Abstract
Aims
The aim of this study was to assess the use and factors associated with the misuse of gabapentin and pregabalin in the general French population, through a cohort study in the EGB (General Sample of Beneficiaries), a national representative sample of the French general population.
Methods
New users of gabapentin and pregabalin were identified from June 2006 to December 2014, and new users of duloxetine served as control group. Misuse was defined as a use of higher daily doses than recommended. Cox proportional hazard regression models were performed to identify associated factors of misuse.
Results
Misuse was more frequent in the 8692 new users of pregabalin (12.8%) than in the 1963 gabapentin (6.6%) or the 3214 duloxetine new users (9.7%) (P < 0.001). Factors associated with misuse were pregabalin (hazard ratio [HR] 1.48; 95% confidence interval [CI] [1.29–1.69]), age (HR[18–45] versus > 70 years 1.98 [1.70–2.31] and HR[58–70] versus > 70 years 1.25 [1.06–1.47]), multiple prescribers (HR2 or 3 versus 1 prescriber 1.29 [1.15–1.45]; HR4 or more versus 1 prescriber 1.54 [1.30–1.83]), cancer (1.28 [1.11–1.47]), multiple sclerosis (1.53 [1.07–2.18]), neuropathy (1.85 [1.19–2.89]), depression (1.26 [1.07–1.49]) and methadone (2.61 [1.16–5.84]). After this first episode of drug misuse, 11.6% of gabapentin and 10.7% of pregabalin misusers developed a primary addiction.
Conclusion
In a cohort of new users, misuse is more likely to occur in new users of pregabalin, with different associated factors of misuse compared to gabapentin and duloxetine. Health professionals and prescribers must be aware of this misuse potential, which could lead to abuse and dependence.
Keywords: abuse, addiction, gabapentin, misuse, pregabalin
What is already known about this subject
There have been numerous documented cases of gabapentin abuse, dependence and withdrawal in the literature.
First signals of pregabalin abuse were identified in Europe some years after marketing authorization with increasing reports from the 2010s.
The epidemiology of misuse of gabapentinoids needs further appropriate assessment, focusing on a better investigation of their addictive liability in the context of real life.
What this study adds
In a representative sample of the French population, misuse (use of higher dose than recommended) was twice as high in pregabalin users compared to gabapentin users, and significantly higher than in duloxetine users (as controls), and was associated with young age, number of different prescribers and initial exposure to methadone maintenance treatment for opioid dependence.
Health professionals and prescribers must be aware of the abuse potential of gabapentinoids in a population of vulnerable patients.
1. INTRODUCTION
Pregabalin and gabapentin, originally presented as anticonvulsants, are now increasingly prescribed for a range of clinical conditions, in particular for chronic pain.1, 2 These drugs selectively bind to the α2‐δ subunit of voltage‐gated calcium channels in central nervous system neuronal tissues.3 They inhibit the release of excitatory neurotransmitters, possibly accounting for the antinociceptive, anticonvulsant, anxiolytic and sleep‐modulating activities of gabapentinoids. Although they may present with a low addictive liability potential at therapeutic dosages compared to other drugs, they may be used to achieve euphoric and dissociative effects similar to those of traditional recreational drugs.4, 5, 6
Gabapentin was first approved in France in 1994 for the treatment of partial seizures, and secondarily extended to post‐zoster and neuropathic pain in 2000. Gabapentin has been suggested as a treatment option for alcohol and substance abuse, but it should be monitored because of potential deleterious consequences.7 A history of alcohol or substance abuse appears to be a significant part of a patient's medical history when evaluating the risk for gabapentin addiction and dependence.8, 9, 10, 11, 12, 13 In the US, gabapentin is not nationally scheduled, but several states have chosen to schedule it (e.g., Kentucky, Tennessee, West Virginia). In the UK, both gabapentin and pregabalin will be classified as Class C in April 2019.14 However, risk of misuse, abuse or dependence with gabapentin is not mentioned in the French Summary Product Characteristics (SPC).
Pregabalin was first authorized in 2004 in the European Union (EU) and available in France in 2006, for epilepsy, neuropathic pain, with approval in generalized anxiety disorder given in 2006. In the US, pregabalin is initially a Schedule V drug (i.e., drugs with limited but recognized potential for abuse), whereas in Europe this potential was considered negligible. First signals of abuse were identified some years after marketing authorization with increasing reports from 2008 onwards,5, 15, 16, 17 with history of substance use disorders recognized as a risk factor for misuse or abuse of pregabalin.18, 19, 20, 21 The risk of misuse, abuse and dependence with pregabalin has been added in the SPC in the EU as a special warning and precautions for use in patients with a history of substance abuse.
The epidemiology of misuse and abuse of gabapentinoids needs further appropriate assessment, focusing on a better investigation of their addictive liability in the context of real life. However, to the best of our knowledge, only the patterns of pregabalin use have been investigated in the general population,22, 23 without any other control group.24 Thus, the aim of this study was to investigate in the French general population the patterns of use and misuse of pregabalin and of gabapentin, in comparison with duloxetine, an antidepressant drug also approved for neuropathic pain, not suspected of an abuse liability.
2. METHODS
The study was conducted following the STROBE statement adapted for observational studies using routinely‐collected health data, i.e. the REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement.25 The study complied with the requirements of the European Network of Centres for Pharmacovigilance and Pharmacoepidemiology (ENCePP) and received an ENCePP seal (study reference number: ENCePP/SDPP/10298), with the complete study protocol recorded in the EU Post Authorization Studies Register available on the ENCePP website (http://www.encepp.eu). The pre‐registered hypothesis is presented in this study protocol.
2.1. Study design and participant selection
We performed a retrospective, national cohort study of new users of pregabalin, gabapentin and duloxetine. This cohort was built with data recorded in the Echantillon Généraliste des Bénéficiaires (General Sample of Beneficiaries, EGB), a permanent 1/97th sample of subjects covered by the French Health Insurance Scheme and consists of more than 700 000 beneficiaries.26, 27, 28 All subjects aged 18 years and over covered by the General Health scheme (around 80% of the population), with at least one reimbursement of one of the three drugs from 15 June 2006 (first reimbursement of pregabalin in France) to 31 December 2012 were eligible. This last date of inclusion was chosen to ensure at least 2 years of follow‐up for all included patients at the time of the analysis, giving an analytic dataset with data from June 2006 to December 2014. The date of cohort entry was that of the first reimbursement of one of the three drugs. To ensure selection of new users, patients with at least one reimbursement of any of the three studied drugs before this date were excluded. Patients with only a single reimbursement were not included. The study was initially planned to include new users of clonazepam as positive controls, because of the well‐known potential of clonazepam abuse and misuse in France.29 Because of changes in health policies regarding this drug, its use decreased in France during the study time frame,30, 31 leading us to choose duloxetine as a control for neuropathic pain.32 To avoid a potential selection bias in the identification of new users of gabapentinoids (suggested as potentially effective to reduce benzodiazepine consumption), previous clonazepam use was an exclusion criterion.
