Abstract
Production of green chemicals and biofuels in biorefineries is the potential alternative for petrochemicals and gasoline in transitioning of petro-economy into bioeconomy. However, an efficient biomass pretreatment process must be considered for the successful deployment of biorefineries, mainly for use of lignocellulosic raw materials. However, biomass recalcitrance plays a key role in its saccharification to obtain considerable sugar which can be converted into ethanol or other biochemicals. In the last few decades, several pretreatment methods have been developed, but their feasibility at large-scale operations remains as a persistent bottleneck in biorefineries. Pretreatment methods such as hydrodynamic cavitation, ionic liquids, and supercritical fluids have shown promising results in terms of either lignin or hemicellulose removal, thus making remaining carbohydrate fraction amenable to the enzymatic hydrolysis for clean and high amount of fermentable sugar production. However, their techno-economic feasibility at industrial scale has not been yet studied in detail. Besides, nanotechnological-based technologies could play an important role in the economically viable 2G sugar production in future. Considering these facts, in the present review, we have discussed the existing promising pretreatment methods for lignocellulosic biomass and their challenges, besides this strategic role of nano and biotechnological approaches towards the viability and sustainability of biorefineries is also discussed.
Keywords: Biorefineries, Biomass pretreatment, Nanomaterials, Cell wall modification, Biomass hydrolysis, Cellulosic sugars
Introduction
Increasing global demand for energy and various other economic, environmental and political issues have promoted intensive research in recent years with the aim to produce renewable fuels and chemicals from lignocellulosic materials (Gupta and Verma 2015; dos Santos et al. 2016; Aditiya et al. 2016). Among the available alternatives, 2G ethanol is considered as important fuel, because it has ability to transform fossil energy-based economy to a bioeconomy. Considering the importance of ethanol, scientific community across the globe developed a great interest in this fuel which can be clearly seen by a quick search in the scientific database, ™Web of Science (Thomson Reuters 2019), which indicated about 3500–8000 documents (including original articles, reviews, proceeding papers, books, book chapters, etc.) were published between 2014 and 2018 with an keyword “ethanol” associated with words as “cellulose” or “biomass”. Although different results can be obtained using diverse combinations of search words, in-depth analysis of information from this database (using its own tools) indicated a linear growth in the number of citations per year of publications in this area in last 5 years.
Different sources of biomass such as forest residues, agricultural residues, and other by-products are commonly used for energy production around the world. Different agricultural crops in particular countries like sugarcane (Brazil), corn (US), sweet sorghum (US), rice (China), and wheat (European Union) generate high amount of by-products which are potential sources for 2G ethanol production (Table 1). Some of them have been used in the first-generation biorefineries to produce sugar, alcohol, and electricity, e.g., sugarcane, which is extensively produced in Brazil, and Brazil is largest producer about 635.51 million of tons; sugarcane is harvested in 2018/2019 season with an 0.4% growth as compared to production obtained in 2017/2018 season (Conab 2019). Considering the generation of 140 kg of bagasse (dry mass) per ton of sugarcane in sugar and alcohol industry, about 90 million tons of this by-product is available in Brazil per year. The most of this amount is used to satisfy the steam and energy demands in industries, but about 10% of this mass would be available to produce ethanol or other products in a 2G biorefinery (Carpio and Souza 2017).
Table 1.
Producers of some potential crops for use as source of second-generation ethanol
| Biomass | Main producer/crop season | References |
|---|---|---|
| Sugarcane | Brazil (635 million of tons)—2018/2019 | Conab (2019) |
| Corn | US (370.961 million of tons)—2018 | NCGA (2018) |
| Sorghum | US (9 millions of tons)—2018/2019 | USDA (2019) |
| Rice | China (148 millions of tons)—2018/2019 | USDA (2019) |
| Wheat | European union (137.6 millions of tons)—2018/2019 | USDA (2019) |
Considering the bioethanol as a real alternative to fossil fuels and several world agreements to combat climate change (e.g., Paris agreement) are focused on the reduction of greenhouse gas emission, and hence, it is expected that the global demand for ethanol is going to increase. Therefore, the production 2G ethanol has been emerged as a promising research area which will help to develop the renewable fuels and also mitigate the growing global energy demand.
The complete process involved in the production of 2G ethanol includes three important steps: pretreatment, enzymatic hydrolysis, and fermentation. Pretreatment is required to modify biomass structure and composition to allow high efficiency in the subsequent enzymatic hydrolysis of cellulose (Njoku et al. 2012; Cardona et al. 2014; Zabed et al. 2016). After pretreatment, the carbohydrate-rich portion is subjected to enzymatic hydrolysis to get fermentable sugars and the final step deals with fermentation of sugars to ethanol and other high value chemicals using appropriate microorganisms (Zabed et al. 2016; Cavalett et al. 2017). The fermentable monomeric sugars can be obtained from hemicellulosic or cellulosic fraction. The hemicellulosic fraction is usually rich in pentoses and can be hydrolysed during the pretreatment or even in the enzymatic hydrolysis step, depending on the technological choice. Indeed, dilute acid pretreatment of hemicellulose results in a xylose-rich hydrolysate along with some inhibitors (Santos et al. 2014). Steam explosion-based pretreatments in the presence or absence of acid catalysts also can hydrolyze hemicellulose into sugar monomers. If un-hydrolyzed hemicellulose remains in the pretreated solid material or in the liquid fraction (oligosaccharides having xylo or cello-oligosaccharides), it can be further enzymatically hydrolyzed by xylanolytic enzymes, which are commonly present in commercial cellulolytic preparations (Terán-Hilares et al. 2017a). Released sugars, both from cellulose or hemicellulose, besides the non-sugar fraction lignin, can also be used for the production of other interesting biomolecules or for energy applications, in a wider concept called “biorefinery”, which includes multi-product industrial installations (dos Santos et al. 2016).
Recently, some commercial 2G ethanol manufacturers have set up production facilities in Brazil, USA, China, Italy, India, and Spain (dos Santos et al. 2016; Chandel et al. 2018). However, the process technology used in these industrial installations is still in the development stage and has several bottlenecks for the deployment at large scale of the economic production of 2G ethanol and other valuable biochemicals (Sandford et al. 2016). Particularly, the pretreatment step has important challenges to be overcome to allow a viable and sustainable biorefinery.
Various pretreatment methods have been extensively studied and found to be efficient at the laboratory which mainly includes physical, chemical or physico-chemical pretreatments (steam explosion, dilute acid, alkaline, organosolv, etc.), biological, and a combination of two or more methods (Alvira et al. 2010; Antunes et al. 2017). However, the use of these pretreatment methods at large scale is still a challenge. Actually, pretreatment is a persistent bottleneck for lignocellulosic biorefineries mainly considering the high recalcitrance of the lignocellulosic material, cost of the process, and operational complexities (Jönsson and Martín 2016). Deconstruction or modification of the biomass to increase enzymatic digestibility usually is performed under severe conditions and consuming large amounts of reagents, which makes the process more expensive (Yoo et al. 2017). Other problems constantly observed in the current biorefineries include operational issues involving the mechanical section; e.g., in case of sugarcane bagasse, the high absorptivity of the material results in a viscous suspension with fiber and silica, turning complex the transport or drained, leading to erosion clogging of pipes and valves, and thread locking (Marques 2018).
Within this context, the present review aimed to critically discuss some selected current promising pretreatment methods that deserve to be targeted for intensive research work and exploring their possible contribution at lignocellulosic biorefineries. Moreover, the emerging role of nano- and biotechnology-based methods for pretreatment of lignocellulosic biomass was discussed.
Biomass recalcitrance and compositional/structural changes promoted by pretreatment
Prior to the selection of a particular method for biomass pretreatment, the knowledge about the chemical composition of the lignocellulosic biomass is of great importance. These materials are composed by three main fractions: cellulose, hemicellulose, and lignin, along with some fractions like ash and extractives. Although the percent composition of these three main fractions varies with the species of plant, as well as with the growth environment and processing conditions, the major portion is composed of cellulose (Canilha et al. 2012). Cellulose is composed of glucose units linked by β-1,4-glycosidic bonds, containing amorphous regions with large voids and other irregularities associated with dense and crystalline regions, resulting in a strong and resistant structure (Coseri 2017). Hemicellulose is formed by groups of branched polysaccharides, mainly consisting of pentose, hexose, and uronic acid residues (Antunes et al. 2017). Hemicellulose has an amorphous structure which can be easily hydrolysed and showed a low degree of polymerization (~ 100–200) as compared to cellulose (1000–50,000). Lignin is a complex amorphous polyphenolic macromolecule which is covalently linked to hemicellulose, inter-connected with cellulose by hydrogen bonds, thus, contributing to strengthen the connection among cell wall fractions (Chandel et al. 2012).
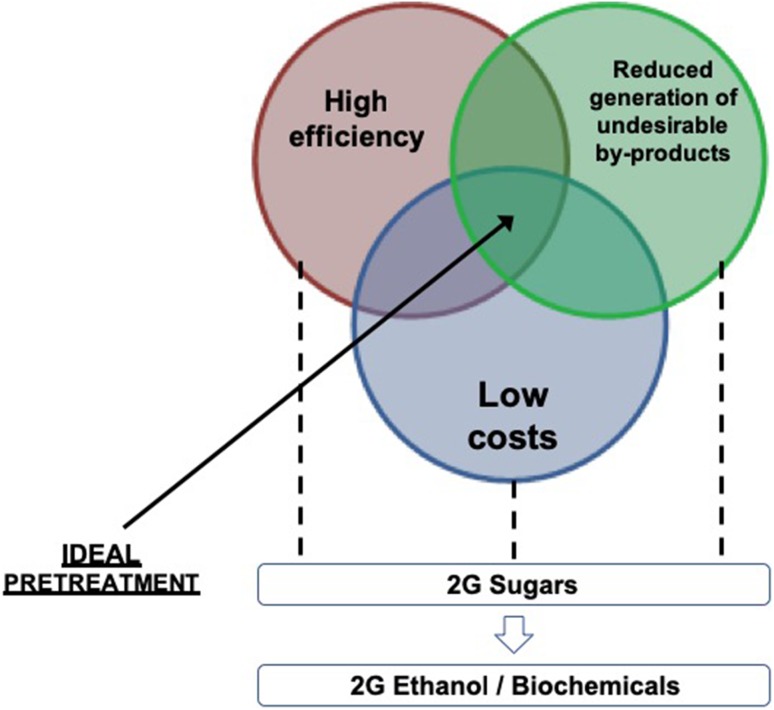
For decades, researchers are trying to develop methods to depolymerize carbohydrates polymeric fractions into fermentable sugars with high efficiency and minimum generation of inhibitors at lower costs (Fig. 1) (Grethlein et al. 1984; Canilha et al. 2012; Chandel et al. 2012; Raza-Amin et al. 2017). Pretreatment methods which are performed at high temperature, high pressure, and very low pH are mostly responsible for the generation of inhibitors like phenolics and other products (some acids, furfural, and 5-hydroxymethylfurfural) which are responsible for sugar degradation (Chornet and Overend 1988; Kapdan et al. 2011). Moreover, such toxic inhibitors affect subsequent step of enzymatic hydrolysis. This problem is more common in dilute acid pretreatments (Antunes et al. 2014).
