Abstract
During recent years, the preclinical stage of Alzheimer's disease (AD) has become a major focus of research. Continued failures in clinical trials and the realization that early intervention may offer better therapeutic outcome triggered a conceptual shift from the late-stage AD pathology to the early-stage pathophysiology. While much effort has been directed to understand the factors initiating AD, little is known about the principle basis underlying the disease progression at its early stages. In this perspective, we suggest a hypothesis to explain the transition from ‘silent’ signatures of aberrant neural circuit activity to clinically evident memory impairments. Namely, we propose that failures in firing homeostasis and imbalance between firing stability and synaptic plasticity in cortico-hippocampal circuits represent the driving force of early disease progression. We analyze the main types of possible homeostatic failures and provide the essential conceptual framework for examining the causal link between dysregulation of firing homeostasis, aberrant neural circuit activity and memory-related plasticity impairments associated with early AD.
Network hyperactivity and impaired synaptic plasticity as early signatures of AD
There is a growing consensus that understanding the preclinical stages of AD is pivotal for design of successful approaches to delay and even reverse the transition from normal brain physiology to cognitive impairments. More than two decades ago, amyloid-β (Aβ) dyshomeostasis has been proposed as the major initiating factor of AD, upstream of alterations in other proteins and diverse cell types1, 2. Until now, none of the Aβ-targeted phase 3 clinical trials have shown benefits in AD, facilitating a search for alternative triggers and drives of AD pathogenesis3–5. While it is conceivable that the complexity of the downstream pathogenic processes increases after the disease initiation5, the common rules and unifying principles underlying memory impairments in the early AD phase remain elusive. Before discussing the basic regulatory mechanisms, let us start from describing the earliest, AD-related changes in the functions of neural circuits.
It has long been proposed that changes in synaptic transmission provide a physiological substrate for learning, memory and a wide range of neurocomputations6. Electrophysiological studies in numerous AD models provide compelling evidence for impairments of distinct forms of hippocampal synaptic plasticity7. A large body of data has accumulated on the role of familial AD (fAD) mutations and Aβ in short-term synaptic plasticity and Hebbian-like, long-term plasticity in the form of long-term potentiation (LTP) and depression (LTD). Acute application of small Aβ oligomers, extracted from cerebral cortex of AD patients, typically results in a disruption of LTP and an increase of LTD8, 9. Inhibition of Aβ degradation by neprilysin causes reduction in short-term synaptic facilitation, shifting hippocampal synapses towards low-pass filters10. In addition to Aβ, other cleavage products of APP processing11, 12 and the full-length APP itself13, 14, may regulate synaptic transmission and plasticity under physiological and pathological conditions. Furthermore, a wide range of synaptic plasticity deficits has been documented in transgenic mouse models expressing single or multiple mutations in genes that cause autosomal-dominant, early-onset fAD - amyloid precursor protein (APP), presenilin-1 (PSEN1) and PSEN2. Although significant variability features distinct models and experimental conditions15, functional changes in the intra-hippocampal and cortico-hippocampal pathways typically precede the appearance of pathological aggregates in distinct fAD models16.
In addition to synaptic plasticity deficits, emerging evidence points to functional alterations in the network activity of specific brain circuits (for review see 16). Electrophysiological studies show numerous EEG abnormalities in AD patients17 and epileptiform activity in amnestic mild-cognitive impairment (MCI) patients that precede or coincide with cognitive decline18, 19. Crucially, patients with epileptiform activity display faster decline of their cognitive abilities18, 20. Moreover, many PSEN1 fAD mutations lead to seizures21, some of them in adolescence, preceding cognitive decline by a decade22. Furthermore, clinically silent hippocampal seizures and epileptiform spikes have recently been detected using intracranial recordings in two patients at the early stages of sporadic AD23. In addition to epileptiform activity detected by electrophysiological recordings in temporal or temporo-frontal lobes during resting state, functional MRI (fMRI) studies demonstrate task-related hippocampal hyperactivation in patients with MCI24, in PSEN1 mutation carriers 30 years before the diagnosis25, and in young asymptomatic carriers of the major risk factor for AD, APOE ε426–28. Aberrant activity of hippocampal and cortical circuits also features numerous distinct fAD mouse models29–35. Imbalance of excitation-to-inhibition (E/I) due to interneuron dysfunction has emerged as a potential driver of AD-related network and cognitive dysfunctions16, 29, 31. Notably, low-dose of atypical antiepileptic drug levetiracetam has been shown to reduce hyperactivity and improved memory in amnestic MCI patients23, 24 and fAD mouse models36. Whether subclinical epileptic-like spikes and seizures represent a typical signature of early AD phase and whether rescue of this abnormal network activity can slow down cognitive decline remains to be determined in future longitudinal studies.
An interplay between firing homeostasis and synaptic plasticity
Why is the activity of cortico-hippocampal circuits destabilized in early AD stages? It is widely accepted that homeostatic system allows central neural circuits to buffer acute and chronic stresses, safeguarding us from hyperactivity and seizures. The instability of spiking properties and the lack of compensation for hyperactivity, induced by distinct triggers, points to malfunction of homeostatic control system at the level of cortico-hippocampal circuits. Thus, understanding the principles underlying stabilization of activity in neuronal populations is essential for determining whether malfunction of firing homeostatic machinery is at the core of the disease progression.
The concept of homeostasis has a long history in physiology, starting from the work of Claude Bernard in the middle of 19th century on the stability of the ‘milieu inte´rieur’, the underlying principle of what Walter Canon would later term ‘homeostasis’. Nearly two decades after Bernard and Canon, James Hardy proposed a model in which homeostatic mechanisms maintain physiological variables with an acceptable range by comparing the actual value of the variable to a desired value called ‘set point’37. However, the research of neuronal homeostasis began only in the end of the 20th century, from the pioneering works of Eve Marder, Larry Abbott and colleagues on the mechanisms maintaining stable excitability properties of neurons38 and of Gina Turrigiano, Sasha Nelson and colleagues on synaptic scaling mechanism39 via regulation of AMPA receptor turnover at synapses40 to maintain neural functions. These studies facilitated the discovery of diverse homeostatic adaptations in a form of negative feedback control that appear to stabilize basic functions of neural circuits41–43.
While most studies on neuronal homeostasis are based on the theoretical guidelines of control theory, implementing these concepts on the complexity of the CNS circuits is quite challenging (see Box 1). Some key questions have remained unanswered. To mention only few: what are the cellular and network properties that are actively controlled by the homeostatic system, what is the spatial scale of this control and how the sensitivity of homeostatic system to perturbations is regulated. Answering these questions is absolutely critical for delineating the role of neuronal homeostasis in the progression of AD.
Box 1. The basics of homeostatic control – not so basic after all.
While it is a well-known fact that the healthy brain functions in a narrow range of activity between status epilepticus and coma, how neural circuits, composed from highly dynamic and heterogeneous individual components, maintain stable activity over long timescales or adjust their properties to constantly changing environments, remains obscure. A number of models adopted engineering control theory to physiological regulation in general95 and to neuronal activity regulation in particular42. According to control theory, homeostatic system is based on several principle features: (1) a set-point that defines the output of the system; (2) sensors that detect a deviation from a set point; (3) a negative feedback loop to retarget precisely a set point via homeostatic effectors (Fig. 1a). Extensive research lead to compelling evidence on a wide repertoire of possible homeostatic processes that may counteract the instability. These stabilizing mechanisms, including adjustments of synaptic strength, excitation-to-inhibition balance and intrinsic excitability, have been collectively termed homeostatic aplasticity96. While the concept of homeostasis is relatively straightforward for a simple mechanical system such as thermostat, for complex CNS networks several key questions remain open:
(1) What are the variables that undergo homeostatic regulation?
