Abstract
Aims
A population kinetic model was developed for the body fluid shifts occurring when 20% albumin is given by intravenous infusion. The aim was to study whether its efficacy to expand the plasma volume is impaired after major surgery.
Methods
An intravenous infusion of 3 mL/kg 20% albumin over 30 minutes was given to 15 volunteers and to 15 patients on the 1st day after major open abdominal surgery. Blood samples and urine were collected during 5 hours. Mixed‐effect modelling software was used to develop a fluid volume kinetic model, using blood haemoglobin and urine excretion the estimate body fluid shifts, to which individual‐specific covariates were added in sequence.
Results
The rise in plasma albumin expanded the plasma volume in excess of the infused volume by relocating noncirculating fluid (rate constant k 21), but it also increased losses of fluid from the kinetic system (k b). The balance between k 21 and k b maintained the rise in plasma albumin and plasma volume at a virtual steady‐state for almost 2 hours. The rate constant for urinary excretion (k 10) was slightly reduced by the preceding surgery, by a marked rise in plasma albumin, and by a high preinfusion urinary concentration of creatinine. The arterial pressure, body weight, and plasma concentrations of C‐reactive protein and shedding products of the endothelial glycocalyx layer (syndecan‐1, heparan sulfate, and hyaluronic acid) did not serve as statistically significant covariates.
Conclusions
There were no clinically relevant differences in the kinetics of 20% albumin between postoperative patients and volunteers.
Keywords: albumin, fluid kinetics, heparan sulfate, hyperoncotic, syndecan‐1
What is already known about this subject
Intravenous infusions of 5% and 20% albumin have long been used for plasma volume support in clinical practice.
No kinetic model exists for the body fluid shifts occurring from infusion of 20% albumin.
The induced plasma volume expansion is believed to be impaired by trauma and inflammation.
What this study adds
A population kinetic model for the intravenous use of 20% albumin was developed.
Only minor differences in kinetics were found between volunteers and patients who had undergone major abdominal surgery.
No effect on the kinetics was found from arterial pressure, markers of inflammation or endothelial shedding products.
1. INTRODUCTION
The use of albumin as a plasma volume expander in surgery and intensive care has undergone a revival in recent years. Albumin does not have the adverse effects on kidney function that occur with artificial colloids in septic patients,1 and it also has a much‐appreciated scavenger effect.2 However, many questions remain regarding its use.
First, the kinetics of crystalloid fluid depends on age, mean arterial pressure,3 rate of infusion4 and sex,5 but the relevance of these factors to the kinetics of colloid fluids is not known. Second, the revised Starling equation questions the ability of hyperoncotic albumin to recruit interstitial fluid to the plasma.6 Third, the transcapillary permeability for albumin is increased by injury to the endothelial glycocalyx layer, which occurs in response to ischaemia, inflammation and hypervolaemia.7, 8 Such injury, of shedding, is then likely to shorten the duration of the plasma volume expansion from albumin,9 because capillary leakage of albumin governs the intravascular persistence of the infused fluid volume.10 However, the extent of the effects of inflammation and shedding on the clinical efficacy of albumin is unknown.
In the present report, we developed a population kinetic model for fluid shifts to examine the body's handling of the 20% albumin. The hypothesis was that the plasma volume expansion is poorer and its duration shorter after major surgery than in healthy volunteers, particularly in subjects with biochemical evidence of shedding.
2. METHODS
This study was based on 30 strictly controlled infusion experiments, 15 in volunteers and 15 in postoperative patients, which were part of a larger effort to evaluate the clinical use of 20% albumin.
2.1. Ethics approval and consent to participate
The protocol was approved by the Regional Ethics Committee of Stockholm on April 28, 2015 (Application 2014/2146–31/1, officer in charge Pierre Lafolie) and by the Medical Products Agency in Uppsala, Sweden (Dnr 5.1–2015 − 21356). Registration of the study was done with Eudra CT number 2016–001135‐20 on April 28, 2015, as well as at ClinicalTrials.gov as NCT02556580 on September 22, 2015.
All subjects gave their approval for participation after being informed about the study's purpose.
A third study arm comprising the use of 20% albumin during surgery was not performed due to current limited availability of lengthy operations with minor blood loss in our hospitals.
2.2. Volunteers
Healthy volunteers were studied between September and December 2016 at Linköping University Hospital in Linköping, Sweden. The participants arrived at the hospital ward between 07.00 and 09.00. They had fasted from midnight except for 1 sandwich and 1 glass (2 dL) of clear liquid ingested 1.5 hours prior to their arrival at the ward. Volunteers rested supine on a bed for 30 minutes and also voided before the experiment began. A cannula was placed in the cubital vein of each arm, 1 for infusion of albumin and the other for blood sampling. The participants remained in the supine position and their bodies were covered in blankets to ensure good thermal comfort. The arm used for blood sampling was placed on a body‐warm heating pad. The systolic, diastolic and mean arterial pressures were measured with a stethoscope and a blood pressure cuff at 0, 60 and 300 minutes. The volunteers voided freely, lying on the lateral supine position, and the volumes were summarized at the end of the experiment.
