Abstract
Background
Coronary artery disease continues to be the most important cause of morbidity and mortality. Obstructive sleep apnea (OSA) is independently associated with subclinical atherosclerosis. In this study, we aimed to assess the relationship between the presence of coronary plaques and OSA and between coronary plaque burden and the severity of OSA according to plaque type.
Methods
In this cross-sectional study, we enrolled 214 consecutive patients who were divided into four groups of 43 patients (age: 52.3 ± 6.4 years) without OSA, 51 patients (age: 53.9 ± 6.7 years) with mild OSA, 40 patients (age: 55.2 ± 5.9 years) with moderate OSA, and 80 patients (age: 54.9 ± 7.2 years) with severe OSA according to the apnea-hypopnea index (AHI). We performed coronary computed tomographic angiography (CCTA) and evaluated plaque positivity, the presence of non-calcified/mixed plaques, and total stenosis score for each group.
Results
The prevalence of non-calcified/mixed plaques was three times higher in the severe OSA (41.3%) group and two times higher in the moderate OSA (30.0%) group compared to the patients without OSA (14.0%). When the four groups were examined in terms of plaque burden, the total stenosis score was found to increase with the presence and severity of OSA (0.27 ± 0.85, 1.07 ± 2.44, 1.75 ± 2.85, and 2.55 ± 3.96 respectively, p = 0.001). In addition, AHI and age were independent predictors of the presence of non-calcified/mixed plaques (p < 0.001 and p = 0.007, respectively).
Conclusions
The presence of coronary artery plaques, especially non-calcified/mixed plaques, and coronary artery stenosis as measured by CCTA was significantly associated with the severity of sleep-disordered breathing in symptomatic patients at low to intermediate risk of coronary artery disease. Prospective studies are needed to establish the relationship between plaque burden and OSA.
Keywords: Sleep disorder, Coronary plaque burden, Coronary artery disease, Subclinical atherosclerosis, Non-calcified plaque
INTRODUCTION
Coronary heart diseases are considered to be the most important cause of death throughout the world, especially in Western countries, despite technological improvements, new drugs and an increasing level of awareness.1,2 Besides the known main risk factors for cardiovascular disease such as hypertension, diabetes mellitus, hypercholesterolemia, age, smoking, obesity, age and male gender, obstructive sleep apnea (OSA) has been reported to be associated with several cardiac and vascular pathologies.3-5 OSA is characterized by repetitive upper airway collapse during sleep resulting in intermittent arterial oxygen desaturation and sympathetic over-activity, with an approximate prevalence in the range of 3% to 7% of the general population.6,7
Studies have determined that OSA may adversely affect coronary artery disease, and a relationship between OSA and a history of myocardial infarction (MI)/acute coronary syndrome has been reported, with odds ratios mostly in the range of 4.1 to 4.5 in both women and men.8,9 Moreover, a prospective observational study with 10 years of follow up by Marin et al. showed that patients with untreated severe OSA suffered more commonly from MI and stroke than patients without OSA.10
There are also studies showing an association between OSA and subclinical atherosclerosis. Tanrıverdi et al. demonstrated that OSA is independently associated with subclinical atherosclerosis in peripheral and carotid arteries.11 Another study reported more signs of subclinical atherosclerosis in patients with severe OSA than in both normal individuals and patients with mild-moderate OSA.12 Nevertheless, relatively few studies have studied the association between OSA and atherosclerosis in the coronary arteries. In addition, these studies were based on calcified plaques, and the results suggested that the presence and severity of OSA were correlated with the presence and extent of calcified plaques.13,14 However, non-calcified atherosclerotic plaques, which can be identified using multi detector-row computed tomography, have a higher tendency to rupture and may play a more critical role in MI than calcified plaques.15-18
To the best of our knowledge, no previous study has investigated the relationship between the severity of OSA and coronary atherosclerosis as indicated by the presence of non-calcified coronary plaque detected by coronary computed tomography angiography (CCTA) in a large population. The lack of studies on newly diagnosed OSA patients with soft coronary plaques, which have been reported to be more likely to cause acute coronary syndrome, makes this study unique and valuable. In this study, we aimed to assess the impact of OSA on subclinical coronary atherosclerosis by determining the presence of coronary plaques and classifying them according to the type of plaque in patients without a history of cardiovascular disease. We also focused on the extent of coronary artery disease according to the severity of OSA.
