Abstract
Ce-doped LaMnO3 perovskite ceramics (La1−xCexMnO3) were synthesized by sol-gel based co-precipitation method and tested for the oxidation of benzyl alcohol using molecular oxygen. Benzyl alcohol conversion of ca. 25–42% was achieved with benzaldehyde as the main product. X-ray diffraction (XRD), thermogravimetric analysis (TGA), BET surface area, transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), temperature-programmed reduction (H2-TPR), temperature-programmed oxidation (O2-TPO), FT-IR and UV-vis spectroscopic techniques were used to examine the physiochemical properties. XRD analysis demonstrates the single phase crystalline high purity of the perovskite. The Ce-doped LaMnO3 perovskite demonstrated reducibility at low-temperature and higher mobility of surface O2-ion than their respective un-doped perovskite. The substitution of Ce3+ ion into the perovskite matrix improve the surface redox properties, which strongly influenced the catalytic activity of the material. The LaMnO3 perovskite exhibited considerable activity to benzyl alcohol oxidation but suffered a slow deactivation with time-on-stream. Nevertheless, the insertion of the A site metal cation with a trivalent Ce3+ metal cation led to an enhanced in catalytic performance because of atomic-scale interactions between the A and B active site. La0.95Ce0.05MnO3 catalyst demonstrated the excellent catalytic activity with a selectivity of 99% at 120 °C.
Subject terms: Organocatalysis, Catalyst synthesis
Introduction
Presently, perovskite-based materials are gaining immense popularity in the field of material science due to their extraordinary optical, electro-magnetic properties. Perovskite materials mostly applied for removing common exhaust pollutants including carbon monoxide, hydrocarbon, ammonia oxidation, water dissociation, and NOx, etc.1–3. Amongst different perovskite, Mn-containing oxide materials have been growing a considerable interest from the researchers because of the large specific external area, high thermo-chemical durability and extraordinary catalytic performance even at environmental conditions2–9. These excellent physicochemical properties of Mn-based perovskite materials made them an ideal candidate for their applications in the decomposition of customary use pollutants including carbon monoxide, NOx, and poisonous hydrocarbons. In this regard, various types of catalytic conversion technologies were developed4,5,8,10,11. Besides that, in order to make the catalytic combustion widely applicable, the development of reliable technologies is highly desirable. Amongst various catalytic active perovskite materials, lanthanide (Ln3+) ion substituted perovskite demonstrated superior activities4–8,10,12. Such materials revealed higher catalytic activity and superior thermal stability for hydrocarbon combustion than their respective un-substituted perovskites2,7,10.
Owing to the outstanding catalytic activity of perovskite-type oxide ABO3, where A is 12 coordinated and larger cation in size, whereas B is 6 fold coordination and smaller cation in size with oxygen anion. The partial co-doping of the A-site by the transition metal ions with dissimilar valance generate a structural defect because of bond stretching and amend the valence of the B-site to meet the chemical charge balance of the perovskite structure; actually, it is the prime origin for extraordinary catalytic oxidation performance of the ABO3 based oxides. Therefore, doping of similar valence state ions at A or B sites might be altered the crystal structure, geometrical symmetry and disturb the oxidation states of the cations without altering the structure. Besides that, the variation of Mn4+/Mn3+ ratio has the main effect on the catalytic activities of ABO3 materials. The partial doping of Ce3+ ion into LaMnO3 altered the catalytic activity because of an increase in specific surface area, surface defects, oxygen mobility, and redox ability. Ceria has the capability to absorb and release the oxygen vacancies, and these oxygen species play a crucial role in the overall catalytic activities of the CeO2-based perovskites13–18. Owing to the oxidation state transformation behavior of ceria between Ce3+ and Ce4+ dependent on the O2 partial pressure in the nearby atmosphere13,14. Usually, the redox behavior of Ce3+ is determined by morphology, size, and dissemination of oxygen species as the utmost appropriate surface defects13. This unique property of Ce3+ revealed high thermo-chemical robustness and large O2 species movement, and thus displays improved performance in catalytic oxidation of hydrocarbons and nitrogen oxides. So far, nonstoichiometric perovskite materials demonstrated some specific physical properties including evolution in surface defects, oxygen ion mobility, and redox property.
In this article, we proposed the synthesis of Ce3+ ion substituted LaMnO3 nanoparticles via sol-gel based co-precipitation process. We inspected the impact of Ce3+ ion doping in LaMnO3 nanoparticles on physiochemical properties and oxidation performance of C6H5CH2OH to C6H5CHO. For characterization various techniques were applied including X-ray diffraction pattern (XRD), transmission electron microscope (TEM), energy dispersive x-ray analysis (EDX), N2 adsorption, Fourier transform infrared (FTIR), optical absorption (UV-Vis), thermogravimetric analysis (TGA), temperature program reduction (TPR), temperature program oxidation (TPO) and X-ray photoelectron spectroscopy (XPS) techniques. These techniques revealed the role of Ce3+ ion substitution on the crystal structure, crystallinity, surface properties, thermal stability, optical, redox behavior, oxygen adsorption properties and catalytic activities of the as-prepared nonstoichiometric LaMnO3 materials.
Experimental Section
Synthesis of perovskites (La1−xCexMnO3)
Analytical grade chemicals were procured and used directly without any extra distillation. In a typical synthesis of LaMnO3 perovskite, 4.3 g La(NO3)3.6H2O (99.99%), and 2.4 g Mn(NO3)3.3H2O (99.99%, BDH Chemicals Ltd, UK), were dissolved in 50 ml H2O along with C6H8O7.H2O (E-Merck, Germany). Citric acid was used as a chelating agent for complexation with lanthanum and manganese nitrates. The resulting mixed aqueous solution was magnetically stirred on a hot plate at 100 °C until the transparent solution was achieved. Aqueous ammonia solution was quickly added to precipitation under constant mechanical stirring. The occurrence of the willing product was dried at 100 °C for overnight and further annealed at 700 °C in the air for 5 hrs. A similar procedure was repeated for synthesis of La1−xCexMnO3 oxides (x = 0.05, 0.07 and 0.10 mol %).
Catalyst characterization
Powder X-ray diffraction measurement was performed on a PANalytical X’PERT (X-ray diffractometer) furnished with Ni filter and using CuKα (λ = 1.5406 Å). Morphology was obtained from Field emission Transmission Electron Microscope (FE-TEM, JEM-2100F JEOL, Japan) furnished with energy dispersive x-ray analysis (EDX) functioned at an accelerating voltage of 200 kV. Thermal analysis was measured on (TGA/DTA Mettler, Toledo, AG, Analytical CH-8603, Schwerzenbach, Switzerland). UV/Vis absorption spectra were measured by using Perkin-Elmer Lambda-40 Spectrophotometer. Fourier transforms Infrared (FT-IR) spectra were recorded on Perkin-Elmer 580B IR spectrometer. Temperature program reduction (TPR) and Temperature program oxidation (TPO) spectra were recorded on chemisorption Micromeritics AutoChem model 2910 analyzer furnished with a thermal conductivity indicator. Before the experiment, 100 mg material sample was treated with 10 vol % O2/He stream at 500 °C for 30 min to get complete oxidation. Then materials were cooled at room temperature and a mixture of 10 vol% H2/Ar gas with flow rate 20 mL/min was introduced and the reactor was heated from ambient temperature to 900 °C and maintained this temperature up to 20 min. For the O2- TPO experiments, helium(He, 30 mL/min) gas was applied for drying the perovskite samples at 150 °C and cooled down to room temperature, followed by an increase of temperature under O2/He (30 mL/min) flow with a temperature slope of 10 °C/min to 900 °C on the same instrument. The textural properties of the perovskites were recorded on a Micromeritics TriStar 3000 BET Analyzer, taking a value of 0.162 nm2 for the cross-sectional area of the N2 molecule adsorbed at 77 K. Powder samples were dried and degassed by heating gently to 90 °C for 1 h, then at 200 °C for 3 h under flowing N2 before measurement. The free space in each sample tube was determined with He, which was assumed not absorb.
