Abstract
Purpose:
Overdose from alcohol and/or drugs kills tens of thousands of Americans annually, with a large number of deaths attributed to opioid pain medications. Addiction treatment patients are known to be at high risk for overdose; however, the relationship between pain and overdose history within this group is understudied, especially in relation to alcohol overdoses. In the present study we evaluated whether non-fatal overdose history was more likely among addiction treatment patients with pain, and examined the characteristics of overdoses among those with and without pain.
Methods:
We analyzed cross-sectional data from 739 patients at a large residential addiction treatment center (Median age = 37, 25.7% female). We used two stepped binary regression models to evaluate whether demographics, pain type (chronic, acute, or both), depression symptoms, and opioid misuse were associated with lifetime history of non-fatal (1) alcohol and (2) drug overdose (lifetime yes/no) and conducted follow-up analyses examining overdose characteristics.
Results:
In adjusted analyses, history of chronic pain (OR = 2.60; 95% CI [1.59, 4.27]) and illicit drug use (OR = 1.99; 95% CI [1.07, 3.68]) were associated with an increased likelihood of non-fatal alcohol overdose. Opioid misuse (OR = 3.11; 95% CI [2.51, 3.86]), depression symptoms (OR = 1.33; 95% CI [1.14, 1.55]), and younger age (OR = 0.96; 95% CI [0.94, 0.97]) were associated with increased likelihood of drug overdose. Those with pain reported a higher number of lifetime alcohol overdoses and were more likely to combine numerous drugs with alcohol prior to overdose.
Conclusions:
Pain conditions may play an under-recognized role in the overdose epidemic, particularly alcohol-related overdose. Addiction treatment and overdose prevention interventions should incorporate appropriate assessment and treatment of pain including education about the risks of polysubstance use, particularly combining alcohol with sedatives and prescription pain relievers.
Keywords: Overdose, Chronic Pain, Acute Pain, Alcohol Use Disorder, Substance Use Disorder
Introduction
Drug and Alcohol Overdose
Drug overdose (OD) is the leading cause of accidental death in the United States (Centers for Disease Control and Prevention, 2016). During 2015, drug OD accounted for 52,404 deaths, of which 33,091 (63.1%) involved an opioid (Rudd et al., 2016). The incidence of fatal alcohol OD, approximately 2,221 annually, is substantially lower than that for drug OD (Kanny et al., 2015). However, alcohol is a major contributor to fatal and non-fatal OD when combined with other addictive drugs, particularly other central nervous system depressants (Witkiewitz and Vowles, 2018). Alcohol is involved in 1 in 4 drug OD-related emergency department visits and overdose deaths (Substance Abuse and Mental Health Services Adminstration, 2013; Jones et al., 2014). Because alcohol is the most commonly used substance in the US (Center for Behavioral Health Statistics and Quality, 2016) with rates of alcohol use, heavy drinking, and alcohol use disorders (AUD) rising sharply since 2001 (Grant et al., 2017), there is a need to more closely study alcohol use in relation to the US’s growing OD problem.
Chronic and Acute Pain
Pain conditions are common among patients with substance use disorder (SUD) and AUD. Recent data from primary care patients indicates that 87% of patients with AUD/SUD reported chronic pain, and 50% reported severe chronic pain (Alford et al., 2016). Individuals with pain report a higher propensity for misusing legal and illegal substances with analgesic effects (Trafton et al., 2004) and more severe addictive drug use, alcohol use, and depressive symptomology (Potter et al., 2008; Jakubczyk et al., 2016a). Given the link between pain and addictive drug use among individuals with SUD/AUD, it may be that those with a history of pain and AUD/SUD are at higher risk for OD; however, very few studies have examined this link, with none focusing on alcohol OD. One study suggested that chronic pain was potentially linked to higher likelihood of non-fatal drug OD through the mediating influence of opioid misuse (Bonar et al., 2014), and another found pain predicted drug OD post-SUD treatment (Britton et al., 2010).
Most research examining the relationship between pain and SUD/AUD focuses on chronic pain. However acute pain, including surgical pain, may contribute to OD risk. Patients with chronic pain report ‘self-medicating’ with alcohol and drugs with analgesic effects, as well as misusing opioid pain relievers (Alford et al., 2016). Acute surgical and non-surgical pain is often managed with prescribed opioid pain relievers, increasing access and exposure. Patients may increase their OD risk when combining prescribed pain relievers with alcohol or other addictive drugs, they may have cross-tolerance to opioid pain relievers due to long term substance or alcohol use, and those in recovery are at risk for relapse if prescribed these medications (Askay et al., 2009). Acute surgical pain is an established gateway for opioid pain reliever misuse, even among opioid naïve patients (Brummett et al., 2017). However, prior work has not examined acute pain and OD risk among those in SUD/AUD treatment despite their potential correlation.
