Abstract
OBJECTIVE:
Previous studies of breast cancer survival have not considered specific depots of adipose tissue such as subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT).
METHODS:
We assessed these relationships among 3,235 women with stage II/III breast cancer diagnosed between 2005–2013 at Kaiser Permanente Northern California and 2000–2012 at the Dana Farber Cancer Institute. SAT and VAT areas (cm2) were calculated from routine CT scans within 6 (median: 1.2) months of diagnosis, covariates were collected from electronic health records, and vital status was assessed by death records. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox regression.
RESULTS:
SAT and VAT ranged from 19.0–891 cm2 and 0.484–454 cm2, respectively. SAT was related to increased risk of death [127 cm2 increase, HR (95% CI): 1.13 (1.02, 1.26)] but no relationship with VAT [78.18 cm2 increase: 1.02 (0.91, 1.14)]. An association with VAT was noted among women with stage II cancer [stage II: 1.17 (0.99, 1.39); stage III: 0.90 (0.76, 1.07); p-interaction: <0.01]. Joint increases in SAT and VAT were associated with mortality above either alone [simultaneous 1-SD increase: 1.19 (1.05, 1.34)].
CONCLUSIONS:
SAT may be an underappreciated risk factor for breast cancer-related death.
INTRODUCTION
The relationship between excess adiposity and survival among women with breast cancer has been widely studied, yet findings have been inconsistent.1–3 Most researchers have considered mortality in relation to body mass index (BMI, kg/m2), a measure of overall body size, with several authors noting that among women with breast cancer, the nadir of the BMI-mortality relationship can appear to be in the overweight range.3 These findings suggest that the ideal weight for breast cancer survivors is greater than that recommended for the general population, contributing to confusion around clinical and public health messaging.4 These inconsistencies may be due to the fact that weight and BMI are inadequate proxies for adiposity that do not distinguish between muscle and adipose tissue, nor differentiate specific depots of adipose tissue,5 (e.g. visceral vs. subcutaneous) which have different physiological effects.5,6
Fat deposited around the abdominal organs, visceral adipose tissue (VAT), has multiple effects. VAT is a source of endogenous estrogen and pro-inflammatory cytokines, and may decrease synthesis of sex hormone binding globulin (SHBG).6,7 Furthermore, higher VAT has been associated with hyperinsulinemia. Thus, the metabolic environment associated with high VAT may encourage tumor progression and negatively influence survival among cancer patients.8 In most large epidemiologic studies, VAT is assessed indirectly through anthropometric measures of central adiposity,9 such as waist circumference (WC) or waist-to-hip ratio (WHR). Although some reports have suggested a positive relationship between measures of central adiposity and breast cancer-related mortality,10–14 these associations have been inconsistent in magnitude, with some reporting only modest relationships12,14 and others strong associations.10,11,13 Additionally, the relationship between central adiposity and breast cancer survival may be limited to certain subgroups based on menopausal status or tumor type.10,14
Although anthropometric measures of central adiposity are positively correlated with VAT, they also reflect abdominal subcutaneous adipose tissue (SAT). SAT is adipose tissue stored beneath the skin, and appears to be related to a more favorable metabolic profile,9,15 in particular SAT stored around the hips and thighs. However, some studies have suggested there are notable metabolic differences between SAT stored in the hips and thighs compared to the trunk.5 While anthropometric measures of central adiposity do not distinguish between abdominal VAT and SAT, imaging techniques such as computed tomography (CT) offer more accurate means of assessing the relevant dimensions of adiposity and muscle.5,9,16
Understanding how specific fat depots are related to breast cancer survival may help to clarify the relationship between excess adiposity and mortality, and more accurately identify patients at high risk of death, than using weight or BMI alone. However, few studies have considered direct measures of VAT vs. SAT and, to our knowledge, none have focused on women with non-metastatic breast cancer. Although a recent paper from our group reported that increased total adiposity and low muscle mass measured by CT scans is associated with poorer survival, and outperforms BMI, that analysis did not distinguish between SAT and VAT depots.17 In the current study, we consider the relationships between CT-derived measures of SAT and VAT on overall mortality in a large cohort of women recently diagnosed with non-metastatic breast cancer.
METHODS
Study Population
Our analysis used data from the Breast Cancer-Sarcopenia and Near Term Survival (B-SCANS) cohort study, derived from electronic medical record data from Kaiser Permanente Northern California (KPNC) and Dana Farber Cancer Institute (DFCI). This study included women older than 18 years of age, with no prior history of cancer, who were diagnosed with stage II or III invasive breast cancer and had an abdominal or pelvic computed tomography (CT) scan within 6 months of diagnosis and prior to any chemotherapy or radiation therapy (n=3,706). Subjects from KPNC included those members diagnosed between 2005–2013 and data from DFCI included patients diagnosed between 2000 and 2012. Exclusions in the total cohort included 303 patients without a valid weight measure around the CT scan, 120 patients whose scans were either unavailable or unreadable due to image quality issues, and 42 patients with BMI < 18.5 at time of scan. We also excluded 23 women with missing data on race, 11 with missing surgery type and 7 with missing ER or PR status. This left 3,235 patients in the final analytic sample. Vital status data for KPNC members was verified from various sources, including the electronic medical record, California state death records, or Social Security Death Index, and for DFCI patients from the electronic medical record and the National Death Index. The study was approved by the KPNC and DFCI institutional review boards.
