Abstract
The presence of psychosis is associated with a more rapid decline in Alzheimer's disease (AD), but the impact of paranoid (persecutory delusions) and misidentification (misperceptions and/or hallucinations) subtypes of psychosis on the speed of decline in AD is still unclear. We analyzed data on Alzheimer's Disease Neuroimaging Initiative 2 participants with late mild cognitive impairment or AD, and we described individual trajectories of Alzheimer's Disease Assessment Scale–Cognitive Subscale scores using a semimechanistic logistic model with a mixed effects–based approach, which accounted for dropout and adjusted for baseline Mini Mental State Examination scores. The covariate model included psychosis subtypes, age, gender, education, medications, and Apolipoprotein E epsilon 4 (Apo‐e ε4 genotype). We found that the Alzheimer's Disease Assessment Scale–Cognitive Subscale rate of increase was doubled in misidentification (β r,misid_subtype = 0.63, P = 0.031) and mixed (both subtypes; β r,mixed_subtype = 0.70, P = 0.003) when compared with nonpsychotic (or paranoid) patients, suggesting that the misidentification subtype may represent a distinct AD sub‐phenotype associated with an accelerated pathological process.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The psychosis phenotype in Alzheimer's disease (AD) predicts an accelerated speed of cognitive and functional declines, but it is unclear if phenomenological differences in psychosis subtypes have distinct disease trajectories.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Does the presence of paranoid (persecutory delusions) or misidentification (misperceptions and/or hallucinations) subtypes of psychosis have an impact on the speed of cognitive decline in early AD?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The misidentification subtype (alone or in conjunction with paranoid delusions) is associated with a faster speed of AD progression. These findings may reflect additional AD (or other) pathology in functional networks that are involved in the perception and contextual association of visual stimuli.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
☑ These findings suggest that treatment approaches addressed to AD patients with misidentification psychotic subtype should aim to target functional networks involved in the processing of sensory stimuli to improve psychotic symptoms and to slow down the rate of disease progression.
Psychosis symptoms (delusions, hallucinations) are common in Alzheimer's disease (AD)1 and manifest early in the illness course. They are associated with an accelerated speed of cognitive and functional declines and precipitate earlier institutionalization.2 Research that elucidates the pathophysiology of AD psychosis and its relationship with disease progression could help to direct future treatment strategies. Factor analysis of AD psychosis symptoms has identified the following two broad categories: a “paranoid” subtype, characterized by delusions of theft, harm, and abandonment, and a “misidentification” subtype, composed of misperceptions, misidentification delusions, and visual or auditory hallucinations.3, 4 Studies that have investigated the phenotypic aspects of AD psychosis subtypes have reported greater performance deficits on tests of visual sustained attention and visuoperceptual function,5 reduced volume in functional networks involved in perception and context‐based recognition of visual stimuli,6, 7 and greater hippocampal/limbic pathology at postmortem,8, 9, 10 but only in association with the misidentification subtype. It is unclear whether AD psychosis subtypes are part of the same biological continuum,3, 4 with misperceptions emerging later in the AD process,4, 11 or whether the two subtypes have distinct disease course trajectories.
This study aimed to investigate longitudinal data from the Alzheimer's Disease Neuroimaging Initiative (ADNI) with the following objectives:
To describe the trajectory of cognitive decline, indexed by the rate of increase in Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog)12 scores in early AD using a mixed effects–based approach.13
To test the hypothesis that the misidentification subtype would be associated with a faster rate of cognitive decline.
