Abstract
Context
18F-FDOPA PET/CT accurately localizes pheochromocytoma in patients with an established biochemical diagnosis. However, cut-off 18F-FDOPA levels of standardized uptake values (SUVmax) for both normal adrenal glands and pheochromocytoma are lacking.
Objective
Objectives of this study were to determine (1) reference maximum standardized uptake values (SUVmax) for normal adrenal 18F-DOPA tracer uptake and (2) the optimal diagnostic approach for pheochromocytoma localization by using 18F-DOPA SUVmax across a series of cut-off points: the affected adrenal gland (inter-individual analysis), the difference in SUVmax between the affected adrenal gland and the contralateral normal adrenal gland (intra-individual analysis), or a combination of these two.
Patients and methods
All patients with histologically confirmed pheochromocytoma diagnosed at our center between November 2009 and December 2017 were retrospectively analysed. Only those patients who underwent an 18F-FDOPA PET/CT-scan for localization purposes before adrenalectomy were included for further analysis. The control group consisted of patients who underwent 18F-FDOPA PET/CT for other indications and who had no genetic susceptibility for developing a pheochromocytoma. SUVmax of the volume of interest surrounding the adrenal glands was determined on EARL reconstructed images. Receiver operating characteristic (ROC) analysis was performed for adrenal gland SUVmax and intra-individual difference in SUVmax between affected and normal adrenal gland. In addition, binary logistic regression was performed for ROC analysis of the combined parameters.
Results
In total, 47 histologically confirmed pheochromocytomas were diagnosed in 45 patients, and 245 disease control patients were identified. In the control group, no statistical differences between the SUVmax of left and right adrenal glands were observed, and uptake values in both adrenal glands correlated significantly with each other (r = 0.865, p < 0.001). Median (range) adrenal gland SUVmax in pheochromocytomas and in the control group was 12 (2.6–50) and 2.9 (1.1–6.6), respectively (p < 0.001). ROC analysis revealed 93% sensitivity and 85% specificity at an SUVmax cut-off value of 4.1 (area under the curve (AUC) = 0.951), and 93% sensitivity and 96% specificity at an intra-individual SUVmax difference between the affected and normal adrenal gland of 1.0 (AUC = 0.992). The combination of both variables increased the AUC to 0.995.
Conclusions
18F-FDOPA PET/CT distinguishes pheochromocytoma from normal adrenal glands with the highest diagnostic accuracy when combining the SUVmax of the affected adrenal gland with the difference in SUVmax between affected and normal adrenal gland.
Keywords: 18F-FDOPA, Pheochromocytoma, Adrenal glands, Normative data
Introduction
Pheochromocytoma are rare neuroendocrine tumours originating from chromaffin tissue in the adrenal medulla, which demonstrate hypersecretion of catecholamines [1, 2]. Patients with pheochromocytoma characteristically have episodes of increased plasma catecholamine levels, which cause symptoms such as headaches, palpitations, anxiety and diaphoresis. Furthermore, paroxysmal or persistent hypertension, may occur [1]. The introduction of 6-[18F]-L-fluoro-L-3, 4-dihydroxyphenylalanine (18F-FDOPA) positron emission tomography with complementary computed tomography (PET/CT) provided a new approach of detecting and visualizing neuroendocrine tumours, compared to metaiodobenzylguanidine scintigraphy [3, 4]. 18F-FDOPA, in structure similar to L-DOPA, is the precursor of catecholamines [5]. It enters neuroendocrine cells by the large amino acid transporter (LAT1/CD98) and is subsequently converted by aromatic L-amino acid decarboxylase in 18F-fluorodopamine and 18F-fluoronorepinephrine [5–7]. Several studies have demonstrated that 18F-FDOPA PET/CT is well suited for the localization and visualization of pheochromocytoma [3, 7, 8]. However, differentiation between pheochromocytoma and normal adrenal gland can be difficult, since there is a large variability in physiological 18F-FDOPA uptake by normal adrenal gland tissue. In addition, (non-secreting) pheochromocytoma may show only faint 18F-FDOPA accumulation [9].
