Abstract
Heart failure is a growing epidemic, especially in Taiwan because of the aging population. The 2016 Taiwan Society of Cardiology – Heart Failure with reduced Ejection Fraction (TSOC-HFrEF) registry showed that the guideline-recommended therapies were prescribed suboptimally both at the time of hospital discharge and during follow-up. We, therefore, conducted this 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure to reinforce the importance of new diagnostic and therapeutic modalities of heart failure.
The 2019 focused update discusses new diagnostic criteria, pharmacotherapy, non-pharmacological management, and certain co-morbidities of heart failure. Angiotensin receptor neprilysin inhibitor and If channel inhibitor is introduced as new and recommended medical therapies. Latest criteria of cardiac resynchronization therapy, implantable cardioverter-defibrillator, heart transplantation, and ventricular assist device therapy are reviewed in the non-pharmacological management chapter. Co-morbidities in heart failure are discussed including chronic kidney disease, diabetes, chronic obstructive pulmonary disease, and sleep-disordered breathing. We also explain the adequate use of oxygen therapy and non-invasive ventilation in heart failure management. A particular chapter for chemotherapy-induced cardiac toxicity is incorporated in the focused update to emphasize the importance of its recognition and management. Lastly, implications from the TSOC-HFrEF registry and post-acute care of heart failure are discussed to highlight the importance of guideline-directed medical therapy and the benefits of multidisciplinary disease management programs.
With guideline recommendations, we hope that the management of heart failure can be improved in our society.
Keywords: Biomarkers, Cardiac resynchronization therapy, Cardio-oncology, Co-morbidities, Guidelines, Heart failure, Pharmacotherapy, Post-acute care, Transplantation, Ventricular assist device
The Taiwan Society of Cardiology (TSOC) Heart Failure Committee provides periodic reviews of new data to produce focused updates that address clinically essential advances in heart failure (HF) management. This 2019 Focused Update deals with the following topics: (1) Diagnosis: echocardiography; (2) Diagnosis: biomarkers; (3) Pharmacotherapy: angiotensin converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs)/angiotensin receptor neprilysin inhibitor (ARNI); (4) Pharmacotherapy: beta-blockers; (5) Pharmacotherapy: mineralocorticoid receptor antagonists; (6) Pharmacotherapy: If channel inhibitors; (7) Non-pharmacological management: cardiac resynchronization therapy and implantable cardioverter-defibrillators; (8) Non-pharmacological management: surgery; (9) Co-morbidities in HF: chronic kidney disease, diabetes, chronic obstructive pulmonary disease, sleep-disordered breathing; (10) Oxygen therapy in acute HF; (11) Chemotherapy-induced cardiac toxicity; (12) Implications from the Taiwan Society of Cardiology – Heart Failure with reduced Ejection Fraction (TSOC-HFrEF) registry; and (13) Post-acute care of HF.
DIAGNOSIS – ECHOCARDIOGRAPHY
Echocardiography is a term encompassing all cardiac ultrasound imaging techniques. We will focus on the use of three-dimensional (3D) echocardiography, tissue Doppler imaging (TDI), deformation imaging (strain and strain rate) and transthoracic echocardiography in the current guidelines to carefully assess the myocardial systolic and diastolic function of both left and right ventricles.
Assessment of systolic function, classification of heart failure
To assess systolic function, we recommend the modified biplane Simpson’s rule. Left ventricular ejection fraction (LVEF) should be obtained from apical four- and two-chamber views. Contrast agents can also add to the diagnostic accuracy for patients with poor quality images.1 In contrast, the Teichholz and Quinones methods of calculating LVEF from linear dimensions are not recommended in the setting of HF, especially for those with regional wall motion abnormalities. In recent years, some studies have shown that 3D echocardiography, tissue Doppler parameters (such as S wave) and deformation imaging techniques (strain and strain rate) can be used to detect subtle, earlier changes in some HF patients and they are suggested in selected cases.2,3 In a retrospective study enrolling 330 HFrEF Taiwanese patients, the authors assessed the predictive value of the ratio of transmitral early filling velocity (E) to early diastolic tissue velocity (E′) and the early diastolic strain rate (E′sr). They concluded that the E/E′sr ratio was better able to predict the prognosis of HFrEF than the E/E′ ratio. In addition, combined assessments of global longitudinal strain and E/E′sr by speckle-tracking longitudinal strain could facilitate risk stratification of these patients.4
In patients with clinical HF, the definition of HF with preserved ejection fraction (HFpEF) varies widely in previous studies.5-7 In most patients, abnormalities of systolic and diastolic dysfunction coexist. Because ejection fraction (EF) is the most common selection criteria in clinical trials, echocardiographic EF is considered necessary to classify patients with HF. In the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) HF guidelines, HF was classified as HFrEF, HFpEF, and borderline HFpEF according to an EF ≤ 40%, 41~49% and ≥ 50%, respectively, with one subcategory of "HFpEF, improved" to describe a subset of HFrEF patients with improvement or recovery in EF above 40% after treatment.8 In the 2016 European Society of Cardiology (ESC) HF guidelines, "gray zone" HF (EF between 40~49%) was defined as HF with mid-range ejection fraction (HFmrEF).9 HfmrEF has been suggested to be a transitional zone for HFpEF and HFrEF in some recent studies.10,11 In the current guidelines, we also define patients with HF as HFpEF, HFmrEF, and HFrEF according to LVEF < 40%, 40% to 49%, and LVEF ≥ 50% (Table 1).
Table 1. Types of heart failure.
| Types of heart failure | HFpEF | HFmrEF | HFrEF |
| Clinical expression | Symptoms and/or signs | Symptoms and/or signs | Symptoms and/or signs |
| Echocardiographic ejection fraction | LVEF ≥ 50% | LVEF between 40 and 49% | LVEF < 40% |
| Objective evidence | Elevated natriuretic peptides* and echocardiographic cardiac structural change or diastolic dysfunction# | Elevated natriuretic peptides* and echocardiographic cardiac structural change or diastolic dysfunction# |
* B-type natriuretic peptide > 100 pg/mL and/or N-terminal pro-B type natriuretic peptide > 300 pg/mL. # Refer to Figure 1 for structural and function change and diastolic dysfunction grading.
HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced EF; LVEF, left ventricular ejection fraction.
Evaluation of diastolic function
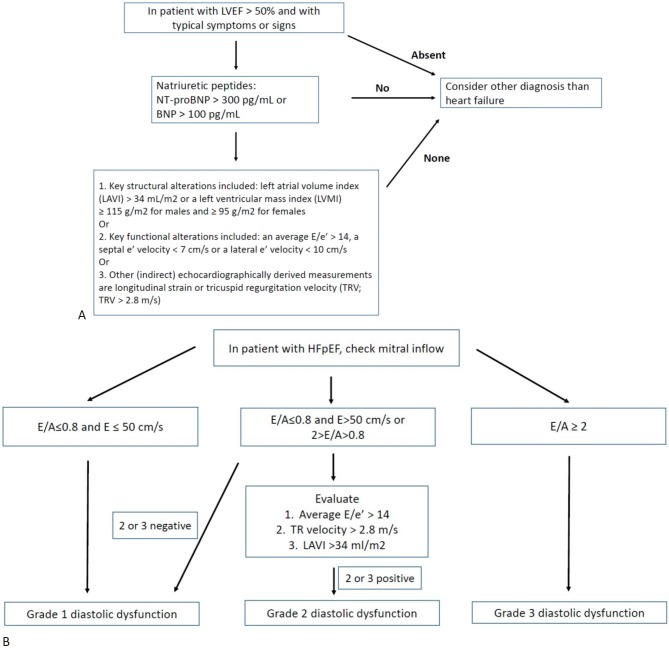
After an initial clinical diagnosis of HFpEF, further objective evidence of echocardiographic cardiac dysfunction is required to validate the diagnosis. Patients with suspected HFpEF or HFmrEF should have the following objective structural and/or functional alterations of the heart:
• Key structural alterations including left atrial volume index (LAVI) > 34 mL/m2 or a left ventricular (LV) mass index ≥ 115 g/m2 for males and ≥ 95 g/m2 for females.9
• Key functional alterations including an average E/e’ > 14, a septal e’ velocity < 7 cm/s or a lateral e’ velocity < 10 cm/s.12
• Other indirect echocardiographically derived measurements including longitudinal strain or tricuspid regurgitation velocity (TRV; TRV > 2.8 m/s).12
• A recent recommendation from the American Society of Echocardiography and the European Association of Cardiovascular Imaging has focused on the assessment of diastolic dysfunction in HFpEF.12 There are three types of abnormal filling patterns recognized conventionally in patients in sinus rhythm.
• When the mitral inflow pattern shows an E/A ratio ≤ 0.8 as well as a peak E velocity of ≤ 50 cm/s, then mean left atrial pressure is considered low. The corresponding grade of diastolic dysfunction is grade I.
• When the mitral inflow pattern shows an E/A ratio ≥ 2, mean left atrial pressure is elevated and is considered to be grade III diastolic dysfunction.
• When mitral inflow shows an E/A ≤ 0.8 and a peak E velocity > 50 cm/s, or if the E/A ratio is > 0.8 but < 2, other criteria should be evaluated including peak TRV > 2.8 m/s, average E/e’ > 14 or LAVI > 34 mL/m2. In patients in whom one of the three main criteria is not available, the ratio of pulmonary vein peak systolic to peak diastolic velocity or systolic time velocity integral to diastolic time-velocity integral < 1 supports the presence of elevated LV filling pressure. If these three parameters are available and none or only one exceeds the cutoff value, the patient is considered to have grade I diastolic dysfunction. If two of the three or all three parameters exceed the cutoff values, then the patient is considered to have grade II diastolic dysfunction. Otherwise, the diastolic dysfunction grade cannot be evaluated and should not be reported.
A diagnostic algorithm for HFpEF and diastolic cardiac dysfunction is shown in Figure 1A, and the grade of diastolic dysfunction is shown in Figure 1B. A recent retrospective study including 451 Taiwanese HFpEF patients evaluated their risks of outcomes based on the 2016 and 2009 diastolic dysfunction grading algorithm. After a follow-up period of 2,976 days, the net reclassification index increased significantly after grading with the 2016 algorithm (10.6%, p < 0.001). Therefore, the 2016 diastolic dysfunction grading algorithm appears to improve the prognostic value in Taiwanese patients with HFpEF.
Figure 1.
(A) Diagnosis of heart failure with preserved ejection fraction. (B) Grading of diastolic dysfunction. BNP, B-type natriuretic peptide; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro B-type natriuretic peptide.
Evaluation of right ventricular function and pulmonary artery pressure
Echocardiography should also address right ventricular (RV) size and function, as well as right atrial size and dimensions.8 RV function is a useful parameter to predict mortality and morbidity in patients with HF.13,14 To measure RV function, the following parameters are especially useful:
• Tricuspid annular plane systolic excursion (TAPSE; abnormal TAPSE < 17 mm indicates RV systolic dysfunction).
• Tissue Doppler-derived tricuspid lateral annular systolic velocity (s′) (s′ velocity < 9.5 cm/s indicates RV systolic dysfunction).1,15,16
• RV fractional area change, which is expressed as a percentage change in the RV chamber area from end-diastole to end-systole, rather than changes in volume.16
• Systolic pulmonary artery pressure derived from an optimal recording of the maximal systolic tricuspid pressure gradient.
• Estimation of right atrial or central venous pressure (CVP) based on inferior vena cava size and its breathing-related collapse.
For patients with severe HF and cardiologists with experience in 3D echocardiography, 3D measurements of RV volume may be more accurate and clinically relevant.3 Newer techniques to assess RV function include 3D speckle-tracking echocardiography, pulsed-wave TDI, color TDI, and strain imaging.16,17
Transesophageal echocardiography and stress echocardiography
Transesophageal echocardiography is recommended in patients with an inadequate thoracic echo window, in patients with complicated valvular disease which cannot be distinguished from transthoracic echo or does not match the patients’ symptoms using transthoracic echo alone, in suspected aortic dissection, suspected endocarditis or congenital heart disease, and to rule out intracavitary thrombi in patients with atrial fibrillation (AF) requiring cardioversion. Stress echocardiography, on the other hand, can be used to assess the severity of ischemic heart disease and myocardial viability.18 Stress echocardiography can also detect exercise diastolic dysfunction for HFpEF patients with an inconclusive diagnosis at rest.19
DIAGNOSIS – BIOMARKERS
Routine diagnostic evaluations for HF should include laboratory tests, including biomarkers for HF, which can be used to assist in the diagnosis and as prognostic predictors. The use of biomarkers to diagnose HF is more convenient than echocardiography as a first line tool at outpatient service or emergency departments. However, these biomarkers can also be elevated in conditions other than HF. Therefore, biomarkers should be used cautiously and be limited to exclusion, especially in patients with atypical presentations.
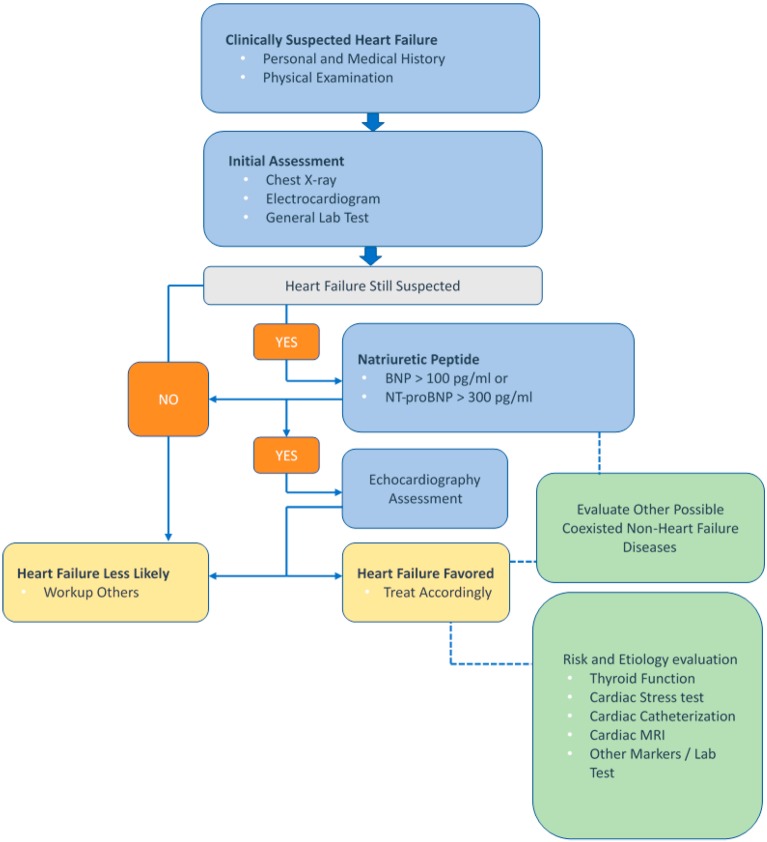
We recommend measuring B-type natriuretic peptide (BNP) or N-terminal proB-type natriuretic peptide (NT-proBNP) to assist in confirming or excluding the diagnosis of HF (Figure 2). Natriuretic peptides (NPs) are biomarkers associated with stretched myocardial myocytes20 which can counteract stress by inducing vasodilation, natriuresis, diuresis, and inhibition of cardiac and vascular myocyte growth. Evidence from some large cohort studies supports the use of NPs, especially BNP and NT-proBNP,21 to predict and diagnose new-onset HF. Other studies also support the potential for predicting the prognosis of HF including hospitalization and overall mortality. Recent studies from Taiwan with regards to patients with acute decompensated HF have reported that a higher BNP was associated with worse function class and a two-fold increased risk of in-hospital mortality.22 In patients with HFpEF, elevated NP levels have also been shown to be a marker associated with a poor prognosis, including mortality and HF-related hospitalization.23,24
Figure 2.
Algorithm of B-type natriuretic peptide (BNP) or N-terminal proB-type natriuretic peptide (NT-proBNP) to assist in the differential diagnosis of HF. HF, heart failure; MRI, magnetic resonance imaging.
We also suggest the use of NPs as a prognostic predictor to monitor the effectiveness of HF therapy before hospital discharge. However, the effectiveness and benefits of serial follow-up measurements or targeting a specific NP reduction as a treatment goal are still unclear. Although some smaller studies have shown an improvement in clinical outcomes,25,26 further studies are required to elucidate the benefits.
Besides HF, ischemic heart disease, uncontrolled hypertension, increasing age, renal dysfunction, anemia, pulmonary diseases, and sepsis can also increase NP levels. The grey zone of BNP as a diagnostic tool for HF is 100-400 pg/mL, and 300-450 pg/mL for NT-proBNP. In elderly patients (age > 75 years), the grey zone of NT-proBNP can be extended to 300-1800 pg/mL.27 Because the level can also be elevated in various conditions other than HF, NPs are preferred as an exclusion tool in first-line screening. A normal concentration in an untreated patient has a high negative predictive value for the diagnosis of HF. Moreover, the level of NPs can be lower in patients with a higher body mass index (BMI) (> 35 kg/m2) due to increased clearance receptors in adipocytes,28 thus the use of NPs in these groups of patients should be applied with caution. For patients receiving treatment with ARNI, NP-proBNP is preferred to evaluate the patient’s prognosis because the mechanism of action of ARNI elevates the level of BNP.
