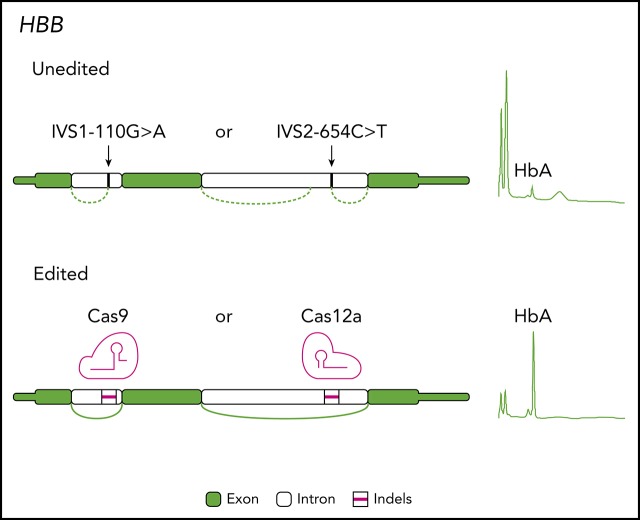
There is a Blood Commentary on this article in this issue.
Key Points
Efficient aberrant splice site disruption by Cas9 and Cas12a RNPs in β-thalassemia HSPCs.
Splice site disruption by indels results in penetrant restoration of β-globin expression.
Abstract
The thalassemias are compelling targets for therapeutic genome editing in part because monoallelic correction of a subset of hematopoietic stem cells (HSCs) would be sufficient for enduring disease amelioration. A primary challenge is the development of efficient repair strategies that are effective in HSCs. Here, we demonstrate that allelic disruption of aberrant splice sites, one of the major classes of thalassemia mutations, is a robust approach to restore gene function. We target the IVS1-110G>A mutation using Cas9 ribonucleoprotein (RNP) and the IVS2-654C>T mutation by Cas12a/Cpf1 RNP in primary CD34+ hematopoietic stem and progenitor cells (HSPCs) from β-thalassemia patients. Each of these nuclease complexes achieves high efficiency and penetrance of therapeutic edits. Erythroid progeny of edited patient HSPCs show reversal of aberrant splicing and restoration of β-globin expression. This strategy could enable correction of a substantial fraction of transfusion-dependent β-thalassemia genotypes with currently available gene-editing technology.
Visual Abstract
Introduction
Therapeutic genome editing is a promising treatment modality for inherited blood disorders in which genetic modification of autologous hematopoietic stem cells (HSCs) would result in durable correction of the hematopoietic system.1 Gene editing is a byproduct of endogenous DNA damage repair pathways, such as homologous recombination (HR), nonhomologous end joining, and microhomology-mediated end joining, acting on double-strand breaks produced by programmable nucleases.2 HR enables the precise templated repair of mutations. However, the required codelivery of an exogenous donor template, competing nontemplated mutagenic repair, and cell-cycle–dependent activity are challenges to achieving therapeutic HR in quiescent HSCs.3-5 Nonhomologous end joining–based genetic disruption is a highly efficient and simple approach suitable when elimination of a functional sequence element will achieve a desired therapeutic outcome. Recently, we have shown that the erythroid enhancer of BCL11A represents a therapeutic target for efficient genetic disruption by Cas9 in human HSCs with subsequent derepression of fetal hemoglobin (HbF) level.6
The β-thalassemias are a genetically heterogeneous set of conditions in which various mutations at HBB result in partial (β+) or complete (β0) loss of β-globin expression.7 Several of the most common mutant alleles disrupt HBB splicing through the creation of aberrant splice sites. For example, IVS1-110G>A (HBB:c.93-21G>A, rs35004220) is one of the most common mutations throughout the Mediterranean and Middle East and is the most prevalent mutation in Cyprus.8 This mutation generates a de novo splice acceptor site in HBB intron-1 that leads to an aberrant messenger RNA (mRNA) that includes 19 nt prior to the start of exon 2 resulting in a premature stop codon.9 IVS2-654C>T (HBB:c.316-197C>T, rs34451549) is among the most frequent β-thalassemia mutations in east Asia.10 This mutation creates a de novo splice donor site in HBB intron-2, resulting in an aberrant β-globin mRNA containing an additional 73-nt exon that produces a premature stop codon.11,12
Study design
Protein purification
Protein purification for 3xNLS–Streptococcus pyogenes Cas9 (SpCas9) and LbCas12a-2xNLS used a common protocol. The generation and characterization of the 3xNLS-SpCas9 and LbCas12a-2xNLS constructs have been recently described (Wu et al6; Pengpeng Liu, K.L., Masahiro Shin, Feston Idrizi, Samantha Kwok, Benjamin Roscoe, Esther Mintzer, Sneha Suresh, Kyle Morrison, Josias B. Frazao, Mehmet Fatih Bolukbasi, Jeremy Luban, Lihua Julie Zhu, Nathan D. Lawson, and S.A.W., manuscript submitted November 2018). The 3xNLS-SpCas9 (#114365) and LbCas12a-2xNLS (#114366) expression plasmids are each available from Addgene. The pET21a plasmid backbone (Novagen) is used to drive the expression of each protein. The plasmid expressing 3xNLS-SpCas9 (or LbCas12a-2xNLS) was transformed into Escherichia coli Rosetta (DE3) pLysS cells (EMD Millipore) for protein production. Cells were grown at 37°C to an optical density 600 nm (OD600) of ∼0.2, then shifted to 18°C and induced at an OD600 of ∼0.4 for 16 hours with isopropyl β-d-1-thiogalactopyranoside (IPTG; 1 mM final concentration). Following induction, cells were pelleted by centrifugation and then resuspended with nickel-NTA buffer (20 mM tris(hydroxymethyl)aminomethane [TRIS] plus 1 M NaCl plus 20 mM imidazole plus 1 mM Tris(2-carboxyethyl)phosphine [TCEP], pH 7.5) supplemented with HALT protease inhibitor cocktail, EDTA-free (100×; ThermoFisher), and lysed with the M-110s Microfluidizer (Microfluidics) following the manufacturer’s instructions. The protein was purified from the cell lysate using nickel-NTA resin, washed with 5 volumes of nickel-NTA buffer, and then eluted with elution buffer (20 mM TRIS, 500 mM NaCl, 500 mM imidazole, 10% glycerol, pH 7.5). The 3xNLS-SpCas9 (or LbCas12a protein) was dialyzed overnight at 4°C in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 500 mM NaCl, 1 mM EDTA, 10% glycerol, pH 7.5. Subsequently, the protein was step-dialyzed from 500 mM NaCl to 200 mM NaCl (final dialysis buffer: 20 mM HEPES, 200 mM NaCl, 1 mM EDTA, 10% glycerol, pH 7.5). Next, the protein was purified by cation exchange chromatography (5 mL HiTrap-S column, buffer A: 20 mM HEPES pH 7.5 + 1 mM TCEP, buffer B: 20 mM HEPES pH 7.5 + 1 M NaCl + 1 mM TCEP, flow rate 5 mL/min, column volume 5 mL) followed by size-exclusion chromatography on a Superdex-200 (16/60) column (isocratic size-exclusion running buffer = 20 mM HEPES pH 7.5, 150 mM NaCl, 1 mM TCEP for 3xNLS-SpCas9 [or 20 mM HEPES pH 7.5, 300 mM NaCl, 1 mM TCEP for LbCas12a-2xNLS]). The primary protein peak from the size-exclusion chromatography was concentrated in Ultra-15 centrifugal filters (Ultracel −30K; Amicon) to a concentration of ∼100 μM, based on absorbance at 280 nm. The purified protein quality was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis/Coomassie staining to be >95% pure, and protein concentration was quantified with the Pierce BCA Protein Assay kit (ThermoFisher Scientific).
Synthesis of IVS1-110A and IVS2-654T specific guide RNAs
Synthetic single guide RNA (sgRNA) to target SpCas9 to the IVS1-110A mutation site and the AAVS1 control site were synthesized by Synthego with end protection containing the following guide sequences: GGGUGGGAAAAUAGACUAAU and CUCCCUCCCAGGAUCCUCUC (hg19 chr19:55 626 932-55 626 951). Synthetic LbCas12a CRISPR RNAs (crRNAs) to the IVS2-654C>T mutation (rs34451549T) and rs1609812T and the AAVS1 control site were synthesized by Integrated DNA Technologies (IDT) with proprietary modifications to each end of the crRNA (AITR1 on 5′ end and AITR2 on 3′ end):
the LbCas12a rs34451549T/rs1609812T crRNA sequence is /AlTR1/rUrArArUrUrUrCrUrArCrUrArArGrUrGrUrArGrArUrUrArUrGrCrArGrArArArUrArUrUrGrCrUrArUrUrArCrC/AlTR2/ and the LbCas12a AAVS1 crRNA sequence (hg19 chr19:55 627 140-55 627 161) is /AlTR1/rUrArArUrUrUrCrUrArCrUrArArGrUrGrUrArGrArUrUrCrUrGrUrCrCrCrCrUrCrCrArCrCrCrCrArCrArGrUrG/AlTR2/
CD34+ HSPC isolation, RNP electroporation, and culture
Healthy human CD34+ hematopoietic stem and progenitor cells (HSPCs) from mobilized peripheral blood of deidentified healthy donors were obtained from Fred Hutchinson Cancer Research Center (Seattle, WA). CD34+ HSPCs of β-thalassemia patients were isolated from nonmobilized peripheral blood following Boston Children’s Hospital Institutional Review Board approval and patient informed consent. CD34+ HSPCs were enriched using the Miltenyi CD34 Microbead kit (Miltenyi Biotec). CD34+ HSPCs were cultured with X-VIVO 15 (Lonza) supplemented with 100 ng mL−1 human stem cell factor (SCF), 100 ng mL−1 human thrombopoietin, and 100 ng mL−1 recombinant human Flt3 ligand. After 24 hours of culture, HSPCs were electroporated with SpCas9 ribonucleoprotein (RNP) or LbCas12a RNP. Electroporation was performed using Lonza 4D Nucleofector (V4XP-3032 for 20-μL Nucleocuvette Strips) following the manufacturer’s instructions. The RNP complex was prepared by mixing SpCas9 (100 pmol) and sgRNA (300 pmol, OD-based quantification) or LbCas12a (400 pmol) and crRNA (400 pmol, OD-based quantification) and incubating for 15 minutes at room temperature immediately before electroporation. HSPCs (50K) resuspended in 20 μL of P3 solution were mixed with RNP and transferred to a cuvette for electroporation with program EO-100. The electroporated cells were resuspended with X-VIVO media with cytokines and changed into erythroid differentiation medium (EDM) 24 hours later for in vitro differentiation. EDM consisted of Iscove modified Dulbecco medium supplemented with 330 μg/mL human holotransferrin, 10 μg/mL recombinant human insulin, 2 IU/mL heparin, 5% human solvent detergent-pooled plasma AB, 3 IU/mL erythropoietin, 1% l-glutamine, and 1% penicillin/streptomycin. During days 0 to 7 of erythroid culture, EDM was further supplemented with 10−6 M hydrocortisone (Sigma-Aldrich), 100 ng mL−1 human SCF, and 5 ng mL−1 human interleukin-3 (R&D