Methods
Participants
Data were obtained from ADNI, a multicenter longitudinal study that collected clinical, neuroimaging, neuropsychological, and blood/cerebrospinal data from healthy controls and those with both mild cognitive impairment (MCI) and early AD with the aim of identifying markers of AD progression (http://adni.loni.usc.edu). MCI was classified as early or late MCI (LMCI) based on a cutoff for objective memory impairment determined using the Wechsler Memory Scale Logical Memory II (http://adni.loni.usc.edu). The data of interest (downloaded January 18, 2016) included all participants diagnosed with AD and LMCI at baseline and those who developed MCI and AD during the observation period, but excluded those who subsequently reverted to healthy controls or early MCI as they did not show a cognitive decline during the time accordingly to AD National Institute on Aging and Alzheimer’s Association (NIA‐AA) criteria.14 The analysis was restricted to ADNI 2 (ADNI2) participants, as full Neuropsychiatric Inventory15 data (which were not included in earlier phases of the ADNI) were required to assign subtypes.3, 4, 5 Participants were coded as psychotic if symptoms were coded as present (scores of 1 or more on delusions or hallucinations domains) either at baseline or any follow‐up visit. The paranoid subtype included items 1, 2, 3, 7 from the delusions domain (“In danger/others are planning to hurt him or her,” “Others are stealing from him or her,” “Spouse is having an affair,” “Family members plan to abandon him or her”); the misidentification subtype included items 4, 5, 6, 8 from the delusions domain (“Unwelcome guests are staying in his or her house,” “His or her spouse or others are not who they claim to be,” “His or her house is not his or her own,” “Television/magazine figures are present in his or her home”); and items 1, 2, 3 were included from the hallucinations domain (“He or she can hear voices,” “Talks to people who are not there,” “Seeing things not seen by others”). Those who were coded positive on items from both the paranoid and misidentification symptoms were described as “mixed,” and a nonpsychotic phenotype was assigned if no items were coded positive at any of the visits. Cognitive and functional statuses were measured using the ADAS‐cog, Mini Mental State Examination (MMSE),16 Clinical Dementia Rating Scale,17 and Functional Activities Questionnaire18 scores. Assessments were carried out at baseline, 6 months, 12 months, and annually thereafter. The longest observation period was 4 years, corresponding to a baseline and four or five follow‐up visits. Between‐AD and psychosis subtypes differences for age, gender, education, Apolipoprotein E epsilon 4 (Apo‐e ε4) genotype, MMSE, Clinical Dementia Rating Scale, Functional Activities Questionnaire, ADAS‐cog, and Neuropsychiatric Inventory total scores at baseline were analyzed using chi‐squared tests and analyses of variance. Data collection and sharing in ADNI were approved by the institutional review boards of each participating institution, and written informed consent was obtained from all participants.
ADAS‐cog score trajectory model
The nonlinear trajectory of cognitive decline was described using the general logistic function model (an equation based on Richard's function)19, 20, 21 below:
The model comprised the following three parameters: baseline ADAS‐cog scores (ADAS‐cog0), rate (slope) of increase in ADAS‐cog scores (r), and a shape parameter (α), which controls an inflection point in the trajectory beyond which the rate of decline slows. Higher values of r and α indicate a faster cognitive decline and steeper trajectory, respectively.
Nonlinear mixed effects modeling was used to explore sources of variability in the trajectory of decline separating interindividual variability and residual unexplained variability.13 Interindividual random effects and residual unexplained errors were assumed to be independent. A log transformation of the observations ADAS‐cogij of subject i at time t ij and model predictions was used to ensure positivity, and we used an additive error model as follows:
This allows the standard deviation of the residual errors , which follow a Gaussian, to be expressed as a coefficient of variation on predicted ADAS‐cog scores.
Interindividual random effects were estimated on ADAS‐cog0, r, and α. A probit‐normal transformation was used for ADAS‐cog0 to ensure that predicted individual values were between 0 and 70, as follows:
with the population value for ADAS‐cog0, the inverse cumulative distribution function (quantile function) associated with the standard normal distribution N(0,1), and following a normal distribution of mean 0 and standard deviation
For r and α, a log‐normal transformation was used to ensure positivity, as follows:
with and the population value for r and , respectively, and and following normal distributions of mean 0 and standard deviations and , respectively. All covariate–parameter associations were modeled using a linear regression on the random effect scale, e.g.:
with covi the value for the covariate of interest for subject i and the effect coefficient of cov on parameter r. Paranoid, misidentification, and mixed subtypes were each compared with the nonpsychotic phenotype, and other covariates previously shown to have an effect on disease progression parameters (baseline age, baseline MMSE score, gender, education, presence of Apo‐e ε4 alleles, age, baseline use of cognitive enhancers)20, 21 were tested on parameters ADAS‐cog0 and r with a Wald test at level 0.05. Continuous covariates were centered on the mean and gender, Apo‐e ε4 genotype, and use of cognitive enhancers were encoded as categorical covariates, with male gender, not being a carrier of Apo‐e ε4, and no medication used as reference values.
Dropout model
A survival (time‐to‐event) model was used to fit attrition rate using a Weibull baseline hazard function22 as risk was expected to change over time. This model includes a shape parameter, k, which when greater than 1 indicates an increase in attrition rate over time, when equal to 1 indicates that attrition is constant over time (such as in the exponential model), and when < 1 indicates that the attrition rate decreases with time, and a scale factor λ, with 1/λ corresponding to the mean time before dropout if k is equal to 1:
An effect of the current predicted ADAS‐cog score was tested on the hazard function of dropout, h(t) as follows:
with the effect coefficient of the unobserved ADAS‐cog score value on the risk of dropout, corresponding to a missing not‐at‐random mechanism.
Dropout and ADAS‐cog models were jointly estimated.
Model evaluation and predictions
Model parameters were estimated using the stochastic approximation to the expectation maximization algorithm.23 The appropriateness of base and covariate models were evaluated using goodness‐of‐fit plots (e.g., visual predictive check) and metrics (standard errors and Bayesian information criteria).
Parameter fixed effect estimates were used to plot typical ADAS‐cog trajectories for each subtype, accounting for other significant covariate effects.