2.3. Patients
The patients were studied between February and November 2016 at Karolinska University Hospital in Solna, Sweden. The infusion study began at 07.00 on the morning after elective major abdominal surgery, which had lasted for 5.9 ± 1.6 hours. The perioperative blood loss was 700 mL (median, interquartile range 363–800 mL) and the perioperative fluid therapy had been managed by a goal‐directed protocol based on measurements of cardiac stroke volume with CardioQ oesophageal Doppler equipment (Deltex Medical, Chichester, UK). All efforts had been made to maintain normovolaemia during the night before the experiments, and no colloid fluid was infused within 6 hours prior to the study.
All patients lay supine in their beds during the experiments. Blood sampling was made via an arterial catheter that had been placed for clinical purposes on the day of the surgery. The arterial pressures were measured with an invasive haemodynamic monitor (Datex Ohmeda S/5, Helsinki, Finland) at the time of each blood sampling, and the urine was collected via an indwelling catheter. Each patient had ongoing epidural analgesia with a constant‐rate infusion of ropivacaine 2 mg/mL and sufentanil 0.5 μg/mL, as well as a slow intravenous infusion of buffered glucose 25 mg/mL to prevent hypoglycaemia.
2.4. Biochemical analyses
Each subject received 3 mL/kg of 20% albumin (sodium concentration 140–160 mmol/L) delivered at a constant rate over 30 minutes with an infusion pump. Blood (9 mL) was drawn at 0, 10, 20, 30, 40, 50, 60, 75, 90, 120, 150, 180, 210, 240 and 300 minutes after the start of the albumin infusion. Urine samples were taken for analysis of creatinine and osmolality at time 0, 60 and 300 minutes.
The blood haemoglobin (Hb) and plasma albumin concentrations were analysed in the hospital's central laboratory with a coefficient of variation (CV%) of 0.8%, based on duplicate samples taken at baseline.
The plasma colloid osmotic pressure (COP) was analysed at the burn care unit at Linköping University Hospital on an Osmomat 050 instrument (Gonotec, Berlin) with a CV% of 1.9%, as given by our duplicate samples.
The plasma concentrations of shedding products from the glycocalyx layer (syndecan‐1, heparan sulfate and hyaluronic acid) were measured on blood samples collected at 0, 60, and 300 minutes at the Research Unit at Södertälje Hospital. Commercially available ELISA kits were used. Details are given elsewhere.11 COP and the shedding products were analysed twice on each sample and the mean used in further calculations.
2.5. Volume kinetics
Our analysis of 20% albumin was based on fluid volume kinetics, which lacks an easily grasped concentration concept with which to work as almost all the infused fluid, and also 80–85% of the blood, consist of water. The haemodilution used here is the inverse of any rise in blood water concentration, so volume kinetics analyse the body fluid volumes that equilibrate more or less instantly with the infused fluid (here called V c). The analysis has only a few requirements, and the reported results then rest mainly on the assumptions made in the model. One basic requirement is a lack of capillary leakage of Hb molecules and an overall even distribution of water volumes in V c during study.
2.6. Base model
Population (mixed effects) kinetics include the traditional fixed model parameters used in pharmacokinetics, but adds submodels for the individual‐specific variations in these components. For the traditional components, we applied a 1‐volume kinetic model that has been used previously for colloid fluid; the volume infused into the plasma (V c) is irreversibly eliminated (k 10, rate constant) in proportion to the expansion of V c. 12 The differential equation is:
| (1) |
where v c is the expanded volume of V c.
The Hb‐derived fractional plasma dilution was used to indicate the expansion of V c resulting from the infusion, thereby serving as a dependent variable in the calculations. The fractional dilution provides a linear relationship between the added fluid volume and the concentration of a marker in an expandable fluid space.13 Hence:
| (2) |
Symbols in capital letters denote baseline values, and Hct is the haematocrit. A minor correction was made for the effects of blood sampling on the plasma dilution.13
These fixed model parameters (V c and k 10) were estimated simultaneously for all 30 experiments using the Phoenix software for nonlinear mixed effects, version 8.0 (Pharsight, St. Louis, MO, USA) and the additive model for the within‐subject variability. The urine volume was used as a second dependent variable whenever measured, which was an average of 2.6 times per experiment. We stabilized the kinetic model by setting k 10 to equal the urinary excretion divided by the area under the volume–time curve.
The naive pooled search routine was used to find starting estimates for the parameters in this so‐called base model. The estimates were made more precise by applying the first order conditional estimation of Lindstrom–Bates and the more slowly working, but usually better, extended least‐squares search routine. The influence of modifications in the base model was tested sequentially, as guided by a reduction of the residual error (−2 LL = log likelihood) for the curve‐fit by >3.8 points, which represents P < .05 (> 6.6 points represents P < .01).
