Abstract
Sympathetic overactivity, an essential mechanism of hypertension, in driving sustained hypertension derives mostly from its effects on renal function. Percutaneous renal denervation (RDN) is designed to disrupt renal afferent and efferent sympathetic nerves to achieve sustained blood pressure (BP) reduction. Since 2017 onward, all three proof-of-concept, sham-controlled RDN trials demonstrated that RDN achieved consistent and clinically meaningful BP reductions [approximately 10 mmHg in office systolic BP (SBP) and 6-9 mmHg in 24-hour SBP] compared to sham operation in patients with mild to moderate or uncontrolled hypertension. There were no serious adverse events. The registry data in Taiwan showed similar 24-hour BP reductions at 12 months following RDN. The Task Force considers RDN as a legitimate alternative antihypertensive strategy and recommends 1) RDN should be performed in the context of registry and clinical studies (Class I, Level C) and 2) RDN should not be performed routinely, without detailed evaluation of various causes of secondary hypertension and renal artery anatomy (Class III, Level C). RDN could be performed in patients who fulfill either of the following BP criteria: 1) office BP ≥ 150/90 mmHg and daytime ambulatory SBP ≥ 135 mmHg or diastolic BP (DBP) ≥ 85 mmHg, irrespective of use of antihypertensive agents (Class IIa, Level B), or 2) 24-hour ambulatory SBP ≥ 140 mmHg and DBP ≥ 80 mmHg, irrespective of use of antihypertensive agents (Class IIa, Level B), with eligible renal artery anatomy and estimated glomerular filtration rate ≥ 45 mL/min/1.73 m2. Five subgroups of hypertensive patients are deemed preferred candidates for RDN and dubbed "RDN i2": Resistant hypertension, patients with hypertension-mediated organ Damage, Non-adherent to antihypertensive medications, intolerant to antihypertensive medications, and patients with secondary (2ndary) causes being treated for ≥ 3 months but BP still uncontrolled. The Task Force recommends assessment of three aspects, dubbed "RAS" (R for renal, A for ambulatory, S for secondary), beforehand to ascertain whether RDN could be performed appropriately: 1) Renal artery anatomy eligibility assessed by computed tomography or magnetic resonance renal angiography if not contraindicated, 2) genuine uncontrolled BP confirmed by 24-hour Ambulatory BP monitoring, and 3) Secondary hypertension identified and properly treated. After the procedure, 24-hour ambulatory BP monitoring, together with the dose and dosing interval of all BP-lowering drugs, should be obtained 6 months following RDN. Computed tomography or magnetic resonance renal angiography should be obtained 12 months following RDN, given that renal artery stenosis might not be clinically evident.
Keywords: Blood pressure, Catheter ablation, Hypertension, Nerve, Renal artery
1. INTRODUCTION
1.1 Hypertension control and renal denervation
Hypertension is the most important preventable cause of cardiovascular disease and all-cause mortality worldwide.1 A plethora of epidemiological studies and pharmacological intervention trials have demonstrated that lower blood pressures (BP) (down to the level of < 120/80 mmHg of office BP) are associated with lower premature morbidity and mortality.2-4 There are numerous effective and well-tolerated lifestyle interventions and drugs that can achieve clinically meaningful BP reductions. Nevertheless, the nationwide BP control rates remain poor worldwide and were around 30-40% in Taiwan.5 Just like many other chronic diseases, the unsatisfactory nationwide control rates of hypertension are ascribed to 1) unawareness of the disease, 2) inadequacy of current therapeutic strategies, and 3) poor lifestyle/medication adherence. Based on the National Reimbursement Claims Database in Taiwan from 2001 to 2007, the medication adherence, defined as having medications refilled for ≥ 80% of days in the year after initiation of antihypertensive treatment, was only 18.6%.6 Although the reasons for non-adherence are multifactorial, the experience (adverse events or fear of future adverse events due to antihypertensive medications) and expectations (wishing not dependent on life-long medical therapy) of hypertensive patients are essential and should be taken into account through shared decision making between patients and healthcare professionals.7 Instead, the quest for a short-term and safe treatment strategy that can achieve long-term BP reductions is genuine, thus setting the stage for device-based renal artery denervation (RDN) therapy as an alternative or complementary BP-lowering strategy.
Sympathetic overactivity, sodium/volume overload, and activation of the renin-angiotensin system have long been recognized as the three fundamental pathogenic mechanisms of essential hypertension.8 Numerous studies have shown that sympathetic activities are higher in hypertensive than in normotensive populations. Many believe that the effects of sympathetic overactivity in driving sustained hypertension derive from its effects on renal function, whereas sympathetic effects on systemic hemodynamics are responsible largely for transient BP elevation.9 Percutaneous RDN is thus designed to disrupt renal afferent and efferent sympathetic nerves, which modulate central sympathetic outflow and renal physiology, to achieve sustained BP reduction. Before the advent of antihypertensive medications, surgeons in the 1950s performed thoracolumbar sympathectomy for severe and malignant hypertension, in which renal sympathetic denervation was achieved inadvertently. This treatment strategy not only reduced BP, but also reduced mortality dramatically.10 Since 2009, percutaneous RDN via radiofrequency ablation of renal arteries had been shown to be effective in reducing office BP in patients with resistant hypertension in mostly open-label, single-arm studies.11,12 A serious setback occurred in 2014 with the publication of SYMPLICITY HTN-3 study which showed that RDN was no more effective than a sham procedure.13 Despite a number of shortcomings in SYMPLICITY HTN-3,14 this study triggered the evolution of RDN in almost every aspect, from study design to ablation strategies.
From 2017 onwards, three carefully designed, randomized sham-controlled RDN trials (SPYRAL HTN-OFF MED, SPYRAL HTN-ON MED, and RADIANCE-HTN SOLO), with blood and urine sampling to ensure adherence with "on drug" or "off drug" designs (except RADIANCE-HTN SOLO), ambulatory BP as the primary endpoint, and next-generation catheters capable of four-quadrant energy delivery, have been published.15-18 All three trials showed similar and clinically meaningful BP reductions [approximately 10 mmHg in office systolic BP (SBP) and 6-9 mmHg in 24-hour ambulatory SBP] in patients with mild or moderate hypertension 2-6 months following RDN,18 irrespective of whether radiofrequency energy with dedicated branch artery ablation or ultrasound energy was applied.19 Furthermore, there were no serious adverse events in these three trials. In summary, the three proof-of-concept studies demonstrated the short-term efficacy and safety of newer-generation RDN.
It is well recognized by the Task Force that it is not yet possible to fully understand the long-term durability and safety of newer-generation RDN, the potential interaction with medications, and the generalizability to the heterogeneous hypertension population. However, it would not be in the best interest of the public health to prevent patient access to these devices for these concerns, given the prevalent non-adherence to antihypertensive medications and the unsatisfactory hypertension control rates in Taiwan and the rest of the world.20 RDN was approved by the Taiwan Food and Drug Administration in 2013. Based on all the above information, the Task Force decided to publish the Consensus Statement on Renal Denervation for the Management of Arterial Hypertension to 1) define reasonable indications and clinical context for performing RDN (Table 1, Figure 1), 2) provide pre- and post-RDN assessment algorithms (Figure 2), and 3) identify important investigational topics to further refine and reassure RDN.
Table 1. Clinical context and indications for renal denervation.
| Recommendations | Class* | Level# |
| Clinical context | ||
| Renal denervation should be performed in the context of registry and clinical studies | I | C |
| Renal denervation should not be performed without detailed evaluation of various causes of secondary hypertension and renal artery anatomy | III | C |
| Blood pressure indications | ||
| Renal denervation can be performed in patients with office SBP ≥ 150 mmHg and DBP ≥ 90 mmHg and daytime ambulatory SBP ≥ 135 mmHg or DBP ≥ 85 mmHg, irrespective of use of anti-hypertensive agents, and with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 | IIa | B |
| Renal denervation can be performed in patients with 24-hour ambulatory SBP ≥ 140 mmHg and DBP ≥ 80 mmHg, irrespective of use of antihypertensive agents, and with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 | IIa | B |
| Patient indications | ||
| Patients been treated with maximally tolerated doses of BP-lowering drugs of ≥ 3 classes for ≥ 1 month and fulfilling either of the above BP criteria, with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 | IIa | B |
| Patients with hypertension-mediated organ damage or established cerebro- or cardio-vascular disease been treated with maximally tolerated doses of BP-lowering drugs of ≥ 3 classes (or fewer if intolerable) for ≥ 1 month and fulfilling either of the above BP criteria, with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 | IIa | C |
| Patients intolerant or non-adherent to antihypertensive agentsand fulfilling either of the above BP criteria, with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 | IIa | B |
| Patients with secondary causes of hypertension (excluding obstructive sleep apnea) been de-finitively treated for ≥ 3 months and fulfilling either of the above BP criteria, with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 | IIb | C |
| Patients with obstructive sleep apnea been definitively treated for ≥ 3 months and fulfilling either of the above BP criteria, with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 | IIa | B |
BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
* Class of recommendation, # Level of evidence.
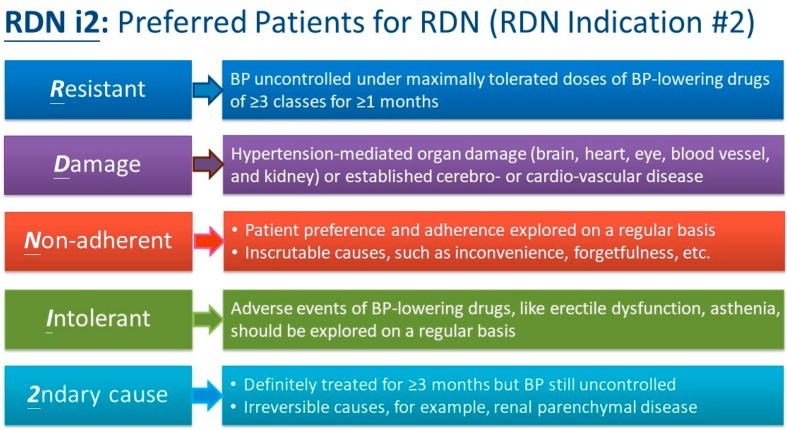
Figure 1.
Five subgroups of hypertensive patients considered preferred candidates for renal denervation: RDN i2. BP, blood pressure; RDN, renal denervation.
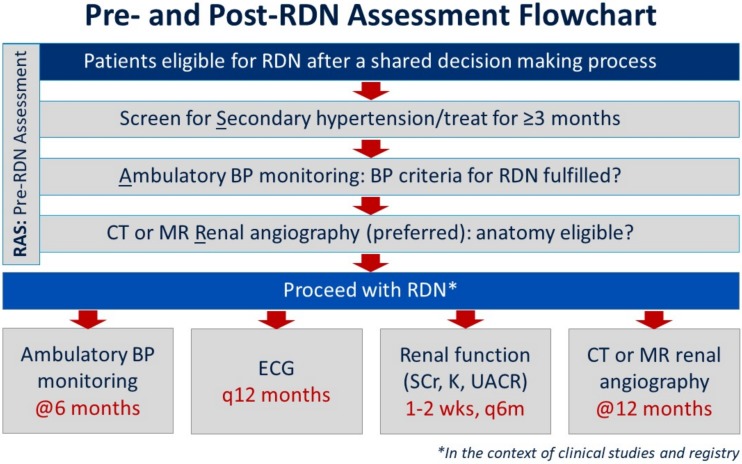
Figure 2.
Pre- and post-renal denervation assessment flowchart. BP, blood pressure; CT, computed tomography; ECG, electrocardiography; K, potassium; MR, magnetic resonance; RDN, renal denervation; SCr, serum creatinine; UACR, urinary albumin:creatinine ratio.
1.2 Guidelines/focused update/consensus development
Taiwan Hypertension Guidelines and related works (Focused Update/Consensus) evaluate and integrate available evidence with the purpose of assisting healthcare professionals in constructing the best management strategies for each individual patient. To ensure guidelines remain updated, new publications are regularly reviewed on a yearly basis. Publication of potentially practice-changing study results will prompt assessment by the Task Force to determine whether a focused update or consensus should be issued if the designated time (approximately 5-year cycles) for full guideline revisions is not reached.
Members of this Task Force were jointly selected by the Taiwan Hypertension Society (THS) and the Hypertension Committee of Taiwan Society of Cardiology (TSOC) to represent professionals from a broad array of backgrounds. The class of recommendation (COR) and level of evidence (LOE) were graded according to predefined scales as modified from the latest American and European guidelines for the management of arterial hypertension (Tables 2 and 3).3,4 The THS/TSOC Guidelines/ Focused Update/Consensus undergo extensive review by the Task Force and external experts and are approved by all Task Force members. The guidelines and related works were developed independently without any involvement from the industry. The Task Force members’ comprehensive disclosure information is shown at the end of this Consensus. The THS/TSOC Hypertension Guidelines/Focused Update/Consensus represent the official position of the THS and TSOC.
Table 2. THS/TSOC classes of recommendations (updated March 2019).
| Classes of recommendations | Definition | Suggested phrases |
| Class I (Benefit >>> Risk) | Evidence and/or general agreement that a given treatment of procedure is beneficial, useful, and effective | • Is recommended |
| • Is indicated | ||
| • Should be performed | ||
| Class II | Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given treatment or procedure | |
| Class IIa (Benefit >/>> Risk) | Weight of evidence/opinion is in favor of usefulness/efficacy | • Is probably recommended |
| • Should be considered | ||
| • Can be performed | ||
| Class IIb (Benefit ≥ Risk) | Usefulness/efficacy is less well established by evidence/opinion | • May/might be considered |
| • May/might be reasonable | ||
| • May/might be performed | ||
| Class III (Benefit ≤ Risk) | Evidence or general agreement that the given treatment or procedure is not useful/effective, and in some cases may be harmful | • Is not recommended |
| • Is not indicated | ||
| • Should not be performed |
THS, Taiwan Hypertension Society; TSOC, Taiwan Society of Cardiology.
Table 3. THS/TSOC levels of evidence (updated Mar 2019).
| Level A | Data derived from multiple (≥ 2) RCTs, or meta-analyses of high-quality RCTs |
| Level B | Data derived from a single RCT, large non-randomized studies, meta-analyses of moderate-quality RCTs or non-randomized studies |
| Level C | Subgroup analyses, post-hoc analyses, retrospective studies, cohort studies, registries, small studies, or consensus of expert opinion |
RCT, randomized controlled trial; THS, Taiwan Hypertension Society; TSOC, Taiwan Society of Cardiology.
Adherence to guidelines and related works can be improved by shared decision making between healthcare professionals and patients, with patient engagement in choosing strategies based on individual preferences, values, and associated conditions. Guidelines and related works should not override clinical judgement, which is the right and responsibility of healthcare professionals. It is also the responsibility of healthcare professionals to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2. PATHOPHYSIOLOGIC AND ANATOMIC BACKGROUND FOR RENAL DENERVATION
Consensus statements
• The effects of sympathetic overactivity in driving sustained hypertension derive mostly from its effects on renal function, whereas sympathetic effects on systemic hemodynamics are responsible for transient BP elevation.
• The renal sympathetic nerve activity regulates renal hemodynamics and excretory function in a dose-dependent manner.
• The renal nerves in the distal segments of main renal artery and branches were closer to the vascular lumen.
• Approximately half of living patients who underwent thoracolumbar sympathectomy for severe hypertension had a significant lowering of BP throughout the 5-year follow-up period.
• Extra renal arteries include accessory and polar arteries.