Systems) as EDM-1. During days 7 to 11 of culture, EDM was supplemented with 100 ng mL−1 human SCF only as EDM-2. During days 11 to 18 of culture, EDM had no additional supplements as EDM-3. Globin gene expression, hemoglobin high-performance liquid chromatography (HPLC), enucleation percentage, and cell size were assessed on day 18 of erythroid culture. For clonal liquid culture, edited CD34+ HSPCs were sorted into 150 μL of EDM-1 in 96-well round-bottom plates (Nunc) at 1 cell per well using FACSAria II. The cells were changed into EDM-2 media 7 days later in 96-well flat-bottom plates (Nunc). After an additional 4 days of culture, cells were changed into 150 μL to 500 μL of EDM-3 at a concentration of 1 M/mL for further differentiation. After additional 7 days of culture, one-tenth of the cells were harvested for genotyping analysis, one-tenth of cells were harvested for RNA isolation with an RNeasy Micro kit (Qiagen), and the remaining cells were processed by Hemolysate reagent (Helena Laboratories).
Sequence analysis
Indel frequencies were measured from cells cultured in EDM 5 days after electroporation. Briefly, genomic DNA was extracted using the Qiagen Blood and Tissue kit. The HBB locus was amplified with KOD Hot Start DNA polymerase and corresponding primers (supplemental Table 5, available on the Blood Web site) using the following cycling conditions: 95° for 3 minutes; 35 cycles of 95° for 20 seconds, 60° for 10 seconds, and 70° for 10 seconds; 70° for 5 minutes. Resulting polymerase chain reaction (PCR) products were subjected to Sanger or Illumina deep sequencing. For the IVS1-110 target site analysis, a nested PCR approach was used, with the first round of 10 cycles, and then 1/10 dilution used as template for 35-cycle second-round PCR. The deep sequencing data were analyzed by CRISPResso2 software.13,14 We predicted nuclease cleavage positions to be between positions 3 and 4 counting from the NGG protospacer adjacent motif (PAM) for SpCas9 and between positions 20 and 21 counting from the TTTV PAM for LbCas12a. After alignment, the guide and predicted cleavage site were identified, and the window set around the cleavage site to determine whether the read was modified from the reference sequence. We used a minimum alignment identity of 60% and window size of 2 bp around the cleavage site for SpCas9 and 8 bp around the cleavage site for LbCas12a to account for the known staggered cleavage of the latter nuclease. We manually removed HBD-aligning reads and collapsed read counts by mutations so that reads with the same mutations adjacent the cleavage sites but differences in nonadjacent regions were classified as the same allele. These latter differences are mainly due to sequencing errors or trimming artifacts and not genome editing.
Amplicon sequences were aligned to respective pathogenic (IVS1-110G>A or IVS2-654C>T) reference sequences in CRISPResso2 to generate nucleotide quilts and allele plots. To determine allele-specific editing efficiency, we aligned reads to both pathogenic (IVS1-110G>A or IVS2-654C>T) and nonpathogenic (IVS1-110G or IVS2-654C) reference sequences. Many edited reads aligned equivalently to both reference alleles, so we regarded all edited reads as a single category and calculated editing efficiency using the number of unedited reads for each reference sequence. To account for all edited reads, we split reads with ambiguous alignments in CRISPResso2, which allocates ambiguously aligned reads equally toward both reference sequences. The total read count was then corrected by subtracting double-counted ambiguous alignments from the pool of edited reads. The percentage of unedited nonpathogenic (IVS1-110G and IVS2-654C), unedited pathogenic (IVS1-110G>A and IVS2-654C>T), and edited reads for each treatment and donor replicate were calculated by dividing the respective read counts by the total read number and multiplying by 100. We then calculated the percentage of edited reads for each reference allele and treatment by subtracting the percentage of unedited targeted (IVS1-110A and IVS2-654T RNPs) amplicons from the percentage of unedited control (sgAAVS1) amplicons. The percentage of edited reads was then divided by the percentage of unedited control reads and multiplied by 100 to find the editing efficiency of the IVS1-110A and IVS2-654T RNPs for their respective target sequences.
Gene expression
RNA isolation with RNeasy columns (Qiagen), reverse transcription with the iScript cDNA synthesis kit (Bio-Rad), and reverse transcription–quantitative PCR (RT-qPCR) with iQ SYBR Green Supermix (Bio-Rad) were performed to determine globin gene expression. Primers are listed in supplemental Table 5.
