Abstract
Early identification of severe acute pancreatitis (SAP) is critical for clinical decision-making. The apolipoprotein B-to-apolipoprotein A1 ratio (ApoB/A1 ratio) reflects the balance between pro-inflammation and anti-inflammation in vivo. This study investigated the association between serum ApoB/A1 ratio at admission and acute pancreatitis (AP) severity. A total of 375 patients with first attack of AP were retrospectively recruited from January 2014 to December 2017. The severity of AP was assessed at admission based on the 2012 revised Atlanta Classification. Serum lipids levels were tested on the first 24 h of hospitalization, of which the correlations with clinical features or scoring systems were also measured. The ApoB/A1 ratio markedly increased across disease severity of AP. The ApoB/A1 ratio, expressed as both quartile and continuous variables, was significantly associated with a high risk of SAP, even after adjustment for other conventional SAP risk factors. The ApoB/A1 ratio positively correlated with the revised 2012 Atlanta Classification, Ranson score, Bedside Index for Severity in AP score, Modified Computed Tomography Severity Index score, and Acute Physiology and Chronic Health Evaluation II score for AP severity. The optimal cut-off value of ApoB/A1 ratio for detecting SAP was 0.88, with a sensitivity of 83.08% and a specificity of 69.03%. Serum ApoB/A1 ratio at admission is closely correlated with disease severity in patients with AP and can serve as a reliable indicator for SAP in clinical setting.
Subject terms: Predictive markers, Acute pancreatitis
Introduction
Acute pancreatitis (AP) is a common and serious digestive disease that represents a sudden inflammation of the pancreatic gland. During the past two decades, the incidence of AP has sharply increasing, the total mortality rate of which is approximately 3.8–7% in China1. Currently, the 2012 revised Atlanta criteria, reported by the Acute Pancreatitis Classification Working Group, are widely adopted worldwide in daily clinical practice and have redefined the severity of AP2. Most AP patients show a self-limiting course and recovery well with conservative treatment. However, up to 20% AP cases suffer a severe form of the illness, and 10–30% of them experience a fatal outcome3. Given that the inflammatory process in AP differs from the disease severity, an important aspect of managing AP is the classification of disease severity at an early stage. Recognizing AP patients with severe disease could help clinical decision-making with regard to transfer to the intensive care unit (ICU) and early initiation of effective therapy, so that the incidence of complications and the possible mortality can be decreased to the greatest extent possible4. Many scoring systems and biomarkers have been applied to predict AP severity, but they are inadequate in clinical practice, especially at the time of diagnosis and at first admission5. Thus, early prediction of severe AP (SAP) remains a challenge, and simple, fast, accurate predictors for the early detection of patientsat high risk of SAP are urgently needed.
Abnormal lipid metabolism can be found at the early phase of AP6. Khan7,8 reported the concentrations of serum lipids, such as total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), exceed the normal limits in patients with AP within the 2 days of admission. The possible explanations include the following: (1) excessive release of inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α), influences lipid synthesis in the liver9; (2) increased capillary permeability causes a redistribution of lipid profiles from the endovascular to the extravascular compartment10. Lipid disorder is not only the etiology of AP but also the result of systemic stress response and islet function injury after AP, suggesting its potential to predict AP severity.
Apolipoprotein A1 (ApoA1) is the major component of HDL that drives the reverse transport of cholesterol from extrahepatic tissues to the liver, and plays an essential role in protecting the arteries11. Similar to C-reactive protein (CRP), ApoA1 is an important acute phase protein. The physiological functions of ApoA1 in vivo include preventing the interaction between T lymphocytes and macrophages as well as inhibiting the production of various inflammatory factors, thereby restricting the “waterfall” effect of inflammation and playing an antioxidant role12. Moreover, apolipoprotein B (ApoB) is the main structure of low density lipoprotein (LDL), intermediate density lipoprotein (IDL), and very-low-density lipoprotein (VLDL). ApoB can promote lipoprotein entering the vascular wall and stimulate the phagocytosis of macrophages, thus inducing inflammation13. ApoB is a risk factor for rheumatoid arthritis, arteriosclerosis, and coronary heart disease (CHD)14,15. Thus, ApoA1 and ApoB reflect the changes in anti-inflammation and pro-inflammation in vivo, respectively.
ApoB-to-ApoA1 ratio (ApoB/A1 ratio) is a composite index that comprehensively reflects the lipid metabolism balance and inflammatory status in the human body. ApoB/A1 ratio has been widely used to predict cardiovascular disease and metabolic syndrome16. Aside from its easy operation and low price, ApoB/A1 ratio is also superior in clinical settingover other composite indexes, such as TC/HDL-C, LDL-C/HDL-C, and non HDL-C/HDL-C ratio17. Considering the significant effect of systemic inflammatory response on the pathophysiological progress of AP, we hypothesized that ApoB/A1 ratio could serve as a reliable inflammatory predictor of AP severity. Therefore, we conducted this observational study to investigate the correlation between AP severity and ApoB/A1 ratio at admission.
Results
Baseline characteristics
A total of 375 patients were included in this study. These cases consisted of 230 men and 145 women with a mean age of 51.78 years (range 25–74 years). The most common cause of AP was gallstones in 272 cases (72.53%). According to the revised 2012 Atlanta criteria, 200 patients (53.33%) were diagnosed with mild AP (MAP), 110 (29.33%) with moderately severe AP (MSAP), and 65 (17.44%) with SAP. The median length of hospital stay was 11 days. One hundred and eight patients were admitted to the ICU, and 11 patients died within 28 days after admission.
Baseline characteristics of the patients with MAP, MSAP, and SAP are shown in Table 1. The patients were classified according to disease severity. The levels of neutrophil, CRP, LDH, amylase, APACHE II score, Ranson score, hospital stays, and ICU admission significantly increased, whereas the level of serum ALB significantly decreased with the increment of disease severity (P < 0.05). The SAP group had higher levels of glucose, BUN, Ranson score, BISAP score, MCTSI score, 28-days mortality, and lower levels of calcium than the MAP group (P < 0.05). The SAP cohort also had higher levels of BUM, BISAP score, MCTSI score, and 28-days mortality than the MSAP cohort (P < 0.05). The levels of Ranson and MCTSI scores were much greater, whereas the level of calcium was much lower in the MSAP group than in the MAP group (P < 0.05).
Table 1.