Body Composition Assessment
Body composition measures were assessed by centrally trained researchers from CT scans obtained during routine clinical care, using the scan that was taken within six months and nearest to diagnosis, and prior to chemotherapy or radiation treatment (median: 1.2 months, range: ±5.9 months). Our analysis did not include stage I patients because they do not routinely receive CT scans, and thus those who did might be a non-representative sample of those patients. From the CT scan, we measured body composition at the third lumbar vertebra (L3), and calculated cross-sectional area in centimeters squared (cm2) of adipose tissue (SAT, VAT) and skeletal muscle index (SMI, cm2 from rectus abdominus, erector spinae muscles, quadratus lumborum, psoas, and internal, transverse and external oblique muscle groups divided by height in m2) by tissue-specific Hounsfield Unit ranges using SliceOmatic Software version 5.0 (TomoVision, Montreal, Quebec, Canada).18 These quantities have been previously shown to be valid proxies for whole body volumes of muscle and adipose tissue.19 The coefficients of variation (CV%) for 30 images randomly selected and colored by both readers were 0.79 and 6.72, and 0.66, for SAT, VAT, and SMI, respectively.
Covariate Assessment
Data on relevant covariates, including demographic characteristics, height, weight, disease stage, tumor characteristics, smoking, and treatment were obtained from the medical records or Cancer Registry. For height and weight, we used the measurement at the clinical visit closest to the scan [mean (range), 0.0 (−5.8 to 3.3) months].
Statistical Analysis
We report counts and percentages, and means and standard deviations, for categorical and continuous characteristics, respectively, by groups defined by quartiles of subcutaneous and visceral fat among those who died. We also calculated Kaplan-Meier survival functions over the follow-up separately for these groups. Follow-up time accrued beginning at the time of CT scan and continued until the earlier of: 1) death from any cause, or 2) July 15, 2016 (KPNC cohort) or last date of contact (DFCI cohort), the latter for censored observations. Median follow-up time was 6.30 years (range 0.041–12.6 years) for the KPNC cohort and 8.5 years (range 0.27–16.5 years) for the DFCI group. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated with Cox proportional hazards models allowing for left truncation with baseline hazard stratified by study site. Because there are no established cutpoints for these measures of body composition, we considered SAT and VAT as continuous variables. To allow for nonlinear relationships between adiposity measures and mortality we expressed them with restricted quadratic spline coding and evaluated nonlinearity with the likelihood ratio test of the higher order spline terms.20 Knot points for each variable were at quartiles of its distribution among those who died in order to allow for sufficient number of events in each region. All models included both measures of adiposity and were additionally adjusted for potential confounders determined from a directed acyclic graph from those variables believed to be associated with body composition and mortality and not lie on the causal pathway between the exposures and outcome: age at diagnosis (continuous, restricted quadratic spline with knots at quintiles), smoking status, race, tumor grade, surgery, estrogen- or progesterone-receptor (ER/PR) status, HER-2 status, skeletal muscle area (continuous), and the portion of BMI not included in muscle or fat (continuous). Treatment modalities were not considered as potential confounders given the timing of the CT scan preceded treatment for most women, and thus it could not influence body composition. The nonlinear spline terms for SAT and VAT did not achieve statistical significance (p-nonlinearity, SAT: 0.59; VAT: 0.36) so we present models with these variables expressed linearly in the log-hazard scaled to represent a 1-standard deviation (1-SD) increase in the corresponding measure. Effect modification was examined through Wald tests on the multiplicative interaction term. We considered the potential for these associations to vary across site through interaction terms between each body fat measure and site. Since body composition near diagnosis could reflect the consequences of more aggressive disease, thus making it appear that low body fat is related to increased risk for death close to diagnosis, we evaluated interactions between each body composition variable and an indicator that follow-up time was >1 years post diagnosis. We also evaluated heterogeneity between SAT and VAT associations over levels of age (dichotomized as <55 years vs. ≥ 55 years), stage (II vs. III), low muscle mass (defined as muscle area divided by squared height in meters < 40), ER/PR status (negative vs. positive), and Her-2 status (negative/indeterminate vs. positive) in separate models. Age was dichotomized at 55 years as a proxy for menopausal status, which was not available from the medical record. We also considered the joint effects of SAT and VAT by the multiplicative interaction between these variables, reporting associations for 1-SD increases in these variables relative to their mean value.
Cause of death was not available for the DFCI portion of the cohort, but we additionally considered these relationships with regard to breast cancer specific mortality in the KPNC sample. Cause-specific hazard ratios were estimated by censoring those subjects who died from causes other than breast cancer, as well as those alive at the end of follow-up in the KPNC sample (July 15, 2016).
