Abstract
Background
Controlling modifiable risk factors (MRFs) in patients with cardiovascular diseases has been shown to be effective in reducing re-hospitalization rates. The aim of this study was to investigate the rates of controlled MRFs and clinical outcomes after pharmacist interventions in patients with myocardial infarction (MI) after hospital discharge.
Methods
This prospective randomized clinical study was conducted at one medical center in Taiwan, and enrolled patients with MI from January 1, 2012 to December 31, 2014. Patients received medication reconciliation and education from a pharmacist before hospital discharge. The intervention group (IG) received continuous consultations from the pharmacist after discharge, whereas the control group (CG) did not. Primary outcomes included achieving blood pressure < 140/70 mmHg, low-density lipoprotein-cholesterol (LDL-C) < 70 mg/dL, and hemoglobin A1c (HbA1c) < 7% targets. The secondary outcome was major adverse cardiac events (MACEs), defined as re-hospitalization due to MI, unstable angina and stroke.
Results
Two hundred and eight patients completed the study protocol (106 in the IG and 102 in the CG). The rate of achieving blood pressure goal was similar between the two groups. More patients in the IG achieved LDL-C and HbA1c goals than those in the CG at 1 year and 2 years post discharge. However, there was no significant difference in the cumulative incidence of MACEs between the two groups (5.7% vs. 9.8%) (p = 0.262). Diabetes was the only independent predictor of re-hospitalization due to a MACE.
Conclusions
Pharmacist interventions led to a higher rate of optimal controlled MRFs but did not significantly reduce the MACE rate in the patients with MI.
Keywords: Clinical outcome, Myocardial infarction, Pharmacist intervention
INTRODUCTION
Cardiovascular disease is the leading cause of death worldwide, and the prevalence of cardiovascular disease is still increasing.1,2 Hypertension, dyslipidemia and diabetes mellitus (DM) are modifiable risk factors (MRFs), and aggressively treating these MRFs has been shown to reduce rates of morbidity and mortality in patients with cardiovascular diseases.3-8 To this end, several evidence-based guidelines recommend treatment strategies to control MRFs to improve long-term outcome in patients with coronary artery disease and myocardial infarction (MI).9-17 Despite major advances in the medications used to treat cardiovascular diseases such as statins, beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and antiplatelet agents, the prevalence of poorly controlled MRFs in patients with MI remains high worldwide,18-20 and a significant gap exists between evidence-based therapy and "real-world" clinical practice.21-24
Recently, pharmacist interventions including patient education, consultation and medication reconciliation have been shown to improve compliance to medications, control of MRFs, and rates of achieving blood pressure (BP), low-density lipoprotein-cholesterol (LDL-C) and hemoglobin A1c (HbA1c) goals, and to reduce overall readmission rates.25-33 Such pharmacist interventions were applied in patients with acute myocardial infarction (AMI) in the Taiwan Clinical Performance Indicator (TCPI) core measures system (as described in the Methods). In addition to the correlation between MRFs and cardiovascular disease-related mortality and morbidity, the effect of guideline-recommended management strategies to control MRFs in patients with MI has been shown to reduce cardiovascular events.9-15 However, whether add-on pharmacist interventions can lead to better rates of achieving optimal MRF control and reducing cardiovascular events in patients with MI is still unclear. Therefore, the aim of this study was to investigate the effect of continuous multifaceted patient-centered pharmacist interventions after discharge on achieving clinical practice guideline goals and reducing the rate of hospital readmissions for cardiovascular diseases.
METHODS
Study design and setting
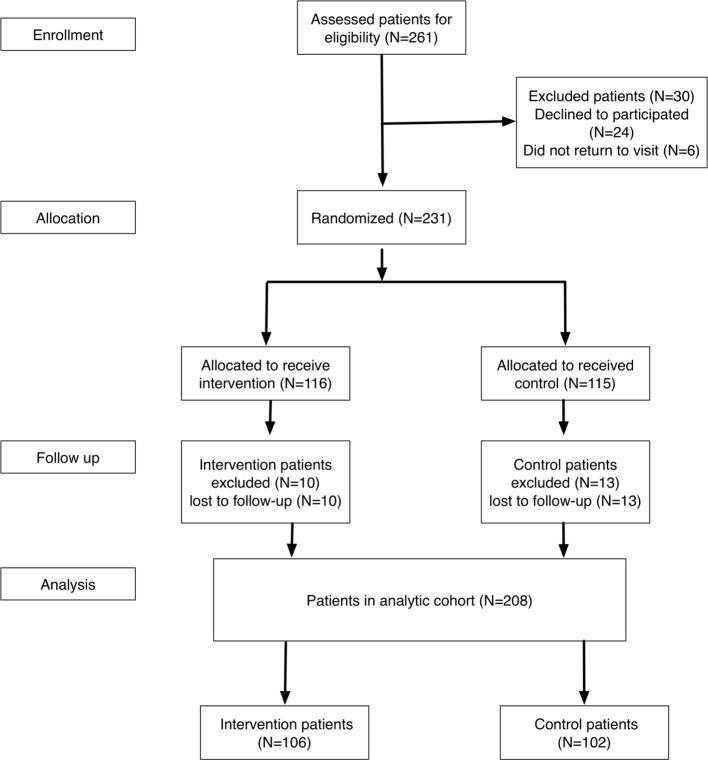
This prospective randomized clinical study was conducted at Chang Gung Memorial Hospital, a medical center in southern Taiwan, from January 1, 2012 to December 31, 2014. We enrolled all patients admitted to our hospital with MI as the principle diagnosis from January 1, 2012 to December 31, 2012. The eligible patients were stratified by age (≥ 65 years and < 65 years) and sex, and were then randomized at a 1:1 ratio into the intervention group (IG) or the control group (CG) (Figure 1). MI was defined as having elevated biomarkers for myocardial necrosis and clinical evidence including prolonged signs/symptoms of ischemia (> 30 minutes) or electrocardiographic ST-segment changes during the initial 24 hours of admission.34,35 The exclusion criteria were patients: (1) admitted for a primary non-cardiac diagnosis who developed MI as a secondary condition (such as perioperative MI); (2) discharged to a nursing home for long-term health care; (3) with irreversible, non-cardiac medical conditions (such as malignancy) which affected the 12-month survival rate or participation in the study; (4) who did not have access to a telephone for communication purposes.