MATERIALS AND METHODS
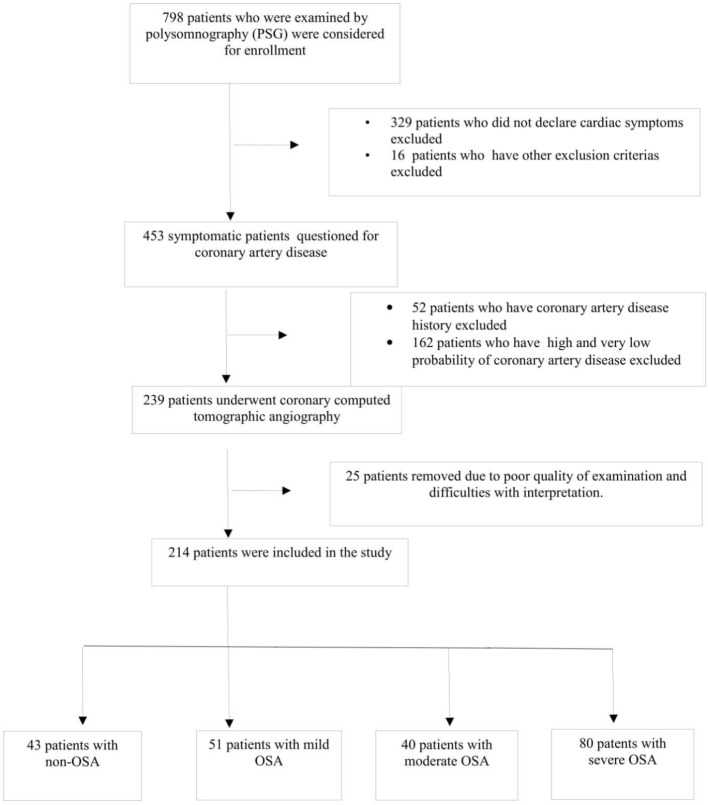
Between January 2011 and December 2014, 798 patients were examined by polysomnography for the first time with a suspicion of OSA, and they were evaluated regarding eligibility to participate in this study. The patients who were suitable for CCTA were selected. When the decision was made to perform CCTA, the patients were screened as follows. First, asymptomatic patients and patients with a history of coronary artery disease were excluded from the study. Second, patients with either a very low pretest likelihood of coronary stenosis or a high pretest likelihood of coronary stenosis were also excluded. Consequently, the study population consisted of symptomatic patients who were at a low to intermediate risk of coronary artery disease (CAD) after initial risk stratification who underwent CCTA, including patients with equivocal stress test results or patients who were unable to perform a stress test. Subsequently, 25 patients were removed from the study due to the poor quality of examinations and difficulties with interpretation. In the final analysis, we followed a 2 × 2 factorial design to create groups according to the presence of OSA and its severity, and 214 patients were divided into four groups including 43 patients (age: 52.3 ± 6.4 years) without OSA, 51 patients (age: 53.9 ± 6.7 years) with mild OSA, 40 patients (age: 55.2 ± 5.9 years) with moderate OSA, and 80 patients (age: 54.9 ± 7.2 years) with severe OSA according to the apnea-hypopnea index (AHI) (Figure 1).
Figure 1.
Flow-diagram showing patient-recruitment based on inclusion and exclusion criteria. After removing patients who do not meet criteria, a total of 214 patients with obstructive sleep apnea (OSA) included in the study and they were divided into four groups according to the presence of OSA and severity.
The major exclusion criteria for the study were: a history of symptomatic CAD, history of coronary revascularization, patients who were not suitable for CCTA according to current guidelines, the presence of heart failure, valvular heart disease, renal or hepatic dysfunction, CCTA images of poor quality, acute/chronic inflammatory conditions and neoplastic disease. In addition, subjects with known sleep disorders and breathing on continuous positive airway pressure (CPAP) were also excluded from the study.
Data on patient demographic parameters, past medical histories, cholesterol, hemograms and medical therapies were collected. Blood lipid levels and other measurements were performed as per standard procedures. Risk factors were categorized as having or not having the illness. Smoking habits were recorded according to patient statements. Hypertension was definedas systolic blood pressure above 140 and diastolic blood pressure above 90 mmHg or having used antihypertensive drugs for longer than two weeks. Hypercholesterolemia was defined as an low density lipoprotein (LDL) cholesterol level above 130 or having used antilipidemic medication. Diabetes mellitus (DM) was defined as a fasting glucose level above 126 mg/dL or having used insulin or oral agents for more than two weeks. Informed consent was obtained from all patients, and the study was approved by the local ethics committee. The study was performed according to the Declaration of Helsinki.
Overnight sleep study
An overnight polysomnography procedure was performed with a 16-channel Embla system (Medcare Inc., Iceland) and continuous sleep technician monitoring. The system uses two channels of electrooculogram and four channels of electroencephalography (with electrodes placed at C4-A1, C3-A2, O2-A1 and O1-A2), and also measures sub mental electromyography (EMG), tibial EMG, oronasal airflow, pulse oximeter oxygen saturation, thoracic and abdominal movements, body position, tracheal sounds and electrocardiography. Following the American Academy of Sleep Medicine 2007 rules,19 an apnea event was defined as an 80% decrease in the amplitude of airflow during sleep, with a hypopnea event defined as the combination of a 30% reduction in airflow with a 4% reduction in oxygen saturation. A minimum event duration of 10 seconds was required for apnea or hypopnea. We also recorded the baseline, minimum oxygen saturation (MOS), mean oxygen saturation (MeOS), total sleep time and sleep stages.
