Abstract
Background
Low adoption of lung cancer screening is potentially caused by inadequate access to a comprehensive lung cancer screening registry (LCSR), currently a requirement for reimbursement by the Centers for Medicare and Medicaid Services. However, variations in LCSR facilities have not been extensively studied.
Methods
We applied a hierarchical clustering method to a comprehensive database integrating state-level LCSR facility density, defined as the number of facilities per 100,000 at-risk persons, lung cancer outcomes including mortality and stage-specific incidence, and socioeconomic and behavioral factors.
Results
We found three distinct clusters of LCSR facilities roughly corresponding to the northern (cluster 1), southeastern (cluster 2), and southwestern (cluster 3) states. The southeastern states had the lowest total number of facilities (67 ± 44 in cluster 2, 74 ± 69 in cluster 1, 80 ± 100 in cluster 3), the slowest increase in facilities (23 ± 20 in cluster 2, 26 ± 28 in cluster 1, 27 ± 32 in cluster 3) between 2016 and 2018, and the highest lung cancer burden and current smokers. They ranked second in terms of facility density (2.9 ± 1.0 in cluster 3, 3.8 ± 1.3 in cluster 2, 6.3 ± 2.8 in cluster 1) and increase in facility density (1.1 ± 0.3 in cluster 3, 1.3 ± 0.7 in cluster 2, 2.5 ± 2.5 in cluster 1).
Conclusions
We found substantial state-level variability in LCSR facilities tied to lung cancer burden, socioeconomic characteristics, and behavioral characteristics. Given the known risk factors of lung cancer, correcting a suboptimal distribution of screening programs will likely lead to improved lung cancer outcomes.
Key Words: geographic disparities, health service access, lung cancer screening, Medicare, registry
Abbreviations: BRFSS, Behavioral Risk Factor Surveillance System; CMS, Centers for Medicare and Medicaid Services; IQR, interquartile range; LCSR, Lung Cancer Screening Registry
FOR EDITORIAL COMMENT, SEE PAGE 883
Lung cancer screening, despite being recommended by the US Preventive Services Task Force in 2013 and by multiple organizations, has been poorly adopted.1 An analysis of US population-based data comparing self-reported lung cancer screening among eligible current and former smokers before and after approval of national recommendations found that lung cancer screening did not significantly change: approximately 3.3% pre- and 3.9% postrecommendations.2 Some of the reasons cited for low adoption include lack of physician awareness and knowledge of the indications for screening, caution related to high false-positive rate, barriers to initiating shared decision-making within a brief clinical encounter, and lack of knowledge regarding how to refer patients.3, 4
Low adoption of lung cancer screening is potentially also because of inadequate access to a comprehensive lung cancer screening program, currently a requirement for reimbursement by the Centers for Medicare and Medicaid Services (CMS).5 A comprehensive lung cancer screening program must include a reading radiologist with specific training criteria (involving board certification and a history of supervision and interpretation of at least 300 chest CT scans), use of a standardized lung nodule identification system, providing smoking cessation interventions, and data submission to a CMS-approved lung cancer screening registry. Optimally, comprehensive lung cancer screening programs are distributed in accordance to screening needs, given the wide geographic variability in rates of smoking across the United States.6 However, there is also known geographic variation in access to health care because of provider supply and availability that are not accounted for, which is important when considering developing a new screening center and evaluating existing screening facilities.7
The importance of the location and accessibility of cancer screening facilities in increasing cancer prevention and control has been demonstrated in breast and colorectal cancers, but remain to be explored for lung cancer.8, 9, 10 In this study, we conducted a cluster analysis to examine the similarity and dissimilarity among the current lung cancer screening facilities across the United States in the context of lung cancer incidence and mortality and socioeconomic environment. Identifying variations of geographic regions may inform state- and regional-level policy agencies regarding the allocations of resources to improve lung cancer detection and mortality.
Methods
Data Sources
We constructed a database by integrating several data sets.
