Abstract
Human salivary gland (SG) branching morphogenesis is an intricate mechanism divided into stages, prebud, initial bud, pseudoglandular, canalicular, and terminal bud, to form the final lobular structure of the organ. The coordination of molecular cascades, including cell proliferation and apoptosis, are fundamental to this process. The intrinsic apoptosis pathway appears to be important in the early phases of ductal cavitation and luminisation; however, the role of the extrinsic apoptosis pathway has still to be determined. Questions remain as to whether the latter mechanism participates in the maintenance of the ductal lumen; therefore, the present study investigated the expression of proteins Prostate apoptosis response‐4 (Par‐4), Fas cell surface death receptor (Fas), Fas ligand (FasL), pleckstrin homology‐like domain family A member 1 (PHLDA1), caspase‐3, B‐cell CLL/lymphoma 2 (Bcl‐2), survivin, Ki‐67, mucin 1 (MUC1), and secreted protein acidic and cysteine‐rich (SPARC) during distinct phases of human SG development (50 specimens). This strategy aimed to draw an immunomorphological map of the proteins involved in apoptosis, cell proliferation, and tissue maturation during the SG branching morphogenesis process. Par‐4 was positive at all stages except the pre‐acinar phase. Fas and FasL were expressed in few cells. PHLDA1 was expressed in all phases but not in the terminal bud. Bcl‐2 expression was mainly negative (expressed in few cells). Survivin showed a cytoplasmic expression pattern in the early phases of development, which changed to a predominantly nuclear expression during development into more differentiated structures. Ki‐67 was expressed mainly at the pseudoglandular stage. MUC1 was positive in the pseudoglandular stage with a cytoplasmic pattern in regions of early luminal opening. Immunostaining for SPARC and caspase‐3 was negative. Our results suggest that proteins associated with the regulation of extrinsic and intrinsic apoptosis contribute to apoptosis during specific phases of the early formation of SGs in humans.
Keywords: apoptosis, development, immunohistochemistry, Ki‐67, morphogenesis, Par‐4, salivary glands
Introduction
Human salivary glands (SGs) are complex structures comprising a system of ducts and acini that originate during embryonic development (Cutler, 1990; Harunaga et al. 2011). Their branching morphogenesis involves growth, proliferation, differentiation, migration, and cell death. These glands are formed in gradual stages, termed the prebud, initial bud, pseudoglandular, canalicular, and terminal bud (Tucker, 2007; Teshima et al. 2016a,b; de Paula et al. 2017). From the primitive oral cavity, epithelial cells bud and internalise, forming branched solid cords (Melnick & Jaskoll, 2000; Patel et al. 2006, 2011). The ductal system formation involves the elimination of central cells of the solid cylindrical cords, which are then converted into a luminised tube; some studies suggest that apoptosis regulates this process (Melnick & Jaskoll, 2000; Lubarsky & Krasnow, 2003; Andrew & Ewald, 2010; Teshima et al. 2016a, b).
Apoptosis is a genetically programmed process of cell death which plays an important role in physiological and pathological processes (Elmore, 2007). Two pathways, intrinsic and extrinsic, can trigger apoptosis. B‐cell CLL/lymphoma 2 (Bcl‐2) family proteins regulate the intrinsic pathway: stimuli from pro‐apoptotic molecules induce mitochondria to release cytochrome‐c into the cytoplasm, where it associates with apoptotic peptidase activating factor 1 (APAF1). This complex, coupled with caspase‐9, activates caspase‐3, leading to cell death. The extrinsic pathway is induced by tumour necrosis factor (TNF)‐family members; these recognise specific ligands, such as Fas associated via death domain (FADD), which then recruit caspase‐8 and thereby activate caspase‐3 (Elmore, 2007; Taylor et al. 2008; Pereira & Amarante‐Mendes, 2011; Suzanne & Steller, 2013; Flusberg & Sorger, 2015).
Some studies have demonstrated the mechanism of apoptosis during SG development in animal models (Melnick & Jaskoll, 2000; Melnick et al. 2001; Teshima et al. 2016a). During mouse SG development, the death of central epithelial cells has been reputed to be an important event for the luminal opening and ductal system formation (Tucker, 2007; Andrew & Ewald, 2010; Teshima et al. 2016a). However, for human SG development, few studies have addressed the role of apoptosis: Teshima et al. (2016b) reported proteins of the intrinsic apoptosis pathway in the early stages of human salivary gland development, but there is little information on the roles of extrinsic apoptotic pathways in salivary gland formation.
