Abstract
Microvesicles (MVs), which are cell‐derived membrane vesicles present in body fluids, are closely associated with the development of malignant tumours. Saliva, one of the most versatile body fluids, is an important source of MVs. However, the association between salivary MVs (SMVs) and oral squamous cell carcinoma (OSCC), which is directly immersed in the salivary milieu, remains unclear. SMVs from 65 patients with OSCC, 21 patients with oral ulcer (OU), and 42 healthy donors were purified, quantified and analysed for their correlations with the clinicopathologic features and prognosis of OSCC patients. The results showed that the level of SMVs was significantly elevated in patients with OSCC compared to healthy donors and OU patients. Meanwhile, the level of SMVs showed close correlations with the lymph node status, and the clinical stage of OSCC patients. Additionally, the ratio of apoptotic to non‐apoptotic SMVs was significantly decreased in OSCC patients with higher pathological grade. Consistently, poorer overall survival was observed in patients with lower ratio of apoptotic to non‐apoptotic SMVs. In conclusion, the elevated level of SMVs is associated with clinicopathologic features and decreased survival in patients with OSCC, suggesting that SMVs are a potential biomarker and/or regulator of the malignant progression of OSCC.
Keywords: apoptosis, biomarker, microvesicles, oral squamous cell carcinoma, saliva
1. INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common subtype of head and neck squamous cell carcinoma.1 Although the treatment of OSCC has made progresses recently, the prognosis of OSCC is still poor, with high rate of local recurrence and a 5‐year survival rate at 50%.2 Thus, more mechanisms behind the development of OSCC need to be unmasked.
Microvesicles (MVs) are submicron (100‐1000 nm) membrane vesicles secreted by cells.3 In addition to various biological functions, MVs also possess significant potential to serve as biomarkers for malignant tumours.4 It has been well‐established that MVs are present and elevated in the blood of patients with various diseases, including diabetes, cardiovascular diseases and cancers.5, 6, 7 Our previous studies have shown that the level of circulating MVs (CMVs) in peripheral blood is significantly elevated in patients with OSCC compared to healthy donors, and it is positively correlated with the clinical features of OSCC patients.8, 9
In addition to the plasma, increased levels of MVs have been reported in other types of body fluids, such as urine in patients with bladder cancer,10 ascites in patients with ovarian carcinoma11 and pleural fluids in patients with lung cancer.12 Previous studies have confirmed that MVs are present in the saliva of healthy donors.13 However, the change in salivary MVs (SMVs) during the development of OSCC is still unclear. Since the primary tumour is directly immersed in the salivary milieu in OSCC patients, tumour cells may potentially release MVs into the saliva. Moreover, previous studies have demonstrated that MVs produced upon cell apoptosis (known as apoptotic MVs) or cell activation might possess different pathophysiological functions.14, 15, 16 Because suppression of apoptosis plays an important role in the development of cancer, the level of apoptotic MVs,17, 18 which is usually a reflection of the level of cell apoptosis, has been linked to the development of cancer.19, 20, 21 For instance, a previous study showed that the level of apoptotic MVs in peripheral blood possessed diagnostic significance in lung cancer.22 However, the biomarker potential of SMVs and apoptotic SMVs in OSCC patients remains to be clarified.
In the present study, the level of total SMVs and the proportion of apoptotic SMVs were evaluated and compared among healthy donors, patients with oral ulcer (OU) and patients with OSCC. In addition, the correlations of SMVs with the clinicopathologic features and prognosis of OSCC patients was examined.
2. MATERIALS AND METHODS
2.1. Isolation of SMVs and CMVs
This study was carried out according to the guidelines approved by the review board of the ethics committee of the Hospital of Stomatology, Wuhan University. Meanwhile, the study was performed according to the World Medical Association Declaration of Helsinki and the National Institutes of Health guidelines regarding to the use of clinical tissues. Written informed consent was obtained from all the subjects in our research. The volunteers were required to stop from performing oral hygiene procedures, smoking, drinking and eating at least 1 hour before saliva collection. Unstimulated saliva sample (5 mL) was collected between 9 AM and 10 AM according to a previously established protocol.23 For CMVs collection, 5 mL of venous citrated blood from OSCC patients or healthy donors was obtained. Then SMVs and CMVs from healthy donors, OU patients and OSCC patients were purified by a standard differential centrifugation protocol8, 24, 25 as described in the supplementary materials.
