Abstract
Aims
Sodium glucose co‐transporter‐2 inhibitors have been shown to reduce cardiovascular events and heart failure in type 2 diabetic (T2D) patients with high cardiovascular risk. Dipeptidyl peptidase‐4 inhibitors showed neutral effects and may increase risk of heart failure. We aimed to compare cardiometabolic effects of dapagliflozin and vildagliptin in T2D patients with coronary artery disease (CAD).
Methods
Forty‐nine T2D patients with CAD were randomly assigned to dapagliflozin (n = 25) or vildagliptin (n = 24) for 6 months in a double‐blind fashion. Cardiometabolic parameters were collected at baseline and at the end of treatments.
Results
Mean age was 63.2 ± 7.9 years (female 46.9%). Baseline characteristics did not differ between two groups. At 6 months, HbA1C significantly decreased in both dapaglifozin and vildagliptin groups (0.6 ± 1.0% vs 0.8 ± 1.4%, P = 0.22, respectively). There was no difference between the changes in lipid profiles. Body mass index decreased in patients receiving dapagliflozin, whereas it increased in those receiving vildagliptin (−1.27 [95% confidence interval −2.01, −0.53] vs 1.72 [0.72, 2.72] kg, P < 0.001). The reduction in systolic blood pressure and high‐sensitivity troponin T was observed in the dapagliflozin group (−9.87 [−18.00, −1.15] mmHg and 2.49 [−4.50, −0.47] pg/mL) but not in vildagliptin group (−1.97 [−9.42, 5.48] mmHg and 1.98 [−0.02, 3.97] pg/mL). The mean haemoglobin increased in the dapagliflozin group, whereas the mean platelet volume increased in the vildagliptin group. There was no significant change in the inflammatory markers in both the groups.
Conclusions
The extraglycaemic effects of dapagliflozin and vildagliptin on cardiometabolic parameters in T2D with CAD were different. The more favourable effects of dapagliflozin compared to vildagliptin may have explained the cardiovascular benefits observed only in sodium glucose co‐transporter‐2 inhibitors.
Keywords: coronary artery disease, dapagliflozin, diabetes, dipeptidyl peptidase‐4 inhibitors, sodium glucose co‐transporter‐2 inhibitors, vildagliptin
Abbreviations
- ACS
Acute coronary syndrome
- CAD
Coronary artery disease
- DPP‐4
Dipeptidyl peptidase‐4
- GLP‐1
Glucagon like peptide‐1
- SGLT2
Sodium glucose co‐transporter 2
What is already known about this subject
Glucose lowering agents have different effects on cardiovascular outcomes in type 2 diabetic (T2D) patients with high cardiovascular risk.
Sodium glucose co‐transporter‐2 inhibitors have been shown to reduce cardiovascular events and heart failure in T2D patients with high cardiovascular risk.
Dipeptidyl peptidase‐4 inhibitors showed neutral effects and may increase risk of heart failure.
What this study adds
There are differences in cardiometabolic profile between sodium glucose co‐transporter‐2 inhibitors and dipeptidyl peptidase‐4 inhibitors in T2D patients with coronary artery disease.
Dapagliflozin possesses favourable effects including body weight reduction, blood pressure reduction, increased haemoglobin and high‐sensitivity cardiac troponin reduction.
Vildagliptin had neutral effect on haemodynamic profiles but increased mean platelet volume.
1. INTRODUCTION
Diabetes is associated with 2‐ to 4‐fold increase in the risk of atherosclerotic cardiovascular events including coronary artery disease (CAD), cerebrovascular disease and peripheral arterial disease1, 2 Furthermore, diabetic patients who had myocardial infarction had worse prognosis compared to patients with either diabetes or myocardial infarction.3 Multifaceted interventions including tight glycaemic control and blood pressure control have been shown to reduce microvascular complications such as nephropathy or retinopathy.4, 5 However, the effect of intensive glucose lowering on cardiovascular outcomes has not yet been clearly elucidated. It has been described that the duration of diabetes and comorbidities may have accounted for those heterogeneous outcomes. In addition, the effects of glucose lowering agents on cardiometabolic profile may have contributed to the differences in cardiovascular outcomes.
Dipeptidyl peptidase‐4 (DPP‐4) is an enzyme that degrades hormone, leading to decreased insulin secretion and increase glucagon release. Inhibition of DPP‐4 can improve glycaemic control with some favourable effects on atherosclerotic biomarkers, including anti‐inflammation and antiproliferation.6, 7 However, DPP‐4 inhibitors including saxagliptin, sitagliptin and alogliptin showed neutral effects on cardiovascular outcomes in patients with high atherosclerotic risk or with acute coronary syndrome (ACS).8, 9, 10 In addition, there are concerns that some DDP‐4 inhibitors may increase risk of heart failure (HF), particularly in patients with elevated B‐type natriuretic peptide at baseline, previous history of HF or chronic kidney disease.11
The sodium glucose co‐transporter‐2 (SGLT2) protein is a high‐capacity molecule responsible for the majority of glucose reuptake from renal proximal tubule.12 SGLT2 inhibition using empagliflozin or canagliflozin has been shown to improve cardiovascular outcomes in patients with high cardiovascular risk or with established CAD.13, 14 In addition to glucose‐lowering effects, SGLT2 inhibitors exert additional effects on some cardiometabolic parameters such as body weight and blood pressure by effecting a decrease in them.13, 14 Although the benefits and/or safety of both DPP‐4 inhibitors and SGLT2 inhibitors in patients with CAD have been demonstrated, a comparison of the effects between DPP‐4 inhibitors and SGLT2 inhibitors on the cardiometabolic parameters has never been carried out before in this group of patients. Therefore, this study was conducted to compare the effects of SGLT2 inhibitors (dapagliflozin) with the effects of DPP‐4 inhibitors (vildagliptin) on the cardio‐metabolic parameters in type 2 diabetic patients with CAD.
2. METHODS
2.1. Study design
This was a prospective randomised double‐blinded study. The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines, and was approved by the Institutional Research Board of Faculty of Medicine, Chiang Mai University. All patients provided written informed consent before the study entry. The study was registered in ClinicalTrials.gov, the identification number is NCT03178591.
