Abstract
Disorders of the skeleton are frequently accompanied by bone pain and a decline in the functional status of the patient. Bone pain occurs following a variety of injuries and diseases including bone fracture, osteoarthritis, low back pain, orthopedic surgery, fibrous dysplasia, rare bone diseases, sickle cell disease and bone cancer. In the past 2 decades, significant progress has been made in understanding the unique population of sensory and sympathetic nerves that innervate bone and the mechanisms that drive bone pain. Following physical injury of bone, mechanotranducers expressed by sensory nerve fibres that innervate bone are activated and sensitized so that even normally non‐noxious loading or movement of bone is now being perceived as noxious. Injury of the bone also causes release of factors that; directly excite and sensitize sensory nerve fibres, upregulate proalgesic neurotransmitters, receptors and ion channels expressed by sensory neurons, induce ectopic sprouting of sensory and sympathetic nerve fibres resulting in a hyper‐innervation of bone, and central sensitization in the brain that amplifies pain. Many of these mechanisms appear to be involved in driving both nonmalignant and malignant bone pain. Results from human clinical trials suggest that mechanism‐based therapies that attenuate one type of bone pain are often effective in attenuating pain in other seemingly unrelated bone diseases. Understanding the specific mechanisms that drive bone pain in different diseases and developing mechanism‐based therapies to control this pain has the potential to fundamentally change the quality of life and functional status of patients suffering from bone pain.
Keywords: aging, neuropathic, NGF, nociceptors, paediatric, TrkA
Introduction
The study of chronic bone pain usually focuses on common diseases such as osteoarthritis, low back pain and osteoporosis induced bone fracture. All of these diseases increase with the age‐related decline in the mass, quality and strength of the skeleton 1. However, there are a host of other adult diseases that generate chronic bone pain as well as over 500 human genetic disorders of bone and cartilage (Figure 1) that frequently present when the patients are young 2. Genetic diseases of the bone/joint that are accompanied by chronic bone pain include; osteogenesis imperfecta, Engelmann disease, Danlos syndrome, fibrous dysplasia, Paget's disease, sickle cell disease and juvenile arthritis 3, 4. While many of the 500 adult and genetic diseases of bone and joint are individually rare disorders, when combined they become a very significant number of patients who suffer from chronic bone pain throughout their life 2, 5.
Figure 1.
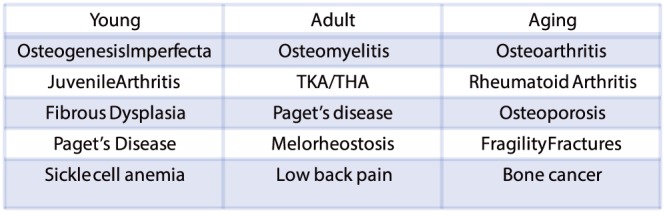
A partial list of human disorders across the lifespan that are frequently accompanied by bone pain. For an extensive list of diseases that are frequently accompanied by bone pain: http://www.rightdiagnosis.com/symptoms/bone_pain/common.htm; in children, http://www.nof.org/articles/5; and for a list of rare (orphan) bone diseases of bone and joint http://www.usbji.org/projects/RBDPN_op.cfm?dirID=252
Currently, the most common classes of pharmacological agents used to treat bone pain are nonsteroidal anti‐inflammatory drugs (NSAIDs) and opiates 5, 6, 7, 8. However, while NSAIDs (including ibuprofen, COX‐2 inhibitors, naproxen, and diclofenac) can be effective in the short‐term relief of bone pain, when used over an extended period they can have unwanted and severe renal, hepatic, and gastrointestinal side effects 9. In light of these issues with NSAIDs, it has now become more common for opiates to be used to control long term moderate‐to‐severe bone pain. However, recent data have suggested that although opiates can be useful in controlling nonmalignant bone pain for 2–3 months, long‐term use (>2–3 months) is associated with reduced functional status and decreased likelihood of returning to work, as well as potential development of dependence, constipation and respiratory depression 10, 11, 12. In older individuals, opiates are also more likely to induce dizziness, vertigo and cognitive clouding all of which increases the likelihood of falling which can result in bone fracture 13, 14.
In light of the side effect profile of NSAIDs and opiates, new mechanism‐based analgesics that relieve bone pain with a lower side effect profile are clearly needed. To develop such analgesics, rodent models of malignant and nonmalignant bone pain were developed 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28. These models of bone pain were then utilized to define the mechanisms that drive bone pain, test whether new mechanism‐based therapies could relieve bone pain and translate promising candidates into human clinical trials 29. The present review summarizes these results from preclinical and human studies. These data suggest that developing a deeper understanding of the mechanisms of one type of bone pain frequently provides unexpected insight and analgesic therapies for a variety of disorders of the skeleton.
The innervation of normal bone
There is a very tight regulation of the sensory and sympathetic innervation of the normal skeleton 30, 31, 32, 33, 34, 35. For example, whereas the articular cartilage is completely lacking in any blood vessels or nerve fibres 31, 32, 35 the periosteum has a remarkably dense sensory and sympathetic innervation (Figure 2). The bone marrow and mineralized bone are also innervated by sensory and sympathetic nerve fibres with the approximate density (per unit area) in the periosteum, bone marrow and cortical bone being 100:2:0.1 31, 35. While much of this analysis was performed on the young, adult and aging mouse femur, previous studies have noted a similar density, morphology and general organization of sensory and sympathetic nerve fibres in the rat calvaria and mandible 36, tibia 37 as well as human bones 38, 39, 40.
Figure 2.