All analyses were performed using R statistical software.21 Statistical significance was established with 2-sided tests with 0.05 significance level.
RESULTS
Characteristics of the study population are presented in Table 1. Among the 3,235 women included in our analysis, 708 died over the follow-up. Individuals in the lower quartiles of SAT, and particularly VAT, were younger than those in the upper categories. Those with higher SAT were more likely to be African-American and less likely to be Asian or Pacific Islander than those with lower SAT. Conversely, individuals with greater VAT were more likely to be Hispanic than those with less VAT. Those with stage III disease tended to have higher levels of both measures of adiposity (mean SAT stage II: 239.6 cm2, stage III: 256.8 cm2; mean VAT stage II: 95.5 cm2, stage III: 105.1 cm2) and to have similar amounts of muscle mass (mean SMI stage II: 43.2 cm2/m2; stage III, 43.7 cm2/m2). Positive ER/PR tumor status was more common among those with greater VAT, but was similar across quartiles of SAT. The unadjusted Kaplan-Meier curves (Figure 1) illustrate a lower probability of survival among those with 336–891 cm2 (highest quartile) SAT (panel A) and with 163–454 cm2 (highest quartile) VAT (panel B), with similar survival among the other groups defined by quartiles of each variable. The correlation between SAT and VAT was 0.61.
Table 1.
Characteristics of B-SCANS study population stratified by quartiles of subcutaneous fat and visceral fat.
| Quartiles of Subcutaneous Adipose Tissue (cm2) | Quartiles of Visceral Adipose Tissue (cm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category boundaries | [19, 160] | (160, 238] | (238, 336] | (336, 891] | [0.484, 43.2] | (43.2, 94.6] | (94.6, 163] | (163, 454] | Total |
| N | 854 | 897 | 790 | 694 | 952 | 845 | 808 | 630 | 3,235 |
| Deaths | 177 | 177 | 177 | 177 | 177 | 177 | 177 | 177 | 708 |
| Age [mean (sd)] | 52.20 (12.95) | 54.42 (11.98) | 54.72 (10.82) | 55.29 (10.86) | 48.63 (11.62) | 53.37 (11.28) | 56.62 (10.74) | 60.09 (10.15) | 54.1 (11.8) |
| Race | |||||||||
| White/non-Hispanic | 627 (74.2) | 615 (69.3) | 559 (71.7) | 476 (69.3) | 696 (74.0) | 573 (68.3) | 518 (65.2) | 490 (78.4) | 2,277 (71.2) |
| Black | 30 ( 3.6) | 50 ( 5.6) | 50 ( 6.4) | 100 (14.6) | 57 ( 6.1) | 70 ( 8.3) | 72 ( 9.1) | 31 ( 5.0) | 230 ( 7.2) |
| Hispanic | 36 ( 4.3) | 68 ( 7.7) | 96 (12.3) | 80 (11.6) | 49 ( 5.2) | 70 ( 8.3) | 93 (11.7) | 68 (10.9) | 280 ( 8.8) |
| Asian/Pacific Islander | 152 (18.0) | 154 (17.4) | 75 ( 9.6) | 31 ( 4.5) | 139 (14.8) | 126 (15.0) | 111 (14.0) | 36 ( 5.8) | 412 (12.9) |
| Other | 9 | 10 | 10 | 7 | 11 | 6 | 14 | 5 | 36 |
| Smoking Status | |||||||||
| Never | 527 (63.0) | 566 (64.2) | 433 (55.9) | 388 (57.2) | 589 (63.4) | 520 (63.0) | 487 (61.4) | 318 (51.0) | 1,914 (60.4) |