• Any renal artery with a diameter between 3 and 8 mm should be ablated by radiofrequency energy delivery to achieve optimal RDN results.
2.1 Experience from thoracolumbar sympathectomy
Although hypertension is a multifactorial disease, the sympathetic nervous system has been recognized to play an important role in the pathogenesis of primary (essential) hypertension and in certain forms of secondary hypertension.21 It is generally believed that the effects of sympathetic overactivity in driving sustained hypertension originate from its effects on various renal physiologic functions.8 Neurosurgeon A. W. Adson at Mayo Clinic was the first to treat malignant hypertension by bilateral renal surgical denervation in 1925. Because such an approach done via renal decapsulation or resection of tissue along the renal arteries alone had only a modest and short-lived effect, more radical forms of surgical sympathectomy were developed in the 1930s. Neurosurgeon Max Peet at the University of Michigan introduced the procedure of thoracolumbar splanchnicectomy to remove dorsal ganglia from T9 to T12. If the anatomy was feasible, T8 and L1/2 ganglia were removed as well. In 1953, Smithwick reported on outcomes of 1266 cases treated with thoracolumbar sympathectomy (T8/9 through L1/2) for severe hypertension who had been followed for 5 to 14 years. Overall, the mortality at 5 years was 19% in the sympathectomy group and 54% for those treated medically, but at a cost of significantly increased morbidity, including postural hypotension, syncope, incontinence, and impotence.10 It is noteworthy that approximately 45% of living operated patients had a significant lowering of BP throughout the 5-year follow-up period, while the rest 55% had no change or an increase in BP. The lessons learned from the historical experience with surgical sympathectomy are important as contemporary RDN is accomplished in a less invasive and more focused manner. The procedure of RDN is designed to attenuate the renal sympathetic activity by ablating the peri-arterial adventitial afferent and efferent sympathetic nerves using various methods like radiofrequency or ultrasonic energy, or chemical neurotoxins (ethanol, guanethidine, etc.) through intra-arterial, trans-urethral/trans-pelvic, or external approach. Various animal models have shown that selective RDN prevents or delays the onset of or ameliorates the magnitude of hypertension.22
2.2 Renal sympathetic nerves
The efferent sympathetic innervation of kidney arises from preganglionic neurons in the intermediate-lateral column of the spinal cord (T10-L2). The postganglionic fibers from celiac, mesenteric, aorticorenal and splanchnic ganglia enter the kidneys, providing a network of nerve fibers primarily innervating the cortex but extending into medulla.23,24 The renal sympathetic nerve activity regulates renal hemodynamics and excretory function in a dose-dependent way. Low levels of renal sympathetic nerve activity (RSNA) increase renin release by stimulation of β1-adrenoreceptor on the juxtaglomerular cells.25,26 At slightly higher levels of stimulation, the increase in renin secretion is accompanied by increased renal tubular sodium reabsorption and decreased urinary sodium excretion via α1B-adrenoceptor.27 Higher levels of RSNA decrease renal blood flow and glomerular filtration rate via stimulation of α1A-adrenoceptor.28-30 Release of renin also promotes activation of the renin-angiotensin-aldosterone system. This in turn increases the activity of the sympathetic nervous system through the release of angiotensin II, increases sodium and water resorption, and induces renal vasoconstriction, thus resulting in elevation of BP.
Afferent renal nerve fibers are predominantly located in the renal pelvis and to a lesser degree in the renal cortex. The afferent signals are transmitted through dorsal ganglia (T6-L4) to neurons in the ipsilateral posterior gray column of the spinal cord. The afferent fibers project to the autonomic central nuclei (providing central sympathetic output and resulting in increased sympathetic activity in various targets such as vasculature, heart, and other organs)31-33 and to the contralateral kidney (allowing cross-talk between the two kidneys for regulation of renal hemodynamics).34 Renal afferent nerve activity is modulated by two types of receptors: 1) mechanoreceptors, which are activated by changes in hydrostatic pressure and renal vasculature pressure, and 2) chemoreceptors that are activated by ischemia and changes in the chemical environment of the renal interstitium.35
Renal sympathetic nerve fibers following the renal arteries to the kidneys are located predominantly in the tunica adventitia and outer media of renal arteries.36,37 It has long been believed that the sympathetic nerves were evenly distributed around the circumference of the renal artery, similar to "basket-weave plexus" described by Page and Heuer in 1935.38,39 However, in a human post-mortem study, Atherton et al. reported a higher number of nerve bundles in more distal segments of renal arteries.37 In contrast, another human study by Sakakura et al.40 and a porcine model by Tellez et al.41 found fewer nerves surrounded the renal artery in the distal segments compared with the proximal and middle segments. Atherton et al. reported 49.3% of the nerve fibers distributed within 1.0 mm distance from the lumen and 90.5% within 2.0 mm, while Tellez et al. found only 45% of nerves within 2.0 mm distance.41 Sakakura et al. reported 50th percentile of the distance from lumen to nerves was 2.44 mm, whereas the 75th and 90th percentiles were 4.28 mm and 6.39 mm, respectively.40 Despite the discrepancies, all three studies showed that nerves in the distal segments of main renal artery and branches were closer to the vascular lumen. The most recent detailed anatomical analysis of human cadavers by Mompeo et al. highlighted several of these histological findings including the distal convergence of renal nerve fibers with the main artery, the closer proximity of renal nerves to the renal arterial lumen in distal sections, and the similar distribution pattern of renal nerves with extra renal arteries.42 These observations support the potential advantages of performing renal denervation beyond the main bifurcation and also of achieving ablation in all directions surrounding the vessel. The change in ablation strategy was further supported by the work of Mahfoud et al., which showed that combined ablations of main renal artery plus branches produced the greatest reductions in renal norepinephrine content and cortical axon density compared with conventional treatment of main renal artery alone.43
2.3 Renal arteries
The renal arteries normally originate from the lateral part of the abdominal aorta at the L1 and L2 levels immediately below the level of origin of the superior mesenteric artery.44,45 The orifice of the right renal artery is located on the anterolateral wall of the aorta and that of the left in a more dorsolateral location. The right renal artery is little longer and lies at a higher level than left renal artery.46 Renal arteries are usually 4-6 cm in length and 5-7 mm in diameter. In approximately 70% of individuals, the kidney is supplied by single renal artery.47 Renal artery variations, ranging between 20 and 30%, are divided into 2 groups: early division (branching of the main renal arteries into segmental branches more proximally than the renal hilus level) and extra renal arteries (ERA). ERA include accessory (entering the kidneys from the hilus with the main renal artery) and polar arteries (entering the renal parenchyma directly from the renal cortex away from the hilum).48 Data on the outcomes of RDN for the accessary renal artery are limited. Id et al. reported that the efficacy of RDN was less pronounced in patients with accessory/polar arteries compared with those without.49 Likewise, greater BP reduction was observed in the denervated accessory artery group compared with the incompletely denervated accessory group. The most plausible explanation is incomplete denervation due to inability to ablate all accessory renal arteries or due to limited catheter manipulation in the typically smaller accessory renal arteries. In the SPYRAL HTN-OFF and HTN-ON MED studies, the Spyral multielectrode catheter (Medtronic, Galway, Ireland) was used to target all accessible renal arteries, including branch arteries and accessory arteries with a diameter greater than 3 mm and less than 8 mm. Based on the positive results and the lack of major adverse events in the SPYRAL HTN-OFF and HTN-ON MED studies, any renal artery with a diameter between 3 and 8 mm should be ablated by radiofrequency energy delivery to achieve optimal RDN results.
3. CLINICAL RESULTS OF RENAL DENERVATION
Consensus statements
• All three feasibility, proof-of-concept trials using the new generation RDN devices (SPYRAL HTN-OFF, SPYRAL HTN-ON, and RADIANCE-HTN SOLO) demonstrated that RDN, achieved by either radiofrequency or ultrasound energy delivery, resulted in consistent and clinically meaningful BP reductions (approximately 10 mmHg in office SBP and 6-9 mmHg in ambulatory SBP) compared to sham operation in patients with mild to moderate or uncontrolled hypertension.
• According to data from registry and clinical studies in Taiwan, Korea, and Japan, RDN could achieve sustained and even more pronounced BP reductions at 12 months in Asian hypertensive patients compared to Caucasians.
• RDN was associated with a < 1% rate of vascular access site complication and renal artery injury, as well as no excess risks of renal dysfunction or hypotension. To reliably assess new-onset renal artery stenosis following ablations in the distal segment of main renal artery and branches, computed tomography (CT) or magnetic resonance (MR) renal angiography should be routinely performed at 12 months following RDN.
3.1 Trials
The technologies applied in currently published single-arm RDN studies include using intra-arterial catheter to deliver either radiofrequency or ultrasound energy through the arterial wall, transurethral catheters to ablate the renal pelvis, external devices to focus the ultrasound energy around the renal artery, or intra-arterial catheter to deliver neurotoxins in the peri-arterial space in patients with resistant hypertension. Nevertheless, only the intra-arterial radiofrequency ablation technology has been extensively studied in clinical trials (Table 4). The most seminal trials with radiofrequency ablation RDN include the SYMPLICITY HTN-1, HTN-2 and HTN-3 trials. Following the publication of the neutral SYMPLICITY HTN-3 trial, evolution in every aspect of RDN from study designs to ablation strategies occurred. From 2017 onwards, the three so-called RDN 2.0 trials (SPYRAL HTN-OFF MED, SPYRAL HTN-ON MED, and RADIANCE-HTN SOLO), with sham-control, ambulatory BP measurements as the endpoint, and blood and urine sampling to ensure drug adherence (except RADIANCE-HTN SOLO), published and opened a new chapter for RDN.
Table 4. Published trials of renal denervation for arterial hypertension in alphabetical order (updated March 2019).
| Trials | N | RDN system | Trials | N | RDN system |
| Randomized sham-controlled trials | Non-randomized trials | ||||
| RADIANCE-HTN SOLO | 146 | ParadiseTM (ReCor Medical) | ACHIEVE | 96 | ParadiseTM (ReCor Medical) |
| RADIANCE REINFORCE | 51 | VessixTM (Boston Scientific) | EncoreD | 109 | Symplicity FlexTM (Medtronic) and others |
| ReSET | 69 | Symplicity FlexTM (Medtronic) | EnligHTN 1 | 46 | EnligHTNTM (St. Jude Medical) |
| SYMPLICITY-FLEX | 71 | Symplicity FlexTM (Medtronic) | EnligHTN 2 | 133 | EnligHTNTM (St. Jude Medical) |
| SPYRAL HTN-OFF MED | 80 | Symplicity SpyralTM (Medtronic) | EnligHTN 3 | 39 | EnligHTNTM (St. Jude Medical) |
| SPYRAL HTN-ON MED | 80 | Symplicity SpyralTM (Medtronic) | GLOBAL SYMPLICITY REGISTRY | 2583 | Symplicity FlexTM (Medtronic) |
| Symplicity SpyralTM (Medtronic) | |||||
| Symplicity HTN-3 | 535 | Symplicity FlexTM (Medtronic) | Heidelberg Registry | 63 | Symplicity FlexTM (Medtronic) |
| WAVE IV | 81 | Surround SoundTM (Kona Medical) | Irish Registry | 31 | Symplicity FlexTM (Medtronic) |
| Subtotal | 1113 | Kazakhstan Registry | 63 | Symplicity FlexTM (Medtronic) | |
| Randomized controlled trials (no sham control) | Portugal Registry | 31 | Symplicity FlexTM (Medtronic), EnligHTNTM (St Jude), OneShotTM (Covidien) | ||
| DENERHTN* | 106 | Symplicity FlexTM (Medtronic) | RAPID | 50 | OneShotTM (Covidien) |
| DENERVHTA# | 27 | Symplicity FlexTM (Medtronic) | Reduce HTN | 146 | VessixTM (Boston Scientific) |
| INSPIReD | 15 | EnligHTNTM (St Jude) | SPYRAL FIM | 50 | Symplicity SpyralTM (Medtronic) |
| PRAGUE 15# | 106 | Symplicity FlexTM (Medtronic) | Swedish Registry | 252 | Symplicity FlexTM (Medtronic), Symplicity SpyralTM (Medtronic), EnligHTNTM (St Jude), OneShotTM (Covidien), VessixTM (Boston Scientific), ParadiseTM (ReCor Medical) |
| RADIOSOUND# | 120 | ParadiseTM (ReCor Medical) versus Symplicity SpyralTM (Medtronic) | SYMPLICITY HTN-1 | 50 | Symplicity FlexTM (Medtronic) |
| RDN OSA | 60 | Symplicity FlexTM (Medtronic) | TREND Registry | 191 | Symplicity FlexTM (Medtronic), Symplicity SpyralTM (Medtronic), EnligHTNTM(St Jude), |
| RDN OSLO# | 19 | Symplicity FlexTM (Medtronic) | UK Registry | 253 | SymplicityFlexTM (Medtronic), SymplicitySpyralTM (Medtronic), VessixTM (Boston Scientific), EnligHTNTM (St Jude), OneShotTM (Covidien) |
| SYMPATHY | 139 | Symplicity FlexTM (Medtronic) | Subtotal | 4186 | |
| SYMPLICITY HTN-Japan | 41 | Symplicity FlexTM (Medtronic) | |||
| Symplicity HTN-2 | 106 | Symplicity FlexTM (Medtronic) | |||
| Subtotal | 739 | ||||
| Total | 6038 |
* Standardized stepped-care antihypertensive control; # Active comparator control. RDN, renal denervation.
The SYMPLICITY HTN-1 is the first proof-of-concept single-arm RDN study conducted in Australia and Europe.50 It is an open-label study and enrolled 50 patients with a mean office BP of 177/101 mmHg and a mean 4.7 antihypertensive medications at baseline. It showed RDN, by using a unipolar intravascular catheter, achieved significant and sustained office BP reductions of 27/17 mmHg at 12 months. After publication of the initial 12-month results of RDN, the extended SYMPLICITY HTN-1 study enrolled 153 patients, with 88 patients having complete data at 36 months.12 Significant office BP reductions of 32/14 mmHg were noted at 36 months. Only one new renal artery stenosis requiring stenting and three deaths unrelated to RDN occurred.
The SYMPLICITY HTN-2 is the first randomized, parallel group, open-label study in patients with resistant hypertension (SBP ≥ 160 mmHg, or ≥ 150 mmHg if with type 2 diabetes mellitus) and receiving ≥ 3 antihypertensive medications.11 Exclusion criteria included history of a prior renal artery intervention, main renal arteries < 4 mm in diameter, or < 20 mm in length and hemodynamically or anatomically significant renal artery abnormalities, and baseline estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2. 106 (56%) of 190 patients screened for eligibility were randomly allocated to RDN (n = 52) or control (n = 54) groups. At 6 months, office BP was reduced by 33/11 mmHg in the RDN group as compared to the control group. At 36-month post-procedure, office BP reductions remained durable and were of 33/14 mmHg.51 Procedural complications included one hematoma and one renal artery dissection. Later complications included two cases of acute renal failure, which were fully resolved, 15 hypertensive events requiring hospitalization, and three deaths.
