Abstract
Aims
Voxelotor (previously GBT440) is a haemoglobin (Hb) modulator that increases Hb‐oxygen affinity, thereby reducing Hb polymerization and sickling of red blood cells (RBCs), being developed as a once‐daily oral drug to treat sickle cell disease (SCD). This first‐in‐human study evaluated the safety, tolerability, pharmacokinetics and pharmacodynamics of voxelotor in healthy volunteers and SCD patients.
Methods
A total of 40 healthy volunteers (100, 400, 1000, 2000 or 2800 mg) and 8 SCD patients (1000 mg) were randomly assigned to a single dose of voxelotor once daily (n = 6 per group) or placebo (n = 2 per group). Twenty‐four healthy volunteers received multiple doses of voxelotor once daily for 15 days (300, 600 or 900 mg, n = 6 per group) or placebo (n = 2 per group).
Results
Voxelotor was well tolerated and exhibited a linear pharmacokinetic profile and a half‐life ranging from 61 ± 7 h to 85 ± 7 h. High partitioning into the RBC compartment provides evidence of highly specific binding to Hb. Voxelotor exhibited a concentration‐dependent left‐shift of oxygen equilibrium curves. Percent Hb modification following 900 mg voxelotor for 15 days was 38 ± 9%. Terminal half‐life of voxelotor in SCD patients (50 ± 3 h) was shorter than in healthy volunteers. Evaluation of erythropoietin, exercise testing, and haematologic parameters were consistent with normal oxygen delivery during both rest and exercise.
Conclusion
This first‐in‐human study demonstrates voxelotor was well tolerated in SCD patients and healthy volunteers and established proof of mechanism on increasing Hb‐oxygen affinity.
Keywords: clinical trial, pharmacokinetics, pharmacodynamics, phase I, sickle cell disease, voxelotor
What is already known about this subject
Sickle cell disease affects millions of people worldwide and treatment remains a serious unmet need.
Current approved treatments for sickle cell disease do not target the underlying pathophysiology of the disease.
Haemoglobin oxygen affinity modulation to inhibit HbS polymerization is a promising and potentially disease‐modifying strategy for treating SCD.
What this study adds
Once‐daily dosing with voxelotor increases Hb oxygen affinity into a range that may be therapeutic for SCD patients.
Voxelotor once‐daily oral dosing demonstrated linear pharmacokinetics with dose‐dependent increases in haemoglobin‐oxygen affinity.
Voxelotor was well tolerated with no dose‐limiting toxicities or indications of tissue hypoxia.
1. INTRODUCTION
Sickle cell disease (SCD) is an autosomal recessive disorder caused by a mutation in the β‐chain of haemoglobin (Hb) that leads to production of sickle haemoglobin (HbS).1 When deoxygenated, HbS polymerizes and deforms red blood cells (RBCs) into a sickle shape, leading to permanent cell membrane damage,1, 2, 3 painful vaso‐occlusive crisis, end‐organ damage and dysfunction, and early death.1, 3 SCD affects approximately 100 000 people in the United States and millions worldwide.4, 5 Current approved treatments for SCD include hydroxyurea 6 and L‐glutamine.7 To date, no drugs have been approved that target the underlying mechanism of SCD. Hydroxyurea is not effective in some patients and has significant safety concerns, including potential embryofetal toxicity and myelosuppression. L‐glutamine, recently approved, demonstrated only a modest effect in prevention of vaso‐occlusive crisis without improvement in haematologic parameters, and does not target the pathophysiologic mechanism of disease.8, 9 Therefore, a chronic preventive treatment remains a serious unmet need.
Because oxygenated Hb is a potent inhibitor of HbS polymerization, increasing the proportion of oxygenated HbS may provide a disease‐modifying approach to SCD.10, 11, 12 Previous Hb allosteric modifiers such as 5‐hydroxymethylfurfural (5‐HMF),13 tucaresol,14, 15 and valerosol (BW12C)16 have been investigated. These agents (tucaresol and valerosol) demonstrated that increasing the Hb‐oxygen affinity at 20–30% Hb modification inhibited RBC sickling in vitro and reduced haemolysis in SCD patients without compromising oxygen delivery to tissues.17, 18 However, poor pharmaceutical properties (5‐HMF),17 lack of specificity, and immunogenicity (tucaresol18) prevented further development. Because a positive pharmacodynamic (PD) effect was observed with 20–30% Hb modification, this target modification is expected to provide therapeutic effect. This also aligns with studies in individuals with compound heterozygosity for HbS and pancellular hereditary persistence of fetal Hb, where 20–30% pancellular fetal Hb conferred significant clinical benefits.19
Voxelotor (previously GBT440) is a first‐in‐class oral therapy developed for the treatment of SCD by modulating the Hb affinity for oxygen. Voxelotor forms a reversible covalent bond with the N‐terminal valine of the α‐chain of Hb, resulting in an allosteric modification of Hb20 and eliciting an increase in oxygen affinity.21 Because oxygenated HbS does not polymerize, voxelotor may prevent sickling of RBCs and potentially interrupt the molecular pathogenesis of the disease.
Following promising preclinical data, an “umbrella protocol” (GBT440‐001, NCT02285088) for a phase 1/2 study was designed to evaluate the safety, tolerability, pharmacokinetics (PK), PD, PK‐PD relationship, and effect of voxelotor on cardiovascular parameters at rest and during exercise following single and multiple doses of voxelotor administered to healthy volunteers and SCD patients. Dose selection and dose escalation were pharmacologically guided based on analysis of PD effect among cohorts.
Here, we report results of single and multiple doses of voxelotor in healthy volunteers and a single dose in SCD patients. Results of multiple dosing in SCD patients are published elsewhere.22
2. METHODS
GBT440‐001 was an integrated phase 1/2, two‐centre, randomized, double‐blind, placebo‐controlled, three‐part study of voxelotor in healthy volunteers and SCD patients. Part A, a single ascending dose (SAD) design, and part B, a multiple ascending dose (MAD) design, included both healthy volunteers and SCD patients. Part C included only SCD patients. The study was conducted in accordance with Good Clinical Practice guidelines and in conformity with the ethical principles of the Declaration of Helsinki and was compliant with all relevant country‐specific laws and regulations. The study protocol and all other appropriate study‐related information were reviewed and approved by an independent ethics committee.
2.1. Subjects
Healthy volunteers aged 18–55 years weighing between 50 kg and 110 kg at screening and SCD patients (HbSS, HbS/β0 thalassemia, HbS/β+ thalassemia or HbSC) aged 18–60 years weighing >50 kg were included. For SCD patients, Hb genotype was confirmed at screening, and concurrent use of hydroxyurea was allowed if dose was stable for 3 months before entry. Key exclusion criteria for the healthy volunteers included the use of prescription drugs within 4 weeks of first dosing or the use of over‐the‐counter medications (excluding routine vitamins) within 7 days of first dosing. Key exclusion criteria for SCD patients included Hb levels <60 g L−1 or >104 g L−1 or transfusion or hospitalization within 30 days of screening. Full details of the inclusion and exclusion criteria for healthy volunteers and SCD patients are provided in Table S1. All subjects provided written informed consent before the commencement of any study‐related procedures.
2.2. Study design and treatment
An objective of this study was to demonstrate drug safety at high target percent Hb modification of ~40% (RBC concentration of 675 μg mL−1 or 2 mM) in healthy volunteers, the level at which there was evidence of a compensatory increase in cardiac output with exercise from prior studies with other agents with similar mechanisms.15 To dose‐escalate safely and effectively, a pharmacologically guided approach was implemented (Figure 1). The initial dose of 100 mg was selected based on allometric scaling of animal data to human to provide approximate 15‐fold margin below no‐observed‐adverse‐effect level of a 28‐day rat toxicology study and to be below the anticipated pharmacologically active dose (≤5% Hb modification). Following the initial dose of 100 mg, subsequent doses of voxelotor were administered to target 5%, 10%, 20% and 30% Hb modification. The first MAD dose (300 mg) was initiated as soon as safety was demonstrated in the single dose subjects at ~20% Hb modification (2000 mg). The final MAD dose was targeted at 40% Hb modification. The multiple dose study in SCD patients was initiated following demonstration of safety in healthy volunteers at equivalent percent Hb modification.