Lung Cancer Screening Program
We obtained the list of screening facilities participating in the Lung Cancer Screening Registry (LCSR), coordinated by the American College of Radiology, which included 3,728 facilities as of February 26, 2018,11 and geocoded each facility by its address.12 We excluded 22 facilities located in Puerto Rico and Guam from the current analysis. The LCSR is the only lung cancer screening registry approved by the CMS and enables lung cancer screening programs to monitor and certify the quality of their program and obtain reimbursement.
Socioeconomic and Health Characteristics
We extracted socioeconomic factors using the 2010 to 2014 American Community Survey 5-Year Estimates: percentage of people ≥ 65 years of age; percentage of non-Hispanic white, non-Hispanic black, and Hispanics; percentage of the population ≥ 25 years of age with high school degree or higher and with bachelor’s degree or higher; percentage of limited English-speaking households; median household income; Gini index of income inequality; percentage of people ≥ 65 years of age below the poverty level; and percentage of people with private insurance and without health insurance coverage.
We defined the at-risk population as current smokers ≥ 45 years of age from the Behavioral Risk Factor Surveillance System (BRFSS) conducted by the Centers for Disease Control and Prevention. The age category was chosen based on data availability. For each state, we extracted the following variables: prevalence of current smokers among age groups 45 to 64 years and ≥ 65 years, and prevalence of former smokers. We also extracted the proportion of older adults ≥ 65 years of age who were up to date on a set of core preventative measures: an influenza vaccination in the last year; a pneumococcal vaccination ever; a fecal occult blood test within the past year, a sigmoidoscopy within the last 5 years and an fecal occult blood test within the last 3 years, or a colonoscopy within the previous 10 years; and a mammogram in the past 2 years (women only). We used the 3-year estimate by averaging three BRFSS cycles from 2012, 2013, and 2014.
Lung Cancer Data
We obtained 2012 to 2014 age-adjusted lung cancer incidence rates using combined data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program for the age group 50 to 79 years. We categorized lung cancer incidence into four summary stages: in situ/localized only, regional, distant, and other (unknown/unstaged/unspecified). We also obtained the age-adjusted lung cancer-specific mortality rate for the age group 50 to 79 years during 2012 to 2014.
Screening Density
We calculated each state’s lung screening facility density, defined as the number of facilities per 100,000 at-risk persons. We obtained total active physicians per 100,000 persons from the Association of American Medical Colleges based on 2014 data.
Statistics
We applied a hierarchical clustering method to identify natural groupings of the 50 states and the District of Columbia based on the aforementioned variables. All variables were normalized at the same scale using z scores. Differences in variables among the identified cluster groups were examined using the Kruskal-Wallis test, and statistically significant level was set as 0.002 after adjusting for multiple testing (0.05/25 variables). We tested spatial autocorrelation of the lung cancer screening facility density of the contiguous US states using Moran’s I and compared with the simulated random distribution (Monte Carlo simulation n = 999) to estimate statistical significance. We compared the changes in the number of lung cancer screening facilities and differences in facility density measures reported in previously published 2016 LCSR data.13 We also investigated the relationships between lung cancer screening facility density and lung cancer incidence and mortality. All analyses were conducted in SAS (v9.4; SAS Institute) and R (V3.5.0; The R Foundation).