Fig. 1.
Desirable trinomial characteristic for 2G ethanol and various biochemical productions from lignocellulosic biomass under biorefinery concept
The different pretreatment approaches result in diverse structural changes in the morphology of biomass and in its composition, reducing its recalcitrance. For example, steam explosion pretreatment results in more disorganization of biomass structure, which is characterized by loosening of the fibrous network due to the solubilization of cell wall components. The most exposed cell wall structure likely allows greater cellulose accessibility to cellulase (Auxenfans et al. 2017).
One important goal of many pretreatment approaches is lignin and/or hemicellulose removal. This effect is generally obtained using chemical pretreatment; Terán-Hilares et al. (2017a) tested successive alkaline pretreatment (70 °C for 4 h with 0.3 M NaOH solution) aimed to enzymatic saccharification of sugarcane bagasse in a packed bed flow-through column reactor. After pretreatment, authors observed 45% reduction in lignin content of the material, showing 49.2% and 57.0% of hemicellulose and cellulose digestibility, respectively. During enzymatic hydrolysis (performed with 17% of initial solid loading during 48 h at 50 °C, with 20 floating point unit (FPU) of enzym/g of biomass). In that work, around 32.2% of hemicellulose was also removed along with lignin during the process. In another work, Mirmohamadsadegh et al. (2016) studied the effect of mild sodium carbonate (Na2CO3) pretreatment on enzymatic hydrolysis of different feedstocks, such as corn stover, Miscanthus, and switchgrass. Those authors observed the removal of 40–59% lignin from these biomasses, with removal values of only 10–14% for xylan and less than 5% for a cellulosic fraction. In that work, after Na2CO3 pretreatment, authors verified increase (i.e., 25%, 21%, and 15%) in the crystallinity of corn stover, switchgrass, and Miscanthus, respectively, attributing this fact to the removal of lignin, partial hemicellulose solubilization, and cellulose retention. As amorphous fractions of the material are easier to remove during pretreatment, the total crystallinity of the material will increase, although the crystallinity of remaining cellulose can be lower if compared to untreated biomass, and, thus, the pretreatment resulting in higher enzymatic digestibility (Driemeier et al. 2011).
The study of biomass characteristics and its changes during pretreatment, as well as the understanding of the interaction of variables (e.g.: lignin removal and increasing of surface area) are fundamental for the development of new technicals and conditions as well as process optimization of known methodologies. Usually, the crystallinity index (CI) is one of the most applied methods to verify changes in the biomass crystallinity related to the pretreatments. However, some authors reported that this method could not be effective due to the difficulties to distinguish the specific crystallinity of the cellulose and total biomass. This fact was discussed by Driemeier et al. (2011), which observed the evolution of cellulose crystals from sugarcane bagasse after pretreatment by hydrothermal, dilute acid or steam explosion methods, and soda delignification. Those authors observed a decrease in crystal-to-cellulose ratio after pretreatment, an effect opposite to preferential removal of non-crystalline cellulose. The observed behavior was explained by a cellulose partial decrystallization or more defective crystallites as a result of the treatments. As an alternative to evaluate the effect of pretreatment, Bernardinelli et al. (2015) demonstrated the application of cross polarization by multiple contact periods (Multi-CP) to obtain quantitative 13 °C solid-state nuclear magnetic resonance (SSNMR) spectra to evaluate raw and pretreated sugarcane bagasse. This method was reported as more feasible to unravel different pretreatments action in biomass cell wall digestion changing cellulose ultrastructure. Actually, aiming to increase scientific comprehension of biomass recalcitrance, researchers have studied changes in the structural morphology of biomass along with lignocellulosic pretreatments by different strategies.
Chandel et al. (2014) evaluated sequential acid–base pretreatment, aiming to first obtain hemicellulosic hydrolysate, followed by lignin solubilization of remaining solid portion by alkali treatment. Thus, cellulose in the remaining portion was cleaved in hexose monomers sugars by enzymatic hydrolysis. A large number of structural changes were observed in biomass along pretreatments using different physical analysis. For instance, after acid hydrolysis, 92.78% hemicellulose was removed, increasing the cellulose amount and, hence, the crystallinity of the sample. By light microscopic analysis, thin shape of particles and more cylindrical shape (20 μm size), as well as the relocation of lignin portion on the surface, were found compared to native sugarcane bagasse. In addition, the relation of cellulose/hemicellulose bands was verified by Raman spectroscopy.
Even by studying the modifications in composition and structure of biomass due to pretreatment, there is not a perfect and unique indicator of biomass recalcitrance or to be used as a predictor of pretreatment success. However, there are some tries in this way. Costa et al. (2013) reported a study about enzymatic hydrolysis of internodes of sugarcane hybrids with varying lignin contents. Those authors observed a correlation between the chemical composition and the microscopy characteristics of the hybrid sugarcane internode fractions with the efficiency of the enzymatic hydrolysis. A quadratic polynomial equation was successfully adjusted when enzymatic hydrolysis yield was plotted against a number calculated by dividing the ratio between the contents of “glucan” and “hemicellulose + total lignin” by the “% area occupied by the vascular bundles”. In another work (Costa et al. 2016), the same research group observed that, besides the content of each biomass fraction, hemicellulose composition is an important characteristic of sugarcane to be considered regarding its recalcitrance. As stated by the authors, in the different sugarcanes evaluated, high arabinose/xylose ratios in pith regions of the low-lignin content hybrids seemed to decrease cellulose–hemicellulose interaction, increasing its enzymatic digestibility. Mixed-linkage glucan (MLG) content was also pointed as contributing to the differential distribution of recalcitrance among internode regions evaluated in sugarcane hybrids. By plotting enzymatic digestibility of internodes of six different sugarcane hybrids against MLG content, a good data fitting for a quadratic model was obtained. Regarding pretreated biomass, this research group recently published a work showing a good data fitting for enzymatic digestibility of glucan of three different samples of sugarcane hybrids pretreated by dilute acid pretreatment (preceded or not by a delignification step) when plotted against the ratio between the contents of “glucan” and “glucurono-arabinoxylans + lignin”.
Current physico-chemical promising strategies for biomass pretreatment
Despite the diversity of known options for biomass pretreatment and the current concern pertaining to the requirement of an effective and non-costly process implementation at industrial use, new alternatives have been reported. Within this context, recent physico-chemical pretreatment methods with potential application at large scale are briefly discussed.
Hydrodynamic cavitation
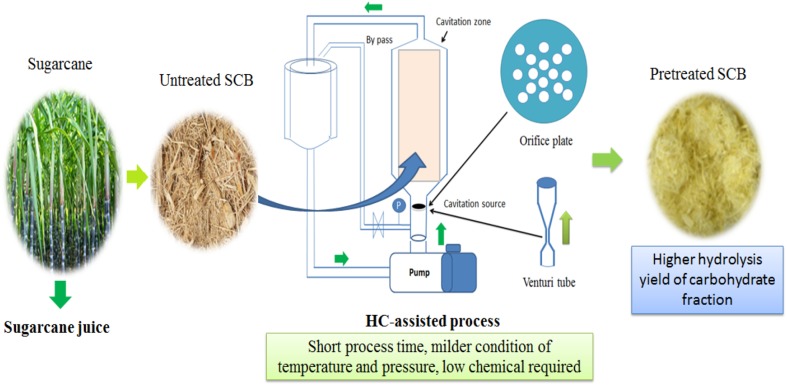
Hydrodynamic cavitation (HC) has been extensively used to accelerate the chemical reaction in different process areas (Gogate and Pandit 2000; Gogate 2016). In recent years, HC was also reported for pretreatment of different lignocellulosic biomass (sugarcane bagasse, corn stover, and reed) with the promising results (Kim et al. 2015; Nakashima et al. 2016, Terán-Hilares et al. 2017a, b). The main results corresponded to high lignin removal and an increase in the porosity of the material, resulting in improved enzymatic digestibility yield of carbohydrate fractions present in pretreated lignocellulosic biomass (Terán-Hilares et al. 2017a; Badve et al. 2014). HC processes are more advantageous as compared to the traditional methods showing short processing time, low energy consumption, and low chemical catalyst requirement (Gogate and Pandit 2000). In addition, the simplicity of the system and possibility to be adapted in the continuous process turn it highly attractive for scaling up (Terán-Hilares et al. 2017b). As shown in Fig. 2, the configuration of HC system for biomass pretreatment process is comprised by a pump, a cavitation device (orifice plate or venture tube), the cavitation zone, and a tank for recirculation of solution.
Fig. 2.
Schematic representation of hydrodynamic cavitation reactor used for pretreatment of sugarcane bagasse (SCB)
Cavitation phenomenon corresponds to generation, growing, and collapse of microbubbles which are produced when the pressure of fluid is dropped below the solution vapor pressure, followed by a fluid pressure recovery (Gogate and Pandit 2005). This phenomenon can be produced when fluid flow through a constriction device such as venture tube or orifice plate (Gogate and Pandit 2005; Patil et al. 2016). Drastic conditions are achieved inside the bubbles, reaching temperatures of about 10000 K and pressure about 1000 atm, releasing a high amount of energy in localized points named “hot spots” (Gogate and Pandit 2005; Saharan et al. 2013). In addition, water molecules are dissociated inside the cavities, resulting in potential oxidative radicals (OH·, H·, HOO·, and O2·) that are released into the medium during bubbles collapse (Badve et al. 2014). These oxidative radicals lead to the oxidation and degradation of lignin, resulting in higher removal of lignin fraction, thus favoring high enzymatic yields of hydrolysis of remaining glucan.
Higher enzymatic hydrolysis yield after HC pretreatment of biomass can also be attributed to the mechanical effect of HC. Actually, shockwaves and microjets (with high velocities, as 100 m/s) are generated after bubble collapse, producing degradation of organic molecules and small perforation on the surface of biomass, increasing the porosity of the pretreated material, and, consequently, enhancing the enzyme accessibility to the cellulosic and hemicellulosic fraction (Terán-Hilares et al. 2017a; E-PIC 2016).