2.2. Characterization and quantification of SMVs and CMVs
According to our previous studies,8, 24 transmission electron microscopy (TEM), dynamic light scattering, carboxyfluorescein succinimidyl ester (CFSE) labeling and flow cytometry were first performed to characterize the purified SMVs and CMVs. Then, the concentrations of MVs were analysed by the count of “events” using flow cytometer. Briefly, identical volumes of a MV sample and the Flow‐count fluorospheres (Beckman Coulter; 10 μm in diameter) were mixed for detection. Since Flow‐count fluorospheres have a known concentration, the concentrations of MVs can be calculated as the following formula: (total number of events for the sample/total number of events for Flow‐count fluorospheres) × Flow‐Count fluorospheres assayed concentration. Following fluorochrome‐coupled antibodies and their corresponding isotypes were used for the analyses of MV phenotype: phycoerythrin (PE) labeled anti‐Annexin V, PE labeled anti‐CD31, FITC labeled anti‐CD41b, PE labeled anti‐EpCAM. All these antibodies were purchased from BD Biosciences. With regard to incubation buffers, Annexin V Binding Buffer containing calcium (BD Biosciences) was used for PE‐labeled Annexin V, and PBS was used for other antibodies recognizing cellular origin markers.
2.3. Immunohistochemistry
Based on previously described procedures,26, 27, 28 the samples from OSCC patients were fixed in 4% paraformaldehyde and embedded in paraffin. Immunohistochemistry was performed as described in the supplementary materials.
2.4. Statistical analysis
All data in our study were shown as Mean ± SD of three independent experiments. Student's t test, one‐way analysis of variance and Spearman's rank correlation test were used for statistical analysis. A value of P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Clinical characteristics and follow‐up of the patients with OSCC
In our present study, the age distribution and gender ratio were similar among the healthy donors, patients with OU and patients with OSCC, with no significant difference (P > 0.05). All cases of OSCC patients were free of any other systemic disease including diabetes and cardiovascular disorders. Up to April 2018, the 65 OSCC patients were followed up from 2 to 45 months. The overall survival rate was 84.6%, the disease‐free survival rate was 76.9% and the recurrence rate was 21.5%.
3.2. The morphology, size and zeta potential of SMVs are in line with the features of MVs and are not changed in OSCC patients
As revealed by TEM images (Figure 1A), SMVs purified from healthy donors (HD‐SMV), OU patients (OU‐SMVs) and OSCC patients (OSCC‐SMVs) were membrane‐limited vesicles presenting round or slightly elongated shapes with diameters between 100 nm and 1000 nm. The flow cytometry (Figure S1) and dynamic light scattering (Figure 1B) analyses demonstrated that the purified SMVs had a size distribution ranging from 100 nm to 1000 nm, showing no significant difference among healthy donors, OU patients and OSCC patients. Moreover, the zeta potentials of HD‐SMV, OU‐SMVs and OSCC‐SMVs showed the similar distribution, ranging from −28 mV to −40 mV (Figure 1C). Additionally, the CFSE labeling assay showed that the purified SMVs could be successfully labeled by CFSE, indicating their membrane‐bound structures (Figure 1D). The flow cytometric results further showed that the majority of particles within the SMV samples were positive for CFSE staining (Figure 1E), suggesting the purity of the isolated SMVs. These above data demonstrate that the purified SMVs are in line with the features of MVs, and some of the basic properties of SMVs, such as physical morphology, size distribution and zeta potential, are not significantly changed in OSCC patients.
Figure 1.