Comparison of clinical characteristics, laboratory parameters, clinical outcomes and scoring systems between subgroups in AP patients.
| Characteristics | MAP | MSAP | SAP | P 1 | P 2 | P 3 |
|---|---|---|---|---|---|---|
| No. of patients | 200 | 110 | 65 | |||
| Age, year | 51.36 ± 13.95 | 52.06 ± 14.22 | 53.79 ± 15.64 | 0.681 | 0.236 | 0.441 |
| Male sex, n (%) | 118 (59.0) | 68 (61.8) | 44 (67.7) | 0.628a | 0.212a | 0.434a |
| Etiology, n (%) | 0.137a | 0.278a | 0.885a | |||
| Gallstones | 152 (76.0) | 75 (68.2) | 45 (69.2) | |||
| Other | 48 (24.0) | 35 (31.8) | 20 (30.8) | |||
| Smoking habit, n (%) | 56 (28.0) | 36 (32.7) | 25 (38.5) | 0.383a | 0.112a | 0.442a |
| BMI, kg/m2 | 24.11 ± 4.55 | 24.42 ± 4.68 | 24.87 ± 4.83 | 0.574 | 0.252 | 0.536 |
| WBC, ×109/L | 12.24 ± 4.98 | 12.68 ± 5.15 | 13.05 ± 5.55 | 0.471 | 0.270 | 0.645 |
| Neutrophil, ×109/L | 9.11 ± 3.75 | 11.25 ± 4.34 | 13.47 ± 6.08 | <0.001 | <0.001 | 0.001 |
| Lymphocyte, ×109/L | 1.06 ± 0.66 | 1.10 ± 0.62 | 0.99 ± 0.74 | 0.612 | 0.460 | 0.290 |
| RBC, ×109/L | 4.72 ± 0.71 | 4.65 ± 0.70 | 4.58 ± 0.63 | 0.396 | 0.159 | 0.520 |
| HGB, g/L | 143.45 ± 27.33 | 141.21 ± 25.15 | 138.50 ± 24.52 | 0.521 | 0.230 | 0.543 |
| PLT, ×109/L | 187.65 ± 66.24 | 182.39 ± 62.71 | 176.74 ± 63.15 | 0.494 | 0.237 | 0.575 |
| CRP, mg/L | 71 (18–126) | 145 (96–237) | 212 (158–356) | <0.001 c | <0.001 c | <0.001 c |
| ALT, U/L | 98 (5–831) | 106 (10–883) | 113 (12–1055) | 0.514c | 0.309c | 0.665c |
| AST, U/L | 125 (15–921) | 133 (21–835) | 129 (20–847) | 0.599c | 0.827c | 0.842c |
| LDH, U/L | 262.29 ± 150.75 | 323.84 ± 162.38 | 455.93 ± 204.65 | 0.002 | <0.001 | <0.001 |
| Glucose, mmol/L | 7.98 ± 4.23 | 8.22 ± 4.67 | 9.43 ± 4.88 | 0.652 | 0.024 | 0.085 |
| ALB, g/L | 37.53 ± 6.36 | 35.19 ± 5.77 | 33.26 ± 5.45 | 0.002 | 0.001 | 0.046 |
| Amylase, U/L | 524 (56–3146) | 685 (89–3313) | 892 (84–3607) | 0.037 c | <0.001 c | 0.042 c |
| BUN, mmol/L | 4.44 ± 1.68 | 4.83 ± 1.74 | 5.99 ± 2.92 | 0.096 | <0.001 | <0.001 |
| Calcium, mmol/L | 2.17 ± 0.27 | 2.09 ± 0.25 | 2.01 ± 0.30 | 0.013 | 0.002 | 0.059 |
| TC, mmol/L | 3.86 ± 1.90 | 4.05 ± 2.12 | 4.08 ± 2.14 | 0.426 | 0.444 | 0.924 |
| TG, mmol/L | 2.98 ± 1.81 | 3.64 ± 2.78 | 4.26 ± 3.07 | 0.020 | 0.001 | 0.096 |
| HDL-C, mmol/L | 1.29 ± 0.26 | 1.25 ± 0.31 | 1.17 ± 0.29 | 0.231 | 0.003 | 0.069 |
| LDL-C, mmol/L | 2.95 ± 1.45 | 3.21 ± 1.69 | 3.12 ± 1.43 | 0.151 | 0.434 | 0.706 |
| ApoA1, g/L | 1.10 ± 0.46 | 1.12 ± 0.51 | 0.95 ± 0.43 | 0.720 | 0.026 | 0.021 |
| ApoB, g/L | 0.72 ± 0.48 | 0.88 ± 0.50 | 0.96 ± 0.61 | 0.009 | 0.001 | 0.317 |
| ApoB/A1 ratio | 0.66 ± 0.41 | 0.81 ± 0.43 | 1.02 ± 0.62 | 0.006 | <0.001 | 0.004 |
| APACHE II score (at 24 h) | 4.0 (3.0–6.0) | 9.00 (4.0–10.0) | 13.0 (8.0–19.0) | <0.001 c | <0.001 c | 0.001 c |
| Ranson score (at 48 h) | 2.0 (1.0–2.0) | 4.0 (2.0–6.0) | 5.0 (4.0–7.0) | <0.001 c | <0.001 c | 0.056c |
| BISAP score | 1.0 (0.0–1.0) | 1.0 (1.0–2.0) | 2.5 (2.0–3.0) | 0.847c | <0.001 c | <0.001 c |
| MCTSI score | 2 (1–3) | 5 (2–7) | 6 (4–8) | 0.002 c | <0.001 c | 0.528c |
| Hospital stay, days | 8 (6–13) | 23 (11–33) | 41 (22–64) | <0.001 c | <0.001 c | <0.001 c |
| Intensive care unit, n (%) | 15 (7.5) | 42 (38.2) | 51 (78.5) | <0.001 a | <0.001 a | <0.001 a |
| 28 days mortality, n (%) | 0 (0.0) | 1 (0.9) | 10 (15.4) | 0.355b | <0.001 b | <0.001 b |
Continuous variables are described as means ± standard deviations (normal distribution) or median and interquartile range (abnormal distribution). Categorical variables are described as N (%).
Notes: P1, MAP vs. MSAP; P2,MAP vs. SAP; P3, MSAP vs. SAP.
aStatistical results based on χ2 analysis; bStatistical results based on Fisher’s exact test; cStatistical results based on Kruskall-Wallis test; the others were based on one way variance analysis and least-significance difference t test.
MAP, mild acute pancreatitis; MASP, moderately severe acute pancreatitis; SAP, severe acute pancreatitis; BMI, body mass index; WBC,white blood cell; RBC, red blood cell; HGB, hemoglobin; PLT,platelets; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; ALB, albumin; BUN, blood urea nitrogen; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB/A1 ratio, apolipoprotein B-to-apolipoprotein A1 ratio; APACHE II, acute physiology and chronic health evaluationII; BISAP, besides index for severity in acute pancreatitis; MCTSI, modified computed tomography severity index.