Objectives
Our primary objective was to establish whether acute and/or chronic pain were related to increased likelihood of non-fatal alcohol and drug OD in a SUD/AUD treatment sample. We expect that those with a history of acute and/or chronic pain are more likely to report non-fatal alcohol or drug OD (examined separately). To test this hypothesis, this study evaluated whether alcohol and drug OD were more likely among those with pain (chronic, acute, or both, compared to none). We included psychosocial and demographic factors that could contribute to the hypothesized pain-OD link including history of depression, age, and sex. Given the scarcity of research on this topic, follow-up analyses described OD characteristics (frequency, substances involved) among those with and without pain.
Methods
Study population and recruitment procedures
This study utilized cross-sectional data from patients completing screening measures for a randomized controlled trial of a prescription opioid OD intervention (N = 817). Data were collected from patients attending drug and alcohol residential rehabilitation in Michigan, which serves patients predominantly from the Flint and Detroit, MI, areas. Patients at the residential treatment center were approached between October 2014 and January 2016, asked to provide informed consent, and self-administered questionnaires to identify their eligibility for enrollment into the parent trial. Patients were eligible for screening if they were 18 years of age or older and able to provide informed consent. In total, 739 respondents had complete data on the primary variables included in our analyses. The Michigan Medicine institutional review board approved all study procedures.
Measures
The screening questionnaire consisted of self‐administered, pen-and-paper surveys measuring demographics, pain, mental and physical health functioning, and substance use history. The following measures were included in the present analyses:
Overdose.
The Overdose Experiences, Self and Witnessed - Alcohol (OESWA) was used to assess alcohol OD. This measure was adapted from a previous study (Tracy et al., 2005) and revised to assess alcohol OD. Participants were provided with a description/definition of alcohol OD: “The following questions are about times you drank too much alcohol. This is sometimes called ‘passing out,’ ‘blacking out,’ or ‘alcohol poisoning.’” They then reported the lifetime frequency of this event. Response options ranged from ‘0’ to ‘6 or more.’ Participants reported when their last OD took place, what happened (e.g., “I called 911”), and what other addictive drugs they took. The Alcohol OD outcome variable was dichotomized to represent ‘0’ or ‘1 or more’ lifetime alcohol ODs.
The Overdose Experiences, Self and Witnessed – Drug (OESWD) was used to assess drug OD. This measure was adapted from a previous study (Tracy et al., 2005). Participants were provided with a description/definition of drug OD: “The following questions are about experiences taking too much drugs or medications/pills. This is sometimes called ‘poisoning,’ ‘nodding out,’ or an ‘overdose’ or ‘OD’”. They then reported the lifetime frequency of this event (‘0’ to ‘6 or more’). Additional questions assessed which substances were used prior to the OD, and what events happened after (e.g. “I was admitted to the hospital.”). The drug OD was dichotomized to represent ‘0’ or ‘1 or more’ lifetime drug ODs.
Alcohol and Drug Use.
Lifetime history items from the self-reported Addiction Severity Index (ASI; McLellan et al., 1980) were used to classify participants into the alcohol OD risk group (lifetime history of alcohol use) or drug OD risk group (lifetime history of heroin, methadone, non-prescribed opioids/narcotic analgesics, barbiturates, sedatives, hypnotics, tranquilizers, amphetamines, ecstasy, cannabis, hallucinogens, PCP, ketamine, inhalants, or ‘any other drugs’). Six items from the Current Opioid Misuse Measure (COMM; Butler et al., 2007; Ashrafioun et al., 2015) assessed opioid misuse thoughts and behaviors in the 30 days prior to treatment. Response choices range from 0 (never) to 4 (very often). The 6-item COMM (see Price et al., 2011) demonstrated excellent internal consistency (α = .97). This measure was modeled as an ordinal variable to represent None (score=0), Mild (Score 1–9), Moderate (Score 10–19), and Severe (Score 20–30).
Pain.
Pain was coded into four categories based off of self-reported responses to questions about chronic and acute pain. The category “Chronic pain only” was defined as answering ‘yes’ to “Have you been told by a doctor that you have chronic pain?” or some positive response to the question “In what area of your body have you felt chronic pain in the last 6 months or longer?” This question and response options were based on the Michigan Body Map (Brummett et al., 2016). “Acute pain only” was defined as answering ‘yes’ to “Have you experienced acute pain or surgery in the last 6 months?” The category “both chronic and acute pain” was defined as answering in the affirmative to both pain categories. “No pain” was defined as answering no to all questions regarding pain.
Depression.
The patient health questionnaire-9 (PHQ; Kroenke et al., 2001) was administered to assess past 2-week depressive symptom severity (α = 0.90). This was modeled as an ordinal variable representing increasing severity of depression symptoms: Minimal (Score=0–4), Mild (5–9), Moderate (10–14), Moderately Severe (15–19), and Severe (20–27).
Data Analysis
From the overall sample (n = 739), two sub-samples were constructed: (1) patients at-risk for alcohol OD, as defined by having answered “yes” to lifetime alcohol use, and (2) those at-risk for drug OD, as defined by answering “yes” to any lifetime addictive drug use on the Addiction Severity Index (ASI) or having a history of prescription opioid pain reliever misuse. Our final alcohol-restricted sample included 713 respondents and our final drug-restricted sample included 684 respondents. Descriptive statistics were generated to describe the alcohol- and drug-restricted samples (Table 1). We compared those who had and had not experienced an OD for both types using chi-square tests for categorical variables and Wilcoxon t-tests for continuous variables.