Results
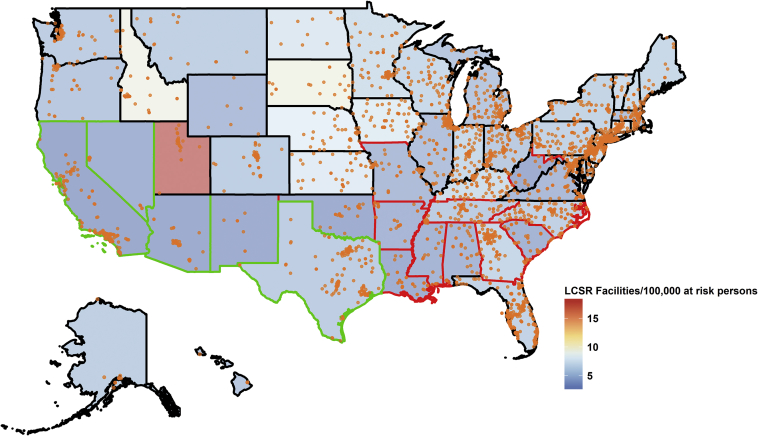
The locations of the 3,728 LCSR facilities are shown in Figure 1 along with the facility densities for each state. The median lung cancer screening facility density is 5 (interquartile range [IQR], 3.4-6.0) per 100,000 at-risk persons, without statistically significant spatial autocorrelation (Moran’s I P = .20). Florida has the highest number of lung cancer screening facilities (n = 294, density = 5.3 per 100,000 at-risk persons) and also the highest proportion of population ≥ 65 years of age. The District of Columbia has the lowest number of lung cancer screening facilities (n = 5, density = 3.1 per 100,000 at-risk persons). The lowest and highest density of lung cancer screening facilities were found in California and Utah, respectively (2.1 vs 18.3 per 100,000 at-risk persons, n = 144 vs n = 55). Utah also has the lowest lung cancer incidence, the lowest lung cancer mortality rate, and the lowest prevalence for current and former smokers. Kentucky has the highest lung cancer incidence of local and regional stages (72 and 156 per 100,000 persons, respectively) and lung cancer deaths (209.3 per 100,000 persons). West Virginia has the highest localized lung cancer incidence (55.5 per 100,000 persons).
Figure 1.
Locations of lung cancer screening facilities (N = 3,706) participating in the LCSR and facility density per 100,000 at-risk persons who were current smokers age ≥ 45 y. The 50 states and the District of Columbia were grouped into three clusters, shown as three different colors for the state boundaries: cluster 1 in black (Alaska, Colorado, Connecticut, Delaware, Florida, Hawaii, Iowa, Idaho, Illinois, Indiana, Kansas, Massachusetts, Maryland, Maine, Michigan, Minnesota, Montana, North Dakota, Nebraska, New Hampshire, New Jersey, New York, Ohio, Oregon, Pennsylvania, Rhode Island, South Dakota, Utah, Virginia, Vermont, Washington, Wisconsin, and Wyoming), cluster 2 in red (Alabama, Arkansas, Georgia, Kentucky, Louisiana, Missouri, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, and West Virginia), and cluster 3 in green (Arizona, California, District of Columbia, New Mexico, Nevada, and Texas). LCSR = Lung Cancer Screening Registry.
We identified three clusters (Fig 1), including 33 states in clusters 1, 12 states in cluster 2, and 6 states in cluster 3. Overall, states (Alabama, Arkansas, Georgia, Kentucky, Louisiana, Missouri, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, and West Virginia in cluster 2) with the largest lung cancer burden, indicated by the stage-specific incidence and/or overall mortality and current smokers, had the lowest current total number of LCSR facilities (67 ± 44 in cluster 2, 74 ± 69 in cluster 1, 80 ± 100 in cluster 3). States in cluster 2 ranked second in terms of the LCSR density (2.9 ± 1.0 in cluster 3, 3.8 ± 1.3 in cluster 2, 6.3 ± 2.8 in cluster 1). Detailed clustering tree diagram and canonical discriminant analysis results showing the separation of the clusters are provided as e-Figures 1 and 2.
