Abstract
Honey bees learn to associate sugars with odorants in controlled laboratory conditions and during foraging. The memory of these associations can be impaired after exposure to contaminants such as pesticides. The sub-lethal effects of acaricides such as 5-methyl-2-(propan-2-yl)-phenol (thymol) introduced into colonies to control varroa mites are of particular concern to beekeeping, due to detrimental effects of some acaricides on bees. Here we assess whether various odorant/sugar pairs are identically memorized in a differential appetitive olfactory conditioning experiment and whether this learning is affected by thymol exposure. Responses to odorants in retrieval tests varied according to the sugar they were paired with, a property called congruency. Interestingly, congruency was altered by pre-exposure to some thymol concentrations during retrieval tests, although electroantennography recordings showed it left odorant detection intact. This highlights the importance of taking into account subtle effects such as odor/sugar congruency in the study of the effect of pesticides on non-target insects, in addition to the simpler question of memory impairment.
Subject terms: Classical conditioning, Behavioural ecology
Introduction
Among the factors implicated in the decline of honey bees (Apis mellifera, Hymenoptera, Apidae) particularly observed in North America and Europe, chemical agents are in the foreground because of their multiple effects on learning1,2, sensory abilities3 and foraging4,5. Olfactory conditioning is an efficient tool to explore whether the alteration of the neurophysiological processes underlying bee learning and memory6–10 by exposure to pesticides2,3,11 is involved in this decline. This is particularly relevant to the pesticides that interfere with γ-aminobutyric acid (GABA) transmission. Among them the acaricide thymol is widely applied inside hives to control infestation with varroa mites (Varroa destructor, Acari, Mesostigmata) so that any sub-lethal effect of this intra-colony pesticide is of particular concern for beekeeping12–15. We previously reported a loss of specificity of olfactory memory in honey bees following topical application of thymol16. This effect on cognition appears similar to those reported for the GABA ligands fipronil3 or picrotoxin17, and thymol potentiates the GABA response in Drosophila melanogaster18.
In our previous work, bees were trained to associate a single odorant, either 2-hexanol or 1-nonanol, with sucrose as a reward, following classical conditioning procedures16. This work pointed to a thymol effect on specificity of memory, which can be confirmed by using differential conditioning. Indeed, such protocols are specifically designed to evaluate discrimination and generalization of odorants, as animals learn to respond to an odor and to ignore another one19. Moreover, in natura, honey bees encounter a wide variety of floral bouquets and they feed on nectar, which is mainly composed of sucrose but also contains other sugars such as fructose although in a lower proportion20,21. In addition to nectar, fructose is an important food source for bees as it is one of the main sugars in honey20,22–26, which in contrast to nectar does not include sucrose.
As a result, we studied the effect of thymol on differential olfactory conditioning. Bees readily learn to associate floral odors with food, and it has been demonstrated that odor/food associations performed while flying freely to natural or artificial sucrose food sources can be transferred to the restrained conditions, and vice-versa27–30.
In laboratory conditions tethered bees are often trained to associate alcohols such as 1-hexanol or 1-octanol with a sucrose solution. This type of protocol relies on the proboscis extension response (PER): when a sugar solution touches its antennae, the bee reflexively extends its main mouthpart, the proboscis. The PER can be conditioned with Pavlovian conditioning during which bees associate an odorant or conditioned stimuli (CS) in the terminology of Pavlovian conditioning, and a sucrose reward or unconditioned stimulus (US). Following this training, presentation of the odorant alone becomes sufficient to elicit the PER31. Recent reports indicate that the nature of the sugar used as US during training affects the robustness of memory: bees develop little long-term memory when 1-hexanol is associated with fructose32 whereas sucrose and glucose led to higher conditioned PER. Moreover, all odorants are not learnt equally well8,33. This suggests some odor/sugar combinations are easier to memorize than others because bees have experienced some odorant molecules of floral bouquets with a sweet taste in nectar. The perceived “harmonious” combination, a phenomenon called congruency, helps bees to represent the food in its context. In humans for instance, perceived odor-taste congruence influences intensity and pleasantness34,35 as well as memory36,37.
The objective of this study was to determine if the effect of thymol on bee’s memory was generalizable to other olfactory and gustatory stimuli combinations32 and whether it affected congruency, a parameter not previously taken into account in the study of bee memory. We used either sucrose or fructose and 4 odorants (1-hexanol, 2-hexanol, 1-octanol or 2-octanol) with and without thymol treatment. These odorants are present in floral38,39 and bee pheromone compounds40. Fructose could be better associated with the odorants present inside the hive (such as pheromone components 1-hexanol, 1-octanol) than sucrose because it is the main sugar of honey and it is less abundant than sucrose in nectars. From this, it could be envisaged that fructose is congruent with the odorants of the colony and sucrose with floral odorants.
Results
Thymol does not affect odor detection
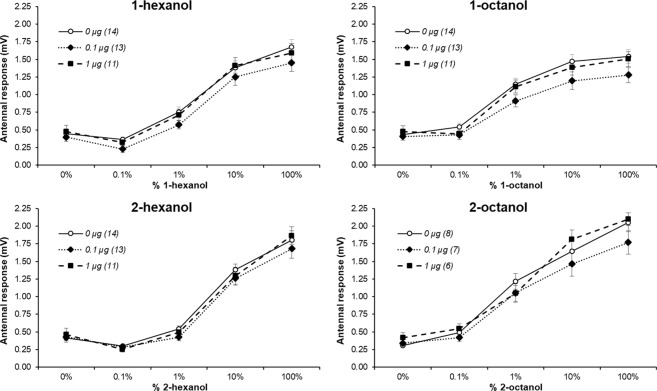
First, as a control experiment, we checked whether thymol affects odorant detection. To do so, 1 µl of a thymol solution dissolved in 1% ethanol was applied topically on the dorsal thorax 3 hours before the recordings (11 bees received 1 µg/µl, 13 bees received 0.1 µg/µl and 14 control bees received 0 µg/µl). We recorded electroantennograms to establish dose-response curves for each of the four odorants used later (1-hexanol, 2-hexanol, 1-octanol and 2-octanol diluted in mineral oil at concentrations of 0%, 0.1%, 1%, 10% and 100%). Electrophysiological recordings of the antennae revealed an expected significant concentration-dependent increase of the response for each of the four odors (Fig. 1, repeated measurement in a mixed model: ps < 0.0001 for the concentration effect in the four models). Conversely, there was no effect of thymol treatment or interaction between thymol treatment and odor concentration (mixed model, ps ≥ 0.175 for the thymol effect and the interaction thymol*concentration in the four models). This indicates that thymol does not affect odorant detection at the antenna level.