Figure 1.
Study enrollment flow chart. A total of 231 patients were randomized into the intervention group (n = 116) or control group (n = 115).
The study protocol was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB-100-3000A3 and 103-7130D). Patients with an estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 at admission day were defined as having chronic kidney disease.
Study protocol and procedures
All of the enrolled patients had AMI and were referred to the TCPI care management program. The TCPI is a system used to assess clinical care in Taiwan and to identify areas where improvements in quality can be made. This system has been used by pharmacists for patients with AMI. Once a patient had been identified, the cardiac unit nurse notified the clinical pharmacist, and a schedule to complete consultations with the pharmacist was established within 3 days. The pharmacist then met with the patient for an initial interview consisting of general counseling with regards to cardiovascular diseases, education (monitoring their disease, reinforcing compliance to medications, therapeutic lifestyle changes, and how to take the medications), medication reconciliation and evaluation. Each interview lasted for approximately 1 hour, and the pharmacist recorded the details of the interview with each patient. The pharmacist was an experienced clinical pharmacist with over 10 years of experience in providing consultations at the study hospital.
All patients were given an illustrated booklet before being discharged containing general information on the process and risk factors for cardiovascular diseases, and emphasizing the importance of achieving recommended targets for BP, LDL-C, and HbA1c. The booklet also contained information about pharmacologic management as outlined in current practice guidelines.9,11,14
Because medications for chronic illnesses are refilled for a maximum of 3 months as per the Taiwan National Health Insurance (NHI) reimbursement policy, the patients in the IG received detailed follow-up by the pharmacist every 3 months by telephone or in face-to-face visits and comprehensive chart reviews. The follow-up rate was 100% in the IG (106 patients). The regular contact and chart reviews were intended to provide adequate opportunities for the patients to ask questions, assess the patients’ medication knowledge, discuss laboratory results, reinforce the importance of compliance with the medication regimen and achieving clinical targets, assess accuracy of the medications, appropriately monitor medication therapy and evidence-based chronic disease state management. Interventional feedback was provided to the patient’s physician and recommendations were made for any identified drug therapy problems. The patients assigned to the CG received no further contact with the study pharmacist. All data collection was based on a review of the patients’ medical records and interviews.
The goals for BP, LDL-C, and HbA1c were: BP < 140/ 90 mmHg (for patients with DM or chronic kidney disease < 130/80 mmHg);14 LDL-C < 70 mg/dL;9 and HbA1c < 7%.11 If any of these risk factors were uncontrolled, the pharmacist alerted the patient by telephone, and their physician was notified through the patient’s electronic medical records and by telephone for the patients in the IG.
Sample size and outcome measures
The study included consecutive patients with MI at a single facility between January 2012 and December 2012. The estimated sample size of 106 study patients in each group was based on an effective size with α = 0.05, a power of 80%, anticipation of a 14.0% major adverse cardiovascular event (MACE) rate in the CG and 4.0% in the IG for the primary objective of this study. A 10% dropout rate was considered in both groups. Therefore, we screened 117 patients in each group. The primary end-points were differences in modifiable cardiovascular disease risk factors [systolic and diastolic BP, total cholesterol (TC), LDL, high density lipoprotein (HDL), triglycerides (TG), and HbA1c in those with DM] before (initial) and after (final) the study in both groups. The secondary end-point was MACEs including re-hospitalization for stroke, MI and unstable angina via the emergency department after hospital discharge. Stroke was defined as sudden onset of the loss of global or focal cerebral function persisting for more than 24 h. Unstable angina was defined as clinical evidence including prolonged signs/symptoms of ischemia (< 30 minutes) or without changes in electrocardiographic ST-segment and elevated biomarkers for myocardial necrosis. MI was defined as elevated biomarkers for myocardial necrosis and clinical evidence including prolonged signs/symptoms of ischemia (> 30 minutes) or changes in electrocardiographic ST-segment.
The clinical outcomes were classified and adjudicated by a cardiologist (Dr Tsai) and pharmacist (Pharm Chiu) based on a review of the electronic medical records at our center from January 1, 2012 to December 31, 2014.