Cavitation phenomenon and efficiency of the process are strongly influenced by different properties, such as density and viscosity of fluid, temperature of process, chemical catalyst (alkaline, oxidant agents, etc.), particle size, and configuration of the system (number and diameter of orifices or throat size in tube) (Terán-Hilares et al. 2017b; Ozoneck 2012). In a recent study reported by Terán-Hilares et al. (2017b), sugarcane bagasse pretreatment was carried out at 70 °C (temperature controlled during all process) using low alkaline concentration (0.3 M). In that work, 559 g of sugars/kg of biomass (corresponding to 93% and 94% of cellulose and hemicellulose enzymatic hydrolysis yield, respectively) was obtained pretreating the bagasse with NaOH assisted by HC (70 °C, 3 bar of inlet pressure) for 20 min.
The kind of device used for cavitation generation also influences directly on pretreatment efficiency. For example, HC system comprising of a venture tube was used by Nakashima et al. (2016) and Madison et al. (2017). As reported by Nakashima et al. (2016), 275 g of sugars/kg was produced from corn stover pretreated with sodium percarbonate-HC (Na2CO3, 0.4 mol/L; H2O2, 0.6 mol/L, 1 h at 30 °C). Madison et al. (2017) reported the production of 325 g of sugars/kg of biomass by enzymatic hydrolysis of sugarcane bagasse (SCB) pretreated in a sequential method using calcium hydroxide and HC (first alkaline process at 100 °C for 2 h, followed by HC process at 22 °C for 120 min). These values were lower than the obtained in HC system based in orifice plate previously reported by Kim et al. (2015), Terán-Hilares et al. (2017a, b)and Terán-Hilares et al. (2017b). The differences in sugar recovery can be attributed to the cavitation intensity that is higher in system based on orifice plate than venturi tube (Moholkar and Pandit 2001). However, venture tube-based devices can be successfully used for other applications that require lower cavitation intensity.
Thus, this technology has been proven as a promising approach, which can be applied for pretreatment process at large-scale operations. However, additional studies are required to optimize the system design. Advantages, such as high efficiency using low chemical catalyst concentration, low energy consumption, high solid content, and short process time, can turn this method attractive for intensification of pretreatment process.
Supercritical fluid pretreatment
Supercritical fluid (SF)-based pretreatments have been found to be an attractive alternative due to competitive economic and environmental performance, as demonstrated in the study of Daza Serna et al. (2016), presenting real possibilities for application at large-scale operations.
Mainly considering last years, SF have been reported as a promising and green technology for pretreatment of different lignocellulosic biomass (Narayanaswamy et al. 2011; Benazzi et al. 2013; Liang et al. 2017). However, this technology has been evaluated from some decades. SF pretreatment using ammonium–water mixture and alcohols was initially reported by Reyes et al. (1989) and Bludworth and Carl Knopf (1993) for lignin extraction from wood. Other alternative, supercritical CO2 was reported for pretreatment of commercial cellulose (Zheng et al. 1995), SCB (Zheng et al. 1998) and wheat straw (Morais et al. 2014; Silva et al. 2014; Daza Serna et al. 2016).
CO2 is one of the mostly used SF, because it is non-toxic, recyclable, and eco-friendly besides has low cost and low critical temperature (30.95 °C) and pressure (1070.37 psi). Pretreatment process using supercritical CO2 (scCO2) disrupt the crystalline structure of biomass concomitantly with an efficient removal of lignin from the biomass in turn ameliorating the cellulose conversion (Liang et al. 2017; McHardy and Sawan 1998). This kind of pretreatment method showed potential towards their use at high solid loading and, simultaneously, the use of CO2 could reduce the greenhouse impacts on the atmosphere.
For the pretreatment process, the moisture of the biomass is considered very important, because carbonic acid can be generated in combination with scCO2, acidifying the medium and promoting the hemicellulose hydrolysis. In the work of Daza Serna et al. (2016), rice husk was subjected to scCO2 pretreatment at 80 °C and 270 bar and the moisture content was adjusted at 75% using water:ethanol mixture as co-solvent. In that condition, 90.6% of lignin removal and 55% of enzymatic hydrolysis yield were achieved. Those authors also stated the presence of ethanol as polar co-solvent contributed to the pretreatment efficiency by cleavage the lignocellulosic matrix.
scCO2 was also reported by Phan and Tan (2014) for pretreatment of SCB in a sequential scCO2 and alkaline hydrogen peroxide process. In that study, 97.5% of glucose was recovered after enzymatic hydrolysis of SCB pretreated at the following conditions: 80% of moisture content first submitted to scCO2 during 40 min at 15.6 MPa and 186.85 °C, followed by the H2O2 process during 9 h, 1% v/v, pH 11.5, and 333 K. In that process, the highest yield of cellulose hydrolysis was attributed to destruction of the rigid structure of SCB, exhibiting the cellulose fiber and, thus, increasing the accessibility for enzyme.
Ionic liquids (ILs)
Pretreatment of lignocellulosic biomass using ionic liquids (ILs) is considered a relevant method due to their “green” properties as recyclability, chemical stability, non-flammability, non-volatibility, thermal stability (up to 400 °C), and capability to dissolve solutes of varying polarity. Currently, different ILs have been proposed for application in pretreatment due to their potential to dissolve all macromolecular fractions of biomass (Zhang et al. 2015a, b; Mohtar et al. 2017).
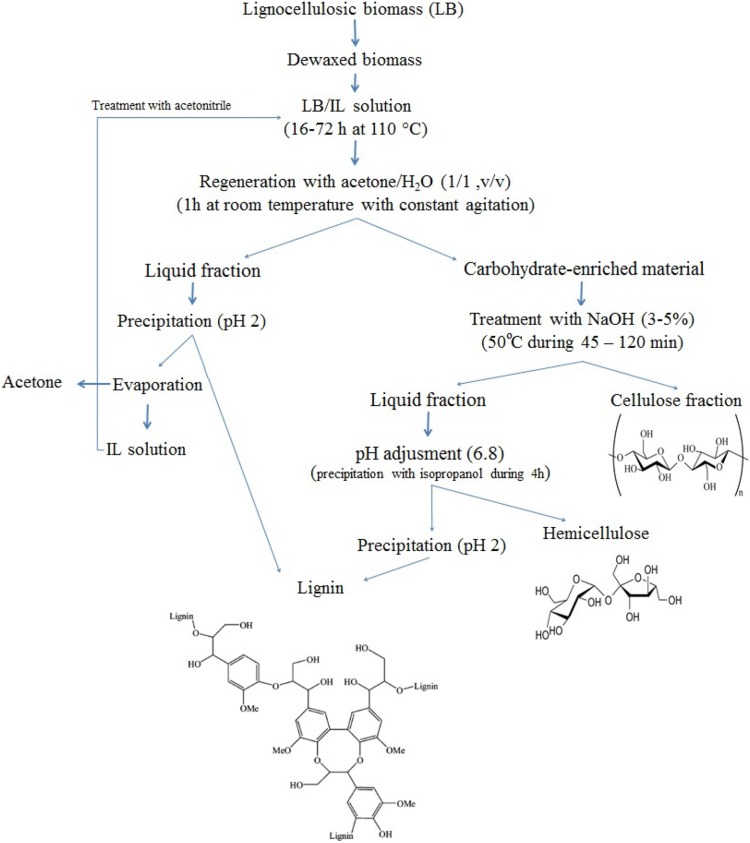
In a recent study, Mohtar et al. (2017) used imidazolium-based ([(C3N2)Xn]+) ionic liquid (1-butyl-3-methylimidazolium chloride [bmim][Cl]) for complete dissolution of oil palm empty fruit bunch and each fraction of the material was separated in sequential steps, achieving recovery yields respect to total biomass (%wt.) of 52.72%, 27.17%, and 16.82% for cellulose, hemicellulose, and lignin, respectively. In Fig. 3, the procedure used to solubilize each fraction of lignocellulosic biomass and recovery of the ILs is schematically shown (Mohtar et al. 2017, Liu et al. 2017; Sun et al. 2009; Lan et al. 2011).
Fig. 3.
Schematic representation of a sample procedure for fractionation of lignocellulosic biomass using ionic liquid (IL) procedure based on the work of Mohtar et al. (2017), Sun et al. (2009)and Lan et al. (2011)
For complete dissolution of biomass by ILs, usually, higher temperatures are required (more than 100 °C) and increased processing time. Aiming to overcome these disadvantages, the use of ultrasound to assist the ILs process was reported as an interesting strategy by Zhang et al. (2015a, b). In that study, fractionation of corn stover into cellulose, hemicellulose, and lignin was successfully performed in ultrasound-assisted ILs (1-H-3-imidazolium chloride [HMIM Cl]) at a low reaction temperature of 70 °C for 3 h followed by alkaline extraction. After enzymatic hydrolysis of the cellulose fraction, 92.5% of conversion yield of cellulose in glucose was achieved in 72 h.
Although IL-mediated pretreatment of biomass has been reported during many years and considered as one of the most attractive technologies for the treatment of lignocellulosic biomass, the evaluated approaches have not been yet adequate for use at large scale. Cost of ILs is the major impeding factor in their deployment for pretreatment and, in this way, the in-house synthesis of low-cost ILs coupled with their recovery for reuse could be disruptive pretreatment process at large-scale operations. ILs can be recycled by addition of salt or reused in additional repeated process; however, its efficiency decreased over subsequent cycles (Mohtar et al. 2017; Liu et al. 2017). Currently, an alternative method is used which can replace the use of aprotic ionic liquids (e.g., N, N-dimethylformamide (DMF), dimethylsulphoxide (DMSO), N,N-dimethylacetamide (DMA), 1,3-dimethyl-2-imidazolidinone (DMI) and 1-butyl-3-methylimidazolium chloride [bmim][Cl]) by protic ionic liquids (e.g., pyridinium formate ([Py][For]), pyridinium acetate ([Py][Ac]), pyridinium propionate ([Py][Pro]), and triethylammonium hydrogen sulfate [TEA][HSO4]), which are comparatively cheaper and easier to produce. For example, a low-cost triethylammonium hydrogen sulfate [TEA][HSO4], a protic ionic liquid, was used to solubilize grass Miscanthus x giganteus (Brandt-Talbot et al. 2017). In that study, 85% of lignin and 100% of hemicellulose were solubilized into the ILs’ solution, resulting in a glucose recovery of 77% from the cellulose fraction after the subsequent enzymatic hydrolysis. Regarding other application of ILs for production of novel biomass-derived chemicals and material, additional information can be found in the review work of Yoo et al. (2017).