The sensory and sympathetic innervation of normal bone. Micro‐computed tomography image of the adult (4‐month‐old) mouse femur (A) and a schematic (B) showing the location of sensory nerve fibres in the normal bone. Confocal microscopic images showing the sensory and sympathetic innervation in the periosteum (C) and the cortical bone (D) of the mouse femur in young (10‐day‐old), adult (4‐month‐old) and aging (24‐month‐old) mice. Note that in normal bone, sensory and sympathetic nerve fibres in the periosteum, cortical bone and marrow each have a unique organization and all contain sensory neurons that can transmit nociceptive stimuli from bone. Abbreviations: C, cambium layer of the periosteum; CB, cortical bone; CD31, platelet endothelial cell adhesion molecule that is expressed by endothelial cells; CGRP, calcitonin gene related peptide (labels small diameter sensory nerve fibers); DAPI, 4′,6‐diamidino‐2‐phenylindole (a counterstain that labels the nucleus of all cells); DIC, differential interference contrast that illustrates cortical bone; HC, cortical pores in mice that correspond to Haversian canals in humans; TH, tyrosine hydroxylase (labels sympathetic nerve fibers)
Within each compartment of bone there is also tight regulation of nerves in terms of density, phenotype and morphology. For example, in the periosteum of the mouse femur, > 90% of nerve fibres are present in the cambium layer with fewer than 10% being present in the fibrous layer 31. Previous studies have suggested that most of the nerve fibres in bone are tropomyosin receptor kinase A positive (TrkA+), are thinly or unmyelinated, and have conduction velocity characteristics of A‐delta and C‐fibres 16, 17, 18, 37, 41, 42, 43, 44, 45. In the periosteum, the A‐delta and C‐sensory nerve fibres are arranged in a fishnet‐like pattern, which appears to be designed to act as a neural net to detect mechanical injury or distortion of the underlying cortical bone 31, 46. In contrast, sympathetic nerve fibres in the periosteum usually have a characteristic corkscrew‐like morphology and are almost invariably tightly associated with blood vessels 31, 46.
In the cortical bone, the great majority of sensory and sympathetic nerve fibres are largely confined to the vascularized cortical pores in mice 31, 47 and vascularized Haversian canals in humans 38, 39, 40. In the bone marrow, the sensory nerve fibres are linear in appearance, whereas the sympathetic nerve fibres again have a corkscrew shaped pattern as they tightly wrap around blood vessels 31, 39. Thus, in terms of location, density and morphology, both sensory and sympathetic nerve fibres are tightly regulated in the normal, uninjured bone. However, as discussed below, injury or disease of the bone can induce a remarkable and highly ectopic reorganization of both sensory and sympathetic nerve fibres.
Acute activation of mechano‐ and chemo‐sensitive nociceptors in bone
Following acute physical injury to bone, mechanosensitive nociceptors that innervate bone are activated and sensitized (Figure 3). Normally, these mechanosensitive nociceptors in the bone are silent 48, 49, 50, 51. However, when these sensory nerve fibres are mechanically distorted (such as occurs following bone fracture) nociceptors that innervate bone respond within seconds 52, 53 and signal this mechanical distortion from the bone to the spinal cord and higher centres of the brain 48, 49. These mechanically sensitive sensory nerve fibres will continue to rapidly discharge until they are returned to their original position, which is usually when the bone is reset and stabilized by a rod or cast 49. Unfortunately, in bone diseases where there is inherent instability of the bone (such as in unhealed fractures in the elderly or bone cancer) these mechanosensitive sensory nerve fibres in bone will be activated whenever the weakened bone is moved or loaded 7, 49. Previous reports have also suggested that increased intraosseous pressure in the bone marrow can drive bone pain by stimulating mechanosensitive nociceptors that innervate the bone 18, 41, 49, 50.
Figure 3.
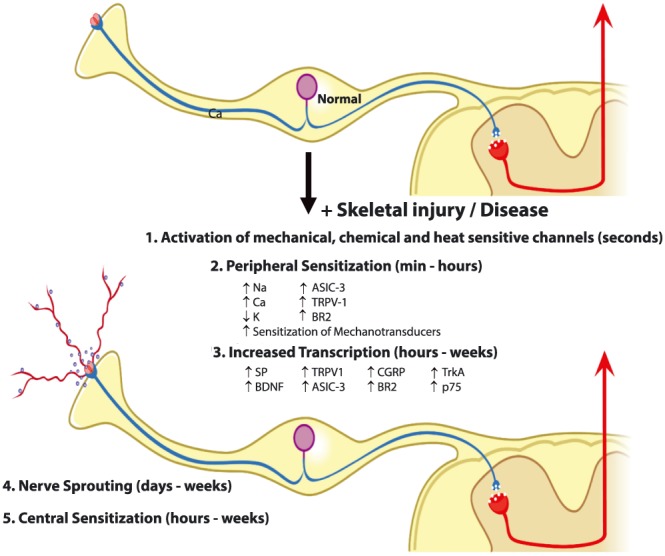
Mechanisms that contribute to acute and chronic bone pain. An evolving set of mechanisms drives acute and chronic bone pain. Bone pain involves nociceptor activation, sensitization, transcriptional changes, ectopic sprouting and central sensitization in the spinal cord and brain. If rapid and effective bone healing occurs (such as following bone fracture in the young), these events return to pre‐fracture levels with resolution of the bone pain. However, if rapid and effective healing does not occur (such as in bone cancer or non‐healed fractures in the aged) these mechanisms can synergistically increase with time, resulting in chronic bone pain. Abbreviations: ASIC‐3, acid sensing ion channel‐3; BDNF, brain‐derived neurotrophic factor; BR2, bradykinin receptor‐2; CGRP, calcitonin gene related peptide; Ca, calcium channels; K, potassium channels; Na, sodium channels; p75, low‐affinity nerve growth factor receptor; SP, substance P; TrkA, Tropomyosin receptor kinase A (high affinity nerve growth factor receptor; TRPV‐1, transient receptor potential vanilloid‐1. Adapted from Enomoto et al. (2018) 120
Bone pain induced by trauma (i.e. bone fracture) is at least initially driven by mechanical distortion of mechanosensitive sensory nerve fibres in the bone. However, in other diseases such as osteogenesis imperfecta or bone cancer, osteoclast induced acidosis can also play a significant role in driving bone pain. Studies have shown that many sensory nerve fibres that innervate bone express acid sensing ion channels including TRPV1, ASIC1 and ASIC3 16, 54 that will be readily activated by a pH of 3–4, which is the extracellular pH generated by osteoclasts when resorbing bone. One of the best examples of therapies initially used to treat one bone pain and then relieving another bone pain are bisphosphonates and Denosumab which both target osteoclasts. Both were originally developed to treat osteoporosis and were later shown to also relieve bone cancer pain 55, 56, 57, 58, 59, 60. Since this original observation, bisphosphonates or Denosumab have also been shown to attenuate bone pain in human patients with; osteogenesis imperfecta, juvenile Paget's disease, fibrous dysplasia, aneurysmal bone cyst and complex regional pain syndrome 5, 59, 61, 62, 63, 64, 65. Mechanistically, what these diseases share in common is excessive osteoclast activity which generates a low pH that will stimulate acid sensing ion channels expressed by sensory nerve fibres that innervate bone 54, 59, 60. These results emphasize the point that therapies that attenuate one type of bone pain that is driven by a specific mechanism (here acidosis) may also be effective in attenuating pain in other bone diseases, which are driven by similar mechanisms.