Characterization of salivary microvesicles (SMVs) derived from healthy donors (HD), patients with oral ulcer (OU) and patients with oral squamous cell carcinoma (OSCC). (A) Transmission electron microscopy (TEM) images of SMVs purified from healthy donors (HD‐SMV), OU patients (OU‐SMVs) and OSCC patients (OSCC‐SMVs). The size distribution of SMVs was analysed based on the TEM images. (B) Dynamic light scattering was performed to assess the size distribution of SMVs derived from healthy donors (HD‐SMV), OU patients (OU‐SMVs) and OSCC patients (OSCC‐SMVs). (C) Dynamic light scattering was carried out to detect the zeta potential of SMVs derived from healthy donors (HD‐SMV), OU patients (OU‐SMVs) and OSCC patients (OSCC‐SMVs). (D) Fluorescence images showing the labeling of SMVs by the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE). (E) Representative flow cytometry dot‐plots showing the percentages of CFSE‐labeled SMVs. Data were expressed as Mean ± SD
3.3. The level of SMVs is elevated in OSCC patients
For quantifying the SMVs and CMVs, flow cytometry using flow‐count fluorospheres was performed. Representative images of flow cytometry for detecting SMVs and CMVs were shown in Figure 2A,B respectively. The fluorescent beads for counting were shown as the gated dot‐plots in the red window, while the SMVs or CMVs were shown in the blue window. The quantification results showed that the number of SMVs was ranged from 147 to 4704 events/μL (Mean ± SD: 1801 ± 1172 events/μL) in healthy donors, ranged from 523 to 4041 events/μL (Mean ± SD: 1791 ± 1207 events/μL) in patients with OU, and ranged from 65 to 35774 events/μL (Mean ± SD: 3758 ± 6119 events/μL) in patients with OSCC. Significant differences were found between OSCC patients and OU patients or healthy donors in terms of the level of SMVs (Figure 2C). In addition, the result also showed that the OSCC cases presenting as a non‐healing ulcer had higher levels of SMVs compared with the OU cases (Figure S2, P < 0.001). However, there was no significant difference between healthy donors and patients with OU in the level of SMVs (Figure 2C). Moreover, as shown in Figure 2D, OSCC patients with or without lymphatic metastasis showed an obviously higher level of SMVs than the healthy donors. Meanwhile, OSCC patients with higher clinical stage (Stage III + IV) had significantly higher level of SMVs than the healthy donors (Figure 2E, P < 0.01). Our previous studies have revealed that the level of CMVs in peripheral blood is also significantly increased in OSCC patients when compared with healthy donors. Here, we further investigated the potential correlation or difference in the levels of SMVs and CMVs in OSCC patients. As shown in Figure 2F, the level of CMVs was significantly elevated in OSCC patients compared to healthy donors (P < 0.001). Nevertheless, there was no significant correlation between the increased levels of SMVs and CMVs in OSCC patients (Figure S3A). In addition, our results revealed that the majority of OSCC‐CMVs were CD31+CD41b+ platelet‐derived MVs, while the majority of OSCC‐SMVs were positive for EpCAM, indicating the origin of epithelial cells (Figure S3B). These results demonstrate that the level of SMVs is elevated concomitant with the level of CMVs in patients with OSCC. However, the cellular origins of SMVs and CMVs in OSCC patients might be significantly different.
Figure 2.

Quantification of the level of salivary microvesicles (SMVs) and circulating microvesicles (CMVs) in healthy donors (HD), patients with oral ulcer (OU) and patients with oral squamous cell carcinoma (OSCC). (A) Representative flow cytometry dot‐plots showing the gated SMVs (blue window) purified from healthy donors (HD‐SMV), OU patients (OU‐SMVs) and OSCC patients (OSCC‐SMVs) as well as the fluorescent beads for counting (red window). (B) Representative flow cytometry dot‐plots showing the gated CMVs (blue window) purified from healthy donors (HD‐CMVs) and OSCC patients (OSCC‐CMVs) as well as and the fluorescent beads for counting (red window). (C) Comparison of the levels of SMVs in healthy donors (HD‐SMV), patients with oral ulcer (OU‐SMVs) and patients with oral squamous cell carcinoma (OSCC‐SMVs). (D) Comparison of the levels of SMVs in healthy donors (HD‐SMV) and OSCC patients with or without lymphatic metastasis. (E) Comparison of the levels of SMVs in healthy donors (HD‐SMV), and OSCC patients with lower clinical stage (Stage I + II) or higher clinical stage (Stage III + IV). (F) Comparison of the levels of CMVs in healthy donors (HD‐CMVs) and patients with oral squamous cell carcinoma (OSCC‐CMVs). (G) Representative immunostaining of VEGF‐C in OSCC patients with different levels of SMVs. (H) Comparison of the levels of VEGF‐C in OSCC patients with different levels of SMVs. (I) Spearman's rank correlation test was performed to determine the correlation between the level of SMVs and the intratumoral expression level of VEGF‐C in OSCC patients. Data were shown as the Mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001
3.4. Increased SMVs are correlated with the clinicopathologic features of OSCC patients
Next, associations between the elevated level of SMVs and the clinicopathologic features of OSCC patients were investigated. As shown in Figure 2D, the level of SMVs in OSCC patients with lymph node metastasis was markedly up‐regulated compared to patients without lymph node metastasis (P < 0.01). Moreover, as shown in Figure 2E, the level of SMVs in patients with higher clinical stage (Stage III + IV) was obviously elevated when compared to that in patients with lower clinical stage (Stage I + II, P < 0.01). To verify the association between increased SMVs and the lymph node metastasis of OSCC, we then evaluated the correlation of the level of SMVs with the expression level of vascular endothelial growth factor C (VEGF‐C), one of the major pro‐lymphangiogenic growth factors, in the tumour samples of OSCC patients. The representative immunohistochemical images are shown in Figure 2G. The semi‐quantitative results revealed that the expression level of VEGF‐C in the tumour samples was markedly increased in the patients with higher levels of SMVs when compared to those with lower levels of SMVs (Figure 2H). Importantly, the correlation analysis showed that the intratumoral expression level of VEGF‐C in OSCC patients was positively associated with the level of SMVs (Figure 2I). These above results suggest that the increased SMVs could be a potential biomarker of clinicopathologic features of OSCC patients.
