion experiments. This work was funded by NIH R00DC012065, NIH R01MH109690, NSF IOS-1555933, the Klingenstein Foundation, the McKnight Foundation, the Sloan Foundation (K.I.N.), and the Leon Levy Foundation (M.P.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Abraira VE, and Ginty DD (2013). The sensory neurons of touch. Neuron 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Salvado E, Licata A, Connor EG, Mchugh MK, King BMN, Stavropoulos N, Crimaldi JP, and Nagel KI (2018). Elementary sensory-motor transformations underlying olfactory navigation in walking fruit-flies. BioRxiv 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo AW, and Wilson RI (2017). Active Mechanisms of Vibration Encoding and Frequency Filtering in Central Mechanosensory Neurons. Neuron 96, 446–460.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell WJ, and Kramer E (1979). Search and Anemotactic Orientation of Cockroaches. J. Insect Physiol 25, 631–640. [Google Scholar]
- Budick S. a, Reiser MB, and Dickinson MH (2007). The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J. Exp. Biol 210, 4092–4103. [DOI] [PubMed] [Google Scholar]
- Camhi JM, and Johnson EN (1999). High-frequency steering maneuvers mediated by tactile cues: antennal wall-following in the cockroach. J. Exp. Biol 202, 631–643. [DOI] [PubMed] [Google Scholar]
- Chang AEB, Vaughan AG, and Wilson RI (2016). A Mechanosensory Circuit that Mixes Opponent Channels to Produce Selectivity for Complex Stimulus Features. Neuron 92, 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CT, Kennedy JS, and Ludlow AR (1983). Finding of a sex pheromone source by gypsy moths released in the field. Nature 303, 804–806. [Google Scholar]
- Flugge C (1934). Geruchliche Raumorientierung von Drosophila melanogaster. Zeitschrift Fur Verleichende Physiol. 20, 463–500. [Google Scholar]
- Frechter S, Bates AS, Tootoonian S, Dolan M-J, Manton JD, Jamasb A, Kohl J, Bock D, and Jefferis GSXE (2018). Functional and Anatomical Specificity in a Higher Olfactory Centre. BioRxiv 336982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, and Robert D (2002). The mechanical basis of Drosophila audition. J. Exp. Biol 205, 1199–1208. [DOI] [PubMed] [Google Scholar]
- von Hadeln J, Althaus V, Häger L, and Homberg U (2018). Anatomical organization of the cerebrum of the desert locust Schistocerca gregaria. Cell Tissue Res. 1–24. [DOI] [PubMed] [Google Scholar]
- Homberg U (1985). Interneurones of the central complex in the bee brain (Apis mellifera, L.). J. Insect Physiol 31, 251–261. [Google Scholar]
- Homberg U (1994). Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J. Comp. Physiol. A 175, 597–610. [Google Scholar]
- Hsu CT, and Bhandawat V (2016). Organization of descending neurons in Drosophila melanogaster. Sci. Rep 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen EV, Dacke M, Heinze S, and el Jundi B (2017). Anatomical organization of the brain of a diurnal and a nocturnal dung beetle. J. Comp. Neurol 525, 1879–1908. [DOI] [PubMed] [Google Scholar]
- Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, et al. (2014). A systematic nomenclature for the insect brain. Neuron 81, 755–765. [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TTB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. (2012). A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Rep. 2, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C (1855). Auditory Apparatus of the Culex Mosquito. J. Cell Sci 3, 97–102. [Google Scholar]
- Kamikouchi A, Shimada T, and Ito KE (2006). Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol 499, 317–356. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, and Ito K (2009). The neural basis of Drosophila gravity-sensing and hearing. Nature 458, 165–171. [DOI] [PubMed] [Google Scholar]
- Kennedy JS, and Marsh D (1974). Pheromone-regulated anemotaxis in flying moths. Science 184, 999–1002. [DOI] [PubMed] [Google Scholar]
- Konishi M (2003). Coding of Auditory Space. Annu. Rev. Neurosci 26, 31–55. [DOI] [PubMed] [Google Scholar]
- Koyama M, Minale F, Shum J, Nishimura N, Schaffer CB, and Fetcho JR (2016). A circuit motif in the zebrafish hindbrain for a two alternative behavioral choice to turn left or right. Elife 5, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, and Sane SP (2015). Antennal Mechanosensors and Their Evolutionary Antecedents In Advances in Insect Physiology, vol. 49 (Academic Press; ), pp. 59–99. [Google Scholar]
- Lacey ES, Ray A, and Cardé RT (2014). Close encounters: Contributions of carbon dioxide and human skin odour to finding and landing on a host in Aedes aegypti. Physiol. Entomol 39, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JS-Y, Lo S-J, Dickson BJ, and Chiang a.-S. (2012). Auditory circuit in the Drosophila brain. Proc. Natl. Acad. Sci 109, 2607–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimon G, Straw AD, and Dickinson MH (2010). Active flight increases the gain of visual motion processing in Drosophila. Nat. Neurosci 13, 393–399. [DOI] [PubMed] [Google Scholar]
- Matsuo E, Seki H, Asai T, Morimoto T, Miyakawa H, Ito K, and Kamikouchi A (2016a). Organization of projection neurons and local neurons of the primary auditory center in the fruit fly Drosophila melanogaster. J. Comp. Neurol 524, 1099–1164. [DOI] [PubMed] [Google Scholar]