Figure 1.
Effect of thymol on the bee olfaction. Electroantennogram responses in mV (mean with standard error) for increasing concentrations of 1-hexanol, 2-hexanol, 1-octanol and 2-octanol diluted in mineral oil after exposure to thymol. Each curve corresponds to a pre-exposure to a thymol concentration; values in parenthesis are the sample size (the same animals were used but some were not tested with 2-octanol).
Performance during training
Other thymol-treated bees were submitted to a differential conditioning protocol during which an odorant was paired with a sugar (CS+) whereas another odorant was not (CS−). This protocol assesses whether animals are able to discriminate between the two odorants. Two pairs of odorants were used: 1-hexanol vs 1-octanol, and 2-hexanol vs 2-octanol (see details of groups in Table 1). The sugar paired with the CS+ was either sucrose or fructose. There were 8 learning trials (4 with CS+ and 4 with CS−) in a pseudo-random sequence (see methods). Table 1 reports the sample size in each treatment combination; within a treatment, they vary between training and retrieval tests as some bees died or became unresponsive to sugar. Training data were analyzed with generalized estimating equations (GEE), using the following factors: trial as a repeated measurement (random factor), thymol treatment, sugar and odor. One analysis was done for CS+, and another one for CS−.
Table 1.
Number of bees of each experimental group.
| Odor (CS+/CS− combinaisons)s | Sugar (US) | Thymol treatment | Bee numbers | ||
|---|---|---|---|---|---|
| Learning | Test 1 h | Test 24 h | |||
|
1-hexanol CS+, 1-octanol CS− |
Fructose | 0 µg | 36 | 36 | 22 |
| Sucrose | 39 | 38 | 24 | ||
|
1-octanol CS+, 1-hexanol CS− |
Fructose | 0 µg | 27 | 27 | 20 |
| Sucrose | 23 | 23 | 18 | ||
|
2-hexanol CS+, 2-octanol CS− |
Fructose | 0 µg | 37 | 37 | 32 |
| Sucrose | 37 | 37 | 36 | ||
|
2-octanol CS+, 2-hexanol CS− |
Fructose | 0 µg | 22 | 22 | 19 |
| Sucrose | 22 | 22 | 19 | ||
|
1-hexanol CS+, 1-octanol CS− |
Fructose | 0.1 µg | 34 | 34 | 21 |
| Sucrose | 41 | 41 | 23 | ||
|
1-octanol CS+, 1-hexanol CS− |
Fructose | 0.1 µg | 25 | 25 | 16 |
| Sucrose | 29 | 28 | 19 | ||
|
2-hexanol CS+, 2-octanol CS− |
Fructose | 0.1 µg | 34 | 34 | 28 |
| Sucrose | 40 | 40 | 35 | ||
|
2-octanol CS+, 2-hexanol CS− |
Fructose | 0.1 µg | 21 | 21 | 14 |
| Sucrose | 22 | 22 | 19 | ||
|
1-hexanol CS+, 1-octanol CS− |
Fructose | 1 µg | 32 | 32 | 21 |
| Sucrose | 42 | 42 | 28 | ||
|
1-octanol CS+, 1-hexanol CS− |
Fructose | 1 µg | 26 | 26 | 21 |
| Sucrose | 30 | 30 | 22 | ||
|
2-hexanol CS+, 2-octanol CS− |
Fructose | 1 µg | 34 | 33 | 31 |
| Sucrose | 32 | 32 | 28 | ||
|
2-octanol CS+, 2-hexanol CS− |
Fructose | 1 µg | 20 | 20 | 19 |
| Sucrose | 20 | 20 | 14 | ||
For each experiment, bees were pre-treated with one dose of thymol (0, 0.1 or 1 µg). Then they were conditioned with one of four odorants (1-hexanol, 1-octanol, 2-hexanol or 2-octanol; CS+), paired to either fructose or sucrose as unconditioned stimulus (US). During learning, another odor was used as CS− (see methods). Some bees were lost during the 1 h or 24 h test because they stopped responding to sugar or died.
During training (Fig. 2) the rate of PER performance by bees increased in response to the CS+ (i.e. the trained odor) during the four trials (GEE, trial effect, p < 0.0001) whereas the PER rate in response to the CS− was constant or even slightly decreasing (GEE, trial effect, p = 0.091). This indicates animals learnt to specifically recognize the odorant associated with sugar. For CS+, neither thymol treatment, sugar used, odorant employed nor interactions between these factors or with the trial factor affected the PER rate (GEE: p = 0.059 for 2-hexanol compared to 1-hexanol, p = 0.096 for 2-octanol compared to 1-hexanol, ps ≥ 0.132 for all other odor comparisons, factors thymol and sugar, and all interactions). For CS−, these effects were not significant either (GEE: p = 0.088 for the interaction 2-octanol*thymol 0.1 µg, p = 0.059 for the interaction 2-octanol*thymol 1 µg, ps ≥ 0.102 for all other interactions and for the factors odor, thymol and sugar). As there was no significant interaction between sugar and odorant factors we concluded that there was no difference in congruency between the odorants relatively to the two sugars during olfactory conditioning.
Figure 2.