3.5. The ratio of apoptotic to non‐apoptotic SMVs is decreased in patients with poorly differentiated OSCC
Annexin V has been frequently used as a marker of MVs from apoptotic cells, especially when analysing the MVs in human body fluids.6, 29, 30 As shown in Figure 3A, the percentages of apoptotic and non‐apoptotic OSCC‐SMVs were analysed based on gating of CFSE (indicative of SMVs) and Annexin V (indicative of apoptotic origin). The results showed that the percentages of apoptotic (CFSE+Annexin V+) SMVs in healthy donors, OU patients and OSCC patients were 45.1 ± 14.0%, 42.6 ± 8.2% and 49.2 ± 20.5%, and the percentages of non‐apoptotic SMVs (CFSE+Annexin V−) SMVs were 54.9 ± 14.0%, 57.4 ± 8.2% and 50.8 ± 20.5% respectively (Figure 3B). There was no significant difference in the ratio of Annexin V+ to Annexin V− SMVs among healthy donors, OU patients and OSCC patients (data not shown). These results suggest that apoptotic and non‐apoptotic SMVs are almost in equal proportion, with no significant change in OSCC patients. By contrast, we found that the majority of CMVs in OSCC patients were negative for Annexin V staining (Figure 3C). To investigate the significance of apoptotic SMVs in OSCC, we then examined the association of the ratio of Annexin V+ to Annexin V− OSCC‐SMVs with clinicopathologic features of OSCC patients. Of interest, flow cytometric results (Figure 3D) showed that the percentage of Annexin V+ SMVs was significantly decreased in OSCC patients with a higher pathological grade (III) when compared to those with lower pathological grades (I and II, P < 0.01), consistent with the observation by fluorescence microscope (Figure 3E). These results suggest that the proportion of apoptotic SMVs is decreased while the proportion of non‐apoptotic SMVs is increased in OSCC patients with a higher pathological grade. Correspondingly, as shown in Figure 3F, the ratio of Annexin V+ to Annexin V− OSCC‐SMVs had a negative correlation with pathological grade (P < 0.01). These above data demonstrate that the ratio of apoptotic to non‐apoptotic OSCC‐SMVs is decreased in poorly differentiated OSCC, suggesting that the formation mechanism of SMVs in terms of apoptosis is altered during the malignant progression of OSCC.
Figure 3.