Other potential pretreatment methods
Pretreatment methods such as steam explosion (STEX), extractive ammonia (EA), co-solvent-enhanced lignocellulosic fractionation (CELF), acidified mixtures of glycerol carbonate (GC), and glycerol have been found to be promising for 2G sugar production.
Currently, STEX pretreatment is mostly used in the pilot, demo, and even commercial plants in one stage or two stages. In one stage, biomass is initially treated with saturated steam for a definite time (~ 15–20 min, ~ 10–15 bar). Saturated steam is penetrated inside the fiber due to high vapor-phase diffusion. Biomass is heated to condensation of steam, and thus, microporous structure is concomitantly soaked in water which leads to the solubilisation of acetyl groups which catalytically acts on hemicellulose yielding xylose monomers and oligosaccharides along with some inhibitors generation. At the end of STEX, the sudden drop of pressure leads to the explosion of biomass structure causing defibrillation and break down of fibers. STEX biomass is amenable for subsequent cellulases action converting cellulose, oligosaccharides, and remaining hemicellulose into sugar monomers (Pielhop et al. 2016). In two stages, biomass is first pretreated at mild conditions followed by harsh conditions (high steam pressure and temperature) for the maximum solubilisation of hemicellulose constituent of the cell wall. The catalytic agent such as SO2 or sulfuric acid may also have added during STEX facilitating the solubilisation of hemicellulose into sugars.
EA-based pretreatment is also considered as a promising approach which changes cellulose crystallinity (Brandt-Talbot et al. 2017). EA pretreatment is also effective to convert native crystalline cellulose (CI) to a highly digestible cellulose (CIII) allomorph with approximately 45% lignin extraction from native corn stover. This pretreatment significantly cut down the enzyme amount required for hydrolysis biomass into fermentable sugars with desired yield (Sousa et al. 2016).
Moreover, Nguyen et al. (2015) developed a new pretreatment method called co-solvent-enhanced lignocellulosic fractionation (CELF) to produce low-cost 2G sugars. In this method, tetrahydrofuran was mixed with dilute acid which helped to recover approximately 95% yields of sugar monomers from corn stover after enzyme hydrolysis. Another promising pretreatment technology, “green solvent” (acidified mixtures of glycerol carbonate and glycerol) can be preferred for biomass pretreatment. Zhang et al. (2013) demonstrated pretreatment of sugarcane bagasse using the “green” solvent” at 90 °C for only 30 min, and found glucan digestibility of 90% and a glucose yield of 80%. Presently, these pretreatment technologies have potential to be explored at industrial scale as they can be scaled up at industries for the 2G sugar recovery from biomass. Table 2 summarizes some technical conditions used in pretreatment and hydrolysis of biomass showing 2G sugar recovery and, subsequently, use of these sugars for the production of various bioproducts.
Table 2.
Some technical conditions used in pretreatment and hydrolysis of biomass showing 2G sugar recovery and subsequently use of these sugars for the production of various bioproducts
| Substrate, pretreatment type, conditions |
Enzymatic hydrolysis conditions | Sugars released | Product | References |
|---|---|---|---|---|
| Sugarcane bagasse, steam explosion (autohydrolysis), 195 °C, 18 atm, 7.5 min | 12 wt% TS (total solids), 62.5 mg/g of Cellic CTec2 (novozymes) to dry mass | Separate hydrolysis and fermentation (SHF): glucose 57.8 g/L | Ethanol, yield: 43.6%, productivity: 0.31 g/L h | Neves et al. (2016) |
| Simultaneous saccharification and fermentation (SSF): glucose 54.9 g/L | Ethanol, yield: 34.2%, productivity: 0.39 g/L h | |||
| Corn stover supercritical fluids, pressure of 3500 psi, temperature of 150 °C), time of 1 h and moisture content of 75% | 100 mg of a dried biomass sample, 50 U of cellulase and 20 U of β-glucosidase 47 °C for 24 h | 30% of glucose yield, mg/100 mg of biomass | – | Narayanaswamy et al. (2011) |
| Switchgrass supercritical fluids, pressure of 3200 psi, temperature of 150 °C, time of 1 h | 14% of glucose yield, mg/100 mg of biomass | |||
| Sugarcane bagasse, hydrodynamic cavitation, flow rate of the NaOH solution (4 m3/h) with a fluid velocity through the orifice plate of 88 m/s and cavitation number of 0.07 | 5% solids loading, 20 FPU of Cellic CTec2 (novozyme)/g of dry pretreated SCB. 50 °C and 150 rpm | 62.33% of total carbohydrate fractions hydrolyzed | Ethanol yield of 0.48 g/g of glucose and xylose | Terán-Hilares et al. (2017a, b) |
| Corn stover, co-solvent-enhanced lignocellulosic fractionation (CELF), THF, water (1:1), 0.5% sulfuric acid, 5% corn stover, 340 °C, 3 min | 5 mg protein (accelerase 1500)/g gluca, SSF, 15.5 wt% solids loadings (corresponding to 11 wt% glucan loadings), Saccharomyces cerevisiae D5A | 95% theoretical yield of glucose, xylose and arabinose | Ethanol (58.8 g/l), yield 89.2% | Nguyen et al. (2015) |
| Corn stover, AFEX, gaseous ammonia added at a 0.6 g/g biomass ratio to a packed bed with corn stover previously heated to 100 °C by steam injection, 30–150 min | 7% loading, 14.7 mg protein/g glucan Cellic CTec3 and 14.9 mg protein/g glucan Cellic HTec3 (novozymes) | 79% glucan conversion | Ethanol yield of 98% | Uppugundla et al. (2014) |
| Extractive ammonia (EA), corn stover, 100 g, Liquid ammonia 600 g, 120 °C, 30 min | 30 mg enzyme (protein) per g glucan loading, 25% solids loading, 72 h hydrolysis | > 80% glucan conversion, > 70% xylan conversion | – | Sousa et al. (2016) |
| Paddy straw (rice variety pusa 2511) biological pretreatment, white-rot fungus, Trametes hirsute | Hydrolysis with enzyme accelerase 1500 at loading (0.5 mL enzyme g−1 of glucan) 50 °C 72 h, 150 rpm | Saccharification efficiency of 76.5% | Ethanol yield of 28% | Arora et al. (2016) |
Biological pretreatment methods: harnessing the potential of fungi and bacteria
The conventional methods used for the pretreatment of biomass are energy intensive and also have many other limitations. Within this context, novel biological pretreatment methods can be a good sustainable alternative for the efficient treatment of biomass (Chandel et al. 2013). In general, biological pretreatment methods involve the use of different microorganisms such as bacteria, fungi, and actinomycetes and their enzymes for the degradation/hydrolysis of various constituents (lignin, cellulose, hemicellulose, and polyphenols) of biomass (Maurya et al. 2015; Sindhu et al. 2016). The main advantages of biological methods are low energy demand and mild conditions required (Akhtar et al. 2016).
Various microorganisms have the potential towards the enzymatic degradation of plant cell wall. Indeed, plant cell wall degradation is the primary event when any saprophytic bacterium or fungus invades the plant. Ochiai et al. (2007) demonstrated that saprophytic Bacillus subtilis strain 168 degrade the plant cell wall polysaccharides such as pectin and polygalacturonan, and utilized for their growth. In another study, plant cell wall-degradation efficacy of various members of bacterial genera, viz., Pectobacterium, Klebsiella, Serratia, Enterobacter, Citrobacter, Providencia, and Pseudomonas, causing soft rot of orchid was evaluated. It was reported that above-mentioned bacteria secreted different enzymes such as pectate lyase, polygalacturonase, cellulase, and protease which are responsible for the degradation of plant cell wall (Joko et al. 2014).
Recently, Pathak and Navneet (2017) reviewed the association of both bacteria and fungi with the degradation of different polymers including those ones of plant cell wall. The bacteria such as Pseudomonas aeruginosa, P. stutzeri, Streptomyces badius, S. setonii, Rhodococcus ruber, Comamonas acidovorans, Clostridium thermocellum, Butyrivibrio fibrisolvens, etc., and fungi like Aspergillus niger, A. flavus, Fusarium lini, Pycnoporus cinnabarinus, and Mucor rouxii were reported as the most prevalent species among their respective community.
Ramos et al. (2016) demonstrated in vitro production of various enzymes like pectinases, cellulases, hemicellulases, and laccase by several isolates of fungus Macrophomina phaseolina recovered from corn stem in Argentina and evaluated their plant cell wall-degrading efficacy. Pectinases were produced in higher concentration, followed by cellulases and hemicellulases. In addition, all those isolates have been reported to possess significant cell wall-degrading activity. In another study, Kaur and Aggarwal (2017) screened five isolates of pathogenic Alternaria, i.e., A. alternata, A. macrospora MKP2, A. macrospora MKP4, Alternaria sp. PMK1, and Alternaria sp. PMK2, isolated from leaves of Parthenium plant for their lignocellulolytic activities. All the isolates reported to produce different lignocellulolytic enzymes such as cellulase, laccase, and lignin peroxidase, having potential activity towards degradation of polymers available in the host plants’ cell wall. Therefore, such fungi can be effectively used in the management of Parthenium weed.
Enzymes produced by Aspergillus were also involved in degradation of plant cell wall polysaccharides (de Vries and Visser 2001). Similarly, Cong et al. (2017) demonstrated that Aspergillus sydowii strain MS-19 isolated from Antarctic region was a rich source of lignocellulosic enzymes having potential plant cell wall-degrading activity. Apart from these, some of other fungi such as Trichoderma reesei (Saloheimo et al. 2002), Neurospora crassa (Tian et al. 2009), and Rhizopus oryzae (Lara-Marquez et al. 2011) were also successfully exploited. In addition, Kubicek et al. (2014) reviewed that many fungi secreting various lignocellulosic enzymes responsible for the degradation of polymers present in the plant cell wall. Above-mentioned studies confirmed that different lignocellulolytic enzymes secreted by bacteria and fungi play an important role in the degradation of plant cell wall. In fact, there is a pressing need to screen microbes of different groups to search for potential cell wall-degrading microbes.