Figure 1.
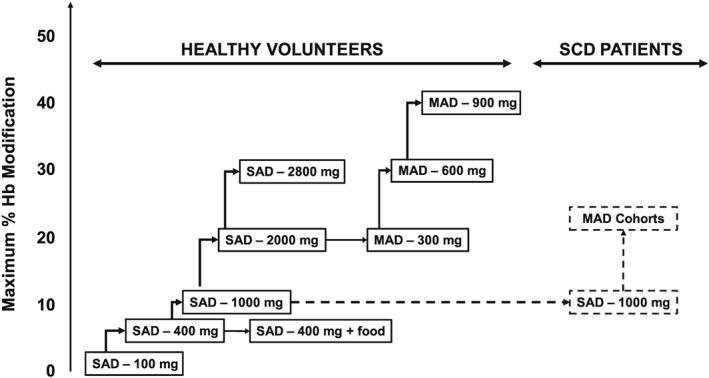
Study design and dose‐escalation scheme. Stepwise dose‐escalation scheme for healthy volunteers and SCD patients. In each cohort, eight subjects were randomized to receive voxelotor (n = 6) or placebo (n = 2). Following the first dose, subsequent doses were escalated to reach 5%, 10% and 20% Hb modification. The first MAD cohort started after equivalent Hb modification was achieved in the single‐dose cohort. The maximum target for the MAD cohort was 40% Hb modification. Food effect was evaluated at 400 mg. Single dose in SCD subjects was initiated at 1000 mg, and the data were used to estimate MAD doses at targeted 20% and 30% Hb modification
2.3. Safety
Safety and tolerability were monitored at regular intervals from predose to a follow‐up visit (27 days following completion of single doses and 45 days following completion of multiple doses). Safety assessments included physical examinations, vital signs, electrocardiograms, clinical laboratory assessments, incidence of clinical adverse events and concomitant medications. Any clinically significant abnormal laboratory test results were followed until resolved or stabilized. No formal power calculations were performed. The sample size was based on the desire to obtain adequate safety, tolerability, PK and PD data to achieve the objectives of the study whilst exposing as few subjects as possible to study medication and procedures.
2.3.1. Part A: SAD
In each dose cohort, healthy volunteers were randomized to voxelotor (n = 6) or placebo (n = 2). Two sentinel subjects per cohort were dosed (one randomized to voxelotor and one randomized to placebo). Providing there were no serious or unexplained safety issues, the remainder of the cohort were dosed 48 h later. A minimum of 10 days was allowed between the last dose administered in one cohort and the first dose administered in the next cohort. The starting dose was 100 mg, and subsequent dose escalations for healthy volunteer cohorts were 400, 1000, 2000 and 2800 mg. Six SCD patients were administered a single dose of 1000 mg voxelotor and two patients received placebo following completion of the 1000 mg cohort in healthy volunteers. Voxelotor or placebo was administered orally with at least 240 mL water, after an overnight fast of approximately 8–10 h.
The effect of food on PK and PD was assessed in a nonrandomized, crossover design in the cohort of healthy volunteers who received 400 mg voxelotor. Subjects returned to the study centre after a washout period (minimum of 28 days after the first dose of study medication) to receive a second single dose of the same treatment (400 mg voxelotor or placebo) 30 min after the start of a low‐fat breakfast.
2.3.2. Part B: MAD
Healthy volunteers in all cohorts were randomized 3:1 to voxelotor or placebo. Three cohorts were enrolled to receive 15 days of treatment with voxelotor doses of 300, 600 and 900 mg once daily or matching placebo. Cohorts were dosed sequentially, with a minimum of 10 days between the last dose of the one cohort and the first dose of the next cohort.
2.4. Pharmacokinetics
Serial blood samples were obtained for PK analyses for the determination of voxelotor concentrations in whole blood, plasma and RBCs. Samples were analysed using a validated liquid chromatography–tandem mass spectrometry method. Analytical ranges were 10–10 000 ng mL−1 and 5–5000 ng mL−1 for blood and plasma samples, respectively. Percent coefficients of variation (%CV) for lower limit of quantitation for intra‐ and inter‐run was <11.8% for the whole blood method and <6.9% for the plasma method. Concentration of voxelotor in RBC was calculated from23:
2.5. Pharmacodynamics
Percent Hb modification was determined by measuring the oxygen equilibrium curves (OECs) and changes in Hb‐oxygen affinity using a TCS Hemox Analyzer. Clinical samples collected in sodium citrate vacutainers were kept at 4°C and analysed according to the method previously described21 to obtain p20 and p50 values (partial pressure of O2 at which Hb is 20% or 50% saturated with O2). Delta p20 (∆ p20) and delta p50 (∆ p50) values were obtained by subtracting the day −1 from the sample value.
2.6. Haematology and serum erythropoietin
Erythrocyte, reticulocyte, white blood cell and platelet counts were determined with a Siemens Advia 2120i (Siemens Healthcare Diagnostics, Surrey, UK). Serum erythropoietin (EPO) was measured by a chemiluminescent assay on a Siemens Immulite XPi (Siemens Healthcare Diagnostics, Surrey, UK).
2.7. Exercise testing
Exercise testing was performed based on the Young Men's Christian Association submaximal cycle ergometer test protocol.24 Heart rate, respiratory rate, blood pressure and pulse oximetry (oxygen saturation) were evaluated.
2.8. Statistical analysis
Demographic data were summarized by dose. PK, PD and safety data were summarized by dose and presented as summary statistics.
PK parameters, including maximum concentration (C max), time to reach maximum concentration (T max), area under the concentration–time curve extrapolated to infinity following a single dose (AUCinf) or over 24‐h dose interval at steady‐state for multiple dosing (AUC0–24) and elimination half‐life (T 1/2), were derived using noncompartmental methods with Phoenix® WinNonlin® Version 6.4 (Certara, Princeton, NJ, USA).
p20 and p50 values between doses were compared using Sidak's multiple comparisons tests to measure statistical significance. PK/PD correlations were determined using linear regression.
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,25 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.26
3. RESULTS
3.1. Demographics and baseline characteristics
A total of 40 healthy volunteers were randomized to receive a single dose of voxelotor (100, 400, 1000, 2000 or 2800 mg; n = 6 per group) or placebo (n = 2 per group). Six SCD patients received 1000 mg voxelotor and two received placebo. A total of 24 healthy volunteers were randomized to receive multiple doses of voxelotor (300, 600 or 900 mg; n = 6 per group) or placebo (n = 2 per group; total n = 8). The demographics for these subjects are summarized in Table 1. Mean age and body mass index were similar across the dosing groups. There was no apparent impact of race on any PK or PD parameters of voxelotor.
Table 1.