2.7. Covariate analysis
The base model was then refined to become the full model by adding individual‐specific covariates to the fixed parameters. The search for covariates was performed in a sequential fashion and guided by plots of random effects (eta plots). The decision to include a covariate was based on a statistically significant reduction of the −2 LL for the curve‐fit, whereby its confidence interval (CI) should not include 1.0, and that the interindividual variability should be <50%.
Sixteen potential covariates were examined. Age, body weight, body mass index and serum creatinine were entered as a continuous covariate once for each subject. Whether the subject was a volunteer or postoperative patient was entered as a categorical covariate.
The following parameters were entered 15 times for each subject, and served as time‐varying covariates: plasma albumin concentration; change in plasma albumin from baseline; amount of infused albumin; COP (volunteers), and the 3 arterial pressures (patients).
The following parameters were entered 3 times per subject (at 0, 60 and 300 min): arterial pressures (volunteers) and the plasma concentrations of syndecan‐1, hyaluronic acid, heparan sulfate and C‐reactive protein, as well as the COP (patients) and the change in COP from baseline.
The finally reported full block model considers correlations between these random effects, which yields more realistic data for simulation purposes.
2.8. Model performance
The goodness‐of‐fit of the model was illustrated by comparing the measured with the predicted plasma dilutions, without and with inclusion of covariates.
The performance of the model was studied by predictive checks based on 1000 simulations using a built‐in function in the Phoenix nonlinear mixed effects software. Close agreement between the percentiles for the predictive check (predicted) and the original data (observed) is evidence of good model performance and indicates that the model is robust.
2.9. Simulations and statistics
Computer simulations using MATLAB R2013b (Math Works Inc., Natick, MA) were used to illustrate the influence of the covariates on the flow of fluid in and out from the central body fluid space (V c). The best estimates of the model parameters and their covariates were entered into the solutions (solved by optODE) to the differential equations describing the kinetic model.
The results are reported as the mean (95% CI). Changes in haemodynamic parameters during the experiments were assessed by repeated‐measures ANOVA and the paired t test. Correlations between parameters were studied by simple linear regression, where r = correlation coefficient. P < .05 was considered significant.
A quantification of the recruited fluid volumes from extravascular and intracellular spaces at 300 minutes of the volunteer experiments has recently been reported elsewhere.13
3. RESULTS
The kinetic analysis comprised 447 data points collected from the 30 subjects. Six volunteers (40%) and 13 patients (87%) were females. The mean age was 31 (24–38) years for the volunteers and 65 (58–73) years for the patients (P < .001), but their body weights were similar, at 76 (69–83) and 73 (65–81) kg, respectively. All data are shown in the Supporting Information.
3.1. Base model
The curve‐fit was improved by separating the elimination from the plasma (V c) into urinary excretion (k 10) and capillary leakage (k b, evaporation included).
The delayed peak in plasma dilution was more adequately captured when adding an absorptive flow (recruitment, k 21) to the plasma (V c).
The finally developed base model could then be expressed by the following differential equation (Figure 1A):
| (3) |
where the measured urine volume equals k 10 (v c – V c) and 0.15 BW is 15% of the body weight, which is an estimate of the interstitial fluid volume.
Figure 1.

A, Schematic drawing of the finally developed base model. B, the measured plasma dilution in all 30 subjects (green = volunteers, red = postoperative, open circles = females, stars = males) and the modelled curve according to the base model (thick line)
Figure 1 shows the curve‐fit derived by the parameters in the base model alone. The rate constants k 21 and k b were found to correlate closely (r = 0.93; P < 001).
3.2. Covariate analysis
Improvement of the curve‐fit by inclusion of individual‐specific factors (covariates) was tested by adding each in sequence to arrive at a final (full) model. Figure 2 shows the distribution of some parameters that were considered for this purpose.
Figure 2.