| Former | 235 (28.1) | 242 (27.5) | 253 (32.6) | 229 (33.8) | 253 (27.2) | 230 (27.9) | 230 (29.0) | 246 (39.5) | 959 (30.3) |
| Current | 74 ( 8.9) | 73 ( 8.3) | 89 (11.5) | 61 ( 9.0) | 87 ( 9.4) | 75 ( 9.1) | 76 ( 9.6) | 59 ( 9.5) | 297 ( 9.4) |
| Unknown | 18 | 16 | 15 | 16 | 23 | 20 | 15 | 7 | 65 |
| Stage | |||||||||
| II | 559 (65.5) | 543 (60.5) | 454 (57.5) | 388 (55.9) | 621 (65.2) | 501 (59.3) | 475 (58.8) | 347 (55.1) | 1,944 (60.1) |
| III | 295 (34.5) | 354 (39.5) | 336 (42.5) | 306 (44.1) | 331 (34.8) | 344 (40.7) | 333 (41.2) | 283 (44.9) | 1,291 (39.9) |
| Tumor Grade | |||||||||
| Well differentiated | 88 (10.8) | 82 ( 9.5) | 63 ( 8.4) | 68 (10.2) | 82 ( 8.9) | 65 ( 8.1) | 88 (11.4) | 66 (11.0) | 301 ( 9.7) |
| Moderately differentiated | 343 (41.9) | 348 (40.5) | 310 (41.1) | 260 (39.0) | 364 (39.4) | 314 (39.2) | 325 (42.2) | 258 (42.9) | 1,261 (40.7) |
| Poor/Undifferentiated | 387 (47.3) | 430 (50.0) | 381 (50.5) | 338 (50.8) | 478 (51.7) | 423 (52.7) | 358 (46.4) | 277 (46.1) | 1,536 (49.6) |
| Unknown | 36 | 37 | 36 | 28 | 28 | 43 | 37 | 29 | 137 |
| Surgery | |||||||||
| None | 43 ( 5.0) | 48 ( 5.4) | 50 ( 6.3) | 36 ( 5.2) | 48 ( 5.0) | 54 ( 6.4) | 40 ( 5.0) | 35 ( 5.6) | 177 ( 5.5) |
| Lumpectomy | 249 (29.2) | 305 (34.0) | 281 (35.6) | 257 (37.0) | 268 (28.2) | 302 (35.7) | 308 (38.1) | 214 (34.0) | 1,092 (33.8) |
| Mastectomy | 562 (65.8) | 544 (60.6) | 459 (58.1) | 401 (57.8) | 636 (66.8) | 489 (57.9) | 460 (56.9) | 381 (60.5) | 1,966 (60.8) |
| ER/PR status | |||||||||
| Both negative | 217 (25.4) | 242 (27.0) | 209 (26.5) | 188 (27.1) | 262 (27.5) | 249 (29.5) | 208 (25.7) | 137 (21.7) | 856 (26.5) |
| Either positive | 637 (74.6) | 655 (73.0) | 581 (73.5) | 506 (72.9) | 690 (72.5) | 596 (70.5) | 600 (74.3) | 493 (78.3) | 2,379 (73.5) |
| HER-2 Status | |||||||||
| Negative | 596 (69.8) | 657 (73.2) | 572 (72.4) | 552 (79.5) | 657 (69.0) | 613 (72.5) | 611 (75.5) | 498 (79.0) | 2,377 (73.5) |
| Positive | 209 (24.5) | 191 (21.3) | 167 (21.1) | 106 (15.3) | 248 (26.1) | 182 (21.5) | 154 (19.0) | 89 (14.1) | 673 (20.8) |
| Equivocal | 49 ( 5.7) | 49 ( 5.5) | 51 ( 6.5) | 36 ( 5.2) | 47 ( 4.9) | 51 ( 6.0) | 44 ( 5.4) | 43 ( 6.8) | 185 ( 5.7) |
| Muscle area in cm2 [mean (sd)] | 105.24 (16.12) | 110.86 (16.86) | 117.95 (17.73) | 129.77 (21.37) | 108.28 (16.36) | 111.83 (17.90) | 117.65 (19.36) | 126.86 (22.64) | 115.17 (20.01) |
| BMI [mean (sd)] | 22.19 (2.55) | 26.01 (3.02) | 29.62 (3.42) | 36.57 (5.74) | 22.92 (3.22) | 26.69 (3.88) | 30.22 (5.13) | 35.35 (6.08) | 28.15 (6.37) |
Figure 1.
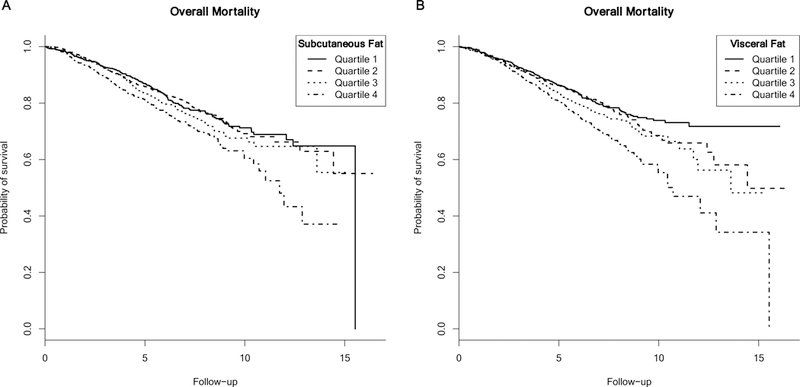
Kaplan-Meier curves for the relationship between subcutaneous adipose tissue (panel A), visceral adipose tissue (panel B) and mortality.