It is reasonable to assume that cell-type or circuit-specific functional demands determine the type of properties that are most strictly regulated. Thus, understanding the functional role of each component of the system is vital for our understanding of the specific variables that are controlled by homeostatic machinery. While mean firing rate and firing synchrony of spontaneous spiking have been shown to be under homeostatic control, whether homeostatic CNS machinery keeps other aspects of activity, such as excitation-to-inhibition ratio97 or average synaptic weight across the dendritic tree98, remains an open question.
(2) Does homeostatic regulation operate at the level of single neurons or/and neuronal population?
Do they operate at a single neuron or neuronal population level? Due to the technical challenge of monitoring the activity of the same neurons at extended timescales, there is no consensus on this question. Long-term in vivo electrophysiological recordings in the monocular zone of primary visual cortex demonstrate a remarkable stability at the level of individual neurons46. However, recently developed optical systems that enable monitoring of neuronal activity at long timescales in deep brain structures of freely moving mice revealed a remarkable degree of instability in the coding of space at the level of individual neurons, while invariant spatial representations at the behavioral level99. Similarly, Ca2+ imaging data provide further support for a stable population motor code with unstable firing patterns of individual neurons in the pre-motor area100. Notably, long-term electrophysiological and optical recordings ex vivo support the idea that single-neuron variability is an intrinsic property of the network44. As the cell-autonomous and network-wide levels of regulation are not mutually exclusive, understanding the interactions between different regulation scales and possible competing hierarchy will be essential in understanding destabilization of neural circuits. Moreover, determining the mechanisms regulating network-wide stability are critical for coping with functional instability of inter-connected networks.
(3) Does susceptibility to perturbations depend on the functional requirements of neural circuits?
Why do hippocampal circuits become dysfunctional in amnestic MCI associated with AD, while the primary sensory cortices remain fully functional until late stages? One possibility is that the specific functional requirements of the hippocampus may limit its homeostatic capacity and create circuit-specific vulnerability. Specifically, the unique role of the hippocampus in learning and memory may represent a challenge for the homeostatic regulatory system. The presence of functional adult neurogenesis in the dentate gyrus and of the requirement for the maintenance of plasticity in hippocampal networks throughout life may pose an overwhelming challenge to the homeostatic regulatory systems to stabilize this hub of plasticity. If this is the case, the same perturbation would result in a restoration of function in less plastic structures, while leading to a pathology in the hippocampus.
Recent studies suggest that mean firing rate, reflecting an average level of spontaneous spiking activity, is under homeostatic control in central neural circuits ex vivo44 and in vivo45–47. Moreover, firing synchrony is under homeostatic control as well, at least in ex vivo hippocampal networks44. If firing stability is indeed under homeostatic control, what are the mechanisms that operate to preserve this function under a constantly changing environment? One of the most important lessons we learn from computational and experimental studies on neural homeostasis is the realization that the same stable properties of neural networks can arise from multiple molecular configurations43. The ability of different mechanisms to yield the same output, termed degeneracy, has been proposed as a ubiquitous biological property and a feature of the system’s complexity48. Thus, a large number of solutions, regulating synaptic and intrinsic membrane properties, can generate similar ongoing firing properties following environmental, genetic or learning-based perturbations.
The problem arises when the same mechanisms that are used by neural circuits to maintain stability, can be also used to encode new information. This would mean that some adaptive solutions may interfere with distinct plasticity forms. For example, multiplicative synaptic scaling39, operating at the level of AMPARs abundance at spines40, has been proposed to uniformly adjust postsynaptic strength across the synapses. In this case, the relative differences in synaptic weights are preserved. If activity-dependent regulation of AMPAR number is within the dynamic range (far from saturation or quiescence), this mechanism may preserve the memory-related Hebbian plasticity and information processing between synaptic connections49. However, if the number of AMPARs reach saturation or quiescence (silent synapses), it can limit Hebbian-like LTP/LTD mechanisms. In addition, presynaptic homeostatic adaptations50–55 ultimately affect short-term synaptic plasticity, thus leading to deficits in synaptic computations6 and in memory functions56. Synaptic adaptations include also structural changes at the level of spine number57. Finally, homeostatic changes in intrinsic excitability are widely documented in various neuronal circuits following a variety of manipulations44, 58, 59. The changes in intrinsic excitability do not induce a gross deformation in firing properties, but tune the sensitivity of neurons to the incoming input. Intrinsic plasticity may involve changes in gain or threshold, in spike frequency adaptation, synaptic integration, local dendritic excitability, temporal firing patterns, and resonance characteristics, thus impacting multiple forms of plasticity60. Moreover, relative intrinsic excitability of a neuron at the time of learning has been suggested to determine its chance to participate in a given memory61. Therefore, modulation of intrinsic excitability of a neuron during resting state can regulate memory allocation.
All these considerations suggest that homeostatic processes, enabling stable firing properties, may preserve some functions of circuits, while altering others. The resultant output depends on the type, magnitude and duration of a perturbation and functional organization of the specific neural circuits. Based on these parameters, some adaptive mechanisms employed by circuits to stabilize certain network behaviors may critically impact memories that are stored within these circuits. Here, we define firing homeostasis as a maintenance of mean firing rate and firing pattern at the level of neuronal population during spontaneous neuronal activity. Firing homeostasis is typically a slow process, taking days for reaching an original set-point44, 45, 62. Therefore, in many cases, ongoing neuronal activity remains unbalanced during many hours following a perturbation. The change in the history of ongoing spiking activity is known to be an important factor modulating numerous synaptic and intrinsic plasticity forms63, phenomenon collectively called ‘metaplasticity’64. Indeed, impairments of synaptic plasticity and reduction in synapse density represent the prominent features of early AD phases7. Yet, our understanding of the balance and imbalance between Hebbian and homeostatic processes is still in its infancy.
The FHP hypothesis
Nervous systems are not always capable of maintaining optimal output. On the one hand, some perturbations (classified as perturbations type I, Fig. 1b) cause changes in synaptic or intrinsic mechanisms that are not essential for homeostatic control and thus induce a compensatory response that restores network functions. On the other hand, other perturbations (type II, Fig. 1c) impair the core homeostatic machinery and thus remain uncompensated or their compensation leads to suboptimal or even pathological function65. Does AD-associated pathophysiology stem from a failure of the core homeostatic machinery?
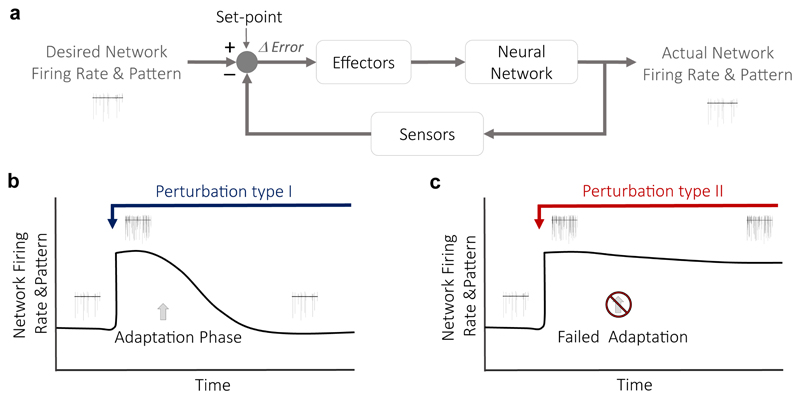
Figure 1. Firing homeostasis and its failure.
