Abstract
Several dietary factors have been suspected to play a role in the development of advanced islet autoimmunity (IA) and/or type 1 diabetes (T1D), but the evidence is fragmentary. A prospective population-based cohort of 6081 Finnish newborn infants with HLA-DQB1-conferred susceptibility to T1D was followed up to 15 years of age. Diabetes-associated autoantibodies and diet were assessed at 3- to 12-month intervals. We aimed to study the association between consumption of selected foods and the development of advanced IA longitudinally with Cox regression models (CRM), basic joint models (JM) and joint latent class mixed models (JLCMM). The associations of these foods to T1D risk were also studied to investigate consistency between alternative endpoints. The JM showed a marginal association between meat consumption and advanced IA: the hazard ratio adjusted for selected confounding factors was 1.06 (95% CI: 1.00, 1.12). The JLCMM identified two classes in the consumption trajectories of fish and a marginal protective association for high consumers compared to low consumers: the adjusted hazard ratio was 0.68 (0.44, 1.05). Similar findings were obtained for T1D risk with adjusted hazard ratios of 1.13 (1.02, 1.24) for meat and 0.45 (0.23, 0.86) for fish consumption. Estimates from the CRMs were closer to unity and CIs were narrower compared to the JMs. Findings indicate that intake of meat might be directly and fish inversely associated with the development of advanced IA and T1D, and that disease hazards in longitudinal nutritional epidemiology are more appropriately modeled by joint models than with naive approaches.
Subject terms: Type 1 diabetes, Risk factors
Introduction
Finland has the highest incidence of type 1 diabetes (T1D) in the world in children aged less than 15 years with the average incidence of 62.5 per 100,000 person-years during 2006–20111. The incidence doubled during 1980–20052 but reasons for the increase remain unclear. The highest increase in incidence rates were seen among the youngest children3, which could imply the role of early exposures in tahe disease process. Although infant-feeding patterns in relation to T1D have been studied broadly4,5, including the finding of no T1D-protective effect of weaning to hydrolyzed infant formula compared to a conventional cow’ s milk based formula in a large randomized controlled trial4, knowledge of food consumption during childhood and the risk of T1D is limited and studies with longitudinal exposure assessment are scarce5.
Longitudinal associations between exposures and disease are often investigated with a Cox regression model (CRM) including longitudinal measurements as a time-dependent covariate, which is defined as for example, a step function of last recorded values6. The associated crude approximation and disregard of measurement error and/or within individual variation can lead to biased estimates and standard errors7. Joint models for longitudinal and time-to-event data7–9 have been developed to address this challenge. They can map the relationship of the hazard to the true, unobserved trajectory of the longitudinal measurement10. The basic joint model (JM) allows this by estimating a linear mixed effects model and a relative risk model and coupling the submodels together by a joint likelihood7. Another variant is the joint latent class mixed model (JLCMM)11,12. This model can capture the possible heterogeneity of the population by finding latent classes of trajectories for longitudinal measurements associated with the risk of the event. The two submodels are tied together only via class membership.
Two recent T1D studies have implemented variants of joint models13,14. Recently, joint models were used to study the association between gluten intake and the risk of islet autoimmunity (IA)15. Otherwise, joint models have been little used in longitudinal nutritional data analysis so far16,17 even though they have a great potential. Joint models can (i) identify individual exposure trajectories even when exposure is observed only at some time points and may include missing values, (ii) smooth out measurement error and (iii) have the potential by JLCMMs to detect periods of sensitivity and risk groups.
First, we set out to assess the association between longitudinal consumption of meat, fish, and eggs during childhood and the risk of advanced IA and T1D in a population-based cohort of young children with HLA-DQB1-conferred susceptibility to T1D. Second, we provide a case investigation of whether associations can be more efficiently identified and more appropriately modeled with joint models (JM and JLCMM) than with the CRM.
Material and Methods
Subjects
This study is part of the Finnish prospective population-based Type 1 Diabetes Prediction and Prevention (DIPP) birth cohort study18. Newborn infants born in the Tampere and Oulu University Hospitals between September 1996 and September 2004 were screened for HLA-DQB1-conferred susceptibility to T1D using cord blood samples. Infants carrying increased genetic susceptibility (HLA-DQB1*02/0302 heterozygous and DQB1*0302/x-positive subjects [x stands for homozygosity or a neutral allele]) were monitored for diabetes-associated autoantibodies and growth up to the age of 15 years or until the manifestation of T1D. The DIPP Nutrition Study includes detailed measurements of childhood food consumption, with 6081 at-risk children (78% of invited children).
The study adheres to the Declaration of Helsinki. The following local ethics committees approved the study protocol: The ethical committee of University of Oulu, the Joint Commission on Ethics of the Turku University and the Turku University Hospital, the ethical committee of the city of Oulu, the ethical committee of the Pirkanmaa Hospital District, and the ethical committee of the University Hospital of Tampere. Parents gave their written informed consent for genetic testing of the newborn infant and for participation in the follow-up.
In the present analysis, the inclusion criteria was having at least one 3-day food record before the last autoantibody measurement, and having at least one autoantibody measurement and/or T1D diagnosis. Among the 5545 children who fulfilled the criteria the median number of autoantibody measurements was 14 (interquartile range, IQR: 6–18) and 3-day food records 5 (IQR: 3–8) per child. Of the 5545 children, 5506 were eligible for the energy-adjusted analyses. The excluded 39 children had no growth data for the first year of life, and therefore calculation of total energy intake was not possible for them during that period. Furthermore, as they had no food record data after the first year, they could not be included to the energy-adjusted analyses. The data used in the present analysis were updated in 2017.
Dietary assessment
Three-day food records were collected at the age of 3 and 6 months and at 1, 2, 3, 4, and 6 years19. For some children, records were also collected at the age of 5 years although it was not the scheduled measurement point. Families recorded all foods and drinks the child had consumed with amount, brand, recipe, and preparation method. Portion size was estimated either with household measures or by using a food portion picture booklet. Trained study nurses checked the food records and a trained nutritionist entered the data19.