Statistical analysis
We used a per-protocol approach for all analyses. Data were presented as the mean and standard deviation for normally distributed continuous variables, and proportions for categorical variables. Baseline characteristics and study results were compared using the chi-square test for categorical data, and the independent t-test, paired t test and analysis of cluster structure variability (ANOCVA) were used to compare continuous data between the two groups. The Kaplan-Meier method was used to determine the cumulative incidence of MACEs in both groups, and differences between groups were tested using the log-rank test. Cox regression analysis was used to determine the independent predictors for MACEs. A p value < 0.05 was considered to indicate statistical significance. All data processing and analyses were conducted using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patients
A total of 261 patients were screened, of whom 24 were excluded due to refusing to participate and six who were lost to follow-up. The remaining 231 patients were randomized into the IG (n = 116) and CG (n = 115). Of these patients, 8.6% (10/116) were excluded in the IG (all of whom were lost to follow-up), and 11.3% (13/115) were excluded in the CG (all of whom were also lost to follow-up). These 23 patients were not enrolled in our study for further analysis. Because medications for chronic illnesses are refilled for a maximum of 3 months as per the Taiwan NHI reimbursement policy, all patients received follow-up at least every 3 months. The remaining 208 patients (106 in the IG and 102 in the CG) with a 100% follow-up rate were entered into the analysis (Figure 1). The last patient was enrolled on December 31, 2012, and follow-up was completed on December 31, 2014. All patients were followed for a minimum of 2 years. The pharmacist followed up the medical records and laboratory results to educate the patients on the importance of compliance with the medication regimen and achieving clinical targets. The pharmacist also assessed the accuracy of medications and appropriate monitoring of medications in the patients in the IG. The follow-up rate with the pharmacist at each 3-month period was 100%.
Baseline comparisons
There were no significant differences in sex, age, education status, smoking history, renal function or body mass index between the two groups. With regards to MRFs, there were no significant differences in hypertension, dyslipidemia or DM between the two groups. More than 30% of the patients had one or two MRFs with a similar distribution between the two groups. Most of the patients did not have a coexisting disease, however in those who did, chronic kidney disease was the most common (IG 19.8% vs. CG 19.6%). There were no significant differences in the rates of medication use including antiplatelets (aspirin or clopidogrel), beta-blockers, ACEIs/ARBs and statins between the two groups at discharge (Table 1).
Table 1. Baseline demographic and clinical characteristics.
| Intervention group (n = 106) | Control group (n = 102) | p value | |
| Male (%) | 88 (83%) | 87 (85.3%) | 0.65 |
| Age, years | 60.1 ± 12.4 | 61.5 ± 12.2 | 0.41 |
| ≥ 65 years | 36 (34%) | 40 (39.2%) | 0.43 |
| BMI (kg/m2) | 21.3 ± 4.3 | 22.0 ± 6.8 | 0.353 |
| Literacy rate (%) | 97 (91.5%) | 88 (86.3%) | 0.23 |
| Smoking (%) | 43 (40.6%) | 51 (50%) | 0.22 |
| Creatinine (mg/dL) | 1.59 ± 2.12 | 1.47 ± 1.69 | 0.662 |
| eGFR | 57.75 ± 13.13 | 66.75 ± 13.21 | 0.330 |
| Hypertension | 78 (73.6%) | 74 (72.5%) | 0.87 |
| Hyperlipidemia | 81 (76.4%) | 75 (73.5%) | 0.73 |
| Diabetes | 35 (33%) | 36 (35.3%) | 0.729 |
| CVA | 6 (5.7%) | 5 (4.9%) | 0.81 |
| CKD | 21 (19.8%) | 20 (19.6%) | 0.97 |
| Drug therapy | |||
| Aspirin | 96 (90.6%) | 90 (88.2%) | 0.59 |
| Clopidogrel | 103 (97.2%) | 102 (100%) | 0.26 |
| Dual antiplatelet | 93 (87.7%) | 91 (89.2%) | 0.74 |
| β-blockers | 87 (82.1%) | 84 (82.4%) | 0.96 |
| ACEIs | 39 (36.8%) | 39 (38.2%) | 0.83 |
| ARBs | 51 (48.1%) | 47 (46.1%) | 0.77 |
| ACEIs or ARBs | 93 (87.7%) | 89 (87.3%) | 0.92 |
| Statins | 92 (86.8%) | 84 (82.4%) | 0.38 |
ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; MRF, modifiable risk factor.
Changes in blood pressure, lipid profile and hemoglobin A1c
Of the 152 patients with hypertension, there were no significant differences in systolic BP (SBP) or diastolic BP (DBP) at baseline, 1 year and 2 years after discharge. Of the 156 patients with hyperlipidemia, there were no significant differences in serum LDL-C, TC, HDL and TG between the two groups. However, the IG had a significantly lower serum LDL-C and higher HDL level than the CG at 1 year and 2 year after discharge. Among the 71 patients with DM, there were no significant differences in mean HbA1c between the two groups. However, the HbA1c level was lower in the IG than in the CG at 1 year and 2 years post discharge (Table 2). In addition, there were higher rates of prescriptions for medications for hypoglycemia and dyslipidemia in the IG (Table 3). Table 3 also lists the different kinds and changes of medication in both groups. There were higher rates of multiple kinds of hypoglycemic drugs in the IG, however these changes were reversed in the CG. This finding indicated that the pharmacist interventions were effective in achieving optimal blood sugar control by adjusting the kinds of drugs. Increases in the kinds of drugs used to achieve optimal blood pressure and lipid level were also found. The changes in medications indicated that the pharmacist interventions were beneficial in increasing the percentage of optimal MRF control rate by notifying the cardiologist to adjust medications.