(a) A classic scheme of a homeostatic controller42. In this case, the output of the network is the mean firing rate that is monitored by sensors and maintained at a set-point value by negative feedback mechanisms mediated via effectors. Any deviation from the desired firing rate is sensed as the difference between the desired output (the set-point) and the actual output. The error signal is then corrected via the activity of effectors. (b) Monitoring the activity of the same neurons for a long time enables to test if the mean firing rate in the network is stable. When a constant perturbation is introduced to elevate firing rates (blue arrow), homeostatic mechanisms are activated to adapt the system to the perturbation (adaptation phase). This type I perturbation relates to changes in non-essential, regulatory homeostatic components. It induces compensatory mechanisms that re-normalize firing rates, despite of the continued interference. (c) Under pathological conditions (perturbation type II, red arrow), homeostatic mechanisms fail to re-normalize firing rates, leaving the network in a hyperactive state due maladaptive responses. Type II perturbation relates to impairments of the core homeostatic machinery.
We view AD pathophysiology as a network state that represents a common end point for distinct initial triggers, instead of a single-cause derived dysfunction. Based on this assumption, we propose that dysregulation of firing stability in cortico-hippocampal circuits and imbalance between firing stability and synaptic plasticity represent the major cause of memory impairments in early AD. This theory, that we refer to as the failure of firing homeostasis and plasticity (FHP) hypothesis, delineates possible mechanisms underlying the transition from ‘silent’ pathophysiological features to memory impairments at the early AD stages. At later disease stages, we hypothesize that firing homeostasis failure triggers a vicious cycle that dysregulates the whole integrative homeostatic network, driving Alzheimer’s degeneration66.
In this perspective, we provide a conceptual and experimental framework essential for examining the casual link between homeostatic control system, firing stability and synaptic plasticity and their possible impairments in AD. While focusing on AD as an example of the most common type of late-life dementia, we believe the logic may be applicable to other types of neurodegenerative disorders accompanied by aberrant spiking activity and plasticity impairments. The type of insults and the circuitry that become vulnerable are expected to be disease-specific.
Utilizing basic concepts of control theory and integrating them into known biological and pathophysiological processes yields strong predictions that can be verified experimentally (as described in the next section). To remove ambiguity that can arise from the complexity of these concepts, we propose the following simple criteria to assess the validity of the FHP theory:
Detectability: A defective homeostatic mechanism should be detectable in the hippocampal and associated cortical circuits that display vulnerability in early AD stages, irrespective of the initial triggers.
Reversibility: Restoration of this specific homeostatic function and stability – plasticity balance leads to amelioration of the pathophysiology and memory deficits.
Mimicry: Targeting of key molecules to interfere with specific homeostatic functions should lead to synaptic plasticity deficits, memory impairments and the disease progression in specific neural circuits.
These criteria are critically important to determine whether deficits in homeostatic systems are necessary and sufficient for initiation of pathophysiology associated with neurodegeneration. Detecting impaired homeostatic mechanisms is the first and the most crucial step in assessing the HFP hypothesis. Thus, it will be the main focus of the experimental framework we propose.
Categorization of failures in homeostatic control system
Typically, fAD cases emerge during the fifth decade of life, whereas sporadic, late-onset AD cases do not exhibit symptoms earlier than the seventh decade. Why do cognitive symptoms appear late in life? This question is still puzzling researchers. We propose that homeostatic systems actively suppress deviations from normal brain activity induced by genetic or environmental changes during healthy aging, while they fail in AD. The failures in firing homeostasis and synaptic plasticity represent the major cause of aberrant neuronal activity and memory impairments at early AD stages. Here we analyze conceptual and experimental frameworks essential to examine the FHP hypothesis on the basis of control theory and outline three general types of homeostatic failures that may underlie AD-related hyperactivity (Fig. 2):
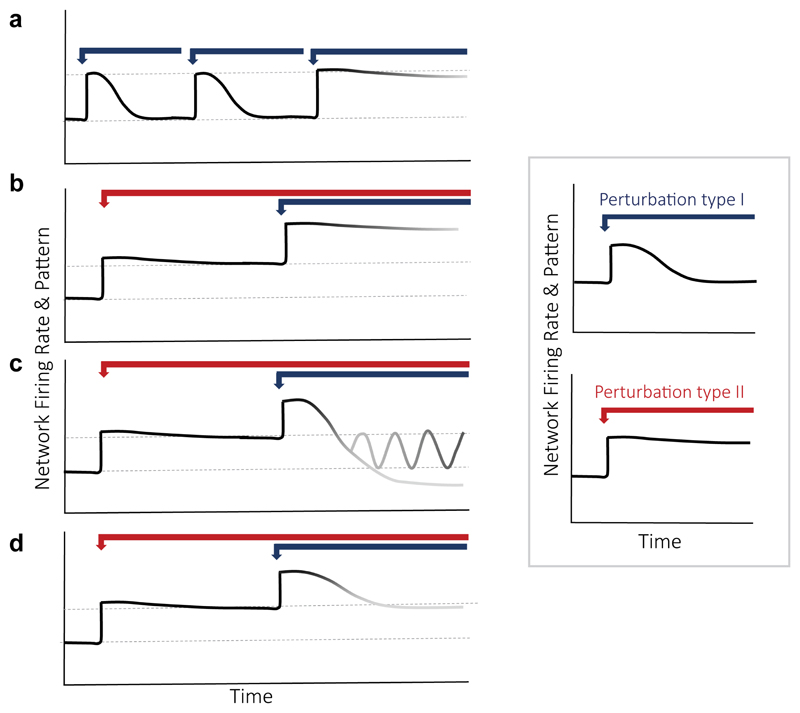
Figure 2. Experimental framework to investigate firing homeostasis failures.
Here we aim to investigate the effect of impairing core homeostatic machinery (perturbation type II) on firing stabilization following hyperactivity. (a) Accumulation of insults: A system that suffers multiple type 1 insults may initially be able to compensate, while it may eventually fail due to a restriction of the solution space following new insults. (b) Regulation is abolished: In this case, when type I is introduced in the presence of type II perturbation, the network does not compensate for the change in firing. This indicates type II restricts type I-induced homeostatic mechanisms and abolishes regulation of firing rates. (c) Regulation fails to reach the set-point: In the more complicated scenario, the network may overshoot, for example under malfunctioning of error signal estimations. The network may also enter an oscillation state if the kinetics of compensatory mechanisms is altered by type II perturbation. (d) Set-point is changed: In this example, when type I perturbation is introduced, homeostatic compensation mechanisms are still active, yet they trigger a compensation to the new steady-state level that type II perturbation imposed, indicating that type II affects firing set-point establishment. Inset: Perturbation type I (blue arrow), acutely augmenting spiking activity without impairing the essential elements of homeostatic system, induces homeostatic compensatory mechanisms that re-normalize firing rates to a set-point level (top panel). Perturbation type II (red arrow) affects mean firing rates without inducing a compensatory homeostatic response (bottom panel), indicating that type II is involved in regulation of firing rate stability.
