Abstract
Three-dimensional (3D) printing has rapidly evolved, with major applications in the field of medicine. One of the greatest advances leading to 3D bioprinting was the development of biomaterials, cells and supporting components for the fabrication of functional living tissues. Several different methods and techniques of 3D bioprinting are briefly described in this review article, and applications of 3D printing for the fabrication of artificial blood vessels and grafts are presented. Advances in additive manufacturing techniques, medical imaging modalities, biomaterials and cellular engineering will lead to further developments in the fabrication of patient-specific vascular tissue constructs. Future multidisciplinary research and innovations are expected to further transform the fields of tissue engineering and regenerative medicine.
Keywords: Additive manufacturing, Artery, Tissue engineering, Vascular graft
INTRODUCTION
Three-dimensional (3D) printing has attracted increasing interest during the last decades and is already used in various industrial sectors for the rapid and easy fabrication of complex structures and materials, exceeding several limitations of conventional manufacturing techniques. 3D printing, also known as additive manufacturing has also led to remarkable advances in the healthcare sector (3D bioprinting), especially in regenerative medicine, as it facilitates on-demand "printing" of cells, tissues and organs. These technological advances have led to the creation of new scientific fields such as "tissue engineering".
Developments in 3D bioprinting have been mostly motivated by the limited availability of organs globally,1 which are needed for the rehabilitation of lost or failed organs and tissues. The most challenging and demanding applications for engineered tissues include the skin,2,3 cartilage,4 hard tissues such as bones,5 cardiac tissue,6 and vascular grafts.7
Medical modelling is another field where 3D printing is increasingly being applied. In clinical practice, 3D printed models have been shown to be useful for surgical planning and medical education.8 The use of 3D printing to develop tools to assist and improve medical procedures has a long history,9 and the Centre for Devices and Radiological Health at the Food and Drug Administration has reviewed and cleared several 3D printed medical devices over the last 10 years.9,10
In the present review article, we aimed to briefly describe the origins and basic principles of 3D bioprinting, highlighting a few key developments in the fabrication of blood vessels and vascular grafts.
A brief history of 3D printing
In the 15th century, the development of the printing (press) industry enabled the fast reproduction of text and images and the widespread dissemination of information, which had a tremendous social, political and financial impact. Five centuries later, 3D printing technology is a new revolution with an expected ground breaking contribution to industry and to other fields including medicine. After the first developments in 3D printing, which was described as "stereolithography" by Charles W. Hull11 in the early 1980s, new methods and techniques for the construction of 3D objects have been developed and used for educational, research and even clinical purposes. Initially, stereolithography, also termed photo-solidification, optical fabrication or resin printing, was used to form 3D structures using sequentially printed thin layers of a material processed by ultraviolet light. Various additive manufacturing techniques have since been developed for the automated production of personalized, computer-modelled tissue replicas and even organs.
3D BIOPRINTING AND TISSUE ENGINEERING
In general, biofabrication can be defined as "the automated generation of biologically functional products with structural organization from living cells, bioactive molecules, biomaterials, cell aggregates such as microtissues, hybrid cell-material constructs through bioprinting or bioassembly".12,13 The definition of biofabrication has been further refined to include "bioprinting" and "bioassembly" as complementary parts of the biofabrication process.13 A general overview of 3D printing processes is shown in Figure 1.
Figure 1.