Sensitization of nociceptors innervating bone
A second mechanism that plays a significant role in amplifying both acute and chronic bone pain (Figure 3) is sensitization of bone nociceptors (i.e. sensory neurons that detect noxious stimuli in bone). Agents that have been reported to sensitize bone nociceptors include bradykinin, endothelins, epidermal growth factor, glial cell line‐derived neurotrophic factor, histamine, nerve growth factor (NGF), prostaglandins, tumour necrosis factor and vascular endothelial growth factor 25, 66, 67, 68, 69, 70, 71, 72, 73. Probably the best studied factor that has been examined in bone pain in both preclinical and human clinical studies is NGF. Once NGF is released in the injured bone, it binds to TrkA+ receptors that are expressed by many bone nociceptors 43, 74, 75, 76. Binding of NGF to TrkA+ results in the phosphorylation and sensitization of a variety of receptors and ion channels expressed by nociceptors 9, 16, 77, 78, 79, 80, 81, 82, 83. These include ion channels that respond to acid (TRPV1 and ASIC3), receptors that bind prostaglandins and bradykinin, and mechanotransducers expressed by sensory nerve fibres 84. Following this NGF induced sensitization, there is an enhanced response of nociceptors to even small amounts of acid released by osteoclasts as well as prostaglandins and bradykinin released by injured tissues 84, 85. In addition, excitation and firing of mechanosensitive nociceptors is magnified following low dose treatment of NGF in both animals and humans, thus increasing the signalling of noxious input to the spinal cord and brain 26, 77.
Transcriptional changes in sensory neurons
It is now well established from preclinical and clinical studies that the peripheral release of NGF and activation of TrkA play important roles in driving bone pain 9, 19, 23, 26, 84, 86, 87, 88. Once NGF is released in bone, it binds to its cognate receptor TrkA and is retrogradely transported as a complex back to the cell body of the sensory neuron located in the dorsal root ganglion 84. When the NGF‐TrkA complex reaches the nucleus of the sensory neuron, there is significant alterations in several genes that are important for the detection and signalling of noxious bone stimuli 16, 44, 84, 89. Thus, NGF: induces upregulation of the ion sensing channels TRPV1 and ASIC3; and increases the expression of neurotransmitters including substance P, calcitonin gene‐related peptide, brain‐derived neurotrophic factor, and a variety of sodium and calcium channels that regulate the excitability of bone nociceptors 84, 90. Recent data also suggest that even low doses of NGF can increase the electrophysiological excitability of human sensory neurons for weeks 77 suggesting that long term transcriptional mechanisms may be involved in these NGF induced changes.
Therapies targeting NGF (Figure 5) have been shown to relieve pain in a variety of human skeletal pathologies including osteoarthritis 86, 91, 92, 93, low back pain 87, 94 and bone cancer pain 95, 96. Presumably, blockade of NGF will be contraindicated in young patients with skeletal pain as NGF is involved in the growth and survival of the developing sensory and sympathetic nervous system in the young 9. However, many individuals with genetic disorders of the bone and joint (such as fibrous dysplasia) not only have significant pain when they are young but continue to have chronic skeletal pain throughout their adult life 65. Whether targeting blockage of NGF or its cognate receptor TrkA will block pain in adults with genetic disorders of the bone, complex regional pain syndrome and other painful bone diseases, has yet to be determined.
Figure 5.
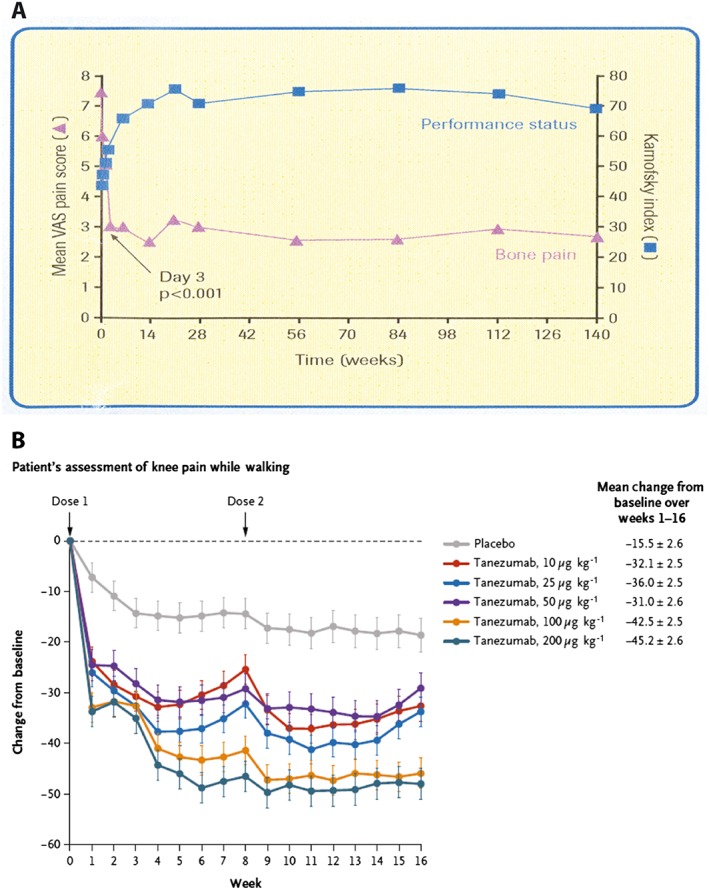
Data showing that blocking osteoclast activity or nerve growth factor can attenuate human bone pain. Data from a human trial (A) showing a reduction in bone pain and an increase in functional status of patients with breast cancer metastases to bone following administration of a bisphosphonate (ibandronate), which targets osteoclasts and reduces bone acidosis. Recent data have demonstrated that bisphosphonates can also reduce pain in osteogenesis imperfect, Paget's disease, fibrous dysplasia and complex regional pain syndrome. Results from a human clinical trial (B) showing that anti‐nerve growth factor (tanezumab) can reduce chronic osteoarthritis pain. Anti‐NGF therapy has also been shown to attenuate pain in patients with low back pain and in bone cancer patients with high pain and low opiate use. These data suggest therapies that attenuate one type of bone pain may be effective in reducing pain in seemingly unrelated bone diseases if the diseases share a common mechanism(s) that is driving the pain. Data in (A) are from Ringe et al. (2007) and Body et al. (2004) 59, 60 and in (B) from Lane et al. (2010) 121. VAS, visual analogue scale
Ectopic sprouting in sensory and sympathetic nerve fibres
The fourth mechanism that may be involved in driving skeletal pain is ectopic nerve sprouting (Figure 4). Following injury or disease, several neurotrophic factors including NGF, glial cell line‐derived neurotrophic factor, vascular endothelial growth factor and epidermal growth factor are released by stromal and inflammatory cells and can induce nerve sprouting, resulting in hyperinnervation of the marrow, mineralized bone and periosteum 68, 96, 97, 98, 99, 100, 101, 102, 103. It should be noted that nerve sprouting has been observed even in bone fractures that are undergoing normal healing 104. This nerve sprouting can be observed in the normal callus and probably is important in causing the patient to guard the injured limb and refrain from excessive use of the fractured bone until it heals. As the fractured bone normally heals and the callus is resorbed, these newly sprouted nerve fibres are pruned back so that, when the bone fully heals, the innervation of bone returns to its normal state.
