Abstract
Objective:
Connective tissue growth factor (CTGF) is an important regulator of fibrogenesis in many organs. This study evaluated the interrelationship among adipose tissue CTGF expression, fat mass and insulin resistance in people.
Methods:
We determined: 1) CTGF gene expression in human subcutaneous preadipocytes before and after inducing adipogenesis; 2) relationships among abdominal subcutaneous adipose tissue CTGF gene expression, body fat mass and indices of insulin sensitivity, including the hepatic insulin sensitivity index and the hyperinsulinemic-euglycemic clamp procedure in conjunction with stable isotope glucose tracer infusion, in 72 people who had marked differences in adiposity and insulin sensitivity; 3) localization of CTGF protein in subcutaneous adipose tissue; and 4) effect of progressive (5%, 11%, and 16%) weight loss on adipose tissue CTGF gene expression.
Results:
CTGF was highly expressed in preadipoocytes, not adipocytes. Adipose tissue CTGF gene expression was strongly correlated with body fat mass and both skeletal muscle and liver insulin sensitivity and CTGF positive cells were predominantly found in areas of fibrosis. Progressive weight loss caused a stepwise decrease in adipose tissue CTGF gene expression.
Conclusions:
We conclude that increased CTGF expression is associated with adipose tissue expansion, adipose tissue fibrosis, and multi-organ insulin resistance in people with obesity.
Keywords: Obesity, glucose metabolism, insulin sensitivity, adipose tissue fibrosis
Introduction
An increase in adipose tissue mass often causes insulin resistance, which is a key contributor to obesity-related metabolic diseases, such as metabolic syndrome and type 2 diabetes (1). The expansion of adipose tissue and the progression from a lean to an obese phenotype requires remodeling of extracellular matrix (ECM) proteins to provide mechanical support for adipocyte hyperplasia and hypertrophy (2, 3, 4, 5). However, excessive production of fibrillar ECM proteins can cause adipose tissue fibrosis, which can have adverse effects on adipose tissue and whole-body metabolic function (2, 3, 4, 5). The results from studies conducted in genetically modified rodent models have shown alterations in fibrogenic pathways in adipose tissue, such as hypoxia-inducible factor 1alpha (HIF1α) (6), endotrophin (7), tenomodulin (8), and focal adhesion kinase (FAK) (9), affect the progression of obesity-induced insulin resistance and glucose intolerance. These findings underscore the potential importance of adipose tissue fibrogenesis and fibrosis in the pathogenesis of obesity-related metabolic dysfunction.
Connective tissue growth factor (CTGF, also known as CCN2) is a matricellular protein that is involved in regulating many biological processes, including fibrogenesis in multiple organs (10, 11). Overexpression of CTGF increases ECM production and decreases ECM degradation, and can cause fibrosis or enhance the susceptibility to fibrosis in lung, kidney, liver, and skin in mice (12, 13, 14, 15). In people, increased CTGF expression has been detected in the liver, heart, kidney, and lungs of patients with fibrosis in those organs (16, 17, 18, 19). Data from studies conducted in high-fat diet-induced and genetically obese mice demonstrate the increase in fat mass is associated with an increase in adipose tissue Ctgf gene expression and fibrosis (20, 21). In addition, Ctgf expression is suppressed during mouse adipogenesis and CTGF inhibits adipogenic differentiation in mouse preadipocytes (22, 23).
The major purpose of the present study was to evaluate the interrelationships among adipose tissue CTGF gene expression, adiposity and insulin resistance in people. We hypothesized that adipose tissue CTGF expression progressively increases with increasing adiposity and insulin resistance and decreases with progressive weight loss. Accordingly, we evaluated CTGF expression in subcutaneous abdominal adipose tissue in men and women who had a large range in body fat mass and insulin sensitivity, and in a subset of people with obesity during progressive diet-induced weight loss with concomitant changes in body fat mass and insulin sensitivity. In addition, to determine the primary source of CTGF expression in adipose tissue, we evaluated CTGF expression in human preadipocytes, mature adipocytes, and stromal vascular fraction (SVF) cells isolated from subcutaneous abdominal adipose tissue.
