Abstract
Riemerella anatipestifer ATCC11845 (RA ATCC11845) is naturally competent. However, the genes involved in natural transformation in this species remain largely unknown. Bioinformatic analysis predicts that DprA of RA (DprARa) has three domains: a sterile alpha motif (SAM), a Rossmann fold (RF) domain and a Z-DNA-binding domain (Zα). Inactivation of dprA abrogated natural transformation in RA ATCC11845, and this effect was restored by the expression of dprA in trans. The dprA with SAM and RF domains of Streptococcus pneumoniae and the dprA with RF and Zα domains of Helicobacter pylori was able to restore natural transformation in the RA ATCC11845 dprA mutant. An Arg123 mutation in the RF domain of R. anatipestifer was not able to restore natural transformation of the RA ATCC11845 dprA mutant. Furthermore, DprAR123E abolished its ability to bind DNA, suggesting that the RF domain is essential for the function of DprA. Finally, the dprA of Fusobacterium naviforme which has not been reported to be natural competent currently was partially able to restore natural transformation in RA ATCC11845 dprA mutant. These results collectively suggest that DprA has a conserved evolutionary mechanism.
Keywords: Riemerella anatipestifer, dprA, natural competence, EMSA, evolution
Introduction
Natural transformation involves the acquisition of naked DNA by a bacterium from the extracellular environment and integrated DNA into their genome, and genetic competence is the ability to undergo natural transformation (Chen and Dubnau, 2004). At present, at least 82 bacterial species have been shown to be naturally transformable (Johnston et al., 2014; Mell and Redfield, 2014). The process of natural transformation involves two steps: DNA uptake and DNA processing (Johnston et al., 2014). Gram-positive and Gram-negative bacteria rely on highly similar DNA-uptake systems (Johnston et al., 2014). Transformable Gram-negative species use proteins that are related to those involved in the assembly of type II secretion systems and type IV pili (T4P) (Hobbs and Mattick, 1993; Dubnau, 1999). It has been proposed that structure similar to T4P known as a competence pseudopilus participates in DNA transport in Gram-positive bacteria (Dubnau, 1999). Once dsDNA has entered the periplasm, one strand of the dsDNA is degraded, and the other strand is internalized via a ComEC transmembrane channel (Draskovic and Dubnau, 2005). After internalization, the transforming ssDNA is bound to the transformation-dedicated recombination mediator protein (RMP) DprA, which loads the homologous recombinase RecA to promote homologous recombination (Mortier-Barriere et al., 2007; Yadav et al., 2013).
Riemerella anatipestifer (R. anatipestifer, RA) is a Gram-negative bacterium that causes septicemic diseases in ducks, geese, turkeys, and other birds (Huang et al., 2017). At present, at least 21 serotypes of R. anatipestifer have been identified (reference). The extensive use of antibiotics for the treatment and prevention of serositis and septicemia has resulted in multidrug resistance in R. anatipestifer (Zhong et al., 2009; Luo et al., 2015, 2018; Huang et al., 2017; Zhang et al., 2017; Zhu et al., 2018), and at least 33 relevant bacterial genomes with genome sizes ranging from 2.09 to 2.43 Mb have been sequenced from different isolates (Wang X. et al., 2014; Song et al., 2016; Zhu et al., 2016). Sequence analysis of RA ATCC11845, RA CH-1, and RA CH-2 showed that RA CH-1 is 140,000 bp larger than the two other strains and there are so many resistance genes in this region (Wang X. et al., 2014). However, the reason for this genome diversity and the mechanisms underlying multidrug resistance remain largely unknown. In our previous study, we showed that R. anatipestifer is naturally competent (Liu et al., 2017). At present, the machinery involved in the taking up of exogenous dsDNA involves T4P or T4SS (Johnston et al., 2014). However, no homologs of T4P and T4SS were found in the genome of R. anatipestifer through sequence comparison. Several genes which was annotated as recA, dprA, comM, and comEC, respectively, in the genome of R. anatipestifer are predicted to be involved in transporting DNA across inner membrane and homologous recombination (Liu et al., 2017). Bioinformatic analysis showed that the gene RA0C_1073 of RA ATCC11845 encodes a putative DprA with low identity to the DprA found in other bacteria. In addition, RA ATCC11845 is the first bacteria in Flavobacteriaceae that has been reported to have natural competence. In this study, we sought to provide a functional characterization of DprA and its mechanism in the natural transformation of RA ATCC11845. This information will be helpful for determining the mechanism of natural transformation in bacteria occurring in R. anatipestifer and other members of Flavobacteriaceae.
Materials and Methods
Bacterial Strains, Plasmids and Primers
The bacterial strains and plasmids used in this study are shown in Table 1. The primers are shown in Table 2.
Table 1.
Strains and plasmids used in this study.
| Riemerella anatipestifer strains | Genotype or description | Source or references |
|---|---|---|
| RA ATCC11845 | RA ATCC11845, KmR | Laboratory collection |
| RA ATCC11845ΔdprA::Erm | RA ATCC11845ΔdprA, ErmR | This study |
| RA ATCC11845 (pLMF03::dprA) | RA ATCC11845 carrying pLMF03::dprA, AmpR CfxR | This study |
| RA ATCC11845 (pLMF03::dprAR123E) | RA ATCC11845 carrying pLMF03::dprAR123E, AmpR CfxR | This study |
| RA ATCC11845 (pLMF03::dprA-SAM-RF) | RA ATCC11845 carrying pLMF03::dprA-SAM-RF, AmpR CfxR | This study |
| RA ATCC11845 (pLMF03::dprA-RF-Zα) | RA ATCC11845 carrying pLMF03::dprA-RF-Zα, AmpR CfxR | This study |
| RA ATCC11845 (pLMF03::Sp-dprA) | RA ATCC11845 carrying pLMF03::Sp-dprA, AmpR CfxR | This study |
| RA ATCC11845 (pLMF03::Hp-dprA) | RA ATCC11845 carrying pLMF03::Hp-dprA, AmpR CfxR | This study |
| RA ATCC11845 (pLMF03::Fn-dprA) | RA ATCC11845 carrying pLMF03::Fn-dprA, AmpR CfxR | This study |
| RA ATCC11845ΔdprA::Erm (pLMF03::dprA) | RA ATCC11845ΔdprA carrying pLMF03::dprA, ErmR AmpR CfxR | This study |
| RA ATCC11845ΔdprA::Erm (pLMF03::dprAR123E) | RA ATCC11845ΔdprA carrying pLMF03::dprAR123E, ErmR AmpR CfxR | This study |
| RA ATCC11845ΔdprA::Erm (pLMF03::dprA-SAM-RF) | RA ATCC11845ΔdprA carrying pLMF03::dprA-SAM-RF, ErmR AmpR CfxR | This study |