Catalytic studies
Liquid-phase oxidation of benzyl alcohol was carried out in a glass vessel equipped with a magnetic stirrer, reflux condenser, and thermometer. Briefly, a mixture containing benzyl alcohol (2 mmol), toluene (10 mL) and the perovskite (0.3 g) was vigorously stirred in a three-necked round-bottomed flask (100 mL) and then heated up to 120 °C. The O2-gas was introduce in the reaction mixture through bubbling to start the oxidation experiment with a 20 mL/min flow rate. After completion of reaction solid catalyst extracted from the solution by centrifugation and reaction mixture was analyzed by gas chromatography to examine the conversion of the alcohol and product selectivity by (GC, 7890 A) Agilent Technologies Inc, equipped with a flame ionization detector (FID) and a 19019S-001 HP-PONA column.
The specific activity of the catalyst was calculated using the equation
| 1 |
The turnover number and turnover frequency of the catalyst were calculated using
| 2 |
| 3 |
Results and Discussion
Crystallographic and morphological structure
Figure 1 demonstrates the XRD pattern to observe the chemical composition, crystallographic structure and grain size of the as-synthesized perovskite. As observed in Fig. 1. the distinct diffraction lines of perovskite in XRD pattern can be assigned to the (012), (110), (104), (202), (024), (122), (116), (214), (018), (208) and (128) lattice planes, which are attributed to the hexagonal structure of LaMnO3 nanoparticles(Fig. 1) (JCPDS card No. 032-0484)6,19. Any other diffraction line associated with MnO or CeO2 is not identified over the whole XRD range specifies the homogeneous dispersion into the crystal lattice and formation of perfect single phase LaMnO3 perovskite. An observed diffraction line at 30.27° corresponds to La2O3, which is weaker than the reflection lines of LaMnO3 perovskite. All diffractograms of the perovskite materials revealed the similar trigonal symmetry in the crystallographic space group with marginally dissimilar cell parameters. As shown in Fig. 1 diffraction lines in trivalent Ce3+ substituted perovskite are slightly shifted towards longer angle along with reduced intensity in respect to the un-substituted LaMnO3 perovskite, it could be due to the effect of Ce3+ ion doping into the crystal matrix. Owing to the small radius of Ce3+ ions, they are highly mobile and easily migrate from surface to crystal lattice within the crystal matrix of perovskite materials at environment conditions13,14,20. The broadening of reflection lines in perovskite materials suggested the nanocrystalline nature of the as-prepared nanomaterials. As shown in Fig. 1, on substituted of small radius Ce3+ (1.25 Å) in place of La(1.27 Å), the reflection lines slightly shifted to higher 2θ, signifying that the crystal arrangement becomes distorted13,21, resulting the transformation is occurring in the symmetry of crystallographic structure7,10,22. The experimentally calculated lattice parameters for LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3, and La0.90Ce0.10MnO3 are a = 5.527 Å, 5.463 Å, 5.449 Å and 5.436 Å, respectively, are decreased on increasing the substitution concentrations of the Ce3+ ion into the LaMnO3 crystal lattice in respect to un-substituted LaMnO3 perovskite. These variations in lattice parameters and shifts in peak positions endorse the substitution of modified ions into the crystal lattice structure.
Figure 1.

X-ray diffraction pattern of LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3 and La0.90Ce0.10MnO3 nanoparticles.
TEM micrograph clearly shows the irregular hexagonal structure, smooth surface, uncontrolled size, highly aggregated, well-distributed nanoparticles. Figure 2a illustrates the typical image of Ce3+ ion substituted LaMnO3 perovskite nanoproduct with size ranging from 25–31 nm. Energy dispersive x-ray analysis in Fig. 2b revealed the existence of all substituted elements including La3+, Mn3+, Ce3+ and oxygen elements in the as-prepared LaMnO3 perovskite. The appearance of intense peaks of Cu2+ and C belong to the carbon coated copper grid. It confirmed the efficacious doping of Ce3+ into the crystal matrix.
Figure 2.
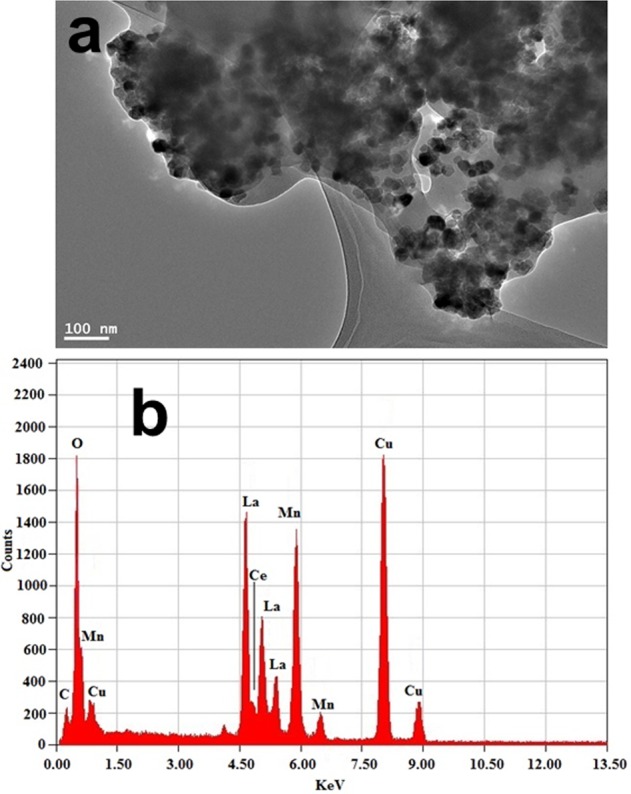
(a) TEM image and (b) EDX analysis of LaMnO3, nanoparticles.
Textural properties and thermal stability
The structural parameters after calcination of Ce substituted LaMnO3 catalysts, Specific surface area (BET), pore volume (PV) and average pore size (PD) are summarized in Table 1. The PV and PD were obtained from the adsorption branch of the respective N2 isotherm by put on the BJH method. Surface area (Single point BET and Multipoint BET), PV and PD drop with increasing Ce ion concentrations from 5 to 10 mol% (Table 1).
Table 1.