Software
Demographic and clinical data were analyzed using SPSS 23 ( IBM Corporation, Armonk, NY, www.spss.com). The data set was prepared using R (version 3.2.1), and the Monolix software was used for model fit and evaluation (version 2016 R1; www.lixoft.eu).
Results
Demographic and clinical characteristics
The ADAS‐cog trajectories from 528 participants are shown in spaghetti plots in Figure 1 a,b on the natural and on the log scales. In median, the patients attended three visits ranging from one to six visits over 4 years (1,783 total observations). Demographic information about the patients is summarized in Table 1 . The sample consisted of 212 AD, 239 LMCI, and 29 early MCI patients at baseline. A total of 48 healthy controls converted to LMCI or AD during the observation period. Attrition rate was high (92%), with only 42 participants remaining at the final follow‐up visit (year 4). Possible explanations for the high attrition are (i) worsening of cognitive and functional impairments of the patients (tested via the effect of predicted current ADAS‐cog scores on the hazard of dropout), (ii) the occurrence of an age‐related disease, and (iii) death. Of note, vital status was not informed in the ADNI2 data set. There were 96 patients with psychosis symptoms (38 paranoid, 29 misidentification, and 29 mixed) who did not differ significantly from nonpsychotic patients in terms of age and education (Table 1 ). At baseline there were 38 patients with psychosis, and 33 patients developed psychosis at the first follow‐up visit, 17 patients at the second follow‐up visit, 7 at the third follow‐up visit, and 1 at the fourth follow‐up visit. A gender difference was found across psychotic status (62% male nonpsychotic vs. 61% paranoid vs. 38% misid. vs. 48% mixed). Participants with psychosis symptoms were more cognitively and functionally impaired than the nonpsychotic patients at baseline (see cognitive and functional scores mean (± SD) in Table 1 and Figure 2 ). In post hoc analyses on the basis of subtype, only the mixed subtype had significantly higher baseline ADAS‐cog scores (P = 0.001) and lower baseline MMSE scores (P = 0.001) when compared with the nonpsychotic group, whereas the mixed and misidentification subtypes had significantly higher baseline Clinical Dementia Rating Scale (P = 0.042 and P < 0.001, respectively) when compared with the nonpsychotic group (Figure 2 ).
Figure 1.

Individual Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog) score trajectories over time of patients included in the Alzheimer's Disease Neuroimaging Initiative 2 data set on the (a) natural and (b) log scales and (c) visual predictive check of the final covariate model of ADAS‐cog score trajectories over time on the log scale. The shaded areas correspond to the 95% confidence intervals around the 5th, 50th, and 95th model predicted percentiles, and the solid lines correspond to 5th, 50th, and 95th percentiles of the observed data.
Table 1.
Demographic and clinical characteristics at baseline (n = 528)
| Nonpsychotic, n = 432 | Paranoid, n = 39 | Misid., n = 29 | Mixed, n = 29 | Test | df | P value | |
|---|---|---|---|---|---|---|---|
| Mean (± SD) | |||||||
| Age, years | 75.9 (± 7.9) | 76 (± 7.2) | 74.3 (± 8.3) | 74.3 (± 8.3) | F = 0.4 | 3 | 0.734 |
| Education, years | 16 (± 2.7) | 15.9 (± 2.8) | 15.1 (± 2.4) | 16.1 (± 2.9) | F = 0.9 | 3 | 0.421 |
| ADAS‐cog | 14.4 (± 8.7) | 16.9 (± 7.8) | 17.6 (± 9.9) | 20.8 (± 9.9) | F = 6.2 | 3 | < 0.001 |
| MMSE | 25.7 (± 3.9) | 24.6 (± 3.2) | 24.1 (± 5.6) | 22.7 (± 4.3) | F = 6.5 | 3 | < 0.001 |
| NPI | 5.4 (± 7.1) | 12 (± 10.8) | 10.1 (± 9.6) | 14.6 (± 12.6) | F = 21 | 3 | < 0.001 |
| CDR | 0.6 (± 0.3) | 0.7 (± 0.3) | 0.8 (± 0.5) | 1 (± 0.5) | F = 11.8 | 3 | < 0.001 |
| FAQ | 7.1 (± 7.7) | 11.4 (± 7.6) | 13.2 (± 8.6) | 17.1 (± 8.2) | F = 21.2 | 3 | < 0.001 |
| Number (%) | |||||||
| Gender, male | 267 (62) | 24 (61) | 11 (38) | 14 (48) | χ 2 = 8.3 | 3 | 0.04 |
| Apo‐e ε4 noncarrier | 208 (48) | 10 (25) | 11 (38) | 8 (27) | χ 2 = 11.7 | 3 | < 0.001 |
| Cognitive enhancer not prescribed | 379 (88) | 32 (82) | 21 (72) | 24 (83) | χ 2 = 6.4 | 3 | 0.092 |
ADAS‐cog, Alzheimer's Disease Assessment Scale‐Cognitive Subscale; CDR, Clinical Dementia Rating Scale; df, degrees of freedom; FAQ, Functional Activities Questionnaire; Misid., misidentification; MMSE, Mini Mental State Examination; NPI, Neuropsychiatric Inventory.