When the patients were divided into quartiles based on the levels of ApoB/A1 ratio (Table 2), the prevalence of SAP significantly increased across quartiles. BMI, CRP, neutrophil, LDH, amylase, BUN, TG, LDL-C, ApoB, APACEH II score, Ranson score, BISAP score, CTSI score, hospital stay, ICU admission, and 28-days mortality increased, whereas ALB, HDL-C, and ApoA1 decreased across quartiles.
Table 2.
Baseline characteristics of AP patients according to ApoB/A1 ratio quartiles.
| Characteristics | Quartile 1 (0.19–0.60) | Quartile 2 (0.61–0.80) | Quartile 3 (0.81–0.98) | Quartile 4 (0.99–1.67) | P |
|---|---|---|---|---|---|
| No. of patients | 94 | 93 | 94 | 94 | |
| SAP, n (%) | 5 (5.3) | 12 (12.9) | 20 (21.3) | 28 (29.8) | <0.001 |
| Age, year | 52.18 ± 14.31 | 51.72 ± 14.15 | 53.25 ± 15.57 | 53.59 ± 15.63 | 0.743 |
| Male sex, n (%) | 55 (58.5) | 56 (60.2) | 59 (62.8) | 60 (63.8) | 0.875a |
| Etiology, n (%) | 0.197a | ||||
| Gallstones | 62 (66.0) | 66 (71.0) | 69 (73.4) | 75 (79.8) | |
| Other | 32 (34.0) | 27 (29.0) | 25 (26.6) | 19 (20.2) | |
| BMI, kg/m2 | 23.41 ± 3.02 | 23.85 ± 3.24 | 24.57 ± 3.95 | 25.13 ± 4.36 | 0.008 |
| Smoking habit, n (%) | 24 (25.5) | 27 (29.0) | 31 (33.0) | 35 (37.2) | 0.342a |
| WBC count, ×109/L | 11.77 ± 4.52 | 12.48 ± 5.17 | 12.89 ± 5.33 | 13.42 ± 5.68 | 0.169 |
| Neutrophil count, ×109/L | 9.08 ± 3.66 | 10.37 ± 4.05 | 11.94 ± 4.55 | 13.56 ± 6.10 | <0.001 |
| Lymphocyte count, ×109/L | 1.08 ± 0.64 | 1.11 ± 0.67 | 1.04 ± 0.61 | 1.01 ± 0.62 | 0.719 |
| RBC count, ×109/L | 4.79 ± 0.73 | 4.68 ± 0.71 | 4.66 ± 0.71 | 4.55 ± 0.68 | 0.144 |
| HGB, g/L | 141.27 ± 26.48 | 145.23 ± 27.36 | 139.84 ± 24.99 | 140.77 ± 25.23 | 0.512 |
| PLT, ×109/L | 186.54 ± 60.66 | 180.55 ± 67.48 | 177.97 ± 64.24 | 173.59 ± 61.15 | 0.562 |
| CRP, mg/L | 76 (19–124) | 125 (81–234) | 171 (123–288) | 222 (145–381) | <0.001 c |
| ALT, U/L | 101 (6–754) | 99 (8–821) | 105 (15–972) | 116 (12–1061) | 0.492c |
| AST, U/L | 121 (15–831) | 129 (25–874) | 132 (20–968) | 130 (18–912) | 0.740c |
| LDH, U/L | 267.06 ± 148.42 | 316.63 ± 158.71 | 391.14 ± 172.45 | 469.89 ± 211.31 | <0.001 |
| Glucose, mmol/L | 7.72 ± 3.65 | 8.55 ± 4.79 | 8.26 ± 4.67 | 9.33 ± 4.54 | 0.093 |
| ALB, g/L | 37.76 ± 6.42 | 36.24 ± 5.85 | 34.89 ± 5.33 | 32.63 ± 5.35 | <0.001 |
| Amylase, U/L | 525 (50–2907) | 671 (81–3233) | 797 (74–3452) | 875 (88–3666) | <0.001 c |
| BUN, mmol/L | 4.35 ± 1.37 | 4.77 ± 1.52 | 5.16 ± 1.81 | 6.13 ± 2.99 | <0.001 |
| Calcium, mmol/L | 2.13 ± 0.28 | 2.10 ± 0.25 | 2.07 ± 0.24 | 2.06 ± 0.26 | 0.239 |
| TC, mmol/L | 3.84 ± 1.88 | 4.03 ± 2.10 | 3.97 ± 2.06 | 4.12 ± 2.20 | 0.822 |
| TG, mmol/L | 2.84 ± 1.72 | 3.25 ± 1.92 | 3.69 ± 2.68 | 4.21 ± 2.98 | <0.001 |
| HDL-C, mmol/L | 1.27 ± 0.32 | 1.22 ± 0.27 | 1.20 ± 0.25 | 1.16 ± 0.24 | 0.048 |
| LDL-C, mmol/L | 2.71 ± 1.33 | 2.95 ± 1.51 | 3.14 ± 1.54 | 3.39 ± 1.91 | 0.027 |
| ApoA1, g/L | 1.24 ± 0.55 | 1.18 ± 0.52 | 1.07 ± 0.45 | 0.93 ± 0.40 | <0.001 |
| ApoB, g/L | 0.68 ± 0.47 | 0.79 ± 0.49 | 0.88 ± 0.57 | 0.98 ± 0.62 | 0.002 |
| APACHE II score (at 24 h) | 3.0 (2.0–6.0) | 6.0 (3.0–10.0) | 10.0 (6.0–15.0) | 13.0 (8.0–19.0) | <0.001 c |
| Ranson score (at 48 h) | 2.0 (1.0–2.0) | 2.0 (1.0–2.0) | 4.0 (1.0–5.0) | 5.0 (3.0–7.0) | <0.001 c |
| BISAP score | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | 2.0 (1.0–2.0) | 2.5 (1.0–3.0) | <0.001 c |
| CTSI score | 2 (1–3) | 4 (1–6) | 5 (2–7) | 6 (3–8) | <0.001 c |
| Hospital stay, days | 7 (5–11) | 13 (8–27) | 26 (14–40) | 45 (20–68) | <0.001 c |
| Intensive care unit, n (%) | 11 (11.7) | 18 (19.4) | 34 (36.2) | 45 (47.9) | <0.001 a |
| 28 days mortality, n (%) | 0 (0.0) | 0 (0.0) | 2 (2.1) | 5 (5.3) | 0.019 b |
Continuous variables are described as means ± standard deviation (normal distribution) or median and interquartile range (abnormal distribution). Categorical variables are described as N (%).
aStatistical results based on χ2 analysis; bStatistical results based on Fisher’s exact test; cStatistical results based on Kruskall-Wallis test; the others were based on one way variance analysis.