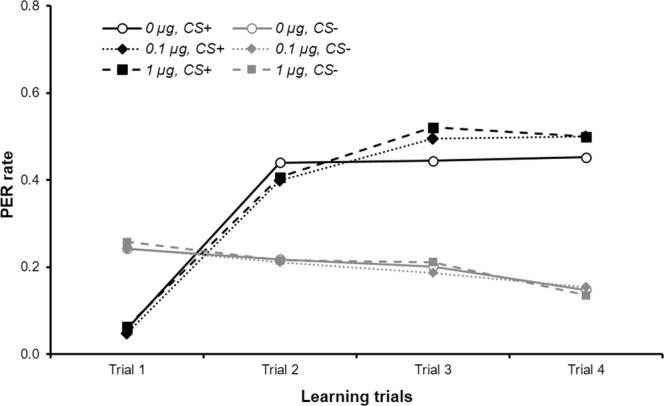
Bee PER rate during the learning trials. Each curve corresponds to the response to the CS+ or the CS− for different thymol treatments. As there was neither significant difference between the different odorants and sugars, nor any interactions, the odorant and sugar groups were pooled. A total of 725 bees were used (20–42 in each combination odorant/sugar/treatment, see Table 1). The initial high response rate to CS− is a usual observation in differential conditioning and corresponds to generalization from CS+.
Thus, in spite of the thymol treatment, the bees could undergo Pavlovian conditioning with the same level of PER for all the odorant/sugar combinations tested. Moreover their capacity to discriminate between the two odorants was not affected by thymol.
Retrieval test 1 hour after training
One hour after training animals were presented again with the CS+ and the CS− without sugar. As shown in Table 2, there was no significant change in the performance between the last learning trial and the retrieval test (McNemar test, adjusted ps ≥ 0.116 for all odor/sugar/thymol combinations); this indicated the performance did not vary between training and retrieval. Performance during the retrieval was then analyzed by comparing PER rate with a logistic regression, using the factors odor, sugar, thymol treatment and their interaction.
Table 2.
PER rate to CS+ during the last acquisition trial, 1-hour and 24-hour retrieval tests.
| Odors | Sugar (US) | Thymol treatment | Trial 4 | Test 1 h | Test 24 h |
|---|---|---|---|---|---|
|
1-hexanol CS+, 1-octanol CS− |
Fructose Sucrose |
0 µg | 0.42 | 0.42 | 0.50 |
| 0.44 | 0.50 | 0.67 | |||
|
1-octanol CS+, 1-hexanol CS− |
Fructose Sucrose |
0 µg | 0.41 | 0.41 | 0.25 |
| 0.57 | 0.65 | 0.61 | |||
|
2-hexanol CS+, 2-octanol CS− |
Fructose Sucrose |
0 µg | 0.51 | 0.35 | 0.38 |
| 0.49 | 0.46 | 0.47 | |||
|
2-octanol CS+, 2-hexanol CS− |
Fructose Sucrose |
0 µg | 0.36 | 0.32 | 0.37 |
| 0.41 | 0.36 | 0.58 | |||
|
1-hexanol CS+, 1-octanol CS− |
Fructose Sucrose |
0.1 µg | 0.59 | 0.59 | 0.71 |
| 0.49 | 0.54 | 0.70 | |||
|
1-octanol CS+, 1-hexanol CS− |
Fructose Sucrose |
0.1 µg | 0.56 | 0.56 | 0.44 |
| 0.52 | 0.54 | 0.68 | |||
|
2-hexanol CS+, 2-octanol CS− |
Fructose Sucrose |
0.1 µg | 0.41 | 0.41 | 0.39 |
| 0.58 | 0.53 | 0.57 | |||
|
2-octanol CS+, 2-hexanol CS− |
Fructose Sucrose |
0.1 µg | 0.43 | 0.48 | 0.36 |
| 0.36 | 0.36 | 0.58 | |||
|
1-hexanol CS+, 1-octanol CS− |
Fructose Sucrose |
1 µg | 0.59 | 0.50 | 0.48 |
| 0.60 | 0.69 | 0.64 | |||
|
1-octanol CS+, 1-hexanol CS− |
Fructose Sucrose |
1 µg | 0.69 | 0.73 | 0.57 |
| 0.43 | 0.40 | 0.73 | |||
|
2-hexanol CS+, 2-octanol CS− |
Fructose Sucrose |
1 µg | 0.44 | 0.39 | 0.42 |
| 0.44 | 0.34 | 0.50 | |||
|
2-octanol CS+, 2-hexanol CS− |
Fructose Sucrose |
1 µg | 0.40 | 0.40 | 0.42 |
| 0.30 | 0.30 | 0.36 |
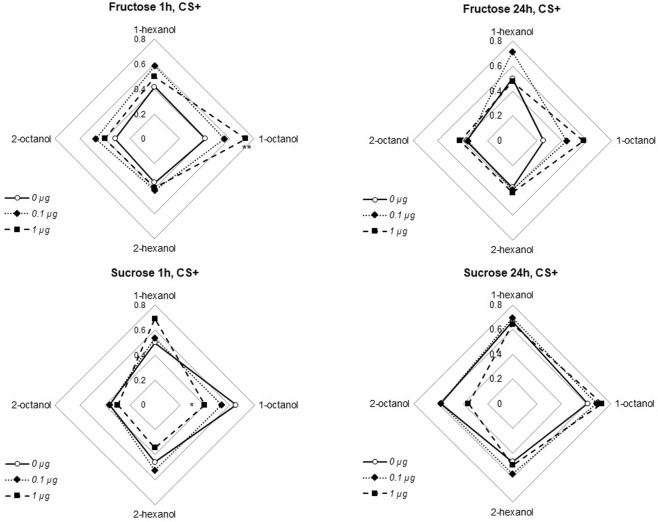
During this retrieval test (Fig. 3), the main factors have no significant effects, neither odorant used (logistic regression, comparisons between odors: ps ≥ 0.249), sugar used (logistic regression, comparisons between sugars: p = 0.473) nor thymol doses (logistic regression, comparisons between thymol doses: ps ≥ 0.085). However, there was a significant increase of conditioned responses specifically for 1-octanol with fructose in animals treated with 1 µg thymol (logistic regression, significant interaction 1-octanol * 1 µg thymol * Fructose: p = 0.007). Interestingly the same dose of thymol significantly decreased the response to 1-octanol with sucrose (logistic regression, interaction 1-octanol * 1 µg thymol: p = 0.013). This suggests 1 µg thymol affected congruency level between the sugar used and 1-octanol but not the three other odorants. No other interactions was significant (logistic regression, interaction 2-hexanol*thymol 1 µg, p = 0.059; all other interactions, ps ≥ 0.177).
