Abstract
Bone disease is a frequent event in cancer patients, both due to cancer spread to bone and to cancer therapies. Bone is the organ most frequently affected by metastatic disease when considering the two most frequent cancers in the Western world (breast and prostate cancers). Bone metastases can have a substantial detrimental effect on patients' quality of life, as well as significant morbidity due to complications collectively known as skeletal‐related events (SREs), which include hypercalcaemia, pathological fractures, spinal cord compression, and need of radiotherapy or surgery to the bone. These have been successfully mitigated with the development of bone‐targeted agents (BTAs; bisphosphonates and denosumab), focused on inhibiting osteoclast activity. The potential direct antitumour effect of bisphosphonates, as well as the impact of osteoclast inhibition with subsequent decrease in bone metabolism, have also propelled investigation on the role of BTAs in preventing cancer relapse in bone. In this review, the authors aimed to discuss the role of BTAs in the treatment and prevention of bone metastases, as well as their potential value in preventing cancer treatment‐induced bone loss (CTIBL). The review will focus on breast and prostate cancers, with the aim of providing the most relevant clinical data emerging from bench to bedside translational research in the field of cancer‐induced bone disease.
Keywords: bisphosphonates, bone metastases, bone‐targeted agents, denosumab, Ra‐223
Introduction
In Oncology, the goal of treatments with the potential to change the odds of success between cancer and host competition is mostly dependent on the setting in which they are used. Most of the drugs that can be used in the metastatic disease may provide benefit in the palliative setting but will not cure the patient from cancer. However, under certain circumstances, the same drug(s) used after surgery (i.e. in the adjuvant setting) may prevent cancer relapse for a percentage of patients and offer them a chance of cure.
Because bone is the organ most frequently affected by metastatic disease when considering the two most frequent cancers in the Western world − breast and prostate cancer 1 – it becomes clear why efforts in Oncology research have been largely committed to unraveling the role of bone‐targeted agents (BTAs) responsible for inhibition of osteoclast activity in the treatment and prevention of bone metastases (i.e. in the palliative and adjuvant settings, respectively).
It is widely accepted that bone microenvironment is key for cancer cells to successfully thrive in bone 2. After the visionary ‘seed and soil’ theory of metastization proposed by Stephen Paget in 1889 2, 3, the work by Gregory Mundy and Theresa Guise was critical to set the foundations for the pathophysiology of bone metastases model known as ‘the vicious cycle’ 4, 5. According to this model, cancer cells in bone are able to stimulate osteoclast activity leading to bone osteolysis and, in turn, cancer cells will receive a positive feedback from humoral factors released by the bone matrix during bone destruction and altered remodelling, favouring tumour growth. This concept led to the hypothesis of testing the value of BTAs to treat and prevent bone metastases and, ultimately, to prove that a host‐directed therapy (i.e. one targeting osteoclasts and not directly cancer cells) could impact on the outcomes of patients with cancer.
Bone loss is a frequent event in cancer patients, often as a result of cancer therapies – known as cancer treatment‐induced bone loss (CTIBL). Indeed, drugs used in cancer treatments (e.g. endocrine therapy, androgen‐deprivation therapy and chemotherapy) may induced bone loss and hence increase the risk for fractures 6.
In this review, the authors aimed to discuss the role of BTAs in the treatment and prevention of bone metastases, as well as their potential value in preventing CTIBL. The review will focus on breast and prostate cancer, with the aim of providing the most relevant clinical data emerging from bench to bedside translational research in the field of cancer‐induced bone disease.
Classes of BTAs in clinical use for cancer treatment
Osteoclast inhibitors
The current use of BTAs in clinical practice is mainly focused on the inhibition of osteoclast activity by bisphosphonates (BPs) or by denosumab, a fully humanized monoclonal antibody that binds to the receptor activator of nuclear factor kappa‐Β ligand (RANKL), leading to osteoclast inhibition.
BPs display a high affinity for calcium ions, and therefore attach to the hydroxyapatite on bone surface, particularly those undergoing active resorption. All BPs are antiresorptive drugs that block pathologic bone resorption by inducing osteoclast apoptosis. Because of their antiresorptive properties, BPs are used to prevent the hyperactivation of osteoclasts associated with the presence of cancer cells in bone or in the setting of CTIBL.
During bone resorption, BPs are internalized by bone‐resorbing osteoclasts, inhibiting osteoclast function 7. At the cellular level, second‐ and third‐generation nitrogen‐containing BPs impair the mevalonate pathway by inhibiting the farnesyl diphosphate (FDP) synthase. Consequently, the prenylation of small GTPase signalling proteins is inhibited, ultimately leading to apoptosis. Examples of alkyl‐amino BPs are pamidronate, alendronate, ibandronate, whereas heterocyclic BPs include risendronate and zoledronic acid (ZA). First‐generation non‐nitrogen‐containing BPs (etidronate, clodronate [CLO], tiludronate) lead to intracellular accumulation of cytotoxic non‐hydrolysable adenosine triphosphate (ATP) analogues. In both circumstances, bone resorption is severely impaired.
Pamidronate and ZA have been approved by both the European Medicines Agency (EMA) (or local European authorities) and the US Food and Drug Administration (FDA) for the treatment of skeletal metastases from breast cancer and multiple myeloma. CLO is not approved in the US but is available in Europe for the treatment of skeletal metastases from breast cancer. Ibandronate is also an option for breast cancer. ZA is the only BP approved for the treatment of metastatic castration‐resistant prostate cancer (mCRPC) and is also approved for use in other solid tumours, as well as in multiple myeloma.
The receptor activator of nuclear factor kappa B (NFκB) RANK is a transmembrane receptor expressed on the surface of osteoclast precursor cells, and its ligand RANKL is produced by osteocytes, osteoblasts and bone marrow stromal cells. Binding of RANKL to RANK leads to stimulation of RANK signalling, regulating osteoclast differentiation, activity and survival. The soluble decoy receptor for RANKL, osteoprotegerin (OPG), is produced by mature osteoblasts and stromal cells, and binds RANKL, blocking osteoclast differentiation 8, 9. The anti‐RANKL monoclonal antibody denosumab is the most potent osteoclast inhibitor and is approved for use in patients with bone metastases in all solid tumours.
Other BTAs
The radiopharmaceutical agent radium‐223 (Ra‐223) is considered by several authors as a BTA. Ra‐223 has a high affinity to bone and significantly reduces the incidence of bone complications in patients with prostate cancer and bone metastases. Additionally, due to its alpha‐emitter properties Ra‐223 causes direct cancer cell death by radiation‐induced double strand breaks. Ra‐223 is probably the first BTA, with unknown target, to show effectiveness in the targeting of osteoblast hyperactivity (as in prostate cancer‐associated blastic bone metastases). The most accurate biomarker to predict response to Ra‐223 in patients with prostate cancer and bone metastases is the decline of alkaline phosphatase, a biomarker of osteoblast activity 10.