Coronary computed angiography examination
CCTA images were obtained using a dual-source CT system (Definition Flash, Siemens Medical Solution, Forchheim, Germany) with 2 × 128 slices and a rotation time of 280 milliseconds. The tube current was set at 180-300 mAs. A 0.6-mm slice collimation was satisfied. Non-ionic contrast material (Iomero 400 mgI/mL; Bracco, Milan, Italy) at a dose of 80-100 mL was used at a rate of 5 mL/s in the antecubital vein. A bolus tracking technique was used, and images were obtained during a single breath-hold of 6 seconds. Unless the participants were hypotensive, nitrate intolerant or had taken sildenafil, tadalafil, or vardenafil within 24-48 hours of the study, they were given nitroglycerine sublingually to avoid coronary artery spasms and to dilate the coronary arteries to increase visualization. Participants with initial heart rates of ≥ 65 beats per minute were given diltiazem or beta-blockers if there were no contraindication to these drugs.
Image analysis
One experienced radiologist blinded to the clinical properties of the subjects analyzed the scans on a three-dimensional workstation (Syngo; Siemens Healthcare, Erlangen, Germany). All datasets were evaluated based on the modified 15-segment model of the American Heart Association.20 Plaques were defined as 1 mm2 structures within or adjacent to a vessel lumen that could be distinguished from the lumen and the surrounding pericardial tissue. Each coronary segment was classified as being either normal or containing non-calcified/mixed plaques or calcified plaques. Plaques without any calcification or with < 50% calcium were defined as the non-calcified/mixed type, and plaques with > 50% of the plaque area occupied by calcified tissue were defined as the calcified type.20 Plaque and stenosis scores were calculated according to the Society of Cardiovascular Computed Tomography guidelines for the interpretation and reporting of CCTA.21 Segment stenosis was defined as no plaque (0 points), minimal stenosis of 1-24% (1 point), mild stenosis of 25-49% (2 points), moderate stenosis of 50-69% (3 points), and severe stenosis of ≥ 70% (4 points). Coronary stenosis was reported by selecting the most narrowed area, even if the plaque was eccentric. The extent of atherosclerosis was expressed as the total stenosis score (TSS), and was evaluated by summing the plaque size scores for all assessable coronary segments with any plaque (either calcified or non-calcified) up to a maximum score of 60.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences version 20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA). Visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests) were used to determine the normal distribution of the variables. Descriptive analyses are presented as means and standard deviations. Categorical variables are expressed as numbers and percentages. Numerical variables were compared using the Student’s t-test, one-way ANOVA, the Kruskal-Wallis test or the Mann-Whitney U test. The Mann-Whitney U test or Tukey’s test was used to determine the significance of pairwise differences. Bonferroni correction was used to adjust for multiple comparisons. Categorical data were compared with a chi-squared test. In addition, stepwise multivariate logistic regression analysis, which included variables with p values less than 0.10 in the univariate analysis, was carried out to identify the independent predictors of coronary plaque positivity. Nagelkerke r-squared values for logistic regression were recorded. Receiver operating characteristic (ROC) curves for potential risk factors to distinguish non-calcified/mixed plaque were drawn. Youden’s index was used to derive the best cut-offs. The area under the ROC curves (AUC) was recorded. An overall 5% type-I error level was used to infer statistical significance, and a p-value less than 0.05 was accepted as indicating statistical significance.
RESULTS
The study sample consisted of 214 patients. The patients were separated into four groups according to their AHI score as follows: 43 patients (mean age: 52.3 ± 6.4 years) without OSA, 51 patients (mean age: 53.9 ± 6.7 years) with mild OSA, 40 patients (mean age: 55.2 ± 5.9 years) with moderate OSA, and 80 patients (mean age: 54.9 ± 7.2 years) with severe OSA. Other than the polysomnography parameters and body mass index, there were no significant differences in baseline characteristics among the four groups (Table 1).
Table 1. Baseline characteristics of study population, mean ± SD or n (%).