Figure 4.
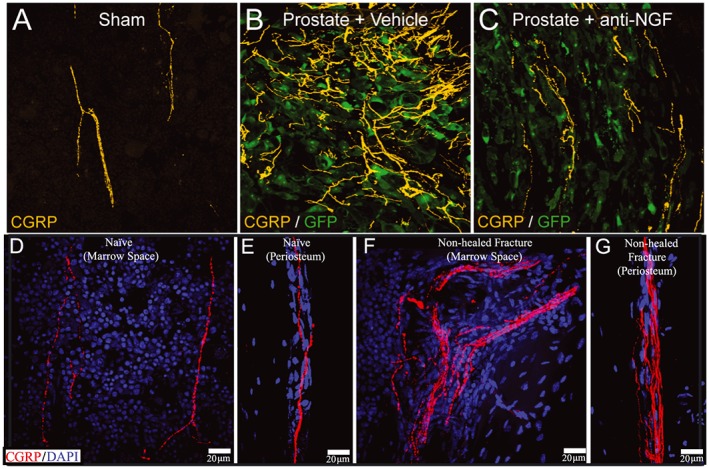
Images of nerve sprouting in malignant and non‐malignant bone pain. As seen in (A) sensory nerve fibres (bright yellow) in the normal bone marrow have a regular and linear appearance. Following invasion of the bone marrow (B) by prostate cancer cells (green) there is marked increase in the density due to the ectopic sprouting of sensory nerve fibres. Prior administration of anti‐nerve growth factor (NGF) before tumour invasion of the bone largely blocks this ectopic nerve sprouting (C). Similar sprouting of sensory nerve fibres is also observed in non‐malignant pain states following injury of bone. (D, E) Sensory nerve fibres (red) in the normal marrow and periosteum respectively. (F, G) Increased sensory innervation of the marrow (F) and periosteum (G) in a bone at 6 months postfracture when effective bone healing has not occurred. Abbreviations: anti‐NGF, a monoclonal antibody that binds to nerve growth factor (NGF); CGRP, calcitonin gene related peptide (labels small diameter sensory nerve fibers); DAPI, 4′,6‐diamidino‐2‐phenylindole (a counterstain that labels the nucleus of all cells); GFP, green fluorescent protein that is expressed by prostate cancer cells
However, in cases of injured or diseased bone where normal or rapid bone healing does not occur, this ectopic nerve sprouting is not pruned back and now the injured or diseased aspects of the bone are hyperinnervated 28, 68, 96, 98, 99, 100, 101, 102, 103. Now, normally non‐noxious loading or movement of the bone may be perceived as a noxious event as the bone is not only weaker than healthy bone but the weak bone is hyperinnervated making detection of any mechanical distortion of the weakened bone more likely. Skeletal diseases where ectopic sprouting has been observed include bone cancer 27, 99, 100, unhealed bone fracture 7, osteoarthritis 28, 101, 105 and the degenerated vertebral disc 102, 103, 106, 107. Whether significant and unwanted sprouting of sensory and sympathetic nerve fibres also occurs in patients with genetic disorders of the skeleton, has yet to be determined. However, if ectopic nerve sprouting in bone does occur in these conditions, it may provide insight into the mechanisms that drive the transition from acute to chronic skeletal pain in these patients 9, 108.
Central sensitization
The fifth mechanism that participates in driving bone pain is changes that occur in the spinal cord and brain, which cause central sensitization that amplifies the perception and severity of pain 89, 109, 110, 111, 112. Central sensitization is thought to occur when the chemical, electrophysiological and pharmacological systems that transmit and modulate pain are altered in both the spinal cord and higher centres of the brain 113, 114, 115, 116, 117, 118, 119. These changes cause an exaggerated perception of painful stimuli so that normally mild painful stimuli are perceived as highly painful (hyperalgesia) and normal nonpainful stimuli such as normal loading or use of joint or bone is now perceived as a painful event (allodynia). Additionally, central sensitization also contributes to the phenomenon of referred pain, where areas adjacent to the initial injury become hypersensitive. A common example of this is in whiplash injuries to the neck where, following injury to one cervical vertebra, nearby areas such as the shoulder, arm and back also become hypersensitive 116.