2.2. Study patients
Adult patients (age ≥ 18 y), male or non‐child bearing potential female with type 2 diabetes who had glycated haemoglobin (HbA1c) level within 6.5–9.0% and an established CAD were eligible for the study. Established CAD was defined as stable angina with >70% stenosis of at least 1 major epicardial artery from coronary angiogram or coronary computed tomography angiography, or post myocardial infarction (>30 days) with at least one non–infarct‐related artery stenosis (>70% stenosis) from coronary angiogram. Patients with following the conditions were excluded from the study: significant renal function (estimated glomerular filtration rate <30 mL/min); significant hepatic impairment or ALT/AST elevations beyond 2 times of upper normal limit or known hepatic failure; planned coronary intervention or planed surgical intervention (percutaneous coronary intervention or coronary artery bypass grafting); recent (<30 d) ACS; hypersensitivity to either of the study drug components; history of lactic acidosis or diabetic ketoacidosis; current treatment with insulin or GLP‐1 agonist, DPP‐4 inhibitors or SGLT2 inhibitors; inability to comply with study protocol; active malignancy other than basal cell carcinoma; clinically advanced congestive HF (New York Heart Association class III‐IV); recent HF decompensation (<3 months); chronic inflammation (i.e. inflammatory bowel disease, lupus, inflammatory arthritis, rheumatoid arthritis) or chronic infection (i.e. chronic diabetic foot infection); and pregnancy, lactation or child‐bearing potential.
2.3. Study procedure
The eligible patients were randomly assigned in a 1:1 ratio to receive either 10 mg of dapagliflozin or 50–100 mg of vildagliptin (according to glomerular filtration rate). Randomisation was performed with the use of a computer‐generated random‐sequence number in block‐of‐4 randomisation manner. The treatment of CAD and other atherosclerotic risk factors were optimised before the enrolment. Background therapy of CAD and diabetes was to remain unchanged during follow‐up period unless there were adverse effects related to medications. After the randomisation visit, patients were followed at 4, 12 and 24 weeks for the end of treatment visit. During these visits, both clinical and adverse events were monitored, medication compliance was verified, and any hypoglycaemic events were evaluated.
2.4. Study outcomes
The study outcomes included changes in the haemodynamic biomarkers, metabolic biomarkers and inflammatory biomarkers at 6 months. The secondary outcome comprised major adverse cardiovascular events including all causes of death, nonfatal myocardial infarction, nonfatal stroke or HF hospitalisation during the 6 months.
2.5. Measurement of biomarkers
Sitting blood pressure (BP) and pulse rate were determined after 10 minutes of rest during clinic visits using automated BP device (OMRON BP monitor). The average of 3 consecutive measurements were recorded. At baseline visit, the BP reading was obtained from both arms and the arm with higher BP reading was used in the subsequent visits. Body weight, height and waist–hip ratio were determined with standard procedure before meal.
Fasting blood sample was collected after an overnight fasting of at least 12 h. Blood glucose, HbA1C, lipid profile, high‐sensitivity troponin T (hs‐TnT), high‐sensitivity C‐reactive protein (hs‐CRP) and N‐terminal B type natriuretic peptide (NT‐proBNP) were determined with standard hospital clinical chemistry analysers. Plasma glucose was measured with enzymatic reference method with hexokinase using the cobas c702 (Roche Diagnostic). HbA1c was measured with turbidimetric inhibition immunoassay using cobas c502. Lipid profile was measured with enzymatic colorimetric using the cobas c702. NT‐proBNP and hs‐TnT were measured with electrochemiluminescence technique using the cobas e801(Roche Diagnostic). Hs‐CRP was evaluated with particle‐enhanced immunoturbidimetric assay using the cobas c502 system (Roche Diagnostic).
Commercially available quantitative sandwich enzyme‐linked immunosorbent assay (ELISA) kits were used for plasma tumour necrosis factor‐α (TNF‐α), interleukin‐10 (IL‐10) and 8‐iso‐prostaglandin analysis, in accordance with protocols provided by the test manufacturer. Plasma was collected and kept frozen at −80°C immediately until analysis. Plasma TNF‐α, IL‐10 and 8‐iso‐prostaglandin levels were determined using the TNF‐α human ELISA kit (KHC3011, Thermo Fisher Scientific, MA, USA), IL‐10 human ELISA kit (KHC0101, Thermo Fisher Scientific) and OxiSelect™ 8‐iso‐Prostaglandin F2α ELISA kit (STA‐337, Cell Biolabs, Inc.).
2.6. Statistical analysis
The data analysis for safety and secondary outcomes was based on intention‐to‐treat analysis defined as all randomised patients who received at least a single dose of study medication. The data analysis for the primary outcomes was based on per protocol analysis including only patients who had baseline as well as 6 months outcomes measurements. The categorical data are presented as n (%) and compared between groups with Fisher's exact test. The continuous data are presented as mean ± SD or medial interquartile range and compared between groups with Student t‐test or nonparametric test where appropriate. The changes in the continuous data within the group were analysed using the paired t‐test, and the mean changes in each group were compared between groups using the nonparametric test.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,15 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.16, 17
3. RESULTS
A total of 49 patients were enrolled into the study and 43 patients completed the study (21 and 22 patients in dapagliflozin and vildagliptin respectively; Figure 1). The baseline characteristics are presented in Table 1. The mean age was 63.22 ± 7.91 years and 46.90% of patients were female. Hypertension, dyslipidaemia, HF and chronic kidney disease were presented in, respectively, 77.60%, 89.80%, 24.50% and 12.20% of the patients.
Figure 1.
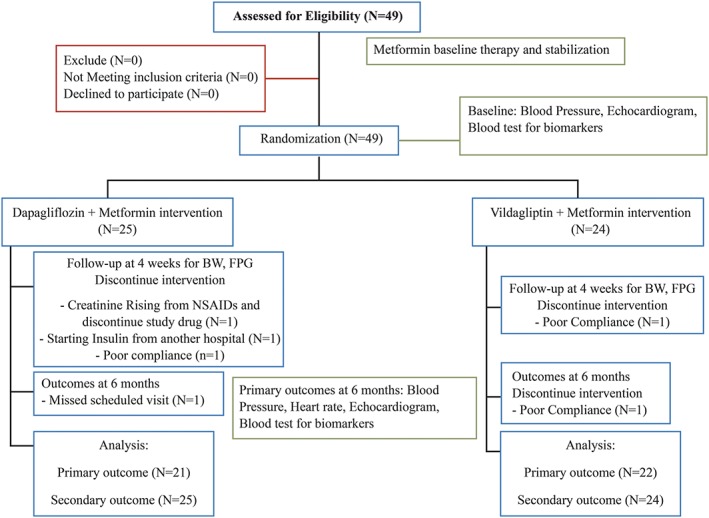
Patients flow diagram. BW, body weight, FPG, fasting plasma glucose; NSAIDs, nonsteroidal inflammatory drugs
Table 1.