Figure 3.
PER rates for the CS+ during the retrieval tests performed 1 hour and 24 hours after the training reported in Fig. 2 (the corresponding CS− data are in Fig. 4). The upper radar plot shows scores of bees trained with fructose the lower plot bees trained with sucrose; plots on the left are for 1-hour retrieval tests, and plots on the right for 24-hour retrieval tests. The curves correspond to the different thymol treatment. The tip of the plots corresponds to the odorants used as CS+. Stars denote significant interaction between 1 µg thymol treatment, fructose and 1-octanol (logistic regression, **p < 0.010) or 1 µg thymol and 1-octanol (logistic regression, *p < 0.050); this kind of interaction is the hallmark of congruency alteration.
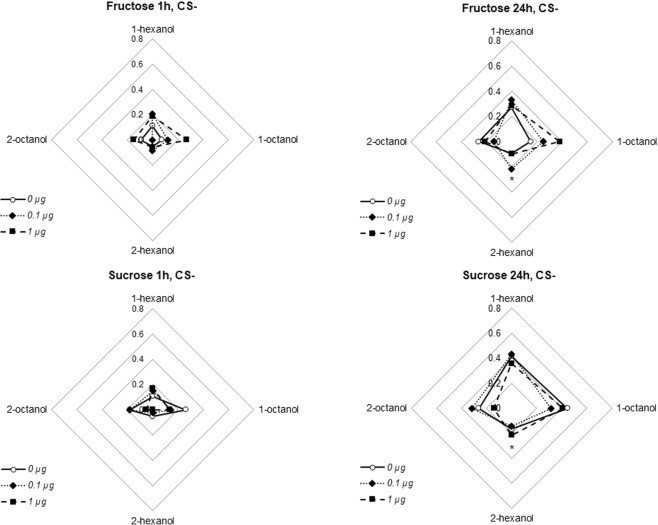
There was no significant effect of the three factors or their interactions on the response level to the CS− (logistic regression, p ≥ 0.122 for all comparisons and interactions; Fig. 4). Moreover, the PER rate for CS+ was always significantly higher than for CS− (McNemar’s test comparing PER rates to CS+ and CS−: ps ≤ 0.046 in all cases). As expected, animals hardly respond to the CS− during the retrieval test, confirming the specificity of the learning and the absence of generalization; this means that thymol does not increase generalization in these experimental conditions. However, as for CS+, the response rate to CS− seems to depend both on the odor presented as CS− and sugar associated with CS+. In the sucrose group the control response rate was 26% for 1-octanol and only 7% for the same odorant in the control fructose group. Moreover, 1 µg thymol treatment decreased the response to 1-octanol with sucrose as US and increased the response to the same odors when CS+ was paired to fructose. Even though no significant difference was observed (due to the low number of animals responding to CS−), responses to 1-octanol are consistent whether it is used as CS+ or CS−.
Figure 4.
PER rates for the CS− during the retrieval tests performed 1 hour and 24 hours after the training reported in Fig. 2 (the corresponding CS+ data are in Fig. 3). Star denotes a significantly lower PER rate in response to 2-hexanol, irrespective of the sugar used or the thymol treatment (logistic regression, *p < 0.050). Other details are as in Fig. 3.
Retrieval test 24 hours after training
One day after training, a new retrieval test was done again as previously. There was no significant change in the PER rate in response to CS+ between the last learning trial and this 24-hour retrieval test (Table 2; McNemar test, adjusted ps ≥ 0.102 for all odor/sugar/thymol combinations), suggesting animals did not forgot the task. Moreover, neither the main effects (odor employed as CS+, thymol treatment or sugar used) nor their interactions were significant (logistic regression, ps ≥ 0.109; Fig. 3).
Similarly, there was no significant effect of any main factors (odor, thymol or sugar) or their interactions on the response level to the CS− (logistic regression, ps ≥ 0.298, Fig. 4), except for a significantly lower PER rate in response to 2-hexanol (logistic regression, p = 0.037).
Even though the response rate was always lower for CS− than for CS+, in some cases the performance was not specific to CS+ in the control group; details and statistics are provided in Table 3. As animals did discriminate during the 1 h retrieval test, this is a case of dissociation between medium-term (1 h) and long-term (24 h) memory trace. Interestingly, 0.1 and 1 µg thymol restored the discrimination (Table 3) for 1-hexanol/fructose and 1-octanol/sucrose, and 1 µg thymol also restored discrimination for 1-octanol/fructose and 2-octanol/fructose but also prevented it for 2-octanol/sucrose. Overall we observed that long-term memory of odorant/sugar associations could be altered by thymol for some odorant/sugar combinations.
Table 3.
Comparisons between CS+ and CS− for each combination during the 24-hour retrieval test (p-values from McNemar’s test).