BTAs in the treatment of bone metastases
Whereas the normal physiologic process of bone depends on a strict balance between bone resorption by osteoclasts and new bone formation by osteoblasts, cancer cells growing on bone disrupt this balance, favouring bone resorption. However, osteoblastic bone metastases are the most prevalent form of bone metastases in prostate cancer. In this case, together with the increase in bone resorption due to osteoclastogenesis, also osteoblasts are activated by prostate cancer cells, leading to the accumulation of immature unmineralized bone (osteoid).
Bone metastases are frequent in solid tumours and half of these patients develop one or more complications, collectively termed skeletal‐related events (SRE). SREs include bone pain, hypercalcaemia, fractures, spinal cord compression, need of radiotherapy for pain, and need of surgery for pathological fractures 10. SREs cause significant morbidity, as well as reduced performance status, quality of life (QoL) and survival 11, 12. Symptomatic skeletal events (SSEs) differ from the latter for only including symptomatic pathologic fractures.
Real‐world evidence continues to support the relevance of SREs as a prevalent clinical event in patients with bone metastases across different solid tumour types 13. The cumulative incidence of SREs at 24 months in a 15‐year study in two large US health systems was 54.2% in breast cancer, 41.9% in prostate cancer, and 47.7% in lung cancer 13.
Bisphosphonates
By disrupting the ‘vicious cycle’ of bone metastases, BPs are able to prevent bone complications associated with tumour‐induced bone osteolysis 13. Following this rational, ZA, ibandronate, pamidronate and CLO were studied and approved for prevention of skeletal complications in breast cancer patients with bone metastases 14. However, ZA is the only BP approved for the treatment of bone metastases across all tumour types.
Since the early days of BP investigation in this setting, phase 3 trials assessing their impact on the incidence of SREs have used as endpoints the proportion of patients with at least one SRE, time to the first SRE, and skeletal morbidity rate (mean rate of SREs per person‐year). Such trials have sought to compare treatment with BPs versus placebo or treatments with two different BPs 14. Another approach used to capture the impact of BPs in the incidence of SREs was the Andersen‐Gill method, which is a more sensitive means of reporting treatment effects by adjusting for variability in event rates over time and gives an intensity/hazard ratio for recurrent events assuming that events are independent 15.
The efficacy of different BPs in the treatment of patients with breast cancer was assessed in a Cochrane review, which confirmed the value of this drug class to prevent SREs associated with bone metastases 16.
Two phase 3 clinical trials compared the efficacy of different BPs to prevent SREs in patients with breast cancer and bone metastases 17, 18. In a double‐blind randomized trial, intravenous (IV) ZA at 4 or 8 mg was compared to IV pamidronate at 90 mg, and no statistically significant difference was found between these agents concerning number of SREs or time to first SRE 18. In the non‐inferiority ZICE trial, oral ibandronate at 50 mg daily was compared with IV ZA at 4 mg every 3–4 weeks (q3–4w) in patients with breast cancer and bone metastases 17. In this study, the authors could not reject the hypothesis that ibandronate was inferior to ZA, with an annual rate of SREs of 0.499 (95% CI 0.454–0.549) for ibandronate and 0.435 (0.393–0.480) for ZA.
IV nitrogen‐containing BPs are considered the most potent BPs and, among these, ZA combines potency with a shorter time of infusion (15 min). However, the incidence of osteonecrosis of the jaw (ONJ) and renal deterioration rate, two of the most worrisome complications of BPs, are higher with IV compared with oral nitrogen‐containing BPs or non‐nitrogen‐containing BPs, as CLO 19.
The role of BPs in the treatment of patients with prostate cancer and bone metastases is limited to mCRPC, with ZA being the only approved BP in this setting. In a randomized placebo‐controlled trial, 643 patients with mCRPC were randomly assigned to IV ZA at 4 or 8 mg or placebo q4w, with a significant reduction in the rate of SREs (49% vs. 38%, P = 0.0029) and an increase in the median time to first SRE in favour of 4 mg ZA 20, 21.
The role of BPs, including ZA, in the castration‐sensitive setting of advanced prostate cancer with bone metastases is yet to be determined. The CALGB 90202 trial, prematurely terminated due to sponsor support withdrawal, reported a median time to first SRE of 31.9 months for ZA vs. 29.8 months for placebo (hazard ratio [HR] 0.97; P = 0.39) 22.
In other, non‐breast or prostate, solid tumours, ZA is the only BP approved to prevent SREs. In the ZA 011 trial, patients were randomly assigned to IV ZA at 8 or 4 mg or placebo q3w with concomitant antineoplastic therapy 23. Incidence of SREs was reduced in both ZA groups compared with placebo (38% for 4 mg and 35% for 8/4 mg ZA vs. 44% for placebo; P = 0.127 and P = 0.023 for 4 and 8/4 mg groups, respectively) 23, 24. Additionally, ZA significantly increased time to first SRE in the 4 mg group (median of 230 vs. 163 days for placebo; P = 0.023). The currently approved dosage of IV ZA to prevent SREs in patients with bone metastases is 4 mg q3–4w.
Denosumab
In two large phase 3 trials, denosumab was superior to ZA in patients with bone‐metastatic disease from breast and prostate cancer 25, 26. Denosumab superiority was evident in the time to first and subsequent SREs, both in breast and prostate tumours. In breast cancer, denosumab delayed time to first SRE compared to ZA (32.4 vs. 26.4 months; HR 0.82; P = 0.01) 26.
In another phase 3 trial comparing denosumab and ZA in patients with bone metastases from multiple myeloma or solid tumours other than breast or prostate, denosumab was non‐inferior to ZA in delaying time to first SRE (20.6 vs. 16.3 months; HR 0.84; P = 0.0007), but not statistically superior to ZA in delaying time to first SRE (P = 0.06) 27.
Approximately 25% of patients receiving nitrogen‐containing BPs typically experience events like self‐limiting bone pain and flu‐like symptoms after the first infusion 28, 29.
In an integrated analysis of 5723 patients from three randomized phase 3 trials, denosumab safety profile on renal function was better than that of ZA, not requiring dose adjustments or renal monitoring 30. But the incidence of hypocalcaemia was higher with denosumab than with ZA (3.1% vs. 1.3% for grade 3–4 toxicities), although most cases were asymptomatic. Importantly, the risk of developing hypocalcaemia was 40% lower in patients treated with denosumab who reported taking calcium/vitamin D supplements at any time during the study, highlighting the importance of adhesion to calcium supplementation in patients receiving denosumab 31.