After adjusting for patient and tumor characteristics, a 1-SD (126.99 cm2) increase in SAT was associated with greater risk of death from any cause [HR: 1.13 (1.02, 1.26)], while a 1-SD (78.18 cm2) increase in VAT was not [HR: 1.02 (0.91, 1.14)] (Table 2). These relationships appeared to be consistent across study site (p-interaction, SAT: 0.71, VAT: 0.60) and over the follow-up period (p-interaction with >1 year follow-up time indicator, SAT: 0.97, VAT: 0.52). Associations between both SAT and VAT and mortality were also similar across categories of age (p-interaction, SAT: 0.54, VAT: 0.14), muscle area (p-interaction, SAT: 0.73, VAT: 0.50), ER/PR status (p-interaction, SAT: 0.49, VAT: 0.84), and HER-2 status (p-interaction, SAT: 0.67, VAT: 0.93), as was the SAT-mortality association across stage (p-interaction: 0.22). However, there was evidence of heterogeneity of the VAT-mortality relationship by stage (p-value: <0.01), with a steeper positive relationship between VAT and mortality among those classified as stage II [per 78.18 cm2 increase in VAT, HR: 1.17 (0.99, 1.39)] compared to a slight inverse relationship among those classified as stage III [HR: 0.90 (0.76, 1.07)]. The relationships between SAT, VAT and breast cancer-specific mortality in the KNPC portion of the cohort are presented in Table 3. Associations were generally similar in magnitude but estimates were less precise due to the reduced number of events when restricting to specific causes of death among a subset of the study sample. We did not observe evidence of heterogeneity of the association between SAT or VAT and breast cancer-specific mortality by any of the covariates considered.
Table 2.
Hazard ratios (HR) and 95% confidence intervals (CI) for change in subcutaneous fat and visceral fat and all-cause mortality.
| Subcutaneous Fat (per 126.99 cm2 increase) | Visceral Fat (per 78.18 cm2 increase) | |||||
|---|---|---|---|---|---|---|
| Deaths | Person-years | HR* (95% CI) | p-interaction | HR* (95% CI) | p-interaction | |
| Overall | 710 | 22,302.41 | 1.13 (1.02, 1.26) | N/A | 1.02 (0.91, 1.14) | N/A |
| Age | 0.54 | 0.14 | ||||
| < 55 years | 278 | 12,054.07 | 1.08 (0.90, 1.31) | 1.16 (0.90, 1.50) | ||
| ≥ 55 years | 432 | 10,247.34 | 1.15 (0.98, 1.34) | 0.98 (0.85, 1.14) | ||
| Stage | 0.22 | <0.01 | ||||
| II | 329 | 14,065.94 | 1.07 (0.90, 1.27) | 1.17 (0.99, 1.39) | ||
| III | 381 | 8,236.47 | 1.19 (1.02, 1.38) | 0.90 (0.76, 1.07) | ||
| Muscle | 0.73 | 0.50 | ||||
| SMI > 40 cm2/m2 | 423 | 14,680.11 | 1.14 (0.99, 1.32) | 1.05 (0.90, 1.22) | ||
| SMI < 40 cm2/m2 | 287 | 7,622.29 | 1.11 (0.90, 1.36) | 0.98 (0.80, 1.21) | ||
| ER/PR status | 0.49 | 0.84 | ||||
| Negative | 241 | 5,560.63 | 1.18 (0.98, 1.41) | 1.01 (0.81, 1.25) | ||
| Positive | 469 | 16,641.78 | 1.11 (0.95, 1.28) | 1.03 (0.89, 1.19) | ||
| Her-2 Status | 0.67 | 0.93 | ||||
| Negative/Indeterminate | 605 | 17,421.89 | 1.15 (1.01, 1.30) | 1.02 (0.89, 1.16) | ||
| Positive | 105 | 4,880.52 | 1.08 (0.77, 1.50) | 1.01 (0.70, 1.43) | ||
Baseline hazard was stratified by study site, and model included both adiposity measures and were adjusted for age at diagnosis, smoking, race, tumor grade, surgery, estrogen receptor status, HER-2 status, skeletal muscle index (SMI), partitioned BMI and height.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for change in subcutaneous fat and visceral fat and breast-cancer specific mortality in Kaiser Permanente Northern California sample.
| Subcutaneous Fat (per 126.99 cm2 increase) | Visceral Fat (per 78.18 cm2 increase) | |||||
|---|---|---|---|---|---|---|
| Deaths | Person-years | HR* (95% CI) | p-interaction | HR* (95% CI) | p-interaction | |
| Overall | 462 | 15,214.65 | 1.18 (1.02, 1.37) | N/A | 0.92 (0.78, 1.08) | N/A |
| Age | 0.44 | 0.28 | ||||
| < 55 years | 160 | 7,513.92 | 1.11 (0.86, 1.43) | 1.05 (0.74, 1.49) | ||
| ≥ 55 years | 302 | 7700.731 | 1.22 (0.99, 1.51) | 0.88 (0.72, 1.08) | ||
| Stage | 0.79 | 0.16 | ||||
| II | 191 | 9,163.87 | 1.16 (0.90, 1.50) | 1.03 (0.79, 1.33) | ||
| III | 271 | 6,050.78 | 1.20 (0.98, 1.46) | 0.86 (0.69, 1.07) | ||
| Muscle | 0.38 | 0.51 | ||||
| SMI > 40 cm2/m2 | 277 | 10,325.01 | 1.15 (0.95, 1.39) | 0.95 (0.77, 1.16) | ||
| SMI < 40 cm2/m2 | 185 | 4,889.64 | 1.29 (0.96, 1.75) | 0.86 (0.64, 1.17) | ||
| ER/PR status | 0.25 | 0.52 | ||||
| Negative | 155 | 3,731.10 | 1.29 (1.01, 1.65) | 0.87 (0.65, 1.16) | ||
| Positive | 307 | 11,483.55 | 1.12 (0.92, 1.37) | 0.95 (0.77, 1.16) | ||
| Her-2 Status | 0.68 | 0.87 | ||||
| Negative/Indeterminate | 396 | 12,095.14 | 1.20 (1.01, 1.43) | 0.91 (0.76, 1.10) | ||
| Positive | 66 | 3,119.51 | 1.11 (0.72, 1.73) | 0.94 (0.58, 1.52) | ||
Baseline hazard was stratified by study site, and model included both adiposity measures and were adjusted for age at diagnosis, smoking, race, tumor grade, surgery, estrogen receptor status, HER-2 status, skeletal muscle index (SMI), partitioned BMI and height.