(1) Maladaptive feedback response to a perturbation. Much effort has been devoted to identifying the primary synaptic and neuronal changes initiating AD-related dysfunctions of neural circuits. While numerous homeostatic molecular players have been implicated in AD pathogenesis (summarized in Table 1), very little is known about the role of compensatory homeostatic mechanisms and their failures in development of aberrant brain activity and cognitive deficits associated with AD. One possibility is that mutations associated with early-onset AD target the key players in the homeostatic machinery, thus interfering with proper homeostatic compensation (Fig. 2a,b). For example, PSEN1 mutation M146V or PSEN1 knockout impairs postsynaptic scaling in hippocampal neurons67. Another attractive possibility, is dysregulation of master transcriptional regulators, such as Repressor Element-1 Silencing Transcription Factor (REST). It has been shown that downregulation of REST, associated with MCI and AD68, impairs presynaptic and intrinsic homeostatic mechanisms in response to hyperactivity in neural networks69, 70. Thus, REST may represent a core regulatory element of homeostatic effectors essential for normal aging. An alternative possibility is that excessive or insufficient homeostatic adjustments occur due to deficits in the regulatory feedback mechanisms activated by the initial perturbation (Fig. 2c). For example, an integral feedback loop involving NF-κB, polo-like kinases (Plks), and GTPase-activating protein (SPAR) have been implicated in limiting overshooting and enabling refinement of homeostatic adjustments to elevated activity71. In this study, deficiency of NF-κB produced exaggerated homeostatic reductions in the size and density of dendritic spines, synaptic AMPA receptors and excitatory synaptic currents in response to chronic increase in neuronal excitation. Indeed, an overshoot in synaptic scaling has recently been reported in the presence of oligomeric Aβ in response to chronic inactivity in vitro and to sensory deprivation in vivo72. As synaptic dysregulation is at the heart of AD pathophysiology, imprecise synaptic scaling may result in a pathological compensation of firing rate. However, how big the contribution of synaptic scaling to firing homeostasis still remains unknown.
Table 1. Putative mechanisms underlying homeostatic failures in early AD.
| Molecular target | Mode of action | Relevance to neural homeostasis | Relevance to AD |
|---|---|---|---|
| Presenilin 1 | Catalytic subunit of γ-secretase complex Regulation of Ca2+ release from ER |
PS1 deletion and early-onset AD M146V PS1 mutation disrupt synaptic scaling | Target of the majority of early-onset AD mutations; the last step in the APP cleavage, determines the length of Aβ and its biophysical properties |
| BACE1 | secretase cleaving APP at β site | BACE1 KO mice display lack of synaptic scaling to visual experience in primary sensory cortex and increased excitatory basal synaptic transmission | APP processing, Aβ production |
| REST | Transcriptional repressor of neuronal genes during embryonic development | Reduction in excitatory presynaptic strength and in intrinsic excitability to hyperactivity | Its expression is downregulated in MCI and AD, in comparison to normal aging |
| TNF-α | Releasable by glia cytokine | Postsynaptic up-scaling to inactivity | Increases Aβ production and inhibits the secretion of sAPP Increases BACE1 expression and suppresses Aβ degradation by microglia |
| BDNF | Activity-dependent, neuron-derived releasable modulator | Postsynaptic scaling and E/I balance Presynaptic adaptation |
Early BDNF treatment ameliorates neuronal loss in AD mouse model Interaction between BDNF and APOE polymorp hism affects memory decline in preclinical AD |
| CDK5 | Proline-directed serine/threonine kinase | Synaptic scaling, presynaptic adaptation | CDK5 hyperactivation promotes neurodegeneration |
| Arc | Immediate early gene product | Synaptic scaling | Regulates activity-dependent Aβ production Reduction of Arc mRNA in the dentate gyrus of AD mice Deregulation of Arc in the vicinity of amyloid plaques disrupts responses to visual stimuli in the visual cortex |
| NPTX2 | Immediate early gene product | NPTXs regulate synaptic scaling of excitatory synapses on PV interneurons | NPTX2 is downregulated in human AD brains and reduction in its expression contributes to aberrant brain activity in AD model mice |
| mTOR | serine/threonine protein kinase | TSC-mTOR signaling regulates inhibition-excitation balance and firing rate without altering homeostatic responses mTOR regulates presynaptic homeostatic adaptations |
Genetic and pharmacological reduction of mTOR signaling ameliorates AD-related pathology and cognitive decline in transgenic AD models |
| CaMKK2 | Ca2+/calmodulin-dependent and serine/threonine protein kinase | STO-609, a CaMKK2 inhibitor, occludes synaptic scaling | STO-609, a CaMKK2 inhibitor, rescues Aβ-induced spine loss |
| CaMKII | Ca2+-calmodulin-dependent kinase II | Presynaptic and postsynaptic adaptations | p(T286)-αCaMKII is reduced at synaptic locations in hippocampus of AD patients and the degree of p(T286)-αCaMKII loss at synaptic locations correlates with severity of the disease |
| CaN | Calcineurin, Ca2+-calmodulin-dependent protein phosphatase | Inhibition of CaN activity causes homeostatic synaptic plasticity via retinoic acid | CaN is hyperactivated in AD |
| Voltage-gated calcium channels | Ion channel | L-type VGCC mediates presynaptic adaptation and postsynaptic scaling | APP regulates L-type VGCC in interneurons |
| RyR | Ca2+ release from ER | Synaptic scaling | Increase in RyR-mediated Ca2+ release from ER causes dysregulation of Ca2+ homeostasis in AD models |
| STIM2-SOC-CaMKII | Ca2+ homeostasis | Spine stability | Downregulation of STIM2 causes spine loss in AD mice |
| Retinoic acid | Transcriptional activator during brain development, synaptic strength modulation | Synaptic scaling | Retinoic acid rescues AD-like pathology in mouse model |
| GABA(B)R | GPCR | presynaptic and postsynaptic adaptations, firing rate homeostasis | APP is a core molecule of the presynaptic GABA(B)R macromolecular complex Regulates the Aβ40/42 ratio during spike bursts |
| Adenosine receptors | GPCR | Sleep homeostasis, anti-epileptic effect by increased extracellular adenosine | A2AR are overexpressed in the hippocampus of AD patients and AD mice, mediate LTP and memory impairments A1R regulates the Aβ40/42 ratio during spike bursts |
See Supplementary Table 1 for supporting references.
A defect in compensatory mechanisms at the level of intrinsic excitability presents another example of maladaptive feedback response that could shift the network into a hyperactive state. If the remaining adaptive synaptic mechanisms are only able to partially compensate for a perturbation, this may lead to functional changes that arise only under specific functional demands, leading to context-specific memory failures. Over longer periods of time, this chronic dysregulation of firing and hyperactivity (even if mild and context-specific) may then cause an over-activation of the remaining functional homeostatic mechanisms, leading to a gradual, but persistent, synaptic loss. Indeed, Aβ accumulation triggers endocytosis of AMPA receptors73 and ubiquitination of the GluA1 receptor subunit74, leading to spine loss8, 75. The synapse weakening and elimination may present a compensatory mechanism which is insufficient to re-normalize hyperactivity induced by Aβ at short timescales10, 13, 14.
In future, we need to determine whether misregulation of the core molecular homeostatic machinery (classified as type II perturbations) causes AD-related firing destabilization66. Systematic screen of the candidates implicated in homeostatic feedback responses and in AD (Table 1), including early-onset fAD mutations as well as late-onset AD genetic risk factors76, will help to assess the role of the genetic and environmental AD risk factors in these processes. The molecular targets that are required for firing rate re-normalization will be selected for identification of the mechanisms underlying the lack of firing compensation. Furthermore, it will be critical to identify the necessary and sufficient adaptive mechanisms enabling firing homeostasis. Whether compensation at the level of a particular adaptive mechanism is sufficient to maintain firing stability or a combination of several adaptive mechanisms is required? If spine loss represents a homeostatic response serving to counteract hyperactivity, therapeutic strategies aiming to rescue spine loss would exacerbate hyperactivity and accelerate cognitive decline. Thus, the balance between different levels of compensation and distinct functional outcomes must be addressed.
(2) Impairments of set-point regulation. An alternative hypothetical possibility is that hippocampal hyperactivity relates to elevation in the firing set-point in prodromal AD stages. Theoretically, impairments of set-point regulation represent a special case of homeostatic machinery failure (Fig. 2d). This type of error does not represent incapability to compensate. Rather, it relates to a systematic deviation from the physiological boundaries that enable optimal functioning of the system. Chronic homeostatic disorders may result from locking the system in a stable pathological state. As a result, all the compensatory mechanisms start acting in reference to this pathological set-point value, being detrimental for circuit’s functioning. Notably, therapeutic approaches at the level of homeostatic effectors might be ultimately ineffective when the system is trying to actively re-establish a pathological steady-state value of output.