SAP, severe acute pancreatitis; BMI, body mass index; WBC, white blood cell; RBC, red blood cell; HGB, hemoglobin; PLT,platelets; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; ALB, albumin; BUN, blood urea nitrogen; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low -density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB/A1 ratio, apolipoprotein B-to-apolipoprotein A1 ratio; APACHE II, acute physiology and chronic health evaluationII; BISAP, besides index for severity in acute pancreatitis; CTSI, CT severity index.
Association between serum lipid parameters and AP severity
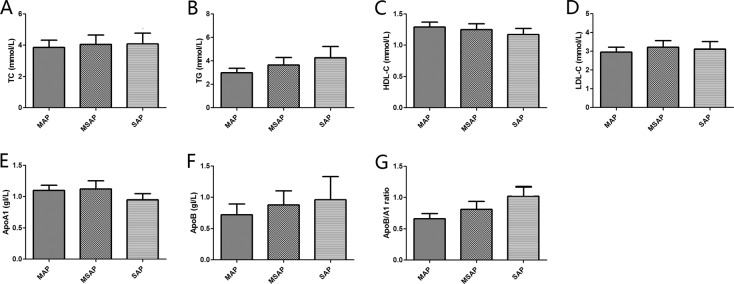
The association between serum lipid parameters and disease severity of AP was also demonstrated in Table 1 and Fig. 1. The levels of TC and LDL-C showed no difference among the AP patients with different levels of disease severity based on the 2012 revised Atlanta Classification. The level of ApoB/A1 ratio markedly increased across disease severity. The serum levels of TG, HDL-C, ApoA1, and ApoB were significantly higher in the patients with SAP than in those with MAP. The level of ApoA1 in the SAP group was much higher than that in the MSAP group. Simultaneously, the MSAP group had higher levels of TG and ApoB compared with the MAP group.
Figure 1.
Serum lipidslevels in different AP severities according to the revised 2012 Atlanta Classification. (A) Serum TC levels in different AP severities. (B) Serum TG levels in different AP severities. (C) Serum HDL-C levels in different AP severities. (D) Serum LDL-C levels in different AP severities. (E) Serum apoA1 levels in different AP severities. (F) Serum apoB levels in different AP severities. (G) Serum ApoB/A1 ratio levels in different AP severities. Abbreviation: AP, acute pancreatitis; MAP, mild acute pancreatitis; MSAP, mild severe acute pancreatitis; SAP, severe acute pancreatitis; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB/A1 ratio, apolipoprotein B-to-apolipoprotein A1 ratio.
The correlation between serum lipid parameters and scoring systems in AP patients was exhibited in Table 3. The ApoB/A1 ratio positively correlated with the revised 2012 Atlanta criteria (r = 0.424, P < 0.001), Ranson score (r = 0.310, P < 0.001), BISAP score (r = 0.188, P = 0.034), MCTSI score (r = 0.201, P = 0.014), and APACHE II score (r = 0.504, P < 0.001). The serum level of ApoB was also positively related to the revised 2012 Atlanta criteria (r = 0.185, P = 0.038), MCTSI score (r = 0.230, P < 0.001), and APACHE II score (r = 0.221, P = 0.003). Serum ApoA1 level was inversely correlated with the BISAP score (r = −0.260, P < 0.001). HDL-C level was also inversely related to the revised 2012 Atlanta criteria (r = −0.168, P = 0.041), BISAP score (r = −0.228, P = 0.002), and APACHE II score (r = −0.329, P < 0.001).
Table 3.
Correlation between serum lipid parameters and scoring systems in AP patients.
| Variable | Statistics | Atlanta 2012 | Ranson | BISAP | MCTSI | APACHE II |
|---|---|---|---|---|---|---|
| TC | R | 0.071 | 0.110 | 0.155 | 0.082 | 0.034 |
| P | 0.313 | 0.144 | 0.065 | 0.267 | 0.618 | |
| TG | R | 0.123 | 0.049 | −0.100 | 0.163 | 0.197 |
| P | 0.095 | 0.447 | 0.188 | 0.045 | 0.022 | |
| HDL-C | R | −0.168 | −0.119 | −0.228 | −0.052 | −0.329 |
| P | 0.041 | 0.102 | 0.002 | 0.408 | <0.001 | |
| LDL-C | R | −0.043 | 0.189 | 0.105 | 0.094 | 0.079 |
| P | 0.555 | 0.035 | 0.163 | 0.191 | 0.281 | |
| ApoA1 | R | −0.077 | −0.020 | −0.260 | −0.085 | −0.112 |
| P | 0.286 | 0.874 | <0.001 | 0.255 | 0.140 | |
| ApoB | R | 0.185 | 0.135 | 0.127 | 0.230 | 0.221 |
| P | 0.038 | 0.071 | 0.088 | <0.001 | 0.003 | |
| ApoB/A1 ratio | R | 0.424 | 0.310 | 0.188 | 0.201 | 0.504 |
| P | <0.001 | <0.001 | 0.034 | 0.014 | <0.001 |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low -density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB/A1 ratio, apolipoprotein B to apolipoprotein A1 ratio; APACHE II, acute physiology and chronic health evaluation II; BISAP, besides index for severity in acute pancreatitis; MCTSI, modified CT severity index.
Risk of SAP according to ApoB/A1 ratio
The association between SAP and ApoB/A1 ratio was analyzed by logistic regression analyses. Univariate logistic regression analyses demonstrated that neutrophil, CRP, LDH, glucose, ALB, amylase, BUN, calcium, TG, HDL-C, ApoA1, ApoB, and ApoB/A1 ratio (continuous variable) were significant risk factors of SAP (Supplementary Table 1). A crude model of univariate logistic regression analysis also showed that a high interquartile of ApoB/A1 ratio was dramatically related to a high risk of SAP (Table 4). Multivariate logistic regression analyses indicated that AP patients with a high ApoB/A1 ratio, expressed both as quartile and continuous variables, are prone to suffer a high risk of SAP, even after adjustment for age, sex, etiology of gallstones, BMI, smoking habit, WBC, neutrophil, lymphocyte, RBC, HGB, PLT, CRP, ALT, AST, LDH, glucose, ALB, amylase, BUN, calcium, TC, TG, HDL-C, LDL-C, ApoA1, or ApoB (Table 4, Model 1~4). In the fully adjusted model, the OR (95% CI) of ApoB/A1 ratio for risk of SAP was 4.87 (2.99–11.79) when ApoB/A1 ratio was analyzed as a continuous variable (Table 4, Model 4).