Various white-rot fungi such as Phanerochaete chrysosporium, Ceriporia lacerata, Cyathus stercolerus, Ceriporiopsis subvermispora, Pycnoporus cinnarbarinus, Pleurotus ostreaus, and P. chrysosporium have been the most commonly used for the pretreatment of different lignocellulosic materials (Chandel et al. 2013; Maurya et al. 2015). These fungi have the ability to produce lignin-degrading enzymes i.e., lignin peroxidases and manganese-dependent peroxidases, which possess high delignification efficiency.
According to Akhtar et al. (2016), white-rot fungi such as Phanerochaete chrysosporium, Pycnoporus cinnabarinus, Phlebia spp., Echinodontium taxodii, Irpex lacteus, and Pycnoporus sanguineus showed high lignin degradation ability. Whereas, some of these fungi like Ceriporiopsis subvermispora, Phlebiabrevispora, P. floridensis, P. radiata, Echinodontium taxodii, Gonoderma sp., Oxysporus sp., Trametes versicolor, Pleurotussajor-caju, and Trichoderma reesei demonstrated ability towards the carbohydrate degradation. Similarly, brown-rot fungi including Serpula lacrymans, Coniophora puteana, Meruli poria incrassata, Laetoporus sulphureus, and Gleophyllum trabeum have been used in biodegradation of many cellulosic materials. Apart from these, some other bacteria like Bacillus circulans and Sphingomonas paucimobilis, Cellulomonas, Zymomonas spp., Bacillus macerans, Cellulomonas cartae, and C. uda have also been used in degradation and hydrolysis of different lignocellulosic biomasses.
Oliva-Taravilla et al. (2015) demonstrated the degradation of wheat straw using laccase enzymes produced from a fungus Pycnoporus cinnabarinus which can be further used for the production of bioethanol. In 2015, Xu et al. (2015) screened different white- rot fungi for their ligninolytic activity as a biological pretreatment of lignocellulosic biomass (barley straw). Furthermore, they emphasized the development of low-cost laccase production method from Tinea versicolor BBEL0970 and efficient biological pretreatment of barley straw by this fungus.
In addition to above-mentioned advantages of biological pretreatments, there are some disadvantages which mainly include problems of contamination and microbial mutation apart from these microbial growths requires specific conditions and downstreaming is comparatively complex.
Nanotechnological strategies for biomass pretreatment
The widespread applications of nanotechnology in biofuel production have been now investigated. Nanotechnology may offer easy, eco-friendly, efficient, and economically viable approaches for the production of biofuel from inexpensive lignocellulosic biomass (Rai et al. 2016). Similarly, recent studies have proposed that the extensive use of nanotechnology in general and nanomaterials in particular for the pretreatment of various lignocellulosic biomasses is most promising. Use of nanotechnology in the development of viable pretreatment method could be a decisive role in 2G sugars production. In this context, e.g., Wang (2012) developed a fast and efficient nano-scale shear hybrid alkaline (NSHA) pretreatment method for corn stover employing modified Taylor–Couette reactor using sodium hydroxide at room temperature, with a 2-min retention time and a 12,500 s−1 shear rate. Pretreatment results showed removal of hemicellulose and lignin from original material, leaving an up to 82% of cellulose content in the remaining solid. NSHA pretreatment synergistically disrupted the recalcitrance of biomass and generated nano-scale polysaccharide aggregates which can be easily digested to sugar monomers by the synergetic action of cellulases enzymes. Indeed, NSHA pretreatment approach showed an approximately fourfold and fivefold increase in enzymatic conversion of cellulose and hemicellulose into sugars using immobilized cellulase.
In despite of promising possibilities, until now, reports are very scanty on biomass pretreatment using nanoparticles as the implication of nanotechnology in biomass refinery is in the nascent stage. Hydrolysis of lignocellulosic materials using nanoparticles or nanomaterials can be achieved through two ways: (1) the immobilization of various enzymes on nanomaterials by physical adsorption or by covalent bonding, and (2) using functionalized nanoparticles. Both these approaches are briefly discussed here.
As earlier discussed, enzymatic hydrolysis is one of the most important steps in biofuel production, but the stability of enzymes involved in the process is a major concern and nanotechnology can play a crucial role in the stabilization of these biocatalysts. In this context, Srivastava et al. (2016) demonstrated that functionalization of cellulase enzyme involved in the hydrolysis of lignocellulosic biomass with zinc oxide nanoparticles provides thermal and pH stability of crude cellulase isolated from Aspergillus fumigatus AA001. They further reported that the nano-functionalized enzymes showed thermal stability at 65 °C up to 10 h and pH stability in the alkaline pH range. The enzyme retained 53% of its relative activity at pH 10.5. Similarly, immobilization of cellulase on modified Fe3O4 magnetic nanosphere by electrostatic binding can also increase the stability of enzyme and retain about 87% native activity (Zhang et al. 2015a, b). Moreover, cellulase immobilized on silver or gold nanoparticles can be reused for about six times with retaining of 73–78% activity. Thus, immobilization of enzymes on various nanomaterials is believed to be effectively used for the hydrolysis of different lignocellulosic materials.
In another study, Huang et al. (2015) demonstrated the immobilization of cellulase recovered from Aspergillus niger on β-cyclodextrin-conjugated magnetic nanoparticles by simple approaches such as silanization and reductive amidation. Furthermore, they studied the comparative hydrolysis of rice straw using free as well as immobilized cellulase, the results showed that immobilized cellulase produced the higher concentration of glucose after yield as compared to free cellulase, and one more added advantage is that immobilized cellulase can be recovered up to 85% by applying magnetic field and reused for continuous hydrolysis. A similar approach has been proposed in which two types of cellulases (β-glucosidase A and cellobiohydrolase D) were immobilized on superparamagnetic nanoparticles and reused for the cellulosic biomass conversion (Song et al. 2016). Recently, a novel magnetic cross-linked cellulase aggregate was developed and efficiently applied for bioconversion of lignocellulosic biomass. Immobilized cellulase showed very high stability and function at the wide range of temperature and pH as compared to free cellulase. Immobilized enzyme could be reused up to six consecutive cycles of lignocellulosic biomass hydrolysis and retained about 74% of its activity (Jia et al. 2017).
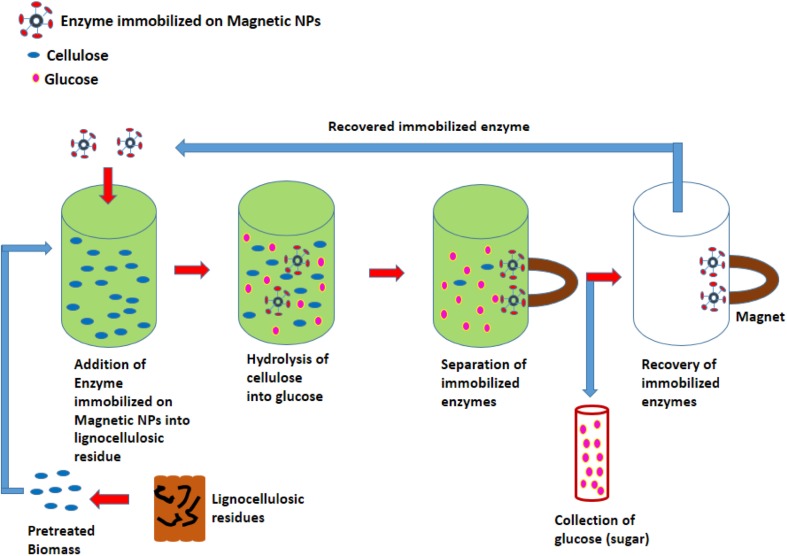
Figure 4 shows the schematic representation of hydrolysis of lignocellulosic biomass using cellulases immobilized on magnetic nanoparticles and their recovery with the help of magnetic field.
Fig. 4.
Schematic representation of hydrolysis of lignocellulosic biomass using cellulases immobilized on magnetic nanoparticles and its easy recovery by applying magnetic field
Similarly, Li et al. (2016) reviewed the role of various nanomaterials in the selective conversion of cellulose as well as cellobiose into sugar (glucose) through different types of reactions including hydrolysis, isomerization, dehydration, hydrogenation, oxidation, hydrogenation, dehydration, gasification, pyrolysis, etc. Magnetic carbonaceous acid nanoparticles synthesized by pyrolysis from homogeneous mixtures of glucose and magnetic Fe3O4 nanoparticles followed by sulfonation can be potentially used for the hydrolysis of plant wastes like bagasse, Jatropha, and Plukenetia hulls in microwave reactors. The obtained results showed a significant level of hydrolysis of biomass (Su et al. 2015).
Other nanomaterials like mesoporous silica and carbon nanoparticles can be effectively support the immobilization of various chemical catalysts, which further can be used for hydrolysis of the cellulosic materials. Lai (2015) reviewed that various solid acid catalysts such as heteropoly acids, sulfonated resins, sulfonated carbon, sulfonic acid functionalized silica, and many other can be supported on mesoporous nanoparticles, and used for hemicellulose and/or cellulose hydrolysis. Liu and Zhang (2016) proposed the concept of nanoparticle-mediated transformation of biomass, so that it could be used for various purposes including biofuel production. They suggested that magnetic nanoparticles can be used as a bridge to fill up the gap between heterogeneous and homogeneous catalysts and effectively used for the conversion of biomass into valuable chemicals. Similarly, Silva et al. (2017) also proposed that the immobilization of cellulase could be an economical alternative for cost reduction of enzyme application in the hydrolysis of lignocellulosic materials.
Another important nanotechnological approach is the use of different acid functionalized nanoparticles for the pretreatment of lignocellulosic biomass. Gill et al. (2007) synthesized two types of acid functionalized magnetic nanoparticles, i.e., silica-coated magnetic nanoparticles functionalized with alkylsulfonic acid (AS-SiMNPs) and perfluoroalkylsulfonic acid (PS-SiMNPs). In addition, they suggested that the hydronium ions (H3O+) formed on the surface of these magnetic nanoparticles lead to promotion and conversion of cellulose into glucose through the hydrolysis reaction. Pena et al. (2011) demonstrated the synthesis of silica-protected cobalt-spinel ferrite nanoparticles having magnetic properties. These nanoparticles were further acid functionalized with perfluoroalkylsulfonic acid, alkylsulfonic acid, and butylcarboxylic acid, and used for breaking β-(1 → 4) glycosidic bonds in cellobiose. About 78% conversion of cellobiose was reported in case of nanoparticles functionalized with alkylsulfonic and these nanoparticles could be reused after their separation using the magnetic field. However, when the efficacy of these acid functionalized nanoparticles was evaluated in hemicellulose conversion, about 66.3% and 61.0% conversion was reported using nanoparticles functionalized with perfluoroalkylsulfonic acid and alkylsulfonic acid, respectively (Pena et al. 2012). In another study, about 96.0% conversion of cellobiose into glucose was reported using silica-coated nanoparticles functionalized with propyl-sulfonic acid groups (Pena et al. 2014).