| Variables | Non OSA (n = 43) | Mild OSA (n = 51) | Moderate OSA (n = 40) | Severe OSA (n = 80) | p |
| Age, years | 52.2 ± 6.9 | 53.9 ± 6.7 | 55.2 ± 5.9 | 54.9 ± 7.2 | 0.152 |
| Male, n (%) | 33 (76.7) | 34 (66.7) | 26 (65.0) | 62 (77.5) | 0.336 |
| HL, n (%) | 21 (48.8) | 33 (64.7) | 26 (65.0) | 42 (52.5) | 0.250 |
| DM, n (%) | 7 (16.3) | 6 (11.8) | 9 (22.5) | 19 (23.8) | 0.333 |
| HT, n (%) | 13 (30.2) | 15( 29.4 ) | 15( 37.5) | 30 (37.5) | 0.705 |
| Smoking, n (%) | 17 (37.5) | 23 (45.1) | 20 (50.0) | 44 (55.0) | 0.390 |
| BMI, kg/m2* | 31.2 ± 3.9 | 32.6 ± 4.2 | 34.5 ± 6.9 | 35.3 ± 5.6 | < 0.001 |
| AHI, events/hr# | 4.0 ± 2.9 | 9.8 ± 5.2 | 21.8 ± 4.1 | 43.5 ± 12.9 | < 0.001 |
| MOS, %† | 89.6 ± 1.8 | 84.5 ± 5.2 | 81.7 ± 4.9 | 76.7 ± 5.7 | < 0001 |
| MeOS, %‡ | 95.5 ± 1.2 | 94.2 ± 1.65 | 92.7 ± 2.1 | 88.4 ± 5.1 | < 0.001 |
| Total sleep time | 395.8 ± 12.8 | 396.2 ± 29.7 | 394.3 ± 34.1 | 397.6 ± 28.5 | 0.939 |
| Non-Rem St. 1, %§ | 17.2 ± 2.6 | 17.2 ± 2.5 | 17.9 ± 2.1 | 18.5 ± 2.2 | 0.004 |
| Non-Rem St. 2, %§ | 47.2 ± 3.0 | 45.6 ± 3.0 | 45.7 ± 3.4 | 45.1 ± 3.5 | 0.014 |
| Non-Rem St. 3, %§ | 14.6 ± 3.5 | 13.8 ± 3.2 | 11.6 ± 2.1 | 10.1 ± 2.5 | < 0.001 |
| Rem, %§ | 17.9 ± 3.4 | 17.8 ± 4.0 | 17.0 ± 3.6 | 16.6 ± 2.7 | 0.141 |
| Antiplatelet use, % | 6 (14.0) | 6 (11.8) | 7 (17.5) | 10 (12.5) | 0.861 |
| Antihypertensive use, % | 9 (20.9) | 15 (29.4) | 14 (35.0) | 21 (26.3) | 0.530 |
| Statin use, % | 6 (14.0) | 14 (27.5) | 13 (32.5) | 21 (26.6) | 0.239 |
| Glucose, mg/dl | 114.8 ± 31.1 | 116.7 ± 47.7 | 108.3 ± 16.3 | 119.6 ± 37.2 | 0.402 |
| HDL, mg/dl | 44.2 ± 12.1 | 42.4 ± 7.7 | 44.4 ± 9.6 | 42.4 ± 10.1 | 0.669 |
| LDL, mg/dl | 133.7 ± 35.5 | 141.5 ± 35.6 | 142.5 ± 36.2 | 132.3 ± 31.1 | 0.279 |
| Creatinine, mg/dl | 0.84 ± 0.49 | 0.73 ± 0.16 | 0.78 ± 0.19 | 0.86 ± 0.40 | 0.206 |
AHI, apnea-hypopnea index; BMI, body-mass index; DM, diabetes mellitus; HDL, high-density lipoprotein; HL, hyperlipidemia; HT, hypertension; LDL, low-density lipoprotein; MeOS, mean oxygen saturation; MOS, minumum oxygen saturation; OSA, obstructive sleep apnea; St, stage; SD, standard deviation.
* p values ≤ 0.05 for comparisons between non-OSA with moderate or severe OSA. # All pairwise p values are ≤ 0.05. † All pairwise p values are ≤ 0.05 except comparisons between mild OSA and moderate OSA. ‡ p values ≤ 0.05 for all comparisons except mild OSA and moderate or non-OSA. § Percentage of total sleep time.
There were significant differences among the four groups in plaque positivity, the presence of non-calcified/mixed plaques, and total stenosis score (p < 0.001, p = 0.003, and p = 0.001, respectively) (Table 2). However, despite the increase in the presence of calcific plaques with OSA severity, this increase did not reach statistical significance. Nevertheless, when we compared the patients in two groups (no OSA/mild OSA vs. moderate/severe OSA), the incidence of calcified plaques was statistically higher in the moderate/severe OSA group (p = 0.033) (Table 2-footnote).
Table 2. The comparison of the coronary arteries evaluation in the multi-detector computed tomography of the patients according to OSA presence and severity.
| Non OSA (n = 43) | Mild OSA (n = 51) | Moderate OSA (n = 40) | Severe OSA (n = 80) | p | |
| Plaque positivity (%)* | 10 (23.3) | 16 (31.4) | 21 (52.5) | 50 (62.5) | < 0.001 |
| Non calcified/mixed plaque (%)# | 6 (14.0) | 9 (17.6) | 12 (30.0) | 33 (41.3) | 0.003 |
| Calcified plaque (%)† | 4 (9.3) | 7 (13.7) | 9 (22.5) | 19 (23.8) | 0.163 |
| TSS‡ | 0.27 ± 0.85 | 1.07 ± 2.44 | 1.75 ± 2.85 | 2.55 ± 3.96 | 0.001 |
OSA, obstructive sleep apnea; TSS, total stenosis score.