Figure 2.
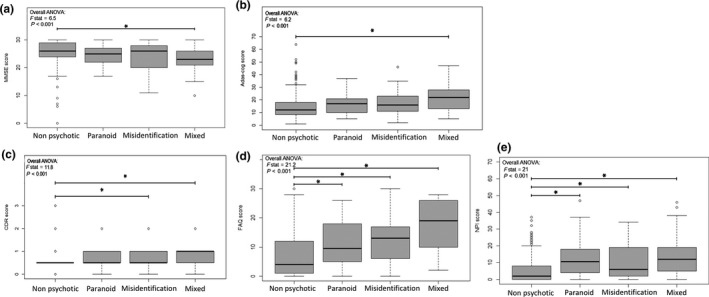
Box plots of (a) Mini Mental State Examination (MMSE) , (b) Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog), (c) Clinical Dementia Rating Scale, (CDR) (d) Functional Activities Questionnaire, (FAQ) and (e) Neuropsychiatric Inventory (NPI) scores at baseline in nonpsychotic Alzheimer’s disease (AD) and in each psychotic AD subtype (paranoid, misidentification, and mixed). An overall comparison was performed using analysis of variance (ANOVA). Black lines indicate post hoc pairwise comparisons of interest. *Significant difference, P < 0.05.
ADAS‐cog trajectory model
Baseline model
Parameter estimates for the base model (without covariates) and for the final model (including covariates) are shown in Table 2 . The final model estimated an inflection point when ADAS‐cog scores reached 38.2 with 95% confidence interval = 32.9–41.8, beyond which the rate of decline slows.
Table 2.
Parameter estimates, relative standard errors (RSE in %) and 95% confidence intervals (95% CI) for models of cognitive decline trajectory in Alzheimer's Disease Neuroimaging Initiative 2 participants without and with covariates
| Without covariate | With covariate | P value | |||||
|---|---|---|---|---|---|---|---|
| Estimate | RSE | 95% CI | Estimate | RSE | 95% CI | ||
| ADAS‐cog trajectory model | |||||||
| Typical value (fixed effects) | |||||||
| ADAS‐cog0 | 13.60 | 3 | 12.80/14.40 | 13.80 | 2 | 13.26/14.34 | — |
| β ADAS‐cog0,MMSE | ne | ne | ne | −0.09 | 4 | −0.10/−0.08 | < 0.001 |
| r | 0.14 | 19 | 0.09/0.19 | 0.07 | 15 | 0.05/0.09 | — |
| β r,misid subtype | ne | ne | ne | 0.63 | 46 | 0.06/1.19 | < 0.031 |
| β r,mixed subtype | ne | ne | ne | 0.70 | 34 | 0.23/1.16 | < 0.003 |
| β r,1 Allele Apo‐e ε4 | ne | ne | ne | 0.76 | 21 | 0.45/1.07 | < 0.001 |
| α | 1.09 | 46 | 0.11/2.07 | 1.54 | 27 | 0.73/2.35 | — |
| IIV | |||||||
| ω ADAS‐cog0 | 0.42 | 4 | 0.39/0.45 | 0.25 | 4 | 0.23/0.27 | — |
| ω r (%) | 67 | 15 | 47/87 | 0.66 | 12 | 0.50/0.82 | — |
| ω α (%) | 176 | 19 | 110/242 | 145 | 14 | 105/185 | — |
| RUV | |||||||
| σ (%) | 26.1 | 2 | 25.08/27.12 | 26 | 2 | 25/27 | — |
| Dropout model | |||||||
| Typical values (fixed effects) | |||||||
| λ | 4.14 | 7 | 3.58/4.70 | 3.99 | 5 | 3.60/4.38 | — |
| k | 2.02 | 6 | 1.79/2.25 | 2.03 | 5 | 1.83/2.23 | — |
| β h0,ADAS‐cog | 0.05 | 14 | 0.04/0.06 | 0.04 | 10 | 0.03/0.05 | < 0.001 |
| Bayesian information criterion | 3,562 | — | — | 3,066 | — | — | — |
ADAS‐cog0, Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog) score at baseline; IIV, interindividual variability expressed in standard deviation or coefficient of variation (%); k, shape parameter in the Weibull hazard model; ne, not estimated; r, rate of decline/disease progression; RUV, residual unexplained variability expressed in coefficient of variation (%); α, shape parameter controlling the inflection point of the decline slope; β ADAS‐cog0, MMSE, effect size of baseline Mini Mental State Examination score on ADAS‐cog (Mini Mental State Examination score at baseline was centered around the mean); β h0,ADAS‐cog, effect of current predicted ADAS‐cog score on the baseline hazard; β r,Allele Apo‐e ε4, effect size of Apo‐e ε4 allele carrier on r (Apo‐e ε 4 allele carrier status was compared with the reference “not carrier”); β r,misid subtype, effect size of misidentification subtype on r; β r,mixed subtype, effect size of mixed subtype on r (misidentification and mixed subtypes were compared with the reference “nonpsychotic (and paranoid)”); λ, scale parameter in the Weibull hazard model.