Table 4.
Risk for severe acute pancreatitis according to ApoB/A1 ratio.
| Quartiles of ApoB/apoA1 ratio | Continuous variable OR (95% CI) | P value | |||||
|---|---|---|---|---|---|---|---|
| Quartile 1 (0.19–0.60) | Quartile 2 (0.61–0.80) | Quartile 3 (0.81–0.98) | Quartile 4 (0.99–1.67) | P for trend | |||
| Crude model | 1 (reference) | 2.48 (1.31–5.97) | 3.75 (1.88–7.05) | 5.34 (3.54–9.34) | <0.001 | 7.73 (3.85–12.61) | <0.001 |
| Model 1a | 1 (reference) | 2.16 (1.26–5.22) | 3.23 (1.63–6.95) | 4.95 (3.15–8.21) | <0.001 | 6. 64 (4.08–10.25) | <0.001 |
| Model 2b | 1 (reference) | 1.82 (1.20–4.78) | 2.66 (1.57–5.49) | 4.58 (3.00–7.32) | <0.001 | 5.79 (3.62–8.44) | <0.001 |
| Model 3c | 1 (reference) | 1.73 (1.16–4.24) | 2.31 (1.39–4.86) | 4.11 (2.76–6.98) | <0.001 | 5.01 (3.21–7.88) | <0.001 |
| Model 4d | 1 (reference) | 1.44 (1.09–3.33) | 2.14 (1.30–4.11) | 3.67 (2.35–5.91) | 0.008 | 4.27 (2.99–6.79) | <0.001 |
ApoB/A1 ratio, apolipoprotein B-to-apolipoprotein A1 ratio; OR, odds ratio; CI, confidence interval.
aModel 1 was adjusted for age, sex, etiology of gallstones, body mass index, and smoking habit.
bModel 2 was additionally adjusted for white blood cell, neutrophil, lymphocyte, red blood cell, hemoglobin, and platelets.
cModel 3 was additionally adjusted for C-reactive protein, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, glucose, albumin, amylase, blood urea nitrogen, and calcium.
dModel 4 was additionally adjusted for total cholesterol, triglyceride, high density lipoprotein cholesterol, low density lipoprotein cholesterol, apolipoprotein A1, and apolipoprotein B.
Predicting value of ApoB/A1 ratio for AP severity
We assessed the predictive ability of each lipid parameter for SAP using ROC analysis. The optimal cut-off value of ApoB/A-I ratio to predict SAP was 0.88 with sensitivity and specificity of 83.08% and 69.03%, respectively (Table 5; Fig. 2). The AUC of ApoB/A-I ratio was 0.812 (95% CI: 0.769–0.851, P < 0.001), which was significantly higher than those of other lipid parameters, such as TC (Z = 6.848, P < 0.001), TG (Z = 4.503, P < 0.001), HDL-C (Z = 2.022, P = 0.043), LDL-C (Z = 6.919, P < 0.001), ApoA1 (Z = 3.670, P < 0.001), and ApoB (Z = 4.412, P < 0.001) (Table 5).
Table 5.
Performance of serum lipids for predicting SAP.
| Variable | AUC (95% CI) | P value | Cut-off | Sensitivity | Specificity | PLR | NLR |
|---|---|---|---|---|---|---|---|
| TC, mmol/L | 0.553 (0.502–0.605) | 0.160 | 4.05 | 43.08 | 63.55 | 1.18 | 0.90 |
| TG, mmol/L | 0.635 (0.584–0.684) | 0.002 | 3.97 | 46.15 | 86.13 | 3.33 | 0.97 |
| HDL-C, mmol/L | 0.706 (0.657–0.752) | <0.001 | 1.23 | 76.92 | 54.37 | 1.69 | 0.42 |
| LDL-C, mmol/L | 0.531 (0.479–0.583) | 0.457 | 3.10 | 33.85 | 41.52 | 1.33 | 0.89 |
| ApoA1, g/L | 0.692 (0.642–0.738) | <0.001 | 1.03 | 80.00 | 58.39 | 1.92 | 0.34 |
| ApoB, g/L | 0.653 (0.602–0.701) | <0.001 | 0.91 | 64.62 | 59.03 | 1.58 | 0.60 |
| ApoB/A1 ratio | 0.812 (0.769–0.851) | <0.001 | 0.88 | 83.08 | 69.03 | 2.68 | 0.25 |
SAP, severe acute pancreatitis; AUC, area under curve; PLR, positive likelihood ratio; NLR, negative likelihood ratio; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB/A1 ratio, apolipoprotein B-to-apolipoprotein A1 ratio.
Figure 2.
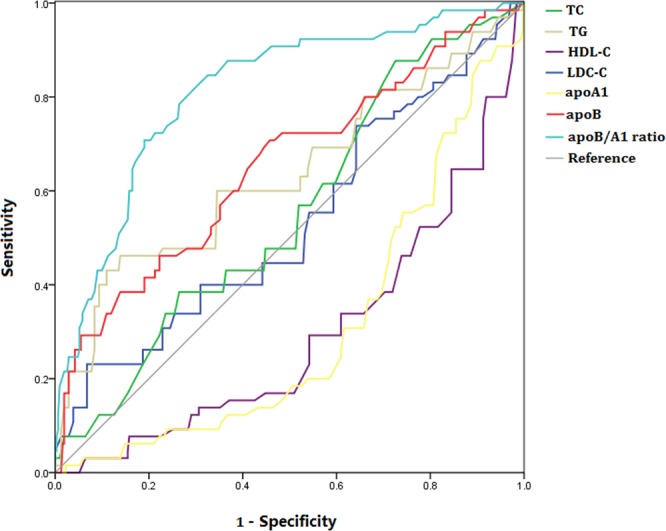
Receiver operating characteristics curves of serum lipids for predicting severe acute pancreatitis. Abbreviation: TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoB/A1 ratio, apolipoprotein B-to-apolipoprotein A1 ratio.
Discussion
Due to the clinical features of AP, potential patients with SAP should be recognized to ensure that they receive intensive care and adequate treatment in a timely manner. Earlier efforts to classify the AP severity resulted in the original 1992 Atlanta Classification, which had identified two subgroups of AP, “mild” and “severe”, and recommended the clinical treatment for each category18. However, emerging evidence has revealed that most AP patients who fell in between the two categories based on the 1992 Severity Classification often suffered relatively good outcomes and positive response to less aggressive treatment than those with severe disease, thus necessitating the substantial revision of this classification system19. In 2012, a revised version of the Atlanta Classification has been established by adding a third category defined as “moderately severe”2. According to a single-center research in Chinese, 649 patients with “severe” disease defined by the 1992 Atlanta Classification were included, and subsequently regrouped as “MSAP” and “SAP” according to the revised 2012 Atlanta Classification20. When compared with the MSAP group, the Ranson score, APACHEII score, BISAP score, MCTSI score, Marshall score, incidence of organ failure, average length of stay, and hospital mortality of the SAP group significantly increased, which indicated that the revised 2012 Atlanta Classification could more precisely reflect AP severity. Therefore, our study explored the value of ApoB/A1 ratio in predicting AP severity based on the revised 2012 Atlanta Classification.