Baseline characteristics of the patients receiving dapagliflozin and vildagliptin
| Total (n = 49) | Dapagliflozin (n = 25) | Vildagliptin (n = 24) | P‐value | |
|---|---|---|---|---|
| Age (y) | 63.22 ± 7.91 | 62.60 ± 8.27 | 63.88 ± 7.65 | .578 |
| Female (n, %) | 23 (46.90) | 11 (44.00) | 12 (50.00) | .778 |
| Hypertension (n, %) | 38 (77.60) | 18 (72.00) | 20 (83.30) | .496 |
| Dyslipidaemia (n, %) | 44 (89.80) | 21 (84.00) | 23 (95.80) | .349 |
| Chronic renal disease (n, %) | 6 (12.20) | 2 (8.00) | 4 (16.70) | .417 |
| Heart failure (n, %) | 12 (24.50) | 5 (20.00) | 7 (29.20) | .520 |
| Prior myocardial infarction (n, %) | 33 (67.30) | 15 (60.00) | 18 (75.00) | .364 |
| Coronary revascularisation (n, %) | 35 (71.40) | 17 (68.00) | 18 (75.00) | .754 |
| Medication | ||||
| Aspirin (n, %) | 46 (93.90) | 23 (92.00) | 23 (95.80) | >.999 |
| Clopidogrel (n, %) | 21 (42.90) | 11 (44.00) | 10 (41.70) | >.999 |
| Beta‐blocker (n, %) | 45 (91.80) | 23 (92.00) | 22 (91.70) | >.999 |
| ACEi (n, %) | 26 (53.10) | 12 (48.00) | 14 (58.30) | 0.571 |
| ARB (n, %) | 14 (28.60) | 7 (28.00) | 7 (29.20) | >.999 |
| CCB (n, %) | 11 (22.40) | 6 (24.00) | 5 (20.80) | >.999 |
| Nitrate (n, %) | 11 (22.40) | 5 (20.00) | 6 (25.00) | 0.742 |
| Diuretic (n, %) | 24 (49.00) | 12 (48.00) | 12 (50.00) | 1.000 |
| Statin (n, %) | 48 (98.00) | 24 (96.00) | 24 (100.00) | 1.000 |
| Sulfonylureas (n, %) | 37 (75.50) | 20 (80.00) | 17 (70.80) | 0.520 |
| Metformin (n, %) | 44 (89.80) | 23 (92.00) | 21 (87.50) | 0.667 |
| Thiazolidinediones (n, %) | 5 (10.20) | 2 (8.00) | 3 (12.50) | 0.480 |
Data are mean ± standard deviation or n (%).
ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CCB, calcium channel blocker.
The mean body weight, body mass index (BMI), waist circumference, systolic BP (SBP) and diastolic BP were 63.06 ± 11.93 kg, 25.28 ± 3.06 kg/m2, 90.57 ± 9.89 cm, 124.50 ± 17.88 mmHg and 67.97 ± 8.66 mmHg, respectively (Table 2). The mean HbA1c, total cholesterol, low‐density lipoprotein–cholesterol, high‐density lipoprotein–cholesterol and triglyceride were 8.21 ± 1.27%, 153.10 ± 35.73 mg/dL, 91.74 ± 37.20 mg/dL, 45.88 ± 13.70 mg/dL and 168.07 ± 65.43 mg/dL, respectively. The baseline characteristics were not significantly different between two treatment groups.
Table 2.
Baseline characteristics of cardiometabolic parameters of the patients receiving dapagliflozin and vildagliptin
| Total (n = 49) | Dapagliflozin (n = 25) | Vildagliptin (n = 24) | P value | |
|---|---|---|---|---|
| Body weight (kg) | 63.06 ± 11.93 | 63.70 ± 10.44 | 62.38 ± 13.50 | .705 |
| BMI (kg/m2) | 25.28 ± 3.06 | 25.63 ± 3.00 | 24.90 ± 3.158 | .408 |
| Waist circumference (cm) | 90.57 ± 9.89 | 89.86 ± 8.27 | 91.46 ± 11.77 | .610 |
| Waist–hip ratio | 0.96 ± 0.08 | 0.95 ± 0.06 | 0.97 ± 0.10 | .542 |
| Systolic blood pressure (mmHg) | 124.50 ± 17.88 | 124.75 ± 20.63 | 124.24 ± 14.92 | .921 |
| Diastolic blood pressure (mmHg) | 67.97 ± 8.66 | 67.39 ± 7.58 | 68.57 ± 9.79 | .639 |
| Fasting plasma glucose (mg/dL) | 143.49 ± 35.20 | 148.56 ± 42.29 | 138.21 ± 25.75 | .305 |
| HbA1c (%) | 8.21 ± 1.27 | 8.17 ± 1.41 | 8.25 ± 1.13 | .811 |
| Total cholesterol (mg/dL) | 153.10 ± 35.73 | 144.86 ± 33.23 | 162.15 ± 36.99 | .121 |
| LDL cholesterol (mg/dL) | 91.74 ± 37.20 | 82.20 ± 30.45 | 101.27 ± 41.42 | .090 |
| HDL cholesterol (mg/dL) | 45.88 ± 13.70 | 47.10 ± 16.75 | 44.67 ± 10.05 | .573 |
| Triglyceride (mg/dL) | 168.07 ± 65.43 | 163.00 ± 66.09 | 173.14 ± 65.99 | .621 |
| Creatinine (mg/dL) | 1.05 ± 0.34 | 1.01 ± 0.29 | 1.10 ± 0.39 | .378 |
| eGFR (mL/min/1.73m2) | 69.99 ± 20.65 | 72.11 ± 19.59 | 67.79 ± 21.90 | .471 |
| NT‐proBNP (pg/mL) | 672.18 ± 1359.04 | 399.94 ± 493.10 | 955.76 ± 1853.75 | .154 |
| hs‐TnT (pg/mL) | 15.80 ± 12.29 | 14.54 ± 9.46 | 17.10 ± 14.78 | .477 |
| hs‐CRP (mg/L) | 1.52 ± 1.75 | 1.55 ± 1.78 | 1.48 ± 1.79 | .913 |
| Left ventricular ejection fraction (%) | 57.07 ± 14.64 | 57.18 ± 16.28 | 56.95 ± 13.06 | .957 |
All data are means ± standard deviation. BMI, body mass index; HbA1c, haemoglobin A1c; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; hs‐TnT, high‐sensitivity troponin T; hs‐CRP, high‐sensitivity C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, how‐density lipoprotein.