Hemoglobin HPLC
Hemolysates were prepared from erythroid cells after 18 days of differentiation using Hemolysate reagent (Helena Laboratories) and analyzed with a D-10 Hemoglobin Analyzer (Bio-Rad). Because the D-10 Hemoglobin Analyzer is not calibrated to measure HbA2/Lepore/hemoglobin E (HbE), we calculated hemoglobin percentages from areas under the curve (AUCs) measured from HPLC traces in ImageJ (version 2.0.0-rc-68/1.52i). If the hemoglobin A (HbA) peak exceeded the HPLC trace boundaries (eg, the sgIVS1-110G>A RNP-edited sample from the β+βLepore donor), the HbA AUC was extrapolated by dividing the HbF AUC by the HbF percentage calculated by the D-10 Hemoglobin Analyzer and multiplying the difference by the HbA percentage calculated by the D-10 Hemoglobin Analyzer. HbF, HbA, and HbA2/Lepore/HbE percentages were then calculated from the summed AUCs of the 3 peaks.
Flow cytometry
For HSC immunophenotyping, CD34+ HSPCs were incubated with Pacific Blue anti-human CD34 antibody (BioLegend), phycoerythrin/Cy5 anti-human CD38 (BioLegend), allophycocyanin anti-human CD90 (BioLegend), allophycocyanin-H7 mouse anti-human CD45RA (BD Biosciences), and Brilliant Violet 510 anti-human lineage cocktail (BioLegend). Cell sorting was performed on a FACSAria II machine (BD Biosciences). For enucleation analysis, cells were stained with 2 μg mL−1 of the cell-permeable DNA dye Hoechst 33342 (Life Technologies) for 10 minutes at 37°C. The Hoechst 33342–negative cells were further gated for cell-size analysis with forward scatter area parameter. Relative cell size was calculated as median forward scatter area of test samples as compared with healthy donor cells.
Analysis of linkage between IVS2-654C>T and rs1609812
Samples from either individuals or family members underwent β-globin gene nucleotide sequencing at the Hemoglobin Diagnostic Reference Laboratory, Boston Medical Center. Individuals and families with at least 1 member found to have IVS2-654C>T mutation were further examined for their genotype at rs1609812.
Analysis of splice sites
A matrix of base frequencies for >20 000 splice sites from the human genome15 was used to generate a TRANSFAC format matrix. Weblogo3.0 (http://weblogo.threeplusone.com/create.cgi) was used to build a probability sequence logo for consensus splice acceptor and consensus splice donor sequences.16
Analysis of off-target sites
The Cas-OFFinder17 Web tool was used with default settings, including no bulges, to identify all genomic sites with up to 3 mismatches sharing canonical PAM with indicated sgRNA. In addition to sgIVS1-110A and crIVS2-654T, several other guide RNAs considered as therapeutic candidates for hemoglobinopathy therapeutic editing were evaluated, including HBB sg10,18,19 HBG1 promoter gRNA-1,20 and BCL11A enhancer #1617.21
Imaging flow cytometry
In vitro–differentiated D18 erythroid cells stained with Hoechst 33342 were resuspended with 150 μL of Dulbecco phosphate buffered saline for analysis with Imagestream X Mark II (Merck Millipore). Well-focused Hoechst-negative single cells were gated for circularity analysis with IDEAS software. Cells with circularity score above 15 were further gated to exclude cell debris and aggregates. No fewer than 2000 gated cells were analyzed to obtain a median circularity score.
Results and discussion
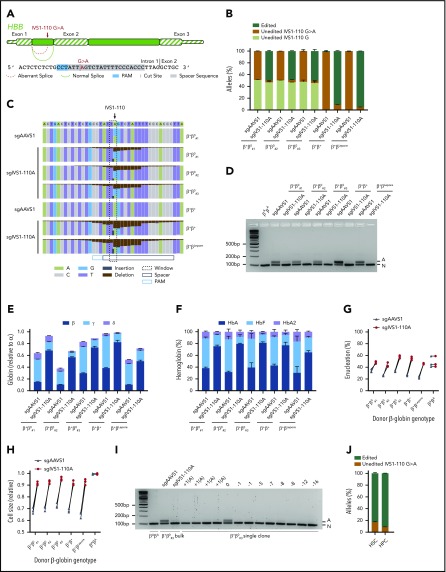
Recently, we optimized conditions for high-efficiency SpCas9 RNP editing of CD34+ HSPCs by electroporation.6 We hypothesized that RNP electroporation of patient CD34+ HSPCs could introduce high-efficiency indels disrupting the aberrant splice sites, abrogating abnormal splicing and restoring β-globin expression. For the IVS1-110G>A target site, we identified a suitable SpCas9 NGG PAM that would direct double-strand break formation directly adjacent to the de novo splice acceptor site (Figure 1A). We isolated nonmobilized peripheral blood CD34+ HSPCs from 5 transfusion-dependent β-thalassemia subjects carrying at least 1 HBB IVS1-110G>A allele. Three of these subjects were compound heterozygous for the IVS1-110G>A allele and a HBB null allele (β+β0#1, β+β0#2, β+β0#3), 1 was homozygous for IVS1-110G>A (β+β+), and 1 was hemizygous for IVS1-110G>A with an HBB-HBD Lepore-Boston-Washington deletion of the other allele (β+βLepore) (supplemental Table 1). We electroporated CD34+ HSPCs from each of these donors with RNP composed of SpCas9-3xNLS protein and a chemically protected sgRNA complementary to the IVS1-110G>A mutant sequence (sgIVS1-110A). Then, we subjected the electroporated cells to an 18-day 3-phase erythroid maturation protocol.22 We found that SpCas9 mutagenesis was highly efficient, with mean 93.0% indel frequency at the IVS1-110G>A alleles within the treated population (Figure 1B-C; supplemental Figure 1A). The SpCas9 RNP preferentially targeted the IVS1-110G>A allele over the IVS1-110G allele due to a single-base mismatch between the guide and target sequence. In the 3 evaluable compound heterozygous subjects (β+β0#1, β+β0#2, β+β0#3), the IVS1-110G allele was inefficiently edited with mean 6.3% indel frequency despite nearly complete editing of IVS1-110G>A.