Methods
Study subjects
A total of 72 people (14 men and 58 women) (50 Caucasian, 18 African American, 4 Native American) who had a wide range in body fat mass and insulin sensitivity participated in this study (Table 1). All subjects had participated in studies that involved percutaneous abdominal subcutaneous adipose tissue biopsies and assessments of body composition and insulin sensitivity (24, 25, 26, 27, 28). A subset of participants was studied before and after progressive 5%, 11% and 16% diet-induced weight loss (n=9) or before and after 6 months of weight maintenance (n=9) (27). No subject had evidence of diabetes or other serious illnesses, and did not take any medications that affect insulin action. All participants provided written informed consent before their participation in all studies that were approved by the Human Research Protection Office of Washington University School of Medicine in St. Louis, MO, USA.
Table 1.
Body composition and metabolic characteristics of the study participants
| Mean ± SE | Range | |
|---|---|---|
| Age (yr) | 45 ± 1 | 20–66 |
| Body mass index (kg/m2) | 37.6 ± 1.1 | 19.3–68.6 |
| Fat-free mass (FFM) (kg) | 56.1 ± 1.4 | 36.9–87.4 |
| Fat mass (kg) | 49.7 ± 2.3 | 8.1–123.1 |
| Fat mass (% body weight) | 45.6 ± 1.0 | 11.8–59.7 |
| Glucose (mg/dL) | 96 ± 1 | 81–121 |
| Insulin (mU/L) | 18.7 ± 1.3 | 2.2–50.9 |
| Free fatty acids (μmol/L)* | 576 ± 19 | 320–1016 |
| Triglyceride (mg/dL)* | 132 ± 8 | 31–327 |
| HDL-cholesterol (mg/dL)* | 45 ± 2 | 24–97 |
| LDL-cholesterol (mg/dL)* | 109 ± 4 | 59–240 |
| HOMA-IR | 4.4 ± 0.3 | 0.5–12.4 |
| HISI [100/(μmol/kg FFM/min x mU/L)] | 0.56 ± 0.07 | 0.13–3.07 |
| Glucose Rd during insulin infusion (μmol/kg FFM/min) | 53 ± 3 | 17–131 |
| Glucose Rd during insulin infusion (% increase ) | 217 ± 18 | 16–731 |
N=71
HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; HISI, hepatic insulin sensitivity index; Glucose Rd, glucose rate of disappearance from plasma.
Experimental protocol
This study was conducted in the Clinical and Translational Research Unit at Washington University School of Medicine in St. Louis, MO. Total fat mass and fat-free mass (FFM) were measured by using dual-energy X-ray absorptiometry (Lunar iDXA; GE Healthcare, Milwaukee, WI). Subjects were given a standard meal at ~1900 h. The following morning, after subjects fasted for approximately 12 h overnight, a hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically labeled glucose tracer infusion, was performed to evaluate insulin sensitivity, as described previously (24, 25, 26, 27). During the clamp procedure, insulin was infused at a rate of 50 mU/m2 per minute, to achieve typical postprandial plasma insulin concentrations and stimulate plasma glucose disposal. Euglycemia (~100 mg/dL) was maintained by variable rate infusion of 20% dextrose enriched to approximately 2.5% with [6,6-2H2]glucose.
Adipose tissue biopsies were obtained during the basal stage of the clamp procedure. Periumbilical abdominal subcutaneous adipose tissue was obtained by needle biopsy for histological assessments and then additional tissue was aspirated through a 4-mm liposuction cannula (Tulip Medical Products, San Diego, CA) to assess gene and protein expression, as described previously (24, 25, 26, 27). Tissue samples were immediately rinsed with ice-cold saline, and flash frozen in liquid nitrogen until subsequent Real time PCR analysis.
CTGF gene expression in human subcutaneous preadipocytes, stromal vascular fraction (SVF) cells and adipocytes
Primary human subcutaneous preadipocytes were purchased from Lonza (#PT-5020; Walkersville, MD). Human preadipocytes were treated with differentiation medium (#PT-8002; Lonza), and differentiated to mature adipocytes as described previously (29). Total RNA was extracted from preadipocytes immediately before and 8 days after induction of adipogenesis. We also evaluated CTGF gene expression reported in SVF cells and adipocytes isolated from subcutaneous adipose tissue samples in publicly available microarray datasets obtained from the Gene Expression Omnibus (GEO) database (GSE8995; https://www.ncbi.nlm.nih.gov/geo/) (30).
