Abstract
The biological effects of the bisphosphonates (BPs) as inhibitors of calcification and bone resorption were first described in the late 1960s. In the 50 years that have elapsed since then, the BPs have become the leading drugs for the treatment of skeletal disorders characterized by increased bone resorption, including Paget's disease of bone, bone metastases, multiple myeloma, osteoporosis and several childhood inherited disorders. The discovery and development of the BPs as a major class of drugs for the treatment of bone diseases is a paradigm for the successful journey from “bench to bedside and back again”. Several of the leading BPs achieved “blockbuster” status as branded drugs. However, these BPs have now come to the end of their patent life, making them highly affordable. The opportunity for new clinical applications for BPs also exists in other areas of medicine such as ageing, cardiovascular disease and radiation protection. Their use as inexpensive generic medicines is therefore likely to continue for many years to come. Fifty years of research into the pharmacology of bisphosphonates have led to a fairly good understanding about how these drugs work and how they can be used safely in patients with metabolic bone diseases. However, while we seemingly know much about these drugs, a number of key aspects related to BP distribution and action remain incompletely understood. This review summarizes the existing knowledge of the (pre)clinical and translational pharmacology of BPs, and highlights areas in which understanding is lacking.
Keywords: Bisphosphonates, bone, osteoporosis, pharmacology
1. INTRODUCTION
Bisphosphonates (BPs, Figure 1) were first synthesized about a century ago by German chemists as antiscaling compounds.1 In 2019, it will be 50 years since the first publications on the biological effects of the BPs.2, 3, 4 Since then, the BPs have evolved to become one of the most successful and widely used groups of drugs in the world, dramatically improving the treatment of Paget's disease of bone, osteoporosis, metastatic bone disease, multiple myeloma and several rare bone diseases.5, 6 BPs are currently also being explored for use in various other disease areas, including non‐skeletal applications such as neurodegenerative diseases.7 There is also a renewed interest in using the bisphosphonates as bone‐targeting carriers for other drugs such as antibiotics, hormones and anti‐cancer drugs.8, 9, 10
Figure 1.
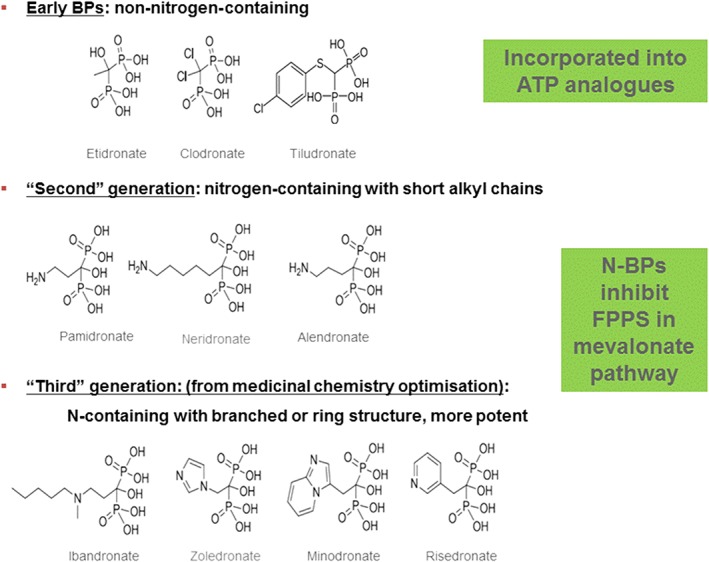
Ten BPs that have been approved for clinical use in various countries and working by two different biochemical mechanisms, incorporation into ATP analogues or inhibition of farnesyl pyrophosphate synthase in the mevalonate pathway
Quite a bit is known about the preclinical, translational and clinical pharmacology of BPs and many excellent reviews have covered this most interesting field.11, 12, 13, 14, 15, 16, 17 There is a vast literature about BPs with more than 24,000 references listed in PubMed alone. However, while we seemingly know much about these drugs, there remain a number of key aspects of BP distribution and action that we do not fully understand. This review summarizes the existing knowledge and highlights areas in which understanding is lacking.
2. PHARMACOKINETICS
All oral BPs have a low and highly variable bioavailability. Oral BPs are taken up in the stomach, duodenum and ileum13 through a mechanism of paracellular and active transport.13, 18 BPs can potentially damage epithelial layers during absorption, which has been demonstrated in in vitro absorption models, animals, healthy volunteers and patients.18, 19, 20 Some of the toxic effects on the GI tract, including the oesophagus, have been linked to a direct damaging effect from the BPs as acids, but the nitrogen‐containing BP drugs' cellular mechanism of action such as FPP‐synthase inhibition, as well as their effects on mitochondrial superoxide production and lipid peroxidation, may also play a role in the GI toxicity.21, 22 At present, no pharmaceutical company has been successful in developing a prodrug that substantially improves the low bioavailability of BPs, in which approximately 99% of any orally administered BP is excreted unchanged into the faeces. This number increases to 100% if the drug is ingested with calcium‐ or magnesium‐containing foods, drinks or drugs.23 In contrast, the low absorption of oral BPs seems to increase to some extent when co‐ingested with gastric pH‐raising drugs such as ranitidine23 and omeprazole. Absorption of BPs has also been increased by enhancers such as caproic acid, and the interference by food can be minimized by slow release formulations that include ethylenediaminetetraacetic acid (EDTA) to chelate divalent cations.24
In the circulation, BPs are bound to plasma and serum proteins. Binding is lower in plasma which has been attributed to endogenous displacers. Moreover, binding seems to depend on the BP, its concentration, pH, calcium and species studied, and has been reported to range as widely as 5–90%.13 It is currently not known whether plasma protein binding plays any role in the pharmacokinetics of BPs, including their renal excretion and delivery to and resorption from bone tissue, nor if plasma protein binding changes during any kind of pathophysiological process.
BPs are found in the liver after oral and parenteral administration, but the drugs typically do not undergo any first phase or second phase metabolism. The only metabolism that has ever been described for BPs is intracellular transformation to cytotoxic ATP analogues of the non‐nitrogen‐containing BPs etidronate, clodronate and tiludronate, which is fundamental to the mechanism by which these BPs inhibit osteoclast‐mediated bone resorption.25 Most circulating BP goes either to calcified tissue or is eliminated via the kidneys, predominantly through glomerular filtration. Indeed, renal clearance of the drugs correlates closely with creatinine clearance, as has been demonstrated for different BPs in different patient populations26, 27, 28 (Figure 2). Indeed, renal clearance was an essential part of the PK model for zoledronic acid on which the US Food and Drug Administration (FDA) based their dose recommendations for patients with renal impairment.29 None of the FDA‐approved BPs have been approved for use in patients with a creatinine clearance below 35 mL/min, which precludes use in patients with severe renal impairment who may benefit from anti‐resorptive treatment when found to have (bone biopsy‐proven) high bone turnover bone loss due to secondary hyperparathyroidism. Retrospective analyses of randomized controlled trials with BPs have looked at patients with low glomerular filtration rates and have shown that the fracture benefit persists in this subset. However, to qualify for these trials, these patients did not have intrinsic renal disease and rather, probably had age‐related decline in renal function.30, 31 Little is also known about the clearance of BPs during various forms of renal replacement therapy. While some BPs have been shown to be cleared by haemodialysis,32, 33, 34, 35 there is limited data for most BPs, particularly in the setting of newer dialysers. In lieu of this small body of data, the general recommendation for the use of BPs in patients on renal replacement therapy is to decrease the BP dose by 50%, increase the infusion time of intravenously administered BPs, and limit the overall number of doses provided.35 One may consider providing the intravenous BP dose prior to a dialysis session, which is likely to result in elimination of part of the administered BP. The complexity of establishing a correct dosing regimen for BPs is increased even more by the effects of both renal impairment and dialysis on biochemical markers of bone turnover.35
Figure 2.