After the success of SYMPLICITY HTN-1 and -2, SYM-PLICITY HTN-3 was initiated to verify RDN efficacy in a more rigorous manner.13 It included 530 patients with resistant hypertension in the USA, with a sham procedure applied in the control group to minimize potential biases and confounders from the open-label studies. Unlike most of the prior smaller-sized, open-label studies, the SYMPLICITY HTN-3 failed its primary efficacy end point, defined as the 6-month change in office SBP (-14.1 mmHg) from baseline compared with sham control [-14.1 mmHg vs. -11.7 mmHg; between-group difference, -2.4 mmHg (95% confident interval, -6.9 to 2.1)] with the superiority margin of 5 mmHg. The differences in ambulatory BP measurements between both RDN and sham-controlled groups did not reach statistical significance as well. Likewise, the 12-month follow-up results showed no further reductions in 24-hour ambulatory BP measurements.52
Because of the disappointing results in SYMPLICITY HTN-3, the efficacy of RDN was questioned. However, additional analyses identified several potential confounders which may have contributed to the results. These confounders include sectors regarding patient selection (less response in patients with isolated systolic hypertension, possibly due to increased vascular stiffness rather than sympathetic hyperactivity as the primary mechanism of hypertension), medication adherence and adjustment (not strictly defined/checked and the Hawthorne effect, thus marked BP reductions in the sham group), and procedural issues (only 5% received bilateral complete circumferential ablations).53 To deal with all these concerns, future RDN clinical trials including a run-in period, medication washout, and evaluation of medication adherence were recommended.54 Together with the development of newer-generation RDN intra-arterial catheters (Medtronic’s Symplicity Spyral RDN catheter and ReCor Medical’s Paradise RDN catheter), the carefully designed, proof-of-concept SPYRAL HTN-OFF MED, SPYRAL HTN-ON MED and RADIANCE-HTN SOLO studies were conducted in hypertensive patients not confounded by medication therapy (mild to moderate hypertension with urine sampling to assure medication adherence) to reliably determine the effectiveness and safety of RDN prior to initiation of a larger complex pivotal study.
SPYRAL HTN-OFF MED15 is a multicenter, single-blind, randomized sham-controlled trial, and enrolled 80 patients with office SBP between 150 mmHg and 180 mmHg and 24-hour ambulatory SBP between140 mmHg and 170 mmHg, after 3-4 weeks of medication washout, in the USA, Europe, Japan and Australia. Eligible patients were drug-naïve or able to discontinue existing antihypertensive medications, with drug testing during the screening. The primary effectiveness endpoint is the change in 24-hour ambulatory BP at 3 months between RDN and sham control groups. Office and 24-hour ambulatory BP decreased significantly from baseline to 3 months in the RDN group (-10.0/-5.3 mmHg and -5.5/-4.8 mmHg for office and 24-hour ambulatory BP, respectively), while no significant changes were seen in the sham-control group. There were no adverse events in either group. The differences in results between SYMPLICITY HTN-3 and SPYRAL HTH-OFF MED have been attributed to the newer design of catheter, distal and branch artery ablation strategy, a longer run-in period to minimize regression to the mean bias, and elimination of confounders related to medication use. One recent meta-analysis of radiofrequency ablation-based RDN trials with < 10% unplanned changes in antihypertensive medications during the follow-up periods showed consistent and statistically significant office and ambulatory BP changes in the RDN group compared to the control group.55
RADIANCE-HTN SOLO16 is another multicenter, international, single-blind, randomized, sham-controlled trial done in the USA and Europe with use of endovascular ultrasound RDN catheter to ablate the main renal artery. The inclusion criteria, slightly different form the SPYRAL HTN trials, were daytime ambulatory BP between 135/85 mmHg and 170/105 mmHg after a 4-week discontinuation of up to two antihypertensive medications. The reduction in daytime ambulatory SBP at 2 months, which was its primary endpoint, was greater with RDN (n = 74) than with sham procedure (n = 72) (-8.5 mmHg and -2.2 mmHg for RDN and sham groups, respectively).
SPYRAL HTN-ON MED17 is a parallel study of SPYRAL HTN-OFF MED study to justify the role of RDN in daily practice. Its inclusion criteria were the same as SPYRAL HTN-OFF MED, but on one to three antihypertensive medications with stable doses for at least 6 weeks. To confirm the adherence of medication, drug testing was applied before and during the study. It showed RDN (n = 38) significantly reduced office and 24-hour BP in uncontrolled hypertensive patients prescribed up to three antihypertensive medications, compared to a sham operation (n = 42) at 6 months (-9.4/-5.2 mmHg and -9.0/-6.0 mmHg for office and 24-hour ambulatory BP, respectively). No major adverse events occurred. All these three feasibility, proof-of-concept studies demonstrate that RDN, achieved by either radiofrequency or ultrasound energy delivery, resulted in similar and clinically meaningful BP reductions (approximately 10 mmHg in office SBP and 6-9 mmHg in ambulatory SBP) compared to sham operation in patients with mild to moderate or uncontrolled hypertension.18 The consistent BP-lowering efficacy and safety will be conclusively evaluated by well-designed larger pivotal trials. Key results from the three RDN 2.0 trials are summarized in Table 5.
Table 5. Summary of three second-generation renal denervation randomized sham-controlled proof-of-concept trials.
| SPYRAL HTN-OFF MED | RADIANCE-HTN SOLO | SPYRAL HTN-ON MED | |
| Patients (n) | 80 | 146 | 80 |
| Age (years) | 54 | 54 | 53 |
| System applied | SPYRAL (Medtronic) | PARADISE (ReCor Medical) | SPYRAL (Medtronic) |
| Inclusion criteria | Office SBP ≥ 150 and < 180 mmHg, office DBP ≥ 90 mmHg, and 24-h SBP ≥ 140 and < 170 mmHg, no antihypertensive meds | Daytime ambulatory BP ≥ 135/85 mmHg and < 170/105 mmHg, no anti-hypertensive meds | Office SBP ≥ 150 and < 180 mmHg, office DBP ≥ 90 mmHg; and 24-h SBP ≥ 140 and < 170 mmHg, 1-3 antihypertensive meds |
| Baseline office BP (mmHg)* | 162/101 ± 7/7 | 155/100 ± 14/8 | 164/101 ± 7/7 |
| Baseline 24-h BP (mmHg)* | 153/99 ± 8/8 | 144/88 ± 9/6 | 152/98 ± 7/8 |
| No. of antihypertensive meds* | 0 | 0 | 000.2.2 ± 0.9 |
| Change 24-h SBP vs. sham (mmHg), # months of f/u | -4.6 (-9.2 to 0.1), 3 | -4.1 (-7.1 to -1.2), 2 | -7.0 (-12.0 to -2.1), 6 |
| Change 24-h SBP vs. baseline (mmHg), # months of f/u | -5.5 (-9.1 to -2.0), 3 | -7.0 (-9.0 to -5.0), 2 | -9.0 (-12.7 to -5.3), 6 |
| Change office SBP vs. sham (mmHg), # months of f/u | -7.1 (-13.2 to -1.1), 3 | -6.5 (-11.3 to -1.8), 2 | -6.6 (-12.4 to -0.9), 6 |
| Change office SBP vs. baseline (mmHg), # months of f/u | -10.0 (-15.1 to -4.9), 3 | -10.8 (-13.9 to -7.7), 2 | -9.4 (-13.5 to -5.3), 6 |
| Major adverse events (%) | 0 | 0 | 0 |
BP, blood pressure; DBP, diastolic blood pressure; f/u, follow-up; SBP, systolic blood pressure.
* Mean ± standard deviation, # Mean (95% confidence interval).
3.2 Registries
There are several RDN registries ongoing (Table 4). The Global SYMPLICITY Registry (GSR) is the largest prospective, open-label, single-arm, multicenter registry of patients undergoing RDN by using the Medtronic’s Symplicity catheters. It has been conducted to evaluate the procedural and long-term safety and effectiveness of RDN in real-world patients. The enrolling is ongoing with close to 3000 patients from 134 centers in Canada, western Europe, Latin America, eastern Europe, South Africa, Middle East, Asia (including Taiwan), Australia, and New Zealand.56
Besides GSR, there are several national registries in different countries. The investigator-initiated Austrian Transcatheter Renal Denervation (TREND) registry includes 407 patients in 14 Austrian centers from April 2011 to September 2014. Results from the TREND registry showed that RDN significantly decreased 24-hour ambulatory BP by 11/6, 8/4, 8/5, and 10/6 mmHg after 2-6 weeks, 3, 6 and 12 months, respectively.57 In one Sweden-based registry, RDN also showed sustained office and ambulatory SBP reductions of 8 mmHg up to 36 months.58
Among Asian populations, the Korean registry, a substudy of GSR (GSR Korea), was recently published.59 Given the differences in baseline characteristics between Korean (n = 93) and Caucasian (n = 169) patients, like lower baseline office SBP, lower body mass index, and differences in medications, propensity score adjustment was performed to fairly compare the changes in office BP after RDN between both groups. After propensity score matching, office BP changes at 6 months were similar between both populations, whereas office BP reductions at 12 months were greater among Korean patients compared to Caucasian patients (Korean, 27.2/13.9 mmHg vs. Caucasian, 20.1/7.6 mmHg, p < 0.001).59 This more pronounced and sustained efficacy implies that RDN is a safe and effective antihypertensive treatment for Asian patients.
In Taiwan, a total of 180 patients underwent RDN till February 2019, of which 89 cases were treated by using the Spyral catheter. The average baseline office BP was 170/93 mmHg, with an average number of 4.8 antihypertensive medications. The mean office SBP reductions were 26, 29, and 26 mmHg at 3, 6, and 12 months, respectively. The mean 24-hour ambulatory SBP reduction was 8 mmHg at 12 months, which is consistent with the registry data worldwide.57-59
These registries also demonstrated additional benefits of RDN besides BP reductions. According to results of the 12-month follow-up from GSR,60 RDN also reduced heart rates throughout the 12-month period.
It’s still an unresolved issue that what kind of patients benefits most from RDN. In TREND registry, baseline 24-hour ambulatory BP was the best predictor for the BP-lowering effects of RDN.57 Pooled data from SYMPLICITY HTN-3 and GSR showed that BP reductions following RDN among patients with isolated systolic hypertension [SBP ≥ 140 mmHg and diastolic BP (DBP) ≤ 90 mmHg] were less pronounced than the reductions in patients with combined systolic-diastolic hypertension.61 However, the two groups of patients were not well matched demographically as combined systolic-diastolic hypertensive patients were younger, healthier and had both higher baseline SBP and heart rate as compared to the isolated systolic hypertension group.
3.3 Safety
Considering the location of target nerves and how the procedures are performed, the major safety events with RDN can be categorized into four sectors: vascular access complications, renal artery injury, renal dysfunction, and blood pressure-related events. According to when the events would occur, the major safety events can be further classified into 1) peri-procedural events, mainly represented by access site complications (e.g. hematomas and aneurysms) and renal events (e.g. renal artery dissection or perforation, renal artery embolism and infarction); and 2) longer-term events, like symptomatic hypotension, hypertensive emergencies, deterioration of renal function, and development of renal artery stenosis. In addition to clarifying how often and how severe the adverse events may occur, another consideration is to determine when is more appropriate and how to assess the occurrence of adverse events.
Safety data for RDN come from experimental studies, mostly uncontrolled safety trials of the various RDN systems, clinical studies, and registries. The majority of data are from studies using the Symplicity catheter, thus might not be generalizable to other RDN systems. Most trials had a follow-up duration of 6 months, and only the SYMPLICITY HTN-1 and two other studies reported a follow-up period of up to 3 years.
In the SYMPLICITY HTN-1 trial,50 1 out of the 45 patients undergoing RDN had renal dissection upon placement of the catheter and thus needed stenting, while another had a pseudoaneurysm treated with antibiotics and analgesics. Short-term (14-30 days) angiograms and 6-month MR angiograms did not show any irregularities at the sites of treatment. Renal function, available in 25 patients at both baseline and 6-month follow-up, was stable. In the 153 patients included in the extended SYMPLICITY HTN-1 study, a total of three patients, all treated with an 8F guide, developed a pseudoaneurysm that was managed conservatively.62 Renal artery imaging with MR angiography, CT angiography or duplex at 6 months (in 81 patients) did not show any new stenosis, but there was one case of progression of a pre-existing proximal stenosis away from the energy application sites that was successfully stented. Other events included flank pain, transient dizziness and pitting edema as well as two deaths considered unrelated to the procedure. The final 3-year report of the study, on 88 patients, documented a new 80% stenosis of the right renal artery in need of stenting at 24 months, as well as 4 hypotensive episodes, 13 hypertensive episodes, and 3 deaths.12 The eGFR decreased from 83.6 ml/min/1.73 m2 at baseline to 74.3 ml/min/1.73 m2 at 36 months.
In the randomized SYMPLICITY HTN-2 trial of 106 patients,11 only minor peri-procedural events were noted including one pseudoaneurysm, one case of symptomatic hypotension in need of reduction of antihypertensive drugs, one urinary tract infection, a case of paresthesia and a case of back pain that were resolved after 1 month. Seven patients had intra-procedural bradycardia requiring atropine. At 6 months, imaging in 43 patients showed one possible progression of a previous atherosclerotic lesion not located at an ablation site, which required no intervention. After the initial 6-month follow-up, a renal artery dissection prior to catheter insertion requiring stenting was noted in the crossover group. Up to 1 year, there were a total of 9 hypertensive events and 2 hypotensive events requiring hospitalization.63 Between 12 and 36 months of follow-up and out of 70 patients that underwent RDN, there were only a few hypertensive and hypotensive events as well as three deaths unrelated to the procedure.51 Renal function was stable in both intervention and control groups at 6 months and no change in mean eGFR was recorded at the 36-month follow-up.
In the first single-blind, randomized sham-controlled SYMPLICITY HTN-3 study of the efficacy and safety of RDN using the Symplicity catheter in 535 patients with drug-resistant hypertension,13 the overall number of adverse events was very low, and no significant differences were noted between groups. Rates of major adverse events did not significantly differ between the denervation group (1.4%) and the control group (0.6%). The documented access site complication rate of 0.3% was rather low and may be attributed to the special care used for study patients. In clinical routine, an average complication rate would be expected to be at about 1.3%.65 Kidney function did not differ between groups at any time point. Regarding an increase in creatinine by 50% compared to baseline, it was observed in 5 cases out of 352 (1.4%) in the RDN group and in 1 case out of 171 (0.6%) in the control group.
In contrast to the main renal artery ablations in the SYMPLICITY HTN-1 to HTN-3 trials, ablations of both main and branch renal arteries were performed, with an average total ablation of 44 points, in the SPYRAL HTN-OFF MED and HTN-ON MED studies. It is noteworthy that there were no adverse events in either SPYRAL HTN-OFF MED or SPYRAL HTN-ON MED study.15,17
In an initial report of the real-world ALSTER BP registry of 93 patients that underwent RDN with the Symplicity catheter, renal function was stable during the 6-month follow-up period.66 One renal artery dissection prior to insertion of the ablation catheter was recorded. There was one case of a small kidney infarct potentially associated with a thrombus at the ablation sites, even though the patient was on dual antiplatelet therapy.
In the open-label, multicenter Global SYMPLICITY Registry, data for the first 1,000 enrolled patients at 6 months were available.67 Even though underreporting of adverse events may have been possible, there were only 6 peri-procedural adverse events related to the procedure, including four vascular access site complications (0.34%) and two renal artery dissections that were stented. Other events included 5 hospitalizations for hypertensive emergency (0.5%), 6 for atrial fibrillation (0.6%), 7 strokes (0.7%), 4 hospitalizations for new-onset heart failure (0.4%), 7 myocardial infarctions (0.7%) and 2 cases of new-onset end-stage kidney disease (0.2%) that were considered unrelated to the procedure.