The data entry and calculation of intakes of meat, fish, and eggs were done with in-house software by using an annually updated national food composition database20. The definition of meat includes red meat, viscera, poultry, meat products, and sausages. Fish includes fish, fish products, and seafood. The food composition database and connected software enabled the summarization of the intake of each food from different food items, for example, the amount of eggs coming from a cake. The dietary data were used up to the detection of advanced IA.
Total energy intake was calculated based on food records and breastfeeding. For those who were breastfed, total energy intake was estimated based on age, bodyweight, and expected energy deposition needed for growth21.
Laboratory methods
Of the four T1D–associated autoantibodies analyzed, islet cell antibodies (ICA) were used as the primary screening tool. When a child seroconverted to positivity for ICA for the first time, all of the child’ s preceding (starting from birth) and subsequent samples were analyzed for insulin autoantibodies (IAA), glutamic acid decarboxylase antibodies (GADA), and islet antigen 2 antibodies (IA-2A). The ICAs were quantified by a standard indirect immunofluorescence method and IAA, GADA, and IA-2A with specific radiobinding assays as described previously22. Transplacentally transferred autoantibodies were excluded from the analyses. The endpoint of advanced IA was defined as repeated positivity for ICA together with at least one other diabetes-associated autoantibody (IAA, GADA or IA-2A), or T1D diagnosis. Additional analyses were performed with T1D alone as an endpoint. Data of T1D diagnosis was obtained from the national pediatric diabetes registry.
Sociodemographic and perinatal characteristics
Information on each child’s sex, maternal vocational education, and diabetes in a first-degree relative (i.e., familial diabetes) was collected with a structured questionnaire completed by parents after the birth of the child. Information on perinatal characteristics was received from the Medical Birth Registry.
Statistical analysis
We used and evaluated the performance of three different statistical models for studying the association between the absolute daily consumption of meat, fish, and eggs, and the risk of advanced IA. The models were the CRM, in which the longitudinal consumption was used as a piecewise constant step function, the JM with a current value association structure, and the JLCMM. Multiply imputed mean food intakes of the 3-day periods were used in the CRMs23, whereas in the joint models daily intakes were used. Times-to-event for children with advanced IA were set to the middle of the time interval between the last advanced IA-negative and the advanced IA-positive (including T1D diagnosis) measurement. JMs for all the foods and JLCMM for fish were also ran with T1D to investigate consistency between alternative endpoints.
Each of the models was fitted as an univariate model with consumption only and as an adjusted model incorporating important confounding factors identified by a stepwise selection strategy6. In addition, CRMs and JMs were fitted as energy-adjusted models with consumption relative to total energy intake. In the energy-adjusted models, mean food intakes in grams (g) were divided by mean total energy intakes in megajoules (MJ) over the 3-day period for CRM, and daily food intakes were divided by daily total energy intakes for JM. The selected confounding factors were sex of the child, genetic risk of the child and familial diabetes. Maternal vocational education was not included in the final models, because the number of children with advanced IA was very small for some of the factor value combinations. The confounders were used in the survival parts of the models.
We assume that the reader is familiar with the well-known CRM, but we briefly describe the JM and the JLCMM7. We then describe the implementation of submodels for food trajectories and our sensitivity analyses. Analyses were implemented with R, by using the jointModel function from the JM package24, Jointlcmm function from the lcmm package25, and the coxph function from the survival package.
The basic joint model
A JM consists of two submodels fitted simultaneously: a linear mixed effects model and a relative risk model, in which the hazard depends on the modeled food consumption. Let mi(t) denote the true and unobserved time trajectory of the food consumption and let yi(t) denote the observed food consumption for child i at time t.
The two submodels were of the form:
| 1 |
where β0 and b0i denote the fixed part and subject-specific random part of the intercepts, respectively, and βk and bki denote the fixed effects and subject-specific random effects parts of the regression parameters, respectively. The covariance matrix of the normally distributed random effects was diagonal. Bk(t) denotes the value of k th B-spline basis function for a piecewise cubic polynomial spline at time t, and εi(t) are normally distributed independent errors with mean 0 and variance σ2. In the survival model denotes the history of the longitudinal process until t, and wi denotes a vector of baseline covariates with a corresponding vector of regression parameters γ. The submodels are combined via the covariate mi(t), and α denotes the corresponding regression parameter. The h0(t) denotes the baseline hazard at time t which was set as a piecewise constant with knots at the ages of 1.99 and 3.9926–28. Based on the basic structure of the JM, the time-to-event data were used only up to the last point of food consumption measurement (6 years). To enable comparison, the same time period was used in the CRMs.
Joint latent class mixed model
A JLCMM consists of three submodels: the linear mixed effects model, the relative risk model and a multinomial logistic model for the latent class probabilities. The model assumes the food trajectories and hazards arise from several underlying classes, and each child belongs to each particular class with some probability. Compared to a JM, latent class -specific parameters were added to the longitudinal submodel, and the baseline hazard in the survival submodel was set as class-specific with an additional knot at 5.99 years. The two submodels were connected via class membership. Standard errors of piecewise hazard ratios were obtained with the delta method, and the overall hazard ratio was obtained with a JLCMM assuming baseline hazards to be proportional over the entire time period. The effects of the baseline covariates were assumed to be the same across classes. The multinomial logistic regression submodel included the intercept only to ensure that class membership probabilities were based solely on the food consumption profiles.
The submodels were of the form:
| 2 |
where g = 1, …, G denotes the latent class, and λg defines class membership probabilities. The submodels are combined by the class indicator ci. The time-to-event data were used up to the age of 15 years. In the JLCMM, detection of underlying classes, their distinct trajectories and inference on the parameters of the class-specific hazards are of interest.
Choosing the number of latent classes is a central issue in JLCMMs, which is often based on the Bayesian information criterion (BIC)29,30. It has also been recommended that the choice of the number of latent classes should, besides the BIC, be based on a good discrimination between classes, correct predictions, satisfactory conditional independence and meaningful latent classes, in order to use the solution with the fewest classes that provides a satisfactory fit to the data11,12,31. Therefore, we fitted the models with 1–4 latent classes (when converged) for each food item, and based the decision on the recommendations32. BIC curves for the JLCMMs can be found as Supplementary Fig. S1.