The relationship between creatinine clearance and renal clearance of Pamidronate. From Berenson et al.26 © 1997 American College of Clinical Pharmacology. Reproduced with permission
BPs bind to hydroxyapatite crystals in bone, which are especially available for binding at sites with high bone turnover.2 BPs bind strongly at sites of new mineral deposition at the “calcification front” in osteoid. They also bind well to resorption sites, presumably because calcium phosphate minerals are exposed during resorption. BPs also bind to sites with little bone turnover, albeit much less avidly.36, 37 In the skeleton, more BP is taken up by trabecular bone than cortical bone, which most likely reflects the higher rate of turnover and greater surface area available in trabecular bone.38 The distribution of BPs within the skeleton is therefore not homogeneous.38 BP binding is even higher when there is locally increased bone turnover such as occurs in metastatic bone disease or Pagetic lesions, which is one of the reasons why skeletal lesions in each condition can be detected using 99mTc coupled to BPs,39 as occurs in the clinical use of bone scanning. The reasons why such scans are positive in bone metastases at both predominantly lytic and blastic metastatic sites, but negative in the case of lytic lesions from multiple myeloma (MM)—a disease for which BPs are highly effective—is unclear.40 One hypothesis is that BP binding (or only 99mTc‐BP) is somehow related to osteoblastic bone alkaline phosphatase activity, which is one of the key differences between bone metastases from solid tumours and MM‐related bone lesions. Bone formation is suppressed in myeloma, and this may also contribute to the observed phenomenon by reducing the amount of newly deposited mineral available to which BPs can adsorb. An additional potential aetiology is that the ionic bond which links 99mTc to the BP is dissociated under the acidic environment present at osteolytic sites, leading 99mTc to disperse from the BP at these surfaces.
The distribution of BP to bone and the distribution between resorbing and quiescent bone is also intriguing and seems to differ from BP to BP.36, 37 Some BPs bind more strongly to hydroxyapatite than others, and it may be that the differences in distribution between resorbing bone and quiescent bone is related to this property. In addition, more recently, differences in BP binding properties were shown to determine not only the extent to which a single BP dose would bind to the skeleton,41 but also how well BPs penetrate into the canalicular network of bone. The binding affinities of BPs for hydroxyapatite vary somewhat depending on the assay method used.17 In general, however, BPs with weaker bone‐binding properties such as risedronate appear to penetrate deeper into the bone than stronger binding BPs such as alendronate, and were shown to have a more pronounced access to osteocytes in bone.42 This phenomenon may help to explain why both drugs have similar anti‐fracture activity, despite the clinically used dose of risedronate being only about half that of alendronate, and the former having an intrinsically ~45 times greater potency for inhibiting osteoclast‐mediated bone resorption.43 Interestingly, in patients, weekly oral alendronate (70 mg) treatment decreases bone turnover markers to a larger extent than weekly oral risedronate (35 mg).44
After a single intravenous BP dose, negligible amounts are deposited in non‐calcified tissue. Accordingly, if patients do not have any extensive extra‐skeletal calcifications, then nearly all of the BP is either bound to the skeleton or rapidly excreted into the urine. The total amount retained by the skeleton in patients can therefore be estimated from an intravenous dose and the amount that ends up in the urine. This technique was originally developed with 99mTc‐BP by Fogelman et al. with validation by comparing the so‐called whole body retention (= Dose − amount in 24 h urine) with bone scintigrams in patients.39 This approach has subsequently also been used to determine the amount of BP delivered to the skeleton in a number of clinical pharmacology studies with pharmacologically active BPs such as alendronate, pamidronate, olpadronate, zoledronate and ibandronate.28, 45, 46, 47, 48 It was also shown that this whole body (or rather skeletal) retention of BPs correlated with the binding properties of the BPs41 (see also Table 1), but that it was also determined by the extent of initial disease (eg number of bone metastases in metastatic bone disease or bone turnover marker levels in either metastatic bone disease or Paget's disease of bone)46, 47, 49 (Figure 3). These interesting observations make intrinsic sense. Moreover, many studies suggest that it is not possible to saturate the skeleton with BPs. Thus, skeletal retention of BPs during monthly administration of BP does not seem to change during treatment despite a decrease in bone turnover, and therefore a decrease in available hydroxyapatite in metastatic lesions.47 This intriguing observation might suggest that whole body retention determined from urinary excretion of BPs is a rather crude measurement and that it does not tell us everything about the actual distribution of the BP at the skeleton, including at sites of bone metastases. It also nicely demonstrates that we do not fully understand the clinical pharmacokinetics (PK) of BPs.
Table 1.
Mean kinetic binding affinity KL/106L/mol for hydroxyapatite for various BPs, calculated from slopes of Langmuir adsorption isotherm plots,41 in relation to mean whole body retention (WBR24h(%)) of these BPs in patients as described in the literature15, 41
| K L /10 6 L/mol | WBR 24h (%) | |
|---|---|---|
| Clodronate | 0.72 | 20 |
| Risedronate | 2.19 | 35 |
| Ibandronate | 2.36 | 54 |
| Alendronate | 2.94 | 55 |
| Zoledronate | 3.47 | 62 |
Figure 3.

Whole body retention (WBR) of the BP olpadronate in patients with Paget's disease of bone correlates with pretreatment renal function (Clcr) as well as pre‐treatment rate of bone turnover, biochemically assessed (uNTx/Cr). From46
After binding to the bone, BPs are either released again by dissolution, or are covered within new bone that overlays resorption sites for later release into the circulation during osteoclast‐mediated bone resorption, either directly or via osteoclasts.36, 37 As illustrated in Figure 4 using fluorescently labelled compounds, BPs undergo endocytosis by osteoclasts during bone resorption.50 Intracellularly, the nitrogen (N)‐containing BPs inhibit farnesyl pyrophosphate synthase (FPPS), which ultimately leads to the loss of function of the OCs.52, 53, 54, 55 Some of the BP is released into the circulation during resorption from bone, and some may be released after apoptosis and death of OCs, although there is no data to our knowledge on the actual contribution of either process to the long‐term elimination of BPs in urine. BPs released from bone may undergo re‐uptake onto bone surfaces, a process that may depend on their relative mineral affinity. BPs are detected in urine for years after treatment discontinuation.45, 56 Interestingly, the renal excretion of alendronate lasted longer than risedronate, perhaps reflecting its higher bone affinity and consequent greater recycling back into the skeleton.57
Figure 4.