| RA ATCC11845ΔdprA::Erm (pLMF03::dprA-RF-Zα) | RA ATCC11845ΔdprA carrying pLMF03::dprA-RF-Zα, ErmR AmpR CfxR | This study |
| RA ATCC11845ΔdprA::Erm (pLMF03::Sp-dprA) | RA ATCC11845ΔdprA carrying pLMF03::Sp-dprA, ErmR AmpR CfxR | This study |
| RA ATCC11845ΔdprA::Erm (pLMF03::Hp-dprA) | RA ATCC11845ΔdprA carrying pLMF03::Hp-dprA, ErmR AmpR CfxR | This study |
| RA ATCC11845ΔdprA::Erm (pLMF03::Fn-dprA) | RA ATCC11845ΔdprA carrying pLMF03::Fn-dprA, ErmR AmpR CfxR | This study |
| Escherichia coli strains | Genotype or description | Source or references |
| DH5α | E. coli DH5α, cloning host cell | Laboratory collection |
| XL1-blue | E. coli XL1-blue, cloning host cell | Laboratory collection |
| Rosetta | E. coli Rosetta, expressing host cell | Laboratory collection |
| Rosetta (pET30a) | E. coli Rosetta carrying pET30a, KmR | This study |
| Rosetta (pET30a::dprA-s) | E. coli Rosetta carrying pET30a::dprA, KmR | This study |
| Rosetta (pET30a::dprAR123E-s) | E. coli Rosetta carrying pET30a::dprAR123E-s, KmR | This study |
| S17-1 | Thi-1 thr leu tonA lac Y supE recA::RP4-2-Tc::Mu KmR | Miller and Mekalanos, 1988 |
| S17-1 (pLMF03::dprA) | S17-1 carrying pLMF03::dprA, AmpR CfxR | This study |
| S17-1 (pLMF03::dprAR123E) | S17-1 carrying pLMF03::dprAR123E, AmpR CfxR | This study |
| S17-1 (pLMF03::dprA-SAM-RF) | S17-1 carrying pLMF03::dprA-SAM-RF, AmpR CfxR | This study |
| S17-1 (pLMF03::dprA-RF-Zα) | S17-1 carrying pLMF03::dprA-RF-Zα, AmpR CfxR | This study |
| S17-1 (pLMF03::Sp-dprA) | S17-1 carrying pLMF03::Sp-dprA, AmpR CfxR | This study |
| S17-1 (pLMF03::Hp-dprA) | S17-1 carrying pLMF03::Hp-dprA, AmpR CfxR | This study |
| S17-1 (pLMF03::Fn-dprA) | S17-1 carrying pLMF03::Fn-dprA, AmpR CfxR | This study |
| Plasmids | Genotype or description | Source or references |
| pET30a | pBR322 lacZ, IPTG-inducible promoter, KmR | Laboratory collection |
| pET30a::dprA-s | pET30a carrying dprA adding his tag from RA ATCC11845, KmR | This study |
| pET30a::dprAR123E-s | pET30a carrying Arg123 site-directed mutant dprA adding his tag of RA ATCC11845, KmR | This study |
| pLMF03 | B739_0921 promoter, oriColE1, ori pRA0726, AmpR CfxR | Liu et al., 2016 |
| pLMF03::dprA | pLMF03 carrying dprA from RA ATCC11845, AmpR CfxR | This study |
| pLMF03::dprAR123E | pLMF03 carrying Arg123 site-directed mutant dprA of RA ATCC11845, AmpR CfxR | This study |
| pLMF03::Sp-dprA | pLMF03 carrying dprA from S. pneumoniae, AmpR CfxR | This study |
| pLMF03::Hp-dprA | pLMF03 carrying dprA from H. pylori, AmpR CfxR | This study |
| pLMF03::Fn-dprA | pLMF03 carrying dprA from F. naviforme, AmpR CfxR | This study |
| pLMF03::dprA-SAM-RF | pLMF03 carrying SAM and RF domains of dprA from RA ATCC11845, AmpR CfxR | This study |
| pLMF03::dprA-RF-Zα | pLMF03 carrying RF and Zα domains of dprA from RA ATCC11845, AmpR CfxR | This study |
AmpR, ampicillin resistance; KmR, kanamycin resistance; CfxR, cefoxitin resistance.
Table 2.
Primers used in this study.
| Primers | Sequence | Organism |
|---|---|---|
| 16SrRNAP1 | CGAAAGTGATAAGTTAGCCACCT | RA ATCC11845 |
| 16SrRNAP2 | GCAGCACCTTGAAAATTGTCC | RA ATCC11845 |
| dprA upP1 | ACAAGGGGTGGCTATGGCGGCAAGTC | RA ATCC11845 |
| dprA upP2 | TAAGACTGGAAAGTGGTAACTAGCGCCTTGCCAT | RA ATCC11845 |
| ErmP1 | GCAAGGCGCTAGTTACCACTTTCCAGTCTTACG | RA ATCC11845 |
| ErmP2 | GTAATTTTTCAACGACTTTGAACTACGAAGGATGAAATTTTT | RA ATCC11845 |
| dprA downP1 | TCCTTCGTAGTTCAAAGTCGTTGAAAAATTACTTTTTTAAAA | RA ATCC11845 |
| dprA downP2 | TGCTTGGCAGAATCTCATAATTTCCATATCCGA | RA ATCC11845 |
| rpsLP1 | ATGCCTACTATTCAACAATTAG | RA ATCC11845 |
| rpsLP2 | TTACTTTTTAGCATCTTTAGGACGC | RA ATCC11845 |
| dprA CompP1 | CATGCCATGGCAATGGTAAATGCGGAAGAAATT | RA ATCC11845 |
| dprA CompP2 | CCGCTCGAGCTAAATGATAGAATATCTCCTCCCA | RA ATCC11845 |
| dprA-RFP1 | CATGCCATGGCAATGATTAAAAACGAAATAAAAAT | RA ATCC11845 |
| dprA-RFP2 | CCGCTCGAGCTAAAAAAGCTCCAAAACTTTAGA | RA ATCC11845 |
| Sp-dprAP1 | CATGCCATGGCAATGGAGTTATTTATGAAAATCACAA | S. pneumoniae D39 |
| Sp-dprAP2 | CCGCTCGAGTTAAAATTCAAATTCCGCAAG | S. pneumoniae D39 |
| Hp-dprAP1 | CATGCCATGGCAGTGAATCAACGAATGAAAAGCC | H. pylori 26695 |
| Hp-dprAP2 | CCGCTCGAGTCACGCTAACACCACAATGTGA | H. pylori 26695 |
| Fn-dprAP1 | CATGCCATGGCAATGGAGCTGACGAATCCACTTGG | synthesized |
| Fn-dprAP2 | GCTCTAGAGCTTAAGGATGAAAGCGGGCACAG | synthesized |
| dprA∗P1 | AGTATTGTTGGGACGGAAAATGCCACTGCTTAT | RA ATCC11845 |
| dprA∗P2 | AGCAGTGGCATTTTCCGTCCCAACAATACTAAT | RA ATCC11845 |
| dprAExP1 | GGGAATTCCATATGGTAAATGCGGAAGAAATT | RA ATCC11845 |
| dprAExP2his | CCGCTCGAGCTAGTGGTGGTGGTGGTGGTGAATGATAGAATATCTCCTCCCA | RA ATCC11845 |
| RA-ssDNA1 | TAGGCTCTGCTAAGGAAGCGTGGGGTCTGTCTAAGTTGGA | RA ATCC11845 |
| RA-ssDNA2 | AAAAACTACGGAACTGACTAAAGGCAGAAAAACTAAACGG | RA ATCC11845 |
| EC-ssDNA | CTCAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATAC | E. coli XL1-blue |
| dprA qRTP1 | TCCGATGTTTGAGGCAATTTG | RA ATCC11845 |
| dprA qRTP2 | TGCAAGTTTGGTTAGCGAGGTAG | RA ATCC11845 |
| recA qRTP1 | CTTAGGATAACCGCCTACTC | RA ATCC11845 |
| recA qRTP2 | CTTAGGATAACCGCCTACTC | RA ATCC11845 |
Media and Growth Conditions
Riemerella anatipestifer was routinely cultured in GC broth (GCB) with agitation (Liu et al., 2017) or on LB plates supplemented with 5% sheep blood at 37°C (Liu et al., 2018a). GCB agar plates were prepared by using GCB supplemented with 1.5% agar. Escherichia coli strains were grown on LB agar at 37°C. When required, antibiotics were added at the following final concentrations (μg/ml): erythromycin (Erm), 1; cefoxitin (Cfx), 1; and streptomycin (Str), 100 for R. anatipestifer and ampicillin (Amp), 100 for E. coli.
Natural Transformation
Natural transformation was performed as described previously with slight modification (Liu et al., 2017). Briefly, the single colony was isolated on LB agar supplemented with 5% sheep blood at 37°C for 24 h. The eugonic cells were collected from the plate into GCB medium (Liu et al., 2017) and checked for OD600. Then, the bacteria were adjusted to an OD600 of 1. The bacterial suspensions (0.3 ml) were transferred to sterilized tubes, and 1 μg of plasmid DNA or genomic DNA was added to the tube. After an additional incubation for 1 h at 37°C, the bacterial cultures were serially diluted and plated onto GCB agar plates or LB agar supplemented with 5% sheep blood with or without antibiotics. Transformation frequencies were calculated as the CFU ml−1 on selective plates divided by the CFU ml−1 on non-selective plates (Kristensen et al., 2012).