Cardiac troponins should also be sampled in patients with suspected or newly diagnosed HF. Cardiac troponins are an established marker of cardiac injury. Several factors are associated with elevated troponins, including subendocardial ischemia, cardiomyocyte necrosis, cardiomyocyte damage from inflammatory cytokines, oxidative stress, apoptosis, and leakage of troponins from the cytosolic pool due to increased membrane permeability.29 For patients with newly diagnosed HF, troponins can be measured to evaluate the possible etiology and also to predict the prognosis. Patients with acute coronary syndrome-induced HF should consider revascularization. Nevertheless, cardiac troponins can also be elevated in patients with myocarditis and severe HF. An elevated cardiac troponin level in HFrEF patients is significantly associated with mortality and cardiovascular (CV) events.30-32 However, data on the prognostic value in patients with HFpEF are limited.33,34
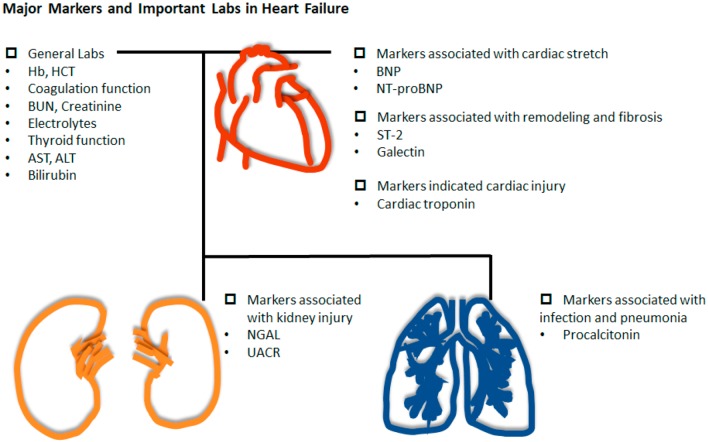
In addition to NPs and cardiac troponins, other markers are also associated with HF (Figure 3). Markers of cardiomyocyte remodeling such as ST-2 and Galectin-3 have been shown to be predictors and markers of HF. An elevated level of soluble ST-2 suggests decreased cardiac protection in cardiac injuries. Studies have also shown that ST-2 can be an independent marker to predict HF hospitalizations and mortality.35 Among patients with acute myocardial infarction (AMI), a high serum ST-2 level has also been shown to be a predictor of HF.36 Macrophages secrete Galectin-3, and this is associated with cardiac fibrosis. Accordingly, studies have suggested that elevated Galectin-3 could be a prognostic indicator of HF. Urinary albumin to creatinine ratio (UACR) and neutrophil gelatinase-associated lipocalin (NGAL) have been used as markers for kidney injury. Recent studies have also suggested that UACR and NGAL can be markers to assess the prognosis of HF.33,36 These kidney biomarkers can reflect nephrotoxic injury and systemic endothelial dysfunction. Although the direct mechanism in HF is unclear, these markers may be an early indicator of kidney injury in HF.37 Furthermore, the occurrence of pneumonia in patients with acute HF is a commonly discussed issue, and initiating appropriate antibiotic therapy is essential. Procalcitonin is a valuable diagnostic marker for infection in the setting of acute exacerbations of HF. The combination of multiple biomarkers may have potential benefits for the diagnosis and prognostic prediction for patients with HF. However, further validation for clinical cohorts is required.
Figure 3.
Key biomarkers and necessary laboratory parameters in the differential diagnosis of HF. ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; Hb, hemoglobin; HCT, hematocrit; HF, heart failure; NGAL, neutrophil gelatinase-associated lipocalin; NT-proBNP, N-terminal proB-type natriuretic peptide; UACR, urinary albumin to creatinine ratio.
PHARMACOTHERAPY – ANGIOTENSIN CONVERTING ENZYME INHIBITORS/ANGIOTENSIN RECEPTOR BLOCKERS/ANGIOTENSIN RECEPTOR NEPRILYSIN INHIBITOR
HF is the final common pathway of various cardiac diseases and is characterized by high morbidity and mortality. A major issue in the treatment of HF is cardiac remodeling after either acute or chronic myocardial injury.38 The renin-angiotensin-aldosterone (RAA) system is deeply involved in cardiac remodeling. Numerous large clinical trials have demonstrated that successfully blocking the RAA axis both reduces morbidity and also improves the survival of HF patients.
Although ACEIs and ARBs have been incorporated into guidelines of international cardiology societies, including the ACC/AHA and ESC, for the management of HF, the prescription rate of RAA system blockers is relatively low. According to the TSOC registry,39 the prescription rates of ACEIs and ARBs are 27.5% and 34.6%, respectively. The combined prescription rate of 62.1% for either an ACEI or ARB is lower than that in Western countries. The doses of both ACEIs and ARBs should be up-titrated to the target doses used in randomized controlled trials (RCTs) if tolerable.40
The development of ARNI has caused a paradigm shift from "add-on" to "replacement" in RAA axis blockers. Although pivotal trials for sacubitril/valsartan are still lacking, the overwhelming superiority of ARNI to ACEIs in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial41 led to the incorporation of ARNI into the revised ESC guidelines for HF in 2016.42
Angiotensin converting enzyme inhibitors
Multiple large-scale RCTs have clearly established the benefits of angiotensin converting enzyme inhibition in patients with mild, moderate, or severe symptoms of HF and in patients with or without coronary artery disease (CAD) [Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS): enalapril, 1987; Studies of Left Ventricular Dysfunction (SOLVD): enalapril, 1991; Survival And Ventricular Enlargement trial (SAVE): captopril, 1992; Acute Infarction Ramipril Efficacy study (AIRE): ramipril, 1993; Trandolapril Cardiac Evaluation study (TRACE): trandolapril 1995; Assessment of Treatment with Lisinopril and Survival trial (ATLAS): lisinopril, 1999].
No significant differences among the available ACEIs have been reported with regards to their effects on symptoms or survival. (ACC/AHA 2017)
• ACEIs reduce morbidity and mortality in HFrEF.
• ACEIs should be started at low doses and titrated upward to doses shown to reduce the risk of CV events in clinical trials.
• ACEIs can produce angioedema and should be given with caution to patients with low systemic blood pressure, renal insufficiency, or elevated serum potassium ([K] > 5.0 mEq/L). The dose of ACEIs should be reduced or held temporarily if serum K > 5.5 mEq/L and be discontinued if K > 6.0 mEq/L.
• If maximal doses are not tolerable, moderate doses should be tried; abrupt withdrawal of ACE inhibition can lead to clinical deterioration and should be avoided. (ACC/AHA 2017)
• Although the use of an ARNI instead of an ACEI for HFrEF is superior, for the patients for whom ARNI is not appropriate, the continued use of an ACEI for all classes of HFrEF remains strongly advised. (ACC/AHA 2017)
Angiotensin receptor blockers
ACEIs are associated with side effects including cough and angioedema, which may compromise its clinical implication. Moreover, escape phenomenon with an elevation in angiotensin II levels may be detected 3 to 6 months after the initiation of ACEI treatment. ARBs were developed with the rationale that angiotensin II production continues in the presence of ACE inhibition, driven through alternative enzyme pathways. ARBs do not inhibit kininase and are associated with a much lower incidence of cough and angioedema than ACEIs.
The findings of multiple large-scale RCTs have shown that long-term therapy with ARBs reduces mortality and morbidity, especially in ACEI-intolerant patients. [Evaluation of Losartan in The Elderly II study (ELITE II): losartan, captopril, 2000; Valsartan in Heart Failure trial (Val-HeFT): valsartan, 2001;43,44 Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity trial (CHARM): candesartan, 2003;45,46 Heart failure Endpoint evaluation of AII-Antagonist Losartan study (HEAAL): losartan, high vs. low dose 200947]. However, ARBs have no beneficial effects on mortality when combined with ACEIs, and may increase the risk of hypotension or hyperkalemia (VAL-HeFT: valsartan/ACEI, ACEI, 2001;43 CHARM add-on: candesartan/ACEI, ACEI, 2003;48 VALsartan In Acute myocardial InfarctioN Trial (VALIANT): valsartan, captopril, or both, 2003; ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET): telmisartan, ramipril, or both, 200849).
• An ARB is recommended to reduce the risk of HF hospitalization and CV death in symptomatic patients intolerant to ACEIs (because of cough or angioedema); (Class A, Level I for ACC/AHA 2017; Class A, Level B for ESC 2016).
• An ARB may be considered to reduce the risk of HF hospitalization and death in patients who are symptomatic despite treatment with a beta-blocker who are unable to tolerate a mineralocorticoid receptor antagonist (MRA) (Class IIb, Level C for ESC 2016).
• Patients already tolerating ARBs for other indications may be continued on ARBs if they subsequently develop HF.
• ARBs should be started at low doses and titrated upward, with an attempt to use doses shown to reduce the risk of CV events in clinical trials.
• ARBs should be given with caution to patients with low systemic blood pressure, renal insufficiency, or elevated serum potassium (> 5.0 mEq/L). The dose of ARBs should be reduced or held temporarily if serum K > 5.5 mEq/L and be discontinued if K > 6.0 mEq/L.
Angiotensin receptor neprilysin inhibitor
The benefits of ACEIs regarding decreased morbidity and mortality have been shown consistently for HF patients across the clinical spectrum, from asymptomatic to severely symptomatic. Similar benefits have been shown for ARBs in populations with mild-to-moderate HF who are unable to tolerate ACEIs.
In ARNI, a single molecule with dual action, the ARB valsartan, blocks the action of angiotensin II at AT1 receptors, thus inhibiting activation of the RAA system and preventing vasoconstriction, renal sodium and fluid retention and cardiac remodeling. On the other hand, the active metabolite in sacubitril LBQ657 inhibits neprilysin and thereby increases NPs, which in turn leads to vasodilation.
In the PARADIGM-HF study, patients with mild-to-moderate HF characterized by either (1) a mildly elevated BNP (> 150 pg/mL) or NT-proBNP (≥ 600 pg/mL), or (2) BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL with a prior hospitalization in the preceding 12 months who were able to tolerate both a target dose of enalapril (10 mg twice daily) and then subsequently an ARNI (sacubitril/valsartan; 200 mg twice daily), were randomized. Compared with the enalapril group, sacubitril/valsartan significantly reduced the combined risk of the primary endpoint (death from a CV cause or first hospitalization for HF) [21.8% vs. 26.5%; hazard ratio (HR) 0.80, 95% confidence interval (CI) 0.73-0.87; p < 0.001]. In particular, the risk of CV death was reduced by 20%, death due to worsening HF by 21%, and sudden cardiac death (SCD) by 20%.
Sacubitril/valsartan therapy is recommended to replace ACEI therapy to further reduce the risk of HF hospitalization and mortality in ambulatory HFrEF patients who remain symptomatic despite optimal therapy with an ACEI, a beta-blocker, and an MRA, and who fit trial criteria.
• The use of an ARNI is associated with hypotension and a low-frequency incidence of angioedema.
• The target dose is 97/103 mg twice daily. Clinical experience will provide further information about the optimal titration and tolerability of ARNI, particularly regarding blood pressure, adjustments in concomitant HF medications, and the rare complication of angioedema.
• ARNI should not be administered concomitantly with an ACEI or within 36 hours of the last dose of an ACEI.
• ARNI should not be administered to patients with a history of angioedema.
Recently, the comParIson Of sacubitril/valsartaN versus Enalapril on Effect on nt-pRo-bnp in patients stabilized from an acute Heart Failure episode trial (PIONEER-HF)50 showed promising results in HFrEF patients who were hospitalized for acute decompensated HF. The initiation of sacubitril/valsartan therapy after hemodynamic stabilization resulted in a significantly greater reduction in NT-proBNP concentration than enalapril therapy, with no significant difference in the rate of renal dysfunction, symptomatic hypotension, hyperkalemia, or angioedema. However, the role of ARNI in the setting of acute HF should be confirmed in a more extensive prospective study.
PHARMACOTHERAPY – BETA-BLOCKERS
Beta-blockers are recommended as first-line therapy for HF. The mortality and morbidity in patients with HF resulting from LV systolic dysfunction have been shown to be reduced by three beta-blockers (bisoprolol, carvedilol, and metoprolol succinate).51-54 Nebivolol, a beta-blocker with vasodilating properties, has been shown to be effective and well-tolerated in older patients with HF.55 Beta-blockers have also been shown to improve LV function and outcomes in Taiwanese studies,56,57 and also in long-term hemodialysis patients with HF in a National Health Insurance Research Database study.58
The TSOC-HFrEF multicenter registry collected data from 21 medical centers or teaching hospitals in Taiwan, and showed that only 59.6% of patients with HF received beta-blocker therapy at discharge,39 which is lower than in Northern America Organized Program To Initiate lifesaving treatMent In HospitaliZEd Patients with Heart Failure registry (OPTIMIZE-HF)59 and Europe ESC Heart Failure Pilot survey (ESC-HF Pilot)60 studies. At 12 months of follow-up, the prescription rate increased to 66.3%,61 and the percentage of patients receiving > 50% of the target dose of beta-blockers increased from 20.6% at discharge to 26.3% at 1-year follow-up.51 However, these results were lower than in the QUALIFY global survey, which reported that the percentages of patients receiving the target dose and > 50% of the target dose of beta-blockers were 14.8% and 51.8%, respectively.62 The mean heart rate in the TSOC-HFrEF registry at 1-year follow-up was 80.7 ± 16.0 bpm, indicating that there was still a need for further drug up-titration or medications.51,61 A multidisciplinary disease management program reported an increase in beta-blocker prescription rate to 77% at discharge in a Taiwan single-center study.63
The prevalence of chronic obstructive pulmonary disease (COPD) and/or asthma in the TSOC-HFrEF registry was 11%,62 which is lower than the 31% in the Acute Decompensated HEart failure national REgistry (ADHERE) and 19% in the EuroHeart Failure Survey II (EHFS-II).64,65 Cardioselective β-blockers, including bisoprolol, carvedilol, and metoprolol, were recommended for patients with coexisting HF and COPD in the 2015 Taiwan cardiologist-pulmonologist consensus handbook and previous studies.66-71 In the Val-HeFT study, cardioselective β-blockers were shown to have a better 23-month mortality rate than non-selective β-blockers66,70 in patients with coexisting HF and COPD. In a Taiwan nationwide study, beta-blockers were shown to reduce mortality, HF exacerbations, and the need for hospitalization in patients with coexisting HF and COPD.72 Moreover, beta-blockers were not shown to be associated with COPD exacerbations.72 However, the suboptimal use of beta-blockers has also been shown in patients with concurrent HF and COPD in Taiwan.72,73
PHARMACOTHERAPY – MINERALOCORTICOID RECEPTOR ANTAGONISTS
• MRAs are recommended in patients with chronic symptomatic HFrEF and New York Heart Association (NYHA) functional class II-IV who are already receiving ACEIs or ARBs and beta blockers to reduce mortality and HF hospitalization.
• In patients following an AMI who have reduced LV function and develop symptoms of HF or have a history of diabetes, treatment with MRAs in addition to optimal medical therapy is recommended to reduce mortality and hospitalizations from a CV cause.
• MRAs should be avoided in patients with advanced chronic kidney disease (CKD) (creatinine > 2.5 mg/dL or estimated glomerular filtration rate [eGFR] < 30 mL/min/ 1.73 m2) or hyperkalemia (potassium level > 5.0 mEq/L).
The benefits of MRA treatment in patients with HFrEF were investigated in two landmark studies: the Randomized Aldactone Evaluation study (RALES)74 and the Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure trial (EMPHASIS-HF).75
The RALES trial randomly assigned patients with NYHA functional class III or IV and an LVEF of no more than 35% who had been treated with an ACEI or loop diuretic to receive spironolactone (25 mg daily) or placebo. After a mean follow-up of 24 months, patients in the spironolactone group showed a 30% reduction in all-cause mortality compared with the placebo group, as well as 29% reduction in SCD and a 35% reduction in the frequency of hospitalizations for worsening HF. The EMPHASIS-HF trial enrolled patients with mild symptoms (NYHA functional class II) and an LVEF of no more than 35% to receive eplerenone (a selective MRA) or placebo, in addition to recommended optimal medical therapy (an ACEI, an ARB, or both and a beta-blocker). After a mean follow-up period of 21 months, the eplerenone group showed a 37% reduction in the composite of CV death or hospitalization for HF as well as a 24% reduction in all-cause mortality.
The effects of MRAs on morbidity and mortality among patients with AMI complicated by LV dysfunction and HF were evaluated in the Eplerenone post-acute myocardial infarction Heart failure Efficacy and SUrvival Study (EPHESUS).76 Patients with LV dysfunction (LVEF ≤ 40%) following AMI who developed symptoms of HF or had a history of diabetes mellitus were randomly assigned to receive eplerenone (25 mg per day initially, titrated to a maximum of 50 mg daily) or placebo in addition to optimal medical therapy (ACEIs, ARBs, diuretics and beta-blockers). After a mean follow-up duration of 16 months, the rates of all-cause mortality, CV death, and hospitalizations for CV events were significantly lower in the eplerenone group.
Of note, patients with hyperkalemia (defined as a serum potassium level > 5.0 mEq/L) or advanced CKD (defined as a serum creatinine concentration > 2.5 mg/dL or eGFR < 30 mL/min/1.73 m2) were all excluded from these randomized trials to avoid life-threatening hyperkalemia in patients with HFrEF.
Dosages of mineralocorticoid receptor antagonists and laboratory monitoring
Spironolactone and eplerenone should be initiated at a dose of 25 mg daily and up-titrated to 50 mg daily after 4~8 weeks. In patients at risk of hyperkalemia or worsening renal function (patients aged ≥ 75 years, with diabetes mellitus, or eGFR < 60 mL/min/1.73 m2),77 an initial regimen of spironolactone 25 mg or eplerenone 25 mg every other day is advised.
The most significant risk related to MRA treatment is hyperkalemia (defined as a potassium level more than 5.5 mEq/L), which occurred in 19.0% of the spironolactone group in the RALES trial and 11.8% of the eplerenone group in the EMPHASIS-HF trial. The development of hyperkalemia is associated with morbidity and mortality.78 However, the treatment benefits of spironolactone were maintained at least until the potassium level exceeded 5.5 mEq/L, and this benefit lost statistical significance as the potassium level approached 6.0 mEq/L.79 Routine follow-up of potassium level and renal function is recommended 1 week and 1 month after starting or increasing the dose of MRAs. Subsequent monitoring should occur at least monthly for the first 3 months and every 3-6 months thereafter according to the baseline renal function. Patients should be educated to avoid foods high in potassium once potassium levels are higher than 5.0 mEq/L. The dose of MRAs should be reduced if potassium levels rise above 5.5 mEq/L. If potassium levels rise above 6.0 mEq/L, MRAs should be withheld. The potassium level should be rechecked within 3-7 days, and MRAs should only be restarted if the follow-up potassium level is less than 5.0 mEq/L.