Impairments in firing set-point regulation may explain why hyperactivity is not compensated by diverse homeostatic mechanisms. Surprisingly, our understanding of firing set-point regulation is still rudimentary. A possible candidate is the mechanistic target of rapamycin (mTOR) pathway that has emerged as a critical integrator of neuronal activity and synaptic inputs that in turn regulate many cell biological processes77. Thus, it is not surprising that mTOR is implicated in a myriad of disorders including autism, epilepsy and AD78. Importantly, dysregulation of mTOR pathway increases the excitation-to-inhibition ratio, leading to hippocampal hyperexcitability79 (see Table 1). Remarkably, rapamycin treatment slowed aging in mice80, reduced seizure frequency and enhanced survival in a mouse model of tuberous sclerosis complex81 and improved cognitive impairments in AD mouse model82. It remains to be determined whether an increase in firing set-point contributes to hyperactivity in early AD stages. Assuming that compensatory responses and set-points are separately controlled, two conditions must be met for identifying bona fide machinery underlying set-point establishment (Fig. 2d): (1) inhibition or knockdown of the key set-point machinery should cause a stable change in the controlled variable such as mean firing rate or firing synchrony without inducing a compensatory response; (2) known activity perturbations that induce firing renormalization under control conditions are not impaired following modulation of set-point. Discovering the mechanisms that regulate firing set-points in specific neural circuits may open a new therapeutic possibility for AD and other disorders characterized by aberrant neuronal activity.
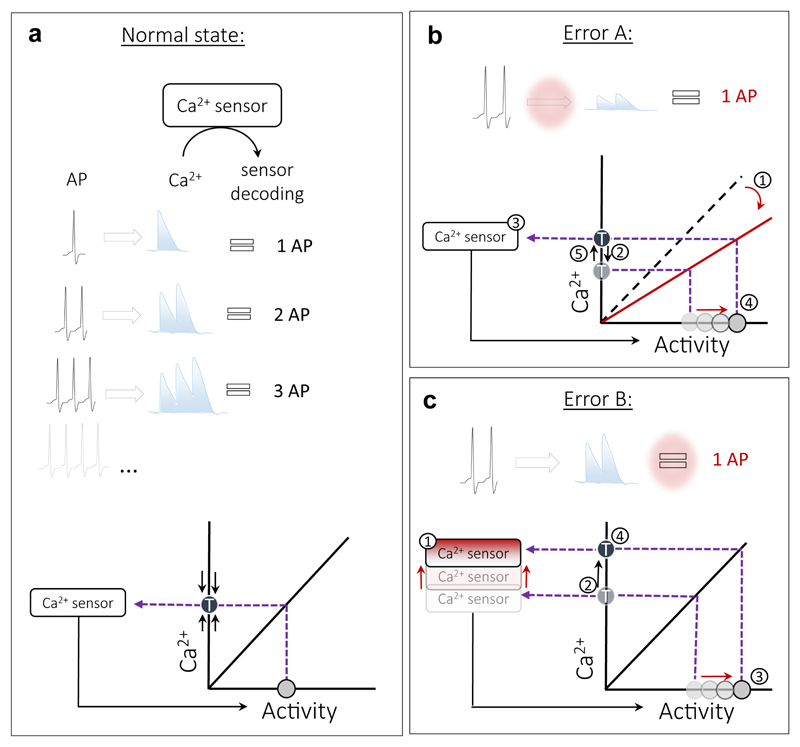
(3) Impairments of sensors, detecting deviation from a set-point. Understanding the mechanism by which sensors are activated is a fundamental open question in the field. Previous studies proposed that spiking activity may be translated to changes in the intracellular Ca2+ levels which are controlled by a putative Ca2+ sensor. CaMK4 has been proposed to sense Ca2+ and trigger postsynaptic scaling in a cell-autonomous manner83, 84. Ca2+ sensor sensitivity and subsequent changes in the steady-state levels of transcriptional complexes have been suggested to induce changes in cell-autonomous regulation of firing set-point65. However, very little is known about the mechanisms that govern this regulation and how they may lead to pathology. Moreover, the sensors that enable firing homeostasis at the level of the population remain unknown. As biological sensors are assumed to use a proxy to measure the controlled variable, Ca2+ sensors may translate spiking activity to the downstream effectors that enable firing homeostasis under physiological conditions (Fig. 3a). Pathological states may be caused by activation of a sensor by incorrect information. Such incidents can occur if the sensed factor is partially decoupled from the controlled variable. For example, cytosolic Ca2+ levels can become partially decoupled from firing rates if Ca2+ homeostasis is impaired or Ca2+ levels exceed the dynamic range of Ca2+ sensors (Fig. 3b). While dysregulation of Ca2+ homeostasis is a prominent feature of AD85, how it affects the coupling of Ca2+ to spiking activity has not been addressed. Another possibility is that the sensor itself develops a malfunction (Fig. 3c), in which case its activity level can be specifically targeted to restore homeostasis. In addition to Ca2+ sensors, these dysfunctions are also applicable for other types of sensors such as metabolic sensors, the sensors that govern protein quality control and immune responses. Sensors impairments may underlie reduction in the threshold to seizures observed in different types of AD model mice and increase in incidence of seizures in AD patients16.
Figure 3. Decoupling of Ca2+ sensors from spiking activity / stability.
(a) Coupling of spiking activity to Ca2+ sensor under physiological conditions. Top: Spiking activity produces changes in a sensed factor (Ca+2 for example). A sensor detects changes in Ca+2 and ‘translates’ spike-evoked Ca+2 transients to downstream effectors that then regulate spiking activity according to this information. Bottom: An example of a linear transfer function linking spiking activity to Ca+2 levels. The sensor corrects any deviation from the target Ca+2 level (T) by adjusting spiking activity. (b) In a pathological setting, the same spiking activity may produce less Ca2+, changing the slope of a transfer function. Ca2+ levels drop (1) even though spiking levels remain the same (2). The activated sensor (3) elevates spiking activity (4) in order to maintain the target Ca2+ levels (5), leading to a new hyperactive steady-state. (c) In another pathological setting, the sensitivity of the Ca+2 sensor to Ca2+ is reduced (1), shifting the target Ca2+ levels upwards (2). Excessive spiking activity is then produced (3) to maintain the new higher target Ca2+ levels (4).
To determine whether sensors or sensed factors are decoupled from the controlled variable, two parameters should be measured in wild-type versus AD models: (i) the dynamic range of a putative sensor; (ii) the transfer function between the changes in the sensed factor and the output which is under homeostatic control, such as mean firing rate. As highly sensitive Ca2+ indicators and other signaling molecules targeted to specific compartments are now widely available, evaluating the coupling between these moieties and a homeostatic function may provide better understanding of the mechanisms underlying AD-related impairments of sensors’ activity.
Disruption of stability – plasticity balance in early AD as a possible path to pathology
The early clinical AD stages are characterized by pure memory deficits that can be caused by the primary impairments of synaptic plasticity (with secondary compensatory problems) or by the primary failures in firing homeostasis (with secondary plasticity dysfunctions). Recent data in fAD mouse models led to inconclusive results regarding the temporal sequence of events15. It is still not clear whether synaptic plasticity abnormalities precede, coincide or follow the changes in the basal synaptic and intrinsic membrane properties that shape ongoing spiking activity. Our study using pharmacological inhibition of Aβ degradation via neprilysin may provide some clues on the sequence of pathophysiological events. Acute inhibition of neprilysin in wild-type, but not in APP lacking neurons, lead to a mild, ~50% increase in the extracellular Aβ levels, resulting in an increase of glutamate release probability, of the E/I ratio and spontaneous firing rate10. However, chronic (48 hr) neprilysin inhibition caused a reduction in the number of functional synapses10 and in the LTP magnitude (Abramov and Slutsky, unpublished data). Based on these results, we proposed that an increase in ongoing neuronal activity might represent a basic feature of the early pathological phase that leads to a compensatory synapse weakening, elimination and plasticity deficits at the later AD stages.