3.1. Effects of dapagliflozin and vildagliptin on metabolic and haemodynamic biomarkers
During 6 months of treatment, HbA1C was observed to have significantly decreased in both the groups (−0.63 ± 1.04% and − 0.84 ± 1.43%, P = 0.22, for dapagliflozin and vildagliptin, respectively) and the change was not different between the groups. There was no significant change in the lipid profiles of the two groups (Table 3).
Table 3.
Changes in haemodynamic and metabolic parameters during treatment with dapagliflozin and vildagliptin
| Total (n = 43) | Dapagliflozin (n = 21) | Vildagliptin (n = 22) | P value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | ||
| Body weight (kg) | 63.64 ± 12.39 | 63.91 ± 12.41 | 64.57 ± 10.94 | 63.30 ± 10.58 | 62.78 ± 13.83 | 64.48 ± 14.17 | |
| Change from baseline | 0.26 (−0.50, 1.02) | −1.27 (−2.01, −0.53)** | 1.72 (0.72, 2.72)** | <.001*** | |||
| BMI (kg/m2) | 25.37 ± 3.14 | 25.49 ± 3.24 | 25.92 ± 2.95 | 25.43 ± 2.97 | 24.84 ± 3.28 | 25.55 ± 3.55 | |
| Change from baseline | 0.12 (−0.19, 0.43) | −0.49 (−0.80, −0.18)** | 0.71 (0.30, 1.12)** | <.001*** | |||
| WC (cm) | 91.44 ± 9.91 | 91.38 ± 8.65 | 90.74 ± 8.36 | 89.63 ± 7.35 | 92.22 ± 11.58 | 93.32 ± 9.73 | |
| Change from baseline | −0.06 (−1.79, 1.67) | −1.11 (−3.03, 0.82) | 1.09 (−1.97, 4.16) | .436 | |||
| SBP (mmHg) | 124.98 ± 18.51 | 119.15 ± 12.75 | 125.65 ± 21.98 | 115.78 ± 11.41 | 124.33 ± 14.98 | 122.36 ± 13.37 | |
| Change from baseline | −5.83 (−11.24, −0.42)* | −9.87 (−18.00, −1.75)** | −1.97 (−9.42, 5.48) | .166 | |||
| DBP (mmHg) | 68.48 ± 9.02 | 65.74 ± 9.79 | 67.76 ± 8.17 | 63.79 ± 6.52 | 69.17 ± 9.90 | 67.59 ± 11.98 | |
| Change from baseline | −2.74 (−5.60, 0.11) | −3.97(−8.26, 0.32) | −1.58 (−5.66, 2.51) | .636 | |||
| HR (beats/min) | 73.93 ± 8.33 | 73.05 ± 10.02 | 74.00 ± 8.51 | 72.77 ± 1.57 | 73.86 ± 8.34 | 73.33 ± 9.67 | |
| Change from baseline | −0.88 (−3.39, 1.63) | −1.23 (−4.63, 2.18) | −0.52 (−4.53, 3.49) | .567 | |||
| FBS (mg/dL) | 148.00 ± 35.78 | 115.80 ± 33.04 | 154.19 ± 42.27 | 116.86 ± 35.22 | 141.50 ± 26.96 | 114.70 ± 31.47 | |
| Change from baseline | −32.20 (−45.30, −19.09)* | −37.33 (−56.60, −18.07)** | −26.80 (−46.00, −7.60)** | .389 | |||
| HbA1c (%) | 8.18 ± 1.25 | 7.45 ± 1.03 | 8.11 ± 1.36 | 7.48 ± .98 | 8.25 ± 1.18 | 7.41 ± 1.10 | |
| Change from baseline | −0.74 (−1.12, −0.35)* | −0.63 (−1.10, −0.16)** | −0.84 (−1.48, −0.21)** | .220 | |||
| Cholesterol (mg/dL) | 153.55 ± 36.60 | 146.79 ± 38.09 | 146.55 ± 33.79 | 142.80 ± 34.55 | 161.33 ± 38.95 | 151.22 ± 42.23 | |
| Change from baseline | −6.76 (−17.47, 3.95) | −3.75 (−16.17, 8.67) | −10.11 (−29.38, 9.16) | .303 | |||
| HDL‐C (mg/dL) | 46.55 ± 14.18 | 44.26 ± 8.73 | 47.47 ± 17.59 | 43.53 ± 1.16 | 45.63 ± 10.10 | 45.00 ± 7.23 | |
| Change from baseline | −2.29 (−6.20, 1.62) | −3.95 (−11.34, 3.45) | −0.63 (−4.01, 2.74) | .908 | |||
| LDL‐C (mg/dL) | 92.26 ± 38.72 | 86.95 ± 31.25 | 82.72 ± 31.54 | 84.35 ± 29.27 | 101.80 ± 43.49 | 89.55 ± 33.67 | |
| Change from baseline | −5.31 (−16.65, 6.03) | 1.63 (−10.35, 13.61) | −12.25 (−32.24, 7.74) | .341 | |||
| Triglyceride (mg/dL) | 163.92 ± 63.55 | 174.16 ± 89.32 | 164.79 ± 68.58 | 163.89 ± 86.50 | 163.05 ± 59.98 | 184.42 ± 93.24 | |
| Change from baseline | 10.24 (−19.41, 39.88) | −0.90 (−43.83, 42.04) | 21.37 (−23.13, 65.36) | .452 | |||