Figure 1.
Therapeutic gene editing of IVS1-110G>A. (A) Schema of IVS1-110G>A mutation within HBB intron 1 and therapeutic editing strategy. (B) Indicated donors and sgRNAs used for therapeutic editing. Five days after RNP electroporation, amplicon deep sequencing was performed on the SpCas9-treated cells. Following sequence analysis, alleles were classified as edited, unedited IVS1-110G>A or unedited IVS1-110G. (C) Nucleotide quilt showing indels and substitutions at each position around IVS1-110 for indicated donors and SpCas9 RNP treatment groups. β+β0#1 with sgAAVS1 shown as a representative example of an unedited IVS1-110G>A heterozygous donor and β+β+ with sgAAVS1 as a representative example of an unedited IVS1-110G>A homozygous/hemizygous donor. (D) RT-PCR from erythroid progeny with primers spanning the exon 1 to exon 2 junction, demonstrates abrogation of aberrant (A) and increase in normal (N) splicing after therapeutic editing. (E) RT-qPCR of globin genes shows increase in β-globin relative to α-globin expression in erythroid progeny after therapeutic editing. (F) Hemoglobin HPLC shows increase in the HbA fraction after therapeutic editing. (G-H) Flow cytometry shows increase in enucleation fraction and cell size of enucleated erythroid cells after therapeutic editing. (I) RT-PCR from clonal erythroid progeny with primers spanning the exon 1 to exon 2 junction. Indel length of edited IVS1-110G>A allele depicted for individual clones. (J) Fluorescence-activated cell sorting (FACS) of CD34+CD38+ HPC- or CD34+CD38−CD90+CD45RA− HSC-enriched populations 2 hours after therapeutic editing of the β+β+ donor, which was 24 hours after CD34+ HSPC isolation. Indel analysis was performed 5 days after sorting.
To test whether genetic disruption of IVS1-110G>A within CD34+ HSPCs is sufficient to restore β-globin splicing and expression, we analyzed globin gene and hemoglobin protein expression following erythroid differentiation. We performed RT-PCR of β-globin mRNA, spanning the exon 1 to exon 2 junction, followed by gel electrophoresis (Figure 1D). From a healthy donor sample, we observed a single band of the expected size (101-bp amplicon). However, for each of the 5 patients, we found a lower mobility amplicon representing the expected aberrant splice product (118-bp amplicon). After SpCas9 sgIVS1-110A RNP editing, in each of the 5 patient donors, we observed a disappearance of the aberrant splice product and an increase in the intensity of the normal splice product. To quantify these changes, we performed RT-qPCR with a β-globin primer pair specific to the normally spliced isoform. We observed that the expression of β-globin relative to α-globin increased from 20.8% in the sgAAVS1 controls compared with 66.2% in the sgIVS1-110A RNP-edited samples (Figure 1E). Hemoglobin quantification via HPLC showed a corresponding increase in the fraction of HbA from 36.4% to 75.6% after sgIVS1-110A RNP editing (Figure 1F; supplemental Figure 2). We hypothesized that restoration of globin chain balance would improve the quality of terminal erythroid maturation in vitro. We found, for each of the 5 β-thalassemia patient donors, that therapeutic editing restored the enucleation fraction and cell size to the normal range for differentiated erythroid cells, whereas the same editing had no effect on healthy donor differentiated erythroid cells (Figure 1G-H).
To correlate the genotype of individual edited alleles with β-globin expression, we sorted individual cells from donor β+β0#3 for clonal erythroid liquid culture following SpCas9 sgIVS1-110A RNP electroporation of CD34+ HPSCs. We performed paired Sanger genotyping and RT-PCR from 13 clones. In each clone, we found that the IVS1-110G allele was unedited. In 1 clone, the IVS1-110G>A allele was unedited and the aberrant splicing product remained. In each of the other 12 clones, a single indel was present in the IVS1-110G>A allele, ranging in length from a 1-bp insertion to a 16-bp deletion. In these 12 edited clones, the aberrant splice product was absent and only the normal splice product remained (Figure 1I). These results demonstrate that even a +1(A) insertion adjacent to the IVS1-110G>A mutation was sufficient to restore normal β-globin splicing, consistent with nucleotide preference of the consensus splice acceptor site (supplemental Figure 1A).23
Because CD34+ HSPCs are a heterogeneous population of cells,24 of which the majority are committed progenitors, we evaluated the editing in CD34+CD38+ hematopoietic progenitors (HPCs) as compared with an HSC-enriched CD34+CD38−CD90+CD45RA− immunophenotype population (supplemental Figure 3). We sorted the HSC and HPC populations 2 hours after SpCas9 RNP editing, which was performed 24 hours after CD34+ HSPC isolation. We found that indel frequencies were similar in the HSC-gated population (83.1%) as compared with the HPC-gated population (91.1%), indicating that this strategy could efficiently generate therapeutic indels in HSCs.