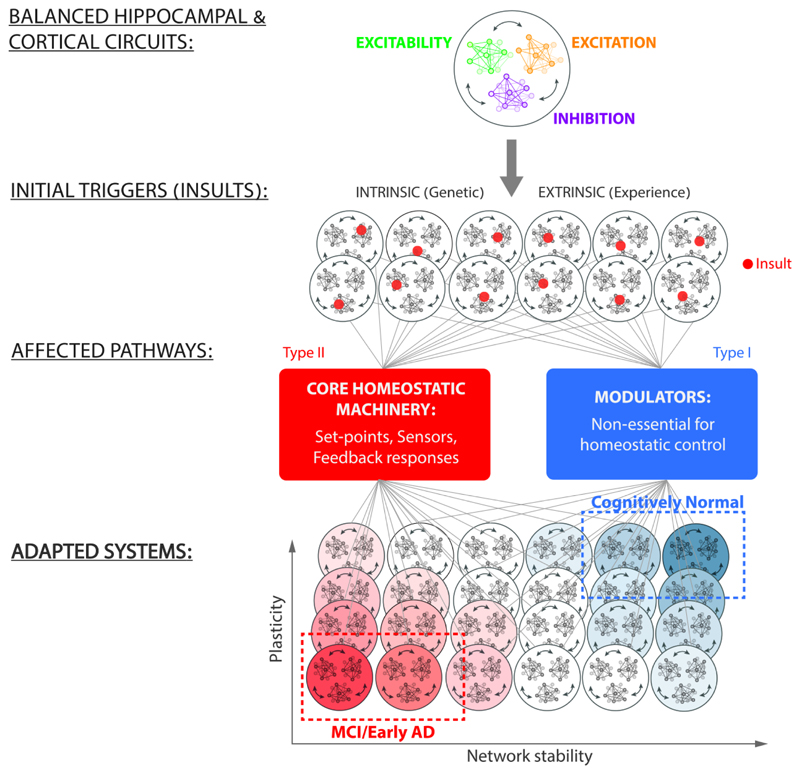
According to the FHP hypothesis, a large number of diverse insults, either intrinsic or extrinsic, may disturb the components of homeostatic regulatory system and plasticity mechanisms (Fig. 4). While very important pieces of information regarding the early AD phase are still missing, the effects these insults produce on homeostatic regulation may be categorized into two main types of impairments, depending on the kind of insult, genetic background and life experience. One – that does not target essential components of homeostatic control - induces a wide spectrum of adaptive solutions that enable firing stabilization and preserve cognitive functions. The second type of impairments targets the core homeostatic machinery at the level of sensors, effectors or set-point that are essential for firing homeostasis66. This type of deficits induces maladaptive solutions that diminish homeostatic capacity of the system, leading to AD-related cognitive impairments. Within the spectrum of early AD states, a fraction of patients may show no obvious changes in rates and patterns of ongoing spikes, but display plasticity-related memory problems due to a limited solution space (in comparison to a large number of adaptive solutions available in cognitively normal individuals). In these cases, reduced homeostatic capacity may result in fragile synaptic plasticity. Thus, plasticity impairments and excessive synaptic elimination at the early disease stages may represent a trade-off, resulting from the system's efforts to maintain firing stability44. On the other hand, another fraction of early AD patients may display primary dysfunctions at the level of the core homeostatic machinery, leading to ‘silent’ epileptiform spikes and seizures and subsequent cognitive decline.
Figure 4. FHP hypothesis: possible transitions from normal to early AD states.
A fully functional homeostatic controller enables a balance between excitatory synaptic drive (excitation), inhibitory synaptic drive (inhibition) and intrinsic excitability. Genetic, pharmacological and experience-dependent life events can trigger malfunction at a particular node (red dot) in the network, affecting firing stability. Depending on the initial state of the regulatory system and the type of insult inflicted, a subset of solutions become maladaptive, resulting in cognitive impairments at the early AD stages, while the majority retain normal cognitive function. According to the FHP hypothesis, the insults that impair the core homeostatic machinery reduce the homeostatic capacity of the network and lead to a spectrum of maladaptive responses, resulting in early AD. Within the AD subset of solutions, not all have the same functional features. Some might manifest it hyperactivity, while others might lead to impaired plasticity, and these dysfunctions may extensively overlap. On the other side of the spectrum are insults affecting mechanisms that are non-essential for homeostatic response. These lead to a spectrum of adaptive solutions that enable functional re-normalization and preserve cognitive function.
What might be putative cellular malfunctions that mediate imbalance between firing stability and synaptic plasticity? Interestingly, fAD mutations in the PSEN1, the catalytic subunit of γ-secretase86, regulate not only LTP87, but also neurogenesis88 and homeostatic scaling89. Moreover, conditional PSEN1 deletion in the CA3 hippocampal area leads to impairments in neurotransmission, short-term synaptic facilitation and LTP90. As PSEN1 mutations increase the incidence of epilepsy in AD patients91, this enzyme may represent the key candidate for stability - plasticity imbalance in the rare, early-onset fAD cases. Another potential candidate that may be involved in firing dysregulation in the most common, sporadic AD form is mTOR, which is hyperactivated in AD92. Notably, mTOR is known to regulate presynaptic homeostatic adaptations93, E/I ratio and spontaneous firing rate79, protein-synthesis dependent long-term plasticity and hippocampus-dependent learning and memory functions94. These are but a few examples of mechanisms that may cause stability - plasticity imbalance underlying memory impairments in AD.
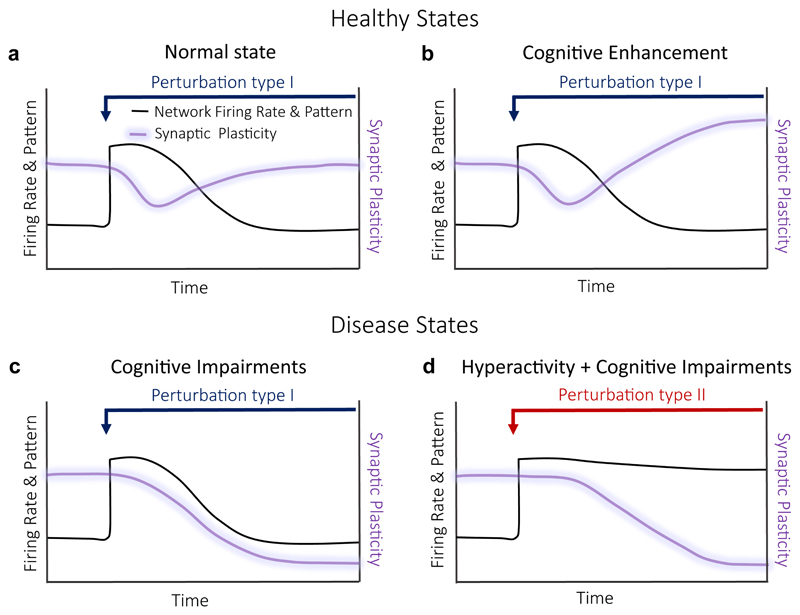
It is important to take into a consideration a wide spectrum of adaptive and maladaptive solutions that may be induced in response to distinct types of perturbations. Circuits that are capable of maintaining firing stability and synaptic plasticity remain in a healthy state (Fig. 5a). Moreover, in some cases, circuits may achieve firing stability through adaptive mechanisms that enhance synaptic plasticity. This may even lead to cognitive enhancement (Fig. 5b). Conversely, in other cases, circuits may compromise synaptic plasticity in order to maintain firing stability (Fig. 5c). Such a trade-off between plasticity and stability may be the earliest hallmark of AD. An alternative track towards memory impairment is characterized by the failure at both fronts: firing stability and plasticity (Fig. 5d). As patients with hyperactivity have been shown to undergo faster cognitive decline20, it would be important to explore if the loss of plasticity and stability together increases the chance for MCI-to-AD transitions. Taken as a whole, the FHP hypothesis suggests that the early AD phase may represent the “price” for a successful effort or the result of a failed attempt to maintain firing stability.