| GFR (mL/min/1.73m2) | 72.43 ± 19.62 | 70.52 ± 20.53 | 74.32 ± 17.48 | 74.66 ± 17.53 | 70.45 ± 21.93 | 66.17 ± 22.92 | |
| Change from baseline | −1.91 (−6.28, 2.45) | 0.34 (−7.34, 8.02) | −4.28 (−8.77, 0.21) | .251 | |||
| Haemoglobin (g/dL) | 12.30 ± 1.71 | 12.73 ± 2.04 | 12.59 ± 1.10 | 13.57 ± 1.51 | 12.01 ± 2.01 | 11.91 ± 2.18 | |
| Change from baseline | 0.43 (0.10, 0.75)* | 0.98 (0.54, 1.42)** | −0.10 (−0.48, 0.29) | .001*** | |||
| Mean platelet volume (fL) | 8.56 ± 0.10 | 8.84 ± 1.25 | 8.98 ± 1.05 | 8.89 ± 1.05 | 8.15 ± 0.74 | 8.79 ± 1.07 | |
| Change from baseline | 0.28 (−0.06, 0.62) | −0.09 (−0.59, 0.40) | 0.64 (0.19, 1.10)** | .011*** | |||
| NT‐proBNP (pg/mL) | 645.19 ± 1406.99 | 314.47 ± 335.06 | 433.94 ± 516.52 | 309.83 ± 314.01 | 837.23 ± 1882.13 | 318.70 ± 360.46 | |
| Change from baseline | −330.72 (−733.34, 71.90) | −124.12 (−284.01, 35.78) | −518.54 (−1296.14, 259.07) | .465 | |||
| hs‐TnT (pg/mL) | 16.15 ± 13.09 | 16.00 ± 14.88 | 14.67 ± 10.31 | 12.18 ± 9.67 | 17.50 ± 15.30 | 19.48 ± 17.91 | |
| Change from baseline | −0.15 (−1.68, 1.38) | −2.49 (−4.50, −0.47)** | 1.98 (−0.02, 3.97) | .002*** | |||
Data are means ± standard deviation or (95% confidence interval of the difference). P < .05 was considered significant. BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; FBS, fasting blood sugar; HbA1c, haemoglobin A1c; GFR, glomerular filtration rate; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; hs‐TnT, high‐sensitivity troponin T; HDL‐C, high‐density lipoprotein–cholesterol; LDL‐C, low‐density lipoprotein–cholesterol.
P < .05; comparison between before and after treatment in all subject.
P < .05; comparison between before and after treatment in each group.
P < .05; comparison between dipeptidyl peptidase‐4 inhibitor and sodium glucose co‐transporter‐2 inhibitor groups.
Body weight and body mass index were found to have significantly reduced in the dapagliflozin group (−1.27 ± 1.61, P = .002, and − 0.49 ± 0.67, P = .003, for body weight and BMI, respectively). In contrast, significant increase in these parameters was found in the vildagliptin group (1.72 ± 2.25 kg, P = 0.002, and 0.71 ± 0.93 kg/m2, P = .002, for body weight and BMI, respectively). Early reduction in the body weight and the BMI was demonstrated from 4 weeks of treatment in the dapagliflozin group (−1.09 ± 1.07 kg and 0.45 ± 0.47 kg/m2, P < .001, for both comparisons). There was no significant change observed in the waist circumference in either of the groups (Table 3 and Figure 2).
Figure 2.
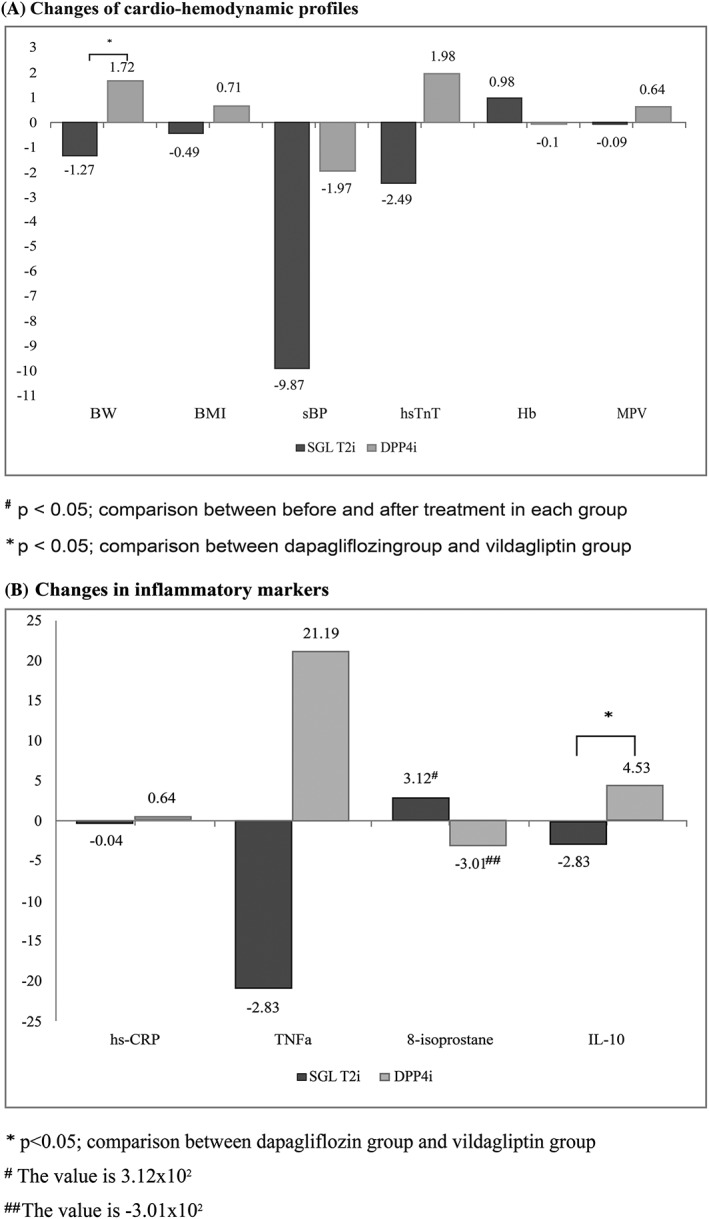
Changes of cardiometabolic parameters. A, Changes of cardiohaemodynamic profiles B, changes in inflammatory markers
The SBP was significantly decreased in the dapagliflozin group but did not change in the vildagliptin group (−9.87 ± 17.86 mmHg, P = .02, and − 1.97 ± 16.80 mmHg, P = .58, for dapagliflozin and vildagliptin, respectively). The diastolic BP and heart rate were not found to have significantly changed in either group. The mean haemoglobin was significantly increased in the dapagliflozin group (0.98 ± 0.97 mg/dL, P < .001), whereas no change was seen in the vildagliptin group (−0.10 ± 0.87 mg/dL, P = .61). The glomerular filtration rate and NT‐proBNP did not change significantly in either of the groups.