Table 1.
Univariate Relationships among Overdose Categories, Demographics, and Outcome Variables
| Lifetime Alcohol Overdose | Lifetime Drug Overdose | |||||
|---|---|---|---|---|---|---|
| Variable Name |
Yes
N=593 |
No
N=120 |
p- value |
Yes N=365 |
No N=319 |
p-value |
| Sex (Female) | 156 (86.67%) | 24 (13.33%) | 0.149 | 117 (63.93%) | 66 (36.07%) | 0.001 |
| Age (Median and IQR) | 37.00 (28, 47) | 41.50 (31, 51) | 0.023 | 32.00 (26, 43) | 42.00 (33, 50) | <.001 |
| Race | ||||||
| White | 424 (71.50%) | 64 (53.33%) | <0.001 | 289 (79.18%) | 185 (57.99%) | <.001 |
| Black | 113 (19.06%) | 44 (36.67%) | 46 (12.60%) | 103 (32.29%) | ||
| Other | 56 (9.44%) | 12 (10.00%) | 30 (8.22%) | 31 (9.72%) | ||
| Ethnicity | ||||||
| Hispanic | 34 (5.73%) | 7 (5.83%) | 0.966 | 21 (5.75%) | 15 (4.70%) | 0.539 |
| Non-Hispanic | 559 (94.27%) | 113 (94.17%) | 344 (94.25%) | 304 (95.30%) | ||
| Court Mandated Treatment | 563 (83.04%) | 115 (16.96%) | 0.556 | 351 (53.51%) | 305 (46.49%) | 0.589 |
| Lifetime Alcohol Use (Yes) | -- | -- | -- | 361 (54.53%) | 301 (45.47%) | 0.003 |
| Lifetime Illicit Drug Use (Yes) | 554 (85.10%) | 97 (14.90%) | <0.001 | -- | -- | -- |
| Lifetime Tobacco Use (Yes) | 548 (84.44%) | 101 (15.56%) | 0.003 | 352 (55.00%) | 288 (45.00%) | 0.002 |
| Depression | ||||||
| None | 176 (29.68%) | 55 (45.83%) | 0.001 | 72 (19.73%) | 135 (42.32%) | <0.001 |
| Mild | 151 (25.46%) | 35 (29.17%) | 91 (24.93%) | 88 (27.59%) | ||
| Moderate | 128 (21.59%) | 16 (13.33%) | 91 (24.93%) | 54 (16.93%) | ||
| Moderate-Severe | 84 (14.17%) | 6 (5.00%) | 64 (17.53%) | 29 (9.09%) | ||
| Severe | 54 (9.11%) | 8 (6.67%) | 47 (12.88%) | 13 (4.08%) | ||
| Opioid Misuse | ||||||
| None (Score 0) | 248 (41.82%) | 71 (59.17%) | <0.001 | 76 (20.82%) | 206 (64.58%) | <0.001 |
| Low (Score 1–9) | 116 (19.56%) | 27 (22.25%) | 70 (19.18%) | 79 (24.76%) | ||
| Medium (Score 10–19) | 139 (23.44%) | 17 (14.17%) | 126 (34.52%) | 31 (9.72%) | ||
| High (Score 20–30) | 90 (15.18%) | 5 (4.17%) | 93 (25.48%) | 3 (0.94%) | ||
| Pain Category | ||||||
| Both Chronic and Acute Pain | 97 (16.36%) | 20 (16.67%) | <0.001 | 74 (20.27%) | 45 (14.11%) | 0.003 |
| Chronic Pain Only | 317 (53.46%) | 39 (32.50%) | 195 (53.24%) | 154 (48.28%) | ||
| Acute Pain Only | 36 (6.07%) | 5 (4.17%) | 22 (6.03%) | 18 (5.64%) | ||
| No Pain | 143 (24.11%) | 56 (46.67%) | 74 (20.27%) | 102 (31.97%) | ||
Next, we modeled the odds of OD via a stepped model building approach with multivariable logistic regression to examine the impact of adjustment on the observed associations. We used Akaike information criteria (AIC) to examine relative model fit for each step of the logistic regression analysis. A lower AIC is indicative of a better fit. Age and sex (males as reference group) were included in all models. Depression and opioid misuse were each transformed into five and four categories, respectively, and modeled as ordinal variables. This was done to improve fit statistics and aid in interpretation of results. We included lifetime illicit substance use in the alcohol model to account for correlation. When examining the effect of pain, ‘no pain’ was the referent group for all models. Odds ratios and their 95% confidence intervals with two-tailed tests at a significance threshold of 0.05 are reported.