Although European Association of Research for Life (EARL) based standardized uptake value (SUV) determinations is common practice in 18F labelled flourodesoxyglucose imaging, studies using EARL-based SUV measurements in 18F-FDOPA imaging are rather scarce. These EARL are considered helpful in comparing SUV measurements between different centres, both in clinical settings and in multicentre trials. As of yet, cut-off values for physiological 18F-FDOPA uptake in adrenal glands have not been established. In addition, EARL-based SUV measurements of 18F-FDOPA accumulation in neither normal adrenal glands nor pheochromocytoma have been defined.
Therefore, the aim of this study was to describe reference ranges for 18F-FDOPA uptake by normal adrenal glands, and to establish EARL-based cut-off values for 18F-FDOPA uptake which can be used to reliably discriminate between a pheochromocytoma and normal adrenal gland tissue.
Subjects and methods
All patients who received a histological diagnosis of pheochromocytoma in our hospital between November 2009 and December 2017 were included in this retrospective study. 18F-FDOPA-PET/CT scans were acquired in all patients. In pheochromocytoma patients, only the 18F-FDOPA-PET/CT scan for localization purposes before adrenalectomy and for possible metastases was used for this retrospective analysis. In disease control subjects who underwent multiple 18F-FDOPA PET/CT scans, only the first 18F-FDOPA PET/CT scan was analysed.
The disease control group consisted of patients who underwent 18F-FDOPA PET/CT for other indications (including neuroendocrine (carcinoid) tumours, medullary thyroid carcinoma, hyperinsulinaemia, and pancreatic isletcell tumours). Disease control subjects were not actively screened for germline mutations, but excluded from this group in case of known genetic susceptibility for developing a pheochromocytoma. These subjects were retrieved from a previously collected database of patients who underwent 18F-FDOPA PET/CT scanning in our centre [10, 11]. This database contained 338 patients with two adrenal glands, who had received carbidopa pre-treatment before 18F-FDOPA PET/CT acquisition. Patients scanned because of the biochemical suspicion of harbouring a pheochromocytoma but in whom no pheochromocytoma was detected (n = 90), as well as carriers of germ-line mutations predisposing for the development of pheochromocytoma (n = 3), were excluded from the database, resulting in 245 patients constituting the disease control group. As previously reported, steal phenomenon was considered non-existing [11].
Additional laboratory test results of the biochemical analysis (i.e. plasma (nor)metanephrine levels) were retrieved from the electronic patient charts.
18F-FDOPA PET/CT scanning and analysis
All patients were pre-treated with carbidopa, and received a median dose of 150 mg (range 75–150 according to body weight). In all patients, a low dose CT scan was performed for attenuation correction and anatomical localization. All 18F-FDOPA PET/CT images were acquired from top of the skull through mid-thigh 60 ± 6 min after intravenous administration of a standard dose of 200 MBq 18F-FDOPA on a Biograph mCT camera (Siemens Medical Systems, Knoxville, TN, USA). All patients fasted for 6 h and were allowed to continue all medication. Acquisition was performed in seven bed positions of 2 min emission time for patients between 60 and 90 kg. Patients with a body weight less than 60 kg and more than 90 kg body weight were scanned with 1 min and 3 min per bed position, respectively. Raw data were reconstructed through ultra high definition (Siemens) and according to guideline-based standardized EARL algorithms [12, 13] for SUV calculations, respectively [10]. Primary interpretation during clinical assessment was not taken into account for the purpose of this retrospective study. The adrenal uptake of 18F-FDOPA was measured using manually drawn spherical volumes of interests (VOIs) on EARL reconstructed PET images, to analyze tracer uptake in the left and right adrenal gland, using Siemens Syngo.via software version VB10. Uptake was expressed as maximum standardized uptake values (SUVmax). Adrenal lesions were excluded from further analysis if, for example, the liver masked the adrenal uptake values. Image analysis was performed by MS, supervised by experienced nuclear medicine physicians (WN and AG), with 10 and 15 years’ experience in reading 18F-FDOPA PET/CT scans, respectively. Readers were not blinded for clinical history or previous imaging findings.