A systematic review of 12 studies of RDN with five different catheters on 506 patients reported a total of five procedural complications (< 1%) including one renal artery dissection and four pseudoaneurysms at the site of arterial puncture (all from the initial SYMPLICITY registry).68 In another meta-analysis of nine studies on 1096 patients, adverse events were rare and were represented mainly by femoral access site complications, while renal function was preserved during the respective follow-ups.69 In a meta-analysis by the European Network Coordinating Research on Renal Denervation (ENCOReD) Consortium of seven randomized trials of RDN with the Symplicity catheter, a total of 985 patients were studied that had been randomized to either RDN (n = 588) or control (n = 397).70 Major adverse events were documented in 56 RDN patients and 29 controls (9.9% and 7.4%, respectively, p = 0.20). The pooled estimate of renal function assessed with eGFR over 6 months did not differ between groups. Based on recent meta-analyses of 52 studies and a qualitative review of an additional 14 studies reporting on 2898 patients in total,71 the authors concluded that renal function does not significantly change up to at least 9 months after RDN.
In summary, the accumulated evidence suggests that RDN was associated with a < 1% rate of vascular access site complication and renal artery injury, as well as no excess risks of renal dysfunction. To reliably assess new-onset renal artery stenosis following ablations in the distal segment of main renal artery and branches, CT or MR renal angiography should be routinely performed at 12 months following RDN.
4. CLINICAL CONTEXT AND INDICATIONS FOR RENAL DENERVATION
Consensus statements
• RDN should be done in the context of registry and clinical studies, unless more reassuring results from larger, pivotal, newer-generation RDN trials are available.
• RDN should not be done routinely, without detailed evaluation of various causes of secondary hypertension and renal artery anatomy.
• RDN could be done in patients who fulfill either of the following 2 BP criteria: 1) office BP ≥ 150/90 mmHg and daytime ambulatory SBP ≥ 135 mmHg or DBP ≥ 85 mmHg and 2) 24-hour ambulatory SBP ≥ 140 mmHg and DBP ≥ 80 mmHg, irrespective of use of antihypertensive agents, and with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2.
• Five subgroups of hypertensive patients [Resistant hypertension, patients with blood pressure-mediated vasculature or organ Damage, Non-adherent to antihypertensive medications, intolerant to antihypertensive medications, secondary (2ndary) causes definitively treated but hypertension still uncontrolled], dubbed "RDN i2", are considered the preferred candidates for RDN.
4.1 Clinical context
Given the global burden of hypertension (average 20-25% prevalence in adult populations), the unsatisfactory hypertension control rate worldwide (generally < 50%), and the widespread non-adherence to long-term antihypertensive medications (> 50% in treated hypertensive patients as seen in other chronic diseases), the pursuit of a procedure that can safely and effectively achieve long-term, clinically meaningful BP reductions19,72 is desperately awaited. The recently published 3 proof-of-concept RDN 2.0 trials, with a total of 150 patients undergoing newer-generation RDN by distal and four-quadrant radiofrequency or ultrasound energy ablations, consistently demonstrated a clinically meaningful and 24-hour sustained BP reduction (nearly 10 mmHg in office SBP and 6-9 mmHg in 24-hour ambulatory SBP) 2-to-6 months following the procedure, with no renal artery injury or renal function deterioration.15-18 The Task Force recognizes the potential morbidity/mortality benefits of newer-generation RDN for the management of arterial hypertension, but also acknowledges there are unresolved issues like uncertain long-term durability and safety of these therapies, lack of reliable procedural markers to indicate successful denervation, and lack of reliable predictors to identify potential responders to RDN. The Task Force thus recommends 1) RDN should be done in the context of registry and clinical studies, unless more reassuring results from larger, pivotal, newer-generation RDN trials are available (COR I, LOE C), and 2) RDN should not be done routinely, without detailed evaluation of various causes of secondary hypertension and renal artery anatomy (COR III, LOE C) (Table 1).
4.2 Indications
4.2.1 #1 Blood pressures
The majority of RDN trials published before the SPYRAL HTN-OFF MED study enrolled patients with resistant hypertension, conventionally defined as office SBP ≥ 140 mmHg or DBP ≥ 90 mmHg despite ≥ 3 classes of antihypertensive medications, including a diuretic agent.73 The labelled indications for RDN in Taiwan, primarily based on the inclusion criteria of SYMPLICITY HTN-1 to HTN-3, is simplified as office SBP ≥ 160 mmHg under ≥ 3 maximally tolerated antihypertensive medications irrespective of which classes in use. Despite DBP ≥ 90 mmHg under ≥ 3 maximally tolerated antihypertensive medications is not included in the indications for RDN in Taiwan, there is evidence indicating that isolated systolic hypertension, reflected by increased pulse wave velocity and central pulse pressure, is related to increased aortic stiffness and is associated with a milder response to RDN.74,75 Therefore, DBP, at least office DBP, might be considered while selecting patients for RDN.
In the three recently published RDN 2.0 trials, patients enrolled had mild to moderate combined hypertension, rather than those with resistant hypertension. The summarized inclusion criteria regarding office BP in all three trials are office SBP ≥ 150 mmHg and DBP ≥ 90 mmHg, irrespective of use of antihypertensive agents. Ambulatory BP monitoring was routinely obtained to ensure the diagnosis of hypertension. Since the ambulatory BP monitoring may influence the quality of sleep and disturb nighttime BP, the Task Force recommends using daytime ambulatory BP to ascertain hypertension. The summarized inclusion criteria regarding daytime ambulatory BP in all three trials are ambulatory SBP ≥ 135 mmHg or DBP ≥ 85 mmHg, irrespective of use of antihypertensive agents. The diagnosis of combined hypertension was mainly based on office BP rather than ambulatory BP in both SPYRAL HTN-OFF MED and HTN-ON MED trials.
Based on all the evidence, the Task Force recommends RDN could be done in patients who fulfill the BP criteria as: office BP ≥ 150/90 mmHg and daytime ambulatory SBP ≥ 135 mmHg or DBP ≥ 85 mmHg, irrespective of use of antihypertensive agents, with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 (COR IIa, LOE B) (Table 1).
Masked hypertension is defined in people whose BP is normal in the office, but is elevated at the out-of-office setting when measured by either home BP monitoring or ambulatory BP monitoring.1 Masked hypertension is present in ~15% of patients with normal office BP, and is associated with higher prevalence of metabolic risk factors and hypertension-mediated organ damage.1 Masked uncontrolled hypertension, defined as treated patients in whom the office BP appears controlled to recommended BP targets, but BP is uncontrolled according to home or ambulatory BP measurements, occurs in ~30% of treated hypertensive patients.76 Meta-analyses and registry-based studies have shown that cardiovascular morbidity and mortality are substantially higher in masked hypertension compared with normotension, and even greater than that of sustained hypertension, irrespective of whether antihypertensive treatment was initiated.77,78 The Task Force recognizes the clinical and prognostic significance of masked hypertension and masked uncontrolled hypertension, which could be overlooked if we recommend both office BP and ambulatory BP should fulfill the BP inclusion criteria as the only indicated clinical scenario.
The definition of hypertension by 24-hour ambulatory BP is SBP ≥ 130 mmHg and DBP ≥ 80 mmHg in Taiwan and European hypertension guidelines.1,4 The ambulatory BP criteria for SPYRAL HTN trials and RADIANCE-HTN SOLO are 24-hour SBP ≥ 140 mmHg and < 170 mmHg and daytime BP ≥ 135/85 mmHg and < 170/105 mmHg, respectively.16,17 To incorporate the combined hypertension phenotype and the intersection of all RDN 2.0 trials, the Task Force therefore recommends RDN could also be done in patients who fulfill the BP criteria as: 24-hour ambulatory SBP ≥ 140 mmHg and DBP ≥ 80 mmHg, irrespective of use of antihypertensive agents, with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2(COR IIa, LOE B) (Table 1).
4.2.2 #2 patients
Just like antihypertensive medications, the strongest predictor of BP reductions following RDN was baseline SBP in the post-hoc analyses of SYMPLICITY HTN-3 trial.53 Likewise, the clinical benefits of each 10 mmHg SBP reductions were directly proportional to baseline cardiovascular risks.19 Patients with either of the 2 features could obtain greater benefits from any BP-lowering strategy (COR IIa, LOE B). On the other hand, given the well-documented clinical benefits of BP-lowering drugs in hypertensive patients with established cerebro- or cardiovascular disease,19,79 BP-lowering drugs should be routinely prescribed and optimized first to achieve BP targets. Once BP targets are difficult to achieve sustainably, RDN could serve as a useful complementary BP-lowering strategy (COR IIa, LOE C). Since the time taken for the full BP-lowering effects of antihypertensive medications to develop is approximately 1 month80 and the relatively high inherent risks with uncontrolled hypertension, the Task Force recommends ≥ 1 month as the observation period for the efficacy of BP-lowering medications in patients with hypertension-mediated organ damage or atherosclerotic cerebro- or cardiovascular disease (Table 1).
Uncontrolled hypertension is very often caused by non-adherence to antihypertensive medications, either by intention (patient preference) or forgetfulness. The impact of non-adherence to long-term hypertension control is huge and often underestimated,6,81 which is reflected in the great difference in hypertension control rates between hospitalized patients and general populations. To curtail this gap, patient preference, with regard to long-term medication therapy, should be carefully explored and considered in determining which antihypertensive strategies should be provided on a regular basis.7 Patients who are intolerant or non-adherent to antihypertensive agents, no matter due to what reasons, fulfilling the above BP criteria with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2 could be managed by RDN (COR IIa, LOE B). The positive results of both SPYRAL HTN-OFF MED and RADIANCE-HTN SOLO trials further certify this approach.
Since hypertension is a multifactorial disease, the presence of secondary causes of hypertension does not guarantee the identified secondary causes are the only causes of hypertension of a given individual. Therefore, the presence of secondary causes of hypertension should not be viewed as a contraindication for RDN. In patients with documented secondary causes of hypertension, including obstructive sleep apnea,82-84 and having been treated for ≥ 3 months, RDN could be done if their ambulatory and/or office BP fulfill the above-mentioned BP indications (COR IIb, LOE C). More detailed descriptions are shown in section 5.
Considering these potential clinical and behavioral predictors of patients who will benefit most from RDN, the Task Force summarized the five subgroups of hypertensive patients [Resistant hypertension, patients with blood pressure-mediated vasculature or organ Damage, Non-adherent to antihypertensive medications, intolerant to antihypertensive medications, secondary (2ndary) causes treated but hypertension still uncontrolled], dubbed "RDNi2", as the preferred candidates for RDN (Figure 1). To facilitate memorizing, the acronym "RDNi2" could be regarded as "renal denervation indication #2" that exactly fits the #2 category, patients, of the indications of RDN recommended in the THS/TSOC Consensus.
5. PRE-RENAL DENERVATION ASSESSMENT
Consensus statements
• The presence of secondary causes of hypertension might not be viewed as an absolute contraindication for RDN. Further, in patients with documented secondary causes of hypertension, RDN could be done if ambulatory BP and/or office BP remain uncontrolled after ≥ 3 months of definitive treatment.
• Chronic kidney disease stages 3b and 4 (eGFR 15-44 mL/min/1.73 m2) is not an absolute contraindication for RDN, which should be performed with caution and in the context of registry and clinical studies.
• Either diluted iodinated contrast with dose restriction or CO2 angiography could be used as the imaging modality for RDN in patients with advanced chronic kidney disease (Stages 3b, 4, and 5).
• Radiofrequency ablation should be performed in renal artery segments > 2 mm away from the stented or stenotic segments.
Once RDN is considered a potential treatment strategy for a given patient with hypertension, assessment of the following three aspects should be done beforehand to ascertain whether RDN could be performed appropriately (Figure 2). The three aspects are 1) Renal artery anatomy eligibility: assessed by CT or MR renal angiography if not contraindicated; 2) genuine uncontrolled BP: confirmed by 24-hour Ambulatory BP monitoring; and 3) Secondary hypertension identified and properly treated. To facilitate memorizing, the acronym "RAS" (R for renal, A for ambulatory, S for secondary) was designated. In this section, essential issues regarding assessment of secondary hypertension and how to apply RDN in patients with coexisting secondary causes of hypertension are detailed.
5.1 Screening for secondary hypertension
Secondary hypertension is defined as elevated systemic arterial BP due to an identifiable cause.1,3 Hypertension with secondary causes can coexist with essential hypertension, with which residual hypertension remains after the pathogenetic causes are identified and removed.85
The overall prevalence of secondary hypertension is 5 to 10% in hypertensive patients.86 In patients with resistant hypertension, the prevalence of secondary hypertension is significantly higher (up to 20 to 35%).87,88 In a retrospective study of Savard et al., 200 patients with resistant hypertension were screened for RDN in a tertiary center, 113 patients (56.5%) were diagnosed with hypertension due to secondary causes.89 Simplified classification into common secondary causes and uncommon causes is suggested by guidelines with cut-off prevalence value of 1% in hypertensive patients (Table 6).3
Table 6. Causes of secondary hypertension and diagnostic screening tests.
| Prevalence in hypertensive patients | Prevalence in resistant hypertensive patients | Screening tests | Additional/confirmatory tests | |
| Common causes | ||||
| Renal parenchymal disease | 1-2% | 2-10% | Serum creatinine, renal ultrasound, urinalysis | Specific tests to evaluate cause of renal disease (toxin, biopsy) |
| Renal artery stenosis | 1-10% | 5-34% | Renal duplex, or CT or MR renal angiography | Intra-arterial renal angiography |
| Primary aldosteronism | 5-8% | 17-23% | Plasma aldosterone concentration, plasma renin activity, and plasma aldosterone/renin ratio | Oral sodium loading test, intravenous saline infusion test, or captopril suppression test; Adrenal CT or MR scan, adrenal vein sampling; Adrenal NP-59 scintigraphy |
| Obstructive sleep apnea | 5-10% | 25-50% | Epworth Sleepiness Score, overnight oximetry | Polysomnography |
| Drug or alcohol induced | 2-4% | - | Urinary/hair drug screen (illicit drugs) | Response to withdrawal of suspected agent |
| Uncommon causes | ||||
| Pheochromocytoma | 0.1-0.6% | < 1% | 24-h urinary fractionated metanephrines or plasma metanephrines | CT or MR scan of abdomen/pelvis |
| Cushing’s syndrome | < 0.1% | < 1% | Overnight 1-mg dexamethasone suppression test, 24-h urinary free cortisol excretion, midnight salivary cortisol | Low-dose dexamethasone suppression test |
| Hypothyroidism | < 1% | 1-3% | Thyroid stimulating hormone, free thyroxine | None |
| Hyperthyroidism | < 1% | 1-3% | Thyroid stimulating hormone, free thyroxine | Radioactive iodine uptake and scan |
| Aortic coarctation | 0.1% | < 1% | Rib notching on chest X-ray, ankle-brachial index, echocardiogram | Thoracic and abdominal CT or MR angiogram |
| Primary hyperparathyroidism | Rare | Rare | Serum calcium | Serum parathyroid hormone |
CT, computed tomography; MR, magnetic resonance.