ONJ is a relevant and potentially serious concern associated with osteoclast‐targeting therapies such as ZA and denosumab 32. ONJ usually manifests as a late adverse event (AE), contrarily to hypocalcaemia, which is an early AE. Median time to ONJ in patients receiving ZA or denosumab is 15 months 33, but the risk of ONJ increases with treatment duration. Therefore, many clinicians consider stopping BPs or denosumab after 24 months of therapy. In patients receiving treatment at monthly intervals, the incidence of ONJ is similar with ZA and denosumab (1–10%).
Other BTAs
As previously mentioned, Ra‐223 is approved for the treatment of mCRPC with bone metastases, being reserved for patients without significant extra‐skeletal disease.
The pivotal phase 3 ALSYMPCA trial was a randomized, double‐blind, placebo‐controlled trial of Ra‐223 in 921 patients with symptomatic mCRPC and two or more bone metastases 34. Men with visceral metastases were excluded from the study. Compared with placebo, Ra‐223 was associated with a 30% reduction in the risk of death, longer time to first SSE, and a lower risk of radiotherapy requirement for bone pain and spinal cord compression. Furthermore, a significantly higher percentage of patients receiving Ra‐223 had a meaningful improvement in QoL according to the Functional Assessment of Cancer Therapy‐Prostate (FACT‐P) total score during the period of drug administration (25% vs. 16% with placebo; P = 0.002).
A recent review of Ra‐223 data was carried out by the EMA's Pharmacovigilance Risk Assessment Committee (PRAC), after results from the phase 3 double‐bind clinical trial ERA 223, evaluating Ra‐223 or placebo, each in combination with abiraterone plus prednisone, in patients with mCRPC with bone metastases 35. The study was prematurely unblinded due to observation of more fractures and deaths in the Ra‐223 treatment arm. Based on this, the combination of Ra‐223 with abiraterone is now contraindicated 36.
Results from the ERA 223 study indicate that manipulating bone microenvironment can have a positive but also a negative impact on patient outcomes. However, a subgroup analysis of this study suggests that the detrimental effect of combining Ra‐223 with abiraterone is not observed in patients concomitantly receiving an anti‐osteoclast inhibitor 35.
BTAs are frequently administered as part of the management of patients with bone metastases to delay or prevent SREs. In breast cancer, several BPs and denosumab are approved to treat bone metastases, whereas in prostate cancer the only osteoclast‐inhibitors approved are denosumab or zoledronic acid (Figure 1).
Figure 1.
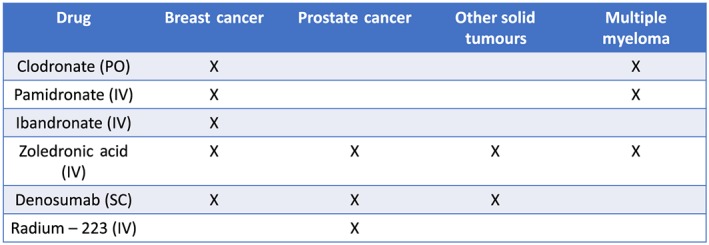
Current approval status of bone‐targeted agents for skeletal‐related complications in the oncology setting
Future trends
Several questions remain unanswered regarding the use of BTAs in the treatment of bone metastases. Two of the most relevant are whether it is possible to de‐escalate the BTA regimen while maintaining effectiveness with a lower the incidence of ONJ, and what is the optimal duration of BTA therapy.
ZOOM and OPTIMIZE‐2 trials evaluated the de‐escalation of ZA from an every 4‐week to an every 12‐week regimen in patients treated with ZA for approximately 12 months, showing that this is a possible treatment option for these patients 37, 38. This approach was also supported by a meta‐analysis in which patients receiving ZA every 12 weeks had a similar risk of SRE as those receiving ZA every 4 weeks 39. The 12‐week administration was subsequently incorporated in the latest guidelines 40.
Since patients with multiple bone metastases and pain have the highest risk for SREs during the first 6–12 months of treatment 30, when ZA is the BP of choice it seems reasonable to start treatment with a monthly regimen for the first 12 months, and then de‐escalate to an every 12‐week regimen. Importantly, the de‐escalation approach is not yet ascertained for patients receiving denosumab. McClung et al. 41 reported results from an observational 1‐year period after up to 8 years of denosumab treatment in a phase 2 study and showed that eight patients (9.8%) ‐ all having at least one predisposing risk factor ‐ experienced 17 fractures. This included seven patients who experienced one or more vertebral fractures. Clinicians should be aware of the prodromes for atypical fracture of the femur in this context.
Regarding BTA treatment duration, although international guidelines recommend maintaining treatment until evidence of a substantial decline in patient's general performance status or even indefinitely, clinicians often stop BTAs after 2 years. Indeed, pivotal trials arbitrarily determined a BP treatment duration of approximately 2 years, and a denosumab treatment duration of up to 3 years. The rational to stop or de‐escalate BTAs after 2 years is mostly dependent on the increased risk of ONJ with prolonged treatment duration.
BTAs for prevention of bone metastases
BTAs have been investigated in clinical trials to assess the impact of osteoclast inhibition and subsequent decrease in bone metabolism in the prevention of bone metastases (Table 1).
Table 1.