Patient baseline and demographic data
| Part |
A Healthy volunteers Single ascending dose |
A Sickle cell disease Single dose | B Healthy volunteers Multiple ascending dose | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxelotor (mg) or matched placebo | Placebo | 100 | 400 | 1000 | 2000 | 2800 | Placebo | 1000 | Placebo | 300 | 600 | 900 |
| n | 10 | 6 | 6 | 6 | 6 | 6 | 2 | 6 | 6 | 6 | 6 | 6 |
| Age, median (range), y | 34 (22–44) | 33 (19–50) | 33 (24–40) | 33 (27–43) | 36 (27–48) | 35 (28–51) | 27 (20–34) | 31 (25–47) | 34 (22–48) | 30 (23–54) | 38 (23–49) | 40 (27–54) |
| Male sex, n (%) | 10 (100) | 6 (100) | 6 (100) | 6 (100) | 5 (83.3) | 6 (100) | 1 (50) | 3 (50) | 6 (100) | 6 (100) | 6 (100) | 6 (100) |
| Race, n (%) | ||||||||||||
| Asian | 0 | 1 (16.7) | 0 | 2 (33.3) | 0 | 1 (16.7) | 0 | 0 | 1 (16.7) | 0 | 0 | 2 (33.3) |
| Black | 1 (10%) | 0 | 1 (16.7) | 1 (16.7) | 0 | 2 (33.3) | 2 | 6 | 0 | 1 (16.7) | 0 | 0 |
| White | 9 (90%) | 3 (50) | 4 (66.7) | 3 (50) | 6 | 2 (33.3) | 0 | 0 | 5 (83.3) | 5 (83.3) | 6 (100) | 4 (66.7) |
| Other | 0 | 2 (33.3) | 1 (16.7) | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| BMI median (range) | 24.7 (22.4–28.1) | 25.7 (24.4–28.5) | 25.3 (21.7–26.8) | 24.7 (22.4–28.3) | 25.1 (22.1–28.2) | 28.5 (19.7–35.3) | 25.8 (25.3–26.2) | 21.9 (20.2–23.9) | 26.5 (22.4–27.5) | 26.5 (22.6–29.5) | 24.9 (20.4–30) | 26.1 (23.2–30.7) |
BMI, body mass index.
3.2. Safety and tolerability
Following single doses, voxelotor was well tolerated up to and including the highest dose evaluated (2800 mg); i.e., a maximum tolerated single dose was not determined. Five treatment‐emergent adverse events (TEAEs; diarrhoea, upper respiratory tract infection, arthralgia, headache and rash) were reported in >1 healthy volunteer (Table 2). Of these, diarrhoea and headache were considered related to study drug. Diarrhoea occurred only at 2800 mg. In SCD patients receiving a single dose of 1000 mg voxelotor, four TEAEs (diarrhoea, nausea, vomiting and pain) were reported in >1 SCD patient (Table 3). Of these, diarrhoea, nausea and vomiting were considered related to study drug. All AEs reported following a single dose of voxelotor in healthy volunteers and SCD patients were grade 1 (mild).
Table 2.
TEAEs occurring in >1 healthy volunteer receiving a single dose of voxelotor or placebo
| Single ascending dose in healthy volunteers | |||||||
|---|---|---|---|---|---|---|---|
| 100 mg | 400 mg | 1000 mg | 2000 mg | 2800 mg | All | Placebo | |
| Preferred term | n = 6 | n = 6 | n = 6 | n = 6 | n = 6 | n = 30 | n = 10 |
| Diarrhoea | 3 | 3 | |||||
| Upper respiratory tract infection | 1 | 1 | 2 | 1 | |||
| Arthralgia | 1 | 1 | 2 | ||||
| Headache | 1 | 1 | 2 | 1 | |||
| Rash (all types) | 1 | 1 | 2 | 1 | |||
All AEs were grade 1.
Table 3.
TEAEs occurring in >1 SCD patient receiving a single dose of voxelotor or placebo
| Patients with SCD (1000 mg) | Placebo | |
|---|---|---|
| Preferred term | n = 6 | n = 2 |
| Diarrhoea | 3 | 0 |
| Nausea | 2 | 0 |
| Vomiting | 2 | 0 |
All AEs were grade 1.
Following multiple doses, voxelotor was well tolerated up to and including the highest dose evaluated (900 mg daily for 15 days); i.e., a maximum tolerated multiple dose was not determined. Five TEAEs (abdominal pain, diarrhoea, gastroenteritis, dizziness and headache) were reported in ≥10% of healthy volunteers (Table 4). Headache, diarrhoea and abdominal pain were considered related to study drug. Most AEs were grade 1 in subjects receiving voxelotor; all AEs were grade 1 in subjects receiving placebo. Three subjects who received voxelotor experienced grade 2 (moderate) events: one at 300 mg and two at 900 mg. No dose‐limiting toxicities were identified. Two subjects discontinued study drug because of TEAEs; one subject receiving 600 mg discontinued on Day 12 because of a grade 1 rash and one subject receiving 900 mg discontinued on Day 7 because of a grade 2 headache along with grade 1 diarrhoea and abdominal pain. The rash was a typical and uncomplicated maculopapular rash. These events resolved without treatment except for headache, which was treated with paracetamol.
Table 4.
TEAEs occurring in >1 healthy volunteer receiving 15 days of voxelotor or placebo
| Voxelotor |
Placebo n = 6 |
||||
|---|---|---|---|---|---|
| Preferred term |
300 mg n = 6 |
600 mg n = 6 |
900 mg n = 6 |
All n = 18 |
|
| Abdominal pain | 0 | 0 | 2 (33.3) | 2 (11.1) | 0 |
| Diarrhoea | 0 | 0 | 3 (50.0) | 3 (16.7) | 0 |
| Gastroenteritis | 1 (16.7)a | 0 | 1 (16.7)a | 2 (11.1) | 0 |
| Dizziness | 2 (33.3) | 0 | 0 | 2 (11.1) | 0 |
| Headache | 1 (16.7)a | 0 | 4 (66.7)a | 5 (27.8) | 0 |
All AEs were grade 1 except for grade 2 gastroenteritis and grade 2 headache (2 of 4 events, grade 2).
A theoretical concern with Hb‐oxygen affinity modifiers is that they could reduce tissue oxygen delivery per unit volume of blood, resulting in a compensatory increase in EPO with subsequent reticulocytosis, increased RBC mass and increased cardiac output under conditions of exercise stress.18, 27 To evaluate this concern, several specific assessments were performed including evaluation of reticulocyte counts, EPO levels and haemodynamic response to submaximal exercise testing. After multiple doses in healthy volunteers up to 900 mg daily, which achieved approximately 40% Hb modification, there was no evidence of a treatment‐related increase in reticulocyte count or Hb level, EPO remained in the normal range (Figure 2), and there was no increase in resting or peak exercise heart rate (data not shown).
Figure 2.
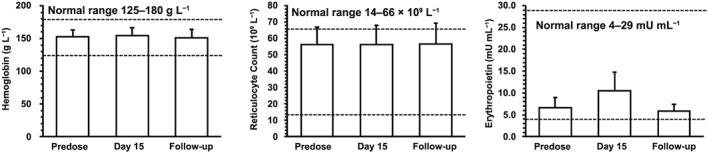
Hb, reticulocyte count, and EPO levels following 900 mg voxelotor once daily for 15 days in healthy volunteers. Hb, reticulocyte count, and EPO values in healthy volunteers at predose, Day 15, and follow‐up (~45 days after the last dose). Data are shown as mean ± standard deviation
3.3. Pharmacokinetics
RBC and plasma concentration–time profiles of voxelotor following single dose (100–2800 mg) and multiple doses (300–900 mg), Day 15, in healthy volunteers are presented in Figure 3. Comparison of RBC concentration–time profiles between healthy volunteers and SCD patients following 1000 mg are shown in Figure 4. PK parameters are summarized in Table 5. Following single dose administration to healthy volunteers, voxelotor was rapidly absorbed into the plasma with median T max < 4 h at doses <2000 mg. At 2800 mg, T max occurred later (7 h), likely because of continuing absorption of high drug load. Later T max was observed in RBC (17–24 h). C max and AUC of RBC and plasma increased proportionally with voxelotor dose for single or multiple doses. The exposure of voxelotor at steady‐state accumulated as predicted based on the single‐dose data. Following a daily dose of voxelotor at 300, 600 and 900 mg for 15 days, at steady state, the respective mean RBC C max were 225, 462 and 688 μg mL−1 and RBC AUC(0–24) were 4950, 9610 and 14 000 μg*h mL−1. The accumulation was approximately six‐fold from that of Day 1 exposure. T 1/2 of voxelotor ranged from 61 to 85 h. Plasma concentration declined in parallel to RBC concentration. Voxelotor had high specific binding to RBC as evidenced by the RBC/plasma ratio ranging from 67:1 to 111:1. Inter‐subject variability for C max and AUC values was low at doses ≤1000 mg for both single (%CV ≤ 22%) and multiple dosing at steady‐state (%CV ≤ 15%). However, at 2000 and 2800 mg, the %CV for C max was 22–36% and for AUC was approximately 28% for both doses. The higher inter‐subject variability at 2000 and 2800 mg was likely caused by variability in dissolution of voxelotor in the gut of individual subjects at high drug loads due to its low solubility (~43 μg mL−1 in simulated intestinal fluid). At the same 1000 mg single dose, inter‐subject variability for C max and AUC of SCD patients (30–33%) was higher than that in healthy volunteers (10–17%), possibly due to the higher variability in the hematocrit values (0.20–0.28) of SCD patients compared to healthy volunteers (0.40–0.45).