Spline-based food trajectory modeling
In the longitudinal submodels of the joint models we used flexible piecewise cubic polynomial spline functions to allow flexible individual food trajectories. B-spline cubic basis functions33 were used and the number of knots was set to q = 2 after finding a balance that allowed sufficient flexibility and avoided overfitting. To fully reach the potential flexibility of splines, knots were not placed equally or only on the measuring points34,35. We considered all four equispaced knot locations between each measurement36 and defined that there must be at least 2 measurements before the first knot, after the second knot, and between the knots. Knot searching was done by fitting linear mixed effects models with piecewise cubic polynomial splines using all knot combinations satisfying the criteria, and selection was done based on BIC. With the chosen criteria, knots were located in the middle parts of the age range and reasonably far apart, resulting in pleasing fits.
Sensitivity analyses
Sensitivity of the results for the assignment of times-to-event for children with advanced IA was assessed by running a multiple imputation approach to interval-censored data37,38. We also verified standard error estimates for the baseline hazards in the JLCMM for fish consumption with the delete-5 jackknife39. To investigate the robustness of the results to the choices of the number and the places of the knots in the baseline hazard, unadjusted JMs for meat, fish and eggs and unadjusted JLCMM for fish were ran with one knot removed and one additional knot, with each time interval including the same number of events. The sensitivity analyses were performed for the models with the advanced IA endpoint.
Results
Baseline characteristics and their association with the risk of advanced IA and T1D
Among the 5545 children with increased genetic risk of T1D, a total of 348 (6.3%) reached the endpoint of advanced IA during the first 15 years of life at a median age of 3.5 years (IQR: 1.8–6.6). Of them, 246 (4.4%) reached it before the age of 6 years at a median age of 2.5 years (IQR: 1.3–3.6). By the age of 15 years, 43 got T1D diagnosis before the autoantibody criterion was fulfilled and by the age of 6 years 34. A total of 195 (3.5%) progressed to T1D during the first 15 years of life at a median age of 6.4 years (IQR: 4.2–10.0) and 88 (1.6%) before the age of 6 years. Of the children, 92% were followed for autoantibodies up to 1 year, 84% up to 2 years, 68% up to 6 years and 33% up to 15 years of age. The median follow-up time was 10 years (IQR: 3.1–14.9). Boys, children with high HLA-DQBI-conferred risk, and children with a diabetic first-degree relative were at higher risk of advanced IA and T1D (Table 1). Maternal vocational education was associated with a child’s lower risk of advanced IA but the association was not so strong with T1D (Table 1). Genetic risk of the child and familial diabetes were not highly associated (see Supplementary Table S1). Kaplan-Meier curves (overall, and by sex, genetic risk and familial diabetes) for advanced IA can be found as Supplementary Fig. S2.
Table 1.
Baseline characteristics of the children, and their association to the advanced islet autoimmunity (IA) and type 1 diabetes (T1D) based on the 15-year follow-up.
| Characteristic | N (%) | Advanced islet autoimmunity | Type 1 diabetes | ||||
|---|---|---|---|---|---|---|---|
| n (%) | HR (95% CIs)1 | P | n (%) | HR (95% CIs)1 | P | ||
| Sex of the child | |||||||
| Boy | 2950 (53.2) | 212 (7.2) | 1 | 115 (3.9) | 1 | ||
| Girl | 2595 (46.8) | 136 (5.2) | 0.72 (0.56, 0.93) | 0.012 | 80 (3.1) | 0.76 (0.57, 1.01) | 0.062 |
| Genetic risk | |||||||
| Moderate (DQB1*0302/x)2 | 4457 (80.4) | 228 (5.1) | 1 | 123 (2.8) | 1 | ||
| High (DQB1*02/0302) | 1088 (19.6) | 120 (11.0) | 1.95 (1.49, 2.55) | <0.001 | 72 (6.6) | 2.49 (1.86, 3.33) | <0.001 |
| Familial diabetes | |||||||
| No | 5001 (90.2) | 299 (6.0) | 1 | 161 (3.2) | 1 | ||
| Yes | 329 (5.9) | 41 (12.5) | 2.13 (1.45, 3.12) | <0.001 | 29 (8.8) | 2.87 (1.93, 4.27) | <0.001 |
| Missing information | 215 (3.9) | 8 (3.7) | 0.30 (0.10, 0.92) | 0.036 | 5 (2.3) | 0.30 (0.09, 1.04) | 0.057 |
| Mother’s vocational education | |||||||
| None | 354 (6.4) | 33 (9.3) | 1 | 17 (4.8) | 1 | ||
| Vocational school or course | 1465 (26.4) | 81 (5.5) | 0.45 (0.29, 0.72) | <0.001 | 40 (2.7) | 0.55 (0.31, 0.96) | 0.037 |
| Secondary vocational education | 2357 (42.5) | 137 (5.8) | 0.38 (0.25, 0.59) | <0.001 | 74 (3.1) | 0.63 (0.37, 1.07) | 0.088 |
| University studies or degree | 1210 (21.8) | 88 (7.3) | 0.57 (0.36, 0.89) | 0.014 | 57 (4.7) | 1.00 (0.58, 1.71) | 0.991 |
| Missing information | 159 (2.9) | 9 (5.7) | 1.58 (0.55, 4.47) | 0.393 | 7 (4.4) | 2.47 (0.79, 7.67) | 0.119 |
1Estimates are hazard ratios from the Cox proportional hazards model including all the four baseline factors in the table.
2x not equal to *02, *0301, or *0602.