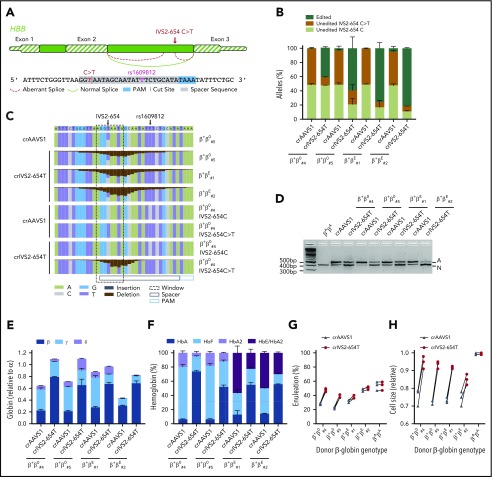
For IVS2-654C>T, there is no suitable NGG PAM neighboring the pathogenic mutation to target SpCas9 cleavage directly to the aberrant splice site. However, a TTTV PAM is appropriately positioned to target cleavage by Lachnospiraceae bacterium ND2006 Cas12a/Cpf1 (LbCas12a)25 to the mutation (Figure 2A). We identified 4 donors with transfusion-dependent β-thalassemia who carried the IVS2-654C>T mutation. Two of these subjects were compound heterozygous for IVS2-654C>T and a HBB null mutation (β+β0#4, β+β0#5) and 2 were compound heterozygous for the IVS2-654C>T mutation and an HbE mutation (β+βE#1, β+βE#2). One of these 4 subjects, β+β0#4, was also heterozygous for the common single-nucleotide polymorphism rs1609812 that overlaps the LbCas12a guide RNA sequence whereas the other 3 subjects were rs1609812-T/T homozygotes. Because Cas12a has been reported to be exquisitely specific, with even a single mismatch to the guide sequence being able to prevent cleavage,26 we determined the linkage of the IVS2-654C/T and rs1609812-C/T variants. We queried a set of 32 IVS2-654C>T alleles that had been ascertained through clinical sequencing for which linkage could be assigned. We found in each case IVS2-654T and rs1609812-T were found on the same haplotype, indicating perfect linkage disequilibrium between IVS2-654T and rs1609812-T (D′ = 1) (supplemental Table 2). Consistent with this analysis, deep sequencing confirmed that IVS2-654T was coinherited with rs1609812-T in the β+β0#4 donor.
Figure 2.
Therapeutic gene editing of IVS2-654C>T. (A) Schema of IVS2-654C>T mutation and therapeutic editing strategy. Cut site is shown at midpoint of expected Cas12a staggered cleavage. (B) Indicated donors and crRNAs used for therapeutic editing. Five days after RNP electroporation, amplicon deep sequencing was performed on the LbCas12a-treated cells. Following sequence analysis, alleles were classified as edited, unedited IVS2-654C>T, or unedited IVS2-654C. (C) Nucleotide quilt showing indels and substitutions at each position around IVS2-654 for indicated donors and LbCas12a RNP treatment groups. β+β0#5 with sgAAVS1 shown as a representative example of an unedited IVS2-654C>T heterozygous donor with rs1609812-T/T. β+β0#4 shown as a donor in which the IVS2-654C/rs1609812-C and IVS2-654C>T/rs1609812-T alleles could be distinguished. (D) RT-PCR from erythroid progeny with primers spanning the exon 2 to exon 3 junction, demonstrates abrogation of aberrant (A) and increase in normal (N) splicing after therapeutic editing. (E) RT-qPCR of globin genes shows increase in β-globin relative to α-globin expression in erythroid progeny after therapeutic editing. (F) Hemoglobin HPLC shows increase in the HbA fraction after therapeutic editing. (G-H) Flow cytometry shows increase in enucleation fraction and cell size of enucleated erythroid cells after therapeutic editing.
We electroporated CD34+ HSPCs from each donor with LbCas12a RNP composed of LbCas12a protein and a crRNA complementary to the IVS2-654C>T mutant sequence (crIVS2-654T) and rs1609812-T. The cells were then subjected to erythroid differentiation culture. Editing by LbCas12a was efficient, with mean 76.6% indel frequency at the IVS2-654C>T alleles (Figure 2B-C; supplemental Figure 1B). The LbCas12a RNP was able to distinguish against alleles with IVS2-654C/rs1609812-C genotype (2.9% indels) but not against alleles with IVS2-654C/rs1609812-T genotype (66.4% indels). Each of the frequent indels at IVS2-654C>T were deletions overlapping the mutation that disrupt the aberrant splice donor site (supplemental Figure 1B).