* p values ≤ 0.05 for comparisons between non-OSA with moderate or severe OSA amd between mild OSA with severe OSA. # p values ≤ 0.05 for comparisons between non-OSA and mild OSA with severe OSA. † When divided 2 groups as non-OSA/mild OSA and moderate/severe OSA, there is statistical significiance between the groups. (Non-mild OSA 11.7% vs. moderate-severe OSA 23.3%, p = 0.033). ‡ p values ≤ 0.05 for comparisons between non-OSA with moderate or severe OSA.
Figure 2 shows plaque positivity and the distribution according to the severity of OSA. It was found that the severity of OSA was associated with a higher incidence of non-calcified/mixed plaques.
Figure 2.
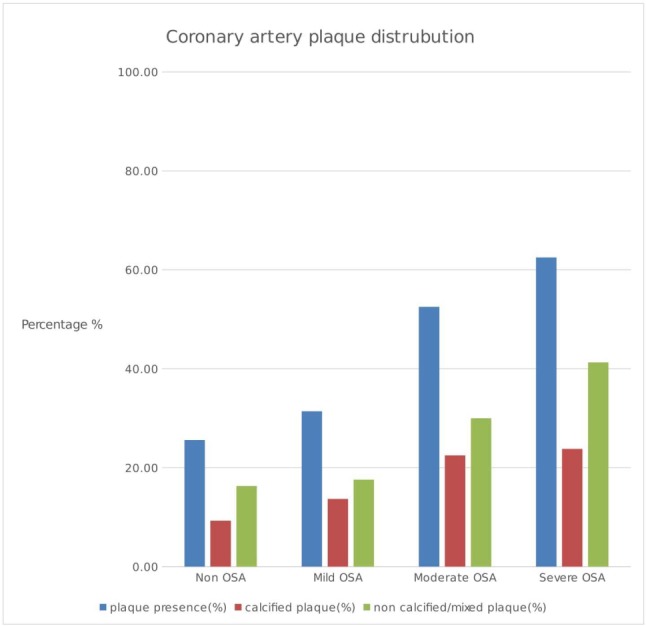
Bar graph showing total plaque percentage and plaque distribution in the groups according to the presence and severity of OSA.
Independent from age, one unit increase in AHI score was associated with a 6% increased odds of a non-calcified/mixed plaque. Similarly, independent of other risk factors, the patients with severe OSA had a 3.8 times increased odds of a non-calcified/mixed plaque compared to those without OSA (Table 3).
Table 3. Multivariate logistic regression analysis for potential predictors of non-calcified/mixed plaque presence.
| Univariate analysis | Multivariate analysis† | |||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age, years | 1.081 (1.033-1.132) | 0.002 | 1.067 (1.019-1.131) | 0.007 |
| HT, yes | 1.362 (0.742-2.502) | 0.319 | ||
| HL, yes | 1.439 (0.779-2.658) | 0.245 | ||
| Smoking, yes | 1.860 (1.027-3.369) | 0.041 | 1.369 (0.669-2.801) | 0.390 |
| DM, yes | 2.161 (1.070-4.364) | 0.032 | 0.925 (0.380-2.253) | 0.864 |
| Male, yes | 1.545(0.800-2.987) | 0.195 | ||
| BMI, kg/m2 | 1.051 (0.996-1.108) | 0.058 | 1.001 (0.940-1.066) | 0.976 |
| AHI, events/hour* | 1.057 (1.040-1.074) | < 0.001 | 1.064 (1.042-1.086) | < 0.001 |
| MOS, %* | 0.903 (0.821-0.992) | 0.001 | ||
| non-OSA# | Ref. | Ref. | Ref. | Ref. |
| mild OSA# | 1.102 (0.373-3.256) | 0.860 | 2.075 (0.503-8.549) | 0.312 |
| moderate OSA# | 2.207 (0.768-6.329) | 0.142 | 1.314 (0.284-6.067) | 0.727 |
| severe OSA# | 3.611 (1.413-9.096) | 0.006 | 3.889 (1.459-10.402) | 0.007 |
AHI, apnea-hypopnea index; BMI, body-mass index; HT, hypertension; HL, hyperlipidemia; DM, diabetes mellitus; MOS, minimum oxygen saturation; CI, confidence interval; OR, odds ratio.
* AHI and MOS were highly correlated, and Nagelkerke R squares for AHI and MOS were 29% and 21% respectively, therefore, we included AHI in the full model. # These groups were included in a second model instead of AHI and MOS. † Nagelkerke R square of the full model was 35.3%.
The discriminatory value of AHI score for the presence of non-calcified/mixed plaques was assessed by ROC curve analysis, and revealed a sensitivity of 51.6%, specificity of 92.6%, and a cut-off value > 37.0 [AUC 0.750; 95% confidence interval (CI) 0.687-0.807; p < 0.001] (Figure 3).
Figure 3.