Table 2. Changes in blood pressure, lipid profile and hemoglobin A1c levels within both groups before and after study.
| Intervention group (n = 106) | Control group (n = 102) | p value* | |
| SBPat baseline (mmHg) | 128.5 ± 17.6 | 128.2 ± 17.1 | 0.856 |
| DBP at baseline (mmHg) | 72.8 ± 10.7 | 71.8 ± 9.8 | 0.472 |
| SBP at 1 year | 131.9 ± 17.6 | 133.0 ± 18.1 | 0.496 |
| DBP at 1 year | 73.1 ± 11.5 | 73 ± 11.7 | 0.783 |
| SBP at 2 years | 133 ± 18.1 | 133.3 ± 20.9 | 0.275 |
| DBP at 2 years | 72.3 ± 10.7 | 73.1 ± 11.7 | 0.644 |
| Lipid profile | |||
| LDL-C at baseline (mg/dL) | 112.2 ± 35.4 | 113.3 ± 34.5 | 0.507 |
| LDL-C after 1 year | 77.71 ± 24.3 | 88.8 ± 34.5 | 0.023* |
| LDL-C after 2 years | 79.1, 23.5 | 84.1, 28.3 | 0.043* |
| HDL-C at baseline (mg/dL) | 43.4 ± 11.6 | 46.5 ± 21.4 | 0.451 |
| HDL-C after 1 year | 49.4 ± 12.4 | 49.3 ± 11.3 | 0.005* |
| HDL-C after 2 years | 50.6, 14.7 | 49.3, 12.7 | 0.001* |
| TC at baseline (mg/dL) | 176.3 ± 43.3 | 178.2 ± 38.6 | 0.426 |
| TC after 1 year | 148.6 ± 31.3 | 159.5 ± 43.9 | 0.021* |
| TC after 2 years | 151.4, 30.1 | 154.5, 34.9 | 0.420 |
| HbA1c at baseline | 6.58 ± 1.48 | 6.66 ± 1.46 | 0.695 |
| HbA1c after 1 year | 6.32 ± 0.91 | 6.57 ± 1.23 | 0.028* |
| HbA1c after 2 years | 6.373 ± 0.94 | 6.77 ± 1.33 | 0.003* |
* Analysis of cluster structure variability (ANOCVA).
BP, blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein-cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
1 year: measurements at 1 year after discharge; 2 years: measurements at 2 years after discharge.
# There were 78 patients and 74 patients with hypertension in control group and intervention group respectively. There were 35 patients and 36 patients with diabetes mellitus in control group and intervention group respectively. There were 81 patients and 75 patients with hyperlipidemia in control group and intervention group respectively.
Table 3. Medication use and changes.
| Medications | Baseline IG | Final IG | Baseline CG | Final CG |
| Hypoglycemic medications | ||||
| 0 kind | 68.9% (73) | 67% (71) | 68.6% (70) | 67.6% (69) |
| 1 kind | 18.9% (20) | 16% (17) | 14.7% (15) | 19.6% (20) |
| 2 kinds | 10.4% (11) | 13.2% (14) | 10.8% (11) | 7.8% (8) |
| 3 kinds | 1.9% (2) | 2.8% (3) | 5.9% (6) | 4.9% (5) |
| 4 kinds | 0% (0) | 0.9% (1) | 0% (0) | 0% (0) |
| Patients with any changes in medication | 19.8% (21) | 19.6% (20) | ||
| Hypertension medications | ||||
| 0 kind | 6.6% (7) | 5.7% (6) | 1% (1) | 2.9% (3) |
| 1 kind | 22.6% (24) | 28.3% (30) | 13.7% (14) | 15.7% (16) |
| 2 kinds | 50.9% (54) | 43.4% (46) | 57.8% (59) | 59.8% (61) |
| 3 kinds | 16.0% (17) | 17.9% (19) | 19.6% (20) | 18.6% (19) |
| 4 kinds | 3.8% (4) | 2.8% (3) | 6.9% (7) | 6.9% (7) |
| 5 kinds | 0% (0) | 1.9% (2) | 1% (1) | 0% (0) |
| Patients with any changes in medication | 47.2% (50) | 39.2% (40) | ||
| Lipid medications | ||||
| 0 kind | 12.3% (13) | 10.4% (11) | 17.6% (18) | 18.6% (19) |
| 1 kind | 86.8% (92) | 87.7% (93) | 78.4% (80) | 75.5% (77) |
| 2 kinds | 0.9% (1) | 1.9% (2) | 3.9% (4) | 5.9% (6) |
| Patients with any changes in medication | 10.4% (11) | 25.5% (26) |
CG, control group; IG, intervention group.