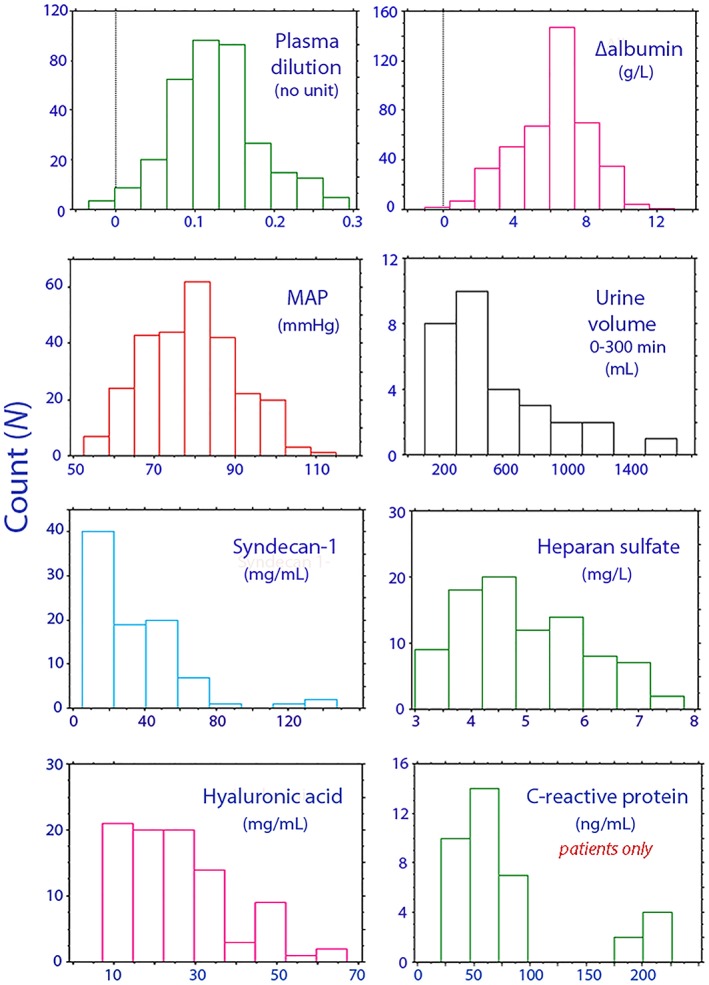
Distribution of key variables in the kinetic analysis. The plot of C‐reactive protein shows data for postoperative patients only, as the volunteers had very low concentrations
The change in serum albumin from baseline (∆alb) improved the curve‐fit when used as a co‐variate to k 10, k b, and k 21. The rate constant for the urinary excretion (k 10) was also affected by renal fluid conservation when the infusion started (expressed as a high U‐creatinine), and by the postoperative setting per se. How the precision of the model improved by adding these covariates is shown in Table 1.
Table 1.
Key features of the search protocol used to find the final population kinetic model
| Optimization routine | Added covariate | Target parameter | LL | ‐2(LL) | AIC |
|---|---|---|---|---|---|
| Base model | |||||
| FOCE LB | 396 | −791 | −775 | ||
| FOCE ELS | 396 | −794 | −744 | ||
| Covariates | |||||
| FOCE ELS | 415 | −830 | −810 | ||
| ∆ alb | k 10 | 418 | −837 | −815 | |
| ∆ alb | k 21 and k b | 428 | −856 | −830 | |
| U‐creatinine | k 10 | 433 | −866 | −838 | |
| Postoperative | k 10 | 437 | −873 | −843 | |
| Full block model | All above | 475 | −951 | −909 | |
AIC = Akaike information criterion; FOCE ELS = forward conditional extended least squares search routine; FOCE LB = Lindstrom–Bates search routine; LL = log likelihood.
Figure 3 shows how much better the kinetic model could predict the 2 dependent variables (plasma dilution and the urinary excretion) when covariates were considered.
Figure 3.
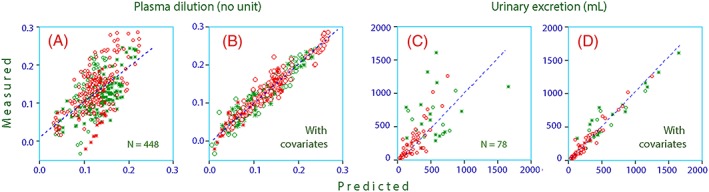
Predicted vs measured plasma dilution for the population (30 patients) without A, and with B, covariates. Predicted vs measured urinary excretion without C, and with D, covariates. Green = volunteers, red = postoperative, open circles = females, stars = males
The parameters that were affected by covariates had the following form (Table 2):
| (4) |
| (5) |
| (6) |
where the mean ∆alb was 5.73 g/L and mean U‐creatinine was 11.1 mmol/L.
Table 2.
Population kinetic parameters in the final model
| Covariate | Covariate model | Best estimate | 2.5% CI | 97.5% CI | CV% | |
|---|---|---|---|---|---|---|
| Kinetic parameter | ||||||
| tvVc (mL) | ‐ | 2,924 | 2,139 | 3,710 | 13.7 | |
| tvk 10 (10−3 min−1) | ‐ | 6.9 | 4.9 | 8.9 | 14.9 | |
| tvk b (10−3 min−1) | ‐ | 30.1 | 21.7 | 38.6 | 14.3 | |
| tvk 21 (10−4 min−1) | ‐ | 15.4 | 9.7 | 21.1 | 18.9 | |
| Covariate effects | ||||||
| tvk 10 | ∆Alb | Linear | −0.12 | −0.23 | −0.01 | −48.3 |
| tvk b | ∆Alb | Linear | 0.14 | 0.10 | 0.18 | 15.2 |
| tvk 21 | ∆Alb | Linear | 0.12 | 0.09 | 0.15 | 13.6 |
| tvk 10 | U‐creatinine | Power | −0.56 | −0.71 | −0.40 | −13.8 |
| tvk 10 | Postoperative | Exponential | −0.64 | −1.09 | −0.19 | −35.5 |
CI = confidence interval; CV = coefficient of variation (interindividual); tv = typical value.