Figure 3.
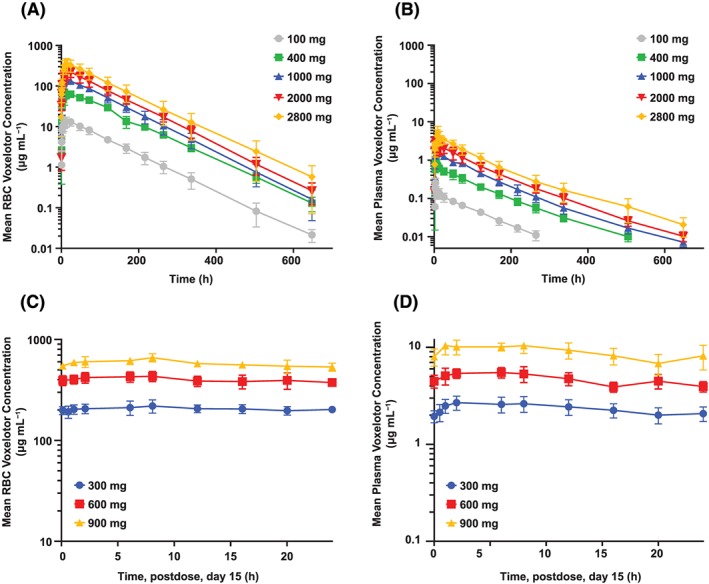
RBC and plasma voxelotor concentration–time profiles after single and multiple doses in healthy volunteers. Healthy volunteers (n = 6/cohort) received single dose of voxelotor at 100, 400, 1000, 2000 and 2800 mg. Concentration–time profiles in RBC (A) and plasma (B) show linear increase in exposure across these doses. Following daily dose at 300, 600 and 900 mg voxelotor for 15 days, RBC (C) and plasma (D) profiles on Day 15 also proportionally increase. Data are shown as mean ± standard deviation
Figure 4.
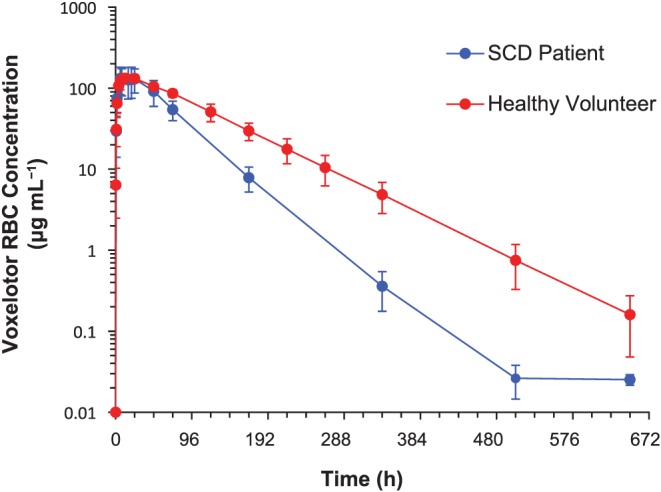
RBC concentration–time profiles of voxelotor following 1000‐mg single dose in healthy volunteers and SCD patients. RBC concentration–time profiles in healthy volunteers (n = 6) and SCD patients (n = 6) receiving 1000 mg voxelotor single dose. Similar C max was achieved but AUCinf in healthy volunteers was higher than in SCD patients because of longer T 1/2. Data are shown as mean ± standard deviation
Table 5.
RBC and plasma voxelotor PK parameters in healthy volunteers and SCD patients
| Single dose | Multiple doses | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers | SCD | Healthy volunteers | |||||||
| Dose, mg | 100 | 400 | 1000 | 2000 | 2800 | 1000 | 300 | 600 | 900 |
| RBC PK parameters | |||||||||
| T max, h | 18.0 (12.0–24.1) | 24.0 (8.0–24.1) | 18.0 (8.0–24.0) | 16.6 (12.6–25.0) | 17.1 (9.0–25.2) | 14.0 (6.0–25.6) | 6.0 (1.0–12.0) | 8.0 (6.0–20.0) | 8.0 (2.0–8.0) |
| C max, μg mL−1 | 13.8 ± 3.48 | 77.3 ± 31.4 | 136 ± 16.1 | 222 ± 67.5 | 377 ± 137 | 146 ± 46.6 | 225 ± 29.1 | 462 ± 59.8 | 688 ± 25.7 |
| AUCa, μg*h mL−1 | 1540 ± 343 | 8080 ± 902 | 15 800 ± 2430 | 24 200 ± 5860 | 38 800 ± 13 400 | 10 000 ± 2860 | 4950 ± 520 | 9610 ± 1000 | 14 000 ± 612 |
| T 1/2, h | 64.7 ± 2.60 | 68.6 ± 5.61 | 61.1 ± 7.30 | 63.6 ± 6.64 | 63.6 ± 11.0 | 49.7 ± 2.87 | 80.3 ± 1.89 | 76.5 ± 3.79 | 85.1 ± 7.43 |
| RAUC(0–24h) | – | – | – | – | – | – | 6.09 ± 0.687 | 6.16 ± 1.91 | 6.42 ± 1.05 |
| Plasma PK parameters | |||||||||
| T max, h | 2.0 (1.0–2.0) | 2.0 (1.0–8.0) | 4.0 (2.0–6.0) | 2.5 (2.5–8.5) | 7.1 (3.1–9.1) | 2.0 (1.3–2.2) | 4.0 (1.0–12.0) | 6.0 (1.0–6.0) | 2.0 (1.0–8.0) |
| Cmax, μg mL−1 | 0.269 ± 0.0819 | 1.14 ± 0.502 | 2.21 ± 0.388 | 3.39 ± 0.731 | 6.31 ± 2.29 | 2.94 ± 0.747 | 2.91 ± 0.410 | 5.78 ± 0.834 | 11.4 ± 1.70 |
| AUCa, μg*h mL−1 | 14.8 ± 3.20 | 74.5 ± 16.8 | 166 ± 17.1 | 266 ± 74.5 | 433 ± 121 | 134 ± 27.3 | 56.6 ± 8.70 | 113 ± 13.2 | 215 ± 32.7 |
| RBC/plasma ratio | 104 ± 14.9 | 111 ± 17.6 | 95.3 ± 9.00 | 92.0 ± 8.86 | 87.9 ± 7.44 | 75.0 ± 15.7 | 88.0 ± 4.85 | 85.0 ± 4.77 | 66.6 ± 11.1 |
Data are presented as mean ± standard deviation for C max, AUC, T 1/2, RBC/plasma ratio, RAUC(0–24h) or median (range) for T max. For multiple dose, PK parameters on day 15 are presented.
AUCinf value for single dose and AUC(0–24h) value on Day 15 for multiple doses are shown.