Textural properties of the Ce doped catalysts (La1−xCexMnO3).
| Nominal Composition | Single point BET (m2/g) | Multi point BET (m2/g) | Pore volume (cm3/g) | Pore size (A) |
|---|---|---|---|---|
| LaMnO3 | 7.754 | 8.34 | 0.0013 | 18.63 |
| La0.95Ce0.05MnO3 | 7.22 | 7.79 | 0.0011 | 18.61 |
| La0.93Ce0.07MnO3 | 7.30 | 7.75 | 0.0012 | 18.60 |
| La0.90Ce0.10MnO3 | 6.54 | 6.93 | 0.0011 | 18.59 |
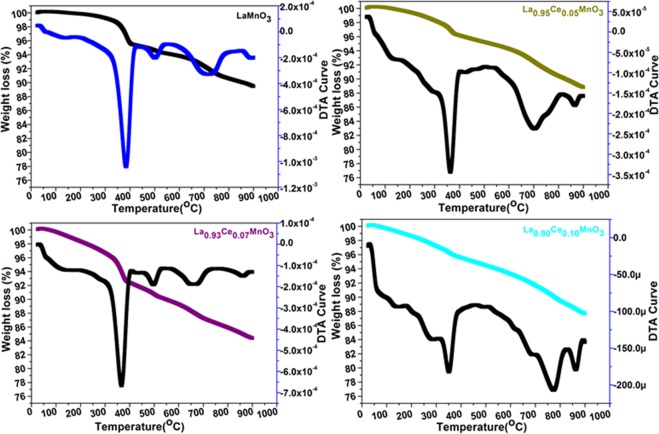
Thermogravimetric (TGA) analysis of the as-prepared LaMnO3 perovskite and Ce-substituted materials exhibit a similar decomposition trend in all thermograms (Fig. 3). TGA spectra were recorded from 0–900 °C in N2-atmosphere with a heating rate of 10 °C/min (Fig. 3). First big exothermic peak (DTA) in all samples are observed at around 400 °C resemble the crystalline H2O molecules or complexation form surface attached organic impurities. The surface attached OH groups or organic moieties are coordinated to the central metal ion in different attachment form in the existing complex precursor system23,24. Generally, -OH groups attached on the surface of metal ions in two forms either terminal Ln-OH or in the bridge from Ln-(OH)-Mn25. In both cases, the dissociation of surface OH groups contrasts from each other depending on the surrounding chemical environment. So that, the reduction ii molar mass occurs in a rather varied range of temperature. No decomposition peaks signifying further crystallization are found in TGA, specifying that the perovskite materials are in crystalline form, as verified by XRD results. All four thermograms illustrate the sluggish weight loss (~6–8%) in between 400–900 °C, which is assigned to the removal or combustion of carbon dioxide at high temperature.
Figure 3.
Thermogravimetric analysis of LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3 and La0.90Ce0.10MnO3 nanoparticles.
Optical properties
Figure 4 displays the infrared spectra of the as-synthesized LaMnO3 and different Ce ion substituted LaMnO3 perovskite nanoparticles. All samples exhibited a diffused band in between 3160–3653 cm−1 assigned to the νO–H stretching vibration originating from surface adsorbed H2O molecules (Fig. 4)25. Two additional strong intensity infrared bands are observed positioned at 1486 and 1375 cm−1 attributed to the δOH and γOH vibrational modes of H2O molecules. These observed infrared spectral results are in accord with TGA observations. The observed infrared band at 644 cm−1 is allotted to the νM-O stretching vibrational mode which certified the formation of metal oxide framework26,27.
Figure 4.
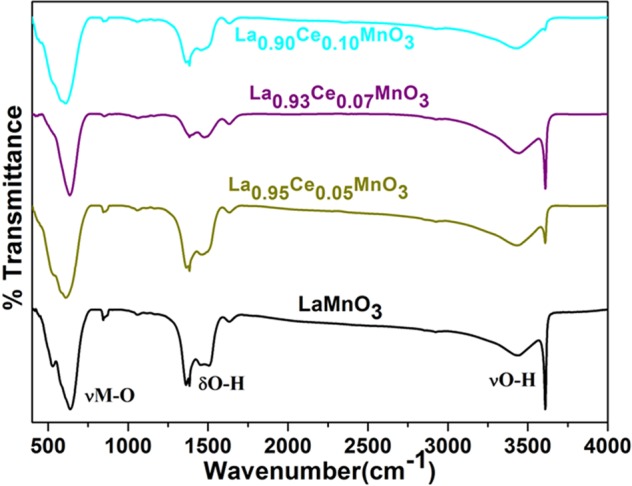
FTIR spectra of LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3 and La0.90Ce0.10MnO3 nanoparticles.
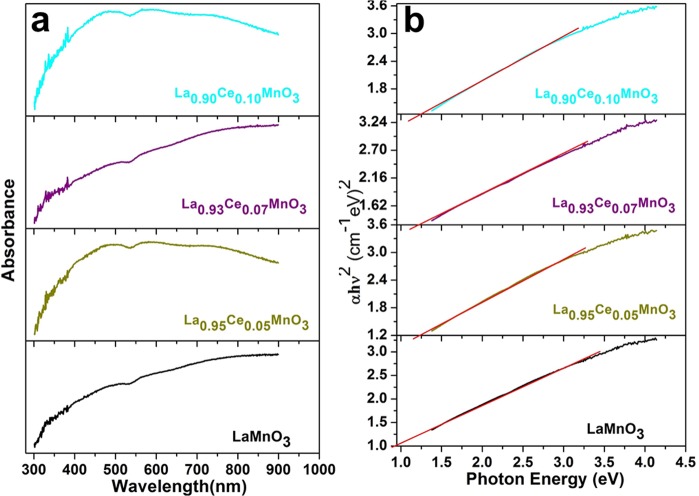
Optical absorption spectra were carried out to determine the optical characteristics of the as-synthesized perovskites (Fig. 5a,b). The direct energy band gap (Eg) is estimated by fitting the absorption spectral data to the straight transition equation by extrapolating the linear portions of the curve into αhν = A(hν − Eg)½, where α is optical absorption coefficient, hν is the photon energy, Eg is the direct bandgap and A is constant (Fig. 5b)25,28,29. The experimentally assessed direct energy band gaps of all perovskite nanomaterials are 1.15, 1.31, 1.34 and 1.32 eV for LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3, and La0.90Ce0.10MnO3 perovskites, respectively. An observed increase band gap energy with increasing the Ce3+ ion substitution quantity into the LaMnO3 crystal lattice, which is attributable to the Burstein-Moss effect28,30–32.
Figure 5.
(a) UV/Vis absorption spectra and (b) The plot of (αhν)2 vs. photon energy(hν) LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3 and La0.90Ce0.10MnO3 nanoparticles.