Rates of modifiable risk factor goals achieved after discharge
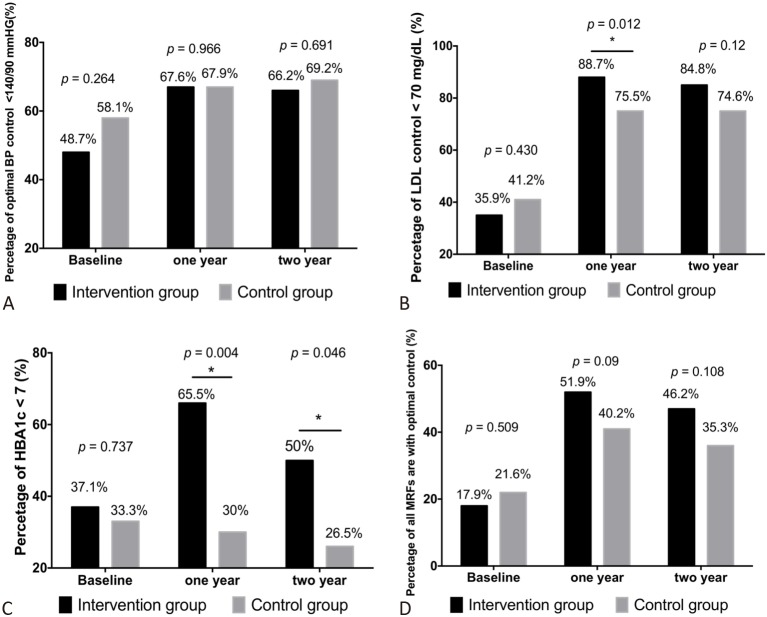
There were no significant differences in the proportion of patients who achieved BP goals at baseline, 1 year and 2 years post discharge between the two groups (Figure 2A). However, the overall increase in patients achieving the BP goal was higher in the IG (17.5%; from 48.7% to 66.2%) than in the CG (11.1%; from 58.1% to 69.2%). In addition, the rate of achieving the lipid goal (LDL-C < 70 mg/dL) increased in both groups from baseline to 1 year and 2 years post discharge. Although the rate of achieving the lipid goal was lower in the IG at baseline, the rates at 1 year and 2 years post discharge were significantly higher in the IG than in the CG (Figure 2B).
Figure 2.
Modifiable risk factor goals. Patients with myocardial infarction were randomized and analyzed into two groups. the intervention group (n = 106) and control group (n = 102). The percentage of patients with optimal blood pressure, low-density lipoprotein cholesterol (LDL-C), and glycosylated hemoglobin (HbA1c) control and all risk factors with optimal control were assessed at baseline, 1 year and 2 years post discharge. (A) There were no significant differences between the groups in optimal blood pressure (BP) control (< 140/90 mmHg) at any time point. (B) The intervention group had a higher rate of reaching the recommended target for LDL-C (< 70 mg/dL) than the control group at 1 year and 2 years post discharge. (C) The intervention group had a higher rate of reaching the recommended target for glycemic control (HbA1c < 7.0%) than the control group at 1 year and 2 years post discharge. (D) All risk factors were defined as blood pressure, low-density lipoprotein cholesterol and glycosylated hemoglobin. The rate of all modified risk factors (MRFs) with optimal control (BP control (< 140/90 mmHg), LDL-C (< 70 mg/dL) and HbA1c < 7.0%) were similar between the intervention and control group at baseline. However, the intervention group had a higher all MRFs with optimal control rate than the control group at 1 year and 2 years post discharge. * Indicated p < 0.05.
There were significant differences in the proportions of patients who achieved the HbA1c target (HbA1c < 7%) between the two groups at 1 year and 2 years post discharge (all p < 0.05), with an increase of 28.4% (from 37.1% to 65.5%) in the IG and a decrease of 3.3% (from 33.3% to 30%) in the CG at 1-year post discharge. In addition, the rate of achieving the HbA1c goal was higher in the IG than in the CG at 2 years post discharge (Figure 2C). Although there were no significant differences in the overall rates of controlled optimal MRFs between the two groups, the rates were higher in the IG than in the CG at 1 year and 2 years post discharge (Figure 2D).
Cardiovascular events post discharge
Kaplan-Meier analysis showed no significant difference in the cumulative incidence of MACEs between the CG and the IG (Figure 3). The rate of re-admission for MI was slightly higher in the CG than in the IG. However, there were no significant differences in the rates of re-admission for stroke or unstable angina after hospital discharge between the two groups. There was also no significant difference in the rate of MACEs between the CG (9.8%) and IG (5.7%) (Table 4). Multivariate Cox regression analysis for MACEs showed that DM was the only an independent predictor of MACEs post discharge in the patients with MI (Table 5).
Figure 3.
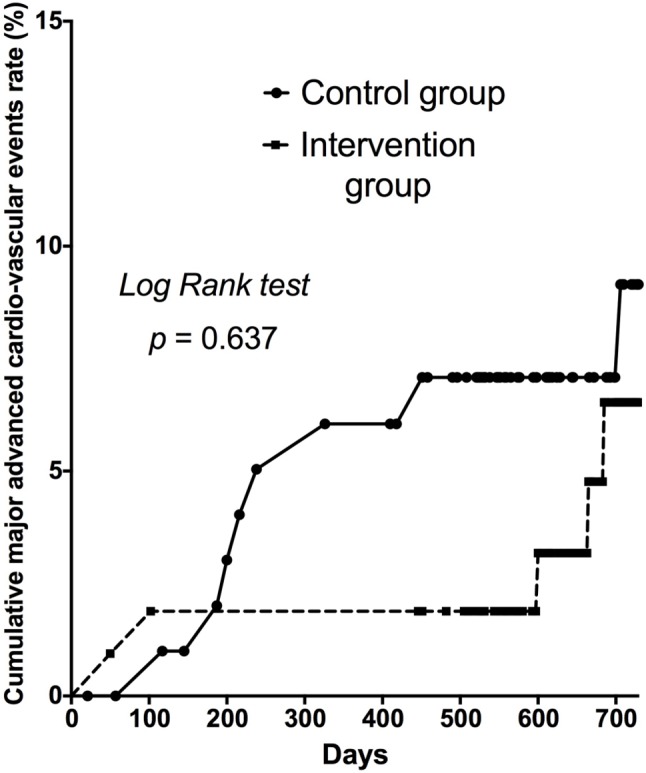
Kaplan-Meier cumulative major adverse cardiac events curve. Kaplan-Meier survival analysis showed that the patients in the control group were associated with a higher rate of major adverse cardiovascular events compared to those in the intervention group (9.8% versus 5.7%, p = 0.637). Major adverse cardiac events were defined as re-hospitalization due to myocardial infarction, unstable angina and stroke.