While considering RDN therapy, a careful evaluation of secondary hypertension is crucial, especially in those with a treatable cause, such as primary aldosteronism, drug or alcohol-induced hypertension, renal artery stenosis, obstructive sleep apnea, or other endocrine hypertension. Given that the presence of secondary causes of hypertension does not preclude the coexistence of essential hypertension and certain secondary causes are not definitively treatable (for example, renal parenchymal disease), the Task Force deems that the presence of secondary causes of hypertension might not be viewed as an absolute contraindication for RDN. The Task Force recognizes there is limited evidence about the effects of RDN in these subgroups of hypertensive patients. Considering the clinical impacts of uncontrolled hypertension, for patients with documented secondary causes of hypertension, RDN might be considered if ambulatory BP and/or office BP remain uncontrolled after ≥ 3 months of definitive treatment. The designation of 3 months as the observational period to gauge treatment efficacy is based on 1) the time needed for the BP-lowering effects of antihypertensive medications to develop fully (~1 month),80 2) the BP-lowering response of RDN, as one form of definitive treatment, to be clinically evident (~3 months),15-17 and 3) the time needed to show a clinically meaningful impact with a given (~4 mmHg) SBP difference (3 months).90
5.1.1 Renal parenchymal disease
Renal parenchymal disease is a common cause of secondary hypertension in adult hypertensive patients.1,3,85 Bilateral abdominal masses palpated during physical examination warrants survey of polycystic kidney disease. Serum creatinine concentration and urinalysis (protein, erythrocytes, and leukocytes) are the best screening tests for renal parenchymal disease.1,3,85 Renal ultrasound measurement of kidney morphology, size, shape, cortical thickness, echogenicity and identification of abnormal masses or urinary tract pathology can further provide etiology and pathogenesis. Other tests to evaluate causes of renal disease would be indicated if specific renal disease suspected.
Although renal parenchymal disease is one cause of secondary hypertension, it is not an absolute contraindication for RDN. Safety and effectiveness of RDN have been reported in stage 3 or 4 chronic kidney disease patients in small single-center, non-randomized studies.91,92
5.1.2 Renovascular disease
Renovascular disease results from narrowing of renal artery (renal artery stenosis) causing restricted renal perfusion. The most common cause is atherosclerotic disease (90%) in adult patients, while non-atherosclerotic disease (fibromuscular dysplasia, the most common) prevails in young adults.3
Clinical conditions suggesting renal artery stenosis include abdominal bruits, sign and symptoms of peripheral vascular disease, and multiple risk factors contributing generalized atherosclerosis. Resistant hypertension, recent onset or progression of severe hypertension, recent renal function deterioration, acute renal function deterioration after the use of renin-angiotensin system blockers, and flash pulmonary edema are other clues indicating renal artery stenosis. These are also factors predicting responses after renal artery revascularization.85
Renal artery stenosis could be reliably identified by CT or MR renal artery angiography, whereas renal duplex ultrasound serves as a screening tool with limited accuracy.
Current guidelines recommend optimization of medical therapy for hypertension and risk factors for patients with atherosclerotic renal artery stenosis because previous studies failed to show advantages with endovascular intervention.3,93 Revascularization with angioplasty or stenting could be considered if failed medically controlled renal artery stenosis (refractory hypertension, worsening renal function, or intractable heart failure) and in patients with non-atherosclerotic renal artery stenosis, including fibromuscular dysplasia.3
With the recent development of device-based RDN for hypertension management, more patients underwent workups for renal artery status. However, patients with significant renal artery stenosis were generally excluded from previous RDN trials.11,12,64,94 The practice of RDN in patients with renal artery stenosis is discussed in section 5.2.2.
5.1.3 Primary aldosteronism
Primary aldosteronism refers to a group of disorders caused by inappropriate production of aldosterone not regulated by or responding with sodium status and renin-angiotensin system. It is a common cause of secondary hypertension and the prevalence ranges from 1.4% to 23% based on different clinical characteristics and selection criteria. In resistant hypertension group, the prevalence of primary aldosteronism is higher, from 17% to 23%.3,87,95-97 In Asia, the prevalence of primary aldosteronism in resistant hypertension is 7.1% among the surveyed 1656 patients in China.98 The two most common causes of primary aldosteronism are aldosterone-producing adenoma (30-40%) and bilateral adrenal hyperplasia (60-70%).95,96,99
Patients with primary aldosteronism would present with hypertension and hypokalemia (approximately 40%), with or without metabolic alkalosis and hypernatremia.96,99 Other symptoms and signs include resistant hypertension, muscle weakness, constipation, and fatigue.99 Compared with patients with essential hypertension with similar BP levels, patients with primary aldosteronism had larger artery stiffness, myocardial tissue fibrosis, endothelial cell dysfunction, and resistance vessel remodeling.99
According to the consensus of Taiwan Society of Aldosteronism, screening of primary aldosteronism is recommended in patients with resistant hypertension.96,99 The plasma aldosterone-renin ratio (ARR), plasma aldosterone concentration divided by plasma renin activity, is currently the best method for screening primary aldosteronism.99 Patients should be prepared for ARR measurement with hypokalemia correction and unrestricted salt intake. Several categories of antihypertensive medications may interfere with ARR data interpretation. Diuretics, including mineralocorticoid receptor antagonists (spironolactone, eplerenone) and amiloride, significantly compromise the test results. This kind of medication should be discontinued at least 4 weeks before testing. Angiotensin-receptor blockers, angiotensin-converting enzyme inhibitors, beta-blockers, and central alpha-2 agonists (clonidine and methyldopa) need to be held at least 2 weeks before testing. Use of neutral antihypertensive drugs such verapamil, hydralazine, or doxazosin are recommended during the preparation for ARR measurement.99
Blood samples collected in the morning are recommended. The most commonly used cutoff value is > 30 (or 35, according to the Consensus in Taiwan) when plasma aldosterone concentration reported in ng/dL and plasma renin activity in ng/mL/h.96,99 Confirmatory tests for primary aldosteronism include oral salt-loading test, intravenous saline infusion test, and captopril suppression test.99
Once diagnosis of primary aldosteronism is confirmed, imaging of bilateral adrenal glands with CT or MR and referral to a specialist for adrenal venous sampling are suggested to determine the source of the increased aldosterone production.3,95,96,99 For unilateral increased aldosterone production, mostly seen in aldosterone-producing adenoma, unilateral laparoscopic adrenalectomy is recommended with improvement of BP achieved in all recipients and half completely cured of hypertension. For bilateral increased aldosterone production and those unilateral producers unsuitable for operation, spironolactone or eplerenone is suggested. RDN could still be considered if hypertension remains ≥ 3 months after the commencement of surgical or medical treatment for primary aldosteronism.
5.1.4 Obstructive sleep apnea
Obstructive sleep apnea is caused by recurrent and intermittent upper airway collapse during sleep, inducing apnea or hypopnea, hypoxemia, and sleep disruption. This chronic medical condition correlates with systemic diseases, including hypertension, coronary and cerebrovascular diseases, heart failure, and atrial fibrillation.100-103
Obstructive sleep apnea is highly prevalent in hypertensive adults, especially with resistant hypertension with prevalence ranging from 60 to 80%.104-106 Patients with obstructive sleep apnea would present with obesity, large neck, and macroglossia and complain of daytime somnolence, restless sleep, choking episodes and snoring during sleep, witnessed apneas, nocturia, irritability, and decreased libido. Ambulatory BP monitoring often shows nocturnal non-dipping pattern, elevated daytime BP, tachycardia and/or bradycardia in patients with obstructive sleep apnea.
Obstructive sleep apnea could be screened with Berlin Questionnaire or Epworth Sleepiness scale. Once positive, the gold standard diagnostic tool of polysomnography can be used. The severity of obstructive sleep apnea is determined by the apnea-hypopnea index.3,85
Several previous studies have shown that treatment with continuous positive airway pressure in patients with obstructive sleep apnea and hypertension could only reduce office SBP by 2-3 mmHg.3
5.1.5 Drugs or alcohol
Medication history should be carefully reviewed since BP is affected by numerous substances, including prescription medications, over-the-counter medications, herbals, and food substances.88
5.1.6 Uncommon causes
Secondary hypertension manifesting as refractory hypertension could be caused by differential diagnoses other than those mentioned above, which are much rarer in prevalence.1,3 Secondary hypertension can be divided into several disease categories: endocrine disorders,107 renal disorders, neurological disorders, acute stress,108 drug-induced hypertension, genetic disorders, and miscellaneous. When faced with symptoms and signs of hypertensive disease combined with clinical presentation indicating other organ-specific disease such as endocrine (eg. hyperparathyroidism) or neurological disorders (eg. intracranial tumor), alertness should be kept by clinician and timely consultation with specialist for specific screening and confirmation studies is warranted.
5.2 Renal denervation in patients with secondary causes of hypertension
5.2.1 Renal parenchymal disease
The majority of studies evaluating RDN excluded individuals with an eGFR of < 45 mL/min/1.73 m2.13,15 However, a parallel relationship between sympathetic overdrive and the severity of renal parenchymal disease, chronic kidney disease, has been demonstrated.109 Considering the high prevalence of hypertension in patients with chronic kidney disease and the relationship between sympathetic overactivity and activation of renin-angiotensin system,110 the so-called "neurogenic hypertension" phenotype should be prevalent in patients with chronic kidney disease and hypertension.111 This makes an argument that RDN could be effective in patients with advanced chronic kidney disease. Statistically significant BP lowering and slightly increased eGFR have been demonstrated in patients with stage 3 or 4 chronic kidney disease in small single-center, non-randomized studies.91,92,112 Renal function seemed to be not aggravated by the procedure in patients with chronic kidney disease stage 3 or 4. Nevertheless, the amount of contrast medium used during the newer-generation RDN emphasizing distal, circumferential, and multiple ablations could be greater compared to conventional main artery ablation strategy. It may be a concern particularly for patients with an eGFR of 15-44 mL/min/1.73 m2. To avoid contrast-induced nephropathy, either diluted iodinated contrast with dose restriction or CO2 angiography could be used as the imaging modality for RDN.113 The Task Force agrees that chronic kidney disease stages 3b and 4 (eGFR 15-44 mL/min/1.73 m2) is not an absolute contraindication for RDN, but should be performed with caution and in the context of registry and clinical studies (COR IIb, LOE C) (Table 7).
Table 7. Renal denervation in special clinical scenarios.
| Recommendations | Class* | Level# |
| Renal denervation might be performed with caution in patients with chronic kidney disease stages 3b and 4 (eGFR 15-44 mL/min/1.73 m2) who fulfill either of the BP indications for renal denervation, with eligible renal artery anatomy, and in the context of registry and clinical studies | IIb | C |
| Radiofrequency renal denervation should not be performed in stenotic or stented segments of renal artery | III | C |
| Concurrent renal artery revascularization for significant renal artery stenosis and renal denervation for plaque-free segments in uncontrolled hypertensive patients might be rational | IIb | C |
BP, blood pressure; eGFR, estimated glomerular filtration rate.
* Class of recommendation; # Level of evidence.
5.2.2 Renovascular disease
Renal artery stenosis is often caused by atherosclerosis and fibromuscular dysplasia. The former causes the majority and usually involves the proximal third of the renal artery.114 Autopsy studies showed that the distance from lumen to nerve was longer in the proximal segment of the renal arteries.115 A case report had documented the destruction of the peri-arterial nerves located within 2 mm from the luminal surface 12 days after radiofrequency RDN. In other words, the thermal injury did not penetrate > 2 mm from the luminal surface.116 Given the thickness of atheromatous plaque, the efficacy of radiofrequency ablation, and the potential risk of inducing plaque progression, the Task Force recommends that radiofrequency ablation should be prohibited in stenotic segment of renal artery (COR III, LOE C) (Table 7). To avoid the inadvertent ablation of diseased renal artery segment, pre-RDN CT or MR angiography is vital since it can provide detailed information regarding the distribution and severity of plaques. More clinical evidence was needed for newer devices and procedures.
Two randomized trials have shown that the benefits of renal artery revascularization in patients with significant renal artery stenosis were over-estimated.117 However, concurrent renal artery revascularization for significant renal artery stenosis and RDN for plaque-free segments in uncontrolled hypertensive patients might be rational.
Some patients have received radiofrequency RDN in their stented artery, according to case reports. However, the penetration of destruction caused by radiofrequency denervation was limited,116 and the energy was further interfered by metallic stent struts. Safety of renal denervation in stented renal arteries was reported in a porcine model, but the efficacy was only achieved when radiofrequency ablation was delivered to segments not stented.118 The Task Force recommends that radiofrequency ablation should be done in renal artery segments > 2 mm away from the stented segment (COR III, LOE C) (Table 7).
5.2.3 Obstructive sleep apnea
In the hypothesis-generating post-hoc analysis of SYMPLICITY HTN-3, RDN reduced the 6-month office SBP in subjects with self-reported obstructive sleep apnea (-17.0 ± 22.4 vs. -6.3 ± 26.1 mmHg, p = 0.01), compared with sham control, but not in subjects without obstructive sleep apnea (-14.7 ± 24.5 vs. -13.4 ± 26.4 mmHg).82 In the GSR subgroup analysis,84 among 1868 patients, self-reported obstructive sleep apnea occurred in 205 patients. RDN significantly reduced office and ambulatory BP by 14 ± 25.3 and 4.9 ± 18.0 mmHg, respectively. The extent of BP lowering was similar between patient with obstructive sleep apnea and those without. It was not affected by treatment with continuous positive airway pressure or not. In addition to one small case series,119 one randomized study enrolling 60 patients with true resistant hypertension coexisting with moderate-to-severe obstructive sleep apnea (apnea/hypopnea index ≥ 15) showed that, in the RDN group, reductions in office and ambulatory BP were sustained and were accompanied by significant improvement in echocardiographic global longitudinal strain and clinical severity of OSA at 6 months.83 Thus, the Task Force recommends that, for patients with obstructive sleep apnea and having been treated for ≥ 3 months who still fulfill the RDN indications, RDN should be considered a rational antihypertensive treatment option (COR IIa, LOE B) (Table 7).
6. RENAL DENERVATION PROCEDURES WITH RADIOFREQUENCY ABLATIONS
Consensus statements
• A single electrode radiofrequency ablation impacts approximately 25% of the circumference of the renal artery. Thus, ablation attempts in at least 4 distinct quadrants of the artery are required to provide a complete circumferential treatment.
• An optimized procedural technique with special emphasis on distal renal artery, branch artery, and accessory artery is critical to reducing variability in patient response and gaining maximal BP-lowering effect with RDN.
• For cases with tortuous and angulated renal arteries, a guidewire with strong support, good torquability, and a non-traumatic tip, such as Grand Slam (Asahi Intecc, Aichi, Japan) or Thunder (Medtronic, MN, USA), is very helpful for advancing the radiofrequency catheter to achieve comprehensive branch ablations.
• For cases with downward ostium, ostial renal artery stenosis, or excessive aortorenal angle, transradial or transbrachial approach might be more appropriate for RDN.
6.1 Four-quadrant ablations
It has been assumed that the less-than-expected efficacy outcomes in the RDN arm in SYMPLICITY HTN-3 may have been due to incomplete denervation. One key insight from HTN-3 was that ablations in a four-quadrant pattern bilaterally resulted in a larger drop in office and ambulatory SBP and heart rate.53 Pre-clinical histologic analysis of ablated nerves in normotensive pigs has shown that a single electrode radiofrequency ablation impacts approximately 25% of the circumference of the artery.120 Thus, ablation attempts in at least 4 distinct quadrants of the artery (superior, inferior, anterior, and posterior quadrants) are required to provide a complete circumferential treatment. The four-electrode Symplicity Spyral catheter was designed to assist in achieving four-quadrant ablations by delivering the radiofrequency energy and was used in the SPYRAL HTN clinical trial program.
The Spyral catheter has a self-expanding helical design to conform to a wide range of artery shapes and sizes (3 to 8 mm in diameter).