Studies investigating BTAs in the prevention of bone metastases from breast and prostate cancer (adapted from Casimiro et al. 75)
| Drug | Number of patients | Bone recurrence | Disease recurrence | Cancer mortality |
|---|---|---|---|---|
| Breast cancer | ||||
| BPs (EBCTCG) 46 | Overall n = 18 766 | HR 0.83 (95% CI 0.73–0.94);P = 0.004 | HR 0.94 (95% CI 0.87–1.01);P = 0.08 | HR 0.91 (95% CI 0.83–0.99);P = 0.04 |
| Postmenopausal n = 7388 | ||||
| HR 0.72 (95% CI 0.60–0.86);P = 0.0002 | HR 0.86 (95% CI 0.78–0.94);P = 0.002 | HR 0.82 (95% CI 0.73–0.93);P = 0.002 | ||
| Denosumab (ABCSG‐18) 50, 51 | Postmenopausal; n = 3425 | HR 0.97 95% CI 0.82–1.14,P = 0.70 | HR 0.82 (95% 0.69–0.98,Cox P = 0.026) | HR 1.03 95% CI 0.85–1.25 |
| Overall n = 4509 Postmenopausal; n = 2149 | ||||
| HR 1.04 95% CI 0.91–1.19,P = 0.57 | ||||
| (D‐CARE) 52 | ||||
| Prostate cancer | ||||
| Zoledronic acid (ZEUS) 57 | High risk disease; 1393 | 14.7 vs. 13.2% in the control group at 4.8 year; (log‐rank: P = 0.65) | 116 vs. 122 deaths in the control group; log‐rank P = 0.76 | |
| (RADAR) 58 | Locally advanced disease treated with RT and ADT ± ZA; n = 1071 | No difference in prostate cancer‐specific mortality | ||
Preclinical data on the potential direct antitumour effect of BPs, particularly nitrogen‐containing ones, has raised expectations of a role for BPs in preventing cancer relapse 42, 43. Either through inhibition of the bone remodelling cycle, or through other potential mechanisms – as a cytotoxic effect on cancer cells, inhibition of angiogenesis or expansion of antitumoral immune cells − BPs became natural candidates to test in the adjuvant setting 42. Additionally, the pharmacodynamic profile of BPs has a favourable long‐lasting effect on bone. This provided a strong reasoning to target dormant residual cancer cells surviving in the bone microenvironment or bone marrow after adjuvant chemotherapy or endocrine therapy with these agents.
Several studies have been conducted in the adjuvant setting aiming to improve recurrence and survival rates by integrating BPs in conventional adjuvant regimens 44, 45. However, results have been inconsistent or conflicting regarding the value of BPs in preventing breast cancer relapse.
This question was clarified in a study conducted by the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) 46. This large patient‐level meta‐analysis of trials of adjuvant amino and non‐amino BPs included 18 776 women with breast cancer. An 18% reduction in the risk of death from breast cancer in the subset of postmenopausal women (HR 0.82 [95% CI 0.73–0.93]; P = 0.002) was observed. This effect seemed to stem mainly from the 28% reduction in the risk of bone recurrence (HR 0.72 [95% CI 0.60–0.86]; P = 0.0002) rather than from extra‐skeletal recurrences. Given these results, several guidelines now advise on the use of ZA and CLO as adjuvant treatment of postmenopausal women with breast cancer 47, 48.
Assessment of the biomarker for bone metastasis MAF status in patients from the phase 3 AZURE trial showed that MAF positivity was predictive of a detrimental effect of adjuvant ZA treatment in non‐postmenopausal women with early breast cancer with more relapses outside bone (HR for extraskeletal recurrences, 6.92; 95% CI 2.44–19.6) 49. These results could explain why only postmenopausal woman achieved benefit with adjuvant ZA in AZURE. However, there is no valid mechanistic explanation for this observation yet.
Denosumab was also investigated as an adjuvant treatment in breast cancer, with the two large phase 3 trials ABCSG‐18 50, 51 and D‐CARE 52 evaluating whether denosumab could have a role in the adjuvant setting in this tumour type.
Results from the ABCSG‐18 study, in 3425 postmenopausal patients with early hormone receptor‐positive breast cancer receiving aromatase inhibitors (AIs) with or without denosumab (60 mg every 6 months), were recently presented 51. After a median follow‐up of 72 months, the disease‐free survival (DFS) was significantly improved in the denosumab arm (HR 0.823, 95% CI 0.69–0.98, P = 0.026) in a descriptive analysis not controlling for multiplicity.
The D‐CARE trial randomized 4509 patients with early breast cancer to standard loco‐regional and (neo)adjuvant therapy plus either subcutaneous (SC) denosumab 120 mg or matching placebo monthly × 6 then 3 monthly for up to 5 years 52. The primary endpoint was bone metastasis‐free survival (BMFS), defined as the first bone metastatic event confirmed by central imaging review or death from any cause. DFS and overall survival (OS) were secondary endpoints. No benefit for the addition of denosumab was seen after a median follow‐up of 67 months that allowed for the full 5 years of treatment in all patients (HR for BMFS [597 events] 0.97; 95% CI 0.82–1.14; P = 0.70). Also, no benefit was observed on DFS (HR 1.04, 95% CI 0.91–1.19, P = 0.57). However, exploratory analysis of time to bone metastases as first recurrence suggested benefit for denosumab (HR 0.76, 95% CI 0.59–0.97), and time to on‐study fracture prior to bone recurrence was also reduced with denosumab (HR 0.76, 95% CI 0.63–0.92). Interestingly, denosumab did not improve BMFS, DFS or OS in the postmenopausal subgroup.
In contrast to the ABCSG‐18 study, the D‐CARE study recruited patients with a very high risk of distant relapse. It is possible that the rate of distant relapse in visceral organs (such as liver or lung) and earlier death of those patients did not allow differences in BMFS, the primary endpoint of the D‐CARE study, to be seen. Bone metastases usually occur later in time when compared to visceral metastases.
Recent attention to a potential role of the RANK/RANKL axis on breast carcinogenesis prompt the development of new clinical trials in the (neo)adjuvant setting. RANKL is the paracrine mediator of progesterone mitogenic action in mammary epithelium and progesterone could also drive mammary stem cell (MaSC) expansion, correlated with increase in RANKL within ER+/PR+ luminal cells 53, 54. There is also preclinical evidence that RANK/RANKL signalling has a role in BRCA1‐associated tumours 55, and RANKL inhibition was further shown to reduce proliferation in BRCA1‐mutated human breast tissue 56.
In prostate cancer, adjuvant BTAs have no well‐established role. The adjuvant role of ZA in prostate cancer was tested in the ZEUS and RADAR trials. The ZEUS phase 3 trial recruited 1433 patients with high‐risk non‐metastatic prostate cancer to receive treatment with or without every 3‐month ZA and found no differences in the proportion of patients developing bone metastases (17.1 vs. 17.0%; P = 0.95) 57. The RADAR study recruited 1071 men with locally advanced prostate cancer to receive treatment with radiotherapy plus 6 or 18 months of androgen deprivation therapy (ADT) with or without 18 months of ZA and also found no differences in prostate cancer‐specific survival between groups 58.
Bone metastases from prostate cancer are often blastic, reflecting a strong paracrine interplay between cancer cells and osteoblasts. This may be a possible explanation for the lack of efficacy of anti‐osteoclast agents as the only BTA to prevent disease relapse in bone. It is yet unknown whether a BTA such as Ra‐223 alone or in combination with an anti‐osteoclast agent could be a valid option to target micrometastases in bone and thus prevent cancer relapse.