There are several central molecules involved with the extrinsic apoptotic pathway. Prostate apoptosis response‐4 (Par‐4) is a pro‐apoptotic molecule ubiquitously expressed in normal tissues and cell types, primarily in the cytoplasm. The ability of Par‐4 to induce apoptosis is associated with its nuclear translocation (Goswami et al. 2006; Zhao & Rangnekar, 2008; Shrestha‐Bhattarai & Rangnekar, 2010). Par‐4 inhibits the nuclear factor kappa B (NF‐κB) pathway and activates the extrinsic death pathway by enabling Fas cell surface death receptor (Fas) and Fas ligand (FasL) to be trafficked to the plasma membrane. Par‐4 also promotes the downregulation of the anti‐apoptotic gene Bcl‐2 at the transcriptional level (Zhao & Rangnekar, 2008).
The aim of this study was to determine whether the extrinsic apoptosis pathway contributes to salivary gland morphogenesis, especially in the maintenance of the ductal lumen. The strategy for this study was to draw a morphological immunomap for the expression of Par‐4 and its possible associated proteins related to apoptosis [Fas, FasL, pleckstrin homology‐like domain family A member 1 (PHLDA1), caspase 3, survivin, and Bcl‐2], cell proliferation (Ki‐67), and tissue remodelling and maturation [secreted protein acidic and cysteine‐rich (SPARC) and mucin 1 (MUC1)].
Materials and methods
Tissue samples
Salivary gland specimens were dissected from 50 postmortem human foetuses (from natural miscarriages) at 10–25 weeks of gestation (Table 1), obtained from the Medical School of the University of São Paulo with the permission of the Ethical Committee of the Institution. The specimens were collected from the tongue, lips, hard palate, sublingual (the floor of the mouth), parotid, and submandibular glands. Foetal SG fragments were paraffin‐embedded and stained with haematoxylin and eosin to analyse their morphology and determine the developmental stage. The A.C.Camargo Cancer Center Ethics Committee (Protocol number 1578/11F) approved the study.
Table 1.
Description of specimens regarding gestational age and site
| Gestational age (weeks) | Number of samples | Anatomical site |
|---|---|---|
| 10 | 2 | Palate, submandibular |
| 11 | 1 | Tongue |
| 12 | 6 | Tongue, parotid, palate, mandibular, submandibular |
| 13 | 7 | Tongue, lip, palate, parotid |
| 15 | 1 | Palate |
| 16 | 1 | Tongue |
| 18 | 5 | Tongue, lip, palate, submandibular |
| 19 | 5 | Tongue, parotid, mandibular, palate |
| 20 | 8 | Tongue, lip, parotid, floor of the mouth (sublingual) |
| 21 | 11 | Tongue, submandibular, lip, parotid, floor of the mouth (sublingual) |
| 22 | 1 | Tongue |
| 23 | 1 | Tongue |
| 25 | 1 | Tongue |
Immunohistochemistry
Serial sections of 3‐μm of developing SGs were deparaffinised, re‐hydrated, and submitted to antigen retrieval, as described in Table 2. The sections were incubated in 3% aqueous hydrogen peroxide for 15 min to quench endogenous peroxidase activity and then in Protein Block Serum‐Free (Dako, Carpinteria, CA, USA) for 20 min at room temperature to suppress nonspecific binding of subsequent reagents. Thereafter, the serial sections were incubated with primary antibodies for 16 h at 4 °C (Table 2). The antigen‐antibody complexes were visualised using Advance system (Dako) and incubated with 3′3 diaminobenzidine tetrachloride (DAB; Dako) for 5 min. The sections were counterstained with Mayer's haematoxylin, dehydrated, and mounted with a glass coverslip and xylene‐based mountant. Positive controls were used according to the manufacturer's recommendations. Analysis of the results was performed using a conventional optical microscope considering the location of the protein expression (membrane, cytoplasm or nucleus) and the distribution of the staining (diffuse or focal).
Table 2.