Redox properties (TPR/TPO)
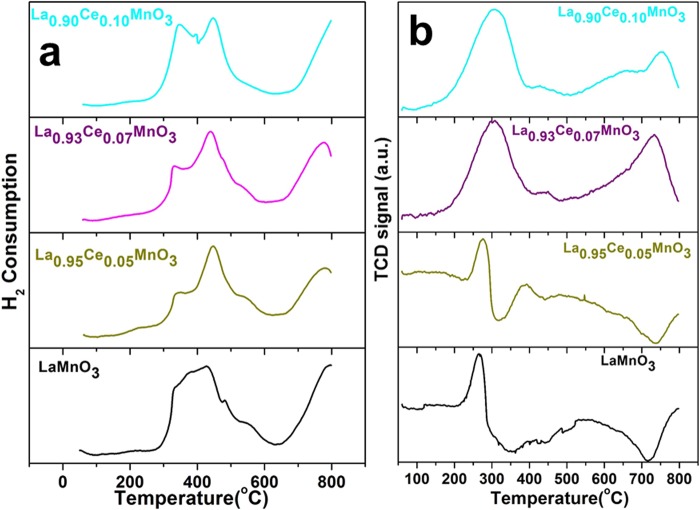
Redox properties of the as-prepared LaMnO3 perovskite and their Ce3+ ion substituted LaMnO3 perovskites are determined by H2-TPR and the observed results are presented in Fig. 6a and tabulated in Table 2. TPR and TPO studies are performed to examine the role of Ce3+ ion-doping on redox behavior of LaMnO3 perovskite within the range from 50–800 °C. The TPR spectra were recorded within the temperature range from 50 to 800 °C temperature. TPR spectra exhibited two typical characteristic reduction peaks, first one in between 280–600 °C and second started from 645 °C5. The observed peak at low reduction temperature (280–600 °C) is correspond to the reduction of Mn4+ to Mn3+ and elimination of surface adsorbed oxygen vacancies, and the second reduction band is observed at a higher temperature (645 °C), which correspond to the reduction of Mn3+ to Mn2+ 4,6,7,33,34. The first broadband occurred at lower reduction temperature indicate the largest H2-consumption, it suggesting the better initiative catalytic activities of LaMnO3 perovskite at a lower temperature. The higher oxidation state of Mn3+/4+ ions is accountable for more oxygen species because of lacking ligand amounts of Mn3+/4+ ion. The occurrence of Mn4+ ion is associated with the fact that Mn3+ has a permitted electron, and have the ability to adsorb molecular O2 and convert it into an electrophilic form6. Reversed transformation of manganese ion oxidation states is observed by the TPO analysis (Fig. 6b), in which the oxidation peak at low temperature (205–310 °C) suggest the transition of Mn2+ to Mn3+ and the oxidation peak at 445–717 °C exhibit the oxidation from Mn3+ to Mn4+. These observations are in accord with published reports4,5,34.
Figure 6.
(a) Temperature program reduction and (b) Temperature program oxidation spectra of LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3 and La0.90Ce0.10MnO3 nanoparticles.
Table 2.
H2 consumption of La1−xCexMnO3 perovskite oxide.
| Catalysts | Tmax1 (°C) | H2-uptake (cm³/g STP) | Tmax2 (°C) | H2-uptake (cm³/g STP) | Tmax3 (°C) | H2-uptake (cm³/g STP) | Total uptake (cm³/g STP) |
|---|---|---|---|---|---|---|---|
| LaMnO3 | 400 | 35.16 | 775.0 | 43.65 | 78.81 | ||
| La0.95Ce0.05MnO3 | 343.3 | 11.54 | 447.0 | 72.13 | 775.6 | 2.24 | 85.91 |
| La0.93Ce0.07MnO3 | 330.7 | 0.880 | 438.4 | 3.85 | 771.2 | 0.36 | 5.09 |
| La0.90Ce0.10MnO3 | 345.6 | 98.51 | 450.2 | 17.40 | 895.8 | 84.31 | 200.22 |
Additionally, the H2-TPR profile shape of LaMnO3 is altered after doping of different Ce3+ ion concentrations into the LaMnO3 crystal lattice as seen in Fig. 6a. The incorporation of Ce3+ ion into the LaMnO3 matrix strongly modified the reduction behavior of LaMnO3 perovskite. As shown in Fig. 6a, the Ce3+ ions-substituted sample revealed three peaks at 330–345, 440–450 and ~800 °C, the first band looks very minute and the second band occurs very robustly35. The occurrence of two peaks in Ce3+ ion substituted LaMnO3 TPR profiles indicates the existence of at least two species in the LaMnO3 crystal lattice, which became stronger and shifted towards high temperature after increasing the doping concentrations of Ce3+. An observed band between 330–345 °C, ascribed to the replacement of Mn2+ by Ce3+ in LaMnO3 crystal matrix. Because of this charge disparity lattice alteration would arise that promote to the construction of La-O-Mn–O–Ce solid solution form, resulting the reactive O2 vacancies are produced that may be reduced simply at low temperature. Generally, the elimination of oxygen vacancies at low temperatures associated with higher oxygen mobility (oxygen reacts more easily) and oxygen reactivity4,6. An observed reduction band at 448 °C ascribed to the dissociation of powerfully interactive MnO2 type with Ce3+ supports, whereas weak intensity reduction band observed at ~800 °C consigned to the high-temperature dissociation band because of bulk MnO2 24. Owing to the variation in balance of both metal (Mn3+/4+ and Ce3+/4+) cations from 4+ to 3+ or from 3+ to 2+, the up-down swings of O2 imperfections escorted with valence alteration is observed6,35. Therefore, the high O2 storage capacity of 10 mol% Ce substituted LaMnO3 perovskite because of the simultaneous occurrence of transportable O2 vacancies and analogous (Mn2+/3+/4+/Ce3+/4+) redox couples. Consequently, the La0.90Ce0.10MnO3 sample revealed an excellent catalytic activity at a lower temperature, so that, the highest redox properties, these results are in accord with previous literature reports7,24,33. Comparatively the intensity of the high-temperature components is remarkably varied on increasing the Ce ion concentrations, whereas peak positions (decomposition temperature) are almost similar. It suggested the similar type of species is reduced at the same temperature, which enhanced by Ce3+ ion substitution.
As shown in Fig. 6a, La0.90Ce0.10MnO3 sample revealed high reducibility at high temperature. So that, the replacement of La3+ by Ce3+ ion would effect in enhanced concentrations of Mn3+ ions and oxygen vacancies because of charge discrepancy accomplished by oxidation of Mn2+ to Mn3+ and by the construction of an oxygen-deficient perovskite La0.90Ce0.10MnO3, which would enhance the reducibility character of the perovskite. These observations are well consistent with XRD and XPS results, in which non-Ce ion substituted Mn2+ species are oxidized and transform into Mn3+ valence states. It inferred that the reducibility behavior of the perovskites in the following sequence LaMnO3 ≤ La0.95Ce0.05MnO3 ≤ La0.90Ce0.10MnO3 ≤ La0.90Ce0.10MnO3, according to the H2 consumption at 446 °C and 800 °C. Generally, oxygen species are attached with metal ion into two different bonding forms including non-crystalline and crystalline bonding forms. In the non-crystalline bonding form, the oxygen species are present in the outer coordination sphere and is referred to as surface adsorbed oxygen species. Whereas in case of crystalline bonding form, the oxygen species entered into the inner coordination sphere and compensate its valence state. These crystalline form oxygen species can be typically eliminated in metal oxide products at higher temperature36,37.
Temperature program oxidation or desorption was performed to evaluate the catalytic affinity towards oxygen. Figure 6b illustrates the TPO profile of the as-prepared LaMnO3 and different Ce3+ ion concentration substituted LaMnO3 perovskites. The TPO- profile of blank LaMnO3 perovskite in Fig. 6b, illustrate three oxygen desorption regions, at three different temperatures including 266, 533 and ~799 °C, respectively. An observed first band at 266 °C is attributed to the weakest oxygen vacancies (superficial O2 species), which are physiochemically adsorbed/chemisorbed O2 species and are eliminated at low-temperature. The appearance of broadband between 350–725 °C assigned to the non-stoichiometric oxygen (interfacial oxygen) vacancies and reduction of Mn4+ to Mn3+, which are desorbed at high temperature. Whereas the oxygen vacancies desorbed at a higher temperature (≥725 °C) can be attributed to the relocation of lattice O2 in the bulk perovskite phase and reduction of Mn3+ to Mn2+ 7,10,33,35. Generally, surface adsorbed O2 vacancies desorbed at low temperatures and interfacial oxygen in non-stoichiometric form desorbing at high temperature23–25,33,35,36,38.