BTAs for prevention of cancer treatment‐induced bone loss
The diagnosis of cancer is in itself a risk factor for bone loss. However, cancer treatment is also acknowledged as a major driver of bone loss, leading to clinically relevant outcomes of bone frailty even in the absence of bone metastases. In this review, the authors will focus on bone loss associated with therapies frequently used to treat cancer patients, herein designated cancer treatment‐induced bone loss (CTIBL) 59.
Drugs used in cancer treatment (like hormone therapy, ADT and chemotherapy) may induce bone resorption, leading to bone loss and a consequently higher risk of bone fractures. Consequently, it is critical to identify which patients are at risk and which preventive measures should be adopted 6.
It is also important to acknowledge the risk for bone loss in other commonly used cancer treatments. For instance, glucocorticoids are used in Oncology as an integral part of antiemetic chemotherapy regimens, as preventive medications for infusion reactions, and as analgesics. In patients with brain primary and secondary tumours, glucocorticoids are used to manage neurological symptoms associated with peritumoral oedema. Long‐term treatment with glucocorticoids can be responsible of iatrogenic osteoporosis.
Chemotherapy is by itself a risk factor for bone loss, independently of subsequent secondary ovarian failure due to chemotherapy. In postmenopausal women, data suggest that patients receiving adjuvant chemotherapy can lose 1–10% of bone mass within 1 year of chemotherapy 60. Although the World Health Organization (WHO) provides a clinical tool – the Fracture Risk Assessment tool (FRAX) – to evaluate the 10‐year probability of major osteoporotic fractures based on several risk factors, anticancer treatments were not included as a specific risk factor 6, 61.
Changes in bone mineral density (BMD) and fracture rates are regarded as the main outcomes to consider in clinical studies of CTIBL. Until now, a dual energy x‐ray absorptiometry (DEXA) scan of the hip, lumbar spine, and femoral neck is the best way to determine BMD and a predictor of fracture risk. The necessity of evaluating fracture risk by integrating the determination of BMD is reflected in several guidelines 62, 63. Periodic BMD evaluations are recommended in women with iatrogenic ovarian failure (those that have treatment‐related amenorrhoea for more than 1 year should have BMD assessed), in postmenopausal women treated with AIs, and in all breast cancer patients with fracture risk factors. BMD detection is also recommended in men on ADT. Long‐term ADT is associated with significant and progressive BMD decline and fracture events significantly correlate with shorter survival in men with prostate cancer 64.
Prevention and treatment of CTIBL
Adopting healthy lifestyle measures, such as performing weight exercises, avoiding alcohol and tobacco, limiting caffeine consumption, and achieving and/or maintaining a normal body weight, are beneficial to prevent CTIBL 65. Supplementation with calcium (1200 mg day−1) and vitamin D (800 IU) to reach serum levels of at least 30 ng ml−1 of serum vitamin D should also be assured to maintain a healthy bone turnover. Furthermore, maintaining such levels is known to reduce the risk for hip fractures in elderly women 64, 65.
BTAs in CTIBL
Several traditional BTAs (BPs and denosumab) have been investigated to treat patients at risk for bone loss or bone fracture 51, 66, 67, 68, 69, 70. In some of these studies, cancer relapse was a secondary endpoint. Oral BPs such as CLO, alendronate, risedronate and ibandronate can be used to prevent osteoporosis. Concerning oral BPs, patients should be instructed to take these medications on an empty stomach, with plenty of water and in the upright position. Among oral BPs, CLO showed efficacy in preventing bone loss in premenopausal breast cancer patients with chemotherapy‐induced ovarian failure 68.
ZA is the most extensively studied IV BP in clinical trials of CTIBL prevention. One relevant aspect of the BP strategy in this setting was provided by the N03CC (Alliance) trial 66. This study enrolled 551 postmenopausal women with breast cancer who completed tamoxifen and were undergoing daily letrozole treatment, who were randomized to upfront (n = 274) or delayed (n = 277) IV ZA 4 mg every 6 months. In the delayed arm, ZA was initiated for post‐baseline BMD T‐score < −2.0 or fracture. Incidence of a 5% decrease in total lumbar spine BMD at 5 years was 10.2% in the upfront vs. 41.2% in the delayed arm (P < 0.0001). Forty‐one patients in the delayed arm were eventually started on ZA. However, the number of patients with fractures was not significantly different between the two arms: 24 vs. 25 patients in the upfront and delayed arms, respectively. This could be explained by the fact that 41 patients in the delayed arm were “rescued” for having started ZA.
Denosumab at 60 mg every 6 months is approved to increase bone mass in patients with increased risk of fractures in several indications, including postmenopausal women with osteoporosis, men receiving ADT for non‐metastatic prostate cancer, and women receiving adjuvant AIs for breast cancer 71.
More recently, updated results of the ABCSG 18 study again reported an important fracture reduction with adjuvant SC denosumab at 60 mg every 6 months in postmenopausal breast cancer patients receiving AIs 51. In men receiving ADT for non‐metastatic prostate cancer, denosumab at 60 mg every 6 months was associated with an increase in BMD and a reduction in new vertebral fractures 72. Table 2 depicts some of the most representative studies using BTAs to prevent CTIBL.
Table 2.
Trials of antiresorptive agents for preventing CTIBL in postmenopausal women with breast cancer (adapted from Hadji et al. 62)
| Antiresorptive agent (trial) | n | BMaD study, n | Dosing | Treatment duration, years |
|---|---|---|---|---|
| Zoledronic acid (ZO‐FAST) | 1065 | 1065 | 4 mg iv q6mo | 5 |
| Zoledronic acid (Z‐FAST) | 602 | 602 | 4 mg iv q6mo | 5 |
| Zoledronic acid (E‐ZO‐FAST) | 527 | 527 | 4 mg iv q6mo | 5 |
| Zoledronic acid (N03CC) | 558 | 395 | 4 mg iv q6mo | 5 |
| Denosumab (HALT‐BC) | 252 | 252 | 60 mg sc q6mo | 2 |
| Denosumab (ABCSG‐18) | 3425 | 1872 | 60 mg sc q6mo | 5 |
| Denosumab 72 | 1468 | 1468 | 60 mg sc q6mo | 2 |
| Risedronate (SABRE) | 154 | 111 | 35 mg po/week | 2 |
| Risedronate | 87 | 87 | 35 mg po/week | 5 |
| Clodronate | 61 | 61 | 1600 mg po/day | 3 |
| Risedronate (ARBI) | 213 | 70 | 35 mg po/week | 2 |
| Risedronate (IBIS‐II) | 613 | 59 | 35 mg po/week | 5 |
| Ibandronate (ARIBON) | 131 | 50 | 150 mg po/month | 2 |
| Risedronate | 118 | 11 | 35 mg po/week | 1 |
BMaD: bone mass density; iv: intravenous; sc: subcutaneous; po: per os; q6month: every 6 months
The incidence of ONJ associated with the use of BTAS in CTIBL is very low, with no cases reported at the ABCSG‐18 study. The main reason for this is that in CTIBL, BTA regimens are less intensive due to the most potent anti‐osteoclast inhibitors (ZA and denosumab) and prescribed every 6 months instead of monthly as used to prevent SREs in patients with bone metastases.