Table 4. Cumulative incidence of MACEs requiring emergency department visits.
| Intervention group (N = 106) | Control group (N = 102) | p value | |
| Myocardial infarction | 0% (0) | 4.9% (5) | 0.076 |
| Stroke | 0% (0) | 2.0% (2) | 0.538 |
| Unstable angina | 5.7% (6) | 2.9% (3) | 0.591 |
| MACEs | 5.7% (6) | 9.8% (10) | 0.262 |
MACEs, major adverse cardiac events.
Table 5. Univariate and multivariate Cox regression analyses for MACEs requiring emergency department visits.
| Variable | HR | 95% CI | p value | HR | 95% CI | p value |
| Age | 1.016 | 0.963-1.071 | 0.571 | |||
| Male gender | 0.440 | 0.187-1.034 | 0.060 | 0.515 | 0.444-3.521 | 0.673 |
| BMI | 0.978 | 0.762-1.225 | 0.860 | |||
| Obesity | 1.552 | 0.656-3.669 | 0.317 | |||
| Creatinine | 0.867 | 0.487-1.554 | 0.628 | |||
| Smoking | 0.525 | 0.259-1.065 | 0.074 | 0.909 | 0.404-2.047 | 0.818 |
| DM | 2.704 | 1.366-5.352 | 0.004 | 4.243 | 1.039-4.591 | 0.039 |
| HTN | 2.203 | 0.848-5.772 | 0.105 | |||
| CVA history | 6.198 | 1.286-29.864 | 0.002 | 2.690 | 0.788-9.175 | 0.114 |
| CKD history | 2.082 | 1.025-4.229 | 0.043 | 1.603 | 0.767-3.352 | 0.210 |
| Baseline LDL | 1.003 | 0.985-1.021 | 0.778 | |||
| Baseline HDL | 0.997 | 0.953-1.043 | 0.997 | |||
| Baseline TC | 1.001 | 0.985-1.017 | 0.892 | |||
| Pharmacist interventions | 0.996 | 0.499-1.909 | 0.991 |
BMI, body mass index; BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; CVA, cerebrovascular accident; DM, diabetes mellitus; HR, hazard ratio; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein-cholesterol; MACEs, major adverse cardiac events; TC, total cholesterol.
DISCUSSION
Main findings
In this study, we investigated whether multifaceted patient-centered pharmacist interventions could improve clinical outcomes in patients with MI by improving the MRF control rate. There are two main findings in this study. First, pharmacist interventions effectively improved the rates of achieving MRF goals, especially with regards to lipid and sugar control. Second, pharmacist interventions did not significantly reduce the rate of long-term MACEs in the patients with MI enrolled in the TCPI care management program.
Utilization of guideline-recommended medications
The utilization rates of guideline-recommended medications in this single center prospective randomized control trial were higher than those reported in a previous nationwide survey in Taiwan8 for beta-blockers (82% vs. 45.7%), renin-angiotensin system inhibitor drugs (87% vs. 58.93%), statins (84% vs. 49.5%), aspirin (89.5 vs. 91.6%) and clopidogrel (98.6 vs. 94.4%), but similar to a secondary prevention clinical trial.36 These findings suggest that prescriptions for guideline-recommended medications in patients with MI in Taiwan are inconsistent, and that there is still scope for improvement in the management of patients with MI after discharge.8,18,19
Impact of pharmacist interventions on prescription patterns
There were no significant differences in the rates of prescriptions for anti-platelet medications including aspirin monotherapy or dual therapy, beta-blockers, renin-angiotensin system inhibitors and statins between the two groups. The rate of change of lipid medications in the CG was higher than that in the IG, and the greatest change in lipid medications was to reduce the dose of lipid medications due to insurance limitations. An increase in the dosage and kinds of lipid medications was noted in the IG, because the pharmacist would notify the in-charge cardiologist to maintain and achieve the optimal lipid level. Although the rate of change of lipid medication was higher in the CG than in the IG, the greatest change was a reduction in the lipid dosage. In addition, the rate of not using lipid medications was higher in the CG (18%) compared to the IG (10%). These changes may explain why the IG had a better lipid control rate and lower lipid values. The IG was prescribed with more hypoglycemic medications than the CG (Table 3). The pharmacist provided data with regards to the control of MRFs to the patients and their physicians, and the higher rates of prescriptions in the IG may reflect the effect of these interventions.
Effectiveness of the pharmacist interventions on controlling modifiable risk factors
There was no significant difference in the rate of optimal BP control between the two groups, and about 67% of the patients achieved the target BP recommended according to the JNC 7 guidelines, which is consistent with previous studies.15-17,31,32 However, our results demonstrated that the pharmacist interventions were effective in reducing the serum LDL-C level at 1 year and 2 years post discharge. More importantly, the percentage of patients achieving the LDL-C goal (< 70 mg/dL) was significantly higher in the IG than in the CG. This effect was persistent and remained at 2 years post discharge. Of note, the HbA1c level and percentage of patients with an HbA1c < 7% were similar between the two groups at baseline, however the IG had a higher rate of HbA1c < 7% at 1 year and 2 years post discharge, which is also consistent with previous studies.26,27 This may have been due to the pharmacist interventions, in which the pharmacist provided recommendations to the physicians with regards to controlling lipid levels and hypoglycemia with changes to the patients’ medications via electronic medical records for the patients in the IG.