RNA isolation and Real time PCR
Total RNA was isolated from frozen subcutaneous adipose tissue samples and human preadipocytes by using RNeasy Mini Kit (#74104; Qiagen, Chatsworth, CA) as previously described (24, 25, 26, 27, 29). Gene expression was determined by using an ABI 7500 real-time PCR system (Invitrogen, Carlsbad, CA) and Fast SYBR Green Master Mix (#4385618, Invitrogen) by using the following primers: CTGF (accession number NM_001901) 5’-CAGCATGGACGTTCGTCTG-3’ (forward) and 5’-AACCACGGTTTGGTCCTTGG-3’ (reverse); adiponectin (ADIPOQ) (accession number NM_001177800) 5’-GGCTTTCCGGGAATCCAAGG-3’ (forward) and 5’-TGGGGATAGTAACGTAAGTCTCC-3’ (reverse); ribosomal protein (RPLP0) (accession number NM_053275) 5’-GTGATGTGCAGCTGATCAAGACT-3’ (forward) and 5’-GATGACCAGCCCAAAGGAGA-3’ (reverse). The expression of each gene was normalized with RPLP0 expression.
Western blot analysis
Frozen subcutaneous adipose tissue was homogenized in ice-cold Cell Lysis Buffer (#9803; Cell Signaling Technology, Beverly, MA) containing proteinase inhibitors. Extracted protein samples were loaded onto 12.5% polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA), separated by SDS-PAGE, and then transferred to Immun-Blot polyvinylidene difluoride membranes (#1620177; Bio-Rad). The following antibodies were used for protein detection: rabbit monoclonal anti-human CTGF antibody (#10095; Cell Signaling Technology), rabbit monoclonal anti-human GAPDH antibody (#5174; Cell Signaling Technology), and horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (#7074; Cell Signaling Technology). Blots were developed by using the ECL Select Western Blotting Detection Reagent (#RPN2235; GE Healthcare Life Sciences, Piscataway, NJ). Densitometry was quantified using the ImageJ software (NIH ImageJ 1.47; http://imagej.nih.gov/ij).
Immunostaining
Subcutaneous adipose tissue samples were fixed in formalin and embedded in paraffin. Paraffin sections were stained with Masson’s trichrome for collagen. Immunostaining was performed by using goat polyclonal anti-human CTGF antibody (AF660; R&D Systems, Minneapolis, MN), biotin-conjugated anti-goat antibody (#BA-9500; Vector Laboratories, Burlingame, CA), and Vectastain ABC kit (Vector Laboratories) as previously described (31). A negative control was obtained by omitting the primary antibody.
Calculations
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as the product of fasting plasma insulin (in mU/L) and glucose (in mg/dL) concentrations divided by 22.5 (32). Endogenous glucose rate of appearance (Ra) in plasma during basal conditions and insulin infusion was calculated by dividing the glucose tracer infusion rate by the average plasma glucose tracer-to-tracee ratio during isotopic steady-state conditions of the basal and clamp periods, respectively, after adjustment for exogenous glucose infusion during insulin infusion. During insulin infusion, glucose rate of disappearance (Rd) from plasma was assumed to equal the sum of endogenous glucose Ra and the rate of infused glucose. Hepatic insulin sensitivity was assessed by using the hepatic insulin sensitivity index (HISI), calculated as 100/[basal glucose Ra (in μmol/kg FFM/min) x fasting plasma insulin concentration (in mU/L)]. Skeletal muscle insulin sensitivity was assessed as the relative increase in glucose Rd during insulin infusion (%).
Statistical analyses
Gene expression data were log-transformed for the statistical assessments. Differences between two groups were determined by using Student’s unpaired or paired t test. Pearson’s correlation analysis was used to examine correlations between outcomes of interest. The effect of weight loss on adipose tissue CTGF expression was evaluated by using repeated analysis of variance (ANOVA). Data are presented as means ± standard error of the mean (SEM). A P-value less than 0.05 was considered statistically significant.
Results
CTGF is highly expressed in human subcutaneous preadipocytes, not mature adipocytes.
A marked increase in adiponectin (ADIPOQ) confirmed successful differentiation of human subcutaneous preadipocytes into mature adipocytes 8 days after induction of adipogenesis. CTGF gene expression was markedly suppressed after induction of adipogenesis (Figure 1A). We next analyzed the publicly available microarray dataset (GSE8995) obtained from studies that evaluated transcriptional differences between adipocytes and preadipocytes containing stromal vascular fraction (SVF) cells isolated from human subcutaneous adipose tissue samples (30). CTGF was highly expressed in SVF cells, compared with subcutaneous adipocytes, whereas ADIPOQ showed the opposite expression pattern (Figure 1B).