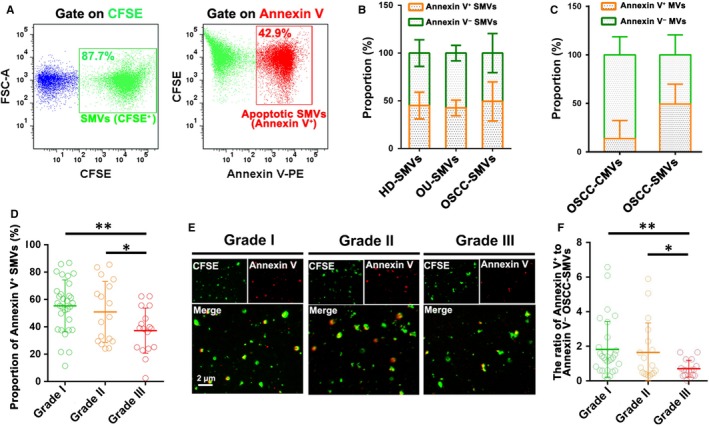
Quantitative analysis of the ratio of apoptotic to non‐apoptotic salivary microvesicles (SMVs). (A) Representative flow cytometry dot‐plots showing the gating strategy for apoptotic SMVs. (B) Quantitative analysis of the proportion of apoptotic and non‐apoptotic SMVs in healthy donors (HD‐SMV), patients with oral ulcer (OU‐SMVs) and patients with oral squamous cell carcinoma (OSCC‐SMVs). (C) Quantitative analysis of the proportion of apoptotic and non‐apoptotic OSCC‐CMVs and OSCC‐SMVs. (D) Quantitative analysis of the proportion of apoptotic SMVs in OSCC patients with different pathological grades. (E) Fluorescence images showing apoptotic SMVs (CFSE+Annexin V+) and non‐apoptotic SMVs (CFSE+Annexin V−) in OSCC patients with different pathological grades. (F) Quantitative analysis of the ratio of Annexin V+ to Annexin V− OSCC‐SMVs in OSCC patients with different pathological grades. Data were expressed as Mean ± SD. *P < 0.05, **P < 0.01
3.6. Prognostic significance of SMVs in OSCC patients
To further determine the prognostic values of SMVs, patients with OSCC were divided into two groups based on either the level of OSCC‐SMVs or the ratio of Annexin V+ to Annexin V− SMVs. Kaplan‐Meier analysis revealed that the overall survival, disease‐free survival and recurrence‐free survival curves stratified but showed no significant difference according to the level of total OSCC‐SMVs (Figure 4A‐C). Also, these survival curves stratified based on the ratio of Annexin V+ to Annexin V− OSCC‐SMVs (Figure 4D‐F). With a statistical significance, OSCC patients with a higher ratio of Annexin V+ to Annexin V− OSCC‐SMVs were predicted to have a better outcome in terms of overall survival (P < 0.05). These above results suggest the prognostic value and biomarker potential of SMVs, especially the ratio of Annexin V+ to Annexin V− SMVs.
Figure 4.
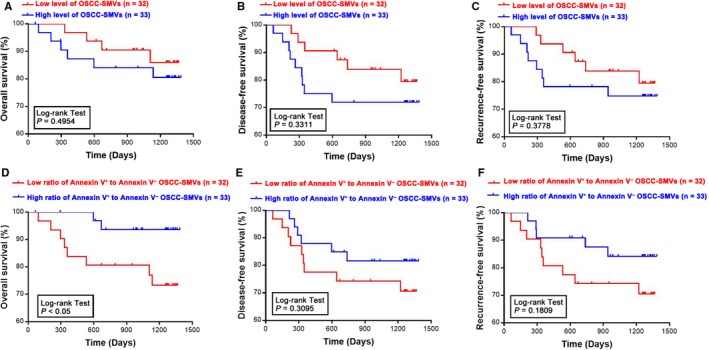
Prognostic values of salivary microvesicles (SMVs) in oral squamous cell carcinoma (OSCC) patients. Kaplan‐Meier analyses of the overall survival, disease‐free survival and recurrence‐free survival of OSCC patients were performed according to the level of total SMVs (A‐C) in patients with OSCC (OSCC‐SMVs) or the ratio of Annexin V+ to Annexin V− OSCC‐SMVs (D‐F). n: the number of subjects in the group
4. DISCUSSION
Previous studies have showed that the level of MVs, which are submicron (100‐1000 nm) membrane vesicles released by cells upon apoptosis or activation, is elevated in various human body fluids due to the development of malignant tumours.11, 31, 32 Although the presence of MVs in saliva of healthy donors has been reported by a previous study,13 the association between SMVs and OSCC is still unknown. In the present study, we find that the level of SMVs is significantly up‐regulated in patients with OSCC compared to healthy donors and OU patients. Meanwhile, the level of SMVs shows positive correlation with the clinicopathologic features of OSCC patients. Moreover, the ratio of Annexin V+ to Annexin V− SMVs, which represents the ratio of apoptotic SMVs and non‐apoptotic SMVs, is significantly decreased in patients with poorly differentiated OSCC. More importantly, OSCC patients with higher ratio of apoptotic to non‐apoptotic SMVs show better overall survival than patients with lower ratio of apoptotic to non‐apoptotic SMVs.