Conclusions
Bone metastases and SREs are common in patients with advanced solid tumours, and in those who experience SREs the occurrence of pathologic fractures is correlated with a lower survival rate. Understanding the mechanisms associated with increased bone resorption prompt the discovery of the potential benefits of a class of agents known as BTAs.
BPs were the first BTAs to be investigated, becoming the standard of care for SRE prevention in patients with metastatic bone disease. Further knowledge on the role of RANK/RANKL axis on bone osteoclast activation and in the pathophysiology of bone metastases led to the development of a new agent acting as potent osteoclast inhibitor, denosumab. Denosumab provides an effective option for the prevention of SREs in patients with advanced cancer and bone metastases, and more recent investigation suggests that inhibition of RANKL with denosumab may be an interesting strategy to prevent breast cancer in mutated BRCA1 carriers.
An agent capable of acting as a BTA – Ra‐223 – is now approved for the treatment of prostate cancer patients with bone metastases. Importantly, this radiopharmaceutical agent may also act as an anticancer agent by delivering alpha radiation to bone metastases and interfering with the hyperactivation of osteoblasts (a typical feature of bone metastases in prostate cancer).
Investigating a possible role for BTAs in the adjuvant setting was a logical step in cancer research to prevent cancer relapse after surgery. But the need to properly select younger breast cancer women who are candidates for adjuvant BPs is a relevant issue. Although adjuvant BPs are currently reserved for postmenopausal women (either for physiological or surgical reasons or due to ovarian function suppression), extending these agents to younger women is an attractive strategy, namely for those patients at significant risk of (early or late) relapse.
The possible detrimental effect of BPs in premenopause are a matter of concern. If patients with MAF‐positive breast cancer are acknowledged to have an increased relapse risk outside of bone due to BPs, they will be ineligible for BPs, including if the purpose is to prevent osteoporosis and fractures, two common issues in the CTIBL setting.
The role of denosumab in the adjuvant setting remains unclear. The D‐CARE study failed to place this agent as an option for preventing cancer relapse and the ABCSG‐18 study is currently the only large trial attesting its use after surgery in postmenopausal women with breast cancer selected to receive AIs.
Until now, there is no evidence for the use of BTAs in prevention of relapse in prostate cancer or any other solid tumours (Figure 2).
Figure 2.
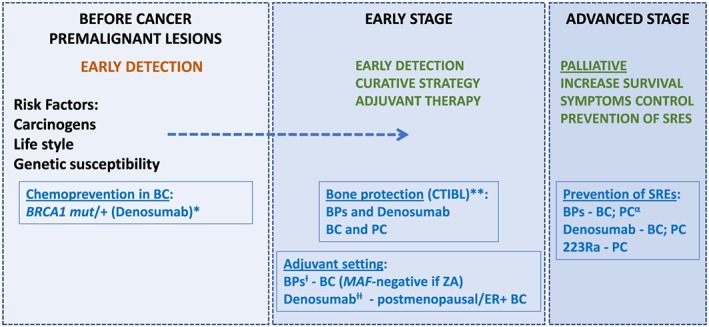
Natural history of breast and prostate cancer: possible time points of clinical intervention and/or research for bone‐targeted agents. BC, breast cancer; BRCA1, breast cancer 1; BPs, bisphosphonates; CTIBL, cancer treatment‐induced bone loss; ER+, estrogen receptor‐positive; MAF, MAF BZIP transcription factor; PC, prostate cancer; ZA, zoledronic acida Investigational data only.b Clinical evidence for bone mineral density (BMD) preservation and decreased incidence of fractures (denosumab).c In postmenopausal women or associated with ovarian suppression. MAF‐negative may have a role in selection of BC patients to ZA.d According to ABCSG‐18 study (only in postmenopausal women treated with aromatase inhibitors).e In PC, ZA is the approved BP with strongest evidence and indication
In September 2011, denosumab was FDA‐approved to treat bone loss in women taking aromatase inhibitors as part of their breast cancer treatment.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 73 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 74.
Competing Interests
L.C. has received research funding from Amgen, Novartis, and Roche; and speakers fee from Amgen, Novartis, and Roche. The other authors have no competing interests to declare.
Dionísio M. R., Mansinho A., Abreu C., Cavaco‐Silva J., Casimiro S., and Costa L. (2019) Clinical and translational pharmacology of drugs for the prevention and treatment of bone metastases and cancer‐induced bone loss, Br J Clin Pharmacol, 85, 1114–1124. doi: 10.1111/bcp.13852.