Figure 1. CTGF is highly expressed in human subcutaneous preadipocytes, compared to mature adipocytes.
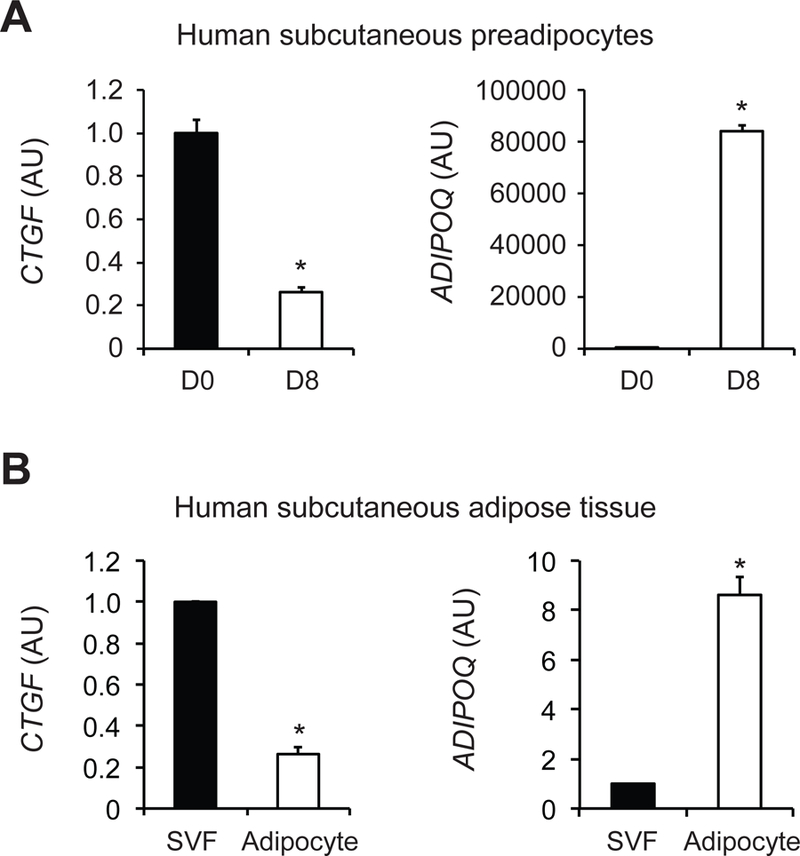
(A) Gene expression of connective tissue growth factor (CTGF) and adiponectin (ADIPOQ) was determined in human subcutaneous preadipocytes before (D0) and 8 days (D8) after induction of adipogenesis (n=4). (B) Microarray dataset obtained from the Gene Expression Omnibus (GEO) database (GSE8995) were used to evaluate CTGF and ADIPOQ expression in stromal vascular fraction (SVF) cells and adipocytes isolated from human subcutaneous adipose tissue samples (n=6). Data were log-transformed for the statistical analysis and back-transformed for presentation. Data are means ± SEM. *Value significantly different from D0 or SVF value, P<0.001.
Adipose tissue CTGF gene expression is associated with measures of adiposity and insulin resistance.
Adipose tissue CTGF gene expression correlated strongly with body mass index (BMI) (r=0.69, P<0.001) and body fat mass (r=0.67, P<0.001) (Figures 2A and 2B). Adipose tissue CTGF gene expression also correlated with different indices of insulin action, including fasting plasma insulin concentration (r=0.54, P<0.001), HOMA-IR (r=0.58, P<0.001), the hepatic insulin sensitivity index (HISI) (r=−0.61, P<0.001), and insulin-stimulated increase (%) in glucose rate of disappearance (Rd) (r=−0.61, P<0.001) (Figures 2C to 2F). The strong correlations among adipose tissue CTGF expression and measures of adiposity and insulin resistance were maintained even when data from men and women were analyzed separately (Supplemental Table 1).
Figure 2. Adipose tissue CTGF gene expression is associated with adiposity and insulin resistance.