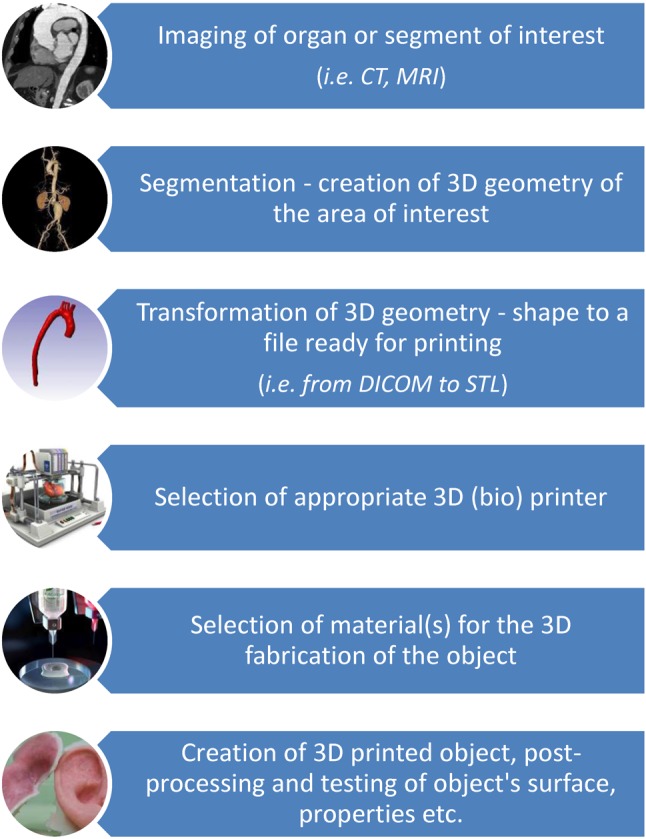
Schematic general overview of 3D (bio) printing processes.
A major challenge for 3D printing technologies is the construction of medical devices and biological tissues and organs. Bioprinting is defined as the positioning of biochemicals, biological materials, and living cells for the generation of bioengineered structures (i.e. additive manufacturing) of biological and biologically relevant materials with the use of computer-aided transfer and build-up processes.7. Typical 3D printing techniques have further evolved with the creation of sacrificial resin molds for the formation of 3D scaffolds from biological materials.14 The development of solvent-free, aqueous-based systems have facilitated the printing of biomaterials into 3D scaffolds that can be used for transplantation with or without seeded cells.14,15 The next milestone was 3D bioprinting, namely the formation of tissue engineered structures.
Bioengineering techniques for the construction of artificial blood vessels have been studied by several groups.16 Successful tissue regeneration has been associated with the seeding of cells, the scaffolds and the construction technologies. 3D printing and electrospinning technologies can promote the rapid and accurate fabrication of scaffolds for cellularization with vascular progenitors or mixtures of mature human vascular cells17 to yield bioengineered blood vessels. The progress in 3D bioprinting is driven by the increasing need for alternative construction approaches to develop tissues and even organs, since the available conventional methods are not capable of fabricating materials and constructs with the necessary structural, mechanical and biological properties such as biocompatibility and complexity.
Basic principles of 3D bioprinting
In general, 3D bioprinting is based on the layer-by-layer precise positioning of biological constituents, biochemicals and living cells, by spatial control of the placement of functional constituents of the fabricated 3D structure.14 3D bioprinting is based on three fundamental approaches: (i) biomimicry or biomimetics, (ii) autonomous self-assembly, and (iii) mini-tissue building blocks, as described extensively elsewhere.14
Briefly, biomimicry or biomimetics (from the Greek words bios, which means life, and mimesis, which means to imitate) is defined as the process of examining nature itself, its systems, processes and elements in order to be inspired and stimulated for the optimal solution of human problems. Incorporation of biomimetic components into a bioprinted construct has a dynamic effect on the attachment, migration, proliferation and function of both endogenous and exogenous cells.14 Materials have a major influence on cell attachment as well as cell size and shape, thus enabling the control of proliferation and differentiation of cells in a scaffold.14 Moreover, nanoscale features such as ridges, steps and grooves can also influence cell attachment, proliferation and cytoskeletal assembly.14,18 Beyond these factors, cell shape and differentiation can be also modulated by the 3D environment in a tissue-engineered construct.19 A biomimicry approach in 3D bioprinting of materials and constructs (i.e. tissues) with specific physiological functions and properties entails a deep understanding of the natural tissue-specific composition of the tissue of interest.14
Autonomous self-assembly, similarly to embryonic organ development, is a process that replicates a specific organ or tissue in-vitro. Early cellular components of a developing tissue produce extracellular matrix components and appropriate cell signals that lead to autonomous organization and patterning of the desired tissue.14 Using autonomous self-assembly it is feasible to use cells for histogenesis and also to modulate the composition, localization, functional and structural properties of the tissue as previously described.20 A solid understanding of the mechanisms of embryonic organogenesis and the ability to manipulate the conditions that regulate these embryonic mechanisms is essential to successfully achieve this goal.