Laboratory analysis
Plasma free metanephrine levels were determined using high-pressure liquid chromatography tandem mass spectrometry (LC-MS/MS) with online solid-phase extraction [14]. Laboratory results were determined within 3 months before or 3 months after the 18F-FDOPA PET/CT scan, in our in-house laboratory facilities only.
Statistical analysis
Results are expressed as median and ranges. Only nonparametric tests were used to compare variables between patient groups. Receiver operating characteristic (ROC) analysis was performed for adrenal gland SUVmax, and intra-individual difference in SUVmax between affected and normal adrenal gland. The highest SUVmax of both adrenal glands was used. The patients with bilateral pheochromocytoma were counted twice for inter-individual analysis, and excluded for intra-individual analysis. In addition, binary logistic regression was performed for ROC analysis of the combined parameters. Statistical analysis was performed using the SPSS package version 23 (IBM). A two-sided P value <0.05 was considered significant.
Ethical consideration
According to the Dutch Medical Research Involving Human Subject Act, the local medical ethical committee (i.e. Medical Ethics Review Board of the University Medical Center Groningen, METc UMCG, number 201800952) exempted approval without additional procedures. No additional informed consent was required. Patient information was anonymized before data analysis.
Results
Patient characteristics
Characteristics of the study population are presented in Table 1. In total, 47 histologically confirmed pheochromocytomas were diagnosed in 45 patients. Patients with pheochromocytoma were younger than disease controls, both for the entire population and in females only.
Table 1.
Characteristics of control subjects and patients harbouring a pheochromocytoma
| Characteristic | Controls (N = 245) | Patients with pheochromocytoma (N = 45) | p value | |
|---|---|---|---|---|
| Gender | Female | 132 | 24 | NS |
| Male | 113 | 21 | NS | |
| Age (years) | Median (range) of total population | 61 (20–82) | 50 (11–77) | P = 0.005 |
| Female | 61 (20–81) | 53 (11–77) | P = 0.016 | |
| Male | 60 (20–82) | 55 (27–80) | NS | |
| Plasma Metanephrine (nmol/L) | Median (range) | N/A | 1.2 (0.070–38) | N/A |
| Plasma Normetanephrine (nmol/L) | Median (range) | N/A | 3.0 (0.58–78) | N/A |
Data presented as absolute values, or median (range). NS non-significant, N/A not applicable
Histopathologic analysis after adrenalectomy confirmed the presence of a pheochromocytoma in 45 patients, of which 24 were left sided and 23 were right sided (Fig. 1). Two patients had synchronous bilateral pheochromocytoma. Of these 45 patients, DNA analysis revealed germ-line mutations in the following genes: MEN2A in seven patients, SDHA in four patients, MAX in two patients, and NF-1 in one patient. In three patients, no DNA analysis was performed. In 28 patients, no germ-line mutation could be confirmed.
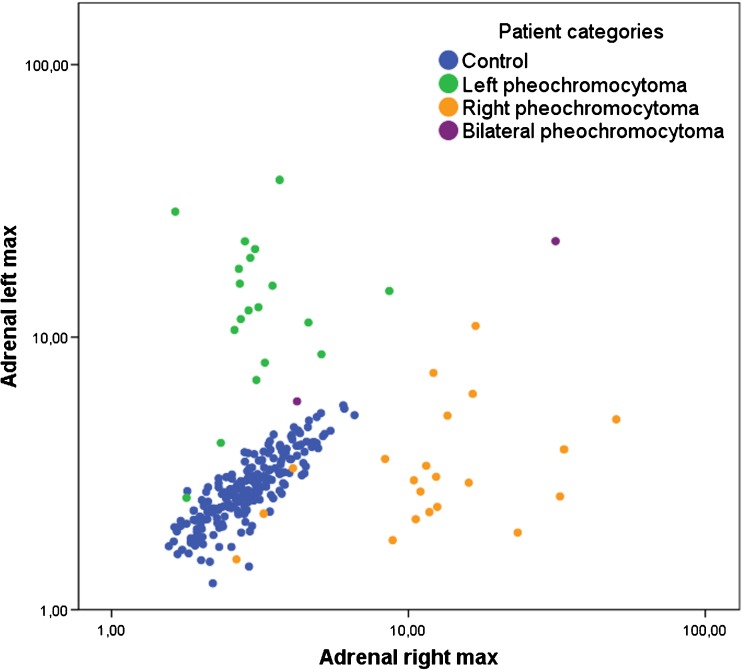
Fig. 1.