Following single‐dose administration of 1000 mg to SCD patients, voxelotor was rapidly absorbed into the plasma with median T max of 2 h and C max of 2.9 μg mL−1. In RBCs, median T max of voxelotor was 14 h and C max was 146 μg mL−1. The AUC ratio in RBCs over plasma was 75:1. Terminal T 1/2 was 50 h. This long T 1/2 suggests suitability for once‐daily dosing.
The effect of food on the PK of voxelotor was evaluated by comparing exposure following a 400 mg single dose under fasted vs. fed (low‐fat breakfast) conditions. The absorption rate and exposure were similar under these two conditions. The T max, C max and AUCinf in RBCs under fasted conditions were 24 h, 77.3 μg mL−1 and 8080 μg*h mL−1, respectively; and under fed conditions were 10 h, 74.4 μg mL−1 and 8690 μg*h mL−1, respectively. Relative bioavailability of voxelotor under fed condition was 108% (90% confidence interval = 100.73%, 115.82%) compared with the fasted condition, indicating that a low‐fat breakfast had no significant effect on voxelotor exposure.
3.4. Pharmacodynamics
Blood samples collected from volunteers at various timepoints during both SAD and MAD studies were used to determine changes in Hb‐oxygen affinity via OEC measurement. As the dose increased, both the p20 and p50 values decreased indicating an increase in Hb‐oxygen affinity (Table 6). Figure 5A shows representative OECs at 24 h for the three MAD cohorts following the dose on Day 15. Following daily dosing of placebo, 300, 600 and 900 mg voxelotor for 15 days, the mean p50s and p20s were 30.7, 29.9, 24.3 and 24.6 mm Hg and 16.2, 14.1, 9.4 and 6.3 mm Hg, respectively. A summary of the p20 and p50 changes observed at C min after 15 days of dosing is shown in Figures 5B and 5C. Due to the biphasic nature of the OECs generated by voxelotor, the changes in p20 value compared with Day −1 were more prominent and significant (Figure 5B) than the changes in p50 value (Figure 5C), especially at the lowest dose (300 mg) with voxelotor RBC concentrations <0.75 mM (equivalent to <15% Hb modification, Figures 5 and 7D). Based on a previously established in vitro correlation (Figure S1, Table S2), percent Hb modification was calculated from the ∆p20 values and are summarized in Table 6. The mean percent Hb modifications at 24 h following a single dose of 100, 400, 1000, 2000 and 2800 mg voxelotor were 1.4%, 3.4%, 5.9%, 7.9% and 23.6%, respectively. The mean percent Hb modifications following 300, 600 and 900 mg day−1 on Day 15 were 10.9%, 24.1% and 38.3%, respectively.
Table 6.
PD parameters in healthy volunteers and SCD patients
| A (healthy volunteers, single ascending dose)a | A (SCD, single dose) | B (healthy volunteers, multiple ascending dose)c | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosing duration, days | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 15 | 15 | 15 |
| Dose, mg | Placebo | 100 | 400 | 1000 | 2000 | 2800 | Placebob | 1000 | Placebo | 300 | 600 | 900 |
| p50, mm Hg | 30.1 ± 1.7 | 26.0 ± 1.7 | 30.5 ± 1.7 | 29.6 ± 1.0 | 28.2 ± 1.2 | 27.4 ± 2.0 | 37.1 | 33.5 ± 2.8 | 30.7 ± 2.2 | 29.9 ± 1.7 | 24.3 ± 1.5 | 24.6 ± 2.5 |
| p20, mm Hg | 15.9 ± 1.1 | 12.7 ± 1.5 | 15.6 ± 0.9 | 14.5 ± 0.7 | 13.4 ± 0.8 | 10.0 ± 3.1 | 19.9 | 16.8 ± 2.6 | 16.2 ± 1.3 | 14.1 ± 1.0 | 9.4 ± 1.1 | 6.3 ± 0.8 |
| % Hb mod | −0.3 ± 4.1 | 1.4 ± 1.4 | 3.4 ± 2.2 | 5.9 ± 4.4 | 7.9 ± 3.9 | 23.6 ± 8.6 | −3.1 | 7.1 ± 10.8 | −1.0 ± 4.8 | 10.9 ± 3.6 | 24.1 ± 2.4 | 38.3 ± 8.6 |
Data are presented as mean ± standard deviation.
Data from 24 h post dose.
n = 1; day −1 data were not usable.
Data from 24 h post final dose, C min.
Figure 5.
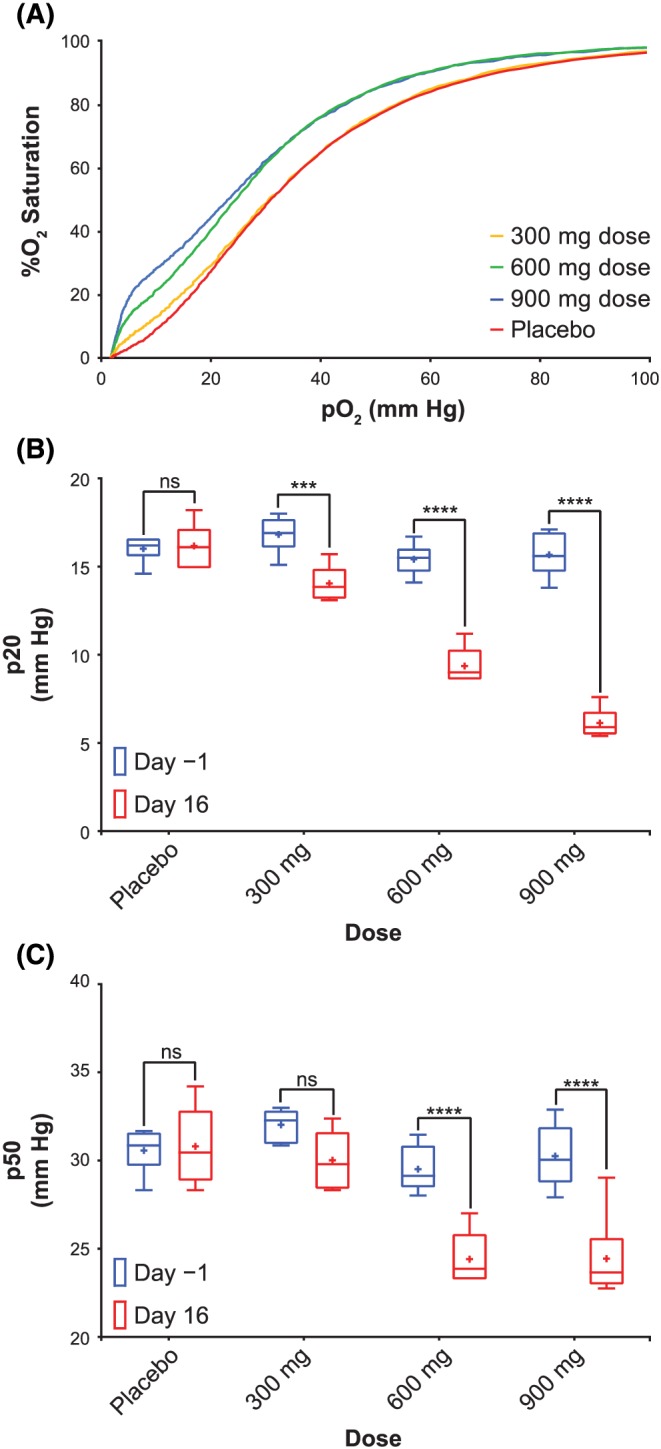
PD at steady state in healthy volunteers. A, Selected median OECs from subjects at C min after 15 days of dosing. A summary of the p20 (B) and p50 (C) values observed in healthy volunteers at C min after 15 days of dosing. A dose–dependent decrease in p20 and p50 was observed, showing that increasing levels of drug leads to increased Hb‐oxygen affinities. Sidak's multiple comparisons tests were used to measure statistical significance
Figure 7.