References
- 1. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27: 165–176. [DOI] [PubMed] [Google Scholar]
- 2. Paget S. The distribution of secondary growths in cancer of the breast. The Lancet 1889; 133: 571–573. [PubMed] [Google Scholar]
- 3. DeVita VT, Rosenberg SA. Two hundred years of cancer research. N Engl J Med 2012; 366: 2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2011; 11: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mundy GR. Mechanisms of bone metastasis. Cancer 1997; 80 (Suppl. 8): 1546–1556. [DOI] [PubMed] [Google Scholar]
- 6. D'Oronzo S, Stucci S, Tucci M, Silvestris F. Cancer treatment‐induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treat Rev 2015; 41: 798–808. [DOI] [PubMed] [Google Scholar]
- 7. Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res 2006; 12: 6222s–6230s. [DOI] [PubMed] [Google Scholar]
- 8. Trouvin A‐P, Goëb V. Receptor activator of nuclear factor‐κB ligand and osteoprotegerin: maintaining the balance to prevent bone loss. Clin Interv Aging 2010; 5: 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003; 423: 337–342. [DOI] [PubMed] [Google Scholar]
- 10. Broder MS, Gutierrez B, Cherepanov D, Linhares Y. Burden of skeletal‐related events in prostate cancer: unmet need in pain improvement. Support Care Cancer 2015; 23: 237–247. [DOI] [PubMed] [Google Scholar]
- 11. Yong M, Jensen AØ, Jacobsen JB, Nørgaard M, Fryzek JP, Sørensen HT. Survival in breast cancer patients with bone metastases and skeletal‐related events: a population‐based cohort study in Denmark (1999–2007). Breast Cancer Res Treat 2011; 129: 495–503. [DOI] [PubMed] [Google Scholar]
- 12. Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA, et al The significance of skeletal‐related events for the health‐related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005; 16: 579–584. [DOI] [PubMed] [Google Scholar]
- 13. Oster G, Lamerato L, Glass AG, Richert‐Boe KE, Lopez A, Chung K, et al Natural history of skeletal‐related events in patients with breast, lung, or prostate cancer and metastases to bone: a 15‐year study in two large US health systems. Support Care Cancer 2013; 21: 3279–3286. [DOI] [PubMed] [Google Scholar]
- 14. Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, et al Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 2007; 19: 420–432. [DOI] [PubMed] [Google Scholar]
- 15. Costa L. Which bisphosphonate to treat bone metastases? Lancet Oncol 2014; 15: 15–16. [DOI] [PubMed] [Google Scholar]
- 16. Pavlakis N, Schmidt RL, Stockler MR. Bisphosphonates for breast cancer In: Cochrane Database of Systematic Reviews, Vol. (3): CD003474, 2005. Available at http://doi.wiley.com/10.1002/14651858.CD003474.pub2 (last accessed 21 November 2018). [DOI] [PubMed] [Google Scholar]
- 17. Barrett‐Lee P, Casbard A, Abraham J, Hood K, Coleman R, Simmonds P, et al Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: a randomised, open label, non‐inferiority phase 3 trial. Lancet Oncol 2014; 15: 114–122. [DOI] [PubMed] [Google Scholar]
- 18. Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double‐blind, comparative trial. Cancer J 2001; 7: 377–387. [PubMed] [Google Scholar]
- 19. Aapro M, Saad F, Costa L. Optimizing clinical benefits of bisphosphonates in cancer patients with bone metastases. Oncologist 2010; 15: 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al A randomized, placebo‐controlled trial of zoledronic acid in patients with hormone‐refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002; 94: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 21. Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al Long‐term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone‐refractory prostate cancer. J Natl Cancer Inst 2004; 96: 879–882. [DOI] [PubMed] [Google Scholar]
- 22. Smith MR, Halabi S, Ryan CJ, Hussain A, Vogelzang N, Stadler W, et al Randomized controlled trial of early zoledronic acid in men with castration‐sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol 2014; 32: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double‐blind, randomized trial – the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol 2003; 21: 3150–3157. [DOI] [PubMed] [Google Scholar]
- 24. Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, et al Long‐term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors. Cancer 2004; 100: 2613–2621. [DOI] [PubMed] [Google Scholar]
- 25. Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al Denosumab versus zoledronic acid for treatment of bone metastases in men with castration‐resistant prostate cancer: a randomised, double‐blind study. Lancet 2011; 377: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stopeck AT, Lipton A, Body J‐J, Steger GG, Tonkin K, de Boer RH, et al Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double‐blind study. J Clin Oncol 2010; 28: 5132–5139. [DOI] [PubMed] [Google Scholar]
- 27. Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al Randomized, double‐blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 28. European Medicines Agency . Zometa Summary of Product Characteristics [online]. 2015. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000336/WC500051730.pdf (last accessed 27 January 2019).
- 29. Body JJ. Dosing regimens and main adverse events of bisphosphonates. Semin Oncol 2001; 28 (4 Suppl. 11): 49–53. [DOI] [PubMed] [Google Scholar]
- 30. Lipton A, Fizazi K, Stopeck AT, Henry DH, Brown JE, Yardley DA, et al Superiority of denosumab to zoledronic acid for prevention of skeletal‐related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012; 48: 3082–3092. [DOI] [PubMed] [Google Scholar]
- 31. Body J‐J, Bone HG, de Boer RH, Stopeck A, Van Poznak C, Damião R, et al Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer 2015; 51: 1812–1821. [DOI] [PubMed] [Google Scholar]
- 32. Body J‐J. Bisphosphonates for malignancy‐related bone disease: current status, future developments. Support Care Cancer 2006; 14: 408–418. [DOI] [PubMed] [Google Scholar]
- 33. Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active‐controlled phase III trials in cancer patients with bone metastases. Ann Oncol 2012; 23: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 34. Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, et al Alpha emitter radium‐223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 35. Smith MR, Parker C, Tombal BF, Miller K, Saad F, Fang F, et al ERA 223: a phase 3 trial of radium‐223 dichloride (Ra‐223) in combination with abiraterone acetate (abiraterone) and prednisone in the treatment of asymptomatic or mildly symptomatic chemotherapy‐naïve patients (pts) with bone predominant metastatic castration‐resistant prostate cancer (mCRPC). J Clin Oncol 2015; 33 (Suppl. 15): abstr TPS508. [Google Scholar]
- 36. European Medicines Agency . EMA restricts use of prostate cancer medicine Xofigo 2018. [online]. Available at http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Xofigo/human_referral_prac_000071.jsp&mid=WC0b01ac05805c516f (last accessed 27 January 2019).