6.2 Distal and branch artery ablations
Recent histologic analyses indicated that renal nerves may have a positional bias,121,122 suggesting that nerves surrounding the distal main and branch renal arteries are closer to the arterial wall than those in the proximal main renal artery. Targeting renal ablation in the distal main and branch arteries increased the amount of non-functioning nerves and reduced renal norepinephrine content in animal studies.115,123
Likewise, renal denervation in patients with accessory renal arteries that were not denervated provided less reduction of BP than it provided in treated patients without accessory vessels, suggesting that it would be beneficial to include accessory renal arteries, if accessible, as targets of the denervation procedure.124 Altogether, the increased knowledge of renal nerve anatomy suggests that an optimized procedural technique with special emphasis on distal renal artery, branch artery, and accessory artery is critical to reducing variability in patient response and gaining maximal BP-lowering effect with RDN.
A dedicated RDN ablation strategy focusing on both main and distal renal arteries was adopted in the SPYRAL HTN program. The average number of ablations was 43.8 in SPYRAL HTN-OFF MED and 45.9 in SPYRAL HTN-ON MED. For cases with tortuous and angulated renal arteries, a guidewire with strong support, good torquability, and a non-traumatic tip, such as Grand Slam (Asahi Intecc, Aichi, Japan) or Thunder (Medtronic, MN, USA), is very helpful for advancing the radiofrequency catheter to achieve comprehensive branch ablations. The location of renal artery ablation should not exceed the inner border of renal parenchyma (Figure 3).
Figure 3.
Inner border of renal parenchyma in renal angiogram. The red dotted line shows the inner border of renal parenchyma. The blue dots indicate ablation points.
6.3 Vascular access
Data from numerous RDN clinical trials have demonstrated that both renal arteries can be successfully accessed via the transfemoral approach.11,15,64,125 The procedural success rate is almost one hundred percent, with acceptable safety profile. However, for patients who have calcified, tortuous, dissected, or infrarenal aneurysmal abdominal aorta, transfemoral approach for any endovascular procedure becomes more difficult or with greater intrinsic risk than those with relatively normal aortic anatomy. Besides, downward ostium, ostial renal artery stenosis, and excessive aortorenal angle might make catheter engagement to the orifice of renal arteries challenging.126-128 Repeated testing with contrast medium and manipulation of guide catheter increases not only the risk of contrast-induced nephropathy, but also embolic event or renal artery injury.129 In these circumstances, transradial or transbrachial approach might be rational for RDN.130,131
There are few published case reports regarding transbrachial, transradial, or transulnar RDN till now.132-135 Considering the size of vascular access and RDN catheter, six- and seven-French guide catheter is suggested for transradial and transbrachial approach, respectively.136 The Spyral catheter is 6-Fr. in diameter with a working length of 117 cm. It is thus possible to engage bilateral renal artery ostia by using a 90-cm 6 or 7-Fr. guide catheter via transbrachial approach. In this way, the catheter protruding length of 27 cm is long enough for performing radiofrequency ablation along the main and distal renal artery. Additionally, left brachial arterial access is more preferable than right one because of shorter brachio-ostial length and less tortuosity.137
Transradial approach for renal angiography or intervention could be considered if the patient’s body height is < 170 cm. In contrast, transbrachial approach is more appropriate for those with height ≥ 170 cm.
6.4 Medications
Common medications available for the procedure include, but are not limited to: heparin, fentanyl/morphine/ultiva, midazolam, propofol, nitroglycerin, and Atropine. Heparin should be given from 5000 to 7500 IU, according to the body size of patients. Fentanyl, morphine, midazolam, and possibly propofol could be given before and during the procedure at proceduralist’s discretion. If vasospasm is noted during the procedure, 100~200 U nitroglycerin could be given.
7. POST-RENAL DENERVATION ASSESSMENT
Consensus statements
• 24-hour ambulatory BP monitoring, together with the dose and dosing interval of all BP-lowering drugs, should be obtained 6 months following RDN.
• Electrocardiogram should be obtained 12 months, and then yearly, following RDN since the QRS voltage change is a surrogate of left ventricular mass, which is closely related to long-term BP burden and of prognostic significance.
• Renal function assessment, including serum creatinine, eGFR, and potassium and urine dipstick or albumin: creatinine ratio, should be obtained 1-2 weeks and every 6 months following RDN.
• Given that more aggressive and distal ablation strategy is adopted by newer-generation RDN, CT or MR renal angiography should be obtained at 12 months following RDN to evaluate whether there is any evidence of renal artery stenosis, which might not be evident clinically.
7.1 Blood pressures
In addition to office BP measurement, patient should be instructed to do home BP monitoring before and after RDN. The importance and necessity of home BP monitoring should be emphasized to all RDN patients, since it is the cornerstone for achieving optimal BP control. Details of how and when to obtain adequate home BP measurements are shown in TSOC/THS 2015 Hypertension Guidelines.1
After hospital discharge, patients should be followed at outpatient clinic within 1-2 weeks. Notably, BP changes rarely become evident immediately after the procedure. It often takes several weeks to months before an apparent BP reduction occurs. According to experience from the 3 newer-generation RDN trials, the Task Force recommends that 24-hour ambulatory BP monitoring should be obtained 6 months following RDN (Figure 2) since it seems to be the most appropriate time point for physicians to judge whether the given patient is responsive to RDN or not.17 Since medication adjustment can influence the BP-lowering effect,55 the Task Force recommends to document the dose and dosing interval of all BP-lowering drugs used whenever ambulatory BP monitoring is obtained. It is worthwhile to obtain ambulatory BP monitoring one to 3 months after RDN to detect whether there is any difference in early-phase response following RDN between populations. After 6 months, ambulatory BP monitoring could be performed on a yearly basis to assess the long-term durability of RDN.
Safety concerns regarding potential orthostatic effects of RDN can largely be ruled out due to the lack of occurrence of orthostasis in the large RDN trials and a small clinical trial.138 It is worthwhile to ask patients to measure their standing BP no matter whether they are symptomatic, given that most orthostatic hypotension cases are asymptomatic.1
7.2 Heart rate and electrocardiogram
In the SYMPLICITY HTN-3 trial,13 there was no significant between-group difference in the change in heart rate from baseline to 6 months. Nevertheless, among 46 patients in SYMPLICITY HTN-2 trial selected for extended investigation of cardiopulmonary exercise tests, results showed that heart rate at rest was decreased after RDN by 4 bpm, whereas maximum heart rate and heart rate increase during exercise were not different.139 In another study of 136 patients with resistant hypertension who underwent RDN, heart rate was reduced by 2.1 bpm after 6 months. The heart rate-slowing effect was more evident in patients whose baseline heart rate was > 71 bpm. The PR interval was prolonged by 11.3 and 10.3 msec at 3 and 6 months after RDN, respectively.140 Thus, heart rate measurement should be obtained together with BP measurement. Electrocardiogram should be obtained 12 months, and then yearly, following RDN (Figure 2) since the QRS voltage change is a surrogate of left ventricular mass, which is closely related to long-term BP burden and of prognostic significance.141,142
7.3 Renal function
Concerns have been raised that RDN might negatively influence renal function. In the SYMPLICITY HTN-1 to HTN-3 trials,12,51,52 there was no significant deterioration of renal function in RDN groups. Nonetheless, SYMPLICITY HTN-3 reported 5 cases receiving RDN and 1 case in the sham group had an increase in serum creatinine levels of greater than 50% from baseline.13 In the SPYRAL HTN-OFF MED and HTN-ON MED trials, there was no increase in serum creatinine of more than 50% from baseline despite a mean of 251 ml of contrast was used in the RDN group.15,18 Factors possibly influence renal function following RDN include contrast media, renal artery injury, BP changes during or after the procedure, and distal embolization.56 The temporal change of renal function would hint us the possible etiologies. For example, in contrast-induced nephropathy, serum creatinine generally peaks at 3 to 5 days and returns to baseline by 7 to 10 days.143 Immediate change of renal function might be observed during hypotensive episodes or significant renal artery complications. Renal function assessment, including serum creatinine, eGFR, and potassium and urine dipstick or albumin: creatinine ratio, should be obtained 1-2 weeks and every 6 months following RDN (Figure 2).
7.4 Renal artery and vascular access
RDN-related vascular complications are rare, even during long-term follow-up, and include renal artery dissection, stenosis, and vascular access complications. However, one case of accelerated progression of renal artery stenosis after RDN was reported which suggested radiofrequency ablations may accelerate the progression of pre-existing renal artery atherosclerotic lesions.144 Given that more aggressive and distal ablation strategy is adopted by newer-generation RDN, the Task Force recommends that CT or MR renal angiography should be obtained 12 months following RDN to evaluate whether there is any evidence of renal artery stenosis, which might not be evident clinically (Figure 2). In patients with procedure-related renal artery injury, unexplained deterioration of renal function, or deteriorating hypertension, follow-up renal artery imaging, like duplex ultrasound, CT or MR angiography with contrast enhancement should be arranged to elucidate renal artery status.
8. FUTURE PERSPECTIVES
Consensus statement
• The unmet needs with regard to RDN include uncertainty about clinical predictors of response, procedural endpoints indicating the success of RDN, and non-invasive indicators for reinnervation later on.
To make RDN a more individual-oriented and goal-directed procedure, there are at least three fundamental issues to be solved: 1) to identify the predictors of response, 2) to identify the procedural endpoint which can reliably indicate the success of RDN, and 3) to identify non-invasive indicators for reinnervation during follow-up.
First, regarding the predictors of response, previous studies have shown that pre-RDN BP level,145 diastolic BP variability,146 combined systolic-diastolic hypertension,61 24-hour ambulatory heart rate,147 renal artery vasodilatation,148 central pulse pressure,75 and impaired cardiac baroreflex sensitivity149 were potential predictors of RDN responders. Despite impaired baroreflex sensitivity improved by RDN, it was not correlated with BP reductions.150 All the other predictors are not direct measures of sympathetic activity. Some of the predictors need to be obtained invasively. Although the muscle sympathetic nerve activity and heart rate variability are related to sympathetic activity, they were not changed by RDN in some studies.151,152 In summary, the ideal predictor, which should be non-invasively accessible, related to sympathetic activity, and reproducibly correlated with BP-lowering response to RDN, is still lacking.
Second, regarding the procedural endpoint, one study group applied high-frequency stimulation to renal artery to ascertain whether RDN was successful or not in 13 patients with paroxysmal or permanent atrial fibrillation and resistant hypertension.153 The change in BP response following high-frequency stimulation was used to determine the procedural success of RDN. Despite the successful result of this small study, the mechanism of BP response by high-frequency stimulation remains controversial.154 Moreover, the BP responses following high-frequency renal artery stimulation are heterogenous and could be site-dependent.155 In a recent study, Sakaoka et al.156 used intravascular ultrasound and optical frequency domain imaging to facilitate the RDN procedure in a porcine model. Positive correlations in measured dimensions of ablations between histopathology and intravascular imaging results were demonstrated. However, it did not guarantee that there was functional change in sympathetic tone simultaneously. It seems that the BP response to high-frequency stimulation of renal artery before and after RDN could serve as a rational procedural endpoint and needs further verification. Without a definite procedural endpoint indicative of immediate success of RDN, it would be hard to compare the BP-lowering efficacy between different RDN technologies.157
Third, there are no non-invasive and reproducible parameters capable of detecting reinnervation for post-RDN follow-up. Reinnervation has been demonstrated after RDN in animal and human studies.158-160 However, a recent sheep model of hypertensive renal disease showed maintenance of the BP-lowering effect of RDN to at least 30 months.161 Histology, tissue noradrenaline level, muscle sympathetic nerve activity, renal sympathetic nerve activity, and the afferent and efferent responses to electrical stimulation of renal nerves could be used to detect reinnervation following RDN. Yet, all of these methods are invasive and hard to perform in clinical practice. One novel method, called neuECG, is developed to record skin sympathetic nerve activity and electrocardiogram simultaneously.162,163 The skin sympathetic nerve activity recorded by neuECG might be able to identify the potential responder of RDN, to evaluate the sympathetic activity change immediately after RDN, and to detect reinnervation.
9. CONCLUSIONS
Sympathetic overactivity is one of the essential mechanisms of hypertension. Sympathetic overactivity in driving sustained hypertension derives mostly from its effects on renal function, whereas sympathetic effects on systemic hemodynamics are responsible largely for transient BP elevation. Percutaneous RDN is designed to disrupt renal afferent and efferent sympathetic nerves, which modulate central sympathetic outflow and renal physiology, to achieve sustained BP reduction. Since 2017 onward, all three feasibility, proof-of-concept, sham-controlled RDN trials demonstrated that RDN resulted in consistent and clinically meaningful BP reductions (approximately 10 mmHg in office SBP and 6-9 mmHg in ambulatory SBP) compared to sham operation in patients with mild to moderate or uncontrolled hypertension, irrespective of whether radiofrequency energy with dedicated branch artery ablation or ultrasound energy was applied. The accumulated evidence suggests that RDN was associated with a < 1% rate of vascular access site complication and renal artery injury, as well as no excess risks of renal dysfunction. In Taiwan, a total of 180 patients underwent RDN till February 2019. The mean 24-hour ambulatory SBP reduction was 8 mmHg at 12 months, consistent with the registry data worldwide.
Considering all the available evidence and the suboptimal long-term control rate of hypertension worldwide, the Task Force agrees that RDN is a legitimate alternative antihypertensive strategy and recommends 1) RDN should be done in the context of registry and clinical studies, unless more reassuring results from larger, pivotal, newer-generation RDN trials are available (COR I, LOE C), and 2) RDN should not be done routinely, without detailed evaluation of various causes of secondary hypertension and renal artery anatomy (COR III, LOE C). The Task Force recommends RDN could be done in patients who fulfill either of the 2 BP criteria: 1) office BP ≥ 150/90 mmHg and daytime ambulatory SBP ≥ 135 mmHg or DBP ≥ 85 mmHg, irrespective of use of antihypertensive agents (COR IIa, LOE B), or 2) 24-hour ambulatory SBP ≥ 140 mmHg and DBP ≥ 80 mmHg, irrespective of use of antihypertensive agents (COR IIa, LOE B), with eligible renal artery anatomy and eGFR ≥ 45 mL/min/1.73 m2. Five subgroups of hypertensive patients who are preferred candidates for RDN are identified and dubbed "RDN i2": Resistant hypertension, patients with blood pressure-mediated vasculature or organ Damage, Non-adherent to antihypertensive medications, intolerant to antihypertensive medications, and patients with secondary (2ndary) causes being treated for ≥ 3 months but hypertension still uncontrolled.
The Task Force recommends assessment of three aspects, dubbed "RAS" (R for renal, A for ambulatory, S for secondary), should be done beforehand to ascertain whether RDN could be performed appropriately: 1) Renal artery anatomy eligibility: assessed by CT or MR renal angiography if not contraindicated, 2) genuine uncontrolled BP: confirmed by 24-hour Ambulatory BP monitoring, and 3) Secondary hypertension identified and properly treated. After the procedure, the Task Force recommends that 24-hour ambulatory BP monitoring should be obtained 6 months following RDN. Renal function assessment, including serum creatinine, eGFR, and potassium and urine dipstick or albumin: creatinine ratio, should be obtained 1-2 weeks and every 6 months following RDN. The Task Force recommends that CT or MR renal angiography should be obtained 12 months following RDN to evaluate whether there is any evidence of renal artery stenosis, which might not be clinically evident.