Primary serum, clones, source, titer, and antigen retrieval
| Primary serum | Clones | Source | Titer | Antigen retrieval |
|---|---|---|---|---|
| Bcl‐2 | 124 | Dako | 1 : 200 | EDTA/Tris, pH 9.0 |
| Cleaved Caspase‐3 | Polyclonal | Cell Signaling | 1 : 1000 | Citrate buffer, pH 6.0 |
| Survivin | Polyclonal | Neomarkers | 1 : 350 | Citrate buffer, pH 6.0 |
| Par‐4 | A‐10 | Santa Cruz | 1 : 700 | Citrate buffer, pH 6.0 |
| MUC1 | Ma552 | Novocastra | 1 : 500 | Citrate buffer, pH 6.0 |
| PHLDA1 | M‐20 | Santa Cruz | 1 : 100 | Citrate buffer, pH 6.0 |
| SPARC | Polyclonal | Chemicon | 1 : 6000 | Trilogy* |
| Fas | EPR5700 | LSBio | 1 : 700 | Trilogy |
| FasL | Polyclonal | LSBio | 1 : 700 | Trilogy |
| Ki‐67 | 30‐9 | Roche | Ready to use | Automated protocol (Ventana, Roche) |
Fas, Fas cell surface death receptor; FasL, Fas ligand; Par‐4, prostate apoptosis response‐4; PHLDA1, pleckstrin homology‐like domain family A member 1; Bcl‐2, B‐cell CLL/lymphoma 2; MUC1, mucin 1; SPARC, secreted protein acidic and cysteine‐rich.
Trilogy, solution that allows deparaffinisation and antigen retrieval in a one‐step procedure.
Results
The expression of Par‐4 and proteins associated with apoptosis (Fas, FasL, PHLDA1, Bcl‐2, and survivin), cell proliferation (Ki‐67), and tissue remodelling and maturation (MUC1) were detected during the various phases of glandular morphogenesis (initial bud, pseudoglandular, canalicular, and terminal bud). No SPARC or cleaved caspase‐3 expression was detected in the parenchyma of any glandular specimens. The results are described in Table 3 and illustrated in Figs 1, 2, 3, 4.
Table 3.
Expression of Fas, FasL, Par‐4, PHLDA1, caspase‐3, Ki‐67, Bcl‐2, Survivin, MUC1, and SPARC proteins in different stages of human salivary gland morphogenesis
| Pro‐apoptotic | Anti‐apoptotic | Remodelling/maturation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fas | FasL | Par‐4 | PHLDA1 | Caspase‐3 | Ki‐67 | Bcl‐2 | Survivin | MUC1 | SPARC | |
| Initial bud | + | + | ++ | ++ | 0 | + | + | ++ | 0 | 0 |
| Pseudoglandular | 0 | 0 | + | + | 0 | ++ | + | ++ | ++ | 0 |
| Canalicular | 0 | 0 | + | + | 0 | + | 0 | + | ++ | 0 |
| Terminal bud | 0 | 0 | 0 | 0 | 0 | + | 0 | + | ++ | 0 |
(++) Strong expression; (+) weak expression; (0) no expression; Fas, Fas cell surface death receptor; FasL, Fas ligand; Par‐4, prostate apoptosis response‐4; PHLDA1, pleckstrin homology‐like domain family A member 1; Bcl‐2, B‐cell CLL/lymphoma 2; MUC1, mucin 1; SPARC, secreted protein acidic and cysteine‐rich.
Figure 1.
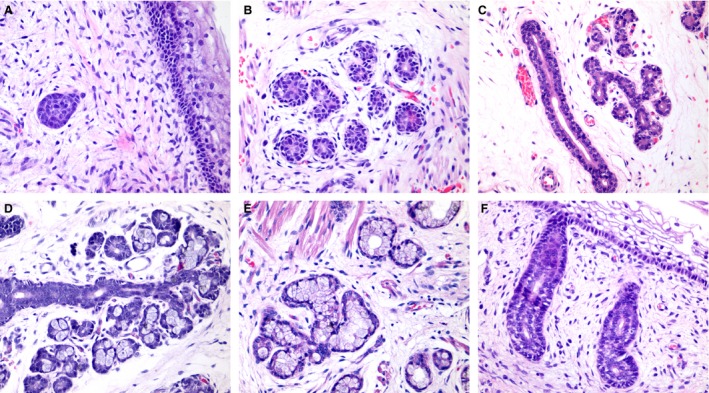
Histological aspects of human salivary glands morphogenesis. (A) Initial bud stage showing a solid group of epithelial cells. (B) Pseudoglandular stage showing the early luminal opening. (C) Canalicular stage showing branched luminal structures. (D) Terminal bud with the presence of early secretory units in branched structures. (E) Well‐differentiated secretory units. (F) Excretory duct connecting to the mucosa. Magnifications A–E = 400 ×.
Figure 2.