RT-PCR of β-globin, spanning the exon 2 to exon 3 junction, followed by gel electrophoresis, demonstrated the expression of normal and aberrant splice products in the differentiated erythroid cells from each affected donor (Figure 2D). From a healthy donor sample, we observed only a single band of the expected size (395-bp amplicon). In the unedited patient samples, we observed an additional band demonstrating the expected aberrant splice product (468-bp amplicon). After crIVS2-654T RNP editing, in each of the 4 patient donors, we observed a reduction of the aberrant splice product and reciprocal increase of the normal splice product. We performed RT-qPCR with a β-globin primer pair specific to the normally spliced isoform to quantify the increase in properly spliced product. After editing, we observed that the expression of β-globin relative to α-globin increased from 25.5% in crAAVS1-treated control to 70.1% in crIVS2-654T RNP-edited samples (Figure 2E). Hemoglobin quantification via HPLC showed an increase in the fraction of HbA from 9.9% to 59.1% after IVS2-654T editing (Figure 2F; supplemental Figure 2). For each of the 4 β-thalassemia patient donors, therapeutic editing of IVS2-654C>T by LbCas12a restored the enucleation fraction and cell size toward the normal range, whereas the same editing had no effect on healthy donor cells (Figure 2G-H).
In this study, we demonstrate that CRISPR-Cas RNP electroporation of CD34+ HSPCs is an efficient strategy to disrupt aberrant splice site mutations. We apply this approach to yield phenotypic rescue of 2 common β-thalassemia mutations, IVS1-110G>A and IVS2-654C>T. These are among the most frequent mutations in specific populations affected by β-thalassemia, namely individuals of Mediterranean or east Asian ancestry, respectively. The SpCas9 cleavage site adjacent to IVS1-110G>A is 16 bases 3′ of the canonical branchpoint sequence,27,28 so the majority of indels would not be expected to impact splicing. Indeed, we find that each of the commonly observed SpCas9-induced indels adjacent to the aberrant IVS1-110G>A splice acceptor site, including the frequent +1(A) insertion, restores normal splicing to β-globin. The overall efficiency of indels in CD34+ HSPCs plus the penetrance of splice site disruption indicate the robustness of this therapeutic-editing strategy. We note that the frequency of genomic sites with closely matching sequences to the sgIVS1-110A and crIVS2-654T guide sequences is of similar magnitude as other guides considered for therapeutic gene editing (supplemental Table 3),18-21 although thorough empirical evaluation of potential off-target genotoxicity would be required prior to clinical translation.
This is the first description to our knowledge of efficient RNP editing in CD34+ HSPCs with the Cas12a nuclease platform. We speculate that further optimization of Cas12a RNP composition might lead to even higher editing frequencies, analogous to the iterative improvements of SpCas9 RNP editing in CD34+ HSPCs reported over recent years.5,6 Although the efficiency of mutagenesis by LbCas12a was modestly lower than SpCas9, the indels that were produced in HSPCs were almost exclusively deletions that span the target mutation and aberrant splice site. This property of Cas12a proteins to produce slightly longer deletions and fewer insertions as compared with SpCas9 may make them especially useful for the targeted disruption of genomic elements.29 Furthermore, we found that the IVS2-654C>T mutation was in perfect linkage disequilibrium with the T allele at the common single-nucleotide polymorphism rs1609812, suggesting that a universal guide RNA design complementary to rs1609812-T could be used to target the IVS2-654C>T allele in the majority of affected individuals.
Alternative genetic therapies for the β-hemoglobin disorders have largely focused on globin gene addition, induction of HbF, or repair of the sickle hemoglobin mutation.30 A challenge to the development of gene repair approaches for the β-thalassemias has been the apparent need to develop individual repair strategies for each mutation in addition to intrinsic challenges of therapeutic homologous recombination.31,32 Here, we propose that aberrant splice site disruption could be a simple and efficient strategy for β-thalassemia patients carrying at least 1 aberrant splice site mutation. Even monoallelic restoration of normal β-globin splicing in a subset of HSCs could be sufficient to convert transfusion-dependent β-thalassemia to an asymptomatic hematologic condition.33 We anticipate that this aberrant splice site disruption approach could be extended to additional mutations, disorders, and editing systems (supplemental Table 4).
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
Z. Herbert of Molecular Biology Core Facilities at Dana-Farber Cancer Institute aided with amplicon deep sequence library generation and sequencing. C. Brugnara and E. Esrick of Boston Children’s Hospital helped with patient recruitment. P. Liu of University of Massachusetts Medical School designed the SpCas9 and LbCas12a constructs that were used for IVS-I and IVS-II editing, respectively. J. Desimini offered graphical assistance. The authors appreciate helpful discussions with S. Orkin, M. Hossain, M. Harris, C. Ma, K. Clement, and G. Lettre. The authors are indebted to the Boston Children’s Hospital clinical staff, and especially thalassemia patients, for participation.
L.P. was supported in part by the National Institutes of Health, National Human Genome Research Institute grant R00HG008399. S.A.W. was supported in part by National Institutes of Health, National Institute of Allergy and Infectious Diseases grant R01AI117839 and National Institutes of Health, National Institute of General Medical Sciences grant 1R01GM115911. D.E.B. was supported in part by National Institutes of Health, National Heart, Lung, and Blood Institute grants DP2OD022716 and P01HL032262, the Doris Duke Charitable Foundation, and the Burroughs Wellcome Fund.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The amplicon sequence data reported in this article have been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession number SRP182804).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.X. performed experiments, assisted by K.L., Q.Y., J.Z., Y.W., and C.B.; K.L. produced Cas9 and Cas12a proteins; Q.Y., A.H.S., and L.P. performed sequence analysis; H.-Y.L. and D.H.K.C. performed linkage analysis; S.A.W. and D.E.B. conceived and supervised the study; and S.X., S.A.W., and D.E.B. wrote the manuscript, with input from all authors.