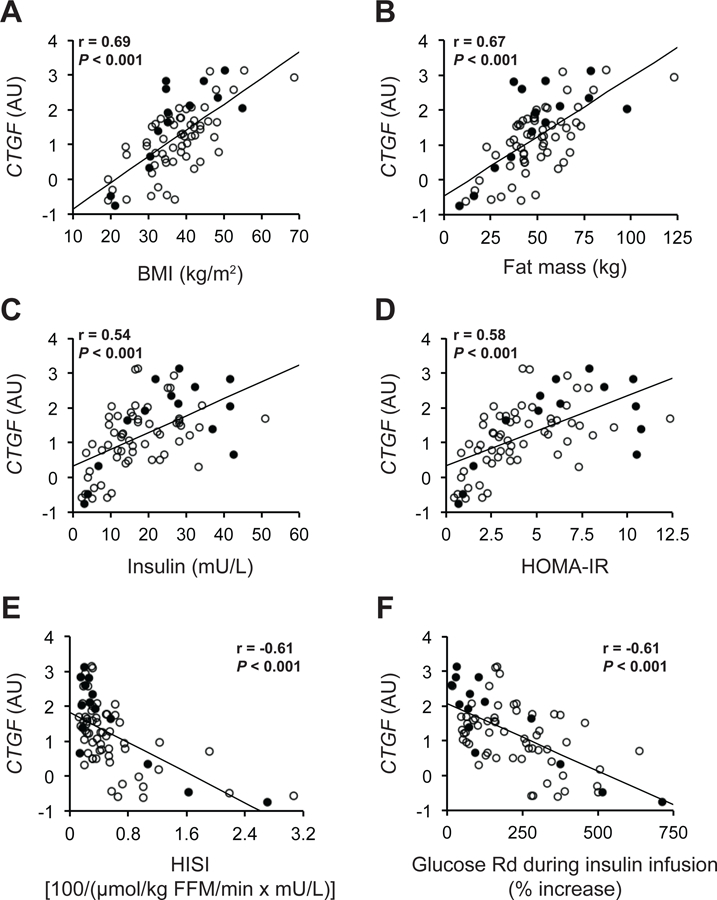
Relationships between adipose tissue CTGF gene expression and body mass index (BMI) (A), fat mass (B), fasting plasma insulin concentration (C), Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (D), hepatic insulin sensitivity index (HISI) (E), and insulin-stimulated increases (%) in glucose rate of disappearance (Rd) (F) in men (black circles, n=14) and women (white circles, n=58). Pearson’s correlation coefficient (r) and P values for all subjects (n=72) are provided in each plot.
Increased CTGF protein expression is associated with adipose tissue fibrosis.
Consistent with the CTGF gene expression data (Figure 2), adipose tissue CTGF protein expression was increased in subjects with obesity compared with those who were lean (Figure 3A). Adipose tissue interstitial fibrosis, assessed by using Masson’s trichrome collagen staining, and CTGF positive cells in the of interstitial fibrosis, assessed by immunostaining, were also increased in subjects with obesity compared with those who were lean (Figure 3B). No signal was detected in the negative control (without the anti-CTGF antibody).
Figure 3. Increased CTGF protein expression is associated with adipose tissue fibrosis.
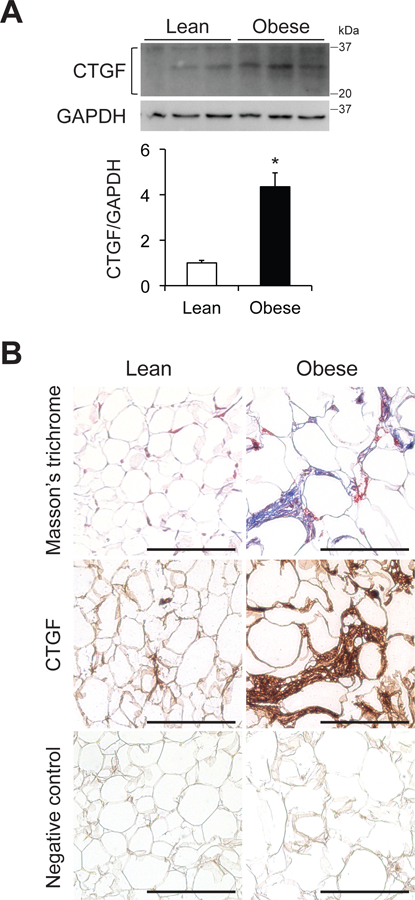
(A) Western blot analysis of CTGF in subcutaneous abdominal adipose tissue and bar graph showing CTGF and its fragments (between 20 and 37 kDa) normalized to GAPDH in participants who were lean or obese (n=4–5 per group). Data are means ± SEM. *Value significantly different from lean value, P<0.01. (B) Masson’s trichrome staining and CTGF immunostaining of subcutaneous abdominal adipose tissue obtained from people who are lean and those with obesity. A negative control for CTGF staining was obtained by omitting the primary antibody. Scale bar: 500 μm.