As seen in Fig. 6b, when the Ce3+ ion is replaced in the La3+ site of LaMnO3 perovskite a charge balance is desired to attain the neutrality of the perovskite. It can either achieved by O2 defects or the swing of the Mn ion towards higher valance states (Mn3+ to Mn4+). As illustrated in Fig. 6b, on the substitution of 5 mol% Ce3+ ion doping the strong low-temperature peak is shifted towards slightly higher temperature, which corresponds to surface desorbed oxygen species. While high-temperature peak assigned to interfacial oxygen species is split into two peaks observed at 390 and 490 °C. However, on increasing the substitution concentration of Ce3+ ion in LaMnO3 crystal lattice, the low temperature desorption peaks are moved towards higher temperature with significant enhanced integral area, indicating the homogeneous substitution of Ce3+ ion into crystal lattice which increase the oxygen ion mobility of both surface (superficial) oxygen species and non-stoichiometric (interfacial) lattice oxygen species, it could be due to the effect of small ionic size Ce3+ ion substitution13,24,25. As observed previously, the Ce3+/4+ ions have high oxygen species motilities because of their multiple oxidation states. The high-temperature O2 desorption of LaMnO3 is typically denoted to as the removal of non-stoichiometric surplus oxygen. It could be due to the creation of Mn3+ in LaMnO3 to reduce the Jahn–Teller distortion, although the charge stability advocates that Mn should be in 3+ oxidation state. In La0.90Ce0.10MnO3 the Mn3+ state is highly stable because of the existence of Ce3+ ions in the crystal lattice (charge compensation)33.
XPS studies
The surface chemical components, phase purity, and their oxidation states are inspected by XPS analysis. Figures 7 and 8 demonstrated the XPS spectra of La(3d & 4d), Mn(2p) and O(1 s) for the different Ce ion concentration substituted perovskites. XPS spectra of the La 3d in the LaMnO3 and LaxCe1−xMnO3 displayed two binding energies (BE) bands located at 844 and 860 eV which correspond to the La 3d5/2 and La 3d3/2, respectively. The existence of these valence band indicates that lanthanum in La3+ ion form(Fig. 7a)1. Additionally, each band has additional satellite band along with core band, owing to the relocation of electrons from O2p to the vacant orbital of La 5 f orbital. These observations are similar to the previous values observed for La2O3 1,39, it suggested the trivalent state of La3+ ions in the perovskite materials. The increased La 4d binding energy is interpreted as due to the displacement of the electron density toward nearest neighbors. The oxygen (O1s) signal in XPS spectra shows two peaks, the first one is centered at 531 eV and second at around 436 eV in La0.95Ce0.05MnO3 sample (Fig. 7b). As shown in Fig. 7b, the low BE band is due to the lattice oxygen, whereas broader band with high BE band is associated with the surface adsorbed oxygen or surface hydroxyl groups. Peng et al. observed that the surface adsorbed O2 is the most active oxygen because of higher mobility in respect of lattice oxygen, which plays a crucial role in conversion process through migration from the surface to lattice sites1,3,13.
Figure 7.
(a) XPS analysis of the La 3d3/2&5/2 and (b) O1s spectra recorded for the LaMnO3, La0.95Ce0.05MnO3, La0.93Ce0.07MnO3 and La0.90Ce0.10MnO3 nanoparticles.
Figure 8.

XPS analysis of the Mn 2p1/2&3/2 spectra recorded for the La0.95Ce0.05MnO3, La0.93Ce0.07MnO3 and La0.90Ce0.10MnO3 nanoparticles.
As seen in Fig. 7b, on increasing the dopant concentration (Ce3+ ions) the peaks are varied along with broadening, it indicates the existence of several types of oxygen vacancies such as oxygen of hydroxyl (–OH−)/carbonate(–CO32−) groups on the surface of matrices2,7,8,10 and it is in accord with the TPO results. According to the TPO results the observed low-temperature desorption band(surface O2 species) is directly related to the quantity of O2 species are in very small, while the high quantity of O2 species evolved at a higher temperature(chemisorbed O2 species). An observed an increase in core-level binding energy indicates that all of the cations in the samples (La, Ce, and Mn) are bonded to the oxygen. Most importantly, we are unable to observe the Ce ion peak in the current perovskites matrixes due to the Ce ion in LaCeMnO3 perovskites are mostly in the tetravalent state40.
An observed XPS peak located at around 655 eV is assigned to 2p1/2 of Mn ions, although the band of Mn 2p3/2 is composed of multiple bands it implies the presence of multivalence states such as Mn2+ (641), Mn3+(644) and Mn4+(648) (Fig. 8)41–46. Qureshi et al. observed that the splitting in Mn 2p peak is due to the asymmetric nature of the metal, which suggests Mn exists in the mixed valence state46,47. However, satellite structure at higher BE divided by ~4 eV, it could be due to the strong columbic interaction in between hybridization of Mn 3d electrons and other valence sub-shells42,44,47. No Mn 2p3/2 band for Mn (~639 eV) is detected in the spectrum, it implies that no metallic form of Mn is presented in the as-prepared perovskites (Fig. 8). The impact of the catalytic activity on MnOx is related to its oxidation states which are MnO2 > Mn2O3 > MnO as reported by Thirupathi & Smirniotis4,10,48,49. According to them, MnO2 is a highly reactive compound in all Mn-based compounds including MnO2, Mn5O8, Mn2O3, and Mn3O4. Therefore, Mn4+ has higher catalytic performance, and this resembled the finest catalytic denitration activity of La90Ce10MnO3. The peaks of the Mn 2p1/2 and Mn 2p3/2 of the applied materials are moved towards longer BE, observed at ~2 eV and 3 eV, respectively. As shown in Fig. 8, the binding energies are significantly varied upon increasing the Ce ion concentration into the perovskite matrix, it indicates the variation in valence states of Mn ions.