2.2. Measures and outcomes
Because the terminology for drug misuse, abuse and related events has been evolving over the last decades, we used the consensus definitions of a recent review of the Analgesic, Anesthetic, and Addiction Clinical Trials, Translations, Innovations, Opportunities, and Networks (ACTTION) group.33 The ACTTION group proposed a misuse‐event indicator, defined as any intentional therapeutic use of a drug product in an inappropriate way (misuse excludes events corresponding to the definition of an abuse‐event indicator, i.e. any intentional, nontherapeutic use of a drug product or substance, even once, for the purpose of achieving a desirable psychological or physiological effect). Regarding data available in the EGB, we defined misuse as the use of a daily dose exceeding the maximum recommended daily dose in Europe: according to the SPC, this maximum daily dose is 600 mg for pregabalin, 3600 mg for gabapentin and 120 mg for duloxetine. For each patient in the cohort, the daily dose used was estimated within a treatment sequence, by dividing the total amount of dispensed drug by the duration of the treatment sequence. This duration was the time elapsed between the first and the last dispensing to which the last number of dispensed defined daily doses of the drug (DDD) was added. Because in France prescription drugs are dispensed in community pharmacies for a period of 28 days, a difference between two consecutive dispensing exceeding 35 days (28 days + a grace period of one week) was considered as a treatment interruption. When a treatment sequence interruption occurred, a new treatment sequence began on the date of subsequent dispensing. Thus, misuse was identified through the computation of the mean daily dose received within a treatment sequence.
Time to event was defined as the time from inclusion date to the time of the first dispensing of the treatment sequence with misuse. Computation of treatment sequences and all the methods are described in detail in the study protocol, available at http://www.encepp.eu. Thus, the main analysis was based on two main outcomes: Occurrence of misuse and time from first prescription to misuse. After the first episode of misuse, subsequent treatment sequences with misuse were computed.
Additional analysis was carried out on a secondary subset of the initial cohort, corresponding to the population of patients without any mention of comorbidities related to substance disorders at baseline (see definitions in Supplementary Table S1), and developing an episode of misuse after the first prescription. For this additional analysis the outcome of interest was a diagnosis of primary addiction after the first episode of misuse. This event corresponded to the first date of hospitalization with a diagnosis of substance disorders after the first episode of misuse, and/or the first date of a prescription for a drug to treat a substance disorder.
2.3. Covariates and potential confounders
Potential covariates associated with misuse were identified a priori and included: age, sex, comorbidities and other medications at baseline, first prescriber specialty and number of different prescribers. Baseline comorbidities were derived from hospital diagnoses or health insurance coverage for long‐term diseases (LTD) classified according to the International Classification of Disease 10th revision (ICD‐10).27, 28 Drugs were categorized through the Anatomical Therapeutic Chemical (ATC) classification. Pain‐related and psychiatric comorbidities and psychoactive drugs of interest were defined by specific codes described in Supplementary Table S1. These covariates were ascertained using the presence of these selected codes in the 12 months before cohort entry (i.e., first dispensing of gabapentin, pregabalin or duloxetine).
2.4. Statistical analysis
The baseline characteristics of the study population were analysed by drug group. Categorical variables were presented as number and percentage and continuous variables as mean and standard deviation (SD), and compared using Chi2 test with 2 degrees of freedom and Kruskal–Wallis test. Subjects were censored at death date, last dispensing date or 31 December 2014. In the main analysis, time to misuse was investigated through Kaplan–Meier estimates. Factors associated with misuse were investigated through a Cox proportional hazard regression model for the whole cohort (using duloxetine as reference), and for each drug separately. Covariates known to increase the risk of misuse and those associated in the univariate analysis (P‐value < 0.20) were selected for inclusion in the multivariate model, and secondarily excluded by a backward stepwise procedure, retaining variables associated with a P‐value of <0.05. The proportional hazards assumption was checked using interaction with time. Adjusted hazard ratios (HR) and their 95% confidence interval were provided. For the additional analysis, time to primary addiction from date of the first episode of misuse was investigated through Kaplan–Meier estimates, with a log‐rank test.
2.5. Ethics approval and consent to participate
National Institute for Health and Medical Research (INSERM) agreement for the research protocol was given in May 2014. Neither ethics committee authorization nor request to national commissions for individual data protection is required according to French law to access this kind of anonymous and restricted access database. Access to EGB is possible only through a secured connection to a specific server. Data are accessible online, and are analysed by the software SAS Enterprise Guide version 4.3 (SAS Institute Inc., Cary, NC, USA).
2.6. Availability of data and material
The authors are restricted from sharing the data underlying this study because publicly sharing EGB data is forbidden by law according to the French national data protection agency (Commission Nationale de l'Informatique et des LIbertés, CNIL); regulatory decisions AT/CPZ/SVT/JB/DP/CR05222O of 14 June 2005 and DP/CR071761 of 28 August 2007. To request data access, please contact the National Institute for Health Data (Institut National des Données de Santé, INDS) (website: http://www.indsante.fr/).
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,34 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.35
3. RESULTS
3.1. Cohort description
Of the 715 000 beneficiaries present in the EGB in 2015, 13 869 (1.9%) met criteria for inclusion in our study. The study cohort consisted of 8692 (62.7%) new users of pregabalin, 1963 (14.2%) of gabapentin and 3214 (23.2%) of duloxetine (Table 1). The cohort included a majority of women (61.7%), with subjects older in the gabapentin group and younger in the duloxetine group (60.4 ± 16.8 years vs 51.6 ± 15.8). Two‐thirds of the cohort presented at least one comorbidity and one‐third were hospitalized at least once during the year before cohort entry (Table 1). Cancer, diabetes and spine and joint diseases were more frequently observed in the pregabalin and gabapentin groups, whereas psychiatric diseases were more frequent in the duloxetine group. Substance‐related disorders were mostly observed in the gabapentin group (4.3%). More than 80% of subjects were exposed to at least one other psychoactive drug: weak and strong opioid analgesics were mostly observed in the pregabalin and gabapentin groups, antidepressants, antipsychotics and benzodiazepines were mostly observed in the duloxetine group. Less than 1% of patients were exposed to drugs for opiate maintenance (Table 1).