Consumption of meat, fish and eggs
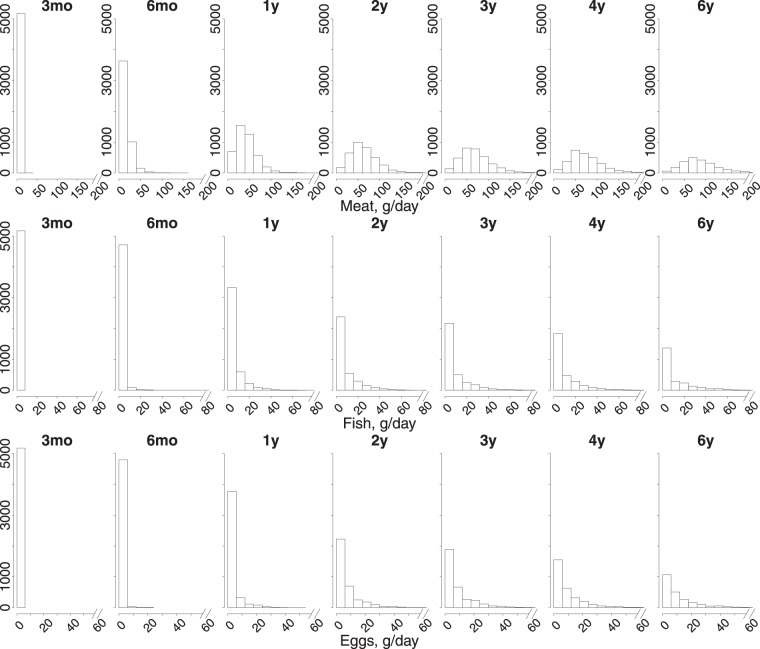
At 3 months of age the selected foods had not been consumed at all. The consumption of meat, fish and egg increased by age. At 6 years, 99.4% of children with completed 3-day food record reported the use of meat, 49.8% reported that of fish and 91.9% reported that of eggs with mean consumption of 88 g/day (SD: 61), 11 g/day (SD: 27) and 10 g/day (SD: 19), respectively. The distributions of the average daily consumption of the foods by age from the 3-day food records are presented in Fig. 1.
Figure 1.
The distribution of daily consumption of meat and meat products, fish and fish products and eggs in grams by age from 3-day food records.
Food consumption and the risk of advanced IA and T1D
Based on the JM, the consumption of meat was marginally associated with advanced IA: the hazard ratio was 1.05 (95% CI: 0.99, 1.12) per 10 g increment in the consumption in the model adjusted for selected confounding factors, and the energy-adjusted hazard ratio was 1.04 (95% CI: 1.00, 1.07) per 1 g/MJ increment in the consumption (Table 2). The results with T1D as an endpoint were consistent with these results (Table 3). The CRM, however, did not reveal this association although estimates from both models were consistent in direction. The JM gave slightly wider confidence intervals than the CRM. Perceptible latent classes for meat consumption and the risk of advanced IA were not identified with the JLCMM.
Table 2.
Risk of advanced islet autoimmunity (IA) associated with the consumption of meat and meat products, fish and fish products and eggs based on the Cox regression model (CRM) and the basic joint model (JM).
| Food consumption | Unadjusted1,2 | P | Adjusted1,2,3 | P | Energy-adjusted1,4 | P |
|---|---|---|---|---|---|---|
| Cox regression model | ||||||
| Meat and meat products | 1.01 (0.96, 1.05) | 0.798 | 1.01 (0.96, 1.05) | 0.831 | 1.00 (0.98, 1.02) | 0.877 |
| Fish and fish products | 0.99 (0.86, 1.13) | 0.840 | 0.99 (0.86, 1.14) | 0.838 | 0.99 (0.94, 1.05) | 0.815 |
| Eggs | 1.08 (0.91, 1.28) | 0.385 | 1.08 (0.91, 1.29) | 0.373 | 1.04 (0.96, 1.12) | 0.379 |
| Basic joint model | ||||||
| Meat and meat products | 1.06 (1.00, 1.12) | 0.054 | 1.05 (0.99, 1.12) | 0.091 | 1.04 (1.00, 1.07) | 0.050 |
| Fish and fish products | 1.14 (0.91, 1.43) | 0.261 | 1.12 (0.89, 1.41) | 0.318 | 1.09 (0.95, 1.24) | 0.210 |
| Eggs | 1.22 (0.94, 1.57) | 0.132 | 1.23 (0.95, 1.59) | 0.119 | 1.16 (1.01, 1.33) | 0.031 |
1Values are hazard ratios with 95% CIs in parentheses.
2Per 10 grams increment in the consumption of the particular food.
3Models adjusted for sex of the child, genetic risk of the child and familial diabetes.
4Per 1 gram/megajoule increment in the consumption of the particular food.
Table 3.
Risk of type 1 diabetes (T1D) associated with the consumption of meat and meat products, fish and fish products and eggs based on the basic joint model (JM).
| Food consumption | Unadjusted1,2 | P | Adjusted1,2,3 | P | Energy-adjusted1,4 | P |
|---|---|---|---|---|---|---|
| Meat and meat products | 1.13 (1.03, 1.24) | 0.011 | 1.13 (1.02, 1.24) | 0.015 | 1.09 (1.02, 1.17) | 0.010 |
| Fish and fish products | 1.20 (0.85, 1.70) | 0.296 | 1.18 (0.83, 1.68) | 0.354 | 1.11 (0.87, 1.42) | 0.398 |
| Eggs | 0.64 (0.24, 1.71) | 0.377 | 0.67 (0.26, 1.73) | 0.409 | 0.74 (0.34, 1.63) | 0.458 |
1Values are hazard ratios with 95% CIs in parentheses.
2Per 10 grams increment in the consumption of the particular food.
3Models adjusted for sex of the child, genetic risk of the child and familial diabetes.
4Per 1 gram/megajoule increment in the consumption of the particular food.