Catalytic reaction
The prepared materials were exposed to catalytic assessment and the conversion of benzyl alcohol into benzaldehyde is taken up as a typical reaction. It was observed that the prepared catalysts are active against the substrate benzyl alcohol. Adding Ce in the LaMnO3 catalyst is found to impact on catalytic aerobic oxidation of benzyl alcohol due to the synergetic effect between Ce3+/4+ and Mn3+/4+ ions. The C6H5CHO is the core constituent, with an insignificant quantity of C6H5COOH as a byproduct. The perovskite LaMnO3 is found to yield a 29% benzaldehyde within 12 hours, while conversion yield is improved on increasing the Ce ion substitution concentration in the perovskite, as shown in Table 3 (Fig. 9). As demonstrated in Fig. 9, on the substitution of 0.05% Ce in the La0.95Ce0.05MnO3 catalyst yielded 10% more benzaldehyde i.e. 40% which is better than their parent or blank perovskite. Further modification of the catalyst with further increase in the percentage content of Ce in the catalytic system, yielded La0.93Ce0.07MnO3 and La0.9Ce0.1MnO3 respectively, it indicates that the catalytic activity decreases as the % of Ce3+ ion concentration increase in the catalyst composition. The catalyst La0.93Ce0.07MnO3 and La0.9Ce0.1MnO3 yielded 37% and 32% oxidation product, i.e. benzaldehyde, respectively. Furthermore, the selectivity towards benzaldehyde was found to be >99% in all the cases. The graphical representation of the results obtained for all the catalysts tested is given in Fig. 9. When the catalytic activity is compared to the external area of the as-synthesized perovskite, it was observed that the catalyst La0.95Ce0.05MnO3 which displayed the best catalytic performance has a surface area of 7.7922 m2/g, and it found to be lower than the surface area of the perovskite LaMnO3 i.e. 8.3410 m2/g, which yielded a 29% benzaldehyde within 12 hours lower than the catalyst La0.95Ce0.05MnO3 which yielded a 40% benzaldehyde. However, as the % of Ce in the catalyst composition is increased in the perovskites i.e. La0.93Ce0.07MnO3 and La0.9Ce0.1MnO3 the surface area further decreases to 7.7554 and 6.9371 respectively and the catalytic performance also depreciates. This indicates that the catalytic activity is not only dependent on the specific surface area it also depends on the doping concentration of the Ce3+ ion in the materials. An un-doped perovskite possesses Mn in +3 state, while upon the inclusion of the Ce3+ ions and the Mn oxidation state +4 (excess) and +2 is obtained as indicated by the XPS. Noticeably, Ce3+ ion concentration plays a crucial part in the enhancement of the catalytic performance as it induces a high surface oxygen mobility than their un-doped perovskite, and the Mn oxidation state +4 (excess) and +2 is obtained, which enhances the surface redox properties of the perovskites as confirmed by the XPS. However, further increase of the Ce3+ ions in the perovskite was found to result in the diminution in the catalytic performance, it specifies may be the depreciation in Mn4+ and Mn2+ sites and increase in the Mn3+ ion. Apart from the oxidation states of Mn, the decrease in the La3+ which results due to the increase of Ce3+ in the catalytic system may also be accountable for the depreciation in the catalytic activity. The specific catalytic activity of the as-designed materials is calculated based on the turnover number and turnover frequency as presented in Table 3. From the values obtained, it is found that the catalyst La0.95Ce0.05MnO3 has the highest TON and TOF among all the catalysts prepared. Further studies are determined in order to optimize the reaction temperature for the best catalytic performance, the catalyst La0.95Ce0.05MnO3, is utilized for the oxidation of C6H5CH2OH at various temperatures ranging from 40 °C to reflux temperature, and it was found that the catalyst performance is best at the reflux temperature, while at other temperatures, a slight decrease in catalytic performance was observed, observed results are illustrated in Fig. 10.
Table 3.
Aerobic oxidation of benzyl alcohol employing La1−xCexMnO3 catalysts.
| Entry | Catalyst | Conv. (%) | Sel. (%) | TON | TOF (h−1) |
|---|---|---|---|---|---|
| 1 | LaMnO3 | 29.18 | >99 | / | / |
| 2 | La0.95Ce0.05MnO3 | 40.25 | <99 | 1127.92 | 93.99 |
| 3 | La0.93Ce0.07MnO3 | 36.71 | <99 | 734.79 | 61.23 |
| 4 | La0.9Ce0.1MnO3 | 32.27 | <99 | 450.15 | 37.51 |
Figure 9.
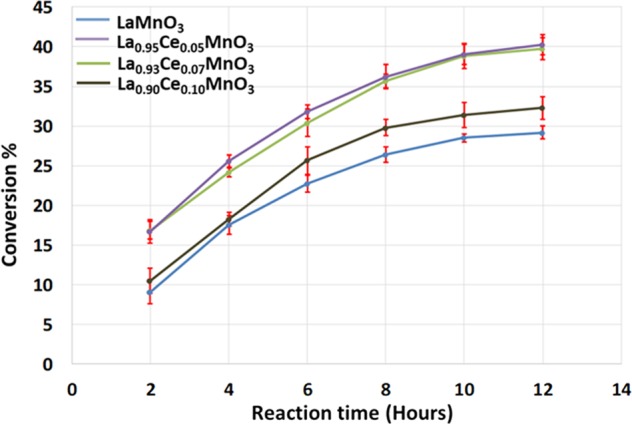
Graphical illustration of the kinetic of the reaction carried out using La1−xCexMnO3 catalysts. Conditions: catalyst = 0.3 g, T = 393 K, benzyl alcohol = 2 mmol, toluene = 10 mL, O2 flow rate = 10 cm3min−1, reaction time = 12 h.
Figure 10.
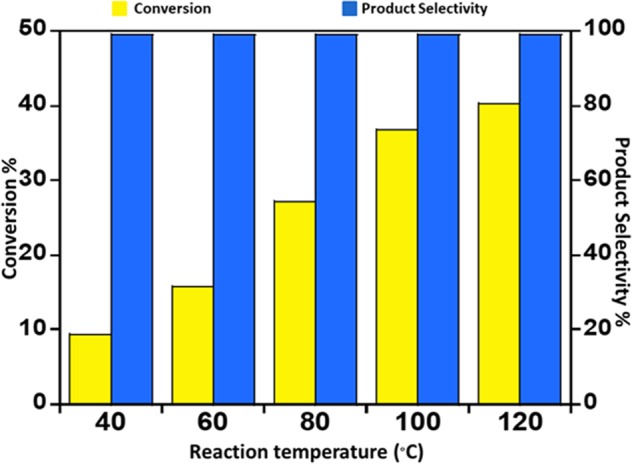
Graphical illustration of catalytic activity of La1−xCexMnO3 as a function of reaction temperature.
Conclusions
We successfully synthesized and characterized the Ce3+ ion substituted lanthanum magnetite perovskites materials by co-precipitation method and applied for conversion of benzyl alcohol into benzaldehyde. Chemical composition and phase purity of the as-synthesized materials were validated from XRD, EDX, TGA and FTIR analysis. The values of optical energy band gaps were varied because of discrepancy in the grain size of the perovskite materials. The increase in doping quantity of Ce3+ ions altered the redox (TPR and TPO) behavior of the perovskite oxides. The insertion of co-dopant Ce3+ ion in perovskite lattice enhanced the quantity of Mn4+ and chemisorbed oxygen positions on the surface of perovskite lattice to increase the catalytic performance. The XPS spectra of La 3d, Mn 2p, and O 1 s clearly revealed the influence of Ce ion substitution, which confirms the transformation of the Mn oxidation state from 3+ to 4+ due to the substitution of trivalent Ce3+ ions at the La3+ site in LaMnO3 perovskite. The surface Ce3+ ion in the perovskite matrix simplifies in oxidation and reduction of oxygen species which stimulates the oxy-dehydrogenation of benzyl alcohol to benzaldehyde. The Mn 2p3/2 core level XPS analysis suggests that due to oxygen vacancies, Mn2+ ions were generated from the Mn3+ transformation in perovskites. It is observed that La0.95Ce0.05MnO3 catalyst shows the highest TON and TOF among all prepared perovskites. According to our observed results the Ce3+ ion -doped LaMnO3 materials could serve as potential heterogeneous catalysts for hydrocarbon conversion. Besides that, trivalent cerium ion doping stimulate the synergistic effect within the crystal lattice along with different transition metal ions as co-catalysts to enhance the performance of the heterogeneous Fenton/perovskite process, an interesting point that merits further investigation.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research King Saud University, Riyadh for funding this work through Research Group No. RG-1439-089.