Comparison between the maximum standard uptake values (SUVmax) of the right and left adrenal gland for the control group and the patients with pheochromocytoma (logarithmic scale)
18F-FDOPA PET/CT findings
Patients with pheochromocytoma
Mean time between 18F-FDOPA PET/CT scan and adrenalectomy was 2 (0–6) months. Adrenal gland SUVmax of pheochromocytoma was higher than SUVmax of control adrenal glands: 12 (2.6–50) vs 2.9 (1.1–6.6), (p < 0.001). The difference in SUVmax of the pheochromocytoma compared to the contralateral normal adrenal gland was 14 (2.2–50). This difference in SUVmax was statistically different from the SUVmax difference between ipsilateral and contralateral adrenal gland in the control group (p < 0.001). No correlation was found between plasma (nor)metanephrines, and adrenal glands SUVmax.
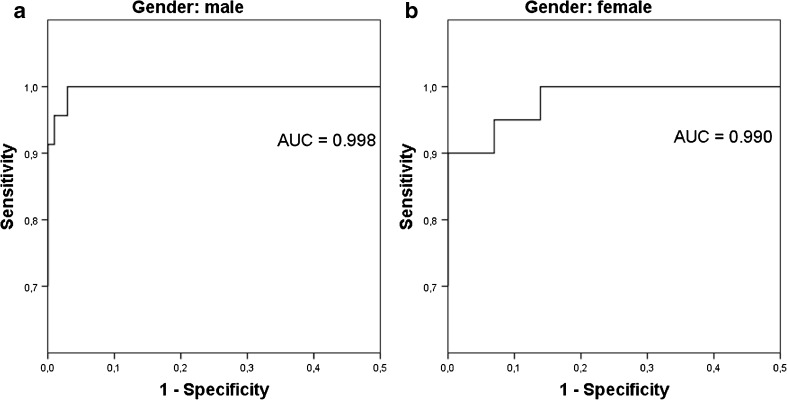
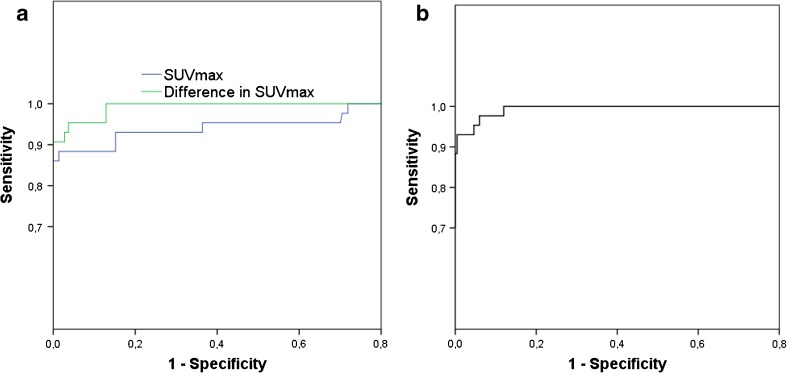
Due to the overlap in SUVmax ranges between pheochromocytoma and disease control patients, receiver operating characteristic (ROC) analysis was used to determine cut-off values. The ROC analysis demonstrated that a cut-off SUVmax of 4.1 resulted in 93% sensitivity and 85% specificity for diagnosing pheochromocytoma, with an area under the curve (AUC) of 0.951 (Fig. 3A). In females a cut-off SUVmax of 4.0 resulted in 90% sensitivity and 80% specificity, whereas in males a cut-off SUVmax of 4.1 showed 96% sensitivity and 89% specificity. In addition, ROC analysis was performed to determine a cut-off value regarding the difference in SUVmax between the pheochromocytoma and the normal contralateral adrenal gland (Fig. 2A). An intra-individual difference in adrenal gland SUVmax of 1.0 resulted in 93% sensitivity and 96% specificity for the diagnosis of pheochromocytoma (AUC = 0.992), and 100% sensitivity and 97% specificity in males only. In females, an intra-individual difference of 0.78 resulted in 95% sensitivity, and 96% specificity. Using binary logistic regression for ROC analysis of the combined parameters increased the AUC to 0.995 (Fig. 2B), and 0.998 and 0.990 for males and females, respectively (Fig. 3).