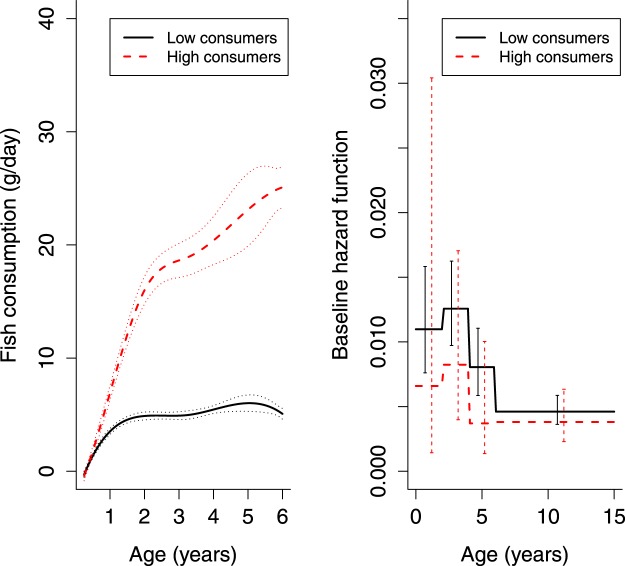
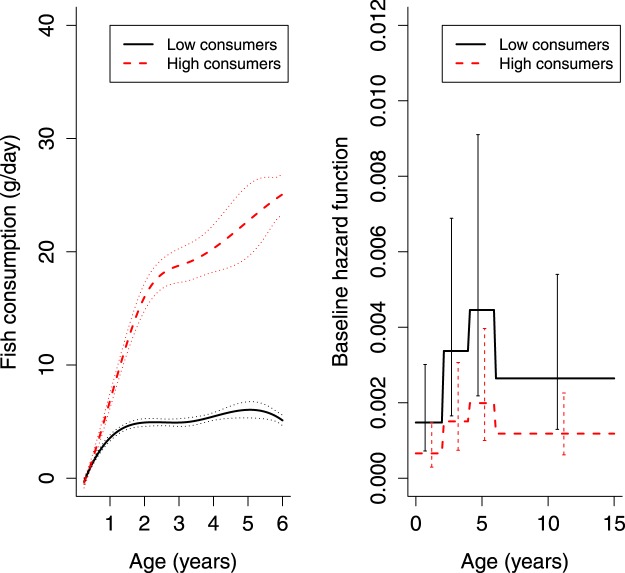
The consumption of fish was not associated with advanced IA in either the JM or the CRM (Table 2), or with T1D in the JM (Table 3). The hazard ratio estimates from the JM and CRM were not consistent in direction but their confidence intervals were not mutually exclusive. However, JLCMM found two perceptible latent classes in the consumption trajectories of fish and the risk of advanced IA (Fig. 2). Similar latent classes were found with the T1D endpoint (Fig. 3).
Figure 2.
Two latent classes with different fish consumption trajectories (with 2.5% and 97.5% percentiles of the Monte Carlo approximation of the posterior distribution), and their associated baseline hazards (with 95% CIs) of advanced islet autoimmunity (IA).
Figure 3.
Two latent classes with different fish consumption trajectories (with 2.5% and 97.5% percentiles of the Monte Carlo approximation of the posterior distribution) and their associated baseline hazards (with 95% CIs) of type 1 diabetes (T1D), based on the joint latent class mixed model (JLCMM) assuming baseline hazards of the latent classes to be proportional over the entire time period.
Low fish-consumer class included children consuming very little fish: the approximate consumption of 5 g/day over the entire time period starting from the age of 1 year. High fish-consumer class showed a steep age gradient in fish consumption from no consumption to an average of 25 g/day at 6 years. The risk of advanced IA among high fish consumers tended to be lower over the entire period compared to low fish consumers. Piecewise hazard ratios were consistently below one, but none of them alone were significant (Table 4). The number of children with advanced IA was small among high fish consumers in some intervals, which resulted in extremely large standard errors both from model-based and jackknife-based estimation. The overall hazard ratio of advanced IA for high fish consumers, compared to low fish consumers, was 0.68 (95% CI: 0.44, 1.05) (Table 4) and of T1D 0.45 (95% CI: 0.23, 0.86) in the models adjusted for selected confounding factors. The number of the children with T1D was 15 (1.6%, N = 941) among the high fish consumers and 180 (3.9%, N = 4604) among the low consumers. The distributions of the confounding factors in the two latent classes of the fish consumption and the risk of advanced IA can be found as Supplementary Table S2 and the average consumption of meat and eggs in the two latent classes as Supplementary Fig. S3. The Kaplan-Meier curves for latent classes can be found as Supplementary Fig. S4. We also performed Fisher’ s exact test as in30 for the number of children with advanced IA by the obtained grouping, which signaled an association (P < 0.001).
Table 4.
The results of the joint latent class mixed model (JLCMM) for fish and fish products consumption: The number of the children reaching the endpoint of advanced islet autoimmunity (IA), and piecewise and overall hazard ratios with 95% CIs for the baseline hazard of high fish consumers in relation to the baseline hazard of low fish consumers.
| Age interval | Children with advanced IA | Unadjusted2 | P | Adjusted2,3 | P | |
|---|---|---|---|---|---|---|
| Low consumers N = 46191 (83.3%) | High consumers N = 9261 (16.7%) | |||||
| 0–24 | 96 | 3 | 0.60 (0.10, 3.73) | 0.62 (0.11, 3.67) | ||
| 2–44 | 87 | 9 | 0.66 (0.28, 1.55) | 0.65 (0.27, 1.53) | ||
| 4–64 | 46 | 5 | 0.46 (0.15, 1.41) | 0.45 (0.15, 1.38) | ||
| 6–154 | 84 | 18 | 0.83 (0.45, 1.53) | 0.81 (0.44, 1.49) | ||
| overall5 | 313 (6.8%) | 35 (3.8%) | 0.69 (0.45, 1.05) | 0.085 | 0.68 (0.44, 1.05) | 0.082 |
1Number of the children is for the unadjusted model, similar in the adjusted model.
2Values are hazard ratios with 95% CIs in parentheses.
3Model adjusted for sex of the child, genetic risk of the child and familial diabetes.
4Estimates of standard errors obtained with delta method.
5Estimates and P-values obtained from the joint latent class mixed model assuming baseline hazards of the latent classes to be proportional over the entire time period.
The CRM or JM did not suggest consistent associations between egg consumption and advanced IA although the energy-adjusted hazard ratio signaled a marginally increased risk association (Table 2). However, the hazard ratio estimates from the JM and CRM were consistent in direction. The estimate from the CRM was closer to unity compared to the JM, and the JM gave wider confidence intervals. The results with T1D as an endpoint did not suggest any associations either, and the hazard ratio estimates were not consistent in direction with the advanced IA results (Table 3). Perceptible latent classes for egg consumption and the risk of advanced IA were not identified with JLCMM.