PHARMACOTHERAPY – If CHANNEL INHIBITORS
Ivabradine is a new therapeutic agent that explicitly inhibits ion movement through the f-channel, thereby inhibiting the If current in the sinoatrial node slowing diastolic depolarization, the sole effect being heart rate reduction, without altering other cardiac functions.80
The Systolic Heart failure treatment with the IF inhibitor ivabradine Trial (SHIFT) demonstrated the efficacy of ivabradine in reducing the composite endpoint of CV death or HF hospitalization. Ivabradine reduced the composite endpoint for HF in patients with symptomatic HFrEF and LVEF ≤ 35%, in sinus rhythm and with a heart rate ≥ 70 beats bpm who had been hospitalized for HF within the previous 12 months, receiving treatment with an evidence-based dose of beta-blockers (or maximum tolerated dose), an ACEI (or ARB), and an MRA.81 Heart rate reduction with ivabradine has been shown to be safe in severe HF and to improve clinical outcomes independently of disease severity.82 Patients receiving ivabradine have been shown to spend fewer days in the hospital as they benefit from a reduction in recurrent hospitalizations,83 which is an essential marker of prognosis and remains a primary objective to reduce healthcare costs. The initiation of ivabradine before discharge has been shown to reduce the risk of rehospitalization during the vulnerable phase after hospitalization for HF.83
Ivabradine treatment is associated with a marked reduction in LV volume and a significant improvement in LVEF, therefore suggesting that it modifies disease progression in patients with HF.84 A great deal of clinical evidence has shown that the use of ivabradine can address unmet needs in the management of systolic HF, as it improves symptoms, increases exercise capacity, improves the quality of life, prevents re-hospitalization, and prolongs survival.85
Ivabradine should be considered to attenuate the risk of CV death and HF hospitalization in HFrEF patients (LVEF ≤ 35%) with NYHA functional class II to IV in sinus rhythm and a resting heart rate ≥ 70 bpm who are receiving a maximal dose of beta-blockers or cannot tolerate or have contraindications for a beta-blocker after receiving an ACEI (or ARB) and an MRA. A high resting heart rate is not only a well-validated risk marker but also a modifiable risk factor in HF.81,86 The magnitude of heart rate reduction with a beta-blocker plus ivabradine, rather than background beta-blocker dose, primarily determines the subsequent effect on outcomes,87 since a substantial proportion of patients with HF cannot tolerate the doses of beta-blockers used in large clinical trials. The most common reasons for patients not receiving target doses include hypotension and fatigue, and contraindications to beta-blockers such as asthma, frequent hypoglycemic episodes or others. Therefore, patients who cannot tolerate optimal beta-blocker doses may benefit from the addition of ivabradine.81,88
Although both beta-blockers and ivabradine are known to reduce resting heart rate, beta-blockers likely reduce ventricular arrhythmias by blocking beta-1 receptors throughout the myocardium. It is therefore likely that beta-blockers have a more pronounced benefit by reducing sudden death,52,89 whereas ivabradine has an isolated effect on sinoatrial nodal tissue and increases diastolic time without affecting blood pressure,90 resulting in improvements in myocardial perfusion and stroke volume and maintaining cardiac output.91 Ivabradine has been shown to have a significant effect on pump failure death with no effect on SCD; these differences in effect indicate that combining the two may result in further benefits and cancel unwanted effects.92 However, bradycardia has been reported to found more common in ivabradine-treated patients.93 Moreover, in a meta-analysis study, patients receiving ivabradine were shown to have more AF than controls.94 Close follow-up is therefore suggested to monitor these effects.
NON-PHARMACOLOGICAL MANAGEMENT – CARDIAC RESYNCHRONIZATION THERAPY & IMPLANTABLE CARDIOVERTER-DEFIBRILLATORS
Cardiac resynchronization therapy for HF
Cardiac resynchronization therapy (CRT) has been shown to improve cardiac performance in appropriately selected patients and to improve symptoms and well-being95-97 and reduce morbidity and mortality.98 Of the improvements in quality-adjusted life years with CRT among patients with moderate to severe HF, two-thirds may be attributed to improved quality of life and one-third to increased longevity.99 The indications are listed in Figure 4.
Figure 4.
Indications for cardiac resynchronization therapy. AF, atrial fibrillation; CLBBB, complete left bundle branch block; CRT, cardiac resynchronization therapy; Fc, functional class; HFrEF, heart failure with reduced ejection fraction; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; QRSd, QRS duration; RVP, right ventricular pacing.
Left ventricular dysfunction
Only the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION)100 and the CArdiac REsynchronization in Heart Failure (CARE-HF)101,102 trials have compared the effect of CRT to guideline-directed medical therapy (GDMT). Most other trials have compared CRT therapy with defibrillation backup (CRT-D) to implantable cardioverter defibrillators (ICDs), and a few have compared CRT-pacemaker (CRT-P) to backup pacing. Most studies of CRT have specified that the LVEF should be < 35%, but the RAFT103 and MADIT-CRT104,105 trials specified an LVEF < 30%, while the REVERSE106-108 trial specified < 40% and the BLOCK-HF109 trial < 50%. Relatively few patients with an LVEF of 35-40% have been randomized. However an individual participant data meta-analysis suggested no reduction in the effect of CRT in this group. The results of CRT trials about remodeling and HF events support a standard threshold of 35% to achieve benefits from CRT in patients with NYHA functional class II through IV HF symptoms.110
QRS morphology and duration
The prevalence of mechanical dyssynchrony has been documented in 40% of patients with dilated cardiomyopathy and QRS duration ≥ 120 ms, and up to 70% of patients with QRS duration ≥ 150 ms and intraventricular mechanical delay, as identified by several echocardiographic techniques.111,112 The COMPANION100 and CARE-HF trials101,102 included patient with a QRS duration ≥ 120 ms, and LVEF ≤ 35% and compared GDMT to CRT pacing therapy without backup defibrillation (CRT-P) and to CRT-D. Both CRT-P and CRT-D reduced the risk of the primary composite endpoint by approximately 20% compared with GDMT alone. The CARE-HF trial enrolled subjects with a QRS duration ≥ 150 ms (89% of the patients) or a QRS duration 120 to 150 ms with echocardiographic evidence of dyssynchrony (11% of the patients) and was the first study to show a significant (36%) reduction in death rate. The prospective EchoCRT trial113,114 suggested possible harm from CRT in patients with a QRS duration < 130 ms, and therefore CRT is not recommended if the QRS duration is < 130 ms in the ESC guidelines.98,113,114 However, randomization in the Echo-CRT trial was not stratified by QRS duration and only in subgroup analysis so that unmeasured residual confounding was possible.114
QRS morphology has also been associated with a beneficial response to CRT. Several studies have shown that patients with left bundle branch block (LBBB) morphology are more likely to respond favorably to CRT, whereas there is less certainty about patients with non-LBBB morphology.98,115 Therefore, the Taiwan National Health Insurance Administration only reimburses CRT for patients with a QRS duration ≥ 120 ms, LBBB, and LV dysfunction.
HFrEF with ventricular pacing dependent
When LVEF is reduced, RV pacing may exacerbate cardiac dyssynchrony. LV dyssynchrony can be prevented by CRT, which might improve patient outcomes.109,116-118 CRT rather than RV pacing is recommended for patients with HFrEF regardless of NYHA functional class who are indicated for ventricular pacing in order to reduce morbidity.109 Upgrading to CRT should be considered in patients with HF and a high proportion of RV pacing despite optimal medical therapy.
Cardiac resynchronization in patients with atrial fibrillation
A subgroup analysis of patients with AF from the RAFT study found no benefit from CRT-D compared with ICD, although less than half of the patients had > 90% biventricular capture.119 Observational studies have reported that when biventricular capture is < 98%, the prognosis of patients with CRT declines.116 Large observational studies have investigated the optimal level of biventricular pacing percentage and found that a higher percentage is associated with more obvious CRT benefits. Optimal CRT benefits have been observed with a biventricular pacing percentage as close to 100% as possible.120-123
The roles of imaging tests
Not all patients respond favorably to CRT.95 Several characteristics can predict improvements in morbidity and mortality, and the extent of reverse remodeling is one of the most important mechanisms of action of CRT. Patients with an ischemic etiology have been shown to have less improvement in LV function due to myocardial scar tissue, which is less likely to undergo favorable remodeling.124 Imaging tests with echocardiography for dyssynchrony have not yet been shown to be of value in selecting patients for CRT.125 Patients with extensive myocardial scarring have been shown to have less improvement in LV function with CRT.126-128 Optimizing the site of the LV lead can be achieved using imaging studies.128,129 Pacing thresholds are higher in scarred myocardium and, if possible, placing the pacing lead in such regions should be avoided.130,131
Recommendations
• CRT is indicated for patients with LV dysfunction (LVEF ≤ 35%), LBBB (QRS ≥ 120 ms) and HF NYHA functional class II-IV.
• CRT is indicated for patients with HFrEF and RV pacing dependent regardless of functional class.
• High biventricular pacing percentage (≥ 98%) is beneficial in patients with CRT and AF.
• Imaging studies can provide information regarding optimal sites for the LV lead.
Implantable cardioverter-defibrillators
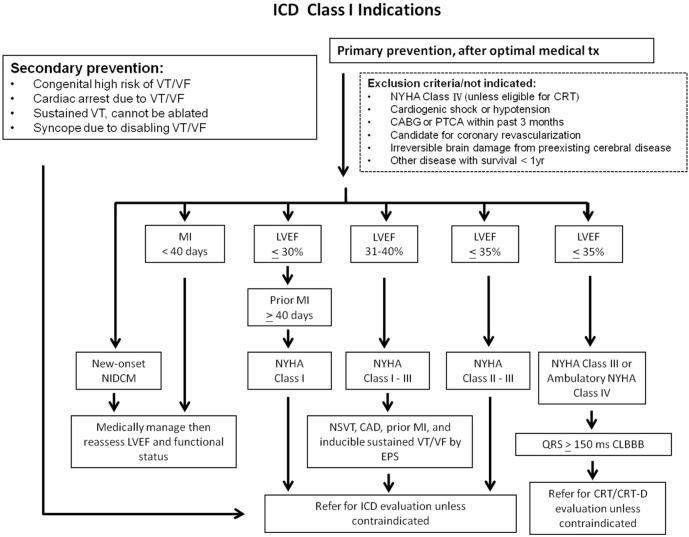
A high proportion of deaths among patients with HF, especially those with milder symptoms, occur suddenly and unexpectedly. Many of these are due to electrical disturbances, including ventricular arrhythmias, bradycardia, and asystole, although some are due to coronary, cerebral or aortic vascular events. Treatments that improve or delay the progression of cardiovascular disease (CVD) will reduce the annual rate of sudden death. ICDs are effective in preventing bradycardia and correcting potentially lethal ventricular arrhythmias. Some antiarrhythmic drugs may also reduce the rates of tachyarrhythmias and sudden death. However they do not reduce overall mortality and may actually increase it. Indications are listed in the algorithm in Figure 5.
Figure 5.
ICD class I indications. CABG, coronary artery bypass graft; CAD, coronary artery disease; CLBBB, complete left bundle branch block; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy defibrillator; EPS, electrophysiologic study; ICD, implantable cardioverter-defibrillator; LVEF left ventricular ejection fraction; MI, myocardial infarction; NIDCM, non-ischemic dilated cardiomyopathy; NSVT, non-sustained ventricular tachycardia; NYHA, New York heart association; PTCA, percutaneous transluminal coronary angioplasty; tx, treatment; VF, ventricular fibrillation; VT, ventricular tachycardia.
Secondary prevention of sudden cardiac death
Compared with amiodarone treatment, ICDs reduce mortality in survivors of cardiac arrest and in patients who have experienced sustained symptomatic ventricular arrhythmias. Therefore, the Taiwan National Health Insurance Administration reimburses indications for secondary prevention. An ICD is recommended in such patients when the intent is to increase survival; the decision to implant should take into account the patient’s wishes and their quality of life, the LVEF (survival benefit is uncertain when the LVEF is > 35%) and the absence of other diseases likely to cause death within the following year.132-134
Primary prevention of sudden cardiac death
Although amiodarone may have been shown to reduce mortality in older trials of HF,135,136 contemporary studies conducted since the widespread introduction of beta-blockers suggest that it does not reduce mortality in patients with HFrEF.137-139 Dronedarone140,141 and class I antiarrhythmic agents140,142 should not be used to prevent arrhythmias in this population. Some guideline-recommended therapies including beta-blockers, MRAs, sacubitril/valsartan, and CRT-Ps have been shown to reduce the risk of sudden death.143
Sudden death has been shown to be strongly reduced by beta-blockers (41-65%).54,144,145 However, ACEIs and ARBs do not fully suppress aldosterone synthesis and do not provide significant benefits with regards to a decrease in SCD. MRAs prevent SCD by controlling potassium loss, blocking the effect of aldosterone on the formation of collagen, and by increasing the myocardial uptake of norepinephrine, which decreases sympathetic activation.146 Spironolactone treatment has been shown to result in a 31% reduction in cardiac death, and eplerenone treatment has been shown to result in a reduction in death from CV causes or hospitalization for CV events (relative risk, 0.83; 95% CI, 0.72-0.94; p = 0.005). A reduction in sudden death from cardiac causes (relative risk, 0.79; 95% CI 0.64-0.97; p = 0.03) has also been reported.147,148
An ICD can reduce the rate of SCD in patients with symptomatic ventricular arrhythmia.149,150 In patients with moderate or severe HF, a reduction in sudden death may be partially or wholly offset by an increase in death due to worsening HF.137 In patients with mild HF (NYHA functional class II-III), an ICD will prevent about two deaths per year for every 100 devices implanted.137 On average, patients with ischemic heart disease are at a greater risk of sudden death than patients with dilated cardiomyopathy, and, therefore, although the relative benefits are similar, the absolute benefit is greater in patients with ischemic heart disease.150 Patients with a longer QRS duration may also benefit more from an ICD. However, these patients should often receive a CRT device.137,151 ICD therapy is not recommended in patients with NYHA functional class IV with severe symptoms refractory to pharmacological therapy who are not candidates for CRT, a ventricular assist device or cardiac transplantation, because such patients have a very limited life expectancy and are likely to die from pump failure. Patients with serious co-morbidities who are unlikely to survive for more than 1 year are unlikely to obtain substantial benefits from an ICD.152-156
Compared with traditional pharmacological therapy, several large RCTs including the Multicenter Automatic Defibrillator Implantation Trial (MADIT), MADIT II, and Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) all showed significant benefits and cost-effectiveness in the primary prevention of SCD by ICD implantation in patients with HFrEF.157 Subgroup analysis of the MADIT and MADIT II trials also showed the same outcome of primary prevention of SCD with ICDs in an Asian population.158,159 Of 313 Taiwanese patients without ICD implantation who satisfied the MADIT II criteria, 152 (49%) died after 4.60 ± 4.31 years of follow-up. Of these patients, 68 (45%) died of SCD, similar to the conventional group in the MADIT II study (51%), and survival during the first 2 years in this cohort was inferior to the conventional group in the MADIT II study.158 Two other RCTs showed no benefits in patients who had an ICD implanted within 40 days after myocardial infarction (MI).160,161 Although sudden arrhythmic deaths were reduced, this was offset by an increase in non-arrhythmic deaths. Accordingly, an ICD is contraindicated during this period. A wearable defibrillator may be considered if the patient is deemed to be at high risk of ventricular fibrillation, although evidence from randomized trials is lacking.162-164
Recommendations
• Secondary prevention is indicated and reimbursed by the Taiwan National Health Insurance Administration.
• SCD is an important issue for patients with LV dysfunction, especially for those after an MI. An ICD is recommended.
• SCD can be reduced with MRAs, beta-blockers, and ARNI rather than ACEIs/ARBs.
• Anti-arrhythmic agents (amiodarone, dronedarone) cannot decrease the incidence of SCD in HF.
NON-PHARMACOLOGICAL MANAGEMENT – SURGERY
Guidelines for heart transplantation listing were established in 2006 and modified in 2016; the two versions of the guidelines are compared with the indications in Taiwanin Table 2. More recent studies have adopted stricter cardiopulmonary stress tests and emphasized the importance of anaerobic threshold to ensure the accuracy of the test results.
Table 2. Comparison of the Taiwan and International Society for Heart and Lung Transplantation 2006 vs. 2016 guidelines for heart transplantation listing165.
| 2006 Guideline recommendations | 2016 Guideline recommendations | |
| Heart transplantation indicationsin Taiwan166 | 1.1. Cardiopulmonary stress testing to guide transplant listing | |
| HF and maximal VO2 < 10 mL/Kg/min. | A maximal cardiopulmonary exercise test is defined as one with a respiratory exchange ratio (RER) > 1.05 and achievement of an anaerobic threshold on optimal pharmacologic therapy (Class I, Level of Evidence: B). | Continuing approval without change. |
| NYHA functional class IV and maximal VO2 < 14 mL/Kg/min. | In patients intolerant of a β-blocker, a cutoff for peak oxygen consumption (VO2) of ≤ 14 mL/kg/min should be used to guide listing (Class I, Level of Evidence: B). | The presence of a CRT device does not alter the current peak VO2 cutoff recommendations (Class I, Level of Evidence: B). |
| Congestive HF | In the presence of a β-blocker, a cutoff for peak VO2 of ≤ 12 mL/kg/min should be used to guide listing (Class I, Level of Evidence: B). | Continuing approval without change. |
| Radionucleotide examination (RNA) LVEF < 20%, maximal medical treatment for more than 6 months with persisted HF symptoms (medications including ACEIs, digoxin, diuretics). | ||
| Severe mitral regurgitation with radionucleotide examination (RNA) LVEF < 25%. | ||
| Severe ischemic heart disease with radionucleotide examination (RNA) LVEF < 20%, thallium scan and cardiac catheterization revealing non-suitable viable revascularizable myocardium. | In young patients (< 50 years) and women, it is reasonable to consider using alternate standards in conjunction with peak VO2 to guide listing, including percent of predicted (≤ 50%) peak VO2 (Class IIa, Level of Evidence: B). | Continuing approval without change. |
| NYHA functional class IV, continuous use of dopamine or dobutamine > 5 mcg/Kg/min for more than 7 days, with radionucleotide examination (RNA) LVEF < 25% or cardiac index < 2.0 L/min/m2. | In the presence of a sub-maximal cardio-pulmonary exercise test (RER < 1.05), use of ventilation equivalent of carbon dioxide (VE/VCO2) slope of > 35 as a determinant in listing for transplantation may be considered (Class IIb, Level of Evidence: C). | Continuing approval without change. |
| HF depending on mechanical support such as extracorporeal membrane oxygenation (ECMO) and ventricular assist device. | In obese (BMI > 30 kg/m2) patients, adjusting peak VO2 to lean body mass may be considered. A lean body mass-adjusted peak VO2 of < 19 mL/kg/min can serve as an optimal threshold to guide prognosis (Class IIb, Level of Evidence: B). | Continuing approval without change. |
| Recurrent symptomatic ventricular arrhythmia which cannot be adequately controlled. | Listing patients based solely on the criterion of a peak VO2 measurement should not be performed (Class III, Level of Evidence: C). | Continuing approval without change. |
| Other end-stage HF, cannot be treated by conventional operation methods. | ||
| 1.2. Use of heart failure prognosis scores | ||
| In circumstances of ambiguity (e.g., peak VO2 > 12 and < 14 mL/kg/min), anHF Survival Score (HFSS) may be considered, and it may add discriminatory value to determining theprognosis and guide listing for transplantation for ambulatory patients (Class IIb, Level of Evidence: C). | HF prognosis scores should be performed along with a cardiopulmonary exercise test to determine the prognosis and guide listing for transplantation for ambulatory patients. An estimated 1-year survival as calculated by the Seattle HF Model (SHFM) of < 80% or an HF Survival Score (HFSS) in the high/medium risk range should be considered as reasonable cutoff points for listing (Class IIb, Level of Evidence: C). Listing patients solely on the criteria of HF survival prognostic scores should not be performed (Class III, Level of Evidence: C). | |
| 1.3. Role of diagnostic right-heart catheterization | ||
| Right heart catheterization (RHC) should be performed on all candidates in preparation for listing for cardiac transplantation and annually until transplantation (Class 1, Level of Evidence: C). | RHC should be performed in all adult candidates in preparation for listing for cardiac transplantation and periodically until transplantation (Class 1, Level of Evidence: C). Periodic RHC is not advocated for routine surveillance in children (Class III, Level of Evidence: C). | |
| RHC should be performed at 3- to 6-month intervals in listed patients, especially in the presence of reversible pulmonary hypertension or worsening HF symptoms (Class I, Level of Evidence: C). | Continuing approval without change. | |
| A vasodilator challenge should be administered when the pulmonary artery systolic pressure is ≥ 50 mmHg and either the transpulmonary gradient is ≥ 15, or the pulmonary vascular resistance (PVR) is > 3 Wood units while maintaining a systolic arterial blood pressure > 85 mmHg (Class I, Level of Evidence: C). | Continuing approval without change. | |
| When an acute vasodilator challenge is unsuccessful, hospitalization with continuous hemodynamic monitoring should be performed, as the PVR will often decline after 24 to 48 hours of treatment consisting of diuretics, inotropes and vasoactive agents such as inhaled nitric oxide (Class I, Level of Evidence: C). | Continuing approval without change. | |
| If medical therapy fails to achieve acceptable hemodynamics, and if the left ventricle cannot be effectively unloaded with mechanical adjuncts, including an intra-aortic balloon pump (IABP) and/or LV assist device (LVAD), it is reasonable to conclude that pulmonary hypertension is irreversible (Class IIb, Level of Evidence: C). | If medical therapy fails to achieve acceptable hemodynamics and if the left ventricle cannot be effectively unloaded with mechanical adjuncts, including an IABP and/or LVAD, it is reasonable to conclude that the pulmonary hypertension is irreversible. After LVAD, reevaluation of hemodynamics should be done after 3 to 6 months to ascertain reversibility of pulmonary hypertension (Class IIA, Level of Evidence: C). |
ACEIs, angiotensin converting enzyme inhibitors; CRT, cardiac resynchronization therapy; HF, heart failure; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
The current trend is more toward durable mechanical support. The suggested indications and contraindications for mechanical support are shown in Table 3.