Adipose tissue CTGF expression decreased progressively with progressive weight loss
Adipose tissue CTGF gene expression decreased progressively with progressive 5%, 11% and 16% weight loss (P=0.02), whereas adipose tissue CTGF gene expression was not significantly different after, compared with before, 6 months of weight maintenance (Figure 4).
Figure 4. Effect of diet-induced progressive weight loss on adipose tissue CTGF gene expression in people with obesity.
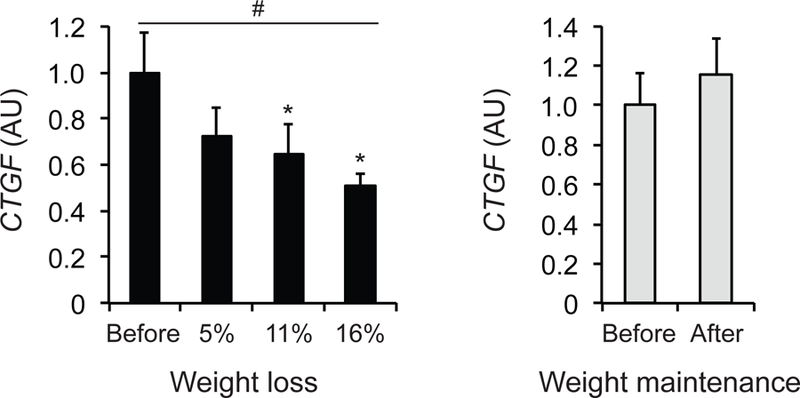
Adipose tissue CTGF gene expression was determined before and after progressive 5%, 11%, and 16% weight loss or before and after 6 months of weight maintenance in people with obesity (n=9 per group). Data were log-transformed for statistical analysis and back-transformed for presentation. Data are means ± SEM. #Significant linear effect of time during progressive weight loss, P<0.05. *Value significantly different from before value, P<0.05.
Discussion
In the present study, we evaluated the potential importance of adipose tissue CTGF in regulating metabolic health by assessing subcutaneous abdominal adipose tissue CTGF expression in a large cohort of men and women who had a large range in in body fat mass and insulin sensitivity, and in a subgroup of people with obesity before and during progressive weight loss. In addition, we assessed CTGF gene expression in preadipocytes, mature adipocytes, and SVF cells isolated from human subcutaneous abdominal adipose tissue. Our data demonstrate that: 1) CTGF gene expression is highly expressed in preadipocytes and SVF cells, compared with mature adipocytes; 2) adipose tissue CTGF expression is positively correlated with body fat mass; 3) adipose tissue CTGF expression is associated with indices of whole-body, liver and skeletal muscle insulin resistance; 4) CTGF positive cells were detected predominantly in adipose tissue areas of fibrosis in people with obesity; and 5) progressive weight loss progressively decreases adipose tissue CTGF expression in people with obesity. These findings suggest that increased adipose tissue CTGF expression is involved in the pathogenesis of obesity-induced insulin resistance in people, presumably mediated by increasing adipose tissue fibrosis.
We found a strong correlation between adipose tissue CTGF expression and both body fat mass and markers of insulin resistance in both men and women. In addition, we found adipose tissue CTGF gene expression progressively decreased with progressive weight loss. These results are in concert with previous findings demonstrating an increase in adipose tissue ECM remodeling pathways and fibrosis in people with obesity and a decrease in adipose tissue gene expression of other markers of fibrogenesis after weight loss (24, 27, 33, 34, 35). Tissue expression of CTGF is a robust marker of fibrosis and contributes to fibrogenesis in many organ systems. Data from previous studies have found tissue CTGF expression is up-regulated in people with fibrotic diseases, including cirrhosis (16), cardiac fibrosis (17), crescentic glomerulonephritis (18), idiopathic pulmonary fibrosis (19), and systemic sclerosis (36). In addition, pharmacological blockade or genetic deletion of CTGF ameliorates the progression of fibrosis in various mouse organs (10, 11, 37, 38, 39, 40). Together, these findings support the concept that adipose tissue fibrosis is involved in the pathogenesis of insulin resistance associated with obesity in people, which has previously been demonstrated in rodent models (2, 5).