Sensitivity analyses
Adjustment of the models for sex, genetic risk and familial diabetes did not change the estimates substantially in any of the models and results were not sensitive to energy adjustment, regardless of the outcome. Estimates of the hazard ratios were not sensitive to interval censoring although their standard errors increased a little after the multiple imputation approach. The choices of the number and the places of the knots in the baseline hazard had little effect on the hazard ratio estimates but did not change the interpretation of the results. Reducing the number of the knots slightly weakened, and adding slightly strengthened the findings. For confounding factors, hazard ratios with 95% CIs from the adjusted joint models with the advanced IA endpoint are presented as Supplementary Table S3.
Discussion
We examined the association between the early consumption of meat, fish, and eggs, and the risk of advanced IA in children with increased HLA-conferred genetic susceptibility to T1D by using a CRM, JM, and JLCMM. The associations of these foods to the risk of T1D were also studied by using the JM and JLCMM. The JM suggested marginal association between higher meat consumption and increased risk of advanced IA, but this could not be verified with a CRM. The JLCMM suggested a group of children with high fish consumption profile, which was marginally associated with lower risk of advanced IA. This association was not identified by the JM or the CRM. The higher meat consumption was also associated to the higher risk of T1D based on the JM, and the JLCMM suggested the similar fish-consumer classes as with the advanced IA endpoint, with a protective association of high fish consumption to the risk of T1D. No consistent evidence of an association between egg consumption and the risk of advanced IA or T1D was found.
The major strengths of the study are a large study population and a long follow-up that together enabled great statistical power; in addition, the collection of dietary information took place before the development of advanced IA excluding reporting bias. From a methodological viewpoint, use of different statistical models enabled a comparison and brought different perspectives to the content.
The major limitations of the study are that the study population consisted of at-risk children, and it is unclear whether our epidemiological findings apply to the general population. Additionally, we were only able to investigate the foods separately. Simultaneous investigation could bring further insight by identification of additive and interaction effects. However, software for joint models for multivariate longitudinal data is still lacking40.
The CRM has previously been found to be far too rough in the modeling of association between a longitudinal exposure and time-to-event endpoint, and to have a tendency to underestimate the association parameter41,42. Our results support these findings as the estimates from the CRMs were all attenuated towards unity compared to the JM. The JM gave wider confidence intervals than the CRM mostly based on its ability to take within individual day-to-day variation of food consumption into account. Another major advantage of the joint models compared to the CRM is the built-in handling of incomplete data. The computational burden and the convergence problems might be the practical limitations of the wider use of joint models, particularly with JLCMMs. However, the marginal associations would have not been found without the use of joint models.
Despite the lack of evidence of an association between fish intake and risk of advanced IA or T1D based on the CRM or JM, the JLCMM revealed groups with higher intake of fish and marginally lower risk of advanced IA and T1D. A relatively small intake of fish might be the reason that an association was not observed when the exposure was considered as a continuous one in the JM. Instead, the sufficient use of fish might be important considering the advanced IA and T1D risk. The children in the high fish-consumer JLCMM-classes used fish on average 70 g/week at 1 year with an increasing pattern to 175 g/week at 6 years (Fig. 2). Taking into consideration the portion sizes, the classes identified children who complied with the Finnish dietary recommendations for children43 which instruct to eat fish 2–3 times a week. The marginal finding of higher fish consumption being associated with lower risk of advanced IA supports two previous prospective observations of a protective association between fish-derived fatty acid status and IA development44,45. The potential benefits of fish consumption may be related to the n-3 fatty acids, which play role in the development and function of the immune system and inflammatory reactions46.
Child’s meat consumption has not been related to advanced IA or T1D development in any prospective setting. Maternal meat consumption during lactation was associated with child’s increased risk of T1D in a prospective cohort27. One case-control study and an ecological correlational analysis suggest that meat consumption is associated with increased risk of TID47,48. The potential mechanism of action of high meat consumption is not yet known. Findings relating advanced glycosylation end product and nitrite or N-nitroso compound intake to the disease process led to interest in processed meat products49–51 in the disease etiology. Also, heme iron and proteins of meat may play a role52. Human gut microbiota may have an important role in the development of T1D and meat consumption is known to affect the gut microbiota52,53.
Together with other known health-benefits of fish and fish-derived fats and the health risks related to high meat consumption, our results are in line with the current Finnish dietary recommendations43. Future prospective studies should repeat the analyses. The role of fish and fish oil consumption in the prevention of T1D could be clarified in a trial setting.
In conclusion, our findings from this study suggest that a child’s intake of meat might be directly, and fish inversely, related to the development of advanced IA and T1D. Disease hazards in longitudinal nutritional epidemiology are more appropriately and efficiently modeled by joint models than with naive approaches.
Supplementary information
Supplementary information for A Joint Modeling Approach for Childhood Meat, Fish and Egg Consumption and the Risk of Advanced Islet Autoimmunity
Acknowledgements
This work was supported by the Academy of Finland (Grants 63672, 68292, 79685, 79686, 80846, 114666, 126813, 129492, 139391, 201988, 210632, 276475, 308066); European Foundation for the Study of Diabetes; the Finnish Diabetes Association; the Finnish Diabetes Research Foundation; the Juho Vainio Foundation; the Juvenile Diabetes Research Foundation International (Grants 4-1998-274, 4-1999-731, 4-2001-435); the Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital (Grants 9E082, 9F089, 9G087, 9H092, 9J147, 9K149, 9L042, 9L117, 9M036, 9M1140, 9N086, 9P057, 9R055, 9S074); Oulu University Hospital Research Funds; Turku University Hospital Governmental Grant; the European Union (Grant BMH4-CT98-3314); the Novo Nordisk Foundation; the Academy of Finland (Center of Excellence in Molecular Systems Immunology and Physiology Research 2012–2017, Decision No. 250114); Special Research Funds for University Hospitals in Finland; and the Sigrid Juselius Foundation.