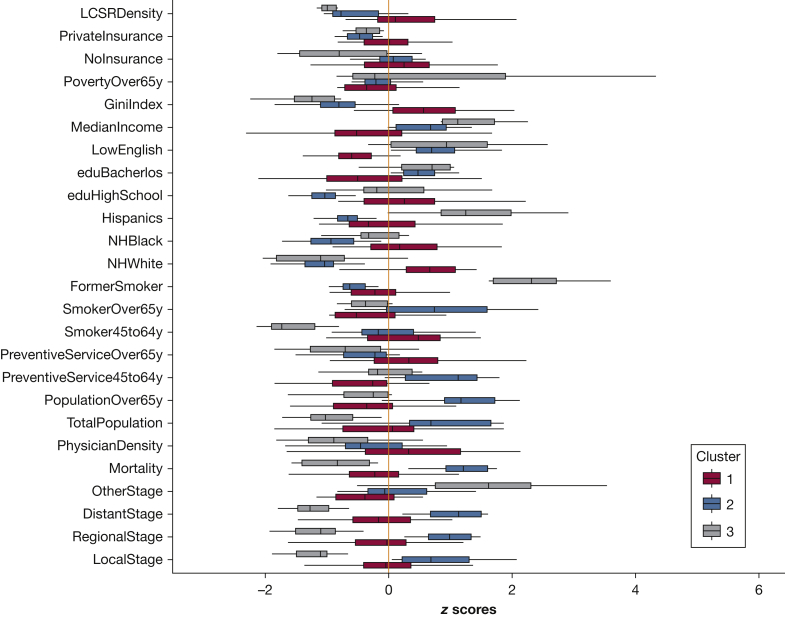
We found significant (P < .002) differences in all these variables across the three clusters (Fig 2), except for active physician rate, state population, prevalence of up-to-date core preventative measures among the age group 45 to 64 years old, and prevalence of former smokers. There were no discernable linear relationships between lung cancer screening facility density and lung cancer incidence and mortality (e-Fig 3).
Figure 2.
Variations of lung cancer data, behavioral measures, sociodemographic variables, physician, and lung cancer screening facility measures according to the three identified clusters. LocalStage, RegionalStage, DistantStage, and OtherStage are summary stages of lung cancer incidence rate (age-adjusted). eduBachelor = Bachelor’s degree or higher; eduHighSchool = high school degree or higher; FormerSmoker = former smoker; GiniIndex = Gini index of income inequality; LCSRDensity = number of state-level lung cancer screening registry facilities per 100,000 at-risk persons; LowEnglish = limited English-speaking households; MedianIncome = median household income; Mortality = lung cancer mortality rate (age-adjusted); NHBlack = non-Hispanic black; NHWhite = non-Hispanic white; NoInsurance = without health insurance coverage; PhysicianDensity = total active physicians per 100,000 persons; PopulationOver65y = population aged ≥ 65 y; PovertyOver65y = aged ≥ 65 y that are below poverty level; PreventiveService45to64y = up to date with core preventative measures among the age group 45 to 64 y; PreventiveServiceOver65y = up to date with core preventative measures among age group ≥ 65 y; PrivateInsurance = with private insurance; Smoker45to64y = current smoker among age group 45 to 64 y; SmokerOver65y = current smoker among age group ≥ 65 y; TotalPopulation = total population.
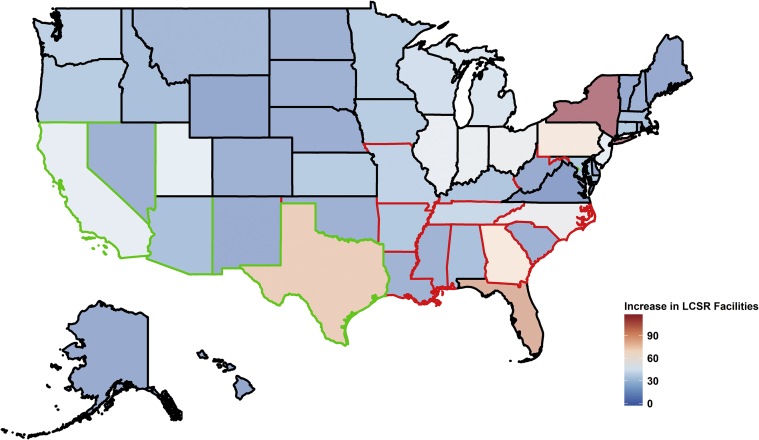
The number of lung cancer screening facilities increased from 2,423 in November 201613 to 3,706 in February 2018, with an average increase of 26 ± 26 (median, 17; IQR, 8-38) (Table 1). The largest increase was seen in New York (Δ = 117) (Fig 3). The increase of lung cancer screening facilities was larger in states belonging to clusters 1 and 3 than cluster 2. States in cluster 2 ranked second in terms of the increase in LCSR density (1.1 ± 0.3 in cluster 3, 1.3 ± 0.7 in cluster 2, 2.5 ± 2.5 in cluster 1). We compared our LCSR density, recalculated density based on the previously reported rate of screening eligible individuals and their density,13 and found similar geospatial variations (eg, cluster 2 ranked second in terms of facility density followed by cluster 3 states) (e-Fig 4, e-Tables 1 and 2).