Figure 5. Balance of firing stability – synaptic plasticity and its disruption in early AD stages.
(a) Normal healthy state: A perturbation type I results in a transient increase in firing rates (black line) with concomitant reduction in synaptic plasticity (purple line). The adaptive mechanisms induced by a perturbation result in re-normalization of both, firing rates and synaptic plasticity. (b) Cognitive enhancement: Adaptive mechanisms induced by a perturbation type I to re-normalize firing rates, increase some types of synaptic plasticity. An example: a decrease in release probability in response to hyperactivity, resulting in increase of synaptic facilitation. (c) Cognitive impairments: Adaptive mechanisms to perturbation type I cause reduction in synaptic plasticity as the price for firing stability. (d) Cognitive impairments: Adaptive mechanisms to perturbation type II cause hyperactivity with subsequent reduction in synaptic plasticity.
Future Challenges
While current experimental evidence based on electrophysiological and imaging studies in human and AD mouse models supports the core idea behind the FHP hypothesis, direct experimental proof is needed. Exciting discoveries on the role of stability – plasticity imbalance in early AD development are ahead of us. Many basic questions still remain unresolved: How properties of single synapses shape the behavior of neural networks and vice versa at long timescales? What are the building blocks of the core homeostatic machinery? How do they interact with memory-related plasticity mechanisms? Do fAD mutations induce dysfunctions in the core homeostatic machinery? Answering these open questions may pave a new road for understanding the principle basis of the early-phase AD in the next decade.
Acknowledgements
We thank Dr. Yuval Nir and I.S. lab members for thoughtful comments on the manuscript. This work was supported by research grants to I.S from the European Research Council starting (281403) and consolidator (724866) grants, the Legacy Heritage Biomedical Program of the Israel Science Foundation (1849/17), the Israel Science Foundation (398/13) and the Binational Science Foundation (2013244). I.S. is grateful to Sheila and Denis Cohen Charitable Trust and Rosetrees Trust of the UK for their support.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Molecular Medicine. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small SA, Duff K. Linking Aβ and Tau in Late-Onset Alzheimer's Disease: A Dual Pathway Hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 5.De Strooper B, Karran E. The Cellular Phase of Alzheimer’s Disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 6.Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 7.Mucke L, Selkoe DJ. Neurotoxicity of Amyloid-beta Protein: Synaptic and Network Dysfunction. Cold Spring Harbor Perspectives in Medicine. 2012 doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankar GM, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008 doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62 doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramov E, et al. Amyloid-[beta] as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 11.Muller UC, Zheng H. Physiological Functions of APP Family Proteins. Cold Spring Harbor Perspectives in Medicine. 2012;2 doi: 10.1101/cshperspect.a006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willem M, et al. η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526:443. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel H, et al. APP Homodimers Transduce an Amyloid-β-Mediated Increase in Release Probability at Excitatory Synapses. Cell Reports. 2014;7:1560–1576. doi: 10.1016/j.celrep.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, et al. Human brain-derived Aβ oligomers bind to synapses and disrupt synaptic activity in a manner that requires APP. The Journal of Neuroscience. 2017 doi: 10.1523/JNEUROSCI.2009-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchetti C, Marie H. Hippocampal synaptic plasticity in Alzheimer’s disease: what have we learned so far from transgenic models? Reviews in the Neurosciences. 2011;373 doi: 10.1515/RNS.2011.035. [DOI] [PubMed] [Google Scholar]
- 16.Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17:777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong J. EEG dynamics in patients with Alzheimer's disease. Clinical Neurophysiology. 2004;115:1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Vossel KA, Beagle AJ, Rabinovici GD, et al. SEizures and epileptiform activity in the early stages of alzheimer disease. JAMA Neurology. 2013:1–9. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cretin B, et al. Epileptic Prodromal Alzheimer's Disease, a Retrospective Study of 13 New Cases: Expanding the Spectrum of Alzheimer's Disease to an Epileptic Variant? Journal of Alzheimer's disease : JAD. 2016;52:1125–1133. doi: 10.3233/JAD-150096. [DOI] [PubMed] [Google Scholar]
- 20.Vossel KA, et al. Incidence and impact of subclinical epileptiform activity in Alzheimer's disease. Annals of Neurology. 2016;80:858–870. doi: 10.1002/ana.24794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann DMA, Pickering-Brown SM, Takeuchi A, Iwatsubo T, members of the Familial Alzheimer’s Disease Pathology Study, G Amyloid Angiopathy and Variability in Amyloid β Deposition Is Determined by Mutation Position in Presenilin-1-Linked Alzheimer’s Disease. The American Journal of Pathology. 2001;158:2165–2175. doi: 10.1016/s0002-9440(10)64688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moehlmann T, et al. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Aβ42 production. Proceedings of the National Academy of Sciences. 2002;99:8025–8030. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam AD, et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer's disease. Nat Med. 2017;23:678–680. doi: 10.1038/nm.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker A, et al. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mondadori CRA, et al. Enhanced brain activity may precede the diagnosis of Alzheimer's disease by 30 years. Brain : a journal of neurology. 2006;129:2908–2922. doi: 10.1093/brain/awl266. [DOI] [PubMed] [Google Scholar]
- 26.Filippini N, et al. Distinct patterns of brain activity in young carriers of the APOE--µ4 allele. Proceedings of the National Academy of Sciences. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunz L, et al. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer's disease. Science. 2015;350:430–433. doi: 10.1126/science.aac8128. [DOI] [PubMed] [Google Scholar]
- 28.Bookheimer SY, et al. Patterns of Brain Activation in People at Risk for Alzheimer's Disease. New England Journal of Medicine. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verret L, et al. Inhibitory Interneuron Deficit Links Altered Network Activity and Cognitive Dysfunction in Alzheimer Model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minkeviciene R, et al. Amyloid beta-Induced Neuronal Hyperexcitability Triggers Progressive Epilepsy. The Journal of Neuroscience. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busche MA, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 34.Busche MA, et al. Critical role of soluble amyloid-b for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall AM, et al. Tau-Dependent Kv4.2 Depletion and Dendritic Hyperexcitability in a Mouse Model of Alzheimer's Disease. The Journal of Neuroscience. 2015;35:6221–6230. doi: 10.1523/JNEUROSCI.2552-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez PE, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proceedings of the National Academy of Sciences. 2012;109:E2895–E2903. doi: 10.1073/pnas.1121081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardy JD. Control of heat loss and heat production in physiologic temperature regulation. Harvey Lect. 1953;49:242–270. [PubMed] [Google Scholar]
- 38.LeMasson G, Marder E, Abbott L. Activity-dependent regulation of conductances in model neurons. Science. 1993;259:1915–1917. doi: 10.1126/science.8456317. [DOI] [PubMed] [Google Scholar]