Table 1.
Baseline characteristics of the cohort of new users of pregabalin, gabapentin, and duloxetine (estimated through data recorded within 12 months before the first dispensing)
| Pregabalin | Gabapentin | Duloxetine | ||
|---|---|---|---|---|
| n = 8692 | n = 1963 | n = 3214 | P‐value | |
| Age (mean [SD]) | 58.2 (16.0) | 60.4 (16.8) | 51.6 (15.8) | <0.001 |
| Men | 3567 (41) | 827 (42.1) | 921 (28.7) | <0.001 |
| At least one hospitalization | 2941 (33.8) | 856 (43.6) | 776 (24.1) | <0.001 |
| At least one comorbidity | 5092 (58.6) | 1301 (66.3) | 1659 (51.6) | <0,0001 |
| Cancer | 1420 (16.3) | 402 (20.5) | 281 (8.7) | <0.001 |
| Diabetes | 1494 (17.2) | 321 (16.4) | 322 (10.0) | <0.001 |
| Joints diseases | 621 (7.1) | 130 (6.6) | 138 (4.3) | <0.001 |
| Spine diseases | 789 (9.1) | 166 (8.5) | 110 (3.4) | <0.001 |
| Multiple sclerosis | 79 (0.9) | 35 (1.8) | 19 (0.6) | <0.001 |
| Neuropathy | 56 (0.6) | 27 (1.4) | 11 (0.3) | <0.001 |
| Epilepsy | 87 (1.0) | 60 (3.1) | 36 (1.1) | <0.001 |
| Substance disorders | 221(2.5) | 85 (4.3) | 104 (3.2) | <0.001 |
| Schizophrenia | 78 (0.9) | 25 (1.3) | 74 (2.3) | <0.001 |
| Depressive disorders | 469 (5.4) | 140 (7.1) | 543 (16.9) | <0.001 |
| Bipolar disorders | 66 (0.8) | 8 (0.4) | 124 (3.9) | <0.001 |
| Anxiety disorders | 145 (1.7) | 40 (2.0) | 117 (3.6) | <0.001 |
| Personality disorders | 140 (1.6) | 36 (1.8) | 185 (5.8) | <0.001 |
| At least one psychoactive drug | 7370 (84.8) | 1608 (81.9) | 2868 (89.2) | <0.001 |
| Strong opioid analgesics | 881 (10.1) | 205 (10.4) | 122 (3.7) | <0.001 |
| Weak opioid analgesics | 5741 (66.0) | 1191 (60.6) | 1452 (45.1) | <0.001 |
| Benzodiazepines | 3883 (44.6) | 898 (45.7) | 2226 (69.2) | <0.001 |
| Antidepressant drugs | 2494 (28.6) | 593 (30.2) | 2075 (64.5) | <0.001 |
| Antipsychotic drugs | 403 (4.63) | 110 (5.6) | 542 (16.8) | <0.001 |
| Other antiepileptic drugs | 1276 (14.6) | 346 (17.6) | 457 (14.2) | 0.002 |
| Psychostimulant drugs | 102 (1.1) | 25 (1.2) | 67 (2.0) | <0.001 |
| Opiate maintenance drugs | 32 (0.36) | 11 (0.5) | 32 (0.99) | <0.001 |
| Buprenorphine for opiate maintenance | 24 (0.27) | 7 (0.3) | 22 (0.68) | 0.006 |
| Methadone for opiate maintenance | 10 (0.11) | 5 (0.2) | 11 (0.34) | 0.03 |
| Length of follow‐up (months) (mean [SD]) | 18.2 (20.9) | 14.3 (20.4) | 17.2 (18.6) | <0.001 |
| Death during follow‐up | 836 (9.6) | 304 (15.5) | 149 (4.6) | <0.001 |
The mean exposure duration to the study drugs was 17.4 months, with the longest duration in the pregabalin group (Table 1). The mean number of treatment sequences was 4.6 with a mean duration of 61.1 days (Table 2). For the three drugs, general practitioners were the main prescribers (79.6%). During the follow‐up, 15.5% of gabapentin subjects (304), 9.6% (836) of pregabalin subjects and 4.6% (149) of duloxetine subjects died (Table 1).
Table 2.
Patterns of use of pregabalin, gabapentin and duloxetine after inclusion in the cohort
| Pregabalin | Gabapentin | Duloxetine | ||
|---|---|---|---|---|
| n = 8692 | n = 1963 | n = 3214 | P‐value | |
| Number of treatment sequences (mean [SD]) | 4.5 (4.7) | 4.6 (5.5) | 4.8 (4.4) | <0.001 |
| Duration of treatment sequences (mean [SD]) in days | 53.4 (79.9 | 51.4 (77) | 87.6 (18.9) | <0.001 |
| Overall daily dose (mean [SD]) in DDD | 1.11 (0.63) | 1.07 (0.49) | 1.11 (0.61) | — |
| Daily dose during misuse sequences (mean [SD]) in DDD | 3.60 (1.64) | 3.44 (1.53) | 2.93 (0.99) | — |
| Different prescribers (mean [SD]) | 1.7 (1) | 1.7 (1) | 1.8 (1.2) | 0.01 |
| First prescriber | ||||
| General practice | 7092 (81.6) | 1552 (79.1) | 2402 (74.7) | <0.001 |
| Bone, joint and spine specialist | 838 (9.6) | 132 (6.7) | 27 (0.84) | <0.001 |
| Psychiatry | 53 (0.6) | 17 (0.9) | 639 (19.9) | <0.001 |
| Neurology | 298 (3.4) | 125 (6.4) | 46 (1.4) | <0.001 |
| Oncology | 65 (0.7) | 11 (0.6) | 2 (0.0) | <0.001 |
| Surgery/anaesthesiology | 111 (1.3) | 41 (2.1) | 15 (0.5) | <0.001 |
| Others | 235 (2.7) | 85 (4.3) | 83 (2.6) | <0.001 |
| Misuse (first event identified) | 1112 (12.8) | 130 (6.6) | 317 (9.7%) | <0.001 |
3.2. Main outcome
Misuse concerned 1112 (12.8%, 95% confidence interval [CI] [12.1–13.5]) subjects in the pregabalin group, 130 (6.6%, [5.6–7.8]) in the gabapentin group, and 313 (9.7%, [8.7–10.8]) in the duloxetine group, with a significant difference between the groups (log‐rank test: P < 0.001, Figure 1).