Construction of the RA ATCC11845 dprA Mutant
The RA ATCC11845 dprA mutant was constructed using the natural transformation method as described previously (Liu et al., 2017). Briefly, the upstream sequence (∼620 bp) and the downstream sequence (∼620 bp) of the dprA gene were amplified using the primers dprA upP1 and dprA upP2 or dprA downP1 and dprA downP2, respectively (Table 2). The 994-bp ErmR cassette was amplified from the RA-CH-1 strain (Luo et al., 2015) (Liao plos one) using the primers ErmP1 and ErmP2 (Table 2). The resulting PCR fragments were ligated using the overlapped PCR method (Xiong et al., 2006). The fused PCR fragments were purified by TianGEN (TIANGEN, Beijing, China) and introduced into the wild-type strain by natural transformation. The transformants were selected on blood agar plates supplemented with Erm (1 μg/ml). The gene-deletion mutant strain was identified by PCR by amplifying the conserved 16S rRNA gene of R. anatipestifer using the primers 16S rRNAP1 and 16S rRNAP2 to verify whether the mutant is R. anatipestifer, and the dprA gene was amplified using the primers dprA Comp P1 and dprA Comp P2.
Preparation of Transformation DNA (tDNA)
Streptomycin-resistant RA ATCC11845 cells were obtained by plating 108 wild-type cells on LB agar supplemented with 5% sheep blood containing 100 μg/ml streptomycin. Streptomycin-resistant clones were streaked for isolation on fresh medium, and the rpsL of each clone was PCR-amplified using the primers rpslP1 and rpslP2 (Table 2) and then sent for sequencing. Point mutations in rpsL conferring streptomycin resistance were identified by comparison to the wild type rpsL. Genomic DNA containing the rpsL mutant was extracted using the TIANamp Bacterial DNA Kit (TIANGEN, Beijing, China) and was used as the transformed DNA (tDNA). The concentration of the genomic DNA was measured by a Nanodrop2000.
Construction of the Recombinant Vectors for Complementation
The insert fragment was cloned into the shuttle plasmid pLMF03, which contain high expression promoter, for complementation as described in previous study (Liu et al., 2016). Briefly, the entire coding region of dprA was amplified from RA ATCC11845 chromosomal DNA using the primers dprA Comp P1 and dprA Comp P2 (Table 2). The domain of dprA-SAM-RF and dprA-RF-Zα for dprA was amplified from RA ATCC11845 chromosomal DNA using the primers dprA Comp P1 and dprA-RF P2, dprA-RF P1 and dprA Comp P2, respectively (Table 2). The entire coding region of Sp-dprA and Hp-dprA was amplified from the S. pneumoniae D39 strain and the Helicobacter pylori 26695 strain using the primers Sp-dprAP1 and Sp-dprAP2, Hp-dprAP1 and Hp-dprAP2, respectively (Table 2). The entire coding region of Fn-dprA of Fusobacterium naviforme was synthesized according to the sequence (NCBI: EI53_RS03865) and amplified using the primers Fn-dprAP1 and Fn-dprAP2 (Table 2). The PCR products were purified, digested by the corresponding restriction endonuclease, and ligated into the plasmid pLMF03 to generate the plasmid pLMF03::dprA, pLMF03::dprA-SAM-RF, pLMF03::dprA-RF-Zα, pLMF03::Sp-dprA, pLMF03::Hp-dprA, and pLMF03::Fn-dprA.
Construction of a Site-Directed Mutant Recombination Vector
Site-directed mutagenesis was carried out by overlap PCR to substitute the arginine residue (R) at position 123 of the R. anatipestifer dprA with glutamic acid (E). The desired mutant DNA fragment was obtained by two rounds of PCR. Briefly, the first 389 bp of the RA ATCC11845 dprA gene was amplified from genomic DNA using the primer dprA Comp P1, which contains a Nco I restriction enzyme site, and the primer dprA∗ P2, which contains mutant nucleotides (TTC). The second 758-bp fragment of the dprA gene was amplified from genomic DNA using the primer dprA∗ P1, which contains mutant nucleotides (GAA), and the primer dprA Comp P2, which contains an XhoI restriction enzyme site. The two fragments were then ligated by overlap PCR (Xiong et al., 2006). The resulting amplicon was digested by Nco I/XhoI and cloned into pLMF03 (also digested with NcoI and XhoI) to generate the pLMF03::dprAR123E plasmid. The ligation products were introduced into E. coli strain DH5α cells using the calcium chloride method, and transformants were selected on LB plates containing Amp (100 μg/ml final concentration). The presence of the correct inserts was confirmed by PCR and sequencing (BGI, Guangzhou, China).
Construction of RA ATCC11845ΔdprA::Erm Containing pLMF03::dprA, pLMF03::dprA-SAM-RF, pLMF03::dprA-RF-Zα, pLMF03::Sp-dprA, pLMF03::Hp-dprA, pLMF03::Fn-dprA, or pLMF03::dprAR123E Plasmids
Because we failed to introduce the plasmid pLMF03 into RA ATCC11845ΔdprA::Erm using either natural transformation or conjugation, the plasmid was introduced into RA ATCC11845 before dprA was knocked out. Briefly, the pLMF03::dprA, pLMF03::dprA-SAM-RF, pLMF03::dprA-RF-Zα, pLMF03::Sp-dprA, pLMF03::Hp-dprA, pLMF03::Fn-dprA, and pLMF03::dprAR123E plasmids was introduced into RA ATCC11845, respectively, as described previously (Huang et al., 2017). The transconjugants were selected on blood plates supplemented with Cfx (1 μg/ml). The RA ATCC11845 (pLMF03::dprA), RA ATCC11845 (pLMF03::dprA-SAM-RF), RA ATCC11845 (pLMF03::dprA-RF-Zα), RA ATCC11845 (pLMF03::Sp-dprA), RA ATCC11845 (pLMF03::Hp-dprA), RA ATCC11845 (pLMF03::Fn-dprA), and RA ATCC11845 (pLMF03::dprAR123E) strains were identified by PCR analysis. Subsequently, the dprA gene was deleted as described above (Liu et al., 2017). The transformants were selected on blood plates supplemented with Cfx (1 μg/ml) and Erm (1 μg/ml). The strains, which were designed RA ATCC11845ΔdprA::Erm (pLMF03::dprA), RA ATCC11845ΔdprA::Erm (pLMF03::dprA-SAM-RF), RA ATCC11845ΔdprA::Erm (pLMF03::dprA-RF-Zα), RA ATCC11845ΔdprA::Erm (pLMF03::Sp-dprA), RA ATCC11 845ΔdprA::Erm (pLMF03::Hp-dprA), RA ATCC11845ΔdprA:: Erm (pLMF03::Fn-dprA), and RA ATCC11845ΔdprA::Erm (pLM F03::dprAR123E), respectively, were identified by PCR analysis.
Construction of the Recombinant Vector for Expression
The complete dprA and dprAR123E genes were amplified by PCR from RA ATCC11845 chromosomal DNA and the recombination vector pLMF03::dprAR123E using the primers dprAExP1 and dprAExP2his (Table 2). The resulting PCR product was purified, digested with NdeI and XhoI, and ligated into the pET30a plasmid (also digested with NdeI and XhoI) to generate pET30a::dprA-s and pET30a::dprAR123E-s plasmids. The ligation products were introduced into E. coli strain DH5α cells using the calcium chloride method, and transformants were selected on LB plates containing Kan (50 μg/ml final concentration). The presence of the correct inserts was confirmed by PCR and sequencing (BGI, Guangzhou, China).
Expression and Purification of DprA and DprR123E His-Tagged Proteins
The E. coli Rosetta (pET30a::dprA-s) and E. coli Rosetta (pET30a::dprAR123E-s) strains were grown overnight in LB medium containing Kan (50 μg/ml). Stationary-phase cultures were diluted to an OD600 of 0.05 in 500 ml of LB medium containing Kan (50 μg/ml) and then incubated with shaking at 37°C until the culture density reached an OD600 of 0.6. The cells were then induced with 0.05 mM isopropyl b-D-1-thiogalactopyranoside (IPTG) and reincubated at 25°C. The cells were harvested by centrifugation for 10 min at 8,000 rpm at 4°C, and the resulting pellet was resuspended in 50 ml of binding buffer I (50 mM Tris–HCl, 250 mM NaCl, 0.05% Triton, pH 8.0) containing lysozyme (1 mg/ml final concentration) and DNase I (1 U/ml final concentration). The bacteria were lysed by freezing and thawing the cells at least three times. The suspension was then centrifuged at 12,000 rpm for 30 min at 4°C, and the supernatant was collected. The supernatant was mixed with 250 μl of Ni-NTA-agarose beads according to the manufacturer’s instructions. Purified protein was dialyzed twice against a buffer containing 50 mM Tris–HCl to eliminate any residual imidazole. The protein was stable for at least 1 month when kept at −80°C in 20% glycerol. Protein concentrations were determined using the BCA method with bovine serum albumin as the standard.