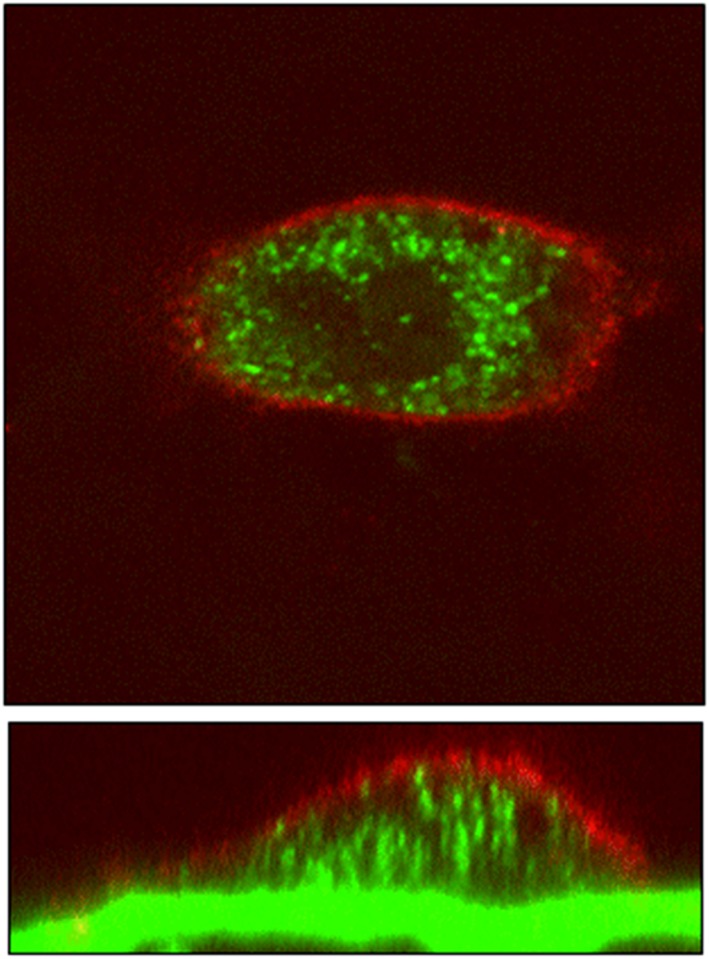
BP uptake into osteoclast. Rabbit osteoclasts were seeded onto dentine discs that had been precoated with 100 mM fluorescently labelled alendronate (green) and incubated for 18 h. Cells were then fixed, and actin stained with TRITC‐phalloidin (red). Cells were examined by light scatter confocal microscopy (LSCM)50, 51
From the PK point of view, bone is not considered a well‐mixed compartment, which is one of the prerequisites for compounds to demonstrate first‐order pharmacokinetics.58 Some investigators have therefore advocated for long‐term decreases in bone, serum and urine concentrations of BPs to be better described by power functions rather than exponential decrease pharmacokinetics.58 However, most investigators ignore this and ultimately describe the long‐term PK of BPs with exponential kinetics, as exemplified by studies with intravenous alendronate in women and intravenous pamidronate in children, which both demonstrated long‐term terminal half‐lives of 1–10 years for BPs.45, 56 The 10‐year half‐life number is often quoted, but might be misleading as shorter term kinetics cannot predict longer term half‐lives with much confidence. Moreover, BPs retained in bone are likely to be pharmacologically inactive until released, just like other bone‐seeking substances stored in the skeleton, eg strontium, lead and mercury. Long‐term bone, serum and urinary PK thus show multiple phases, with longer half‐lives the further removed from BP administration, such that a comprehensive description of BP PK is usually provided by multiple compartment mathematical PK models (see also the paper by Riggs et al. in this Themed Issue).
Long‐term PK might also be determined by the binding properties of the BPs (in conjunction with their potency), which might to some extent explain the differences in loss of the anti‐resorptive effect after treatment discontinuation with alendronate or risedronate. In a head‐to‐head study comparing the effect of stopping alendronate, risedronate or ibandronate, all bone turnover markers (BTMs) increased after treatment withdrawal, but remained below the pretreatment baseline with less suppression of BTMs for the risedronate group as compared to alendronate and ibandronate up to 48 weeks following treatment discontinuation.59 The only head‐to‐head study comparing the PK of BPs in humans used accelerator mass spectrometry to demonstrate that after intravenous administration, whole body retention (WBR) of risedronate was less than alendronate [51.0 vs 55.5%, respectively after 24 h; 33.8 vs 44.9%, respectively after 4 weeks].60 This difference in retention might explain the difference in loss of the anti‐resorptive effect between alendronate and risedronate, albeit the differences in both WBR and BTM levels seem relatively small. Interestingly, the change in bone mineral density (BMD) 96 weeks after treatment discontinuation with alendronate, risedronate or ibandronate was the same,59 again illustrating our incomplete understanding of the clinical and translational pharmacology of BPs.
3. PHARMACODYNAMICS
The effects of BPs have been evaluated using a variety of preclinical in vitro and in vivo models in many animal species over the many decades since the first descriptions of their biological effects. Rodents are commonly used but many other species have been utilized including pigs, dogs and monkeys. Endpoints include BMD, HRpQCT, mineral, hormone and BTM measurements as well as biomechanical strength testing. There are different models for different diseases such as ovariectomized mice to study the effect of BPs (and other drugs) on oestrogen‐deficiency‐related bone loss in vivo. An example of an in vitro study includes the effect of BP incubation on 45Ca release from 17‐day‐old fetal mouse metatarsals cultured for several days, which has been used to assess the potency of different BPs.61 It is important to realize that different models lead to different values of eg EC50 and LED (lowest effective dose). However, in general the ranking of these potency parameters is similar across most models. In addition, this ranking translates for the most part to humans.
A landmark discovery in the 1990s, more than two decades after their first clinical use, was the elucidation of how BPs act biochemically within cells. For the N‐containing BPs such as alendronate, risedronate, ibandronate and zoledronate, the inhibition of osteoclast‐mediated bone resorption52, 53, 54, 55 is mediated via inhibition of the enzyme, farnesylpyrophosphate (FPP) synthase (FPPS), an essential enzyme of the mevalonate pathway (Figure 5). Inhibition of this enzyme leads to a decrease in FPP, and also geranylgeraniol pyrophosphate (GGPP), which leads to a decreased prenylation of signal transduction proteins such as Rac, Ras and Rho, which in turn leads to a loss of function of the osteoclasts. Co‐crystallization of the FPPS enzyme with BPs allowed the exact nature of binding of BPs within the enzyme to be determined by X‐ray crystallography.62 This knowledge has led to the design of even more potent and selective BPs such as OX14, a recently described potential candidate for clinical development, which was also selected to have relatively low and potentially advantageous skeletal binding properties.63 Interestingly, BPs have also been shown to work on other bone cells, osteoblasts as well as osteocytes, by preserving their viability.64 Especially for the latter, penetration into the osteocyte canalicular network is important which is better for low‐affinity BPs.42 Although further studies are needed, this effect of BPs might be mediated through opening of Connexin 43 hemichannels by BPs and subsequent activation of ERKs which can activate anti‐apoptotic signalling pathways.65
Figure 5.

The nitrogen containing bisphosphonates (N‐BPs) work on the mevalonate pathway of cholesterol biosynthesis, just like statins. By inhibiting the enzyme, farnesyl pyrophosphate synthase (FPPS), they interfere with intracellular signalling that requires prenylated proteins
With respect to cancer, the effect of BPs on metastatic bone disease is mostly related to their ability to decrease osteoclast‐mediated bone resorption. However, some BPs also seem to have direct anti‐tumour activity. In preclinical models zoledronate was shown to inhibit cell migration, invasion and metastasis66, 67, 68 and several clinical studies have shown a beneficial effect for adjuvant use of the drug in elderly postmenopausal women66, 69 in addition to a role in preventing bone metastases (see also papers in this Themed Issue by Dionisio et al.70 and Chukir et al.71). Zoledronate might exert its anti‐tumour effects through the miR‐21/PTEN/Akt signalling and Activin signalling pathways.72, 73
The potential actions of BPs in other indications such as for neurodegenerative diseases are intriguing but are not yet fully explored. Their ability to inhibit calcium crystal growth as well as to inhibit FPP synthase in the mevalonate pathways has been implicated but, in reality, this needs to be studied more extensively. BPs, especially etidronate, have been shown to prevent but not reverse ectopic mineralization in a mouse model of pseudoxanthoma elasticum (PXE), a rare disease in which an ABCC6 mutation leads to insufficient circulating pyrophosphate and subsequent soft tissue mineralization.74 It is, however, not known if bisphosphonate treatment might help PXE patients.