Author Contributions
R.V., J.I., J.T., M.K. and S.M.V. designed the DIPP study and gathered the data. S.M.V. designed and was responsible for the conduct of the dietary part of the study. M.Å. was responsible for the processing of the food consumption data. E.S. and H.-M.T. analyzed the data. J.N. and J.P. supervised the statistical analysis. All the authors participated in interpreting the results and writing the manuscript. E.S. took the main responsibility for writing the manuscript as the first author. All authors reviewed and approved the submitted version of the manuscript.
Data Availability
Data supporting the findings of this study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44196-1.
References
- 1.Harjutsalo V, Sund R, Knip M, Groop P. Incidence of type 1 diabetes in Finland. JAMA. 2013;310:427–428. doi: 10.1001/jama.2013.8399. [DOI] [PubMed] [Google Scholar]
- 2.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. The Lancet. 2008;371:1777–1782. doi: 10.1016/S0140-6736(08)60765-5. [DOI] [PubMed] [Google Scholar]
- 3.Patterson Christopher C, Dahlquist Gisela G, Gyürüs Eva, Green Anders, Soltész Gyula. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. The Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 4.Writing Group for the TRIGR Study Group. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: The TRIGR randomized clinical trial. JAMA319, 38–48 (2018). [DOI] [PMC free article] [PubMed]
- 5.Virtanen SM. Dietary factors in the development of type 1 diabetes. Pediatr. Diabetes. 2016;17:49–55. doi: 10.1111/pedi.12341. [DOI] [PubMed] [Google Scholar]
- 6.Collett, D. Modelling Survival Data in Medical Research, Third Edition. Chapman & Hall/CRC Texts in Statistical Science (CRC Press, 2015).
- 7.Rizopoulos, D. Joint models for longitudinal and time-to-event data: With applications in R (CRC Press, 2012).
- 8.Wulfsohn MS, Tsiatis AA. A Joint Model for Survival and Longitudinal Data Measured with Error. Biom. 1997;53:330–339. doi: 10.2307/2533118. [DOI] [PubMed] [Google Scholar]
- 9.Henderson R, Diggle P, Dobson A. Joint modelling of longitudinal measurements and event time data. Biostat. 2000;1:465–480. doi: 10.1093/biostatistics/1.4.465. [DOI] [PubMed] [Google Scholar]
- 10.Tsiatis AA, DeGruttola V, Wulfsohn MS. Modeling the Relationship of Survival to Longitudinal Data Measured with Error. Applications to Survival and CD4 Counts in Patients with AIDS. J. Am. Stat. Assoc. 1995;90:27–37. doi: 10.1080/01621459.1995.10476485. [DOI] [Google Scholar]
- 11.Lin H, Turnbull BW, McCulloch CE, Slate EH. Latent class models for joint analysis of longitudinal biomarker and event process data: application to longitudinal prostate-specific antigen readings and prostate cancer. J. Am. Stat. Assoc. 2002;97:53–65. doi: 10.1198/016214502753479220. [DOI] [Google Scholar]
- 12.Proust-Lima C, Séne M, Taylor JM, Jacqmin-Gadda H. Joint latent class models for longitudinal and time-to-event data: A review. Stat. Methods Med. Res. 2014;23:74–90. doi: 10.1177/0962280212445839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Köhler M, et al. Flexible Bayesian additive joint models with an application to type 1 diabetes research. Biom. J. 2017;59:1144–1165. doi: 10.1002/bimj.201600224. [DOI] [PubMed] [Google Scholar]
- 14.Köhler M, et al. Joint modeling of longitudinal autoantibody patterns and progression to type 1 diabetes: results from the TEDDY study. Acta Diabetol. 2017;54:1009–1017. doi: 10.1007/s00592-017-1033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LUND-BLIX NICOLAI A., DONG FRAN, MARILD KARL, BARON ANNA E., SEIFERT JENNIFER A., WAUGH KATHLEEN, JONER GEIR, STORDAL KETIL, TAPIA GERMAN, STENE LARS C., JOHNSON RANDI K., REWERS MARIAN, NORRIS JILL. Gluten Intake and Risk of Islet Autoimmunity and Progression to Type 1 Diabetes in Children at Increased Risk of Disease. Diabetes. 2018;67(Supplement 1):136–OR. doi: 10.2337/db18-136-OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekinci EI, et al. Relationship Between Urinary Sodium Excretion Over Time and Mortality in Type 2 Diabetes. Diabetes Care. 2014;37:e62–e63. doi: 10.2337/dc13-1947. [DOI] [PubMed] [Google Scholar]
- 17.Fischer K, et al. Prospective Associations between Single Foods, Alzheimer’s Dementia and Memory Decline in the Elderly. Nutr. 2018;10:852. doi: 10.3390/nu10070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kupila A, et al. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetol. 2001;44:290–297. doi: 10.1007/s001250051616. [DOI] [PubMed] [Google Scholar]
- 19.Virtanen SM, et al. Food consumption and advanced b cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: a nested case-control design. The Am. J. Clin. Nutr. 2012;95:471–478. doi: 10.3945/ajcn.111.018879. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Welfare. Fineli - Finnish food composition database [Online; Stand 5. April 2019] (2019).
- 21.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (The National Academies Press, Washington, DC, 2005).