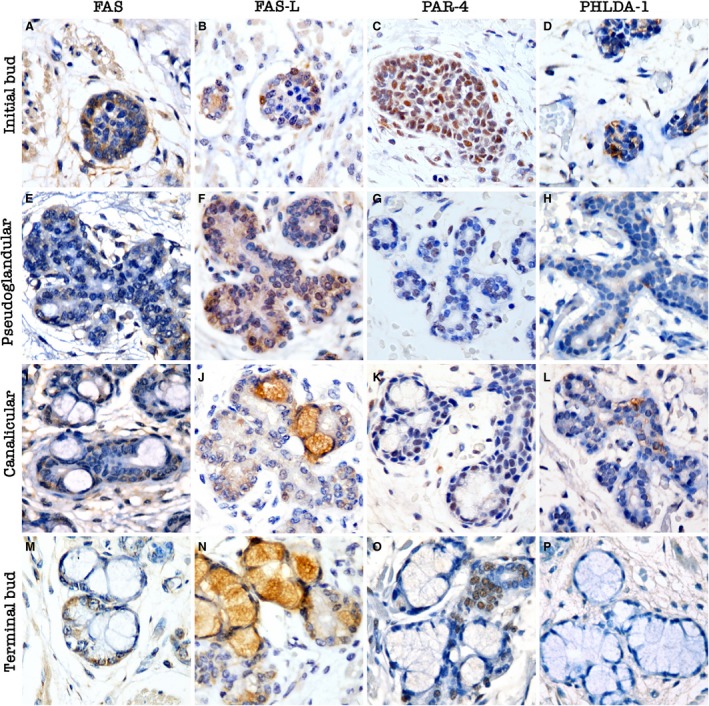
Pro‐apoptotic protein expression during human salivary gland development. Initial bud stage: (A) membrane Fas cell surface death receptor (Fas) expression in solid epithelial buds; (B) membrane Fas ligand (FasL) positivity in rare cells; (C) nuclear and cytoplasmic prostate apoptosis response‐4 (Par‐4) expression; (D) cytoplasmic pleckstrin homology‐like domain family A member 1 (PHLDA1) expression in epithelial buds. Pseudoglandular stage: (E) membrane Fas expression in few cells, especially in branching areas; (F) membrane FasL expression in early luminal structures; (G) nuclear Par‐4 expression, especially in the periphery of the structures; (H) cytoplasmic PHLDA1 expression, especially in branching areas and in early luminal structures. Canalicular stage: (I) membrane Fas expression in few cells of the intercalated ducts; (J) membrane FasL expression in few cells; (K) nuclear Par‐4 expression in branched areas and in intercalated ducts near the primitive pre‐acinar structures; (L) cytoplasmic PHLDA1 in branched areas and luminal structures. Terminal bud stage: (M) membrane Fas expression in a few cells of the intercalated ducts; (N) absence of membrane FasL expression; (O) nuclear Par‐4 expression near pre‐acinar structures in the intercalated ducts and branched areas; (P) absence of PHLDA1 expression. Magnifications A–P = 400 ×.
Figure 3.
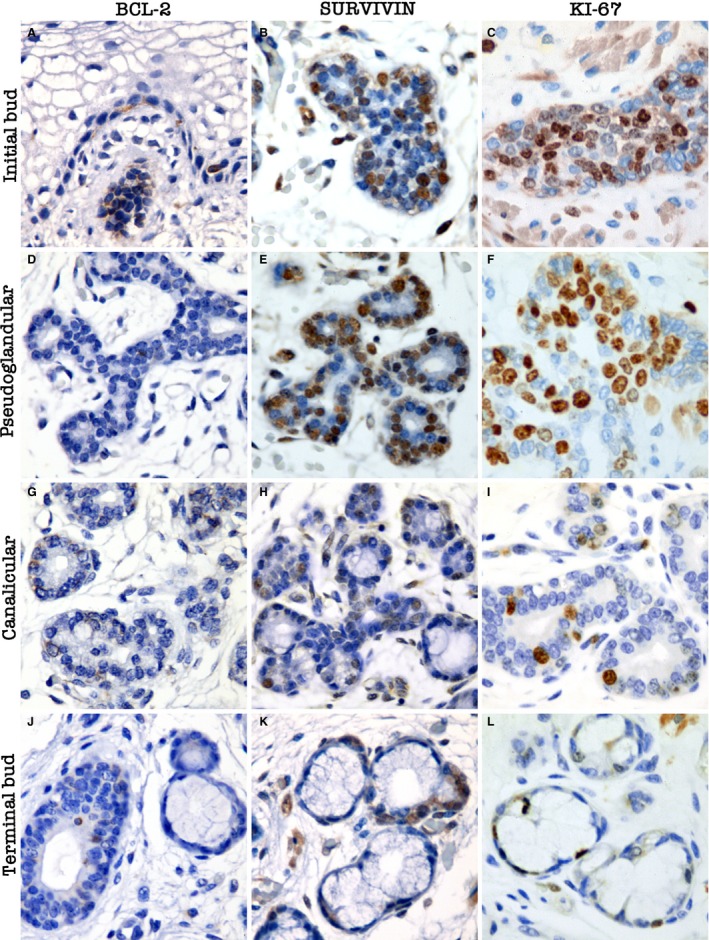
Expression of anti‐apoptotic and proliferation proteins during human salivary gland development. Initial bud stage: (A) cytoplasmic Bcl‐2 expression in the solid bud of epithelial cells and in few cells in the epithelium; (B) nuclear and cytoplasmic survivin expression, especially in the basal portion of the bud; (C) nuclear Ki‐67 expression in the solid bud of epithelial cells and poorly differentiated areas. Pseudoglandular stage: (D) cytoplasmic Bcl‐2 expression in few cells of branched areas; (E) prominent nuclear survivin expression in branching areas and in luminal structures; (F) prominent nuclear Ki‐67 expression, especially in branching areas and luminal structures. Canalicular stage: (G) cytoplasmic B‐cell CLL/lymphoma 2 (Bcl‐2) expression in a few cells, especially in poor