A potential challenge for translating animal experiments to humans is the difference in bone modelling and bone remodelling between various animals and humans.75 However, while acknowledging some of the differences, most models have been quite predictive for calculating a safe initial dose for first‐in‐human (FIH) studies, most of which were conducted 20–30 years ago.76, 77, 78, 79 Further tailoring of the dose regimen, though, is taking place in humans based on PK and pharmacodynamic (PD) data generated in phase 1 studies.76, 79, 80
The most important clinical outcome parameters for BP treatment are reduction of fracture risk in osteoporosis, skeletal‐related events (SREs) in metastatic bone disease and more disease‐specific parameters in other diseases such as pain and deformation in Paget's disease of bone, or number of fractures and bone pain in osteogenesis imperfecta. Surrogate parameters that can be assessed include BMD, HRpQCT, indentation and biochemistry, including minerals, hormones and biochemical markers of bone turnover (BTMs). In humans, as well as animals, these surrogate parameters are the PDs of the BPs. Measurements such as BMD have an excellent correlation with fracture risk but increase quite slowly over 3–12 months before reaching significant changes from baseline. In contrast, BTMs respond sooner and to a greater extent, even within days, depending on the dose, the mode of administration and the potency of the BP. The changes in BTMs correlate with actual rates of bone remodelling as determined from bone biopsies but also correlate with bone loss and fracture risk.81, 82, 83, 84, 85 BTMs are therefore used in phase 1 studies to identify the most appropriate dose, usually the one that maximally suppresses BTM in the majority of patients.84 BPs inhibit osteoclast‐mediated bone resorption and therefore the most important and most rapidly responding BTMs are the biochemical markers of bone resorption such as CTX and NTX, which can be measured in serum as well as urine.84 Markers of bone formation such as BALP, PINP and OC change more slowly during BP therapy as a result of coupling between bone formation and resorption, which is typically decreased by BPs.84
The BTMs in combination with BMD have been used to identify the most appropriate dose regimens for pivotal phase 3 trials.84, 86 In addition, they have been used for so‐called bridging trials, phase 2 studies to seek regulatory approval for slightly different indications. An example is seeking approval for corticosteroid‐induced osteoporosis for a BP that has been approved for postmenopausal osteoporosis. This is typically granted when the drug has similar effects on BMD and BTMs in the other indication. In recent years, the combination of BTMs and BMD has been used to identify new dose regimens such as 70 mg once weekly instead of 10 mg daily for oral alendronate, 35 mg once weekly instead of 5 mg daily for oral risedronate, 150 mg monthly instead of 2.5 mg daily for oral ibandronate for osteoporosis, and 4 mg q3 months instead of 4 mg monthly for zoledronate in metastatic bone disease from breast cancer.87, 88, 89
The search for alternative dose regimens has either been part of attempts to improve adherence to BPs or it has been spurred by the fear of serious side effects of BPs such as osteonecrosis of the jaw (ONJ) and atypical femur fractures (AFFs), the incidence of which seems to be related to long‐term use of relatively high doses. Several studies have explored the potential use of BTMs in predicting which patients might be at risk for these side effects.90, 91, 92 Unfortunately, most of these studies into the relationships between BTM levels and serious side effects have been characterized by suboptimal study designs and we therefore still do not know if BTMs are useful or not, although it seems unlikely.
BTMs, in combination with BMD, are used successfully to monitor so‐called drug holidays from BPs.59, 93, 94, 95, 96 These “drug holidays” aim to avoid administering BPs continuously for more than a few years. And during these breaks in treatment, the BTMs, especially together with BMD measurements, signal when the anti‐resorptive and possibly anti‐fracture effect clearly wears off, and when BP or alternate therapy might be reinstated. BTMs in combination with BMD measurements have also provided information on sequential treatment with anabolic therapy such as teriparatide in patients who have either been or who continue to use BP therapy.97 However, although useful, this is also one of the examples in which histomorphometric analysis of bone biopsy samples provide evidence of different effects on different bone envelopes that is not easily captured by either BMD or BTMs, such as the block of teriparatide‐induced increase in cortical porosity by concurrent alendronate use,97 illustrating limitations of the use of BTMs and BMD. Such information on the effect of BPs and other drugs on different bone envelopes is ultimately relevant for anti‐fracture efficacy, and is thus not captured by BTMs and BMD, nor fully by histomorphometry, but might be partially captured by use of HRpQCT.98
4. INTEGRATED PHARMACOKINETICS AND PHARMACODYNAMICS
Most of the effects observed in patients can be explained by a combination of disease, extent of the disease, disease progression, co‐medication, mode of administration of the BP, dose regimen, binding affinity and potency of the BPs, pretreatment rate of bone turnover, BTMs, BMD, fracture risk and renal function. Much of these observations have been captured using mathematical models that simultaneously describe PK and PD of the drugs, with PD either restricted to bone resorption markers or expanded with BTMs, BMD and fracture risk. Some models include effects of co‐medication such as the effects of calcium and vitamin D supplementation on BTMs and BMD and fracture risk, while others also include renal function (affecting both PK and PD) and disease progression.15, 48, 99, 100, 101, 102, 103, 104, 105 Several mathematical modelling and simulation approaches are described elsewhere in this Themed Issue (Riggs and Cremers), but it is important to realize that any type of simulation using these type of models remains a simulation, even if all or most available data on the drug and the disease has been incorporated.
4.1. Future directions and opportunities for novel applications
The field of bisphosphonate research is still being pursued actively, although the big pharma companies are no longer involved. Attempts to improve formulations continue, and there is interest in extending the use of BP drugs to new areas, including target and release strategies with BP‐drug conjugates to deliver known actives to treat skeletal‐related diseases.9, 10 There are many unmet medical needs that might be aided by these drugs, not only in cancer, but also in fracture healing, implant fixation and osteoarthritis to name just a few. There is currently much emphasis based on the management of rare diseases, where BPs would be affordable compared with the usually extremely high costs of other new treatments for rare diseases.
New bisphosphonates are being studied. Ox‐14 is a novel highly potent patented BP.63 Lidadronate (formerly known as IG9402) is an amino analogue of olpadronate of particular interest because it enhances bone mass without apparently inhibiting bone resorption, indicating a different mode of action than the classical one through the mevalonate pathway.64
One of the most exciting new directions is the increasing recognition that bisphosphonates can exert a range of important non‐skeletal effects that may be of clinical benefit. The placebo‐controlled trial by Lyles et al.106 demonstrated that yearly administration of 5 mg of zoledronate reduced mortality by 28% and a recent placebo‐controlled prospective study by Reid et al.107 also showed this mortality benefit, albeit non‐significant, plus a reduction in cancers and a numerical, but non‐significant reduction in cardiovascular outcomes, as has also been seen in more than 10 observational studies (eg by Wolfe et al.108). We need to improve our basic understanding of the mechanisms underlying these fascinating effects. Recent studies show that BPs can extend life span in models of progeria,109, 110 and can protect stem cells from radiation damage.111
These observations offer us an opportunity for the ‘re‐purposing’ of bisphosphonates for new indications. The wide range of new potential medical uses includes effects on T cells, protozoan parasites, tissue regeneration, radioprotection and extension of life span. Bisphosphonates have an excellent safety profile established over many decades, and new and even more potent compounds with lower bone affinity could be developed for these novel non‐skeletal applications.