Table 1.
Distribution of the Variables Used in This Study
| Variables | Mean ± SD | Min (ST)-Max (ST) | Median |
|---|---|---|---|
| Lung cancer incidence (per 100,000 persons aged 50-79 y) | |||
| In situ/localized | 38 ± 8.3 | 16 (Utah) to 56 (West Virginia) | 39 |
| Regional | 44 ± 10.3 | 18 (Utah) to 72 (Kentucky) | 45 |
| Distant | 96 ± 20.2 | 46 (Utah) to 156 (Kentucky) | 94 |
| Other | 7 ± 4.7 | 2 (Wisconsin) to 24 (Nevada) | 6 |
| Lung cancer mortality (per 100,000 persons aged 50-79 y) | 131 ± 28.6 | 57 (Utah) to 209 (Kentucky) | 127 |
| Active physicians (per 100,000 persons) | 270 ± 97.3 | 185 (Mississippi) to 849 (District of Columbia) | 255 |
| State population, No. | 5,578,690 ± 6,029,632 | 480,910 (District of Columbia) to 31,716,199 (California) | 4,101,984 |
| Population aged ≥ 65 ya | 14 ± 1.7 | 9 (Alaska) to 18 (Florida) | 14.2 |
| Preventive service usea | |||
| 45-64 y | 23 ± 4.1 | 15 (Nevada) to 32 (Massachusetts) | 23.0 |
| ≥ 65 y | 43 ± 3.9 | 36 (Alaska) to 51 (North Carolina) | 43.9 |
| Current smokersa | |||
| 45-64 y | 20 ± 3.8 | 11 (Utah) to 29 (West Virginia) | 20.2 |
| ≥ 65 y | 9 ± 1.7 | 4 (Utah) to 14 (Nevada) | 8.8 |
| Former smokersa | 25 ± 2.6 | 17 (Utah) to 31 (Maine) | 25.2 |
| Race/ethnicitya | |||
| Non-Hispanic white | 70 ± 16.1 | 23 (Hawaii) to 94 (Maine) | 0.7 |
| Non-Hispanic black | 11 ± 10.8 | 0.4 (Montana) to 49 (District of Columbia) | 0.1 |
| Hispanic | 11 ± 10 | 1.3 (West Virginia) to 47 (New Mexico) | 0.1 |
| Educationa | |||
| ≥ High school | 88 ± 3.1 | 82 (California) to 92 (Montana) | 88.8 |
| ≥ Bachelor’s degree | 29 ± 6 | 19 (West Virginia) to 53 (District of Columbia) | 27.8 |
| Limited English language householda | 3 ± 2.2 | 0.3 (West Virginia) to 9.6 (California) | 2.3 |
| Median household income | $54,131 ± $9,005 | $39,464 (Mississippi) to $74,149 (Maryland) | $52,400 |
| Gini index of income inequality | 0.5 ± 0.02 | 0.4 (Alaska) to 0.5 (District of Columbia) | 0.5 |
| Below poverty among those aged ≥ 65 ya | 9 ± 1.9 | 5 (Alaska) to 14 (District of Columbia) | 8.4 |
| No health insurancea | 13 ± 4 | 4 (Massachusetts) to 22 (Texas) | 12.9 |
| Private insurancea | 68 ± 5.8 | 55 (New Mexico) to 80 (North Dakota) | 68.3 |
| Screening facility densityb | 5 ± 2.7 | 2 (California) to 18 (Utah) | 5.0 |
| No. of screening facilities | 73 ± 67 | 5 (District of Columbia) to 294 (Florida) | 55 |
| Increase in the No. of screening facilities | 26 ± 26 | −3 (Virginia) to 117 (New York) | 17 |
| Increase in screening facility density | 2.0 ± 2.1 | −0.13 (Virginia) to 14.9 (Utah) | 1.6 |
Sample size = 51 (50 states and the District of Columbia). All variables except for the number of screening facilities, the increase in the number of screening facilities, and the increase in screening facility density were used in the cluster analysis. Max = maximum; Min = minimum; ST = state.