Similarly, Duque (2013) synthesized silica-coated nanoparticles which were functionalized with three different acids such as perfluoropropyl-sulfonic acid, carboxylic acid, and propyl-sulfonic acid. Those authors reported that propyl-sulfonic and perfluoropropyl-sulfonic acid functionalized nanoparticles catalyzed the hydrolysis of cellobiose significantly. These nanoparticles also showed potential to solubilize wheat straw hemicelluloses. A glucose yield of 90% and 58% of the hemicellulosic sugars could be obtained using these nanoparticles. In another study, Wang et al. (2015) also demonstrated that sulfonic acid functionalized silica-coated crystalline Fe/Fe3O4 core/shell magnetic nanoparticles exhibited excellent stability and showed significant catalytic activity toward the production of biofuel.
Overall viewpoint of biomass pretreatment technologies: drawbacks and challenges
A number of pretreatment technologies are developed for the accessibility of cellulase enzymes to remaining carbohydrate in the substrate, yielding clean 2G sugars with desired yields. Pretreatment approaches such as steam explosion, supercritical fluids, hydrodynamic cavitation, afex/extractive ammonia, and biological pretreatment showed the promising results. However, these methods also need to be deeply investigated for their feasibility at industrial scale along with a concrete techno-economic analysis. Table 3 shows the merits and demerits of various pretreatment technologies in addition to their overall impact on capital expenses and operational expenses.
Table 3.
Features of different biomass pretreatment technologies and their impact on capital and operational expense
| Pretreatment type | Pros | Cons | Capital expenses (capex) | Operational expenses (opex) | Overall potential |
|---|---|---|---|---|---|
| Steam explosion |
Fast reaction rates Reproducibility results at scale-up operations Ease product recovery |
Requirement of high pressure and temperature Energy intensive Process complexity, inhibitor generation |
+++ | ++ | ++ |
| Supercritical fluids |
High yield of lignin removal Possibility to work with high solid loading Use of CO2 impact on greenhouse gasses |
High process complexity Difficulties in scale-up Requirement of specific reactors and vessels |
+++ | +++ | ++ |
| Hydrodynamic cavitation |
Fast reaction time High productivity and yield of 2G sugars Ease working with high substrate loading |
Scale-up needs careful investigations Difficulties to maintain process conditions High energy intensive process |
+++ | ++ | +++ |
| AFEX/extractive ammonia |
High yields of 2 g sugars Fast and specific process Ease process scale-up No inhibitor generation |
Requirement of specific reactors for high temperature and pressure Operational difficulties Environmental concerns |
++ | ++ | ++ |
| Ionic liquids (ILs) |
High sugars recovery yields Specific reaction rate Possibility to recycling of ILs |
High cost of ILs Availability of ILs to be deployed at large scale |
+++ | + | +++ |
| Biological pretreatment |
No need of harsh chemicals, temperature and pressure Ease process configuration Minimum waste discharge, low carbon footprint, green process No inhibitor generation |
Slow process Low specificity Difficulties to maintain microrganisms growth Difficulties to scale-up |
+ | + | + |
+ Low effect, ++ medium effect, +++ high effect
Cellulosic ethanol production at large scale is challenging due to the biomass complexity, harvesting, and transportation of biomass from farm to factory. Smooth biomass supply chain is utmost important for the holistic success of biorefinery. Broadly, biomass supply chain includes following key steps: biomass collection from farm, storage, compaction, transportation, and biomass processing at the biorefinery (Sultana et al. 2010). Besides the logistic challenges, biomass cleaning and desizing are important steps before pretreatment. Challenges to be overcome also must consider the overall process in an industrial biorefinery besides logistic issues. The collected biomass from agricultural farms usually has contaminants, stone and soil, which can affect the pretreatment process largely in terms of sugars recovery, inhibiting the pretreatment reaction and choking the reactor lines. In addition, these issues greatly impact on operational and capital cost for the overall biomass conversion process (Balan 2014). In general, majority of feedstock needs to be desized to have maximum uniformity in biomass to have smooth transfer of biomass to reactors from pipelines. Indeed, non-uniformity of biomass feedstock can create the problem in biomass transfer by choking the lines eventually impairing the smooth processing of biomass at large scale. Interrupted biomass processing at large-scale operations causes low sugars recovery with high processing cost (Chandel et al. 2018).
Conclusions
Production of the second-generation sugars from lignocellulosic biomass has a central role in the development of biorefineries. These sugars are considered as renewable building block for the sustainable production of biofuels and chemical commodities or specialities. However, for their production in a biorefinery, pretreatment is an inevitable step in the process. Biomass recalcitrance plays a pivotal role in pretreatment efficiency. Removal of hemicellulose and lignin, besides structural modification in biomass, is goals of pretreatment technologies to turn the carbohydrate fraction accessible for enzymatic hydrolysis of biomass. Each pretreatment method has their own advantages, but also challenges to be overcome. Traditional and more studied technologies have shown promising results and have been evaluated in large scale, but they present a number of disadvantages as inhibitor production in dilute acid and steam explosion, or high reagent consumption in alkaline pretreatment. In this context, options as hydrodynamic cavitation, supercritical fluids, and ionic liquids have proven their applicability for 2G sugar production, although the viability of these processes in large scale needs more studies. Biological pretreatment methods are green and eco-friendly, but they are inherently slow and difficult to scale up. Recently, nanotechnology-based pretreatment methods have also been found to be significant for the hydrolysis of a variety of lignocellulosic materials, and hence, in near future, they could play a remarkable role in the desired success of biorefineries. Thus, although with decades of studies and still representing a persistent bottleneck for biorefineries viability, novel alternatives have emerging last years and this research field remains as a exciting research area, requiring creativity and inventive capacity to overcome the challenges and to contribute with the transition to a sustainable bioeconomy.
Acknowledgements
The authors gratefully acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil, processes number 154193/2018-6 and 449609/2014-6), FAPESP-São Paulo Research Foundation (#2014/27055-2;#2016/10636-8;#2016/23758-4;#2017/11086-4) and CAPES, by the financial resources.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
References
- Aditiya HB, Mahlia TMI, Chong WT, Nur H, Sebayang AH. Second generation bioethanol production: a critical review. Renew Sust Energy Rev. 2016;66:631–653. [Google Scholar]
- Akhtar N, Gupta K, Goyal D, Goyal A. Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ Prog Sustain Energy. 2016;35(2):489–511. [Google Scholar]
- Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol. 2010;101:4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- Antunes FAF, Chandel AK, Milessi TSS, Santos JC, Rosa CA, DA Silva SS. Bioethanol production from sugarcane bagasse by a novel Brazilian pentose fermenting yeast Scheffersomyces shehatae UFMG-HM 52.2: evaluation of fermentation medium. Int J Chem Eng. 2014;2014:1–8. [Google Scholar]
- Antunes FAF, Santos JC, Cunha MAA, Brumano LP, Milessi TSS, Teran-Hilares R, et al. Biotechnological production of xylitol from biomass. In: Fang Z, Smith Q, et al., editors. Biofuels and biorefineries. 1. Singapore: Springer Nature; 2017. pp. 311–342. [Google Scholar]
- Arora A, Priya S, Sharma P, Sharma S, Nain L. Evaluating biological pretreatment as a feasible methodology for ethanol production from paddy straw. Biocat Agric Biotechnol. 2016;8:66–72. [Google Scholar]
- Auxenfans T, Crônier D, Chabbert B, Paës G. Understanding the structural and chemical changes of plant biomass-following steam explosion pretreatment. Biotechnol Biofuels. 2017;10:36. doi: 10.1186/s13068-017-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badve MP, Gogate PR, Pandit AB, Levente C. Hydrodynamic cavitation as a novel approach for delignification of wheat straw for paper manufacturing. Ultrason Sonochem. 2014;21:162–168. doi: 10.1016/j.ultsonch.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Balan V. Current challenges in commercially producing biofuels from lignocellulosic biomass. ISRN Biotechnol. 2014;2014:1–31. doi: 10.1155/2014/463074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazzi T, Calgaroto S, Rosa CD, Oliveira JV, Mazutti MA. Hydrolysis of sugarcane bagasse using supercritical carbon dioxide to obtain fermentable sugars. J Chem Technol Biotechnol. 2013;88:1766–1768. [Google Scholar]
- Bernardinelli OD, Lima MA, Rezende CA, Polikarpov I, Azevedo ER. Quantitative 13C MultiCP solip-state NMR as a tool for evaluation of cellulose crystallinity index measured directly inside sugarcane biomass. Biotechnol Biofuels. 2015;8:110. doi: 10.1186/s13068-015-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludworth J, Carl Knopf F. Reactive extraction of lignin from wood using supercritical ammonia-water mixtures. J Supercrit Fluids. 1993;6:249–254. [Google Scholar]
- Brandt-Talbot A, Gschwend FJV, Fennell PS, Lammens TM, Tan B, Weale J, et al. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017;19:1–26. [Google Scholar]
- Canilha L, Chandel AK, Milessi TSS, Antunes FAF, Freitas WLC, Felipe MGA, et al. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J Biomed Biotechnol. 2012;2012:1–15. doi: 10.1155/2012/989572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona E, Rios J, Peña J, Rios L. Effects of the pretreatment method on enzymatic hydrolysis and ethanol fermentability of the cellulosic fraction from elephant grass. Fuel. 2014;118:41–47. [Google Scholar]