As shown in Table 4, there was a suggestion of a synergistic effect between SAT and VAT for all-cause mortality (p-interaction: 0.04). Hazard ratios for individual changes in SAT and VAT from the model that included the multiplicative interaction were of similar magnitude to the main effects model [1-SD increase in SAT alone HR: 1.12 (0.99, 1.27); per 1-SD increase in VAT alone: 0.99 (0.87, 1.14)], but we observed a somewhat greater than multiplicative effect for increases in both measures simultaneously [HR: 1.19 (1.05, 1.34)]. When considering breast cancer-specific deaths, we did not observe evidence of interaction between SAT and VAT (p-interaction: 0.46).
Table 4.
Hazard ratios (HR) and 95% confidence intervals (CI) for joint effects of subcutaneous fat and visceral fat and all-cause and breast cancer-specific mortality.
| HR* (95% CI) | ||
|---|---|---|
| SAT/VAT Combination | All-cause mortality | Breast cancer mortality† |
| SAT increase only (126.99 cm2), VAT at mean | 1.12 (0.99, 1.27) | 1.17 (0.99, 1.39) |
| VAT increase only (78.18 cm2), SAT at mean | 0.99 (0.87, 1.14) | 0.90 (0.75, 1.09) |
| Both SAT (126.99 cm2) and VAT (78.18 cm2) increase | 1.19 (1.05, 1.34) | 1.10 (0.92, 1.31) |
| p-interaction | 0.04 | 0.46 |
Reference level for both variables is their respective means (246.49 cm2 for SAT and 99.29 cm2 for VAT). Baseline hazard was stratified by study site, and model included multiplicative interaction between both adiposity measures and was adjusted for age at diagnosis, smoking, race, tumor grade, surgery, estrogen receptor status, HER-2 status, skeletal muscle area, partitioned BMI and height.
Limited to the Kaiser Permanente Northern California portion of the cohort.
DISCUSSION
To our knowledge this is the first analysis to consider the independent and synergistic effects of specific fat depots in relation to survival in a large sample of women with non-metastatic breast cancer. We demonstrated that in addition to considering total adiposity, assessment of individual fat depots will provide key clinical information. Contrary to our a priori hypothesis, we found that elevated SAT was associated with worse survival among all women, but a deleterious relationship with VAT was only observed for women with stage II cancer.
To date, few researchers have considered the relationship between SAT and cancer-related mortality, but a recent study of 1,746 patients with gastrointestinal, respiratory, or kidney cancers reported that low SAT was related to poor survival.22 However, that analysis did not include any breast cancer patients, and the majority of the patients in that study had advanced stage disease. In contrast to our results, a small study of 172 women with metastatic breast cancer failed to find an association between SAT and mortality.23 Compared to the limited prior research on SAT, the relationship between VAT and mortality has been considered more frequently. According to a recent review of 22 studies of CT determined VAT and cancer survival, greater VAT tended to be associated with poor survival among those with colorectal and pancreatic cancers, but improved survival among those with kidney cancer.24 However, our observation that greater VAT was related to poor survival among only women with stage II, but not stage III, breast cancer is not corroborated by the only report among women with breast cancer, limited to those with advanced stage disease, that found an positive association between VAT and mortality.23
Our findings suggest that elevated SAT, in particular that around the abdomen, may be an underappreciated risk factor for mortality among women with breast cancer. Although CT imaging has been shown to be well-correlated with total body volumes of subcutaneous and visceral adipose tissue,25 scans at the L3 vertebra, which we used for this analysis, would capture SAT in the trunk, specifically the abdomen, more than other areas. Greater abdominal SAT may reflect a phenotype with unique effects in the breast as abdominal SAT is correlated with breast adipose tissue more strongly than either VAT or gluteofemoral SAT.26,27 Breast adipose tissue is a site for crown-like structures of the breast (CLS-B), macrophage-infiltrated adipocytes which produce a milieu of inflammatory cytokines and encourage endogenous estrogen production,28 which provide an environment thought to encourage tumor growth and development. Besides the local influences of CLS-B, research has also shown that even among ideal weight women (18.5 ≤ BMI < 25), CLS-B are associated with systemic metabolic dysfunction, including higher circulating leptin, insulin and triglycerides29 which could also influence survival.