Proportions.
Rate per 100,000 at-risk persons, defined as current smokers aged ≥ 45 y.
Figure 3.
Increase in the number of lung cancer screening facilities participating in the LCSR between February 26, 2018 (N = 3,706) and November 18, 2016 (n = 2,423). The 50 states and the District of Columbia were grouped into three clusters, shown as three different colors for the state boundaries: cluster 1 in black (Alaska, Colorado, Connecticut, Delaware, Florida, Hawaii, Iowa, Idaho, Illinois, Indiana, Kansas, Massachusetts, Maryland, Maine, Michigan, Minnesota, Montana, North Dakota, Nebraska, New Hampshire, New Jersey, New York, Ohio, Oregon, Pennsylvania, Rhode Island, South Dakota, Utah, Virginia, Vermont, Washington, Wisconsin, and Wyoming), cluster 2 in red (Alabama, Arkansas, Georgia, Kentucky, Louisiana, Missouri, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, and West Virginia), and cluster 3 in green (Arizona, California, District of Columbia, New Mexico, Nevada, and Texas). LCSR = Lung Cancer Screening Registry.
Discussion
Our analysis demonstrates substantial variation in lung cancer screening programs availability. Based on lung cancer burden, lung cancer screening facilities, and socioeconomic characteristics, we identified three clusters roughly corresponding to the northern, southeastern, and southwestern states. In addition to the geographic distribution, these cluster patterns also reveal some underlying differences in lung cancer burden, behavior, preventive factors, and socioeconomic factors that may be of interest to develop targeted lung cancer prevention and control strategies at the national and state level. Overall, states (Alabama, Arkansas, Georgia, Kentucky, Louisiana, Missouri, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, and West Virginia) in cluster 2 had the highest lung cancer burden, the lowest current total number of LCSR facilities, and the slowest increase in LCSR facilities between 2016 and 2018. Given the high lung cancer incidence and mortality rates, and the high prevalence of current smokers in cluster 2, the lung cancer screening facility density may appear to be inadequate among these states. Although cluster 3 states had the lowest lung cancer incidence and mortality, lung cancer screening facility density in this group of states is below the national average, which may be appropriate.
We applied the most updated status of LCSR facility distribution in the United States and found the median lung cancer facility density is 5 (IQR, 3.4-6.0) per 100,000 at-risk persons. Although the optimal number and the number of lung cancer screening programs per 100,000 at-risk persons are unknown, our findings suggest that certain states (eg, those within cluster 2) are below the national average and may suffer from poor access to health care and fewer opportunities to benefit from lung cancer screening. This result is consistent with findings from a previous study using 2016 LCSR data,13 or using a recalculated density measure based on the reported rate of screening eligible individuals in the previous study.13
Our results also indicate an increasing trend in LCSR density in the United States and a varied increase across states. Most notably, cluster 2 states had the slowest increase in LCSR facilities (23 ± 20 in cluster 2, 26 ± 28 in cluster 1, 27 ± 32 in cluster 3) between 2016 and 2018, suggesting a potential growth space of the LCSR in these states given their lung cancer burden. It is unclear, from this study, whether states with moderate to low lung cancer burden have a sufficient total number and density of LCSR facilities that meet their screening needs. We also investigated the correlations between lung cancer screening density and lung cancer data. Similar to a previous preliminary study, we found no significant correlations between number of lung cancer screening facilities and lung cancer incidence or mortality.13 Our analysis additionally suggests that the relationships are likely nonlinear, and the magnitude and the direction of the relationships may differ across subgroups of states. It is also likely that it is too early to observe the effect of the LCSR programs on subsequent lung cancer burden, which requires future investigation with longitudinal data.