- Carpio LGT, Souza FS. Optimal allocation of sugarcane bagasse for producing bioelectricity and second generation ethanol in Brazil: scenarios of cost reductions. Renew Energ. 2017;111:771–780. [Google Scholar]
- Cavalett O, Chagas MF, Junqueira TL, Watanabe MDB, Bonomi A. Environmental impacts of technology learning curve for cellulosic ethanol in Brazil. Ind Crops Prod. 2017;106:31–39. [Google Scholar]
- Chandel AK, Giese EC, Antunes FFA, Oliveira IS, Silva SS. Pretreatment of sugarcane bagasse and leaves: Unlocking the treasury of “Green currency”. In: Zhen F, editor. Pretreatment techniques for biofuels and biorefineries. Beijing: Springer-Verlag, Springer Asia Limited; 2012. pp. 369–391. [Google Scholar]
- Chandel AK, Silva SS, Singh OV. Detoxification of lignocellulose hydrolysates: Biochemical and metabolic engineering toward white biotechnology. BioEnergy Res. 2013;6:388–401. [Google Scholar]
- Chandel AK, Antunes FAF, Anjos V, Bell MJV, Rodrigues LN, Polikarpov I, et al. Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels. 2014;7:63. doi: 10.1186/1754-6834-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel AK, Garlapati VK, Singh AK, Antunes FAF, Silva SS. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour Technol. 2018;264:370–381. doi: 10.1016/j.biortech.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Chornet E, Overend RP (1988) Phenomenological kinetics and reaction engineering aspects of steam/aqueous treatments. In: Focher B, Marzetti A, Crescenzi V (eds) Steam explosion techniques—fundamentals and industrial applications : proceedings of the international workshop on steam explosion techniques: fundamentals and industrial applications, Milan, Italy. CRC Press pp 21–58
- Conab-Companhia Nacional de abastecimento (2019) BOLETIM DA SAFRA DE GRÃOS. 6º Levantamento-Safra 2018/19. Available in https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos. Accessed 6 Apr 2019
- Cong B, Wang N, Liu S, Liu F, Yin X, Shen J. Isolation, characterization and transcriptome analysis of a novel antarctic Aspergillus sydowii strain MS-19 as a potential lignocellulosic enzyme source. BMC Microbiol. 2017;17:129. doi: 10.1186/s12866-017-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coseri S. Cellulose: To depolymerize… or not to? Biotechnol Adv. 2017;35:251–266. doi: 10.1016/j.biotechadv.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Costa THF, Masarin F, Bonifácio TO, Milagres AMF, Ferraz A. The enzymatic recalcitrance of internodes of sugar cane hybrids with contrasting lignin contents. Ind Crops Prod. 2013;51:202–211. [Google Scholar]
- Costa THF, Vega-Sanchez ME, Milagres AMF, Scheller HV, Ferraz A. Tissue-specific distribution of hemicelluloses in six different sugarcane hybrids as related to cell wall recalcitrance. Biotechnol Biofuels. 2016;9:99. doi: 10.1186/s13068-016-0513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daza Serna LV, Orrego Alzate CE, Cardona Alzate CA. Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour Technol. 2016;199:113–120. doi: 10.1016/j.biortech.2015.09.078. [DOI] [PubMed] [Google Scholar]
- de Vries RP, Visser J. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev. 2001;65(4):4497–4522. doi: 10.1128/MMBR.65.4.497-522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos LV, Grassi MCB, Gallardo JCM, Pirolla RAS, Calderon LL, Carvalho-netto OV, et al. Second-generation ethanol: the need is becoming a reality. Ind Biotechnol. 2016;12(1):40–57. [Google Scholar]
- Driemeier C, Pimenta MTB, Rocha GJM, Oliveira MM, Mello DB, Maziero P, et al. Evolution of cellulose crystals during prehydrolysis and soda delignification of sugarcane lignocellulose. Cellulose. 2011;18:1509–1519. [Google Scholar]
- Duque LEP (2013) Acid-functionalized nanoparticles for biomass hydrolysis PhD thesis submitted to Department of Biological & Agricultural Engineering College of Engineering, Kansas State University Manhattan, Kansas
- E-PIC S.r.l (2016) Biomass pretreatment (BIOGAS) [Internet]. Mongrando (BI). http://www.epic-srl.com/en/cavitation-technologies/biomass-pretreatment-biogas
- Gill CS, Price BA, Jones CW. Sulfonic acid-functionalized silica-coated magnetic nanoparticle catalysts. J Catal. 2007;251:145–152. [Google Scholar]
- Gogate PR. Greener processing routes for reactions and separations based on use of ultrasound and hydrodynamic cavitation. In: Stefanidis G, Stankiewicz A, editors. Alternative energy sources for green chemistry. Cambridge: Thomas Graham House, RSC Publishing Blog; 2016. [Google Scholar]
- Gogate PR, Pandit AB. Engineering design methods for cavitation reactors II: hydrodynamic cavitation. AIChE J. 2000;46:1641–1649. [Google Scholar]
- Gogate PR, Pandit AB. A review and assessment of hydrodynamic cavitation as a technology for the future. Ultrason Sonochem. 2005;12:21–27. doi: 10.1016/j.ultsonch.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Grethlein HE, Allen DC, Converse AO. A comparative study of the enzymatic hydrolysis of acid-pretreated white pine and mixed hardwood. Biotechnol Bioeng. 1984;26:1498–1505. doi: 10.1002/bit.260261215. [DOI] [PubMed] [Google Scholar]
- Gupta A, Verma JP. Sustainable bio-ethanol production from agro-residues: a review. Renew Sust Energy Rev. 2015;41:550–567. [Google Scholar]
- Huang PJ, Chang KL, Hsieh JF, Chen ST. Catalysis of rice straw hydrolysis by the combination of immobilized cellulase from Aspergillus niger on β-cyclodextrin-Fe3O4 nanoparticles and ionic liquid. BioMed Res Int. 2015;40:9103. doi: 10.1155/2015/409103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Zhang W, Yang Z, Yang X, Wang N, Yu X. Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules. 2017;22:269. doi: 10.3390/molecules22020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joko T, Subandi A, Kusumandari N, Wibowo A, Priyatmojo A. Activities of plant cell wall-degrading enzymes by bacterial soft rot of orchid. Arch Phytopathol Plant Protect. 2014;47(10):1239–1250. [Google Scholar]
- Jönsson LJ, Martín C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol. 2016;199:103–112. doi: 10.1016/j.biortech.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Kapdan IK, Kargi F, Oztekin R. Effects of operating parameters on acid hydrolysis of ground wheat starch: maximization of the sugar yield by statistical experiment design. Starch-Stärke. 2011;63:311–318. [Google Scholar]
- Kaur M, Aggarwal NK. Screening of alternaria pathogens associated with Parthenium hysterophorus for the production of lignocellulolytic enzymes. Bioeng Biosci. 2017;5(1):14–23. [Google Scholar]
- Kim I, Lee I, Hwang T, Han JI. Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour Technol. 2015;192:335–339. doi: 10.1016/j.biortech.2015.05.038. [DOI] [PubMed] [Google Scholar]
- Kubicek CP, Starr TL, Glass NL. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol. 2014;52:427–451. doi: 10.1146/annurev-phyto-102313-045831. [DOI] [PubMed] [Google Scholar]
- Lai CY. Mesoporous materials-based catalysts for chemical hydrolysis of polysaccharides. Thermodyn Catal. 2015;6:3. [Google Scholar]
- Lan W, Liu CF, Sun RC. Fractionation of bagasse into cellulose, hemicelluloses, and lignin with ionic liquid treatment followed by alkaline extraction. J Agric Food Chem. 2011;59:8691–8701. doi: 10.1021/jf201508g. [DOI] [PubMed] [Google Scholar]
- Lara-Marquez A, Zavala-Paramo MG, Lopez-Romero E, Camacho HC. Biotechnological potential of pectinolytic complexes of fungi. Biotechnol Lett. 2011;33:859–868. doi: 10.1007/s10529-011-0520-0. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang Q, Riisager A, Yang S. Catalytic valorization of cellulose and cellobiose with nanoparticles. Curr Nanosci. 2016;11(1):1–14. [Google Scholar]
- Liang J, Chen X, Wanga L, Weia X, Wang H, Lu S, et al. Subcritical carbon dioxide-water hydrolysis of sugarcane bagasse pith for reducing sugars production. Bioresour Technol. 2017;228:147–155. doi: 10.1016/j.biortech.2016.12.080. [DOI] [PubMed] [Google Scholar]
- Liu B, Zhang Z. Catalytic conversion of biomass into chemicals and fuels over magnetic catalysts. ACS Catal. 2016;6(1):326–338. [Google Scholar]
- Liu Z, Longfei L, Cheng L, Airong X. Saccharification of cellulose in the ionic liquids and glucose recovery. Renewable Energy. 2017;106:99–102. [Google Scholar]
- Madison MJ, Coward-Kelly G, Liang C, Karim N, Falls M, Holtzapple MT. Mechanical pretreatment of biomass-part i: Acoustic and hydrodynamic cavitation. Biomass Bioenergy. 2017;98:135–141. [Google Scholar]
- Marques F (2018) Bioenergia. Obstaculos no caminho. Available in: http://revistapesquisa.fapesp.br/2018/06/18/obstaculos-no-caminho/. Accessed 25 March 2018
- Maurya DP, Singla A, Negi S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech. 2015;5:597–609. doi: 10.1007/s13205-015-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy J, Sawan SP. Supercritical fluid cleaning: fundamentals, technology and applications. New Jersey: Noyes Publications; 1998. [Google Scholar]
- Mirmohamadsadegh S, Chen Z, Wan C. Reducing biomass recalcitrance via mild sodium carbonate pretreatment. Bioresour Technol. 2016;209:386–390. doi: 10.1016/j.biortech.2016.02.096. [DOI] [PubMed] [Google Scholar]
- Moholkar VS, Pandit AB. Modeling of hydrodynamic cavitation reactors: a unified approach. Chem Eng Sci. 2001;56:6295–6302. [Google Scholar]
- Mohtar SS, Busu TNZTM, Noor AMM, Shaari N, Mat H. An ionic liquid treatment and fractionation of cellulose, hemicellulose and lignin from oil palm empty fruit bunch. Carbohydr Polym. 2017;166:291–299. doi: 10.1016/j.carbpol.2017.02.102. [DOI] [PubMed] [Google Scholar]
- Morais ARC, Mata AC, Bogel-Lukasik R. Integrated conversion of agroindustrial residue with high pressure CO2 within the biorefinery concept. Green Chem. 2014;16:4312–4322. [Google Scholar]
- Nakashima K, Evi Y, Shibasaki-Kitakawa N, Soyama H, Yonemoto R. Hydrodynamic cavitation reactor for efficient pretreatment of lignocellulosic biomass. Ind Eng Chem Res. 2016;55:1866–1871. [Google Scholar]