Author Contributions
Anees Ansari, synthesized the material and wrote the manuscript, N., Ahmad, M., Alam(TGA, FTIR), S.M., Ramay(XRD, UV/Vis), A, Ahmad(BET), B.F., Alrayes(XRD), A.R., Albadri(XPS) and A., Al-Enizi, help in characterization. S.F., Adil, M., Assal and A.R., Alwarthan applied material for conversion process. All authors reviewed and approved the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feng NJ, et al. Facile synthesis of three-dimensionally ordered macroporous silicon-doped La0.8K0.2CoO3 perovskite catalysts for soot combustion. Catalysis Science & Technology. 2016;6:7718–7728. doi: 10.1039/c6cy00677a. [DOI] [Google Scholar]
- 2.Lee YN, Lago RM, Fierro JLG, Gonzalez J. Hydrogen peroxide decomposition over Ln(1-x)A(x)MnO(3) (Ln = La or Nd and A = K or Sr) perovskites. Applied Catalysis a-General. 2001;215:245–256. doi: 10.1016/S0926-860x(01)00536-1. [DOI] [Google Scholar]
- 3.Peng Y, Si WZ, Li JH, Crittenden J, Hao JM. Experimental and DFT studies on Sr-doped LaMnO3 catalysts for NOx storage and reduction. Catalysis Science & Technology. 2015;5:2478–2485. doi: 10.1039/c5cy00073d. [DOI] [Google Scholar]
- 4.Zhang CH, et al. Relationship between catalytic deactivation and physicochemical properties of LaMnO3 perovskite catalyst during catalytic oxidation of vinyl chloride. Applied Catalysis B-Environmental. 2016;186:173–183. doi: 10.1016/j.apcatb.2015.12.052. [DOI] [Google Scholar]
- 5.Zou GJ, Chen L, Wang XL. Properties and Catalytic Performance for Methane Combustion of LaMnO(3) Perovskite Prepared in Oil-Water Two-Phase System. Catal Lett. 2008;126:96–99. doi: 10.1007/s10562-008-9582-6. [DOI] [Google Scholar]
- 6.Miniajluk N, Trawczynski J, Zawadzki M. Properties and catalytic performance for propane combustion of LaMno(3) prepared under microwave-assisted glycothermal conditions: Effect of solvent diols. Appl Catal a-Gen. 2017;531:119–128. doi: 10.1016/j.apcata.2016.10.026. [DOI] [Google Scholar]
- 7.Lee YN, et al. Surface properties and catalytic performance for ethane combustion of La1-xKxMnO3+ delta perovskites. Applied Catalysis a-General. 2001;207:17–24. doi: 10.1016/S0926-860x(00)00610-4. [DOI] [Google Scholar]
- 8.Alifanti M, Kirchnerova J, Delmon B. Effect of substitution by cerium on the activity of LaMnO3 perovskite in methane combustion. Applied Catalysis a-General. 2003;245:231–243. doi: 10.1016/S0926-860x(02)00644-0. [DOI] [Google Scholar]
- 9.Kamata K. Perovskite Oxide Catalysts for Liquid-Phase Organic Reactions. B Chem Soc Jpn. 2019;92:133–151. doi: 10.1246/bcsj.20180260. [DOI] [Google Scholar]
- 10.Ponce S, Pena MA, Fierro JLG. Surface properties and catalytic performance in methane combustion of Sr-substituted lanthanum manganites. Applied Catalysis B-Environmental. 2000;24:193–205. doi: 10.1016/S0926-3373(99)00111-3. [DOI] [Google Scholar]
- 11.Royer S, et al. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem Rev. 2014;114:10292–10368. doi: 10.1021/cr500032a. [DOI] [PubMed] [Google Scholar]
- 12.Zhu HY, Zhang PF, Dai S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. Acs Catal. 2015;5:6370–6385. doi: 10.1021/acscatal.5b01667. [DOI] [Google Scholar]
- 13.Patil, S., Seal, S., Guo, Y., Schulte, A. & Norwood, J. Role of trivalent La and Nd dopants in lattice distortion and oxygen vacancy generation in cerium oxide nanoparticles. Applied Physics Letters88, 10.1063/1.220795 (2006).
- 14.Deshpande Sameer, Patil Swanand, Kuchibhatla Satyanarayana VNT, Seal Sudipta. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Applied Physics Letters. 2005;87(13):133113. doi: 10.1063/1.2061873. [DOI] [Google Scholar]
- 15.Solanki PR, et al. Nanostructured cerium oxide film for triglyceride sensor. Sensors and Actuators B-Chemical. 2009;141:551–556. doi: 10.1016/j.snb.2009.05.034. [DOI] [Google Scholar]
- 16.Kaushik Ajeet, Solanki Pratima Rathee, Ansari Anees Ahmad, Ahmad Sharif, Malhotra Bansi Dhar. A nanostructured cerium oxide film-based immunosensor for mycotoxin detection. Nanotechnology. 2009;20(5):055105. doi: 10.1088/0957-4484/20/5/055105. [DOI] [PubMed] [Google Scholar]
- 17.Ansari Anees A., Solanki Pratima R., Malhotra B. D. Sol-gel derived nanostructured cerium oxide film for glucose sensor. Applied Physics Letters. 2008;92(26):263901. doi: 10.1063/1.2953686. [DOI] [Google Scholar]
- 18.Ansari AA, Kaushik A, Solanki PR, Malhotra BD. Sol-gel derived nanoporous cerium oxide film for application to cholesterol biosensor. Electrochemistry Communications. 2008;10:1246–1249. doi: 10.1016/j.elecom.2008.06.003. [DOI] [Google Scholar]
- 19.Mefford JT, Hardin WG, Dai S, Johnston KP, Stevenson KJ. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat Mater. 2014;13:726–732. doi: 10.1038/NMAT4000. [DOI] [PubMed] [Google Scholar]
- 20.Fan Q, et al. Catalytic oxidation of diesel soot particulates over Ag/LaCoO3 perovskite oxides in air and NOx. Chinese Journal of Catalysis. 2016;37:428–435. doi: 10.1016/S1872-2067(15)61000-2. [DOI] [Google Scholar]
- 21.Thanneeru R, Patil S, Deshpande S, Seal S. Effect of trivalent rare earth dopants in nanocrystalline ceria coatings for high-temperature oxidation resistance. Acta Mater. 2007;55:3457–3466. doi: 10.1016/j.actamat.2007.01.043. [DOI] [Google Scholar]
- 22.Lu YJ, Dai QG, Wang XY. Catalytic combustion of chlorobenzene on modified LaMnO3 catalysts. Catal Commun. 2014;54:114–117. doi: 10.1016/j.catcom.2014.05.018. [DOI] [Google Scholar]
- 23.Parchur AK, Prasad AI, Ansari AA, Rai SB, Ningthoujam RS. Luminescence properties of Tb3+-doped CaMoO4 nanoparticles: annealing effect, polar medium dispersible, polymer film and core-shell formation. Dalton T. 2012;41:11032–11045. doi: 10.1039/c2dt31257c. [DOI] [PubMed] [Google Scholar]
- 24.Ansari AA, et al. Synthesis, Structural and Optical Properties of Mn-Doped Ceria Nanoparticles: A Promising Catalytic Material. Acta Metallurgica Sinica-English Letters. 2016;29:265–273. doi: 10.1007/s40195-016-0387-0. [DOI] [Google Scholar]
- 25.Ansari AA, et al. Effect of cobalt doping on structural, optical and redox properties cerium oxide nanoparticles. Phase Transitions. 2016;89:261–272. doi: 10.1080/01411594.2015.1116532. [DOI] [Google Scholar]
- 26.Ansari AA, Singh SP, Singh N, Malhotra BD. Synthesis of optically active silica-coated NdF3 core-shell nanoparticles. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy. 2012;86:432–436. doi: 10.1016/j.saa.2011.10.063. [DOI] [PubMed] [Google Scholar]
- 27.Ansari AA, Yadav R, Rai SB. Enhanced luminescence efficiency of aqueous dispersible NaYF4:Yb/Er nanoparticles and the effect of surface coating. Rsc Adv. 2016;6:22074–22082. doi: 10.1039/c6ra00265j. [DOI] [Google Scholar]
- 28.Sasikala C, et al. Transition metal titanium (Ti) doped LaFeO3 nanoparticles for enhanced optical structural and magnetic properties. J Alloy Compd. 2017;712:870–877. doi: 10.1016/j.jallcom.2017.04.133. [DOI] [Google Scholar]
- 29.Ansari AA, Singh SP, Malhotra BD. Optical and structural properties of nanostructured CeO2:Tb3+ film. Journal of Alloys and Compounds. 2011;509:262–265. doi: 10.1016/j.jallcom.2010.07.009. [DOI] [Google Scholar]
- 30.Janbutrach Yutana, Hunpratub Sitchai, Swatsitang Ekaphan. Ferromagnetism and optical properties of La1 − xAlxFeO3 nanopowders. Nanoscale Research Letters. 2014;9(1):498. doi: 10.1186/1556-276X-9-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad AA, Khan W, Dhiman P, Naqvi AH, Singh M. Structural, Optical and Magnetic Properties of Perovskite (La1−xSrx)(Fe1−xNix)O-3, (x = 0.0, 0.1 & 0.2) Nanoparticles. Electron Mater Lett. 2013;9:77–81. doi: 10.1007/s13391-012-2103-1. [DOI] [Google Scholar]
- 32.Xu, X. Z., Zhang, X. Y. & Wu, Y. L. Folic acid-conjugated GdPO4:Tb3+@SiO2 Nanoprobe for folate receptor-targeted optical and magnetic resonance bi-modal imaging. Journal of Nanoparticle Research18, 10.1007/s11051-016-3649-x (2016).