While we do not yet know the specific mechanisms that generate central sensitization following injury to bone, what is known is that injury to skeleton seems to be much more effective at inducing central sensitization as compared to injury to skin or muscle 49, 110, 118. As noted by Woolf and Wall in 1986 “ …a twisted ankle invokes relatively little destruction of tissue and elicits an abrupt localized stabbing pain that dies down quickly but is followed by a prolonged period of spreading, poorly localized deep pain, and tenderness that affects reflexes and gait. In contrast, localized skin damage produces an acute burst of pain that gradually dies down over minutes but is associated with a spatially restricted response of flair, wheal and surrounding tenderness” 110. Just why injury to the skeleton is so effective at producing central sensitization remains unclear 49, 117, 118, 119, 120, 121 although the skeleton is innervated by a very distinct population of primary afferent neurons that may be uniquely effective at inducing central sensitization in the spinal cord and higher centres of the brain.
Conclusions and limitations
In the past 2 decades there has been a remarkable increase in our understanding of the specific mechanisms that drive bone pain. The specific nerve fibres that innervate the bone have begun to be defined as well as the remarkable changes these nerves can undergo in injury and disease. Mechanisms that drive bone pain are now known to include: activation of mechanotranducers and acid sensing ion channels expressed by sensory nerve fibres; sensitization, transcriptional changes and ectopic sprouting of sensory nerve fibres; and central sensitization involving both the spinal cord and brain. What is also clear is that insight into mechanisms that generate bone pain in one disease can be very useful in understanding the mechanisms that generate pain in other bone diseases (Figure 5).
While significant progress has been made, we are only beginning to understanding how nerves and bone interact and modulate each other. Importantly, it can still be very challenging to fully control most chronic bone pain. However, if new mechanism‐based therapies that better control bone pain can be developed, they have the potential to fundamentally change the quality of life and functional status of patients suffering from skeletal pain.
Competing Interests
There are no competing interests to declare.
Research supporting this manuscript was funded by NIH grants CA154550, CA157449, and NS023970 to Patrick Mantyh. Dr Mantyh has served as a consultant and/or received research grants from Abbott (Abbott Park, IL), Adolor (Exton, PA), Array Biopharma (Boulder, CO), Johnson and Johnson (New Brunswick, NJ), Merck (White Plains, New York), Pfizer (New York, NY), Plexxikon (Berkley, CA), Rinat (South San Francisco, CA, and Roche (Palo Alto, CA).
Mantyh P. W. (2019) Mechanisms that drive bone pain across the lifespan, Br J Clin Pharmacol, 85, 1103–1113. doi: 10.1111/bcp.13801.
References
- 1. Mitchell SAT, Majuta LA, Mantyh PW. New insights in understanding and treating bone fracture pain. Curr Osteoporos Rep 2018; 16: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarthy EF. Genetic diseases of bones and joints. Semin Diagn Pathol 2011; 28: 26–36. [DOI] [PubMed] [Google Scholar]
- 3. Wynne‐Davies R, Gormley J. The prevalence of skeletal dysplasias. An estimate of their minimum frequency and the number of patients requiring orthopaedic care. J Bone Joint Surg Br 1985; 67: 133–137. [DOI] [PubMed] [Google Scholar]
- 4. Clinch J, Eccleston C. Chronic musculoskeletal pain in children: assessment and management. Rheumatology (Oxford) 2009; 48: 466–474. [DOI] [PubMed] [Google Scholar]
- 5. Boyce AM. Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep 2017; 15: 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frost CO, Hansen RR, Heegaard AM. Bone pain: current and future treatments. Curr Opin Pharmacol 2016; 28: 31–37. [DOI] [PubMed] [Google Scholar]
- 7. Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain 2014; 155: 2323–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gossai N, Hilgers MV, Polgreen LE, Greengard EG. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer 2015; 62: 1078–1080. [DOI] [PubMed] [Google Scholar]
- 9. Mantyh PW. The neurobiology of skeletal pain. Eur J Neurosci 2014; 39: 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am 2009; 91: 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013; 154 (Suppl. 1): S94–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Webster BS, Choi Y, Bauer AZ, Cifuentes M, Pransky G. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine (Phila Pa 1976) 2014; 39: 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol 2006; 41: 1080–1093. [DOI] [PubMed] [Google Scholar]
- 14. Svensson H, Olofsson E, Karlsson J, Hansson T, Olsson LE. A painful, never ending story: older women's experiences of living with an osteoporotic vertebral compression fracture. Osteoporos Int 2016; 27: 1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freeman KT, Koewler NJ, Jimenez‐Andrade JM, Buus RJ, Herrera MB, Martin CD, et al A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology 2008; 108: 473–483. [DOI] [PubMed] [Google Scholar]
- 16. Nencini S, Ringuet M, Kim DH, Chen YJ, Greenhill C, Ivanusic JJ. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain 2017; 13: 1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aso K, Izumi M, Sugimura N, Okanoue Y, Ushida T, Ikeuchi M. Nociceptive phenotype alterations of dorsal root ganglia neurons innervating the subchondral bone in osteoarthritic rat knee joints. Osteoarthr Cartil 2016; 24: 1596–1603. [DOI] [PubMed] [Google Scholar]
- 18. Nencini S, Ivanusic J. Mechanically sensitive Adelta nociceptors that innervate bone marrow respond to changes in intra‐osseous pressure. J Physiol 2017; 595: 4399–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Majuta LA, Longo G, Fealk MN, McCaffrey G, Mantyh PW. Orthopedic surgery and bone fracture pain are both significantly attenuated by sustained blockade of nerve growth factor. Pain 2015; 156: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwei MJ, Honore P, Rogers SD, Salak‐Johnson JL, Finke MP, Ramnaraine ML, et al Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 1999; 19: 10886–10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, et al Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain 2002; 99: 397–406. [DOI] [PubMed] [Google Scholar]