Figure 1.
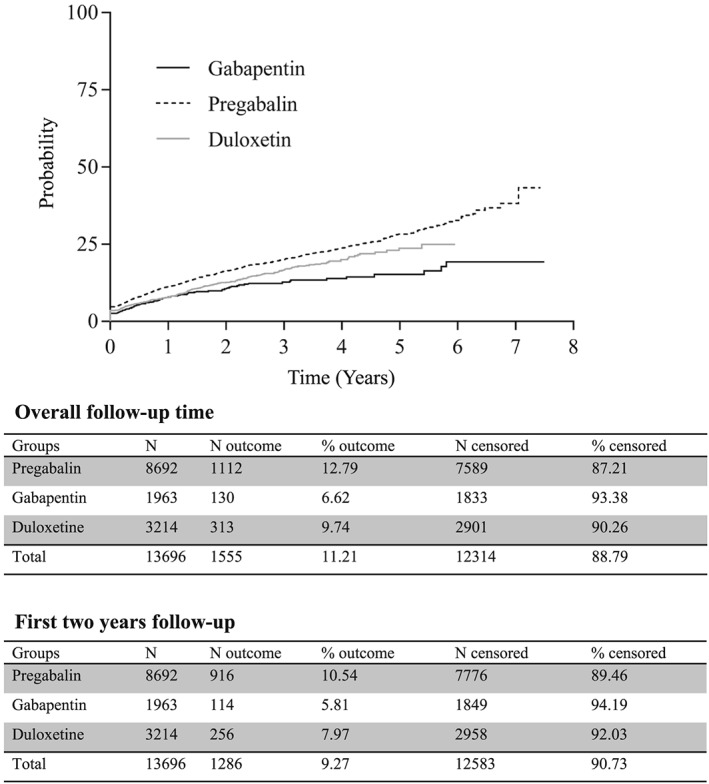
Cumulative incidence of misuse over time (years) according to the use of pregabalin, gabapentin or duloxetine (new users), presented as Kaplan‐Meier curves. Number of outcomes and censored subjects during the overall observation period, and within the first two years after study entry
Most events (82.7%) occurred within the first 2 years of prescribing. One‐third of treatment sequences with misuse were followed by at least one other sequence with misuse (Table 3). After excluding subjects with any previous history of substance use disorders before cohort entry, 1331 patients experienced a first sequence of misuse with one of the three drugs. They correspond to 11.3% of pregabalin users (960 among 8471 new users of pregabalin with no previous history of substance disorders), 5.9% of gabapentin (112 out of 1879) and 8.3% of duloxetine users (259 out of 3110). This population of misusers included 839 women (63%) and the mean age was 54.2 ± 15.7 years. In this population, the likelihood of developing a primary addiction to any substance after a first episode of misuse was 11.6% (n = 13) for gabapentin, 10.7% (n = 103) for pregabalin and 7.3% (n = 19) for duloxetine (log rank test: P = 0.0019, Supplementary Figure S1).
Table 3.
Number and duration of treatment sequences with or without misuse for pregabalin, gabapentin and duloxetine, presented as mean (SD) and median [interquartile range]
| Pregabalin | Gabapentin | Duloxetine | P‐value | |
|---|---|---|---|---|
| Number of subjects with a misuse sequence | 1112 | 130 | 313 | |
| Patients with only one sequence of misuse | 69.5% | 66.1% | 69.3% | 0.730 |
| Number of treatment sequences after the first misuse sequence | 3.9 (5.2) 2[0–35] | 5.3 (6.3) 3[0–34] | 3.7 (4.2) 3[0–29] | 0.040 |
| Ratio of misuse sequences over other treatment sequences (after the first misuse) | 0.18 (0.24) 0[0–1] | 0.21 (0.26) 0.09[0–0.83] | 0.16 (0.14) 0[0–1] | 0.130 |
| Duration of the first misuse sequence (days) | 33.8 (101) 14[1.7–1893] | 23.5 (36) 15[5–245] | 51.7 [94.8) 14[3.5–1079] | <0.001 |
| Duration of the following treatment sequences without misuse (days) | 59.4 (77.9) 36.7[4.7–1343] | 53.9 (81.6) 37[5–1471] | 78.4 (85.7) 55[14–698] | <0.001 |
| Duration of the following treatment sequences with misuse (days) | 37.1 (69.9) 14[4.7–1343] | 29.9 (44.5) 20[5–322] | 40.5 (73.7) 14[14–614] | <0.001 |
3.3. Factors associated with misuse
Considering the whole cohort of new users, the risk of misuse was significantly associated with pregabalin (adjusted HR 1.48, 95% CI [1.29–1.69]), age (HR[18‐45] versus > 70 years 1.98 [1.70–2.31] and HR[58–70] versus > 70 years 1.25 [1.06–1.47], number of different prescribers, presence of cancer, multiple sclerosis, neuropathy and depressive disorders (Table 4). Methadone was also associated with misuse (HR 2.61 [1.16–5.84]). In the stratified analysis by drug groups, age and number of different prescribers were significantly associated with misuse for all drug groups. For the pregabalin group, cancer, multiple sclerosis, neuropathy and personality disorders were also associated with misuse, whereas methadone was strongly associated with an adjusted HR of 4.01 [1.49–10.81]. These statistically significant associations were not observed with gabapentin and duloxetine; only baseline exposure to psychostimulants was associated with duloxetine misuse (1.91 [1.07–3.43]).
Table 4.