Another potential explanation is that SAT in the trunk, particularly around the abdomen, appears to have different systemic metabolic effects compared to SAT in the gluteofemoral region.30 SAT in the gluteofemoral region is homogeneous, and related to a relatively favorable metabolic profile,30 but abdominal SAT may have metabolic effects similar to, and independent from, VAT.31 These observations may be explained by the different sub-depots of abdominal SAT, which may be separated into compartments that are anterior and posterior to the fascia superficialis: superficial SAT (sSAT) and deep SAT (dSAT), respectively.32,33 These sub-depots differ in their fatty acid composition34 as well as their anatomic location. Abdominal sSAT is metabolically more similar to gluteofemoral SAT than dSAT or VAT as it has less potential for insulin resistance than other depots.35 Conversely, researchers have demonstrated that dSAT has systemic effects similar to VAT.32,35 Thus, SAT around the abdomen may influence tumor promotion and cancer survival through mechanisms similar to those related to VAT.
VAT is often cited as the relevant fat depot in disease etiology because of its systemic effects on hyperinsulinemia, inflammation, and endogenous estrogen synthesis.36,37 We observed a trend toward greater risk of death with increasing levels of VAT only among women with stage II disease, but a slightly lower risk of death with increasing VAT among women with stage III disease. The opposite pattern was observed for SAT, with a somewhat more pronounced association among women with advanced stage disease. The potential mechanism for these observations is unclear, and additional research should seek to clarify this finding. In contrast to our previous results regarding overall adiposity,17 we did not find evidence of heterogeneity of the association of either of these specific fat measures according to level of muscle area. Low muscle mass is independently related to poor insulin sensitivity38 and inflammation,39 and has been linked to poor survival.17,40 The findings from our two studies taken together may suggest that in the presence of low muscle, the overall burden of body fat may be more important than the effect of either of the adipose tissue compartments individually.
Strengths of our study include a large sample size with long-term follow-up using resources from two large healthcare systems, allowing for detailed characterization of patient-level and tumor data. Importantly, we were able to use clinically-derived CT scans to assess measures of specific aspects of body composition that had not been previously considered in relation to breast cancer mortality, instead of more gross anthropometric measures such as waist circumference or BMI. However, our analysis has a few limitations. First, our analysis relied on cross-sectional CT images at the L3 vertebra to assess body composition which may reflect adipose tissue in a single anatomical area rather than whole-body volumes. This may have resulted in some mis-characterization of body composition since it does not capture the total volume in each region, and does not include adiposity in certain areas, such as around the hips and thighs. However, prior studies have shown assessments from scans at the L3 vertebra to be reasonable proxies of overall body composition.19 We note that the CV for the agreement of VAT area across readers was somewhat higher than for SAT in our data, which could suggest some potential for greater measurement error in VAT. However, mean VAT area is much lower than mean SAT, thus errors of similar absolute magnitude would yield larger CVs for the former. Nevertheless, the CV for both measures was low, indicating good agreement between readers. Additionally, our study used single assessments of body composition which would not capture its longitudinal trajectory over the follow-up period. Clarifying how changes and trends in body composition are related to mortality among breast cancer survivors would provide important insight. We did not have data on cause of death in the DFCI group, which precluded us from considering breast-cancer specific mortality. Finally, although we adjusted for tumor characteristics and stage of disease we cannot rule out the possibility that these associations could be a consequence of disease progression and not causal.
CONCLUSION
CT-scans are routinely performed in clinical care of cancer patients, and thus these measures of body composition are easily obtained and provide a more refined measure of obesity than BMI. In addition to providing improved prognostic indicators, aspects of body composition may yield new insight the relationship between adiposity and cancer-related mortality. Our findings regarding the relationship between specific adipose tissue depots and survival among women with breast cancer suggests that there may be distinct relationships between mortality and abdominal SAT and VAT. Future work should aim to clarify the relationship between abdominal SAT, breast adipose tissue, and mortality. If abdominal SAT is, in fact, a marker of breast adipose tissue and CLS-B infiltration, then CT-assessments of abdominal SAT may be useful to identify women for whom interventions to ameliorate the inflammatory effects of excess breast adipose tissue would be most beneficial.
What is already known about this subject?
The relationship between body mass index (BMI) and cancer survival has been inconsistent.
Specific adipose tissue depots may have different relationships with mortality but measures of overall adiposity are unable to distinguish them.
Measures of body composition from computed tomography (CT) scans offer an opportunity to clarify the relationship between adipose tissue depots and cancer survival.
What does this study add?
Among women with nonmetastatic breast cancer, subcutaneous adipose tissue (SAT) appears to be associated with greater overall mortality, independent of other measures of body composition.
In stratified analyses, greater VAT was related to poorer survival only among women with earlier stage disease.
There appears to be a synergistic effect between increases in SAT and VAT and overall mortality.