- 22. Sabino MA, Mantyh PW. Pathophysiology of bone cancer pain. J Support Oncol 2005; 3: 15–24. [PubMed] [Google Scholar]
- 23. Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, et al A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res 2005; 65: 9426–9435. [DOI] [PubMed] [Google Scholar]
- 24. Halvorson KG, Sevcik MA, Ghilardi JR, Rosol TJ, Mantyh PW. Similarities and differences in tumor growth, skeletal remodeling and pain in an osteolytic and osteoblastic model of bone cancer. Clin J Pain 2006; 22: 587–600. [DOI] [PubMed] [Google Scholar]
- 25. Sevcik MA, Ghilardi JR, Halvorson KG, Lindsay TH, Kubota K, Mantyh PW. Analgesic efficacy of bradykinin B1 antagonists in a murine bone cancer pain model. J Pain 2005; 6: 771–775. [DOI] [PubMed] [Google Scholar]
- 26. Koewler NJ, Freeman KT, Buus RJ, Herrera MB, Jimenez‐Andrade JM, Ghilardi JR, et al Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res 2007; 22: 1732–1742. [DOI] [PubMed] [Google Scholar]
- 27. Bloom AP, Jimenez‐Andrade JM, Taylor RN, Castaneda‐Corral G, Kaczmarska MJ, Freeman KT, et al Breast cancer‐induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain 2011; 12: 698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jimenez‐Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther 2012; 14: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fillingim RB, Bruehl S, Dworkin RH, Dworkin SF, Loeser JD, Turk DC, et al The ACTTION‐American pain society pain taxonomy (AAPT): an evidence‐based and multidimensional approach to classifying chronic pain conditions. J Pain 2014; 15: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gajda M, Litwin JA, Cichocki T, Timmermans JP, Adriaensen D. Development of sensory innervation in rat tibia: co‐localization of CGRP and substance P with growth‐associated protein 43 (GAP‐43). J Anat 2005; 207: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience 2018; 387: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hukkanen M, Konttinen YT, Rees RG, Santavirta S, Terenghi G, Polak JM. Distribution of nerve endings and sensory neuropeptides in rat synovium, meniscus and bone. Int J Tissue React 1992; 14: 1–10. [PubMed] [Google Scholar]
- 33. Hill EL, Elde R. Calcitonin gene‐related peptide‐immunoreactive nerve fibers in mandibular periosteum of rat: evidence for primary afferent origin. Neurosci Lett 1988; 85: 172–178. [DOI] [PubMed] [Google Scholar]
- 34. Hill EL, Elde R. Distribution of CGRP‐, VIP‐, D beta H‐, SP‐, and NPY‐immunoreactive nerves in the periosteum of the rat. Cell Tissue Res 1991; 264: 469–480. [DOI] [PubMed] [Google Scholar]
- 35. Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002; 113: 155–166. [DOI] [PubMed] [Google Scholar]
- 36. Hukkanen M, Platts LA, Corbett SA, Santavirta S, Polak JM, Konttinen YT. Reciprocal age‐related changes in GAP‐43/B‐50, substance P and calcitonin gene‐related peptide (CGRP) expression in rat primary sensory neurones and their terminals in the dorsal horn of the spinal cord and subintima of the knee synovium. Neurosci Res 2002; 42: 251–260. [DOI] [PubMed] [Google Scholar]
- 37. Ivanusic JJ. Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J Comp Neurol 2009; 517: 276–283. [DOI] [PubMed] [Google Scholar]
- 38. Gronblad M, Liesi P, Korkala O, Karaharju E, Polak J. Innervation of human bone periosteum by peptidergic nerves. Anat Rec 1984; 209: 297–299. [DOI] [PubMed] [Google Scholar]
- 39. Miller MR, Kasahara M. Observations on the innervation of human long bones. Anat Rec 1963; 145: 13–23. [Google Scholar]
- 40. Ralston HJ 3rd, Miller MR, Kasahara M. Nerve endings in human fasciae, tendons, ligaments, periosteum, and joint synovial membrane. Anat Rec 1960; 136: 137–147. [DOI] [PubMed] [Google Scholar]
- 41. Furusawa S. A neurophysiological study on the sensibility of the bone marrow. Nihon Seikeigeka Gakkai Zasshi 1970; 44: 365–370. [PubMed] [Google Scholar]
- 42. Aso K, Ikeuchi M, Izumi M, Sugimura N, Kato T, Ushida T, et al Nociceptive phenotype of dorsal root ganglia neurons innervating the subchondral bone in rat knee joints. Eur J Pain 2014; 18: 174–181. [DOI] [PubMed] [Google Scholar]
- 43. Castaneda‐Corral G, Jimenez‐Andrade JM, Bloom AP, Taylor RN, Mantyh WG, Kaczmarska MJ, et al The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase a. Neuroscience 2011; 178: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nencini S, Ivanusic JJ. The physiology of bone pain. How much do we really know? Front Physiol 2016; 7: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakada S, Taguchi S. Electrophysiological studies on the free‐fiber ending units of the cat mandibular periosteum. Bull Tokyo Dent Coll 1971; 12: 175–197. [PubMed] [Google Scholar]
- 46. Martin CD, Jimenez‐Andrade JM, Ghilardi JR, Mantyh PW. Organization of a unique net‐like meshwork of CGRP+ sensory fibers in the mouse periosteum: implications for the generation and maintenance of bone fracture pain. Neurosci Lett 2007; 427: 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jilka RL. The relevance of mouse models for investigating age‐related bone loss in humans. J Gerontol A Biol Sci Med Sci 2013; 68: 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sakada S, Shimizu T. Electrophysiological studies of the free‐fiber endings in the temporal fascia of the cat. Bull Tokyo Dent Coll 1974; 15: 245–259. [PubMed] [Google Scholar]
- 49. Haegerstam GA. Pathophysiology of bone pain: a review. Acta Orthop Scand 2001; 72: 308–317. [DOI] [PubMed] [Google Scholar]
- 50. Seike W. Electrophysiological and histological studies on the sensibility of the bone marrow nerve terminal. Yonago Acta Med 1976; 20: 192–211. [PubMed] [Google Scholar]
- 51. Schmidt RF, editor Silent and active nociceptors: structure, functions, and clinical implications. Proceedings of the 7th World Congress on Pain, 1994; 1994: IASP press.