An essential factor in tissue engineering is the scaffold, namely a 3D, highly porous substrate. Cells are expanded in culture and then transferred to the scaffold.20 The scaffold offers a surface upon which cells adhere, multiply, thrive, and produce the extracellular matrix of structural and functional proteins and saccharides that create the living tissue.20 The biological properties of the cells are modulated and controlled by the composition of the scaffold material as well as its internal architecture (dimensions of the struts, walls, pores, or channels).20 A schematic representation of some common methods used for bioprinting as described by Li et al.1 is shown in Figure 2.
Figure 2.
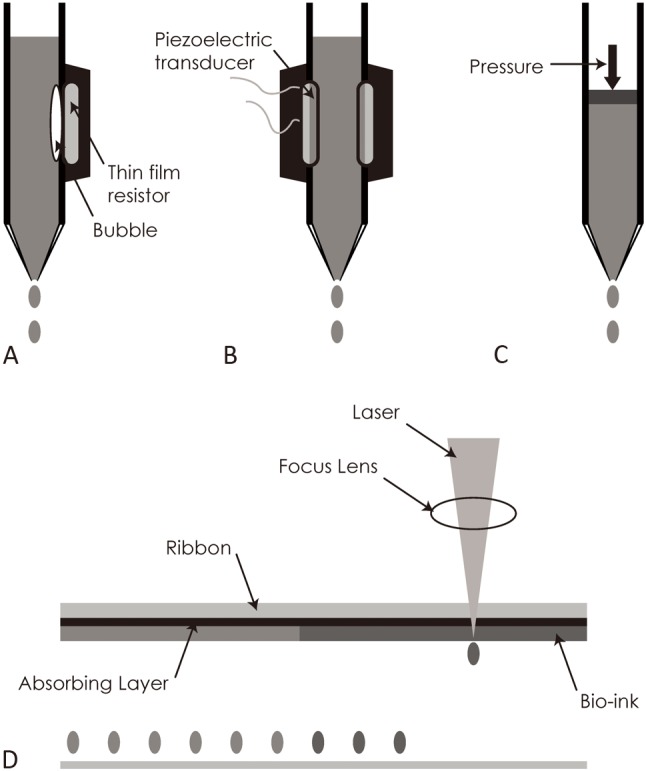
Schematic drawing of common bioprinting techniques based in the description of Li et al.1 (A) Thermal inkjet-based bioprinting technology employs an electric current pulse that impulses the thin film resistor, then produces bubbles that generate a pressure pulse that propels the ink droplet onto the substrates. (B) A piezoelectric transducer generates a pulse that creates transient pressure, resulting in droplet ejection. (C) Pressure-assisted bioprinting uses solutions, pastes, or dispersions as biomaterials, which are extruded by pressure in the form of a continuous filament through a microscale nozzle orifice or a microneedle. (D) Laser based bioprinting consists of three parts: a pulsed laser source, a ribbon and a receiving substrate. The lasers irradiate the ribbon, causing the liquid biological materials to evaporate and reach the receiving substrate in droplet form.
The philosophy beyond inkjet-based bioprinting is similar to that of conventional inkjet printing (i.e. desktop inkjet printers). Printing is achieved through a non-contact process and specifically by depositing precise tiny droplets of "bioink" onto a hydrogel substrate or culture dish.1 This type of bioprinting can be performed using thermal or piezoelectric actuator methods as shown in Figure 2.
The pressure-assisted bioprinting technique relies on the extrusion of selected biomaterials usually formulated as pastes, solutions or dispersions.1,21 These biomaterials are extruded by coordinating the motion of pneumatic pressure or plunger- or screw-based pressure in the form of a continuous filament through a microscale nozzle orifice or a microneedle onto a stationary substrate. After the layer-by-layer deposition of biomaterial, complete 3D patterns and constructions are finally formed.1 A schematic representation of the material extrusion technique is illustrated in Figure 3.
Figure 3.