There has been a growing interest in geospatial approaches across the cancer continuum, notably by the National Cancer Institute, which has advocated for more efforts to address the complex relationships between place and health.14, 15, 16, 17 Geospatial approaches are particularly expedient for identifying potential interventions to address disparities in health care. For example, in a study of digital mammography adoption and distribution in New York State, researchers found that affluent areas were more likely to be among the early adopters of the technology.8 Another study found that areas with low mammography facility density were associated with areas with high proportion of blacks, low educational attainment, and high rate of late-stage breast cancer in eight US states.18 Similarly, investigators used geospatial mapping to understand availability to colorectal screening services in a high-risk population in Florida, finding an inverse relationship between percentage of black population and colonoscopy resources.10 Our study adds to this important research agenda by offering a timely examination of the current landscape of national lung cancer screening services, which similarly shows initial adoption of lung cancer screening technologies in urban centers. The overall increase in lung cancer facilities across the US states is encouraging. However, the growth of lung cancer facilities is not distributed according to lung cancer incidence and mortality. In addition, we demonstrated that the grouping of these states also tied to the similarities and dissimilarities in a range of sociodemographic factors. Detailed investigations of underlying reasons of these changes and in-depth evaluations how these changes impact on lung cancer screening uptake and the subsequent cancer incidence and mortality are warranted. Compared with other cancer screening programs, lung cancer screening is still in its early stage. As the adoption of lung cancer screening practices increases, further studies are needed to evaluate the detailed geospatial distribution of lung cancer screening facilities and its changes over time to identify whether the screening needs of at-risk populations in a particular region are being met. Another area of future research is investigating the accessibility to these lung cancer screening facilities. As shown in Figure 1, locations of individual facilities tend to cluster in urban areas within a state, which may pose a greater burden for rural residents, furthering the potential for geographic disparity. Such variations in facility accessibility may also be widespread, even within states with moderate-to-high numbers of facilities, and warrants further investigation at refined geographic scales.
There are several strengths and limitations to note. Our findings are limited by the use of self-reported measures of smoking, which may lead to a biased estimate of the screening eligible population. Additionally, we used an approximation of the at-risk or screening eligible population because we did not have specific pack-year history. Nonetheless, BRFSS is a nationally representative survey that provides the nation’s premier information regarding health risk behaviors and chronic health conditions, and use of preventive services at the state and local level. Additionally, our study is cross-sectional, which limits our ability to examine changes in lung cancer incidence/mortality in relation to changes in lung cancer screening facility density. Because our analysis is primarily exploratory in nature, it is not suitable for causal inferences. Additionally, we cannot assume that availability of lung cancer screening services is synonymous with appropriate management of radiologic findings. However, our analysis is strengthened by the incorporation of multiple data sets in a novel fashion using a statistically sound clustering method and is valuable for providing insights for further hypothesis testing. Additionally, we have refined our estimates of lung cancer incidence by stage, a key outcome of interest as we consider how the availability of lung cancer screening affects the incidence of early stage cancer.
Conclusions
In our study we determined that lung cancer screening programs are suboptimally distributed. We suspect that this distribution partly contributes to the slow adoption of lung cancer screening among eligible current and former smokers in the United States. Although increased awareness (societal and among physicians) and decreased stigma related to tobacco will improve lung cancer screening rates, we will be unable to fully achieve the expected gains from lung cancer screening until there is improved access and referrals to high-quality lung cancer screening programs.
Acknowledgments
Author contributions: All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. B. L. designed the study. M. S. K. and B. L. performed the literature search. B. L. performed the statistical analyses. M. S. K. and B. L. wrote the manuscript draft. M. S. K., J. W., E. T., and B. L. critically revised the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. S. K.'s work was supported in part by a National Institute's of Health grant. J. W. is a member of the research board of EHE International; has received consulting honorarium from Sanofi, Quintiles, AstraZeneca, and Merck; and a research grant from Aventis Pharmaceutical and Quorum. None declared (E. T., B. L.).