5. CONCLUSION
Bisphosphonates (BPs) are well‐established as the leading drugs for the treatment of skeletal disorders characterized by increased bone resorption, including Paget's disease, bone metastases, myeloma, osteoporosis and several childhood inherited disorders.
The major BPs were formerly available as branded drugs but are now generic and inexpensive. The range of clinically successful BPs include etidronate, alendronate, risedronate, pamidronate, ibandronate and zoledronate, which is arguably the optimal drug for clinical use at present.
However, despite nearly 50 years of use in biological systems, it would be naive to suggest that we know everything about BPs and metabolic bone diseases and that we can therefore predict pretty much every scenario. Despite our extensive knowledge of the clinical and translational pharmacology of BPs, we continue to need clinical studies supported by preclinical and translational research to fully investigate old and new ideas, and the challenges associated with these interesting compounds.
There have been recent setbacks for several other medicines in development for metabolic bone diseases, resulting in abandoning their costly development, eg for the SERM, lasofoxifene and the cathepsin K inhibitor, odanacatib. BPs are therefore likely to continue to be used as the major drugs to treat disorders of bone resorption for several decades to come. Studies to extend and optimize their use are still needed, however.
COMPETING INTERESTS
There are no competing interests to declare.
Cremers S, Drake MT, Ebetino FH, Bilezikian JP, Russell RGG. Pharmacology of bisphosphonates. Br J Clin Pharmacol. 2019;85:1052–1062. 10.1111/bcp.13867
Contributor Information
Serge Cremers, Email: sc2752@cumc.columbia.edu.
R. Graham G. Russell, Email: graham.russell@ndorms.ox.ac.uk.
REFERENCES
- 1. Widler L, Jahnke W, Green JR. The chemistry of bisphosphonates: From antiscaling agents to clinical therapeutics. Anticancer Agents Med Chem. 2012;12(2):95‐101. [DOI] [PubMed] [Google Scholar]
- 2. Fleisch H, Russell RG, Francis MD. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo . Science. 1969;165(3899):1262‐1264. [DOI] [PubMed] [Google Scholar]
- 3. Francis MD, Russell RG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo . Science. 1969;165(3899):1264‐1266. [DOI] [PubMed] [Google Scholar]
- 4. Fleisch H, Russell RG, Simpson B, Muhlbauer RC. Prevention by a diphosphonate of immobilization "osteoporosis" in rats. Nature. 1969;223(5202):211‐212. [DOI] [PubMed] [Google Scholar]
- 5. Burr D, Russell G. Foreword: bisphosphonates. Bone. 2011;49(1):1 10.1016/j.bone.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 6. Corral‐Gudino L, Tan AJ, Del Pino‐Montes J, Ralston SH. Bisphosphonates for Paget's disease of bone in adults. Cochrane Database Syst Rev. 2017;12:CD004956 10.1002/14651858.CD004956.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zameer S, Najmi AK, Vohora D, Akhtar M. Bisphosphonates: Future perspective for neurological disorders. Pharmacol Rep. 2018;70(5):900‐907. 10.1016/j.pharep.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 8. Farrell KB, Karpeisky A, Thamm DH, Zinnen S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 2018;9:47‐60. 10.1016/j.bonr.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sedghizadeh PP, Sun S, Junka AF, et al. Design, synthesis, and antimicrobial evaluation of a novel bone‐targeting bisphosphonate‐ciprofloxacin conjugate for the treatment of osteomyelitis biofilms. J Med Chem. 2017;60(6):2326‐2343. 10.1021/acs.jmedchem.6b01615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang H, Xiao L, Tao J, et al. Synthesis of a bone‐targeted bortezomib with in vivo anti‐myeloma effects in mice. Pharmaceutics. 2018;10(3):154 10.3390/pharmaceutics10030154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19(6):733‐759. 10.1007/s00198-007-0540-8 [DOI] [PubMed] [Google Scholar]
- 12. Russell RG. Bisphosphonates: The first 40 years. Bone. 2011;49(1):2‐19. 10.1016/j.bone.2011.04.022 [DOI] [PubMed] [Google Scholar]
- 13. Lin JH. Bisphosphonates: A review of their pharmacokinetic properties. Bone. 1996;18(2):75‐85. [DOI] [PubMed] [Google Scholar]
- 14. Drake MT, Cremers SC. Bisphosphonate therapeutics in bone disease: The hard and soft data on osteoclast inhibition. Mol Interv. 2010;10(3):141‐152. 10.1124/mi.10.3.5 [DOI] [PubMed] [Google Scholar]
- 15. Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: Use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 2005;44(6):551‐570. 10.2165/00003088-200544060-00001 [DOI] [PubMed] [Google Scholar]
- 16. Cremers S, Papapoulos S. Pharmacology of bisphosphonates. Bone. 2011;49(1):42‐49. 10.1016/j.bone.2011.01.014 [DOI] [PubMed] [Google Scholar]
- 17. Ebetino FH, Hogan AML, Sun S, et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone. 2011;49(1):20‐33. 10.1016/j.bone.2011.03.774 [DOI] [PubMed] [Google Scholar]
- 18. Twiss IM, Burggraaf J, Schoemaker RC, et al. The sugar absorption test in the evaluation of the gastrointestinal intolerance to bisphosphonates: Studies with oral pamidronate. Clin Pharmacol Ther. 2001;69(6):431‐437. [DOI] [PubMed] [Google Scholar]
- 19. Twiss IM, van den Berk AHM, de Kam ML, et al. A comparison of the gastrointestinal effects of the nitrogen‐containing bisphosphonates pamidronate, alendronate, and olpadronate in humans. J Clin Pharmacol. 2006;46(4):483‐487. 10.1177/0091270006286781 [DOI] [PubMed] [Google Scholar]
- 20. Twiss IM, Pas O, Ramp‐Koopmanschap W, Den Hartigh J, Vermeij P. The effects of nitrogen‐containing bisphosphonates on human epithelial (Caco‐2) cells, an in vitro model for intestinal epithelium. J Bone Miner Res. 1999;14(5):784‐791. 10.1359/jbmr.1999.14.5.784 [DOI] [PubMed] [Google Scholar]
- 21. Suri S, Mönkkönen J, Taskinen M, et al. Nitrogen‐containing bisphosphonates induce apoptosis of Caco‐2 cells in vitro by inhibiting the mevalonate pathway: A model of bisphosphonate‐induced gastrointestinal toxicity. Bone. 2001;29(4):336‐343. [DOI] [PubMed] [Google Scholar]
- 22. Nagano Y, Matsui H, Shimokawa O, et al. Bisphosphonate‐induced gastrointestinal mucosal injury is mediated by mitochondrial superoxide production and lipid peroxidation. J Clin Biochem Nutr. 2012;51:196‐203. 10.3164/jcbn.12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gertz BJ, Holland SD, Kline WF, et al. Studies of the oral bioavailability of alendronate. Clin Pharmacol Ther. 1995;58(3):288‐298. 10.1016/0009-9236(95)90245-7 [DOI] [PubMed] [Google Scholar]