Acknowledgments
Funding: National Cancer Institute Grant R01 CA184953
REFERENCES
- 1.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas 2010;66(1):5–15. [DOI] [PubMed] [Google Scholar]
- 2.Caan BJ, Kwan ML, Shu XO, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev 2012;21(8):1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25(10):1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lajous M, Banack HR, Kaufman JS, Hernan MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med 2015;128(4):334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol 2006;35(1):83–92. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11(1):11–18. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer 2015;15(8):484–498. [DOI] [PubMed] [Google Scholar]
- 8.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev 2012;21(8):1244–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21(6):697–738. [DOI] [PubMed] [Google Scholar]
- 10.Borugian MJ, Sheps SB, Kim-Sing C, et al. Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol 2003;158(10):963–968. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev 2006;15(10):1871–1877. [DOI] [PubMed] [Google Scholar]
- 12.Tao MH, Shu XO, Ruan ZX, Gao YT, Zheng W. Association of overweight with breast cancer survival. Am J Epidemiol 2006;163(2):101–107. [DOI] [PubMed] [Google Scholar]
- 13.George SM, Bernstein L, Smith AW, et al. Central adiposity after breast cancer diagnosis is related to mortality in the Health, Eating, Activity, and Lifestyle study. Breast Cancer Res Treat 2014;146(3):647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control 2015;26(12):1803–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 2008;7(5):410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prado CM, Heymsfield SB. Lean Tissue Imaging A New Era for Nutritional Assessment and Intervention. Journal of Parenteral and Enteral Nutrition 2014;38(8):940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol 2018. [DOI] [PMC free article] [PubMed]
- 18.SliceOmatic [computer program]. Version 5.0 Montreal, Quebec, Canada: TomoVision; 2015. [Google Scholar]
- 19.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97(6):2333–2338. [DOI] [PubMed] [Google Scholar]
- 20.Witte JS, Greenland S. A nested approach to evaluating dose-response and trend. Ann Epidemiol 1997;7(3):188–193. [DOI] [PubMed] [Google Scholar]
- 21.R: A Language and Environment for Statistical Computing [computer program]. Version 3.3.1 Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 22.Ebadi M, Martin L, Ghosh S, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer 2017;117(1):148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase T, Sangai T, Nagashima T, et al. Impact of body fat distribution on neoadjuvant chemotherapy outcomes in advanced breast cancer patients. Cancer Med 2016;5(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J, Mazurak VC, Olobatuyi TA, Caan BJ, Prado CM. Visceral adiposity and cancer survival: a review of imaging studies. Eur J Cancer Care (Engl) 2018;27(2):e12611. [DOI] [PubMed] [Google Scholar]
- 25.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Applied Physiology, Nutrition, and Metabolism 2008;33(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 26.Janiszewski PM, Saunders TJ, Ross R. Breast volume is an independent predictor of visceral and ectopic fat in premenopausal women. Obesity (Silver Spring) 2010;18(6):1183–1187. [DOI] [PubMed] [Google Scholar]
- 27.Schautz B, Later W, Heller M, Muller MJ, Bosy-Westphal A. Associations between breast adipose tissue, body fat distribution and cardiometabolic risk in women: cross-sectional data and weight-loss intervention. Eur J Clin Nutr 2011;65(7):784–790. [DOI] [PubMed] [Google Scholar]
- 28.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med 2015;66:297–309. [DOI] [PubMed] [Google Scholar]
- 29.Iyengar NM, Brown KA, Zhou XK, et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev Res (Phila) 2017;10(4):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond) 2010;34 Suppl 2:S4–17. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997;46(10):1579–1585. [DOI] [PubMed] [Google Scholar]
- 32.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000;278(5):E941–948. [DOI] [PubMed] [Google Scholar]
- 33.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001;50(4):425–435. [DOI] [PubMed] [Google Scholar]
- 34.Lundbom J, Hakkarainen A, Lundbom N, Taskinen MR. Deep subcutaneous adipose tissue is more saturated than superficial subcutaneous adipose tissue. Int J Obes (Lond) 2013;37(4):620–622. [DOI] [PubMed] [Google Scholar]
- 35.Monzon JR, Basile R, Heneghan S, Udupi V, Green A. Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obes Res 2002;10(4):266–269. [DOI] [PubMed] [Google Scholar]
- 36.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444(7121):881–887. [DOI] [PubMed] [Google Scholar]
- 37.Katzmarzyk PT, Heymsfield SB, Bouchard C. Clinical utility of visceral adipose tissue for the identification of cardiometabolic risk in white and African American adults. Am J Clin Nutr 2013;97(3):480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. The Journal of Clinical Endocrinology \& Metabolism 2011;96(9):2898–2903. [DOI] [PubMed] [Google Scholar]
- 39.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 2006;119(6):526 e529–517. [DOI] [PubMed] [Google Scholar]
- 40.Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care 2015;18(6):535–551. [DOI] [PubMed] [Google Scholar]