Note that a high ∆alb increased both the absorption (k 21) and the leakage of fluid from the plasma (k b) while the urinary excretion (k 10) decreased.
The curve‐fits with all covariates entered are plotted in Figure 4A.
Figure 4.
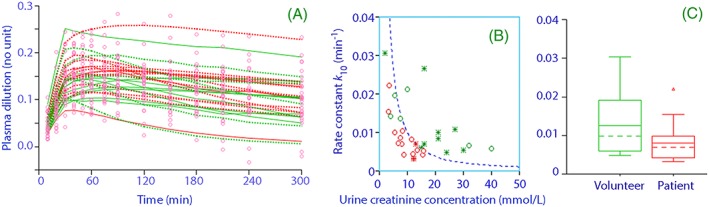
A, Measured plasma dilution in all 30 subjects (points) and the predicted dilution when the individual‐specific covariate effects have been considered (green = volunteer, red = postoperative, dotted line = females, continuous line = male). B, the covariate effect of the preinfusion urinary creatinine concentration on k 10 (green = volunteer, red = postoperative, open circle = female, star = male). C, Box‐plot showing the covariate effect of the preceding surgery on k 10
The distributions of 2 specific covariates are shown in Figures 4B and 4 C.
Certain covariates correlated closely with each other (Figure 5) but we included only the parameter that most effectively improved the precision of the curve‐fit.
Figure 5.
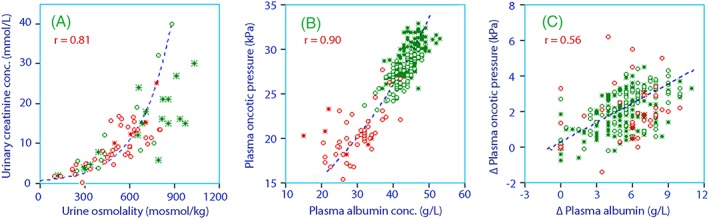
Correlation between A, urine osmolality and the urinary creatinine concentration. B, Plasma albumin and the colloid osmotic pressure C, and the changes in these parameters during the study. Green = volunteers, red = postoperative, open circles = females, stars = males
Factors that did not serve as statistically significant covariates to any of the fixed parameter in the base model (V c, k 10, k b, and k 21) included age, body weight, body mass index, sex, the arterial pressures, the plasma concentrations of C‐reactive protein, and the 3 shedding substances from breakdown of the endothelial glycocalyx layer.
The half‐life of fluid in the plasma (i.e. ln 2/(k b + k 10)) amounted to 41 (30–52) minutes in the volunteers and 73 (60–85) in the patients, but the plasma volume expansion lasted considerably longer due to the ongoing absorption of fluid via k 21 (Figure 4A).
3.3. Simulations and predictive check
Computer simulations were used to illustrate the effect of 20% albumin on the plasma volume expansion in volunteers vs. postoperative patients (Figure 6A) and high vs low preinfusion U‐creatinine level (Figure 6B).
Figure 6.
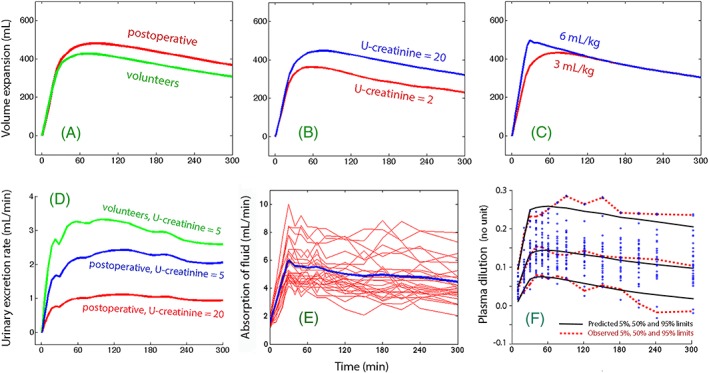
The effect of 20% albumin on the volume expansion of V c (the plasma) depending on A, whether the subjects were volunteers or postoperative patients. B, Urinary concentration of creatinine before the infusion started. C, Infusing the same amount of albumin but dispersed in different volumes of fluid. D, Combined effect of 2 covariates for k 10 on the predicted urinary excretion. E, Rate of absorption of fluid in all subjects (thin lines) and as the mean rate (thick line). F, Predictive check of the kinetic model. The parameter values shown in Table 2 were used for the simulations. In subplots A–D, 1 or 2 covariates values were changed as indicated
Increasing the infused volume had only a short‐lasting impact on the plasma volume expansion, as long as ∆alb was the same (Figure 6C).
The urinary excretion was more strongly affected than the plasma volume expansion by the postoperative setting and the U‐creatinine concentration (Figure 6D).