Recently, our studies have shown that the level of CMVs is elevated and closely associated with the increased procoagulant activity and tumour angiogenesis in OSCC patients.8, 9 Here, we found that the major subpopulations and the formation mechanism (in terms of apoptosis) of SMVs and CMVs in OSCC patients were different. CMVs derived from OSCC patients were almost platelet‐derived MVs, while more than 50% of SMVs derived from OSCC patients showed epithelial origin. Moreover, consistent with a previous report,33 we found that the majority of CMVs derived from OSCC patients were negative for Annexin V staining (indicative of non‐apoptotic MVs), while apoptotic and non‐apoptotic SMVs were almost in equal proportion in OSCC patients. These findings suggest that the levels of SMVs and CMVs are probably elevated in OSCC patients through different mechanisms. Our previous study has revealed that the elevated level of CMVs in OSCC patients shows a close correlation with the secretion of inflammation‐related cytokines by OSCC cells. Although the mechanism behind the elevated level of SMVs in OSCC patients remains to be investigated, it is a reasonable hypothesis that MVs secreted by apoptotic and/or activated OSCC cells or other cells in the tumour microenvironment may contribute to the increase in the level of SMVs. As collection of saliva samples is convenient, noninvasive and low‐cost, SMVs may possess unique advantages over CMVs to serve as potential biomarkers for OSCC.
It has been widely accepted that both cell apoptosis and activation can result in the generation of MVs.29 To investigate the clinical significance of SMVs in terms of apoptosis in OSCC patients, we performed Annexin V staining by flow cytometry, which has been widely used to distinguish between apoptotic (Annexin V+) and non‐apoptotic (Annexin V−) MVs.6, 14, 34 We found that the ratio of apoptotic to non‐apoptotic SMVs possessed a negative correlation with the pathological grade in OSCC patients, and OSCC patients with lower ratio of apoptotic to non‐apoptotic SMVs were predicted to have a worse prognosis. These data suggest that SMVs may be a promising diagnostic and prognostic biomarker for OSCC patients.
So far, VEGF‐C is considered to be a major pro‐lymphangiogenic growth factor that can up‐regulate lymphatic vessel density and promote the tumour cells metastasize to lymphatic node.35, 36 Interestingly, our results revealed that the level of SMVs possessed a positive correlation with the expression level of VEGF‐C in OSCC tumour samples, suggesting a close association between SMVs and the lymph node metastasis of OSCC. Previous studies have documented that platelet‐derived MVs, the main CMVs, could transfer the receptor to cancer cell surface, induce kinase‐dependent protein phosphorylation and elevate the level of the matrix metalloproteinases to enhance cellular invasion.37, 38 In our study, although the precise mechanism behind the promotive effects of SMVs on OSCC cells in the VEGF‐C expression remains to be elucidated, our findings suggest that increased SMVs is not only a potential biomarker of clinicopathologic features of OSCC patients but may be also functionally involved in the development of OSCC, such as lymph node metastasis.
In summary, our present study reports that the level of SMVs is elevated in patients with OSCC, and the increased level of SMVs is closely correlated with the clinical data and outcomes of OSCC patients. Moreover, we also demonstrate that the ratio of apoptotic to non‐apoptotic SMVs is significantly decreased in patients with poorly differentiated OSCC. Importantly, lower ratio of apoptotic to non‐apoptotic SMVs predicts poorer overall survival of OSCC patients. Taken together, this study highlights the biomarker potential and functional significance of SMVs in OSCC.
CONFLICTS OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
WQ Zhong, YF Zhao and G Chen participated in the conception design, data acquisition and analysis, and wrote the manuscript. WQ Zhong, JG Ren and QW Man performed the experiments. XP Xiong, B Liu, ZJ Sun, J Jia and WF Zhang helped with the collection of clinical samples. W Zhang and G Chen revised the manuscript. All authors approve and agree to be responsible for all aspects of this work.
Supporting information
ACKNOWLEDEGEMENT
This research was supported by the National Natural Science Foundation of China to Gang Chen (NO. 81671816) and Yi‐Fang Zhao (NO. 81570994). We are grateful to the Core Facility and Technical Support, Wuhan Institute of Virology, and Juan Min for assistance in flow cytometric analyses.