- 24. McClung MR, Miller PD, Brown JP, et al. Efficacy and safety of a novel delayed‐release risedronate 35 mg once‐a‐week tablet. Osteoporos Int. 2012;23(1):267‐276. 10.1007/s00198-011-1791-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frith JC, Monkkonen J, Auriola S, Monkkonen H, Rogers MJ. The molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: Evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosis. Arthritis Rheum. 2001;44(9):2201‐2210. [DOI] [PubMed] [Google Scholar]
- 26. Berenson JR, Rosen L, Vescio R, et al. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol. 1997;37(4):285‐290. [DOI] [PubMed] [Google Scholar]
- 27. Bergner R, Henrich DM, Hoffmann M, et al. Renal safety and pharmacokinetics of ibandronate in multiple myeloma patients with or without impaired renal function. J Clin Pharmacol. 2007;47(8):942‐950. 10.1177/0091270007301801 [DOI] [PubMed] [Google Scholar]
- 28. Skerjanec A, Berenson J, Hsu CH, et al. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J Clin Pharmacol. 2003;43(2):154‐162. [DOI] [PubMed] [Google Scholar]
- 29. Booth BPR, Ibrahim A, Scher N, et al. Population pharmacokinetic (PPK) modeling and simulation‐derived dosing of intravenous busulfan (Busulfex) in pediatric patients. Clin Pharmacol Ther. 2003;73:66. [Google Scholar]
- 30. Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age‐related reduced renal function as estimated by the Cockcroft and Gault method: A pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20(12):2105‐2115. 10.1359/JBMR.050817 [DOI] [PubMed] [Google Scholar]
- 31. Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate treatment in women with normal to severely impaired renal function: An analysis of the fracture intervention trial. J Bone Miner Res. 2007;22(4):503‐508. 10.1359/jbmr.070112 [DOI] [PubMed] [Google Scholar]
- 32. Bergner R, Dill K, Boerner D, Uppenkamp M. Elimination of intravenously administered ibandronate in patients on haemodialysis: A monocentre open study. Nephrol Dial Transplant. 2002;17(7):1281‐1285. [DOI] [PubMed] [Google Scholar]
- 33. Buttazzoni M, Rosa Diez GJ, Jager V, Crucelegui MS, Algranati SL, Plantalech L. Elimination and clearance of pamidronate by haemodialysis. Nephrol Ther. 2006;11(3):197‐200. 10.1111/j.1440-1797.2006.00569.x [DOI] [PubMed] [Google Scholar]
- 34. Beigel AE, Rienhoff E, Olbricht CJ. Removal of clodronate by haemodialysis in end‐stage renal disease patients. Nephrol Dial Transplant. 1995;10(12):2266‐2268. [DOI] [PubMed] [Google Scholar]
- 35. Miller PD. Is there a role for bisphosphonates in chronic kidney disease? Semin Dial. 2007;20(3):186‐190. 10.1111/j.1525-139X.2007.00271.x [DOI] [PubMed] [Google Scholar]
- 36. Masarachia P, Weinreb M, Balena R, Rodan GA. Comparison of the distribution of 3H‐alendronate and 3H‐etidronate in rat and mouse bones. Bone. 1996;19(3):281‐290. [DOI] [PubMed] [Google Scholar]
- 37. Azuma Y, Sato H, Oue Y, et al. Alendronate distributed on bone surfaces inhibits osteoclastic bone resorption in vitro and in experimental hypercalcemia models. Bone. 1995;16(2):235‐245. [DOI] [PubMed] [Google Scholar]
- 38. Weiss HM, Pfaar U, Schweitzer A, Wiegand H, Skerjanec A, Schran H. Biodistribution and plasma protein binding of zoledronic acid. Drug Metabol Dispos. 2008;36(10):2043‐2049. 10.1124/dmd.108.021071 [DOI] [PubMed] [Google Scholar]
- 39. Fogelman I, Bessent RG, Turner JG, Citrin DL, Boyle IT, Greig WR. The use of whole‐body retention of Tc‐99m diphosphonate in the diagnosis of metabolic bone disease. J Nucl Med. 1978;19(3):270‐275. [PubMed] [Google Scholar]
- 40. Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology clinical practice guidelines: The role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20(17):3719‐3736. 10.1200/JCO.2002.06.037 [DOI] [PubMed] [Google Scholar]
- 41. Nancollas GH, Tang R, Phipps RJ, et al. Novel insights into actions of bisphosphonates on bone: Differences in interactions with hydroxyapatite. Bone. 2006;38(5):617‐627. 10.1016/j.bone.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 42. Roelofs AJ, Stewart CA, Sun S, et al. Influence of bone affinity on the skeletal distribution of fluorescently labeled bisphosphonates in vivo . J Bone Miner Res. 2012;27(4):835‐847. 10.1002/jbmr.1543 [DOI] [PubMed] [Google Scholar]
- 43. Dunford JE, Kwaasi AA, Rogers MJ, et al. Structure‐activity relationships among the nitrogen containing bisphosphonates in clinical use and other analogues: Time‐dependent inhibition of human farnesyl pyrophosphate synthase. J Med Chem. 2008;51(7):2187‐2195. 10.1021/jm7015733 [DOI] [PubMed] [Google Scholar]
- 44. Rosen CJ, Hochberg MC, Bonnick SL, et al. Treatment with once‐weekly alendronate 70 mg compared with once‐weekly risedronate 35 mg in women with postmenopausal osteoporosis: A randomized double‐blind study. J Bone Miner Res. 2005;20(1):141‐151. 10.1359/JBMR.040920 [DOI] [PubMed] [Google Scholar]
- 45. Khan SA, Kanis JA, Vasikaran S, et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res. 1997;12(10):1700‐1707. 10.1359/jbmr.1997.12.10.1700 [DOI] [PubMed] [Google Scholar]
- 46. Cremers SC, Eekhoff MEMW, den Hartigh J, Hamdy NAT, Vermeij P, Papapoulos SE. Relationships between pharmacokinetics and rate of bone turnover after intravenous bisphosphonate (olpadronate) in patients with Paget's disease of bone. J Bone Miner Res. 2003;18(5):868‐875. 10.1359/jbmr.2003.18.5.868 [DOI] [PubMed] [Google Scholar]
- 47. Cremers SC, Papapoulos SE, Gelderblom H, et al. Skeletal retention of bisphosphonate (pamidronate) and its relation to the rate of bone resorption in patients with breast cancer and bone metastases. J Bone Miner Res. 2005;20(9):1543‐1547. 10.1359/JBMR.050522 [DOI] [PubMed] [Google Scholar]
- 48. Pillai G, Gieschke R, Goggin T, Jacqmin P, Schimmer RC, Steimer JL. A semimechanistic and mechanistic population PK‐PD model for biomarker response to ibandronate, a new bisphosphonate for the treatment of osteoporosis. Br J Clin Pharmacol. 2004;58(6):618‐631. 10.1111/j.1365-2125.2004.02224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen T, Berenson J, Vescio R, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42(11):1228‐1236. [DOI] [PubMed] [Google Scholar]
- 50. Coxon FP, Thompson K, Roelofs AJ, Ebetino FH, Rogers MJ. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non‐resorbing cells. Bone. 2008;42(5):848‐860. 10.1016/j.bone.2007.12.225 [DOI] [PubMed] [Google Scholar]
- 51. Coxon FPT, Thompson K, Hughes A, Ebetino FH, Rogers MJ. Resorbing osteoclasts increase the availability of mineral‐bound bisphosphonates to non‐resorbing cells. ASBMR Meeting 2005, Poster, 2005.