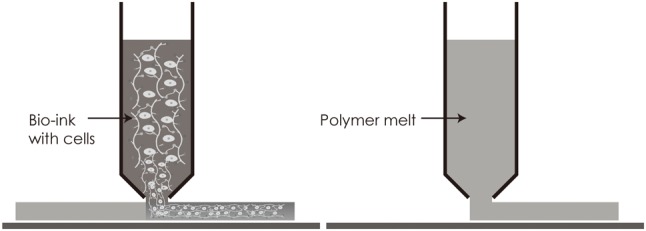
Schematic illustration of material extrusion techniques based on the description of Lee et al.13
Laser-assisted bioprinting is based on the deposition of biomaterials onto a substrate using a laser as the energy source. This technology usually consists of three systems: (i) a pulsed laser source, (ii) a ribbon coated with liquid biological materials that are deposited on the metal film, and (iii) a receiving substrate.1 The laser irradiates the ribbon, leading to evaporation of the liquid biological materials. The receiving substrate contains a biopolymer or cell culture medium to preserve cellular adhesion and sustained growth after transfer of the cells from the ribbon.1 The basic principle of this technique is shown in Figure 4, as described by Lee et al.13
Figure 4.
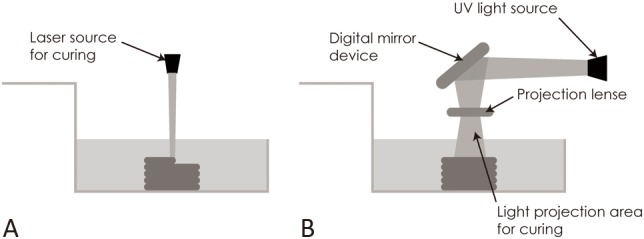
Illustration of (A) stereolithography and (B) digital light processing techniques based on the description of Lee et al.13
BIOPRINTING OF ARTIFICIAL BLOOD VESSELS
Various methodologies and techniques have been proposed in the literature for the bioprinting of artificial blood vessels and vascular grafts using either cellular or acellular materials and procedures. Among the various techniques of 3D bioprinting used for tissue engineering, three main methodologies are used to bioprint artificial vessels: (a) fabrication of bulk matrices with integrated channels as perfusable matrices, (b) patterning of cells into line structures for self-assembly of interconnected vessel systems, and (c) fabrication of free-standing tubular structures, which may serve as artificial vessels.7
Construction of grafts using acellular materials permits the use of a high printing temperature and organic solvents.22 On the other hand, unlike cellular-based techniques, it is quite challenging to achieve spatial control of cell seeding on prefabricated constructs which is possible by using cellular-based techniques. 3D bioprinting using cell-laden materials has been shown to make the incorporation of cells more efficient, increase the initial cell loading density and improve the control of cell distribution, irrespective of whether there are single or multiple cell types.22
In 2005, Kesari and colleagues made one of the first attempts to construct tubular hydrogel structures using drop-on-demand inkjet printing.23 In brief, they created cell structures in which the cells were entrapped within hydrogels and crosslinked on demand. Their study was the first to introduce the use of calcium chloride (CaCl2)-based alginate complex formation in inkjet printing by printing CaCl2 solution into an alginate bath simply using a modified Hewlett Packard (HP697c) ink-jet printer.7,23 Specifically, the authors used ionic crosslinking additives (CaCl2) in saline cell suspensions at hypertonic concentrations, thereby facilitating passage of the cells through the printer nozzles, as well as fast local gelling of the pattern.23 As a pattern of ink was deposited onto the alginate meniscus, ion exchange led to crosslinking of alginate, thus enabling cell encapsulation within the droplets which ranged in diameter from 30 to 50 μm.23
In 2008-2009 this method was further advanced by Nakamura et al.7 In their experimental work they succeeded in fabricating fibers and tubes by ejecting alginate droplets into a solution of CaCl2.24,25 Immediately after contact, well-defined hydrogel spheroids formed by diffusion of Ca-ions into the alginate ink droplets which exerted semi-solid properties.7 The constructed "tubes" had wall thicknesses and inner diameters that could be adjusted from 35 to 40 μm and from 30 to 200 μm, respectively, by varying the diameter of the microgel beads (from 10 to 40 μm, accordingly).7
In 2009, Norotte et al described a rapid, scalable, scaffold-free, extrusion-based technique which was used to fabricate tubular vascular grafts.26 This method was based on a totally biological self-assembly approach implemented via rapid prototyping bioprinting. First, various vascular cell types, such as fibroblasts and smooth muscle cells, were aggregated into discrete units, either multicellular spheroids or cylinders of controllable diameter (ranging between 300-500 μm). These cells were then "printed" layer-by-layer concurrently with agarose rods which were used as a molding template. The post-printing fusion of the discrete units resulted in single- and double-layered small diameter vascular tubes.26 This technique has successfully been used to engineer multi-layer vessels with different shapes and complex hierarchical trees with branching geometries.27