Table 3. Indications and contraindications for mechanical support.
| Durable mechanical support indications and contraindications167-169 |
| Indications: combination of the following: |
| Frequent hospitalizations for HF |
| NYHA functional class IIIB*-IV functional limitations despite maximal therapy |
| NYHA functional class IIIB*-IV |
| Intolerance to neurohormonal antagonists |
| Increasing diuretic requirement |
| Symptomatic despite CRT |
| Inotrope dependence |
| Low peak VO2 (< 14-16) |
| End-organ dysfunction attributable to low cardiac output |
| Contraindications |
| Absolute |
| Irreversible hepatic disease |
| Irreversible neurological disease |
| Medical nonadherence |
| Severe psychosocial limitations |
| Relative |
| Age > 80 years for destination therapy |
| Irreversible renal disease |
| Obesity or malnutrition |
| Musculoskeletal disease that impairs rehabilitation |
| Active systemic infection or prolonged intubation |
| Untreated malignancy |
| Severe peripheral vascular disease |
| Active substance abuse |
| Impaired cognitive function |
| Unmanaged psychiatric disorder |
| Lack of social support |
CRT, cardiac resynchronization therapy; HF, heart failure; NYHA, New York heart association; * NYHA functional class IIIB refers to Interagency registry for mechanically assisted circulatory support (INTERMACS) patient profiles 6 (exertion limited) symptoms.
The suggested timing of mechanical circulatory (MCS) support is based on the INTEragency Registry for Mechanically Assisted Circulatory Support (INTERMACS) patient profiles as shown in Table 4.
Table 4. INTERMACS patient profiles and mechanical support timing170,171.
| Level | Definition | Description | Time to MCS | |
| 1 | “Crash and burn” | Critical cardiogenic shock | Patients with life-threatening hypotension despite rapidly escalating inotropic support, critical organ hypoperfusion, often confirmed by worsening acidosis and/or lactate levels. | Within hours |
| 2 | “Sliding on inotropes” | Progressive decline | Patients with declining function despite intravenous inotropic support may be manifested by worsening renal function, nutritional depletion, and an inability to restore volume balance. | Within a few days |
| 3 | “Dependent stability” | Stable but inotrope dependent | Patients with stable blood pressure, organ function, nutrition, and symptoms on continuous intravenous inotropic support (or a temporary circulatory support device or both), but demonstrating repeated failure to wean from support due to recurrent symptomatic hypotension or renal dysfunction. | Within a few weeks |
| 4 | “Frequent flyer” | Resting symptoms | Patients can be stabilized close to normal volume status but experience daily symptoms of congestion at rest or during activities of daily living (ADL). Doses of diuretics generally fluctuate at very high levels. More intensive management and surveillance strategies should be considered, which may in some cases reveal poor compliance that would compromise outcomes with any therapy. | Within weeks to months |
| 5 | “Housebound” | Exertion intolerant | Comfortable at rest and with ADL but unable to engage in any other activity, living predominantly within the house. Patients are comfortable at rest without congestive symptoms but may have underlying refractory elevated volume status, often with renal dysfunction. | Variable |
| 6 | “Walking wounded” | Exertion limited | Patients without evidence of fluid overload are comfortable at rest, and with ADL and minor activities outside the home but fatigue after the first few minutes of any meaningful activity. | Variable |
| 7 | “Placeholder” | Advanced NYHA functional class III | Includes patients who are without current or recent episodes of unstable fluid balance, living comfortably with meaningful activity limited to mild physical exertion. | Not a candidate for MCS |
INTERMACS, Interagency registry for mechanically assisted circulatory support; MCS, mechanical circulatory support; NYHA, New York Heart Association.
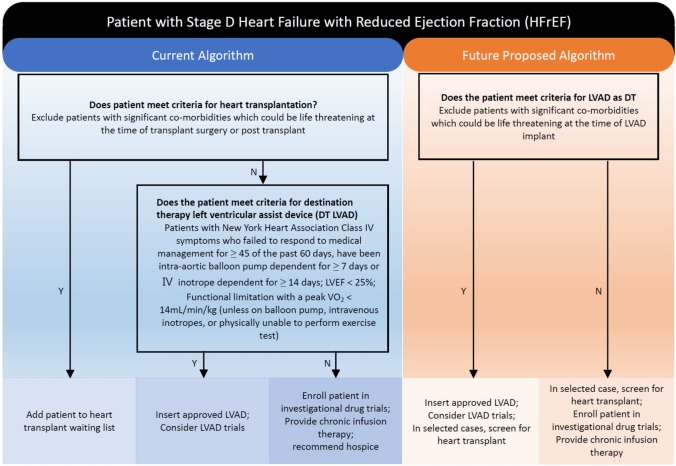
The current algorithm for stage D HF and HFrEF, in which transplantation is the first consideration. In the future, the shortage of organs and improvements in durable LV assist device (LVAD) may change the algorithm (Figure 6).167,168
Figure 6.
Algorithm changes for stage D heart failure. DT, destination therapy; IABP, intra-aortic balloon pump; IV, intravenous; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; VO2, oxygen uptake.
Surgery for heart failure
The standard surgery for stage D HF is still heart transplantation. For stage D HF and HFrEF, temporary or permanent mechanical support can also be considered. Valvular surgery does not provide any survival benefits for patients with stage D HF. In selected patients with stage B and stage C HF, revascularization or valvular surgery is appropriate.172
CO-MORBIDITIES IN HEART FAILURE
Chronic kidney disease
With the implementation of The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines, CKD is clinically defined as an eGFR < 60 mL/min/1.73 m2 and/or the presence of albuminuria (high 30-300 or very high > 300 mg albumin/1 g of urine creatinine) and eGFR < 30 mL/min/1.73 m2 as severe renal dysfunction. Worsening renal functionis defined as an increase in serum creatinine by 25% or 0.3 mg/dL increase or eGFR drop by > 20%.
CKD is one of the most common comorbidities of HF.173 The prevalence of moderate to severe renal dysfunction has been reported to be around 30% to 60%,174 and the incidence of worsening renal function in acute HF has been estimated to be around 45%. The extent of renal dysfunction affects the risk of CVD,175,176 and patients with HF and moderate renal dysfunction have been reported to have a more than 2-fold risk of total mortality.177 The survival of patients on hemodialysis has also been reported to be lower in patients with HF than in those without HF before starting end-stage renal disease (ESRD) therapy.178 Interactions between the heart and kidneys are so tightly involved that they affect the outcome of patients.179,180 Therefore, the term "cardiorenal syndrome" has been coined to indicate the relationship between renal dysfunction and HF.
Many factors contributing to the impairment of renal function in patients with HF have been proposed, including reduced cardiac output, intra-abdominal pressure and CVP, sympathetic overactivity, a maladaptive RAA system, oxidative injury, endothelial dysfunction, and anemia.179,181,182 Reduced cardiac output in HF is considered to be the main factor leading to a decrease in renal function. However, data analysis from the Evaluation Study of Congestive heart failure And Pulmonary artery catheterization Effectiveness trial (ESCAPE) showed that cardiac output was not the only risk factor causing impaired renal function, and a weak correlation between the cardiac index and eGFR was shown.183,184 Nonetheless, CVP level can affect eGFR and mortality. Damman et al. reported that increased CVP was associated with impairment of renal function and independently associated with all-cause mortality during a 10-year follow-up period in patients with CVD.185 In addition, a high CVP level has been reported to be the most important hemodynamic factor leading to renal dysfunction in decompensated patients with advanced HF.185,186 A previous clinical study reported that, when the intra-abdominal venous pressure increased up to approximately 20 mmHg, the GFR decreased by 28%.187 Therefore, the appropriate use of diuretics may reduce renal venous pressure and thus improve GFR. RV dysfunction may also contribute to central venous congestion and impairment of renal function, and thus improving RV function may decrease the extent of renal dysfunction. Along with the hemodynamic factors, HF can activate the RAA system that would subsequently increase sodium reabsorption, water retention, sympathetic overactivity, peripheral vascular contraction, and LV remodeling. Renal function may decline after RAA system activation. Previous studies have shown that ACEIs and ARBs can protect against the deterioration of renal function in diabetic nephropathy.188,189
The metabolism of BNP/NT-proBNP is affected by renal function, and thus caution should be used when using these biomarkers to diagnosis or evaluate patients with acute kidney injury or CKD. However, the level of BNP itself still has a similar diagnostic value to predict LV hypertrophy in dialysis patients as in the general population.190 It is essential to use an echocardiogram to evaluate cardiac function. Foley et al. reported that 73.4% of patients with ESRD before hemodialysis had LV hypertrophy, 35.8% had LV dilatation, and 14.8% had LV dysfunction function.191 The prevalence of LV diastolic dysfunction has been reported to be higher in patients with CKD than in patients without CKD.192 The KDOQI guidelines recommend that echocardiograms should be performed in all patients at the initiation of dialysis, once patients have achieved a dry weight within 1-3 months, and at 3-yearly intervals thereafter.193
Most HF randomized clinical trials have excluded patients with severe renal dysfunction (eGFR < 30 mL/min/1.73 m2). Therefore, there is a lack of evidence-based therapy in these patients. In general, HF patients with eGFR > 30 mL/min/1.73 m2 should receive standard therapy with an ACEI, ARB and MRA.194 Worsening of renal function is often encountered in patients with HF at initiation or up-titration of ACEI or ARB therapy, especially under conditions of dehydration or diuretic usage. It is necessary to increase the frequency of monitoring serum creatinine and electrolytes when up-titrating the doses of ACEIs, ARBs, and diuretics. Although worsening renal function increases mortality in HF patients during therapy, decreasing the doses of ACEIs and ARBs or discontinuing therapy should only be considered when serum creatinine increases or eGFR decreases by more than 30% from the baseline level, since long-term outcomes are not affected by ACEIs or ARBs with a mild decrease in eGFR.195 Furthermore, Brisco et al. reported that an increased serum creatinine level after diuretic therapy did not increase the mortality rate in patients with acute HF.196 In HF patients with renal dysfunction, beta-blockers have been shown to decrease CV mortality by 34% and all-cause mortality by 28%, but increase the risk of hypotension and bradycardia by 5-fold.197 Digoxin should be avoided in patients with acute renal injury and an eGFR < 30 mL/min/1.73 m2. MRAs are also contraindicated in patients with severe renal dysfunction with an eGFR < 30 mL/min/1.73 m2. However, patients with HF undergoing chronic hemodialysis are still recommended to receive ACEI, ARB and beta-blocker therapy.198-200
It is recommended to use loop diuretics to remove fluid overload in acute HF with CKD effectively. Ultrafiltration is considered to those with complications and poor response to high dose diuretics. Theoretically, ultrafiltration therapy could remove fluid faster and sodium efficiently. However, there are potential complications with ultrafiltration therapy including bleeding, hemolysis, hypotension, allergic reaction, air emboli, and worsening renal function. In the Relief for Acutely fluid-overloaded PatIents with Decompensated Congestive Heart Failure trial (RAPID-CHF) and the UltrafiltratioN versus intravenous diuretics for patients hospitaLized for Acute Decompensated heart failure trial (UNLOAD), ultrafiltration therapy could early remove body fluid, improve symptoms and decrease 90-day readmission rate, but no effects on creatinine level and days of hospitalization.201-203 In CArdiorenal REScue Study in acute decompensated Heart Failure (CARESS-HF) trial, it is demonstrated that stepped pharmacologic therapy is preferred to the preservation of renal function and fewer complications than ultrafiltration therapy in acute decompensated HF.204
Diabetes
It is very common for diabetes and dysglycemia in HF patients. Diabetes in HF is associated with poorer LV function and prognosis, and a higher glycohemoglobin (HbA1c) is associated with a higher risk of CV events in HF patients who have not been treated for diabetes.205
It is not clear whether intensive glycemic control alters the risk of CV events, so HF patients with diabetes should be individually assessed for his or her optimal glycemic target for the prevention of macrovascular events.206,207 Metformin is considered to be first-line pharmacological therapy for type 2 diabetes, but it is contraindicated in patients with severe renal or hepatic dysfunction.206-208 Empagliflozin and canagliflozin, inhibitors of sodium-glucose cotransporter 2 (SGLT-2 inhibitors), were shown to significantly reduce CV death and hospitalizations for HF in the Empagliflozin cardiovascular outcome event trial in type 2 diabetes mellitus patients (EMPA-REG OUTCOME)208 and the Canagliflozin cardiovascular assessment study (CANVAS program)209 trials. They should be considered as second-line pharmacological therapy for HF patients with type 2 diabetes. In addition, dapagliflozin reduced the rates of CV death and hospitalizations for HF in the Dapagliflozin Effect on CardiovasculAR Events trial (DECLARE-TIMI 58) trial.210 To date, the DECLARE-TIMI 58 trial is the only diabetic CV outcome trial that has included HF as a primary endpoint. The results of this trial indicated durable protection against HF in those with underlying CVD, and probably in those without a history of CVD. Reliable protection against renal decline was also shown with dapagliflozin, with similar risk estimates in the DECLARE-TIMI 58 trial as previously reported for empagliflozin and canagliflozin in the EMPA-REG OUTCOME and CANVAS program trials.
There is no firm evidence of the additional benefits of long-acting glucagon-like peptide-1 and dipeptidyl peptidase-4 inhibitors (DPP4is) in patients with HF.206,207,211 However, clinicians should be aware of the potential of an increased risk of hospitalization for HF in patients taking DPP4is, particular for saxagliptin, but less for sitagliptin.212-215 Thiazolidinedione can increase the risk of HF and should be avoided in HF patients.206,207 Insulin may exacerbate fluid retention and worsen HF. Sulfonylurea derivatives can also increase the risk of worsening HF.207
Chronic obstructive pulmonary disease
COPD and HF may coexist, and about 20-32% of COPD patients have HF.216 Due to overlap in symptoms and signs, the diagnosis of COPD in a patient with HF may be difficult. BNP/NT-proBNP levels and echocardiography can be used to confirm the diagnosis of HF in COPD patients. COPD should be suspected if HF patients have the following: smoking history; age > 40 years; dyspnea; chronic productive cough; disproportionate dyspnea or body weight loss for an unknown reason; electrocardiogram showing P-pulmonale, atrial flutter, AF or incomplete RBBB; chest X-ray showing increased lung marking, emphysema or hyperinflation; echocardiogram showing right atrial dilation or pulmonary hypertension for an unknown reason. Spirometry should be performed when HF patients have been stable and euvolemic for at least 3 months. If the FEV% (FEV1/FVC) is < 0.7 after inhalation of a bronchodilator, COPD can be diagnosed.217
Beta-blockers are not contraindicated in patients with COPD, and only relatively contraindicated in those with asthma. Starting with a low dose of more selective β1-adrenoceptor antagonists (bisoprolol, metoprolol succinate or nebivolol) combined with close monitoring for signs of airway obstruction is considered to be appropriate to treat COPD patients with HF.207 A study on patients with coexisting COPD and HF using the Taiwan National Health Insurance Research Database showed a dose-response survival benefit of bisoprolol.73 All ACEIs, ARBs, diuretics and MRAs are also suitable treatments for COPD patients with HF.218 Inhaled bronchodilators are preferred for HF patients with COPD rather than the oral forms.219 Long-acting β2-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), or a combination of both have not been associated with an increased risk of CV events.220 Oral corticosteroids can cause sodium and water retention, but is not for inhaled form.221 After LABA or LAMA, combing with low dose theophylline (100-200 mg/day) might be considered for HF patients with COPD. However, the toxicity of theophylline is dose-related and should be closely monitored.219,221
Sleep-disordered breathing
More than one-third of HF patients suffer from sleep-disordered breathing (SDB). The most common types are central sleep apnea (CSA), obstructive sleep apnea (OSA), and a mixed pattern of the two. CSA and HFrEF are closely linked, and OSA is associated with an increased risk of incident HF in men. It is clinically important to distinguish OSA from CSA, given the different responses to treatment. Overnight polysomnography can be used to distinguish the type of sleep apnea in HF patients with suspected SDB or excessive daytime sleepiness.40,207 In patients with CVD and OSA, continuous positive airway pressure (CPAP) may improve sleep quality and daytime sleepiness.222 In patients with paroxysmal AF, the use of CPAP for OSA has been shown to reduce the risk of progressing to more permanent forms.223 Although CPAP, bi-level positive airway pressure (BiPAP) and adaptive servo-ventilation (ASV) can be considered to treat nocturnal hypoxemia in patients with OSA, none of them has been proven to improve the major outcomes in HFrEF.40,206,207,222,224 CPAP has been shown to alleviate CSA, improve LVEF and 6-minute walk test distance, but not to improve the prognosis or the rate of HF-related hospitalizations.225 ASV is not recommended in patients with HFrEF and predominantly CSA due to increases in both all-cause and CV mortality.40,206,207,226
OXYGEN THERAPY IN ACUTE HEART FAILURE
Supplemental oxygen is considered to be a standard treatment for all patients with heart disease, especially for hypoxemia. However, compelling clinical evidence is lacking for the beneficial effect of oxygen therapy in patients with heart disease. It could be life-saving, but it may also be harmful if the inappropriate concentration is given.