- 52. Luckman SP, Hughes DE, Coxon FP, Russell RGG, Rogers MJ. Nitrogen‐containing bisphosphonates inhibit the mevalonate pathway and prevent post‐translational prenylation of GTP‐binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581‐589. 10.1359/jbmr.1998.13.4.581 [DOI] [PubMed] [Google Scholar]
- 53. van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen‐containing bisphosphonates. Biochem Biophys Res Commun. 1999;264(1):108‐111. 10.1006/bbrc.1999.1499 [DOI] [PubMed] [Google Scholar]
- 54. van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Nitrogen‐containing bisphosphonates inhibit isopentenyl pyrophosphate isomerase/farnesyl pyrophosphate synthase activity with relative potencies corresponding to their antiresorptive potencies in vitro and in vivo . Biochem Biophys Res Commun. 1999;255(2):491‐494. 10.1006/bbrc.1999.0224 [DOI] [PubMed] [Google Scholar]
- 55. Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9(32):2643‐2658. [DOI] [PubMed] [Google Scholar]
- 56. Papapoulos SE, Cremers SC. Prolonged bisphosphonate release after treatment in children. N Engl J Med. 2007;356(10):1075‐1076. 10.1056/NEJMc062792 [DOI] [PubMed] [Google Scholar]
- 57. Peris P, Torra M, Olivares V, et al. Prolonged bisphosphonate release after treatment in women with osteoporosis: Relationship with bone turnover. Bone. 2011;49(4):706‐709. 10.1016/j.bone.2011.06.027 [DOI] [PubMed] [Google Scholar]
- 58. Kasting GB, Francis MD. Retention of etidronate in human, dog, and rat. J Bone Miner Res. 1992;7(5):513‐522. 10.1002/jbmr.5650070507 [DOI] [PubMed] [Google Scholar]
- 59. Naylor KE, Bradburn M, Paggiosi MA, et al. Effects of discontinuing oral bisphosphonate treatments for postmenopausal osteoporosis on bone turnover markers and bone density. Osteoporos Int. 2018;29(6):1407‐1417. 10.1007/s00198-018-4460-6 [DOI] [PubMed] [Google Scholar]
- 60. Phipps R, Lindsay R, Burgio D, et al. Head‐to‐head comparison of risedronate and alendronate pharmacokinetics at clinical doses. Bone. 2004;34:S81‐S82. [Google Scholar]
- 61. van Beek ER, Lowik CW, Ebetino FH, Papapoulos SE. Binding and antiresorptive properties of heterocycle‐containing bisphosphonate analogs: Structure‐activity relationships. Bone. 1998;23(5):437‐442. [DOI] [PubMed] [Google Scholar]
- 62. Kavanagh KL, Guo K, Dunford JE, et al. The molecular mechanism of nitrogen‐containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci U S A. 2006;103(20):7829‐7834. 10.1073/pnas.0601643103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lawson MA, Ebetino FH, Mazur A, et al. The pharmacological profile of a novel highly potent bisphosphonate, OX14 (1‐fluoro‐2‐(imidazo‐[1,2‐alpha]pyridin‐3‐yl)‐ethyl‐bisphosphonate). J Bone Miner Res. 2017;32(9):1860‐1869. 10.1002/jbmr.3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone. 2011;49(1):50‐55. 10.1016/j.bone.2010.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti‐apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo . J Bone Miner Res. 2008;23(11):1712‐1721. 10.1359/jbmr.080617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mathew A, Brufsky A. Bisphosphonates in breast cancer. Int J Cancer. 2015;137(4):753‐764. 10.1002/ijc.28965 [DOI] [PubMed] [Google Scholar]
- 67. Boissier S, Ferreras M, Peyruchaud O, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60(11):2949‐2954. [PubMed] [Google Scholar]
- 68. Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: Evidence for synergy with paclitaxel. Br J Cancer. 2001;84(8):1126‐1134. 10.1054/bjoc.2001.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Coleman R, Cameron D, Dodwell D, et al. Adjuvant zoledronic acid in patients with early breast cancer: Final efficacy analysis of the AZURE (BIG 01/04) randomised open‐label phase 3 trial. Lancet Oncol. 2014;15(9):997‐1006. 10.1016/S1470-2045(14)70302-X [DOI] [PubMed] [Google Scholar]
- 70. Dionísio MR, Mansinho A, Abreu C, Cavaco‐Silva J, Casimiro S, Costa L. Clinical and translational pharmacology of drugs for the prevention and treatment of bone metastases and cancer‐induced bone loss. Br J Clin Pharmacol. 2019;85(6):1114‐1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chukir T, Liu Y, Farooki A. Antiresorptive agents' bone‐protective and adjuvant effects in postmenopausal women with early breast cancer. Br J Clin Pharmacol. 2019;85(6):1125‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fragni M, Bonini SA, Bettinsoli P, et al. The miR‐21/PTEN/Akt signaling pathway is involved in the anti‐tumoral effects of zoledronic acid in human breast cancer cell lines. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(5):529‐538. 10.1007/s00210-016-1224-8 [DOI] [PubMed] [Google Scholar]
- 73. Wilson C, Ottewell P, Coleman RE, Holen I. The differential anti‐tumour effects of zoledronic acid in breast cancer—Evidence for a role of the activin signaling pathway. BMC Cancer. 2015;15(1):55 10.1186/s12885-015-1066-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li Q, Kingman J, Sundberg JP, Levine MA, Uitto J. Etidronate prevents, but does not reverse, ectopic mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(−/−)). Oncotarget. 2018;9(56):30721‐30730. 10.18632/oncotarget.10738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jilka RL. The relevance of mouse models for investigating age‐related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1209‐1217. 10.1093/gerona/glt046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adami S, Salvagno G, Guarrera G, et al. Treatment of Paget's disease of bone with intravenous 4‐amino‐1‐hydroxybutylidene‐1,1‐bisphosphonate. Calcif Tissue Int. 1986;39(4):226‐229. [DOI] [PubMed] [Google Scholar]
- 77. Harinck HI, Papapoulos SE, Blanksma HJ, Moolenaar AJ, Vermeij P, Bijvoet OL. Paget's disease of bone: Early and late responses to three different modes of treatment with aminohydroxypropylidene bisphosphonate (APD). Br Med J. 1987;295(6609):1301‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Body JJ, Lortholary A, Romieu G, Vigneron AM, Ford J. A dose‐finding study of zoledronate in hypercalcemic cancer patients. J Bone Miner Res. 1999;14(9):1557‐1561. 10.1359/jbmr.1999.14.9.1557 [DOI] [PubMed] [Google Scholar]
- 79. Berenson JR, Vescio R, Henick K, et al. A phase I, open label, dose ranging trial of intravenous bolus zoledronic acid, a novel bisphosphonate, in cancer patients with metastatic bone disease. Cancer. 2001;91(1):144‐154. [DOI] [PubMed] [Google Scholar]
- 80. Papapoulos SE, Landman JO, Bijvoet OLM, et al. The use of bisphosphonates in the treatment of osteoporosis. Bone. 1992;13(Suppl 1):S41‐S49. [DOI] [PubMed] [Google Scholar]
- 81. Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J. Serum markers of type I collagen formation and degradation in metabolic bone disease: Correlation with bone histomorphometry. J Bone Miner Res. 1993;8(2):127‐132. 10.1002/jbmr.5650080202 [DOI] [PubMed] [Google Scholar]
- 82. Rogers A, Hannon RA, Eastell R. Biochemical markers as predictors of rates of bone loss after menopause. J Bone Miner Res. 2000;15(7):1398‐1404. 10.1359/jbmr.2000.15.7.1398 [DOI] [PubMed] [Google Scholar]