We performed post-hoc analyses examining differences between those who reported pain and no pain among respondents with regard to characteristics of their ODs. We dichotomized this pain variable from the original scale used in the primary analysis due to small cell sizes. We analyzed associations via chi-square tests to assess differences between pain groups and receiving medical attention, defined as responding in the affirmative to questions about calling 911, being taken to the emergency room, or being admitted to the hospital. Average number of substances used during alcohol or drug OD was also compared between pain and no pain groups, analyzed via t-tests. Because of the potential for floor and ceiling effects given the range of allowed responses (0–6) for lifetime number of alcohol and drug ODs, beta regression (Cribari-Neto and Zeileis, 2010) was used to measure the number of ODs by pain group. Beta-regression accounts for heteroskedasticity or skewness frequently observed in health-related outcome data. The outcome variable, OD, was scaled to fit in a range of 0 to 1 and modeled the pain and OD relationship via the beta distribution, allowing for Odds Ratios and their 95% confidence intervals to be calculated. All analyses were carried out in SAS® 9.4 (SAS® Institute, Cary, NC).
Results
Participant Characteristics
The sample (n = 739) was 25.7% female, with a median age of 37 years-old. The study sample was 67.1% (n = 496) White, 23.3% (n = 172) Black, 6.0% (n = 44) Hispanic, and 9.6% (n = 71) represented other races or ethnicities (categories are not mutually exclusive). The majority reported AUD/SUD treatment had been recommended by the criminal justice system (n = 703; 95.1%). Unemployment was high (n = 424; 57.4%), and half the sample 48.9% (n = 361) completed high school/GED, with an additional 35.1% (n = 259) reporting some level of post-graduate education.
Pain was reported by 71.7% (n = 530) of participants; almost half (n = 368; 49.8%) experienced ‘chronic pain only,’ 5.6% (n = 41) endorsed ‘acute pain only,’ and 16.4% (n = 121) endorsed ‘both chronic and acute pain.’ In the alcohol-restricted sample, 83.2% of participants reported one or more alcohol ODs in their lifetime (Table 1). In the drug-restricted sample, 53.4% reported one or more drug OD in their lifetime. There was moderate overlap between these two groups (45.3% of the full sample experienced both an alcohol and drug OD).Table 1 highlights univariate relationships among alcohol and drug OD, sociodemographic factors, and other dependent variables of interest. Alcohol OD was significantly related to age, race, drug use, tobacco use, depression, opioid misuse, and pain. Drug OD was significantly related to sex, age, race, alcohol use, tobacco use, depression, opioid misuse, and pain.
Multivariate Logistic Regression Outcomes:
Alcohol Overdose.
Model 1 included pain, age, and female sex as independent variables. Those who were younger, had ‘chronic pain only,’ or had ‘both chronic and acute pain’ were significantly more likely to have experienced an alcohol OD (see Table 2). In Model 2, depression was added. Those who were younger, had chronic pain only, and had more severe depression were more likely to report an alcohol OD. With the addition of opioid misuse and illicit substance use in Model 3, ‘chronic pain only’ and illicit substance use were significantly associated with alcohol OD. Those who reported ‘chronic pain only’ were 2.6 times more likely to have experienced one or more non-fatal alcohol ODs. Those who had used addictive drugs in their lifetime were almost twice as likely to have experienced an alcohol OD (Figure 1).
Table 2.
Results of Logistic Regression Analyses for Alcohol Overdose
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Independent Variable | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Both Chronic & Acute Pain | 2.16 (1.21, 3.89)* | 1.73 (0.94, 3.19) | 1.30 (0.68, 2.47) |
| Chronic Pain Only | 3.60 (2.26, 5.74)* | 3.05 (1.88, 4.94)* | 2.60 (1.59, 4.27)* |
| Acute Pain Only | 2.65 (0.98, 7.17) | 2.42 (0.89, 6.58) | 2.17 (0.79, 5.95) |
| Age | 0.97 (0.96, 0.99)* | 0.98 (0.96, 0.99)* | 0.99 (0.97, 1.02) |
| Female | 1.23 (0.74, 2.03) | 1.20 (0.72, 1.99) | 1.12 (0.67, 1.87) |
| Depression | 1.25 (1.05, 1.50)* | 1.18 (0.98, 1.42) | |
| Opioid Misuse Measure | 1.26 (1.00, 1.60) | ||
| Illicit Substance Use | 1.99 (1.07, 3.68)* | ||
| AIC: 621.71 | AIC: 617.38 | AIC: 611.76 | |
p < .05; AIC = Akaike information criterion; CI = Confidence Interval
Note. The reference category for pain is the ‘no pain’ group.
Figure 1.