Pharmacist interventions and cardiovascular outcomes
Our results showed that the pharmacist interventions did not significantly reduce the rates of re-hospitalization due to MACEs in the patients with MI. Most previous studies on pharmacist interventions have focused on whether they can effectively reduce risk factors and enhance drug compliance,25-33 however few studies have explored the benefits of pharmacist interventions in reducing readmissions for MACEs.37 Our results showed that the pharmacist interventions improved the MRF control rate, which is in consistent with a previous study.37 In addition, our data showed that diabetes was an independent predictor of MACEs, which is consistent with previous reports.3-6 Our results only demonstrated a mild non-significant decrease in the re-admission rate due to MACEs. There are several possible reasons to explain why improving the MRF control rate did not translate to a reduction in MACEs in this study. First, MACEs were defined as re-admission for stroke, MI and unstable angina. Only absolute changes in LDL/HBA1c were comparatively small, and these small changes may not have been sufficient to result in a significant reduction in MACEs. The pharmacist interventions in our study included oral education for drug adherence. The pharmacist could not ask the cardiologist to change the dose or type of medication, and the dosage of lipid lowering drugs is also restricted by the NHI program in Taiwan. The rate of optimal lipid control can’t persist increase with year reflect this phenomenon. These findings may explain why the pharmacist interventions did not improve the clinical outcome.
Second, the hospital computer system notified the in-charge cardiologist to provide optimal treatment for the patients with MI, including prescribing statins, beta-blockers, and ACEIs/ARBs. All cardiologists in our hospital agreed to achieve the targets established by the TCPI, and the lipid profile, blood pressure and HbA1c were obviously reduced in all patients in this study. The improvements in MRF optimal control rate in the CG also obviously improved, and this improvement may explain the low MACE rate in this study. This may be another reason why add-on pharmacist interventions did not significantly lower the MACE rate in this study.
Third, our pharmacist interventions focused on adherence to medications to control blood pressure, sugar and lipid levels. Other cardiovascular risk factors such as smoking, physical activity and BMI are also all important predictors of MACEs in patients with MI. The pharmacist interventions may have been too simplistic and did not provide sufficient motivation to quit smoking, lower the BMI and enhance physical activity in the IG. This is another important potential explanation of why the pharmacist interventions did not improve the clinical outcomes in this study. A multidisciplinary team with a comprehensive disease management program (including pharmacists, nurses, nutritionists and doctors) should be included in further studies.
Limitations
There are several limitations to this study. First, the sample size was relatively small. In addition, the study was conducted at a single center with a single pharmacist. The study was an open-label design, and none of the participants were blinded to treatment assignment. These factors may have introduced bias and a double blind clinical trial with a larger sample size conducted at multiple centersis warranted to validate our findings. Second, data on blood pressure were recorded from medical records at wards and outpatient clinics, and the effect of white coat hypertension cannot be excluded. Third, some confounders that could have affected BP, lipids and sugar control were not explored in detail. Fourth, MACEs were assessed by only two of the investigators in this open-label study. In addition, the use of unstable angina as a MACE was also prone to bias. However, the patients with unstable angina in this study all received coronary angiography and received coronary interventions if the in-charge cardiologist thought it would be beneficial for the patients. The diagnosis of unstable angina was not only made according to symptoms.
CONCLUSIONS
In this study, continuous pharmacist interventions led to improvements in MRF control and achieving guideline goals. Pharmacist interventions did not reduce readmission rates due to MACEs after hospital discharge in the patients with MI.
Acknowledgments
This study was supported by research grants from Chang Gung Memorial Hospital Grant number: (CMRPG 8B0411-1, CMRPG8B0411-2, CMRPG8E1151, CMRPG 8F0351, CMRPG8B0601, CMRPG8D0231-3 CMRPG8E 1151, CMRPG8F0351, CMRPG8F1681, CMRPG8G1371, CMRPG8G1541 and CMRPG8E0661-3, CMRPG8F1831, CMRPG8E1151, CMRPG8F0351, CMRPG8F1681, CMRPG 8G1371 and CMRPG8G1541) and Ministry of Science and Technology grant number: 104-2314-182A-132 and 104-2314-182A-102-MY2.