Covariate model
The paranoid subtype was found to have an estimate of the rate of progression not significantly different from the nonpsychotic subtype; therefore, paranoid and nonpsychotic subtypes were combined in a reference subtype in the following analyses. When compared with this reference subtype, the misidentification and mixed subtypes had significantly higher estimates of the rate of progression (multiplied by factors 1.87 and 2 with P < 0.031 and < 0.003, respectively).
The presence of at least one allele of the Apo‐e ε4 gene was found to double the rate of progression (P < 0.001), leading to a rate of progression fourfold higher in patients having at least one Apo‐e ε4 allele and being categorized into the misidentification or mixed subtypes. A significant effect of baseline MMSE score was found on the parameter ADAS‐cog0 (P < 0.001), with an ADAS‐cog0 of 7.02 associated with an MMSE score of 30 and an ADAS‐cog0 of 57.50 associated with an MMSE score of 6, accounting for the association between ADAS‐cog0 and MMSE score at baseline lowering the interindividual variability estimates on ADAS‐cog0 by 40%.
Dropout model
The k parameter was estimated > 1 (2.03), confirming that dropout hazard rate increases with time. The current predicted ADAS‐cog score was found to significantly increase the dropout hazard, with a dropout hazard rate at 12 months five times higher for those with an ADAS‐cog score of 50 when compared with those with a score of 13 (β h0,ADAS‐cog = 0.04, P < 0.001).
Model evaluation
Visual predictive checks on log‐transformed ADAS‐cog scores are shown in Figure 1 c. The observed percentiles are adequately included in the predicted bands from model simulations, but on one occasion for the median and upper band and on two occasions for the lower band. Further goodness‐of‐fit plots are provided in the Supplementary Material along with the mlxtran code and the data set.
Model predictions
Model fixed effect estimates were used to plot the ADAS‐cog trajectory for a typical patient from each of the following categories: baseline MMSE scores of 10, 25, or 29; nonpsychotic (or paranoid), misidentification, and mixed subtypes; noncarrier (Figure 3 a) or carrier (Figure 3 b) of at least one Apo‐e ε4 allele.
Figure 3.

Typical Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog) trajectories over 20 years (using model parameter fixed effect estimates) for the nonpsychotic or paranoid (left), misidentification (center), and mixed (right) subtypes for (a) a noncarrier and (b) a carrier of at least one Apo‐e ε 4 allele. The black, green, and red lines correspond to Mini Mental State Examination scores at baselines of 25, 29, and 10, respectively. Vertical lines represent the end of the follow‐up in the current study, i.e., 4 years after inclusion.
Discussion
This study investigated the impact of psychosis subtype on the rate of disease progression/cognitive decline in AD using a semimechanistic model to describe the trajectory of change in ADAS‐cog scores. Consistent with our hypothesis, the misidentification subtype (alone or as part of a mixed subtype) was associated with an increased rate of decline when compared with the nonpsychotic or paranoid subtypes. These findings were not explained by differences in baseline cognitive status in those who presented solely with misidentifications, as ADAS‐cog (and MMSE) were similar across the paranoid, misidentification, and nonpsychotic groups, with only the mixed subtype being more impaired at baseline.
The faster rate of decline in the misidentification subtype might be associated with earlier and greater pathological changes in the functional networks involved in the perception and recognition of visual stimuli. Indeed, previous studies in patients with the misidentification subtype showed poorer performance on neuropsychological tests, which localize to the ventral visual stream,5 and greater volume loss in functionally connected regions,6 including the parahippocampal gyrus, which is involved in the processing and retrieval of contextual memories.24, 25 There is also evidence of greater neurofibrillary tangle burden in the frontal26 and limbic/paralimbic regions including the parahippocampal gyrus8, 9, 26 in AD patients with a history of misidentifications and/or hallucinations. Our findings, supported by those of previous studies, suggest that the misidentification subtype may be a distinct sub‐phenotype of AD and one that is associated with an accelerated cognitive decline.