Multivariate Cox regression models for occurrence of misuse, for the whole cohort, and for each group of new users of pregabalin, gabapentin and duloxetine
| Whole cohort | P‐value | Pregabalin group | P‐value | Gabapentin group | P‐value | Duloxetine group | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Duloxetine (reference) | 1 | — | — | — | ||||
| Pregabalin | 1.48 [1.29–1.69] | <0.001 | — | — | — | |||
| Gabapentin | 0.85 [0.69–1.05] | 0.146 | — | — | — | |||
| Age (reference >70 years) | 1 | 1 | 1 | 1 | ||||
| [18–45] | 1.98 [1.70–2.31] | <0.001 | 2.04 [1.71–2.45] | <0.001 | 2.27 [1.44–3.57] | <0.001 | 1.85 [1.21–2.82] | 0.004 |
| [46–57] | 1.59 [1.36–1.85] | <0.001 | 1.62 [1.36–1.93] | <0.001 | 0.97 [0.57–1.67] | 0.936 | 1.86 [1.21–2.84] | 0.004 |
| [58–70] | 1.25 [1.06–1.47] | 0.005 | 1.22 [1.01–1.47] | 0.031 | 1.20 [0.74–1.96] | 0.448 | 1.51 [0.95–2.39] | 0.075 |
| Number of prescribers (reference 1) | 1 | 1 | 1 | 1 | ||||
| 2–3 prescribers | 1.29 [1.15–1.45] | <0.001 | 1.29 [1.13–1.48] | <0.001 | 1.47 [0.99–2.18] | 0.053 | 1.39 [1.07–1.80] | 0.012 |
| 4 or more | 1.54 [1.30–1.83] | <0.001 | 1.64 [1.34–2.02] | <0.001 | 1.50 [0.84–2.68] | 0.164 | 1.59 [1.13–2.24] | 0.007 |
| General practitioner prescriber | 0.78 [0.69–0.87] | <0.001 | — | 0.52 [0.41–0.66] | <0.001 | |||
| Cancer | 1.28 [1.11–1.47] | <0.001 | 1.31 [1.12–1.54] | <0.001 | — | — | ||
| Multiple sclerosis | 1.53 [1.07–2.18] | 0.017 | 1.99 [1.36–2.91] | <0.001 | — | — | ||
| Neuropathy | 1.85 [1.19–2.89] | 0.006 | 1.81[1.08–3.03] | 0.022 | — | — | ||
| Depressive disorders | 1.26 [1.07–1.49] | 0.005 | — | — | — | |||
| Personality disorders | — | 1.52 [1.04–2.23] | 0.030 | 0.91 [0.27–3.03] | 0.879 | — | ||
| Substance disorders | — | — | 0.96 [0.44–2.11] | 0.934 | — | |||
| Methadone | 2.61 [1.16–5.84] | 0.019 | 4.01 [1.49–10.81] | 0.006 | — | — | ||
| Antipsychotics | — | — | 1.11 [0.55–2.45] | 0.769 | — | |||
| Benzodiazepines | — | 0.96 [0.85–1.09] | 0.580 | 1.07 [0.74–1.54] | 0.713 | — | ||
| Psychostimulants | — | — | — | 1.91 [1.07–3.43] | 0.027 |
4. DISCUSSION
In this large population‐based study of all first‐time pregabalin, gabapentin and duloxetine users in France, we found that misuse was more frequent among pregabalin users (12.8% were dispensed a dose that exceeded the maximum recommended dose), compared to gabapentin (6.6%) and duloxetine users (9.7%). This misuse, whatever the substance, was more likely to occur in young adults, in patients suffering from cancer, multiple sclerosis and neuropathic pain, in patients with psychiatric comorbidities, treated by methadone for opiate maintenance treatment, and in patients visiting a high number of different prescribers. Age and number of prescribers were factors associated with misuse for each drug separately, but only misuse of pregabalin was associated with methadone and personality disorders. After the first episode of misuse, more than 10% of gabapentin and pregabalin misusers, without any notion of substance‐related disorders before cohort entry, developed a primary addiction.
Our study identifies that more than 10% of pregabalin new users and 6% of gabapentin new users have misused the drug within 2 years after the first prescription, i.e. have been prescribed more than the recommended daily dose. We used the definition recommended by the ACTTION network for harmonizing reporting of misuse, abuse and adverse reactions in clinical trials in the context of pain. We did not use the term abuse, as we did not know the intentionality of use at higher dosage than recommended. Thus, this pattern of misuse must be interpreted cautiously because high doses can correspond to different situations.
First of all, this high dose use could be an indicator of lack of effectiveness, as suggested elsewhere,13, 36, 37 explaining the higher frequency observed with pregabalin. For duloxetine, the use of high dose could be related to a lack of effectiveness for neuropathic pain or depression, or the need for high doses regarding the intensity of depressive symptoms. A search of reports of drug abuse and dependence involving duloxetine, gabapentin or pregabalin in Vigibase® (the WHO Pharmacovigilance database38) and corresponding to French spontaneously reported cases which occurred during the study period, identified no cases with duloxetine, five with gabapentin and 17 with pregabalin. The likelihood of duloxetine misuse for its psychoactive effects seems very low.