- 52. Mahns DA, Ivanusic JJ, Sahai V, Rowe MJ. An intact peripheral nerve preparation for monitoring the activity of single, periosteal afferent nerve fibres. J Neurosci Methods 2006; 156: 140–144. [DOI] [PubMed] [Google Scholar]
- 53. Inman VT, deC M, Saunders JB. Referred pain from skeletal structures. J Nerv Ment Dis 1944; 99: 660–667. [Google Scholar]
- 54. Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, et al Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci 2005; 25: 3126–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, et al Osteoprotegerin blocks bone cancer‐induced skeletal destruction, skeletal pain and pain‐related neurochemical reorganization of the spinal cord. Nat Med 2000; 6: 521–528. [DOI] [PubMed] [Google Scholar]
- 56. Luger NM, Honore P, Sabino MA, Schwei MJ, Rogers SD, Mach DB, et al Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res 2001; 61: 4038–4047. [PubMed] [Google Scholar]
- 57. Sevcik MA, Luger NM, Mach DB, Sabino MA, Peters CM, Ghilardi JR, et al Bone cancer pain: the effects of the bisphosphonate alendronate on pain, skeletal remodeling, tumor growth and tumor necrosis. Pain 2004; 111: 169–180. [DOI] [PubMed] [Google Scholar]
- 58. Clohisy DR, Mantyh PW. Bone cancer pain and the role of RANKL/OPG. J Musculoskelet Neuronal Interact 2004; 4: 293–300. [PubMed] [Google Scholar]
- 59. Ringe JD, Body JJ. A review of bone pain relief with ibandronate and other bisphosphonates in disorders of increased bone turnover. Clin Exp Rheumatol 2007; 25: 766–774. [PubMed] [Google Scholar]
- 60. Body JJ, Diel IJ, Bell R, Pecherstorfer M, Lichinitser MR, Lazarev AF, et al Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 2004; 111: 306–312. [DOI] [PubMed] [Google Scholar]
- 61. Chawla S, Henshaw R, Seeger L, Choy E, Blay JY, Ferrari S, et al Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open‐label, parallel‐group, phase 2 study. Lancet Oncol 2013; 14: 901–908. [DOI] [PubMed] [Google Scholar]
- 62. Martin‐Broto J, Cleeland CS, Glare PA, Engellau J, Skubitz KM, Blum RH, et al Effects of denosumab on pain and analgesic use in giant cell tumor of bone: interim results from a phase II study. Acta Oncol 2014; 53: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 63. Grasemann C, Schundeln MM, Hovel M, Schweiger B, Bergmann C, Herrmann R, et al Effects of RANK‐ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget's disease. J Clin Endocrinol Metab 2013; 98: 3121–3126. [DOI] [PubMed] [Google Scholar]
- 64. Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, et al Denosumab treatment for fibrous dysplasia. J Bone Miner Res 2012; 27: 1462–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chapurlat RD, Gensburger D, Jimenez‐Andrade JM, Ghilardi JR, Kelly M, Mantyh P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis 2012; 7 (Suppl. 1): S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nencini S, Ringuet M, Kim DH, Greenhill C, Ivanusic JJ. GDNF, Neurturin, and Artemin activate and sensitize bone afferent neurons and contribute to inflammatory bone pain. J Neurosci 2018; 38: 4899–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gavioli E, Abrams M. Prevention of granulocyte‐colony stimulating factor (G‐CSF) induced bone pain using double histamine blockade. Support Care Cancer 2017; 25: 817–822. [DOI] [PubMed] [Google Scholar]
- 68. Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, et al A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell 2015; 27: 780–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guise TA. Breaking down bone: new insight into site‐specific mechanisms of breast cancer osteolysis mediated by metalloproteinases. Genes Dev 2009; 23: 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, et al Endothelin and the tumorigenic component of bone cancer pain. Neuroscience 2004; 126: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 71. Sin A, Tang W, Wen CY, Chung SK, Chiu KY. The emerging role of endothelin‐1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthr Cartil 2015; 23: 516–524. [DOI] [PubMed] [Google Scholar]
- 72. Hess A, Axmann R, Rech J, Finzel S, Heindl C, Kreitz S, et al Blockade of TNF‐alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A 2011; 108: 3731–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hall BE, Zhang L, Sun ZJ, Utreras E, Prochazkova M, Cho A, et al Conditional TNF‐alpha overexpression in the tooth and alveolar bone results in painful pulpitis and Osteitis. J Dent Res 2016; 95: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jimenez‐Andrade JM, Mantyh WG, Bloom AP, Xu H, Ferng AS, Dussor G, et al A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 2010; 46: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ghilardi JR, Allen CJ, Vigna SR, McVey DC, Mantyh PW. Cholecystokinin and neuropeptide Y receptors on single rabbit vagal afferent ganglion neurons: site of prejunctional modulation of visceral sensory neurons. Brain Res 1994; 633: 33–40. [DOI] [PubMed] [Google Scholar]
- 76. Ghilardi JR, Freeman KT, Jimenez‐Andrade JM, Mantyh WG, Bloom AP, Kuskowski MA, et al Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma‐induced nerve sprouting, neuroma formation and bone cancer pain. Mol Pain 2010; 6: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Obreja O, Rukwied R, Nagler L, Schmidt M, Schmelz M, Namer B. Nerve growth factor locally sensitizes nociceptors in human skin. Pain 2018; 159: 416–426. [DOI] [PubMed] [Google Scholar]
- 78. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 2006; 29: 507–538. [DOI] [PubMed] [Google Scholar]
- 79. Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, et al Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol 1999; 81: 1379–1390. [DOI] [PubMed] [Google Scholar]
- 80. Ghilardi JR, Allen CJ, Vigna SR, McVey DC, Mantyh PW. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: possible site of opiate‐CCK analgesic interactions. J Neurosci 1992; 12: 4854–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mantyh PW, Allen CJ, Rogers S, DeMaster E, Ghilardi JR, Mosconi T, et al Some sensory neurons express neuropeptide Y receptors: potential paracrine inhibition of primary afferent nociceptors following peripheral nerve injury. J Neurosci 1994; 14: 3958–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mantyh CR, Kruger L, Brecha NC, Mantyh PW. Localization of specific binding sites for atrial natriuretic factor in peripheral tissues of the Guinea pig, rat, and human. Hypertension 1986; 8: 712–721. [DOI] [PubMed] [Google Scholar]
- 83. Jimenez‐Andrade JM, Martin CD, Koewler NJ, Freeman KT, Sullivan LJ, Halvorson KG, et al Nerve growth factor sequestering therapy attenuates non‐malignant skeletal pain following fracture. Pain 2007; 133: 183–196. [DOI] [PubMed] [Google Scholar]