- 83. Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: The EPIDOS Prospective Study. J Bone Miner Res. 1996;11(10):1531‐1538. 10.1002/jbmr.5650111021 [DOI] [PubMed] [Google Scholar]
- 84. Cremers S, Garnero P. Biochemical markers of bone turnover in the clinical development of drugs for osteoporosis and metastatic bone disease: Potential uses and pitfalls. Drugs. 2006;66(16):2031‐2058. 10.2165/00003495-200666160-00001 [DOI] [PubMed] [Google Scholar]
- 85. Bauer DC, Garnero P, Harrison SL, et al. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: The MrOS study. J Bone Miner Res. 2009;24(12):2032‐2038. 10.1359/jbmr.090526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Leeming DJ, Alexandersen P, Karsdal MA, Qvist P, Schaller S, Tankó LB. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol. 2006;62(10):781‐792. 10.1007/s00228-006-0174-3 [DOI] [PubMed] [Google Scholar]
- 87. Brown JP, Kendler DL, McClung MR, et al. The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int. 2002;71(2):103‐111. 10.1007/s00223-002-2011-8 [DOI] [PubMed] [Google Scholar]
- 88. Schnitzer T, Bone HG, Crepaldi G, et al. Therapeutic equivalence of alendronate 70 mg once‐weekly and alendronate 10 mg daily in the treatment of osteoporosis. Aging. 2000;12(1):1‐12. [PubMed] [Google Scholar]
- 89. Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1‐year results from the MOBILE study. J Bone Miner Res. 2005;20(8):1315‐1322. 10.1359/JBMR.050313 [DOI] [PubMed] [Google Scholar]
- 90. Peisker A, Raschke GF, Fahmy MD, et al. Cross‐sectional study of four serological bone turnover markers for the risk assessment of medication‐related osteonecrosis of the jaw. J Craniofac Surg. 2018;29:e137‐e140. 10.1097/SCS.0000000000004224 [DOI] [PubMed] [Google Scholar]
- 91. Cremers S, Farooki A. Biochemical markers of bone turnover in osteonecrosis of the jaw in patients with osteoporosis and advanced cancer involving the bone. Ann N Y Acad Sci. 2011;1218(1):80‐87. 10.1111/j.1749-6632.2010.05770.x [DOI] [PubMed] [Google Scholar]
- 92. Thumbigere‐Math V, Michalowicz BS, Hughes PJ, et al. Serum markers of bone turnover and angiogenesis in patients with bisphosphonate‐related osteonecrosis of the jaw after discontinuation of long‐term intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2016;74(4):738‐746. 10.1016/j.joms.2015.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chiha M, Myers LE, Ball CA, Sinacore JM, Camacho PM. Long‐term follow‐up of patients on drug holiday from bisphosphonates: Real‐world setting. Endocr Pract. 2013;19(6):989‐994. 10.4158/EP12425.OR [DOI] [PubMed] [Google Scholar]
- 94. Kong SY, Kim DY, Han EJ, et al. Effects of a “drug holiday” on bone mineral density and bone turnover marker during bisphosphonate therapy. J Bone Metab. 2013;20(1):31‐35. 10.11005/jbm.2013.20.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Roberts J, Castro C, Moore AE, Fogelman I, Hampson G. Changes in bone mineral density and bone turnover in patients on “drug holiday” following bisphosphonate therapy: Real‐life clinic setting. Clin Endocrinol (Oxf). 2016;84(4):509‐515. 10.1111/cen.13012 [DOI] [PubMed] [Google Scholar]
- 96. Liel Y, Plakht Y, Tailakh MA. Bone turnover in osteoporotic women during long‐term oral bisphosphonates treatment: Implications for treatment failure and “drug holiday” in the real world. Endocr Pract. 2017;23(7):787‐793. 10.4158/EP171781.OR [DOI] [PubMed] [Google Scholar]
- 97. Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: Anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res. 2017;32(2):198‐202. 10.1002/jbmr.3051 [DOI] [PubMed] [Google Scholar]
- 98. Lespessailles E, Hambli R, Ferrari S. Osteoporosis drug effects on cortical and trabecular bone microstructure: A review of HR‐pQCT analyses. Bonekey Rep. 2016;5:836 10.1038/bonekey.2016.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Post TM, Cremers SC, Kerbusch T, Danhof M. Bone physiology, disease and treatment: Towards disease system analysis in osteoporosis. Clin Pharmacokinet. 2010;49(2):89‐118. 10.2165/11318150-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 100. Berkhout J, Stone JA, Verhamme KM, Danhof M, Post TM. Disease systems analysis of bone mineral density and bone turnover markers in response to alendronate, placebo, and washout in postmenopausal women. CPT: Pharmacometrics Syst Pharmacol. 2016;5(12):656‐664. 10.1002/psp4.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schmidt S, Post TM, Peletier LA, Boroujerdi MA, Danhof M. Coping with time scales in disease systems analysis: Application to bone remodeling. J Pharmacokinet Pharmacodyn. 2011;38(6):873‐900. 10.1007/s10928-011-9224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Peterson MC, Riggs MM. A physiologically based mathematical model of integrated calcium homeostasis and bone remodeling. Bone. 2010;46(1):49‐63. 10.1016/j.bone.2009.08.053 [DOI] [PubMed] [Google Scholar]
- 103. Riggs MM, Peterson MC, Gastonguay MR. Multiscale physiology‐based modeling of mineral bone disorder in patients with impaired kidney function. J Clin Pharmacol. 2012;52(S1):45S‐53S. 10.1177/0091270011412967 [DOI] [PubMed] [Google Scholar]
- 104. Peterson MC, Riggs MM. Predicting nonlinear changes in bone mineral density over time using a multiscale systems pharmacology model. CPT: Pharmacometrics Syst Pharmacol. 2012;1:e14 10.1038/psp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Eudy‐Byrne RJ, Gillespie W, Riggs MM, Gastonguay MR. A model of fracture risk used to examine the link between bone mineral density and the impact of different therapeutic mechanisms on fracture outcomes in patients with osteoporosis. J Pharmacokinet Pharmacodyn. 2017;44(6):599‐609. 10.1007/s10928-017-9551-z [DOI] [PubMed] [Google Scholar]
- 106. Lyles KW, Colón‐Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. New Engl J Med. 2007;357(18):1799‐1809. 10.1056/NEJMoa074941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Reid IRH, Horne AM, Mihov B, et al. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018;379:2407‐2416. 10.1056/NEJMoa1808082 [DOI] [PubMed] [Google Scholar]
- 108. Wolfe F, Bolster MB, O'Connor CM, Michaud K, Lyles KW, Colón‐Emeric CS. Bisphosphonate use is associated with reduced risk of myocardial infarction in patients with rheumatoid arthritis. J Bone Miner Res. 2013;28(5):984‐991. 10.1002/jbmr.1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Varela I, Pereira S, Ugalde AP, et al. Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging. Nat Med. 2008;14(7):767‐772. 10.1038/nm1786 [DOI] [PubMed] [Google Scholar]
- 110. Gordon LB, Massaro J, D'Agostino RB Sr, et al. Impact of farnesylation inhibitors on survival in Hutchinson‐Gilford progeria syndrome. Circulation. 2014;130(1):27‐34. 10.1161/CIRCULATIONAHA.113.008285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Misra J, Mohanty ST, Madan S, et al. Zoledronate attenuates accumulation of DNA damage in mesenchymal stem cells and protects their function. Stem Cells. 2016;34(3):756‐767. 10.1002/stem.2255 [DOI] [PMC free article] [PubMed] [Google Scholar]