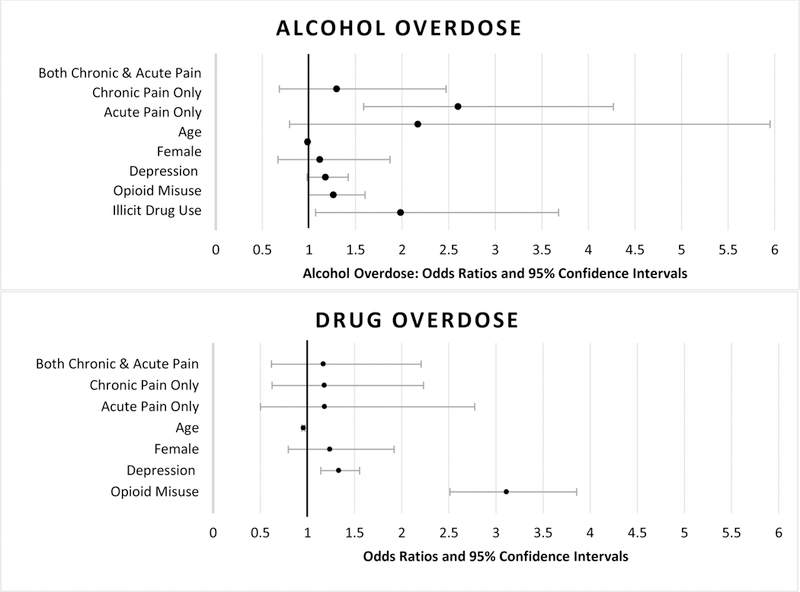
Odds of Alcohol and Drug Overdose in Final Logistic Regression Models
Note. Please see Tables 2 and 3(Model 3 results) for numeric values of Odds Ratios and Confidence Intervals
Drug Overdose.
Model 1 incorporated pain, age and female sex as independent variables (see Table 3). Those who were female, younger, had ‘chronic pain only,’ or had ‘both chronic and acute pain were significantly more likely to have experienced a drug OD. After adding depression in Model 2, results indicated those with more severe depression were also more likely to have experienced a drug OD. With the addition of opioid misuse in Model 3, those who were younger, had more severe depression, and had higher levels of opioid misuse were more likely to have experienced one or more non-fatal drug ODs. In the final model the association between pain and overdose was no longer statistically significant. Every unit increase in depression was associated with a 1.3 times increase in the likelihood of having had a drug OD. Every unit increase in opioid misuse was associated with 3.1 times increase in the likelihood of having had a drug OD. Each one-year increase in participant age was associated with a 0.96 decrease in odds of experiencing a drug OD (Figure 1).
Table 3.
Results of Logistic Regression Analyses for Drug Overdose
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Independent Variable | Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Both Chronic & Acute Pain | 3.53 (2.10, 5.93)* | 2.48 (1.43, 4.29)* | 1.17 (0.62, 2.21) |
| Chronic Pain Only | 2.51 (1.68, 3.74)* | 1.91 (1.25, 2.90)* | 1.18 (0.62, 2.23) |
| Acute Pain Only | 1.66 (0.80, 3.45) | 1.42 (0.67, 3.02) | 1.18 (0.50, 2.78) |
| Age | 0.94 (0.93, 0.96)* | 0.94 (0.93, 0.96)* | 0.96 (0.94, 0.97)* |
| Female | 1.48 (1.02, 2.15)* | 1.49 (1.01, 2.18)* | 1.23 (0.80, 1.92) |
| Depression | 1.54 (1.34, 1.77)* | 1.33 (1.14, 1.55)* | |
| Opioid Misuse Measure | 3.11 (2.51, 3.86)* | ||
| AIC: 871.22 | AIC: 833.16 | AIC: 696.89 | |
p < .05; AIC = Akaike information criterion; CI = Confidence Interval
Note. The reference category for pain is the ‘no pain’ group.
Drug and Alcohol Overdose Characteristics
Overdose characteristics for drugs and alcohol are presented in Table 4. The median number of lifetime alcohol ODs was five. Only 3.7% of participants received medical attention after their most recent alcohol OD. On average, participants reported taking two additional addictive drugs (in addition to alcohol) at their last alcohol OD. Participants most commonly reported concurrently using marijuana (41.0%), prescription sedatives (28.2%), cocaine/crack (26.0%), and prescription opioid pain relievers (25.1%).
Table 4.
Post-Hoc analyses of overdose characteristics for those with and without pain who reported one or more alcohol or drug OD in their lifetime.