Oxygen is an integral part of the maintenance of normal cellular function and tissue survival. Hypoxemia can cause tissue hypoxia that subsequently impairs tissue function. It is reasonable to provide supplemental oxygen in this situation to recover hypoxemia; however excessive oxygen supplementation would have adverse effects on the circulatory system. Most clinical studies on patients with acute coronary syndrome or healthy populations have shown that oxygen therapy decreases cardiac output and stroke volume but increases systemic vascular resistance in normal oxygen saturation (SaO2 > 90%).227-229 Oxygen therapy in patients with ST-segment-elevation MI without hypoxemia may also increase early myocardial injury and MI size.230 A registry-based study did not support the routine use of supplemental oxygen in patients with suspected MI without hypoxemia.231 Another study showed that hyperoxemia increased the generation of reactive oxygen species which then damaged pulmonary alveolar cells in animal studies and that this injury could be alleviated with antioxidants.232 In other animal studies, exposure to a very high oxygen concentration (FIO2 > 0.9) for more than 72-96 hours or even FIO2 > 0.8 for a week induced lung injury.233,234 Therefore, appropriate oxygen supplementation should maintain cellular function and improve tissue hypoxia. Hyperoxemia may cause unnecessary burden and even injury to tissue.
Peripheral pulse oximetry (SpO2) is a standard clinical tool to continuously monitor oxygenation. The exact prevalence of hypoxemia in acute HF is unclear. Theoretically, tissue hypoxia in acute HF results from low cardiac output and decreased tissue perfusion even with normal SpO2. To improve tissue hypoxia in acute HF, it is important to focus on increases in cardiac output and tissue perfusion. Simply providing supplementation with a high oxygen concentration does not seem to be of much help in this condition. Masip et al. demonstrated that measuring baseline oxygen saturation with pulse oximetry in patients with acute HF could enhance the diagnosis of the severity and prognostic implications.235 In their study, baseline oxygen saturation decreased progressively according to the presence and severity of acute HF after acute MI. Another study demonstrated that baseline pO2, pCO2, and pH were not significantly associated with mortality in patients with acute decompensated HF after multivariate analysis.236
A target SpO2 range within 94-98% is recommended in patients with acute HF. In addition, a target SpO2 range of 88-92% should be used for patients at risk of hypercapnic respiratory failure, such as those with COPD.237 A sudden decline in SpO2 ≥ 3% in a patient within the target saturation range and acute illness should prompt a comprehensive assessment. Oxygen supplementation should be considered if the oxygen desaturation is < 94% (or 88% in patients at risk of hypercapnic failure) in patients with acute HF. The routine use of supplemental oxygen in patients without obvious hypoxemia is not recommended.
There is evidence of the efficacy of non-invasive ventilation (NIV) in patients with acute HF and pulmonary edema to reduce the intubation rate and decrease short-term mortality.238,239 However, the routine use of NIV is not advisable. An analysis from the Three Interventions in Cardiogenic Pulmonary Oedema trial showed no differences in intubation rate, 7-day mortality rate, 30-day mortality rate, or admission to an intensive care unit (ICU) among three study groups of CPAP, NIV, or standard oxygen therapy.240 Therefore, NIV (BiPAP or CPAP) is recommended for patients with acute pulmonary edema with a high respiratory rate (> 25 breaths per minute) and persistent systemic hypoxemia (< 90%) despite high-flow oxygen supplementation.241 NIV has the potential risks of worsening hypercapnia, aspiration, right HF, and pneumothorax.
In most acute HF patients, oxygen therapy has to be reduced gradually when they become clinically stable and achieve oxygen saturation above the target range of 94-98% (or 88-92% in patients at risk of hypercapnic failure). Weaning or discontinuing oxygen supplementation should be considered and stopped once a patient is clinically stable on low-concentration oxygen (nasal cannula 2 L/min oxygen). Once the patient can breathe on room air after discontinuing oxygen supplementation, SpO2 and vital signs should be continuously monitored for at least 5 minutes. If oxygen saturation is maintained within the normal range, it is recommended to recheck SpO2 1 hour later.
Recommendations
1. Oxygen therapy is not routinely recommended for patients with acute HF without hypoxemia.
2. Keep SpO2 within 94-98% (88-92% in patients at risk of hypercapnic respiratory failure).
3. Supply oxygen if SpO2 < 94% (88% in patients at risk of hypercapnic respiratory failure).
4. Taper the oxygen concentration if SpO2 > 98% (> 92% in patients at risk of hypercapnic respiratory failure).
5. NIV (BiPAP or CPAP) should not routinely be used (only for patients with acute pulmonary edema with a high respiratory rate (> 25 breaths per minute) and persistent systemic hypoxemia (< 90%) despite high-flow oxygen supplementation.
CHEMOTHERAPY-INDUCED CARDIAC TOXICITY
Introduction
• With advances in cancer therap, the survival of patients with cancer has improved. However, the morbidity and mortality due to treatment side effects have increased.242
• Both traditional, targeted and even immune-therapies can affect the CV system, resulting in hypertension, HF, myocarditis, arrhythmias, vascular disease, and thrombosis.243,244
• Even though the recurrence of cancer causes most cancer-related deaths in cancer survivors, CVD is also responsible for substantial morbidity and mortality.244,245
• According to previous studies, the incidence of chemotherapy-induced cardiotoxicity varies.246,247 In previous retrospective studies, the overall incidence of anthracycline-induced cardiotoxicity is 4-5% for cumulative doses of up to 500 mg/m2, and 11-31% for cumulative doses greater than 500 mg/m2.247-249
• Among the cases of chemotherapy-induced cardiotoxicity, 98% have been reported to occur within the first year and to be asymptomatic.247 However, the effects on the CV system may last for years after exposure, particularly among children.
Mechanism and clinical manifestation
• There are two types of cardiotoxicities (Table 5).
Table 5. Two types of chemotherapy-induced cardiac dysfunction.
| Type I | Type II | |
| Medications | Anthracyclines (Doxorubicin, Idarubicin, Epirubicin) | Monoclonal antibodies (Trastuzumab, Bevacizumab, Pertuzumab) |
| Alkylating agents (Cyclophosphamide, Ifosfamide) | TKIs (Sunitinib, Pazopanib, Sorafenib) | |
| Antimetabolites (Clofarabine) | Protease inhibitors (Carfilzomib, Bortezomib) | |
| Antimicrotubule agents (Docetaxel, Paclitaxel) | ||
| Incidence | 1-48% | 0.2-20% |
| Mechanism | Cellular death | Cellular dysfunction |
| Dose | Dose dependent | Grossly dose independent |
| Pathology | Biopsy findings (+) | Biopsy findings (-) |
| Reversibility | Permanent damage | Mostly reversible |
| Risk factors | Concurrent radiotherapy | Concurrent anthracyclines |
| Age (< 18 or > 65 y/o) | Age | |
| Female | Previous cardiac disease | |
| Previous cardiac disease | Obesity | |
| Hypertension | Genetic factors | |
| Renal failure | ||
| Genetic factors |
• Type I includes anthracyclines (e.g., doxorubicin and epirubicin), alkylating agents, antimetabolites and antimicrotubule agents, which cause permanent cell death and irreversible myocardial damage in a dose-dependent manner.250 For example, doxorubicin has been shown to affect cardiac function mainly through mechanisms that involve the formation of reactive oxygen species, induction of apoptosis, deoxyribonucleic acid (DNA) damage through interactions with topoisomerase II, and inhibition of protein synthesis.250 Subsequently, the production of toxic oxygen-free radicals and the topoisomerase II-beta-doxorubicin-DNA complex can also induce DNA breaks and cell death in cardiomyocytes, resulting in cardiotoxicity.250
• Type II includes monoclonal antibodies, tyrosine kinase inhibitors (TKIs) and protease inhibitors, are their effects are mostly reversible.251 The use of TKIs requires an understanding of mechanism-based (on-target) and bystander-based (off-target) toxicity.251,252 Off-target effects result from non-selective redundancy in essential signaling cascades targeting cancer cell proliferation. Unlike in conventional chemotherapy, off-target toxicity is less common with TKIs.251
Diagnosis
• Cancer therapeutic-related cardiac dysfunction is defined as a reduction of LVEF > 10% to a value below 50%.244,253,254
• Imaging strategies to screen and detect cardiotoxicity include echocardiography, nuclear imaging, and cardiac magnetic resonance examinations.244
• Novel modalities such as speckle-tracking echocardiography have also been used to detect cardiac dysfunction.244 A relative percentage reduction in global longitudinal strain > 15% from baseline may suggest the risk of cardiotoxicity.255
• Biomarkers including troponins and NPs may increase the diagnostic value using the same assays during follow-up measurements.256 The choice of modalities depends on local expertise and availability.
• According to the European Society for Medical Oncology guidelines, patients receiving anthracyclines and/or trastuzumab in an adjuvant setting should receive serial monitoring of cardiac function at baseline, 3, 6 and 9 months during treatment, and then at 12 and 18 months after the initiation of treatment.257 Monitoring should be repeated during or following treatment as clinically indicated.
• Patients with a decreased LVEF at baseline should be evaluated with repeated cardiac imaging 2-3 weeks after showing the initial decrease in LVEF.258 If a reduced LVEF is found at baseline, a multidiscipline consultation between cardiologists and oncologists is required.244
Other cardiac comorbidity associated with chemotherapy
• In addition to HF, chemotherapy is involved in other CV complications such as CAD, arrhythmia, arterial hypertension, valvular disease and venous thrombosis (Table 6).243,244
Table 6. Cancer drug-associated other cardiac comorbidities including arrhythmia, coronary artery disease, and arterial hypertension.
| Arrhythmia | |
| Medications | Risks |
| Anthracyclines, arsenic trioxide, bortezomib, alkylating agents (cisplatin, cyclophosphamide, ifosfamide, melphalan), 5-FU, ifosfamide, IL-2, methotrexate, mitoxantrone, paclitaxel, rituximab, thalidomide. | Bradycardia |
| Anthracyclines, arsenic trioxide, bortezomib, cyclophosphamide, 5-FU, mitoxantrone, rituximab, taxanes, thalidomide. | Atrioventricular block (high risk as a combination of radiotherapy) |
| Alkylating agents, amsacrine, anthracyclines, antimetabolites, bortezomib, IL-2, interferons, paclitaxel | Supraventricular tachycardias (most atrial fibrillation) |
| Alkylating agents, amsacrine, antimetabolites, arsenic trioxide, doxorubicin, interferons, IL-2, methotrexate, paclitaxel, proteasome inhibitors | Ventricular tachycardia/fibrillation |
| Anthracyclines (14%), histone deacetylase inhibitors (depsipeptide, vorinostat) (10-14%), TKIs* (3-15%), arsenic trioxide (35.4%, risk of Torsade de pointes) | QTc prolongation |
| Coronary artery disease | |
| Medications | Risks |
| Fluoropyrimidines (5-FU, capecitabine, gemcitabine) | Symptomatic myocardial ischemia (18%), silent myocardial ischemia (7-10%) |
| VEGF inhibitors# (bevacizumab) | Risk of arterial thrombosis (1.4-3.8%) |
| Radiotherapy | 2-7-fold increased relative risk of myocardial infarction, especially in Hodgkin lymphoma survivors. |
| Dose-dependent | |
| Arterial hypertension | |
| Medications | Risks |
| VEGF inhibitors# | Incidence of HTN up to 23.6% |
| TKIs* | Incidence of HTN up to 15.3-21.6% |
* Tyrosine kinase inhibitors (TKIs), e.g., ponatinib, sorafenib, sunitinib, and ibrutinib; # Vascular endothelial growth factor (VEGF) inhibitors, e.g., bevacizumab. HTN, hypertension.
• For example, kinase inhibitors (e.g., nilotinib or ponatinib) may contribute to hypertension and vascular disease.259
• Angiogenesis-inhibitors (vascular endothelial growth factor [VEGF] inhibitors, e.g., bevacizumab or pazopanib) may induce hypertension, thrombosis, and cardiomyopathy.260
• Proteasome inhibitors (e.g., carfilzomib) are associated with hypertension and thrombosis.261
• Cancer immunotherapies may cause myocarditis and vascular toxicity.262
• Chemotherapeutic agents do not directly affect cardiac valves, but they may be observed in patients post radiotherapy for decades.258,263
• Patients with cancer may experience a broad spectrum of cardiac arrhythmias, including bradyarrhythmias, tachyarrhythmias, and conduction defects at a baseline incidence of 16-36%.264
• Post-chemotherapy, treatment-induced QT prolongation can lead to life-threatening arrhythmias such as torsade de pointes.264 The duration of the QT interval and risk factors for QT prolongation should be monitored before, during and post cancer treatment.244
• Among different drugs, arsenic trioxide, which is mostly used in leukemia and myelomas, has been associated with the high occurrence of QTc prolongation in 26-93% of patients, and subsequent life-threatening ventricular tachyarrhythmias have frequently been reported.265
The management of chemotherapy-induced cardiotoxicity
• Strategies to prevent chemotherapy-induced cardiotoxicity include prevention before treatment, monitoring during treatment and treatment after the development of cardiotoxicity (Figure 7).
Figure 7.
Strategies to reduce chemotherapy-induced cardiotoxicity. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
• Before chemotherapy, the risk factors which increase the incidence of cardiotoxicity should be evaluated and managed carefully. When choosing antineoplastic regimens, analogs (epirubicin, pixantrone) or liposomal formulations can be used instead of doxorubicin in the delivery of continuous infusions (up to 48-96 h) to decrease peak plasma levels.266,267
• Most importantly, the cumulative dose should be carefully monitored to below 400 mg/m2.244 When there is evidence of similar efficacy or superiority with non-anthracycline regimens, they should be considered, particularly in patients with established CV risk factors or previous exposure to anthracyclines.244
• A reliable method to evaluate cardiac function should be performed through periods of treatment and follow-up. Clinicians should be aware of newly developed HF symptoms or asymptomatic reductions in LVEF. A careful review of medications is crucial for further discussion with an oncologist regarding the chemotherapy plan.
• If cardiac dysfunction develops, early interventions for HF management should be initiated, with beta-blockers and ACEIs/ARBs being regarded as the most beneficial medications.244,268
• Chemo- or target therapy regimens should be discontinued if significant complications occur. It remains unclear whether these regimens should be reinstituted once these complications have been managed. The subsequent use of traditional chemotherapies (e.g., fluorouracil) has been associated with an increased risk of serious complications,269 while target therapies (e.g., VEGF inhibitors) should be reinstituted at the same or lower dose to achieve a maximum effect on the tumor once the complications have been controlled.270
Taiwanese data and future perspectives
• Cardio-oncology, a new field in Taiwan with many unmet needs, requires close collaboration between oncologists and cardiologists. With the advances in knowledge in cardio-oncology, some local studies have been performed.
• An increased systemic vascular fluorodeoxyglucose uptake has been observed in serial positron-emission tomography/computed tomography examinations after cisplatin-based concurrent chemoradiotherapy in patients with head/neck cancer.271
• In addition, using speckle-tracking imaging, RV longitudinal strain sensitively has been shown to predict the development of dyspnea in breast cancer patients receiving epirubicin therapy.272 The future points to further joint research to provide evidence-based strategies in cardio-oncology.
IMPLICATIONS FROM THE TAIWAN HFrEF REGISTRY
Although more than 20,000 patients are hospitalized for HF annually, local data of "real-world practice" are scarce. Adherence to guideline-driven HF treatment and multi-disciplinary HF care has not received much attention in Taiwan. Therefore, a nationwide registration program, the Taiwan Society of Cardiology - Heart Failure with reduced Ejection Fraction (TSOC-HFrEF) registry was established to improve awareness of HF management status in Taiwan.
Study design and population
The TSOC-HFrEF registry was a prospective, multicenter, observational survey of patients presenting to 21 hospitals in Taiwan. Hospitalized patients with either acute new-onset HF or acute decompensation of chronic HFrEF were enrolled. Only patients with an LVEF < 40% were enrolled.
Baseline characteristics and management
A total of 1509 patients were enrolled in the registry between May 2013 and October 2014, with a mean age of 64 years (72% were male). The most common etiology of HF was ischemic cardiomyopathy (44.1%), followed by dilated cardiomyopathy (32.9%) and valvular heart disease (7.9%). Diabetes (43.6%), CAD (41.8%), hypertension (34.5%), and chronic renal insufficiency (31.5%) were the most common comorbid conditions. Acute coronary syndrome (31.3%), non-compliance to treatment (24.6%), and concurrent infection (17.0%) were the major precipitating factors for acute decompensation.
After excluding 102 patients who received cardiac implantable electronic device (49 patients with pacemakers, 25 with ICDs and 29 with CRT pacemakers or defibrillators), sinus rhythm was noted in 65.6% and atrial fibrillation/flutter in 27.4% of the patients. Prolonged QRS duration > 120 ms was detected in 25.8% of the patients (25.9% of them showed an LBBB pattern).
The median length of hospital stay was 8 days. During hospitalization, 33% of the patients were admitted to an intensive care unit with a median stay of 4 days. Intravenous diuretics and inotropes were given in 62.6% and 36.5% of the patients, respectively. Mechanical ventilator support for respiratory failure was used in 12.9% of the cases, and including an intra-aortic balloon pump (IABP) and extracorporeal membrane oxygenation (ECMO) were applied in 2.7% and 0.5% of the patients, respectively. A total of 33 patients (2.2%) received either an ICD or CRT during the index hospitalization. At discharge, 62.1% of the patients were prescribed with either an ACEI or ARB, 59.6% with a beta-blocker, and 49% with an MRA.
Patient characteristics, medications, and outcomes of the TSOC-HFrEF registry compared with recent large-scale acute HF registries are shown in Table 7. Several differences were noted among these registries. The patients in Taiwan were younger than those in Western countries, Japan and Korea, but older than those in the Middle East and Africa. Moreover, compared with other cohorts, patients in the TSOC-HFrEF registry were more often male, less frequently had a history of hypertension and more frequently had a history of diabetes mellitus and CKD. The percentage of severe HF (NYHA functional class III-IV) on admission was highest in Taiwan among these registries. Concerning medications at discharge, adherence to using a renin-angiotensin system blocker was lower, but adherence rates to beta-blockers and MRAs were similar compared with the other registries. The utilization of electronic device therapy in Taiwan during the index hospitalization was low compared with that in Europe and Japan.