Hs‐TnT significantly decreased in the dapagliflozin group (−2.49 ± 4.30 pg/mL, P = .02), whereas a nonsignificant increase in hs‐TnT was observed in the vildagliptin group (1.98 ± 4.50 pg/mL, P = .052). The mean platelet volume significantly increased in patients receiving vildagliptin (0.64 ± 0.99 fL, P = .002), whereas no change was seen in patients receiving dapagliflozin (Table 3, Figure 2).
3.2. Effects of dapagliflozin and vildagliptin on inflammatory biomarkers
There was no change in hs‐CRP, TNF‐α and 8‐isoprostane during treatment in both the groups. IL‐10, the anti‐inflammatory marker, was nonsignificantly increased in the vildagliptin group (4.53 ± 10.92 pg/mL, P = .07), whereas there was no change in the dapagliflozin group (−2.83 ± 11.63 pg/mL, P = .29). The changes in IL‐10 were significantly different between the groups in favour of vildagliptin (Table 4 and Figure 2).
Table 4.
Changes in inflammatory biomarkers during treatment with dapagliflozin and vildagliptin
| Total (n = 43) | Dapagliflozin (n = 21) | Vildagliptin (n = 22) | P value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | ||
| TNF‐α | 103.27 ± 209.50 | 103.42 ± 157.41 | 106.67 ± 178.97 | 85.78 ± 164.00 | 99.86 ± 240.91 | 121.05 ± 152.68 | |
| Change from baseline | 0.15 (−79.50, 79.80) | −20.89 (−130.58, 88.80) | 21.19 (−103.92, 146.29) | .600 | |||
| IL‐10 | 5.08 ± 8.25 | 6.02 ± 8.92 | 6.52 ± 9.92 | 3.69 ± 7.52 | 3.72 ± 6.21 | 8.25 ± 9.74 | |
| Change from baseline | 0.94 (−2.76, 4.65) | −2.83 (−8.27, 2.62) | 4.53 (−0.44, 9.50) | .043* | |||
| 8‐isoprostane | 10 005.17 ± 1104.89 | 10 003.62 ± 9647.23 | 8242.86 ± 6487.19 | 8555.64 ± 5546.59 | 11 683.55 ± 14 064.27 | 11 382.64 ± 12 361.98 | |
| Change from baseline | −1.55 (−4876.10, 4873.00) | 312.78 (−2807.40, 3432.96) | −300.91 (−9790.19, 9188.37) | .899 | |||
| hs‐CRP | 1.61 ± 1.83 | 1.91 ± 1.50 | 1.66 ± 1.90 | 1.62 ± 1.56 | 1.56 ± 1.85 | 2.20 ± 1.45 | |
| Change from baseline | 0.30 (−0.36, 0.95) | −0.04 (−0.68,0.60) | 0.64 (−0.61, 1.89) | .652 | |||
Data are means ± standard deviation or (95% confidence interval of the difference), P < 0.05 was considered significant. TNF‐α, tumour necrosis factor‐α; IL‐10, interleukin‐10; hs‐CRP, high‐sensitivity C‐reactive protein.
P < .05; comparison between dipeptidyl peptidase‐4 inhibitor and sodium glucose co‐transporter‐2 inhibitor groups.
3.3. Effects of dapagliflozin and vildagliptin on secondary outcomes and safety
All recruited patients were followed up for secondary outcomes. Only 1 patient in the dapagliflozin group developed major adverse cardiovascular outcomes which was nonfatal myocardial infarction. This patient had prior ACS with multivessel disease and denied coronary revascularisation. There was no death, stroke or HF event. One patient had transient rising serum creatinine because of nonsteroidal inflammatory drug and discontinued dapagliflozin. There was no hypoglycaemic event requiring treatment in either of the groups during the 6‐month follow‐up period.
4. DISCUSSION
Diabetic patients are at risk of atherosclerotic cardiovascular disease and HF as well as microvascular complications.1, 18 Despite the fact that the overall mortality rate in diabetic patients has declined, the risk of mortality in younger diabetic patients with good glycaemic control is still higher than that in nondiabetic population.2 Glucose lowering agents with favourable cardiovascular outcomes should confer an important key to improve long term outcomes in diabetic patients. It has been shown that SGLT2 inhibitors, empagliflozin and canagliflozin, improve cardiovascular outcomes in patients with established cardiovascular disease compared to placebo.13, 14 The risk of HF was also significantly reduced by empagliflozin, canagliflozin and dapagliflozin in patients with established cardiovascular disease or at risk for cardiovascular disease.13, 14, 19 In contrast, DPP‐4 inhibitors such as sitagliptin, alogliptin or saxagliptin had neutral effects on cardiovascular outcome in patients with high risk of cardiovascular disease compared to placebo.8, 10, 11 In addition, there was a concern regarding the risk of hospitalisation from HF from saxagliptin.11 Furthermore, vildagliptin in patients with HF with reduced ejection fraction was associated with an increase in left ventricular volume despite the neutral effect on left ventricular ejection fraction.20 Nevertheless, no direct comparison between therapies with SGLT2 inhibitors and DPP‐4 inhibitors has been investigated previously.
In this study, we demonstrated the significant disparity between the effects of dapagliflozin and vildagliptin on several cardiometabolic parameters in patients with type 2 diabetes and CAD. During 6 months of treatment, dapagliflozin and vildagliptin had the comparable effects on glycaemic control and lipid profile. The effects beyond the glycaemic and lipid parameters probably account for the cardiovascular benefit of SGLT2 inhibitors.
The present study demonstrated that dapagliflozin reduces body weight and SBP. In contrast, vildagliptin was found to increase body weight and it did not change the SBP over a 6‐month follow‐up. The effects of dapagliflozin on blood pressure in this study are in keeping with previous studies that reported significant blood pressure reduction without any effect on the heart rate. The blood pressure‐lowering effect of the SGLT2 inhibitors is attributable to the excretion of sodium and volume as well as the inhibition of the renal renin angiotensin system activation due to enhanced sodium delivery to the juxtaglomerular apparatus.