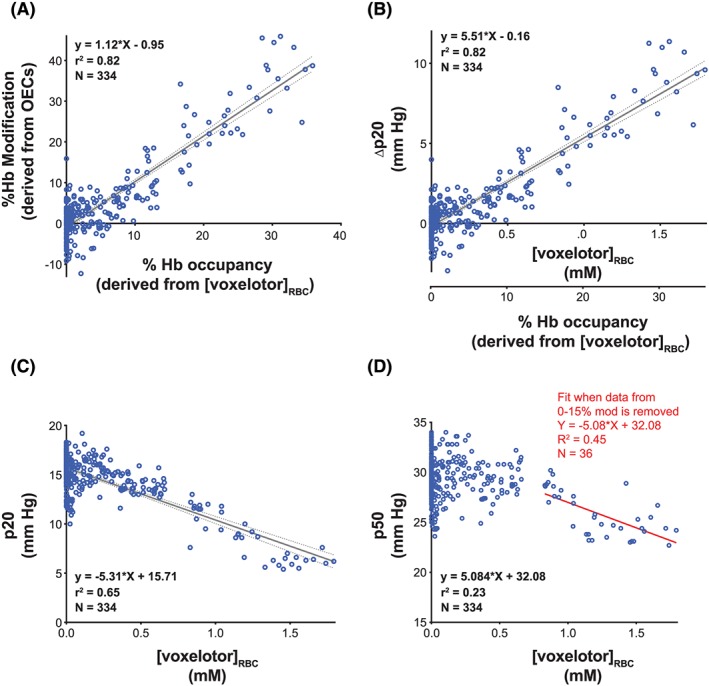
PK/PD correlation in healthy volunteers. A, Linear correlation observed between percent Hb modification derived from OECs and percent Hb occupancy (derived from [voxelotor]RBC) in healthy volunteers. B, Correlation observed between Δp20 and RBC voxelotor concentrations or percent Hb occupancy. C, Correlation observed between p20 and RBC voxelotor concentrations. D, Correlation observed between p50 and RBC voxelotor concentrations
Following a single dose of 1000 mg voxelotor, SCD patients and healthy volunteers achieved similar maximum percentage Hb modification (Table 6). In SCD, because of the anaemia, RBCs tend to have a higher 2,3‐bisphosphoglycerate concentration leading to a lower Hb‐oxygen affinity, and a rightward shifted OEC. Figure 6 shows the OEC for a predosed SCD patient, compared with the 24‐h OEC post 1000 mg voxelotor dose, which exhibits a leftward shifted OEC, trending towards the OEC of a healthy volunteer.
Figure 6.
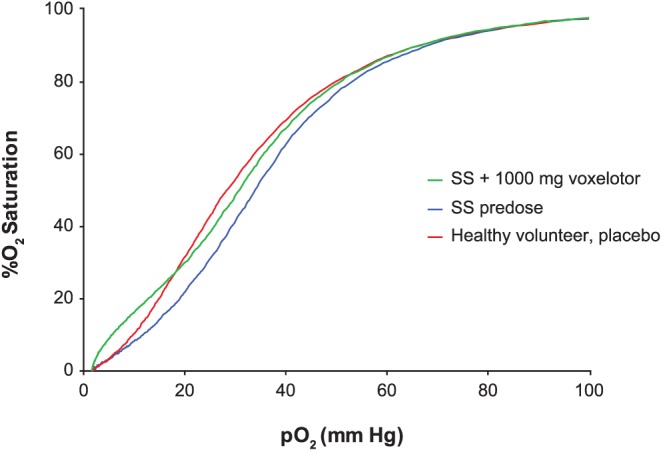
Oxygen equilibrium curves from blood of a voxelotor‐treated SCD patient compared with a healthy volunteer. Representative predose and 24 h postdose OECs of SCD patients who achieved 23% Hb modification on a single 1000‐mg voxelotor dose, compared with the OEC of a healthy volunteer on placebo
3.5. PK/PD correlations
A percent Hb occupancy was calculated as the ratio of the voxelotor concentration to the Hb concentration (estimated at 5 mM) in RBCs.
Figure 7A illustrates that the percent Hb modification (derived from OECs) is highly correlated (R 2 = 0.82) with the percent Hb occupancy (derived from the voxelotor concentration in RBCs). Figure 7B shows the correlation between ∆p20 and RBC voxelotor concentrations in samples from healthy volunteers. These correlations allow for an estimation of the Hb‐oxygen affinity in future studies when only the RBC voxelotor levels are available. Figure 7C shows a linear and robust correlation between p20 values and RBC levels of voxelotor (R 2 = 0.65). As expected, the correlation with p50 values is less robust (R 2 = 0.23) (Figure 7D). However, when data from 0 to 15% Hb modification (derived from OECs) is omitted, the correlation is improved to R 2 = 0.45, indicating that correlations with p50 improve at higher percent Hb modifications.
4. DISCUSSION
This is the first study of voxelotor in healthy volunteers and SCD patients to evaluate safety, tolerability, PK, PD and PK/PD relationship. Voxelotor was administered as a single dose at 100, 400, 1000, 2000 and 2800 mg and once daily for 15 days at 300, 600 and 900 mg to healthy volunteers. The dose increments were determined based on emerging tolerability, PK and PD data. PK and PD following a single dose of 1000 mg voxelotor in SCD patients was also reported. PK profiles in plasma and RBCs were monitored to demonstrate voxelotor binding specificity to Hb and concentrations in the target compartment (RBCs). Following oral administration to healthy volunteers, voxelotor was rapidly absorbed into plasma followed by distribution into RBCs as demonstrated by earlier plasma T max (2–4 h) than RBC T max (16–24 h). Nevertheless, the voxelotor concentration in RBCs reached near C max levels within an hour after administration and continued to increase slowly until the C max was reached. This observation supports previous in vitro data which show that voxelotor has a higher binding affinity for Hb than for plasma proteins.28 These distribution profiles are consistent across animal species tested.21 Voxelotor is mainly eliminated by metabolism in humans with <1% of the dose administered excreted unchanged into urine.29 The T 1/2 of voxelotor (~60 days) is much shorter than the T 1/2 of human RBC,30 implying its binding to Hb is a reversible process and drug can be released from Hb before being further metabolized.
In healthy volunteers, the PK of voxelotor was linear between 100 and 2800 mg. Accumulation of voxelotor was also linear upon multiple dosing. The unique PK properties of voxelotor are critical in binding to high abundance target and achieving the target Hb modification at a reasonable oral dose (e.g., 900 mg day−1 could achieve ~40% Hb modification in healthy volunteers). In addition, the high RBC to plasma partitioning results in relatively low plasma concentrations, thus reducing the risk of dose‐related off‐target toxicities. The high selectivity of voxelotor for Hb with a lack of off‐target safety concerns, together with pharmaceutical properties enabling once daily dosing, represent a significant advance over prior attempts at developing Hb‐modifying agents.18, 31, 32 In SCD patients, voxelotor was also rapidly absorbed into plasma following oral administration, then distributed into RBCs. Although SCD patients achieved the same C max as healthy volunteers, the AUC and T 1/2 of voxelotor in SCD patients were lower than those observed in healthy volunteers. The mean T 1/2 of voxelotor was 50 h in SCD patients compared with 61–85 h in healthy volunteers. These differences are likely due to lower hematocrit and decreased RBC T 1/2 in SCD patients compared with healthy volunteers. As C max levels were similar between SCD patients and healthy volunteers, both exhibited similar percent Hb modifications, indicating the same potency of voxelotor in normal (AA) blood and sickle (SS) blood.