Electrophoretic Mobility Shift Assays (EMSA)
The DNA-binding activity of DprA and DprAR123E was measured using an EMSA Kit (Beyotime, China). RA-ssDNA1 from RA ATCC11845 (coding sequence: TAGGCTCTGCTAAGGAAGCGTGGGGTCTGTCTAAGTTGGA), RA-ssDNA2 from RA ATCC11845 (non-coding sequence: AAAAACTACGGAACTGACTAAAGGCAGAAAAACTAAA CGG), E. coli (EC-ssDNA from E. coli (CTCAGGTGCGAAAGCGTGGGGAGCAAA CAGGATTAGATAC) and dsDNA annealed from RA-ssDNA1 were labeled with biotin using an EMSA Probe Biotin Labeling Kit (Beyotime, China). A 10 μl reaction mixture containing 0.5 μl of DNA substrate (biotin-labeled ssDNA or dsDNA) in binding buffer with 25 mM HEPES (pH 7.0), 150 mM NaCl, 10% glycerol, 1 mM DTT and 0.05% IGEPAL (v/v; Sigma-Aldrich) mixed with the indicated concentrations of DprA and DprAR123E. After 30 min of incubation at 30°C, the samples were electrophoresed on an 8% non-denaturing PAGE in 0.5 × TBE (45 mM Tris-borate, 1 mM EDTA, pH 8.3). A constant voltage of 10 V/cm was applied for 2 h in baths of ice. The gel was transferred to a nylon membrane (Beyotime, China) at 380 mA for 30 min in 0.5 × TBE buffer. Fluorescence detection was performed with streptavidin-conjugated HRP and BeyoECL using a Chemiluminescent EMSA Kit (Beyotime, China) as described in the product specifications.
qRT-PCR
Riemerella anatipestifer ATCC11845 (pLMF03), RA ATCC11845ΔdprA::Erm (pLMF03::dprA), and RA ATCC11845ΔdprA::Erm (pLMF03::dprAR123E) were grown in GCB medium at an initial OD600 = 0.05 at 37°C with shaking at 180 rpm. The cells were harvested after 6 h of incubation. Total RNA was extracted using an RNAprep pure Cell/Bacteria Kit (TIANGEN, China). cDNA was synthesized from each RNA sample as described in a previous study (Liu et al., 2016). Real-time PCR assays were conducted with the primers shown in Table 2. Quantitative PCR was performed in triplicate on deposited samples as described in a previous study (Liu et al., 2016). The RNA quantity was normalized using a probe specific for 16S rRNA. Fold change was calculated as described in a previous study with the delta delta Ct method considering the efficiency of the PCR reaction for each target (Pfaffl, 2001).
Statistics and Software
The results of the transformation experiments were analyzed using GraphPad Prism 7.0 software for Windows (GraphPad Software Inc., La Jolla, CA, United States). The homology analysis of the RF domain of DprA was based on amino acid identity and performed using ClustalX 2.0 or DNAMAN 8.0 (Lynnon Biosoft, ON, Canada). One-way ANOVA followed by Tukey’s multiple-comparison test were used to evaluate statistical significance. A P-value <0.05 was considered significant.
Results
Identification and Sequence Analysis of DprA in R. anatipestifer ATCC11845
In the genome of RA ATCC11845, RA0C_1073 was annotated as a putative DprA. A protein sequence comparison showed that the DprA of R. anatipestifer (DprARa) had low identity with other well-characterized DprA sequences, including 30% identity with the DprA of Neisseria meningitidis (DprANm), 31% identity with the DprA of H. pylori (DprAHp), 37% identity with the DprA of Streptococcus pneumoniae (DprASp) and 36% identity with the DprA of Haemophilus influenzae (DprAHi) The dprA gene (RA0C_1073) is the last gene in a putative operon containing RA0C_1074, RA0C_1075, RA0C_1076, and RA0C_1077 in RA ATCC11845 (Figure 1A), which encode group III truncated hemoglobin, the crossover junction endodeoxyribonuclease RuvC, a hypothetical protein and an Lrp/AsnC family transcriptional regulator, respectively.
FIGURE 1.
Bioinformatics analysis of DprARa. (A) The dprA locus in R. anatipestifer ATCC11845. ORFs are indicated by block arrows and point in the direction of transcription. Names of ORFs are indicated below each arrow. (B) Predicted overall domain structure of DprARa and orthologs of S. pneumonia (DprASp), H. pylori (DprAHp), and Neisseria meningitidis (DprANm). (C) Deduced amino acid sequence alignment of the hallmark RF DprA domains of R. anatipestifer (RA), S. pneumoniae (Sp), and H. pylori (Hp); alignment was performed using ClustalX 2.0 ∗the same amino acid.
Similar to the DprANm (Hovland et al., 2017) and DprAHi (Karudapuram et al., 1995; Karudapuram and Barcak, 1997), DprARa has three domains: a sterile alpha motif (SAM), a Rossmann fold (RF) and a winged-helix DNA-binding motif/Z-DNA-binding domain (Zα) (Figure 1B). In contrast, DprASp has only SAM and RF domains and lacks a Zα domain, and DprAHp has only RF and Zα domains and lacks a SAM domain (Figure 1B). Moreover, among the SAM domains of DprARa, DprANm, DprASp, and DprAHi, the identity ranges from 16 to 17%; while among the Zα domains of DprARa, DprANm, DprAHp, and DprAHi, the identity ranges from 14 to 20%. However, for the RF domain of DprARa, DprANm, DprASp, DprAHp, and DprAHi, the identity ranges from 31 to 37% (Figure 1C).
DprA Is Essential for the Natural Transformation of R. anatipestifer ATCC11845
In H. influenzae, dprA is necessary for the uptake of chromosomal but not plasmid DNA (Karudapuram et al., 1995). In contrast, deletion of dprA in H. pylori had an impact on transformation of both chromosomal DNA and plasmid DNA (Ando et al., 1999). To test the role of dprA in the natural transformation of R. anatipestifer ATCC11845, the mutant strain RA ATCC11845ΔdprA::Erm and a complementation strain were constructed. Transforming genomic DNA containing resistance to streptomycin as a substrate was prepared. The result showed that the transformation frequency of the wild type was 6 (±0.7) × 10−5. However, no transformation was detected (limit-of-detection = 4.2 (±1.5) × 10−10) in the dprA mutant, and the transformation frequency of the complemented strain was 6.3 (±1.9) × 10−5 (Table 3). These results suggested that the dprA gene of RA ATCC11845 is essential for natural transformation when genomic DNA was used as the donor. To examine whether the dprA of RA ATCC11845 was also involved in the uptake of plasmids, the plasmid pLMF03, which carries a cefoxitin resistance (CfxR) marker (Liu et al., 2016), was used as a donor DNA to perform natural transformation. As shown in Table 3, the transformation frequency of the wild-type R. anatipestifer ATCC11845 strain to CfxR was 1.7 (±0.2) × 10−7. Again, no transformation was detected (limit-of-detection = 4.2 (±1.5) × 10−10) in the dprA mutant. These results suggest that the dprA of RA ATCC11845 is essential for the transformation of both genomic DNA and plasmid DNA.
Table 3.
Natural transformation assays performed in RA ATCC11845, RA ATCC11845ΔdprA::Erm, RA ATCC11845ΔdprA::Erm (pLMF03::dprA), and RA ATCC11845ΔdprA::Erm (pLMF03::dprAR123E).
| Strain | Transformation frequency using chromosomal DNA | Transformation frequency using plasmid DNA |
|---|---|---|
| RA ATCC11845 | 6 (±0.7) × 10−5 | 1.7 (±0.2) × 10−7 |
| RA ATCC11845ΔdprA::Erm | <d.l. | <d.l. |
| RA ATCC11845ΔdprA::Erm (pLMF03::dprA) | 6.3 (±1.9) × 10−5 | NA |
| RA ATCC11845ΔdprA::Erm (pLMF03::dprAR123E) | < d.l. | NA |
NA, Not applicable. <d.l., below detection limit. The average detection limit of non-transformable strains was 4.2 (±1.5) × 10−1.