By analyzing human subcutaneous preadipocytes and the published microarray database, we found that, compared with mature adipocytes, CTGF expression was markedly increased in preadipocytes and SVF cells. These findings are consistent with data from previous studies that found Ctgf gene expression is suppressed during mouse adipogenesis and that CTGF inhibits differentiation of mouse 3T3-L1 preadipocytes and mesenchymal stromal cells into mature adipocytes (22, 23). These findings suggest adipose tissue ECM remodeling during adipose tissue expansion is dynamically coordinated by SVF cells, including preadipocytes, fibroblasts, endothelial cells, and macrophages (2, 3, 41). Accordingly, excessive CTGF production by SVF cells can have adverse metabolic effects during weight gain and increased adipose tissue mass by promoting excessive fibrogenesis and inhibiting adipocyte differentiation.
The results from the present study demonstrate a close interrelationship among adipose tissue CTGF expression, adipose tissue fibrosis, body adiposity and multi-organ insulin resistance in people. Additional studies are needed to determine whether increased adipose tissue CTGF expression causes adipose tissue fibrosis and obesity-associated insulin resistance.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
Obesity causes adipose tissue fibrosis and multi-organ insulin resistance.
Connective tissue growth factor (CTGF) is involved in the pathogenesis of fibrosis in several fibrotic diseases.
Adipose tissue Ctgf expression is increased in high-fat diet-induced and genetically obese mice.
What does our study add?
Compared with mature adipocytes, CTGF is highly expressed in human subcutaneous preadipocytes and stromal vascular fraction cells.
Adipose tissue CTGF gene expression is strongly correlated with body fat mass and multi-organ (liver, skeletal muscle) insulin resistance in people.
Progressive weight loss induces a stepwise decrease in adipose tissue CTGF gene expression in people with obesity.
Acknowledgments
The authors thank Dr. David Alpers for insightful discussion; Dr. Adewole Okunade, Shannon Kelly, Michael Franczyk, Jennifer Shew, and Freida Custodio for technical assistance; the staff of the Clinical and Translational Research Unit for their help in performing the studies; and the study subjects for their participation.
Deidentified data that underlie the results reported in this article will be made available to external investigators. Please contact with the corresponding author for the details.
Trial registration: ClinicalTrials.gov Identifier: NCT01184170; NCT01299519; NCT00981500; NCT01104220
Funding: This study was supported by National Institutes of Health grants DK 56341 (Nutrition Obesity Research Center), DK20579 (Diabetes Research Center), DK052574 (Digestive Disease Research Center), RR024992 (Clinical and Translational Science Award), and TR000450 (KL2 Career Developmental Award), and grants from the Pershing Square Foundation, the Barnes Jewish Hospital Foundation, and the Longer Life Foundation.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology 2002;123: 882–932. [DOI] [PubMed] [Google Scholar]
- 2.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 2013;18: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol 2015;208: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope BD, Warren CR, Parker KK, Cowan CA. Microenvironmental Control of Adipocyte Fate and Function. Trends Cell Biol 2016;26: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest 2017;127: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol 2009;29: 4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat Commun 2014;5: 3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senol-Cosar O, Flach RJ, DiStefano M, Chawla A, Nicoloro S, Straubhaar J, et al. Tenomodulin promotes human adipocyte differentiation and beneficial visceral adipose tissue expansion. Nat Commun 2016;7: 10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luk CT, Shi SY, Cai EP, Sivasubramaniyam T, Krishnamurthy M, Brunt JJ, et al. FAK signalling controls insulin sensitivity through regulation of adipocyte survival. Nat Commun 2017;8: 14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramazani Y, Knops N, Elmonem MA, Nguyen TQ, Arcolino FO, van den Heuvel L, et al. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol 2018;68–69: 44–66. [DOI] [PubMed] [Google Scholar]
- 11.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 2012;5: S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S, Platteau A, Chen S, McNamara G, Whitsett J, Bancalari E. Conditional overexpression of connective tissue growth factor disrupts postnatal lung development. Am J Respir Cell Mol Biol 2010;42: 552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonnylal S, Shi-Wen X, Leoni P, Naff K, Van Pelt CS, Nakamura H, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum 2010;62: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int 2008;73: 446–455. [DOI] [PubMed] [Google Scholar]