Absorption of fluid occurred throughout all experiments (Figure 6E).
A predictive check of the performance of the kinetic model is shown in Figure 6F.
4. DISCUSSION
A volume kinetic model was developed for 20% albumin that is applicable to both volunteers and postoperative patients. The 95% confidence interval for the observed plasma dilution was wide, but it was well predicted by the kinetic model (Figure 6F). Although some of the subjects still had very small or pronounced plasma dilution that could not be adequately accounted for (Figure 4A), the inclusion of the individual‐specific covariates resulted in marked improvement of the precision of the kinetic model (Figure 3).
The kinetics of 20% albumin in the 2 groups was strikingly similar, despite differences with regard to age, recent surgery and inflammatory status. The only feature that distinguished the patients was their slightly smaller urinary excretion, which had a limited spill‐over effect on the volume expansion (Figure 6 A). Contrary to our hypothesis, inflammatory markers and shedding products from the endothelial glycocalyx did not influence the rate of elimination of the infused fluid volume. Several parameters known to influence the kinetics of crystalloid fluids, such as age, sex and mean arterial pressure,3, 4, 5 also did not seem to be important.
A central issue in the kinetic analysis was the effect of the acute rise in plasma albumin on the flow of fluid in and out of the compartment with circulating plasma. In our model, the increase in plasma albumin that occurred after just 10 minutes of infusion increased the plasma volume expansion to twice the administered volume. This was explained by absorption of noncirculating fluid that, in turn, reduced plasma albumin by dilution and increased the capillary leakage of fluid. This circuit seemed to continue for several hours and resulted a virtual steady state with regard to plasma volume expansion. The kinetic analysis suggests that 1.5 L of fluid travelled to and from the circulating plasma during the 5‐hour study (Figure 6E). However, capillary leakage of albumin slowly reduced the albumin gradient, allowing the absorption of fluid to eventually subside and the plasma volume to decrease. The explanation is probably that plasma volume expansion increases the capillary leakage of albumin.14
Volume kinetics is a branch of pharmacokinetics where the distribution and elimination of infusion fluids is studied.15 Infusion of fluid volume increases the blood water concentration, which correlates inversely and very strongly with an accompanying decrease in blood Hb. For crystalloids, frequent measurement of the Hb concentration and urinary excretion clearly distinguishes distribution of the infused fluid between 2 body fluid compartments, which are likely to be the plasma and interstitial fluid volumes. For iso‐oncotic colloid fluid only 1 compartment has been used.12 The fluid shifts induced by hyperoncotic albumin are likely to be more complex and have not been modelled previously.
The kinetic model was built to reflect our macroscopic view of body physiology, and the results support that it was applicable for 20% albumin in both volunteers and postoperative patients. The 20% albumin was infused into the circulating blood that occupies an expandable body fluid space, V c. The size of V c was virtually identical to the anthropometric value of the plasma volume of 2.97 L,16 but it serves only as a technical conversion factor between plasma dilution and volume. Hence, dividing all data on plasma dilution (the input variable) by 2 would double V c but not change the values of the rate constants, whereby the predicted fluid distribution would remain the same. The blood volume is not important to the calculations, as haemodilution is determined by how water volumes distribute. The volume of the glycocalyx layer is included in the haemodilution if the blood water distributes there, regardless of whether plasma proteins and erythrocytes are excluded from that layer.
These rate constants are functional parameters aimed to represent absorption (k 21), filtration into tissues (k b), and urinary excretion (k 10). Absorption of fluid into the plasma can occur via the lymph and the glycocalyx layer, but also via fenestrated capillaries in the gastrointestinal tract and glands, although the latter pertains mostly to hydrostatic forces.6 Similarly, capillary filtration of fluid for further delivery to the lymphatics was captured by the rate constant k b, which attained high values.
The urinary excretion, expressed as k 10, did not correlate with the other flow rates, but was affected to a larger extent by individual‐specific covariates. The lower urinary excretion in the postoperative setting can be regarded as a summary effect of unmeasured factors associated with the surgery, including hormonal responses of stress and the fact that the patients received epidural analgesia for pain relief. As for crystalloids,17 renal fluid conservation detected prior to the infusions reduced subsequent urinary excretion.
Combining the covariance effects of fluid conservation shown in Figure 6D with an acute increase of plasma albumin illustrates how oliguria can develop when using hyperoncotic fluids in dehydrated patients.18, 19
The simulations in Figure 6C seem to support the old principle that the amount of infused albumin, rather than its concentration, determines the resulting plasma volume expansion.20 In contrast, the intravascular persistence time of the plasma volume expansion seems to be at least tripled when albumin is administered as a hyperoncotic preparation. The plasma volume decreased with a half‐life of only 2.5 hours in a study of 5% albumin, while plasma albumin attained a steady state rise of 4 g/L for at least 8 hours.10 This means that albumin leakage was followed by a matched capillary leakage of fluid.