In 2010, Cetola et al. proposed a technique for the fabrication of a hybrid vascular graft.28 Specifically, they developed a poly-L-lactide (PLLA)/poly-ε-caprolactone (PCL) scaffold releasing heparin through a combination of electrospinning and fused deposition modelling techniques.28 PLLA/heparin scaffolds were created by electrospinning in a tubular shape. The tubes were then armored with a single coil of PCL on the outer layer to improve mechanical properties. The scaffolds were then seeded with human mesenchymal stem cells and assayed to measure the morphology, mechanical tensile strength, cell viability and differentiation.28 This hybrid graft had a stress-strain profile comparable to a human thoracic artery and maintained the endothelial differentiation and proliferation of the seeded human mesenchymal stem cells.22
Also in 2010, another method was proposed by Skardal and colleagues employing an extrusion-based technique to directly print cellularized tubular tissue constructs made from hyaluronan hydrogels crosslinked with polyethylene glycol.27,29 In their study, new biomaterials were developed and used to fabricate constructs with a shape very similar to a simple form of blood vessel. In addition, the fabricated cell structures were shown to have high viability in culture media for a month.27
In 2015, Itoh et al. used a 3D bioprinter (Cyfuse Biomedical K.K., Japan) for the fabrication of scaffold-free tubular tissues with an inner diameter of 1.5 mm which were cultured in a perfusion system.30 The authors also evaluated blood flow (by ultrasound) and performed histological analysis on the 2nd and 5th days after implantation of the fabricated tubular tissues into the abdominal aortas of F344 nude rats.30 The authors found that all grafts were patent, and remodeling of the tubular tissues (enlargement of the lumen area and thinning of the wall) was observed, while a layer of endothelial cells was confirmed 5 days after implantation.30
Several other cellular and acellular biomaterials and fabrication methodologies for the 3D bioprinting of vascular structures have been developed,7,22,31 and future applications and advancements are anticipated. In addition, new technological developments are anticipated to decrease the cost of 3D bioprinters.32
In some experimental studies the fabrication materials of the scaffolds have contained cells, however several problems remain to be resolved. Since several different cells types exist in blood vessels, including endothelial cells, smooth muscle cells, fibroblasts, connective tissue and others, it is still very difficult in hybrid printing of different cells types to allow for normal growth of multiple cells together.
CONCLUSIONS
Advances in additive manufacturing techniques, medical imaging modalities, biomaterials and cellular engineering will lead to further developments in the fabrication of patient-specific vascular tissue constructs. Considerable challenges remain to be overcome, such as cell and material requirements, tissue maturation and functionality and appropriate vascularization and innervation.14 Future multidisciplinary research and development are expected to further transform the fields of tissue engineering and regenerative medicine.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. J Transl Med. 2016;14:271. doi: 10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cubo N, Garcia M, Del Canizo JF, et al. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication. 2016;9:015006. doi: 10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- 3.Vijayavenkataraman S, Lu WF, Fuh JY. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes. Biofabrication. 2016;8:032001. doi: 10.1088/1758-5090/8/3/032001. [DOI] [PubMed] [Google Scholar]
- 4.Xiongfa J, Hao Z, Liming Z, Jun X. Recent advances in 3D bioprinting for the regeneration of functional cartilage. Regen Med. 2018;13:73–87. doi: 10.2217/rme-2017-0106. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Ao Q, Tian X, et al. 3D bioprinting technologies for hard tissue and organ engineering. Materials (Basel) 2016;9:pii:E802. doi: 10.3390/ma9100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izadifar M, Chapman D, Babyn P, et al. Uv-assisted 3D bioprinting of nanoreinforced hybrid cardiac patch for myocardial tissue engineering. Tissue Eng Part C Methods. 2018;24:74–88. doi: 10.1089/ten.TEC.2017.0346. [DOI] [PubMed] [Google Scholar]