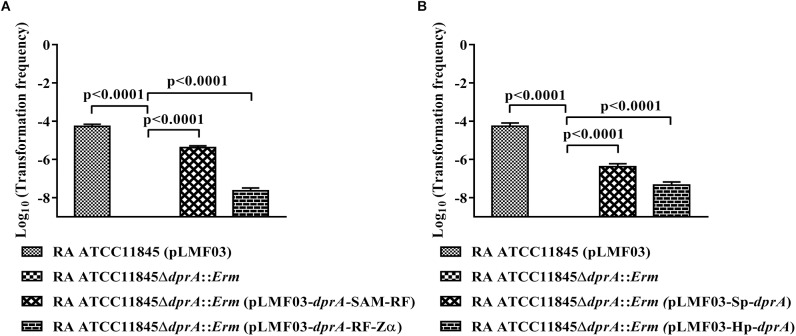
The Importance of Three Domains of DprA in Natural Transformation
As described above, DprARa has three domains, similar to N. meningitidis and H. influenzae, whereas the DprA of H. pylori and S. pneumoniae have only two domains. To investigate the role of each DprA domain in natural transformation, the strain RA ATCC11845ΔdprA::Erm was complemented by a plasmid expressing SAM-RF domains or a plasmid expressing RF-Zα domains, and the natural transformation frequency was measured. As shown in Figure 2A, the transformation frequency of RA ATCC11845ΔdprA::Erm (pLMF03::dprA-SAM-RF) was 4.1 (±0.7) × 10−6, while that of RA ATCC11845ΔdprA::Erm (pLMF03::dprA-RF-Zα) was 2.4 (±0.5) × 10−8. Both partially restored the level of natural transformation in the mutant strain RA ATCC11845ΔdprA::Erm. Interestingly, the transformation frequency of RA ATCC11845ΔdprA::Erm (pLMF03::dprA-SAM-RF) was higher than that of RA ATCC11845ΔdprA::Erm (pLMF03::dprA-RF-Zα). From these results, we predicted that the role of the SAM domain is more important role than that of the Zα domain in the natural transformation of RA ATCC11845.
FIGURE 2.
Complementation of R. anatipestifer ATCC11845ΔdprA::Erm by different domains of DprARa, dprA of S. pneumoniae and dprA of H. pylori. (A) Complementation of R. anatipestifer ATCC11845ΔdprA::Erm by different domains of DprARa. (B) Complementation of R. anatipestifer ATCC11845ΔdprA::Erm by dprA of S. pneumoniae (Sp-dprA) and dprA of H. pylori (Hp-dprA). The Log10 of averages and standard deviations of three independent experiments are shown. The numbers above each data point represent P-values for comparisons (one-way ANOVA followed by Tukey’s multiple-comparison test) of log10 of the average relative transformation frequencies.
To further verify the functions of these three domains in natural transformation, the strain RA ATCC11845ΔdprA::Erm was complemented by either a dprA of S. pneumoniae containing SAM and RF domains or a dprA of H. pylori containing RF and Zα domains, and the natural transformation frequency was measured. As shown in Figure 2B, the transformation frequency of RA ATCC11845ΔdprA::Erm (pLMF03::Sp-dprA) was 3.8 (±0.9) × 10−7, while that of RA ATCC11845ΔdprA::Erm (pLMF03::Hp-dprA) was 2.9 (±0.6) × 10−8. Interestingly, the frequency of complementing in cells the dprA from S. pneumoniae was higher than that observed in cells with the dprA from H. pylori. These results suggest that the role of the SAM domain is more important than that of the Zα domain in natural transformation.
The Amino Acid Arg123 in the RF Domain Is Essential for the Function of DprA in RA ATCC11845
As described above, it has been hypothesized that the RF domain is essential for the function of DprA in natural transformation. Moreover, sequence comparison showed that the amino acid residue Arg123 is one of the most conserved amino acid residues in this domain (Figure 1C). Thus, we chose to explore the function of Arg123 in natural transformation in RA ATCC11845. A mutant dprAR123E (arginine mutated to glutamic acid) construct was developed and cloned into the shuttle plasmid pLMF03 as described in the Section “Materials and Methods”. As shown in Table 3, the plasmid pLMF03::dprAR123E was not able to restore natural transformation to RA ATCC11845ΔdprA::Erm. As a control, dprAR123E was transcribed well in the RA ATCC11845ΔdprA::Erm strain (pLMF03::dprAR123E; Supplementary Figure S1). The results suggested that the RF domain is essential for natural transformation in RA ATCC11845 and that the amino acid Arg123 plays a key role in this domain.
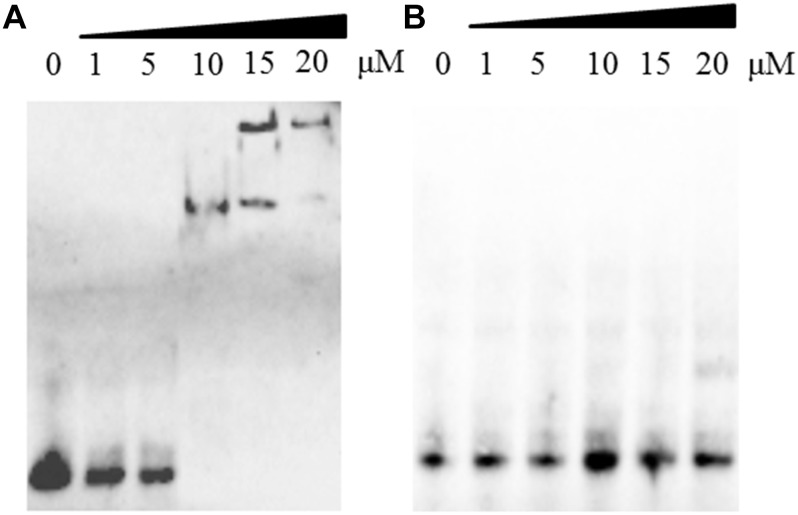
DprAR123E Has a Dramatic Effect on Binding ssDNA and dsDNA
The R123E mutant of DprA abolished the ability of RA ATCC11845 to undergo natural transformation, potentially because the mutant was unable to bind and protect DNA. Thus, we next evaluated whether Arg123 of the DprA domain of RA ATCC11845 affects the ability of the protein to bind to DNA. In these experiments, DprA and DprAR123E were expressed and purified, and EMSA was performed as described in the Section “Materials and Methods”. In this study, we first chose three different sources of single strand DNA (ssDNA): RA-ssDNA1 from the coding sequence of RA ATCC11845, RA-ssDNA2 from the non-coding sequence of RA ATCC11845 and (EC-ssDNA from E. coli. The sequences of those ssDNA were shown in the Section “Materials and Methods.” Single-strand DNA (ssDNA) was synthesized and used as a substrate for EMSA. As shown in Figure 3A–C, all the ssDNA was shifted in the presence of DprA. As the concentration of DprA increased, the formation of protein-DNA complexes was clearly slowed, and this change was associated with a parallel loss of uncomplexed DprA and ssDNA, suggesting that DprA can bind ssDNA and that it has no sequence or species specificity.
FIGURE 3.
The effect of the R123E mutant of DprA on binding to single-strand DNA (ssDNA). All ssDNA was labeled by biotin. The first lane of each picture contains free ssDNA without proteins. Different concentrations of DprA (1–20 μM) were combined with 0.5 μl of DNA substrate (biotin-labeled ssDNA) and incubated for 30 min at 30°C before the mixtures were loaded onto the gel. (A) Interaction between DprA and ssDNA from the R. anatipestifer coding sequence (RA-ssDNA1). (B) Interaction between DprA and ssDNA from the R. anatipestifer non-coding sequence (RA-ssDNA2). (C) Interaction between DprA and ssDNA from E. coli XL1-blue (E. coli-ssDNA). (D) Interaction between DprAR123E and ssDNA from the R. anatipestifer coding sequence (RA-ssDNA1). (E) Interaction between DprAR123E and ssDNA from the R. anatipestifer non-coding sequence (RA-ssDNA2). (F) Interaction between DprAR123E and ssDNA from E. coli XL1-blue (E. coli-ssDNA). Samples were electrophoresed on an 8% non-denaturing PAGE gel and detected by fluorography.