Zhong W‐Q, Ren J‐G, Xiong X‐P, et al. Increased salivary microvesicles are associated with the prognosis of patients with oral squamous cell carcinoma. J Cell Mol Med. 2019;23:4054–4062. 10.1111/jcmm.14291
Wen‐Qun Zhong and Jian‐Gang Ren contributed equally to this work.
Funding information
This work was supported by the National Natural Science Foundation of China to Gang Chen (NO. 81671816) and Yi‐Fang Zhao (NO. 81570994).
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5‐29. [DOI] [PubMed] [Google Scholar]
- 2. Zhong L‐P, Zhang C‐P, Ren G‐X, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up‐front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Loyer X, Vion AC, Tedgui A, Boulanger CM. Microvesicles as cell‐cell messengers in cardiovascular diseases. Circ Res. 2014;114:345‐353. [DOI] [PubMed] [Google Scholar]
- 4. Hoefer IE, Steffens S, Ala‐Korpela M, et al. Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J. 2015;36:2635‐2642. [DOI] [PubMed] [Google Scholar]
- 5. Koga H, Sugiyama S, Kugiyama K, et al. Elevated levels of remnant lipoproteins are associated with plasma platelet microvesicles in patients with type‐2 diabetes mellitus without obstructive coronary artery disease. Eur Heart J. 2006;27:817‐823. [DOI] [PubMed] [Google Scholar]
- 6. Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J. 2011;32:2034‐2041. [DOI] [PubMed] [Google Scholar]
- 7. Laresche C, Pelletier F, Garnache‐Ottou F, et al. Increased levels of circulating microparticles are associated with increased procoagulant activity in patients with cutaneous malignant melanoma. J Invest Dermatol. 2014;134:176‐182. [DOI] [PubMed] [Google Scholar]
- 8. Ren JG, Man QW, Zhang W, et al. Elevated level of circulating platelet‐derived microparticles in oral cancer. J Dent Res. 2016;95:87‐93. [DOI] [PubMed] [Google Scholar]
- 9. Ren JG, Zhang W, Liu B, et al. Clinical significance and roles in angiogenesis of circulating microparticles in oral cancer. J Dent Res. 2016;95:860‐867. [DOI] [PubMed] [Google Scholar]
- 10. Smalley DM, Sheman NE, Nelson K, Theodorescu D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J Proteome Re. 2008;7:2088‐2096. [DOI] [PubMed] [Google Scholar]
- 11. Press JZ, Reyes M, Pitteri SJ, et al. Microparticles from ovarian carcinomas are shed into ascites and promote cell migration. Int J Gynecol Cancer. 2012;22:546‐552. [DOI] [PubMed] [Google Scholar]
- 12. Park JO, Choi D‐Y, Choi D‐S, et al. Identification and characterization of proteins isolated from microvesicles derived from human lung cancer pleural effusions. Proteomics. 2013;13:2125‐2134. [DOI] [PubMed] [Google Scholar]
- 13. Berckmans RJ, Sturk A, van Tienen LM, Schaap MC, Nieuwland R. Cell‐derived vesicles exposing coagulant tissue factor in saliva. Blood. 2011;117:3172‐3180. [DOI] [PubMed] [Google Scholar]
- 14. Jimenez JJ, Jy W, Mauro LM, Horstman LL, Ahn YS. Elevated endothelial microparticles in thrombotic thrombocytopenic purpura: findings from brain and renal microvascular cell culture and patients with active disease. Br J Haematol. 2001;112:81‐90. [DOI] [PubMed] [Google Scholar]
- 15. Combes V, Simon A‐C, Grau G‐E, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277‐287. [DOI] [PubMed] [Google Scholar]
- 17. Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501‐507. [DOI] [PubMed] [Google Scholar]
- 18. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342‐348. [DOI] [PubMed] [Google Scholar]
- 19. Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36:888‐906. [DOI] [PubMed] [Google Scholar]
- 20. Tseng C‐C, Wang C‐C, Chang H‐C, et al. Levels of circulating microparticles in lung cancer patients and possible prognostic value. Dis Markers. 2013;35:301‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aharon A, Brenner B. Microparticles, thrombosis and cancer. Best Pract Res Clin Haematol. 2009;22:61‐69. [DOI] [PubMed] [Google Scholar]