differentiated areas and ductal structures; (H) nuclear and cytoplasmic survivin expression in poorly differentiated and branched areas. Reduced survivin expression in pre‐acinar structures: (I) reduced nuclear Ki‐67 expression in branched areas and open luminal spaces. Terminal bud stage: (J) cytoplasmic Bcl‐2 expression in few cells in excretory ducts. Pre‐acinar structures are negative: (K) nuclear and cytoplasmic survivin expression in intercalated ducts. Pre‐acinar structures are negative: (L) nuclear Ki‐67 expression in a few cells in intercalated ducts. Pre‐acinar structures are negative. Magnifications A–L = 400 ×.
Figure 4.
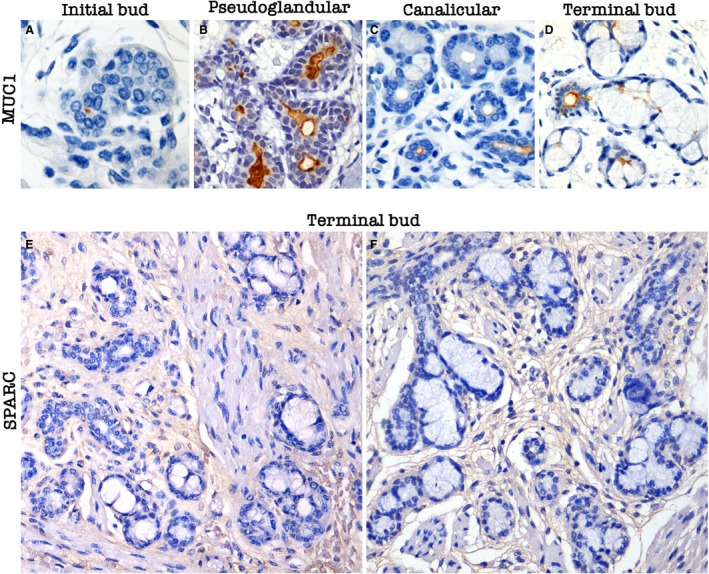
Expression of maturation and tissue remodelling proteins during human salivary gland development. (A) Absence of MUC1 expression in the solid bud of epithelial cells. Note the positivity of the protein in the primitive luminal opening near the button. (B) Prominent MUC1 expression during pseudoglandular stage. (C) MUC1 expression in early luminal regions. (D) MUC1 positivity in the excretory duct near the terminal sac and in the intercalated duct. (E,F) SPARC expression in the stroma during terminal bud stage.
At the early development stages, strong nuclear Par‐4 expression and weak cytoplasmic expression was observed (Fig. 2C). At the branched regions, a nuclear pattern was observed during luminal opening (Fig. 2G,K). More differentiated structures demonstrated nuclear Par‐4 expression in intercalated ducts near the secretory lobules, and cytoplasmic staining was predominant in the excretory ducts (Fig. 2O).
The expression of pro‐apoptotic proteins (Fas, FasL, and PHLDA1) was observed mainly in the early developmental stages. Membrane Fas and FasL were expressed in few cells. The membranous expression of both proteins was observed mainly in the early morphogenesis stages (Fig. 2A,B). In some acinar lobules, FasL staining was observed in the cytoplasm, but was regarded as non‐active protein, as the classical description of active FasL is that in the cell membrane.
Cytoplasmic PHLDA1 expression was found mainly during the early stages of morphogenesis in solid cords of epithelial cells and persisted until luminal opening (Fig. 2D,H).