- 39.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien RJ, et al. Activity-Dependent Modulation of Synaptic AMPA Receptor Accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 41.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 42.Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 43.Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- 44.Slomowitz E, et al. Interplay between population firing stability and single neuron dynamics in hippocampal networks. Elife. 2015;4 doi: 10.7554/eLife.04378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hengen KB, Lambo ME, Van Hooser SD, Katz DB, Turrigiano GG. Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron. 2013;80:335–342. doi: 10.1016/j.neuron.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hengen Keith B, Torrado Pacheco A, McGregor James N, Van Hooser Stephen D, Turrigiano Gina G. Neuronal Firing Rate Homeostasis Is Inhibited by Sleep and Promoted by Wake. Cell. 2016;165:180–191. doi: 10.1016/j.cell.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keck T, et al. Synaptic scaling and homeostatic plasticity in the mouse visual cortex in vivo. Neuron. 2013;80:327–334. doi: 10.1016/j.neuron.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences. 2001;98:13763–13768. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turrigiano GG. The dialectic of Hebb and homeostasis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372 doi: 10.1098/rstb.2016.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 51.Branco T, Staras K, Darcy KJ, Goda Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron. 2008;59:475–485. doi: 10.1016/j.neuron.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 53.Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 54.Laviv T, et al. Basal GABA Regulates GABA(B)R Conformation and Release Probability at Single Hippocampal Synapses. Neuron. 2010;67:253–267. doi: 10.1016/j.neuron.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 55.Jakawich SK, et al. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu W, et al. Distinct Neuronal Coding Schemes in Memory Revealed by Selective Erasure of Fast Synchronous Synaptic Transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirov SA, Harris KM. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nature Neuroscience. 1999;2:878. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- 58.Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- 59.Kim J, Tsien RW. Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron. 2008;58:925–937. doi: 10.1016/j.neuron.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watt AJ, Desai NS. Homeostatic plasticity and STDP: keeping a neuron's cool in a fluctuating world. Frontiers in Synaptic Neuroscience. 2010;2 doi: 10.3389/fnsyn.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogerson T, et al. Synaptic tagging during memory allocation. Nat Rev Neurosci. 2014;15:157–169. doi: 10.1038/nrn3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vertkin I, et al. GABAB receptor deficiency causes failure of neuronal homeostasis in hippocampal networks. Proc Natl Acad Sci U S A. 2015;112:E3291–3299. doi: 10.1073/pnas.1424810112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 64.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 65.O’Leary T, Williams Alex H, Franci A, Marder E. Cell Types, Network Homeostasis, and Pathological Compensation from a Biologically Plausible Ion Channel Expression Model. Neuron. 2014;82:809–821. doi: 10.1016/j.neuron.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frere S, Slutsky I. Alzheimer’s Disease: From Firing Instability to Homeostasis Network Collapse. Neuron. 2018;97:32–58. doi: 10.1016/j.neuron.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 67.Pratt KG, Zimmerman EC, Cook DG, Sullivan JM. Presenilin 1 regulates homeostatic synaptic scaling through Akt signaling. Nat Neurosci. 2011;14:1112–1114. doi: 10.1038/nn.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu T, et al. REST and stress resistance in ageing and Alzheimer/'s disease. Nature. 2014;507:448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pozzi D, et al. REST/NRSF-mediated intrinsic homeostasis protects neuronal networks from hyperexcitability. The EMBO Journal. 2013;32:2994–3007. doi: 10.1038/emboj.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pecoraro-Bisogni F, et al. REST-Dependent Presynaptic Homeostasis Induced by Chronic Neuronal Hyperactivity. Molecular Neurobiology. 2017 doi: 10.1007/s12035-017-0698-9. [DOI] [PubMed] [Google Scholar]
- 71.Mihalas AB, Araki Y, Huganir RL, Meffert MK. Opposing Action of Nuclear Factor κB and Polo-like Kinases Determines a Homeostatic End Point for Excitatory Synaptic Adaptation. The Journal of Neuroscience. 2013;33:16490–16501. doi: 10.1523/JNEUROSCI.2131-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilbert J, et al. β-Amyloid triggers aberrant over-scaling of homeostatic synaptic plasticity. Acta Neuropathologica Communications. 2016;4:131. doi: 10.1186/s40478-016-0398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guntupalli S, et al. GluA1 Ubiquitination Mediates Amyloid-β-induced Loss of Surface AMPA Receptors. Journal of Biological Chemistry. 2017 doi: 10.1074/jbc.M116.774554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 76.Karch CM, Goate AM. Alzheimer’s Disease Risk Genes and Mechanisms of Disease Pathogenesis. Biological psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costa-Mattioli M, Monteggia LM. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci. 2013;16:1537–1543. doi: 10.1038/nn.3546. [DOI] [PubMed] [Google Scholar]
- 78.Lipton Jonathan O, Sahin M. The Neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bateup Helen S, et al. Excitatory/Inhibitory Synaptic Imbalance Leads to Hippocampal Hyperexcitability in Mouse Models of Tuberous Sclerosis. Neuron. 2013;78:510–522. doi: 10.1016/j.neuron.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilkinson JE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeng L-H, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Annals of Neurology. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular Interplay between Mammalian Target of Rapamycin (mTOR), Amyloid-β, and Tau: EFFECTS ON COGNITIVE IMPAIRMENTS. Journal of Biological Chemistry. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ibata K, Sun Q, Turrigiano GG. Rapid Synaptic Scaling Induced by Changes in Postsynaptic Firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 84.Goold CP, Nicoll RA. Single-Cell Optogenetic Excitation Drives Homeostatic Synaptic Depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends in Neurosciences. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Strooper B, Iwatsubo T, Wolfe MS. Presenilins and gamma-Secretase: Structure, Function, and Role in Alzheimer Disease. Cold Spring Harb Perspect Med. 2012;2:a006304. doi: 10.1101/cshperspect.a006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Auffret A, Gautheron V, Mattson MP, Mariani J, Rovira C. Progressive Age-Related Impairment of the Late Long-Term Potentiation in Alzheimer’s Disease Presenilin-1 Mutant Knock-in Mice. Journal of Alzheimer's disease : JAD. 2010;19:1021–1033. doi: 10.3233/JAD-2010-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi SH, et al. Non-Cell-Autonomous Effects of Presenilin 1 Variants on Enrichment-Mediated Hippocampal Progenitor Cell Proliferation and Differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pratt KG, Zimmerman EC, Cook DG, Sullivan JM. Presenilin 1 regulates homeostatic synaptic scaling through Akt signaling. Nat Neurosci. 2011;14 doi: 10.1038/nn.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang C, et al. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vossel KA, et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70 doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bové J, Martínez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nature Reviews Neuroscience. 2011;12:437. doi: 10.1038/nrn3068. [DOI] [PubMed] [Google Scholar]
- 93.Henry FE, et al. Retrograde Changes in Presynaptic Function Driven by Dendritic mTORC1. The Journal of Neuroscience. 2012;32:17128–17142. doi: 10.1523/JNEUROSCI.2149-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoeffer CA, Klann E. mTOR Signaling: At the Crossroads of Plasticity, Memory, and Disease. Trends in neurosciences. 2010;33:67. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiener N. Cybernetics or Control and Communication in the Animal and the Machine. Paris: (Hermann & Cie) & Camb. Mass. (MIT Press); 1948. ISBN 978-970-262-73009-73009. [Google Scholar]
- 96.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 97.Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu G, Tsien RW. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995;375:404–408. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- 99.Ziv Y, et al. Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liberti WA, Iii, et al. Unstable neurons underlie a stable learned behavior. Nat Neurosci. 2016;19:1665–1671. doi: 10.1038/nn.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]