Consistent with the in vitro findings,21 OECs of voxelotor in human subjects exhibited a biphasic profile because of the coexistence of Hb species with varying oxygen affinity. Voxelotor exhibited a concentration‐dependent decrease in p20 and p50 values following the various dosing regimens. The biphasic nature of OECs yields differentiable p20 values at clinical voxelotor concentration ranges, while p50 shift was not significant at low voxelotor concentrations but became more prominent at higher voxelotor concentration; i.e., >0.75 mM (equivalent to ~15% Hb modification). In this study, the changes in p20 values at steady state compared with predose level were significant at all three dose levels, whereas p50 showed a shift at 600 and 900 mg but not at the 300 mg dose. Therefore, consistent with the previously established in vitro model, p20 is more suitable than p50 in assessing the pharmacologic activity of voxelotor at therapeutic doses and RBC concentrations.
There was a linear relationship between ∆p20 measured from samples in this clinical study and percent Hb occupancy. Percent Hb occupancy correlated with percent Hb modification. Therefore, percent Hb occupancy could be utilized as a determinant for the extent of Hb modification in future clinical studies when p20 values are not available.
Evaluation of EPO, exercise testing and haematologic parameters was consistent with maintenance of normal oxygen delivery during rest and exercise up to the exposures achieved in this study, indicating that the percent Hb modification achieved in this study did not impair the ability of Hb to deliver oxygen to tissues.
In this first‐in‐human study, the highest single dose studied in SCD patients (1000 mg) and healthy volunteers (2800 mg) was well tolerated, as was a 15‐day course of 900 mg once daily in healthy volunteers and established proof of mechanism on increasing Hb‐oxygen affinity. Voxelotor PK was linear and predictable across the dose range tested. The drug showed a high binding specificity for Hb, and demonstrated a long half‐life, allowing sustained PD effect with once‐daily dosing.
COMPETING INTERESTS
A.H., M.P., C.W., V.S., D.O. and J.L.‐G. are employees of and have equity ownership in Global Blood Therapeutics. T.M. and E.A. are employees of IQVIA. T.M. owns shares of IQVIA. D.D.G. is an independent consultant.
CONTRIBUTORS
A.H. helped design and plan the study, oversaw PK sample analysis, performed PK/PD data analysis, suggested dose escalation levels between cohorts, and contributed to manuscript writing. M.P. designed and implemented the in vitro model for Hb modification, trained the staff for OEC determinations, troubleshooted and analysed PD data, prepared figures, discussed and interpreted data, and contributed to manuscript writing. C.W. provided input to dose escalation levels and contributed to manuscript writing. V.S. performed experiments to develop and implement the in vitro Hb modification model, trained the staff at the clinical site for OEC determinations, and troubleshooted and analysed PD data. E.A. helped to design and plan the study. D.O. supervised the implementation of the Hb modification model, discussed and interpreted data, and contributed to manuscript writing. D.D.G. was the sponsor's consultant and a member of the safety committee, designed the study, and reviewed and provided input into this publication. T.M. was the principal investigator, was responsible for study conduct under International Conference on Harmonisation Good Clinical Practice and reviewed and provided input into this publication. J.L.‐G. was the sponsor's principal investigator and a member of the safety committee, designed the study, and reviewed and provided input into this publication.
Supporting information
TABLE S1A Inclusion and exclusion criteria for healthy volunteers
TABLE S1B Inclusion and exclusion criteria for patients with SCD
TABLE S1C Inclusion and exclusion criteria for all subjects
TABLE S2 Models and model robustness across four potential parameters
TABLE S3 Models and model robustness across four potential parameters in SS blood
FIGURE S1 OECs were collected from healthy volunteers’ whole blood samples spiked with voxelotor. A total of 105 OECs were used to determine the correlation between p20 (A), p50 (B), ∆p20 (C) and ∆p50 (D) compared with [voxelotor]/(Hct*5 mM). Based upon the correlations, p20 (R 2 = 0.88) and Δp20 (R 2 = 0.87) are the optimal parameters to follow in a clinical setting where voxelotor target dosing is at 40% or below the Hb concentration. Equation of the line, correlation and N values for all four panels can be found in Supplemental Table S2.
FIGURE S2 OECs were collected from healthy volunteers’ whole blood samples spiked with voxelotor. A total of 145 OECs were used to test the robustness of the four parameters evaluated. The four panels show how predictive the model was of predicting the [voxelotor]RBC to [Hb]RBC ratio (also known as the %Hb occupancy). The robustness of the model based upon either p20 (A), p50 (B), ∆p20 (C) or ∆p50 (D) can be assessed. In addition, the direct relationship between percent Hb modification and %Hb occupancy can be obtained from the slope of the line. Based upon the R 2 values and the slope of the lines (found in Supplemental Table S2), it is clear that using p50 is not predictive of the %Hb occupancy.
FIGURE S3 OECs were collected from SCD patients whole blood samples spiked with voxelotor. A toal of 132 OECs were used to determine the correlation between p20 (A), p50 (B), ∆p20 (C) and ∆p50 (D) compared to [voxelotor]/(Hct*5 mM). Based upon the correlations, ∆p20 (R 2 = 0.78) is the optimal parameter to follow in a clinical setting when voxelotor target dosing is at 40% or below the Hb concentration. Equation of the line, correlation and N values for all four panels can be found in Supplemental Table S3. The greater variability in the SCD data is likely due to varying 2,3 DPG levels in SCD patient blood, and therefore using the delta values provides an internal reference.
FIGURE S4 OECs were collected from SCD patients’ whole blood samples spiked with voxelotor. A total of 101 OECs were used to test the robustness of the four parameters evaluated. The four panels show how predictive the four models were of predicting the [voxelotor]RBC to [Hb]RBC ratio (also known as the %Hb occupancy). The robustness of the model based upon p20 (A), p50 (B), ∆p20 (C) and ∆p50 (D) was assessed. In addition, the direct relationship between percent Hb modification and %Hb occupancy can be judged from the slope of the line. Based upon the slope of the lines (found in Supplemental Table S3), it is clear that both p50 and ∆p50 are not predictive of the %Hb occupancy.
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGEMENTS
This study was funded by Global Blood Therapeutics, Inc. Medical writing support and editorial assistance was provided by Matthew Salkovitz, PharmD (ApotheCom, San Francisco, CA), and was supported by funding from Global Blood Therapeutics. Timothy Mant is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London.