- 33.Kumar S, Teraoka Y, Joshi AG, Rayalu S, Labhsetwar N. Ag promoted La0.8Ba0.2MnO3 type perovskite catalyst for N2O decomposition in the presence of O-2, NO and H2O. Journal of Molecular Catalysis a-Chemical. 2011;348:42–54. doi: 10.1016/j.molcata.2011.07.017. [DOI] [Google Scholar]
- 34.Giroir-Fendler A, et al. Synthesis of oxide supported LaMnO3 perovskites to enhance yields in toluene combustion. Appl Catal B-Environ. 2016;180:29–37. doi: 10.1016/j.apcatb.2015.06.005. [DOI] [Google Scholar]
- 35.Zhang CH, et al. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene. Appl Catal B-Environ. 2014;148:490–498. doi: 10.1016/j.apcatb.2013.11.030. [DOI] [Google Scholar]
- 36.Ansari AA, et al. Influence of copper ion doping on structural, optical and redox properties of CeO2 nanoparticles. Journal of Electroceramics. 2016;36:150–157. doi: 10.1007/s10832-016-0018-1. [DOI] [Google Scholar]
- 37.Ansari AA, et al. Physicochemical and Redox Characteristics of Fe Ion-doped CeO2 Nanoparticles. Journal of the Chinese Chemical Society. 2015;62:925–932. doi: 10.1002/jccs.201500195. [DOI] [Google Scholar]
- 38.Ansari AA, Parchur AK, Alam M, Azzeer A. Effect of surface coating on optical properties of Eu3+-doped CaMoO4 nanoparticles. Spectrochim Acta A. 2014;131:30–36. doi: 10.1016/j.saa.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 39.Li TF, Liu JJ, Jin XM, Wang F, Song Y. Composition-dependent electro-catalytic activities of covalent carbon-LaMnO3 hybrids as synergistic catalysts for oxygen reduction reaction. Electrochimica Acta. 2016;198:115–126. doi: 10.1016/j.electacta.2016.02.027. [DOI] [Google Scholar]
- 40.Ben Hammouda S, et al. Reactivity of novel Ceria-Perovskite composites CeO2-LaMO3 (MCu, Fe) in the catalytic wet peroxidative oxidation of the new emergent pollutant ‘Bisphenol F’: Characterization, kinetic and mechanism studies. Applied Catalysis B-Environmental. 2017;218:119–136. doi: 10.1016/j.apcatb.2017.06.047. [DOI] [Google Scholar]
- 41.Xu HM, et al. Enhancement of Ce1−xSnxO2 support in LaMnO3 for the catalytic oxidation and adsorption of elemental mercury. Rsc Advances. 2016;6:63559–63567. doi: 10.1039/c6ra10006f. [DOI] [Google Scholar]
- 42.Huang, Z. M. et al. High performance of Mn-Co-Ni-O spinel nanofilms sputtered from acetate precursors. Scientific Reports5, 10.1038/srep10899 (2015). [DOI] [PMC free article] [PubMed]
- 43.Xiong, G. P., He, P. G., Liu, L., Chen, T. F. & Fisher, T. S. Synthesis of porous Ni-Co-Mn oxide nanoneedles and the temperature dependence of their pseudocapacitive behavior. Frontiers in Energy Research, 10.3389/fenrg.2015.00039 (2015).
- 44.Guo DY, et al. Room temperature ferromagnetism in (Ga1−xMnx)(2)O-3 epitaxial thin films. Journal of Materials Chemistry C. 2015;3:1830–1834. doi: 10.1039/c4tc02833c. [DOI] [Google Scholar]
- 45.Ullattil SG, Periyat P. Green microwave switching from oxygen rich yellow anatase to oxygen vacancy rich black anatase TiO2 solar photocatalyst using Mn(II) as ‘anatase phase purifier’. Nanoscale. 2015;7:19184–19192. doi: 10.1039/c5nr05975e. [DOI] [PubMed] [Google Scholar]
- 46.Patra AS, Gogoi G, Sahu RK, Qureshi M. Modulating the electronic structure of lanthanum manganite by ruthenium doping for enhanced photocatalytic water oxidation. Physical Chemistry Chemical Physics. 2017;19:12167–12174. doi: 10.1039/c7cp01444a. [DOI] [PubMed] [Google Scholar]
- 47.Meng M., Wu S. X., Ren L. Z., Zhou W. Q., Wang Y. J., Wang G. L., Li S. W. Enlarged Mn 3s splitting and room-temperature ferromagnetism in epitaxially grown oxygen doped Mn2N0.86 films. Journal of Applied Physics. 2014;116(17):173911. doi: 10.1063/1.4901210. [DOI] [Google Scholar]
- 48.Kapteijn F, Singoredjo L, Andreini A, Moulijn JA. Activity and Selectivity of Pure Manganese Oxides in the Selective Catalytic Reduction of Nitric-Oxide with Ammonia. Applied Catalysis B-Environmental. 1994;3:173–189. doi: 10.1016/0926-3373(93)E0034-9. [DOI] [Google Scholar]
- 49.Thirupathi B, Smirniotis PG. Nickel-doped Mn/TiO2 as an efficient catalyst for the low-temperature SCR of NO with NH3: Catalytic evaluation and characterizations. Journal of Catalysis. 2012;288:74–83. doi: 10.1016/j.jcat.2012.01.003. [DOI] [Google Scholar]