| Odors | Sugar (US) | Thymol treatment | p-values |
|---|---|---|---|
|
1-hexanol CS+, 1-octanol CS- |
Fructose | 0 µg | 0.059° |
| Sucrose | 0.014* | ||
|
1-octanol CS+, 1-hexanol CS- |
Fructose | 0 µg | 0.317 |
| Sucrose | 0.083° | ||
|
2-hexanol CS+, 2-octanol CS- |
Fructose | 0 µg | 0.003* |
| Sucrose | 0.0009*** | ||
|
2-octanol CS+, 2-hexanol CS- |
Fructose | 0 µg | 0.157 |
| Sucrose | 0.014* | ||
|
1-hexanol CS+, 1-octanol CS- |
Fructose | 0.1 µg | 0.005** |
| Sucrose | 0.014* | ||
|
1-octanol CS+, 1-hexanol CS- |
Fructose | 0.1 µg | 0.083° |
| Sucrose | 0.008** | ||
|
2-hexanol CS+, 2-octanol CS- |
Fructose | 0.1 µg | 0.025* |
| Sucrose | 0.0001*** | ||
|
2-octanol CS+, 2-hexanol CS- |
Fructose | 0.1 µg | 0.083° |
| Sucrose | 0.025* | ||
|
1-hexanol CS+, 1-octanol CS- |
Fructose | 1 µg | 0.046* |
| Sucrose | 0.021* | ||
|
1-octanol CS+, 1-hexanol CS- |
Fructose | 1 µg | 0.046* |
| Sucrose | 0.008** | ||
|
2-hexanol CS+, 2-octanol CS- |
Fructose | 1 µg | 0.002** |
| Sucrose | 0.005** | ||
|
2-octanol CS+, 2-hexanol CS- |
Fructose | 1 µg | 0.046* |
| Sucrose | 0.083° |
Discussion
When effects of thymol on memory were analyzed through differential olfactory conditioning, they were shown to vary according to the CS+/US combination used in the conditioning paradigm (1-octanol/fructose vs 1-octanol/sucrose in the 1-hour retrieval test). Thus, thymol significantly modified bee performances in 1 h retrieval for specific odorant/sugar pairs only. Moreover during long-term memory recall (24 h retrieval test) thymol sometimes facilitated discrimination and sometimes prevented it (see details in Table 3). By contrast, thymol did not affect odorant detection at the antenna level and performance during learning in differential conditioning was intact. This suggests thymol left odorant perception, sugar sensitivity and PER responsiveness intact. Altogether our results suggest thymol did not alter memory as a whole but instead modified specific memory traces.
During 1 h retrieval, pre-treatment with 1 µg thymol induced a change: response to 1-octanol increased relatively to other odors when it had been previously associated with fructose but decreased when associated with sucrose. These differences were not due to fructose itself, as they were not observed during learning or long-term memory for other odorants. During learning, central neural integration of sugar and the learnt odor alter the representation of the odor so that it acquires a positive valence and triggers an appetent behavior (PER). This integrative process seems modified in bees after thymol exposure for some odor/sugar combination. Thus, we propose that pre-training exposure of 1 µg thymol alters congruency between CS+ and US during memory recall (retrieval test).
In humans, odor-sugar pairs were either congruent or incongruent in the context of feeding behavior, demonstrating the role of experience in odor/taste integration41. Odors ranged from highly congruent to highly incongruent with sugar: for example, an odor–induced enhancement of performance is found for a sucrose/strawberry combination but not for the incongruent sucrose/ham35 mixture. Response decrease can be induced by conflicts elicited by the occurrence of incongruence in the trial sequences: stimulus conflicts trigger inhibition or avoidance. Similarly, mood-congruent facilitation can occur during retrieval of emotional information37.
Variations in stability of associative memory indicate congruency is also present in insects. For instance, Simcock et al. reported that bees are good at remembering 1-hexanol associated with sucrose or glucose but not with fructose32, indicating that olfactory memory is affected by sugar identity. In our study, control bees had identical responses to 1-hexanol tested at 1 h when it was paired to sucrose or fructose. However, the response to 1-octanol was higher when paired with sucrose than with fructose. Whether an odor/taste combination is congruent depends on familiarity with them or foraging experience before the laboratory test: bees pre-fed with solutions containing an amino acid were less likely to associate odors with sucrose42. This would explain the contrast between the present results and Simcok et al.’s32. Interestingly, the most available sugar is different for winter and summer bees. Honey, the unique food of winter bees, is rich in fructose and glucose. By contrast, foraging summer bees consume nectar, which is rich in sucrose as well as glucose and fructose20–26. Thus, it would be particularly interesting to compare their responses. This is all the more relevant that anti-varroa treatments such as thymol are often performed in winter.
To account for the differences between the odorants we used as CS+ we propose that thymol exposure highlighted the potential conflict between sucrose, an appetitive stimulus, and 1-octanol and 1-hexanol that are components of the alarm pheromone40. As thymol is a positive allosteric modulator of GABA receptors18 we postulate its effects are mediated through GABA circuits. This hypothesis can be tested by looking at the effects of GABA blockers such as fipronil or picrotoxin, on recovery tests after differential conditioning. We have previously reported that after picrotoxin injection in the antennal lobes, bees fail to discriminate between 1-hexanol and 1-octanol when tested for short-term memory17. Fipronil makes the bees generalize between 1-hexanol and 1-nonanol 24 h and 48 h after learning3. Conversely, in the experiments described in the present paper, control animals failed to discriminate CS+ and CS− during long-term memory tests when 1-hexanol and 1-octanol were paired with fructose, but thymol restored the discrimination. This is fully consistent with our prediction that thymol and picrotoxin should have opposite effects on odorant-sugar associations.
Pesticide impacts in honey bees are not consistent43. Currently bees are exposed to fluctuating environmental conditions which combined with internal factors could affect their physiological and behavioral responses to pesticides. Thus, data obtained for contaminants with olfactory conditioning paradigms in bees can be differently interpreted according to subtle differences in the experimental factors. For instance in laboratory conditions learning performances of honey bees are differentially affected by a pesticide according to the learning task: acute treatment with the miticide coumaphos improved short-term memory in massed conditioned bees but not in spaced conditioning44. Finally it is noteworthy to mention that the alcohols used as CS are not only floral compounds but also alarm pheromone components increasing recruiting40. Thus subtle changes of the congruency in odor-induced taste representation after thymol exposure could significantly impact foraging and intra-hive feeding behaviors in natural conditions.
Material and Methods
Chemicals
Thymol solutions were prepared with thymol powder (99.5%, Sigma. CAS 89-83-8) first dissolved in ethanol and then diluted in deionized water. Final concentrations of thymol were 1 µg/µl, 0.1 µg/µl and 0 µg/µl (control) in water-ethanol. The concentrations used were sub-lethal45.