| Alcohol OD Characteristics | Total N = 593 |
Pain N = 450 |
No Pain N = 143 |
p- value |
|||
|---|---|---|---|---|---|---|---|
| Received medical attention1 at last alcohol OD | 22 | (3.71%) | 20 | (4.44%) | 2 | (1.40%) | 0.241 |
| Mean number of substances used in addition to alcohol at last alcohol OD | 1.86 | ( 2.07) | 2.00 | (2.16) | 1.41 | (1.68) | 0.005 |
| Substances used at last Alcohol OD | |||||||
| Only Alcohol | 125 | (21.08%) | 91 | (20.22%) | 34 | (23.78%) | -- |
| Marijuana | 243 | (40.98%) | 187 | (41.56%) | 56 | (39.16%) | -- |
| Prescription Sedatives | 167 | (28.16%) | 135 | (30.00%) | 32 | (22.38%) | -- |
| Cocaine or Crack | 154 | (25.97%) | 124 | (27.56%) | 30 | (20.98%) | -- |
| Prescription Pain Relievers | 149 | (25.13%) | 130 | (28.89%) | 19 | (13.29%) | -- |
| Street Opioids | 113 | (19.06%) | 87 | (19.33%) | 26 | (18.18%) | -- |
| Energy Drinks | 103 | (17.37%) | 86 | (19.11%) | 17 | (11.89%) | -- |
| Prescription Stimulants | 59 | (9.95%) | 53 | (11.78%) | 6 | (4.20%) | -- |
| Methamphetamines | 45 | (7.59%) | 36 | (8.00%) | 9 | (6.29%) | -- |
| Other3 | 53 | (8.94%) | 48 | (10.67%) | 5 | (3.50%) | -- |
| Drug OD Characteristics |
Total N = 365 |
Pain N = 291 |
No Pain N = 74 |
p- value |
|||
| Received medical attention1 at last Drug OD | 76 | (20.82%) | 64 | (84.21%) | 12 | (15.79%) | 0.379 |
| Mean number of substances at last Drug OD | 2.23 | (1.73) | 2.31 | (1.81) | 1.91 | (1.33) | 0.174 |
| Substances used at last Drug OD | |||||||
| Street Opioids | 193 | (52.88%) | 143 | (49.14%) | 50 | (67.57%) | -- |
| Prescription Sedatives | 127 | (34.79%) | 104 | (35.74%) | 23 | (31.08%) | -- |
| Alcohol | 109 | (29.86%) | 94 | (32.30%) | 15 | (20.27%) | -- |
| Prescription Pain Relievers | 106 | (29.04%) | 91 | (31.27%) | 15 | (20.27%) | -- |
| Marijuana | 83 | (22.74%) | 69 | (23.71%) | 14 | (18.92%) | -- |
| Cocaine or Crack | 86 | (23.56%) | 75 | (25.77%) | 11 | (14.86%) | -- |
| Energy Drinks | 28 | (7.67%) | 24 | (8.25%) | 4 | (5.41%) | -- |
| Prescription Stimulants | 25 | (6.85%) | 21 | (7.22%) | 4 | (5.41%) | -- |
| Methamphetamines | 21 | (5.75%) | 19 | (6.53%) | 2 | (2.70%) | -- |
| Other2 | 30 | (8.22%) | 27 | (9.28%) | 3 | (4.05%) | -- |
Note. All percentages represent fraction of the total n within the column. Each column adds up to more than 100% because categories are not mutually exclusive. All results take into account missing data for each measure Grey area not included in analysis, presented for descriptive purposes only. OD = Overdose
: Defined as having called 911 (either the patient or someone else), being taken to the ER or being admitted to the hospital.
:Other includes Hallucinogens, over the counter medications, inhalants, as well as write-in ‘other’ category.
The median number of lifetime drug ODs was one and 20.8% of participants received medical attention for their most recent drug OD. On average, participants used 2.2 addictive drug classes in combination prior to their most recent drug OD, with the most common substances taken including street opioids (e.g. heroin; 52.9%), prescription sedatives (34.8%), alcohol (29.9%), and prescription pain relievers (29.0%).
Beta regression analysis indicated those with any type of pain had a greater number of alcohol ODs in their lifetime OR = 2.1 (95% CI 1.6 – 2.8), p < .05. There was a significant difference between pain groups and the number of additional substances used at the last alcohol OD; those with any type of pain used an average of 2.0 additional substances while those in the no pain group used an average of 1.4 additional substances (p < 0.05). Pain was not significantly associated with medical attention following alcohol OD. Analyses examining the association between pain and drug OD characteristics were not statistically significant.
Discussion
Overall, these findings indicate that chronic and acute pain are pervasive among this residential treatment sample. Nearly three out of four patients reported chronic or acute pain in the past 6 months or longer. Likewise, alcohol OD, which has received relatively scant attention in the literature compared to drug OD, was very common in our sample. Four out of five participants reported an alcohol OD in their lifetime, and the median number of lifetime alcohol ODs was 5.1 The likelihood of alcohol OD was 2.6 times higher among those with chronic pain. To our knowledge, this is the first study to evaluate the lifetime prevalence of alcohol OD and its correlates in an addiction treatment sample. These findings add to a growing body of literature linking chronic pain to alcohol use and consequences (Rosenblum et al., 2003; Brennan et al., 2011; Brennan and Soohoo, 2013; Macfarlane and Beasley, 2015; Alford et al., 2016; Jakubczyk et al., 2016b) and adds to this literature by highlighting the positive association between non-fatal alcohol OD among those with pain.