- Narayanaswamy N, Faik A, Goetz DJ, Gu T. Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour Technol. 2011;102:6995–7000. doi: 10.1016/j.biortech.2011.04.052. [DOI] [PubMed] [Google Scholar]
- NCGA (2018) The national corn growers association. U.S. corn production 1938–2018. http://www.worldofcorn.com/pdf/WOC-2019.pdf
- Neves PV, Pitarelo AP, Ramos LP. Production of cellulosic ethanol from sugarcane bagasse by steam explosion: Effect of extractives content, acid catalysis and different fermentation technologies. Bioresour Technol. 2016;208:184–194. doi: 10.1016/j.biortech.2016.02.085. [DOI] [PubMed] [Google Scholar]
- Nguyen TY, Cal CM, Kumar R, Wyman CE. Co-solvent pretreatment reduces costly enzyme requirements for high sugar and ethanol yields from lignocellulosic biomass. Chem Sus Chem. 2015;8:1716–1725. doi: 10.1002/cssc.201403045. [DOI] [PubMed] [Google Scholar]
- Njoku SI, Ahring BK, Uellendahl H. Pretreatment as the crucial step for a cellulosic ethanol biorefinery: Testing the efficiency of wet explosion on different types of biomass. Bioresour Technol. 2012;124:105–110. doi: 10.1016/j.biortech.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Ochiai A, Itoh T, Kawamata A, Hashimoto W, Murata K. Plant cell wall degradation by saprophytic Bacillus subtilis strains: Gene clusters responsible for rhamnogalacturonan depolymerization. Appl Environ Microbiol. 2007;73(12):3803–3813. doi: 10.1128/AEM.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva-Taravilla A, Moreno AD, Demuez M, Ibarra D, Tomas-Pejo E, Gonzalez-Fernandez C, et al. Unraveling the effects of laccase treatment on enzymatic hydrolysis of steam-exploded wheat straw. Bioresour Technol. 2015;175:209–215. doi: 10.1016/j.biortech.2014.10.086. [DOI] [PubMed] [Google Scholar]
- Ozoneck J. Application of hydrodynamic cavitation in environmental engineering. New York: CRC Press, Taylor & Francis Group; 2012. [Google Scholar]
- Pathak VM, Navneet Review on the current status of polymer degradation: A microbial approach. Bioresour Bioprocess. 2017;4:15. [Google Scholar]
- Patil PN, Gogate PR, Csoka L, Dregelyi-Kiss A, Horvath M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason Sonochem. 2016;30:79–86. doi: 10.1016/j.ultsonch.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Pena L, Ikenberry M, Ware B, Hohn KL, Boyle D, Sun XS, et al. Cellobiose hydrolysis using acid-functionalized nanoparticles. Biotechnol Bioproc Eng. 2011;16:1214–1222. [Google Scholar]
- Pena L, Ikenberry M, Hohn KL, Wang D. Acid-functionalized nanoparticles for pretreatment of wheat straw. J Biomater Nanobiotechnol. 2012;3:342–352. [Google Scholar]
- Pena L, Hohn KL, Li J, Sun XS, Wang D. Synthesis of propyl-sulfonic acid-functionalized nanoparticles as catalysts for cellobiose hydrolysis. J Biomater Nanobiotechnol. 2014;5:241–253. [Google Scholar]
- Phan DT, Tan CS. Innovative pretreatment of sugarcane bagasse using supercritical CO2 followed by alkaline hydrogen peroxide. Bioresour Technol. 2014;167:192–197. doi: 10.1016/j.biortech.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Pielhop T, Amgarten J, von Rohr PR, Studer MH. Steam explosion pretreatment of softwood: the effect of the explosive decompression on enzymatic digestibility. Biotechnol Biofuels. 2016;9:152. doi: 10.1186/s13068-016-0567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M, Santos JC, Soler MF, Marcelino PRF, Brumano LP, Ingle AP, et al. Strategic role of nanotechnology for production of bioethanol and biodiesel. Nanotechnol Rev. 2016;5(2):231–250. [Google Scholar]
- Ramos AM, Gally M, Szapiroa G, Itzcovicha T, Carabajal M, Levina L. In vitro growth and cell wall degrading enzyme production by Argentinean isolates of Macrophomina phaseolina, the causative agent of charcoal rot in corn. Rev Argent Microbiol. 2016;48(4):267–273. doi: 10.1016/j.ram.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Raza-Amin F, Khalid H, Zhang H, Rahman SU, Zhang R, Liu G, Chen C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express. 2017;7:72. doi: 10.1186/s13568-017-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes T, Bandyopadhyay SS, McCoy BJ. Extraction of lignin from wood with supercritical alcohols. J Supercrit Fluids. 1989;2:80–84. [Google Scholar]
- Saharan VK, Manava R, Aqeel M, Pandit AB. Effect of geometry of hydrodynamically cavitating device on degradation of orange-G. Ultrason Sonochem. 2013;20:345–353. doi: 10.1016/j.ultsonch.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Saloheimo M, Paloheimo M, Hakola S, Pere J, Swanson B, Nyyssönen E, et al. Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur J Biochem. 2002;269:4202–4211. doi: 10.1046/j.1432-1033.2002.03095.x. [DOI] [PubMed] [Google Scholar]
- Sandford K, Chotani G, Danielson N, Zahn JA. Scaling up of renewable chemicals. Curr Opin Biotechnol. 2016;38:112–122. doi: 10.1016/j.copbio.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Santos JC, Marton JM, Felipe MGA. Continuous system of combined columns of ion exchange resins and activated charcoal as a new approach for the removal of toxics from sugar cane bagasse hemicellulosic hydrolysate. Ind Eng Chem Res. 2014;53:16494–16501. [Google Scholar]
- Silva SPM, Morais AR, Bogel-Lukasik R. The CO2-assisted autohydrolysis of wheat straw. Green Chem. 2014;16:238–246. [Google Scholar]
- Silva DF, Carvalho AF, Shinya TY, Mazali GS, Herculano RD, Oliva-Neto P. Recycle of immobilized endocellulases in different conditions for cellulose hydrolysis. Enzyme Res. 2017 doi: 10.1155/2017/4362704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu R, Binod P, Pandey A. Biological pretreatment of lignocellulosic biomass: an overview. Bioresour Technol. 2016;199:76–82. doi: 10.1016/j.biortech.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Song Q, Mao Y, Wilkins M, Segato F, Prade R. Cellulase immobilization on superparamagnetic nanoparticles for reuse in cellulosic biomass conversion. AIMS Bioeng. 2016;3(3):264–276. [Google Scholar]
- Sousa LC, Jin M, Chundawat SPS, Bokade V, Tang X, Azarpira A, et al. Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ Sci. 2016;9:1215–1223. [Google Scholar]
- Srivastava N, Srivastava M, Mishra PK, Ramteke PW. Application of ZnO nanoparticles for improving the thermal and pH stability of crude cellulase obtained from Aspergillus fumigatus AA001. Front Microbiol. 2016;7:514. doi: 10.3389/fmicb.2016.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TC, Fang Z, Zhang F, Luo J, Li XK. Hydrolysis of selected tropical plant wastes catalyzed by a magnetic carbonaceous acid with microwave. Sci Rep. 2015;5:17538. doi: 10.1038/srep17538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana A, Kumar A, Harfield D. Development of agri–pellet production cost and optimum size. Bioresour Technol. 2010;101(14):5609–5621. doi: 10.1016/j.biortech.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Sun N, Rahman M, Qin Y, Maxim ML, Rodriguez H, Rogers RD. Complete dissolution and partial delignification of wood in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009;11:646–655. [Google Scholar]
- Terán-Hilares R, de Almeida GFaria, Ahmed MAjaz, Antunes FAF, da Silva Silvério S, Han J, et al. Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass: A parametric study. Bioresour Technol. 2017;235:301–308. doi: 10.1016/j.biortech.2017.03.125. [DOI] [PubMed] [Google Scholar]
- Terán-Hilares R, Ramos L, Silva SS, Dragone G, Mussatto SI, Santos JC. Hydrodynamic cavitation as a strategy to enhance the efficiency of lignocellulosic biomass pretreatment. Crit Rev Biotechnol. 2017;38:483–493. doi: 10.1080/07388551.2017.1369932. [DOI] [PubMed] [Google Scholar]
- Thomson Reuters (2019) Web of Science, https://www.webofknowledge.com/. Accessed 4 Feb 2019
- Tian C, Beeson WT, Iavarone AT, Sun J, Marletta MA, Cate JHD, et al. Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proc Natl Acad Sci. 2009;106:22157–22162. doi: 10.1073/pnas.0906810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppugundla N, Sousa LC, Chundawat SPS, Yu X, Simmons B, Singh A, et al. A comparative study of ethanol production using dilute acid, ionic liquid and AFEX™ pretreated corn stover. Biotechnol Biofuels. 2014;7:72. doi: 10.1186/1754-6834-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA-United States Department of Agriculture (2019) World agricultural production. Available in https://apps.fas.usda.gov/psdonline/circulars/production.pdf. Accessed 6 Apr 2019
- Wang W (2012) Nanotechnology applications for biomass pretreatment, functional material fabrication and surface modification. Dissertation submitted to Michigan State University
- Wang H, Covarrubias J, Prock H, Wu X, Wang D, Bossmann SH. Acid-functionalized magnetic nanoparticle as heterogeneous catalysts for biodiesel synthesis. J Phys Chem C. 2015;119(46):26020–26028. [Google Scholar]
- Xu C, Singh D, Dorgan KM, Zhang X, Chen S. Screening of ligninolytic fungi for biological pretreatment of lignocellulosic biomass. Can J Microbiol. 2015;61(10):745–752. doi: 10.1139/cjm-2015-0156. [DOI] [PubMed] [Google Scholar]
- Yoo CG, Pu Y, Ragauskas AJ. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr Opin Green Sustain Chem. 2017;5:5–11. [Google Scholar]
- Zabed H, Sahu JN, Boyce AN, Faruq G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew Sustain Energy Rev. 2016;66:751–774. [Google Scholar]
- Zhang Z, Rackemann DW, Doherty WO, O’Hara IM. Glycerol carbonate as a green solvent for pretreatment of sugarcane bagasse. Biotechnol Biofuels. 2013;6:153. doi: 10.1186/1754-6834-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Xu W, Yan P, Liu X, Zhang C. Overcome the recalcitrance of eucalyptus bark to enzymatic hydrolysis by concerted ionic liquid pretreatment. Process Biochem. 2015;50:2208–2214. [Google Scholar]
- Zhang W, Qiu J, Feng H, Zang L, Sakai E. Increase in stability of cellulase immobilized on functionalized magnetic nanospheres. J Magn Magn Mater. 2015;375:117–123. [Google Scholar]
- Zheng Y, Lin H, Tsao GT. Supercritical carbon dioxide explosion as a pretreatment for cellulose hydrolysis. Biotechnol Lett. 1995;17:845–850. doi: 10.1021/bp980087g. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Lin H, Tsao GT. Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnol Prog. 1998;14:890–896. doi: 10.1021/bp980087g. [DOI] [PubMed] [Google Scholar]