- 7.Hoch E, Tovar GE, Borchers K. Bioprinting of artificial blood vessels: current approaches towards a demanding goal. Eur J Cardiothorac Surg. 2014;46:767–778. doi: 10.1093/ejcts/ezu242. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Liu Y, Luo H, et al. Is a three-dimensional printing model better than a traditional cardiac model for medical education? A pilot randomized controlled study. Acta Cardiol Sin. 2017;33:664–669. doi: 10.6515/ACS20170621A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen A, Rybicki FJ. Maintaining safety and efficacy for 3d printing in medicine. 3D Print Med. 2017;3:1. doi: 10.1186/s41205-016-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Prima M, Coburn J, Hwang D, et al. Additively manufactured medical products – the fda perspective. 3D Print Med. 2016;2:1–6. doi: 10.1186/s41205-016-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull CW. Apparatus for production of three-dimensional objects by stereolithography. Google Patents. 1986;US 4575330 A [Google Scholar]
- 12.Groll J, Boland T, Blunk T, et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication. 2016;8:013001. doi: 10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Sing S, Zhou M, Yeong W. 3D bioprinting processes: a perspective on classification and terminology. Int J Bioprint. 2018;4:151. doi: 10.18063/IJB.v4i2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M, Iwanaga S, Henmi C, et al. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication. 2010;2:014110. doi: 10.1088/1758-5082/2/1/014110. [DOI] [PubMed] [Google Scholar]
- 16.Zhang WJ, Liu W, Cui L, Cao Y. Tissue engineering of blood vessel. J Cell Mol Med. 2007;11:945–957. doi: 10.1111/j.1582-4934.2007.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colunga T, Dalton S. Building blood vessels with vascular progenitor cells. Trends Mol Med. 2018;24:630–641. doi: 10.1016/j.molmed.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira AI, Nealey PF, Murphy CJ. Responses of human keratocytes to micro- and nanostructured substrates. J Biomed Mater Res A. 2004;71:369–376. doi: 10.1002/jbm.a.30089. [DOI] [PubMed] [Google Scholar]
- 19.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 20.Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338:921–926. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- 21.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elomaa L, Yang YP. Additive manufacturing of vascular grafts and vascularized tissue constructs. Tissue Eng Part B Rev. 2017;23:436–450. doi: 10.1089/ten.teb.2016.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesari P, Xu T, Boland T. Layer-by-layer printing of cells and its application to tissue engineering. Mater Res Soc Symp Proc. 2005;845:111–117. [Google Scholar]
- 24.Nakamura M, Nishiyama Y, Henmi C, et al. Ink jet three-dimensional digital fabrication for biological tissue manufacturing: analysis of alginate microgel beads produced by ink jet droplets for three dimensional tissue fabrication. J Imaging Sci Technol. 2008;52:1–16. [Google Scholar]
- 25.Nishiyama Y, Nakamura M, Henmi C, et al. Development of a three-dimensional bioprinter: construction of cell supporting structures using hydrogel and state-of-the-art inkjet technology. J Biomech Eng. 2009;131:035001. doi: 10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- 26.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua CK, Yeing WY. Bioprinting. Principles and Applications. Singapore: World Scientific; 2015. [Google Scholar]
- 28.Centola M, Rainer A, Spadaccio C, et al. Combining electrospinning and fused deposition modeling for the fabrication of a hybrid vascular graft. Biofabrication. 2010;2:014102. doi: 10.1088/1758-5082/2/1/014102. [DOI] [PubMed] [Google Scholar]
- 29.Skardal A, Zhang J, Prestwich GD. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials. 2010;31:6173–6181. doi: 10.1016/j.biomaterials.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 30.Itoh M, Nakayama K, Noguchi R, et al. Scaffold-free tubular tissues created by a bio-3d printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One. 2015;10:e0136681. doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinnock CB, Xu Z, Lam MT. Scaling of engineered vascular grafts using 3d printed guides and the ring stacking method. J Vis Exp. 2017:121. doi: 10.3791/55322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McElheny C, Hayes D, Devireddy R. Design and fabrication of a low-cost three-dimensional bioprinter. J Med Device. 2017;11:0410011–0410019. doi: 10.1115/1.4037259. [DOI] [PMC free article] [PubMed] [Google Scholar]