Table 7. Comparison of patients’ characteristics among recent HF registries.
| GWTG* | ESC-HF-LT# | Gulf CARE† | THESUS-HF‡ | ATTEND* | CHINA-HF§ | KorHF* | Hong Kong HF Registry* | TSOC-HFrEF Registry | |
| Patients’ characteristics | |||||||||
| Year of enrollment | 2005~11 | 2011~13 | 2012 | 2007~10 | 2007~11 | 2012~15 | 2004~09 | 2005~12 | 2013~14 |
| Region | USA | Europe | Middle East | Africa | Japan | China | Korea | Hong Kong | Taiwan |
| Patient numbers, n | 15716 | 4449 | 5005 | 1006 | 2585 | 4882 | 1527 | 383 | 1509 |
| Age, years | 79 | 69.4 | 59 | 52.3 | 69.7 | 60 | 69.1 | 72.2 | 63.9 |
| Male | 60% | 62.6% | 63% | 49.2% | 67.9% | 69.8% | 55.9% | 59.8% | 72.4% |
| BMI, kg/m2 | 25.7 | 28.7 | 27 | 25.2 | 23.2 | 23.7 | 23.2 | - | 25.2 |
| LVEF, % | 25 | 40.4 | 35 | 39.5 | - | - | 28.7 | - | 28.5 |
| SBP at admission, mmHg | 132 | 133.5 | 137 | 130.4 | 143.1 | 121 | 129.3 | - | 130.9 |
| HR at admission, bpm | 82 | 90.8 | 97 | 103.7 | 103.7 | 83 | 93.9 | - | 92.7 |
| NYHA III-IV at admission, % | 85.2 | 75 | 34.6 | 84.6 | 85.8 | 59.6 | - | 88.2 | |
| Median length of stay, day | - | 7 | 7 | 21 | 11 | 8 | - | 8 | |
| Hypertension | 73.1% | 65.6% | 61% | 45.4% | 65.2% | 41.2% | 42.0% | 60.3% | 34.5% |
| Diabetes mellitus | 39.3% | 39.0% | 50% | 11.4% | 35.1% | 20.1% | 31.4% | 36.0% | 43.6% |
| Chronic renal failure | 20.9% | 25.3% | 15.0% | 7.7% | 70.2% | 36.4% | 7.3% | 8.9% | 31.5% |
| Coronary artery disease | 58.0% | 53.8% | 47% | 7.7% | 39.8% | 42.9% | 40.1% | 34.2% | 41.8% |
| Atrial fibrillation | 36.1% | 44.0% | 12% | 18.3% | 31.2% | 23.4% | 20.8% | 31.3% | 26.0% |
| COPD | 26.7% | 20.1% | - | - | 10.8% | - | 3.5% | - | 11.0% |
| Guideline-directed therapy at discharge | |||||||||
| ACEI or ARB | 88.0% | 77.0% | 78% | 81% | 81.2% | 67.5% | 68.0% | 68.6% | 62.1% |
| ACEI | - | - | 61% | - | 36.6% | 48.4% | 45.6% | - | 27.5% |
| ARB | - | - | 17% | - | 44.6% | 19.1% | 24.5% | - | 34.6% |
| Beta-blocker | 73.4% | 72.6% | 71% | 30% | 78.9% | 70.0% | 40.9% | 48.2% | 59.6% |
| Aldosterone antagonist | 24.9% | 53.9% | 43% | 72% | 53.9% | 74.1% | 37.5% | 12.2% | 49.0% |
| In hospital implantation of CRT/ICD | 8.8% | 1.7% | - | 9.9% | 1.4% | 3.7% | - | 2.2% | |
| Outcomes after discharge | |||||||||
| Follow-up period | 1 year | 1 year | 1 year | 180 days | 1 year | Ongoing | 1 year | 1 year | 1 year |
| All-cause mortality | 37.5% | 23.6% | 20.2% | 17.8% (180 days) | 24.8% | - | 9.2% | 19.5% | 15.9% |
| Re-hospitalization | 42.4% | 18.7% | 40% | 9.1% (60 days) | 42.4% (death & readmission) | - | 9.8% | NA | 38.5% |
* Subgroup of patients with LVEF < 40%. # Includes 41.6% of patients with LVEF > 40%. † Includes 31% of patients with LVEF > 40%. ‡ Percentage of HFrEF unknown. § Subgroup of patients with LVEF < 45%.
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HFeEF, heart failure with reduced ejection fraction; HR, heart rate; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York heart association; SBP, systolic blood pressure.
Pharmacological therapy: dosage and up-titration
The prescription rate of guideline-recommended medical therapy was suboptimal in the TSOC-HFrEF registry, and the prescription dosage of these medications was also low. Table 8 shows the types and doses of guideline-recommended medications at discharge and after 1 year of follow-up. In the TSOC-HFrEF registry, the proportions of patients at the target dose and ≥ 50% of the target dose were 5% and 24.4% for ACEIs/ARBs, 3.6% and 20.6% for beta-blockers, and 21.6% and 86.2% for MRAs at discharge, respectively, which were significantly lower than those reported in the recent QUALIFY global survey. Kaplan-Meier survival curves showed that patients who received two or three classes of guideline-recommended medications with ≥ 50% of the target dose had a better prognosis than those who received < 50% of the target dose and those who received fewer than two classes of guideline-recommended medical therapy (Figure 8). Current guidelines recommended that these medications should be up-titrated to the maximum tolerated evidence-based doses in order to reduce mortality and morbidity in patients with HFrEF, but at 1 year of follow-up, the prescription rates and dosages of guideline-recommended medications in the TSOC-HFrEF registry were not significantly increased.
Table 8. Prescribed pharmacological treatments for heart failure over time.
| At discharge | At 12 months | |||||
| Rate of use | Dose (mg/day) | ≥ 50% of target dose | Rate of use | Dose (mg/day) | ≥ 50% of target dose | |
| ACEIs/ARBs | 62.1% | 24.4% | 57.7% | 24.3% | ||
| ACEIs | 27.5% | 27.1% | 16.9% | 36.2% | ||
| Ramipril | 33.8% | 4.5 ± 4.2 | 34.6% | 44.2% | 4.6 ± 4.4 | 36.1% |
| Captopril | 30.3% | 29.0 ± 22.9 | 14.8% | 15.3% | 31.1 ± 20.8 | 12.0% |
| Enalapril | 23.6% | 8.1 ± 8.9 | 31.6% | 20.2% | 12.3 ± 11.9 | 54.5% |
| ARBs | 34.6% | 22.3% | 40.8% | 20.0% | ||
| Candesartan | 39.7% | 7.2 ± 5.0 | 14.4% | 32.9% | 6.8 ± 5.2 | 10.0% |
| Valsartan | 35.0% | 114.1 ± 62.0 | 39.0% | 40.3% | 112.4 ± 61.8 | 34.4% |
| Losartan | 16.4% | 41.4 ± 29.1 | 6.0% | 16.5% | 40.5 ± 18.8 | 4.6% |
| Beta-blockers | 59.6% | 20.6% | 66.2% | 26.3% | ||
| Bisoprolol | 57.9% | 2.5 ± 2.0 | 21.7% | 62.0% | 2.7 ± 1.9 | 27.4% |
| Carvedilol | 37.5% | 13.6 ± 14.1 | 20.7% | 33.7% | 15.0 ± 13.5 | 25.3% |
| Metoprolol | 1.3% | 40.0 ± 26.2 | 18.2% | 2.8% | 48.6 ± 36.1 | 27.8% |
| MRAs | 49.0% | 86.2% | 40.8% | 86.6% | ||
| Spironolactone | 98.7% | 28.9 ± 14.2 | 86.0% | 99.5% | 32.8 ± 29.3 | 86.6% |
| Eplerenone | 1.3% | 52.8 ± 19.5 | 100% | 0.5% | 75.0 ± 35.4 | 100% |
| Diuretics | 82.2% | 75.9% | ||||
| Digitalis | 25.9% | 24.0% | ||||
| Nitrate | 36.4% | 32.2% | ||||
| Hydralazine | 4.9% | 4.2% |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist.
Figure 8.
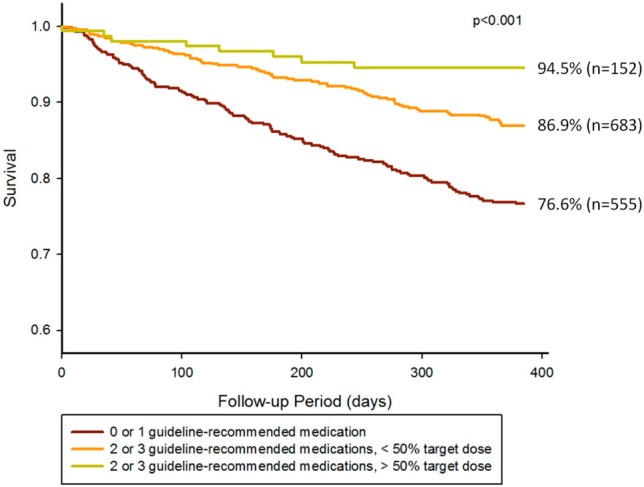
Kaplan-Meier survival curves in registry patients presenting with different types and dosages of guideline-recommended medications. Adapted from “Gap between guidelines and clinical practice in heart failure with reduced ejection fraction: results from TSOC-HFrEF registry”, by Chang HY, Wang CC, Wei J, et al., J Chin Med Assoc 2017;80: 750-7. Adapted with permission.
One-year outcome: comparison with other heart failure registries
The all-cause mortality rate in the TSOC-HFrEF registry was 15.9%, and the CV mortality rate was 10.5% 1 year after hospital discharge. The 1-year re-hospitalization rate for HF was 38.5%, and 9.7% of the patients were admitted more than once within 1 year. Overall, 46.4% of the patients were free from death, hospitalization for HF, implantation of an LVAD and heart transplantation at 1 year. The 1-year mortality rate was comparable to those from Asia registries and better than those from the Middle East and Africa. The older patient populations might explain the high rates of mortality in European and American registries. Nevertheless, the 1-year re-hospitalization rate of 38.5% in Taiwan is very high compared with other registries. Underutilization of guideline-recommended therapies and device therapies, a high prevalence of precipitating factors such as acute coronary syndrome and non-compliance, and proximity to a relatively low-cost healthcare system may contribute to this high rate of re-hospitalization.
Implications of the TSOC-HFrEF registry
The TSOC-HFrEF is the largest Taiwanese database to date involving acute decompensated HFrEF patients. There are several important implications of this registry:
• Significant underutilization of the RAA system blockers at discharge compared to registries in other countries.
• The guideline drugs were used at suboptimal dosages, and there was a reluctance to up-titrate these drugs to the target dose during follow-up.
• The implantation of CRT devices and ICDs remained uncommon.
• The severity of HF in Taiwanese hospitalized patients was high. Acute HF care was aggressive, as one-third of the patients were admitted to an intensive care unit and some of them received invasive cardiopulmonary support. Nevertheless, post-acute HF care did not attract much attention, and the 1-year rehospitalization rate remained high. Prominent efforts should be made to overcome the barriers to guideline adherence, and improve care and reduce the cost of HF.
POST-ACUTE CARE
After discharge for decompensated HF, many patients require post-acute care (PAC) to maximize their functional progress, reduce disability, and make it possible for them to return to their home and community. PAC is also called intermediate care and transitional care. It involves a community-based multidisciplinary team.
The pre-discharge period, post-discharge period, and long-term chronic phase occur after the acute phase. PAC involves these pre- and post-discharge periods for approximately 3 to 6 months (Figure 9).
Figure 9.
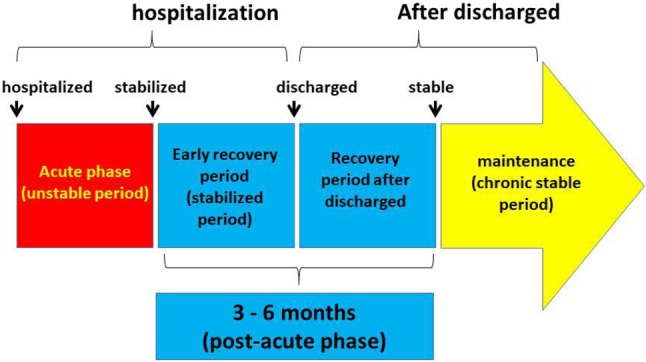
Periods from acute, post-acute to chronic phases.
Figures 10 and Figure 11 depict the recommended items for the pre- and post-discharge periods, respectively.
Figure 10.
Programs before discharge. Cath, cardiac catheterization; COPD, chronic obstructive pulmonary disease; ED, emergency department; EF, ejection fraction; HF, heart failure; ICU, intensive care unit; Lab, laboratory tests; PT, physical therapist; rehab, rehabilitation.
Figure 11.
Programs after discharge. Echo, echocardiograms; HF, heart failure; rehab, rehabilitation.
Annotations:
(* in Figure 10) Contents of education, self-care learning, and a guide to decision making207
(A) Symptom monitoring and self-care
1. Monitor and recognize changes in signs and symptoms.
2. Know how and when to contact a healthcare professional.
3. In line with professional advice, know when to self-manage diuretic therapy and fluid intake. For example, in the case of increasing dyspnea or edema or a sudden unexpected weight gain of > 2 Kg in 3 days, patients may increase their diuretic dose and/or alert their healthcare team.
(B) Diet and alcohol
1. Avoid excessive fluid intake.
2. Recognize the need for altered fluid intake such as:
(1) Increase intake during periods of high heat and humidity, nausea/vomiting.
(2) Fluid restriction of 1.5-2 L/day (including fluid in food) may be considered in patients with severe HF to relieve symptoms and congestion.
3. Monitor body weight and prevent malnutrition.
4. Eat healthily, avoid excessive salt intake (> 5 g/day).
5. Abstain from or avoid excessive alcohol intake.
(C) Psychosocial aspects
1. Depressive symptoms and cognitive dysfunction are found more frequently in people with HF, and that may affect adherence.
2. Recognize psychological problems which may occur in the course of the disease in relation to a changed lifestyle, pharmacotherapy, implanted devices, and others. Patients do not always accept the changes associated with the treatment plan, leading to poor outcomes.
(D) Definition, etiology, and trajectory of HF (including prognosis).
(E) Pharmacological treatment.
(F) Implanted devices and percutaneous/surgical interventions.
(G) Immunization: annual influenza vaccination.
(H) Quit smoking and recreational substance use.
(I) Exercise regularly.
(J) Travel and leisure.
(K) Sleep and breathing: optimize sleep duration and quality.
(L) Sexual activity.
Benefits
A few meta-analyses have consistently shown that multidisciplinary disease management programs (MDP) reduce all-cause and HF-related readmission rates.63,273-275 For example, Roccaforte et al. reported that MDP programs significantly reduced all-cause readmission rates by 24% (odds ratio [OR], 0.76; 95% CI, 0.69-0.94) and HF readmission rates by 42% (OR, 0.58; 95% CI, 0.50-0.67).273 MDP was also associated with a significant reduction in mortality by 20% (OR, 0.80; 95% CI, 0.69-0.93) although less robust, along with improved medication prescription and adherence, improved quality of life, and reduced cost of care.