Alcohol and concurrent drug use were also common contributors to OD, particularly among those with pain. In fact, the vast majority of ‘alcohol ODs’ involved polysubstance use, primarily alcohol combined with marijuana, prescription sedatives, cocaine/crack, and prescription pain relievers (in order of prevalence). Combining alcohol with other depressants such as benzodiazepines and opioid pain relievers increases the likelihood of fatal and non-fatal OD (Witkiewitz and Vowles, 2018). In fact, alcohol involvement greatly increases the likelihood that benzodiazepines and low-potency opioid pain relievers (e.g. hydrocodone) use will lead to Emergency Department admissions and/or death (Jones et al., 2014). While not as lethal, 41% of alcohol ODs involved marijuana. Future research on combination marijuana-alcohol OD (i.e. ‘greening out’) is recommended particularly given the increasing use of marijuana and marijuana-related emergencies in the US (Center for Behavioral Health Statistics and Quality, 2016) .
The likelihood of drug OD was three times more likely with each unit increase in severity of opioid misuse, and one time more likely with each unit increase in depression severity. The odds of drug OD decreased slightly with each year of age. There was no clear link between pain and drug OD likelihood, frequency, or severity. Consistent with prior research (Bonar et al., 2014), the association between pain and drug OD was likely mitigated by opioid misuse in our study.
Drug OD was a lower frequency event relative to alcohol OD; however, medical attention was more likely to follow drug OD. Similar to alcohol OD, most drug ODs included more than one addictive drug class, with the most common being street opioids, followed by prescription sedatives, alcohol, and prescription pain relievers. Central nervous system depressants were the most commonly used and combined addictive drug classes, underscoring the role of high-risk polysubstance use that led to OD in this sample.
Limitations
This research has several limitations. We used a single-site, cross-sectional sample for our analysis. Importantly, timing of overdose relative to the onset and occurrence of pain was not evaluated in our assessment, and thus we do not know if existing pain leads to OD or whether complications from OD leads to new pain problems. In addition, concurrent validity of our overdose assessment could not be established, because there is no ‘gold-standard’ to compare it to. Future longitudinal research is needed to disentangle the relationship between pain and OD likelihood or severity. Another limitation of our assessment is recall bias. Memory of OD and other health problems decay over time and by definition, portions of ODs involving blackouts cannot be recalled. Regarding pain assessment, we used pain categories in our analyses rather than continuous pain severity scores. The number of participants with only acute pain was small, thus limiting our analysis for this category. Alcohol and other drug use commonly co-occurred prior to OD; thus clearly differentiating a true ‘alcohol OD’ from a purely ‘drug OD’ at the event level was not possible and we relied on participants’ categorization of these events. We used medical attention as a proxy for OD severity in our analysis; however, this categorization may not be a clear indicator of severity because it is related to secondary factors such as availability and willingness of others to call for medical help (Tracy et al., 2005).
Conclusions
Chronic pain is associated with poorer substance-related outcomes in individuals treated for SUDs (Caldeiro et al., 2008). Therefore, understanding and treating pain is an important treatment goal for individuals with co-occurring SUD (Caldeiro et al., 2008; Samet and Walley, 2008; Ilgen et al., 2016). In fact, decreases in physical pain following alcohol treatment have been linked to a reduced risk of relapse (Jakubczyk et al., 2016b), and combined pain and addiction treatment can reduce both alcohol use and pain, and improve pain-related functioning (Ilgen et al., 2016). Psycho-social, behavioral, and other non-pharmacological treatments that can address pain and SUD concurrently are recommended. Non-opioid medication treatments are also worth exploring with patients. Our findings highlight that a lifetime history of chronic pain is associated with a lifetime history of one or more alcohol OD (often alcohol combined with other drugs). Given that those with past OD are at higher risk for future OD (Caudarella et al., 2016), treating pain may also prevent future ODs. While this study was likely under-powered to detect theorized associations between acute pain and OD, future research should examine this relationship further given the risk of opioid misuse in those with acute surgical pain (Brummett et al., 2017), potential for cross-tolerance to opioids among those with active substance use disorders, and potential for relapse when individuals in addiction recovery use prescribed narcotics (Askay et al., 2009)
Alcohol OD is not typically discussed in the OD literature and it is less emphasized in the pain literature relative to other addictive drugs (e.g., opioids, marijuana). However, this research indicates that many SUD/AUD patients frequently experience events perceived as severe enough to be viewed as an OD when drinking alcohol. In addition, many of the events classified as an alcohol OD included concurrent drug use. Patients may need education regarding the role of combining substances and how it can increase the risk of OD, particularly when drinking alcohol. This type of information could be delivered through an evidenced-based psychosocial intervention to reduce OD risk behaviors (Bohnert et al., 2016).
Acknowledgments
The authors wish to thank the staff and clients at the addiction treatment facility that hosted our research project. We also wish to thank Laura Thomas and Emily Yeagley for their oversight of the data collection.
Funding: This project was supported by National Institute on Drug Abuse: R34 DA035331. Dr. Fernandez was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism: K23 AA023869.
Footnotes
This may be an underestimate. The overdose frequency question’s response options ranged from ‘0’ to ‘6 or more.‘ 48.83% of the alcohol-restricted sample reported ‘6 or more overdoses.’ This ceiling effect was taken into account in analytic strategy.
Financial Disclosures: The authors report no relevant financial conflicts.