In contrast, the balance between the flows governed by k 21 and k b seems to be essential for the degree and duration of the steady state volume expansion following infusion of 20% albumin, which could also be seen when 20% albumin was infused in septic patients.21
Overlaps between covariates were expected and occurred. Urine osmolality and creatinine are both measures of renal fluid conservation,22 but creatinine was applied in the model as they more strongly improved the curve‐fit. Similarly, plasma albumin and the colloid osmotic pressure were correlated (Figure 5), but plasma albumin was chosen for use. The use of colloid pressure would be more correct from a physiological point of view, but albumin was measured with higher precision and, in the patient group, also more frequently than the colloid osmotic pressure.
4.1. Limitations
Key factors that differed between the subjects such as age, sex, inflammatory markers and shedding products were applied as potential covariates in the kinetic model. All differences between the 2 groups were also lumped together as a surrogate covariate called postoperative status. Still, remarkably small differences in the kinetics of 20% albumin were found between the 2 studied cohorts (Figure 6A), suggesting that 20% albumin provides a stable and long‐lasting plasma volume expansion 1 day after major abdominal surgery as well as it does in healthy volunteers.
The plasma levels of inflammatory markers and shedding products were less elevated than expected after major abdominal surgery. The spread of the data (Figure 2) may then not have been sufficient to disclose true covariance with the kinetic parameters. Moreover, the maximum elevation may not have been found. This point seems to occur 6–8 hours after cardiopulmonary bypass surgery,23 while our measurements were initiated 12–15 hours after the abdominal surgery ended.
The trend in the excluded covariances analyses still deserves a comment. Hyaluronic acid and C‐reactive protein had a 95% CI that did not include 1.0 when tested as covariates to capillary leakage (k b), and so was the case also when syndecan‐1 was tested as a covariate to fluid absorption (k 21), but the precision of the kinetic model did not improve sufficiently to allow the inclusion of these variables as covariates. However, the effect was negative (i.e. high concentrations decreased k b and k 21), which is contrary to the view that shedding speeds up the turnover of fluid. Another piece of evidence also questions this view widespread belief. As repair of an injured glycocalyx requires between 5 and 7 days to be completed,24 the variable postoperative status would be expected to be associated with more rapid capillary leakage (higher k b) even 15 hours after major abdominal surgery, but this was not the case.
Plasma albumin showed a dynamic variation over time, with the highest concentrations at the end of the infusions, as did the arterial pressures and several other potential covariates. These parameters served as time‐varying covariates, which means that the numeric association between a covariate and kinetic parameter was re‐calculated for each 1 of the 448 data points (see Supporting Information). A detailed comparison of nonkinetic data between the cohorts is beyond the scope of the present evaluation, but will be published elsewhere.
5. CONCLUSIONS
A kinetic model for 20% albumin showed a great variability in plasma volume expansion that could partly be explained by the applied individual‐specific covariates. The excess amount of exogenous albumin initiated a lively traffic of fluid to and from the plasma, thereby creating a long‐lasting steady‐state volume expansion that is not seen after infusion of 5% albumin.
No covariance was found between the kinetic parameters and the plasma concentrations of C‐reactive protein and 3 shedding substances that indicate injury to the glycocalyx layer (syndecan‐1, heparan sulfate and hyaluronic acid).
The clinical efficacy of 20% albumin was only affected to a limited extent by recent major open abdominal surgery.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
R.G.H. planned the study, wrote applications, performed the data analysis, and wrote the manuscript. E.H. collected the patient data. M.Z. collected the data on the volunteers. J.H. organized the volunteer study. H.B. organized the patient study.
Supporting information
Data S1.
Additional file 1. This.xls file contains all evaluated data in the study (sex, body weight, plasma dilution over time, urinary excretion, arterial pressures, shedding products etc.) and is the file used as input in the kinetic analysis.
ACKNOWLEDGEMENTS
We acknowledge nurse anaesthetist Sandra Månsson assisted in the collection of data from the postoperative patients. Associate professor Camilla Krizhanovskii and PhD student Stelia Ntika performed the analyses of shedding products. The simulation program for Matlab was created by Alexey Geynts at the Department of Mathematical Sciences at Chalmers University of Technology in Gothenburg, Sweden.
The work was supported by a grant from Mats Kleberg Foundation and local hospital funds.
Hahn RG, Zdolsek M, Hasselgren E, Zdolsek J, Björne H. Fluid volume kinetics of 20% albumin. Br J Clin Pharmacol. 2019;85:1303–1311. 10.1111/bcp.13897
The authors confirm that the Principal Investigator is Robert Hahn. Emma Hasselgren had clinical responsibility for the patients and Markus Zdolsek for the volunteers.