The second interpretation of high dose could be an initial step for drug abuse and dependence, with an intentional misuse for psychoactive effects. It seems possible for gabapentin and pregabalin, with about 10% of misusers developing de novo substance use disorders during follow‐up. In fact, we cannot assess with these data the intentionality of misuse, as the observed results may reflect that medical doctors prescribed daily doses higher than recommended because of insufficient pain relief. However, the observed results may also reflect substance‐seeking behaviour by doctor‐shopping and/or by falsifying prescription forms. Previous studies performed in such databases suggest that deviant behaviour represents an important part of this misuse.29, 39
To the best of our knowledge, this is the first study that has aimed to assess both gabapentin and pregabalin use and misuse at the population level. Only few studies performed among specific populations of opioid addicts have investigated both gabapentin and pregabalin,9, 40 but not in the general population. None of them used a control group with a drug with a low likelihood of misuse. One post‐market pharmacovigilance study examined gabapentin reporting in the FDA Adverse Event Reporting System (FAERS) in comparison to pregabalin and duloxetine, as control drugs for their similar indication in neuropathic pain.32 Even if all drugs produced signals for any abuse‐related and abuse‐specific adverse events in the FAERS, strongest associations were observed with gabapentin as well as with pregabalin for ataxia, drug tolerance and feeling drunk, but not with duloxetine. Two studies performed on prescription databases in Sweden and in Denmark focused only on pregabalin (using the same definition of misuse, daily dose prescribed exceeding the maximum recommended dose), without any control group, and for a shorter study frame.22, 23 They identified a level of misuse similar to that observed in our study, with 8% (in Sweden) to 10% (in Denmark) of misusers. In this Scandinavian context, factors associated with pregabalin misuse were young age, male gender, current or past history of substance abuse and use of benzodiazepines or antipsychotics. Recently, a study in the EudraVigilance database has investigated the part of adverse drug reactions related to abuse and dependence with gabapentin and pregabalin.6 An increasing overall reporting was observed over time, with these adverse drug reactions more frequently reported for pregabalin. These results are consistent with those observed in the French pharmacovigilance experience.36
Factors for vulnerability for misuse observed in this study were consistent with previous findings, including young age and doctor‐shopping behaviour.19 This behaviour indicates a common trait of drug misuse, and could be a prodromal characteristic for prescription drug addiction.41 Our results are contrasted with results observed in the US or in the UK for gabapentin, which seems to be more likely misused in those countries.7, 10, 11, 12, 42, 43, 44 These geographical variations must be interpreted with caution, but could be explained partly by the health professionals' awareness regarding the abuse potential of these drugs. Pregabalin was initially scheduled in the US, whereas it was not in Europe, and the representation of the two drugs might be different, with pregabalin being promoted off‐label for benzodiazepine or alcohol reduction.45, 46, 47, 48 It is also possible that, depending on country, health professionals in charge of opiate addicts switched benzodiazepines, well‐known for their abuse potential in this vulnerable population, for gabapentin in the UK or US8, 9, 11, 42, 49 or for pregabalin in other European countries,18, 21 due to lack of knowledge regarding their psychoactive properties.50 Finally, these discrepancies could be due to the level of use, which is very low for gabapentin compared to pregabalin in France.51 Because the incident use of pregabalin is four times higher than that observed with gabapentin, the likelihood that vulnerable subjects will be exposed to misuse or to other adverse outcomes is higher for pregabalin.51, 52 This situation has been observed previously in similar contexts with other drugs.53
Factors specifically associated with pregabalin were personality disorders and opiate maintenance treatment with methadone. These factors were also identified in Scandinavian studies.22, 23 Two hypotheses may explain the association with methadone (labelled in France only for opiate maintenance): the first explanation is a hyperalgesic condition, which is less probable because this association was not observed with other opioids used as analgesics. The second hypothesis refers to a recreational use of pregabalin in opiate addicts, for its specific psychoactive and euphoric properties, or for a synergic effect with methadone.49 This interpretation must be made with caution as the number of patients treated by methadone in this study was low. Previous exposure to benzodiazepines was not associated with pregabalin misuse, but we have to keep in mind that patients previously exposed to clonazepam were not eligible for our study, probably excluding a large proportion of benzodiazepine abusers.29, 54
Some limitations must be mentioned. In this health reimbursement database, randomly selected subjects from the national healthcare system are prospectively registered and data are collected independently of patients, prescribers and pre‐specified hypothesis. In fact, the EGB is a 1/97th representative sample, with the insurance general scheme representing more than 80% of the whole French population.27 One limitation could be the relative lack of power to identify factors associated with marginal behaviours for drugs used less often than pregabalin. The second limitation is the lack of data regarding the reason for prescribing in case of misuse. However, this limit could be minimized, as patients seeking drugs are not likely to report to their doctor the real aim of drug request (for a medical or a recreational purpose). The lack of information regarding other substance use (alcohol, tobacco and illicit drugs) may be considered as a limit to better characterize factors associated with misuse. Finally, reimbursement data may not reflect the real drug consumption, and drugs illegally obtained on the black market cannot be captured with these data.55 Despite these limitations, these data allowed identification of several signals of drug misuse in the recent past.39, 41, 54, 56
This study provides information at the general population level concerning the magnitude of pregabalin, gabapentin and duloxetine misuse and its consequences. The profile of misusers includes young age, use of multiple prescribers, painful or psychiatric comorbidities and treatment with methadone. In France, the incidence of pregabalin misuse seems to be higher than that observed with gabapentin and duloxetine. The first interpretation of this finding could be the need for higher doses because of a lack of effectiveness of these drugs for pain or other symptoms. The second interpretation of high dose could be an initial step towards drug abuse and dependence, particularly for gabapentin and pregabalin. The huge increase in pregabalin use in Europe and in France pleads for greater vigilance in the general population and specifically among substance use disorder patients, or patients with chronic pain.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
All of the authors contributed: D.D. performed data management and statistical analysis and wrote the first draft of the paper. J.D., S.O., M.L.‐M. and E.J. performed the literature search. All the authors interpreted data, corrected and approved the final version of the article. M.L.‐M. was the study chief investigator, developed the study protocol, planned the statistical analysis, and wrote the article. She is the principal investigator and the scientific guarantor for this study.
Supporting information
TABLE S1
List of comorbidities, coded according to the International Classification of Diseases—10th Revision (ICD‐10), to the categories of Chronic Disease Condition (in French ALD: Affection Longue Durée) and occupational diseases; and drugs coded according to the Anatomical Chemical and Therapeutic (ATC) classification
FIGURE S1
Occurrence of addiction after a first episode of misuse (cumulative incidence of addiction), in the 1331 subjects with no previous history of substance disorders, according to the first used drug (pregabalin [n = 960], gabapentin [n = 112] or duloxetine [n = 259])
ACKNOWLEDGEMENTS
The authors would like to acknowledge Nicolas Savy, Professor of Statistics (Toulouse University), Cyrille Delpierre, Senior Researcher in Epidemiology (INSERM) for their technical comments, Aurore Palmaro, Pharmacoepidemiologist (Toulouse University) for her help in managing the healthcare database and Felicie Pastore, Professor of English (Toulouse University) for English editing. This study was performed in an academic setting, in the context of public drug abuse monitoring, through unrestricted funding of the French Medicine Agency (ANSM) for the French Addictovigilance system. The study received a label of transparency from the European Networks of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCEPP, European Medicine Agency), through the ENCEPP study seal number ENCEPP/SDPP/10298.