The reduction of body weight was observed in the dapagliflozin group since 4 weeks of treatment and was sustained throughout 6 months, which was consistent with previous studies.21 The rapid change of body weight within the 1st month of therapy was possibly due to the effect of SGLT2 inhibitor therapy on the plasma volume reduction in the early phase.13, 14, 22 The negative energy balance from urinary excretion of glucose and reduction in body fat has been taken in to account for the sustained reduction of body weight.23 In contrast to previous report that showed neutral or weight reduction effect of vildagliptin,24 patients receiving vildagliptin in our study had weight gain. Body weight change may be contributed by several factors affecting calorie balance such as life style change or medications. The calorie balance related with baseline glucose and renal threshold has been shown to determine the body weight change during vildagliptin treatment. Patients with negative calorie balance from high plasma glucose at baseline had weight gain during treatment.25
It is well‐established that weight gain and high SPB are the strong risk factors of adverse cardiovascular outcomes. The favourable effects of dapagliflozin on these 2 parameters probably contributed to the cardiovascular benefits of SGLT2 inhibitors shown in clinical studies.
Hs‐TnT is a marker of myocardial injury, which has been shown to be associated with adverse cardiovascular outcomes in CAD and diabetes.26, 27 The hs‐TnT level significantly decreased in patients receiving dapagliflozin but increased in those receiving vildagliptin. Consistent with our findings, canagliflozin has been shown to attenuate the rise of troponin during 104 weeks of follow‐up28 and also significantly reduced cardiovascular events in patients with high cardiovascular risk with longer follow‐up.14 These findings corresponded with the cardiovascular benefits in patients receiving SGLT2 inhibitors.
The mean platelet volume is a marker of platelet reactivity and associated with glycaemic level in diabetic patients. Similar to hs‐TnT, the mean platelet volume is also associated with cardiovascular pathologies including atherosclerotic cardiovascular disease, HF and mortality.29, 30, 31, 32, 33, 34 Our results showed that patients receiving vildagliptin had a significant increase in the mean platelet volume, while those receiving dapagliflozin had a stable mean platelet volume. Although the clinical significance of temporal change of mean platelet volume is still uncertain, the elevation of mean platelet volume as well as hsTn may reflect the potential risk of HF during DPP‐4 inhibitor therapy.
Previous studies have shown that SGLT2 inhibitors decreased the risk of HF hospitalisation. The reduction of plasma volume and blood pressure without increased sympathetic activity are potential mechanism for early and substantial reduction of HF events in patients receiving SGLT2 inhibitors.13, 14, 22, 35, 36 However, we did not find the significant effects of dapagliflozin on NT‐proBNP level. A previous study also demonstrated that NT‐proBNP slightly increased while plasma volume decreased during treatment with dapagliflozin.9 The increase in haemoglobin level was noted in patients receiving dapagliflozin in our study, which is in accordance with previous studies. Several investigators have shown that dapagliflozin increased erythropoietin and reticulocyte count, which resulted in the increased haemoglobin level and the enhanced BNP secretion.22, 37, 38 As a result, BNP or NT‐proBNP may not reflect the adverse HF events and should not be used as a surrogate marker for HF outcomes in patients treating with dapagliflozin.
The anti‐inflammatory effects of vildagliptin and dapagliflozin have been shown in previous studies.6, 39, 40, 41, 42 In our study, the effect of dapagliflozin and vildagliptin on inflammatory markers did not differ significantly. Although the nonsignificantly increased IL‐10 level was found in the vildagliptin treated group and the changes between groups showed favourably effects in vildagliptin group. These findings suggest that anti‐inflammatory properties might not play an important role in cardiovascular benefits of SGLT2 inhibitors.
The findings from our study demonstrated that the effects of dapagliflozin and vildagliptin on extraglycaemic cardiometabolic parameters were different and may contribute to the different in cardiovascular outcomes. The favourable effects of SGLT2 inhibitors on body mass index, BP, platelet activation may contribute to reduction of atherosclerotic cardiovascular events. The reduction of plasma volume and BP without increased sympathetic activity are potential mechanism for early and substantial reduction of HF events in patients receiving empagliflozin and canagliflozin. 13, 14, 19 In addition, our findings support role of extraglycaemic mechanism contributing cardiovascular complications in diabetes.43 These also underlie the importance of multifaceted approach to prevent cardiovascular complication.
This study has a few limitations. The small sample size was a limitation of this study particularly for the secondary outcomes analysis. Studies with larger sample sizes may be warranted to confirm the results of the present study. The per protocol analysis was applied for the primary outcomes, all patients were followed for secondary outcomes. In addition, there was no difference in the characteristics of the patients who did and did not complete treatment. Although the number of recruited patients was limited with regard to stable CAD patients, these findings support the results of the cardiovascular outcome trials of SGLT2 inhibitors and DPP‐4 inhibitors in patients with established atherosclerotic disease and patients at high risk for atherosclerotic disease.
5. CONCLUSIONS
The extraglycaemic effects of dapagliflozin on the cardiometabolic parameters in type 2 diabetes with CAD were different from the extraglycaemic effects of vildagliptin. The more favourable effects of dapagliflozin compared to vildagliptin may explain the benefits of SGLT2 inhibitors and the neutral effect of DPP‐4 inhibitors on cardiovascular outcomes in patients with high cardiovascular risk.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
A.P. designed the study, recruited the patients, performed the statistical analyses, evaluated the results and drafted the paper. W.W., S.G., S.K. and T.J. collected the data and contributed substantially to data preparation and quality assurance. S.C. and N.C. participated in the conception and design of the study, and revised the paper for important intellectual content.
ACKNOWLEDGEMENTS
The authors would like to thank all staffs of Northern Cardiac Center and Division of Cardiology, Department of Internal Medicine, Faculty of Medicine for their assistance throughout this study.
This study was supported by the Thailand Research Fund grants RSA5780040 (A.P.), RSA5780039 (W.W.), RTA6080003 (S.C.C.), and MRG6180239 (S.K.), and the National Science and Technology Development Agency NSTDA Research Chair Grant (N.C.)