- 15.Tong Z, Chen R, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology 2009;50: 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paradis V, Dargere D, Vidaud M, De Gouville AC, Huet S, Martinez V, et al. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology 1999;30: 968–976. [DOI] [PubMed] [Google Scholar]
- 17.Koitabashi N, Arai M, Kogure S, Niwano K, Watanabe A, Aoki Y, et al. Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension 2007;49: 1120–1127. [DOI] [PubMed] [Google Scholar]
- 18.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int 1998;53: 853–861. [DOI] [PubMed] [Google Scholar]
- 19.Baran CP, Opalek JM, McMaken S, Newland CA, O’Brien JM Jr., Hunter MG, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 2007;176: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rancoule C, Viaud M, Gres S, Viguerie N, Decaunes P, Bouloumie A, et al. Pro-fibrotic activity of lysophosphatidic acid in adipose tissue: in vivo and in vitro evidence. Biochim Biophys Acta 2014;1841: 88–96. [DOI] [PubMed] [Google Scholar]
- 21.Tan JT, McLennan SV, Williams PF, Rezaeizadeh A, Lo LW, Bonner JG, et al. Connective tissue growth factor/CCN-2 is upregulated in epididymal and subcutaneous fat depots in a dietary-induced obesity model. Am J Physiol Endocrinol Metab 2013;304: E1291–1302. [DOI] [PubMed] [Google Scholar]
- 22.Tan JT, McLennan SV, Song WW, Lo LW, Bonner JG, Williams PF, et al. Connective tissue growth factor inhibits adipocyte differentiation. Am J Physiol Cell Physiol 2008;295: C740–751. [DOI] [PubMed] [Google Scholar]
- 23.Battula VL, Chen Y, Cabreira Mda G, Ruvolo V, Wang Z, Ma W, et al. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood 2013;122: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley D, Conte C, Mittendorfer B, Eagon JC, Varela JE, Fabbrini E, et al. Gastric bypass and banding equally improve insulin sensitivity and beta cell function. J Clin Invest 2012;122: 4667–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013;145: 366–374 e361–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabbrini E, Yoshino J, Yoshino M, Magkos F, Tiemann Luecking C, Samovski D, et al. Metabolically normal obese people are protected from adverse effects following weight gain. J Clin Invest 2015;125: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab 2016;23: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A 2009;106: 15430–15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi S, Moseley AC, Almeda-Valdes P, Stromsdorfer KL, Franczyk MP, Okunade AL, et al. Diurnal Variation in PDK4 Expression Is Associated With Plasma Free Fatty Acid Availability in People. J Clin Endocrinol Metab 2018;103: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005;54: 2277–2286. [DOI] [PubMed] [Google Scholar]
- 31.Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, et al. NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell Rep 2016;16: 1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28: 412–419. [DOI] [PubMed] [Google Scholar]
- 33.Henegar C, Tordjman J, Achard V, Lacasa D, Cremer I, Guerre-Millo M, et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 2008;9: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010;59: 2817–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasarica M, Gowronska-Kozak B, Burk D, Remedios I, Hymel D, Gimble J, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab 2009;94: 5155–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi-wen X, Pennington D, Holmes A, Leask A, Bradham D, Beauchamp JR, et al. Autocrine overexpression of CTGF maintains fibrosis: RDA analysis of fibrosis genes in systemic sclerosis. Exp Cell Res 2000;259: 213–224. [DOI] [PubMed] [Google Scholar]
- 37.Kubota S, Takigawa M. Cellular and molecular actions of CCN2/CTGF and its role under physiological and pathological conditions. Clin Sci (Lond) 2015;128: 181–196. [DOI] [PubMed] [Google Scholar]
- 38.Ihn H Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol 2002;14: 681–685. [DOI] [PubMed] [Google Scholar]
- 39.Koshman YE, Sternlicht MD, Kim T, O’Hara CP, Koczor CA, Lewis W, et al. Connective tissue growth factor regulates cardiac function and tissue remodeling in a mouse model of dilated cardiomyopathy. J Mol Cell Cardiol 2015;89: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahab NA, Yevdokimova N, Weston BS, Roberts T, Li XJ, Brinkman H, et al. Role of connective tissue growth factor in the pathogenesis of diabetic nephropathy. Biochem J 2001;359: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011;121: 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