Fig. 3.
Combined ROC curve for male (A) and female (B) patients
Fig. 2.
ROC curves for both adrenal gland SUVmax > 4.1, and intra-individual difference in adrenal gland SUVmax > 1.0 (A), and combined ROC curve (B)
Disease control group
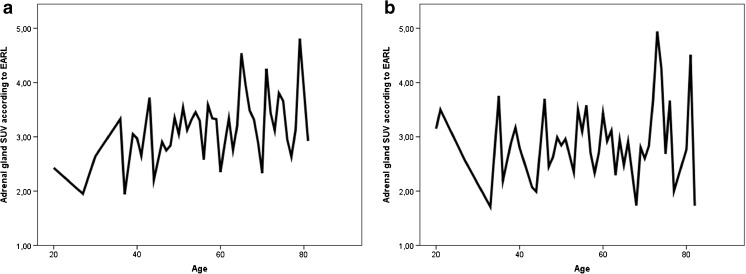
Table 2 summarizes the SUVmax of the disease control group, which are considered normal reference values for future studies. The intra-individual (i.e. left versus right within the same subject) difference in SUVmax between the two adrenal glands was 0.30 (0.010–1.5). Within the whole group of control subjects, SUVmax of either one adrenal gland was significantly correlated to SUVmax of the contralateral adrenal gland (r = 0.86, p < 0.001, Fig. 1). This was consistent in male and female subjects only: r = 0.86 and r = 0.85, respectively, p < 0.001. However, adrenal gland SUVmax was higher in women than in men: 3.1 (1.8–6.6) and 2.8 (1.1–6.1), respectively (p < 0.001). In addition, only in women a statistical significant correlation between SUVmax and increase in age was found (p = 0.024) (Fig. 4).
Table 2.
Comparison of 18F-FDOPA standardized uptake values (SUVmax) in left and right adrenal glands in control groups, with p values
| Measure | Controls | p value | ||
|---|---|---|---|---|
| Total (N = 245) | Males (N = 113) | Females (N = 132) | ||
| Left SUVmax | 2.8 (1.1–5.6) | 2.6 (1.1–5.5) | 3.0 (1.5–5.6) | p < 0.001 |
| Right SUVmax | 2.9 (1.6–6.6) | 2.7 (1.6–6.1) | 3.0 (1.6–6.6) | p < 0.001 |
| Intra-individual difference | 0.30 (0.010–1.5) | 0.30 (0.020–1.5) | 0.30 (0.010–1.4) | NS |
| Correlation coefficient (Spearman’s rho) | 0.86 | 0.85 | 0.86 | p < 0.001 |
Fig. 4.
Line plots of mean standard uptake values (SUVmax) in disease controls (A females, B males) per patient’s age, showing an age increase in SUVmax with increase in age in females only
Discussion
This is the first study to compare EARL based 18F-FDOPA accumulation in a large group of histologically confirmed pheochromocytoma to a large cohort of disease control patients, resulting in reference values for future studies. Based on this retrospective analysis, both inter- and intra-individual adrenal SUV’s on 18F-FDOPA PET/CT show very high test characteristics in distinguishing pheochromocytoma from normal adrenal glands, with an intra-individual difference in SUV of 1.0 showing the highest AUC. Furthermore, it appears that males and females show different normal adrenal gland SUVs, allowing different inter- and intra-individual cut-off values for identifying pheochromocytoma.