Interestingly, DprA of H. pylori binds not only ssDNA but also dsDNA (Dwivedi et al., 2013). However, DprA of S. pneumoniae and Bacillus subtilis has been reported to bind and protect ssDNA but not dsDNA (Mortier-Barriere et al., 2007). To determine whether DprA of R. anatipestifer binds dsDNA, EMSA was performed using both DprA and biotinylated dsDNA. The dsDNA (40 bp) was annealed from RA-ssDNA1. As shown in Figure 4A, the retarded protein-DNA complex became more evident with the increasing of DprA concentration, suggesting that DprA binds dsDNA. As shown in Figure 3D–F, 4B, there is not any retarded protein-DNA complex as the concentration of the DprAR123E was increased as the concentration of DprAR123E increased. Taken together, these results suggest that the Arg123 mutant abolished the ability of DprA to bind and protect DNA.
FIGURE 4.
The effect of the R123E mutant of DprA on binding to double-strand DNA (dsDNA). The dsDNA was annealed from RA-ssDNA1 and labeled with biotin. The first lane of each picture contained free dsDNA without proteins. Different concentrations of DprA (1–20 μM) were combined with 0.5 μl of DNA substrate (biotin-labeled dsDNA) and incubated for 30 min at 30°C before the mixture was loaded on the gel. (A) Interaction between DprA and dsDNA. (B) Interaction between DprAR123E and dsDNA. Samples were electrophoresed on an 8% non-denaturing PAGE gel and detected by fluorography.
DprA Has a Conserved Function in Evolution
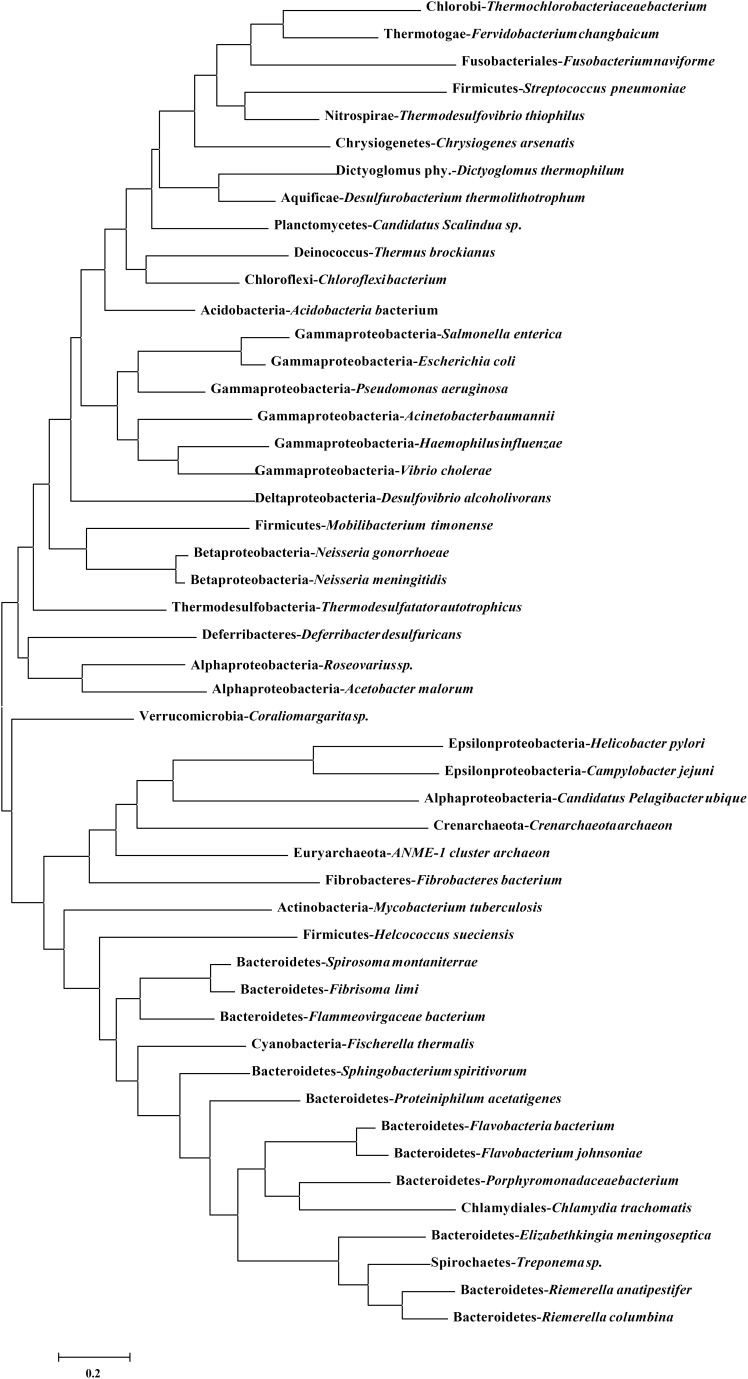
DprA has been found in nearly all the sequenced bacteria, including bacteria currently recognized as non-competent (Supplementary Table S1). The identity ranged from 35 to 63% in Flavobacteriaceae (Supplementary Table S1). Compared to other bacterial genera, for RA ATCC11845, the identity ranged from 28 to 39%, but it shared 59% identity with Spirochaetes. As shown in Figure 5, a phylogenetic analysis of DprA across different bacterial species showed that there were no obvious branches between naturally competent bacteria and non-competent bacteria. It has been speculated that DprA has conserved functions across different bacteria. Thus, we wondered whether dprA from other bacteria would be able to restore natural transformation to RA ATCC11845ΔdprA::Erm. In a proof-of-principle experiment, we chose the DprA of F. naviforme, which has not been reported natural competence currently and has low identity (30%) with the DprA of RA ATCC11845 (Supplementary Figure S3). The dprA of F. naviforme was synthesized and cloned into the shuttle plasmid pLMF03. An RA ATCC11845ΔdprA::Erm construct that expressed pLMF03::Fn-dprA was constructed as described in the Section “Materials and Methods”. As shown in Figure 6, the transformation frequency of RA ATCC11845 (pLMF03) was 2.9 (±0.3) × 10−5, while that of RA ATCC11845ΔdprA::Erm (pLMF03::Fn-dprA) was 4.9 (±0.7) × 10−7. These results suggest that a dprA from F. naviforme, partially restored natural transformation to the mutant RA ATCC11845ΔdprA::Erm.
FIGURE 5.
Phylogenetic analysis of DprA among different bacterial species. A phylogenetic tree was constructed based on amino acid sequences using MEGA6.0 using neighbor-joining method. The sequence information for DprA is listed in Supplementary Table S1.
FIGURE 6.
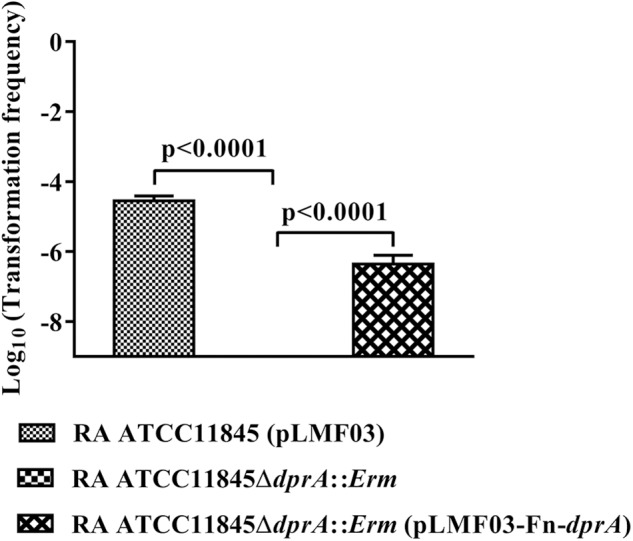
Complementation of R. anatipestifer ATCC11845ΔdprA::Erm by the dprA of the non-competent bacterium F. naviforme. The Log10 of averages and standard deviations of three independent experiments are shown. The numbers above each data point represent P-values for comparisons (one-way ANOVA followed by Tukey’s multiple-comparison test) of log10 of the average relative transformation frequencies.