Conflict-of-interest disclosure: S.X., K.L., S.A.W., and D.E.B. have filed patent applications related to therapeutic gene editing. The remaining authors declare no competing financial interests.
Correspondence: Scot A. Wolfe, Department of Molecular, Cell and Cancer Biology, University of Massachusetts Medical School, Worcester, MA 01605; e-mail: scot.wolfe@umassmed.edu; and Daniel E. Bauer, Division of Hematology/Oncology, Boston Children’s Hospital, 1 Blackfan Circle, Karp 8211, Boston, MA 02115; e-mail: daniel.bauer@childrens.harvard.edu.
REFERENCES
- 1.Hoban MD, Bauer DE. A genome editing primer for the hematologist. Blood. 2016;127(21):2525-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang HHY, Pannunzio NR, Adachi N, Lieber MR. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18(8):495-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohrin M, Bourke E, Alexander D, et al. . Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7(2):174-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese P, Schiroli G, Escobar G, et al. . Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014;510(7504):235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlesworth CT, Camarena J, Cromer MK, et al. . Priming human repopulating hematopoietic stem and progenitor cells for Cas9/sgRNA gene targeting. Mol Ther Nucleic Acids. 2018;12:89-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Zeng J, Roscoe BP, et al. . Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Origa R. Beta-thalassemia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 2018:1-33. [Google Scholar]
- 8.Kountouris P, Lederer CW, Fanis P, Feleki X, Old J, Kleanthous M. IthaGenes: an interactive database for haemoglobin variations and epidemiology. PLoS One. 2014;9(7):e103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spritz RA, Jagadeeswaran P, Choudary PV, et al. . Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci USA. 1981;78(4):2455-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau Y-L, Chan LC, Chan YY, et al. . Prevalence and genotypes of α- and β-thalassemia carriers in Hong Kong -- implications for population screening. N Engl J Med. 1997;336(18):1298-1301. [DOI] [PubMed] [Google Scholar]
- 11.Cheng TC, Orkin SH, Antonarakis SE, et al. . beta-Thalassemia in Chinese: use of in vivo RNA analysis and oligonucleotide hybridization in systematic characterization of molecular defects. Proc Natl Acad Sci USA. 1984;81(9):2821-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takihara Y, Matsunaga E, Nakamura T, et al. . One base substitution in IVS-2 causes a beta +-thalassemia phenotype in a Chinese patient. Biochem Biophys Res Commun. 1984;121(1):324-330. [DOI] [PubMed] [Google Scholar]
- 13.Clement K, Rees H, Canver MC, et al. . CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat Biotechnol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinello L, Canver MC, Hoban MD, et al. . Analyzing CRISPR genome-editing experiments with CRISPResso. Nat Biotechnol. 2016;34(7):695-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma SL, Vega-Warner V, Gillies C, et al. . Whole exome sequencing reveals novel PHEX splice site mutations in patients with hypophosphatemic rickets. PLoS One. 2015;10(6):e0130729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae S, Park J, Kim JS. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30(10):1473-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeWitt MA, Magis W, Bray NL, et al. . Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med. 2016;8(360):360ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dever DP, Bak RO, Reinisch A, et al. . CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539(7629):384-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traxler EA, Yao Y, Wang YD, et al. . A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat Med. 2016;22(9):987-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canver MC, Smith EC, Sher F, et al. . BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527(7577):192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giarratana MC, Rouard H, Dumont A, et al. . Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118(19):5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004;11(2-3):377-394. [DOI] [PubMed] [Google Scholar]
- 24.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218-221. [DOI] [PubMed] [Google Scholar]
- 25.Zetsche B, Gootenberg JS, Abudayyeh OO, et al. . Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strohkendl I, Saifuddin FA, Rybarski JR, Finkelstein IJ, Russell R. Kinetic basis for DNA target specificity of CRISPR-Cas12a. Mol Cell. 2018;71(5):816-824.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruskin B, Krainer AR, Maniatis T, Green MR. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984;38(1):317-331. [DOI] [PubMed] [Google Scholar]
- 28.Pineda JMB, Bradley RK. Most human introns are recognized via multiple and tissue-specific branchpoints. Genes Dev. 2018;32(7-8):577-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swarts DC, Jinek M. Cas9 versus Cas12a/Cpf1: structure-function comparisons and implications for genome editing. Wiley Interdiscip Rev RNA. 2018;9(5):e1481. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari G, Cavazzana M, Mavilio F. Gene therapy approaches to hemoglobinopathies. Hematol Oncol Clin North Am. 2017;31(5):835-852. [DOI] [PubMed] [Google Scholar]
- 31.Xu P, Tong Y, Liu XZ, et al. . Both TALENs and CRISPR/Cas9 directly target the HBB IVS2-654 (C > T) mutation in β-thalassemia-derived iPSCs. Sci Rep. 2015;5(1):12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antony JS, Latifi N, Haque AKMA, et al. . Gene correction of HBB mutations in CD34+ hematopoietic stem cells using Cas9 mRNA and ssODN donors. Mol Cell Pediatr. 2018;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreani M, Testi M, Gaziev J, et al. . Quantitatively different red cell/nucleated cell chimerism in patients with long-term, persistent hematopoietic mixed chimerism after bone marrow transplantation for thalassemia major or sickle cell disease. Haematologica. 2011;96(1):128-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.