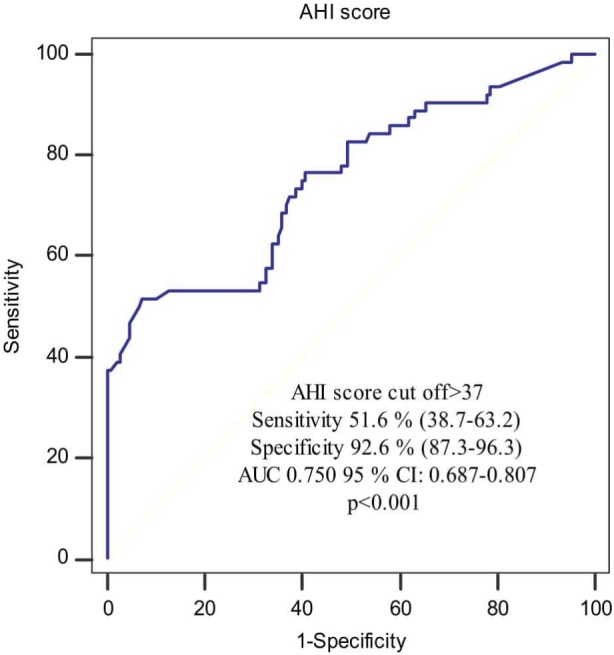
Receiver operating characteristics curve showing the distinguishing ability of apnea-hypopnea index (AHI) score for non-calcified or mixed plaque presence. AHI, apnea-hypopnea index; AUC, area under the ROC curve; CI, confidence interval; ROC, receiver operating characteristics.
DISCUSSION
In this study of newly diagnosed OSA patients, we observed that the presence of coronary plaques and total stenosis score were higher in the patients with moderate or severe OSA compared to those without OSA. In addition, we found a significant relationship between the presence of non-calcified/mixed plaques and severity of OSA, indicating that patients with a higher number of non-calcified/mixed plaques tended to have OSA of greater severity. Our findings suggest that beyond traditional risk factors, OSA severity had an additional effect on subclinical atherosclerosis.
Coronary artery disease is a significant cause of mortality and morbidity worldwide. In both the American Heart Association/American College of Cardiology and European Society of Cardiology guidelines, OSA is highlighted as a potential risk factor for cardiovascular disease.22,23 Therefore, the early recognition of potential atherosclerotic changes in OSA patients may help to perform risk classification and make therapeutic recommendations for affected individuals.
It has been demonstrated that OSA can cause atherosclerosis and exacerbate the disease.24-28 Furthermore, a randomized controlled trial that analyzed the relationship between OSA and subclinical coronary artery disease established that OSA is an independent risk factor for atherosclerosis, and that interventions with CPAP resulted in a significant reduction in carotid intima-media thickness.29 The effect of OSA on accelerating clinical or subclinical atherosclerosis may occur directly by inducing hypertension,30 or indirectly via intermittent hypoxia and endothelial damage.24-28 In the current study, as the study population was composed of patients newly diagnosed with OSA, the effect of CPAP treatment on atherosclerosis was not evaluated. In addition we found no statistically significant difference in the frequency of hypertension among the groups, so the difference in plaque positivity among OSA groups might be related to an indirect effect of OSA on coronary atherogenesis.
It has been shown that acute coronary syndrome is commonly the result of an acute disruption of unstable atherosclerotic plaques, not slowly progressing luminal narrowing.15-18 In addition, various studies have established that the presence of a lipid-rich core or non-calcified type is an essential component of a vulnerable plaque.16,17 Hence, exploring the relationship between non-calcified plaques and OSA can contribute to understanding the impact of OSA on acute coronary syndrome. Many studies have examined the relationship between OSA and acute coronary syndrome, and they have shown that severe untreated OSA is associated with fatal and non-fatal future cardiovascular events in comparison to healthy controls.31-33 In the Sleep Heart Health Study, OSA was an independent predictor of incident coronary artery disease (MI, revascularization procedures, or coronary artery disease-associated death) only in men < 70 years of age, but not in older men or women of any age.34 Since non-calcified/mixed plaques are more clinically significant, we focused on this type of plaque using CCTA in this study. We found that AHI score was an independent predictor of non-calcified mixed plaques [odds ratio (OR) 95% confidence interval (CI) = 1.064 (1.042-1.086) p < 0.001]. These results indicate that newly diagnosed OSA patients are potential candidates for acute coronary syndrome, especially advanced ones.
Coronary artery calcium (CAC) level evaluated with the use of coronary computed tomography is an essential marker that illustrates subclinical atherosclerosis. Kwon and colleagues14 assessed CAC levels among patients with OSA and normal individuals and found a significant difference at baseline, and this difference became statistically more significant after 8 years of follow-up (p = 0.002 and p = 0.001, respectively, adjusted for age, ethnicity, sex, site, income level, educational level, smoking status, and physical activity level). In a study by Matthew, OSA patients were divided into groups according to their AHI and CAC scores. Although there was a difference between the groups, it was not statistically significant, and the p-value was within limits (p = 0.08).35 In a wider population study conducted by Lutsey and colleagues, it was found that the calcium score increased with increasing OSA severity in all models in the patients with CAC > 0 (adjusted for age, race/ethnicity, sex, center, education, body mass index and traditional risk factors). This suggested a significant correlation between the CAC score and AHI as the number of patients increased.36 In the current study, the CAC score was not included in the study design, as we wanted to focus on non-calcific plaques. Moreover, we investigated calcified plaque ratios, and statistical significance was not reached even though there was a difference among the four groups (p = 0.163). However, the difference was significant when the patients were grouped into moderate/severe OSA and non/mild OSA groups (23.3% vs. 11.7%, p = 0.033). This seems to agree with the aforementioned studies. The fact that our study consisted of newly diagnosed OSA patients may explain the differences, and larger studies may clarify this issue.