- 84. Denk F, Bennett DL, McMahon SB. Nerve growth factor and pain mechanisms. Annu Rev Neurosci 2017; 40: 307–325. [DOI] [PubMed] [Google Scholar]
- 85. Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat‐gated ion channels. EMBO J 2005; 24: 4211–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schnitzer TJ, Lane NE, Birbara C, Smith MD, Simpson SL, Brown MT. Long‐term open‐label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthr Cartil 2011; 19: 639–646. [DOI] [PubMed] [Google Scholar]
- 87. Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, et al Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011; 152: 2248–2258. [DOI] [PubMed] [Google Scholar]
- 88. Majuta LA, Mitchell SAT, Kuskowski MA, Mantyh PW. Anti‐NGF does not change physical activity in normal young or aging mice but does increase activity in mice with skeletal pain. Pain 2018; 159: 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sevcik MA, Ghilardi JR, Peters CM, Lindsay TH, Halvorson KG, Jonas BM, et al Anti‐NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005; 115: 128–141. [DOI] [PubMed] [Google Scholar]
- 90. Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 2007; 148: 560–572. [DOI] [PubMed] [Google Scholar]
- 91. Seidel MF, Lane NE. Control of arthritis pain with anti‐nerve‐growth factor: risk and benefit. Curr Rheumatol Rep 2012; 14: 583–588. [DOI] [PubMed] [Google Scholar]
- 92. Seidel MF, Wise BL, Lane NE. Nerve growth factor: an update on the science and therapy. Osteoarthr Cartil 2013; 21: 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tiseo PJ, Kivitz AJ, Ervin JE, Ren H, Mellis SJ. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double‐blind, placebo‐controlled exploratory study in osteoarthritis of the knee. Pain 2014; 155: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 94. Kivitz AJ, Gimbel JS, Bramson C, Nemeth MA, Keller DS, Brown MT, et al Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013; 154: 1009–1021. [DOI] [PubMed] [Google Scholar]
- 95. Sopata M, Katz N, Carey W, Smith MD, Keller D, Verburg KM, et al Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 2015; 156: 1703–1713. [DOI] [PubMed] [Google Scholar]
- 96. Jimenez‐Andrade JM, Ghilardi JR, Castaneda‐Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti‐NGF therapy attenuates tumor‐induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011; 152: 2564–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yasui M, Shiraishi Y, Ozaki N, Hayashi K, Hori K, Ichiyanagi M, et al Nerve growth factor and associated nerve sprouting contribute to local mechanical hyperalgesia in a rat model of bone injury. Eur J Pain 2012; 16: 953–965. [DOI] [PubMed] [Google Scholar]
- 98. Lindsay TH, Jonas BM, Sevcik MA, Kubota K, Halvorson KG, Ghilardi JR, et al Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain 2005; 119: 233–246. [DOI] [PubMed] [Google Scholar]
- 99. Jimenez‐Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, et al Pathological sprouting of adult nociceptors in chronic prostate cancer‐induced bone pain. J Neurosci 2010; 30: 14649–14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mantyh WG, Jimenez‐Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010; 171: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Walsh DA, McWilliams DF, Turley MJ, Dixon MR, Franses RE, Mapp PI, et al Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 2010; 49: 1852–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tolofari SK, Richardson SM, Freemont AJ, Hoyland JA. Expression of semaphorin 3A and its receptors in the human intervertebral disc: potential role in regulating neural ingrowth in the degenerate intervertebral disc. Arthritis Res Ther 2010; 12: R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, et al Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br 1997; 79: 147–153. [DOI] [PubMed] [Google Scholar]
- 104. Hukkanen M, Konttinen YT, Santavirta S, Paavolainen P, Gu XH, Terenghi G, et al Rapid proliferation of calcitonin gene‐related peptide‐immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience 1993; 54: 969–979. [DOI] [PubMed] [Google Scholar]
- 105. Driscoll C, Chanalaris A, Knights C, Ismail H, Sacitharan PK, Gentry C, et al Nociceptive sensitizers are regulated in damaged joint tissues, including articular cartilage, when osteoarthritic mice display pain behavior. Arthritis Rheumatol 2016; 68: 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro . Arthritis Rheum 2002; 46: 2658–2664. [DOI] [PubMed] [Google Scholar]
- 107. Johnson WE, Caterson B, Eisenstein SM, Roberts S. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro . Spine (Phila Pa 1976) 2005; 30: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 108. Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: a prospective study. Pain 2017; 158: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 2017; 18: 113. [DOI] [PubMed] [Google Scholar]
- 110. Woolf CJ, Wall PD. Relative effectiveness of C primary afferent fibers of different origins in evoking a prolonged facilitation of the flexor reflex in the rat. J Neurosci 1986; 6: 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 112. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152 (Suppl. 3): S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mantyh PW. The spinothalamic tract in the primate: a re‐examination using wheatgerm agglutinin conjugated to horseradish peroxidase. Neuroscience 1983; 9: 847–862. [DOI] [PubMed] [Google Scholar]
- 114. Honore P, Rogers SD, Schwei MJ, Salak‐Johnson JL, Luger NM, Sabino MC, et al Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000; 98: 585–598. [DOI] [PubMed] [Google Scholar]
- 115. Sikandar S, West SJ, McMahon SB, Bennett DL, Dickenson AH. Sensory processing of deep tissue nociception in the rat spinal cord and thalamic ventrobasal complex. Physiol Rep 2017; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Graven‐Nielsen T, Arendt‐Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010; 6: 599–606. [DOI] [PubMed] [Google Scholar]
- 117. Arendt‐Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al Sensitization in patients with painful knee osteoarthritis. Pain 2010; 149: 573–581. [DOI] [PubMed] [Google Scholar]
- 118. Yanagisawa Y, Furue H, Kawamata T, Uta D, Yamamoto J, Furuse S, et al Bone cancer induces a unique central sensitization through synaptic changes in a wide area of the spinal cord. Mol Pain 2010; 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Arendt‐Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, et al Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 2018; 22: 216–241. [DOI] [PubMed] [Google Scholar]
- 120. Enomoto M, Mantyh PW, Murrell J, Innes JF, Lascelles DX. Anti‐nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. Vet Rec 2018; 10.1136/vr.104590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010; 363: 1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]