Driot D, Jouanjus E, Oustric S, Dupouy J, Lapeyre‐Mestre M. Patterns of gabapentin and pregabalin use and misuse: Results of a population‐based cohort study in France. Br J Clin Pharmacol. 2019;85:1260–1269. 10.1111/bcp.13892
REFERENCES
- 1. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia—An overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shmagel A, Ngo L, Ensrud K, Foley R. Prescription medication use among community‐based US adults with chronic low back pain: A cross‐sectional population based study. J Pain. 2018;19(10):1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SP, Peters JA, Kelly E, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol. 2017;174:S130‐S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaccara G, Gangemi P, Perucca P, Specchio L. The adverse event profile of pregabalin: A systematic review and meta‐analysis of randomized controlled trials. Epilepsia. 2011;52(4):826‐836. [DOI] [PubMed] [Google Scholar]
- 5. Schifano F, D'Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychother Psychosom. 2011;80(2):118‐122. [DOI] [PubMed] [Google Scholar]
- 6. Chiappini S, Schifano F. A decade of gabapentinoid misuse: An analysis of the European Medicines Agency's 'Suspected Adverse Drug Reactions' database. CNS Drugs. 2016;30(7):647‐654. [DOI] [PubMed] [Google Scholar]
- 7. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid‐related death: A population‐based nested case‐control study. PLoS Med. 2017;14(10):e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: A systematic review. Addiction. 2016;111(7):1160‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: A survey among substance misusers. Eur Addict Res. 2014;20(3):115‐118. [DOI] [PubMed] [Google Scholar]
- 10. Kapil V, Green JL, Le Lait MC, Wood DM, Dargan PI. Misuse of the gamma‐aminobutyric acid analogues baclofen, gabapentin and pregabalin in the UK. Br J Clin Pharmacol. 2014;78(1):190‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buttram ME, Kurtz SP, Dart RC, Margolin ZR. Law enforcement‐derived data on gabapentin diversion and misuse, 2002–2015: Diversion rates and qualitative research findings. Pharmacoepidemiol Drug Saf. 2017;26(9):1083‐1086. [DOI] [PubMed] [Google Scholar]
- 12. Bastiaens L, Galus J, Mazur C. Abuse of gabapentin is associated with opioid addiction. Psychiatr Q. 2016;87(4):763‐767. [DOI] [PubMed] [Google Scholar]
- 13. Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: Do the benefits outweigh the harms? J R Coll Physicians Edinb. 2017;47(4):310‐313. [DOI] [PubMed] [Google Scholar]
- 14.Pregabalin and gabapentin to be controlled as class C drugs. UK government, 2018. https://www.gov.uk/government/news/pregabalin‐and‐gabapentin‐to‐be‐controlled‐as‐class‐c‐drugs. Accessed February 21, 2019.
- 15. Schwan S, Sundstrom A, Stjernberg E, Hallberg E, Hallberg P. A signal for an abuse liability for pregabalin—Results from the Swedish spontaneous adverse drug reaction reporting system. Eur J Clin Pharmacol. 2010;66(9):947‐953. [DOI] [PubMed] [Google Scholar]
- 16. Gahr M, Franke B, Freudenmann RW, Kolle MA, Schonfeldt‐Lecuona C. Concerns about pregabalin: Further experience with its potential of causing addictive behaviors. J Addict Med. 2013;7(2):147‐149. [DOI] [PubMed] [Google Scholar]
- 17. Driot D, Chicoulaa B, Jouanjus E, Dupouy J, Oustric S, Lapeyre‐Mestre M. Pregabalin use disorder and secondary nicotine dependence in a woman with no substance abuse history. Therapie. 2016;71(6):575‐578. [DOI] [PubMed] [Google Scholar]
- 18. Grosshans M, Lemenager T, Vollmert C, et al. Pregabalin abuse among opiate addicted patients. Eur J Clin Pharmacol. 2013;69(12):2021‐2025. [DOI] [PubMed] [Google Scholar]
- 19. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403‐426. [DOI] [PubMed] [Google Scholar]
- 20. Schjerning O, Rosenzweig M, Pottegard A, Damkier P, Nielsen J. Abuse potential of pregabalin: A systematic review. CNS Drugs. 2016;30(1):9‐25. [DOI] [PubMed] [Google Scholar]
- 21. McNamara S, Stokes S, Kilduff R, Shine A. Pregabalin abuse amongst opioid substitution treatment patients. Ir Med J. 2015;108(10):309‐310. [PubMed] [Google Scholar]
- 22. Schjerning O, Pottegard A, Damkier P, Rosenzweig M, Nielsen J. Use of pregabalin—A nationwide pharmacoepidemiological drug utilization study with focus on abuse potential. Pharmacopsychiatry. 2016;49(4):155‐161. [DOI] [PubMed] [Google Scholar]
- 23. Boden R, Wettermark B, Brandt L, Kieler H. Factors associated with pregabalin dispensing at higher than the approved maximum dose. Eur J Clin Pharmacol. 2014;70(2):197‐204. [DOI] [PubMed] [Google Scholar]
- 24. Stannard C. Misuse of gabapentin and pregabalin: A marker for a more serious malaise? Addiction. 2016;111(10):1699‐1700. [DOI] [PubMed] [Google Scholar]
- 25. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante. 2010;58(4):286‐290. [DOI] [PubMed] [Google Scholar]
- 27. Moulis G, Lapeyre‐Mestre M, Palmaro A, Pugnet G, Montastruc JL, Sailler L. French health insurance databases: What interest for medical research? Rev Med Interne. 2015;36(6):411‐417. [DOI] [PubMed] [Google Scholar]
- 28. Palmaro A, Moulis G, Despas F, Dupouy J, Lapeyre‐Mestre M. Overview of drug data within French health insurance databases and implications for pharmacoepidemiological studies. Fund Clin Pharmacol. 2016;30(6):616‐624. [DOI] [PubMed] [Google Scholar]
- 29. Frauger E, Pauly V, Pradel V, et al. Evidence of clonazepam abuse liability: Results of the tools developed by the French Centers for Evaluation and Information on Pharmacodependence (CEIP) network. Fundam Clin Pharmacol. 2011;25(5):633‐641. [DOI] [PubMed] [Google Scholar]
- 30. Benard‐Laribiere A, Noize P, Pambrun E, et al. Trends in incident use of benzodiazepines and Z‐drugs in France from 2006 to 2012: A population‐based study. Pharmacoepidemiol Drug Saf. 2017;26(2):162‐169. [DOI] [PubMed] [Google Scholar]
- 31. Palmaro A, Dupouy J, Lapeyre‐Mestre M. Benzodiazepines and risk of death: Results from two large cohort studies in France and UK. Eur Neuropsychopharmacol. 2015;25(10):1566‐1577. [DOI] [PubMed] [Google Scholar]
- 32. Smith RV. Exploration of the misuse, abuse, and diversion of gabapentin. PhD dissertation, University of Kentucky, 2016.