The greater proportion of women in the misidentification subtype warrants further discussion, as psychosis occurs more frequently in women, and histopathologic studies have shown higher levels of phosphorylated tau in the frontal cortex of women with AD psychosis when compared with their male counterparts.27 However, when gender was incorporated into the model during covariate testing, we could not estimate a significant impact on the rate‐of‐progression parameter. The presence of at least one Apo‐e ε4 allele increased the rate of cognitive decline in all groups. The effect of the presence of Apo‐e ε4 on the rate of disease progression has been already demonstrated in previous AD progression models,20, 21, 28 but here, in contrast to Conrado et al., 21 we did not distinguish between the presence of one or two alleles. The effect of the Apo‐e ε4 genotype on the rate of decline further supports the suggestion that the misidentification subtype has a greater neuropathological burden, as Apo‐e ε4 is involved in the deposition of both neurofibrillary tangles and amyloid29 and regulates Aβ clearance.30, 31 We found an effect of MMSE scores at baseline on ADAS‐cog0, as showed by Ito et al.28 MMSE values are highly correlated to ADAS‐cog values, and this could explain the improved model fit.
There were several study limitations. ADNI represents a highly selective data set, which includes mildly impaired patients with high educational attainments and low vascular burdens. As a result of the mild baseline severity, cognition declined slowly during the 4‐year observation period, which meant that it was not possible to fully capture the nonlinear trajectory of decline as managed in previous studies,19, 20 and our decision to use a nonlinear model was essentially based on its superiority over a linear model in a previous comparative study.20, 21
It is possible that the absence of differences in the rates of decline between the paranoid and nonpsychotic groups reflects the poor sensitivity of the ADAS‐cog to detect deficits in fronto‐executive functioning (subscales largely focus on memory, language, visuospatial, and praxis functions),32 which are perhaps most relevant to the paranoid subtype.4 This could be investigated in future analyses using a similar (mixed effects) based approach to describe changes in digit span, a measure of fronto‐executive function previously shown to decline rapidly in those with psychosis in previous analyses of ADNI data.33 Neither can we completely rule out an effect of gender given the relatively small sample size of psychotic subtypes, and this needs to be explored in a larger sample.
The majority of researchers who have investigated subtype dependency have reported lower MMSE scores in those with the misidentification subtype,4, 11, 34 although this has not been consistently shown.5, 6 In ADNI2 participants, only the mixed group were more impaired using ADAS‐cog and MMSE as markers of cognitive status and disease stage. However, all subtypes were impaired in functional activities when compared with the nonpsychotic group, and it will be important to investigate this further in future studies, as it is possible that specific functional domains may be more sensitive markers of psychosis symptoms.
All patients diagnosed with AD or LMCI during the follow‐up period were included in the study. However, many of the participants were in the early stages of dementia or still diagnosed with MCI, and they would not have progressed far enough through their illness by the end of the 4 years’ follow‐up period to develop psychotic symptoms. It is thus possible that they were assigned a false‐negative psychosis phenotype.
The decision to not use a threshold cutoff for the Neuropsychiatric Inventory scores to define the presence of delusions or hallucinations was based on our previous approach5, 6 and our aim to examine psychosis trait as opposed to state. However, this approach increases the risk of false positives.
The fact that the ADNI2 excluded people who had psychotic symptoms within the previous 3 months or those prescribed antipsychotic or sedative medication limited the sample size of the psychotic group and reduced the power of the study to compare subtypes or to establish any correlation between symptom severity (or antipsychotic use) and cognitive trajectory. Neither was it possible to determine the individual contributions of misidentification phenomena and hallucinations to subtype‐specific differences in rate of decline, as the majority of participants had visual and/or auditory hallucinations (n = 20), with a smaller number having misidentification delusions (n = 6) or both (n = 3).
We cannot rule out the possibility that a proportion of ADNI2 participants assigned to the misidentification subtype represent undiagnosed cases of Lewy body disease given the occurrence of visual hallucinations and misidentifications at a relatively mild stage of disease.26 However, postmortem studies have shown that the early occurrence of these symptoms may also represent a greater expression of AD‐related pathology.26
Given the relatively small sample size of each subtype, our findings should be viewed as preliminary and investigated prospectively in future studies. The development of positron emission tomography ligands that bind to tau and α‐synuclein35, 36 means that it is now possible to simultaneously collect clinical (psychosis subtype, neuropsychological test performance), molecular (pathological, neurochemical), morphological, and functional information in vivo. This approach could be used to further elucidate the pathophysiology of the psychosis subtypes in early AD and other neurodegenerative disorders (Lewy body disease, Parkinson's disease psychosis) in which the misidentification subtype is highly prevalent.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no conflict of interest.
Author Contributions
F.D., S.R., and J.B. wrote the manuscript; F.D., S.R., R.H., and J.B. designed the research; F.D., S.R., and J.B. performed the research; F.D., Y.S., and J.B. analyzed the data; S.R., J.B., R.H., E.M., and C.d.L. contributed new reagents/analytical tools.