- Matsuo E, Seki H, Asai T, Morimoto T, Miyakawa H, Ito K, and Kamikouchi A (2016b). Organization of projection neurons and local neurons of the primary auditory center in the fruit fly Drosophila melanogaster. J. Comp. Neurol 524, 1099–1164. [DOI] [PubMed] [Google Scholar]
- Mech LD (1966). The Wolves of Isle Royale (Fauna of National Parks of the United States), pp. 1–210. [Google Scholar]
- Miller A (1950). The Internal Anatomy and Histology of the Imago of Drosophila Melanogaster In Biology of Drosophila, Demerec M, ed. (John Wiley & Sons, Inc.), pp. 420–531. [Google Scholar]
- Morley EL, Steinmann T, Casas J, and Robert D (2012). Directional cues in Drosophila melanogaster audition: structure of acoustic flow and inter-antennal velocity differences. J. Exp. Biol 215, 2405–2413. [DOI] [PubMed] [Google Scholar]
- Müller M, and Wehner R (2007). Wind and sky as compass cues in desert ant navigation. Naturwissenschaften 94, 589–594. [DOI] [PubMed] [Google Scholar]
- Namiki S, Dickinson MH, Wong AM, Korff W, and Card GM (2017). The functional organization of descending sensory-motor pathways in Drosophila. BioRxiv 231696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada J, and Toh Y (2006). Active tactile sensing for localization of objects by the cockroach antenna. J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol 192, 715–726. [DOI] [PubMed] [Google Scholar]
- Patella P, and Wilson RI (2018). Functional Maps of Mechanosensory Features in the Drosophila Brain. Curr. Biol 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo T-TB, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. (2008). Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci 105, 9715–9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Ngo TTB, Hibbard KL, Murphy C, Jenett A, Truman JW, and Rubin GM (2010). Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BD, Enett A, Hammonds AS, Ngo TTB, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. (2012). Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. U. S. A 109, 6626–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzmann RE, Ridgel AL, and Pollack AJ (2008). Multi-unit recording of antennal mechanosensitive units in the central complex of the cockroach, Blaberus discoidalis. J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol 194, 341–360. [DOI] [PubMed] [Google Scholar]
- Sinclair BJ, and Cumming JM (2006). The morphology, higher-level phylogeny and classification of the Empidoidea (Diptera) In Zootaxa, (Magnolia Press; ), pp. 1–172. [Google Scholar]
- Suver MP, Huda A, Iwasaki N, Safarik S, and Dickinson MH (2016). An Array of Descending Visual Interneurons Encoding Self-Motion in Drosophila. J. Neurosci 36, 11768–11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talay M, Richman EB, Snell NJ, Hartmann GG, Fisher JD, Sorkaç A, Santoyo JF, Chou-Freed C, Nair N, Johnson M, et al. (2017). Transsynaptic Mapping of Second-order Taste Neurons in Flies by trans-Tango. Neuron 96, 783–795.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PS, Blinder P, Migliori BJ, Neev J, Jin Y, Squier JA, and Kleinfeld D (2009). Plasma-mediated ablation: an optical tool for submicrometer surgery on neuronal and vascular systems. Curr. Opin. Biotechnol 20, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AG, Zhou C, Manoli DS, and Baker BS (2014). Neural pathways for the detection and discrimination of conspecific song in D. melanogaster. Curr. Biol 24, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Weir PT, Henze MJ, Bleul C, Baumann-Klausener F, Labhart T, and Dickinson MH (2016). Anatomical Reconstruction and Functional Imaging Reveal an Ordered Array of Skylight Polarization Detectors in Drosophila. J. Neurosci 36, 5397–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, and Laurent G (2005). Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci 25, 9069–9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, and Wehner R (2000). Pinpointing food sources: olfactory and anemotactic orientation in desert ants, Cataglyphis fortis. J. Exp. Biol 203, 857–868. [DOI] [PubMed] [Google Scholar]
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, and Anderson DJ (2009). Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YSW, Graff MM, Bresee CS, Man YB, and Hartmann MJZ (2016). Whiskers aid anemotaxis in rats. Sci. Adv 2, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Related to Figure 7. Confocal image stack of postsynaptic targets of JO-CE neurons using trans-Tango. Green signal shows GFP signal from started line (JO-CE neurons), magenta labels postsynaptic targets, and neuropil is labeled in blue.
Video S2. Related to Figure 7. Confocal image stack of APN2s and their postsynaptic targets using trans-Tango. Green signal shows GFP signal from started line (APN2s), magenta labels postsynaptic targets, and neuropil is labeled in blue.
Video S3. Related to Figure 7. Confocal image stack of APN3s and their postsynaptic targets using trans-Tango. Green signal shows GFP signal from started line (APN3s), magenta labels postsynaptic targets, and neuropil is labeled in blue.
Video S4. Related to Figure 7. Confocal image stack of B1 neurons and their postsynaptic targets using trans-Tango. Green signal shows GFP signal from starter line (B1 neurons), magenta labels postsynaptic targets, and neuropil is labeled in blue.
Data Availability Statement
Data is available at Dryad and analysis software is available at Github: https://github.com/nagellab/Suveretal2019.