(† in Figures 10 and 11) Activity, exercise prescription, and cardiac rehabilitation: recommendations276
1. Exercise training (or regular physical activity) is recommended as being safe and effective for patients with HF who can participate in improving functional status. (Class I, Level of Evidence: A)
2. Cardiac rehabilitation can be useful in clinically stable patients with HF to improve functional capacity, exercise duration, health-related quality of life, and mortality. (Class IIa, Level of Evidence: B)
(‡ in Figure 11) Palliative care207
The primary focus is on improving or maintaining the quality of life of a patient and the patient’s family as much as possible until the patient dies. Hospice care also includes advanced care planning, taking into account preferences for the place of death and resuscitation.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 2.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Badano LP, Tsang W, et al. American Society of E; European Association of E. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr. 2012;25:3–46. doi: 10.1016/j.echo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Chan YH, Lee HF, Wu LS, et al. Ratio of transmitral early filling velocity to early diastolic strain rate predicts outcomes in patients with systolic heart failure. Eur Heart J Cardiovasc Imaging. 2017;18:79–85. doi: 10.1093/ehjci/jew015. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 6.Wu CK, Lee JK, Chiang FT, et al. Plasma levels of tumor necrosis factor-alpha and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med. 2011;39:984–992. doi: 10.1097/CCM.0b013e31820a91b9. [DOI] [PubMed] [Google Scholar]
- 7.Wu CK, Tsai HY, Su MM, et al. Evolutional change in epicardial fat and its correlation with myocardial diffuse fibrosis in heart failure patients. J Clin Lipidol. 2017;11:1421–1431. doi: 10.1016/j.jacl.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 10.Nadruz W, Jr., West E, Santos M, et al. Heart failure and midrange ejection fraction: implications of recovered ejection fraction for exercise tolerance and outcomes. Circ Heart Fail. 2016;9:e002826. doi: 10.1161/CIRCHEARTFAILURE.115.002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji K, Sakata Y, Nochioka K, et al. Characterization of heart failure patients with mid-range left ventricular ejection fraction-a report from the CHART-2 Study. Eur J Heart Fail. 2017;19:1258–1269. doi: 10.1002/ejhf.807. [DOI] [PubMed] [Google Scholar]
- 12.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Zornoff LA, Skali H, Pfeffer MA, et al. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–1455. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 14.Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 15.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786-8. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Horton KD, Meece RW, Hill JC. Assessment of the right ventricle by echocardiography: a primer for cardiac sonographers. J Am Soc Echocardiogr. 2009;22:776–792; quiz 861-2.. doi: 10.1016/j.echo.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Smith BC, Dobson G, Dawson D, et al. Three-dimensional speckle tracking of the right ventricle: toward optimal quantification of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 2014;64:41–51. doi: 10.1016/j.jacc.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 18.Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement--executive summary: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur Heart J. 2009;30:278–289. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 19.Erdei T, Smiseth OA, Marino P, Fraser AG. A systematic review of diastolic stress tests in heart failure with preserved ejection fraction, with proposals from the EU-FP7 MEDIA study group. Eur J Heart Fail. 2014;16:1345–1361. doi: 10.1002/ejhf.184. [DOI] [PubMed] [Google Scholar]
- 20.Jortani SA, Prabhu SD, Valdes R Jr. Strategies for developing biomarkers of heart failure. Clin Chem. 2004;50:265–278. doi: 10.1373/clinchem.2003.027557. [DOI] [PubMed] [Google Scholar]
- 21.McKie PM, Cataliotti A, Sangaralingham SJ, et al. Predictive utility of atrial, N-terminal pro-atrial, and N-terminal pro-B-type natriuretic peptides for mortality and cardiovascular events in the general community: a 9-year follow-up study. Mayo Clin Proc. 2011;86:1154–1160. doi: 10.4065/mcp.2011.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen LJ, Hung CL, Yeh HI, et al. The utilization and prognostic impact of B-type natriuretic peptide in hospitalized acute decompensated heart failure in an Asian population. BMC Cardiovasc Disord. 2016;16:178. doi: 10.1186/s12872-016-0342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleland JG, Taylor J, Freemantle N, et al. Relationship between plasma concentrations of N-terminal pro brain natriuretic peptide and the characteristics and outcome of patients with a clinical diagnosis of diastolic heart failure: a report from the PEP-CHF study. Eur J Heart Fail. 2012;14:487–494. doi: 10.1093/eurjhf/hfs049. [DOI] [PubMed] [Google Scholar]
- 24.Anand IS, Rector TS, Cleland JG, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail. 2011;4:569–577. doi: 10.1161/CIRCHEARTFAILURE.111.962654. [DOI] [PubMed] [Google Scholar]
- 25.Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of in-hospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and Mortality rAtes. Am Heart J. 2014;168:30–36. doi: 10.1016/j.ahj.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Felker GM, Ahmad T, Anstrom KJ, et al. Rationale and design of the GUIDE-IT study: guiding evidence based therapy using biomarker intensified treatment in heart failure. JACC Heart Fail. 2014;2:457–465. doi: 10.1016/j.jchf.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maisel A, Mueller C, Adams K, Jr., et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Daniels LB, Clopton P, Bhalla V, et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J. 2006;151:999–1005. doi: 10.1016/j.ahj.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56:1071–1078. doi: 10.1016/j.jacc.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 31.Pascual-Figal DA, Manzano-Fernandez S, Boronat M, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. 2011;13:718–725. doi: 10.1093/eurjhf/hfr047. [DOI] [PubMed] [Google Scholar]
- 32.Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation. 2012;125:280–288. doi: 10.1161/CIRCULATIONAHA.111.044149. [DOI] [PubMed] [Google Scholar]
- 33.de Boer RA, Nayor M, deFilippi CR, et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3:215–224. doi: 10.1001/jamacardio.2017.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santhanakrishnan R, Chong JP, Ng TP, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail. 2012;14:1338–1347. doi: 10.1093/eurjhf/hfs130. [DOI] [PubMed] [Google Scholar]
- 35.Tung YC, Chu PH. Soluble ST2: a novel prognostic biomarker of heart failure. Acta Cardiol Sin. 2014;30:501–503. [PMC free article] [PubMed] [Google Scholar]
- 36.Iqbal N, Wentworth B, Choudhary R, et al. Cardiac biomarkers: new tools for heart failure management. Cardiovasc Diagn Ther. 2012;2:147–164. doi: 10.3978/j.issn.2223-3652.2012.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palazzuoli A, Beltrami M, Pellegrini M, Nuti R. Natriuretic peptides and NGAL in heart failure: does a link exist? Clin Chim Acta. 2012;413:1832–1838. doi: 10.1016/j.cca.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling - concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 39.Wang CC, Chang HY, Yin WH, et al. TSOC-HFrEF Registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin. 2016;32:400–411. doi: 10.6515/ACS20160704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 41.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 42.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 43.Cohn JN, Tognoni G Valsartan Heart Failure Trial (Val-HeFT) Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 44.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of health care databases to support supplemental indications of approved medications. JAMA Intern Med. 2018;178:55–63. doi: 10.1001/jamainternmed.2017.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–776. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 47.Konstam M, Neaton J, Dickstein K, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. 2009;374:1840–1848. doi: 10.1016/S0140-6736(09)61913-9. [DOI] [PubMed] [Google Scholar]
- 48.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 49.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 50.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 51.Chang HY, Wang CC, Wei J, et al. Gap between guidelines and clinical practice in heart failure with reduced ejection fraction: results from TSOC-HFrEF registry. J Chin Med Assoc. 2017;80:750–757. doi: 10.1016/j.jcma.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Krum H, van Veldhuisen DJ, Funck-Brentano C, et al. Effect on mode of death of heart failure treatment started with bisoprolol followed by Enalapril, compared to the opposite order: results of the randomized CIBIS III trial. Cardiovasc Ther. 2011;29:89–98. doi: 10.1111/j.1755-5922.2010.00185.x. [DOI] [PubMed] [Google Scholar]
- 53.Group M-HS. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 54.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 55.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 56.Hu H, Jui HY, Hu FC, et al. Predictors of therapeutic response to beta-blockers in patients with heart failure in Taiwan. J Formos Med Assoc. 2007;106:641–648. doi: 10.1016/S0929-6646(08)60021-2. [DOI] [PubMed] [Google Scholar]
- 57.Lin TY, Chen CY, Huang YB. Evaluating the effectiveness of different beta-adrenoceptor blockers in heart failure patients. Int J Cardiol. 2017;230:378–383. doi: 10.1016/j.ijcard.2016.12.098. [DOI] [PubMed] [Google Scholar]
- 58.Tang CH, Wang CC, Chen TH, et al. Prognostic benefits of carvedilol, bisoprolol, and metoprolol controlled release/extended release in hemodialysis patients with heart failure: a 10-year cohort. J Am Heart Assoc. 2016;5:e002584. doi: 10.1161/JAHA.115.002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 60.Maggioni AP, Dahlstrom U, Filippatos G, et al. EURObservational research programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15:808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 61.Chang HY, Wang CC, Wu YW, et al. One-year outcomes of acute decompensated systolic heart failure in Taiwan: lessons from TSOC-HFrEF registry. Acta Cardiol Sin. 2017;33:127–138. doi: 10.6515/ACS20170202A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Komajda M, Anker SD, Cowie MR, et al. Physicians’ adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail. 2016;18:514–522. doi: 10.1002/ejhf.510. [DOI] [PubMed] [Google Scholar]
- 63.Mao CT, Liu MH, Hsu KH, et al. Effect of multidisciplinary disease management for hospitalized heart failure under a national health insurance programme. J Cardiovasc Med (Hagerstown) 2015;16:616–624. doi: 10.2459/JCM.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 64.Harjola VP, Follath F, Nieminen MS, et al. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12:239–248. doi: 10.1093/eurjhf/hfq002. [DOI] [PubMed] [Google Scholar]
- 65.Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 66.Diagnosis and treatment of coexistingheart failure and chronic obstructive pulmonary disease. https://www.tspccm.org.tw/media/5631: In: Taiwan cardiologist-pulmonologist consensus handbook; 2016. [Google Scholar]
- 67.Salpeter SR, Ormiston TM, Salpeter EE, et al. Cardioselective beta-blockers for chronic obstructive pulmonary disease: a meta-analysis. Respir Med. 2003;97:1094–1101. doi: 10.1016/s0954-6111(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 68.Au DH, Udris EM, Fan VS, et al. Risk of mortality and heart failure exacerbations associated with inhaled beta-adrenoceptor agonists among patients with known left ventricular systolic dysfunction. Chest. 2003;123:1964–1969. doi: 10.1378/chest.123.6.1964. [DOI] [PubMed] [Google Scholar]
- 69.Hirono O, Kubota I, Minamihaba O, et al. Left ventricular diastolic dysfunction in patients with bronchial asthma with long-term oral beta2-adrenoceptor agonists. Am Heart J. 2001;142:E11. doi: 10.1067/mhj.2001.118117. [DOI] [PubMed] [Google Scholar]
- 70.Hawkins NM, Petrie MC, Macdonald MR, et al. Heart failure and chronic obstructive pulmonary disease the quandary of beta-blockers and beta-agonists. J Am Coll Cardiol. 2011;57:2127–2138. doi: 10.1016/j.jacc.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 71.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 72.Liao KM, Lin TY, Huang YB, et al. The evaluation of beta-adrenoceptor blocking agents in patients with COPD and congestive heart failure: a nationwide study. Int J Chron Obstruct Pulmon Dis. 2017;12:2573–2581. doi: 10.2147/COPD.S141694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su VY, Chang YS, Hu YW, et al. Carvedilol, bisoprolol, and metoprolol use in patients with coexistent heart failure and chronic obstructive pulmonary disease. Medicine (Baltimore) 2016;95:e2427. doi: 10.1097/MD.0000000000002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 75.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 76.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 77.Eschalier R, McMurray JJ, Swedberg K, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization and SurvIval Study in Heart Failure). J Am Coll Cardiol. 2013;62:1585–1593. doi: 10.1016/j.jacc.2013.04.086. [DOI] [PubMed] [Google Scholar]
- 78.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 79.Vardeny O, Claggett B, Anand I, et al. Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7:573–579. doi: 10.1161/CIRCHEARTFAILURE.114.001104. [DOI] [PubMed] [Google Scholar]
- 80.Thollon C, Cambarrat C, Vian J, et al. Electrophysiological effects of S 16257, a novel sino-atrial node modulator, on rabbit and guinea-pig cardiac preparations: comparison with UL-FS 49. Br J Pharmacol. 1994;112:37–42. doi: 10.1111/j.1476-5381.1994.tb13025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 82.Borer JS, Bohm M, Ford I, et al. Efficacy and safety of ivabradine in patients with severe chronic systolic heart failure (from the SHIFT study). Am J Cardiol. 2014;113:497–503. doi: 10.1016/j.amjcard.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 83.Borer JS, Bohm M, Ford I, et al. Effect of ivabradine on recurrent hospitalization for worsening heart failure in patients with chronic systolic heart failure: the SHIFT Study. Eur Heart J. 2012;33:2813–2820. doi: 10.1093/eurheartj/ehs259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tardif JC, O'Meara E, Komajda M, et al. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–2515. doi: 10.1093/eurheartj/ehr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ekman I, Chassany O, Komajda M, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32:2395–2404. doi: 10.1093/eurheartj/ehr343. [DOI] [PubMed] [Google Scholar]
- 86.Bohm M, Borer J, Ford I, et al. Heart rate at baseline influences the effect of ivabradine on cardiovascular outcomes in chronic heart failure: analysis from the SHIFT study. Clin Res Cardiol. 2013;102:11–22. doi: 10.1007/s00392-012-0467-8. [DOI] [PubMed] [Google Scholar]
- 87.Swedberg K, Komajda M, Bohm M, et al. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. doi: 10.1016/j.jacc.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 88.Sarraf M, Francis GS. It is all about heart rate. Or is it? J Am Coll Cardiol. 2012;59:1946–1947. doi: 10.1016/j.jacc.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 89.Pereira-Barretto AC. Addressing major unmet needs in patients with systolic heart failure: the role of ivabradine. Am J Cardiovasc Drugs. 2016;16:93–101. doi: 10.1007/s40256-016-0159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dillinger JG, Maher V, Vitale C, et al. Impact of ivabradine on central aortic blood pressure and myocardial perfusion in patients with stable coronary artery disease. Hypertension. 2015;66:1138–1144. doi: 10.1161/HYPERTENSIONAHA.115.06091. [DOI] [PubMed] [Google Scholar]
- 91.De Ferrari GM, Mazzuero A, Agnesina L, et al. Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail. 2008;10:550–555. doi: 10.1016/j.ejheart.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Volterrani M, Cice G, Caminiti G, et al. Effect of carvedilol, ivabradine or their combination on exercise capacity in patients with heart failure (the CARVIVA HF trial). Int J Cardiol. 2011;151:218–224. doi: 10.1016/j.ijcard.2011.06.098. [DOI] [PubMed] [Google Scholar]
- 93.Fox K, Ford I, Steg PG, et al. Bradycardia and atrial fibrillation in patients with stable coronary artery disease treated with ivabradine: an analysis from the SIGNIFY study. Eur Heart J. 2015;36:3291–3296. doi: 10.1093/eurheartj/ehv451. [DOI] [PubMed] [Google Scholar]
- 94.Tanboga IH, Topcu S, Aksakal E, et al. The risk of atrial fibrillation with ivabradine treatment: a meta-analysis with trial sequential analysis of more than 40000 patients. Clin Cardiol. 2016;39:615–620. doi: 10.1002/clc.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sohaib SM, Finegold JA, Nijjer SS, et al. Opportunity to increase life span in narrow QRS cardiac resynchronization therapy recipients by deactivating ventricular pacing: evidence from randomized controlled trials. JACC Heart Fail. 2015;3:327–336. doi: 10.1016/j.jchf.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 96.Cheng CM, Huang JL, Wu TJ, et al. Comparison of quick optimization of interventricular delay between simple methods: intracardiac electrogram and surface electrocardiogram after cardiac resynchronization therapy. Europace. 2012;14:1317–1323. doi: 10.1093/europace/eus061. [DOI] [PubMed] [Google Scholar]
- 97.Cheng CM, Huang JL, Wu TJ, et al. Novel tips for engaging the coronary sinus guided by right ventricular lead. Europace. 2012;14:1754–1758. doi: 10.1093/europace/eus192. [DOI] [PubMed] [Google Scholar]
- 98.Cleland JG, Abraham WT, Linde C, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–3556. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cleland JG, Calvert MJ, Verboven Y, Freemantle N. Effects of cardiac resynchronization therapy on long-term quality of life: an analysis from the CArdiac Resynchronisation-Heart Failure (CARE-HF) study. Am Heart J. 2009;157:457–466. doi: 10.1016/j.ahj.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 101.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 102.Cleland JG, Daubert JC, Erdmann E, et al. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 103.Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 104.Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 105.Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370:1694–1701. doi: 10.1056/NEJMoa1401426. [DOI] [PubMed] [Google Scholar]
- 106.Linde C, Gold MR, Abraham WT, et al. Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur Heart J. 2013;34:2592–2599. doi: 10.1093/eurheartj/eht160. [DOI] [PubMed] [Google Scholar]
- 107.Daubert C, Gold MR, Abraham WT, et al. Prevention of disease progression by cardiac resynchronization therapy in patients with asymptomatic or mildly symptomatic left ventricular dysfunction: insights from the European cohort of the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial. J Am Coll Cardiol. 2009;54:1837–1846. doi: 10.1016/j.jacc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 108.Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 109.Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 110.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61:e6–e75. doi: 10.1016/j.jacc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Haghjoo M, Bagherzadeh A, Fazelifar AF, et al. Prevalence of mechanical dyssynchrony in heart failure patients with different QRS durations. Pacing Clin Electrophysiol. 2007;30:616–622. doi: 10.1111/j.1540-8159.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 112.Bleeker GB, Schalij MJ, Molhoek SG, et al. Relationship between QRS duration and left ventricular dyssynchrony in patients with end-stage heart failure. J Cardiovasc Electrophysiol. 2004;15:544–549. doi: 10.1046/j.1540-8167.2004.03604.x. [DOI] [PubMed] [Google Scholar]
- 113.Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 114.Steffel J, Robertson M, Singh JP, et al. The effect of QRS duration on cardiac resynchronization therapy in patients with a narrow QRS complex: a subgroup analysis of the EchoCRT trial. Eur Heart J. 2015;36:1983–1989. doi: 10.1093/eurheartj/ehv242. [DOI] [PubMed] [Google Scholar]
- 115.Woods B, Hawkins N, Mealing S, et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101:1800–1806. doi: 10.1136/heartjnl-2015-307634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stavrakis S, Garabelli P, Reynolds DW. Cardiac resynchronization therapy after atrioventricular junction ablation for symptomatic atrial fibrillation: a meta-analysis. Europace. 2012;14:1490–1497. doi: 10.1093/europace/eus193. [DOI] [PubMed] [Google Scholar]
- 117.Brignole M, Botto G, Mont L, et al. Cardiac resynchronization therapy in patients undergoing atrioventricular junction ablation for permanent atrial fibrillation: a randomized trial. Eur Heart J. 2011;32:2420–2429. doi: 10.1093/eurheartj/ehr162. [DOI] [PubMed] [Google Scholar]
- 118.Doshi RN, Daoud EG, Fellows C, et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160–1165. doi: 10.1111/j.1540-8167.2005.50062.x. [DOI] [PubMed] [Google Scholar]
- 119.Leclercq C, Walker S, Linde C, et al. Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. European Heart Journal. 2002;23:1780–1787. doi: 10.1053/euhj.2002.3232. [DOI] [PubMed] [Google Scholar]
- 120.Koplan BA, Kaplan AJ, Weiner S, et al. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009;53:355–360. doi: 10.1016/j.jacc.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 121.Hayes DL, Boehmer JP, Day JD, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8:1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 122.Gasparini M, Auricchio A, Regoli F, et al. Four-year efficacy of cardiac resynchronization therapy on exercise tolerance and disease progression: the importance of performing atrioventricular junction ablation in patients with atrial fibrillation. J Am Coll Cardiol. 2006;48:734–743. doi: 10.1016/j.jacc.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 123.Ousdigian KT, Borek PP, Koehler JL, et al. The epidemic of inadequate biventricular pacing in patients with persistent or permanent atrial fibrillation and its association with mortality. Circ Arrhythm Electrophysiol. 2014;7:370–376. doi: 10.1161/CIRCEP.113.001212. [DOI] [PubMed] [Google Scholar]
- 124.Cleland JG, Mareev Y, Linde C. Reflections on EchoCRT: sound guidance on QRS duration and morphology for CRT? Eur Heart J. 2015;36:1948–1951. doi: 10.1093/eurheartj/ehv264. [DOI] [PubMed] [Google Scholar]
- 125.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 126.Chiang KF, Cheng CM, Tsai SC, et al. Relationship of myocardial substrate characteristics as assessed by myocardial perfusion imaging and cardiac reverse remodeling levels after cardiac resynchronization therapy. Ann Nucl Med. 2016;30:484–493. doi: 10.1007/s12149-016-1083-x. [DOI] [PubMed] [Google Scholar]