Cleaved caspase‐3 expression was not detected in the glandular parenchyma. In contrast, the expression of total caspase‐3 was observed in the early stages during human SG development. Caspase‐7 expression was observed, with a cytoplasmic and nuclear pattern, during early development and in more differentiated stages.
The expression of anti‐apoptotic proteins (Bcl‐2 and survivin) was observed mainly during the early developmental stages of morphogenesis. Bcl‐2 positivity was observed in a cytoplasmic pattern in few cells (Fig. 3A). More differentiated stages presented a focal expression pattern (Fig. 3D,G,J). Survivin expression was observed in all stages of morphogenesis (Fig. 3B,E,H,K). Cytoplasmic staining was observed in the early stages of development, whereas nuclear expression was observed at the later stages.
Nuclear Ki‐67 expression was observed in all stages of differentiation. Solid epithelial buds exhibited strong proliferative activity (Fig. 3C). Predominant Ki‐67‐positive cells were observed in the branched ducts with early luminal opening (Fig. 3F).
MUC1, which is associated with SG maturation, luminal opening, and secretion from SGs, was expressed in a cytoplasmic pattern, mainly at luminal structures. SPARC expression was observed in the stroma surrounding ducts and the secretory units of the SGs in mature developmental stages (terminal bud; Fig. 4).
Discussion
In the present study, the expression of proteins related to apoptosis, cell proliferation, and tissue remodelling and maturation was evaluated in human developing salivary gland specimens.
The pro‐apoptotic proteins Par‐4, Fas, and FasL were observed mainly during the initial stages of human SG development, whereas little Bcl‐2 expression was detected. Par‐4 expression could facilitate Fas/FasL trafficking to the membrane and interrupt anti‐apoptotic Bcl‐2 protein activity (Zhao & Rangnekar, 2008). Teshima et al. (2016b) reported an absence of Bcl‐2 expression during human SG development. Carev et al. (2006) demonstrated Bcl‐2 expression during the early differentiation of tubular nephrons, which contributed to the selective survival of some tubules and gave rise to adult structures.
The PHLDA1 protein has been associated with the cellular death mechanism (Park et al. 1996; Neef et al. 2002), leading to increase levels of caspase‐9 and cleaved poly(ADP‐ribose) polymerase (PARP), allowing the activation of the intrinsic apoptotic pathway (Neef et al. 2002). Our results demonstrated PHLDA1 expression in the early developmental stages of the SGs.
Caspases are fundamental apoptotic regulators that are essential for embryonic development (Boatright & Salvesen, 2003). Our study demonstrated the absence of cleaved caspase‐3 expression in glandular structures during development. In contrast, during mouse SG development, Teshima et al. (2016a) identified apoptotic activity of cleaved caspase‐3 in the early morphogenesis stages, mainly during the formation of the luminal duct cavity. Nedvetsky et al. (2014) inhibited apoptosis by culturing E13.5 submandibular glands (SMGs) with a pan‐caspase inhibitor and demonstrated lumen formation. Furthermore, Bax null SMGs displayed a normal tubular ductal system, suggesting that apoptosis did not mediate lumen formation in SMGs in vivo.
Teshima et al. (2016b) also analysed cleaved caspase‐3 in human SGs and did not observe protein expression in any stages of morphogenesis. Therefore, cleaved caspase‐3 expression might not be a sensitive marker to identify apoptosis activity in human SGs. To confirm these results, we evaluated the expression of total caspase‐3, which was observed in the early stages during human SG development, thus confirming that the protein is present during glandular morphogenesis but is not activated. By contrast, caspase‐7 expression was observed, with a cytoplasmic and nuclear pattern, during early development and in more differentiated stages. Caspase‐7 is also an effector caspase, and its expression could compensate for the absence of cleaved caspase‐3. Using in vivo models, Lakhani et al. (2006) demonstrated that the concomitant deletion of caspase‐3 and caspase‐7 results in impaired morphogenesis, whereas caspase‐7 expression could overcome the absence of caspase‐3. It is important to note that studies using animal models results are restricted to the submandibular salivary gland, whereas in our study we used tissues from both major and minor salivary glands.
The balance between cellular proliferation and apoptosis is essential to maintain homeostasis during organogenesis (Melnick et al. 2001; Lubarsky & Krasnow, 2003; Carev et al. 2006). Survivin is a member of the inhibitor of apoptosis protein (IAP) family which is responsible for regulating cell division and suppressing apoptosis (Johnson & Howerth, 2004). Jaskoll et al. (2001) reported that in mouse embryonic submandibular gland development, survivin translocation into the nucleus is necessary for the induction of cell cycle entry and inhibition of caspase 3‐mediated apoptosis. Our results demonstrated nuclear and cytoplasmic survivin expression, mainly during early human SG morphogenesis. Survivin expression was reduced in more developed structures, suggesting that survivin might allow the survival of epithelial cells and the maintenance of the branched ductal system. Similar results were also observed during human SG development (Teshima et al. 2016b), and in mouse SG and kidney development (Jaskoll et al. 2001; Carev et al. 2006).