References
- Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, and Saitz R. Primary care patient with drug use report chronic pain and self-medicate with alcohol and other drugs. J Gen Intern Med 2016;31:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafioun L, Bohnert ASB, Jannausch M, and Ilgen MA. Evaluation of the current opioid misuse measure among substance use disorder treatment patients. J Subst Abuse Treat 2015;55:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askay SW, Bombardier CH, and Patterson DR. Effect of acute and chronic alcohol abuse on pain management in a trauma center. Expert Rev Neurother 2009;9:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert ASB, Bonar EE, Cunningham R, et al. A pilot randomized clinical trial of an intervention to reduce overdose risk behaviors among emergency department patients at risk for prescription opioid overdose. Drug Alcohol Depend 2016;163:40–7. [DOI] [PubMed] [Google Scholar]
- Bonar EE, Ilgen MA, Walton M, and Bohnert ASB. Associations among pain, non-medical prescription opioid use, and drug overdose history. Am J Addict 2014;23:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL, Schutte KK, SooHoo S, and Moos RH. Painful medical conditions and alcohol use: a prospective study among older adults. Pain Med 2011;12:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PL and Soohoo S. Pain and use of alcohol in later life: prospective evidence from the health and retirement study. J Aging Health 2013;25:656–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton PC, Wines JD, and Conner KR. Non-fatal overdose in the 12 months following treatment for substance use disorders. Drug Alcohol Depend 2010;107:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett CM, Bakshi RR, Goesling J, et al. Preliminary validation of the Michigan Body Map. Pain 2016;157:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain 2007;130:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeiro RM, Malte CA, Calsyn DA, et al. The association of persistent pain with out-patient addiction treatment outcomes and service utilization. Addiction 2008;103:1996–2005. [DOI] [PubMed] [Google Scholar]
- Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, and Hayashi K. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug Alcohol Depend 2016;162:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2016. [Google Scholar]
- Centers for Disease Control and Prevention Underlying cause of death 1999–2015 [CDC web site]. 2016. Available at: https://wonder.cdc.gov/ucd-icd10.html. Accessed May 30, 2017.
- Cribari-Neto F and Zeileis A. Beta Regression in R. J Stat Softw 2010;34:1–24. [Google Scholar]
- Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-Month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013. JAMA Psychiatry 2017;48:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgen MA, Bohnert ASB, Chermack S, et al. A randomized trial of a pain management intervention for adults receiving substance use disorder treatment. Addiction 2016;111: 1385–1393. [DOI] [PubMed] [Google Scholar]
- Jakubczyk A, Ilgen MA, Bohnert ASB, et al. Physical pain in alcohol-dependent patients entering treatment in Poland—prevalence and correlates. J Stud Alcohol Drugs 2016a;76:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubczyk A, Ilgen MA, Kopera M, et al. Reductions in physical pain predict lower risk of relapse following alcohol treatment. Drug Alcohol Depend 2016b;158:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, and Mack KA. Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep 2014;63:881–5. [PMC free article] [PubMed] [Google Scholar]
- Kanny D, Brewer RD, Mesnick JB, Paulozzi LJ, Naimi TS, and Lu H. Vital signs: alcohol poisoning deaths — United States, 2010–2012. Morb Mortal Wkly Rep 2015;65:1238–1242. [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, and Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GJ and Beasley M. Alcohol consumption in relation to risk and severity of chronic widespread pain: results from a UK population-based study. Arthritis Care Res (Hoboken) 2015;67:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, and O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis 1980;168:26–33. [DOI] [PubMed] [Google Scholar]
- Potter JS, Prather K, and Weiss RD. Physical pain and associated clinical characteristics in treatment-seeking patients in four substance use disorder treatment modalities. Am J Addict 2008;17:121–125. [DOI] [PubMed] [Google Scholar]
- Rosenblum A, Joseph H, Fong C, Kipnis S, Cleland C, and Portenoy RK. Prevalence and characteristics of chronic pain among chemically dependent patients in methadone maintenance and residential treatment facilities. JAMA 2003;289:2370–8. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibell JE, and Gladden RM. Increases in drug and opioid overdose deaths-United States, 2000–2015. Morb Mortalitiy Wkly Rep 2016;65:1445–1452. [DOI] [PubMed] [Google Scholar]
- Samet JH and Walley AY. Can one be an expert in addiction medicine without expertise in pain management? Addiction 2008;103:2006–2007. [DOI] [PubMed] [Google Scholar]
- Tracy M, Piper TM, Ompad D, et al. Circumstances of witnessed drug overdose in New York City: implications for intervention. Drug Alcohol Depend 2005;79:181–190. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Oliva EM, Horst DA, Minkel JD, and Humphreys K. Treatment needs associated with pain in substance use disorder patients: Implications for concurrent treatment. Drug Alcohol Depend 2004;73:23–31. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Drug abuse warning network, 2011: National estimates of drug-related emergency department visits. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. [Google Scholar]
- Witkiewitz K and Vowles KE. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: A critical review. Alcohol Clin Exp Res 2018;42:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]