Supporting information
Figure S1. Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog) scores along the time (blue crosses) and model‐based individual fits (green line) of 12 randomly sampled patients included in the Alzheimer's Disease Neuroimaging Initiative data set.
Figure S2. Observed Alzheimer's Disease Assessment Scale‐Cognitive Subscale scores in the Alzheimer's Disease Neuroimaging Initiative data set vs. model population (left) and individual (right) predictions. The pink line represents the identity line and the blue line a spline.
Figure S3. Normalized prediction distribution error vs. time (top) and Alzheimer's Disease Assessment Scale‐Cognitive Subscale individual predictions (center) and normalized prediction distribution error probability density function in black (pdf) overlaid to a Gaussian pdf in green (bottom).
Data S1. Disease progression in psychosis with impact on dropout.
Data S2. Data set description.
Data S3. Data set
References
- 1. Ropacki, S.A. & Jeste, D.V. Epidemiology of and risk factors for psychosis of Alzheimer's disease: a review of 55 studies published from 1990 to 2003. Am. J. Psychiatry 162, 2022–2030 (2005). [DOI] [PubMed] [Google Scholar]
- 2. Koppel, J. , Blaine, S. & Greenwald, B.S. Optimal treatment of Alzheimer's disease psychosis: challenges and solutions. Neuropsychiatr. Dis. Treat. 10, 2253–2262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cook, S.E. et al Psychotic symptoms in Alzheimer disease: evidence for subtypes. Am. J. Geriatr. Psychiatry 11, 406–413 (2003). [PubMed] [Google Scholar]
- 4. Reeves, S.J. , Gould, R.L. , Powell, J.F. & Howard, R.J. Origins of delusions in Alzheimer's disease. Neurosci. Biobehav. Rev. 36, 2274–2287 (2012). [DOI] [PubMed] [Google Scholar]
- 5. Reeves, S.J. , Clark‐Papasavas, C. , Gould, R.L. , Ffytche, D. & Howard, R.J. Cognitive phenotype of psychotic symptoms in Alzheimer's disease: evidence for impaired visuoperceptual function in the misidentification subtype. Int. J. Geriatr. Psychiatry 30, 1147–1155 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McLachlan, E. , Bousfield, J. , Howard, R. & Reeves, S. Reduced parahippocampal volume and psychosis symptoms in Alzheimer's disease. Int. J. Geriatr. Psychiatry 33, 389–395 (2018). [DOI] [PubMed] [Google Scholar]
- 7. Lee, Y.M. et al Decreased gray matter volume is associated with the subtypes of psychotic symptoms in patients with antipsychotic‐naïve mild or moderate Alzheimer's disease: a voxel‐based morphometry study. Psychiatry Res. 249, 45–51 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Forstl, H. , Burns, A. , Levy, R. & Cairns, N. Neuropathological correlates of psychotic phenomena in confirmed Alzheimer's disease. Br. J. Psychiatry 165, 53–59 (1994). [DOI] [PubMed] [Google Scholar]
- 9. Mukaetova‐Ladinska, E.B. , Harrington, C.R. , Roth, M. & Wischik, C.M. Biochemical and anatomical redistribution of tau protein in Alzheimer's disease. Am. J. Pathol. 143, 565–578 (1993). [PMC free article] [PubMed] [Google Scholar]
- 10. Sweet, R.A. et al Psychotic symptoms in Alzheimer's disease are not associated with more severe neuropathologic features. Int. Psychogeriatr. 12, 547–558 (2000). [DOI] [PubMed] [Google Scholar]
- 11. Wilson, R.S. et al Hallucinations, cognitive decline, and death in Alzheimer's disease. Neuroepidemiology 26, 68–75 (2006). [DOI] [PubMed] [Google Scholar]
- 12. Rosen, W.G. , Mohs, R.C. & Davis, K.L. A new rating scale for Alzheimer's disease. Am. J. Psychiatry 141, 1356–1364 (1984). [DOI] [PubMed] [Google Scholar]
- 13. Ette, E.I. , Williams, P.J. & Lane, J.R. Population pharmacokinetics III: design, analysis, and application of population pharmacokinetic Studies. Ann. Pharmacother. 38, 2136–2144 (2004). [DOI] [PubMed] [Google Scholar]
- 14. McKhann, G.M. et al The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 263–269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cummings, J.L. , Mega, M. , Gray, K. , Rosenberg‐Thompson, S. , Carusi, D.A. & Gornbein, J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314 (1994). [DOI] [PubMed] [Google Scholar]
- 16. Folstein, M.F. , Folstein, S.E. & McHugh, P.R. “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975). [DOI] [PubMed] [Google Scholar]
- 17. Morris, J.C. Clinical Dementia Rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 9, 173–176 (1997). [DOI] [PubMed] [Google Scholar]
- 18. Pfeffer, R.I. , Kurosaki, T.T. , Harrah, C.H. Jr , Chance, J.M. & Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 37, 323–329 (1982). [DOI] [PubMed] [Google Scholar]