Acknowledgments
None.
DECLARATION OF CONFLICT OF INTEREST
Tzung-Dau Wang has been on the speakers bureau for AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Novartis, Pfizer, Sanofi, and Takeda. All the other authors have nothing to declare.
REFERENCES
- 1.Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the management of hypertension. J Chin Med Assoc. 2015;78:1–47. doi: 10.1016/j.jcma.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Chiang CE, Wang TD, Lin TH, et al. The 2017 focused update of the guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the management of hypertension. Acta Cardiol Sin. 2017;33:213–225. doi: 10.6515/ACS20170421A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 4.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 5.Beaney T, Schutte AE, Tomaszewski M, et al. May measurement month 2017: an analysis of blood pressure screening results worldwide. Lancet Glob Health. 2018;6:e736–e743. doi: 10.1016/S2214-109X(18)30259-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang TD, Chen YH, Huang CH, et al. Bidirectional adherence changes and associated factors in patients switched from free combinations to equivalent single-pill combinations of antihypertensive drugs. Hypertension. 2014;63:958–967. doi: 10.1161/HYPERTENSIONAHA.113.02455. [DOI] [PubMed] [Google Scholar]
- 7.Schmieder RE, Hogerl K, Jung S, et al. Patient preference for therapies in hypertension: a cross-sectional survey of german patients. Clin Res Cardiol. 2019 doi: 10.1007/s00392-019-01468-0. [DOI] [PubMed] [Google Scholar]
- 8.Mann SJ. Neurogenic hypertension: pathophysiology, diagnosis and management. Clin Auton Res. 2018;28:363–374. doi: 10.1007/s10286-018-0541-z. [DOI] [PubMed] [Google Scholar]
- 9.Esler M. Does increased 24-h ambulatory heart rate identify de facto neurogenic hypertension, and facilitate selection of hypertensive patients for renal denervation? Eur Heart J. 2019;40:752–754. doi: 10.1093/eurheartj/ehz027. [DOI] [PubMed] [Google Scholar]
- 10.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension: results in 1,266 cases. JAMA. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 11.Symplicity HTNI, Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 12.Krum H, Schlaich MP, Sobotka PA, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the symplicity HTN-1 study. Lancet. 2014;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 14.Schmieder RE, Mahfoud F, Azizi M, et al. European Society of Hypertension position paper on renal denervation 2018. J Hypertens. 2018;36:2042–2048. doi: 10.1097/HJH.0000000000001858. [DOI] [PubMed] [Google Scholar]
- 15.Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- 16.Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1. [DOI] [PubMed] [Google Scholar]
- 17.Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6. [DOI] [PubMed] [Google Scholar]
- 18.Sardar P, Bhatt DL, Kirtane AJ, et al. Sham-controlled randomized trials of catheter-based renal denervation in patients with hypertension. J Am Coll Cardiol. 2019;73:1633–1642. doi: 10.1016/j.jacc.2018.12.082. [DOI] [PubMed] [Google Scholar]
- 19.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 20.Wang TD. Our stance towards the 2017 ACC/AHA high blood pressure clinical practice guideline: has the pendulum swung too far? Acta Cardiol Sin. 2018;34:1–3. doi: 10.6515/ACS.201801_34(1).20171218A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 22.Dibona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R245–R253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 23.Dibona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–198. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 24.Barajas L, Liu L, Powers K. Anatomy of the renal innervation: intrarenal aspects and ganglia of origin. Can J Physiol Pharmacol. 1992;70:735–749. doi: 10.1139/y92-098. [DOI] [PubMed] [Google Scholar]
- 25.Zanchetti AS. Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension. Circulation. 1977;56:691–698. doi: 10.1161/01.cir.56.5.691. [DOI] [PubMed] [Google Scholar]
- 26.Keeton TK, Campbell WB. The pharmacologic alteration of renin release. Pharmacol Rev. 1980;32:81–227. [PubMed] [Google Scholar]
- 27.Osborn JL, Francisco LL, DiBona GF. Effect of renal nerve stimulation on renal blood flow autoregulation and antinatriuresis during reductions in renal perfusion pressure. Proc Soc Exp Biol Med. 1981;168:77–81. doi: 10.3181/00379727-168-41238. [DOI] [PubMed] [Google Scholar]
- 28.Armenia A, Munavvar A, Abdullah N, et al. The contribution of adrenoceptor subtype(s) in the renal vasculature of diabetic spontaneously hypertensive rats. Br J Pharmacol. 2004;142:719–726. doi: 10.1038/sj.bjp.0705842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdulla M, Sattar M, Salman I, et al. Effect of acute unilateral renal denervation on renal hemodynamics in spontaneously hypertensive rats. Auton Autacoid Pharmacol. 2008;28:87–94. doi: 10.1111/j.1474-8673.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 30.Sattar MA, Johns EJ. Evidence for an alpha 1-adrenoceptor subtype mediating adrenergic vasoconstriction in wistar normotensive and stroke-prone spontaneously hypertensive rat kidney. J Cardiovasc Pharmacol. 1994;23:232–239. [PubMed] [Google Scholar]
- 31.Liu L, Barajas L. The rat renal nerves during development. Anat Embryol. 1993;188:345–361. doi: 10.1007/BF00185944. [DOI] [PubMed] [Google Scholar]
- 32.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res. 1997;753:102–119. doi: 10.1016/s0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- 33.Ciriello J, de Oliveira CV. Renal afferents and hypertension. Cur Hypertens Rep. 2002;4:136–142. doi: 10.1007/s11906-002-0038-x. [DOI] [PubMed] [Google Scholar]
- 34.Kopp UC, Smith LA, DiBona GF. Renorenal reflexes: neural components of ipsilateral and contralateral renal responses. Am J Physiol-Renal Physiol. 1985;249:F507–F517. doi: 10.1152/ajprenal.1985.249.4.F507. [DOI] [PubMed] [Google Scholar]
- 35.Moss NG. Renal function and renal afferent and efferent nerve activity. Am J Physiol. 1982;243:F425–F433. doi: 10.1152/ajprenal.1982.243.5.F425. [DOI] [PubMed] [Google Scholar]
- 36.Vink A, Schoneveld AH, Poppen M, et al. Morphometric and immunohistochemical characterization of the intimal layer throughout the arterial system of elderly humans. J Anat. 2002;200:97–103. doi: 10.1046/j.0021-8782.2001.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atherton DS, Deep NL, Mendelsohn FO. Micro-anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat. 2012;25:628–633. doi: 10.1002/ca.21280. [DOI] [PubMed] [Google Scholar]
- 38.Page IH, Heuer GJ. The effect of renal denervation on patients suffering from nephritis. J Clin Invest. 1935;14:443. doi: 10.1172/JCI100695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziakas A, Gossios T, Doumas M, et al. The pathophysiological basis of renal nerve ablation for the treatment of hypertension. Curr Vasc Pharmacol. 2014;12:23–29. doi: 10.2174/15701611113119990145. [DOI] [PubMed] [Google Scholar]
- 40.Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. doi: 10.1016/j.jacc.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 41.Tellez A, Rousselle S, Palmieri T, et al. Renal artery nerve distribution and density in the porcine model: biologic implications for the development of radiofrequency ablation therapies. Transl Res. 2013;162:381–389. doi: 10.1016/j.trsl.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Mompeo B, Maranillo E, Garcia-Touchard A, et al. The gross anatomy of the renal sympathetic nerves revisited. Clin Anat. 2016;29:660–664. doi: 10.1002/ca.22720. [DOI] [PubMed] [Google Scholar]
- 43.Mahfoud F, Tunev S, Ewen S, et al. Impact of lesion placement on efficacy and safety of catheter-based radiofrequency renal denervation. J Am Coll Cardiol. 2015;66:1766–1775. doi: 10.1016/j.jacc.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Beregi JP, Mauroy B, Willoteaux S, et al. Anatomic variation in the origin of the main renal arteries: spiral cta evaluation. Eur Radiol. 1999;9:1330–1334. doi: 10.1007/s003300050843. [DOI] [PubMed] [Google Scholar]
- 45.Turba UC, Uflacker R, Bozlar U, Hagspiel KD. Normal renal arterial anatomy assessed by multidetector ct angiography: are there differences between men and women? Clin Anat. 2009;22:236–242. doi: 10.1002/ca.20748. [DOI] [PubMed] [Google Scholar]
- 46.Munnusamy K, Kasirajan SP, Gurusamy K, et al. Variations in branching pattern of renal artery in kidney donors using CT angiography. J Clin Diag Res. 2016;10:AC01. doi: 10.7860/JCDR/2016/16690.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Standring S. Gray’s Anatomy e-book: The Anatomical Basis of Clinical Practice. Elsevier Health Sciences; 2015. [Google Scholar]
- 48.Özkan U, Oguzkurt L, Tercan F, et al. Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diag Interv Radiol. 2006;12:183–186. [PubMed] [Google Scholar]
- 49.Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC: Cardiovasc Interv. 2013;6:1085–1091. doi: 10.1016/j.jcin.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 51.Esler MD, Bohm M, Sievert H, et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the symplicity HTN-2 randomized clinical trial. Eur Heart J. 2014;35:1752–1759. doi: 10.1093/eurheartj/ehu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakris GL, Townsend RR, Flack JM, et al. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: the symplicity HTN-3 trial. J Am Coll Cardiol. 2015;65:1314–1321. doi: 10.1016/j.jacc.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 53.Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the symplicity HTN-3 trial. Eur Heart J. 2015;36:219–227. doi: 10.1093/eurheartj/ehu441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White WB, Galis ZS, Henegar J, et al. Renal denervation therapy for hypertension: pathways for moving development forward. J Am Soc Hypertens. 2015;9:341–350. doi: 10.1016/j.jash.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Liu YM, Lin PL, Liao FC, et al. Effect of radiofrequency-based renal denervation: the impact of unplanned medication change from a systematic review and meta-analysis. Acta Cardiol Sin. 2019;35:144–152. doi: 10.6515/ACS.201903_35(2).20181231A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahfoud F, Bohm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the global symplicity registry. Eur Heart J. 2019;pii:ehz118. doi: 10.1093/eurheartj/ehz118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zweiker D, Lambert T, Steinwender C, et al. Effects of renal denervation documented in the austrian national multicentre renal denervation registry. PLoS One. 2016;11:e0161250. doi: 10.1371/journal.pone.0161250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volz S, Spaak J, Elf J, et al. Renal sympathetic denervation in Sweden: a report from the Swedish registry for renal denervation. J Hypertens. 2018;36:151–158. doi: 10.1097/HJH.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 59.Kim BK, Bohm M, Mahfoud F, et al. Renal denervation for treatment of uncontrolled hypertension in an asian population: results from the global symplicity registry in South Korea (gsr Korea). J Hum Hypertens. 2016;30:315–321. doi: 10.1038/jhh.2015.77. [DOI] [PubMed] [Google Scholar]
- 60.Bohm M, Ukena C, Ewen S, et al. Renal denervation reduces office and ambulatory heart rate in patients with uncontrolled hypertension: 12-month outcomes from the global symplicity registry. J Hypertens. 2016;34:2480–2486. doi: 10.1097/HJH.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 61.Mahfoud F, Bakris G, Bhatt DL, et al. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from symplicity HTN-3 and the global symplicity registry. Eur Heart J. 2017;38:93–100. doi: 10.1093/eurheartj/ehw325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Symplicity HTN-1 Investigators. Catheter-based renal sympathetic denervationfor resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 63.Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for treatment of drug-resistant hypertension clinical perspective: one-year results from the SYMPLICITY HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–2982. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 64.Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Applegate RJ, Sacrinty MT, Kutcher MA, et al. Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc Interv. 2008;1:317–326. doi: 10.1016/j.jcin.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Kaiser L, von Wedel J, Meincke F, et al. Results of the ALSTER BP real-world registry on renal denervation employing the symplicity system. EuroIntervention. 2014;10:157–165. doi: 10.4244/EIJV10I1A24. [DOI] [PubMed] [Google Scholar]
- 67.Böhm M, Mahfoud F, Ukena C, et al. First report of the global SYMPLICITY registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension. 2015;65:766–774. doi: 10.1161/HYPERTENSIONAHA.114.05010. [DOI] [PubMed] [Google Scholar]
- 68.Davis MI, Filion KB, Zhang D, et al. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:231–241. doi: 10.1016/j.jacc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Sun D, Li C, Li M, et al. Renal denervation vs pharmacotherapy for resistant hypertension: a meta-analysis. J Clin Hypertens (Greenwich) 2016;18:733–740. doi: 10.1111/jch.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fadl Elmula FEM, Jin Y, Yang WY, et al. Meta-analysis of randomized controlled trials of renal denervation in treatment-resistant hypertension. Blood Press. 2015;24:263–274. doi: 10.3109/08037051.2015.1058595. [DOI] [PubMed] [Google Scholar]
- 71.Sanders MF, Reitsma JB, Morpey M, et al. Renal safety of catheter-based renal denervation: systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32:1440–1447. doi: 10.1093/ndt/gfx088. [DOI] [PubMed] [Google Scholar]
- 72.Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2:775–781. doi: 10.1001/jamacardio.2017.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- 74.Fengler K, Rommel KP, Hoellriegel R, et al. Pulse wave velocity predicts response to renal denervation in isolated systolic hypertension. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ott C, Schmid A, Toennes SW, et al. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment-resistant hypertension. EuroIntervention. 2015;11:110–116. doi: 10.4244/EIJV11I1A19. [DOI] [PubMed] [Google Scholar]
- 76.Banegas JR, Ruilope LM, de la Sierra A, et al. High prevalence of masked uncontrolled hypertension in people with treated hypertension. Eur Heart J. 2014;35:3304–3312. doi: 10.1093/eurheartj/ehu016. [DOI] [PubMed] [Google Scholar]
- 77.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193–2198. doi: 10.1097/HJH.0b013e3282ef6185. [DOI] [PubMed] [Google Scholar]
- 78.Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med. 2018;378:1509–1520. doi: 10.1056/NEJMoa1712231. [DOI] [PubMed] [Google Scholar]
- 79.Yang TL, Huang CC, Huang SS, et al. Aortic arch calcification associated with cardiovascular events and death among patients with acute coronary syndrome. Acta Cardiol Sin. 2017;33:241–249. doi: 10.6515/ACS20160902A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lasserson DS, Buclin T, Glasziou P. How quickly should we titrate antihypertensive medication? Systematic review modelling blood pressure response from trial data. Heart. 2011;97:1771–1775. doi: 10.1136/hrt.2010.221473. [DOI] [PubMed] [Google Scholar]
- 81.Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. 2013;62:218–225. doi: 10.1161/HYPERTENSIONAHA.113.00687. [DOI] [PubMed] [Google Scholar]
- 82.Kario K, Bhatt DL, Kandzari DE, et al. Impact of renal denervation on patients with obstructive sleep apnea and resistant hypertension-insights from the symplicity HTN-3 trial. Circ J. 2016;80:1404–1412. doi: 10.1253/circj.CJ-16-0035. [DOI] [PubMed] [Google Scholar]
- 83.Warchol-Celinska E, Prejbisz A, Kadziela J, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept phase II trial. Hypertension. 2018;72:381–390. doi: 10.1161/HYPERTENSIONAHA.118.11180. [DOI] [PubMed] [Google Scholar]