DECLARATION OF CONFLICT OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, et al. Executive summary: heart disease and stroke statistics--2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2.Wu TS, Shih FY, Yen MY, et al. Establishing a nationwide emergency department-based syndromic surveillance system for better public health responses in Taiwan. BMC Public Health. 2008;8:18. doi: 10.1186/1471-2458-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 4.Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 5.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 6.Buse JB, Ginsberg HN, Bakris GL, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 7.Chen KC, Yin WH, Wu CC, et al. In-hospital implementation of evidence-based medications is associated with improved survival in diabetic patients with acute coronary syndrome - data from TSOC ACS-DM Registry. Acta Cardiol Sin. 2018;34:211–223. doi: 10.6515/ACS.201805_34(3).20180207B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei CC, Shyu KG, Cheng JJ, et al. Diabetes and adverse cardiovascular outcomes in patients with acute coronary syndrome - data from Taiwan’s Acute Coronary Syndrome Full Spectrum Data Registry. Acta Cardiol Sin. 2016;32:31–38. doi: 10.6515/ACS20150322A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 10.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes A. Summary of revisions to the 2011 clinical practice recommendations. Diabetes Care. 2011;34 Suppl 1:S3. doi: 10.2337/dc11-S003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes A. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection E; Treatment of High Blood Cholesterol in A. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 15.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 16.Li YH, Hsieh IC, Shyu KG, et al. What could be changed in the 2012 Taiwan ST-segment elevation myocardial infarction guideline? Acta Cardiol Sin. 2014;30:360–364. [PMC free article] [PubMed] [Google Scholar]
- 17.Li YH, Wang YC, Wang YC, et al. 2018 Guidelines of the Taiwan Society of Cardiology, Taiwan Society of Emergency Medicine and Taiwan Society of Cardiovascular Interventions for the management of non ST-segment elevation acute coronary syndrome. J Formos Med Assoc. 2018;117:766–790. doi: 10.1016/j.jfma.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Fox KA, Goodman SG, Klein W, et al. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2002;23:1177–1189. doi: 10.1053/euhj.2001.3081. [DOI] [PubMed] [Google Scholar]
- 19.Salomaa V, Paakkonen R, Hamalainen H, et al. Use of secondary preventive medications after the first attack of acute coronary syndrome. Eur J Cardiovasc Prev Rehabil. 2007;14:386–391. doi: 10.1097/01.hjr.0000244573.10229.6e. [DOI] [PubMed] [Google Scholar]
- 20.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan AT, Yan RT, Tan M, et al. Contemporary management of dyslipidemia in high-risk patients: targets still not met. Am J Med. 2006;119:676–683. doi: 10.1016/j.amjmed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Ali MK, Bullard KM, Gregg EW. Achievement of goals in U.S. Diabetes Care, 1999-2010. N Engl J Med. 2013;369:287–288. doi: 10.1056/NEJMc1306652. [DOI] [PubMed] [Google Scholar]
- 23.Egan BM, Li J, Qanungo S, et al. Blood pressure and cholesterol control in hypertensive hypercholesterolemic patients: national health and nutrition examination surveys 1988-2010. Circulation. 2013;128:29–41. doi: 10.1161/CIRCULATIONAHA.112.000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saposnik G, Goodman SG, Leiter LA, et al. Applying the evidence: do patients with stroke, coronary artery disease, or both achieve similar treatment goals? Stroke. 2009;40:1417–1424. doi: 10.1161/STROKEAHA.108.533018. [DOI] [PubMed] [Google Scholar]
- 25.Crowley MJ, Melnyk SD, Ostroff JL, et al. Can group medical clinics improve lipid management in diabetes? Am J Med. 2014;127:145–151. doi: 10.1016/j.amjmed.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Edelman D, Fredrickson SK, Melnyk SD, et al. Medical clinics versus usual care for patients with both diabetes and hypertension: a randomized trial. Ann Intern Med. 2010;152:689–696. doi: 10.7326/0003-4819-152-11-201006010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Cohen LB, Taveira TH, Khatana SA, et al. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educ. 2011;37:801–812. doi: 10.1177/0145721711423980. [DOI] [PubMed] [Google Scholar]
- 28.Carter BL, Rogers M, Daly J, et al. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748–1755. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coons JC, Fera T. Multidisciplinary team for enhancing care for patients with acute myocardial infarction or heart failure. Am J Health Syst Pharm. 2007;64:1274–1278. doi: 10.2146/ajhp060281. [DOI] [PubMed] [Google Scholar]
- 30.Bex SD, Boldt AS, Needham SB, et al. Effectiveness of a hypertension care management program provided by clinical pharmacists for veterans. Pharmacotherapy. 2011;31:31–38. doi: 10.1592/phco.31.1.31. [DOI] [PubMed] [Google Scholar]
- 31.Ripley TL, Adamson PB, Hennebry TA, et al. Collaborative practice model between cardiologists and clinical pharmacists for management of patients with cardiovascular disease in an outpatient clinic. Ann Pharmacother. 2014;48:412–419. doi: 10.1177/1060028013515432. [DOI] [PubMed] [Google Scholar]
- 32.Warden BA, Freels JP, Furuno JP, et al. Pharmacy-managed program for providing education and discharge instructions for patients with heart failure. Am J Health Syst Pharm. 2014;71:134–139. doi: 10.2146/ajhp130103. [DOI] [PubMed] [Google Scholar]
- 33.Arnold ME, Buys L, Fullas F. Impact of pharmacist intervention in conjunction with outpatient physician follow-up visits after hospital discharge on readmission rate. Am J Health Syst Pharm. 2015;72(11 Suppl 1):S36–S42. doi: 10.2146/sp150011. [DOI] [PubMed] [Google Scholar]
- 34.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention and the 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the society for cardiovascular angiography and interventions. Circulation. 2016;133:1135–1147. doi: 10.1161/CIR.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 35.Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–e579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 36.Arnold SV, Spertus JA, Masoudi FA, et al. Beyond medication prescription as performance measures: optimal secondary prevention medication dosing after acute myocardial infarction. J Am Coll Cardiol. 2013;62:1791–1801. doi: 10.1016/j.jacc.2013.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassan Y, Kassab Y, Abd Aziz N, et al. The impact of pharmacist-initiated interventions in improving acute coronary syndrome secondary prevention pharmacotherapy prescribing upon discharge. J Clin Pharm Ther. 2013;38:97–100. doi: 10.1111/jcpt.12027. [DOI] [PubMed] [Google Scholar]