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Dr Kale's work was supported in part by a National Institutes of Health grant (NCI K07CA187071).
Supplementary Data
References
- 1.Moyer V.A., U. S. Preventive Services Task Force Screening for lung cancer: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Fedewa S.A. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017;3(9):1278–1281. doi: 10.1001/jamaoncol.2016.6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triplette M., Kross E.K., Mann B.A. An assessment of primary care and pulmonary provider perspectives on lung cancer screening. Ann Am Thorac Soc. 2018;15(1):69–75. doi: 10.1513/AnnalsATS.201705-392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ersek J.L., Eberth J.M., McDonnell K.K. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–2331. doi: 10.1002/cncr.29944. [DOI] [PubMed] [Google Scholar]
- 5.Center for Medicare and Medicaid Services (CMS). Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). 2015. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed April 29, 2018.
- 6.Loop M.S., Howard G., de Los Campos G. Heat maps of hypertension, diabetes mellitus, and smoking in the continental United States. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.003350. e003350-00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine (U.S.) Committee on Geographic Adjustment Factors in Medicare Payment. Edmunds M., Sloan F.A., Steinwald B. National Academies Press; Washington, DC: 2012. Geographic Adjustment in Medicare Payment. Phase II, Implications for Access, Quality, and Efficiency. [PubMed] [Google Scholar]
- 8.Boscoe F.P., Zhang X. Visualizing the diffusion of digital mammography in New York State. Cancer Epidemiol Biomarkers Prev. 2017;26(4):490–494. doi: 10.1158/1055-9965.EPI-16-0928. [DOI] [PubMed] [Google Scholar]
- 9.Rahman S., Price J.H., Dignan M., Lindquist P.S., Jordan T.R. Access to mammography facilities and detection of breast cancer by screening mammography: a GIS approach. Int J Canc Prev. 2009;2(6):403–413. [PMC free article] [PubMed] [Google Scholar]
- 10.Gwede C.K., Ward B.G., Luque J.S. Application of geographic information systems and asset mapping to facilitate identification of colorectal cancer screening resources. Online J Public Health Inform. 2010;2(1):2893. doi: 10.5210/ojphi.v2i1.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Radiology Lung Cancer Screening Registry. 2018. https://www.acr.org/Practice-Management-Quality-Informatics/Registries/Lung-Cancer-Screening-Registry. Accessed April 6, 2018.
- 12.Texas A&M Geoservices Geocoding services. http://geoservices.tamu.edu/Services/Geocode/ Accessed April 14, 2018.
- 13.Charkhchi P., Kolenic G.E., Carlos R.C. Access to lung cancer screening services: preliminary analysis of geographic service distribution using the ACR Lung Cancer Screening Registry. J Am Coll Radiol. 2017;14(11):1388–1395. doi: 10.1016/j.jacr.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickle L.W. A history and critique of U.S. mortality atlases. Spat Spatiotemporal Epidemiol. 2009;1(1):3–17. doi: 10.1016/j.sste.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Schootman M., Gomez S.L., Henry K.A. Geospatial approaches to cancer control and population sciences. Cancer Epidemiol Biomarkers Prev. 2017;26(4):472–475. doi: 10.1158/1055-9965.EPI-17-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson D.B., Volkow N.D., Kwan M.P., Kaplan R.M., Goodchild M.F., Croyle R.T. Medicine. Spatial turn in health research. Science. 2013;339(6126):1390–1392. doi: 10.1126/science.1232257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dankwa-Mullan I., Perez-Stable E.J. Addressing health disparities is a place-based issue. Am J Public Health. 2016;106(4):637–639. doi: 10.2105/AJPH.2016.303077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tatalovich Z., Zhu L., Rolin A., Lewis D.R., Harlan L.C., Winn D.M. Geographic disparities in late stage breast cancer incidence: results from eight states in the United States. Int J Health Geogr. 2015;14:31. doi: 10.1186/s12942-015-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.