Discussion
Natural transformation is a widely distributed mechanism used by many bacterial genera to acquire DNA and undergo genetic recombination (Johnston et al., 2014). The competence machinery actively processes exogenous dsDNA and takes up internalized ssDNA to replace homologous (or partially homologous) chromosomal sequences via a mechanism catalyzed by RecA and facilitated by accessory factors, such as DprA (Kidane et al., 2012). Although it has been found that R. anatipestifer was natural competent and established multiple genome editing tools using it (Liu et al., 2018b), the mechanism of natural transformation in R. anatipestifer kept largely unknown. This study focused on the characterization of DprA in R. anatipestifer ATCC11845.
DprA is essential for the natural transformation in N. meningitidis, N. gonorrhoeae, S. pneumoniae, and H. influenzae (Karudapuram et al., 1995; Berge et al., 2003; Duffin and Barber, 2016; Hovland et al., 2017). However, in B. subtilis, dprA is not stringently required for DNA transformation as there is some redundancy between the RecF and DprA pathways (Kidane et al., 2009). RA0C_1073 of RA ATCC11845 encodes a hypothetical DprA with low identity to the DprA sequences of other bacteria. To explore whether this gene is involved in natural transformation in RA ATCC11845, a dprA mutant strain was constructed. The transformation frequency of dprA mutant strain was not able to be detected whatever plasmid or chromosomal DNA was used as the donor DNA, suggesting that DprA is essential for the natural transformation of both chromosomal DNA and plasmid DNA in RA ATCC11845, consistent with what has been observed in H. pylori (Ando et al., 1999), but inconsistent with H. influenzae, in which dprA is necessary for chromosomal uptake but not for plasmid (Karudapuram et al., 1995). In the case of H. influenzae, it was hypothesized that the plasmid DNA enters the cytoplasm without cutting and recombination. However, in the case of R. anatipestifer, we predicted that the plasmid DNA enter the cytoplasm as the single strand DNA and was re-circled, since if the circular DNA enters into the cytoplasm directly, the dprA mutant should have no any effect on transformation frequency of plasmid. However, the fact is that the plasmid was not able to be introduced into the dprA mutant by natural transformation after many times attempts. Additionally, we tried many times to introduce the plasmid into dprA mutant by conjugation. It was also failed. Although it is impossible that natural transformation and conjugation use the same pathway, it is predicted that single-stranded linear of the plasmid is transported into the cytoplasm by conjugation and the dprA was required for the recyclizing.
Bioinformatic analysis showed that DprARa has three domains (SAM, RF and Zα domains). To investigate the function of each domain of DprA, complementary assays were performed with SAM-RF domain and RF-Zα domain, respectively. It was showed that the natural transformation frequency was higher when the mutant strain was complemented by SAM-RF domain, than that complemented by RF-Zα domain. Consistently, the dprA of H. pylori (Hp-dprA), which contains SAM and RF domains, was more efficiently to restore the natural transformation of the dprA mutant strain than dprA of S. pneumoniae (Sp-dprA), which contains RF and Zα domain, did. All these results suggest that the SAM domain is more important than Zα domain for natural transformation. It is possible that the SAM domain play more important role than Zα domain in protect ssDNA or promote recombination. In DprASp, the SAM domain plays a role in intracellular signaling and the regulation of competence, in which it plays an important role in shutting down natural competence in this bacterium by interacting with ComE (Mirouze et al., 2013). Compared with S. pneumonia, there are some different traits in R. anatipestifer. First, the natural competence of R. anatipestifer is constitutive. Second, the SAM domain in DprARa lacks the amino acid residues that confer the ability to induce competence in S. pneumoniae (Supplementary Figure S2). Third, R. anatipestifer lacks the ComDE two-component system, which is responsible for regulating natural competence in S. pneumoniae (Martin et al., 2013). The specific function that the SAM domain of RA ATCC11845 plays in natural transformation is unknown at this time. It has been hypothesized that the SAM domain of DprA in R. anatipestifer protects ssDNA from degradation or interacts with RecA to promote homologous recombination.
When the conserved amino acid Arg123 in the RF domain of DprA of RA ATCC11845 was switched to glutamic acid (Glu), the resulting Arg123 mutant completely abolished the ability of RA ATCC11845 to undergo natural transformation, suggesting that RF is essential for natural transformation in R. anatipestifer. It has also been shown that Arg123 is essential for the function of DprARa. To explore whether the abrogation of natural transformation observed in the Arg123 mutant strains was because the mutant was no longer able to bind DNA, EMSA was performed to evaluate the interaction between ssDNA and DprA or DprAR123E. The results showed that DprA binds ssDNA without sequence or species specificity. Moreover, DprA can also bind dsDNA, consistent with H. pylori (Dwivedi et al., 2013). As expected, DprAR123E was no longer able to bind either ssDNA or dsDNA. In this study, it was shown that RF was essential for natural transformation and that the Arg123 site in the RF domain was essential for the functions of DprA in R. anatipestifer ATCC11845, which is consistent with findings in H. pylori. In H. pylori, the 3D structure of DprA was studied, and the results showed that Arg52 is essential for DprAHp to grasp the substrate with high affinity (Wang W. et al., 2014).
Phylogenetic assay showed that the homolog of DprA was distributed in most of sequenced bacterial species, including the bacteria that was not found to be natural competent at present. It was consistent with the previous study which suggested that natural competence is an ancient ancestral trait and is usually to be lost during the evolution (Redfield et al., 2006). This viewpoint was strengthened by the fact that the dprA of F. naviforme, which has not been reported natural competence currently, was able to partially restored natural transformation to R. anatipestifer dprA mutant. It has been reported that DprA interact with RecA to promote recombination (Mortier-Barriere et al., 2007). There is a possible reason for explaining why dprA of F. naviforme only partially restore the ability of natural competence of R. anatipestifer. Taken together, R. anatipestifer is the first bacterium to be identified as a naturally competent species in Flavobacteriaceae. In this study, our experiments involving DprA were helpful for revealing the mechanism of natural transformation in R. anatipestifer, even in Flavobacteriaceae. These findings will also be helpful for improving gene editing methods for R. anatipestifer.