- 37. Hortobagyi GN, Van Poznak C, Harker WG, Gradishar WJ, Chew H, Dakhil SR, et al Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone. JAMA Oncol 2017; 3: 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amadori D, Aglietta M, Alessi B, Gianni L, Ibrahim T, Farina G, et al Efficacy and safety of 12‐weekly versus 4‐weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open‐label, randomised, non‐inferiority trial. Lancet Oncol 2013; 14: 663–670. [DOI] [PubMed] [Google Scholar]
- 39. Ibrahim MFK, Mazzarello S, Shorr R, Vandermeer L, Jacobs C, Hilton J, et al Should de‐escalation of bone‐targeting agents be standard of care for patients with bone metastases from breast cancer? A systematic review and meta‐analysis. Ann Oncol 2015; 26: 2205–2213. [DOI] [PubMed] [Google Scholar]
- 40. Van Poznak C, Somerfield MR, Barlow WE, Biermann JS, Bosserman LD, Clemons MJ, et al Role of bone‐modifying agents in metastatic breast cancer: an American Society of Clinical Oncology–Cancer Care Ontario focused guideline update. J Clin Oncol 2017; 35: 3978–3986. [DOI] [PubMed] [Google Scholar]
- 41. McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM. Observations following discontinuation of long‐term denosumab therapy. Osteoporos Int. 2017; 28: 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clézardin P. Mechanisms of action of bisphosphonates in oncology: a scientific concept evolving from antiresorptive to anticancer activities. Bonekey Rep 2013; 2: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costa L, Harper P, Coleman RE, Lipton A. Anticancer evidence for zoledronic acid across the cancer continuum. Crit Rev Oncol Hematol 2011; 77: S31–S37. [DOI] [PubMed] [Google Scholar]
- 44. Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M, et al Breast‐cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011; 365: 1396–1405. [DOI] [PubMed] [Google Scholar]
- 45. Gnant M, Mlineritsch B, Stoeger H, Luschin‐Ebengreuth G, Knauer M, Moik M, et al Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol 2015; 26: 313–320. [DOI] [PubMed] [Google Scholar]
- 46. Early Breast Cancer Trialists' Collaborative Group (EBCTCG) . Adjuvant bisphosphonate treatment in early breast cancer: meta‐analyses of individual patient data from randomised trials. Lancet 2015; 386: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 47. Hadji P, Coleman RE, Wilson C, Powles TJ, Clézardin P, Aapro M, et al Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European panel. Ann Oncol 2016; 27: 379–390. [DOI] [PubMed] [Google Scholar]
- 48. Dhesy‐Thind S, Fletcher GG, Blanchette PS, Clemons MJ, Dillmon MS, Frank ES, et al Use of adjuvant bisphosphonates and other bone‐modifying agents in breast cancer: a Cancer Care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2017; 35: 2062–2081. [DOI] [PubMed] [Google Scholar]
- 49. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open‐label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol 2017; 18: 1543–1552. [DOI] [PubMed] [Google Scholar]
- 50. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al Adjuvant denosumab in breast cancer (ABCSG‐18): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2015; 386: 433–443. [DOI] [PubMed] [Google Scholar]
- 51. Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, et al Adjuvant denosumab in early breast cancer: disease‐free survival analysis of 3,425 postmenopausal patients in the ABCSG‐18 trial. J Clin Oncol 2018; 36 (Suppl): 500. [Google Scholar]
- 52. Coleman RE, Finkelstein D, Barrios CH, Martin M, Iwata H, Glaspy JA, et al Adjuvant denosumab in early breast cancer: first results from the international multicenter randomized phase III placebo controlled D‐CARE study. J Clin Oncol 2018; 36 (Suppl): 501. [Google Scholar]
- 53. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al Progesterone induces adult mammary stem cell expansion. Nature 2010; 465: 803–807. [DOI] [PubMed] [Google Scholar]
- 54. Fernandez‐Valdivia R, Lydon JP. From the ranks of mammary progesterone mediators, RANKL takes the spotlight. Mol Cell Endocrinol 2012; 357: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sigl V, Owusu‐Boaitey K, Joshi PA, Kavirayani A, Wirnsberger G, Novatchkova M, et al RANKL/RANK control Brca1 mutation‐driven mammary tumors. Cell Res 2016; 26: 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nolan E, Vaillant F, Branstetter D, Pal B, Giner G, Whitehead L, et al RANK ligand as a potential target for breast cancer prevention in BRCA1‐mutation carriers. Nat Med 2016; 22: 933–939. [DOI] [PubMed] [Google Scholar]
- 57. Wirth M, Tammela T, Cicalese V, Gomez Veiga F, Delaere K, Miller K, et al Prevention of bone metastases in patients with high‐risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol 2015; 67: 482–491. [DOI] [PubMed] [Google Scholar]
- 58. Denham JW, Joseph D, Lamb DS, Spry NA, Duchesne G, Matthews J, et al Short‐term androgen suppression and radiotherapy versus intermediate‐term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): an open‐label, randomised, phase 3 factorial. Lancet Oncol 2014; 15: 1076–1089. [DOI] [PubMed] [Google Scholar]
- 59. Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, et al Cancer treatment‐induced bone loss in breast and prostate cancer. J Clin Oncol 2008; 26: 5465–5476. [DOI] [PubMed] [Google Scholar]
- 60. Clines GA, Choksi P, Van Poznak C. Adjuvant endocrine therapy and bone health in breast cancer. Curr Osteoporos Rep 2015; 13: 263–273. [DOI] [PubMed] [Google Scholar]
- 61. Doo L, Shapiro CL. Skeletal manifestations of treatment of breast cancer on premenopausal women. Curr Osteoporos Rep 2013; 11: 311–318. [DOI] [PubMed] [Google Scholar]
- 62. Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE, et al Management of aromatase inhibitor‐associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol 2011; 22: 2546–2555. [DOI] [PubMed] [Google Scholar]
- 63. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol 2014; 25 (Suppl. 3): iii124–iii137. [DOI] [PubMed] [Google Scholar]
- 64. Brufsky AM. Cancer treatment‐induced bone loss: pathophysiology and clinical perspectives. Oncologist 2008; 13: 187–195. [DOI] [PubMed] [Google Scholar]
- 65. Ibrahim T, Mercatali L, Amadori D. Bone and cancer: the osteoncology. Clin Cases Miner Bone Metab 2013; 10: 121–123. [PMC free article] [PubMed] [Google Scholar]
- 66. Wagner‐Johnston ND, Sloan JA, Liu H, Kearns AE, Hines SL, Puttabasavaiah S, et al 5‐year follow‐up of a randomized controlled trial of immediate versus delayed zoledronic acid for the prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen: N03CC (Alliance) trial. Cancer 2015; 121: 2537–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al Denosumab and bone‐metastasis‐free survival in men with castration‐resistant prostate cancer: results of a phase 3, randomised, placebo‐controlled trial. Lancet 2012; 379: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saarto T, Blomqvist C, Välimäki M, Mäkelä P, Sarna S, Elomaa I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol 1997; 15: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 69. Gnant M, Mlineritsch B, Luschin‐Ebengreuth G, Kainberger F, Kässmann H, Piswanger‐Sölkner JC, et al Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early‐stage breast cancer: 5‐year follow‐up of the ABCSG‐12 bone‐mineral density substudy. Lancet Oncol 2008; 9: 840–849. [DOI] [PubMed] [Google Scholar]
- 70. Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, et al Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 2008; 26: 4875–4882. [DOI] [PubMed] [Google Scholar]
- 71. Lipton A, Smith MR, Ellis GK, Goessl C. Treatment‐induced bone loss and fractures in cancer patients undergoing hormone ablation therapy: efficacy and safety of denosumab. Clin Med Insights Oncol 2012; 6: 287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Smith MR, Egerdie B, Toriz NH, Feldman R, Tammela TLJ, Saad F, et al Denosumab in men receiving androgen‐deprivation therapy for prostate cancer. N Engl J Med 2009; 361: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Catalytic receptors. Br J Pharmacol 2017; 174: S225–S271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Casimiro S, Ferreira A, Mansinho A, Alho I, Costa L. Molecular mechanisms of bone metastasis: which targets came from the bench to the bedside? Int J Mol Sci 2016; 17: 1415. [DOI] [PMC free article] [PubMed] [Google Scholar]