Odorants used for olfactory conditioning were 1-hexanol (>99%, Sigma-Aldrich, CAS 111-27-3), 1-octanol (>99.5%. Fluka, CAS 111-87-5), (+−)2-hexanol (>98%. Fluka, CAS 626-93-7) and (+−)2-octanol (97.8%, Sigma, CAS 123-96-6). The sugar solutions used were sucrose 40% w/w (powder, >99.5%, Sigma, CAS 57-50-1) or fructose 40% w/w (powder, 98%, Aldrich, CAS 57-48-7) in deionized water.
Animals
Bees used in these experiments were Apis mellifera (Hymenoptera: Apidae) from the apiary of the Centre de Recherches pour la Cognition Animale in Toulouse (France) or hives in Versailles INRA for electrophysiological experiments. They were fed with their own honey and didn’t receive any treatment except Versailles hives which had received oxalic acid for varroa control the previous winter. PER experiments were performed in 2015 from February to April and from July to September. Electrophysiological recordings were performed between the two PER experiment periods.
Bees were captured before 9:30 AM at the entrance of the hive, cooled in ice until immobile (~5 min) and harnessed in small tubes so that their antennae and proboscis could freely move. After recovering from cooling, they were fed individually with 3 µl of 40% sucrose solution to normalize their feeding motivation and treated with a topical application of 1 µl of one of the thymol solutions (1 µg/µl, 0.1 µg/µl or 0 µg/µl as control) on dorsal thorax. Experiments were conducted more than 3 hours after this treatment to enhance their motivation for sugar; in the meantime they were kept at 28 °C in the dark.
Electrophysiology
One antenna was immobilized horizontally at the level of the pedicel with dental glue. Electrodes were composed of chlorinated silver wires inserted into glass capillaries filled with physiological solution (NaCl 9 g, KCl 0.2 g, glucose 4.36 g in 1 l of distilled water)46. Reference electrode was inserted into the eye and recording electrode capped the tip of the antenna (which was previously cut). The electrodes were connected to an amplifier via a pre-amplifier (NPI electronic). Antennal signals were amplified 1000 times (amplifier ELC-03X, npi electronic), filtered between 0.3 Hz and 500 Hz and digitized at a resolution of 16 bit and 1 kHz sampling rate by a analog-digital conversion card (Data Translation 9800). They were then analysed with MEAD, a dedicated script home-developed with Measure Foundry. The electroantennography set-up was surrounded by a Faraday cage to limit electromagnetic noise.
A main humidified airflow of 70 l/h was directed to the antennae and placed 1 cm away from it to allow the bee to be habituated to airflow mechanical stimulation. The pipette containing the stimulation odorant was connected to a secondary air flow of 10 l/h which was activated during stimulation and added to the main flow. A volume of 5 ml of each odorant solution was deposited on a filter paper in a Pasteur pipette. Fresh stimulus sources were prepared daily before starting experiments and stored until use in a sealed test tube. A new source was used for each bee. An automatic stimulation system (ValveBank. AutoMate Scientific) was used to release a 500 ms odor puff. Each bee was tested with four concentrations (0.1%, 1%, 10%, 100%) of the four odorants diluted in mineral oil as well as control stimulations containing the mineral oil alone. Stimulations were spaced at least 90 s to avoid saturation or sensory adaptation of the antenna stimulated by odorants; exhaustion behind the bee prevented odorant accumulation.
Olfactory conditioning and memory test
We used a differential conditioning procedure19,47. This procedure tested the ability of bees to distinguish between two odorants. Bees were placed in a box with a constant airflow so that any odorant was removed after a trial. The odor stimulations were made with a plastic syringe containing a 1*1 cm square of filter paper with 5 µl of pure odorant; the filter paper was changed every day. Bees were alternately stimulated with two odorants: one odorant (positive conditioned stimulus or CS+) was always associated with the US whereas the second odorant was never associated with the US (negative conditioned stimulus or CS−). To avoid bias caused by the order of appearance we used pseudo-random sequences: either CS+, CS−, CS−, CS+, CS+, CS−, CS−, CS+ or CS−, CS+, CS+, CS−, CS−, CS+, CS+, CS−, with 1 min interval between trials in all cases. Thus four trials were conducted with CS+ and four with CS−. Two pairs of odorant were used as CS+ and CS−: 1-hexanol was paired with 1-octanol and 2-hexanol was paired with 2-octanol. These odorants are often used in bee conditioning experiments8. Sucrose or fructose (40%) was used as US.
During a learning trial, bees were stimulated with odorant for 3 s (CS+ or CS−). The bee antenna was touched with sugar solution 2 s after the beginning of odorant stimulation for the CS+ but not for the CS−. If bees showed PER after antenna stimulation with US they were fed 1 µL of the sugar solution used as US.
Memory retrieval tests were performed 1 h and 24 h after conditioning. During a retrieval test both CS+ and CS− were presented in a random order at 1 min intervals without US and we recorded whether bees showed PER. Bees were then fed with 5 µl of sucrose 40% to test for their motivation and bees non-responding by PER were discarded. Once fed, bees were kept at 28 °C in the dark with 70–80% relative humidity until the 24 h test, which was performed the same way.
Data analysis
Statistics were computed using R Studio 1.1.423 and R3.4.0; α was set to 0.05.
Electrophysiological recordings were analyzed for each odorant using a mixed model; the factors were thymol treatment (0, 0.1 or 1 µg/µl), odorant concentrations (0%, 0.1%, 1%, 10% and 100%, treated as a repeated measurement within each bee, i.e. the factor bee was random) and their interaction. Sample sizes are reported in Fig. 1.
In behavioral experiments, there were 20 to 42 bees (average 30) for each combination odorant/thymol/sugar (Table 1). In each experiment the measure we report is the occurrence of PER to the odorant alone. Bees who didn’t show PER for US stimulations were discarded. As PER is binary data, we used generalized linear models to assess the effects of the three factors (sugar reward [two levels: sucrose or fructose], conditioned odorant [i.e. CS+: either one of 1-hexanol, 1-octanol, 2-hexanol or 2-octanol] and thymol treatment [three levels: 0, 0.1 or 1 µg per animal]) and of their interactions. Performance during retrieval tests was evaluated using logistic regressions. Performance during training was evaluated using generalized estimating equation (GEE), a type of logistic regression used for repeated measurements; in that case, we used trial as a repeated measurement within each bee (i.e. random factor) beside sugar, odorant and thymol factors.