Few studies have evaluated the relationship between calcified plaques and OSA, and we only found two small studies on the presence of non-calcified plaques in patients with OSA. In one of them,37 Sharma et al. showed that, among patients with OSA, 63% had non-calcified/ mixed plaques, as opposed to 16% in the non-OSA group (p < 0.001), with an adjusted odds ratio of 7.00 (1.9, 26.5; p < 0.05). Compared to our study, the Sharma study had fewer patients (n = 81) and the OSA groups were divided into OSA vs. non-OSA, so there was no classification according to the severity of the disease. In addition, they considered only non-calcified plaques, but not calcified plaques. Partly in parallel with that study, we analyzed non-calcified plaques and found that among the patients with severe OSA, 41.3% had non-calcified/mixed plaques, compared to 14% in the non-OSA group (p = 0.007) with an adjusted odds ratio of 3.8. We speculate that as a result of some differences between studies (more patients, more detailed classification of the disease) our odds ratio was smaller but more confident than that provided in Sharma’s study.
In another study, Kent et al.38 performed coronary computed tomographic angiography on 29 OSA patients and compared coronary artery plaque volume between subjects according to AHI scores. They determined that coronary plaque volume was significantly greater in the high AHI group (mean plaque volume 2.6 ± 0.7 mm2 versus 0.8 ± 0.2 mm2; p = 0.017), and they also showed that AHI levels could be used as a significant predictor of plaque volume (standardized b = 0.424; p = 0.027). In the current study, we did not analyze plaque volume, but we calculated the total stenosis score, which reflects plaque burden, and reached similar results to the Kent study (total stenosis score according to the severity of disease, 0.27 ± 0.85/1.07 ± 2.44/1.75 ± 2.85/2.55 ± 3.96, respectively; p = 0.001). Moreover, the patients who underwent CCTA in this study included those who had no prior history of coronary disease and who did not have a high probability of coronary stenosis because of the study design. Therefore, the total stenosis was low as expected. Furthermore, the aim of this study was not to investigate whether there was critical stenosis in the coronaries in these patients. Rather, our aim was to detect the presence of non-calcified/mixt plaques that were more prone to rupture.
When examining other independent predictors of coronary plaques other than parameters related to OSA disease, only age was found to be significant among the traditional risk factors [OR 95% CI = 1.067 (1.019-1.131), p = 0.007]. A likely reason is that the study is not large enough to show this relationship. Another reason may be that this study was not performed in all OSA patients, as patients with coronary artery disease and those at high risk of coronary artery disease were excluded from the study. Especially in this group of patients, the presence of traditional risk factors may have a greater effect. However, as a result, a relationship between the presence and severity of OSA and soft plaques was clearly demonstrated.
Registry data has shown that baseline therapy is associated with a significant reduction in mortality for individuals with non-obstructive coronary artery disease detected on CCTA.39 Thus, the detection of coronary artery disease even in asymptomatic patients with OSA may facilitate lifestyle and pharmacological interventions to improve outcomes in these patients.
Study limitations
There are some limitations to this study. First, this study involved a single center and it was non-randomized, which may have caused selection bias. Second, the cross-sectional design of the study suggests an independent association but cannot establish a causal relationship between OSA and non-calcified plaques. Third, we assessed the severity of coronary atherosclerosis using two parameters (presence of plaques and segment stenosis score). Other parameters such as coronary calcium score or total plaque volume could not be evaluated because of the study design. This may underestimated the burden of coronary atherosclerosis. Another study limitation was the inability to assess the effect of CPAP treatment on the coronary plaques because the study population was composed of newly diagnosed OSA patients. Moreover, patients with a history of coronary artery disease and no CCTA indications were excluded from the study. Therefore, the results may not be generalizable to all OSA patients. However, since there were no differences in baseline characteristics among the groups, it can be assumed that the presence of OSA and its severity were clearly shown in relation to the presence of plaques. Finally, we lacked follow-up data describing future cardiovascular events.
CONCLUSIONS
In summary, this is the first study to show that the incidence of soft coronary plaques increased in proportion to the severity of OSA in patients newly diagnosed with OSA who had cardiac symptoms and who were at a low to intermediate risk of coronary artery disease. In addition, we found that the total stenosis score as measured by CCTA was significantly correlated with the severity of OSA. Therefore, clinicians need to be careful with OSA patients, especially those with severe disease, and we suggest that they should be screened for coronary artery disease. Prospective studies with sleep clinics and community-based populations are needed to establish the relationship between plaque burden and OSA. Since, non-calcified plaques are more prone to acute coronary syndrome, according to larger studies, routine antiplatelet therapy may be considered in moderate-severe OSA patients.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
FUNDING
No funding was received for this research.