- 127.Chiang KF, Hung GU, Tsai SC, et al. Impact of cardiac reverse remodeling after cardiac resynchronization therapy assessed by myocardial perfusion imaging on ventricular arrhythmia. J Nucl Cardiol. 2017;24:1282–1288. doi: 10.1007/s12350-016-0447-x. [DOI] [PubMed] [Google Scholar]
- 128.Cheng CM, Su CS, Chou P, et al. Prediction of both electrical and mechanical reverse remodeling on acute electrocardiogram changes after cardiac resynchronization therapy. Circ J. 2017; 81:1322–1328. doi: 10.1253/circj.CJ-16-1181. [DOI] [PubMed] [Google Scholar]
- 129.Hung GU, Huang JL, Lin WY, et al. Impact of right-ventricular apical pacing on the optimal left-ventricular lead positions measured by phase analysis of SPECT myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2014;41:1224–1231. doi: 10.1007/s00259-014-2693-y. [DOI] [PubMed] [Google Scholar]
- 130.Khan FZ, Virdee MS, Palmer CR, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 131.Saba S, Marek J, Schwartzman D, et al. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ Heart Fail. 2013;6:427–434. doi: 10.1161/CIRCHEARTFAILURE.112.000078. [DOI] [PubMed] [Google Scholar]
- 132.Antiarrhythmics versus Implantable Defibrillators I. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 133.Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 134.Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–2078. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 135.Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350:1417–1424. [PubMed] [Google Scholar]
- 136.Andrey JL, Gomez-Soto FM, Romero SP, et al. Mortality of newly diagnosed heart failure treated with amiodarone A propensity-matched study. Int J Cardiol. 2011;151:175–181. doi: 10.1016/j.ijcard.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 137.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 138.Piepoli M, Villani GQ, Ponikowski P, et al. Overview and meta-analysis of randomised trials of amiodarone in chronic heart failure. Int J Cardiol. 1998;66:1–10. doi: 10.1016/s0167-5273(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 139.Torp-Pedersen C, Metra M, Spark P, et al. The safety of amiodarone in patients with heart failure. J Card Fail. 2007;13:340–345. doi: 10.1016/j.cardfail.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 140.Chatterjee S, Ghosh J, Lichstein E, et al. Meta-analysis of cardiovascular outcomes with dronedarone in patients with atrial fibrillation or heart failure. Am J Cardiol. 2012;110:607–613. doi: 10.1016/j.amjcard.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 141.Kober L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 142.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 143.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed) 2016;69:1167. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 144.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 145.CIBIS-II Investigators. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 146.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 147.Mearns BM. Heart failure: EMPHASIS-HF links eplerenone with reduced risk of new-onset AF. Nat Rev Cardiol. 2012;9:373. doi: 10.1038/nrcardio.2012.68. [DOI] [PubMed] [Google Scholar]
- 148.Schwinger RH. The aldosterone antagonist spironolactone prolongs the survival of chronic heart failure patients. The results of the RALES study. The Randomized Aldactone Evaluation Study. Dtsch Med Wochenschr. 1999;124:987–988. [PubMed] [Google Scholar]
- 149.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Theuns DA, Smith T, Hunink MG, et al. Effectiveness of prophylactic implantation of cardioverter-defibrillators without cardiac resynchronization therapy in patients with ischaemic or non-ischaemic heart disease: a systematic review and meta-analysis. Europace. 2010;12:1564–1570. doi: 10.1093/europace/euq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 152.Miller RJ, Howlett JG, Exner DV, et al. Baseline functional class and therapeutic efficacy of common heart failure interventions: a systematic review and meta-analysis. Can J Cardiol. 2015;31:792–799. doi: 10.1016/j.cjca.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 153.Gialama F, Prezerakos P, Maniadakis N. The cost effectiveness of implantable cardioverter defibrillators: a systematic review of economic evaluations. Appl Health Econ Health Policy. 2014;12:41–49. doi: 10.1007/s40258-013-0069-2. [DOI] [PubMed] [Google Scholar]
- 154.Raphael CE, Finegold JA, Barron AJ, et al. The effect of duration of follow-up and presence of competing risk on lifespan-gain from implantable cardioverter defibrillator therapy: who benefits the most? Eur Heart J. 2015;36:1676–1688. doi: 10.1093/eurheartj/ehv102. [DOI] [PubMed] [Google Scholar]
- 155.Steinberg BA, Al-Khatib SM, Edwards R, et al. Outcomes of implantable cardioverter-defibrillator use in patients with comorbidities: results from a combined analysis of 4 randomized clinical trials. JACC Heart Fail. 2014;2:623–629. doi: 10.1016/j.jchf.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hess PL, Al-Khatib SM, Han JY, et al. Survival benefit of the primary prevention implantable cardioverter-defibrillator among older patients: does age matter? An analysis of pooled data from 5 clinical trials. Circ Cardiovasc Qual Outcomes. 2015;8:179–186. doi: 10.1161/CIRCOUTCOMES.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smith T, Jordaens L, Theuns DA, et al. The cost-effectiveness of primary prophylactic implantable defibrillator therapy in patients with ischaemic or non-ischaemic heart disease: a European analysis. Eur Heart J. 2013;34:211–219. doi: 10.1093/eurheartj/ehs090. [DOI] [PubMed] [Google Scholar]
- 158.Chang PC, Chen WT, Wo HT, et al. Long-term survival of multicenter automatic defibrillator implantation trial (MADIT) II-eligible patients in Taiwan. Acta Cardiol Sin. 2014;30:229–235. [PMC free article] [PubMed] [Google Scholar]
- 159.Siu CW, Pong V, Ho HH, et al. Are MADIT II criteria for implantable cardioverter defibrillator implantation appropriate for Chinese patients? J Cardiovasc Electrophysiol. 2010;21:231–235. doi: 10.1111/j.1540-8167.2009.01609.x. [DOI] [PubMed] [Google Scholar]
- 160.Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 161.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 162.Chung MK, Szymkiewicz SJ, Shao M, et al. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol. 2010;56:194–203. doi: 10.1016/j.jacc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zishiri ET, Williams S, Cronin EM, et al. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol. 2013;6:117–128. doi: 10.1161/CIRCEP.112.973552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Opreanu M, Wan C, Singh V, et al. Wearable cardioverter-defibrillator as a bridge to cardiac transplantation: a national database analysis. J Heart Lung Transplant. 2015;34:1305–1309. doi: 10.1016/j.healun.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 165.Mehra MR. Guidelines for listing candidates for heart transplant: a 10-year update. JAMA Cardiology. 2017;2:98–99. doi: 10.1001/jamacardio.2016.3756. [DOI] [PubMed] [Google Scholar]
- 166.Wang SS, Chou NK. Organ Transplantation. Taipei City, Taiwan, ROC: Ho-Chi; 2014. [Google Scholar]
- 167.Mancini D, Colombo PC. Left ventricular assist devices: a rapidly evolving alternative to transplant. J Am Coll Cardiol. 2015;65:2542–2555. doi: 10.1016/j.jacc.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 168.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. doi: 10.1056/NEJMoa1602954. [DOI] [PubMed] [Google Scholar]
- 169.Cook JL, Colvin M, Francis GS, et al. Recommendations for the use of mechanical circulatory support: ambulatory and community patient care: a scientific statement from the American Heart Association. Circulation. 2017;135:e1145–e1158. doi: 10.1161/CIR.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 170.Cedars A, Vanderpluym C, Koehl D, et al. An Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of hospitalization, functional status, and mortality after mechanical circulatory support in adults with congenital heart disease. J Heart Lung Transplant. 2018;37:619–630. doi: 10.1016/j.healun.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 171.Cowger JA, Stulak JM, Shah P, et al. Impact of center left ventricular assist device volume on outcomes after implantation: an INTERMACS analysis. JACC Heart Fail. 2017;5:691–699. doi: 10.1016/j.jchf.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 173.van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16:103–111. doi: 10.1002/ejhf.30. [DOI] [PubMed] [Google Scholar]
- 174.Adams KF, Jr., Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 175.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 176.Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606–1615. doi: 10.1681/ASN.V1071606. [DOI] [PubMed] [Google Scholar]
- 177.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 178.Harnett JD, Foley RN, Kent GM, et al. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–890. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 179.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 180.Damman K, Valente MA, Voors AA, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 181.Shamseddin MK, Parfrey PS. Mechanisms of the cardiorenal syndromes. Nat Rev Nephrol. 2009;5:641–649. doi: 10.1038/nrneph.2009.156. [DOI] [PubMed] [Google Scholar]
- 182.Testani JM, Khera AV, St John Sutton MG, et al. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol. 2010;105:511–516. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 184.Hanberg JS, Sury K, Wilson FP, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol. 2016;67:2199–2208. doi: 10.1016/j.jacc.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 186.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bradley SE, Bradley GP. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest. 1947;26:1010–1022. doi: 10.1172/JCI101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 189.Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385:2047–2056. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
- 190.Mallamaci F, Zoccali C, Tripepi G, et al. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int. 2001;59:1559–1566. doi: 10.1046/j.1523-1755.2001.0590041559.x. [DOI] [PubMed] [Google Scholar]
- 191.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 192.Lopez B, Gonzalez A, Hermida N, et al. Myocardial fibrosis in chronic kidney disease: potential benefits of torasemide. Kidney Int Suppl. 2008:S19–S23. doi: 10.1038/ki.2008.512. [DOI] [PubMed] [Google Scholar]
- 193.Workgroup K. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45(Suppl):S1–S153. [PubMed] [Google Scholar]
- 194.Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Suppl):S1–S266. [PubMed] [Google Scholar]
- 195.Clark H, Krum H, Hopper I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2014;16:41–48. doi: 10.1002/ejhf.13. [DOI] [PubMed] [Google Scholar]
- 196.Brisco MA, Zile MR, Hanberg JS, et al. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. 2016;22:753–760. doi: 10.1016/j.cardfail.2016.06.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Badve SV, Roberts MA, Hawley CM, et al. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 198.Cice G, Ferrara L, D'Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 199.Winkelmayer WC, Charytan DM, Levin R, Avorn J. Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis. 2006;47:301–308. doi: 10.1053/j.ajkd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 200.Cice G, Di Benedetto A, D'Isa S, et al. Effects of telmisartan added to angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010;56:1701–1708. doi: 10.1016/j.jacc.2010.03.105. [DOI] [PubMed] [Google Scholar]
- 201.Bart BA. Treatment of congestion in congestive heart failure: ultrafiltration is the only rational initial treatment of volume overload in decompensated heart failure. Circ Heart Fail. 2009;2:499–504. doi: 10.1161/CIRCHEARTFAILURE.109.863381. [DOI] [PubMed] [Google Scholar]
- 202.Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46:2043–2046. doi: 10.1016/j.jacc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 203.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 204.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gerstein HC, Swedberg K, Carlsson J, et al. The hemoglobin a1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the candesartan in heart failure: assessment of reduction in mortality and morbidity (charm) program. Arch Intern Med. 2008;168:1699–1704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 206.Ezekowitz JA, O'Meara E, McDonald MA, et al. 2017 comprehensive update of the Canadian Cardiovascular Society Guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 207.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Kardiol Pol. 2016;74:1037–1147. doi: 10.5603/KP.2016.0141. [DOI] [PubMed] [Google Scholar]
- 208.American Diabetes Association. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(Suppl):S64–S74. doi: 10.2337/dc17-S011. [DOI] [PubMed] [Google Scholar]
- 209.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 210.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 211.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 213.Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 Randomized Trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 214.Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–2076. doi: 10.1016/S0140-6736(14)62225-X. [DOI] [PubMed] [Google Scholar]
- 215.Filion KB, Suissa S. DPP-4 inhibitors and heart failure: some reassurance, some uncertainty. Diabetes Care. 2016;39:735–737. doi: 10.2337/dci15-0036. [DOI] [PubMed] [Google Scholar]
- 216.Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2007;49:171–180. doi: 10.1016/j.jacc.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 217.Güder G, Brenner S, Störk S, et al. Chronic obstructive pulmonary disease in heart failure: accurate diagnosis and treatment. Eur J Heart Fail. 2014;16:1273–1282. doi: 10.1002/ejhf.183. [DOI] [PubMed] [Google Scholar]
- 218.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012 The Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 219.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report. Respirology. 2017;22:575–601. doi: 10.1111/resp.13012. [DOI] [PubMed] [Google Scholar]
- 220.Mascarenhas J, Azevedo A, Bettencourt P. Coexisting chronic obstructive pulmonary disease and heart failure: implications for treatment, course and mortality. Curr Opin Pulm Med. 2010;16:106–111. doi: 10.1097/MCP.0b013e328335dc90. [DOI] [PubMed] [Google Scholar]
- 221.Diagnosis and treatment of coexisting heart failure and chronic obstructive pulmonary disease. asthma-copd.tw/images/files/Brochure/0008.pdf: Taiwan cardiologist-pulmonologist consensus handbook; 2016. [Google Scholar]
- 222.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 223.Shukla A, Aizer A, Holmes D, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC: Clinical Electrophysiology. 2015;1:41–51. doi: 10.1016/j.jacep.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 224.Randerath WJ, Nothofer G, Priegnitz C, et al. Long-term auto-servoventilation or constant positive pressure in heart failure and coexisting central with obstructive sleep apnea. Chest. 2012;142:440–447. doi: 10.1378/chest.11-2089. [DOI] [PubMed] [Google Scholar]
- 225.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 226.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sukumalchantra Y, Levy S, Danzig R, et al. Correcting arterial hypoxemia by oxygen therapy in patients with acute myocardial infarction. Effect on ventilation and hemodynamics. Am J Cardiol. 1969;24:838–852. doi: 10.1016/0002-9149(69)90474-3. [DOI] [PubMed] [Google Scholar]
- 228.Mak S, Azevedo ER, Liu PP, Newton GE. Effect of hyperoxia on left ventricular function and filling pressures in patients with and without congestive heart failure. Chest. 2001;120:467–473. doi: 10.1378/chest.120.2.467. [DOI] [PubMed] [Google Scholar]
- 229.Bodetoft S, Carlsson M, Arheden H, Ekelund U. Effects of oxygen inhalation on cardiac output, coronary blood flow and oxygen delivery in healthy individuals, assessed with MRI. Eur J Emerg Med. 2011;18:25–30. doi: 10.1097/MEJ.0b013e32833a295e. [DOI] [PubMed] [Google Scholar]
- 230.Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 231.Hofmann R, James SK, Jernberg T, et al. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377:1240–1249. doi: 10.1056/NEJMoa1706222. [DOI] [PubMed] [Google Scholar]
- 232.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 233.Kistler GS, Caldwell PR, Weibel ER. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967;32:605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pappas CT, Obara H, Bensch KG, et al. Effect of prolonged exposure to 80% oxygen on the lung of the newborn mouse. Lab Invest. 1983;48:735–748. [PubMed] [Google Scholar]
- 235.Masip J, Gaya M, Paez J, et al. Pulse oximetry in the diagnosis of acute heart failure. Rev Esp Cardiol (Engl Ed) 2012;65:879–884. doi: 10.1016/j.recesp.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 236.Minana G, Nunez J, Banuls P, et al. Prognostic implications of arterial blood gases in acute decompensated heart failure. Eur J Intern Med. 2011;22:489–494. doi: 10.1016/j.ejim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 237.O'Driscoll BR, Howard LS, Earis J, Mak V. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir Res. 2017;4:e000170. doi: 10.1136/bmjresp-2016-000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bellone A, Vettorello M, Monari A, et al. Noninvasive pressure support ventilation vs. continuous positive airway pressure in acute hypercapnic pulmonary edema. Intensive Care Med. 2005;31:807–811. doi: 10.1007/s00134-005-2649-6. [DOI] [PubMed] [Google Scholar]
- 239.L'Her E, Duquesne F, Girou E, et al. Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients. Intensive Care Med. 2004;30:882–888. doi: 10.1007/s00134-004-2183-y. [DOI] [PubMed] [Google Scholar]
- 240.Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103:573–581. doi: 10.1093/qjmed/hcq077. [DOI] [PubMed] [Google Scholar]
- 241.Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;5:CD005351. doi: 10.1002/14651858.CD005351.pub3. [DOI] [PubMed] [Google Scholar]
- 242.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 243.Moslehi J, Amgalan D, Kitsis RN. Grounding cardio-oncology in basic and clinical science. Circulation. 2017;136:3–5. doi: 10.1161/CIRCULATIONAHA.117.025393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 245.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 247.Reinbolt RE, Patel R, Pan X, et al. Risk factors for anthracycline-associated cardiotoxicity. Support Care Cancer. 2016;24:2173–2180. doi: 10.1007/s00520-015-3008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kremer LC, van Dalen EC, Offringa M, et al. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19:191–196. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 249.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 250.McGowan JV, Chung R, Maulik A, et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–344. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 252.Hahn VS, Lenihan DJ, Ky B. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665. doi: 10.1161/JAHA.113.000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 254.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 255.Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28:183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 256.Cardinale D, Sandri MT. Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis. 2010;53:121–129. doi: 10.1016/j.pcad.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 257.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl):vii155–vii166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 258.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 259.Moslehi JJ, Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–4218. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Di Lisi D, Madonna R, Zito C, et al. Anticancer therapy-induced vascular toxicity: VEGF inhibition and beyond. Int J Cardiol. 2017;227:11–17. doi: 10.1016/j.ijcard.2016.11.174. [DOI] [PubMed] [Google Scholar]
- 261.Chari A, Hajje D. Case series discussion of cardiac and vascular events following carfilzomib treatment: possible mechanism, screening, and monitoring. BMC Cancer. 2014;14:915. doi: 10.1186/1471-2407-14-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang DY, Okoye GD, Neilan TG, et al. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep. 2017;19:21. doi: 10.1007/s11886-017-0835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hering D, Faber L, Horstkotte D. Echocardiographic features of radiation-associated valvular disease. Am J Cardiol. 2003;92:226–230. doi: 10.1016/s0002-9149(03)00546-0. [DOI] [PubMed] [Google Scholar]
- 264.Tamargo J, Caballero R, Delpon E. Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 2015;38:129–152. doi: 10.1007/s40264-014-0258-4. [DOI] [PubMed] [Google Scholar]
- 265.Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 266.Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med. 1982;96:133–139. doi: 10.7326/0003-4819-96-2-133. [DOI] [PubMed] [Google Scholar]
- 267.Safra T, Muggia F, Jeffers S, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 268.Thakur A, Witteles RM. Cancer therapy-induced left ventricular dysfunction: interventions and prognosis. J Card Fail. 2014;20:155–158. doi: 10.1016/j.cardfail.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 269.Steger F, Hautmann MG, Kolbl O. 5-FU-induced cardiac toxicity--an underestimated problem in radiooncology? Radiat Oncol. 2012;7:212. doi: 10.1186/1748-717X-7-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Maitland ML, Bakris GL, Black HR, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang YC, Hsieh TC, Chen SW, et al. Concurrent chemo-radiotherapy potentiates vascular inflammation: increased FDG uptake in head and neck cancer patients. JACC Cardiovasc Imaging. 2013;6:512–514. doi: 10.1016/j.jcmg.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 272.Chang WT, Shih JY, Feng YH, et al. The early predictive value of right ventricular strain in epirubicin-induced cardiotoxicity in patients with breast cancer. Acta Cardiol Sin. 2016;32:550–559. doi: 10.6515/ACS20151023A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Phillips CO, Wright SM, Kern DE, et al. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 274.Roccaforte R, Demers C, Baldassarre F, et al. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. Eur J Heart Fail. 2005;7:1133–1144. doi: 10.1016/j.ejheart.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 275.Liu MH, Wang CH, Chiou AF, et al. Factors associated with inadequate effectiveness of a multidisciplinary disease management program in heart failure patients stratified by galectin 3 level. Biol Res Nurs. 2017;19:77–86. doi: 10.1177/1099800416659743. [DOI] [PubMed] [Google Scholar]
- 276.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]