Ki‐67 was detected in all stages of SG morphogenesis, mainly at the pseudoglandular stage. The balance between differentiation, maturation, proliferation, and apoptosis is necessary for salivary gland development, and disruption of this compensatory pattern can trigger several disorders in the organ, as suggested in a study regarding kidney morphogenesis (Carev et al. 2006).
Mucins are salivary glycoproteins that are important for the maturation, protection, and homeostatic balance of the oral cavity (Teshima et al. 2011). In addition, MUC1 might be associated with apoptosis regulation because it contributes to cellular death evasion in tumour cells, avoiding the intrinsic apoptotic pathway activation (Nath & Mukherjee, 2014). MUC1 is typically found in the apical portion or luminal cells of glands (Mahomed, 2011; Nath & Mukherjee, 2014). In the present study, MUC1 expression was observed at the beginning of the pseudoglandular stage, mainly in the luminal region. Teshima et al. (2011) also observed MUC1 expression at pseudoglandular stage in early SG morphogenesis.
SPARC is a multifunctional secreted glycoprotein expressed during mammalian development; however, its expression declines in most organs after maturation (Arnold & Brekken, 2009; Morris & Kyriakides, 2014). This stromal protein is important for remodelling of the extracellular matrix and stimulates proliferation, migration, and cellular differentiation (Arnold & Brekken, 2009). In the present study, SPARC expression was observed in the stroma surrounding the ductal and secretory portions. During lung development, in which branching morphogenesis is similar to that in SGs, the absence of SPARC expression results in impaired morphology (Strandjord et al. 1995).
Apoptotic stimuli during human SG morphogenesis seem to be present mainly at the early stages of development. With the results of previous studies, our results showed that the extrinsic mechanism of apoptosis might promote luminisation at the early stages of salivary gland development. Throughout morphogenesis, the distribution of pro‐apoptotic proteins and proteins associated with cell proliferation increase varied. Cell proliferation appears to be predominant at the pseudoglandular stage, whereas expression of pro‐apoptotic proteins was more prominent at the initial bud stage (FAS and PAR4) and the pseudoglandular stage (FasL). Survivin has a dual‐role, controlling both apoptosis and cell proliferation, and was observed during all developmental stages, as was Ki‐67.
Until now, the expression of Par‐4, PHLDA1, SPARC, and Ki‐67 had not been evaluated in salivary gland development; therefore, the present study provided new insights into the mechanism involved in salivary branching morphogenesis. However, other mechanisms, such as cell polarisation, are probably involved in the maintenance of luminal ductal opening and maintenance, in addition to apoptosis.
Understanding the mechanisms underlying salivary gland morphogenesis is important to comprehend tumorigenesis because similarities between the two processes have been suggested (Ma et al. 2010). Molecular and genetic screening of signalling pathways targeting both mechanisms might contribute to the development of novel strategies to identify, prognose, and treat cancer.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
A.N.M.G. performed the research, analysed the data, and wrote the paper; M.A.N. designed the study, analysed the data, and revised the paper; S.V.L. designed the research study, analysed the data, and wrote the paper; C.M.C.C. designed the research study, analysed the data, and wrote the paper.
Acknowledgements
The authors were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; scholarship to ANMG), and by grants 11/02051‐6 and 12/09759‐7 from the São Paulo Research Foundation (FAPESP).