Correct localization of pheochromocytoma is of clinical importance, since adrenalectomy is the only curative treatment. For that purpose, reference values for normal 18F-FDOPA uptake are pivotal in accurately discriminating normal adrenal glands from pheochromocytoma. Several reports have shown that test characteristics of 18F-FDOPA PET/CT, 18F-FDOPA PET alone, and iodine-123 labelled meta-iodobenzylguanidine scintigraphy are not significantly different in the identification of pheochromocytoma [15, 16]. Previous studies reported high test characteristics for 18F-FDOPA PET/CT in the identification of pheochromocytoma, with 84–100% sensitivity and 88–100% specificity [16–18]. However, normal values of 18F-FDOPA uptake have not yet been retrieved from large groups. The most recent study that tried to provide more insight in both normal adrenal gland and pheochromocytoma 18F-FDOPA accumulations, retrospectively analysed 112 18F-FDOPA PET scans [17]. The authors re-assessed 212 adrenal glands, of which 17 were pheochromocytoma (with histology confirmation in six cases). However, neither EARL-based 18F-FDOPA cut-off SUVmax for pheochromocytoma, nor EARL-based normal adrenal gland 18F-FDOPA SUVmax have been reported for males and females, thus far.
Currently, the main role of 18F-FDOPA PET/CT in pheochromocytoma is in localization of the tumour. Future prospective studies are needed to further explore the value of the presented 18F-FDOPA cut-off SUV in patients with a biochemical suspicion of a pheochromocytoma. Additionally, in this retrospective study only adrenal glands from patients with pheochromocytoma and disease control patients were re-assessed. As of yet, little is known of SUVmax in adrenal medullary hyperplasia. The value of 18F-FDOPA PET/CT in distinguishing adrenal hyperplasia from normal adrenal tissue has to be investigated in future studies.
Limitations
In addition to the retrospective character of this study, potentially resulting in selection bias, there are some limitations of this study. Although this study contains a rather large group of pheochromocytoma patients, and a large group of disease control subjects, no power analysis has been performed to solidify the test characteristics.
SUVs of other organs than the adrenal glands have not been re-assessed in this study. However, female control subjects showing higher SUVs than male control subjects is in line with findings from a previous study from our group, in which we studied patients with post gastric bypass surgery hypoglcemia [11]. We are not yet aware of any other organ displaying a difference in SUVmax between males and females. As of yet, the clinical relevance of a significant difference between male and female adrenal gland SUVmax, with higher values in women than in men remains to be determined.
Urinary (nor)metanephrine levels were not retrieved from the patient charts. Recently, however, urinary metanephrine levels showed a strong correlation with total lesion uptake in 56 patients with non-metastatic pheochromocytoma [18]. At present it is unclear if the difference of plasma versus urinary (nor)metanephrine levels is sufficient to explain the difference in correlation.
Conclusion
18F-FDOPA PET/CT accurately distinguishes pheochromocytoma from normal adrenal glands, with the highest diagnostic accuracy when combining the SUVmax of the affected adrenal gland with the difference in SUVmax between affected and normal adrenal gland. In addition, in control subjects, women showed higher adrenal gland 18F-FDOPA accumulation than men. Therefore, we propose different cut-off SUVmax for women and men.