REFERENCES
- 1. Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. New Engl J Med. 2014;370(15):1412‐1421. [DOI] [PubMed] [Google Scholar]
- 2. Faraq E, Ebrahim Z. The perioperative use of albumin In: Faraq E, ed. Kurz, editors: Perioperative fluid management. Cham, Switzerland: Springer; 2016:215‐234. [Google Scholar]
- 3. Hahn RG. Arterial pressure and the elimination of crystalloid fluid: a population‐based study. Anesth Analg. 2017;124(6):1824‐1833. [DOI] [PubMed] [Google Scholar]
- 4. Hahn RG, Drobin D, Zdolsek J. Distribution of crystalloid fluid changes with the rate of infusion: a population‐based study. Acta Anaesthesiol Scand. 2016;60(5):569‐578. [DOI] [PubMed] [Google Scholar]
- 5. Hahn RG. The elimination half‐life of crystalloid fluid is shorter in female than in male volunteers; a retrospective population kinetic analysis. Biol Sex Differ. 2016;7(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384‐394. [DOI] [PubMed] [Google Scholar]
- 7. Rehm M, Bruegger D, Christ F, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;166:1896‐1906. [DOI] [PubMed] [Google Scholar]
- 8. Bertram A, Stahl K, Hegermann J, Haller H. The glycocalyx layer In: Hahn RG, ed. Clinical fluid therapy in the perioperative setting. 2nd ed. Cambridge: Cambridge University Press; 2016:73‐81. [Google Scholar]
- 9. Bashandy GMN. Implications of recent accumulating knowledge about endothelial glycocalyx on anesthetic management. J Anesth. 2015;29(2):269‐278. [DOI] [PubMed] [Google Scholar]
- 10. Hedin A, Hahn RG. Volume expansion and plasma protein clearance during intravenous infusion of 5% albumin and autologous plasma. Clin Sci. 2005;106:217‐224. [DOI] [PubMed] [Google Scholar]
- 11. Nemme J, Hahn RG, Krizhanovskii C, Ntika S, Sobelnikovs O, Vanags I. Minimal shedding of the glycocalyx layer during abdominal hysterectomy. A preliminary report. BMC Anesthesiol. 2017;17(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hahn RG, Bergek C, Gebäck T, Zdolsek J. Interactions between the volume effects of hydroxyethyl starch 130/0.4 and Ringer's acetate. Crit Care. 2013;17(3):R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zdolsek M, Hahn RG, Zdolsek JH. Recruitment of extravascular fluid by hyperoncotic albumin. Acta Anaesthesiol Scand. 2018;62(9):1255‐1260. [DOI] [PubMed] [Google Scholar]
- 14. Renkin EM, Tucker V, Rew K, O'Loughlin D, Wong M, Sibley L. Plasma volume expansion with colloids increases blood‐tissue albumin transport. Am J Physiol. 1992;262(4 Pt 2):H1054‐H1067. [DOI] [PubMed] [Google Scholar]
- 15. Hahn RG. Volume kinetics for infusion fluids. Anesthesiology. 2010;113(2):470‐481. [DOI] [PubMed] [Google Scholar]
- 16. Nadler SB, Hidalgo JU, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224‐232. [PubMed] [Google Scholar]
- 17. Hahn RG, Nyberg Isacson M, Fagerström T, Rosvall J, Nyman CR. Isotonic saline in elderly men: an open‐labelled controlled infusion study of electrolyte balance, urine flow and kidney function. Anaesthesia. 2016;71(2):155‐162. [DOI] [PubMed] [Google Scholar]
- 18. Moran M, Kapsner C. Acute renal failure associated with elevated plasma oncotic pressure. N Engl J Med. 1987;317(3):150‐153. [DOI] [PubMed] [Google Scholar]
- 19. Haskell LP, Tannenberg AM. Elevated urinary specific gravity in acute oliguric renal failure due to hetastarch administration. NY State J Med. 1988;88:387‐388. [PubMed] [Google Scholar]
- 20. Lamke LO, Liljedahl SO. Plasma volume expansion after infusion of 5%, 20% and 25% albumin solutions in patients. Resuscitation. 1976;5(2):85‐92. [DOI] [PubMed] [Google Scholar]
- 21. Margarson MP, Soni NC. Changes in serum albumin concentration and volume expanding effects following a bolus of 20% albumin in septic patients. Br J Anaesth. 2004;92(6):821‐826. [DOI] [PubMed] [Google Scholar]
- 22. Hahn RG, Grankvist N, Krizhanovskii C. Urinary analysis of fluid retention in the general population: a cross‐sectional study. PLoS One. 2016;11(10):e0164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pesonen E, Passov A, Andersson S, et al. Glycocalyx degradation and inflammation in cardiac surgery. J Cardiothorac Vasc Anesth. 2018;2(3):341‐345. [DOI] [PubMed] [Google Scholar]
- 24. Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ Res. 2009;104(11):1318‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Additional file 1. This.xls file contains all evaluated data in the study (sex, body weight, plasma dilution over time, urinary excretion, arterial pressures, shedding products etc.) and is the file used as input in the kinetic analysis.