- 22.Virtanen SM, et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetol. 2006;49:1512–1521. doi: 10.1007/s00125-006-0236-1. [DOI] [PubMed] [Google Scholar]
- 23.Nevalainen J, Kenward MG, Virtanen SM. Missing values in longitudinal dietary data: A multiple imputation approach based on a fully conditional specification. Stat. Medicine. 2009;28:3657–3669. doi: 10.1002/sim.3731. [DOI] [PubMed] [Google Scholar]
- 24.Rizopoulos D. JM: An R package for the joint modelling of longitudinal and time-to-event data. Journal of Statistical Software (Online) 2010;35:1–33. [Google Scholar]
- 25.Proust-Lima C, Philipps V, Liquet B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. Journal of Statistical Software. 2017;78:1–56. doi: 10.18637/jss.v078.i02. [DOI] [Google Scholar]
- 26.Virtanen SM, et al. Early introduction of root vegetables in infancy associated with advanced β-cell autoimmunity in young children with human leukocyte antigen-conferred susceptibility to Type 1 diabetes. Diabet. Medicine. 2011;28:965–971. doi: 10.1111/j.1464-5491.2011.03294.x. [DOI] [PubMed] [Google Scholar]
- 27.Niinistö S, et al. Maternal intake of fatty acids and their food sources during lactation and the risk of preclinical and clinical type 1 diabetes in the offspring. Acta Diabetol. 2015;52:763–772. doi: 10.1007/s00592-014-0673-0. [DOI] [PubMed] [Google Scholar]
- 28.Hakola L, et al. Infant Feeding in Relation to the Risk of Advanced Islet Autoimmunity and Type 1 Diabetes in Children With Increased Genetic Susceptibility: A Cohort Study. Am. J. Epidemiol. 2018;187:34–44. doi: 10.1093/aje/kwx191. [DOI] [PubMed] [Google Scholar]
- 29.Proust-Lima C, Taylor JMG. Development and validation of a dynamic prognostic tool for prostate cancer recurrence using repeated measures of posttreatment PSA: a joint modeling approach. Biostat. 2009;10:535–549. doi: 10.1093/biostatistics/kxp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brombin C, Di Serio C, Rancoita PM. Joint modeling of HIV data in multicenter observational studies: A comparison among different approaches. Stat. Methods Med. Res. 2016;25:2472–2487. doi: 10.1177/0962280214526192. [DOI] [PubMed] [Google Scholar]
- 31.Jacqmin-Gadda H, Proust-Lima C, Taylor JM, Commenges D. Score test for conditional independence between longitudinal outcome and time to event given the classes in the joint latent class model. Biom. 2010;66:11–19. doi: 10.1111/j.1541-0420.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 32.Pohle J, Langrock R, van Beest FM, Schmidt NM. Selecting the Number of States in Hidden Markov Models: Pragmatic Solutions Illustrated Using Animal Movement. J. Agric. Biol. Environ. Stat. 2017;22:270–293. doi: 10.1007/s13253-017-0283-8. [DOI] [Google Scholar]
- 33.De Boor, C. A practical guide to splines, vol. 27 (Springer-Verlag New York, 1978).
- 34.Meyer K. Random regression analyses using b-splines to model growth of Australian Angus cattle. Genet. Sel. Evol. 2005;37:473–500. doi: 10.1186/1297-9686-37-6-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyata S, Shen X. Free-knot splines and adaptive knot selection. J. Jpn. Stat. Soc. 2005;35:303–324. doi: 10.14490/jjss.35.303. [DOI] [Google Scholar]
- 36.Wand MP. A Comparison of Regression Spline Smoothing Procedures. Comput. Stat. 2000;15:443–462. doi: 10.1007/s001800000047. [DOI] [Google Scholar]
- 37.Pan W. A Multiple Imputation Approach to Cox Regression with Interval-Censored Data. Biom. 2000;56:199–203. doi: 10.1111/j.0006-341X.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 38.Read DJS, The Breastfeeding and HIV International Transmission Study Groupa Late Postnatal Transmission of HIV-1 in Breast-Fed Children: An Individual Patient Data Meta-Analysis. The J. Infect. Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 39.Shao, J. & Tu, D. The jackknife and bootstrap (Springer Science & Business Media, 2012).
- 40.Hickey GL, Philipson P, Jorgensen A, Kolamunnage-Dona R. Joint modelling of time-to-event and multivariate longitudinal outcomes: recent developments and issues. BMC Med. Res. Methodol. 2016;16:117. doi: 10.1186/s12874-016-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim JG, Chu H, Chen LM. Basic Concepts and Methods for Joint Models of Longitudinal and Survival Data. J. Clin. Oncol. 2010;28:2796–2801. doi: 10.1200/JCO.2009.25.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sweeting MJ, Thompson SG. Joint modelling of longitudinal and time-to-event data with application to predicting abdominal aortic aneurysm growth and rupture. Biom. J. 2011;53:750–763. doi: 10.1002/bimj.201100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Nutrition Council. Eating together - food recommendations for families with children [Online; Stand 11. September 2018] (2016).
- 44.Niinistö S, et al. Fatty acid status in infancy is associated with the risk of type 1 diabetes-associated autoimmunity. Diabetol. 2017;60:1223–1233. doi: 10.1007/s00125-017-4280-9. [DOI] [PubMed] [Google Scholar]
- 45.Norris JM, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. Jama. 2007;298:1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 46.Innis SM. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern. & Child Nutr. 2011;7:112–123. doi: 10.1111/j.1740-8709.2011.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muntoni S, et al. High meat consumption is associated with type 1 diabetes mellitus in a Sardinian case–control study. Acta Diabetol. 2013;50:713–719. doi: 10.1007/s00592-012-0385-2. [DOI] [PubMed] [Google Scholar]
- 48.Muntoni S, Muntoni S. Epidemiological association between some dietary habits and the increasing incidence of type 1 diabetes worldwide. Annals Nutr. Metab. 2006;50:11–19. doi: 10.1159/000089559. [DOI] [PubMed] [Google Scholar]
- 49.Virtanen SM, et al. Nitrate and nitrite intake and the risk for type 1 diabetes in Finnish children. Diabet. Medicine. 1994;11:656–662. doi: 10.1111/j.1464-5491.1994.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 50.Coughlan MT, et al. Advanced glycation end products are direct modulators of b-cell function. Diabetes. 2011;60:2523–2532. doi: 10.2337/db10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahlquist GG, Blom LG, Persson LA, Sandström AI, Wall SG. Dietary factors and the risk of developing insulin dependent diabetes in childhood. BMJ. 1990;300:1302–1306. doi: 10.1136/bmj.300.6735.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim E, Coelho D, Blachier F. Review of the association between meat consumption and risk of colorectal cancer. Nutr. Res. 2013;33:983–994. doi: 10.1016/j.nutres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 53.Vahed SZ, Sani HM, Saadat YR, Barzegari A, Omidi Y. Type 1 diabetes: Through the lens of human genome and metagenome interplay. Biomed. & Pharmacother. 2018;104:332–342. doi: 10.1016/j.biopha.2018.05.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information for A Joint Modeling Approach for Childhood Meat, Fish and Egg Consumption and the Risk of Advanced Islet Autoimmunity
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author on reasonable request.