Funding
This study was not funded.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Informed consent
According to the Dutch Medical Research Involving Human Subject Act, the local medical ethical committee exempted approval without additional procedures. No additional informed consent was required.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 2.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and Paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–1942. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 3.Hoegerle S, Nitzsche E, Altehoefer C, Ghanem N, Manz T, Brink I, et al. Pheochromocytomas: detection with 18F DOPA whole body PET--initial results. Radiology. 2002;222:507–512. doi: 10.1148/radiol.2222010622. [DOI] [PubMed] [Google Scholar]
- 4.Pacak K, Eisenhofer G, Carrasquillo JA, Chen CC, Whatley M, Goldstein DS. Diagnostic localization of pheochromocytoma: the coming of age of positron emission tomography. Ann NY Acad Sci. 2002;970:170–176. doi: 10.1111/j.1749-6632.2002.tb04423.x. [DOI] [PubMed] [Google Scholar]
- 5.Chondrogiannis S, Marzola MC, Al-Nahhas A, Venkatanarayana TD, Mazza A, Opocher G, et al. Normal biodistribution pattern and physiologic variants of 18F-DOPA PET imaging. Nucl Med Commun. 2013;34:1141. [DOI] [PMC free article] [PubMed]
- 6.Santhanam P, Taïeb D. Role of 18F-FDOPA PET/CT imaging in endocrinology. Clin Endocrinol. 2014;81:189–798. doi: 10.1111/cen.12566. [DOI] [PubMed] [Google Scholar]
- 7.Koopmans KP, Neels ON, Kema IP, Elsinga PH, Links TP, de Vries EG, et al. Molecular imaging in neuroendocrine tumors: molecular uptake mechanisms and clinical results. Crit Rev Oncol Hematol. 2009;71:199–213. [DOI] [PubMed]
- 8.Imani F, Agopian VG, Auerbach MS, Walter MA, Imani F, Benz MR, et al. 18F-FDOPA PET and PET/CT accurately localize pheochromocytomas. J Nucl Med. 2009;50:513–9. [DOI] [PubMed]
- 9.Moreau A, Giraudet AL, Kryza D, Borson-Chazot F, Bournaud-Salinas C, Mognetti T, et al. FDOPA patterns in adrenal glands: a pictorial essay. Clin Nucl Med. 2017;42:379–382. doi: 10.1097/RLU.0000000000001636. [DOI] [PubMed] [Google Scholar]
- 10.Noordzij W, van Beek AP, Tio RA, Van der Horst-Schrivers AN, de Vries EG, van Ginkel B, et al. Myocardial metastases on 6-[18 F] fluoro-L-DOPA PET/CT: a retrospective analysis of 116 serotonin producing neuroendocrine tumour patients. PLoS One. 2014;14(9):e112278. [DOI] [PMC free article] [PubMed]
- 11.van Beek AP, de Heide LJ, van Ginkel B, Slart RH, Van der Horst-Schrivers AN et al. Pancreatic uptake by 18F-FDOPA PET/CT in patients with hypoglycemia after gastric bypass surgery compared with controls with or without carbidopa pretreatment. Clin Nucl Med. 2017;42:163–8. [DOI] [PubMed]
- 12.Boellaard R, Oyen WJ, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-Centre trials. Eur J Nucl Med Mol Imaging. 2008;35:2320–2333. doi: 10.1007/s00259-008-0874-2. [DOI] [PubMed] [Google Scholar]
- 13.Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour PET imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–2354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jong WHA, Graham KS, Van der Molen JC, Links T, Morris MR, Ross A, et al. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC-tandem mass spectrometry. Clin Chem. 2007;53:1684–1693. doi: 10.1373/clinchem.2007.087114. [DOI] [PubMed] [Google Scholar]
- 15.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94:4757–67. [DOI] [PMC free article] [PubMed]
- 16.Luster M, Karges W, Zeich K, Pauls S, Verburg FA, Dralle H, et al. Clinical value of 18F-fluorodihydroxyphenylalanine positron emission tomography/computed tomography (18F-DOPA PET/CT) for detecting pheochromocytoma. Eur J Nucl Med Mol Imaging. 2010;37:484–93. [DOI] [PubMed]
- 17.Moreau A, Giraudet AL, Kryza D, Borson-Chazot F, Bournaud C, Mognetti T, et al. Quantitative analysis of normal and pathologic adrenal glands with 18F-FDOPA PET/CT: focus on pheochromocytomas. Nucl Med Commun. 2017;38:771–9. [DOI] [PubMed]
- 18.Amodru V, Guerin C, Delcourt S, Romanet P, Loundou A, Viana B, et al. Quantitative 18F-DOPA PET/CT in pheochromocytoma: the relationship between tumor secretion and its biochemical phenotype. Eur J Nucl Med Mol Imaging. 2018;45:278–82. [DOI] [PMC free article] [PubMed]