- 84.Linz D, Mancia G, Mahfoud F, et al. Renal artery denervation for treatment of patients with self-reported obstructive sleep apnea and resistant hypertension: results from the global SYMPLICITY registry. J Hypertens. 2017;35:148–153. doi: 10.1097/HJH.0000000000001142. [DOI] [PubMed] [Google Scholar]
- 85.Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–1254. doi: 10.1093/eurheartj/eht534. [DOI] [PubMed] [Google Scholar]
- 86.Mancia G, Fagard R, Narkiewicz K, et al. 2013 esh/esc guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 87.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the council for high blood pressure research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 88.Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2011;2011:236239. doi: 10.4061/2011/236239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Savard S, Frank M, Bobrie G, et al. Eligibility for renal denervation in patients with resistant hypertension: when enthusiasm meets reality in real-life patients. J Am Coll Cardiol. 2012;60:2422–2424. doi: 10.1016/j.jacc.2012.08.1002. [DOI] [PubMed] [Google Scholar]
- 90.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the value randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 91.Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe ckd. J Am Soc Nephrol. 2012;23:1250–1257. doi: 10.1681/ASN.2011111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kiuchi MG, Graciano ML, Carreira MA, et al. Long-term effects of renal sympathetic denervation on hypertensive patients with mild to moderate chronic kidney disease. J Clin Hypertens (Greenwich) 2016;18:190–196. doi: 10.1111/jch.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the enlightn i trial. Eur Heart J. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chao CT, Wu VC, Kuo CC, et al. Diagnosis and management of primary aldosteronism: an updated review. Ann Med. 2013;45:375–383. doi: 10.3109/07853890.2013.785234. [DOI] [PubMed] [Google Scholar]
- 96.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 97.Attar A, Sadeghi AA, Amirmoezi F, Aghasadeghi K. Low dose spironolactone monotherapy in the management of stage i essential hypertension: a pilot randomized, double-blind, placebo-controlled trial. Acta Cardiol Sin. 2018;34:59–65. doi: 10.6515/ACS.201801_34(1).20170903B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sang X, Jiang Y, Wang W, et al. Prevalence of and risk factors for primary aldosteronism among patients with resistant hypertension in china. J Hypertens. 2013;31:1465–1471. doi: 10.1097/HJH.0b013e328360ddf6. [DOI] [PubMed] [Google Scholar]
- 99.Wu VC, Hu YH, Er LK, et al. Case detection and diagnosis of primary aldosteronism - the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116:993–1005. doi: 10.1016/j.jfma.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 100.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep heart health study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 101.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 102.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton health study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 103.Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (cooperation in scientific and technological research) action b26 on obstructive sleep apnea. J Hypertens. 2012;30:633–646. doi: 10.1097/HJH.0b013e328350e53b. [DOI] [PubMed] [Google Scholar]
- 104.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 105.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 106.Muxfeldt ES, Margallo VS, Guimaraes GM, Salles G2. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27:1069–1078. doi: 10.1093/ajh/hpu023. [DOI] [PubMed] [Google Scholar]
- 107.Polat Canbolat I, Belen E, Bayyigit A, et al. Evaluation of daily blood pressure alteration in subclinical hypothyroidism. Acta Cardiol Sin. 2017;33:489–494. doi: 10.6515/ACS20170220B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li YD, Lin TK, Tu YR, et al. Blood pressure reactivity and recovery to anger recall in hypertensive patients with type d personality. Acta Cardiol Sin. 2018;34:417–423. doi: 10.6515/ACS.201809_34(5).20180330A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grassi G, Quarti-Trevano F, Seravalle G, et al. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. doi: 10.1161/HYPERTENSIONAHA.110.164780. [DOI] [PubMed] [Google Scholar]
- 110.Sanders MF, Blankestijn PJ. Chronic kidney disease as a potential indication for renal denervation. Front Physiol. 2016;7:220. doi: 10.3389/fphys.2016.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Esler M. Renal nerve ablation for hypertension: update from an insider. Eur Heart J. 2018;39:4054–4055. doi: 10.1093/eurheartj/ehy717. [DOI] [PubMed] [Google Scholar]
- 112.Ott C, Mahfoud F, Schmid A, et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33:1261–1266. doi: 10.1097/HJH.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 113.Palena LM, Diaz-Sandoval LJ, Candeo A, et al. Automated carbon dioxide angiography for the evaluation and endovascular treatment of diabetic patients with critical limb ischemia. J Endovasc Ther. 2016;23:40–48. doi: 10.1177/1526602815616924. [DOI] [PubMed] [Google Scholar]
- 114.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–442. doi: 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 115.Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. doi: 10.1016/j.jacc.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 116.Vink EE, Goldschmeding R, Vink A, et al. Limited destruction of renal nerves after catheter-based renal denervation: results of a human case study. Nephrol Dial Transplant. 2014;29:1608–1610. doi: 10.1093/ndt/gfu192. [DOI] [PubMed] [Google Scholar]
- 117.Plouin PF, Chatellier G, Darné B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai multicentrique medicaments vs angioplastie (EMMA) study group. Hypertension. 1998;31:823–829. doi: 10.1161/01.hyp.31.3.823. [DOI] [PubMed] [Google Scholar]
- 118.Mahfoud F, Tunev S, Ruwart J, et al. Efficacy and safety of catheter-based radiofrequency renal denervation in stented renal arteries. Circ Cardiovasc Interv. 2014;7:813–820. doi: 10.1161/CIRCINTERVENTIONS.114.001506. [DOI] [PubMed] [Google Scholar]
- 119.Witkowski A, Prejbisz A, Florczak E, et al. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 120.Rippy MK, Zarins D, Barman NC, et al. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100:1095–1101. doi: 10.1007/s00392-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 121.Tzafriri AR, Mahfoud F, Keating JH, et al. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079–1087. doi: 10.1016/j.jacc.2014.07.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahfoud F, Bhatt DL. Catheter-based renal denervation: the black box procedure. JACC Cardiovasc Interv. 2013;6:1092–1094. doi: 10.1016/j.jcin.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Henegar JR, Zhang Y, Hata C, et al. Catheter-based radiofrequency renal denervation: location effects on renal norepinephrine. Am J Hypertens. 2015;28:909–914. doi: 10.1093/ajh/hpu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv. 2013;6:1085–1091. doi: 10.1016/j.jcin.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 125.Silva JD, Costa M, Gersh BJ, Gonçalves L. Renal denervation in the era of HTN-3. Comprehensive review and glimpse into the future. J Am Soc Hypertens. 2016;10:656–670. doi: 10.1016/j.jash.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 126.Bhatt DL. Guide to peripheral and cerebrovascular intervention: remedica. 2004. [Google Scholar]
- 127.Hazirolan T, Öz M, Türkbey B, et al. CT angiography of the renal arteries and veins: normal anatomy and variants. Diagn Interv Radiol. 2011;17:67–73. doi: 10.4261/1305-3825.DIR.2902-09.1. [DOI] [PubMed] [Google Scholar]
- 128.Kawarada O, Yokoi Y, Sakamoto S, et al. Impact of aortorenal morphology on renal artery stent procedures: significance of aortic tortuosity and renal artery derivation. J Endovasc Ther. 2014;21:140–147. doi: 10.1583/13-4455MR.1. [DOI] [PubMed] [Google Scholar]
- 129.Weibull H, Bergqvist D, Jonsson K, et al. Long-term results after percutaneous transluminal angioplasty of atherosclerotic renal artery stenosis—the importance of intensive follow-up. Eur J Vasc Surg. 1991;5:291–301. doi: 10.1016/s0950-821x(05)80513-4. [DOI] [PubMed] [Google Scholar]
- 130.Galli M, Tarantino F, Mameli S, et al. Transradial approach for renal percutaneous transluminal angioplasty and stenting: a feasibility pilot study. J Invasive Cardiol. 2002;14:386–390. [PubMed] [Google Scholar]
- 131.Alli O, Mathew V, From AM, et al. Transradial access for renal artery intervention is feasible and safe. Vasc Endovascular Surg. 2011;45:738–742. doi: 10.1177/1538574411418845. [DOI] [PubMed] [Google Scholar]
- 132.Honton B, Pathak A, Sauguet A, et al. First report of transradial renal denervation with the dedicated radiofrequency IberisTM catheter. EuroIntervention. 2014;9:1385–1388. doi: 10.4244/EIJV9I12A235. [DOI] [PubMed] [Google Scholar]
- 133.Jiang XJ, Dong H, Liang T, et al. First-in-man report of a novel dedicated radiofrequency catheter for renal denervation via the transulnar approach. Eurointervention. 2013;9:684–686. doi: 10.4244/EIJV9I6A111. [DOI] [PubMed] [Google Scholar]
- 134.Dong H, Jiang X, Liang T, et al. Transradial renal denervation for the treatment of resistant hypertension. J Invasive Cardiol. 2014;26:322–327. [PubMed] [Google Scholar]
- 135.Petrov I, Tasheva I, Garvanski I, et al. Comparison of standard renal denervation procedure versus novel distal and branch vessel procedure with brachial arterial access. Cardiovasc Revasc Med. 2019;20:38–42. doi: 10.1016/j.carrev.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 136.Mafeld S, Vasdev N, Haslam P. Renal denervation for treatment-resistant hypertension. Ther Adv Cardiovasc Dis. 2012;6:245–258. doi: 10.1177/1753944712468040. [DOI] [PubMed] [Google Scholar]
- 137.Trani C, Tommasino A, Burzotta F, et al. Transradial renal stenting: why and how. Catheter Cardiovasc Interv. 2009;74:951–956. doi: 10.1002/ccd.22126. [DOI] [PubMed] [Google Scholar]
- 138.Lenski M, Mahfoud F, Razouk A, et al. Orthostatic function after renal sympathetic denervation in patients with resistant hypertension. Int J Cardiol. 2013;169:418–424. doi: 10.1016/j.ijcard.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 139.Ukena C, Mahfoud F, Kindermann I, et al. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2011;58:1176–1182. doi: 10.1016/j.jacc.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 140.Ukena C, Mahfoud F, Spies A, et al. Effects of renal sympathetic denervation on heart rate and atrioventricular conduction in patients with resistant hypertension. Int J Cardiol. 2013;167:2846–2851. doi: 10.1016/j.ijcard.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 141.Wang TD, Sheu WH. From casual blood pressure measurement to long-term blood pressure burden: better elucidation of the association between versatile blood pressures and cardiovascular events. Hypertens Res. 2011;34:49–51. doi: 10.1038/hr.2010.204. [DOI] [PubMed] [Google Scholar]
- 142.Soliman EZ, Ambrosius WT, Cushman WC, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: Sprint (Systolic Blood Pressure Intervention Trial). Circulation. 2017;136:440–450. doi: 10.1161/CIRCULATIONAHA.117.028441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000;11:177–182. doi: 10.1681/ASN.V111177. [DOI] [PubMed] [Google Scholar]
- 144.Pucci G, Battista F, Lazzari L, et al. Progression of renal artery stenosis after renal denervation. Circ J. 2014;78:767–768. doi: 10.1253/circj.cj-13-0997. [DOI] [PubMed] [Google Scholar]
- 145.Fink GD, Phelps JT. Can we predict the blood pressure response to renal denervation? Auton Neurosci. 2017;204:112–118. doi: 10.1016/j.autneu.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Persu A, Gordin D, Jacobs L, et al. Blood pressure response to renal denervation is correlated with baseline blood pressure variability: a patient-level meta-analysis. J Hypertens. 2018;36:221–229. doi: 10.1097/HJH.0000000000001582. [DOI] [PubMed] [Google Scholar]
- 147.Bohm M, Mahfoud F, Townsend RR, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40:743–751. doi: 10.1093/eurheartj/ehy871. [DOI] [PubMed] [Google Scholar]
- 148.Chen W, Du H, Lu J, et al. Renal artery vasodilation may be an indicator of successful sympathetic nerve damage during renal denervation procedure. Sci Rep. 2016;6:37218. doi: 10.1038/srep37218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zuern CS, Eick C, Rizas KD, et al. Impaired cardiac baroreflex sensitivity predicts response to renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2013;62:2124–2130. doi: 10.1016/j.jacc.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 150.Hart EC, McBryde FD, Burchell AE, et al. Translational examination of changes in baroreflex function after renal denervation in hypertensive rats and humans. Hypertension. 2013;62:533–541. doi: 10.1161/HYPERTENSIONAHA.113.01261. [DOI] [PubMed] [Google Scholar]
- 151.Peters CD, Mathiassen ON, Vase H, et al. The effect of renal denervation on arterial stiffness, central blood pressure and heart rate variability in treatment resistant essential hypertension: a substudy of a randomized sham-controlled double-blinded trial (the RESET trial). Blood Press. 2017;26:366–380. doi: 10.1080/08037051.2017.1368368. [DOI] [PubMed] [Google Scholar]
- 152.Vink EE, Verloop WL, Siddiqi L, et al. The effect of percutaneous renal denervation on muscle sympathetic nerve activity in hypertensive patients. Int J Cardiol. 2014;176:8–12. doi: 10.1016/j.ijcard.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 153.Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 154.Madhavan M, Desimone CV, Ebrille E, et al. Transvenous stimulation of the renal sympathetic nerves increases systemic blood pressure: a potential new treatment option for neurocardiogenic syncope. J Cardiovasc Electrophysiol. 2014;25:1115–1118. doi: 10.1111/jce.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fudim M, Sobotka AA, Yin YH, et al. Selective vs. global renal denervation: a case for less is more. Curr Hypertens Rep. 2018;20:37. doi: 10.1007/s11906-018-0838-2. [DOI] [PubMed] [Google Scholar]
- 156.Sakaoka A, Takami A, Onimura Y, et al. Acute changes in histopathology and intravascular imaging after catheter-based renal denervation in a porcine model. Catheter Cardiovasc Interv. 2017;90:631–638. doi: 10.1002/ccd.27158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fengler K, Rommel KP, Blazek S, et al. A three-arm randomized trial of different renal denervation devices and techniques in patients with resistant hypertension (RADIOSOUND-HTN). Circulation. 2019;139:590–600. doi: 10.1161/CIRCULATIONAHA.118.037654. [DOI] [PubMed] [Google Scholar]
- 158.Mauriello A, Rovella V, Borri F, et al. Hypertension in kidney transplantation is associated with an early renal nerve sprouting. Nephrol Dial Transplant. 2017;32:1053–1060. doi: 10.1093/ndt/gfx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Booth LC, Nishi EE, Yao ST, et al. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension. 2015;65:393–400. doi: 10.1161/HYPERTENSIONAHA.114.04176. [DOI] [PubMed] [Google Scholar]
- 160.Booth LC, Nishi EE, Yao ST, et al. Reinnervation following catheter-based radio-frequency renal denervation. Exp Physiol. 2015;100:485–490. doi: 10.1113/expphysiol.2014.079871. [DOI] [PubMed] [Google Scholar]
- 161.Singh RR, McArdle ZM, Iudica M, et al. Sustained decrease in blood pressure and reduced anatomical and functional reinnervation of renal nerves in hypertensive sheep 30 months after catheter-based renal denervation. Hypertension. 2019;73:718–727. doi: 10.1161/HYPERTENSIONAHA.118.12250. [DOI] [PubMed] [Google Scholar]
- 162.Kabir RA, Doytchinova A, Liu X, et al. Crescendo skin sympathetic nerve activity and ventricular arrhythmia. J Am Coll Cardiol. 2017;70:3201–3202. doi: 10.1016/j.jacc.2017.10.065. [DOI] [PubMed] [Google Scholar]
- 163.Doytchinova A, Hassel JL, Yuan Y, et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm. 2017;14:25–33. doi: 10.1016/j.hrthm.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]