The present study found a close association between high ApoB/A1 ratio and the worsening of AP severity, regardless of conventional risk factor. The mechanisms underlying the association between ApoB/A1 ratio and AP severity have been not yet completely understood and are probably multi-factorial. Systematic inflammatory response syndrome (SIRS) and organ dysfunction are the main features in the early phase of AP, whereas pancreatic necrosis and infection in the late stage21. In the traditional view, the main cause of death in AP patients is the complications in the late stage. However, a recent growing number of studies have confirmed that early-phase SIRSs are the main reasons for organ failure and mortality in AP patients22. Thus, the balance between pro-inflammatory and anti-inflammatory status is the key point of severity prediction and clinical therapy23. ApoB facilitates, whereas ApoA1 suppresses systemic inflammatory states. Hence, an increased ApoB/A1 ratio might reflect a predominance of pro-inflammatory effects over anti-inflammatory of lipoprotein lipids, thus contributing to the progress of inflammation and AP severity. Huh24 also found that ApoB/A1 ratio is strongly predictive of SAP independent of the etiology of AP, of which the optimal cut-off value was 1.16 with 53% sensitivity and 93% specificity. Notable, the optimal cut-off value in our research was 0.88 with a relatively high sensitivity of 83.08% and a relatively low specificity of 69.03%, which differed considerably from the previous study. Given that SAP is deeply harmful to the human body and needs prompt treatment, false negative results should be reduced and misdiagnose of SAP should be avoided25. Therefore, we applied a low cutoff value to achieve a high sensitivity.
Another major finding of our study is that the ApoB/A1 ratio was the strongest indicator for SAP compared with the other lipid parameters. Several studies have showed that lipid profiles, including HDL-C, and LDL-C at admission, could predict the development of severe disease and organ failure in AP patients26,27. However, these traditional lipid indexes are not always adequate indicators of dyslipidemia due to the wide alternation of measurement, whereas the lipid-transporting apolipoprotein is relatively more stable. First, each lipid particle, including LDL, VLDL, and IDL, contains only one unit of ApoB. Considering that atherogenic dyslipidemia promotes inflammatory response and causes oxidative stress in pancreas, the predictive value is more related to the number of atherogenic particles but not LDL-C alone, which only measures the amount of cholesterol in LDL particles28. Thus, the presence of a few but large particles is more favorable than the presence of a large number of small particlesat a given LDL-C concentration29. Second, the removal processes of ApoA1 and HDL-C are prone to be affected by plasma transferrin and transferase30. During the alteration of lipid metabolism, the change in HDL-C was more significant than that in ApoA1. Three large cohort studies also exhibited a consistent outcome that both ApoB and ApoA1 had independent and equal predictive values, and ApoB/A1 ratio was the strongest and most specific indicator for cardiovascular disease that was superior to the cholesterol ratios31–33. Therefore, ApoB/A1 ratio demonstrates better superiority over the cholesterol ratios in terms of predictive ability. However, these findings were mainly based on research concerning cardiovascular diseases. Medical evidence for ApoB/A1 ratio in the prediction of AP severity is insufficient. Thus, additional studies are warranted to further confirm the advantage of this parameter.
To date, several scoring systems including Ranson score, BISAP score, MCTSI score, and APACHE II score, have been widely applied to predict AP severity at an early stage. The scoring systems have played a valuable role in the early prediction of AP severity. In our study, the ApoB/A1 ratio was significantly positively correlated with the above-mentioned scoring systems rather than other lipid indexes, which indicated the preponderance of ApoB/A1 ratio on predicting AP severity from another perspective. However, abundant evidence has proven that these scoring systems are unsuitable in clinical practice because of their complex and time-consuming applications34. For example, Ranson score should be obtained within 48 h after admission, and some variables, such as residual alkali and fluid isolation, are not the routine assessment in hospital35. BISAP score is established based on mortality, of which the predicting value for AP severity is relatively low36. MCTSI score is superior to other scoring systems on the assessment of the extent of pancreatic necrosis but fails in some SAP patients with delayed pancreatic necrosis37. APACHE II score was initially designed as an ICU instrument and therefore comprises many variables of medical history and medication details, which may be unavailable if the patients are unconscious, intubated, or transferred fromother medical institutions with insufficient medical records38. By contrast, the examinations of ApoA1 and ApoB are routine blood tests on admission, of which the operation is simple, convenient, and can be carried out in most hospitals. Therefore, the ApoB/A1 ratio may be more applicable to predict AP severity than the scoring systems.
Several limitations of this study should be considered when interpreting the results. First, this study was originated from a single institution in China, and the number of patients was relatively small. Thus, the findings of this study cannot be generalized. Further multicentric studies with large sample sizes are needed to validate the results. Second, given its cross-sectional design, this study could not establish a causal relationship between ApoB/A1 ratio and AP severity. Third, we did not compare ApoB/A1 ratio with other conventional inflammatory biomarkers, such as procalcitonin and IL-6. Fourth, outcomes related to SAP, such as mortality and organ failure, were not examined. AP patients with adverse outcomes would likely benefit from early classification of disease severity. Fifth, our study was conducted in the largest hospital in the region, which was committed to the treatment of critical illness, and thus could lead to a disproportional inclusion of patients with MSAP or SAP. Such selection bias might result in an overestimation of the predictive value of ApoB/A1 ratio.
In conclusion, this study revealed that serum ApoB/A1 ratio at admission is independently associated with disease severity in patients with AP. Pretreatment serum ApoB/A1 ratio can serve as a reliable indicator for SAP in clinical setting, and its application at admission may improve clinical management strategies for patients with AP.
Methods
Patient population
This retrospective observational study included 375 AP patients aged 18–75 years who were consecutively admitted to the Department of Gastroenterology between January 2014 to December 2017. All these patients were diagnosed with a first attack of AP according to typical symptoms, including acute abdominal pain and serum amylase level that was at least three times higher than the upper normal limit or a confirmation of pancreatitis by radiologic findings. The interval between the occurrence of symptoms and on admission was within 24 h. We excluded patients with chronic diseases, such as hypertension, diabetes, liver disease, renal disease, and malignant tumors. We also excluded patients who were pregnant or have taken lipid-lowering drugs within the last 6 months. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University (No: PJ2018-041). The study methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from each patient prior to study enrolment.