- 19. Tsoularis, A. & Wallace, J. Analysis of logistic growth models. Math. Biosci. 179, 21–55 (2002). [DOI] [PubMed] [Google Scholar]
- 20. Samtani, M.N. et al Alzheimer's Disease Neuroimaging Initiative. An improved model for disease progression in patients from the Alzheimer's disease neuroimaging initiative. J. Clin. Pharmacol. 52, 629–644 (2012). [DOI] [PubMed] [Google Scholar]
- 21. Conrado, D.J. , Denney, W.S. , Chen, D. & Ito, K. An updated Alzheimer's disease progression model: incorporating non‐linearity, beta regression, and a third‐level random effect in NONMEM. J. Pharmacokinet Pharmacodyn. 41, 581–598 (2014). [DOI] [PubMed] [Google Scholar]
- 22. Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 18, 293–297 (1951). [Google Scholar]
- 23. Delyon, B. , Lavielle, M. & Moulines, E. Convergence of a stochastic approximation version of the EM algorithm. Ann. Statist. 27, 94–128 (1999). [Google Scholar]
- 24. Turk‐Browne, N.B. , Simon, M.G. & Sederberg, P.B. Scene representations in parahippocampal cortex depend on temporal context. J. Neurosci. 32, 7202–7207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aminoff, E.M. , Kveraga, K. & Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 17, 379–390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferman, T.J. et al Pathology and temporal onset of visual hallucinations, misperceptions and family misidentification distinguishes dementia with Lewy bodies from Alzheimer's disease. Parkinsonism Relat. Disord. 19, 227–231 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koppel, J. Psychotic Alzheimer's disease is associated with gender‐specific tau phosphorylation abnormalities. Neurobiol. Aging 35, 2021–2028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ito, K. et al Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimers Dement. 7, 151–160 (2011). [DOI] [PubMed] [Google Scholar]
- 29. Takeda, M. et al Apolipoprotein E and central nervous system disorders: reviews of clinical findings. Psychiatry Clin. Neurosci. 64, 592–607 (2010). [DOI] [PubMed] [Google Scholar]
- 30. Castellano, J.M. et al Human apoE isoforms differentially regulate brain amyloid‐β peptide clearance. Sci. Transl. Med. 3, 89ra57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hollands, S. et al APOEɛ4 genotype, amyloid, and clinical disease progression in cognitively normal older adults. J. Alzheimers Dis. 57, 411–422 (2017). [DOI] [PubMed] [Google Scholar]
- 32. Verma, N. , Beretvas, S.N. , Pascual, B. , Masdeu, J.C. & Markey, M.K. , Alzheimer's Disease Neuroimaging Initiative . New scoring methodology improves the sensitivity of the Alzheimer's Disease Assessment Scale‐Cognitive subscale (ADAS‐Cog) in clinical trials. Alzheimers Res Ther. 7, 64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koppel, J. , Sunday, S. , Goldberg, T.E. , Davies, P. , Christen, E. & Greenwald, B.S. Psychosis in Alzheimer's disease is associated with frontal metabolic impairment and accelerated decline in working memory: findings from the Alzheimer's Disease Neuroimaging Initiative. Am. J. Geriatr. Psychiatry 22, 698–707 (2014). [DOI] [PubMed] [Google Scholar]
- 34. Perez‐Madriñan, G. et al Alzheimer disease with psychosis: excess cognitive impairment is restricted to the misidentification subtype. Am. J. Geriatr. Psychiatry 12, 449–456 (2004). [DOI] [PubMed] [Google Scholar]
- 35. Kantarci, K. et al AV‐1451 tau and β‐amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann. Neurol. 81, 58–67 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koga, S. , Ono, M. , Sahara, N. , Higuchi, M. & Dickson, D.W. Fluorescence and autoradiographic evaluation of tau PET ligand PBB3 to α‐synuclein pathology. Mov. Disord. 32, 884–892 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐cog) scores along the time (blue crosses) and model‐based individual fits (green line) of 12 randomly sampled patients included in the Alzheimer's Disease Neuroimaging Initiative data set.
Figure S2. Observed Alzheimer's Disease Assessment Scale‐Cognitive Subscale scores in the Alzheimer's Disease Neuroimaging Initiative data set vs. model population (left) and individual (right) predictions. The pink line represents the identity line and the blue line a spline.
Figure S3. Normalized prediction distribution error vs. time (top) and Alzheimer's Disease Assessment Scale‐Cognitive Subscale individual predictions (center) and normalized prediction distribution error probability density function in black (pdf) overlaid to a Gaussian pdf in green (bottom).
Data S1. Disease progression in psychosis with impact on dropout.
Data S2. Data set description.
Data S3. Data set