REFERENCES
- 1.Gaziano JM. Global burden of cardiovascular disease. In Heart disease: a textbook of cardiovascular medicine 6th edition. Edited by: Braunwald E, Zipes DP, Libby P. Philadelphia: WB Saunders Company; 2001. pp. 1–17. [Google Scholar]
- 2.Chu CY, Lin TH, Lai WT. The management and prognostic factors of acute coronary syndrome: evidence from the Taiwan acute coronary syndrome full spectrum registry. Acta Cardiol Sin. 2017;33:329–338. doi: 10.6515/ACS20161205A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 4.Karabag T, Aydın M, Altin R, et al. Evaluation of atrial electromechanical delay and left atrial mechanical function in patients with obstructive sleep apnea: cardiac involvement in patients with obstructive sleep apnea. Wien. Klin. Wochenschr. 2012;124:444–452. doi: 10.1007/s00508-012-0197-y. [DOI] [PubMed] [Google Scholar]
- 5.Lin YS, Liu PH, Chu PH. Obstructive sleep apnea independently increases the incidence of heart failure and major adverse cardiac events: a retrospective population-based follow-up study. Acta Cardiol Sin. 2017;33:656–663. doi: 10.6515/ACS20170825A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488–1495. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 8.D’Alessandro R, Magelli C, Gamberini G, et al. Snoring every night as a risk factor for myocardial infarction: a case-control study. BMJ. 1990;300:1557–1558. doi: 10.1136/bmj.300.6739.1557-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooe T, Rabben T, Wiklund U, et al. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996;101:251–256. doi: 10.1016/S0002-9343(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardio vascular outcomes in men with obstructive sleep apnoeahypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 11.Tanriverdi H, Evrengul H, Kara CO, et al. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: non-invasive indicators of atherosclerosis. Respiration. 2006;73:741–750. doi: 10.1159/000093531. [DOI] [PubMed] [Google Scholar]
- 12.Drager LF, Bortolotto LA, Lorenzi MC, et al. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 13.Sorajja D, Gami AS, Somers VK, et al. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon Y, Duprez DA, Jacobs DR, et al. Obstructive sleep apnea and progression of coronary artery calcium: the multi-ethnic study of atherosclerosis study. J Am Heart Assoc. 2014;3:e001241. doi: 10.1161/JAHA.114.001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virmani R, Burke AP, Kolodgie FD, Farb A. Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol. 2002;15:439–446. doi: 10.1111/j.1540-8183.2002.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmermund A, Erbel R. Unstable coronary plaque and its relation to coronary calcium. Circulation. 2001;104:1682–1687. doi: 10.1161/hc3901.093339. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Virmani R, Younis H, et al. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 18.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson AL, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st Ed. Westchester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 20.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease,Council on Cardiovascular Surgery. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 21.Raff G, Abidov A, Achenbach S, et al. Society of Cardiovascular Computed Tomography guidelines for the interpretation and reporting of coronary computed of tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Perk J, De Backer G, Gohlke H, et al. European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 23.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 25.Jun J, Reinke C, Bedja D, et al. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dematteis M, Julien C, Guillermet C, et al. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008;177:227–235. doi: 10.1164/rccm.200702-238OC. [DOI] [PubMed] [Google Scholar]
- 28.Jun J, Polotsky VY. Metabolic consequences of sleep disordered breathing. ILAR J. 2009;50:289–306. doi: 10.1093/ilar.50.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drager LF, Bortolotto LA, Figueiredo AC, et al. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 31.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 32.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, et al. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156:115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 33.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews KA, Strollo PJ, Hall M, et al. Associations of Framingham risk score profile and coronary artery calcification with sleep characteristics in middle-aged men and women: pittsburgh SleepSCORE study. Sleep. 2011;34:711–716. doi: 10.5665/SLEEP.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutsey PL, McClelland RL, Duprez D, et al. Objectively measured sleep characteristics and prevalence of coronary artery calcification: The Multi-Ethnic Study of Atherosclerosis Sleep Study. Thorax. 2015;70:880–887. doi: 10.1136/thoraxjnl-2015-206871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, Gebregziabher M, et al. Independent association between obstructive sleep apnea and noncalcified coronary plaque demonstrated by noninvasive coronary computed tomography angiography. Clin Cardiol. 2012;35:641–645. doi: 10.1002/clc.22057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kent B, Garvey J, Ryan S, et al. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study. Eur Respir J. 2013;42:1263–1270. doi: 10.1183/09031936.00094812. [DOI] [PubMed] [Google Scholar]
- 39.Chow BJW, Small G, Yam Y, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (COronary CT Angiography EvaluatioN for Clinical Outcomes: an InteRnational Multicenter registry) registry. Arterioscler Thromb Vasc Biol. 2015;4:981–989. doi: 10.1161/ATVBAHA.114.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]