Definition
The disease severity of AP patients was measured at admission based on the 2012 revised Atlanta Classification and was divided into three groups, namely, MAP, MSAP, and SAP2. MAP was defined as the absence of organ failure and local or systemic complications. MSAP was defined as the presence of organ failure that resolved within 48 h and/or local or systemic complications without persistent organ failure. SAP was defined as persistent organ failure (lasting > 48 h). The Bedside Index for Severity in AP (BISAP) was determined within 24 h of admission. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score and the Ranson score were also measured at 24 and 48 h after admission, respectively. Contrast-enhanced computed tomography (CT) was performed within 24 h after admission, and the Modified CT Severity Index (MCTSI) scores were subsequently assessed. Imaging examinations were performed by the same two senior radiologists. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of a person’s height in meters.
Data collection
Demographics (i.e., age, sex, and BMI), etiology (i.e., gallstones or not), and lifestyle factor (smoking habit) were recorded for each patient on admission. Blood samples were collected from patients after overnight fasting on the first 24 h of hospitalization and then analyzed immediately for laboratory tests, including serum lipids [i.e., TC, triglyceride (TG), HDL-C, LDL-C, ApoA1, and ApoB], complete blood count [i.e., white blood cell (WBC), neutrophil, lymphocyte, red blood cell (RBC), hemoglobin (HGB), and platelets (PLT)], and serum biochemical indexes [i.e., CRP, alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), amylase, albumin (ALB), blood urea nitrogen (Bun), and calcium]. The ApoB/A1 ratio was calculated according to the ratio between serum ApoB and ApoA1 concentrations on the first day of hospitalization. Therapeutic regimen and ICU admission were decided by the attending physician, independently from the participants of this study. The length of hospital stays and the number of death with 28 days after admission were also recorded. At the time of data collection, the participants (YF Wang and WK Tan) were blinded to the disease severity and clinical outcomes of patients.
Statistical analysis
Data were recorded into a Microsoft Excel database. Continuous data accorded withnormal distribution were presented as means ± standard deviations (SDs); otherwise, they were presented as medians and interquartile ranges. Categorical variables were described as frequencies and proportions (%). Differences amongmultiple groups were evaluated by one-way variance analysis for continuous variables with normal distribution, nonparametric Kruskall – Wallis test for continuous variables with abnormal distribution, and by Pearson’s χ2 test or Fisher’s exact test for categorical variables. Spearman rank correlation was performed to evaluate the correlation between serum lipid levels and scoring systems. We calculated the odds ratios (ORs) for predicting the risk of SAP by using logistic regression analysis after adjustment for confounding factors. All statistical analyses were conducted by SPSS version 25.0 for windows (SPSS Inc., Chicago, IL), except for the analyses with receiver operating characteristic (ROC) curve.
The area under the ROC curve (AUC) was applied to determine the discriminative values of serum lipid parameters for SAP. It was also used to assess the optimal cut-off by showing the trade-off between sensitivity and specificity. The AUCs were compared using Z test to identify the difference in predictive capability for SAP between serum lipid parameters39. The ROC was analyzed with the STATA 15.0 software (STATA Corp., College Station, TX). Two-sided P values less than 0.05 were regarded as statistically significant.
Supplementary information
Acknowledgements
This study was supported by National Natural Science Foundation of China (No.81702270), Guangdong Province Science and Technology Development of Special Funding Support (No. 2016A020215151), Competitive Project of Financial Special Funds for Science and Technology of Zhanjiang City (2018A01026), and The Affiliated Hospital of Guangdong Medical University Clinical Research Program (LCYJ2019B004).
Author Contributions
Jiayuan Wu, Yufeng Wang and Hongyan Li performed the literature search, data extraction and drafted of the manuscript; Yufeng Wang, Hongyan Li and Wenkai Tan collected the data; Xiaoming Chen and Shicai Ye gave lots of suggestions; Jiayuan Wu, Xiaoming Chen and Shicai Ye designed the study and edited the manuscript as corresponding author. All authors have drafted and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiayuan Wu and Yufeng Wang contributed equally.
Contributor Information
Jiayuan Wu, Email: 87537665@qq.com.
Xiaoming Chen, Email: cxmgood@126.com.
Shicai Ye, Email: caizi23@126.com.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44244-w.
References
- 1.Zhang T, et al. Predicting the severity of acute pancreatitis with red cell distribution width at early admission stage. Shock. 2018;48:551–555. doi: 10.1097/SHK.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 2.Banks PA, et al. Acute Pancreatitis Classification Working G: Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Shah AP, Mourad MM, Bramhall SR. Acute pancreatitis: current perspectives on diagnosis and management. J Inflamm Res. 2018;11:77–85. doi: 10.2147/JIR.S135751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsmak CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 5.Cho SK, Jung S, Lee KJ, Kim JW. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio can predict the severity of gallstone pancreatitis. BMC Gastroenterology. 2018;18:18. doi: 10.1186/s12876-018-0748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bugdaci MS, Sokmen M, Zuhur SS, Altuntas Y. Lipid profile changes and importance of low serum α-lipoprotein fraction (high-density lipoprotein) in cases with acute pancreatitis. Pancreas. 2011;40:1241–1244. doi: 10.1097/MPA.0b013e3182211bbf. [DOI] [PubMed] [Google Scholar]
- 7.Khan J, Nordback I, Sand J. Serum lipid levels are associated with the severity of acute pancreatitis. Digestion. 2013;87:223–228. doi: 10.1159/000348438. [DOI] [PubMed] [Google Scholar]