- 22. Wang C‐C, Tseng C‐C, Hsiao C‐C, et al. Circulating endothelial‐derived activated microparticle: a useful biomarker for predicting one‐year mortality in patients with advanced non‐small cell lung cancer. Biomed Res Int. 2014;2014:173401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, St John MA, Zhou X, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004;10:8442‐8450. [DOI] [PubMed] [Google Scholar]
- 24. Chen G, Zhu J‐Y, Zhang Z‐L, et al. Transformation of cell‐derived microparticles into quantum‐dot‐labeled nanovectors for antitumor siRNA delivery. Angew Chem Int Ed Engl. 2015;54:1036‐1040. [DOI] [PubMed] [Google Scholar]
- 25. Zhao JY, Chen G, Gu YP, et al. Ultrasmall magnetically engineered Ag2Se quantum dots for instant efficient labeling and whole‐body high‐resolution multimodal real‐time tracking of cell‐derived microvesicles. J Am Chem Soc. 2016;138:1893‐1903. [DOI] [PubMed] [Google Scholar]
- 26. Zhong W‐Q, Chen G, Zhang W, et al. Down‐regulation of connexin43 and connexin32 in keratocystic odontogenic tumours: potential association with clinical features. Histopathology. 2015;66:798‐807. [DOI] [PubMed] [Google Scholar]
- 27. Zhong W‐Q, Chen G, Zhang W, et al. M2‐polarized macrophages in keratocystic odontogenic tumor: relation to tumor angiogenesis. Sci Rep. 2015;5:15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu H, Chen G, Zhang W, et al. Overexpression of macrophage migration inhibitory factor in adenoid cystic carcinoma: correlation with enhanced metastatic potential. J Cancer Res Clin Oncol. 2013;139:287‐295. [DOI] [PubMed] [Google Scholar]
- 29. Singh N, Van Craeyveld E, Tjwa M, et al. Circulating apoptotic endothelial cells and apoptotic endothelial microparticles independently predict the presence of cardiac allograft vasculopathy. J Am Coll Cardiol. 2012;60:324‐331. [DOI] [PubMed] [Google Scholar]
- 30. Berezin AE, Kremzer AA, Samura TA, et al. Predictive value of apoptotic microparticles to mononuclear progenitor cells ratio in advanced chronic heart failure patients. J Cardiol. 2015;65:403‐411. [DOI] [PubMed] [Google Scholar]
- 31. Muralidharan‐Chari V, Clancy JW, Sedgwick A, D'Souza‐Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roca E, Lacroix R, Judicone C, et al. Detection of EpCAM‐positive microparticles in pleural fluid: a new approach to mini‐invasively identify patients with malignant pleural effusions. Oncotarget. 2016;7:3357‐3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet‐derived microparticles fail to bind annexin V, lack phospholipid‐dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103:1044‐1052. [DOI] [PubMed] [Google Scholar]
- 34. Dieker J, Tel J, Pieterse E, et al. Circulating apoptotic microparticles in systemic lupus erythematosus patients drive the activation of dendritic cell subsets and prime neutrophils for NETosis. Arthritis Rheumatol. 2016;68:462‐472. [DOI] [PubMed] [Google Scholar]
- 35. Siriwardena BS, Kudo Y, Ogawa I, Udagama MN, Tilakaratne WM, Takata T. VEGF‐C is associated with lymphatic status and invasion in oral cancer. J Clin Pathol. 2008;61:103‐108. [DOI] [PubMed] [Google Scholar]
- 36. Sugiura T, Inoue Y, Matsuki R, et al. VEGF‐C and VEGF‐D expression is correlated with lymphatic vessel density and lymph node metastasis in oral squamous cell carcinoma: Implications for use as a prognostic marker. Int J Oncol. 2009;34:673‐680. [DOI] [PubMed] [Google Scholar]
- 37. Janowska‐Wieczorek A, Wysoczynski M, Kijowski J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752‐760. [DOI] [PubMed] [Google Scholar]
- 38. Zmigrodzka M, Guzera M, Miskiewicz A, Jagielski D, Winnicka A. The biology of extracellular vesicles with focus on platelet microparticles and their role in cancer development and progression. Tumour Biol. 2016;37:14391‐14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