To evaluate whether the different treatments affect odorant discrimination during the retrieval tests we performed McNemar’s test to compare within each combination odorant*sucrose*thymol the response rate between CS+ and CS−. McNemar’s test was also used to compare performance during the 4th learning trial (the last one) and each of the two retrieval tests; in that case, Holm’s correction was used to correct for repeated measurements (Table 2).
Acknowledgements
The authors thank Lucie Hotier for her help with beekeeping. This work was supported by the National Center for Scientific Research and the University Toulouse III- Paul Sabatier.
Author Contributions
C.A. designed experiment and wrote the main manuscript text, C.C. performed electrophysiology, C.C. and L.R. performed behavioral experiments, M.D. carried out the data analysis and prepared the figures. M.D. and M.R. supervised electrophysiology and contributed to the analysis and writing.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El Hassani AK, Dacher M, Gauthier M, Armengaud C. Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera) Pharmacol Biochem Behav. 2005;82:30–39. doi: 10.1016/j.pbb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Decourtye A, et al. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.) Pesticide Biochemistry and Physiology. 2004;78:83–92. doi: 10.1016/j.pestbp.2003.10.001. [DOI] [Google Scholar]
- 3.Aliouane Y, et al. Subchronic exposure of honeybees to sublethal doses of pesticides: effects on behavior. Environ Toxicol Chem. 2009;28:9. doi: 10.1897/08-110.1. [DOI] [PubMed] [Google Scholar]
- 4.Tan K, et al. Imidacloprid alters foraging and decreases bee avoidance of predators. PLoS One. 2014;9:e102725. doi: 10.1371/journal.pone.0102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry M, et al. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science. 2012;336:348–350. doi: 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- 6.Erber J, Masuhr T, Menzel R. Localization of short-term memory in the brain of the bee, Apis mellifera. Physiological Entomology. 1980;5:343–358. doi: 10.1111/j.1365-3032.1980.tb00244.x. [DOI] [Google Scholar]
- 7.Lozano VC, Armengaud C, Gauthier M. Memory impairment induced by cholinergic antagonists injected into the mushroom bodies of the honeybee. J Comp Physiol A. 2001;187:249–254. doi: 10.1007/s003590100196. [DOI] [PubMed] [Google Scholar]
- 8.Guerrieri F, Schubert M, Sandoz JC, Giurfa M. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 2005;3:e60. doi: 10.1371/journal.pbio.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dacher M, Smith BH. Olfactory interference during inhibitory backward pairing in honey bees. PLoS One. 2008;10:e3513. doi: 10.1371/journal.pone.0003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Démares F, et al. Differential involvement of glutamate-gated chloride channel splice variants in the olfactory memory processes of the honeybee Apis mellifera. Pharmacology Biochemistry and Behavior. 2014;124:137–144. doi: 10.1016/j.pbb.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 11.El Hassani AK, Dupuis JP, Gauthier M, Armengaud C. Glutamatergic and GABAergic effects of fipronil on olfactory learning and memory in the honeybee. Invert Neurosci. 2009;9:91–100. doi: 10.1007/s10158-009-0092-z. [DOI] [PubMed] [Google Scholar]
- 12.Boncristiani H, et al. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J Insect Physiol. 2012;58:613–620. doi: 10.1016/j.jinsphys.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Frost EH, Shutler D, Hillier NK. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J Exp Biol. 2013;216:2931–2938. doi: 10.1242/jeb.086538. [DOI] [PubMed] [Google Scholar]
- 14.Dai P, Jack CJ, Mortensen AN, Bustamante TA, Ellis JD. Chronic toxicity of amitraz, coumaphos and fluvalinate to Apis mellifera L. larvae reared in vitro. Sci Rep. 2018;8:5635. doi: 10.1038/s41598-018-24045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregorc Aleš, Alburaki Mohamed, Sampson Blair, Knight Patricia R., Adamczyk John. Toxicity of Selected Acaricides to Honey Bees (Apis mellifera) and Varroa (Varroa destructor Anderson and Trueman) and Their Use in Controlling Varroa within Honey Bee Colonies. Insects. 2018;9(2):55. doi: 10.3390/insects9020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnafe E, et al. Effect of a thymol application on olfactory memory and gene expression levels in the brain of the honeybee Apis mellifera. Environ Sci Pollut Res Int. 2015;22:8022–8030. doi: 10.1007/s11356-014-2616-2. [DOI] [PubMed] [Google Scholar]
- 17.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 18.Priestley CM, Williamson EM, Wafford KA, Sattelle DB. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA(A) receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol. 2003;140:1363–1372. doi: 10.1038/sj.bjp.0705542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis T, et al. Amelα8 subunit knockdown in the mushroom body vertical lobes impairs olfactory retrieval in the honeybee, Apis mellifera. European Journal of Neuroscience. 2012;36:3438–3450. doi: 10.1111/j.1460-9568.2012.08261.x. [DOI] [PubMed] [Google Scholar]
- 20.Baker, H. G. & Baker, I. Floral nectar constituents in relation to pollinator type. Handbook of experimental pollination biology., 117–141 (1983).
- 21.Nepi, M. New perspectives in nectar evolution and ecology: simple alimentary reward or a complex multiorganism interaction? Acta Agrobot. 70, 10.5586/aa.1704 (2017).