- 8.Khan J, et al. Serum lipid and fatty acid profiles are highly changed in patients with alcohol induced acute pancreatitis. Pancreatology. 2012;12:44–48. doi: 10.1016/j.pan.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Hong W, et al. Serum albumin is independently associated with persistent organ failure in acute pancreatitis. Can J Gastroenterol Hepatol. 2017;2017:10. doi: 10.1155/2017/5297143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu KE, Fong Y, Wang D, Chen CF, Yeh DY. Pretreatment of a matrix metalloproteases inhibitor and aprotinin attenuated the development of acute pancreatitis-induced lung injury in rat model. Immunobiology. 2018;223:64–72. doi: 10.1016/j.imbio.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Mangaraj M, Nanda R, Panda S. Apolipoprotein A-I: a molecule of diverse function. Indian J Clin Biochem. 2016;31:253–259. doi: 10.1007/s12291-015-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon EM, Figueroa DM, Barochia AV, Yao X, Levine SJ. High-density lipoproteins and apolipoprotein A-I: potential new players in the prevention and treatment of lung disease. Front Pharmacol. 2016;7:323. doi: 10.3389/fphar.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng M, Rached F, Kontush A, Chapman MJ. Impact of lipoproteins on atherobiology: emerging insights. Cardiol Clin. 2018;36:193–201. doi: 10.1016/j.ccl.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, et al. Apolipoprotein B binds to enolase-1 and aggravates inflammation in rheumatoid arthritis. Ann Rheum Dis. 2018;77:1480–1489. doi: 10.1136/annrheumdis-2018-213444. [DOI] [PubMed] [Google Scholar]
- 15.Rhainds D, Brodeur MR, Tardif JC. Lipids, apolipoproteins, and inflammatory biomarkers of cardiovascular risk: what have we learned? Clinical Pharmacol Ther. 2018;104:244–56. doi: 10.1002/cpt.1114. [DOI] [PubMed] [Google Scholar]
- 16.Kaneva AM, Potolitsyna NN, Bojko ER, Odland JO. The apolipoprotein B/apolipoprotein A-I ratio as a potential marker of plasma atherogenicity. Dis Markers. 2015;2015:591454. doi: 10.1155/2015/591454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jun JE, et al. ApoB/ApoA-I ratio is independently associated with carotid atherosclerosis in type 2 diabetes mellitus with well-controlled LDL cholesterol levels. Korean J Intern Med. 2018;33:138–147. doi: 10.3904/kjim.2017.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley EL., 3rd A clinically based classification system foracute pancreatitis: summary of the International Symposiumon Acute Pancreatitis, Atlanta, Ga, September 11–13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 19.Foster BR, Jensen KK, Bakis G, Shaaban AM, Coakley FV. Revised Atlanta classification for acute pancreatitis: a pictorial essay. Radiographics. 2016;36:675–687. doi: 10.1148/rg.2016150097. [DOI] [PubMed] [Google Scholar]
- 20.Song XM, et al. A single-center study of clinical re-evaluation of the new classification of acute pancreatitis. Chin J Pancreatol. 2014;14:8–11. [Google Scholar]
- 21.Stigliano S, Sternby H, de Madaria E, Capurso G, Petrov MS. Early management of acute pancreatitis: A review of the best evidence. Dig Liver Dis. 2017;49:585–594. doi: 10.1016/j.dld.2017.01.168. [DOI] [PubMed] [Google Scholar]
- 22.Sharma D, et al. Association of systemic inflammatory and anti-inflammatory responses with adverse outcomes in acute pancreatitis: preliminary results of an ongoing study. Dig Dis Sci. 2017;62:3468–3478. doi: 10.1007/s10620-017-4813-6. [DOI] [PubMed] [Google Scholar]
- 23.Cao JQ, Tang LJ. Research progress of systemic inflammatory response syndrome in acute pancreatitis-associated lung injury. Chin J Dig Surg. 2015;14:975–979. [Google Scholar]
- 24.Huh JH, Jung S, Cho SK, Lee KJ, Kim JW. Predictive value of apolipoprotein B and A-I ratio in severe acute pancreatitis. J Gastroenterol Hepatol. 2018;33:548–553. doi: 10.1111/jgh.13860. [DOI] [PubMed] [Google Scholar]
- 25.Karpavicius A, et al. The clinical value of adipokines inpredicting the severity and outcome ofacute pancreatitis. BMC Gastroenterol. 2016;16:99. doi: 10.1186/s12876-016-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou CL, et al. Early prediction of persistent organ failure by serum apolipoprotein A-I andhigh-density lipoprotein cholesterol in patients with acute pancreatitis. Clin Chim Acta. 2018;476:139–145. doi: 10.1016/j.cca.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 27.Hong W, et al. Relationship between low-density lipoprotein cholesterol and severe acute pancreatitis (“the lipid paradox”) Ther Clin Risk Manag. 2018;14:981–989. doi: 10.2147/TCRM.S159387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sniderman AD, Islam S, Yusuf S, McQueen MJ. Discordance analysis of apolipoprotein B and non-high density lipoprotein cholesterol as markers of cardiovascular risk in the INTERHEART study. Atherosclerosis. 2012;225:444–449. doi: 10.1016/j.atherosclerosis.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Ljungberg J, et al. Lipoprotein(a) and the apolipoprotein B/A1 ratio independently associate with surgery for aortic stenosis only in patients with concomitant coronary artery disease. J Am Heart Assoc. 2017;6:e007160. doi: 10.1161/JAHA.117.007160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tremblay AJ, Sniderman AD, Gagne C, Bergeron J, Couture P. Differential impact of plasma triglycerides on HDL-cholesterol and HDL-apoA-I in a large cohort. Clin Biochem. 2007;40:25–29. doi: 10.1016/j.clinbiochem.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Walldius G, et al. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between the plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med. 2004;42:1355–1363. doi: 10.1515/CCLM.2004.254. [DOI] [PubMed] [Google Scholar]
- 32.McQueen MJ, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 33.Canoui-Poitrine F, et al. Relative contribution of lipids and apolipoproteins to incident coronary heart disease and ischemic stroke: the PRIME Study. Cerebrovasc Dis. 2010;30:252–259. doi: 10.1159/000319067. [DOI] [PubMed] [Google Scholar]
- 34.Sreejith V, et al. Comparison of various scoring systems and biochemical markers in predicting the outcome in acute pancreatitis. Pancreas. 2018;47:65–71. doi: 10.1097/MPA.0000000000000957. [DOI] [PubMed] [Google Scholar]
- 35.Khanna AK, et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI scores, IL-6, CRP, andprocalcitonin in predicting severity, organ failure, pancreatic necrosis,and mortality in acute pancreatitis. HPB Surg. 2013;2013:367581. doi: 10.1155/2013/367581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KJ, et al. Comparison of predictive systems in severe acute pancreatitisaccording to the revised Atlanta Classification. Pancreas. 2016;45:46–50. doi: 10.1097/MPA.0000000000000433. [DOI] [PubMed] [Google Scholar]
- 37.Cho JH, Kim TN, Chung HH, Kim KH. Comparison of scoring systems in predicting the severity of acute pancreatitis. World J Gastroenterol. 2015;21:2387–2394. doi: 10.3748/wjg.v21.i8.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JY, et al. Bedside index for severity in acute pancreatitis: comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int. 2013;12:645–650. doi: 10.1016/S1499-3872(13)60101-0. [DOI] [PubMed] [Google Scholar]
- 39.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristics curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.