References
- Andrew DJ, Ewald AJ (2010) Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev Biol 341, 34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SA, Brekken RA (2009) SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal 3, 255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15, 725–731. [DOI] [PubMed] [Google Scholar]
- Carev D, Krnić D, Saraga M, et al. (2006) Role of mitotic, pro‐apoptotic and anti‐apoptotic factors in human kidney development. Pediatr Nephrol 21, 627–636. [DOI] [PubMed] [Google Scholar]
- Cutler LS (1990) The role of extracellular matrix in the morphogenesis and differentiation of salivary glands. Adv Dent Res 4, 27–33. [DOI] [PubMed] [Google Scholar]
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35, 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flusberg DA, Sorger PK (2015) Surviving apoptosis: life‐death signaling in single cells. Trends Cell Biol 25, 446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami A, Ranganathan P, Rangnekar VM (2006) The phosphoinositide 3‐kinase/Akt1/Par‐4 axis: a cancer‐selective therapeutic target. Cancer Res 66, 2889–2892. [DOI] [PubMed] [Google Scholar]
- Harunaga J, Hsu JC, Yamada KM (2011) Dynamics of salivary gland morphogenesis. J Dent Res 90, 1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskoll T, Chen H, Min Zhou Y, et al. (2001) Developmental expression of survivin during embryonic submandibular salivary gland development. BMC Dev Biol 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ME, Howerth EW (2004) Survivin: a bifunctional inhibitor of apoptosis protein. Vet Pathol 41, 599–607. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, et al. (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311, 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA (2003) Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang P, Wang F, et al. (2010) The relationship between early embryo development and tumourigenesis. J Cell Mol Med 14, 2697–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahomed F (2011) Recent advances in mucin immunohistochemistry in salivary gland tumors and head and neck squamous cell carcinoma. Oral Oncol 47, 797–803. [DOI] [PubMed] [Google Scholar]
- Melnick M, Jaskoll T (2000) Mouse submandibular gland morphogenesis: a paradigm for embryonic signal processing. Crit Rev Oral Biol Med 11, 199–215. [DOI] [PubMed] [Google Scholar]
- Melnick M, Chen H, Zhou Y, et al. (2001) Embryonic mouse submandibular salivary gland morphogenesis and the TNF/TNF‐R1 signal transduction pathway. Anat Rec 262, 318–330. [DOI] [PubMed] [Google Scholar]
- Morris AH, Kyriakides TR (2014) Matricellular proteins and biomaterials. Matrix Biol 37, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S, Mukherjee P (2014) MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med 20, 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvetsky PI, Emmerson E, Finley JK, et al. (2014) Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev Cell 30, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef R, Kuske MA, Pröls E, et al. (2002) Identification of the human PHLDA1/TDAG51 gene: down‐regulation in metastatic melanoma contributes to apoptosis resistance and growth deregulation. Cancer Res 62, 5920–5929. [PubMed] [Google Scholar]
- Park CG, Lee SY, Kandala G, et al. (1996) A novel gene product that couples TCR signaling to Fas (CD95) expression in activation‐induced cell death. Immunity 4, 583–591. [DOI] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP (2006) Salivary gland branching morphogenesis. Differentiation 74, 349–364. [DOI] [PubMed] [Google Scholar]
- Patel N, Sharpe PT, Miletich I (2011) Coordination of epithelial branching and salivary gland lumen formation by Wnt and FGF signals. Dev Biol 358, 156–167. [DOI] [PubMed] [Google Scholar]
- de Paula F, Teshima TH, Hsieh R, et al. (2017) Overview of human salivary glands: highlights of morphology and developing processes. Anat Rec (Hoboken) 300, 1180–1188. [DOI] [PubMed] [Google Scholar]
- Pereira WO, Amarante‐Mendes GP (2011) Apoptosis: a programme of cell death or cell disposal? Scand J Immunol 73, 401–407. [DOI] [PubMed] [Google Scholar]
- Shrestha‐Bhattarai T, Rangnekar VM (2010) Cancer‐selective apoptotic effects of extracellular and intracellular Par‐4. Oncogene 29, 3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandjord TP, Sage EH, Clark JG (1995) SPARC participates in the branching morphogenesis of developing fetal rat lung. Am J Respir Cell Mol Biol 13, 279–287. [DOI] [PubMed] [Google Scholar]
- Suzanne M, Steller H (2013) Shaping organisms with apoptosis. Cell Death Differ 20, 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9, 231–241. [DOI] [PubMed] [Google Scholar]
- Teshima THN, Ianez RF, Coutinho‐Camillo CM, et al. (2011) Development of human minor salivary glands: expression of mucins according to stage of morphogenesis. J Anat 219, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima TH, Wells KL, Lourenço SV, et al. (2016a) Apoptosis in early salivary gland duct morphogenesis and lumen formation. J Dent Res 95, 277–283. [DOI] [PubMed] [Google Scholar]
- Teshima TH, Ianez RC, Coutinho‐Camillo CM, et al. (2016b) Apoptosis‐associated protein expression in human salivary gland morphogenesis. Arch Oral Biol 69, 71–81. [DOI] [PubMed] [Google Scholar]
- Tucker AS (2007) Salivary gland development. Semin Cell Dev Biol 18, 237–244. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Rangnekar VM (2008) Apoptosis and tumor resistance conferred by PAR4. Cancer Biol Ther 7, 1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]