- 22.White JW. The Composition of Honey. Bee World. 1957;38:57–66. doi: 10.1080/0005772X.1957.11094976. [DOI] [Google Scholar]
- 23.Lüttge U. Nectar composition and membrane transport of sugars and amino acids: a review on the present state of nectar research. Apidologie. 1977;8:305–319. doi: 10.1051/apido:19770402. [DOI] [Google Scholar]
- 24.Pacini E, Nepi M, Vesprini LJ. Nectar biodiversity: a short review. Plant Syst Evol. 2003;238:7–21. doi: 10.1007/s00606-002-0277-y. [DOI] [Google Scholar]
- 25.De la Barrera E, Nobel PS. Nectar: properties, floral aspects, and speculations on origin. Trends Plant Sci. 2004;9:65–70. doi: 10.1016/j.tplants.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Machado De-Melo Adriane Alexandre, Almeida-Muradian Ligia Bicudo de, Sancho María Teresa, Pascual-Maté Ana. Composition and properties of Apis mellifera honey: A review. Journal of Apicultural Research. 2017;57(1):5–37. doi: 10.1080/00218839.2017.1338444. [DOI] [Google Scholar]
- 27.Gerber Bertram, Wüstenberg Daniel, Schütz Anne, Menzel Randolf. Temporal Determinants of Olfactory Long-Term Retention in Honeybee Classical Conditioning: Nonmonotonous Effects of the Training Trial Interval. Neurobiology of Learning and Memory. 1998;69(1):71–78. doi: 10.1006/nlme.1997.3801. [DOI] [PubMed] [Google Scholar]
- 28.Sandoz JC, Laloi D, Odoux JF, Pham-Delègue MH. Olfactory information transfer in the honeybee: compared efficiency of classical conditioning and early exposure. Anim Behav. 2000;59:1034–1043. doi: 10.1006/anbe.2000.1395. [DOI] [PubMed] [Google Scholar]
- 29.Chaffiol A, Laloi D, Pham-Delègue MH. Prior classical olfactory conditioning improves odour-cued flight orientation of honey bees in a wind tunnel. Journal of experimental biology. 2005;208:3731–3737. doi: 10.1242/jeb.01796. [DOI] [PubMed] [Google Scholar]
- 30.Gil M, De Marco RJ. Olfactory learning by means of trophallaxis in Apis mellifera. Journal of experimental biology. 2005;208:671–680. doi: 10.1242/jeb.01474. [DOI] [PubMed] [Google Scholar]
- 31.Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J Comp Psychol. 1983;97:107–119. doi: 10.1037/0735-7036.97.2.107. [DOI] [PubMed] [Google Scholar]
- 32.Simcock NK, Gray H, Bouchebti S, Wright GA. Appetitive olfactory learning and memory in the honeybee depend on sugar reward identity. J Insect Physiol. 2018;106:71–77. doi: 10.1016/j.jinsphys.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Menzel, R. In Experimental Behavioral Ecology and Sociobiolog (eds Holldobler, B. and Lindau, M.) 55–74 (Sunderland, MA: Sinauer., 1985).
- 34.Amsellem S, Ohla K. Perceived Odor-Taste Congruence Influences Intensity and Pleasantness Differently. Chem Senses. 2016;41:677–684. doi: 10.1093/chemse/bjw078. [DOI] [PubMed] [Google Scholar]
- 35.Schifferstein HN, Verlegh PW. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychologica. 1996;94:87–105. doi: 10.1016/0001-6918(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 36.Barenholtz E, Lewkowicz DJ, Davidson M, Mavica L. Categorical congruence facilitates multisensory associative learning. Psychon Bull Rev. 2014;21:1346–1352. doi: 10.3758/s13423-014-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis PA, Critchley HD, Smith AP, Dolan RJ. Brain mechanisms for mood congruent memory facilitation. Neuroimage. 2005;25:1214–1223. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 38.Knudsen JT, Tollsten L, Bergström LG. Floral scents—a checklist of volatile compounds isolated by head-space techniques. Phytochemistry. 1993;33:253–280. doi: 10.1016/0031-9422(93)85502-I. [DOI] [Google Scholar]
- 39.Knudsen JT, Eriksson R, Gershenzon J, Ståhl B. Diversity and Distribution of Floral Scent. Bot Rev. 2006;72:1–120. doi: 10.1663/0006-8101(2006)72[1:DADOFS]2.0.CO;2. [DOI] [Google Scholar]
- 40.Wager BR, Breed M. Does Honey Bee Sting Alarm Pheromone Give Orientation Information to Defensive Bees? Ann. Entomol. Soc. Am. 2000;93:1329–1333. doi: 10.1603/0013-8746(2000)093[1329:DHBSAP]2.0.CO;2. [DOI] [Google Scholar]
- 41.Small DM, Prescott J. Odor/taste integration and the perception of flavor. J. Exp Brain Res. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- 42.Simcock NK, Gray HE, Wright GA. Single amino acids in sucrose rewards modulate feeding and associative learning in the honeybee. J Insect Physiol. 2014;69:41–48. doi: 10.1016/j.jinsphys.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poquet Y, Vidau C, Alaux C. Modulation of pesticide response in honeybees. Apidologie. 2016;47:412–426. doi: 10.1007/s13592-016-0429-7. [DOI] [Google Scholar]
- 44.Williamson SM, Baker DD, Wright GA. Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honeybee Apis mellifera. Invert Neurosci. 2013;13:63–70. doi: 10.1007/s10158-012-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahlgren L, Johnson RM, Siegfried BD, Ellis MD. Comparative Toxicity of Acaricides to Honey Bee (Hymenoptera: Apidae) Workers and Queens. Journal of Economic Entomology. 2012;105:1895–1902. doi: 10.1603/EC12175. [DOI] [PubMed] [Google Scholar]
- 46.Sandoz JC, Pham-Delègue MH, Renou M, Wadhams LJ. Asymmetrical generalisation between pheromonal and floral odours in appetitive olfactory conditioning of the honey bee (Apis mellifera L.) J Comp Physiol A. 2001;187:559–567. doi: 10.1007/s003590100228. [DOI] [PubMed] [Google Scholar]
- 47.Mauelshagen J. Neural correlates of olfactory learning paradigms in an identified neuron in the honeybee brain. J Neurophysiol. 1993;69:609–625. doi: 10.1152/jn.1993.69.2.609. [DOI] [PubMed] [Google Scholar]