- 33. Smith SM, Dart RC, Katz NP, et al. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154(11):2287‐2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alexander SPH, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol. 2017;174:S1‐S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carlisle B, Federico CA, Kimmelman J. Trials that say "maybe": The disconnect between exploratory and confirmatory testing after drug approval. BMJ. 2018;360:k959. [DOI] [PubMed] [Google Scholar]
- 37. Aagaard L, Hansen EH. Adverse drug reactions reported by consumers for nervous system medications in Europe 2007 to 2011. BMC Pharmacol Toxicol. 2013;14(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caster O, Edwards IR, Noren GN, Lindquist M. Earlier discovery of pregabalin's dependence potential might have been possible. Eur J Clin Pharmacol. 2011;67(3):319‐320. [DOI] [PubMed] [Google Scholar]
- 39. Pauly V, Pradel V, Pourcel L, et al. Estimated magnitude of diversion and abuse of opioids relative to benzodiazepines in France. Drug Alcohol Depend. 2012;126(1–2):13‐20. [DOI] [PubMed] [Google Scholar]
- 40. Lyndon A, Audrey S, Wells C, et al. Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction. 2017;112(9):1580‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nordmann S, Pradel V, Lapeyre‐Mestre M, et al. Doctor shopping reveals geographical variations in opioid abuse. Pain Physician. 2013;16(1):89‐100. [PubMed] [Google Scholar]
- 42. Reeves RR, Ladner ME. Potentiation of the effect of buprenorphine/naloxone with gabapentin or quetiapine. Am J Psychiatry. 2014;171(6):691. [DOI] [PubMed] [Google Scholar]
- 43. Schifano F. Misuse and abuse of pregabalin and gabapentin: Cause for concern? CNS Drugs. 2014;28(6):491‐496. [DOI] [PubMed] [Google Scholar]
- 44. Smith BH, Higgins C, Baldacchino A, Kidd B, Bannister J. Substance misuse of gabapentin. Br J Gen Pract. 2012;62(601):406‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martinotti G, di Nicola M, Frustaci A, et al. Pregabalin, tiapride and lorazepam in alcohol withdrawal syndrome: A multi‐centre, randomized, single‐blind comparison trial. Addiction. 2010;105(2):288‐299. [DOI] [PubMed] [Google Scholar]
- 46. Oulis P, Konstantakopoulos G. Pregabalin in the treatment of alcohol and benzodiazepines dependence. CNS Neurosci Ther. 2010;16(1):45‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bramness JG, Sandvik P, Engeland A, Skurtveit S. Does pregabalin (Lyrica®) help patients reduce their use of benzodiazepines? A comparison with gabapentin using the Norwegian prescription database. Basic Clin Pharmacol Toxicol. 2010;107(5):883‐886. [DOI] [PubMed] [Google Scholar]
- 48. Lapeyre‐Mestre M, Dupui M. Drug abuse monitoring: Which pharmacoepidemiological resources at the European level? Therapie. 2015;70(2):147‐165. [DOI] [PubMed] [Google Scholar]
- 49. Wilens T, Zulauf C, Ryland D, Carrellas N, Catalina‐Wellington I. Prescription medication misuse among opioid dependent patients seeking inpatient detoxification. Am J Addict. 2015;24(2):173‐177. [DOI] [PubMed] [Google Scholar]
- 50. Abrahamsson T, Berge J, Ojehagen A, Hakansson A. Benzodiazepine, z‐drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment—A nationwide register‐based open cohort study. Drug Alcohol Depend. 2017;174:58‐64. [DOI] [PubMed] [Google Scholar]
- 51. Fuzier R, Serres I, Guitton E, Lapeyre‐Mestre M, Montastruc JL, French Network of Pharmacovigilance Centres . Adverse drug reactions to gabapentin and pregabalin: A review of the French pharmacovigilance database. Drug Saf. 2013;36(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 52. Bossard JB, Ponte C, Dupouy J, Lapeyre‐Mestre M, Jouanjus E. Disproportionality analysis for the assessment of abuse and dependence potential of pregabalin in the French pharmacovigilance database. Clin Drug Investig. 2016;36(9):735‐742. [DOI] [PubMed] [Google Scholar]
- 53. Rossow I, Bramness JG. The total sale of prescription drugs with an abuse potential predicts the number of excessive users: A national prescription database study. BMC Public Health. 2015;15(1):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pradel V, Delga C, Rouby F, Micallef J, Lapeyre‐Mestre M. Assessment of abuse potential of benzodiazepines from a prescription database using “doctor shopping” as an indicator. CNS Drugs. 2010;24(7):611‐620. [DOI] [PubMed] [Google Scholar]
- 55. Frauger E, Nordmann S, Orleans V, et al. Which psychoactive prescription drugs are illegally obtained and through which ways of acquisition? About OPPIDUM survey. Fundam Clin Pharmacol. 2012;26(4):549‐556. [DOI] [PubMed] [Google Scholar]
- 56. Ponté C, Lepelley M, Boucherie Q, et al. Doctor shopping of opioid analgesics relative to benzodiazepines: A pharmacoepidemiological study among 11.7 million inhabitants in the French countries. Drug Alcohol Depend. 2018;187:88‐94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1
List of comorbidities, coded according to the International Classification of Diseases—10th Revision (ICD‐10), to the categories of Chronic Disease Condition (in French ALD: Affection Longue Durée) and occupational diseases; and drugs coded according to the Anatomical Chemical and Therapeutic (ATC) classification
FIGURE S1
Occurrence of addiction after a first episode of misuse (cumulative incidence of addiction), in the 1331 subjects with no previous history of substance disorders, according to the first used drug (pregabalin [n = 960], gabapentin [n = 112] or duloxetine [n = 259])
Data Availability Statement
The authors are restricted from sharing the data underlying this study because publicly sharing EGB data is forbidden by law according to the French national data protection agency (Commission Nationale de l'Informatique et des LIbertés, CNIL); regulatory decisions AT/CPZ/SVT/JB/DP/CR05222O of 14 June 2005 and DP/CR071761 of 28 August 2007. To request data access, please contact the National Institute for Health Data (Institut National des Données de Santé, INDS) (website: http://www.indsante.fr/).