Author Contributions
ML, AC, and FB conceived and designed the experiments. LH, XT, DZ, MW, YL, LZ, XC, and YY performed the experiments. LP, MR, JH, RJ, SC, and XZ analyzed the data. BT, YW, QY, and SZ contributed reagents, materials, and analysis tools. LH, ML, FB, and AC wrote the manuscript. All authors have reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Qiong Liu (Nanchang University) and Hebin Liao (North Sichuan Medical College) for generously providing the templates for H. pylori and S. pneumoniae, respectively.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (31572521), the China Agricultural Research System (CARS-42-17), the Sichuan Veterinary Medicine and Drug Innovation Group of China Agricultural Research System (CARS-SVDIP), and a Special Fund for Key Laboratory of Animal Disease and Human Health of Sichuan Province (2016JPT0004).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00429/full#supplementary-material
References
- Ando T., Israel D. A., Kusugami K., Blaser M. J. (1999). HP0333, a member of the dprA family, is involved in natural transformation in Helicobacter pylori. J. Bacteriol. 181 5572–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge M., Mortier-Barriere I., Martin B., Claverys J. P. (2003). Transformation of Streptococcus pneumoniae relies on DprA- and RecA-dependent protection of incoming DNA single strands. Mol. Microbiol. 50 527–536. [DOI] [PubMed] [Google Scholar]
- Chen I., Dubnau D. (2004). DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2 241–249. 10.1038/nrmicro844 [DOI] [PubMed] [Google Scholar]
- Draskovic I., Dubnau D. (2005). Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds. Mol. Microbiol. 55 881–896. 10.1111/j.1365-2958.2004.04430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. (1999). DNA uptake in bacteria. Annu. Rev. Microbiol. 53 217–244. 10.1146/annurev.micro.53.1.217 [DOI] [PubMed] [Google Scholar]
- Duffin P. M., Barber D. A. (2016). DprA is required for natural transformation and affects pilin variation in Neisseria gonorrhoeae. Microbiology 162 1620–1628. 10.1099/mic.0.000343 [DOI] [PubMed] [Google Scholar]
- Dwivedi G. R., Sharma E., Rao D. N. (2013). Helicobacter pylori DprA alleviates restriction barrier for incoming DNA. Nucleic Acids Res. 41 3274–3288. 10.1093/nar/gkt024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs M., Mattick J. S. (1993). Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 10 233–243. 10.1111/j.1365-2958.1993.tb01949.x [DOI] [PubMed] [Google Scholar]
- Hovland E., Beyene G. T., Frye S. A., Homberset H., Balasingham S. V., Gomez-Munoz M., et al. (2017). DprA from Neisseria meningitidis: properties and role in natural competence for transformation. Microbiology 163 1016–1029. 10.1099/mic.0.000489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yuan H., Liu M. F., Zhao X. X., Wang M. S., Jia R. Y., et al. (2017). Type B chloramphenicol acetyltransferases are responsible for chloramphenicol resistance in Riemerella anatipestifer. China Front. Microbiol. 8:297. 10.3389/fmicb.2017.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C., Martin B., Fichant G., Polard P., Claverys J. P. (2014). Bacterial transformation: distribution, shared mechanisms and divergent control. Nat. Rev. Microbiol. 12 181–196. 10.1038/nrmicro3199 [DOI] [PubMed] [Google Scholar]
- Karudapuram S., Barcak G. J. (1997). The Haemophilus influenzae dprABC genes constitute a competence-inducible operon that requires the product of the tfoX (sxy) gene for transcriptional activation. J. Bacteriol. 179 4815–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karudapuram S., Zhao X., Barcak G. J. (1995). DNA sequence and characterization of Haemophilus influenzae dprA+, a gene required for chromosomal but not plasmid DNA transformation. J. Bacteriol. 1773235–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane D., Ayora S., Sweasy J. B., Graumann P. L., Alonso J. C. (2012). The cell pole: the site of cross talk between the DNA uptake and genetic recombination machinery. Crit. Rev. Biochem. Mol. Biol. 47 531–555. 10.3109/10409238.2012.729562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane D., Carrasco B., Manfredi C., Rothmaier K., Ayora S., Tadesse S., et al. (2009). Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells. PLoS Genet. 5:e1000630. 10.1371/journal.pgen.1000630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen B. M., Sinha S., Boyce J. D., Bojesen A. M., Mell J. C., Redfield R. J. (2012). Natural transformation of Gallibacterium anatis. Appl. Environ. Microbiol. 78 4914–4922. 10.1128/AEM.00412-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Huang M., Shui Y., Biville F., Zhu D., Wang M., et al. (2018a). Roles of B739_1343 in iron acquisition and pathogenesis in Riemerella anatipestifer CH-1 and evaluation of the RA-CH-1DeltaB739_1343 mutant as an attenuated vaccine. PLoS One 13:e0197310. 10.1371/journal.pone.0197310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Huang Y., Liu J., Biville F., Zhu D., Wang M., et al. (2018b). Multiple genetic tools for editing the genome of Riemerella anatipestifer using a counterselectable marker. Appl. Microbiol. Biotechnol. 102 7475–7488. 10.1007/s00253-018-9181-4 [DOI] [PubMed] [Google Scholar]
- Liu M., Wang M., Zhu D., Wang M., Jia R., Chen S., et al. (2016). Investigation of TbfA in Riemerella anatipestifer using plasmid-based methods for gene over-expression and knockdown. Sci. Rep. 6:37159. 10.1038/srep37159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhang L., Huang L., Biville F., Zhu D., Wang M., et al. (2017). Use of natural transformation to establish an easy knockout method in Riemerella anatipestifer. Appl. Environ. Microbiol. 83:e127-17. 10.1128/AEM.00127-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Liu M., Wang L., Zhou W., Wang M., Cheng A., et al. (2015). Identification of ribosomal RNA methyltransferase gene ermF in Riemerella anatipestifer. Avian Pathol. 44 162–168. 10.1080/03079457.2015.1019828 [DOI] [PubMed] [Google Scholar]
- Luo H. Y., Liu M. F., Wang M. S., Zhao X. X., Jia R. Y., Chen S., et al. (2018). A novel resistance gene, lnu(H), conferring resistance to lincosamides in Riemerella anatipestifer CH-2. Int. J. Antimicrob. Agents 51 136–139. 10.1016/j.ijantimicag.2017.08.022 [DOI] [PubMed] [Google Scholar]
- Martin B., Soulet A. L., Mirouze N., Prudhomme M., Mortier-Barriere I., Granadel C., et al. (2013). ComE/ComE∼P interplay dictates activation or extinction status of pneumococcal X-state (competence). Mol. Microbiol. 87 394–411. 10.1111/mmi.12104 [DOI] [PubMed] [Google Scholar]
- Mell J. C., Redfield R. J. (2014). Natural competence and the evolution of DNA uptake specificity. J. Bacteriol. 196 1471–1483. 10.1128/JB.01293-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. (1988). A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze N., Berge M. A., Soulet A. L., Mortier-Barriere I., Quentin Y., Fichant G., et al. (2013). Direct involvement of DprA, the transformation-dedicated RecA loader, in the shut-off of pneumococcal competence. Proc. Natl. Acad. Sci. U.S.A. 110 E1035–E1044. 10.1073/pnas.1219868110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier-Barriere I., Velten M., Dupaigne P., Mirouze N., Pietrement O., McGovern S., et al. (2007). A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell 130 824–836. 10.1016/j.cell.2007.07.038 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield R. J., Findlay W. A., Bosse J., Kroll J. S., Cameron A. D., Nash J. H. (2006). Evolution of competence and DNA uptake specificity in the Pasteurellaceae. BMC Evol. Biol. 6:82. 10.1186/1471-2148-6-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. H., Zhou W. S., Wang J. B., Liu M. F., Wang M. S., Cheng A. C., et al. (2016). Genome sequence of Riemerella anatipestifer strain RCAD0122, a multidrug-resistant isolate from ducks. Genome Announc. 4:e00332-16. 10.1128/genomeA.00332-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Ding J., Zhang Y., Hu Y., Wang D. C. (2014). Structural insights into the unique single-stranded DNA-binding mode of Helicobacter pylori DprA. Nucleic Acids Res. 42 3478–3491. 10.1093/nar/gkt1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu W., Zhu D., Yang L., Liu M., Yin S., et al. (2014). Comparative genomics of Riemerella anatipestifer reveals genetic diversity. BMC Genomics 15:479. 10.1186/1471-2164-15-479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong A. S., Yao Q. H., Peng R. H., Duan H., Li X., Fan H. Q., et al. (2006). PCR-based accurate synthesis of long DNA sequences. Nat. Protoc. 1 791–797. 10.1038/nprot.2006.103 [DOI] [PubMed] [Google Scholar]
- Yadav T., Carrasco B., Hejna J., Suzuki Y., Takeyasu K., Alonso J. C. (2013). Bacillus subtilis DprA recruits RecA onto single-stranded DNA and mediates annealing of complementary strands coated by SsbB and SsbA. J. Biol. Chem. 288 22437–22450. 10.1074/jbc.M113.478347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang M. S., Liu M. F., Zhu D. K., Biville F., Jia R. Y., et al. (2017). Contribution of RaeB, a putative RND-type transporter to aminoglycoside and detergent resistance in Riemerella anatipestifer. Front. Microbiol. 8:2435. 10.3389/fmicb.2017.02435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C. Y., Cheng A. C., Wang M. S., Zhu D. K., Luo Q. H., Zhong C. D., et al. (2009). Antibiotic susceptibility of Riemerella anatipestifer field isolates. Avian Dis. 53 601–607. [DOI] [PubMed] [Google Scholar]
- Zhu D. K., Luo H. Y., Liu M. F., Zhao X. X., Jia R. Y., Chen S., et al. (2018). Various profiles of tet genes addition to tet(X) in Riemerella anatipestifer isolates from ducks in China. Front. Microbiol. 9:585. 10.3389/fmicb.2018.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D. K., Yang X. Q., Yang H., Zhou W. S., Song X. H., Wang J. B., et al. (2016). Comparative genomic analysis identifies structural features of CRISPR-Cas systems in Riemerella anatipestifer. BMC Genomics 17:689. 10.1186/s12864-016-3040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.