Phrommintikul A, Wongcharoen W, Kumfu S, et al. Effects of dapagliflozin vs vildagliptin on cardiometabolic parameters in diabetic patients with coronary artery disease: A randomised study. Br J Clin Pharmacol. 2019;85:1337–1347. 10.1111/bcp.13903
The authors confirm that the PI for this paper is Arintaya Phrommintikul and that she had direct clinical responsibility for patients
REFERENCES
- 1. Emerging Risk Factors C, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375(9733):2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720‐1732. [DOI] [PubMed] [Google Scholar]
- 3. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229‐234. [DOI] [PubMed] [Google Scholar]
- 4. Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353(9153):617‐622. [DOI] [PubMed] [Google Scholar]
- 5. Gaede P, Oellgaard J, Carstensen B, et al. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow‐up on the Steno‐2 randomised trial. Diabetologia. 2016;59(11):2298‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Derosa G, Bonaventura A, Bianchi L, et al. Comparison of vildagliptin and glimepiride: effects on glycaemic control, fat tolerance and inflammatory markers in people with type 2 diabetes. Diabet Med. 2014;31(12):1515‐1523. [DOI] [PubMed] [Google Scholar]
- 7. Matsubara J, Sugiyama S, Akiyama E, et al. Dipeptidyl peptidase‐4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti‐inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77(5):1337‐1344. [DOI] [PubMed] [Google Scholar]
- 8. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232‐242. [DOI] [PubMed] [Google Scholar]
- 9. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317‐1326. [DOI] [PubMed] [Google Scholar]
- 10. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 11. Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR‐TIMI 53 randomized trial. Circulation. 2014;130(18):1579‐1588. [DOI] [PubMed] [Google Scholar]
- 12. DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care. 2013;36(10):3169‐3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 14. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 15. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol. 2017;174(Suppl 1):S272‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alexander SPH, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol. 2017;174(Suppl 1):S360‐S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baena‐Diez JM, Penafiel J, Subirana I, et al. Risk of cause‐specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016;39(11):1987‐1995. [DOI] [PubMed] [Google Scholar]
- 19. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJV, Ponikowski P, Bolli GB, et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo‐controlled trial. JACC Heart Fail. 2018;6(1):8‐17. [DOI] [PubMed] [Google Scholar]
- 21. Mishriky BM, Tanenberg RJ, Sewell KA, Cummings DM. Comparing SGLT‐2 inhibitors to DPP‐4 inhibitors as an add‐on therapy to metformin in patients with type 2 diabetes: A systematic review and meta‐analysis. Diabetes Metab. 2018;44(2):112‐120. [DOI] [PubMed] [Google Scholar]
- 22. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15(9):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdul‐Ghani MA, DeFronzo RA. Dapagliflozin for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2013;14(12):1695‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foley JE, Jordan J. Weight neutrality with the DPP‐4 inhibitor, vildagliptin: mechanistic basis and clinical experience. Vasc Health Risk Manag. 2010;6:541‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bluher M, Schweizer A, Bader G, Foley JE. Changes in body weight after 24 weeks of vildagliptin therapy as a function of fasting glucose levels in patients with type 2 diabetes. Vasc Health Risk Manag. 2014;10:661‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Samman Tahhan A, Sandesara P, Hayek SS, et al. High‐sensitivity troponin i levels and coronary artery disease severity, progression, and long‐term outcomes. J Am Heart Assoc. 2018;7(5):e007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Everett BM, Brooks MM, Bhatt DL. Troponin in stable ischemic heart disease and diabetes. N Engl J Med. 2015;373(20):1978‐1979. [DOI] [PubMed] [Google Scholar]
- 28. Januzzi JL Jr, Butler J, Jarolim P, et al. Effects of canagliflozin on cardiovascular biomarkers in older adults with type 2 diabetes. J Am Coll Cardiol. 2017;70(6):704‐712. [DOI] [PubMed] [Google Scholar]
- 29. Endler G, Klimesch A, Sunder‐Plassmann H, et al. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117(2):399‐404. [DOI] [PubMed] [Google Scholar]
- 30. Sansanayudh N, Numthavaj P, Muntham D, et al. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta‐analysis. Thromb Haemost. 2015;114(6):1299‐1309. [DOI] [PubMed] [Google Scholar]
- 31. Tavil Y, Sen N, Yazici H, et al. Coronary heart disease is associated with mean platelet volume in type 2 diabetic patients. Platelets. 2010;21(5):368‐372. [DOI] [PubMed] [Google Scholar]
- 32. Papanas N, Symeonidis G, Maltezos E, et al. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15(8):475‐478. [DOI] [PubMed] [Google Scholar]
- 33. Jiang P, Song Y, Xu JJ, et al. Two‐year prognostic value of mean platelet volume in patients with diabetes and stable coronary artery disease undergoing elective percutaneous coronary intervention. Cardiol J. 2018 10.5603/CJ.a2018.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chung I, Choudhury A, Lip GY. Platelet activation in acute, decompensated congestive heart failure. Thromb Res. 2007;120(5):709‐713. [DOI] [PubMed] [Google Scholar]
- 35. Kosiborod M, Gause‐Nilsson I, Xu J, Sonesson C, Johnsson E. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and concomitant heart failure. J Diabetes Complications. 2017;31(7):1215‐1221. [DOI] [PubMed] [Google Scholar]
- 36. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375(9733):2223‐2233. [DOI] [PubMed] [Google Scholar]
- 37. Piuhola J, Kerkela R, Keenan JI, Hampton MB, Richards AM, Pemberton CJ. Direct cardiac actions of erythropoietin (EPO): effects on cardiac contractility, BNP secretion and ischaemia/reperfusion injury. Clin Sci (Lond). 2008;114(4):293‐304. [DOI] [PubMed] [Google Scholar]
- 38. List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium‐glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32(4):650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase‐IV inhibition. Diabetes Care. 2012;35(10):2076‐2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klempfner R, Leor J, Tenenbaum A, Fisman EZ, Goldenberg I. Effects of a vildagliptin/metformin combination on markers of atherosclerosis, thrombosis, and inflammation in diabetic patients with coronary artery disease. Cardiovasc Diabetol. 2012;11(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leng W, Ouyang X, Lei X, et al. The SGLT‐2 inhibitor dapagliflozin has a therapeutic effect on atherosclerosis in diabetic ApoE(−/−) mice. Mediators Inflamm. 2016;2016:6305735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298‐310. [DOI] [PubMed] [Google Scholar]
- 43. Bornfeldt KE. Does elevated glucose promote atherosclerosis? Pros and cons. Circ Res. 2016;119(2):190‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]