Hutchaleelaha A, Patel M, Washington C, et al. Pharmacokinetics and pharmacodynamics of voxelotor (GBT440) in healthy adults and patients with sickle cell disease. Br J Clin Pharmacol. 2019;85:1290–1302. 10.1111/bcp.13896
REFERENCES
- 1. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561‐1573. [DOI] [PubMed] [Google Scholar]
- 2. Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762‐769. [DOI] [PubMed] [Google Scholar]
- 3. Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: A 4‐decade observational study of 1056 patients. Medicine (Baltimore). 2005;84:36‐76. [DOI] [PubMed] [Google Scholar]
- 4. Ansong D, Akoto AO, Ocloo D, Ohene‐Frempong K. Sickle cell disease: Management options and challenges in developing countries. Mediterr J Hematol Infect Dis. 2013;5. e2013062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hassell KL. Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38(4):S512‐S521. [DOI] [PubMed] [Google Scholar]
- 6. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: The battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3(3):255‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niihara Y, Zerez CR, Akiyama DS, Tanaka KR. Oral L‐glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am J Hematol. 1998;58(2):117‐121. [DOI] [PubMed] [Google Scholar]
- 8. Morrone K, Mitchell WB, Manwani D. Novel sickle cell disease therapies: Targeting pathways downstream of sickling. Semin Hematol. 2018;55(2):68‐75. [DOI] [PubMed] [Google Scholar]
- 9. Niihara Y, Viswanathan K, Miller ST, et al. Phase 3 study of L‐glutamine therapy in sickle cell anemia and sickle β0‐thalassemia subgroup analyses show consistent clinical improvement. Blood. 2016;128:1318.27609540 [Google Scholar]
- 10. Noguchi CT, Rodgers GP, Serjeant G, Schechter AN. Levels of fetal hemoglobin necessary for treatment of sickle cell disease. N Engl J Med. 1988;318(2):96‐99. [DOI] [PubMed] [Google Scholar]
- 11. Oder E, Safo MK, Abdulmalik O, Kato GJ. New developments in anti‐sickling agents: Can drugs directly prevent the polymerization of sickle haemoglobin in vivo? Br J Haematol. 2016;175(1):24‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Safo MK, Kato GJ. Therapeutic strategies to alter the oxygen affinity of sickle hemoglobin. Hematol Oncol Clin North Am. 2014;28(2):217‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdulmalik O, Safo MK, Chen Q, et al. 5‐hydroxymethyl‐2‐furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128(4):552‐561. [DOI] [PubMed] [Google Scholar]
- 14. Arya R, Rolan PE, Wootton R, Posner J, Bellingham AJ. Tucaresol increases oxygen affinity and reduces haemolysis in subjects with sickle cell anaemia. Br J Haematol. 1996;93(4):817‐821. [DOI] [PubMed] [Google Scholar]
- 15. Rolan PE, Parker JE, Gray SJ, et al. The pharmacokinetics, tolerability and pharmacodynamics of tucaresol (589C80; 4[2‐formyl‐3‐hydroxyphenoxymethyl] benzoic acid), a potential anti‐sickling agent, following oral administration to healthy subjects. Br J Clin Pharmacol. 1993;35(4):419‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beddell CR, Goodford PJ, Kneen G, White RD, Wilkinson S, Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br J Pharmacol. 1984;82(2):397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kato GJ, Lawrence MP, Mendelsohn LG, et al. Phase 1 clinical trial of the candidate anti‐sickling agent Aes‐103 in adults with sickle cell anemia. Blood. 2013;122:1009. [Google Scholar]
- 18. Rolan PE, Mercer AJ, Wootton R, Posner J. Pharmacokinetics and pharmacodynamics of tucaresol, an antisickling agent, in healthy volunteers. Br J Clin Pharmacol. 1995;39(4):375‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akinsheye I, Alsultan A, Solovieff N, et al. Fetal hemoglobin in sickle cell anemia. Blood. 2011;118(1):19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Metcalf B, Chuang C, Dufu K, et al. Discovery of GBT440, an orally bioavailable R‐state stabilizer of sickle cell hemoglobin. ACS Med Chem Lett. 2017;8(3):321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oksenberg D, Dufu K, Patel MP, et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half‐life in a murine model of sickle cell disease. Br J Haematol. 2016;175(1):141‐153. [DOI] [PubMed] [Google Scholar]
- 22. Howard J, Hemmaway CJ, Telfer P, et al A phase 1/2 ascending dose study and open label extension study of voxelotor in patients with sickle cell disease. Blood. 2019. 10.1182/blood-2018-08-868893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rowland M, Tozer TN. Clinical Pharmacokinetics and Pharmacodynamics Concepts and Applications. 4th ed. Baltimore, MD: Wolters Kluwer/Lippincot Williams & Wilkins Health; 2011:476‐477. [Google Scholar]
- 24. Golding LA, Myers CR. Y's Way to Physical Fitness: The Complete Guide to Fitness Testing and Instruction. 3rd illustrated ed. Champaign, IL: YMCA of the United States; 1989. [Google Scholar]
- 25. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res. 2018;D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alexander SPH, Kelly E, Marrion NV, et al. The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol. 2017;174:S1‐S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nichols JW, Weber LJ. Comparative oxygen affinity of fish and mammalian myoglobins. J Comp Physiol B. 1989;159(2):205‐209. [DOI] [PubMed] [Google Scholar]
- 28. Patel MP, Siu V, Silva‐Garcia A, Xu Q, Li Z, Oksenberg D. Development and validation of an oxygen dissociation assay, a screening platform for discovering, and characterizing hemoglobin‐oxygen affinity modifiers. Drug Des Devel Ther. 2018;12:1599‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rademacher P, Hutchaleelaha A, Washington C, Lehrer J, Ramos E. Absorption, metabolism and excretion of GBT440, a novel hemoglobin S (HbS) polymerization inhibitor for the treatment of sickle cell disease (SCD), in healthy male subjects. Blood. 2016;128:2487. [Google Scholar]
- 30. Quinlivan EP. Calculation of steady state conditions and elimination kinetics of red blood cell folate in women of childbearing age after daily supplementation with various forms and doses of folate. Am J Clin Nutr. 2008;87(5):1537‐1538. author reply, 1538–1539 [DOI] [PubMed] [Google Scholar]
- 31. Fitzharris P, McLean AE, Sparks RG, Weatherley BC, White RD, Wootton R. The effects in volunteers of BW12C, a compound designed to left‐shift the blood‐oxygen saturation curve. Br J Clin Pharmacol. 1985;19(4):471‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu GG, Pagare PP, Ghatge MS, et al. Design, synthesis, and biological evaluation of ester and ether derivatives of antisickling agent 5‐HMF for the treatment of sickle cell disease. Mol Pharm. 2017;14(10):3499‐3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1A Inclusion and exclusion criteria for healthy volunteers
TABLE S1B Inclusion and exclusion criteria for patients with SCD
TABLE S1C Inclusion and exclusion criteria for all subjects
TABLE S2 Models and model robustness across four potential parameters
TABLE S3 Models and model robustness across four potential parameters in SS blood
FIGURE S1 OECs were collected from healthy volunteers’ whole blood samples spiked with voxelotor. A total of 105 OECs were used to determine the correlation between p20 (A), p50 (B), ∆p20 (C) and ∆p50 (D) compared with [voxelotor]/(Hct*5 mM). Based upon the correlations, p20 (R 2 = 0.88) and Δp20 (R 2 = 0.87) are the optimal parameters to follow in a clinical setting where voxelotor target dosing is at 40% or below the Hb concentration. Equation of the line, correlation and N values for all four panels can be found in Supplemental Table S2.
FIGURE S2 OECs were collected from healthy volunteers’ whole blood samples spiked with voxelotor. A total of 145 OECs were used to test the robustness of the four parameters evaluated. The four panels show how predictive the model was of predicting the [voxelotor]RBC to [Hb]RBC ratio (also known as the %Hb occupancy). The robustness of the model based upon either p20 (A), p50 (B), ∆p20 (C) or ∆p50 (D) can be assessed. In addition, the direct relationship between percent Hb modification and %Hb occupancy can be obtained from the slope of the line. Based upon the R 2 values and the slope of the lines (found in Supplemental Table S2), it is clear that using p50 is not predictive of the %Hb occupancy.
FIGURE S3 OECs were collected from SCD patients whole blood samples spiked with voxelotor. A toal of 132 OECs were used to determine the correlation between p20 (A), p50 (B), ∆p20 (C) and ∆p50 (D) compared to [voxelotor]/(Hct*5 mM). Based upon the correlations, ∆p20 (R 2 = 0.78) is the optimal parameter to follow in a clinical setting when voxelotor target dosing is at 40% or below the Hb concentration. Equation of the line, correlation and N values for all four panels can be found in Supplemental Table S3. The greater variability in the SCD data is likely due to varying 2,3 DPG levels in SCD patient blood, and therefore using the delta values provides an internal reference.
FIGURE S4 OECs were collected from SCD patients’ whole blood samples spiked with voxelotor. A total of 101 OECs were used to test the robustness of the four parameters evaluated. The four panels show how predictive the four models were of predicting the [voxelotor]RBC to [Hb]RBC ratio (also known as the %Hb occupancy). The robustness of the model based upon p20 (A), p50 (B), ∆p20 (C) and ∆p50 (D) was assessed. In addition, the direct relationship between percent Hb modification and %Hb occupancy can be judged from the slope of the line. Based upon the slope of the lines (found in Supplemental Table S3), it is clear that both p50 and ∆p50 are not predictive of the %Hb occupancy.
Supporting information
Supporting information
Supporting information
Supporting information


